- Department of Psychiatry, Renmin Hospital of Wuhan University, Wuhan, China
Objective: Accumulating evidences indicate that gastrin-releasing peptide receptor (GRPR) may contribute to the pathophysiology of depression. However, the mechanism of the involvement of GRPR in the progression of depression remains unclear. Here, we showed the extent to which stress and antidepressant treatment impact GRPR expression, and explored the interactions between 5-HT2a receptor (5-HT2aR) and GRPR at the cellular level.
Methods: The rat depression models were created with chronic unpredictable mild stress (CUMS). Then, these rats were treated with fluoxetine for 4 weeks after CUMS. We measured body weight and performed behavioral tests to determine the effects of stress and fluoxetine on depressive-like behaviors. Real-time PCR and western blotting were used to measure the mRNA and protein expression levels of GRPR in the hypothalamus. Then, Flag-tagged protein (pcmv-Flag-5HT2aR) and Myc-tagged protein (pcmv-Myc-GRPR) expression vectors were constructed, identified, and transfected into human embryo kidney 293 (HEK293) cells. The interaction between 5-HT2aR and GRPR was detected by coimmunoprecipitation and double-label immunofluorescence.
Results: The rats subjected to 4 weeks of CUMS showed depressive-like behaviors, including decreased body weight, sucrose preference, and distance traveled, rearing frequency and velocity in the open field test and increased immobility time in the forced swimming test. Fluoxetine treatment reversed CUMS-induced depressive-like behavior. The mRNA and protein expression of GRPR in the hypothalamus was significantly increased after 4 weeks CUMS exposure, and treatment with fluoxetine reversed these changes. Coimmunoprecipitation showed that 5-HT2aR and GRPR combine with each other in vitro. Immunofluorescence revealed that the 5-HT2aR and GRPR were colocalization in both the cell membrane and cytoplasm.
Conclusion: Our study enhances the understanding of the involvement of GRPR in depression. This study also provides in vitro experimental evidence of the interaction between 5-HT2aR and GRPR, which may play an important role in the pathogenesis of depression.
Introduction
Depression is a common and complex mental disorder. It is associated with enormous adverse effects in humans and high costs to society and healthcare systems (1, 2), and the pathogenesis of depression is unclear. Over the past 30 years, 5-HT has been a major target of antidepressant drugs, such as 5-HT reuptake inhibitors (SSRIs), the antidepressant effects of which may involve many types of 5-HT receptors (3). Dysfunction of the serotonergic system is closely related to the pathogenesis of depression, and several genetic studies have focused on genes encoding 5-HT2a receptor (5-HT2aR) (4, 5). The 5-HT2aR is a subtype of the 5-HT2 receptor and belongs to the seven transmembrane-spanning receptor family, which is coupled via G q/11 to the inositol triphosphate (IP3)/protein kinase C (PKC)/calcium pathway. 5-HT2aR is highly expressed in several brain regions that are mainly involved in the regulation of emotions, such as the hippocampus, the amygdala, the thalamus, and several cortical areas (6). In preclinical studies, 5-HT2aR mRNA and protein expression were shown to be significantly upregulated in the frontal cortex of stressed rats (7). An increasing number of studies have found the antidepressant-like effects of 5-HT2aR selective antagonists in rodents (8–10). Moreover, increased 5-HT2aR density has been confirmed in depressed patients (11). Postmortem studies have also shown increased 5-HT2aR in unmedicated depressed patients (12). Together, these studies highlight the important roles of 5-HT2aR in the pathology of depression.
Gastrin-releasing peptide receptor (GRPR) belongs to the G-protein coupled receptor (GPCR) superfamily and plays a role in several aspects of emotional responses (13). GRPR is a type of bombesin receptor in humans, mice, and rats that consists of 384 amino acids and was cloned from 3T3 cells. GRPR is directly coupled to the Gq type of G protein and is primarily associated with an increased cellular (Ca2+) and activation of the phospholipase C (PLC)/PKC and extracellular signal-regulated protein kinase (ERK)/mitogen-activated protein kinase (MAPK) pathways (14). Gastrin-releasing peptide (GRP) acts by binding to the GRP receptor, and consistent evidence has proposed that GRP might act as a stress mediator. Merali et al. found that chronic restraint exposure is associated with increased levels of GRP in the anterior pituitary (15). Rats given a systemic injection of corticosterone show enhanced release of GRP in the amygdala and medial prefrontal cortex in response to an acute stressor (16). Furthermore, several studies have shown that the dysfunction of the hypothalamic pituitary adrenal (HPA) axis is mainly involved in the course and progression of depression (17). Considerable evidence suggests that the expression of GRPR in stress-related brain areas including the hypothalamus, hippocampus, and amygdala is involved in the regulation of the HPA axis (18, 19). These data demonstrate the critical role of the GRP/GRPR system in the modulation of depressive-like behavior.
Previous studies have shown that GRP binds preferentially to GRPR, which increases 5-HT neuronal activity in the paraventricular nucleus (PVN) (20). In our previous study, we observed that GRPR mRNA and protein levels are markedly increased in the hypothalamus of CUMS-exposed mice and that treatment with fluoxetine reverses these changes (21). SSRIs are effective in the treatment of depression. There are different families and subtypes of 5-HT receptors, and 5-HT2aR may be involved in the antidepressant effects of SSRIs (22). The administration of fluoxetine and a reduction in either 5-HT2AR or GRPR is associated with a reduction in depression behavior. However, little is known about the molecular mechanisms of the interaction between these two important neurotransmitter systems. In this study, we used the chronic unpredictable mild stress (CUMS) to establish a depressive-like phenotype, and treatment with the antidepressant fluoxetine. We performed the behavioral tests to detect the effects of stress and fluoxetine on anhedonia and activity. We measured the mRNA and protein expression levels of GRPR in the hypothalamus. Human embryo kidney 293 (HEK 293) cells have become the mammalian cell line of choice for the production of recombinant proteins because they are easy to culture and exhibit high transfection efficiency. Transient expression in HEK293 cells provides a way of rapidly assessing the protein function. Therefore, in this study, Flag-tagged protein (pcmv-Flag-5HT2aR) and Myc-tagged protein (pcmv-Myc-GRPR) protein expression vectors were constructed, identified, and transfected into HEK293 cells. Coimmunoprecipitation and double immunofluorescence were used to explore the interaction between 5-HT2aR and GRPR at the cellular level.
Material and Methods
Animals
Male Sprague Dawley (SD) rats, weighing 180 g to 200 g, were obtained from the Company of Experimental Animals of Hunan slilaike jingda. The rats were housed in cages and maintained in a standard animal room (12 h/12 h light/dark cycle; 22 ± 2°C; food and water ad libitum). Before the CUMS procedure, the rats were acclimated to the environment for 1 week. All procedures were carried out in accordance with the guidelines of the P.R. China legislation on the ethical care and use of laboratory animals, and the Institutional Animals Care Committee of Renmin Hospital of Wuhan University approved the experimental protocols.
Experimental Groups and Drug Treatment
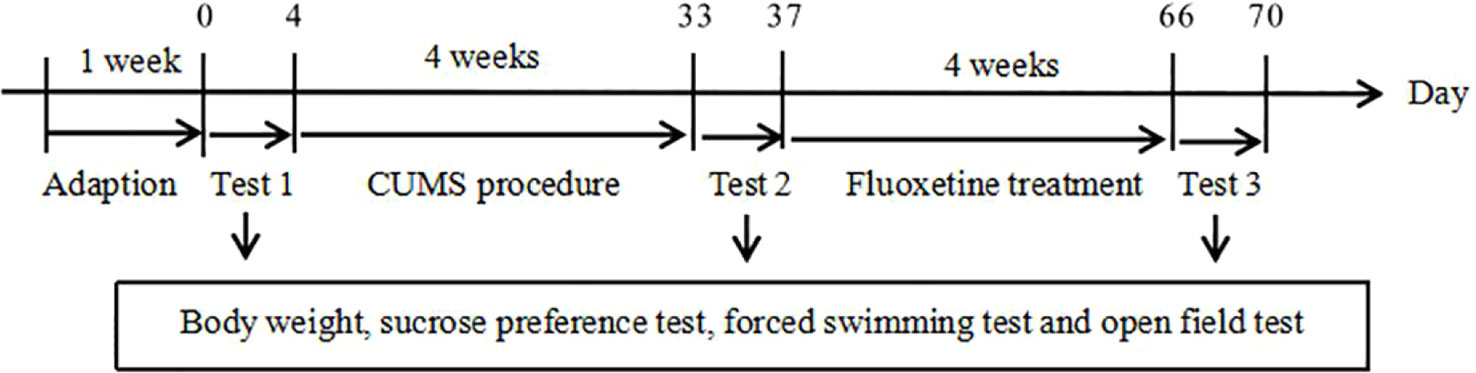
The rats were randomly divided into three groups (n = 10/group): the chronic unpredictable mild stress (CUMS) + normal saline group (CUMS group); the CUMS + fluoxetine group (fluoxetine group); and the control group. At the end of the CUMS procedure, normal saline was administered daily to the CUMS group rats for 4 weeks by intraperitoneal injection. Fluoxetine (Aladdin; F131623) was diluted in normal saline and intraperitoneally administered to the fluoxetine group rats. A previous work showed that a 4-week course of fluoxetine at a dose of 10 mg/kg produces antidepressant effects. The experimental design is shown in Figure 1.
CUMS Procedure
After 1 week of the acclimatization period, the depression model was established by CUMS as described previously with slight modification (23). The rats in the CUMS group and fluoxetine group were subjected to seven different stressors for 4 weeks. They were exposed to food deprivation for 24 h; water deprivation for 24 h; 45° cage tilt for 24 h; swimming in 4°C ice water for 5 min; tail clamping for 3 min; damp sawdust for 24 h; lights on overnight. Each animal received one of these stressors per day and the same stressor was not presented consecutively over 2 days.
Body Weight and Behavioral Tests
We measured the weights of the rats before and after the CUMS procedure, and the rats were again weighed after the fluoxetine treatment. The sucrose preference test, forced swimming test, and open field test were conducted before the CUMS procedure began, at the end of the 4-week CUMS period and after fluoxetine was administered. The sucrose preference test was performed as described previously with minor modifications to quantify anhedonia (24). Before the test, the rats were trained to consume sucrose solution (1%). After adaptation, the rats were deprived of water for 24 h. Subsequently, each rat was given two bottles, one containing 1% sucrose solution and the other containing tap water, for 24 h (the positions of the bottles were changed after 12 h). Sucrose preference was calculated by the following formula: sucrose preference (%) = (sucrose intake/total fluid intake) × 100%. In the forced swimming test, the rats were placed individually in a cylindrical tank that they were unable to exit. The immobility time, the length of time during which the rats remained still without struggling or used only minor movements to keep themselves afloat, was recorded. Each rat was forced to swim for 6 min, and the total time spent immobile during the final 4 min was recorded. The open field test was used to detect the spontaneous activity of the rats. The apparatus consisted of a square 100 × 100 cm area with 35-cm high walls. A rat was placed in the center of the rectangular cage and observed using a video tracking system for 5 min (Ethovision XT 11.5). The parameters that were assessed were the distance traveled, the speed and the frequency of rearing.
Sample Collection
The samples were collected after the final behavioral tests. The hypothalamus was immediately isolated after the rats were sacrificed under deep anesthesia. The samples were stored at −80°C until use.
Plasmid Construction
5-HT2aR and GRPR cDNA sequences were synthesized by the Wuhan Tianyi Huiyuan Company. The 5-HT2aR and GRPR genes were amplified by polymerase chain reaction (PCR), and the following primer sequences were used: 5-HT2aR (forward: 5’-CCCAAGCTTATGGAAATTCTCTGTG-3’; reverse: 5’-CCGGAATTCTCACACACAGCTAACC-3’) and GRPR (forward: 5’-CAGCTCGAGATGGCTCCAAATAAT-3’; reverse: 5’-CCGGAATTCCTAGACATACCCCT-3’). The PCR conditions were as follows: 1 cycle of 95°C for 5 min and then 38 cycles of 95°C for 15 s, 56°C for 15 s, and 72°C for 15 s. The PCR products were analyzed by 1% agarose gel electrophoresis and purified with a DNA purification kit (OMEGA; D2500-01). Then, 5-HT2aR cDNA was subcloned into the pcmv-flag vector at the EcoR I and Hind III sites, and the GRPR cDNA was subcloned into the pcmv-myc vector at the EcoR I and Xho I sites. The plasmids were transformed into DH5α by the heat shock method and extracted with a plasmid mini kit (OMEGA; D6943-01). Finally, the pcmv-Flag-5HT2aR and pcmv-Myc-GRPR plasmids were identified by restriction enzyme digestion.
Cell Culture and Transfection
HEK293 cells were obtained from the microbiology laboratory of the School of Basic Medicine, Wuhan University. The cells were cultured in DMEM (Gibco, 21885108) supplemented with 10% FBS and gentamicin and incubated at 37°C in 5% CO2. The cells were seeded in 10-cm culture dishes 24 h before transfection. The pcmv-Flag-5HT2aR and pcmv-Myc-GRPR plasmids were cotransfected with Lipofectamine 2000 reagent (Thermo,11668019) according to the manufacturer’s instructions. Single transfection of the pcmv-Flag-5HT2aR or pcmv-Myc-GRPR plasmid was performed in the control groups.
RNA Extraction and RT-PCR
Total RNA was extracted from the hypothalamus using Trizol reagent (Ambion, 15596-026) and purified according to the manufacturer’s instructions. The RNA concentration was determined by spectrophotometer at A260/A280 nm. Reverse transcription was performed with mRNA (3 µg) using a first-strand synthesis kit (Thermo, K1691) for cDNA synthesis. cDNAs were subsequently amplified by PCR with the following specific primers: GAPDH (forward: 5’-TGTGAAGCTCATTTCCTGGTATG-3’, reverse: 5’-AGGGCCTCTCTCTTGCTCTC-3’) and GRPR (forward: 5’-GCAGGATTGGCTGCAAACTG-3’, reverse: 5’-ATTGGCCTGACGATGGCTTT-3’). PCR was carried out as follows: 1 cycle of 95°C for 5 min followed by 39 cycles of 95°C for 10 s, 60°C for 30 s, and 72°C for 30 s, and finally 72°C for 10 min. The expression of GRPR mRNA was analyzed by 2-ΔΔCT method, and was normalized to GAPDH as a reference gene. Each experiment had more than three independent replicates.
Protein Extraction and Western Blotting
Western blotting was performed to test the expression of GRPR in the hypothalamus of rats and the cultured HEK 293 cells. Total protein from each group was extracted in 1 ml of RIPA buffer (Beyotime, P0013B) with 1% PMSF and protease inhibitor cocktail. The hypothalamus tissues were homogenized in ice cold RIPA lysis buffer. The HEK293 cells were washed with PBS 48 h after transfection and lysed with ice cold RIPA lysis buffer. Tissues and cells were centrifuged for 15 min at 12,000 rpm at 4°C, and the supernatants were collected and stored at −80°C. The concentration of proteins was determined by the BCA assay (BCA Protein Assay Kit, Thermo,23228). Then, 12% polyacrylamide gel electrophoresis was used to separate the protein samples, and then the proteins were transferred onto PVDF membranes (Merck millipore, ISEQ00010). The PVDF membranes were blocked in 5% non-fat dry milk at room temperature for 1 h. Then, the membranes were incubated with anti-GRPR antibody (1:200) (Santa Cruz, sc-32904), anti-Flag antibody (1:2,000) (Abbkine; A02010), and/or anti-Myc antibody (1:2,000) (Abbkine; A02061) at 4°C overnight. The following day the membranes were washed with TBST for three times. Then the membranes were incubated with secondary antibodies (1:5,000) at room temperature for 2 h. The immunoreactions were visualized with a chemiluminescence kit (Beyotime, P0018AM). GAPDH was used as a loading control to analyze the relative protein expression of GRPR. The intensity of the protein bands was calculated by ImageJ software. Each experiment was performed more than three times.
Coimmunoprecipitation Analysis
The protein samples were preincubated with anti-Flag antibody (1:200) at 4°C overnight. Then, 50% agarose beads were washed with PBS, incubated at 4°C for 2 h, centrifuged to remove the nonspecific binding protein, and added to the samples. Rabbit IgG was used as a negative control. The antigen-antibody complexes proteins were separated using 10% polyacrylamide gel electrophoresis and transferred onto PVDF membranes. The membranes were blocked with 5% non-fat dry milk for 1 h, and were incubated with anti-Myc antibody (1:2,000) at 4°C overnight. Similarly, the protein samples were preincubated with anti-Myc antibody (1:200), and the antigen-antibody complex proteins were detected by anti-Flag antibody (1:2,000). Finally, the immunoreactions were performed with a chemiluminescence kit after incubation with secondary antibodies (1:5,000).
Double-Label Immunofluorescence and Confocal Microscopy
Double-label immunofluorescence was used to analyze 5-HT2aR and GRPR distribution in HEK293 cells. After 48 h of the transfection, HEK293 cells were washed with PBS, fixed with 4% paraformaldehyde, and permeabilized with 0.3% Triton X-100. The cells were blocked with 5% non-fat dry milk for 1 h. Then, the cells were incubated in a mixture of mouse anti-Flag monoclonal antibody (1:200) and rabbit anti-Myc polyclonal antibody (1:200) overnight at 4°C. The second day, the cells were washed with PBS several times and incubated with DyLight488-conjugated goat anti-mouse IgG (Abbkine; A23210) and DyLight594-conjugated goat anti-rabbit IgG (Abbkine; A23420) in the dark at room temperature for 1 h. Nuclei were stained with DAPI for 5 min. After staining, the fluorescence signals were examined with a laser-scanning confocal microscope.
Statistical Analysis
Statistical analysis was performed using SPSS 20.0 software, and all data were reported as the means ± standard error of mean (SEM). The data were analyzed by one-way ANOVA test followed by LSD post hoc test. Statistical significance was set at P < 0.05.
Results
Body Weight and Behavioral Tests
As shown in Figure 2, before the CUMS procedure, no significant difference was observed among the three groups, but the CUMS group and the fluoxetine group were significantly different from the control group following 4 weeks of CUMS. The CUMS group and the fluoxetine group rats showed a lower index of body weight (F = 43.48, P < 0.05) and sucrose preference (F = 102.94, P < 0.05), decreased distance traveled (F = 12.93, P < 0.05), rearing frequency (F = 12.14, P < 0.05) and velocity (F = 17.87, P < 0.05) in the open field test and an increased immobility time in the forced swimming test (F = 35.75, P < 0.05). Compared with the CUMS group, the fluoxetine groups exhibited significantly increased the body weight (F = 120.14, P < 0.05), sucrose preference (F = 68.11, P < 0.05), and the distance traveled (F = 20.34, P < 0.05), rearing frequency (F = 10.48, P < 0.05), and velocity (F = 16.13, P < 0.05) in the open field test and decreased immobility time in the forced swimming test (F = 16.70, P < 0.05).
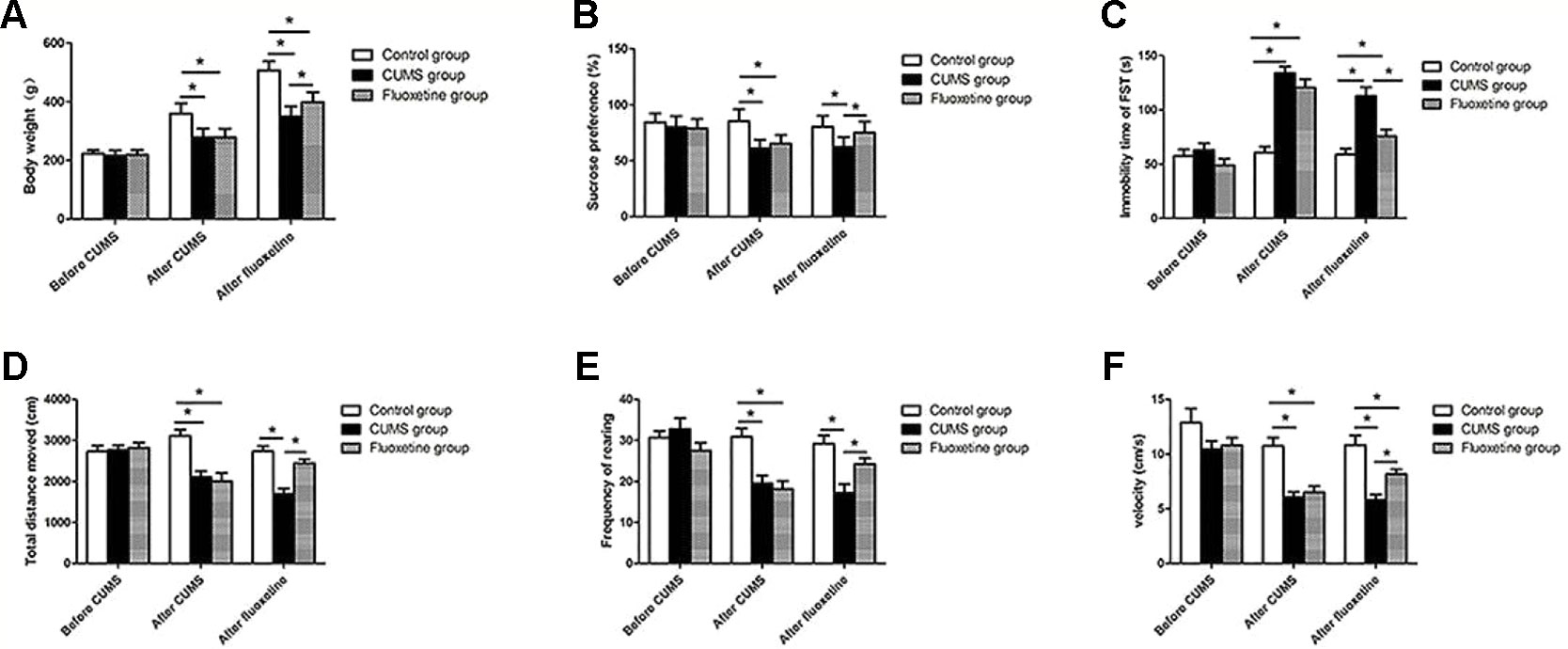
Figure 2 Body weight (A), sucrose preference (B), forced swimming test (C), total distance moved (D), rearing frequency (E), and velocity (F). *P < 0.05.
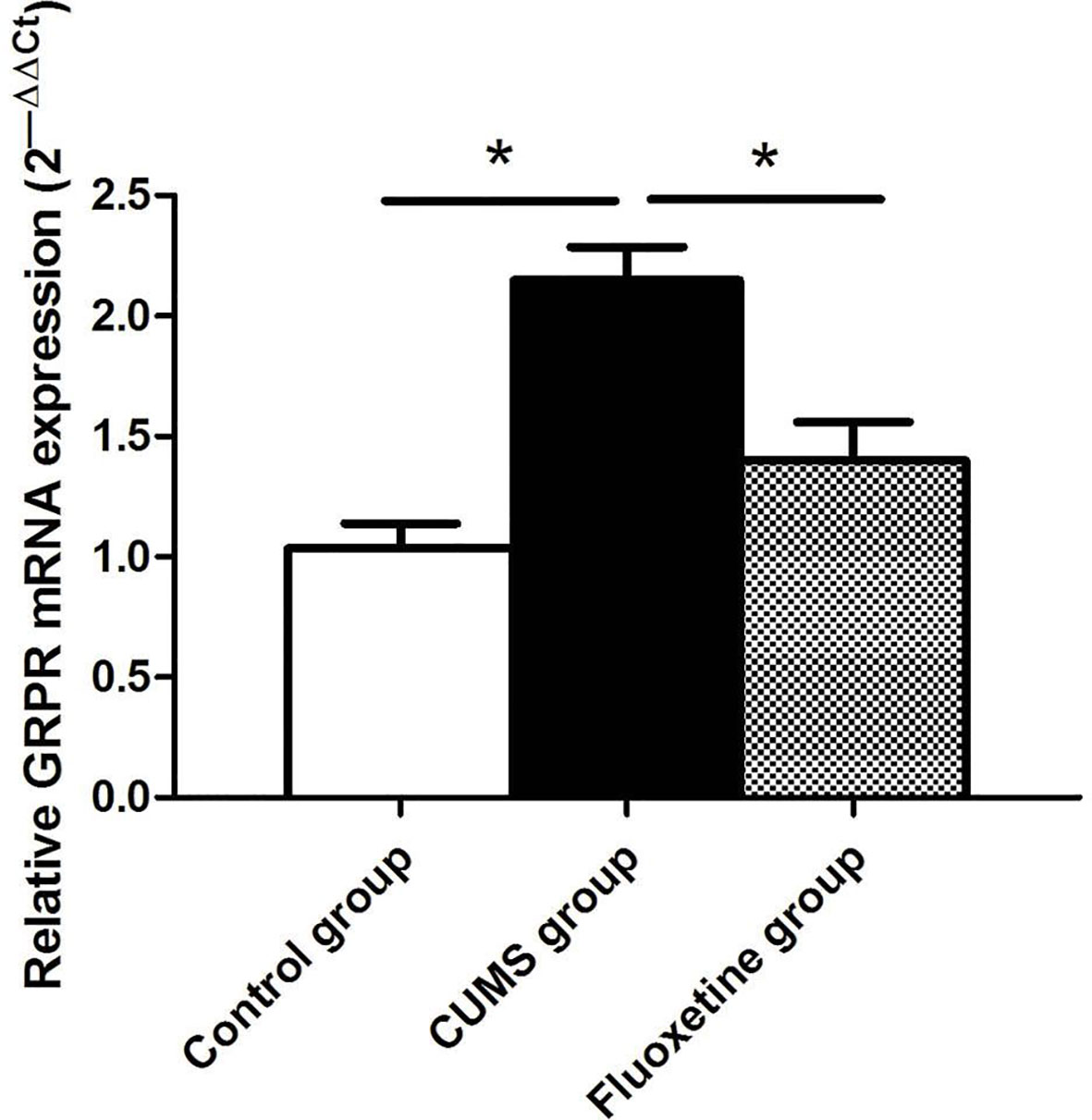
GRPR mRNA Expression
As shown in Figure 3, significant differences in the mRNA expression of GRPR were observed in the hypothalamus of rats between groups. Compared with that in rats from the control group, the mRNA expression of GRPR was significantly increased in rats from the CUMS group, and fluoxetine treatment significantly downregulated the mRNA expression of GRPR in rats from the fluoxetine group (F = 17.32, P < 0.05).
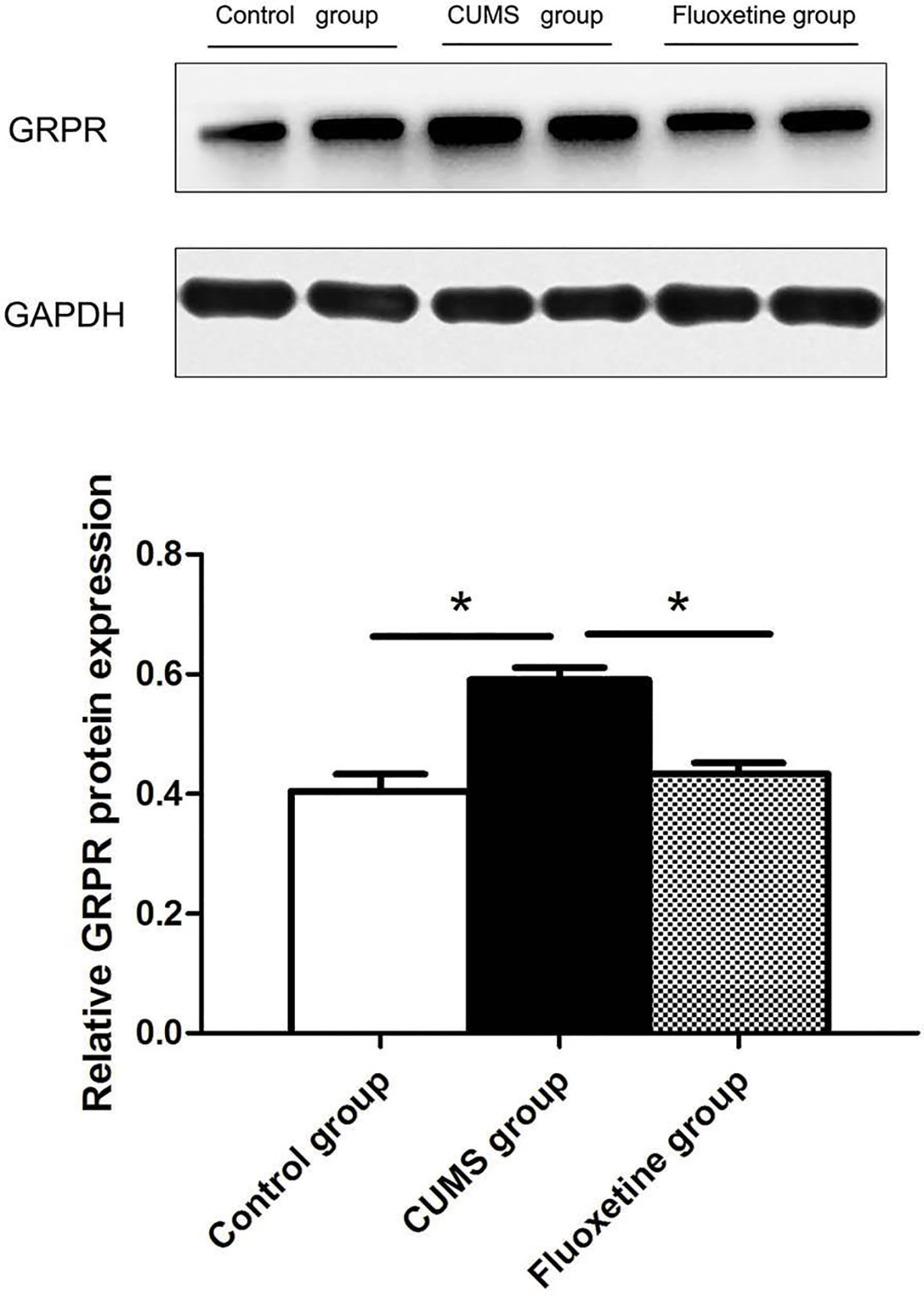
GRPR Protein Expression
Figure 4 shows the analysis of GRPR protein using western blotting in hypothalamic tissue. The GRPR protein level in the CUMS group was significantly increased compared with that in the control group and the fluoxetine group (F = 19.38, P < 0.05).
5-HT2aR Is a GRPR-Interacting Protein In Vitro
To investigate whether 5-HT2aR is functionally associated with GRPR, coimmunoprecipitation was performed in vitro. The pcmv-Flag-5HT2aR and pcmv-Myc-GRPR plasmids were constructed, identified, and cotransfected or singly transfected into HEK293 cells. First, protein from the cotransfected cells was immunoprecipitated with anti-Flag antibody and analyzed by western blotting with anti-Myc antibody. The results showed that a GRPR band was detected in the immunoprecipitate of the anti-Flag antibody. Then, anti-Myc antibody was used for immunoprecipitation, and anti-Flag antibody was used for western blotting. A 5-HT2aR band was detected in the immunoprecipitate of the anti-Myc antibody. However, neither a 5-HT2aR band nor a GRPR band were detected in singly transfected cells immunoprecipitated with antibodies for both proteins. The protein bands were also not present in the immunoprecipitate of rabbit IgG (Figures 5A, B).
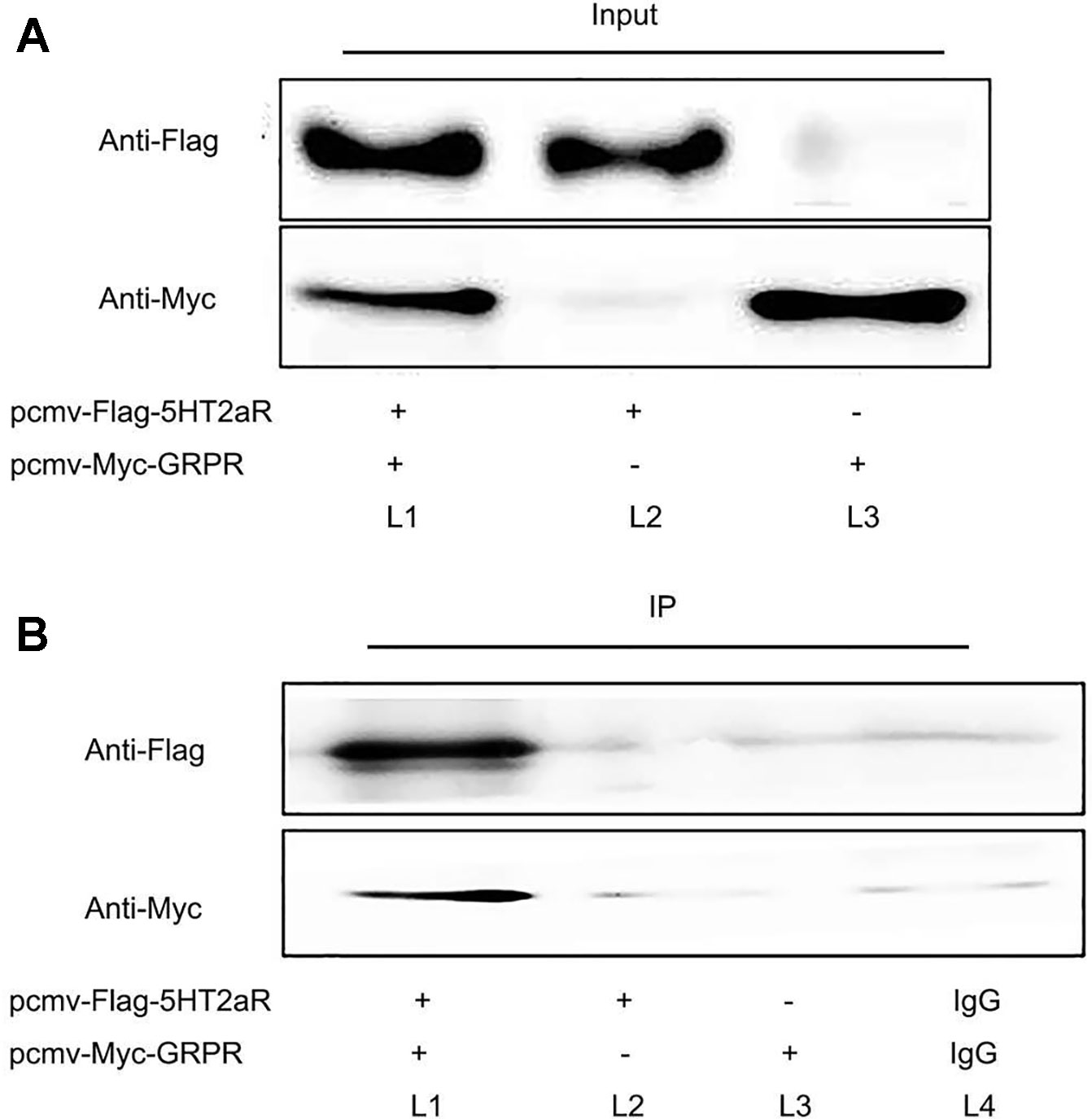
Figure 5 Coimmunoprecipitation of 5-HT2aR and GRPR. Input (A) and IP (B). The pcmv-Flag-5HT2aR and pcmv-Myc-GRPR plasmids were cotransfected into HEK293 cells (L1). The pcmv-Flag-5HT2aR plasmid was singly transfected into HEK293 cells (L2). The pcmv-Myc-GRPR plasmid was singly transfected into HEK293 cells (L3). IgG served as the negative control (L4).
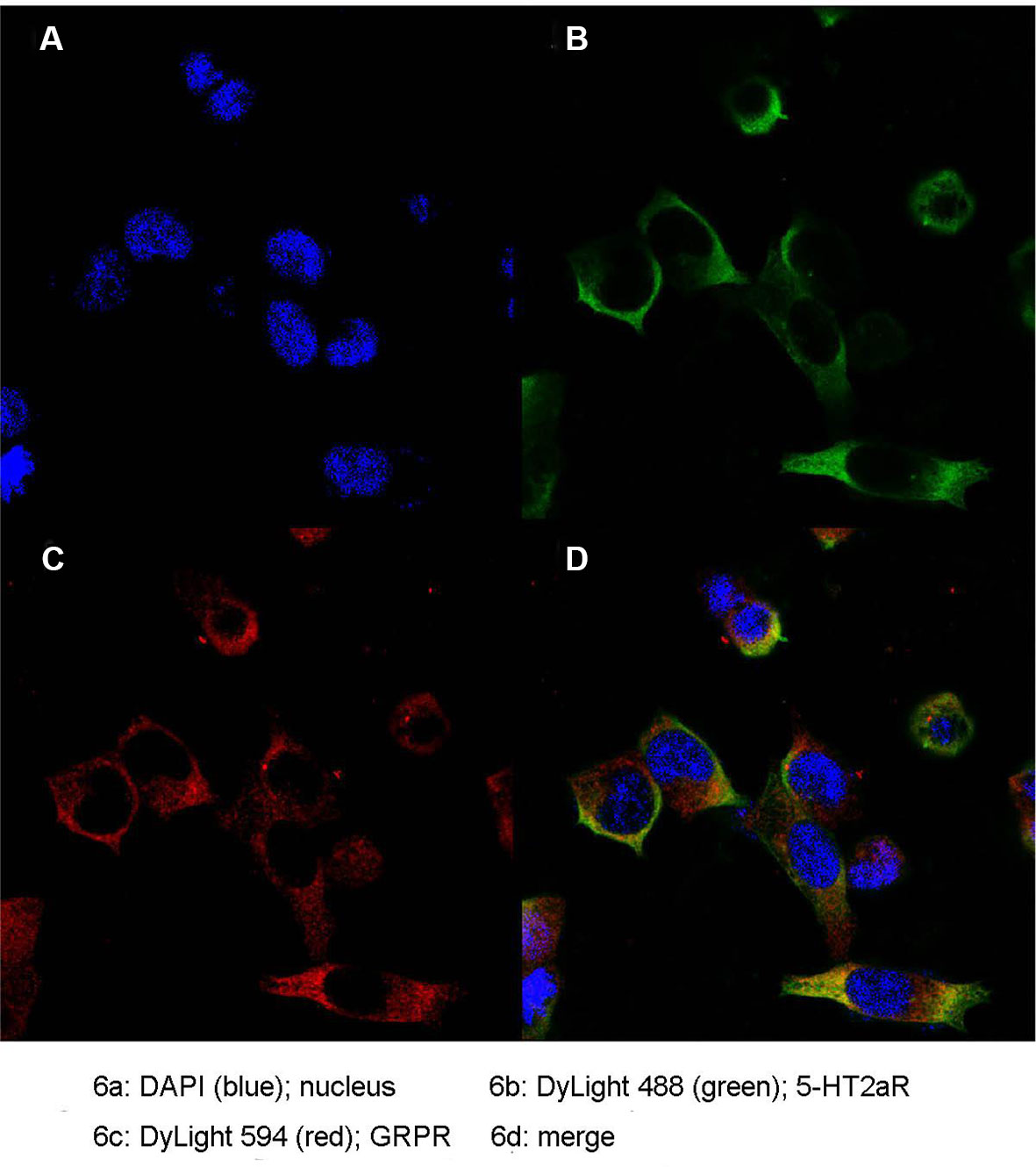
Colocalization of 5-HT2aR and GRPR
To further determine the expression pattern of 5-HT2aR and GRPR, we used the anti-Flag antibody and anti-Myc antibody specifically bound to HEK293 cells cotransfected with pcmv-Flag-5-HT2aR and pcmv-Myc-GRPR plasmids. Double immunofluorescence staining for 5-HT2aR and GRPR revealed that the expression of the two receptors overlapped in HEK293 cells. As revealed by confocal microscopy and shown in Figure 6, 5-HT2aR and GRPR were colocalized in the cytoplasm and membrane of HEK293 cells, as revealed by confocal microscopy.
Discussion
In this study, 4 weeks of CUMS was used to establish a classical model of depression in rats. The results indicated that fluoxetine treatment had an antidepressant-like effect in a CUMS model of depression in rats. Fluoxetine treatment decreased the levels of GRPR mRNA and protein in the hippocampus of stressed rats. Our coimmunoprecipitation results showed that 5-HT2aR and GRPR combine with each other in vitro. Additionally, immunofluorescence results revealed that the 5-HT2aR and GRPR were colocalized in both the cell membrane and cytoplasm. Our findings provide additional research ideas to explore the pathogenesis of targeting GRPR signaling in depression. Our data also supports that a functional interaction between GRPR and 5-HT2aR, were supported at the cellular level.
CUMS is regarded as a valid animal model of depression-like behavior (25). In this study, a classical model of depression was successfully built by 4 weeks of CUMS. Anhedonia is one of the major symptoms of depression, and is manifested as a reduction in interest or pleasure in daily activities. Additionally, CUMS causes many other symptoms of depression, such as decreases in food/water intake, exploratory behaviors, and locomotor activity, and helplessness (26). A reduction in sucrose preference in stressed rats reflects anhedonia, and an increase in immobility time in the forced swimming test indicates a lower desire to escape, which may be similar to the helplessness symptom in depression (27). The lower body weight observed in the stressed rats suggests that CUMS induced a decrease in food/water intake. In the open field test, CUMS significantly reduced the distance moved, velocity, and rearing frequency of rats. These results indicated that stressed rats exhibited less activity and fewer exploratory behaviors in new environments (28). Fluoxetine treatment led to a significant improvement in body weight, sucrose preference, and distance traveled, rearing frequency, and velocity in the open field test, and a decrease in immobility time of the forced swimming test. These findings are in agreement with previous studies and suggest that fluoxetine has antidepressant-like effects.
In this experiment, we showed that the mRNA and protein expression of GRPR in the hypothalamus were significantly upregulated in CUMS rats and that the expression of GRPR was downregulated after fluoxetine treatment. GRP binds preferentially to GRPR and stimulates the release of adrenocorticotropic hormone (ACTH) and corticosterone, and increasing the activity of the HPA axis, evokes behaviors associated with stress (29). GRP and GRPR are present in several brain regions implicated in the stress response, including the amygdala, hippocampus, hypothalamus, and bed nucleus of the stria terminalis, as well as caudal brainstem structures such as the nucleus tractus solitarii, parabrachial nucleus, and locus coeruleus (30). In addition, studies have shown that the locomotor activity and non-aggressive social behaviors are increased in the GRPR-deficient mice (31). In this study, we further confirmed that the mRNA and protein expression of GRPR was increased in CUMS rats. The results also showed that chronic fluoxetine treatment restored the stress-induced increase in GRPR expression. However, the mechanisms by which fluoxetine treatment restores GRPR expression remain unclear.
Fluoxetine is an SSRI and exerts its pharmacological effects through the manipulation of the 5-HT system (32). Fluoxetine selectively block 5-HT transporters, thereby increasing extracellular concentrations of 5-HT at the postsynaptic 5-HT receptors (33). In addition, fluoxetine treatment induces a complex array of neuropharmacological changes, such as a reduction in the density of 5-HT2aR (34). A Japanese cohort study suggested that 5-HT2aR may play an important role in the pathophysiology of the therapeutic response to SSRIs (35). In rats, chronic treatment with citalopram decreases the 5-HT2aR density in the brain cortex (36). Additionally, several studies have found that the CUMS-induced increase in 5-HT2aR expression can be decreased by fluoxetine administration (37, 38). Moreover, Qesseveur et al. explored the genetic variants of the 5-HT2aR gene that affect the therapeutic outcome of antidepressants. The results suggested that the genetic inactivation of the 5-HT 2A receptor may affect the antidepressant effects of SSRIs (39). These findings suggest that 5-HT2aR may be involved in the mechanism of action of antidepressant effect. In our study, we also found that chronic fluoxetine treatment decreased GRPR expression in the hypothalamus of rats. To explore the mechanism underlying the regulation of this response, we used HEK293 cells to explore the interactions between 5-HT2aR and GRPR. We used coimmunoprecipitation to identify the protein-protein interaction between 5-HT2aR and GRPR and used double immunofluorescence staining to study the colocalization of 5-HT2aR and GRPR in vitro. By performing coimmunoprecipitation and double immunofluorescence staining, we confirmed the interaction between 5-HT2aR and GRPR in vitro, which may provide new ideas for the treatment of depression.
One limitation is that our study was only performed in vitro, so it may not fully explain the possible mechanism of the interaction between these two receptors in the pathogenesis of depression. Therefore, it is necessary to confirm this conclusion in patients or animal models of depression. Another limitation is that we only explored the impacts of stress and fluoxetine treatment on GRPR expression. GRPR plays a physiological role by activating the PLC/PKC pathway, has a similar signaling pathway to 5-HT2aR. The molecular and cellular basis for GRPR-5-HT2aR cross-signaling may be an interesting research direction in this field.
In general, this study confirmed a change in GRPR expression in the rat hypothalamus after stress. Additionally, for the first time, this study provides experimental evidence of the interaction between 5-HT2aR and GRPR in vitro. These results highlight the involvement of GRPR in depression, and provide a novel biological target for the treatment of depression.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The animal study was reviewed and approved by The P.R. China legislation on the ethical care and use of laboratory animals, and the Institutional Animals Care Committee of Renmin Hospital of Wuhan University.
Author Contributions
ZL and GW designed and supervised the study. DX carried out the experimental procedures and analyzed the date. ZL and DX interpreted results of experiments and drafted the manuscript. ZL revised the manuscript. All authors provided feedback on manuscript.
Funding
This work was supported by the National Key R&D Program of China (2018YFC1314600) and the National Natural Science Foundation of China (81771472, 81271496, and 30971040).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Kolovos S, Bosmans JE, Riper H, Chevreul K, Coupe V, van Tulder MW. Model-based economic evaluation of treatments for depression: a systematic literature review. Pharmacoecon Open (2017) 1:149–65. doi: 10.1007/s41669-017-0014-7
2. Singh R, Mazi-Kotwal N, Thalitaya MD. Recognising and treating depression in the elderly. Psychiatr Danub (2015) 27 Suppl 1:S231–4.
3. Cowen PJ, Browning M. What has serotonin to do with depression? World Psychiatry (2015) 14:158–60. doi: 10.1002/wps.20229
4. Amidfar M, Kim YK. Recent developments on future antidepressant-related serotonin receptors. Curr Pharm Des (2018) 24:2541–8. doi: 10.2174/1381612824666180803111240
5. Carr GV, Lucki I. The role of serotonin receptor subtypes in treating depression: a review of animal studies. Psychopharmacol (Berl) (2011) 213:265–87. doi: 10.1007/s00213-010-2097-z
6. Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP, et al. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science (2006) 313:536–40. doi: 10.1126/science.1123432
7. Dwivedi Y, Mondal AC, Payappagoudar GV, Rizavi HS. Differential regulation of serotonin (5HT)2A receptor mRNA and protein levels after single and repeated stress in rat brain: role in learned helplessness behavior. Neuropharmacology (2005) 48:204–14. doi: 10.1016/j.neuropharm.2004.10.004
8. Baumeister D, Barnes G, Giaroli G, Tracy D. Classical hallucinogens as antidepressants? A review of pharmacodynamics and putative clinical roles. Ther Adv Psychopharmacol (2014) 4:156–69. doi: 10.1177/2045125314527985
9. Pandey DK, Mahesh R, Kumar AA, Rao VS, Arjun M, Rajkumar R. A novel 5-HT(2A) receptor antagonist exhibits antidepressant-like effects in a battery of rodent behavioural assays: approaching early-onset antidepressants. Pharmacol Biochem Behav (2010) 94:363–73. doi: 10.1016/j.pbb.2009.09.018
10. Zaniewska M, McCreary AC, Wydra K, Filip M. Effects of serotonin (5-HT)2 receptor ligands on depression-like behavior during nicotine withdrawal. Neuropharmacology (2010) 58:1140–6. doi: 10.1016/j.neuropharm.2010.02.006
11. Amidfar M, Kim YK, Colic L, Arbabi M, Mobaraki G, Hassanzadeh G, et al. Increased levels of 5HT2A receptor mRNA expression in peripheral blood mononuclear cells of patients with major depression: correlations with severity and duration of illness. Nord J Psychiatry (2017) 71:282–8. doi: 10.1080/08039488.2016.1276624
12. Shelton RC, Sanders-Bush E, Manier DH, Lewis DA. Elevated 5-HT 2A receptors in postmortem prefrontal cortex in major depression is associated with reduced activity of protein kinase A. Neuroscience (2009) 158:1406–15. doi: 10.1016/j.neuroscience.2008.11.036
13. Roesler R, Schwartsmann G. Gastrin-releasing peptide receptors in the central nervous system: role in brain function and as a drug target. Front Endocrinol (Lausanne) (2012) 3:159. doi: 10.3389/fendo.2012.00159
14. Stangelberger A, Schally AV, Varga JL, Zarandi M, Cai RZ, Baker B, et al. Inhibition of human androgen-independent PC-3 and DU-145 prostate cancers by antagonists of bombesin and growth hormone releasing hormone is linked to PKC, MAPK and c-jun intracellular signalling. Eur J Cancer (2005) 41:2735–44. doi: 10.1016/j.ejca.2005.08.022
15. Merali Z, Kent P, Anisman H. Role of bombesin-related peptides in the mediation or integration of the stress response. Cell Mol Life Sci (2002) 59:272–87. doi: 10.1007/s00018-002-8422-x
16. Merali Z, Anisman H, James JS, Kent P, Schulkin J. Effects of corticosterone on corticotrophin-releasing hormone and gastrin-releasing peptide release in response to an aversive stimulus in two regions of the forebrain (central nucleus of the amygdala and prefrontal cortex). Eur J Neurosci (2008) 28:165–72. doi: 10.1111/j.1460-9568.2008.06281.x
17. Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology (2000) 23:477–501. doi: 10.1016/S0893-133X(00)00159-7
18. Merali Z, Hayley S, Kent P, McIntosh J, Bedard T, Anisman H. Impact of repeated stressor exposure on the release of corticotropin-releasing hormone, arginine-vasopressin and bombesin-like peptides at the anterior pituitary. Behav Brain Res (2009) 198:105–12. doi: 10.1016/j.bbr.2008.10.025
19. Mountney C, Anisman H, Merali Z. In vivo levels of corticotropin-releasing hormone and gastrin-releasing peptide at the basolateral amygdala and medial prefrontal cortex in response to conditioned fear in the rat. Neuropharmacology (2011) 60:410–7. doi: 10.1016/j.neuropharm.2010.10.013
20. Garrido MM, Fuentes JA, Manzanares J. Gastrin-releasing peptide mediated regulation of 5-HT neuronal activity in the hypothalamic paraventricular nucleus under basal and restraint stress conditions. Life Sci (2002) 70:2953–66. doi: 10.1016/S0024-3205(02)01558-8
21. Yao L, Chen J, Chen H, Xiang D, Yang C, Xiao L, et al. Hypothalamic gastrin-releasing peptide receptor mediates an antidepressant-like effect in a mouse model of stress. Am J Transl Res (2016) 8:3097–105.
22. Wilkie MJ, Smith G, Day RK, Matthews K, Smith D, Blackwood D, et al. Polymorphisms in the SLC6A4 and HTR2A genes influence treatment outcome following antidepressant therapy. Pharmacogenomics J (2009) 9:61–70. doi: 10.1038/sj.tpj.6500491
23. Luo DD, An SC, Zhang X. Involvement of hippocampal serotonin and neuropeptide Y in depression induced by chronic unpredicted mild stress. Brain Res Bull (2008) 77:8–12. doi: 10.1016/j.brainresbull.2008.05.010
24. Overstreet DH. Modeling depression in animal models. Methods Mol Biol (2012) 829:125–44. doi: 10.1007/978-1-61779-458-2_7
25. Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacol (Berl) (1997) 134:319–29. doi: 10.1007/s002130050456
26. Abelaira HM, Reus GZ, Quevedo J. Animal models as tools to study the pathophysiology of depression. Braz J Psychiatry (2013) 35 Suppl 2:S112–20. doi: 10.1590/1516-4446-2013-1098
27. Castagne V, Moser P, Roux S, Porsolt RD. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci (2011) Chapter 8:Unit 8.10A. doi: 10.1002/0471142301.ns0810as55
28. Hurley LL, Akinfiresoye L, Kalejaiye O, Tizabi Y. Antidepressant effects of resveratrol in an animal model of depression. Behav Brain Res (2014) 268:1–7. doi: 10.1016/j.bbr.2014.03.052
29. Garrido MM, Martin S, Ambrosio E, Fuentes JA, Manzanares J. Role of corticotropin-releasing hormone in gastrin-releasing peptide-mediated regulation of corticotropin and corticosterone secretion in male rats. Neuroendocrinology (1998) 68:116–22. doi: 10.1159/000054357
30. Ladenheim EE, Behles RR, Bi S, Moran TH. Gastrin-releasing peptide messenger ribonucleic acid expression in the hypothalamic paraventricular nucleus is altered by melanocortin receptor stimulation and food deprivation. Endocrinology (2009) 150:672–8. doi: 10.1210/en.2008-0559
31. Monje FJ, Kim EJ, Cabatic M, Lubec G, Herkner KR, Pollak DD. A role for glucocorticoid-signaling in depression-like behavior of gastrin-releasing peptide receptor knock-out mice. Ann Med (2011) 43:389–402. doi: 10.3109/07853890.2010.538716
32. Dupuis A, Wattiez AS, Pinguet J, Richard D, Libert F, Chalus M, et al. Increasing spinal 5-HT2A receptor responsiveness mediates anti-allodynic effect and potentiates fluoxetine efficacy in neuropathic rats. Evidence for GABA release. Pharmacol Res (2017) 118:93–103. doi: 10.1016/j.phrs.2016.09.021
33. Sanchez C, Hyttel J. Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cell Mol Neurobiol (1999) 19:467–89. doi: 10.1023/A:1006986824213
34. Peroutka SJ, Snyder SH. Long-term antidepressant treatment decreases spiroperidol-labeled serotonin receptor binding. Science (1980) 210:88–90. doi: 10.1126/science.6251550
35. Kishi T, Yoshimura R, Kitajima T, Okochi T, Okumura T, Tsunoka T, et al. HTR2A is associated with SSRI response in major depressive disorder in a Japanese cohort. Neuromol Med (2010) 12:237–42. doi: 10.1007/s12017-009-8105-y
36. Muguruza C, Miranda-Azpiazu P, Diez-Alarcia R, Morentin B, Gonzalez-Maeso J, Callado LF, et al. Evaluation of 5-HT2A and mGlu2/3 receptors in postmortem prefrontal cortex of subjects with major depressive disorder: effect of antidepressant treatment. Neuropharmacology (2014) 86:311–8. doi: 10.1016/j.neuropharm.2014.08.009
37. Abuelezz SA, Hendawy N, Magdy Y. The potential benefit of combined versus monotherapy of coenzyme Q10 and fluoxetine on depressive-like behaviors and intermediates coupled to Gsk-3beta in rats. Toxicol Appl Pharmacol (2018) 340:39–48. doi: 10.1016/j.taap.2017.12.018
38. Yang Y, Hu Z, Du X, Davies H, Huo X, Fang M. miR-16 and fluoxetine both reverse autophagic and apoptotic change in chronic unpredictable mild stress model rats. Front Neurosci (2017) 11:428. doi: 10.3389/fnins.2017.00428
39. Qesseveur G, Petit AC, Nguyen HT, Dahan L, Colle R, Rotenberg S, et al. Genetic dysfunction of serotonin 2A receptor hampers response to antidepressant drugs: a translational approach. Neuropharmacology (2016) 105:142–53. doi: 10.1016/j.neuropharm.2015.12.022
40. Anisman H, Lacosta S, Kent P, McIntyre DC, Merali Z. Stressor-induced corticotropin-releasing hormone, bombesin, ACTH and corticosterone variations in strains of mice differentially responsive to stressors. Stress (1998) 2:209–20. doi: 10.3109/10253899809167284
Keywords: 5-HT2a receptor, gastrin-releasing peptide receptor, chronic unpredictable mild stress, interaction, depression
Citation: Xiang D, Wang H, Sun S, Yao L, Li R, Zong X, Wang G and Liu Z (2020) GRP Receptor Regulates Depression Behavior via Interaction With 5-HT2a Receptor. Front. Psychiatry 10:1020. doi: 10.3389/fpsyt.2019.01020
Received: 08 October 2019; Accepted: 23 December 2019;
Published: 28 January 2020.
Edited by:
Shaohua Hu, Zhejiang University, ChinaReviewed by:
Zhongqiu Zhao, Washington University School of Medicine in St. Louis, United StatesBhaskar Roy, University of Alabama at Birmingham, United States
Marong Fang, Zhejiang University, China
Copyright © 2020 Xiang, Wang, Sun, Yao, Li, Zong, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gaohua Wang, d2doNjQwMkAxNjMuY29t; Zhongchun Liu, emNsaXU2QHdodS5lZHUuY24=
 Dan Xiang
Dan Xiang Gaohua Wang
Gaohua Wang Zhongchun Liu
Zhongchun Liu