- 1Department of Immunology and Immunotherapy, School of Infection, Inflammation and Immunology, College of Medicine and Health, University of Birmingham, Birmingham, United Kingdom
- 2Department of Inflammation and Ageing, School of Infection, Inflammation and Immunology, College of Medicine and Health, University of Birmingham, Birmingham, United Kingdom
Bacterial infections remain a significant cause of morbidity and mortality globally. Compounding the issue is the rise of antimicrobial-resistant strains, which limit treatment options. Macrophages play key roles in the immunity and pathogenicity of intracellular infections, such as those caused by Mycobacterium tuberculosis and Salmonella. Recent advancements have enabled us to better understand how the host orchestrates immune responses to fight these infections and, specifically how the infected cell rewires its metabolism to face this challenge. The engagement of the host cell in specific metabolic pathways impacts cell function and behaviour, and ultimately, infection outcomes. In this perspective, we summarise key findings regarding the metabolic adaptations in macrophages induced by Mycobacterium tuberculosis and Salmonella infections. We also explore how cross-pathogen studies can deepen our insights into infection biology to improve therapeutic design.
Introduction
Bacterial infections are significant contributors to deaths worldwide. The most recent report from the World Health Organization highlights that in 2019, approximately 13.7 million deaths were due to 33 bacterial pathogens, collectively making bacterial infections the second leading cause of death globally (Ikuta et al., 2022). Amongst these, infections caused by intracellular bacteria are major contributors, including Mycobacterium tuberculosis (M.tb), Chlamydia trachomatis, Listeria monocytogenes and Salmonella enterica (World Health Organization, 2024; Kirk et al., 2015). In this review, we focus on M.tb and Salmonella enterica, since together they are responsible for almost 20 million cases yearly, with nearly 1.5 million deaths (World Health Organization, 2023). Moreover, both microorganisms share similarities in the immune response they elicit. For example, the induction of IFN-γ+ CD4 T cells and the formation of granuloma-like structures are hallmark features of infection. An inability to induce these signature responses leads to uncontrolled pathogen growth in both infections (Perez-Toledo et al., 2020; Ramirez-Alejo and Santos-Argumedo, 2014), which highlights the importance of macrophages in the containment of infection.
Elie Metchnikoff discovered macrophages and their phagocytic capacity at the end of the 19th century (Metchnikoff, 1905). Since then, cumulative research has evidenced the vast macrophage heterogeneity. Different macrophage subsets in distinct body locations and at a specific moment will exist within the spectrum of fighters against infection and guardians of homeostasis. Research on how intracellular pathogens infect and survive within macrophages can reveal novel insights into the fundamental biology of such host cells. Lately, the increased interest in understanding the contribution of metabolism in macrophage physiology has also started to shed light on the role of immunometabolism in infection control. Specifically, the process of autophagy enables cell survival and proper cellular function, bridging quality control, cellular metabolism and innate immune defences. Studies in different pathological contexts have started to elucidate how autophagy and glycolysis may modulate each other. In ageing, autophagy can reset glycolysis, boosting the regenerative potential of old hematopoietic stem cells (Dellorusso et al., 2024). Our understanding of the crosstalk between autophagy and metabolic rewiring in infection settings is limited. Studies on viral immunity have reported autophagy positively (Lee et al., 2020) and negatively (Oh et al., 2021) regulating glycolysis. In this paper, we will discuss current advances in metabolism in the context of Salmonella and M.tb infection to try and complement our understanding of infection biology by comparing two microorganisms that seem so different and are yet so similar in the responses they elicit.
M.tb rewires carbohydrate and lipid metabolism in infected macrophages
The lungs of M.tb-infected hosts present high glycolytic activity, evidenced by transcriptomic, proteomic and metabolomic studies quantifying expression of glycolytic enzymes and lactate, the end-product of glycolysis (reviewed in Llibre, Grudzinska et al. (Llibre et al., 2021). Studies using avirulent (HR37Ra, Bacille Calmette-Guerin) or γ-irradiated mycobacteria (e.g. iH37Rv) suggested a shift towards predominantly glycolytic macrophages upon infection (MOI 1-5) (Gleeson et al., 2016; Braverman et al., 2016). This was assessed by bioenergetic profiling (i.e. extracellular flux analysis via the Seahorse platform), and by performing lactate measurements in supernatants as a surrogate of glycolytic activity. More recent studies have shed light on the intricacies and nuances of this macrophage metabolic rewiring (Brown et al., 2025). Glycolysis is critical for effective macrophage TB immunity, and virulent M.tb has evolved specific prevention strategies (Cumming et al., 2018; Hackett et al., 2020; Olson et al., 2021). Mechanistically, glycolysis might be essential to produce specific antimycobacterial molecules such as IL1β or nitric oxide (NO) (Tannahill et al., 2013; Gleeson et al., 2016; Huang et al., 2018; Hackett et al., 2020) or to trigger and/or regulate specific immune defence pathways such as autophagy via lactate (O Maoldomhnaigh et al., 2021; Sun et al., 2023). This glycolytic engagement by the infected macrophage helps the host clear the infection, and the strategies employed by M.tb to circumvent this metabolic shift might be key for mycobacterial persistence.
Lipid metabolism is profoundly reprogrammed upon M.tb infection, inhibiting catabolic pathways and activating de novo lipid synthesis and uptake [reviewed in Laval et al. (Laval et al., 2021)]. This causes the development of a foamy, lipid-rich macrophage phenotype, which is a hallmark of TB granulomas (D’Avila et al., 2006; Peyron et al., 2008). There is strong evidence supporting the use of host lipids (cholesterol and fatty acids) by M.tb to eat (Pandey and Sassetti, 2008), survive (Daniel et al., 2011) and specifically impair key host immune defence mechanisms (e.g. autophagy, phagosome maturation) (Singh et al., 2012; Chandra and Kumar, 2016) (Figure 1). Therefore, on one hand, the mycobacterial-induced host lipid rewiring enables pathogen survival and immune evasion. On the other, lipid droplets and lipid mediators (e.g. prostaglandin E2, Leukotriene B4) are key in generating effective anti-mycobacterial responses within the macrophage (Mayer-Barber et al., 2014; Mayer-Barber and Sher, 2015; Bosch et al., 2020; Sorgi et al., 2020). Furthermore, cholesterol-derived compounds such as oxysterols and vitamin D can restrict mycobacterial growth (Bah et al., 2017; Varaksa et al., 2021; Foo et al., 2022). Thus, the host lipid metabolic adaptation in response to infection is also essential for effective protective responses. The specific factors that tilt the balance in favour of the host or the pathogen are still not fully understood. Other relevant metabolic adaptations (e.g. amino acids, TCA cycle intermediates, ions) are beyond the scope of this Perspective and have been excellently reviewed elsewhere (Neyrolles et al., 2015; Hackett and Sheedy, 2020).
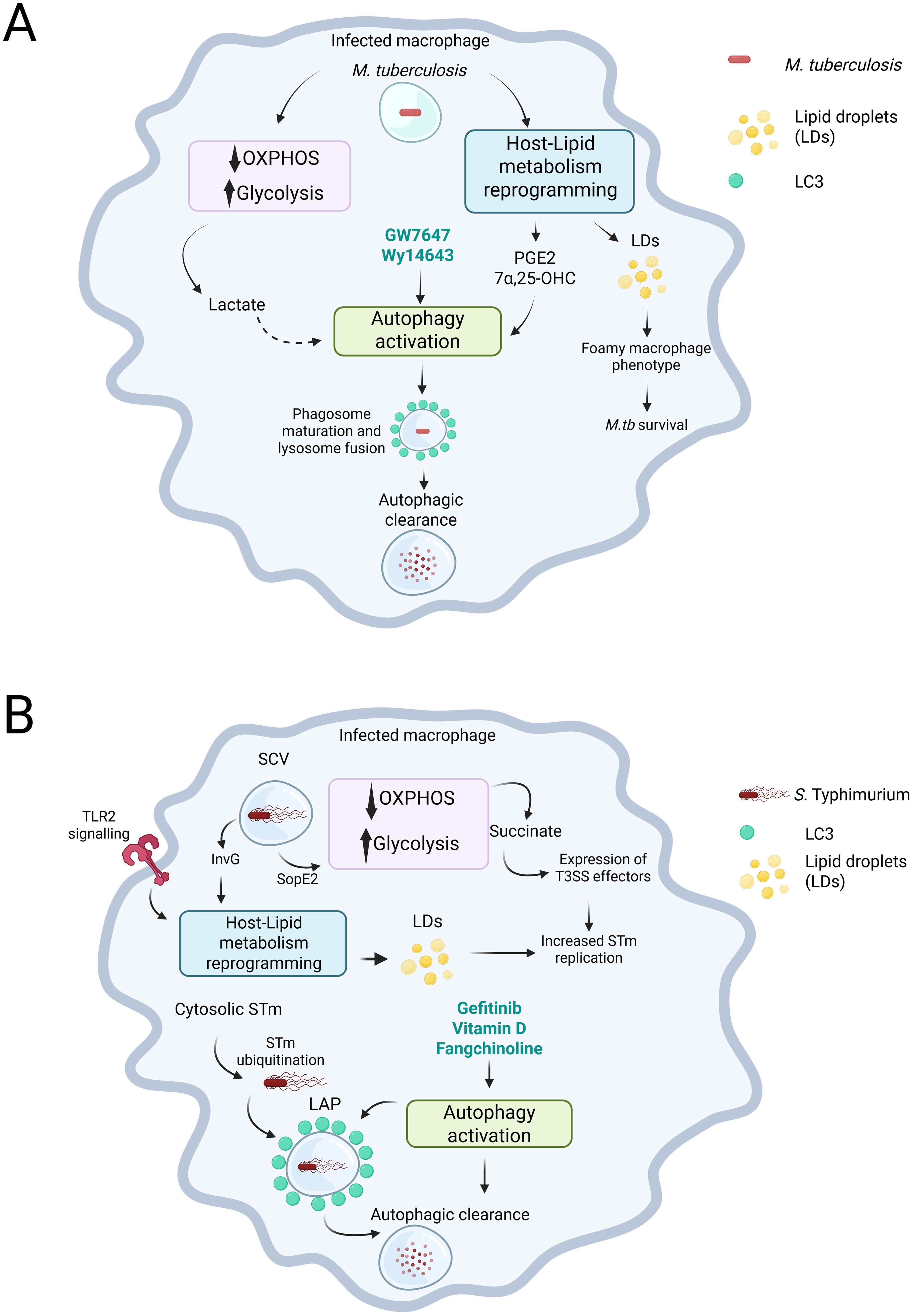
Figure 1. M. tuberculosis (M.tb) and S. Typhimurium (STm) induce metabolic changes in infected macrophages that impact infection outcomes. (A) In macrophages, infection with M.tb decreases the reliance on oxidative phosphorylation (OXPHOS) while enhancing glycolysis. This metabolic shift leads to increased levels of metabolites, such as lactate. Additionally, M.tb infection causes a reprogramming of lipid metabolism in the host. This creates a dichotomy: on one hand, the reprogramming results in the production of lipid droplets, which M.tb can exploit to survive within the infected macrophage. On the other hand, it can lead to the production of prostaglandin E2 (PGE2) or the oxysterol 7-α,25-dihydroxycholesterol (7-α,25-OHC), both of which can promote the activation of autophagy. Moreover, agonists of the PPARα receptor, such as GW7647 and Wy14643, can also stimulate autophagy. Although not directly shown in the context of M.tb infection, lactate has the potential to activate autophagy, which is an important process for bacterial control. (B) In the infected macrophage, STm is located within the Salmonella-containing vacuole (SCV). By expressing type III secretion system (T3SS) effectors, such as SopE2, STm alters the macrophage’s metabolism, shifting it towards glycolysis, which leads to an accumulation of succinate. STm senses the succinate and respond by promoting the expression of additional T3SS effectors to enhance its replication. Additionally, through TLR2 signalling and the action of another T3SS effector, InvG, STm can reprogram the host’s lipid metabolism to exploit it and further boosts its replication. Ultimately, compounds that promote autophagy, such as gefitinib, Vitamin D, and fangchinoline, can promote bacterial killing through the induction of LC3-associated Phagocytosis (LAP). Created in BioRender. Perez toledo, M. (2025) https://BioRender.com/y2oepra.
The new macrophage metabolic landscape triggered by infection results in changes regarding the accumulation and availability of specific metabolites. These are the primary sources of post-translational modifications and epigenetic regulation, which regulate essential cellular processes such as autophagy (Sun et al., 2023; Huang et al., 2024; Shu et al., 2023; Nieto-Torres et al., 2023; Park et al., 2022). Thus, the predominance of specific metabolic pathways will dictate gene expression profiles that may impact cell function and behaviour and, ultimately, infection outcome.
Salmonella rewires carbohydrate and lipid metabolism in infected macrophages
Salmonella enterica are important pathogens for human and animal health. Notably, serovars Typhimurium (STm) and Enteritidis are linked to non-typhoidal salmonellosis (NTS), which occasionally develops into invasive disease (iNTS), especially in young children and immunocompromised individuals, with mortality rates reaching up to 25% (Feasey et al., 2012). As with M. tb, the interaction between STm and macrophages has a profound impact on the successful establishment of infection and the outcome for the host.
Recent research has highlighted how STm affects metabolic reprogramming and the subsequent impacts on infection outcomes. In vitro, through the Salmonella Pathogenicity Island-I (SPI-I) effector SopE2, STm represses serine synthesis in peritoneal macrophages. The result is an increase in glycolysis, which is accompanied by a significant accumulation of glycolytic products, such as pyruvate, lactate, and phosphoglycerates, as well as TCA intermediates, such as fumarate, α-ketoglutarate, and succinate (Jiang et al., 2021). The bacteria can then uptake some of these intermediates and use them as carbon sources, which results in increased intracellular replication. Moreover, this effect has also been shown in vivo, where mice infected with STm and supplemented with lactate showed increased bacterial burdens in the spleen and liver 3 days post-infection (Wang et al., 2023). The authors attributed this effect to a switch of macrophages towards an M2-like anti-inflammatory phenotype, which has been associated with more permissiveness to STm infection (Pham et al., 2020; Panagi et al., 2020). Recent work by Rosenberg et al. reported that succinate, which accumulates due to the macrophage glycolytic shift and the concomitant truncation of the TCA cycle, is sensed by STm and induces the expression of type 3 secretion system (T3SS) effectors, such as sseL, steC, ssrB, and pipB2, resulting in increased bacterial burdens (Rosenberg et al., 2021). However, not everything is bad news for the host. Khatib-Massalha et al. showed that infection with STm increases lactate levels in the bone marrow. This increase leads to increased permeability of the bone marrow, thus increasing neutrophil mobilisation through lactate signalling via the GPR81 receptor (Khatib-Massalha et al., 2020).
Similarly to what has been observed with M.tb infection in macrophages, STm can also interact with the mammalian lipid metabolism to induce the production of lipid droplets (LDs). Experiments with monocyte-derived human macrophages supplemented with oleic acid showed that STm infection induced the production of LDs shortly after infection. However, this did not result in better control of bacterial infection (Bosch et al., 2020). A more recent study elegantly dissected the mechanism of LDs formation following STm infection, which required the T3SS effector invG and TLR2 signalling (Kiarely Souza et al., 2022). Moreover, this work also showed that the bacteria may benefit from forming LDs, as pharmacologically inhibiting key enzymes involved in forming LDs reduced bacterial burdens (Figure 1) (Kiarely Souza et al., 2022).
So far, most of the evidence shown here suggests a deleterious role for macrophages engaging in glycolysis in the outcome of STm infection, contrasting with the existing evidence in M.tb infection. However, this could be the result of an oversimplification of the models used, e.g. Most studies shown here use human or mouse-derived bone marrow macrophages. As not all macrophages are the same (Huang et al., 2018; Gordon and Taylor, 2005; Heieis et al., 2023; Russell et al., 2025), future studies could benefit from considering how the intrinsic differences in macrophage populations can affect their response to metabolic changes induced by pathogens. However, both STm and M.tb appear to exploit the changes in lipid metabolism within infected macrophages to their advantage. Further research on this shared pathway will be crucial for identifying potential targets for infection control.
Autophagy in the context of intracellular pathogens such as STm and M.tb
In the previous sections, we have summarised some key immune-metabolic events in host macrophages after Salmonella and M.tb infection, including changes in glycolysis and lipid metabolism. Immune metabolism comprises the metabolic adaptations of the host cell to face challenges such as infection. Additionally, it covers how cellular engagement on particular metabolic pathways results in the accumulation of specific metabolites (e.g. lactate, succinate, prostaglandins, oxysterols), which can directly act as signalling molecules, fuel, and/or substrates for post-translational and epigenetic modifications. Furthermore, metabolism and immunity converge in essential biological processes to regulate and tackle cellular stress. The process of autophagy perfectly illustrates this. Also known as self-eating, this cellular housekeeping strategy clears the cytoplasm from waste and defective macromolecules and organelles, acting as a quality control for the cell and enabling survival. Autophagy is not only key for metabolic homeostasis but also constitutes an innate immune defence mechanism of paramount relevance for controlling intracellular pathogens, directing them to autophagolysosomal degradation.
The role of autophagy as a key mechanism of M.tb defence within macrophages was first described using a combination of RAW cells (Herb et al., 2024) and murine and human monocyte-derived macrophages (Gutierrez et al., 2004). Since then, different studies have assessed the interplay and function of canonical and non-canonical autophagy in the immune responses of macrophages to M.tb infection. Evidence for both protective (Golovkine et al., 2023) and non-protective (Kimmey et al., 2015) roles of autophagy in the outcome of M.tb infection has been described. Similarly, autophagy has been reported to be an essential mechanism for intracellular control of STm. STm is targeted by autophagy when bacteria escape the Salmonella-containing vacuole (SCV) into the cytosol. This triggers the ubiquitination of cytosolic bacteria and the recruitment of LC3 and other autophagy proteins, restricting intracellular bacterial growth (Birmingham et al., 2006). However, STm can also exploit the autophagy machinery to promote SCV maturation, thus inducing the activation of T3SS effectors that supports bacterial replication (Figure 1) (Kreibich et al., 2015).
The macrophage metabolic rewiring that occurs upon M.tb and Stm infection has the potential to modulate autophagy. For instance, the formation and maturation of autophagosomes, as well as endosome-lysosomal degradation need lactylation of specific core autophagy proteins (e.g. PIK3C3/VPS34, TFEB, a master regulator of autophagy) (Sun et al., 2023; Huang et al., 2024). Although not specifically assessed in the context of infection, lactate has been shown to promote autophagy in different contexts, including retinal degradation (via upregulation of the LCII/II ratio), high intensity interval training (via ERK1/2/mTOR/p70S6K activation, and neurodegeneration (through cytosol acidification) (Zou et al., 2023; Pirani et al., 2024; Fedotova et al., 2022). Distinct lipid mediators also have the potential to modulate autophagy. For example, LTB4 suppressed autophagy in mouse lung macrophages (Zhang et al., 2019a), and PGE2 induced autophagy in PBMCs from healthy donors and TB patients stimulated with M.tb cell lysate (Pellegrini et al., 2021). The oxysterol 7α,25-dihydroxycholesterol (7α,25-OHC) reduced mycobacterial growth through increased autophagy via GPR183 signalling in human monocytes (Bartlett et al., 2020). These examples illustrate how infection triggers host cell’s metabolic adaptations, resulting in changes in the intracellular pool of available metabolites which can regulate key biological/immunological processes such as autophagy.
Researchers from both the TB and Salmonella fields have exploited the zebrafish model to elucidate both mechanisms of bacterial virulence and host protective responses. The autophagy receptors p62 and Optineurin were shown to be essential for protection against mycobacterial infection and to act independently (Zhang et al., 2019b; Xie and Meijer, 2023). However, the same p62 and Optineurin receptors were shown to be interdependent when promoting autophagy in the context of Salmonella infection. Lc3-associated phagocytosis (LAP) was identified as the primary autophagy-related process behind macrophage control for STm infection (Masud et al., 2019a, 2019b). Although the specific role of LAP in the context of mycobacterial infection has not been tested explicitly in the zebrafish model, LAP could not clear M.tb infection in a mouse model of TB (Koster et al., 2017). We can learn much about the biological nuances of crucial immune defence mechanisms (i.e., autophagy) by using the same model but different pathogens or distinct models with the same pathogen. Although further research is needed, distinct roles for specific components of the autophagy machinery might be pathogen-specific, knowledge that could be harnessed therapeutically.
Discussion
The threatening increase in antimicrobial resistance has prompted the search for alternative therapeutic strategies, including host-directed therapies (HDTs). In contrast to conventional antibiotic drugs, which target the pathogen, HDTs modulate specific host factors to pursue various aims. For instance, we might want to dampen inflammation to prevent immune-driven tissue pathology, enhance the bactericidal capacity of the infected macrophage, or disrupt the granuloma structure to expose the bacteria to conventional antibiotic treatments (Young et al., 2020). HDTs have dramatically changed the treatment and outcomes of specific cancers (i.e. checkpoint inhibitors such as anti-PD1 or anti-CTLA4 antibodies) (Robert, 2020); unfortunately, their use in infectious diseases has limitedly reached clinical practice.
The first step in designing and using novel HDTs is to have an accurate mechanistic understanding of the host pathway that needs targeting. For instance, anti-mycobacterial autophagy can be promoted via modulation of different host factors, including nuclear receptors such as peroxisome proliferator-activated receptor (PPAR) and estrogen-related receptor (ERR) (Kim et al., 2019), vitamin D (Yuk et al., 2009; Campbell and Spector, 2012) and AMPK (Yang et al., 2014; Singhal et al., 2014; Parihar et al., 2014; Guerra-De-Blas et al., 2019). Specifically, the compounds GW7647 and Wy14643, which are PPARα activators, have been shown to successfully reduce bacterial burdens in M. tb-challenged bone marrow derived macrophages, presumably through the activation of autophagy and increased phagosome maturation (Kim et al., 2017). Although these specific PPAR agonists have not been evaluated in the context of STm infection, there is evidence that suggests that PPAR inhibition instead of activation results in better bacterial control and reduced immunopathology (Taddeo et al., 2024). However, the use of an inverse agonist of ERRγ (GSK5182) resulted in lower intracellular burdens in STm infected macrophages, although this was the result of iron metabolism modulation, which is out of the scope of our paper (Kim et al., 2014). Vitamin D induced intestinal epithelial autophagy during Salmonella infection (Huang, 2016; Huang and Huang, 2021). The Epidermal growth factor receptor (EGFR) inhibitor Gefitinib reduced Salmonella survival in macrophages and mice via host metabolic reprogramming, including autophagy (Sadhu et al., 2021). Also, fangchinoline, a phytochemical with anti-proliferative properties, promoted Salmonella killing in vitro and in vivo through autophagy (He et al., 2025). While most studies were performed in vitro using a combination of monocytic cell lines and primary mouse or human macrophages, some of them used in vivo models, including mice and zebrafish (reviewed in Adikesavalu et al. and Wu et al. (Wu et al., 2020; Adikesavalu et al., 2021). Furthermore, molecules such as vitamin D and AMPK modulators, such as metformin, have been and continue to be tested in clinical trials. Some (not all) studies show promising results in using these compounds in combination with conventional antibiotic treatment to improve TB outcomes (Salahuddin et al., 2013; Padmapriydarsini et al., 2022; Sutter et al., 2022; Biswal, 2021). Still, more research is needed to find effective HDTs to aid the fight against TB.
The terminology we use in science significantly shapes our understanding of the entities we study. Terms like T cells, B cells, and macrophages can limit our perspective, especially when new cell types are discovered that do not fit into existing classifications. This issue also affects how we approach the study of diseases. In cancer, for instance, concentrating on tumour types based on their tissue location may be less beneficial than focusing on the underlying mechanisms that cause the cancer, such as mutations in the p53 or BRCA genes. Consequently, therapies aimed at addressing the pathological mechanisms driven by mutations in p53 could potentially be applied to various cancer types associated with this same mutation. A comprehensive, mechanistic understanding of the metabolic rewiring undergone by host macrophages upon infection, and how these shape protective responses, is a first key step for the identification of novel host therapeutic targets. Furthermore, the way we use language regarding pathogens and the host cellular pathways they elicit, could also open new avenues for novel treatments. For instance, instead of referring to infections caused by Mycobacteria, Salmonella, Burkholderia, or Leishmania, we could categorise them, as pathogens that promote lipid droplet formation to their advantage, (e.g. STm and M. tb), or as pathogens that are LAP-susceptible or LAP-resistant. This shift in how we use language, driven by a deeper understanding of the fundamental biological changes triggered by infection, could revolutionize the development or repurposing of host-directed therapies for infections that continue to claim millions of lives worldwide each year.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
MP: Conceptualization, Writing – original draft, Writing – review & editing. AL: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adikesavalu, H., Gopalaswamy, R., Kumar, A., Ranganathan, U. D., and Shanmugam, S. (2021). Autophagy Induction as a Host-Directed Therapeutic Strategy against Mycobacterium tuberculosis Infection. Med. (Kaunas) 57. doi: 10.3390/medicina57060522
Bah, S. Y., Dickinson, P., Forster, T., Kampmann, B., and Ghazal, P. (2017). Immune oxysterols: Role in mycobacterial infection and inflammation. J. Steroid Biochem. Mol. Biol. 169, 152–163. doi: 10.1016/j.jsbmb.2016.04.015
Bartlett, S., Gemiarto, A. T., Ngo, M. D., Sajiir, H., Hailu, S., Sinha, R., et al. (2020). GPR183 regulates interferons, autophagy, and bacterial growth during mycobacterium tuberculosis infection and is associated with TB disease severity. Front. Immunol. 11, 601534. doi: 10.3389/fimmu.2020.601534
Birmingham, C. L., Smith, A. C., Bakowski, M. A., Yoshimori, T., and Brumell, J. H. (2006). Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J. Biol. Chem. 281, 11374–11383. doi: 10.1074/jbc.M509157200
Biswal, S. P. I. S. S. B. (2021). Metformin as an adjunct in treatment of Pulmonary Tuberculosis in Non-Diabetic patients. Eur. Respir. J. 58. doi: 10.1183/13993003.congress-2021.OA1601
Bosch, M., Sanchez-Alvarez, M., Fajardo, A., Kapetanovic, R., Steiner, B., Dutra, F., et al. (2020). Mammalian lipid droplets are innate immune hubs integrating cell metabolism and host defense. Science 370. doi: 10.1126/science.aay8085
Braverman, J., Sogi, K. M., Benjamin, D., Nomura, D. K., and Stanley, S. A. (2016). HIF-1alpha is an essential mediator of IFN-gamma-dependent immunity to mycobacterium tuberculosis. J. Immunol. 197, 1287–1297. doi: 10.4049/jimmunol.1600266
Brown, K., Walsh, A., Yennemadi, A., O’Leary, S., O’Sullivan, M., Nadarajan, P., et al. (2025). Mycobacterium tuberculosis induces warburg metabolism in human alveolar macrophages: A transcriptomic analysis. Am. J. Respir. Cell Mol. Biol. 72, 597–602. doi: 10.1165/rcmb.2024-0268LE
Campbell, G. R. and Spector, S. A. (2012). Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PloS Pathog. 8, e1002689. doi: 10.1371/journal.ppat.1002689
Chandra, P. and Kumar, D. (2016). Selective autophagy gets more selective: Uncoupling of autophagy flux and xenophagy flux in Mycobacterium tuberculosis-infected macrophages. Autophagy 12, 608–609. doi: 10.1080/15548627.2016.1139263
Cumming, B. M., Addicott, K. W., Adamson, J. H., and Steyn, A. J. (2018). Mycobacterium tuberculosis induces decelerated bioenergetic metabolism in human macrophages. Elife 7. doi: 10.7554/eLife.39169.018
D’Avila, H., Melo, R. C., Parreira, G. G., Werneck-Barroso, E., Castro-Faria-Neto, H. C., and Bozza, P. T. (2006). Mycobacterium bovis bacillus Calmette-Guerin induces TLR2-mediated formation of lipid bodies: intracellular domains for eicosanoid synthesis in vivo. J. Immunol. 176, 3087–3097. doi: 10.4049/jimmunol.176.5.3087
Daniel, J., Maamar, H., Deb, C., Sirakova, T. D., and Kolattukudy, P. E. (2011). Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PloS Pathog. 7, e1002093. doi: 10.1371/journal.ppat.1002093
Dellorusso, P. V., Proven, M. A., Calero-Nieto, F. J., Wang, X., Mitchell, C. A., Hartmann, F., et al. (2024). Autophagy counters inflammation-driven glycolytic impairment in aging hematopoietic stem cells. Cell Stem Cell 31, 1020–1037, e9. doi: 10.1016/j.stem.2024.04.020
Feasey, N. A., Dougan, G., Kingsley, R. A., Heyderman, R. S., and Gordon, M. A. (2012). Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379, 2489–2499. doi: 10.1016/S0140-6736(11)61752-2
Fedotova, E. I., Dolgacheva, L. P., Abramov, A. Y., and Berezhnov, A. V. (2022). Lactate and pyruvate activate autophagy and mitophagy that protect cells in toxic model of parkinson’s disease. Mol. Neurobiol. 59, 177–190. doi: 10.1007/s12035-021-02583-8
Foo, C. X., Bartlett, S., and Ronacher, K. (2022). Oxysterols in the immune response to bacterial and viral infections. Cells 11. doi: 10.3390/cells11020201
Gleeson, L. E., Sheedy, F. J., Palsson-McDermott, E. M., Triglia, D., O’Leary, S. M., O’Sullivan, M. P., et al. (2016). Cutting edge: mycobacterium tuberculosis induces aerobic glycolysis in human alveolar macrophages that is required for control of intracellular bacillary replication. J. Immunol. 196, 2444–2449. doi: 10.4049/jimmunol.1501612
Golovkine, G. R., Roberts, A. W., Morrison, H. M., Rivera-Lugo, R., McCall, R. M., Nilsson, H., et al. (2023). Autophagy restricts Mycobacterium tuberculosis during acute infection in mice. Nat. Microbiol. 8, 819–832. doi: 10.1038/s41564-023-01354-6
Gordon, S. and Taylor, P. R. (2005). Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964. doi: 10.1038/nri1733
Guerra-De-Blas, P. D. C., Bobadilla-Del-Valle, M., Sada-Ovalle, I., Estrada-Garcia, I., Torres-Gonzalez, P., Lopez-Saavedra, A., et al. (2019). Simvastatin enhances the immune response against mycobacterium tuberculosis. Front. Microbiol. 10, 2097. doi: 10.3389/fmicb.2019.02097
Gutierrez, M. G., Master, S. S., Singh, S. B., Taylor, G. A., Colombo, M. I., and Deretic, V. (2004). Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119, 753–766. doi: 10.1016/j.cell.2004.11.038
Hackett, E. E., Charles-Messance, H., O’Leary, S. M., Gleeson, L. E., Munoz-Wolf, N., Case, S., et al. (2020). Mycobacterium tuberculosis Limits Host Glycolysis and IL-1beta by Restriction of PFK-M via MicroRNA-21. Cell Rep. 30, 124–136. doi: 10.1016/j.celrep.2019.12.015
Hackett, E. E. and Sheedy, F. J. (2020). An army marches on its stomach: metabolic intermediates as antimicrobial mediators in mycobacterium tuberculosis infection. Front. Cell Infect. Microbiol. 10, 446. doi: 10.3389/fcimb.2020.00446
He, M., Wu, H., Xu, T., Zhao, Y., Wang, Z., and Liu, Y. (2025). Fangchinoline eliminates intracellular Salmonella by enhancing lysosomal function via the AMPK-mTORC1-TFEB axis. J. Adv. Res. doi: 10.1016/j.jare.2025.01.015
Heieis, G. A., Patente, T. A., Almeida, L., Vrieling, F., Tak, T., Perona-Wright, G., et al. (2023). Metabolic heterogeneity of tissue-resident macrophages in homeostasis and during helminth infection. Nat. Commun. 14, 5627. doi: 10.1038/s41467-023-41353-z
Herb, M., Schatz, V., Hadrian, K., Hos, D., Holoborodko, B., Jantsch, J., et al. (2024). Macrophage variants in laboratory research: most are well done, but some are RAW. Front. Cell Infect. Microbiol. 14, 1457323. doi: 10.3389/fcimb.2024.1457323
Huang, F. C. (2016). Vitamin D differentially regulates Salmonella-induced intestine epithelial autophagy and interleukin-1beta expression. World J. Gastroenterol. 22, 10353–10363. doi: 10.3748/wjg.v22.i47.10353
Huang, F. C. and Huang, S. C. (2021). Active vitamin D3 attenuates the severity of Salmonella colitis in mice by orchestrating innate immunity. Immun. Inflammation Dis. 9, 481–491. doi: 10.1002/iid3.v9.2
Huang, Y., Luo, G., Peng, K., Song, Y., Wang, Y., Zhang, H., et al. (2024). Lactylation stabilizes TFEB to elevate autophagy and lysosomal activity. J. Cell Biol. 223. doi: 10.1083/jcb.202308099
Huang, L., Nazarova, E. V., Tan, S., Liu, Y., and Russell, D. G. (2018). Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J. Exp. Med. 215, 1135–1152. doi: 10.1084/jem.20172020
Ikuta, K. S. (2022). Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 400, 2221–2248. doi: 10.1016/S0140-6736(22)02185-7
Jiang, L., Wang, P., Song, X., Zhang, H., Ma, S., Wang, J., et al. (2021). Salmonella Typhimurium reprograms macrophage metabolism via T3SS effector SopE2 to promote intracellular replication and virulence. Nat. Commun. 12, 879. doi: 10.1038/s41467-021-21186-4
Khatib-Massalha, E., Bhattacharya, S., Massalha, H., Biram, A., Golan, K., Kollet, O., et al. (2020). Lactate released by inflammatory bone marrow neutrophils induces their mobilization via endothelial GPR81 signaling. Nat. Commun. 11, 3547. doi: 10.1038/s41467-020-17402-2
Kiarely Souza, E., Pereira-Dutra, F. S., Rajao, M. A., Ferraro-Moreira, F., Goltara-Gomes, T. C., Cunha-Fernandes, T., et al. (2022). Lipid droplet accumulation occurs early following Salmonella infection and contributes to intracellular bacterial survival and replication. Mol. Microbiol. 117, 293–306. doi: 10.1111/mmi.v117.2
Kim, D. K., Jeong, J. H., Lee, J. M., Kim, K. S., Park, S. H., Kim, Y. D., et al. (2014). Inverse agonist of estrogen-related receptor gamma controls Salmonella typhimurium infection by modulating host iron homeostasis. Nat. Med. 20, 419–424. doi: 10.1038/nm.3483
Kim, Y. S., Lee, H. M., Kim, J. K., Yang, C. S., Kim, T. S., Jung, M., et al. (2017). PPAR-alpha activation mediates innate host defense through induction of TFEB and lipid catabolism. J. Immunol. 198, 3283–3295. doi: 10.4049/jimmunol.1601920
Kim, Y. S., Silwal, P., Kim, S. Y., Yoshimori, T., and Jo, E. K. (2019). Autophagy-activating strategies to promote innate defense against mycobacteria. Exp. Mol. Med. 51, 1–10. doi: 10.1038/s12276-019-0290-7
Kimmey, J. M., Huynh, J. P., Weiss, L. A., Park, S., Kambal, A., Debnath, J., et al. (2015). Unique role for ATG5 in neutrophil-mediated immunopathology during M. tuberculosis infection. Nature 528, 565–569.
Kirk, M. D., Pires, S. M., Black, R. E., Caipo, M., Crump, J. A., Devleesschauwer, B., et al. (2015). World health organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases 2010: A data synthesis. PloS Med. 12, e1001921. doi: 10.1371/journal.pmed.1001921
Koster, S., Upadhyay, S., Chandra, P., Papavinasasundaram, K., Yang, G., Hassan, A., et al. (2017). Mycobacterium tuberculosis is protected from NADPH oxidase and LC3-associated phagocytosis by the LCP protein CpsA. Proc. Natl. Acad. Sci. U.S.A. 114, E8711–E8720.
Kreibich, S., Emmenlauer, M., Fredlund, J., Ramo, P., Munz, C., Dehio, C., et al. (2015). Autophagy proteins promote repair of endosomal membranes damaged by the salmonella type three secretion system 1. Cell Host Microbe 18, 527–537. doi: 10.1016/j.chom.2015.10.015
Laval, T., Chaumont, L., and Demangel, C. (2021). Not too fat to fight: The emerging role of macrophage fatty acid metabolism in immunity to Mycobacterium tuberculosis. Immunol. Rev. 301, 84–97. doi: 10.1111/imr.v301.1
Lee, Y. R., Wu, S. Y., Chen, R. Y., Lin, Y. S., Yeh, T. M., and Liu, H. S. (2020). Regulation of autophagy, glucose uptake, and glycolysis under dengue virus infection. Kaohsiung J. Med. Sci. 36, 911–919. doi: 10.1002/kjm2.v36.11
Llibre, A., Grudzinska, F. S., O’Shea, M. K., Duffy, D., Thickett, D. R., Mauro, C., et al. (2021). Lactate cross-talk in host-pathogen interactions. Biochem. J. 478, 3157–3178. doi: 10.1042/BCJ20210263
Masud, S., Prajsnar, T. K., Torraca, V., Lamers, G. E. M., Benning, M., van der Vaart, M., et al. (2019a). Macrophages target Salmonella by Lc3-associated phagocytosis in a systemic infection model. Autophagy 15, 796–812. doi: 10.1080/15548627.2019.1569297
Masud, S., van der Burg, L., Storm, L., Prajsnar, T. K., and Meijer, A. H. (2019b). Rubicon-dependent lc3 recruitment to salmonella-containing phagosomes is a host defense mechanism triggered independently from major bacterial virulence factors. Front. Cell Infect. Microbiol. 9, 279. doi: 10.3389/fcimb.2019.00279
Mayer-Barber, K. D., Andrade, B. B., Oland, S. D., Amaral, E. P., Barber, D. L., Gonzales, J., et al. (2014). Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 511, 99–103. doi: 10.1038/nature13489
Mayer-Barber, K. D. and Sher, A. (2015). Cytokine and lipid mediator networks in tuberculosis. Immunol. Rev. 264, 264–275. doi: 10.1111/imr.2015.264.issue-1
Metchnikoff, É. (1905). Immunity in infective diseases (Cambridge, United Kingdom: Cambridge, University Press).
Neyrolles, O., Wolschendorf, F., Mitra, A., and Niederweis, M. (2015). Mycobacteria, metals, and the macrophage. Immunol. Rev. 264, 249–263. doi: 10.1111/imr.2015.264.issue-1
Nieto-Torres, J. L., Zaretski, S., Liu, T., Adams, P. D., and Hansen, M. (2023). Post-translational modifications of ATG8 proteins - an emerging mechanism of autophagy control. J. Cell Sci. 136. doi: 10.1242/jcs.259725
Oh, D. S., Park, J. H., Jung, H. E., Kim, H. J., and Lee, H. K. (2021). Autophagic protein ATG5 controls antiviral immunity via glycolytic reprogramming of dendritic cells against respiratory syncytial virus infection. Autophagy 17, 2111–2127. doi: 10.1080/15548627.2020.1812218
Olson, G. S., Murray, T. A., Jahn, A. N., Mai, D., Diercks, A. H., Gold, E. S., et al. (2021). Type I interferon decreases macrophage energy metabolism during mycobacterial infection. Cell Rep. 35, 109195. doi: 10.1016/j.celrep.2021.109195
O Maoldomhnaigh, C., Cox, D. J., Phelan, J. J., Mitermite, M., Murphy, D. M., Leisching, G., et al. (2021). Lactate alters metabolism in human macrophages and improves their ability to kill mycobacterium tuberculosis. Front. Immunol. 12, 663695. doi: 10.3389/fimmu.2021.663695
Padmapriydarsini, C., Mamulwar, M., Mohan, A., Shanmugam, P., Gomathy, N. S., Mane, A., et al. (2022). Randomized trial of metformin with anti-tuberculosis drugs for early sputum conversion in adults with pulmonary tuberculosis. Clin. Infect. Dis. 75, 425–434. doi: 10.1093/cid/ciab964
Panagi, I., Jennings, E., Zeng, J., Gunster, R. A., Stones, C. D., Mak, H., et al. (2020). Salmonella effector steE converts the mammalian serine/threonine kinase GSK3 into a tyrosine kinase to direct macrophage polarization. Cell Host Microbe 27, 41–53. doi: 10.1016/j.chom.2019.11.002
Pandey, A. K. and Sassetti, C. M. (2008). Mycobacterial persistence requires the utilization of host cholesterol. Proc. Natl. Acad. Sci. U.S.A. 105, 4376–4380. doi: 10.1073/pnas.0711159105
Parihar, S. P., Guler, R., Khutlang, R., Lang, D. M., Hurdayal, R., Mhlanga, M. M., et al. (2014). Statin therapy reduces the mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. J. Infect. Dis. 209, 754–763. doi: 10.1093/infdis/jit550
Park, N. Y., Jo, D. S., and Cho, D. H. (2022). Post-translational modifications of ATG4B in the regulation of autophagy. Cells 11. doi: 10.3390/cells11081330
Pellegrini, J. M., Martin, C., Morelli, M. P., Schander, J. A., Tateosian, N. L., Amiano, N. O., et al. (2021). PGE2 displays immunosuppressive effects during human active tuberculosis. Sci. Rep. 11, 13559. doi: 10.1038/s41598-021-92667-1
Perez-Toledo, M., Beristain-Covarrubias, N., Channell, W. M., Hitchcock, J. R., Cook, C. N., Coughlan, R. E., et al. (2020). Mice deficient in T-bet form inducible NO synthase-positive granulomas that fail to constrain salmonella. J. Immunol. 205, 708–719. doi: 10.4049/jimmunol.2000089
Peyron, P., Vaubourgeix, J., Poquet, Y., Levillain, F., Botanch, C., Bardou, F., et al. (2008). Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PloS Pathog. 4, e1000204. doi: 10.1371/journal.ppat.1000204
Pham, T. H. M., Brewer, S. M., Thurston, T., Massis, L. M., Honeycutt, J., Lugo, K., et al. (2020). Salmonella-driven polarization of granuloma macrophages antagonizes TNF-mediated pathogen restriction during persistent infection. Cell Host Microbe 27, 54–67. doi: 10.1016/j.chom.2019.11.011
Pirani, H., Soltany, A., Hossein Rezaei, M., Khodabakhshi Fard, A., Nikooie, R., Khoramipoor, K., et al. (2024). Lactate-induced autophagy activation: unraveling the therapeutic impact of high-intensity interval training on insulin resistance in type 2 diabetic rats. Sci. Rep. 14, 1108. doi: 10.1038/s41598-023-50589-0
Ramirez-Alejo, N. and Santos-Argumedo, L. (2014). Innate defects of the IL-12/IFN-gamma axis in susceptibility to infections by mycobacteria and salmonella. J. Interferon Cytokine Res. 34, 307–317. doi: 10.1089/jir.2013.0050
Robert, C. (2020). A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 11, 3801. doi: 10.1038/s41467-020-17670-y
Rosenberg, G., Yehezkel, D., Hoffman, D., Mattioli, C. C., Fremder, M., Ben-Arosh, H., et al. (2021). Host succinate is an activation signal for Salmonella virulence during intracellular infection. Science 371, 400–405. doi: 10.1126/science.aba8026
Russell, D. G., Simwela, N. V., Mattila, J. T., Flynn, J., Mwandumba, H. C., and Pisu, D. (2025). How macrophage heterogeneity affects tuberculosis disease and therapy. Nat. Rev. Immunol. 25, 370–384. doi: 10.1038/s41577-024-01124-3
Sadhu, S., Rizvi, Z. A., Pandey, R. P., Dalal, R., Rathore, D. K., Kumar, B., et al. (2021). Gefitinib results in robust host-directed immunity against salmonella infection through proteo-metabolomic reprogramming. Front. Immunol. 12, 648710. doi: 10.3389/fimmu.2021.648710
Salahuddin, N., Ali, F., Hasan, Z., Rao, N., Aqeel, M., and Mahmood, F. (2013). Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis’. BMC Infect. Dis. 13, 22. doi: 10.1186/1471-2334-13-22
Shu, F., Xiao, H., Li, Q. N., Ren, X. S., Liu, Z. G., Hu, B. W., et al. (2023). Epigenetic and post-translational modifications in autophagy: biological functions and therapeutic targets. Signal Transduct Target Ther. 8, 32. doi: 10.1038/s41392-022-01300-8
Singh, V., Jamwal, S., Jain, R., Verma, P., Gokhale, R., and Rao, K. V. (2012). Mycobacterium tuberculosis-driven targeted recalibration of macrophage lipid homeostasis promotes the foamy phenotype. Cell Host Microbe 12, 669–681. doi: 10.1016/j.chom.2012.09.012
Singhal, A., Jie, L., Kumar, P., Hong, G. S., Leow, M. K., Paleja, B., et al. (2014). Metformin as adjunct antituberculosis therapy. Sci. Transl. Med. 6, 263ra159. doi: 10.1126/scitranslmed.3009885
Sorgi, C. A., Soares, E. M., Rosada, R. S., Bitencourt, C. S., Zoccal, K. F., Pereira, P. A. T., et al. (2020). Eicosanoid pathway on host resistance and inflammation during Mycobacterium tuberculosis infection is comprised by LTB(4) reduction but not PGE(2) increment. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165574. doi: 10.1016/j.bbadis.2019.165574
Sun, W., Jia, M., Feng, Y., and Cheng, X. (2023). Lactate is a bridge linking glycolysis and autophagy through lactylation. Autophagy 19, 3240–3241. doi: 10.1080/15548627.2023.2246356
Sutter, A., Landis, D., and Nugent, K. (2022). The potential role for metformin in the prevention and treatment of tuberculosis. J. Thorac. Dis. 14, 1758–1765. doi: 10.21037/jtd-22-39
Taddeo, J. R., Wilson, N., Kowal, A., Beld, J., Klein-Szanto, A., Tukel, C., et al. (2024). PPARalpha exacerbates Salmonella Typhimurium infection by modulating the immunometabolism and macrophage polarization. Gut Microbes 16, 2419567. doi: 10.1080/19490976.2024.2419567
Tannahill, G. M., Curtis, A. M., Adamik, J., Palsson-McDermott, E. M., McGettrick, A. F., Goel, G., et al. (2013). Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 496, 238–242. doi: 10.1038/nature11986
Varaksa, T., Bukhdruker, S., Grabovec, I., Marin, E., Kavaleuski, A., Gusach, A., et al. (2021). Metabolic fate of human immunoactive sterols in mycobacterium tuberculosis. J. Mol. Biol. 433, 166763. doi: 10.1016/j.jmb.2020.166763
Wang, X., Yang, B., Ma, S., Yan, X., Ma, S., Sun, H., et al. (2023). Lactate promotes Salmonella intracellular replication and systemic infection via driving macrophage M2 polarization. Microbiol. Spectr. 11, e0225323. doi: 10.1128/spectrum.02253-23
World Health Organization (2023). Typhoid. Available online at: https://www.who.int/news-room/fact-sheets/detail/typhoid (Accessed 9th May 2025).
World Health Organization (2024). Global Tuberculosis Report. Available online at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2024/tb-disease-burden/1-1-tb-incidence (Accessed February 26 2025).
Wu, S., Shen, Y., Zhang, S., Xiao, Y., and Shi, S. (2020). Salmonella interacts with autophagy to offense or defense. Front. Microbiol. 11, 721. doi: 10.3389/fmicb.2020.00721
Xie, J. and Meijer, A. H. (2023). Xenophagy receptors Optn and p62 and autophagy modulator Dram1 independently promote the zebrafish host defense against Mycobacterium marinum. Front. Cell Infect. Microbiol. 13, 1331818. doi: 10.3389/fcimb.2023.1331818
Yang, C. S., Kim, J. J., Lee, H. M., Jin, H. S., Lee, S. H., Park, J. H., et al. (2014). The AMPK-PPARGC1A pathway is required for antimicrobial host defense through activation of autophagy. Autophagy 10, 785–802. doi: 10.4161/auto.28072
Young, C., Walzl, G., and Du Plessis, N. (2020). Therapeutic host-directed strategies to improve outcome in tuberculosis. Mucosal Immunol. 13, 190–204. doi: 10.1038/s41385-019-0226-5
Yuk, J. M., Shin, D. M., Lee, H. M., Yang, C. S., Jin, H. S., Kim, K. K., et al. (2009). Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 6, 231–243. doi: 10.1016/j.chom.2009.08.004
Zhang, L., Huang, J., Dong, R., Feng, Y., and Zhou, M. (2019a). Therapeutic potential of BLT1 antagonist for COPD: involvement of inducing autophagy and ameliorating inflammation. Drug Des. Devel Ther. 13, 3105–3116. doi: 10.2147/DDDT.S215433
Zhang, R., Varela, M., Vallentgoed, W., Forn-Cuni, G., van der Vaart, M., and Meijer, A. H. (2019b). The selective autophagy receptors Optineurin and p62 are both required for zebrafish host resistance to mycobacterial infection. PloS Pathog. 15, e1007329. doi: 10.1371/journal.ppat.1007329
Keywords: Mycobacterium tuberculosis, Salmonella typhimurium, macrophage, metabolism rewiring, host-direct therapies
Citation: Perez-Toledo M and Llibre A (2025) Lessons from cross-pathogen studies: understanding the metabolic rewiring of macrophages upon infection. Front. Cell. Infect. Microbiol. 15:1584777. doi: 10.3389/fcimb.2025.1584777
Received: 27 February 2025; Accepted: 26 May 2025;
Published: 18 June 2025.
Edited by:
Alexander Gluschko, University Hospital of Cologne, GermanyReviewed by:
Andrea J. Wolf, Cedars Sinai Medical Center, United StatesCopyright © 2025 Perez-Toledo and Llibre. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marisol Perez-Toledo, bS5wZXJlei10b2xlZG9AYmhhbS5hYy51aw==; Alba Llibre, YS5sbGlicmVAYmhhbS5hYy51aw==
 Marisol Perez-Toledo
Marisol Perez-Toledo Alba Llibre
Alba Llibre