- 1Faculty of Medicine, Institute of Medical Science, University of Toronto, Toronto, ON, Canada
- 2Bloorview Research Institute, Holland Bloorview Kids Rehabilitation Hospital, Toronto, ON, Canada
- 3Department of Applied Psychology and Human Development, OISE; University of Toronto, Toronto, ON, Canada
- 4Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada
- 5Map and Data Library, University of Toronto, Toronto, ON, Canada
- 6Department of Pediatrics, University of Toronto, Toronto, ON, Canada
Background: Sex differences in the prevalence of neurodevelopmental disorders such as autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) are well documented, but studies examining sex differences in social and communication function remain limited and inconclusive.
Objectives: The objective of this study is to conduct a meta-analysis of sex differences in social-communication function in children with ASD or ADHD and typically developing controls.
Methods: Using PRISMA, a search was performed on Medline and PSYCHINFO on English-language journals (2000–2017) examining sex differences in social and communication function in ASD and ADHD compared to controls. Inclusion criteria: 1) peer reviewed journal articles, 2) diagnosis of ASD or ADHD and controls, 3) age 6–18 years, 4) measures of social–communication function, and 5) means, standard deviations, and sample sizes reported in order to calculate standardized mean differences (SMD).
Results: Eleven original/empirical studies met inclusion criteria for ASD and six for ADHD. No significant sex differences were found between ASD and controls in social (SMD = −0.43; p = 0.5; CI: −1.58–0.72), or communication function (SMD = 0.86; p = 0.5 CI; −1.57–−3.30) and between ADHD and controls in social function (SMD = −0.68: p = 0.7, CI: −4.17–2.81). No studies evaluated sex differences in communication in ADHD. Significant heterogeneity was noted in all analyses. Type of measure may have partially accounted for some variability between studies.
Conclusions: The meta-analysis did not detect sex differences in social and communication function in children with ASD and ADHD; however, significant heterogeneity was noted. Future larger studies, controlling for measure and with adequate numbers of female participants are required to further understand sex differences in these domains.
Introduction
Rationale
Autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD) are neurodevelopmental disorders, affecting multiple aspects of behavior and cognition (1). Sex differences in prevalence are well documented, but how such sex differences interact/impact core symptom domain phenotypes remains unclear. Given the potential implications for both understanding biology and developing effective interventions, understanding such interactions is critical.
ASD is characterized by deficits in social communication, and repetitive/restricted behaviors, and occurs in approximately 1.5% of children (2, 3). ADHD is characterized by difficulties in attention, hyperactivity, and impulsivity, and has a prevalence of 5–7% in children (4). Comorbidity among these disorders has been reported to be high. The prevalence of comorbid ADHD is reported to be between 30 and 80% in individuals with ASD (5, 6) whereas the presence of ASD is estimated to range between 20 to 50% of individuals with ADHD (7–9). There is also consistent evidence of overlapping behavioral traits, such as inattention, hyperactivity, inhibitory control and other executive functions, repetitive behavior, and social deficits across these disorders, although such symptoms are not always a part of core symptom domains for a specific disorder (6, 10–14).
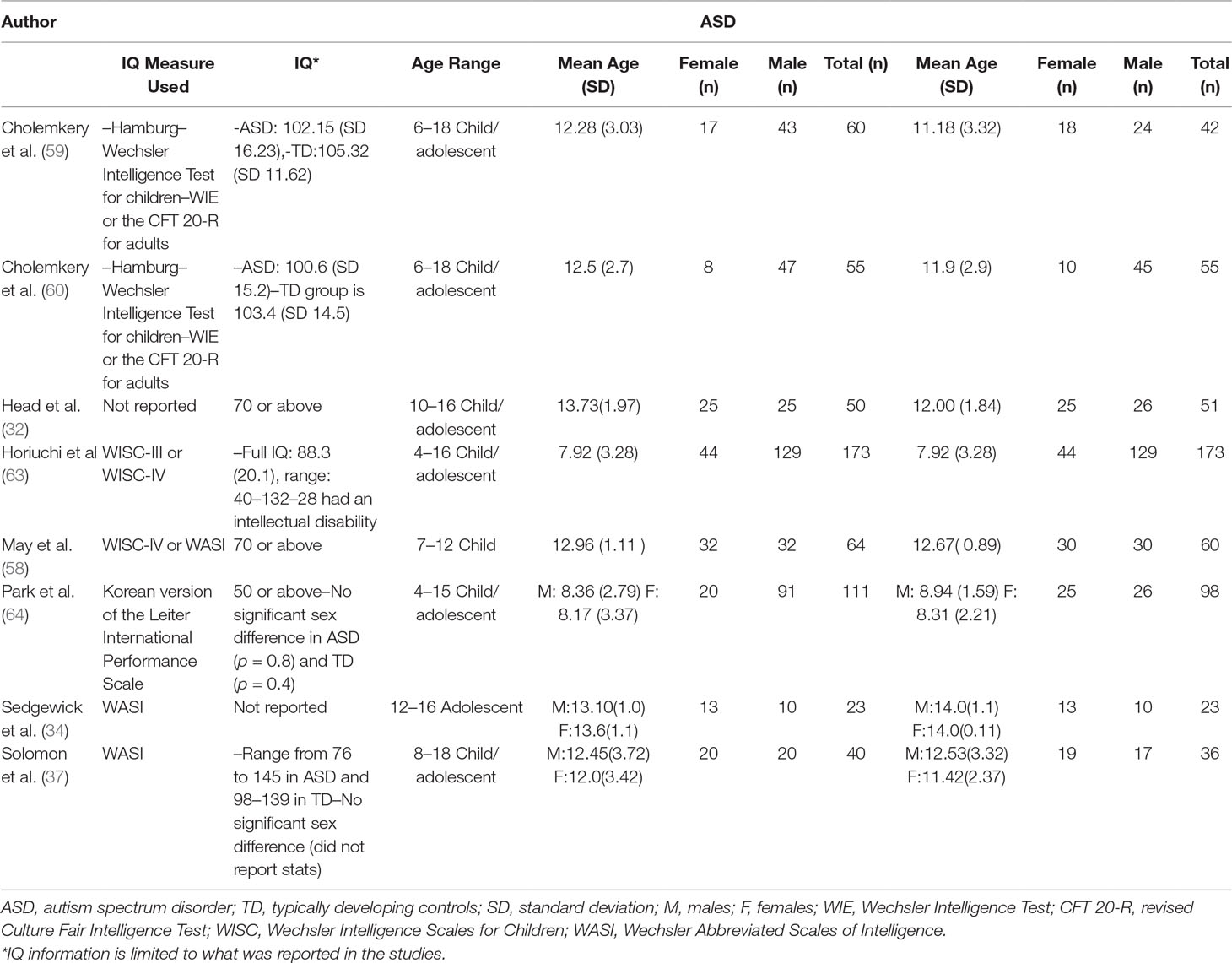
Both ASD and ADHD are characterized by male predominance. The male to female ratio in ASD has been reported to range from 1.33:1 to 16:1 (15–18). IQ has been reported to influence male to female ratios, with higher ratios (10:1) in individuals with higher IQs and lower ratios (2:1) in individuals with comorbid intellectual disability (15, 19). In ADHD, the male to female ratio is reported to vary between 6:1 (clinical samples) and 3:1 (community samples). Considering the differences in the prevalence of these disorders in males and females, it is important to understand how core symptom presentation may vary by sex.
Social communication deficits are a core symptom of ASD (1), but have also been reported in ADHD. For example, studies have found children with ADHD to have impairments in peer relations and poor friendship quality and stability (20, 21). Some research has argued that social difficulties in children with ADHD may result directly from ADHD symptoms (22, 23) rather than reflecting qualitative impairments in social–communicative function that are characteristic of ASD (24). However, in contrast to this hypothesis, several authors report the presence of social and communicative profiles in ADHD that are qualitatively similar to those associated with ASD (25–27). For example, studies that use the Child Communication Checklist and Social Responsiveness Scale have found that children with ADHD are impaired in a similar manner to many children with ASD (28, 29), suggesting that social–communication impairment in ADHD may not entirely result from ADHD symptoms alone as suggested by Huang-Pollack et al. (22); Tseng and Gau (23). Even though social deficits are seen across these neurodevelopmental disorders, and may indeed have similar presentations, it is unclear how/whether sex differences in prevalence and onset observed in these disorders influence severity of social and communication deficits. Investigating such differences will help us understand the experiences and the unique manifestations/needs of males and females diagnosed with different neurodevelopmental disorders.
There have been relatively few studies in ASD examining sex differences in social–communication function, and findings have been inconsistent. Some studies found that females diagnosed with ASD engaged in significantly more social/peer interaction and had better communication skills compared to males (30–34), while others found no significant differences between males and females (18, 35–37), and some reported that adolescent females had more social–communication difficulties than males (38, 39). Previous systematic reviews have attempted to synthesize inconsistent results and have found no significant differences in social communication function in males and females with ASD. However, these reviews did not include studies with a control group against which to compare findings (40, 41).
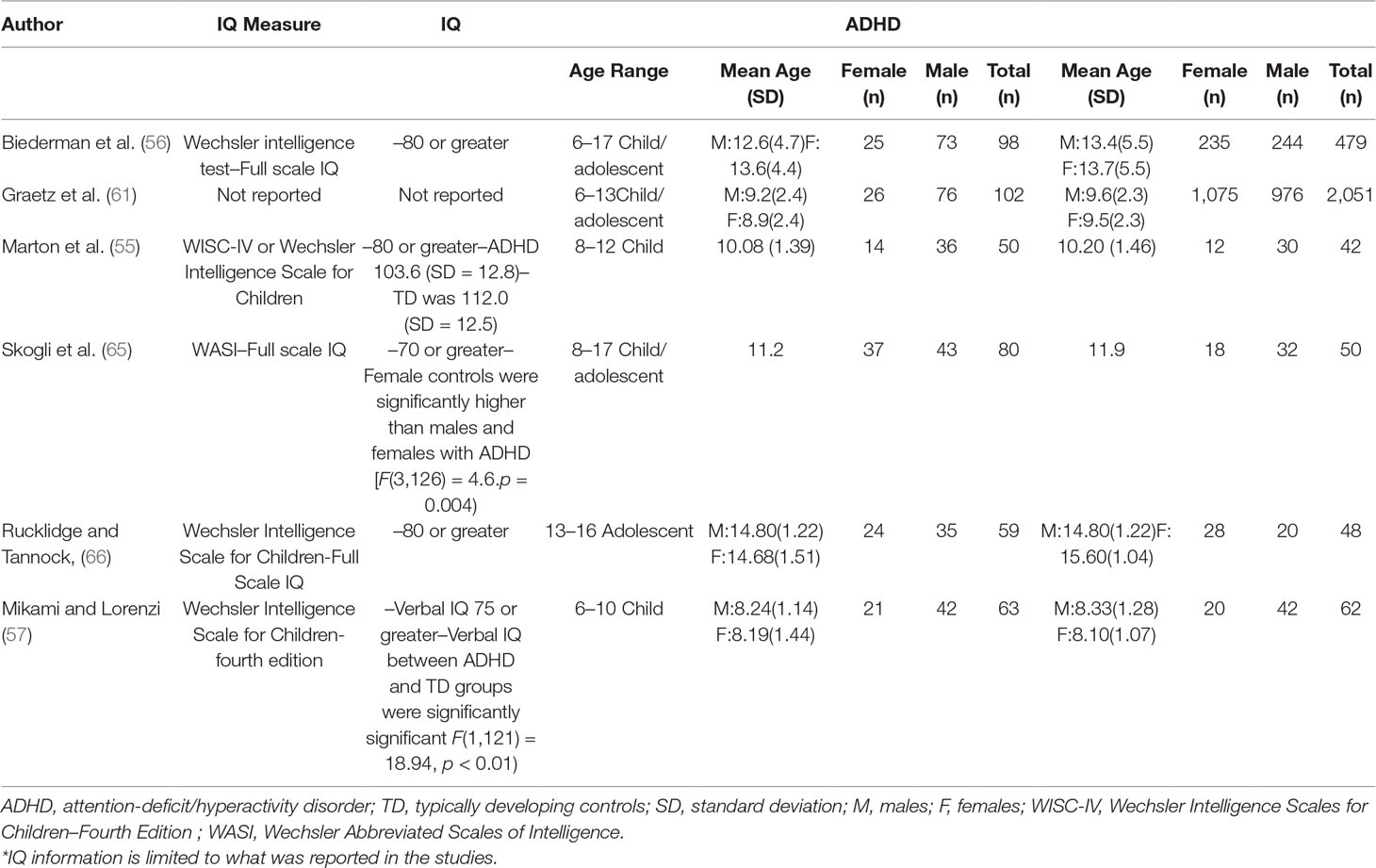
Similarly, evidence for sex differences in social–communication function in ADHD remain inconsistent. Most of the literature on ADHD has focused mostly on males and there is limited information on peer relation and social interaction difficulties in females with ADHD (42). While some studies have documented more deficits in peer interaction in males than females (43, 44), other studies found that females were more likely to be rejected/disliked by peers than males (45, 46). Furthermore, a few studies have reported no sex-related differences in social functioning (47–49). To date, the meta-analyses by Gaub and Carlson (50) and Gershon (51) are the only meta-analyses that have examined sex differences in social functioning in children/adolescents with ADHD. Even though both meta-analyses concluded that there were no differences between males and females with ADHD with respect to social/peer functioning, the analyses lacked typically developing control groups, and were performed more than 15 years ago. Thus, some of the study participants were diagnosed with ADHD based on DSM III criteria, but most importantly no studies from the last 15 years were included.
In summary, although sex differences are well documented in the prevalence of neurodevelopmental disorders, and social deficits are observed across such disorders, there is limited research examining how such sex differences may influence social and communication function. Previous attempts at synthesizing available evidence did not include typically developing control (TD) groups, making it unclear whether observed sex differences are similar to those found in the general population or are specific to a condition. Thus, this meta-analysis will attempt to examine whether there are sex differences in social–communication function between children with ASD and ADHD and controls.
Research Objective
This study will review the current literature in order to examine potential sex differences in social–communication function in children with ASD and ADHD compared to typically developing controls.
Methods
Study Design
The current study is a meta-analysis of the literature that will examine sex differences in social and communication function in children diagnosed with ASD and ADHD compared to controls, followed by a meta-analysis of a subgroup of studies to summarize and quantitatively compare sex differences in social and communication function between children with these developmental disorders and controls.
Search Strategy
A search was performed using OVID Medline and PsychINFO databases for relevant articles in September 2017, on sex differences in social and communication function in ASD and ADHD, using the keywords listed in Table 1. Key search terms and Medical Subject Headings terms (MeSH-used for indexing articles) for Medline and PsychINFO for neurodevelopmental disorders, sex differences and social and communication behaviors were selected with the assistance of an academic librarian (PW). During development of key search terms and MeSH headings, the key words, “social” and “communication” were found to produce a more extensive and broader search as these terms captured a wide range of types of social and communication skills, such as social pragmatic skills, verbal and nonverbal communication.
Participant/Comparators
The inclusion criteria were: 1) peer reviewed journal articles, 2) published in English between the year 2000 and 2017, 3) males and females in the sample, 4) diagnosis of ASD or ADHD by DSM criteria and typically developing controls, 4) age range of 6–18 years old, 5) sex differences between the diagnostic group (i.e., ASD or ADHD) and controls tested using measures of social–communication function, and 6) means, standard deviations, and sample sizes reported in order to calculate standardized mean differences (SMD).
Systematic Review Protocol
Title and abstract of articles were screened for inclusion criteria by two raters (TM, MM). A third rater was consulted in case of discrepancies (EA). In addition, articles were excluded if they were off topic, descriptive studies, did not provide mean scores, standard deviations, and sample sizes for social or communication function for males and females, and/or did not include a typically developing control group. Authors of excluded articles were contacted to request data on control groups for the inclusion in the analysis, but none provide the requested information.
Data Extraction
We used the Quality Assessment Tool for Cohort and Cross-Sectional Studies to assess the quality of the studies (please see Supplemental Tables 1 and 2). Variables extracted for analysis included mean age and standard deviation, sample sizes of males and females with a developmental disorder and controls, type of measure used to assess social and/or communication function, mean scores and standard deviations for females and males on these measures.
Data Analysis
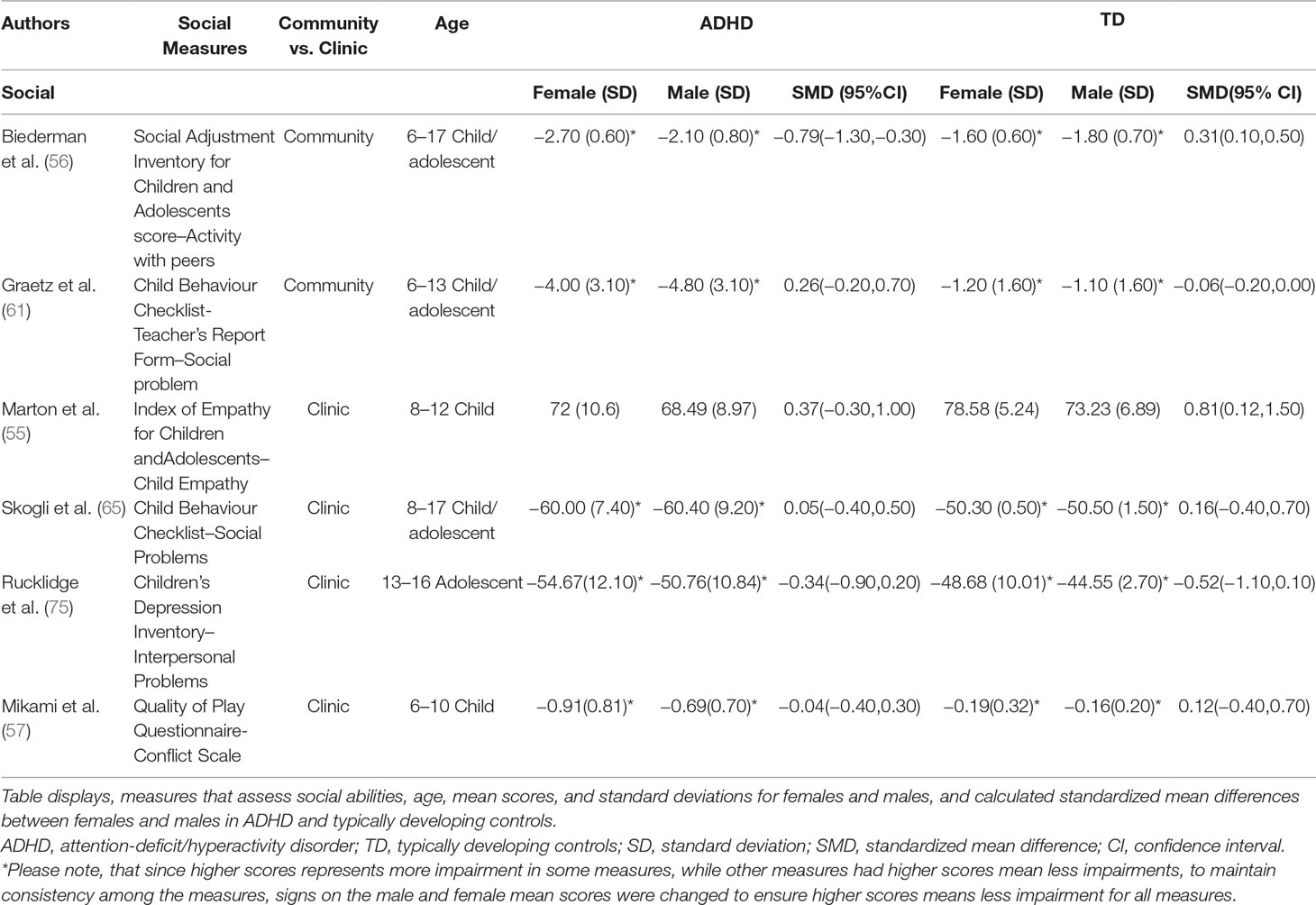
Random-effects meta-analyses were performed using the “metafor” package in R (52, 53; R Project for Statistical Computing, RRID: SCR_001905) for measures of social and communication function in ASD and social function in ADHD. We used a random-effects model to account for variance within and between studies caused by sampling error and other artefacts (54). Standardized mean sex differences for social and communication function were calculated in ASD and for social function in ADHD. We then calculated SMD between groups. Social and communication function were analyzed separately since some studies tested these individually or only tested for one of these. Where tests for heterogeneity were significant, a mixed-effects model was used to test for the effect of the moderator “Measure” (test/instrument used), as well as “Age” [age of participant-categorized into child (6–12 years); child/adolescent (for studies including both children and adolescents); adolescent (12–18 years)]. ASD groups were entered into the analysis first. Positive effect sizes represent females outperforming males more the in ASD relative to controls, while negative effect sizes represent males outperforming females more in ASD relative to controls. Where multiple measures of the same symptoms were used within one study, we report on measures that were commonly used in other studies. A few studies had more than one measure that assessed social and/or communication behaviors. For the ADHD articles, some articles used more than one measure to assess social function (55–57). To maintain consistency across all studies, measures were selected if they assessed social behavior and if they were parent reports (i.e. My Child—55; Social Adjustment Inventory for Children and Adolescents—56; Quality of Play Questionnaire—57). For ASD social function, we found that three studies had reported both the total scores and social communication domain scores of the SRS (58–60). To determine whether we should report the effect size of the total score versus the social communication domain score, the effect sizes of the SRS total scores and social communication domain scores were plotted on to a forest plot and were compared. As both were found to have similar effect sizes and to stay consistent with studies that publish total scores, we decided to use the SRS total scores. Additionally, given that two of the ADHD studies (56 and 61) used community rather than clinic samples, we ran the analyses with and without them. All R scripts for all analyses were borrowed from Dr. Laura Hull (40) and slightly modified with her permission. The R-Script used in the present study is available upon request.
Results
Study Selection and Characteristics
The initial database search identified 2,105 results (Figure 1). Of the 2,105 studies found, 1,805 were excluded based on title review, which led to 300 articles available for abstract review. From those, articles were excluded if they were off topic (n = 109) or were descriptive studies (n = 10). Of the remaining 181 articles, 164 articles were excluded after a thorough examination of the data provided (123 articles did not provide mean scores, standard deviations, and sample sizes for social or communication function/deficit for males and females, 36 articles did not include a typically developing control group while 5 articles did not report social–communication scores on any measures). Only 17 original/empirical studies met the inclusion criteria; 11 studies measuring social–communication function in ASD and 6 studies measuring social function in ADHD. Figure 1 provides a detailed overview of this selection process. A summary of the quality of the studies included is seen in Supplemental Tables 1 and 2. All studies were cross sectional in nature. All but two studies represented clinical samples, which are associated with high risk of bias. Study demographics for ASD and ADHD are presented in and Tables 2 and 3 respectively.
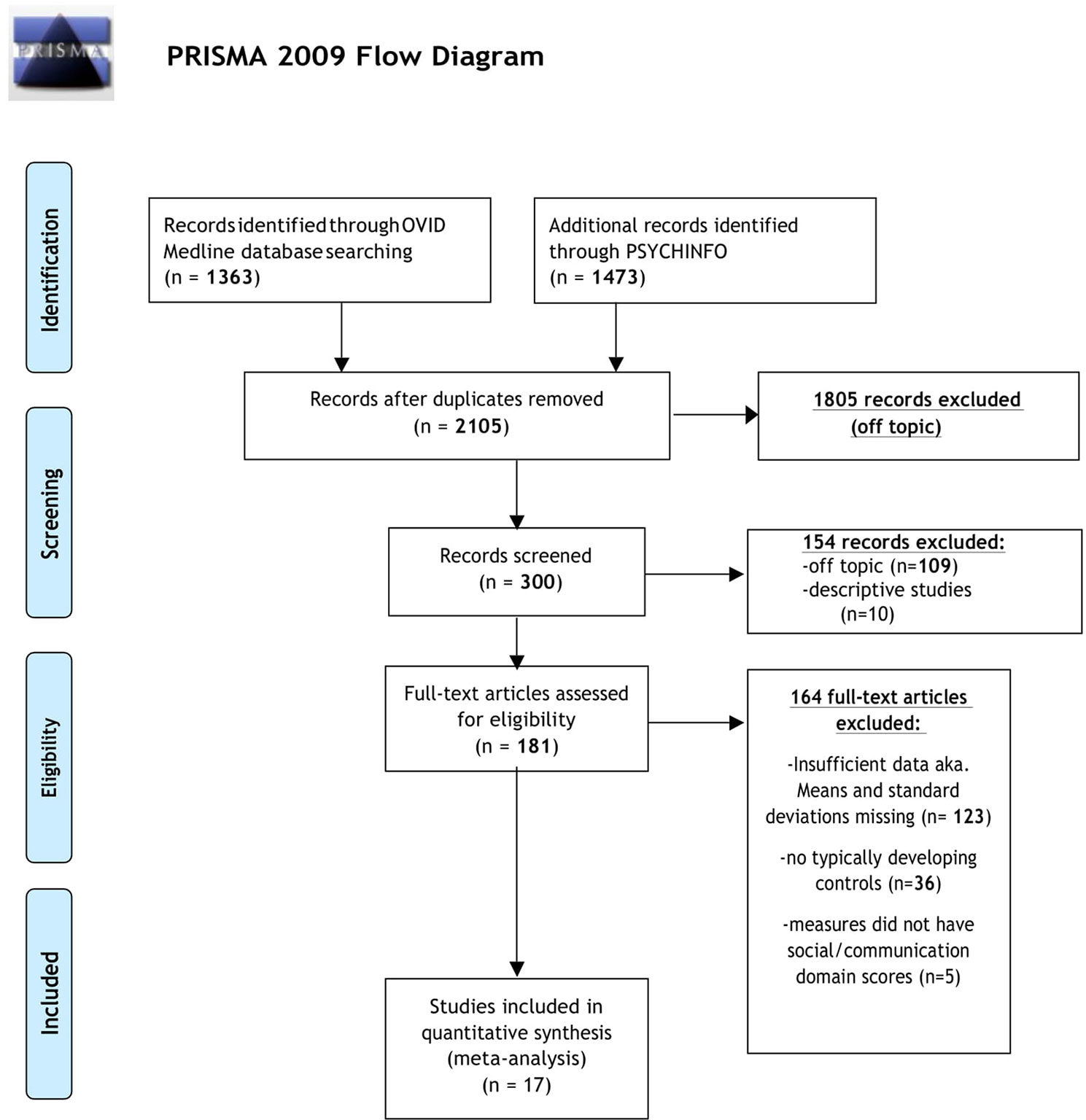
Figure 1 PRISMA flow diagram displaying article selection process. Flow chart from: (62).
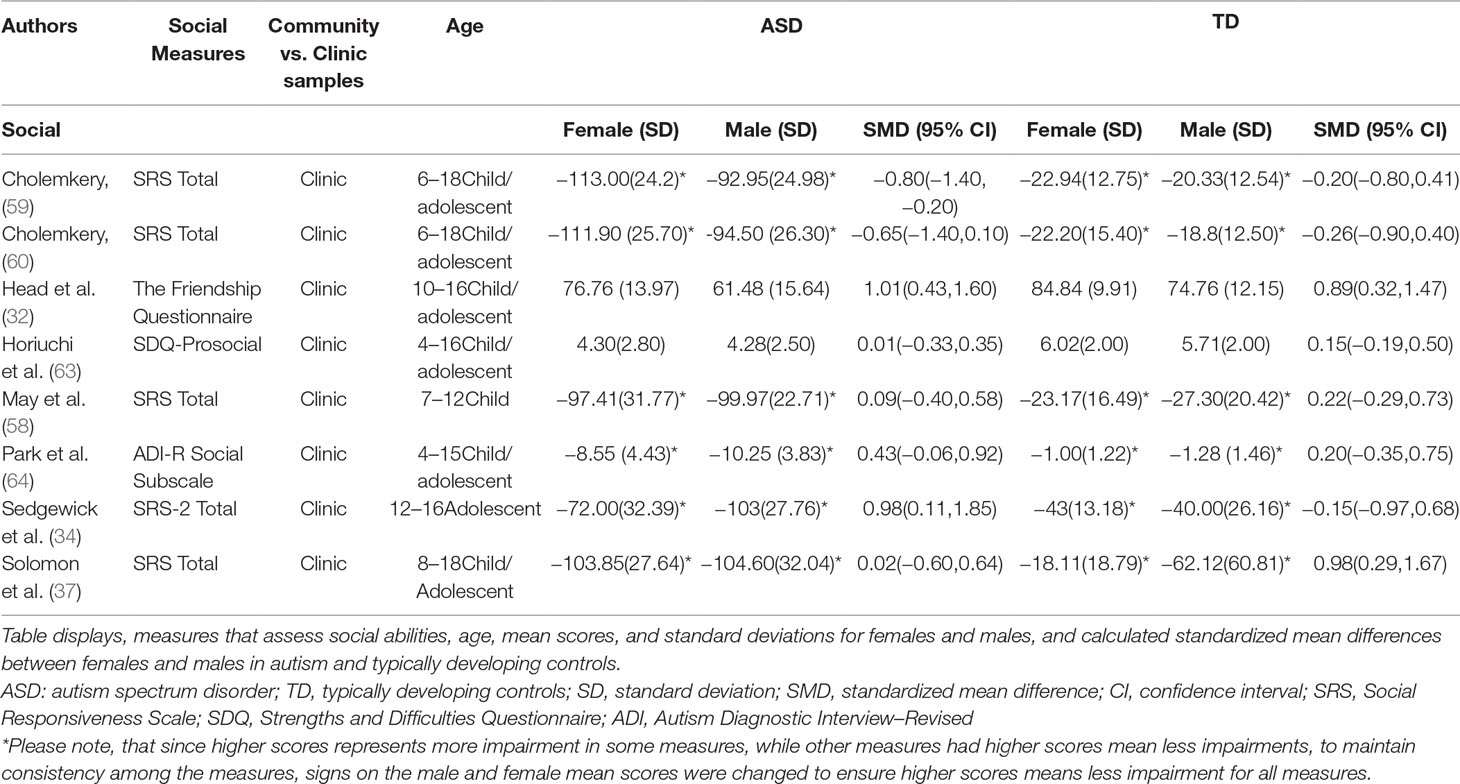
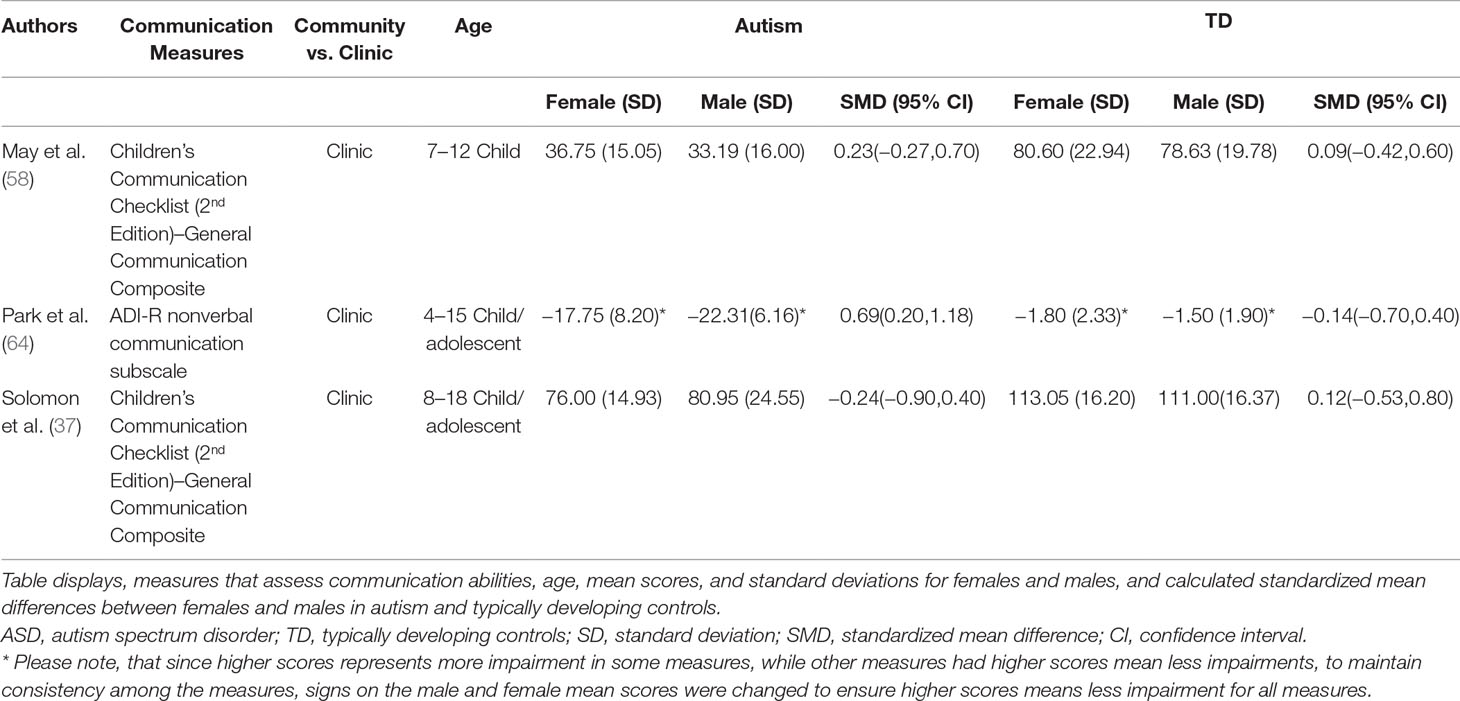
Standardized mean sex differences for social and communication function were calculated in ASD (Tables 4 and 5), and for social function in ADHD (Table 6). SMD were then computed between groups using the “metafor” package in R software (52, 53; R Project for Statistical Computing, RRID: SCR_001905), to yield pooled SMDs; the pooled SMDs are represented in the forest plots in Figures 2–4. Please note that since higher scores represent more impairment in some measures but better abilities in others, signs on scores were changed to ensure higher scores indicate less impairment on all measures. A positive effect size indicates that females outperformed males.
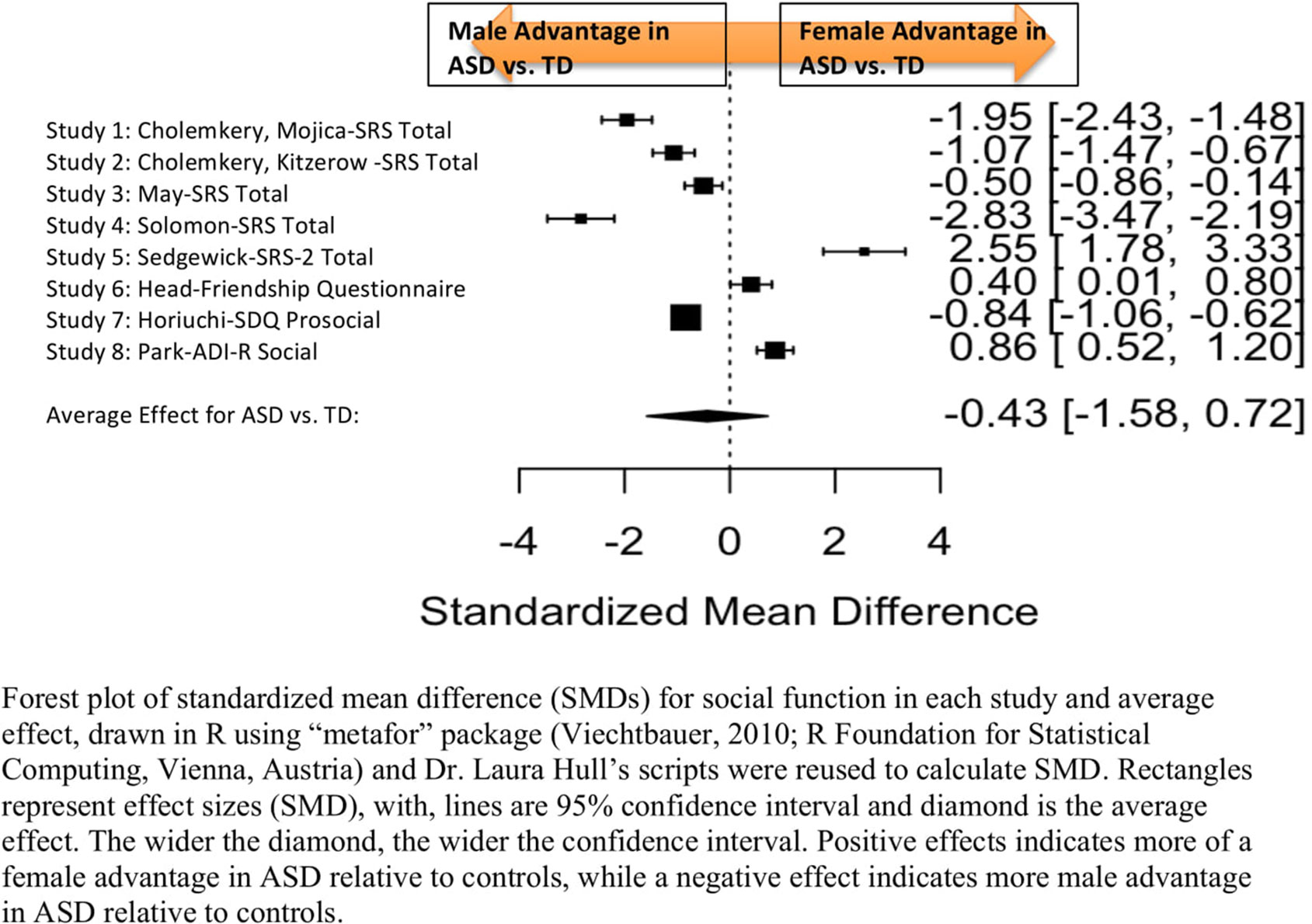
Figure 2 Meta-analysis of studies comparing sex differences in social abilities between ASD and controls. Forest plot of standardized mean difference (SMDs) for social abilities in each study and average effect, drawn in R using “metafor” package (48; R Foundation for Statistical Computing, Vienna, Austria) and Dr. Laura Hull’s scripts were reused to calculate SMD. Rectangles represent effect sizes (SMD), with, lines are 95% confidence interval and diamond is the average effect. The wider the diamond, the wider the confidence interval. Positive effects indicates more of a female advantage in ASD relative to controls, while a negative effect indicates more male advantage in ASD relative to controls.
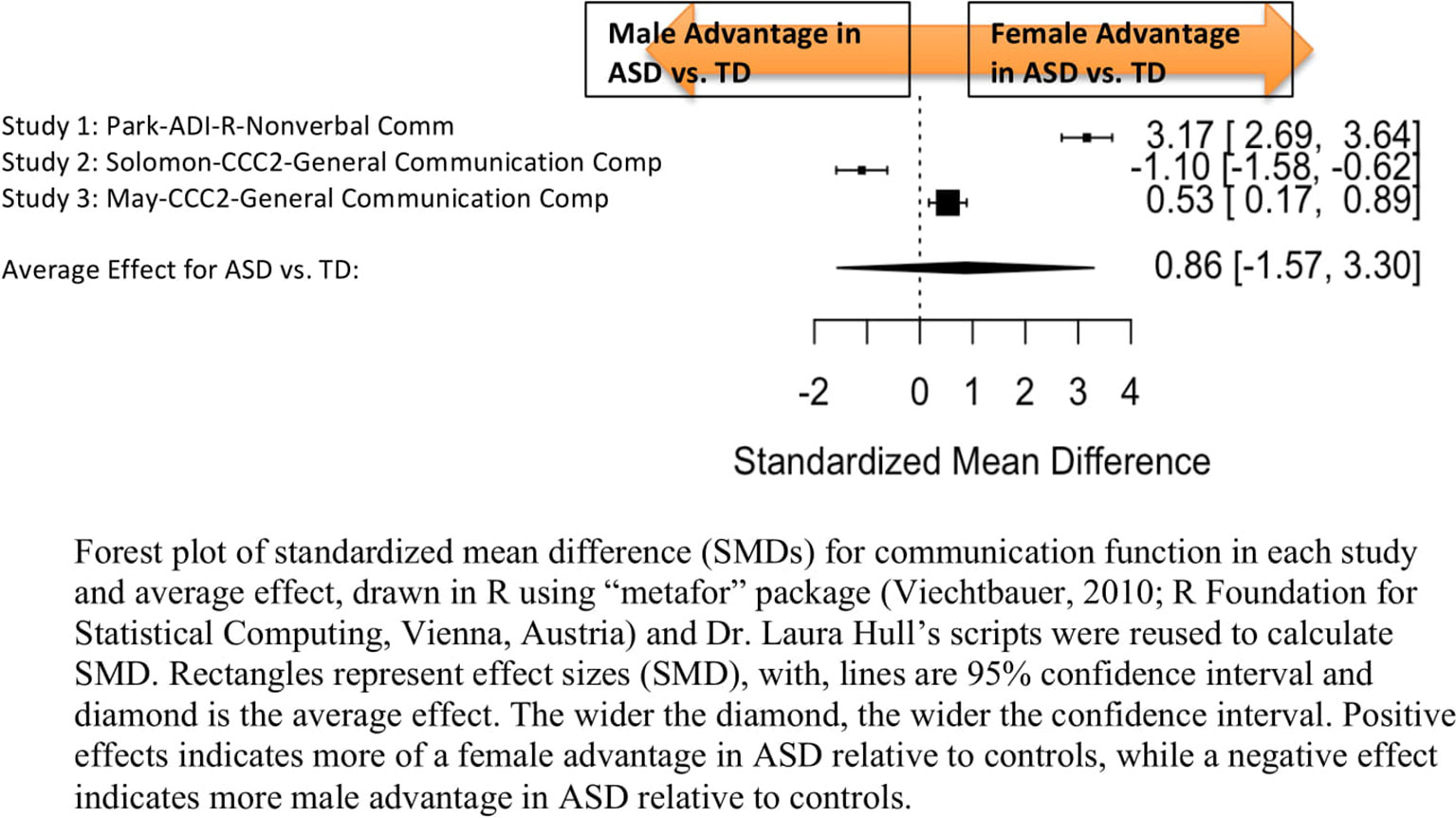
Figure 3 Meta-analysis of studies comparing sex differences in communication abilities between autism spectrum disorder (ASD) and controls. Forest plot of standardized mean difference (SMDs) for communication abilities in each study and average effect, drawn in R using “metafor” package (48; R Foundation for Statistical Computing, Vienna, Austria) and Dr. Laura Hull’s scripts were reused to calculate SMD. Rectangles represent effect sizes (SMD), with, lines are 95% confidence interval and diamond is the average effect. The wider the diamond, the wider the confidence interval. Positive effects indicates more of a female advantage in ASD relative to controls, while a negative effect indicates more male advantage in ASD relative to controls.
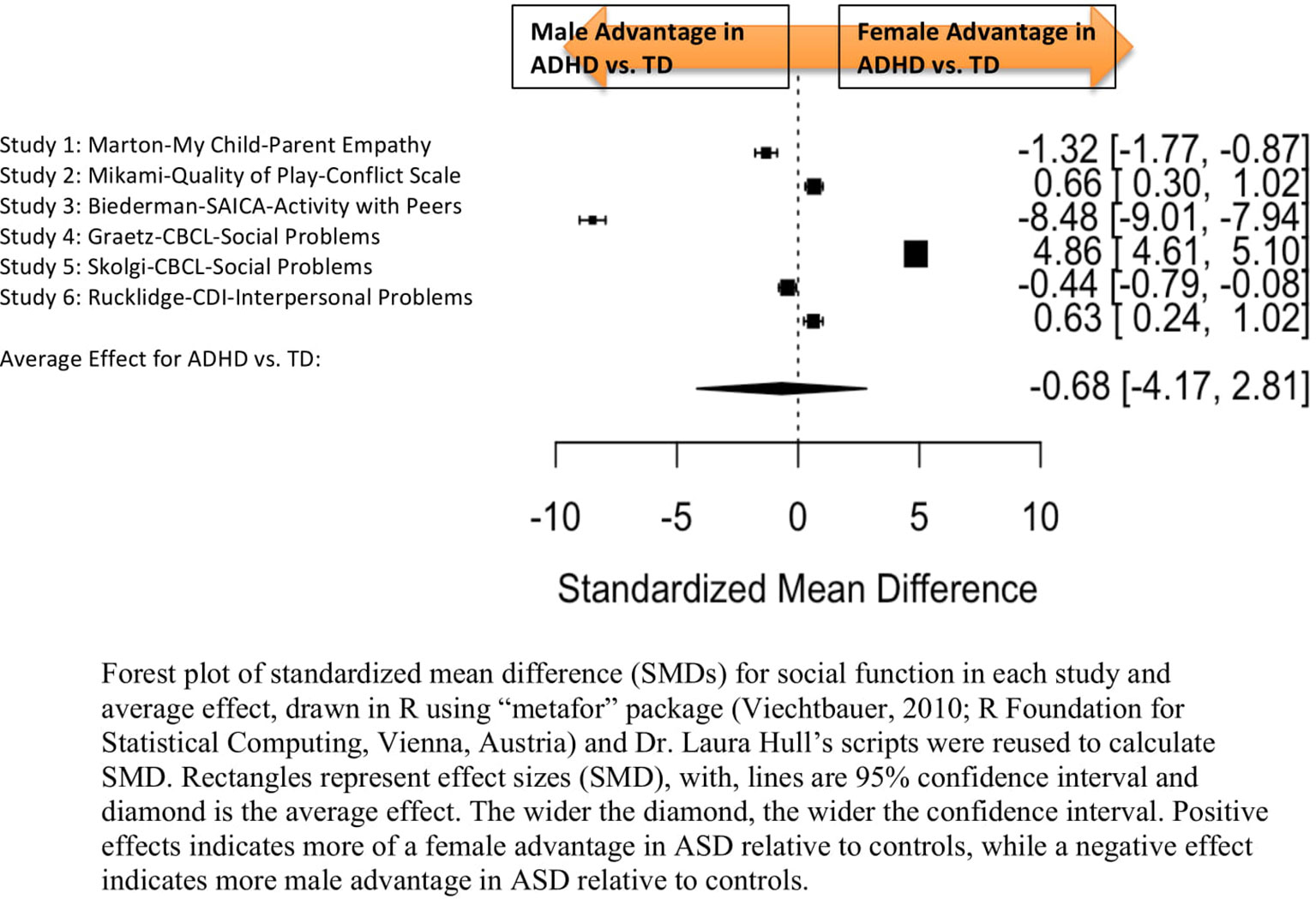
Figure 4 Meta-analysis of studies comparing sex differences in social abilities between attention-deficit/hyperactivity disorder (ADHD) and controls. Forest plot of standardized mean difference (SMDs) for social abilities in each study and average effect, drawn in R using “metafor” package (48; R Foundation for Statistical Computing, Vienna, Austria) and Dr. Laura Hull’s scripts were reused to calculate SMD. Rectangles represent effect sizes (SMD), with, lines are 95% confidence interval and diamond is the average effect. The wider the diamond, the wider the confidence interval. Positive effects indicates more of a female advantage in autism spectrum disorder (ASD) relative to controls, while a negative effect indicates more male advantage in ASD relative to controls.
Synthesized Findings
ASD Social Domain
Main Effects
Table 4 displays the measures used to assess social function, male and female individual mean scores, and the calculated SMD between males and females in ASD and TD groups. No significant sex differences in social function in ASD compared to TD were found (Figure 2) (SMD = −0.43, p-value = 0.5). Of note, no significant sex differences were noted in social function within ASD (Online Resource 1, Supplemental Figure 1) (SMD = 0.13, p = 0.6) or within TD (Online Resource 1, Supplemental Figure 1) (SMD = 0.24, p = 0.1) either. Significant heterogeneity was found in this analysis [Q(df = 9) = 345.45, p < 0.0001], therefore, measure and age were included in the model.
Effect of Measure
Measure was not significant in the random effect model [QM(df = 5) = 0.14, p = 0.7].
Effect of Age
Age was also found not to be significant [QM(df = 3) = 5.88, p = 0.1].
ASD Communication Domain
Main Effects
Table 5 displays the measures used to assess communication function, male and female individual mean scores, and the calculated SMD between males and females in ASD and TD groups. A random-effects meta-analysis revealed no significant sex differences between ASD and TD (Figure 3) (SMD = 0.86, p = 0.5). Of note, no significant sex differences were found within ASD (SMD = 0.25, p = 0.3) or TD (Online Resource 1, Supplemental Figure 2) (SMD = 0.019, p = 0.9) either. Significant heterogeneity was found in this analysis [Q(df = 2) = 155.66, p < 0.0001], therefore, moderators of measure and age were individually evaluated.
Effect of Measure
Measure was found to be significant in the random effect model [QM(df = 2) = 7.58, p = 0.02]. The resulting mixed-effects meta-analysis found significant variation in sex differences for communication function between ASD and TD groups only for the Autism Diagnostic Interview-Revised (ADI-R)–Nonverbal Communication (p = 0.006) in one study. However, the test for residual heterogeneity after including “measure” as a moderator remained significant [QE(df = 1) = 28.23, p < 0.0001], suggesting that other moderators may still be at play.
Effect of Age
Age was found not to be significant in the model [QM(df = 2) = 0.27, p = 0.9].
ADHD Social Domain
Main Effects
Table 6 displays the measures used to assess social function, male and female individual mean scores, and the calculated SMD between males and females in ADHD and TD groups. A random-effects meta-analysis revealed no significant sex difference in social function between ADHD or TD (Figure 4) (SMD = −0.68, p = 0.70). Of note, there were no significant sex differences in social function within ADHD (SMD = −0.038, p = 0.84) and TD (Online Resource 1, Supplemental Figure 3) (SMD = 0.11, p = 0.42) either. Significant heterogeneity was found in this analysis [Q(df = 5) = 2,316.76, p < 0.0001], therefore, moderators of measure and age were included in the model.
Effect of Measure
Measure was found to be significant [QM(df = 5) = 5.48, p = 0.019]. The resulting mixed-effects meta-analysis found a significant variation in sex differences for social function between ADHD and TD groups using the Social Adjustment Inventory for Children and Adolescents–Activity with peers (p = 0.024) in one study but not for the rest of the measures (Child Behavior Checklist–Social Problems (n = 2), Children’s Depression Inventory–Interpersonal Problems (n = 1), My Child–Parent Empathy (n = 1), Quality of Play–Conflict Scale (n = 1)]. Still, the test for residual heterogeneity was significant [QE(df = 1) = 571.57, p < 0.0001] indicating that other moderators, not included in the model, may still be influencing the effect.
Effect of Age
Age was not found to be a significant moderator [QM(df = 3) = 0.19, p = 0.98].
Community Versus Clinic Sample
Graetz (61) and Biederman (56) were the only studies that used community samples instead of clinic samples. When the meta-analysis was conducted excluding the community samples, no significant sex differences emerged [SMD: –0.113; p = 0.8106; confidence interval (−1.04–0.81)].
Discussion
Summary of Findings
This study examined potential sex differences in social and communication function in neurodevelopmental disorders (i.e., ASD and ADHD) and typically developing groups. The meta-analysis found no evidence of sex differences between ASD and TD groups in social or communication function. Still, with only three studies examining sex differences in communication between ASD and TD, the strength of evidence remains limited. There were no studies examining sex differences in communication function for ADHD. We found no sex differences between ADHD and TD groups in social function. However, the type of measure may partly explain some of the heterogeneity across studies in the domain of communication in ASD and social in ADHD, although only a single study in each disorder was found to be a significant source of heterogeneity and as such other unreported characteristics of these studies such as population characteristics and social economic status may have been responsible for the finding. In summary, there were no sex differences found in social–communication function between ASD and TD and ADHD and TD. However, the choice of measure across studies may have influenced results in some domains but this effect was only seen in one study in each case. Also, given there was significant residual heterogeneity, the variability between studies could have been caused by other factors (e.g. socio-economic status, population characteristics).
Several biological theories have attempted to describe/explain sex differences in developmental disorders. According to Eme (67), the sex least frequently affected by the developmental disorder (females) is relatively more severely affected. Eme (67) explained this using two types of models 1) the polygenetic multiple-threshold model, which suggests that females require a higher genetic/environmental load to be affected, 2) constitutional variability model, which proposes that greater genetic variability in males produces higher rates of less severe manifestations of disorders, while females are more likely to be affected in cases where there is a pathological event (e.g. brain damage). This theory is also consistent with other models used to explain sex differences in ASD such as the Genetic Variability Model (68) and Liability Threshold Model (69). The extreme male brain theory (70) suggests that both males and females with ASD present with an “extreme male” profile of good systematizing abilities at the expense of empathizing abilities, so that fewer sex differences in social communication may be predicted (30, 70). Our findings would partially support the extreme male brain theory, as we found no differences between ASD males and females, although we also did not find sex differences in social function and communication in controls. The latter, although consistent with previous systematic reviews in typically development (70, 72), would not be consistent with the extreme male brain theory. Still several limitations of the identified studies preclude strong conclusions.
To explain potential sex differences in ASD, a few social theories have articulated possible scenarios. Holtmann (38) developed a term called the “interpreting bias” which is the difference between observed and expected behaviors. Holtman (38) suggested that despite comparable levels of ASD traits in males and females on direct measures, parents with children with ASD may expect more socially sophisticated behaviors in their daughters than in their sons, and hence will report more social impairment in their girls than in their boys. Similarly, Crick and Zahn-Waxler (73) reported that girls with ASD were perceived by parents as having a greater level of social impairment, despite comparable symptoms reported and directly observed on the ADI-R and Autism Diagnostic Observation Schedule. Another possible explanation about sex differences in ASD is the increase in social demand/complexity with age may differ between boys and girls. McLennan (39) found more impairment with age in girls but not boys, and suggested that as the child transitions into adolescence, social situations may get more diverse and complex for females, as peer activities in typical girls and young women become mostly dependent on communication and interpersonal skills compared to boys who may have other social options that are less verbal and less intensely interactive (e.g. spectator sports and competitive play). Thus, social deficits may become more evident in girls as they transition to adolescence compared to boys. Another key social factor that has been reported to influence sex differences relates to gender specific expectations related to play and social roles. Despite similar amounts of socializing, Kuo et al. (74) found that males with ASD tended to play video games, whereas females with ASD mostly talked with their friends, suggesting that these skills may allow females with ASD to maintain closer and more empathetic friendships, ultimately to interact as expected by their nonautistic female peers. However, our study results cannot at this point inform these theories as we found no consistent sex differences.
In addition, significant heterogeneity was observed among studies. There are several reasons why there may be discrepancies among studies examining sex effects in ASD and ADHD:
1. Measurement issues: Variability in measures that may be capturing unique constructs, or have differences in psychometric properties. For example, Marton et al. (55) used the parent reported measure “My Child” which assesses only empathic ability, while Skolgi et al. (65) and Graetz et al. (61), used the Child Behavior Checklist Social Problems domain which surveys a broader range of social problems. In the ASD communication domain, the ADI-R assesses social and communication symptoms relevant to ASD while the Children’s Communication Checklist-2 assesses communication skills such as language structure, pragmatic skills and communication skills that are not diagnostic specific.
2. Population differences: Most studies included clinical samples, which may be subject to referral and identification bias. Studies of clinical samples may include more severe cases and/or symptoms that draw more attention, potentially influencing the expression of ASD and ADHD in males and females in the results (76). In fact, a meta-analysis by Gaub and Carlson (50) found that clinic referred females significantly differed from nonreferred females with ADHD, such that clinic-referred females exhibited more severe symptoms and disruptive behaviors. Moreover, girls are more likely to have inattentive symptoms/subtype (77), which may go less noticed and be less likely to lead to a referral and/or ADHD diagnosis compared to the other subtypes. Even though the present study used a random effects model to account for such variances, most studies in this meta-analysis are from clinic populations, and so it is possible that sex differences were examined in children who had more disruptive and severe symptoms.
Limitations
There were certain limitations in this study. A key limitation was the small number of studies identified. According to Hunter and Schmidt (54), a meta-analysis based on a small number of studies is more susceptible to second-order sampling errors, which may inflate the observed variance. Moreover, several of the studies had very few females included, and may be underpowered to detect sex differences. Further, the choice of measure was identified to be a potential confounding variable, albeit only in single studies, but the residual heterogeneity remained significant, indicating that there were other confounding variables that were influencing sex differences. Previous studies have implicated IQ, ethnicity, and comorbidities (18), as well as other social and biological factors, (genetic influences, social/cultural environments; 76, 78) in interacting with potential sex differences but we had no access to such data. Also, since most of these studies used parent reported measures, results may have been influenced by parental expectations or biases (i.e., the “interpreting bias” described by 38). Moreover, there was some variability in the types of constructs evaluated by measures used in the meta-analysis; while the majority of measures evaluated deficits, other measures may have measured skills. However, there was no evidence that in this set of studies, sex differences were different across the two constructs.
Future research investigating sex differences across neurodevelopmental disorders should include large cohorts with adequate numbers of female participants with neurodevelopmental disorders and consistent use of measures. Longitudinal designs should be employed to examine sex differences over time. Other moderators such as cognitive abilities, socio-economic status, ethnicity and comorbidities should be explored. Additionally, examining sex differences in community samples would be important in understanding whether there are variations in reported sex differences between clinical versus community samples. Lastly, other biological markers (e.g., genetics, brain) of sex differences should be evaluated.
Implications
Understanding potential sex differences in social and communication outcomes across neurodevelopmental disorders is critical in elucidating the biology of these disorders. In addition, this study suggests that other unidentified factors including potentially IQ and population characteristics may explain the significant heterogeneity observed across the studies and should be included in future studies.
Conclusions
The present study did not identify significant sex differences in social communication between ASD, ADHD, and controls. However, the limited number of studies, small female samples, and heterogeneity of measures/tools used, suggests that conclusions may not be drawn with confidence until larger longitudinal studies that address these issues. We argue that the overlap on the social–communication domains between the two disorders is not well characterized in the current literature and can only be resolved when participants with ASD and ADHD are recruited in single cohorts and evaluated by similar measures to understand whether there are systematic differences in the types of social–communication deficits observed or whether there are overlapping subgroups across both disorders with unique patterns of deficits.
Data Availability Statement
The R-Script used in the present study is available upon request.
Author Contributions
TM contributed to conceptualization, did data analysis, and is primarily responsible for manuscript preparation. AD contributed to the data analysis and manuscript. JB participated in the design, of the study, cosupervised data analytic approaches, and revised and edited manuscript. NM helped with the data analysis and revised manuscript. P-YW is the librarian on the study and assisted with the search, selection, and the process of the systematic review and has revised manuscript. AI has significantly contributed to the manuscript preparation. EA supervised all procedures in this study and manuscript. All authors have read and approved the final manuscript.
Funding
This study was funded by the Ontario Brain Institute−Province of Ontario Neurodevelopmental Disorders (POND) Network (grant number: IDP-PND-2018) and Ontario Graduate Scholarship.
Conflict of Interest
EA has served as a consultant to Roche, has received grant funding from Sanofi Canada and SynapDx, has received royalties from APPI and Springer, and has received kind support from AMO Pharmaceuticals.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2019.00804/full#supplementary-material
References
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: Author (2013). doi: 10.1176/appi.books.9780890425596
2. National Autism Spectrum Disorder Surveillance. NASS. (2018). Autism Spectrum Disorder among children and youth in Canada 2018. A Report of the National Autism Spectrum Disorder Surveillance System. Retrieved from https://www.canada.ca/en/public-health/services/publications/diseases-conditions/autism-spectrum-disorder-children-youth-canada-2018.html.
3. Centers for Disease Control and Prevention (CDC). Prevalence of autism spectrum disorder among children aged 8 years — Autism and developmental disabilities monitoring network, 11 Sites-United States, 2014. (2018).
4. Williamson D, Johnston C. Gender differences in adults with attention-deficit/hyperactivity disorder. Clin Psychol Rev (2015) 40:15–27. doi: 10.1016/j.cpr.2015.05.005
5. Postorino V, Kerns CM, Vivanti G, Bradshaw J, Siracusano M, Mazzone L. Anxiety disorders and obsessive-compulsive disorder in individuals with autism spectrum disorder. Curr Psychiatry Rep (2017) 19(12):92. doi: 10.1007/s11920-017-0846-y
6. Van der Meer JM, Oerlemans AM, van Steijn DJ, Lappenschaar MG, de Sonneville LM, Buitelaar JK, et al. Are autism spectrum disorder and attention-deficit/hyperactivity disorder different manifestations of one overarching disorder? Cognitive and symptom evidence from a clinical and population-based sample. J Am Acad Child Adolesc Psychiatry (2012) 51:1160–1172. e1163. doi: 10.1016/j.jaac.2012.08.024
7. Ronald A, Simonoff E, Kuntsi J, Asherson P, Plomin R. Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. J Child Psychol Psychiatry Allied Disc (2008) 49:535–42. doi: 10.1111/j.1469-7610.2007.01857.x
8. Ames CS, White SJ. Are ADHD traits dissociable from the autistic profile? Links between cognition and behaviour. J Autism Dev Disord (2011) 41, 357–63. doi: 10.1007/s10803-010-1049-0
9. Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, et al. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. J Autism Dev Disord (2006) 36:849–861. doi: 10.1007/s10803-006-0123-0
10. Anholt GE, Cath DC, van Oppen P. Autism and ADHD symptoms in patients with OCD: are they associated with specific OC symptom dimensions or OC symptom severity? J Autism Dev Disord (2010) 40:580–9. doi: 10.1007/s10803-009-0922-1
11. Zandt F, Prior M, Kyrios M. Repetitive behaviour in children with high functioning autism and obsessive compulsive disorder. J Autism Dev Disord (2007) 37:251–9.
12. Baribeau DA, Doyle-Thomas K, Dupuis A, Iaboni A, Crosbie J, McGinn H, et al. Examining and comparing social perception abilities across childhood-onset neurodevelopmental disorders. J Am Acad Child Adolesc Psychiatry (2015) 54(6):479–86. doi: 10.1016/j.jaac.2015.03.016
13. Taurines R, Schwenck C, Westerwald E, Sachse M, Siniatchkin M, Freitag C. ADHD and autism: differential diagnosis or overlapping traits? A selective review. Atten Defic Hyperact Disord (2012) 4(3):115–3.
14. Craig F, Margari F, Legrottaglie AR, Palumbi R, de Giambattista C, Lucia Margari. A review of executive function deficits in autism spectrum disorder and attention-deficit/ hyperactivity disorder. Neuropsychiatr Dis and Treat (2016) 12:1191–202.
15. Fombonne E. Epidemiology of pervasive developmental disorders. Pediatric Res (2009) 65(6):591–8. doi: 10.1203/PDR.0b013e31819e7203
16. Baird G, Simonoff E, Pickles A, Chandler S, Loucas T, Meldrum D. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP). Lancet (2006) 368(9531):210–5. doi: 10.1016/S0140-6736(06)69041-7
17. Wing L, Yeates SR, Brierley LM, Gould J. The prevalence of early childhood autism: comparison of administrative and epidemiological studies. Psychol Med (1976) 6(1):89–100. doi: 10.1017/S0033291700007522
18. Rivet TT, Matson JL. Review of gender differences in core symptomatology in autism spectrum disorders. Res Autism Spectr Disord (2011) 23(3):957–76. doi: 10.1016/j.rasd.2010.12.003
19. Nicholas JS, Charles JM, Carpenter LA, King LB, Jenner W, Spratt EG. Prevalence and characteristics of children with autism-spectrum disorders. Ann Epidemiol (2008) 18(2):130–6. doi: 10.1016/j.annepidem.2007.10.013
20. Abikoff H, Hechtman L, Klein RG, Gallagher R, Fleiss K, Etcovitch J, et al. Social functioning in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Am Acad Child Adolesc Psychiatry (2004) 43:820–9. doi: 10.1097/01.chi.0000128797.91601.1a
21. Blachman DR, Hinshaw SP. Patterns of friendship among girls with and without attention-deficit/hyperactivity disorder. J Abnormal Child Psychol (2002) 30:625–40.
22. Huang-Pollack CI, Mikami AY, Pfiffner L, McBurnett K. Can executive functions explain the relationship between attention deficit hyperactivity disorder and social adjustment? J Abnormal Child Psychol (2009) 37(5):679–91. doi: 10.1007/s10802-009-9302-8
23. Tseng WL, Gau SSF. Executive function as a mediator in the link between attention-deficit/hyperactivity disorder and social problems. J Child Psychol Psychiatry (2013) 54(9):996–1004. doi: 10.1111/jcpp.12072
24. Grzadzinski R, Di Martino A, Brady E, Mairena MA, O’Neale M, Petkova E, et al. Examining autistic traits in children with ADHD: does the autism spectrum extend to ADHD? J Autism Dev Disord (2011) 41(9):1178–91. doi: 10.1007/s10803-010-1135-3
25. Reiersen AM, Constantino JN, Volk HE, Todd RD. Autistic traits in a population-based ADHD twin sample. J Child Psychol Psychiatry Allied Disciplines (2007) 48:464–72. doi: 10.1111/j.1469-7610.2006.01720.x
26. Mulligan A, Anney RJL, O’Regan M, Chen W, Butler L, Fitzgerald M, et al. Autism symptoms in attention-deficit/hyperactivity disorder: a familial trait which correlates with conduct, oppositional defiant, language and motor disorders. J Autism Dev Disord (2009) 39:197–209. doi: 10.1007/s10803-008-0621-3
27. Clark T, Feehan C, Tinline C, Vostanis P. Autistic symptoms in children with attention deficit-hyperactivity disorder. Eur Child Adolesc Psychiatry (1999) 8(1):50–5. doi: 10.1007/s007870050083
28. Geurts HM, Embrechts M. Language Profiles in ASD, SLI, and ADHD. J Autism Dev Disord (2008) 38(10):1931–43. doi: 10.1007/s10803-008-0587-1
29. Bishop DV, Baird G. Parent and teacher report of pragmatic aspects of communication: use of the children’s communication checklist in a clinical setting. Dev Med Child Neurol (2001) 43:809–18. doi: 10.1017/S0012162201001475
30. Baron-Cohen S. The extreme male brain theory of autism. Trends Cogn Sci (2002) 6(6):248–54. doi: 10.1016/S1364-6613(02)01904-6
31. Frazier TW, Georgiades S, Bishop SL, Hardan AY. Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. J Am Acad Child Adolesc Psychiatry (2014) 53:329–340. e1–3. doi: 10.1016/j.jaac.2013.12.004
32. Head AM, McGillivray JA, Stokes MA. Gender differences in emotionality and sociability in children with autism spectrum disorders. Mol Autism (2014) 5(19):1–19. doi: 10.1186/2040-2392-5-19
33. Lai MC, Lombardo MV, Ruigrok AN, Chakrabarti B, Wheelwright SJ, Auyeung B, et al. Cognition in males and females with autism: similarities and differences. PloS One (2012) 7(10):e47198. doi: 10.1371/journal.pone.0047198
34. Sedgewick F, Hill V, Yates R, et al. Gender differences in the social motivation and friendship experiences of autistic and non-autistic adolescents. J Autism Dev Disorders (2016) 46(4):1297–306. doi: 10.1007/s10803-015-2669-1
35. Mandy W, Chilvers R, Chowdhury U, Salter G, Seigal A, Skuse D. Sex differences in autism spectrum disorder: evidence from a large sample of children and adolescents. J Autism Dev Disord (2012) 42(7):1304–13. doi: 10.1007/s10803-011-1356-0
36. Sipes M, Matson JL, Worley JA, Kozlowski AM. Gender differences in symptoms of autism spectrum disorders in toddlers. Res Autism Spectr Disord (2011) 5(4):1465–70. doi: 10.1016/j.rasd.2011.02.007
37. Solomon M, Miller M, Taylor SL, Hinshaw SP, Carter CS. Autism symptoms and internalizing psychopathology in girls and boys with autism spectrum disorders. J Autism Dev Disord (2012) 42(1):48–59. doi: 10.1007/s10803-011-1215-z
38. Holtmann M, Bölte S, Poustka F. Autism spectrum disorders: sex differences in autistic behaviour domains and coexisting psychopathology – ProQuest. Dev Med Child Neurol (2007) 49(5):361–6. doi: 10.1111/j.1469-8749.2007.00361.x
39. McLennan JD, Lord C, Schopler E. Sex differences in higher functioning people with autism. J Autism Dev Disord (1993) 23:217–27. doi: 10.1007/BF01046216
40. Hull L, Mandy W, Petrides KV. Behavioural and cognitive sex/gender differences in autism spectrum condition and typically developing males and females. Autism (2017) 21(6):706–27. doi: 10.1177/1362361316669087
41. Van Wijngaarden-Cremers PJ, Eten E, Groen WB, Van Deurzen PA, Oosterling IJ, Van der Gaat RJ. Gender and age differences in the Core Triad of impairments in Autism Spectrum Disorders: a systematic review and meta-analysis. J Autism Dev Disord (2014) 44(3):627–35. doi: 10.1007/s10803-013-1913-9
42. Thurber JR, Heller TL, Hinshaw SP. The social behaviours and peer expectations of girls with attention deficit hyperactivity disorder and comparison girls. J Clin Child Adolesc Psychol (2002) 31(4):443–52. doi: 10.1207/153744202320802124
43. Thorell LB, Rydell A-M. Behavior problems and social competence deficits associated with symptoms of attention-deficit hyperactivity disorder: Effects of age and gender. Child Care Health Dev (2008) 34:584–95. doi: 10.1111/j.1365-2214.2008.00869.x
44. Pelham WE, Bender ME. Peer relationships in hyperactive children: Description and treatment. In: Gadow KD, Bailer I, editors. Advances in learning and behavioral disabilities. Greenwich, CT: JAI Press (1982). 1:365–436.
45. Diamantopoulou S, Henricsson L, Rydell AM. ADHD symptoms and peer relations of children in a community sample: examining associated problems, self-perceptions, and gender differences. Int J Behavioural Development (2005) 29(5):388–98. doi: 10.1080/01650250500172756
46. Berry CA, Shaywitz SE, Shaywitz BA. Girls with attention deficit disorder: a silent majority? A report on behavioral and cognitive characteristics. Pediatrics (1985) 76(5):801–9.
47. Greene RW, Biederman J, Faraone SV, Monuteaux M, Mick E, DuPre EP, et al. Social impairment in girls with ADHD: patterns, gender comparisons, and correlates. J Am Acad Child Adolesc Psychiatry (2001) 40:704–10. doi: 10.1097/00004583-200106000-00016
48. DeHaas P. Attention styles and peer relationships of hyperactive and normal boys and girls. J Abnormal Child Psychol (1986) 14:457–67. doi: 10.1007/BF00915438
49. DuPaul GJ, Jitendra AK, Tresco KE, Junod REV, Volpe RJ, Lutz JG. Children with attention deficit hyperactivity disorder: are there gender differences in school functioning? School Psych Rev (2006) 35:292–308.
50. Gaub M, Carlson CL. Gender differences in ADHD: a meta-anal- ysis and critical review. J Am Acad Child Adolesc Psychiatry (1997) 36:1036–45. doi: 10.1097/00004583-199708000-00011
51. Gershon J. A meta-analytic review of gender differences in ADHD. J Attention Disord (2002) 5:143–54. doi: 10.1177/108705470200500302
52. R Core Team. R: A Language and Environment for Statistical Processing. Vienna, Austria: R Foundation for Statistical Computing (2013). Available at: http://www.r-project.org/.
53. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Statistical Software (2010) 36(3):1–48. doi: 10.18637/jss.v036.i03
54. Hunter JE, Schmidt FL. Methods of meta-analysis: Correcting error and bias in research findings. Newbury Park, CA: Sage Publications (2004). doi: 10.4135/9781412985031
55. Marton I, Wiener J, Maria R, Moore C, Tannock R. Empathy and social perspective taking in children with attention-deficit/hyperactivity disorder. JAbnor Child Psychol (2009) 37(1):107–18. doi: 10.1007/s10802-008-9262-4
56. Biederman J, Kwon A, Aleardi M, Chouinard VA, Marino T, Cole H, et al. Absence of gender effects Attention Deficit Hyperactivity Disorder: findings in non-referred subjects. Am J Psychiatry (2005) 162:1083–9. doi: 10.1176/appi.ajp.162.6.1083
57. Mikami AY, Lorenzi J. Gender and conduct problems predict peer functioning among children with Attention-Deficit/Hyperactivity Disorder. J Clin Child Adolesc Psychology (2011) 40(5):777–86. doi: 10.1080/15374416.2011.597089
58. May T, Cornish K, Rinehart NJ. Gender profiles of behavioral attention in children with autism spectrum disorder. J Attention Disord (2012) 20:627–35.
59. Cholemkery H, Mojica L, Rohramann S, Gensthaler A, Freitage MC. Can Autism Spectrum Disorders and social anxiety disorders can be differentiated by the social responsiveness scale in children and adolescents? J Autism Dev Disord (2014b) 44:1168–82. doi: 10.1007/s10803-013-1979-4
60. Cholemkery H, Kitzerow J, Rohrmann Freitag MC. Validity of the social responsiveness scale to differentiate between autism spectrum disorder and disruptive behavior disorders. Eur Child Adolesc Psychiatry (2014a) 23:81–93. doi: 10.1007/s00787-013-0427-5
61. Graetz BW, Michael GS, Peter B. Gender differences among children with DSM-IV ADHD in Australia. J Am Acad Child Adolesc Psychiatry (2005) 44(2):159–68. doi: 10.1097/00004583-200502000-00008
62. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred reporting items for systematic reviews and meta- analyses: the PRISMA statement. PLoS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed1000097
63. Horiuchi F, Oka Y, Uno H, Kawabe K, Okada F, Saito I, et al. Age- and sex-related emotional and behavioural problems in children with autism spectrum disorders: Comparison with control children. Psychiatry Clin Neurosciences (2014) 68:542–50. doi: 10.1111/pcn.12164
64. Park S, Cho SC, Cho IH, Kim BN, Kim JW, Shin MS, et al. Sex differences in children with autism spectrum disorders compared with their unaffected siblings and typically developing children. Res Autism Spectr Disorders (2012) 6(2):861–70. doi: 10.1016/j.rasd.2011.11.006
65. Skolgi EW, Teicher MH, Andersen PN, Hovik KT, Oie M. ADHD in girls and boys-gender differences in co-existing symptoms and executive function measures. BMC Psychiatry (2013) 13(298):1–12. doi: 10.1186/1471-244X-13-298
66. Rucklidge JL, Tannock R. Psychiatric, psychosocial, and cognitive functioning of female adolescents with ADHD. J Am Acad Child Adolesc Psychiatry (2001) 40:530–40. doi: 10.1097/00004583-200105000-00012
67. Eme RF. Selective female affliction in the developmental disorders of childhood: a literature review. J Clin Child Psychol (1992) 21:354–64. doi: 10.1207/s15374424jccp2104_5
68. Wing L. Sex ratios in early childhood autism and related conditions. Psychiatry Res (1981) 5(2):129–37. doi: 10.1016/0165-1781(81)90043-3
69. Tsai L, Stewart MA, August G. Implication of sex differences in the familial transmission of infantile autism. J Autism Dev Disord (1981) 11(2):165–73. doi: 10.1007/BF01531682
70. Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord (2004) 34(2):163–75.
71. Hyde JS. The gender similarities hypothesis. Am Psychologist (2005) 60(6):581–92. doi: 10.1037/0003-066X.60.6.581
72. Hyde JS. New directions in the study of gender similarities and differences. Curr Dir Psychol Sci (2007) 16: (5):259–63. doi: 10.1111/j.1467-8721.2007.00516.x
73. Crick NR, Zahn-Waxler C. The development of psychopathology in females and males: current progress and future challenges. Dev Psychopathol (2003) 15:719–42. doi: 10.1017/S095457940300035X
74. Kuo MH, Orsmond GI, Cohn ES, Coster WJ. Friendship characteristics and activity patterns of adolescents with an autism spectrum disorder. Autism (2013) 17(4):481–500. doi: 10.1177/1362361311416380
75. Lehnhardt FG, Falter CM, Gawronski A, Pfeiffer K, Tepest R, Franklin J, Vogeley K. Sex-related cognitive profile in autism spectrum disorders diagnose late in life: implications for the female autistic phenotype. J Autism Dev Disorders (2015) 46(1):139–54. doi: 10.1007/s10803-015-2558-7
76. Kreiser NL, White SW. ASD in females: are we over- stating the gender difference in diagnosis? Clin Child Family Psychol Rev (2014) 17(1):67–84. doi: 10.1007/s10567-013-0148-9
77. Biederman J, Mick E, Faraone SV, Braaten E, Doyle A, Spencer T, et al. Influence of gender on at- tention deficit hyperactivity disorder in children referred to a psychiatric clinic. Am J Psychiatry (2002) 159:36–42. doi: 10.1176/appi.ajp.159.1.36
Keywords: autism spectrum disorder, sex differences, attention-deficit/hyperactivity disorder, meta-analysis, neurodevelopmental disorders, social function
Citation: Mahendiran T, Brian J, Dupuis A, Muhe N, Wong P-Y, Iaboni A and Anagnostou E (2019) Meta-Analysis of Sex Differences in Social and Communication Function in Children With Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder. Front. Psychiatry 10:804. doi: 10.3389/fpsyt.2019.00804
Received: 09 March 2019; Accepted: 08 October 2019;
Published: 04 November 2019.
Edited by:
Roberto Canitano, Siena University Hospital, ItalyReviewed by:
Leonardo Emberti Gialloreti, University of Rome Tor Vergata, ItalyFrancesco Craig, Eugenio Medea (IRCCS), Italy
Copyright © 2019 Mahendiran, Brian, Dupuis, Muhe, Wong, Iaboni and Anagnostou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tania Mahendiran, dGFuaWEubWFoZW5kaXJhbjkyQGdtYWlsLmNvbQ==
 Tania Mahendiran
Tania Mahendiran Jessica Brian2,3
Jessica Brian2,3 Evdokia Anagnostou
Evdokia Anagnostou