- 1Lowe Center for Thoracic Oncology, Dana-Farber Cancer Institute, Boston, MA, United States
- 2Harvard Medical School, Boston, MA, United States
- 3Sylvester Comprehensive Cancer Center, University of Miami Miller School of Medicine, Miami, FL, United States
- 4Department of Medicine, Hospital of the University of Pennsylvania, Philadelphia, PA, United States
- 5University of Illinois College of Medicine Peoria, Peoria, IL, United States
- 6Duke Cancer Center, Duke University School of Medicine, Durham, NC, United States
- 7Division of Hematology and Oncology, UT Southwestern Medical Center, Dallas, TX, United States
- 8Centro de Câncer de Brasília, Instituto Unity de Ensino e Pesquisa, Grupo Oncoclinicas, Brasília, Brazil
- 9Young Lung Cancer Initiative, Raleigh, NC, United States
- 10Department of Medicine, University of California San Francisco, San Francisco, CA, United States
Background: Young patients with lung cancer represent a distinct population, with unique disease and treatment-related characteristics, as well as psychosocial and survivorship needs. Nevertheless, this population remains vastly understudied.
Methods: We review the unique clinicopathological characteristics and needs of young patients with lung cancer, including topics such as incidence rates, diagnostic challenges, genomics, treatment patterns and outcomes, psychosocial needs, fertility and sexual health, and palliative care. We discuss emerging and understudied data, provide recommendations on aspects in which future research is warranted, and advocate for actionable strategies that multi-disciplinary healthcare teams may adopt to provide more personalized and equitable care.
Results: Though epidemiological trends suggest an overall decrease in lung cancer incidence among all age groups, recent increasing incidences have been reported among certain young populations in the U.S., as well as among Hispanic women and women in certain European countries. Young patients are significantly more likely to be female or Asian/Pacific Islander, have no tobacco use history, metastasis to the brain, and a higher frequency of somatic mutations or rearrangements. Diagnostic delays pose a considerable concern to young patients with lung cancer and may contribute to how these patients are more likely to be diagnosed with advanced disease than their older counterparts. However, young patients demonstrate improved survival compared to older patients, underscoring the importance of survivorship care. Young patients are more likely to be diagnosed at a disruptive time in their lives, rendering them with distinct psychosocial needs and financial toxicity. Future data on treatment-related effects on fertility and sexual health for young patients is warranted, as is the data related to complementary medicine use. Training in palliative care and promoting a positive attitude towards supportive care is also essential.
Conclusions: Young patients with lung cancer represent a distinct patient population, necessitating disease management that is markedly different from that of older patients with lung cancer. Future research, some of which are highlighted by this Review, will aid in elucidating risk factors, survival rates, and clinical, genomic, and histopathological characteristics of young-onset lung cancer to improve screening, early detection, prevention, and treatment of this understudied population.
1 Introduction: incidence and etiology of young-onset lung cancer
The incidence of lung cancer in patients under 50 years of age, most of whom have no history of tobacco exposure, is alarming and demands urgent attention by the medical and research community. This review aims to reduce the educational gap in appropriate care delivery for this understudied population by providing a comprehensive analysis of the unique clinicopathological characteristics and needs of young patients with lung cancer from diagnosis to end-of-life care.
Lung cancer remains the leading cause of cancer death worldwide (1). While the incidence of lung cancer has declined from 2012–2021 by 3% per year in men and 1.4% per year in women (2), more people still die from lung cancer annually than colon, breast, and prostate cancers combined (3). In recent years, there has been growing attention on young-onset lung cancer; however, a definitive definition of “young patients” has yet to be established. Proposed age cutoffs include 40, 45, or 50 years, therefore, the age ranges from primary data reported in studies included in this review vary by context.
Data on incidence trends regarding young-onset lung cancer remains an area of active investigation. In recent years, an increasing incidence of lung cancer among patients <50 years old has been reported among Hispanic women (4) and women in France, Italy, and Spain (5); increasing or stable incidence rates have also been reported in China (6) and Hong Kong (7). It is unclear if the incidence of lung cancer in younger patients is increasing globally, however, as most recent data suggests a decrease in lung cancer incidence in all age groups, including in countries such as the United States (U.S.) (8–10). Contemporary, expanded studies in younger age groups, incorporating both histology and race/ethnicity, are needed to definitively answer this question. In China, the age standardized incidence rate (ASIR) per 100,000 individuals was 7.38 in 2019, higher than the 5.30 ASIR in 1990 (6). In the U.S., according to Surveillance, Epidemiology, and End Results (SEER) data from 2017-2021 (11), 0.2% of new lung cancer cases were among 20–34-year-olds, 0.9% in 35-44-year olds, and 21.8% in 45-54-year-olds. Despite overall decreasing incidence in younger populations, slightly increasing incidences were observed for certain populations under 50 years including Non-Hispanic Asian/Pacific Islander females, Non-Hispanic Black men, and Black (including Hispanic) men. Between 1-10% of patients with lung cancer in Latin America (12) are younger than 40, though the incidence of genomic driver mutations vary across countries. For instance, Northern Latin America tends to have higher incidences of EGFR mutations and ALK alterations than the southern part of the region.
Contrary to popular belief, a recent retrospective Chinese review (13) of 82 patients with lung cancer <35-years-old found that nearly all (98.6%) had no family history of lung cancer nor personal history of tobacco use (14) (71.6%). This relative lack of tobacco use among young patients, particularly women, has garnered much attention. Since 2018, higher lung cancer incidence rates that are irrespective of tobacco use have been reported among females aged 35–54 years in the U.S., reversing historically higher rates in men (4). A recent supplementary study (15) found that lung cancer incidence rates in women were equal to or higher than rates in their male counterparts in 40 of 51 states, with statistically significant differences in 20 states; the highest female-to-male incident rate ratios found in North Dakota (1.53), Idaho (1.37), and Wyoming (1.35). Notably, current and ever smoking prevalence in women compared to men was statistically significantly lower or similar in 33 and 34 states, respectively, supporting how higher incidence rates in young women are unexplained by differences in smoking prevalence. However, the impact of second-hand smoke, believed to increase the risk of lung cancer by 24% in non-tobacco users (16), warrants further analysis. With a recent report from the American Cancer Society depicting how women under 50 years old are 82% more likely to develop any cancer than their male counterparts (2), factors unique to women, such as the impact of childbirth and oral contraceptive use (17), are also ripe areas for exploration. Some have further hypothesized that risk factors such as radon gas exposure and air pollution may be contributing to young-onset cases (18, 19), similar to the environmental exposures that may be contributing to the rise in early-onset colorectal cancer cases (20). The observation that younger patients often lack tobacco or identifiable environmental exposure additionally suggests that germline predisposition may contribute to lung cancer development in young people, which is an ongoing area of investigation that is challenging due to the large numbers of individuals required to identify shared but rare genetic changes.
2 Diagnostic challenges
Although the survival rate for metastatic lung cancer has improved over the past four decades, the 5-year disease survival rate remains low at only 9% for non-small cell lung cancer (NSCLC) and 3% small cell lung cancer (SCLC) (21), highlighting the importance of early diagnosis. Indeed, delayed diagnosis of lung cancer poses a significant concern, particularly to young patients, as it may impact disease stage and overall survival (OS) (22). Patients may present with general pulmonary symptoms that lead to misdiagnoses (23), such as allergies, asthma, tuberculosis (24), sarcoidosis (25), viral infections, and pneumonia (26). Additionally, due to the disease’s stigma and association with tobacco use and older age, young patients and individuals with no prior tobacco use may be at greater risk of misdiagnosis due to the perceived low likelihood of developing lung cancer (27). Lung cancer screening guidelines (28) may also impact contribute to diagnostic delays, as recommendations currently only focus on individuals aged 50–80 with a history of at least 20 pack-years of tobacco use, thereby excluding young patients, even if they have extensive exposure to second-hand smoke or other risk factors.
Gender bias may also contribute to diagnostic delays, as women experience significantly longer times to diagnoses than men (29, 30). At least 33% of women newly diagnosed with lung cancer have three or more visits with their general practitioner before being referred for further imaging or biopsy, compared to only 3% of patients with breast cancer (31). Women are also 32% less likely than men to report a lung cancer screening discussion with a primary care provider and 32% less likely than men to be aware of available lung cancer screening tests (32). Further, women are more likely to have additional follow-up visits and a greater number of consultations rather than an immediate diagnostic intervention that could lead to a timely diagnosis (33, 34). Gendered association of certain risk factors such as tobacco (35) may also contribute to delays, as women with a history of tobacco use are still more likely to have a late-stage diagnosis due to delays faced in obtaining appropriate imaging and tissue diagnosis (36).
Diagnostic delays may be one contributing factor to how young patients are more likely than their older counterparts to be diagnosed with later-stage or advanced disease (13, 37–40), with a larger number having node-positive disease (60% vs. 51%) (41). Unsurprisingly, one retrospective analysis of 355 lung cancer cases (42) found young women to be diagnosed at a significantly later stage than other patient populations. Consequently, young individuals often have limited local therapeutic options such as curative-intent surgeries available to them (43). Indeed, a U.S. SEER database review from 2010-2017 (44) found that patients under 50 years old comprised only 6.6% of 33,586 surgically treated patients with NSCLC, similar to how patients under 50 encompassed only 5.0% of 11,663 surgical cases in a 2004 Japanese Lung Cancer Registry Study (44). Further, a nationwide South Korean database study found a significant decrease in the frequency of surgery among individuals aged 20–60 years from 2015 (45). Such data is unfortunate, considering how young patients exhibit significantly enhanced surgical outcomes compared to their older counterparts (44, 46).
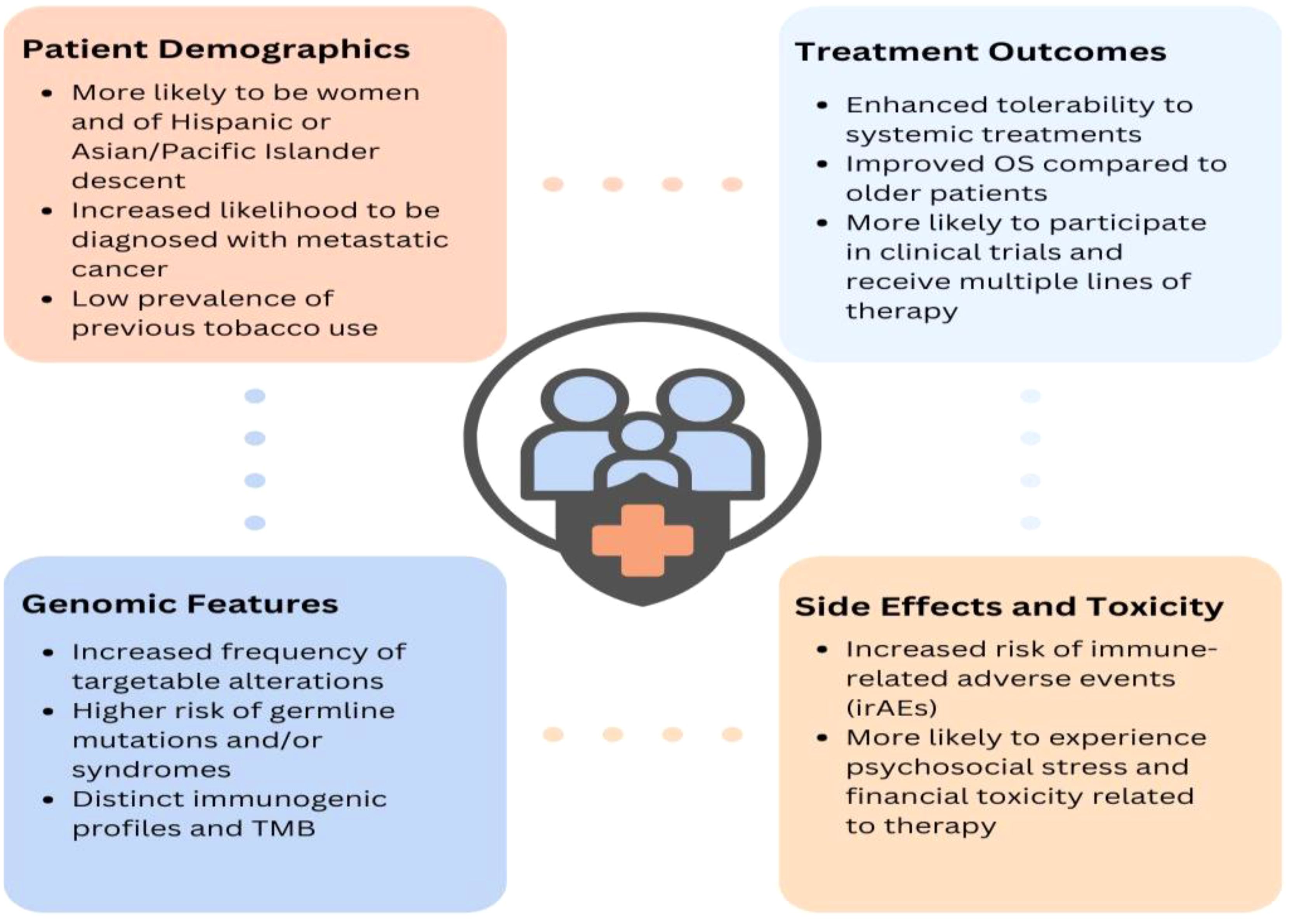
In addition to later-stage diagnoses, there remain numerous other unique clinical characteristics of young lung cancer. A study of 5,657 patients with lung cancer (41) within the National Cancer Data Base found young patients (ages 20-46) to have a significantly higher prevalence of adenocarcinoma than older patients (49% vs. 39%). Young patients with lung cancer are also significantly more likely to be female (4, 47) or Asian/Pacific Islander (48), have no tobacco use history (49), metastasis to the brain (50), and a higher frequency of somatic mutations or rearrangements such as EGFR, ALK, RET, and ROS1 (49, 51, 52) (Figure 1). Indeed, an evaluation of 2,237 patients with a diagnosis of non-small cell lung cancer (NSCLC) from 2002 to 2014 at Dana-Farber Cancer Institute (DFCI) found that tumors from patients <50 at diagnosis were 59% more likely to harbor a targetable alteration (52). Notably, young patients, especially women, demonstrate improved survival (37), particularly in earlier stages (41). This may be attributed to their relative lack of comorbidities, higher frequency of targetable mutations, and capacity to tolerate more extensive surgical interventions and lines of therapy than their older counterparts.
A recent systemic review (53) evaluated diagnostic time intervals and their effect on prognosis of patients with lung cancer. These intervals included time from symptom onset to treatment, symptom onset to diagnosis, first specialist visit to diagnosis, specialist referral to surgery or treatment, and time from diagnosis to treatment. Interestingly, 35% of time intervals studied showed no relationship between waiting time and the disease prognosis, while 37.5% of time intervals studied found a better prognosis with longer time intervals, and 27.5% found a better prognosis with shorter time intervals. To explain these paradoxical results (which have been replicated) (54), it has been suggested that earlier-stage diagnoses may require more testing or evaluation, thereby prolonging the diagnostic interval, but correlating with improved prognosis due to earlier disease stage and more targeted or curative-intent treatment options. While diagnostic time intervals may be an inconsistent measure of prognosis and survival, these studies denote the importance of early detection and intervention, given that earlier stage at diagnosis was a favorable prognostic modifier across studies. Therefore, early detection of lung cancer remains of paramount importance, particularly among young patients who tend to be diagnosed with later-stage disease.
Studies such as the Taiwan Lung Cancer Screening in Never-Smoker Trial (TALENT) (55) and the FANSS study (56) have explored the efficacy of low-dose CT scans among individuals with no tobacco use. Favorable preliminary results reveal diagnoses of early-stage lung cancer with adequate management. Most participants diagnosed with lung cancer in the TALENT study (246/318) had stage I disease. Similarly as promising, in the FANSS study, eight patients with a screening CT scan showing Lung-RADS 3 and 4 (57) lesions remain in close follow-up; three who were diagnosed with invasive adenocarcinoma all underwent surgical resection and are receiving adjuvant targeted therapy. Notably, TALENT also found that individuals with a family history of lung cancer were significantly more likely to develop lung cancer than those without a family history (3.2% vs 2.0%; p<0.001). This risk was positively correlated with the number of first-degree relatives with lung cancer, particularly a mother or sibling. Such remarkable feasibility of early lung cancer detection among non-tobacco users, compounded with newfound knowledge of family history, offers an attractive opportunity for deployment of lung cancer screening among young patients (especially those with a family history of lung cancer), given their historically advanced presentation of disease and likelihood of being excluded from current screening guidelines based on age and lack of tobacco use history. The EQUAL study is also deploying a novel ctDNA assay to detect early-stage EGFRm-NSCLC among non-tobacco using East/Southeast Asian and Hispanic/Latinx individuals who would otherwise be ineligible for lung cancer screening despite being at higher risk for the disease due to their race or ethnicity. Among a sample size of 1,000 40-80-year-olds, the study seeks to recruit 500 40-49-year-olds, most of whom will have a first-degree family history of EGFRm-NSCLC. This recruitment strategy will facilitate a preliminary risk stratification based on family history of EGFR lung cancer (58).
Still, to modify or develop new guidelines, the United States Preventative Services Task Force (USPSTF) first will review nominated topics based on their relevance to prevention and primary care, importance for public health, potential impact, and novel evidence. A draft research plan is then formulated, posted, and commented on, whereafter the USPSTF will refine and finalize the research plan that will guide the development of a draft recommendation statement. Following a 4-week public comment period, the USPSTF will refine, finalize, and post the updated recommendations (59).
3 Genomics of young lung cancer
As previously mentioned, younger age at diagnosis of lung cancer is strongly associated with presence of targetable alterations, making early biomarker testing critical. One global prospective review (49) of patients <40 years old found that of 112 individuals, 84% of those with adenocarcinoma had a targetable alteration, including 85% of those with stage IV disease Descriptive studies of oncogenic genomic alterations in this population have similarly found 52-91% of patients to have targetable alterations (51, 52, 60–69).
In one of the largest retrospective studies analyzing data from over 8,000 patients with NSCLC, the most common single nucleotide variants (SNV) among 189 patients under 50 years old were TP53 (57.1%), KRAS (15.5%), EGFR (21.4%), STK11 (10.1%), SMARCA4 (7.7%), and LRP1B (4.8%) (70, 71). Though specific types of KRAS mutations were not specified, transition mutations such as KRAS G12D mutations are more common in individuals without tobacco use (72) and may therefore be more enriched among young patients. In that same study, the most common fusions or skipping variants among patients 151 patients under 50 years old were ALK (14.6%), RPS6KB1 (3.3%), ROS1 (3.3%), RET (2%), and MET (2%). Indeed, over 20% of young patients have been found to have at least one alterations in ALK (52, 62, 68, 73–75), ROS, RET, or NTRK (76), a frequency nearly four times higher than in older patients (77). Among these, ALK rearrangements are the most commonly identified, with studies globally reporting variable prevalence with rates between 10-41% in young patients and higher rates among young Asians (12, 52, 62, 64, 65, 69). The EML4-ALK fusion, specifically, has been found to be more prevalent in younger patients with lung cancer, though this was a much rarer genetic alteration among all patients studied (70). Other genetic alterations more common among younger patients include RAD51B, CREBBP, LZTR1 SNVs as well as ROS1 and NTRK1 fusions (70). Conversely, KRAS SNVs, particularly KRAS G12C, MET exon 14 skipping variant (70), and BRAF V600E mutations (52), are distinctly more common among older patients.
Studies report conflicting results on whether EGFR mutation frequency in young patients differs compared to older populations. Among 14 studies analyzing EGFR mutation frequency, 7 found no differences between these populations, 3 reported higher rates among younger populations, and 4 reported higher rates among older populations (69). Regardless, rates of EGFR mutations among younger populations remain high, ranging from 12.8%-60%, with higher rates reported in studies conducted among Asians and Hispanic patients (69). Some studies suggest that the type of EGFR mutation may vary between older and younger populations (78), with EGFR exon 19 deletions (49, 70) and exon 20 insertions being more common among young patients, and EGFR L858R and de novo T790M more common among older populations (62, 69, 73, 75, 79, 80). Wu et al. (79, 80) has also described that uncommon EGFR mutations (defined as EGFR mutations other than the tyrosine kinase inhibitor (TKI)-sensitizing EGFR exon 19 deletion or L858R mutations) are more frequent among young patients (18% vs 9%; p = 0.02; 13.7% vs 8.4%; p = 0.03, respectively), suggesting that higher rates of such uncommon EGFR non-canonical mutations in Asian patients diagnosed under 50 years old may lead to lower response rates in these younger populations, at least partly due to limited treatment options compared to individuals with classical TKI-sensitizing EGFR mutation types. Along those lines, Hou et al. noted younger Chinese patients with EGFR mutations’ increased likelihood of harboring concurrent TP53 mutations, a negative prognostic factor for treatment response to EGFR TKIs (13, 81). Young patients with lung cancer may also be more likely to have underlying germline or hereditary TP53 mutations from syndromes such as Li-Fraumeni Syndrome (82), though it is unclear if germline TP53 mutation confers an increased risk of treatment resistance in this context (13).
To further understand disease characteristics and germline contribution among this population, two studies are ongoing at DFCI, one specifically investigating NSCLC or SCLC in those 45 and under (Biology of Young Lung Cancer, NCT05265429), and one studying lung cancers with strong family histories (the INHERIT study) (83), both of which incorporate clinical and exploratory research germline testing in addition to exposure and somatic data. Study results will attempt to aid in improving screening, early detection, prevention, and treatment of patients, as little is currently known regarding the risk factors, survival rates, and clinical, genomic, and histopathological characteristics of young-onset lung cancer. Similarly, the ongoing 23andMe Lung Cancer Genetics Study (84) aims to recruit 10,000 patients with lung cancer across the U.S. to provide further insights genetics of people diagnosed with lung cancer.
Younger patients with lung cancer also have distinct immune profiles and tumor mutational burden (TMB), related to the high prevalence of targetable driver alterations (e.g., EGFR mutations and oncogene fusions) (70). Although tumors that harbor targetable driver alterations are often PD-L1 positive, PD-L1 positivity in these tumors is believed to result from intrinsic oncogene-driven activation of downstream signaling effectors, such as STAT3 and HIF1α, which transcriptionally upregulate PD-L1 expression (85, 86). This mechanism differs from true T cell-mediated immunogenicity via IFNγ signaling, which is typically associated with responsiveness to immunotherapy. One study of 97 patients with NSCLC who were under 65 years old in China found these patients to have a lower expression of immune-related genes indicating reduced immune system activation, suggesting a lower response to immunotherapy (immune checkpoint inhibitors/ICIs), though PD-L1 expression level among younger and older patients with NSCLC was similar (70) (this study did not control for tobacco exposure). This trend was more pronounced among younger males, who had worse survival when receiving pembrolizumab alone in comparison to pembrolizumab with chemotherapy. Although there were no significant differences in the OS and progression free survival (PFS) between younger and older patients and between males and females, younger females had improved outcomes and better PFS when immunotherapy was combined with chemotherapy instead of immunotherapy monotherapy (70). Nevertheless, compared to older females, younger females had a similar outcome when treated with immunotherapy alone (70).
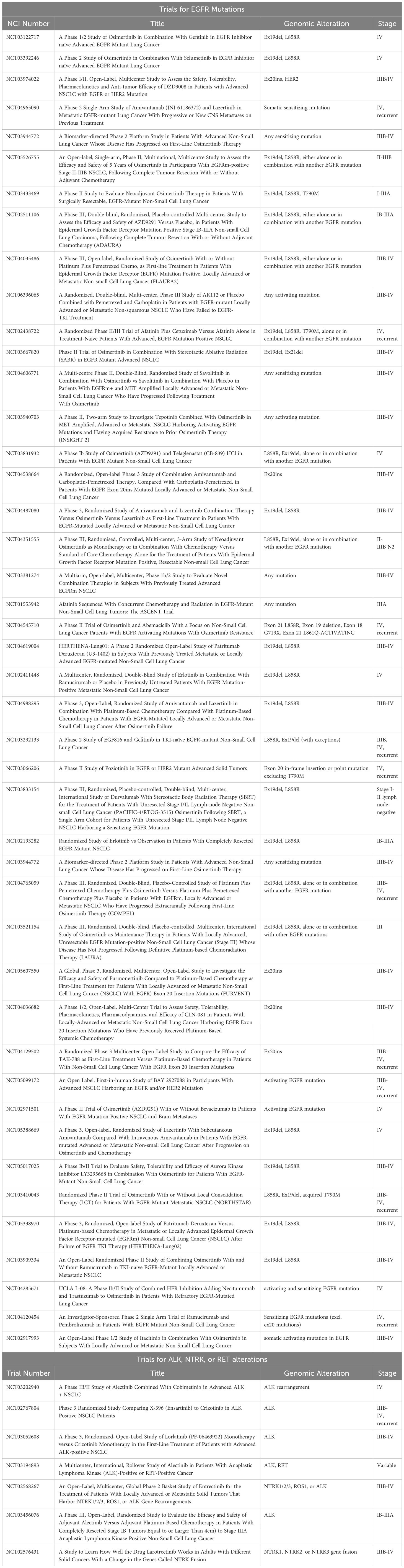
While the aforementioned study also found that both TMB and TIGS scores (signature for tumor inflammation) decreased significantly with decreasing age (p < 2E-4) and were significantly lower in almost all age groups compared against the ≥65 group (70), among patients with high TMB, another study of 93 patients under 65 years old found among them a higher prevalence of KRAS and STK11 co-mutations (71). Mutations in STK11 and KEAP1 generally correlate with worse outcomes among patients with concurrent mutations in KRAS who received ICIs (87, 88), further highlighting the importance of biomarker testing and considering the potential impact of co-mutations in young patients with lung cancer. Patients with concurrent mutations in KEAP1, STK11, SMARCA4 and PBRM1 have also been found to have a low response to immunotherapy, despite having a high TMB (89).More research is warranted to investigate the prognosis of patients with these mutations and to further understand frequent co-mutation patterns in young patients. To aid in this, researchers at DFCI are developing a longitudinal, international cohort study to track cases of young lung cancer globally and use data from the evolving cohort to provide insights into the molecular background, risk factors, optimal treatment, and follow-up care for young patients with lung cancer. The aforementioned Biology of Young Lung Cancer study also seeks to collect EHR data, blood, and tissue samples in patients 45 years old and younger to better estimate lung cancer risks and potential risk factors for the disease, as well as the somatic and germline genetic changes that may be shared among young patients (90). Researchers aim to routinely disseminate interim results of these cohorts through scientific meeting presentations and peer-reviewed publications so that data may serve as an impetus for the implementation of tailored diagnostic, treatment, and management services for young patients with lung cancer across the globe. Numerous other studies are also ongoing to investigate potential risk factors, needs, and clinical outcomes among young patients with lung cancer (Table 1).
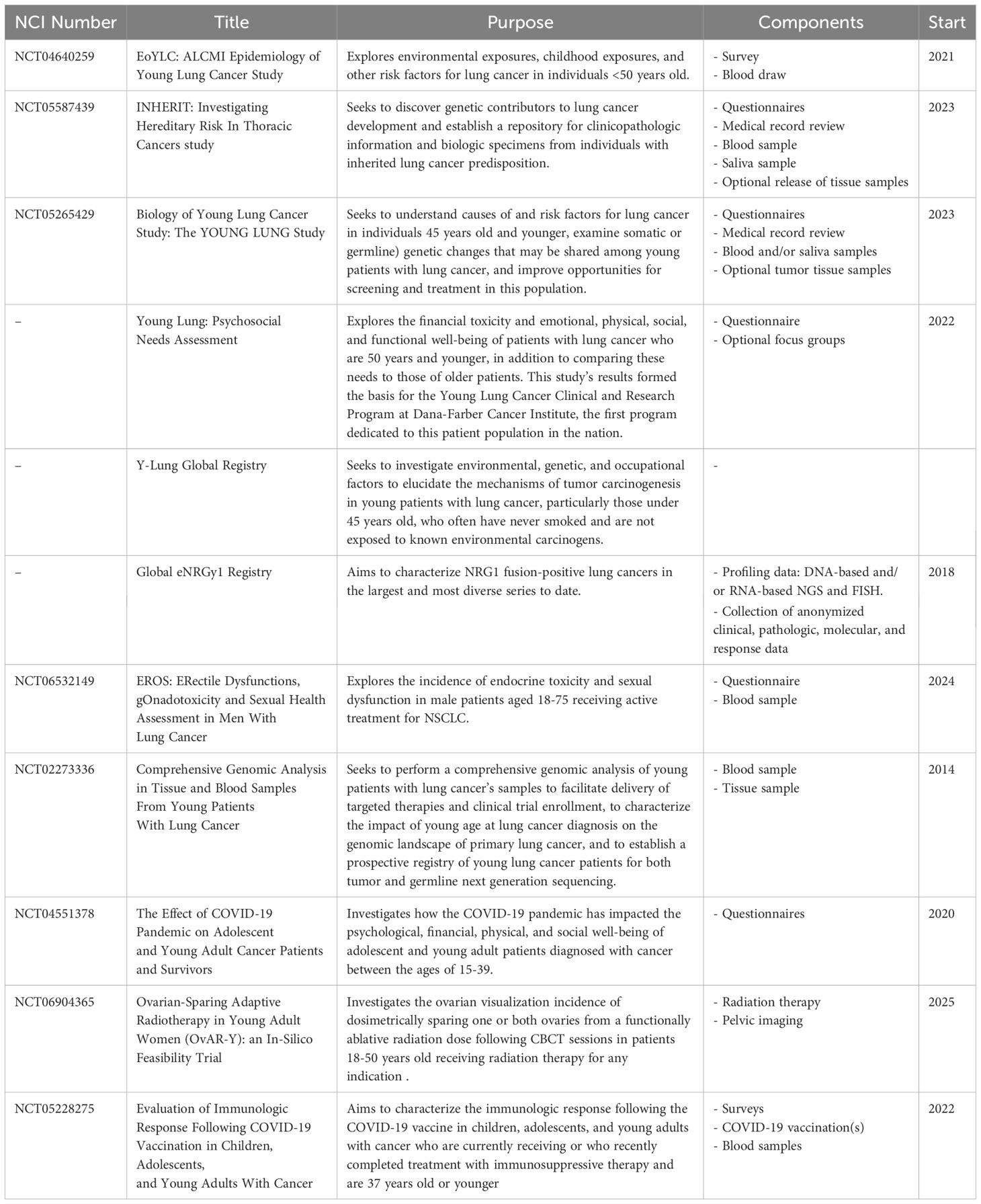
Table 1. Ongoing studies to investigate risk factors, needs, and outcomes among young patients with lung cancer.
4 Treatment of young lung cancer
The unique epidemiologic, biologic, and diagnostic considerations in younger-onset lung cancer highlight the need for tailored treatment approaches (50). Young patients are more likely to be diagnosed with brain metastases (50) and tend to harbor larger tumors, more nodal involvement, and initial diagnosis of metastatic disease (13, 41). However, their significantly higher rates of actionable alterations (49) highlight the crucial need for early genomic testing to guide therapy selection (91–97). Given the significant enrichment for fusion-positive lung cancers in younger populations, RNA-based sequencing is necessary to elucidate targets when DNA-based testing is uninformative, as the genomic breakpoints for novel ALK fusions and more rare fusions (e.g, those in ROS1, TRK, and NRG1) often occur within intronic sequences that are long or contain repetitive elements and are not adequately covered in targeted DNA-based panels. To account for this, RNA-based NGS using anchored multiplex PCR (e.g., Archer FusionPlex) has been used to identify both known and novel fusion partners for ALK, ROS1, RET, NRG1, and TRK. In a study out of MSK-IMPACT using Archer-based technology, a custom RNA-sequencing panel was able to detect fusions as well as MET exon 14 alterations in 14% of cases negative for oncogenic drivers by DNA-based NGS (PMID 31028088). RNA-based sequencing also detects oncogenic fusions in cases where tumor purity may be too low for DNA detection, owing to the high level of expression of fusion proteins.
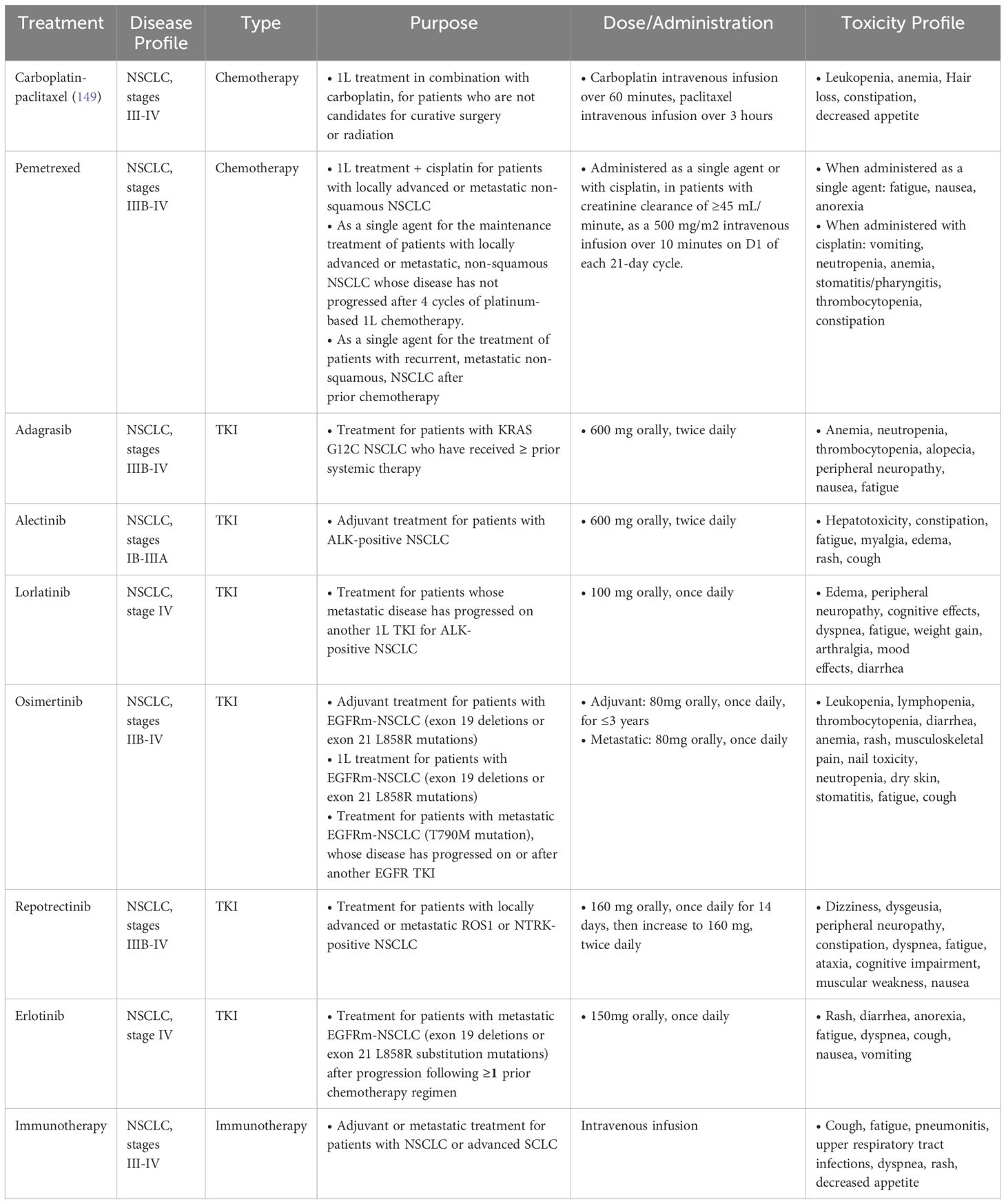
As previously mentioned, analyses continue to demonstrate the improved OS of young patients with lung cancer (41, 50, 67, 98–100). This is likely a result of their higher prevalence of targeted alterations, increased likelihood of receiving aggressive treatments (41), multimodal therapy (41, 98, 101), and surgical resection in early stage and oligometastatic disease (102–107), as well as their receiving of additional lines of treatment given their enhanced performance status (41, 50, 108) and greater likelihood of participating in clinical trials (109). While surgery has historically not been an option for patients with metastatic lung cancer, there has been a shift in those with oligometastatic disease. More recent studies, with a median age of 61, have shown improved PFS and OS in oligometastatic patients (102–104), with significantly improved outcomes (HR 0.4) among patients under 60 years old (105). Older, retrospective analyses suggesting worse outcomes in young patients with targetable alterations (110) are likely due to these studies pre-dating newer generation and highly effective TKIs such as osimertinib, lorlatinib, and repotrectinib [Table 2 (111)].
For those with EGFR-mutant disease, osimertinib, a third generation EGFR TKI, significantly improved OS and progression-free survival (PFS) in newly diagnosed patients, with a median OS of 38.6 months (91) and a median PFS of 18.9 months (112). More recently, osimertinib has been assessed in combination with chemotherapy (platinum agent plus pemetrexed) in the front-line setting. Compared to osimertinib alone, the combination significantly improved PFS [HR for disease progression or death, 0.62; median response duration of 24.0 months vs single agent osimertinib 15.3 months (113)]. A consistent benefit was observed among young and older patients, though individuals under age 65 appeared to derive potentially greater benefit with the combination than with osimertinib alone (HR 0.59 (0.44-0.80) vs 0.68 (0.47-0.98 in those over 65). However, OS data is still immature and requires longer follow up to determine the survival benefit of adding chemotherapy to osimertinib. Other intensification regimens over first-line osimertinib monotherapy have also been developed, including the combination of amivantamab plus lazertininb in the MARIPOSA trial (114), as well as the addition of VEGF inhibitors to osimertinib in the RAMOSE trial (115), both of which significantly prolonged PFS compared to osimertinib alone. Further research to understand the benefits of these intensification regimens is vital, as young patients with lung cancer are more likely to receive and tolerate these combination therapies. To aid in this, numerous clinical trials are ongoing to investigate novel drugs and therapeutic combinations for genomically driven lung cancer (Table 3).
Indeed, intensified treatment approaches are often attractive for young patients, as an improved response in patients with limited or oligometastatic disease could allow local consolidative approaches with surgery and radiation to further improve survival outcomes. Intensification approaches unsurprisingly are associated with higher toxicity and treatment selection should be tailored to patient preferences and characteristics. Though osimertinib itself does not show pharmacokinetic differences between age or sex (116), the addition of chemotherapy unsurprisingly yields higher toxicities among age groups, which must be accounted for (113).
Lorlatinib as first line treatment for ALK-rearranged lung cancer has also shown impressive 5-year outcomes based on phase 3 CROWN study results, with median PFS and median time to intracranial progression still not reached, and a 5 year PFS rate of 60% (117). Patients under 65 years old accounted for 65% (n=193) of the total population and had an improved HR compared to their older counterparts (HR 0.22 vs 0.35). The central nervous system (CNS) protective role of lorlatinib makes it an attractive choice, especially given that young patients are more prone to brain metastases and disease progression in the CNS after treatment initiation, though toxicity often necessitates dose reduction at the outset of treatment. Compared with other ALK and ROS1 inhibitors, lorlatinib is associated with a unique set of spectrum of treatment-related adverse events (trAEs), including hyperlipidemia, peripheral neuropathy, and neurocognitive effects (118) that may render younger patients as being reluctant to begin lorlatinib in the first-line setting, despite its remarkable trial results. In the updated phase 3 CROWN study, grade 3–5 trAEs in the lorlatinib arm occurred in 67% of patients, of which 39%, 21%, and 5% led to temporary drug discontinuation, dose reduction, and permanent drug discontinuation, respectively. Though the majority (86%) of all-causality CNS AEs in the lorlatinib group were grade 1 or 2, and only three patients who experienced CNS trAEs permanently discontinued lorlatinib, CNS AEs occurred in six of nine (67%) of patients with prior brain radiotherapy and in 57 of 140 (41%) patients without prior brain radiotherapy. One recent study (118) of 144 patients with advanced ALK- or ROS1-rearranged NSCLC treated with lorlatinib in the second-line setting or later, too, found that 40% of patients had trAE- related dose reductions, most (59%) owing to neurocognitive AEs or neuropathy.
The TRIDENT-1 trial also showed robust efficacy of repotrectinib in patients with advanced ROS1 rearranged lung cancer, with an overall response rate (ORR) of 79%, median duration of response 34.1 months, and median PFS 35.7 months. The majority of TKI-naïve patients in this trial were 18–65 years old (n=52, 73%) and had an ORR of 81%, compared to the 65-75-year-old patients (n=15, 21%) with ORR 67% (95). In sum, though drugs with enhanced CNS penetration are more likely to be tolerated by young patients, these regimens are often accompanied by a unique set of adverse events, including cognitive changes and mood disorders that that may significantly impact a patient’s quality of life (QOL). Healthcare providers should counsel patients on expectations and consider utilizing a cognitive assessment tool or referral to a psychiatrist or therapist, if warranted (119).
Other more rare oncogenic drivers, such as NRG1 fusions, also recently received FDA approval for the bispecific antibody zenocutuzumab (120). Though this fusion is only found in approximately 0.2% of lung cancers (121), the global eNRGy1 Registry primarily observed its prevalence among individuals without a history of tobacco use (57%) (122), potentially offering an attractive opportunity to explore this fusion’s drug tolerability and clinical outcomes in the context of young patients.
For patients with no actionable mutations, the standard of care in the frontline for metastatic NSCLC includes the combination of chemotherapy and immunotherapy. Several landmark trials assess immunotherapy efficacy with or without chemotherapy in the metastatic setting. Some show a similar degree of benefit for patients across age groups (123–126), with no benefit for younger patients (127, 128), while others have demonstrated a more meaningful benefit in those <65 years (129–136). Immunotherapy is also being incorporated in the perioperative management of resectable NSCLC without detected alterations in EGFR or ALK and have also shown a similar degree of benefit, irrespective of age (137–139).
As previously mentioned, tumors with targetable driver alterations are often PD-L1 positive, likely due to the oncogene-driven activation of downstream signaling effectors and not true T cell-mediated immunogenicity that is typically associated with ICI responsiveness. Therefore, though young patients may derive less benefit from ICIs, and an increased rate of immune-related adverse events (irAEs) in adults 65 years or older (42%) compared to 18–64 year olds (33%) has been observed (140–142), the risks of irAEs for young patients still remain an important consideration (143). Early identification and mitigation of these AEs is particularly important given that young patients are more likely to experience the psychosocial and stress-related effects of therapy (144), as well as long-term AEs (99). Fatigue is commonly reported amongst young patients, with potential to significantly affect long term QOL (145); endocrine toxicity has also been observed to be more common in those under age 65 (142). Our analysis of 231 patients with NSCLC (146) found that women, specifically, were more likely to experience irAEs compared with men, but also significantly more likely to develop endocrinopathies (i.e. hypothyroidism, hypophysitis, type 1 diabetes). As these adverse events tend to have long-term repercussions on fertility and QOL, clinicians must therefore adequately caution and counsel young patients on realistic and lifelong irAE management strategies.
Lastly, despite the remarkable outcomes of precision medicine, attention must situate on how the overall length of life of young patients is still considerably lower than older patients diagnosed at an advanced age. A 2022 review (147) of 177 patients with advanced EGFR or ALK-positive NSCLC who received their first chemotherapy between December 2008- September 2015 found the median OS to be 40.6 months, the three-year survival rate to be 54%, and the five-year survival rate to be 28%. For patients with EGFRm-NSCLC, the median OS was 36.9 months, and for those with ALK-altered disease, the median OS was 55.4 months. Similarly, a 2025 cohort study (148) of 1,323 patients treated with first-line osimertinib found that while 58% of patients survived to 2 years, only 18% survived to 5 years. Even in the remarkable case that a 40-year-old individual with lung cancer survived for a decade, they still would have hardly reached midlife. For these patients, it is not solely the number of life years that is lost, but all that comes with it: pursuing passions, achieving goals, developing relationships, witnessing children’s milestones, advancing within work, and configuring hopes and dreams for the future (149).
5 Psychosocial needs
Young patients with lung cancer are diagnosed at a more disruptive time in their lives than their older counterparts, rendering them with distinct psychosocial needs. These young patients experience higher rates of emotional distress compared to older patients; with over 60% reporting significant distress (150). Though younger age is a strong predictor of heightened emotional challenges, patients with lung cancer in general report higher rates of depression and anxiety than those with other cancers (150–153), perhaps due to the chronic pain and fatigue conferred by the disease, which exacerbate emotional issues (150). Young patients may also be more apt to experience feelings of isolation (154, 155) due to not having peers facing similar challenges and not identifying with older patients whom they may meet throughout their cancer journey. Additionally, younger patients often face greater disease-related stigma (156), leading to feelings of guilt, shame (157), depression, anxiety (158), poorer QOL (159), and higher symptom severity (158); stigma may relate to diagnostic delays, limited use of adjunctive treatment and psychosocial support services, and low clinical trial enrollment (160). Young patients also experience a greater burden of familial responsibilities, as they are more likely to have dependent children and act as caregivers to parents. Further, with advancements in treatment, younger patients may live longer, but face ongoing fears of recurrence and long-term health impacts from their extensive cancer treatments (153). As younger patients are more likely to be on a greater number of lines of treatment, more research is warranted to explore the degree of distress engendered by these additional and/or more aggressive therapies.
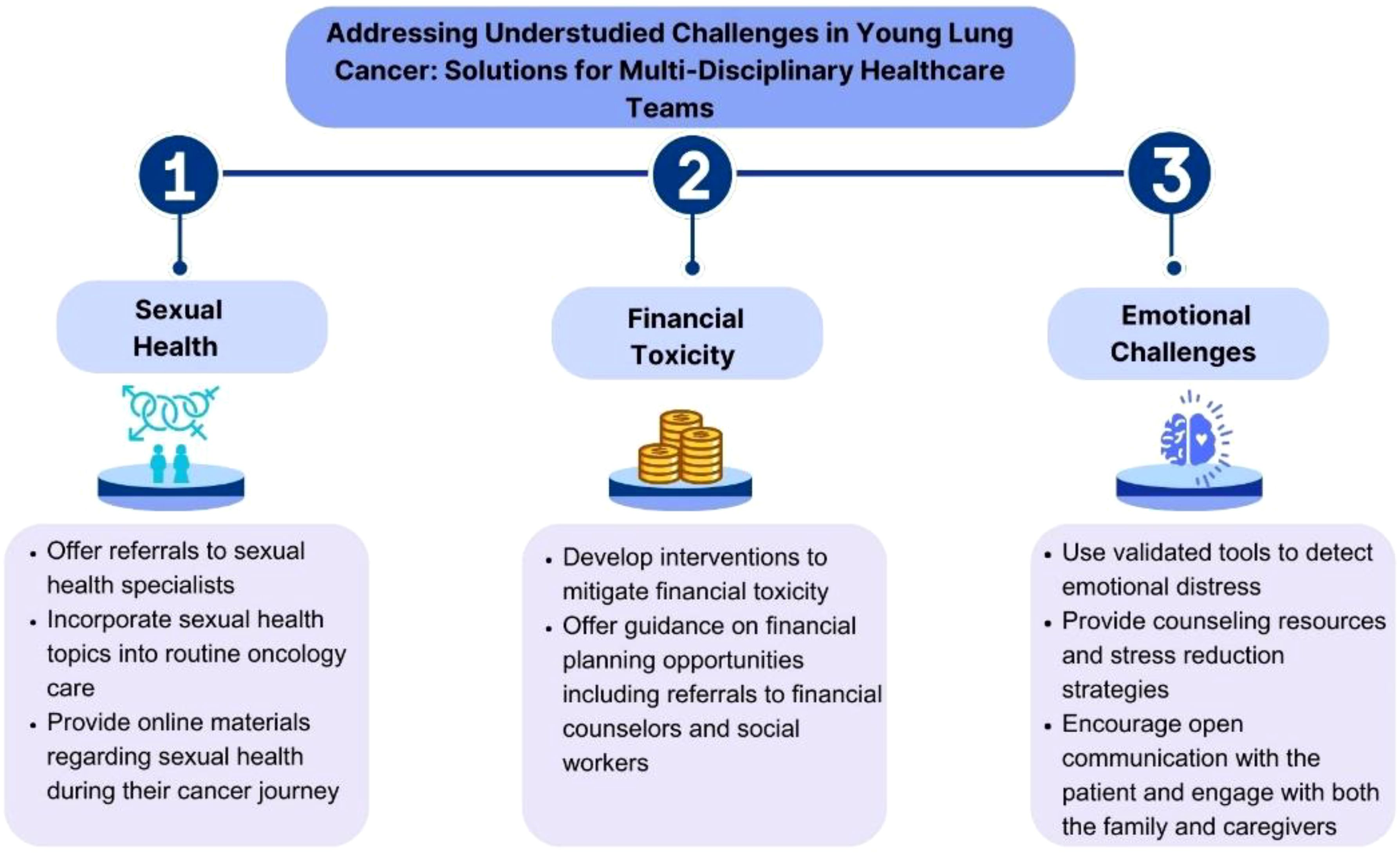
Early and accurate identification of emotional issues, achieved through routine administration of validated tools to detect emotional distress, is crucial (161, 162). Healthcare providers should also be vigilant for changes in mood, withdrawal, or expressions of hopelessness, and encourage patients to openly share their feelings. Insights from family members or caregivers can also provide valuable perspectives. Counseling and psychotherapy (163) may also be helpful, particularly cognitive-behavioral therapy (CBT) interventions, which have shown efficacy in reducing anxiety and depression across various cancer populations (164, 165). Psychoeducational interventions that teach skills such as stress management may be particularly effective when combined with CBT (166, 167). Additionally, mindfulness-based therapies such as meditation and yoga have demonstrated moderate effects in reducing symptoms of depression and anxiety in patients with cancer (168–170) and may resonate well with younger individuals. As social connectedness has been found to mediate the relationship between social isolation and depression among young adult cancer survivors (155), facilitating peer connections through in-person or online support groups tailored for younger patients with cancer may also confer a shared identity and help combat feelings of isolation, with age-specific groups allowing for the sharing of unique concerns (171–173).
Financial toxicity is another significant concern for younger patients, who are often required to continue working for insurance coverage and support children or parents, exacerbating stress and limiting their ability to focus on treatment and recovery (174). More research is needed to identify the unique needs and most effective support for this growing population. The Young Lung Cancer: Psychosocial Needs Assessment at DFCI (174) is the first study to explore the psychosocial needs of young patients with lung cancer; preliminary results revealed how these patients report ample financial toxicity and issues impacting physical, emotional, and functional well-being. Specifically, a majority reported feeling sad or anxious (53%, 59%), and 57% were worried about premature death. Of a maximum score of 44, the mean COST score was 24, consistent with high financial toxicity in this population. Therefore, integrating the psychosocial support strategies into routine care and National Cancer Control Plans is essential for delivering comprehensive cancer care (Figure 2).
6 Fertility, pregnancy, and sexual health
The enhanced overall survival of young patients makes it important to elucidate the effects of treatments on fertility and sexual health. However, most contemporary knowledge has been derived from research conducted in animal models, from data extrapolated from patients with breast cancer (175) (a hormonal dependent cancer with differing clinic-pathologic characteristics), and before the incorporation of novel therapies such as ICIs and TKIs, which young patients are more likely to be on.
A global regulatory filings and public assessment analysis found that for female patients, 58% of ICI treatments represent a fertility risk, compared to only 33% for male patients (176). Animal studies of ICIs have also yielded unfavorable results including diminished ovarian follicular reserve, low maternal weight, excessive rates of abortion (177, 178), stillbirth, and premature delivery (177), compounded with negative long-term consequences such as hypogonadism, hypophysitis, and hypothyroidism (178). Similarly, early-generation TKIs have been found to decrease women’s total follicle count, negatively affect oocyte retrieval, ovarian reserve, reduce embryonic developmental potential, and produce teratogenic effects (179), though data regarding the effects of contemporary TKIs on oocytes and sex hormones is non-existant (180, 181).
A total of 66 cases of lung cancer diagnoses during pregnancy have been reported in the literature. Given the bleak and heterogenous aforementioned therapeutic effects on fertility, it is unsurprising that lung cancer treatment management throughout pregnancy is far from standardized. Though cytotoxic therapy has been commonly used throughout pregnancy, chemotherapy is typically avoided during the first trimester and instead given throughout the second and third trimesters, as risks include spontaneous abortion, congenital abnormalities, and transplacental transmission of taxanes. While some cases report safe TKI use, other cases have ended in spontaneous abortion or resulted in fetal growth restriction, treatment-related malformations, and severe maternal adverse effects. Surgery may be considered in highly specific cases of early-stage lung cancer or as a palliative measure for patients with extensive bone metastasis or those at risk for spinal cord compression. While novel radiation techniques have reduced toxicity to surrounding healthy tissue, routine radiation is not recommended, as some radiation still reaches the fetus. Regarding imaging, PET/MRI is preferred, though doses as low as reasonably possible should still be applied; ultrasound techniques can also be used to evaluate pleural effusions, mediastinal staging, and liver involvement (182). To aid in the development of a systematic and data-based understanding of how to treat pregnant young women with lung cancer, researchers at DFCI launched the International Pregnancy and Lung Cancer Registry to continuously learn about the impact of lung cancer treatment on maternal and fetal outcomes. An initial review of the first 22 cases was recently presented at the 2024 World Conference on Lung Cancer (183); results demonstrated a lack of uniformity in treatments utilized, treatment deferrals, and imaging modalities, highlighting the need for an increased and data-driven understanding to optimally support this vulnerable population of young patients.
Sexual health is another important consideration, as the health effects of sexual activity on psychological wellness have been widely documented (184, 185) and ensuring that young people experience good sexual health is a key public health concern (186, 187). Among a nationally representative sample of U.S. adults (188), high importance of sexual health to QOL was reported by 62% of men and 43% of women, particularly those in their mid-30s to mid-40s. Sexual dysfunction has been related to higher symptom distress (189) and worse functional status in patients with lung cancer, and among young adults with cancer, sexual dysfunction amplifies feelings of isolation and emotional distress (190–193). In our Sexual Health Assessment for Women with Lung Cancer (SHAWL) Study (194), nearly half of patients reported minimal satisfaction with their sex life. The majority (74%) of young patients also indicated having minimal interest in sexual activity, emphasizing the need to integrate sexual health counseling within routine oncology care for young patients with lung cancer.
Despite the importance, oncologists have suboptimal knowledge, practices, and attitudes on fertility preservation and sexual health throughout cancer treatments (14–16). Young women are rarely adequately counseled regarding options for future fertility (195), unlike men, who are frequently advised to preserve sperm. Women have expressed negative sentiments regarding fertility preservation, citing inadequate information and presentation of available options as contributing factors (196, 197). Similarly, in a survey of 120 medical oncologists, 81.5% reported that they discussed sexual function with fewer than half of patients, citing a lack of training as primary cause. Social media is a largely untapped resource that can be used to disseminate accessible information about sexuality to young patients. In addition to offering referrals to sexual health specialists, open-ended discussion frameworks may also serve as a helpful origin source for oncologists to incorporate inclusive sexuality care into their practice (198).
Women, especially those belonging to medically underserved populations such as racial and ethnic minorities, rural residents, or those who are low-income or uninsured, are also disproportionately impacted (199) by the costly and invasive nature of fertility preservation (99), which typically involves hormone stimulation, ultrasounds, bloodwork, and surgical procedures. Ovarian stimulation and egg retrieval cycle may take up to 14 days if successful, potentially delaying the initiation of cancer treatment (200). In contrast, male fertility tends to be a non-invasive, cost-effective, timely, and efficient process. Many insurance plans also do not cover procedural costs, adding to the existing financial strain of cancer treatments. While sperm preservation can cost several hundred dollars, oocyte or embryo cryopreservation can range from $6,000 to $15,000 per cycle, excluding medication and storage fees. A recent study also revealed significant geographic disparities in access to oncology fertility preservation care, only exacerbating the aforementioned disparities (201).
7 Palliative care and end-of-life care
Early integration of palliative care is crucial in the comprehensive management of young patients, aiming to improve their emotional symptoms, QOL, and OS (202). Systematic reviews emphasize the significance of culturally sensitive approaches in end-of-life (EOL) care, respecting patient preferences, maintaining human dignity, and facilitating open communication within family dynamics (203, 204). Research comparing early virtual palliative care versus in-person visits has shown equivalent effects on QOL in patients with advanced NSCLC, highlighting the feasibility and effectiveness of telemedicine in providing supportive care (205). Additionally, stepped-care model, associated with fewer days in hospice, offers a scalable approach to delivering early palliative care and enhancing patient-reported outcomes (206). Despite these advancements, biases against supportive care persist, often resulting in young patients to be referred to hospice only when it is too late to benefit.
Complementary medicine, including cannabis use, has also emerged as a potential adjunctive therapy in managing symptoms and improving QOL for patients with advanced cancer (207). However, ASCO guidelines suggest that evidence still remains insufficient to recommend for or against cannabis in managing cancer treatment-related symptoms, except for alleviating treatment-related nausea or vomiting with dronabinol, nabilone, or a 1:1 THC extract (207) (Figure 3). Indeed, a recent umbrella review (208) of global epidemiological evidence on the cancer risk of cannabis use based upon publications since 2017 found moderate evidence of no statistical association between cannabis smoking and the incidence of lung cancer. However, difficulties remain in quantifying and soliciting data on cannabis use, especially in the context of confounding factors such as tobacco exposure, emphasizing the importance of future research that incorporates cannabis use history when reporting on disease outcomes. Though clinicians are encouraged to inquire about cannabis use, educate patients on risks, and minimize potential harm (207), recommendations specific to young patients with lung cancer are underexplored.
Though evidence has found advance care planning (ACP) to be well-liked among young adults and improve caregiver decisional certainty, ACP among young patients with cancer remains clinically underutilized (209). Advanced directives (AD), a component of ACP, contributes to the attenuation of patient suffering and anxiety, psychological relief and patient satisfaction (210), though AD may be difficult to introduce among young patients. Due in part to the lack of standards to guide the quality, content, approach, and timing of ACP discussions, as well as physician belief that EOL discussions will lead patients to higher levels of stress and discomfort (210), late EOL discussions and completion of AD often occurs during the last 3 months of life or later (211), when aggressive care preferences (associated with worse QOL and worse bereavement adjustment) are more likely (212). Promoting the completion of AD early in the disease trajectory will empower young patients to articulate their care preferences, ensuring alignment with their values during critical moments.
At the moment of death, though optimizing pain management to promote comfort and dignity is crucial, addressing psychological needs through facilitating family presence and providing spiritual and emotional support to both patient and caregivers is also necessary. Early integration of palliative care in the disease trajectory will allow for the comfortable integration of these measures to meet patients’ needs with sensitivity and understanding.
8 Conclusions
Young patients with lung cancer represent a distinct population, with unique disease and treatment-related characteristics and psychosocial and survivorship needs. Young patients with lung cancer are more likely to harbor a targetable oncogenic driver and be diagnosed with advanced disease, highlighting the importance of disease awareness, unbiased attention to symptoms, and early biomarker testing. However, further research is needed to explore the influence of genetic predisposition, comorbidities, geographic location, and other risk factors in the development of lung cancer in young adults. Clinical trials are also crucial to guide evidence-based decisions on therapy selection and customization, the incorporation of targeted therapies, and escalation and de-escalation strategies.
Young patients with lung cancer also demonstrate improved survival, underscoring the need for comprehensive research efforts that evaluate disease-related effects on survivorship needs such as fertility, financial toxicity, and psychosocial well-being. Healthcare providers should screen for distress and implement an integrated approach combining psychotherapies like CBT, mindfulness training, peer support, and targeted psychoeducation, to provide tailored psychological support, combat stigma, and support family dynamics. Training in palliative care and promoting a positive attitude towards supportive care is also essential. In this way, comprehensive cancer care can be tailored to this unique yet understudied patient population.
Author contributions
NF: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LK: Formal Analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. RK: Data curation, Formal Analysis, Investigation, Writing – original draft. JL: Data curation, Formal Analysis, Investigation, Supervision, Validation, Writing – review & editing. BR: Formal Analysis, Investigation, Validation, Writing – review & editing. AM: Writing – review & editing. OF: Writing – review & editing. CM: Writing – review & editing. CO: Data curation, Formal Analysis, Investigation, Writing – original draft. NS: Data curation, Formal Analysis, Investigation, Writing – original draft. DK: Data curation, Formal Analysis, Investigation, Writing – original draft. LA: Data curation, Formal Analysis, Investigation, Writing – original draft. ArS: Data curation, Formal Analysis, Investigation, Writing – original draft. CB: Data curation, Formal Analysis, Investigation, Writing – original draft. BB: Data curation, Formal Analysis, Investigation, Writing – original draft. AV: Writing – review & editing. AlS: Project administration, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Siegel RL, Kratzer TB, Giaquinto AN, Sung H, and Jemal A. Cancer statistics. CA: A Cancer J Clin. (2025) 2025:10–45. doi: 10.3322/caac.21871
4. Jemal A, Miller KD, Ma J, Siegel RL, Fedewa SA, Islami F, et al. Higher lung cancer incidence in young women than young men in the United States. New Engl J Med. (2018) 378:1999–2009. doi: 10.1056/NEJMoa1715907
5. Levi F, Bosetti C, Fernandez E, Hill C, Lucchini F, Negri E, et al. Trends in lung cancer among young European women: the rising epidemic in France and Spain. Int J Cancer. (2007) 121:462–5. doi: 10.1002/ijc.v121:2
6. Long J, Zhai M, Jiang Q, Li J, Xu C, and Chen D. The incidence and mortality of lung cancer in China: a trend analysis and comparison with G20 based on the Global Burden of Disease Study 2019. Front Oncol. (2023) 13:1177482. doi: 10.3389/fonc.2023.1177482
7. Au PCM, Lee AWM, Lee VHF, Wong ICK, Hui YM, and Cheung C-L. The trends in lung cancer prevalence, incidence, and survival in Hong Kong over the past two decades (2002–2021): a population-based study. Lancet Regional Health–Western Pacific. (2024) 45. doi: 10.1016/j.lanwpc.2024.101030
8. Thomas A, Chen Y, Yu T, Jakopovic M, and Giaccone G. Trends and characteristics of young non-small cell lung cancer patients in the United States. Front Oncol. (2015) 5:113. doi: 10.3389/fonc.2015.00113
9. Huang J, Deng Y, Tin MS, Lok V, Ngai CH, Zhang L, et al. Distribution, risk factors, and temporal trends for lung cancer incidence and mortality: a global analysis. Chest. (2022) 161:1101–11. doi: 10.1016/j.chest.2021.12.655
10. LoPiccolo J, Gusev A, Christiani DC, and Jänne PA. Lung cancer in patients who have never smoked—An emerging disease. Nat Rev Clin Oncol. (2024) 21:121–46. doi: 10.1038/s41571-023-00844-0
11. National Cancer Institute Surveillance E, and End Results Program. Lung and bronchus recent trends in SEER age-Adjusted incidence rates, 2000-2021.
12. Corrales-Rodríguez L, Arrieta O, Mas L, Báez-Saldaña R, Castillo-Fernández O, Blais N, et al. An international epidemiological analysis of young patients with non-small cell lung cancer (AduJov-CLICaP). Lung Cancer. (2017) 113:30–6. doi: 10.1016/j.lungcan.2017.08.022
13. Liu B, Quan X, Xu C, Lv J, Li C, Dong L, et al. Lung cancer in young adults aged 35 years or younger: a full-scale analysis and review. J Cancer. (2019) 10:3553. doi: 10.7150/jca.27490
14. Galvez-Nino M, Ruiz R, Pinto JA, Roque K, Mantilla R, Raez LE, et al. Lung cancer in the young. Lung. (2020) 198:195–200. doi: 10.1007/s00408-019-00294-5
15. Jemal A, Schafer EJ, Star J, Bandi P, Sung H, Islami F, et al. Lung cancer incidence rates in young women and men by state in the United States. Int J Cancer. (2025) 156:499–504. doi: 10.1002/ijc.v156.3
16. Possenti I, Romelli M, Carreras G, Biffi A, Bagnardi V, Specchia C, et al. Association between second-hand smoke exposure and lung cancer risk in never-smokers: a systematic review and meta-analysis. Eur Respir Rev. (2024) 33. doi: 10.1183/16000617.0077-2024
17. Institute NC. Oral Contraceptives and Cancer Risk. (2018). Bethesda, MD: National Cancer Institute (NCI)
18. Mezquita L, Barlesi F, Auclin E, Planchard D, Botticella A, Gazzah A, et al. OA09. 06 molecular alterations and estimated indoor radon in NSCLC patients from the French National Cancer Institute registry: Radon France study. J Thorac Oncol. (2018) 13:S342. doi: 10.1016/j.jtho.2018.08.285
19. Garcia M, Garcia de Herreros M, Auclin E, Caravaca G, Sart J, Riudavets M, et al. OA13. 04 prevalence of molecular alterations in NSCLC and Estimated Indoor radon in Europe: RADON EUROPE Study. J Thorac Oncol. (2022) 17:S34–5. doi: 10.1016/j.jtho.2022.07.065
22. Ellis PM and Vandermeer R. Delays in the diagnosis of lung cancer. J Thorac Dis. (2011) 3:183. doi: 10.3978/j.issn.2072-1439.2011.01.01
23. Newman-Toker DE, Schaffer AC, Yu-Moe CW, Nassery N, Saber Tehrani AS, Clemens GD, et al. Serious misdiagnosis-related harms in malpractice claims: the “Big Three”–vascular events, infections, and cancers. Diagnosis. (2019) 6:227–40. doi: 10.1515/dx-2019-0019
24. Rima A, Haryati I, and Maulana S. 458P Lung cancer diagnostic delay secondary to misdiagnosis with lung tuberculosis in Dr. Moewardi Public Hospital pulmonary ward Surakarta. Ann Oncol. (2016) 27:ix147. doi: 10.1016/S0923-7534(21)00616-5
25. Shin H, Kim M-S, Kho B, Park H, Kim T-O, Park C-K, et al. Delayed diagnosis of lung cancer due to misdiagnosis as worsening of sarcoidosis: a case report. BMC Pulmonary Med. (2020) 20:1–4. doi: 10.1186/s12890-020-1105-2
26. Evman S, Bostanci K, and Yuksel M. Infection or Malignancy? Malignant pulmonary mass mimicking pneumonia. Surg J. (2016) 2:e11–3. doi: 10.1055/s-0036-1572359
27. See KC. Lung cancer screening for never smokers: current evidence and future directions. Singapore Med J. (2024) 10:4103. doi: 10.4103/singaporemedj.SMJ-2023-007
28. Force USPST. Lung Cancer: Screening. Rockville, MD: U.S. Preventative Services Task Force (2021).
29. Din NU, Ukoumunne OC, Rubin G, Hamilton W, Carter B, Stapley S, et al. Age and gender variations in cancer diagnostic intervals in 15 cancers: analysis of data from the UK Clinical Practice Research Datalink. PLoS One. (2015) 10:e0127717. doi: 10.1371/journal.pone.0127717
30. Brueckl WM, Wagner H, Wagner M, Wiest GH, and Ficker JH. Delays in the diagnosis and treatment of women with lung cancer: A systematic analysis. Am Soc Clin Oncol. (2015) 33. doi: 10.1200/jco.2015.33.15_suppl.e17740
31. Lyratzopoulos G, Abel GA, McPhail S, Neal RD, and Rubin GP. Measures of promptness of cancer diagnosis in primary care: secondary analysis of national audit data on patients with 18 common and rarer cancers. Br J Cancer. (2013) 108:686–90. doi: 10.1038/bjc.2013.1
32. Warner ET and Lathan CS. Race and sex differences in patient provider communication and awareness of lung cancer screening in the health information National Trends Survey, 2013–2017. Prev Med. (2019) 124:84–90. doi: 10.1016/j.ypmed.2019.05.001
33. Chilet-Rosell E, Parker LA, Hernández-Aguado I, Pastor-Valero M, Vilar J, González-Álvarez I, et al. Differences in the clinical management of women and men after detection of a solitary pulmonary nodule in clinical practice. Eur Radiol. (2020) 30:4390–7. doi: 10.1007/s00330-020-06791-z
34. MacLean A, Hunt K, Smith S, and Wyke S. Does gender matter? An analysis of men's and women's accounts of responding to symptoms of lung cancer. Soc Sci Med. (2017) 191:134–42. doi: 10.1016/j.socscimed.2017.09.015
35. Keenan BP, Barr E, Gleeson E, Greenberg CC, and Temkin SM. Structural sexism and cancer care: the effects on the patient and oncologist. Am Soc Clin Oncol Educ Book. (2023) 43:e391516. doi: 10.1200/EDBK_391516
36. Tolwin Y, Gillis R, and Peled N. Gender and lung cancer—SEER-based analysis. Ann Epidemiol. (2020) 46:14–9. doi: 10.1016/j.annepidem.2020.04.003
37. Dingillo G, Bassiri A, Badrinathan A, Alvarado CE, Sinopoli J, Tapias L, et al. Lung cancer in young patients is associated with more advanced disease but better overall survival. J Surg Res. (2023) 292:307–16. doi: 10.1016/j.jss.2023.08.007
38. Garrana SH, Dagogo-Jack I, Cobb R, Kuo AH, Mendoza DP, Zhang EW, et al. Clinical and imaging features of non–small-cell lung cancer in young patients. Clin Lung Cancer. (2021) 22:23–31. doi: 10.1016/j.cllc.2020.10.012
39. Mhlana N and Koegelenberg C. The impact of age at presentation on lung cancer staging. Afr J Thorac Crit Care Med. (2020) 26:29–31. doi: 10.7196/AJTCCM.2020.v26i2.045
40. Hughes DJ, Kapiris M, Podvez Nevajda A, McGrath H, Stavraka C, Ahmad S, et al. Non-small cell lung cancer (NSCLC) in young adults, age< 50, is associated with late stage at presentation and a very poor prognosis in patients that do not have a targeted therapy option: a real-world study. Cancers. (2022) 14:6056. doi: 10.3390/cancers14246056
41. Arnold BN, Thomas DC, Rosen JE, Salazar MC, Blasberg JD, Boffa DJ, et al. Lung cancer in the very young: treatment and survival in the national cancer data base. J Thorac Oncol. (2016) 11:1121–31. doi: 10.1016/j.jtho.2016.03.023
42. Virally J, Choudat L, Chebbo M, Sartene R, Jagot J-L, Elhadad A, et al. Epidemiology and delays in the management of 355 patients with lung cancer. Rev Des maladies respiratoires. (2006) 23:43–8. doi: 10.1016/S0761-8425(06)71461-9
43. Montagne F, Guisier F, Venissac N, and Baste J-M. The role of surgery in lung cancer treatment: present indications and future perspectives—state of the art. Cancers. (2021) 13:3711. doi: 10.3390/cancers13153711
44. Zhang Z, Huo H, Li F, Miao J, Hu B, and Chen S. Surgical outcomes for non-small cell lung cancer in younger adults: A population-based study. Thorac Cancer. (2024) 15:1218–27. doi: 10.1111/1759-7714.15300
45. Kim D and Lee J-W. Current status of lung cancer and surgery based on studies using a nationwide database. J Chest Surg. (2022) 55:1. doi: 10.5090/jcs.21.105
46. Leo RT, Sugarbaker EA, McAllister M, Singh A, Barcelos RR, Basil Ali A, et al. Young lung cancer patients undergoing surgery: Comparison of clinicopathological characteristics and outcomes in patients aged≤ 50 years versus> 50 years. JTCVS Open. (2024) 24:409–22. doi: 10.1016/j.xjon.2024.12.008
47. Abdennadher M, Dahmane MH, Zair S, Zribi H, Abdelkbir A, Bouassida I, et al. Sex-specificity in surgical stages of lung cancer in young adults. Open Respir Med J. (2023) 17. doi: 10.2174/18743064-v17-230818-2022-20
48. Lee MH, Qureshi MM, Suzuki K, Everett P, Tapan U, and Mak KS. Small cell lung cancer in young patients: trends in sociodemographic factors, diagnosis, treatment, and survival. J Thorac Dis. (2022) 14:2880. doi: 10.21037/jtd-22-210
49. Gitlitz BJ, Novello S, Vavalà T, Bittoni M, Sable-Hunt A, Pavlick D, et al. The genomics of young lung cancer: comprehensive tissue genomic analysis in patients under 40 with lung cancer. JTO Clin Res Rep. (2021) 2:100194. doi: 10.1016/j.jtocrr.2021.100194
50. Suidan AM, Roisman L, Rozenblum AB, Ilouze M, Dudnik E, and Zer A. Lung cancer in young patients: higher rate of driver mutations and brain involvement, but better survival. J Global Oncol. (2019) 5:1–8. doi: 10.1200/JGO.18.00216
51. Pan X, Lv T, Zhang F, Fan H, Liu H, and Song Y. Frequent genomic alterations and better prognosis among young patients with non-small-cell lung cancer aged 40 years or younger. Clin Trans Oncol. (2018) 20:1168–74. doi: 10.1007/s12094-018-1838-z
52. Sacher AG, Dahlberg SE, Heng J, Mach S, Jänne PA, and Oxnard GR. Association between younger age and targetable genomic alterations and prognosis in non–small-cell lung cancer. JAMA Oncol. (2016) 2:313–20. doi: 10.1001/jamaoncol.2015.4482
53. Guirado M, Fernández Martín E, Fernández Villar A, Navarro Martín A, and Sánchez-Hernández A. Clinical impact of delays in the management of lung cancer patients in the last decade: systematic review. Clin Trans Oncol. (2022) 24:1549–68. doi: 10.1007/s12094-022-02796-w
54. Romine PE, Sun Q, Fedorenko C, Li L, Tang M, Eaton KD, et al. Impact of diagnostic delays on lung cancer survival outcomes: A population study of the US SEER-Medicare database. JCO Oncol Pract. (2022) 18:e877–85. doi: 10.1200/OP.21.00485
55. Chang G-C, Chiu C-H, Yu C-J, Chang Y-C, Chang Y-H, Hsu K-H, et al. Low-dose CT screening among never-smokers with or without a family history of lung cancer in Taiwan: a prospective cohort study. Lancet Respir Med. (2024) 12:141–52. doi: 10.1016/S2213-2600(23)00338-7
56. Shum E, Li W, Bell J, Sequist L, Ou S-HI, Goldberg J, et al. OA16. 04 preliminary results from the female Asian nonsmoker screening study (FANSS). J Thorac Oncol. (2023) 18:S81–2. doi: 10.1016/j.jtho.2023.09.085
58. Narjust Florez LT, Kiel L, Kaufman R, Mantz C, Beckwith J, Costa D, et al. The EQUAL study: Utilizing plasma EGFR cfDNA detection as an accessible screening tool for lung cancer in underserved patients ineligible for routine screening. Am Soc Clin Oncol. (2025) 43. doi: 10.1200/JCO.2025.43.16_suppl.TPS3168
60. Cai L, Chen Y, Tong X, Wu X, Bao H, Shao Y, et al. The genomic landscape of young and old lung cancer patients highlights age-dependent mutation frequencies and clinical actionability in young patients. Int J Cancer. (2021) 149:883–92. doi: 10.1002/ijc.v149.4
61. Chen Z, Teng X, Zhang J, Huang K, Shen Q, Cao H, et al. Molecular features of lung adenocarcinoma in young patients. BMC Cancer. (2019) 19:1–7. doi: 10.1186/s12885-019-5978-5
62. Tanaka K, Hida T, Oya Y, Yoshida T, Shimizu J, Mizuno T, et al. Unique prevalence of oncogenic genetic alterations in young patients with lung adenocarcinoma. Cancer. (2017) 123:1731–40. doi: 10.1002/cncr.v123.10
63. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features and gene mutations of lung cancer patients 30 years of age or younger. PLoS One. (2015) 10:e0136659. doi: 10.1371/journal.pone.0136659
64. Catania C, Botteri E, Barberis M, Conforti F, Toffalorio F, De Marinis F, et al. Molecular features and clinical outcome of lung Malignancies in very young people. Future Oncol. (2015) 11:1211–21. doi: 10.2217/fon.15.10
65. VandenBussche CJ, Illei PB, Lin M-T, Ettinger DS, and Maleki Z. Molecular alterations in non–small cell lung carcinomas of the young. Hum Pathol. (2014) 45:2379–87. doi: 10.1016/j.humpath.2014.08.005
66. Luo W, Tian P, Wang Y, Xu H, Chen L, Tang C, et al. Characteristics of genomic alterations of lung adenocarcinoma in young never-smokers. Int J Cancer. (2018) 143:1696–705. doi: 10.1002/ijc.v143.7
67. Subramanian J, Morgensztern D, Goodgame B, Baggstrom MQ, Gao F, Piccirillo J, et al. Distinctive characteristics of non-small cell lung cancer (NSCLC) in the young: a surveillance, epidemiology, and end results (SEER) analysis. J Thorac Oncol. (2010) 5:23–8. doi: 10.1097/JTO.0b013e3181c41e8d
68. Hou H, Zhang C, Qi X, Zhou L, Liu D, Lv H, et al. Distinctive targetable genotypes of younger patients with lung adenocarcinoma: a cBioPortal for cancer genomics data base analysis. Cancer Biol Ther. (2020) 21:26–33. doi: 10.1080/15384047.2019.1665392
69. Viñal D, Martínez D, Higuera O, and De Castro J. Genomic profiling in non-small-cell lung cancer in young patients. A systematic review. ESMO Open. (2021) 6:100045. doi: 10.1016/j.esmoop.2020.100045
70. Wallen ZD, Ko H, Nesline MK, Hastings SB, Strickland KC, Previs RA, et al. Real-world comprehensive genomic and immune profiling reveals distinct age-and sex-based genomic and immune landscapes in tumors of patients with non-small cell lung cancer. Front Immunol. (2024) 15:1413956. doi: 10.3389/fimmu.2024.1413956
71. Ulivi P, Urbini M, Petracci E, Canale M, Dubini A, Bartolini D, et al. Wide next-generation sequencing characterization of young adults non-small-cell lung cancer patients. Cancers. (2022) 14:2352. doi: 10.3390/cancers14102352
72. Ferrara MG, Stefani A, Pilotto S, Carbone C, Vita E, Di Salvatore M, et al. The renaissance of KRAS targeting in advanced non-small-cell lung cancer: new opportunities following old failures. Front Oncol. (2021) 11:792385. doi: 10.3389/fonc.2021.792385
73. Chen L, Hu X, Wu H, Liu J, Mu X, Wu H, et al. Unique profiles of targetable genomic alterations and prognosis in young Chinese patients with lung adenocarcinoma. Pathology-Research Pract. (2019) 215:152407. doi: 10.1016/j.prp.2019.03.035
74. He C-H, Shih J-F, Lai S-L, and Chen Y-M. Non–small cell lung cancer in the very young: Higher EGFR/ALK mutation proportion than the elder. J Chin Med Assoc. (2020) 83:461–5. doi: 10.1097/JCMA.0000000000000311
75. Zhong W, Zhao J, Huang K, Zhang J, and Chen Z. Comparison of clinicopathological and molecular features between young and old patients with lung cancer. Int J Clin Exp Pathol. (2018) 11:1031.
76. Bruno R and Fontanini G. Next generation sequencing for gene fusion analysis in lung cancer: a literature review. Diagnostics. (2020) 10:521. doi: 10.3390/diagnostics10080521
77. Yang S, Song Z, and Cheng G. Genomic alterations and survival in young patients aged under 40 years with completely resected non-small cell lung cancer. Ann Trans Med. (2019) 7. doi: 10.21037/atm.2019.03.39
78. Tang W-F, Qiu Z-B, Chu X-P, Zeng Y-M, Hu Y-B, Tang X, et al. EGFR mutation rates correlate with age at diagnosis and tumor characteristics in patients with pulmonary ground-glass opacities. Ann Surg Oncol. (2024) 32(7):4641–9. doi: 10.1245/s10434-024-16730-7
79. Wu S-G, Liu Y-N, Yu C-J, Yang JC-H, and Shih J-Y. Driver mutations of young lung adenocarcinoma patients with Malignant pleural effusion. Genes Chromosomes Cancer. (2018) 57:513–21. doi: 10.1002/gcc.v57.10
80. Wu S-G, Chang Y-L, Yu C-J, Yang P-C, and Shih J-Y. Lung adenocarcinoma patients of young age have lower EGFR mutation rate and poorer efficacy of EGFR tyrosine kinase inhibitors. ERJ Open Res. (2017) 3. doi: 10.1183/23120541.00092-2016
81. Hou H, Zhu H, Zhao H, Yan W, Wang Y, Jiang M, et al. Comprehensive molecular characterization of young Chinese patients with lung adenocarcinoma identified a distinctive genetic profile. Oncologist. (2018) 23:1008–15. doi: 10.1634/theoncologist.2017-0629
82. Mezquita L, Jové M, Nadal E, Kfoury MK, Morán T, Ricordel C, et al. High prevalence of somatic oncogenic driver alterations in patients with NSCLC and Li-Fraumeni syndrome. J Thorac Oncol. (2020) 15:1232–9. doi: 10.1016/j.jtho.2020.03.005
83. Oxnard GR, Chen R, Pharr JC, Koeller DR, Bertram AA, Dahlberg SE, et al. Germline EGFR mutations and familial lung cancer. J Clin Oncol. (2023) 41:5274–84. doi: 10.1200/JCO.23.01372
85. Koh J, Jang J-Y, Keam B, Kim S, Kim M-Y, Go H, et al. EML4-ALK enhances programmed cell death-ligand 1 expression in pulmonary adenocarcinoma via hypoxia-inducible factor (HIF)-1α and STAT3. Oncoimmunology. (2016) 5:e1108514. doi: 10.1080/2162402X.2015.1108514
86. Shen J, Li S, Medeiros LJ, Lin P, Wang SA, Tang G, et al. PD-L1 expression is associated with ALK positivity and STAT3 activation, but not outcome in patients with systemic anaplastic large cell lymphoma. Modern Pathol. (2020) 33:324–33. doi: 10.1038/s41379-019-0336-3
87. Di Federico A, De Giglio A, Parisi C, and Gelsomino F. STK11/LKB1 and KEAP1 mutations in non-small cell lung cancer: Prognostic rather than predictive? Eur J Cancer. (2021) 157:108–13. doi: 10.1016/j.ejca.2021.08.011
88. Ricciuti B, Arbour KC, Lin JJ, Vajdi A, Vokes N, Hong L, et al. Diminished efficacy of programmed death-(ligand) 1 inhibition in STK11-and KEAP1-mutant lung adenocarcinoma is affected by KRAS mutation status. J Thorac Oncol. (2022) 17:399–410. doi: 10.1016/j.jtho.2021.10.013
89. Marinelli D, Mazzotta M, Scalera S, Terrenato I, Sperati F, D'Ambrosio L, et al. KEAP1-driven co-mutations in lung adenocarcinoma unresponsive to immunotherapy despite high tumor mutational burden. Ann Oncol. (2020) 31:1746–54. doi: 10.1016/j.annonc.2020.08.2105
91. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. New Engl J Med. (2020) 382:41–50. doi: 10.1056/NEJMoa1913662
92. Mok T, Camidge DR, Gadgeel SM, Rosell R, Dziadziuszko R, Kim D-W, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol. (2020) 31:1056–64. doi: 10.1016/j.annonc.2020.04.478
93. Solomon BJ, Bauer TM, Ignatius Ou S-H, Liu G, Hayashi H, Bearz A, et al. Post hoc analysis of lorlatinib intracranial efficacy and safety in patients with ALK-positive advanced non–small-cell lung cancer from the phase III CROWN study. J Clin Oncol. (2022) 40:3593–602. doi: 10.1200/JCO.21.02278
94. Drilon A, Chiu C-H, Fan Y, Cho BC, Lu S, Ahn M-J, et al. Long-term efficacy and safety of entrectinib in ROS1 fusion–positive NSCLC. JTO Clin Res Rep. (2022) 3:100332. doi: 10.1016/j.jtocrr.2022.100332
95. Drilon A, Camidge DR, Lin JJ, Kim S-W, Solomon BJ, Dziadziuszko R, et al. Repotrectinib in ROS1 Fusion–positive non–small-Cell lung cancer. New Engl J Med. (2024) 390:118–31. doi: 10.1056/NEJMoa2302299
96. Zhou C, Solomon B, Loong HH, Park K, Pérol M, and Arriola E. First-line selpercatinib or chemotherapy and pembrolizumab in RET fusion–positive NSCLC. New Engl J Med. (2023) 389:1839–50. doi: 10.1056/NEJMoa2309457
97. Griesinger F, Curigliano G, Thomas M, Subbiah V, Baik CS, Tan DSW, et al. Safety and efficacy of pralsetinib in RET fusion–positive non-small-cell lung cancer including as first-line therapy: update from the ARROW trial. Ann Oncol. (2022) 33:1168–78. doi: 10.1016/j.annonc.2022.08.002
98. Yoneyama R, Saji H, Kato Y, Kudo Y, Shimada Y, Kimura M, et al. Clinicopathological characteristics and treatment strategies for young lung cancer patients. Ann Trans Med. (2019) 7. doi: 10.21037/atm.2019.01.69
99. Laguna JC, Tagliamento M, Lambertini M, Hiznay J, and Mezquita L. Tackling non–small cell lung cancer in young adults: from risk factors and genetic susceptibility to lung cancer profile and outcomes. Am Soc Clin Oncol Educ Book. (2024) 44:e432488. doi: 10.1200/EDBK_432488
100. Voruganti T, Soulos PR, Mamtani R, Presley CJ, and Gross CP. Association between age and survival trends in advanced non–small cell lung cancer after adoption of immunotherapy. JAMA Oncol. (2023) 9:334–41. doi: 10.1001/jamaoncol.2022.6901
101. Gadgeel SM, Ramalingam S, Cummings G, Kraut MJ, Wozniak AJ, Gaspar LE, et al. Lung cancer in patients< 50 years of age: the experience of an academic multidisciplinary program. Chest. (1999) 115:1232–6. doi: 10.1378/chest.115.5.1232
102. Antonoff MB, Deboever N, Werner R, Altan M, Gomez D, and Opitz I. Surgery for oligometastatic non-small cell lung cancer. Amsterdam, Netherlands: Elsevier (2023).
103. Gomez DR, Tang C, Zhang J, Blumenschein GR Jr, Hernandez M, Lee JJ, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non–small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. (2019) 37:1558–65. doi: 10.1200/JCO.19.00201
104. Mitchell KG, Farooqi A, Ludmir EB, Corsini EM, Zhang J, Sepesi B, et al. Improved overall survival with comprehensive local consolidative therapy in synchronous oligometastatic non–small-cell lung cancer. Clin Lung Cancer. (2020) 21:37–46.e7. doi: 10.1016/j.cllc.2019.07.007
105. Opitz I, Patella M, Payrard L, Perentes JY, Inderbitzi R, Gelpke H, et al. Prognostic factors of oligometastatic non-small-cell lung cancer following radical therapy: a multicentre analysis. Eur J Cardio-Thoracic Surg. (2020) 57:1166–72. doi: 10.1093/ejcts/ezz384
106. Khan TM, Verbus EA, Gandhi S, Heymach JV, Hernandez JM, and Elamin YY. Osimertinib, surgery, and radiation therapy in treating patients with stage IIIB or IV non-small cell lung cancer with EGFR mutations (NORTHSTAR). Ann Surg Oncol. (2022) 29:4688–9. doi: 10.1245/s10434-022-11627-9
107. Heeke S, Gandhi S, Tran HT, Lam VK, Byers LA, Gibbons DL, et al. Longitudinal tracking of ALK-positive lung cancer from plasma using circulating-tumor RNA and circulating-tumor DNA in the BRIGHTSTAR clinical trial. J Liquid Biopsy. (2023) 1. doi: 10.1016/j.jlb.2023.100065
108. de Rijke JM, Schouten LJ, ten Velde GPM, Wanders SL, Bollen ECM, Lalisang RI, et al. Influence of age, comorbidity and performance status on the choice of treatment for patients with non-small cell lung cancer; results of a population-based study. Lung Cancer. (2004) 46:233–45. doi: 10.1016/j.lungcan.2004.03.011
109. Murthy VH, Krumholz HM, and Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. (2004) 291:2720–6. doi: 10.1001/jama.291.22.2720
110. Shi J, Li D, Liang D, and He Y. Epidemiology and prognosis in young lung cancer patients aged under 45 years old in northern China. Sci Rep. (2021) 11:6817. doi: 10.1038/s41598-021-86203-4
112. Soria J-C, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. New Engl J Med. (2018) 378:113–25. doi: 10.1056/NEJMoa1713137
113. Planchard D, Jänne PA, Cheng Y, Yang JC-H, Yanagitani N, Kim S-W, et al. Osimertinib with or without chemotherapy in EGFR-mutated advanced NSCLC. New Engl J Med. (2023) 389:1935–48. doi: 10.1056/NEJMoa2306434
114. Cho BC, Lu S, Felip E, Spira AI, Girard N, Lee JS, et al. Amivantamab plus lazertinib for previously untreated EGFR-mutated advanced NSCLC. N. Engl. J. Med. (2024) 391(16):1486–98. doi: 10.1056/NEJMoa2403614
115. Le X, Patel JD, Shum E, Baik C, Sanborn RE, Shu CA, et al. A multicenter open-label randomized phase II study of osimertinib with and without ramucirumab in tyrosine kinase inhibitor–naïve EGFR-mutant metastatic non–small cell lung cancer (RAMOSE trial). J Clin Oncol. (2024) 43:403–11. doi: 10.1200/JCO.24.00533
116. Brown K, Comisar C, Witjes H, Maringwa J, de Greef R, Vishwanathan K, et al. Population pharmacokinetics and exposure-response of osimertinib in patients with non-small cell lung cancer. Br J Clin Pharmacol. (2017) 83:1216–26. doi: 10.1111/bcp.13223
117. Solomon BJ, Liu G, Felip E, Mok TSK, Soo RA, Mazieres J, et al. Lorlatinib versus crizotinib in patients with advanced ALK-positive non–small cell lung cancer: 5-year outcomes from the phase III CROWN study. J Clin Oncol. (2024) 42:3400–9. doi: 10.1200/JCO.24.00581
118. Thummalapalli R, Choudhury NJ, Ehrich F, Beardslee T, Brazel D, Zhang SS, et al. Lorlatinib tolerability and association with clinical outcomes in patients with advanced ALK-or ROS1-rearranged NSCLC: A brief report. JTO Clin Res Rep. (2023) 4:100546. doi: 10.1016/j.jtocrr.2023.100546
119. Reed M, Rosales A-LS, Chioda MD, Parker L, Devgan G, and Kettle J. Consensus recommendations for management and counseling of adverse events associated with lorlatinib: a guide for healthcare practitioners. Adv Ther. (2020) 37:3019–30. doi: 10.1007/s12325-020-01365-3
121. Liu SV. NRG1 fusions: Biology to therapy. Lung Cancer. (2021) 158:25–8. doi: 10.1016/j.lungcan.2021.05.011
122. Drilon A, Duruisseaux M, Han J-Y, Ito M, Falcon C, Yang S-R, et al. Clinicopathologic features and response to therapy of NRG1 fusion–driven lung cancers: The eNRGy1 global multicenter registry. J Clin Oncol. (2021) 39:2791–802. doi: 10.1200/JCO.20.03307
123. Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroǧlu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. (2021) 397:592–604. doi: 10.1016/S0140-6736(21)00228-2
124. Mok TSK, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. (2019) 393:1819–30. doi: 10.1016/S0140-6736(18)32409-7
125. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of PD-L1–selected patients with NSCLC. New Engl J Med. (2020) 383:1328–39. doi: 10.1056/NEJMoa1917346
126. Hellmann MD, Ciuleanu T-E, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. New Engl J Med. (2018) 378:2093–104. doi: 10.1056/NEJMoa1801946
127. Takamori S, Komiya T, and Powell E. Survival benefit from immunocheckpoint inhibitors in stage IV non-small cell lung cancer patients with brain metastases: A National Cancer Database propensity-matched analysis. Cancer Med. (2021) 10:923–32. doi: 10.1002/cam4.v10.3
128. Lichtenstein MRL, Nipp RD, Muzikansky A, Goodwin K, Anderson D, Newcomb RA, et al. Impact of age on outcomes with immunotherapy in patients with non–small cell lung cancer. J Thorac Oncol. (2019) 14:547–52. doi: 10.1016/j.jtho.2018.11.011
129. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non–small-cell lung cancer. New Engl J Med. (2018) 379:2040–51. doi: 10.1056/NEJMoa1810865
130. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. New Engl J Med. (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
131. Paz-Ares LG, Ciuleanu T-E, Cobo M, Bennouna J, Schenker M, Cheng Y, et al. First-line nivolumab plus ipilimumab with chemotherapy versus chemotherapy alone for metastatic NSCLC in CheckMate 9LA: 3-year clinical update and outcomes in patients with brain metastases or select somatic mutations. J Thorac Oncol. (2023) 18:204–22. doi: 10.1016/j.jtho.2022.10.014
132. Corbaux P, Maillet D, Boespflug A, Locatelli-Sanchez M, Perier-Muzet M, Duruisseaux M, et al. Older and younger patients treated with immune checkpoint inhibitors have similar outcomes in real-life setting. Eur J Cancer. (2019) 121:192–201. doi: 10.1016/j.ejca.2019.08.027
133. Nishijima TF, Muss HB, Shachar SS, and Moschos SJ. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: a systematic review and meta-analysis. Cancer Treat Rev. (2016) 45:30–7. doi: 10.1016/j.ctrv.2016.02.006
134. Li J and Gu J. Efficacy of immune checkpoint inhibitors in cancer patients of different ages: a meta-analysis. Future Oncol. (2019) 15:3633–46. doi: 10.2217/fon-2019-0279
135. Wu Q, Wang Q, Tang X, Xu R, Zhang L, Chen X, et al. Correlation between patients’ age and cancer immunotherapy efficacy. Oncoimmunology. (2019) 8:e1568810. doi: 10.1080/2162402X.2019.1568810
136. Huang X-Z, Gao P, Song Y-X, Sun J-X, Chen X-W, Zhao J-H, et al. Efficacy of immune checkpoint inhibitors and age in cancer patients. Immunotherapy. (2020) 12:587–603. doi: 10.2217/imt-2019-0124
137. O'Brien M, Paz-Ares L, Marreaud S, Dafni U, Oselin K, Havel L, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB–IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. (2022) 23:1274–86. doi: 10.1016/S1470-2045(22)00518-6
138. Wakelee H, Liberman M, Kato T, Tsuboi M, Lee S-H, Gao S, et al. Perioperative pembrolizumab for early-stage non–small-cell lung cancer. New Engl J Med. (2023) 389:491–503. doi: 10.1056/NEJMoa2302983
139. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. New Engl J Med. (2022) 386:1973–85. doi: 10.1056/NEJMoa2202170
140. Chen C, Wu B, Zhang C, and Xu T. Immune-related adverse events associated with immune checkpoint inhibitors: an updated comprehensive disproportionality analysis of the FDA adverse event reporting system. Int Immunopharmacol. (2021) 95:107498. doi: 10.1016/j.intimp.2021.107498
141. Wang DY, Salem J-E, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. (2018) 4:1721–8. doi: 10.1001/jamaoncol.2018.3923
142. Samani A, Zhang S, Spiers L, et al. Impact of age on the toxicity of immune checkpoint inhibition. J ImmunoTherapy Cancer. (2020) 8. doi: 10.1136/jitc-2020-000871
143. Johnson DB, Chandra S, and Sosman JA. Immune checkpoint inhibitor toxicity in 2018. JAMA. (2018) 320:1702–3. doi: 10.1001/jama.2018.13995
144. Lawrence L. Early-life cancer diagnosis can have lasting psychological impact. Med Bag. (2023).
145. Lustberg MB, Kuderer NM, Desai A, Bergerot C, and Lyman GH. Mitigating long-term and delayed adverse events associated with cancer treatment: implications for survivorship. Nat Rev Clin Oncol. (2023) 20:527–42. doi: 10.1038/s41571-023-00776-9
146. Duma N, Abdel-Ghani A, Yadav S, Hoversten KP, Reed CT, Sitek AN, et al. Sex differences in tolerability to anti-programmed cell death protein 1 therapy in patients with metastatic melanoma and non-small cell lung cancer: are we all equal? oncologist. (2019) 24:e1148–55. doi: 10.1634/theoncologist.2019-0094
147. Shimamura SS, Shukuya T, Asao T, Hayakawa D, Kurokawa K, Xu S, et al. Survival past five years with advanced, EGFR-mutated or ALK-rearranged non-small cell lung cancer—is there a “tail plateau” in the survival curve of these patients? BMC Cancer. (2022) 22:323. doi: 10.1186/s12885-022-09421-7
148. Sabari JK, Yu HA, Mahadevia PJ, Liu Y, Demirdjian L, Chen YH, et al. Overall survival in EGFR-mutant advanced non-small cell lung cancer treated with first-line osimertinib: A cohort study integrating clinical and biomarker data in the United States. J Thorac Oncol. (2025) S1556–0864. doi: 10.1016/j.jtho.2025.04.010
149. Narjust Florez M, FASCO, Lauren Kiel BS, Rebekah Kaufman MS, Angela Morabito BS, and Courtney Mantz BS. Understanding young lung cancer: the time is now. (2025).
150. Graves KD, Arnold SM, Love CL, Kirsh KL, Moore PG, and Passik SD. Distress screening in a multidisciplinary lung cancer clinic: prevalence and predictors of clinically significant distress. Lung Cancer. (2007) 55:215–24. doi: 10.1016/j.lungcan.2006.10.001
151. Hopwood P, Stephens RJ, and Party BMRCLCW. Depression in patients with lung cancer: prevalence and risk factors derived from quality-of-life data. J Clin Oncol. (2000) 18:893–3. doi: 10.1200/JCO.2000.18.4.893
152. Brintzenhofe-Szoc KM, Levin TT, Li Y, Kissane DW, and Zabora JR. Mixed anxiety/depression symptoms in a large cancer cohort: prevalence by cancer type. Psychosomatics. (2009) 50:383–91. doi: 10.1176/appi.psy.50.4.383
153. Boyes AW, Girgis A, D'Este CA, Zucca AC, Lecathelinais C, and Carey ML. Prevalence and predictors of the short-term trajectory of anxiety and depression in the first year after a cancer diagnosis: a population-based longitudinal study. J Clin Oncol. (2013) 31:2724–9. doi: 10.1200/JCO.2012.44.7540
154. Fox RS, Armstrong GE, Gaumond JS, Vigoureux TFD, Miller CH, Sanford SD, et al. Social isolation and social connectedness among young adult cancer survivors: A systematic review. Cancer. (2023) 129:2946–65. doi: 10.1002/cncr.v129.19
155. Li X, Hathaway CA, Small BJ, Tometich DB, Gudenkauf LM, Hoogland AI, et al. Social isolation, depression, and anxiety among young adult cancer survivors: The mediating role of social connectedness. Cancer. (2024) 130:4127–37. doi: 10.1002/cncr.v130.23
156. Li C, Zeng L, and Zhong J. A study on the status and the correlation of stigma in young patients with lung cancer. Psychology. (2022) 13:816–28. doi: 10.4236/psych.2022.135055
157. LoConte NK, Else-Quest NM, Eickhoff J, Hyde J, and Schiller JH. Assessment of guilt and shame in patients with non–small-cell lung cancer compared with patients with breast and prostate cancer. Clin Lung Cancer. (2008) 9:171–8. doi: 10.3816/CLC.2008.n.026
158. Cataldo JK and Brodsky JL. Lung cancer stigma, anxiety, depression and symptom severity. Oncology. (2013) 85:33–40. doi: 10.1159/000350834
159. Chambers SK, Baade P, Youl P, Aitken JA, Occhipinti S, Vinod S, et al. Psychological distress and quality of life in lung cancer: the role of health-related stigma, illness appraisals and social constraints. Psycho-Oncology. (2015) 24:1569–77. doi: 10.1002/pon.v24.11
160. Hamann HA, Ostroff JS, Marks EG, Gerber DE, Schiller JH, and Craddock Lee SJ. Stigma among patients with lung cancer: A patient-reported measurement model. Psycho-Oncology. (2014) 23:81–92. doi: 10.1002/pon.v23.1
161. Riba MB, Donovan KA, Ahmed K, Andersen B, Braun I, Breitbart WS, et al. NCCN Guidelines® insights: distress management, version 2.2023: featured updates to the NCCN Guidelines. J Natl Compr Cancer Network. (2023) 21:450–7. doi: 10.6004/jnccn.2023.0026
162. Andersen BL, Lacchetti C, Ashing K, Berek JS, Berman BS, Bolte S, et al. Management of anxiety and depression in adult survivors of cancer: ASCO guideline update. J Clin Oncol. (2023) 41:3426–53. doi: 10.1200/JCO.23.00293
163. Alpert O, Siddiqui B, Shabbir Z, Soudan M, and Garren P. The role of psychiatry in quality of life in young patients with non-small cell lung cancer. Brain Behavior Immunity-Health. (2022) 25:100507. doi: 10.1016/j.bbih.2022.100507
164. Zhang L, Liu X, Tong F, Zou R, Peng W, Yang H, et al. Cognitive behavioral therapy for anxiety and depression in cancer survivors: a meta-analysis. Sci Rep. (2022) 12:21466. doi: 10.1038/s41598-022-25068-7
165. Sutanto YS, Ibrahim D, Septiawan D, Sudiyanto A, and Kurniawan H. Effect of cognitive behavioral therapy on improving anxiety, depression, and quality of life in pre-diagnosed lung cancer patients. Asian Pacific J Cancer Prev. (2021) 22:3455. doi: 10.31557/APJCP.2021.22.11.3455
166. Rueda JR, Sola I, Pascual A, and Casacuberta MS. Non-invasive interventions for improving well-being and quality of life in patients with lung cancer. Cochrane Database Systematic Rev. (2011) 2011. doi: 10.1002/14651858.CD004282.pub3
167. Huang C-C, Kuo H-P, Lin Y-E, and Chen S-C. Effects of a web-based health education program on quality of life and symptom distress of initially diagnosed advanced non-small cell lung cancer patients: a randomized controlled trial. J Cancer Educ. (2019) 34:41–9. doi: 10.1007/s13187-017-1263-y
168. Carlson LE, Ismaila N, Addington EL, Asher GN, Atreya C, Balneaves LG, et al. Integrative oncology care of symptoms of anxiety and depression in adults with cancer: society for integrative oncology–ASCO guideline. J Clin Oncol. (2023) 41:4562–91. doi: 10.1200/JCO.23.00857
169. Oberoi S, Yang J, Woodgate RL, Niraula S, Banerji S, Israels SJ, et al. Association of mindfulness-based interventions with anxiety severity in adults with cancer: a systematic review and meta-analysis. JAMA network Open. (2020) 3:e2012598–e2012598. doi: 10.1001/jamanetworkopen.2020.12598
170. Cillessen L, Johannsen M, Speckens AE, and Zachariae R. Mindfulness-based interventions for psychological and physical health outcomes in cancer patients and survivors: a systematic review and meta-analysis of randomized controlled trials. Psycho-oncology. (2019) 28:2257–69. doi: 10.1002/pon.v28.12
171. Mueller J, Davies A, Jay C, Harper S, and Todd C. Evaluation of a web-based, tailored intervention to encourage help-seeking for lung cancer symptoms: a randomised controlled trial. Digital Health. (2020) 6:2055207620922381. doi: 10.1177/2055207620922381
172. Novotny PJ, Smith DJ, Guse L, Rummans TA, Hartmann L, Alberts S, et al. A pilot study assessing social support among cancer patients enrolled on clinical trials: a comparison of younger versus older adults. Cancer Manage Res. (2010) 2:133–42. doi: 10.2147/CMAR.S9668
173. Sansom-Daly UM, Wakefield CE, Ellis SJ, McGill BC, Donoghoe MW, Butow P, et al. Online, group-based psychological support for adolescent and young adult cancer survivors: results from the recapture life randomized trial. Cancers. (2021) 13:2460. doi: 10.3390/cancers13102460
174. Florez N, Kiel L, Horiguchi M, Kaufman R, Haradon D, Sanchez M, et al. Young lung cancer: Psychosocial needs assessment. Am Soc Clin Oncol. (2024) 42. doi: 10.1200/JCO.2024.42.16_suppl.12104
175. Esposito S, Tenconi R, Preti V, Groppali E, and Principi N. Chemotherapy against cancer during pregnancy: A systematic review on neonatal outcomes. Medicine. (2016) 95. doi: 10.1097/MD.0000000000004899
176. Walter JR, Xu S, Paller AS, Choi JN, and Woodruff TK. Oncofertility considerations in adolescents and young adults given a diagnosis of melanoma: fertility risk of Food and Drug Administration–approved systemic therapies. J Am Acad Dermatol. (2016) 75:528–34. doi: 10.1016/j.jaad.2016.04.031
177. Garutti M, Lambertini M, and Puglisi F. Checkpoint inhibitors, fertility, pregnancy, and sexual life: a systematic review. ESMO Open. (2021) 6:100276. doi: 10.1016/j.esmoop.2021.100276
178. Duma N and Lambertini M. It is time to talk about fertility and immunotherapy. oncologist. (2020) 25:277–8. doi: 10.1634/theoncologist.2019-0837
179. Rambhatla A, Strug MR, De Paredes JG, Cordoba Munoz MI, and Thakur M. Fertility considerations in targeted biologic therapy with tyrosine kinase inhibitors: a review. J Assisted Reprod Genet. (2021) 38:1897–908. doi: 10.1007/s10815-021-02181-6
180. da Rosa PRA, De Cesaro MP, Dau AMP, Duggavathi R, Bordignon V, and Dias Gonçalves PB. Reversible meiotic arrest of bovine oocytes by EGFR inhibition and follicular hemisections. Theriogenology. (2017) 99:53–62. doi: 10.1016/j.theriogenology.2017.05.014
181. Nagyova E. Regulation of cumulus expansion and hyaluronan synthesis in porcine oocyte-cumulus complexes during in vitro maturation. Endocr Regul. (2012) 46:225–35. doi: 10.4149/endo_2012_04_225
182. Florez N, Kaufman RA, Yáñez-Sarmiento A, Abioye O, and Kiel LR. When the unimaginable happens: Lung cancer diagnosis during pregnancy. Cancer. (2024) 130:1905–9. doi: 10.1002/cncr.v130.11
183. Florez N, Horiguchi M, Abioye O, Kaufman R, Haradon D, Walton S, et al. P3. 03I. 19 the international pregnancy and lung cancer registry at dana-farber cancer institute. J Thorac Oncol. (2024) 19:S317–8. doi: 10.1016/j.jtho.2024.09.569
184. Carosa E, Benvenga S, Trimarchi F, Lenzi A, Pepe M, Simonelli C, et al. Sexual inactivity results in reversible reduction of LH bioavailability. Int J impotence Res. (2002) 14:93–9. doi: 10.1038/sj.ijir.3900832
185. Ciocca G, Limoncin E, Carosa E, Di Sante S, Gravina GL, and Mollaioli D. Is testosterone a food for the brain? Sexual Med Rev. (2016) 4:15–25. doi: 10.1016/j.sxmr.2015.10.007
186. Brown E, Lo Monaco S, O’Donoghue B, Nolan H, Hughes E, Graham M, et al. Improving the sexual health of young people (under 25) in high-risk populations: a systematic review of behavioural and psychosocial interventions. Int J Environ Res Public Health. (2021) 18:9063. doi: 10.3390/ijerph18179063
187. Mitchell KR, Lewis R, O'Sullivan LF, and Fortenberry JD. What is sexual wellbeing and why does it matter for public health? Lancet Public Health. (2021)6:e608–13. doi: 10.1016/S2468-2667(21)00099-2
188. Flynn KE, Lin L, Bruner DW, Cyranowski JM, Hahn EA, and Jeffery DD. Sexual satisfaction and the importance of sexual health to quality of life throughout the life course of US adults. J sexual Med. (2016) 13:1642–50. doi: 10.1016/j.jsxm.2016.08.011
189. Sarna L. Correlates of symptom distress in women with lung cancer. Cancer Pract. (1993) 1:21–8.
190. Knox MK, Hales S, Nissim R, Jung J, Lo C, Zimmermann C, et al. Lost and stranded: the experience of younger adults with advanced cancer. Supportive Care Cancer. (2017) 25:399–407. doi: 10.1007/s00520-016-3415-8
191. Flynn KE, Jeffery DD, Keefe FJ, Porter LS, Shelby RA, Fawzy MR, et al. Sexual functioning along the cancer continuum: Focus group results from the Patient-Reported Outcomes Measurement Information System (PROMIS®). Psycho-Oncology. (2011) 20:378–86. doi: 10.1002/pon.v20.4
192. Wright J, Duchesne C, Sabourin S, Bissonnette F, Benoit J, and Girard Y. Psychosocial distress and infertility: men and women respond differently. Fertility sterility. (1991) 55:100–8. doi: 10.1016/S0015-0282(16)54067-9
193. Litt MD, Tennen H, Affleck G, and Klock S. Coping and cognitive factors in adaptation to in vitro fertilization failure. J Behav Med. (1992) 15:171–87. doi: 10.1007/BF00848324
194. Florez N, Kiel L, Meza K, Wei Z, Mazzola E, Velazquez AI, et al. Sexual health assessment in women with lung cancer study: sexual health assessment in women with lung cancer. Cancer. (2024) 130:375–84. doi: 10.1002/cncr.v130.3
195. Anderson RA, Clatot F, Demeestere I, Lambertini M, Morgan A, Nelson SM, et al. Cancer survivorship: reproductive health outcomes should be included in standard toxicity assessments. Eur J Cancer. (2021) 144:310–6. doi: 10.1016/j.ejca.2020.11.032
196. Peddie VL, Porter MA, Barbour R, Culligan D, MacDonald G, King D, et al. Factors affecting decision making about fertility preservation after cancer diagnosis: a qualitative study. BJOG. (2012) 119:1049–57. doi: 10.1111/j.1471-0528.2012.03368.x
197. Florez N, Kiel L, Riano I, Patel S, DeCarli K, Dhawan N, et al. Lung cancer in women: the past, present, and future. Clin Lung Cancer. (2023) 25:1–8. doi: 10.1016/j.cllc.2023.10.007
198. Kaufman R, Agrawal L, Teplinsky E, Kiel L, Abioye O, and Florez N. From diagnosis to survivorship addressing the sexuality of women during cancer. Oncologist. (2024) 29(12):1014–23. doi: 10.1093/oncolo/oyae242
201. Peipert BJ, Potapragada NR, Lantos PM, Harris BS, Reinecke J, and Goldman KN. A geospatial analysis of disparities in access to oncofertility services. JAMA Oncol. (2023) 9:1364–70. doi: 10.1001/jamaoncol.2023.2780
202. Temel JS, Greer JA, El-Jawahri A, Pirl WF, Park ER, Jackson VA, et al. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol. (2017) 35:834–41. doi: 10.1200/JCO.2016.70.5046
203. Golmohammadi M, Ebadi A, Ashrafizadeh H, Rassouli M, and Barasteh S. Factors related to advance directives completion among cancer patients: a systematic review. BMC Palliative Care. (2024) 23:3. doi: 10.1186/s12904-023-01327-w
204. Barragan-Carrillo R, Pabon CM, Chavarri-Guerra Y, Soto-Perez-de-Celis E, and Duma N. End-of-life care and advanced directives in Hispanic/Latinx patients: challenges and solutions for the practicing oncologist. Oncologist. (2022) 27:1074–80. doi: 10.1093/oncolo/oyac211
205. Greer JA, Trotter C, Jackson V, Rinaldi S, Kamdar M, El-Jawahri A, et al. Comparative effectiveness trial of early palliative care delivered via telehealth versus in person among patients with advanced lung cancer. Am Soc Clin Oncol. (2024) 42. doi: 10.1200/JCO.2024.42.17_suppl.LBA3
206. Temel JS, Jackson VA, El-Jawahri A, Rinaldi SP, Petrillo LA, Kumar P, et al. Stepped palliative care for patients with advanced lung cancer: A randomized clinical trial. JAMA. (2024) 332:471–81. doi: 10.1001/jama.2024.10398
207. Braun IM, Bohlke K, Abrams DI, Anderson H, Balneaves LG, Bar-Sela G, et al. Cannabis and cannabinoids in adults with cancer: ASCO guideline. J Clin Oncol. (2024) 42:1575–93. doi: 10.1200/JCO.23.02596
208. Nargis N, Westmaas JL, Orr E, Alqahtani MM, Choudhury PP, Islami F, et al. Cancer risk and legalisation of access to cannabis in the USA: overview of the evidence. Lancet Public Health. (2024) 10:e160–4. doi: 10.1016/S2468-2667(24)00223-8
209. Snaman JM, Feifer D, Helton G, Chang Y, El-Jawahri A, Volandes AE, et al. A pilot randomized trial of an advance care planning video decision support tool for adolescents and young adults with advanced cancer. J Natl Compr Cancer Network. (2023) 21:715–23.e17. doi: 10.6004/jnccn.2023.7021
210. Buiar PG and Goldim JR. Barriers to the composition and implementation of advance directives in oncology: a literature review. ecancermedicalscience. (2019) 13. doi: 10.3332/ecancer.2019.974
211. Agarwal R and Epstein AS. Advance care planning and end-of-life decision making for patients with cancer. In: Seminars in oncology nursing. Amsterdam, Netherlands: Elsevier (2018). p. 316–26.
Keywords: young patients, lung cancer, psychosocial, targeted therapy, diagnostic delays
Citation: Florez N, Kiel L, Kaufman R, LoPiccolo J, Ricciuti B, Morabito A, Fakorede O, Mantz C, Olazagasti C, Swami N, Kanan D, Alder L, Sridhar A, Bergerot CD, Bye B, Velazquez AI and Shaw AT (2025) Young lung cancer: from diagnosis to survivorship. Front. Oncol. 15:1570143. doi: 10.3389/fonc.2025.1570143
Received: 03 February 2025; Accepted: 09 June 2025;
Published: 26 June 2025.
Edited by:
Ilit Turgeman, Carmel Medical Center, IsraelReviewed by:
Nemany A.N. Hanafy, Kafrelsheikh University, EgyptRajat Thawani, Oregon Health and Science University, United States
Copyright © 2025 Florez, Kiel, Kaufman, LoPiccolo, Ricciuti, Morabito, Fakorede, Mantz, Olazagasti, Swami, Kanan, Alder, Sridhar, Bergerot, Bye, Velazquez and Shaw. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Narjust Florez, bmFyanVzdF9mbG9yZXpAZGZjaS5oYXJ2YXJkLmVkdQ==
 Narjust Florez1,2*
Narjust Florez1,2* Lauren Kiel
Lauren Kiel Biagio Ricciuti
Biagio Ricciuti Olayinka Fakorede
Olayinka Fakorede Coral Olazagasti
Coral Olazagasti Nishwant Swami
Nishwant Swami Duaa Kanan
Duaa Kanan Laura Alder
Laura Alder Arthi Sridhar
Arthi Sridhar Cristiane Decat Bergerot
Cristiane Decat Bergerot Bianca Bye
Bianca Bye Ana I. Velazquez
Ana I. Velazquez Alice T. Shaw
Alice T. Shaw