- 1Chemical Senses and Mental Health Lab, Department of Psychology, School of Public Health, Southern Medical University (Guangdong Provincial Key Laboratory of Tropical Disease Research), Guangzhou, China
- 2Department of Psychology, Guangdong 999 Brain Hospital, Guangzhou, China
- 3Department of Psychiatry, Zhujiang Hospital, Southern Medical University, Guangzhou, China
- 4Smell and Taste Clinic, Department of Otorhinolaryngology, Technische Universität Dresden, Dresden, Germany
Background: “Early-onset schizophrenia” (EOS) is defined as disease with onset before the age of 18 years. This subset of schizophrenia exhibits worse cognitive function and carries a worse prognosis than adult-onset schizophrenia (AOS). Olfactory impairment has been found in patients with schizophrenia-spectrum disorders. However, most research has focused on olfactory impairment in patients with AOS: olfactory function in EOS is not known. The aim of this study was to investigate the olfactory identification ability in EOS, and its relationship with negative symptoms.
Methods: We compared olfactory function between two independent samples: 40 patients with EOS and 40 age- and sex-matched healthy controls (HCs); as well as 40 patients with AOS and 40 age- and sex-matched HCs. The University of Pennsylvania Smell Identification Test was administered.
Results: The EOS group and AOS group exhibited worse olfactory identification ability than HCs; impairment correlated significantly with negative symptoms. Olfactory identification was worse in patients suffering EOS compared with those suffering AOS.
Conclusion: Olfactory identification impairment may be a trait marker of schizophrenia.
Introduction
Within the sensory system, olfactory neural circuitry is associated most closely with temporo-limbic and frontal-lobe regions. These pathways overlap with brain regions that are typically defective in schizophrenia (1, 2). Several meta-analyses have consistently reported obvious olfactory impairment in patients with schizophrenia, especially in olfactory identification (3, 4). Impairment of olfactory identification has been reported to be related to negative symptomatology in schizophrenia (5–7). In addition, impairment of olfactory identification has been found among first-degree family members of patients with schizophrenia and youths at risk of developing psychosis (4, 7). These aforementioned findings suggest impairment of olfactory function to be a potential trait marker of schizophrenia.
Early-onset schizophrenia (EOS) is a rare, severe, and chronic form of schizophrenia. EOS is defined as disease with an onset before 18 years of age (8, 9). Scholars have reported that patients suffering from EOS exhibit more negative symptoms compared with those with adult-onset schizophrenia (AOS), which is defined as disease with the initial episode presenting after 18 years of age (10, 11). Numerous studies have revealed that patients with EOS show more severe impairment in cognitive function (9, 12), premorbid adjustment (13), and long-term outcome (14, 15) compared with those with AOS. The EOS phenotype is relatively homogenous, and may represent a subgroup with more salient genetic loading less affected by environmental alterations (9, 16, 17). These findings provide a unique opportunity to identify clinically relevant biomarkers in schizophrenia by studying EOS. Most studies regarding olfaction in schizophrenia conducted have focused on patients with AOS.
Only Corcoran and colleagues have examined olfactory function in EOS patients (18). They examined olfactory identification in 26 well-characterized adolescents with early-onset psychosis, including major depression (n = 5), bipolar disorder, mania (n = 3), otherwise unspecified psychosis (n = 5), and schizophrenia or schizoaffective disorder (n = 13). They revealed that deficits in olfactory identification were present in early-onset psychotic disorders (as they are in adults) and were related to cognitive and negative symptoms. However, the sample size was small (only 13 adolescents suffering schizophrenia or schizoaffective disorder). Thus, olfactory identification ability (OIA) in EOS patients is not clear.
Here, we investigated OIA in EOS patients and compared it with that of patients with AOS and healthy controls (HCs). EOS is a more severe phenotype of schizophrenia and shows more impairment in cognitive function compared with AOS, while cognitive function has a strong correlation with olfactory function (19, 20). Hence, we hypothesized that EOS patients and AOS patients would show worse OIA compared with that of HCs, and that impairment would be more severe in EOS patients compared with that in AOS patients. We also examined the relationship between OIA and negative symptoms. We hypothesized that olfactory performance would correlate negatively with negative symptoms in EOS and AOS patients.
Methods
Participants
The study protocol was approved by the ethics committees of Southern Medical University and Guangdong 999 Brain Hospital, both of which are in Guangzhou, China. Participants provided written informed consent to be part of this study.
Patients with schizophrenia were recruited from Zhujiang Hospital (Guangzhou, China), Southern Medical University, and Guangdong 999 Brain Hospital. Forty patients (26 males and 14 females; 20.5 ± 2.5 years) who experienced their first episode at age 13–18 years comprised the EOS group. Forty patients (17 males and 23 females; 25.4 ± 5.2 years) who experienced their first episode after the age of 18 years were assigned to the AOS group. The sample size was calculated by G*Power software (21) (t tests: difference between two independent means (two groups), two tails, α = 0.05, power = 0.8, d = −0.97) and the effect size was used according to a previous meta-analysis (5). Patients were diagnosed by trained psychiatrists according to guidelines set forth in Diagnostic and Statistical Manual of Mental Disorders (5th edition) (22). The Positive and Negative Syndrome Scale (PANSS) was used to identify clinical symptoms (23). Thirty-four patients of EOS were using antipsychotic medication during the experiment [aripiprazole (n = 7), risperidone (n = 15), olanzapine (n = 10), amisulpride (n = 2), Sertraline (n = 6), paliperidone (n = 10), or quetiapine (n = 5)], and 22 patients of AOS were taking antipsychotics [aripiprazole (n = 1), risperidone (n = 3), olanzapine (n = 8), amisulpride (n = 4), Sertraline (n = 3), paliperidone (n = 5), or quetiapine (n = 3)].
Exclusion criteria were: (i) history of brain injury; (ii) presence of other neuropsychiatric disorders; (iii) history of throat or nose diseases; (iv) severe infection with influenza during the week before study commencement; and (v) history of drug abuse.
Eighty healthy participants recruited from universities and nearby communities were divided into two groups. Participants were matched, respectively, with EOS and AOS patients based on sex and age. Identical exclusion criteria were applied to HC participants. Participants did not have a history of neuropsychiatric disorders. Smoking status was also recorded; only two patients in each patient group had a history of tobacco-smoking, and none had such history in the HC group.
Olfactory Identification Ability
The University of Pennsylvania Smell Identification Test (UPSIT) is a standardized forced-choice test, and is one of the most widely used smell tests in olfactory research. It is composed of four test booklets, each containing 10 items, for a total of 40 items (24). Each item includes “scratch and sniff” microencapsulated odors attached to a strip at the bottom of the page. The microcapsule was scratched directly by the examiner with a pencil to optimize performance. Subsequently, participants smelled the odor released from the microcapsules and were asked to choose one of four possible responses. If the participant was unsure of the odor emitted, the examiner scratched the patch again. Each correct response was awarded 1 mark. The total score ranged from 0 mark to 40 marks, with a higher score indicating better OIA.
Self-Reported Hedonic Traits
The Temporal Experience of Pleasure Scale (TEPS) was designed to measure the traits of individuals in anticipatory and consummatory experiences of pleasure (25). We used the Chinese version of the TEPS, which contains 20 self-reported items designed to distinguish the temporal aspects of pleasure. This test was rated using a six-point Likert scale, ranging from 1 (“very false for me”) to 6 (“very true for me”). Higher scores indicated a stronger ability to experience pleasure. The internal consistency of the Chinese version is good, and Cronbach’s α of the total scale is 0.83 (26).
Data Analysis
Comparison of olfactory function (UPSIT) and hedonic traits (TEPS) between patients with EOS, AOS, and their age- and sex-matched HCs was conducted by applying independent t-tests using SPSS v22.0 (IBM, Armonk, NY, USA). In addition, we calculated the effect size (Cohen’s d) to standardize the group difference of respective olfactory function among two samples (sample A: EOS, HCs; sample B: AOS, HCs) (27). In addition, we carried out a 2 × 2 two-way analysis of covariance (ANCONA) to examine the effects of sex and group on OIA in each sample using education as a covariate. Pearson’s correlation was used to assess the relationship between olfactory function and clinical symptoms (PANSS), age, illness duration, chlorpromazine equivalence (CPZ), and hedonic traits for each group. P < 0.05 denoted significance.
Results
Participant Characteristics, Hedonic Traits, and Olfactory Test Performance
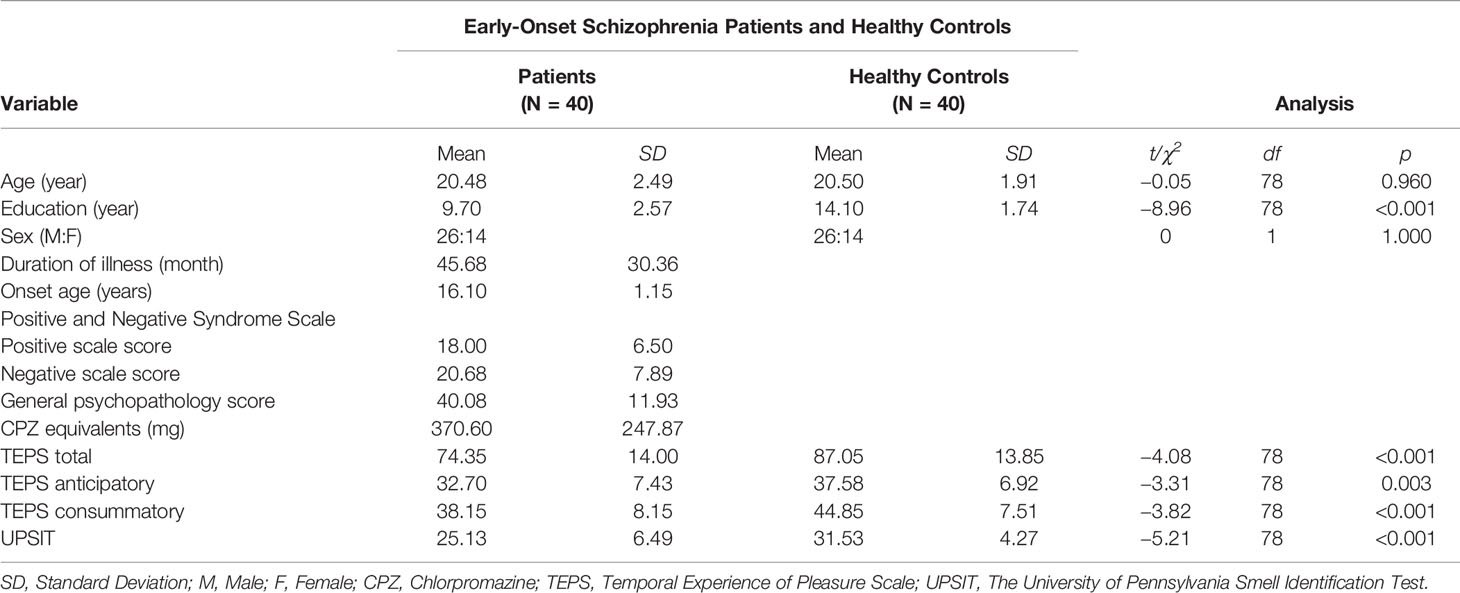
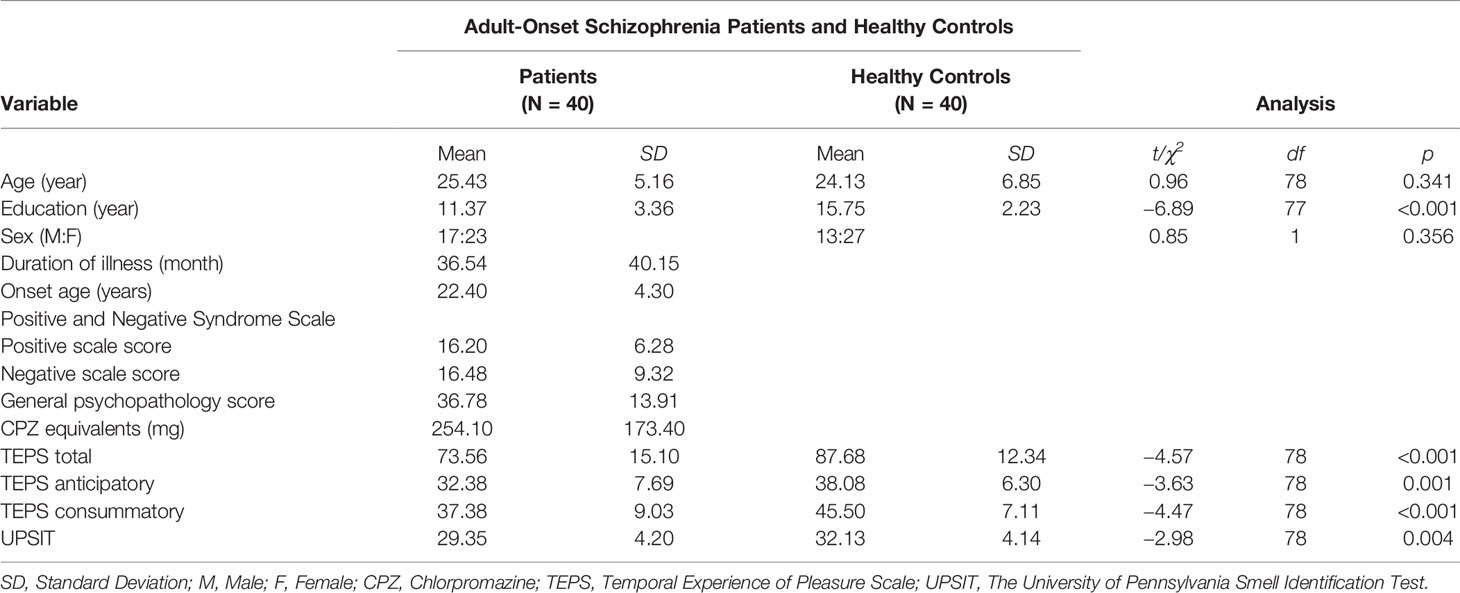
For the complete sample, significant differences were found between EOS, AOS and sex- and age-matched HC participants. The demographic and clinical data of patients are summarized in Tables 1 and 2. Patients in EOS and AOS groups had less educational experience compared with HCs. People in EOS and AOS groups showed significant differences in UPSIT scores compared with HCs (EOS vs. HC: t[78] = −5.21, p < 0.001; AOS vs. HC: t[78] = −2.98, p = 0.004). Moreover, the calculated effect sizes of the two samples were medium-to-large [EOS vs. HC: dA = 1.19, 95% confidence interval (CI) (0.68, 1.63); AOS vs. HC: dB = 0.66, 95% CI (0.21, 1.11)]. Two-way ANCOVA showed that the main effect of sex was not significant [EOS vs. HC: F(1,75) = 3.30, p = 0.73; AOS vs. HC: F(1,75) = 0.42, p = 0.519], whereas the main effect of the group was significant (EOS vs. HC: F(1,75) = 9.27, p = 0.003; AOS vs. HC: F(1,75) = 5.45, p = 0.022) after controlling for educational experience. The interaction of sex and group was not significant in sample A or B (p > 0.05 for all).
Patients with EOS showed a significantly lower score in negative symptoms (t [78] = 2.18, p = 0.033) compared with those with AOS. However, there were no significant differences in positive symptoms or general psychopathology (p > 0.05 for all). CPZ and illness duration did not differ between EOS and AOS groups (p > 0.05 for all).
As shown in Tables 1 and 2, samples A and B differed significantly in terms of TEPS total score (EOS vs. HC: t[78] = −4.08, p < 0.001, dA = 0.91, 95% CI (0.45, 1.37); AOS vs. HC: t[78] = −4.57, p < 0.001, dB = 1.02, 95% CI (0.55, 1.49), respectively), TEPS anticipatory subscale score (EOS vs. HC: t[78] = −3.31, p = 0.003, dA = 0.74, 95% CI (0.28, 1.19); AOS vs. HC: t[78] = −3.63, p = 0.001, dB = 0.68, 95% CI (0.36, 1.27), respectively), and TEPS consummatory subscale score (EOS vs. HC: t[78] = −3.82, p < 0.001, dA = 0.85, 95% CI (0.39, 1.31); AOS vs. HC: t[78] = −4.47, p < 0.001, dB = 1.00, 95% CI (0.53, 1.46).
Correlational Analysis
The OIA of EOS patients correlated significantly with negative symptoms (r = −0.331, p = 0.037) (Figure 1). All clinical-subscale scores of patients with AOS correlated significantly with olfactory function (positive symptoms: r = −0.409, p = 0.009; negative symptoms: r = −0.345, p = 0.029; general psychopathology: r = −0.372, p = 0.018). Moreover, the olfactory function of HCs in sample B correlated significantly with the TEPS anticipation subscale score (r = 0.379, p = 0.016). There were no significant relationships between olfactory function and total or subscale TEPS scores in sample-A HCs and patients with EOS or AOS (p > 0.05). A significant correlation between age and olfactory function in the EOS group (r = −0.334, p = 0.035) was documented. In both patient groups, OIA was not related to age of onset, illness duration, or CPZ (p > 0.05).
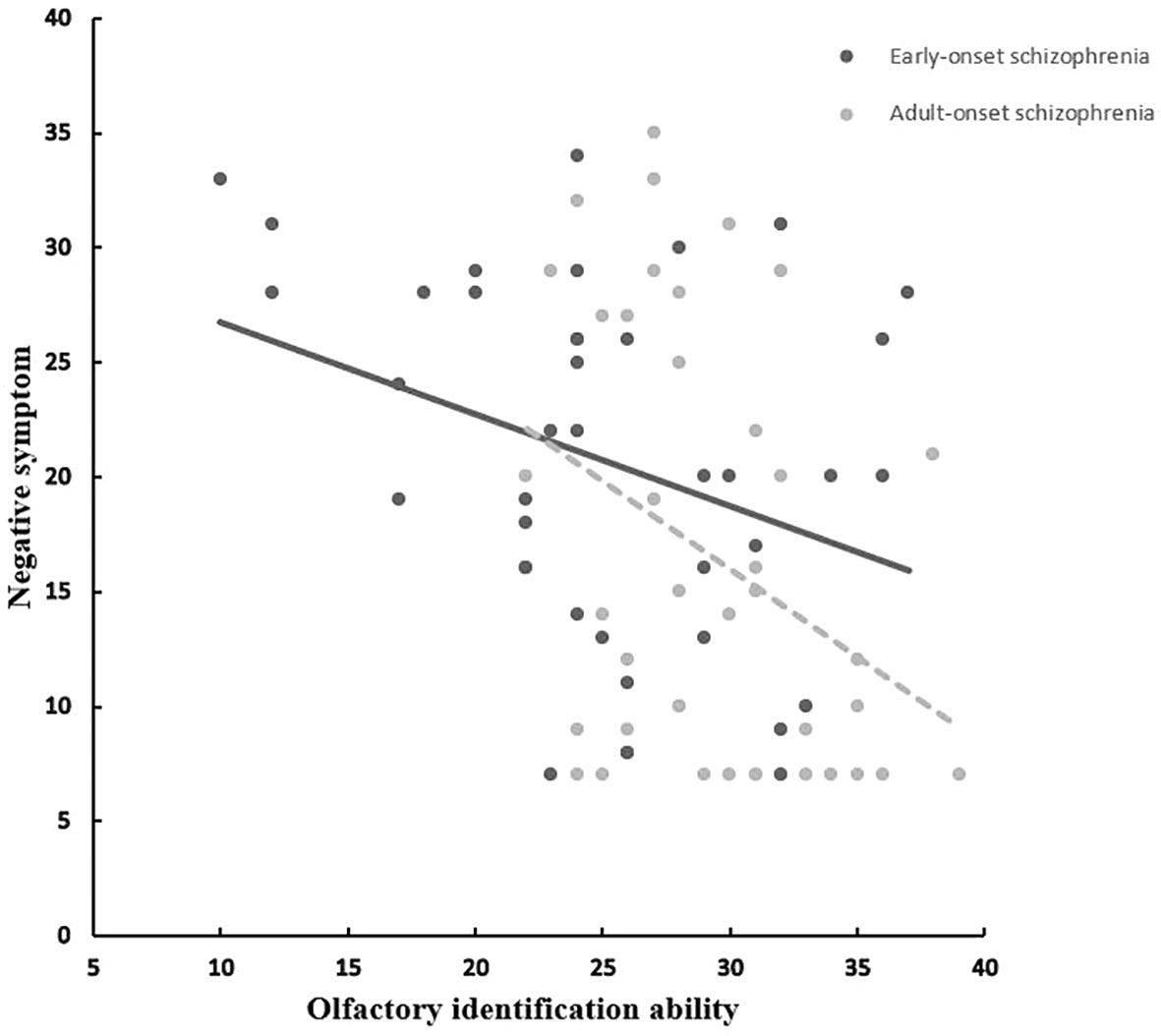
Figure 1 Correlation between olfactory identification ability (UPSIT) and negative symptom in EOS and AOS groups. UPSIT, University of Pennsylvania Smell Identification Test; EOS, Early-onset schizophrenia; AOS, Adult-onset schizophrenia.
Discussion
Here, we aimed to evaluate impairment of olfactory identification in patients with EOS and AOS. Such impairment has been reported in schizophrenia but the age of onset has not been addressed. This was the first study to investigate OIA in adults with EOS.
Our initial hypotheses were confirmed because patients suffering EOS or AOS exhibited significantly worse olfactory identification compared with sex- and age-matched HCs. These findings are in accordance with those of studies that consistently reported obvious olfactory impairment in patients with schizophrenia (3, 4). Furthermore, comparison of the effect size with regard to olfactory-identification impairment revealed differences between EOS (d = 1.19) and AOS (d = 0.66), suggesting impairment of greater severity in the former group of patients. In addition, results revealed that male patients did not differ from female patients with respect to OIA in the EOS group or AOS group, a finding that is consistent with that in other studies (28–30). Notably, Mossaheb et al. (31) found that female patients performed better than male patients in the olfactory-identification test, but the effect was only borderline significant (31).
Notably, there was no difference between patients with EOS and AOS in terms of illness duration or CPZ. Hence, the worse OIA observed in EOS compared with AOS may be attributed (at least in part) to brain maturity. Numerous studies have reported gray-matter volume in the frontal and temporal lobes of patients suffering EOS to be reduced (32–34). Matsumoto (32) reported a positive correlation between the volume of the bilateral superior temporal gyrus and age at disease onset. Although the findings of a longitudinal study revealed that the pattern of abnormal cortical thickness in EOS is similar to that observed in AOS, lower volume has been observed consistently in prefrontal and temporal cortices (35). These brain regions have a crucial role in olfactory function.
We found that only negative symptoms were more severe in EOS compared with AOS. Our findings are consistent with those of other studies, indicating that patients suffering EOS exhibit more negative symptoms than those with AOS (10, 11). In addition, our findings revealed that EOS and AOS patients experienced less pleasure than HCs. These findings are also in accordance with recent data from Zou and colleagues for patients with first-episode schizophrenia and individuals with schizotypy, who were of a similar age to the people evaluated in the present study. They revealed that the patient group suffered a significant loss of pleasure compared that in HCs (7). However, a significant difference in experiencing pleasure among patients suffering EOS and AOS was not observed.
Correlation analyses revealed a significant negative correlation between negative symptoms and OIA in EOS and AOS patients. In contrast with studies investigating individuals with schizotypy and patients with first-episode schizophrenia (7, 36), a significant association between olfactory function and self-reported experience of pleasure in chronically ill patients suffering EOS and AOS was not found. Several scholars have reported OIA to be associated with negative symptoms in adult patients with schizophrenia (5–7). Our findings are consistent with those reported by Corcoran et al. (18), who detailed a relationship between negative symptoms and OIA in early-onset psychosis. Corcoran et al. (18) stated that impairment of olfactory identification is a trait-like marker of vulnerability to schizophrenia and the manifestation of negative symptomology. A possible explanation for this phenomenon is that negative symptoms that occur during childhood and adolescence may affect acquisition of the language skills necessary to identify odors correctly (18).
Interestingly, our study did not reveal a correlation between the age of onset and reduced OIA in patients with EOS or AOS. This observation may be explained by the narrow range of the age of onset in both patient groups (EOS: 16.1 ± 1.5 years; AOS: 22.4 ± 4.3 years). In addition, reduced OIA did not correlate with illness duration or CPZ in either patient group. These findings suggest that the observed OIA reduction reflected olfactory function in schizophrenia itself.
Our study had several limitations. First, we recruited only adults with EOS. Future research should also investigate olfactory function in children and adolescents with EOS. Second, Seckinger suggested that the intelligence quotient (IQ) was related to OIA in schizophrenia (37). However, we did not test IQ due to time constraints, so whether olfactory impairment was because of IQ impairment in EOS was not investigated. Future studies must address this issue. Even so, OIA showed impairment in EOS and AOS after controlling for the educational level in our study. Third, we only assessed OIA in patients with EOS versus those with AOS. Future research should investigate olfactory function in a more differentiated way; that is, adding olfactory threshold and odor-discrimination tests using the Sniffin’ Sticks test (38). In addition, we only recruited EOS and AOS patients according to their first episode information in our study; future study should provide information about other episodes. Finally, this was a cross-sectional study; further longitudinal research is warranted to validate our findings.
In summary, we found the EOS group and AOS group exhibited worse OIA than HCs, and the impairment was correlated significantly with negative symptoms. Furthermore, OIA was worse in patients suffering EOS compared with those suffering AOS. With previous research revealed the olfactory identification impairment in first-episode schizophrenia and individuals with schizotypy (7), this continuity suggests olfactory identification impairment may be a trait marker of schizophrenia and emphasizes the significance of early diagnosis and intervention. Also, special olfactory treatment (e.g., olfactory training) may decrease the negative symptoms in schizophrenia, and future study need to address this issue.
Conclusions
EOS is linked to a more severe deficit in olfactory identification compared with AOS and is associated with the manifestation of negative symptoms. Our findings support olfactory identification impairment may be a trait marker of schizophrenia and also provide a new insight into the early intervention for schizophrenia.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committees of Southern Medical University and the Guangdong 999 Brain Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Z-TL helped design and run the study, analyzed, and interpreted the data, and wrote the manuscript. S-BL recruited the participants, helped run the study, analyzed, and interpreted the data. J-FW recruited the individuals with schizophrenia, provided demographic and symptom information, and helped with manuscript preparation. X-YZ helped with manuscript preparation and provided comments on the final version. TH helped with manuscript preparation and provided comments on the final version. L-QZ helped design and run the study, analyzed and interpreted the data, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant number: 31700963), the Natural Science Foundation of Guangdong Province, China (grant number: 2017A030310069; 2019A1515012135), and the Medical Science and Technology Foundation of Guangdong Province, China (grant number: 201906546). These funding agents had no further role in any aspect of the study or the writing of this paper.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all the participants who attended our study.
References
1. Moberg PJ, Agrin R, Gur RE, Gur RC, Turetsky B II, Doty RL. Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology (1999) 21(3):325–40. doi: 10.1016/s0893-133x(99)00019-6
2. Turetsky B, Cowell PE, Gur RC, Grossman RI, Gur RE. Frontal and temporal lobe brain volumes in schizophrenia: relationship to symptomatology and clinical subtype. Biol Psychiatry (1994) 35(9):723. doi: 10.1016/0006-3223(94)91044-8
3. Cohen AS, Brown LA, Auster TL. Olfaction, “olfiction,” and the schizophrenia-spectrum: an updated meta-analysis on identification and acuity. Schizophr Res (2012) 135(1-3):152–7. doi: 10.1016/j.schres.2011.12.005
4. Moberg PJ, Kamath V, Marchetto DM, Calkins ME, Doty RL, Hahn CG, et al. Meta-analysis of olfactory function in schizophrenia, first-degree family members, and youths at-risk for psychosis. Schizophr Bull (2014) 40(1):50–9. doi: 10.1093/schbul/sbt049
5. Ishizuka K, Tajinda K, Colantuoni C, Morita M, Winicki J, Le C, et al. Negative symptoms of schizophrenia correlate with impairment on the University of Pennsylvania smell identification test. Neurosci Res (2010) 66(1):106–10. doi: 10.1016/j.neures.2009.10.001
6. Strauss GP, Allen DN, Ross SA, Duke LA, Schwartz J. Olfactory hedonic judgment in patients with deficit syndrome schizophrenia. Schizophr Bull (2010) 36(4):860–8. doi: 10.1093/schbul/sbn178
7. Zou LQ, Zhou HY, Lui SSY, Wang Y, Wang Y, Gan J, et al. Olfactory identification deficit and its relationship with hedonic traits in patients with first-episode schizophrenia and individuals with schizotypy. Prog Neuropsychopharmacol Biol Psychiatry (2018) 83:137–41. doi: 10.1016/j.pnpbp.2018.01.014
8. Tamnes CK, Agartz I. White Matter Microstructure in Early-Onset Schizophrenia: A Systematic Review of Diffusion Tensor Imaging Studies. J Am Acad Child Adolesc Psychiatry (2016) 55(4):269–79. doi: 10.1016/j.jaac.2016.01.004
9. Vyas NS, Patel NH, Puri BK. Neurobiology and phenotypic expression in early onset schizophrenia. Early Interv Psychiatry (2011) 5(1):3–14. doi: 10.1111/j.1751-7893.2010.00253.x
10. Diaz-Caneja CM, Pina-Camacho L, Rodriguez-Quiroga A, Fraguas D, Parellada M, Arango C. Predictors of outcome in early-onset psychosis: a systematic review. NPJ Schizophr (2015) 1(1):1–10. doi: 10.1038/npjschz.2014.5
11. Harvey RC, James AC. Shields GE. A Systematic Review and Network Meta-Analysis to Assess the Relative Efficacy of Antipsychotics for the Treatment of Positive and Negative Symptoms in Early-Onset Schizophrenia. CNS Drugs (2016) 30(1):27–39. doi: 10.1007/s40263-015-0308-1
12. White T, Ho BC, Ward J, O’Leary D, Andreasen NC. Neuropsychological performance in first-episode adolescents with schizophrenia: a comparison with first-episode adults and adolescent control subjects. Biol Psychiatry (2006) 60(5):463–71. doi: 10.1016/j.biopsych.2006.01.002
13. Vourdas A, Pipe R, Corrigall R, Frangou S. Increased developmental deviance and premorbid dysfunction in early onset schizophrenia. Schizophr Res (2003) 62(1-2):13–22. doi: 10.1016/s0920-9964(02)00429-2
14. Clemmensen L, Vernal DL, Steinhausen, HC. A systematic review of the long-term outcome of early onset schizophrenia. BMC Psychiatry (2012) 12(1):150. doi: 10.1186/1471-244x-12-150
15. Hollis C. Adult outcomes of child-and adolescent-onset schizophrenia: diagnostic stability and predictive validity. Am J Psychiatry (2000) 157(10):1652–9. doi: 10.1176/appi.ajp.157.10.1652
16. Vyas NS, Gogtay N. Treatment of early onset schizophrenia: recent trends, challenges and future considerations. Front Psychiatry (2012) 3:29. doi: 10.3389/fpsyt.2012.00029
17. Vyas NS, Kumra S, Puri BK. What insights can we gain from studying early-onset schizophrenia? The neurodevelopmental pathway and beyond. Expert Rev Neurother (2010) 10(8):1243–7. doi: 10.1586/ern.10.109
18. Corcoran C, Whitaker A, Coleman E, Fried J, Feldman J, Goudsmit N, et al. Olfactory deficits, cognition and negative symptoms in early onset psychosis. Schizophr Res (2005) 80(2-3):283–93. doi: 10.1016/j.schres.2005.07.028
19. de Nijs J, Meijer JH, de Haan L, Meijer CJ, Bruggeman R, van Haren NEM, et al. Associations between olfactory identification and (social) cognitive functioning: A cross-sectional study in schizophrenia patients and healthy controls. Psychiatry Res (2018) 266:147–51. doi: 10.1016/j.psychres.2018.05.009
20. Takahashi T, Nakamura M, Sasabayashi D, Komori Y, Higuchi Y, Nishikawa Y, et al. Olfactory deficits in individuals at risk for psychosis and patients with schizophrenia: relationship with socio-cognitive functions and symptom severity. Eur Arch Psychiatry Clin Neurosci (2018) 268(7):689–98. doi: 10.1007/s00406-017-0845-3
21. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods (2007) 39(2):175–91. doi: 10.3758/bf03193146
22. APA. Diagnostic and statistical manual of mental disorders (DSM-5®). Washington, DC: American Psychiatric Publishing (2013).
23. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull (1987) 13(2):261–76. doi: 10.1093/schbul/13.2.261
24. Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav (1984) 32(3):489–502. doi: 10.1016/0031-9384(84)90269-5
25. Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: a scale development study. J Res Pers (2006) 40(6):1086–102. doi: 10.1016/j.jrp.2005.11.001
26. Chan RC, Shi YF, Lai MK, Wang YN, Wang Y, Kring AM. The Temporal Experience of Pleasure Scale (TEPS): exploration and confirmation of factor structure in a healthy Chinese sample. PloS One (2012) 7(4):e35352. doi: 10.1371/journal.pone.0035352
27. Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic Press (2013).
28. Kamath V, Turetsky BI, Moberg PJ. Identification of pleasant, neutral, and unpleasant odors in schizophrenia. Psychiatry Res (2011) 187(1-2):30–5. doi: 10.1016/j.psychres.2010.12.011
29. Larsson M, Finkel D, Pedersen NL. Odor identification: influences of age, gender, cognition, and personality. J Gerontol Ser B: psychol Sci Soc Sci (2000) 55(5):P304–P10. doi: 10.1093/geronb/55.5.P304
30. Stuck BA, Frey S, Freiburg C, Hörmann K, Zahnert T, Hummel T. Chemosensory event-related potentials in relation to side of stimulation, age, sex, and stimulus concentration. Clin Neurophysiol (2006) 117(6):1367–75. doi: 10.1016/j.clinph.2006.03.004
31. Mossaheb N, Kaufmann RM, Schlogelhofer M, Aninilkumparambil T, Himmelbauer C, Gold A, et al. The Impact of Sex Differences on Odor Identification and Facial Affect Recognition in Patients with Schizophrenia Spectrum Disorders. Front Psychiatry (2018) 9:9. doi: 10.3389/fpsyt.2018.00009
32. Matsumoto H, Simmons A, Williams S, Hadjulis M, Pipe R, Murray R, et al. Superior temporal gyrus abnormalities in early-onset schizophrenia: similarities and differences with adult-onset schizophrenia. Am J Psychiatry (2001) 158(8):1299–304. doi: 10.1176/appi.ajp.158.8.1299
33. Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry (2005) 10(5):434–49. doi: 10.1038/sj.mp.4001642
34. Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci (2001) 98(20):11650–5. doi: 10.1073/pnas.201243998
35. Greenstein D, Lerch J, Shaw P, Clasen L, Giedd J, Gochman P, et al. Childhood onset schizophrenia: cortical brain abnormalities as young adults. J Child Psychol Psychiatry (2006) 47(10):1003–12. doi: 10.1111/j.1469-7610.2006.01658.x
36. Zou LQ, Geng FL, Liu WH, Wei XH, Jiang XQ, Wang Y, et al. The neural basis of olfactory function and its relationship with anhedonia in individuals with schizotypy: An exploratory study. Psychiatry Res (2015) 234(2):202–7. doi: 10.1016/j.pscychresns.2015.09.011
37. Seckinger RA, Goudsmit N, Coleman E, Harkavy-Friedman J, Yale S, Rosenfield PJ, et al. Olfactory identification and WAIS-R performance in deficit and nondeficit schizophrenia. Schizophr Res (2004) 69(1):55–65. doi: 10.1016/s0920-9964(03)00124-5
Keywords: early-onset, adult-onset, schizophrenia, odor identification, negative symptoms
Citation: Li Z-t, Li S-b, Wen J-f, Zhang X-y, Hummel T and Zou L-q (2020) Early-Onset Schizophrenia Showed Similar but More Severe Olfactory Identification Impairment Than Adult-Onset Schizophrenia. Front. Psychiatry 11:626. doi: 10.3389/fpsyt.2020.00626
Received: 20 December 2019; Accepted: 15 June 2020;
Published: 30 June 2020.
Edited by:
Kiyoto Kasai, University of Tokyo, JapanReviewed by:
Antonio Rampino, University of Bari Aldo Moro, ItalyBartlomiej Stanczykiewicz, Wroclaw Medical University, Poland
Copyright © 2020 Li, Li, Wen, Zhang, Hummel and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lai-quan Zou, em91bHFAc211LmVkdS5jbg==
 Ze-tian Li
Ze-tian Li Shu-bin Li
Shu-bin Li Jin-feng Wen2
Jin-feng Wen2 Thomas Hummel
Thomas Hummel Lai-quan Zou
Lai-quan Zou