- 1McGovern Medical School, UT Houston Health Science Center, Houston, TX, United States
- 2Department of Medical Oncology, Novant Health Cancer Institute – Ballantyne, Charlotte, NC, United States
- 3Division of Radiation Oncology, Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 4Department of Translational Molecular Pathology, Pathology and Laboratory Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 5Division of Cancer Medicine, Department of Genitourinary Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 6Division of Pathology and Laboratory Medicine, Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 7Division of Cancer Medicine, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 8UTHealth Houston Graduate School of Biomedical Sciences (GSBS), Houston, TX, United States
- 9David H. Koch Center for Applied Research of Genitourinary Cancers, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
SMARCB1-deficient renal medullary carcinoma (RMC) is a rare and aggressive kidney cancer defined by the loss of SMARCB1 tumor suppressor and primarily affecting adolescents and young adults with sickle hemoglobinopathies. Approximately 7% of RMC cases, known as renal cell carcinoma unclassified with medullary phenotype (RCCU-MP), lack sickle hemoglobinopathy. RMC does not respond to immune checkpoint inhibitors and antiangiogenic tyrosine kinase inhibitors, with chemotherapy being the main treatment. Here we present the first documented case of RMC diagnosed during pregnancy. A 24-year-old woman presented with right-sided back pain, leading to the discovery of a 6-cm right renal mass. Pathology confirmed RCCU-MP with SMARCB1 loss. With the woman at 16 weeks into pregnancy, imaging revealed metastatic retroperitoneal lymphadenopathy and lung nodules. A chemotherapy regimen of doxorubicin and cyclophosphamide, followed by weekly paclitaxel, was selected for safety in pregnancy. This approach yielded significant tumor shrinkage and alleviated the symptoms, allowing for the safe, preterm delivery of a healthy baby at 33 weeks. Following delivery, the patient received combination chemotherapy and definitive radiation therapy, achieving disease control. At 2 years post-diagnosis, she remains alive, exceeding the median survival for RCCU-MP. This case demonstrates that established chemotherapeutic regimens used in pregnant patients with other cancers can be successfully applied to manage RMC during pregnancy. Our findings underscore the importance of early, aggressive treatment and suggest that a coordinated approach can achieve favorable outcomes for both the mother and the fetus.
1 Introduction
SMARCB1-deficient renal medullary carcinoma (RMC) is a rare but aggressive subtype of renal cell carcinoma (RCC) that primarily affects adolescents and young adults (AYAs) with underlying sickle hemoglobinopathies, such as sickle cell trait and sickle cell disease (1–4). However, approximately 7% of RMC cases are not associated with hemoglobinopathies and are classified as renal cell carcinoma unclassified with medullary phenotype (RCCU-MP) (1, 3, 5). All RMC cases, including RCCU-MP, are defined by the loss of SMARCB1 tumor suppressor protein expression as determined by immunohistochemistry (IHC) (1, 6). In contrast to most other RCCs, RMC is resistant to currently approved immune checkpoint therapies (7) and tyrosine kinase inhibitors (TKIs) targeting the vascular endothelial growth factor (VEGF) pathway (4, 8). Platinum-based cytotoxic chemotherapy is the recommended first-line therapy for RMC (1). Combination chemotherapy with definitive radiation may also be used in selected patients with oligoprogressive or oligometastatic RMC (9). Herein we report the first documented case of RMC during pregnancy in a young woman who was diagnosed with cancer on the same day she discovered she was pregnant. The tailored management and treatment plan enabled a healthy preterm delivery that can guide the clinical approach for future cases of pregnant patients with metastatic RMC.
2 Case presentation
A 24-year-old Caucasian female with no major past medical or surgical history presented to primary care clinic with a 1-week history of right-sided lower back and flank pain, scored 4/10 in severity. She denied fevers, chills, or gross hematuria. In the clinic, a point-of-care urine dipstick showed trace blood and a computed tomography (CT) of the abdomen and pelvis without contrast was performed to rule out kidney stones. The patient was instructed to use ibuprofen and acetaminophen for pain.
The CT of the abdomen and pelvis showed two punctuate stones in the left kidney and, despite being limited by lack of contrast, showed findings suggestive of pyelonephritis and/or a renal abscess. The urine cultures were negative, but the patient was treated empirically with ciprofloxacin due to concern for infection. She was then referred to a urologist, and these findings were attributed at that visit as papillary necrosis secondary to ibuprofen use. A renal ultrasound (US) was ordered for repeat imaging in 3 months.
At 2 weeks later, the flank pain had become bilateral, and the patient began reporting gross hematuria. She underwent renal US which showed a 5.9-cm right renal mass. Subsequent magnetic resonance imaging (MRI) with and without contrast confirmed the presence of a 6-cm mass suspicious for RCC. At 2 weeks later, the patient underwent an enhanced recovery after surgery (ERAS) hand-assisted laparoscopic nephrectomy of her right kidney with no complications. In the morning of her surgery, the patient had a urine pregnancy test that came back positive. The last menstrual period (LMP) was exactly 4 weeks prior. A subsequent US evaluation confirmed this to be an intrauterine pregnancy.
The pathology evaluation of the nephrectomy specimen showed a high-grade carcinoma without sarcomatoid or rhabdoid features, 5.9 cm in greatest dimension and invading the renal sinus and perinephric tissue (pT3a). There was no lymphovascular invasion, and the resection margins were free of tumor. The lymph nodes were not sampled. Morphologically, the malignant cells were epithelioid with large nuclei with vesicular chromatin and variably conspicuous nucleoli and abundant amphophilic cytoplasm. Numerous mitotic figures and apoptotic debris were present (Figure 1A). The IHC showed the malignant cells to be positive for PAX8, CK7, and CK20 (weak) while negative for CD10, p63, GATA-3, and SMARCB1 (INI-1). The hemoglobin electrophoresis came back negative for the sickle cell trait or any other hemoglobinopathy, confirming the diagnosis as RCCU-MP.
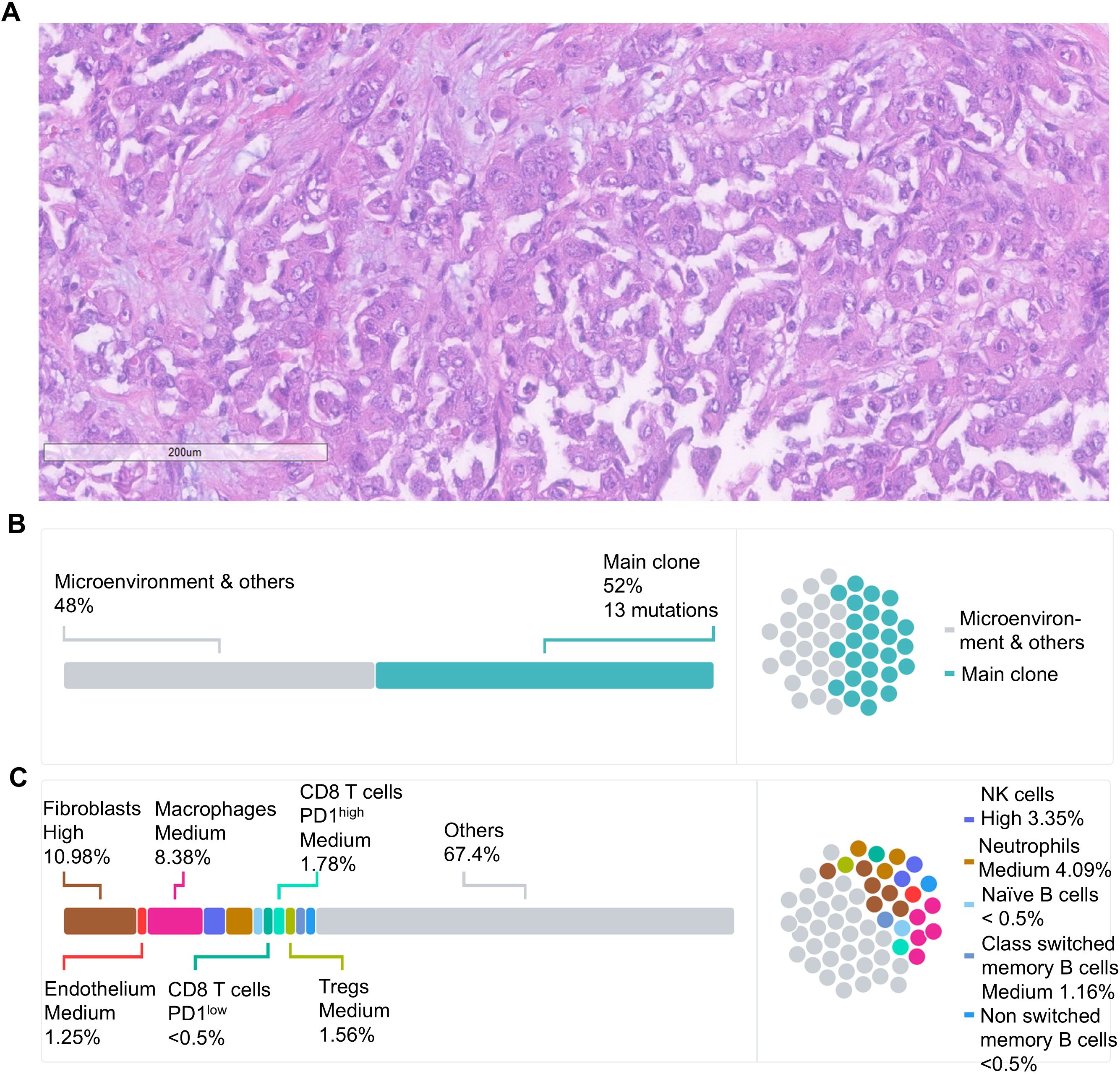
Figure 1. (A) Histological evaluation by hematoxylin and eosin staining showed a high-grade carcinoma without sarcomatoid or rhabdoid features. The malignant cells were epithelioid, featuring large nuclei with vesicular chromatin, nucleoli that ranged from inconspicuous to prominent, and abundant amphophilic cytoplasm consistent with RMC. Scale bar = 200 μm. (B) Schematic representation of the tumor clonal composition based on whole-exome sequencing of the nephrectomy tumor specimen and germline control. Major tumor clones are presented as a percentage from the entire tumor tissue. (C) Schematic representation of the cellular makeup of the tumor micro-environment (TME) based on the RNAseq of the nephrectomy specimen. The composition of malignant and microenvironment compartments is inferred from gene expression profiling as previously described (10).
Whole-exome sequencing (WES) and bulk RNA-sequencing (RNA-seq) were performed on formalin-fixed paraffin-embedded (FFPE) tissue from the nephrectomy specimen as previously described (11, 12). The microsatellite status was stable, and the tumor mutational burden was low at 0.43 mut/Mb, as is typically observed in RMC (6). Somatic missense mutations were identified in 13 genes (Table 1). As is often the case in RMC (6), no SMARCB1 mutations were detected, but there was a homozygous copy number loss of the SMARCB1 gene. The tumor cells comprised a single dominant clone with no detectable subclones (Figure 1B). The RNA-seq revealed a high expression of genes known to be upregulated in RMC such as CD70, EGFR, and MUC16 (6) as well as a moderate expression of PD-L1. Cell content deconvolution using the Kassandra algorithm (10) revealed enrichment for cancer-associated fibroblasts and macrophages in the tumor microenvironment (TME), with few CD8 T cells expressing high PD-1 levels (Figure 1C).
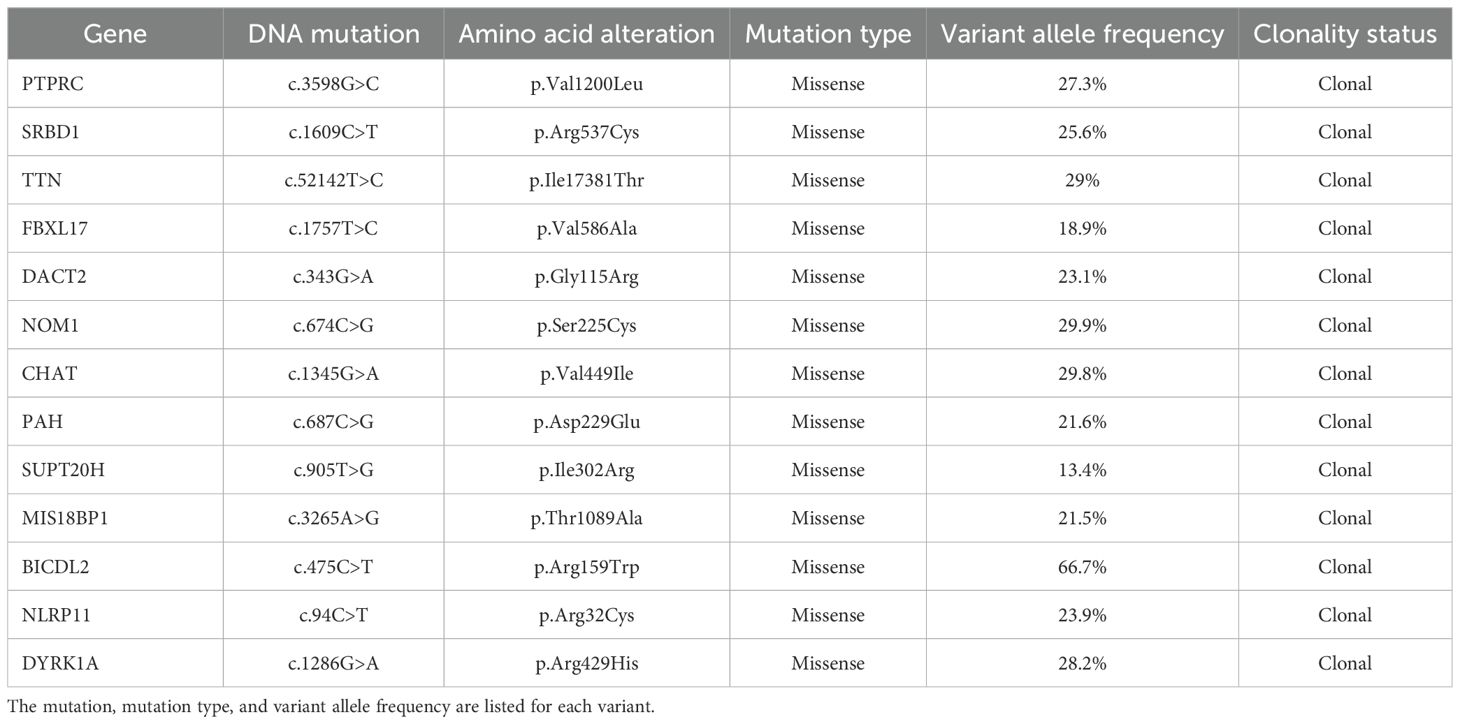
Table 1. List of somatic variants identified according to validated thresholds for FFPE samples based on whole-exome sequencing of the nephrectomy tumor specimen and germline control.
At approximately 6 weeks after the nephrectomy, a repeat MRI of the abdomen and pelvis without contrast revealed enlarged retrocaval and aortocaval lymph nodes up to 4.2 cm in maximal diameter (Figure 2A) consistent with metastatic retroperitoneal lymphadenopathy, the most common site of metastasis in RMC (3). The non-contrast CT of the chest showed enlarging lung nodules of up to 6 mm in diameter suspicious for metastatic disease. No evidence of central nervous system metastasis was noted on MRI of the brain. The patient was in her 16th week of pregnancy. Clinically, she began experiencing worsening right-sided abdominal pain. The decision was made to start chemotherapy consisting of the AC-T regimen (four cycles of doxorubicin plus cyclophosphamide followed by weekly paclitaxel), which has established safety profile in pregnant patients with breast cancer (13, 14). RMC is sensitive to doxorubicin (6, 15) and paclitaxel (6, 16). Although cyclophosphamide is not typically used to treat RMC (3, 16), the loss of SMARCB1 is known to increase replication stress, rendering tumor cells susceptible to alkylating agents such as cyclophosphamide (6, 17). The chemotherapy regimen initially consisted of doxorubicin at 60 mg/m2 given intravenously (IV) in combination with cyclophosphamide at 600 mg/m2 IV every 3 weeks for four cycles. The premedication antiemetics consist of ondansetron at 8 mg IV and famotidine at 20 mg IV. If necessary, fosaprepitant at 150 mg IV and metoclopramide could be added as needed. The baseline transthoracic echocardiogram showed normal left ventricular size and systolic function with left ventricular ejection fraction calculated as 57% using the bi-plane method of disks. The patient’s abdominal pain subsided after two cycles of this chemotherapy regimen and she did not experience any adverse events, while the anatomical scans of the fetus at 20 weeks of gestation showed normal development. The fourth cycle of doxorubicin plus cyclophosphamide was completed at week 25 of pregnancy with re-staging of MRI of the abdomen and pelvis without contrast, showing a significant interval decrease in the size of the retrocaval/retroperitoneal lymphadenopathy (Figure 2B). For example, the confluent retrocaval lymphadenopathy decreased in size from 4.2 × 2.4 cm to 3.1 x× 1.5 cm. A non-contrast CT of the chest also showed a decrease in the size of the multiple bilateral lung nodules. At week 28 (3 weeks after completion of the last cycle of doxorubicin plus cyclophosphamide), weekly paclitaxel at 80 mg/m2 for up to six doses was initiated with the goal to not continue chemotherapy after 34 weeks of pregnancy. The patient received five doses of weekly paclitaxel with no issues. However, at week 33, she experienced a premature rupture of membranes and subsequently had an uncomplicated spontaneous vaginal delivery of a healthy premature baby. Restaging non-contrast MRI of the abdomen and pelvis and CT of the chest, a further slight decrease in metastatic disease is shown (Figure 2C). Following the successful pregnancy, the patient was started on definitive radiation therapy in combination with carboplatin plus paclitaxel for her persistent oligometastatic disease. The timeline of diagnosis and pregnancy are outlined in Figure 3. She remains alive and with good disease control 2 years after her diagnosis.
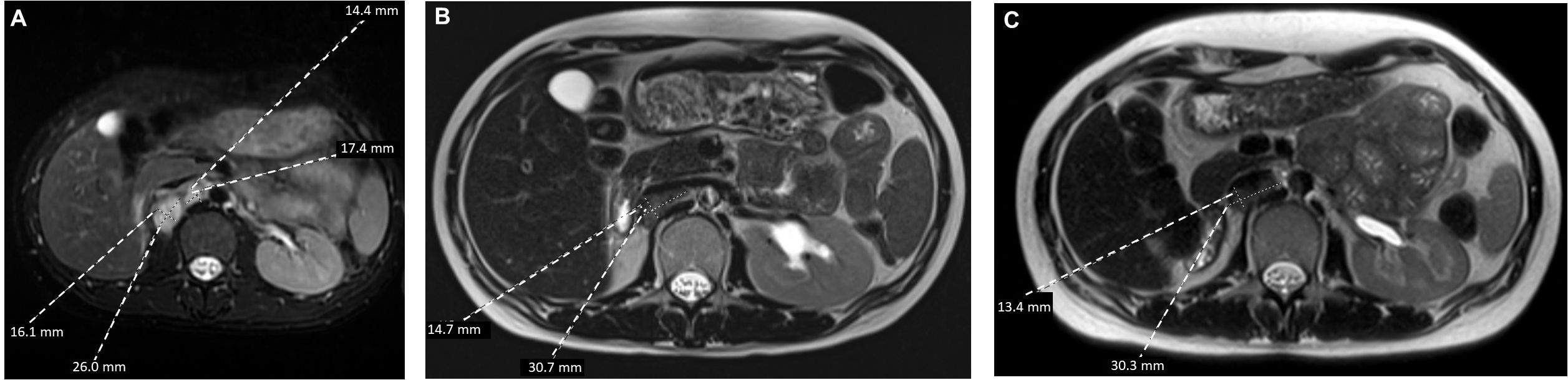
Figure 2. Axial T2-weighted MRI view of the retroperitoneal metastasis (A) at baseline, (B) following four cycles of doxorubicin plus cyclophosphamide, and (C) following six infusions of weekly paclitaxel.
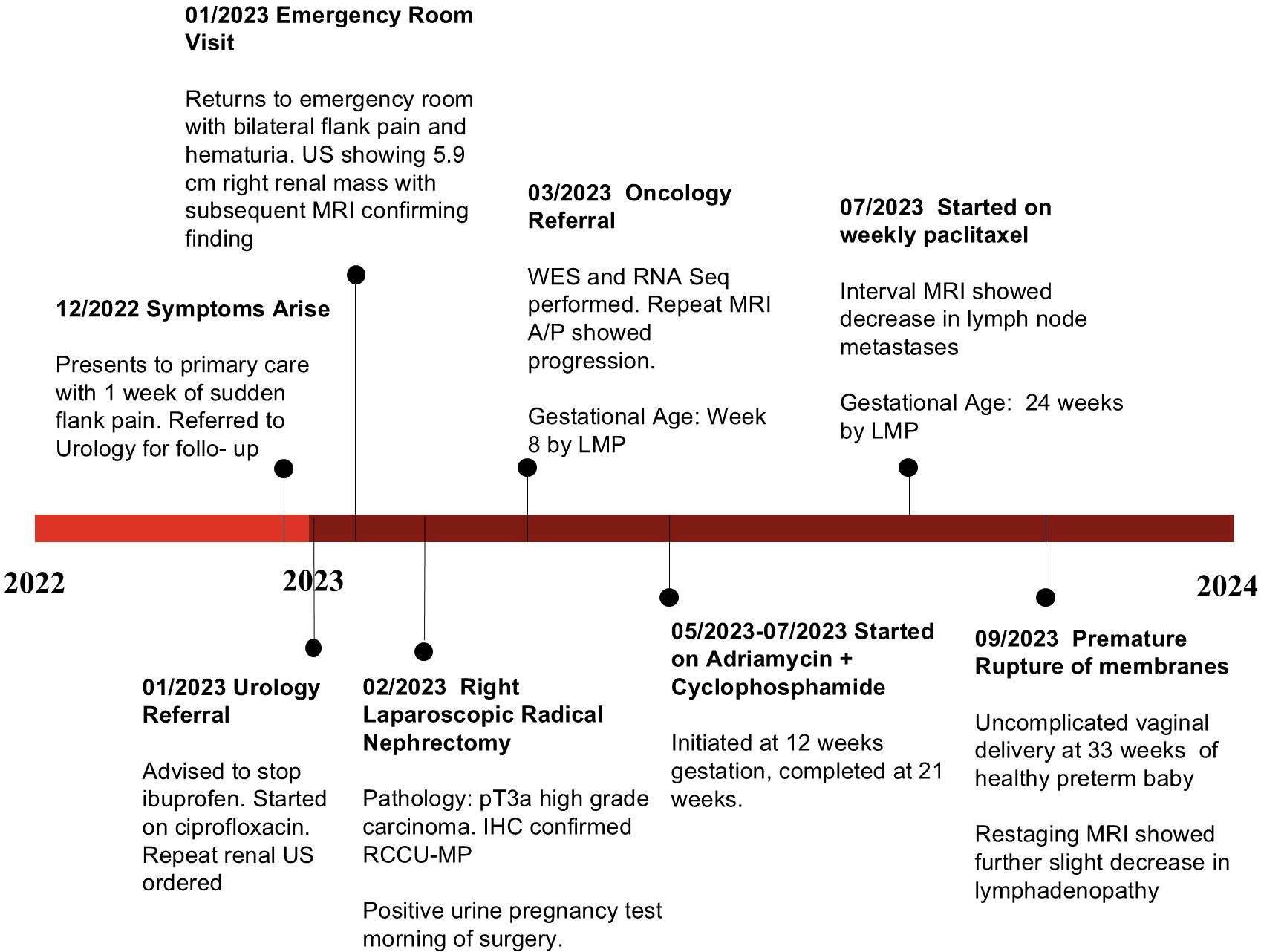
Figure 3. Timeline of the patient’s clinical course from initial symptom onset to delivery, highlighting key diagnostic and therapeutic milestones.
3 Discussion
Although the present case is the first description of successful delivery in a pregnant patient with metastatic RMC, it will not be the last as the median age of RMC diagnosis overlaps with peak fertility age (3). Although rare, RMC is still the third most commonly diagnosed RCC in the AYA population in the United States (18). Our case demonstrates that utilizing established therapies, which have been well tested in pregnant patients with other cancer types (13, 14), can enable patients to continue their intrauterine pregnancy without needing to consider termination. Had the patient not been pregnant, active cytotoxic chemotherapies such as platinum salts, paclitaxel, gemcitabine, or doxorubicin would be deployed (1, 3, 6, 17). While platinum-based chemotherapy is not absolutely contraindicated in pregnancy, it carries the risk of fetal bone marrow suppression and ototoxicity (19). Although carboplatin carries less ototoxicity risk than cisplatin, studies in primates have shown high carboplatin transplacental transfer of carboplatin to the fetus with mean fetal plasma concentrations reaching 57.5% of maternal concentrations (20). Paclitaxel is a therapeutic mainstay in the first-line therapy of RMC and has an excellent safety profile in pregnancy, in part because it is a substrate for p-glycoprotein which protects they fetus by carrying paclitaxel from the fetal to the maternal side of the placental barrier (19). As a result, transplacental transfer of paclitaxel to the fetus is only marginal, with primate studies showing that the mean fetal plasma concentrations are 1.5% of maternal concentrations (20). SMARCB1 loss induces replication stress, making RMC cells susceptible to topoisomerase II inhibitors such as doxorubicin (6, 15, 17) and potentially to alkylating agents such as cyclophosphamide that form DNA crosslinks, blocking the replication fork progression and leading to increased replication stress (21, 22). Nucleoside analogs such as gemcitabine may also target replication stress (6, 15, 17), and while there are case reports of their successful use in pregnancy (23, 24), we chose to avoid it due to its potential for teratogenicity and genotoxicity. Doxorubicin, cyclophosphamide, and paclitaxel are commonly used in pregnant patients with breast cancer, which is the most common solid malignancy treated with cytotoxic chemotherapy in AYA women of childbearing age (25) and therefore has the most extensive and long-term safety data available (13, 14, 26). Studies in primates have shown that mean fetal plasma concentrations compared to maternal concentrations is 7.5% for doxorubicin and 6.3% for cyclophosphamide (27). The ACT regimen we chose is commonly used and easily applied in the community center, thus alleviating the need for the patient to travel to our tertiary center during her pregnancy. Combination chemotherapy with definitive radiation therapy is a promising treatment modality for patients with oligoprogressive or oligometastatic RMC (9). However, such an approach should be deferred until after delivery due to the short- and long-term risks of fetal radiation exposure, particularly in scenarios where the metastatic disease is not located sufficiently far from the fetus (28). Our use of the AC-T chemotherapy regimen during pregnancy produced immediate clinical and radiological responses, allowing for safe delivery followed by the use of combination chemotherapy with definitive radiation. Notably, our patient has survived for longer than 2 years from diagnosis, exceeding the median RCCU-MP overall survival of 19.5 months (3). Given the aggressiveness of RMC, including the RCCU-MP subtype, it is unlikely that either she or her fetus would have survived without timely initiation of chemotherapy during pregnancy.
4 Conclusion
In conclusion, this case report provides a unique account of managing metastatic RMC during pregnancy, emphasizing the importance of a tailored, multidisciplinary approach. The use of well-established chemotherapy regimens with demonstrated safety profiles in pregnant patients, such as the AC-T regimen, allowed for disease control and a successful preterm delivery. This case highlights the potential of leveraging known therapeutic strategies from other malignancies to manage RMC in pregnant patients while minimizing risks to the fetus. The patient’s prolonged survival beyond the typical outcomes for RMC underscores the effectiveness of prompt, innovative management. As more cases emerge, our experience can inform future clinical decisions and improve the outcomes for pregnant patients diagnosed with this aggressive cancer.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
This case report has received waiver of informed consent and authorization by the University of Texas MD Anderson Cancer Center Institutional Review Board (protocol # 2023-1079). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this case report involves the retrospective review of a single patient’s clinical course and does not include identifiable private information. Because no direct patient identifiers (e.g., name, date of birth, medical record number, or images that could reveal identity) are disclosed and the information presented is strictly limited to clinically relevant details, the risk of harm to the individual is minimal. Given these considerations, the IRB has determined that the publication meets the criteria for a waiver of informed consent under applicable federal regulations. Written informed consent was obtained from the patient for the publication of this case report.
Author contributions
PK: Writing – original draft, Writing – review & editing. AK: Writing – review & editing. CT: Writing – review & editing. JC: Writing – review & editing. BC: Writing – review & editing. ST: Writing – review & editing. PR: Writing – review & editing. BL: Writing – review & editing. PM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported in part by the Cancer Center Support Grant to MDACC (grant P30 CA016672) from the National Cancer Institute. Pavlos Msaouel was supported by the National Cancer Institute R37CA288448 and R01CA285454, the Andrew Sabin Family Foundation Fellowship, Gateway for Cancer Research, a Translational Research Partnership Award (KC200096P1) and an Idea Development Award (RA230062) by the United States Department of Defense, an Advanced Discovery Award by the Kidney Cancer Association, a Translational Re-search Award by the V Foundation, the MD Anderson Physician-Scientist Award, donations from the Renal Medullary Carcinoma Research Foundation in honor of Ryse Williams, the Finneran Family Endowment, as well as philanthropic donations by the Chris “CJ” Johnson Foundation, and by the family of Mike and Mary Allen.
Acknowledgments
We acknowledge the support of MD Anderson’s Prometheus informatics system and the Department of Genitourinary Medical Oncology’s Eckstein and Alexander Laboratories in this research.
Conflict of interest
PM has received honoraria for service on a Scientific Advisory Board for Mirati Therapeutics, Bristol Myers Squibb, and Exelixis; consulting for Axiom Healthcare Strategies; non-branded educational programs supported by DAVA Oncology, Exelixis and Pfizer; and research funding for clinical trials from Regeneron Pharmaceuticals, Summit Therapeutics, Takeda, Bristol Myers Squibb, Mirati Therapeutics, Gateway for Cancer Research, and UT MD Anderson Cancer Center.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Msaouel P, Hong AL, Mullen EA, Atkins MB, Walker CL, Lee CH, et al. Updated recommendations on the diagnosis, management, and clinical trial eligibility criteria for patients with renal medullary carcinoma. Clin Genitourin Cancer. (2019) 17:1–6. doi: 10.1016/j.clgc.2018.09.005
2. Msaouel P, Tannir NM, and Walker CLA. Model linking sickle cell hemoglobinopathies and SMARCB1 loss in renal medullary carcinoma. Clin Cancer Res. (2018) 24:2044–9. doi: 10.1158/1078-0432.CCR-17-3296
3. Lebenthal JM, Kontoyiannis PD, Hahn AW, Lim ZD, Rao P, Cheng JP, et al. Clinical characteristics, management, and outcomes of patients with renal medullary carcinoma: A single-center retrospective analysis of 135 patients. Eur Urol Oncol. (2024) 8(2):315–23. doi: 10.1016/j.euo.2024.07.002
4. Msaouel P, Genovese G, and Tannir NM. Renal cell carcinoma of variant histology: biology and therapies. Hematol Oncol Clin North Am. (2023) 37:977–92. doi: 10.1016/j.hoc.2023.04.019
5. Sarkar S, Throckmorton W, Bingham R, Msaouel P, Genovese G, Slopis J, et al. Renal cell carcinoma unclassified with medullary phenotype in a patient with neurofibromatosis type 2. Curr Oncol. (2023) 30:3355–65. doi: 10.3390/curroncol30030255
6. Msaouel P, Malouf GG, Su X, Yao H, Tripathi DN, Soeung M, et al. Comprehensive molecular characterization identifies distinct genomic and immune hallmarks of renal medullary carcinoma. Cancer Cell. (2020) 37:720–734 e713. doi: 10.1016/j.ccell.2020.04.002
7. Nze C, Msaouel P, Derbala MH, Stephen B, Abonofal A, Meric-Bernstam F, et al. Phase II clinical trial of pembrolizumab efficacy and safety in advanced renal medullary carcinoma. Cancers (Basel). (2023) 15(15):3806. doi: 10.3390/cancers15153806
8. Soeung M, Perelli L, Chen Z, Dondossola E, Ho IL, Carbone F, et al. SMARCB1 regulates the hypoxic stress response in sickle cell trait. Proc Natl Acad Sci U.S.A. (2023) 120:e2209639120. doi: 10.1073/pnas.2209639120
9. Mbilinyi RH, Msaouel P, Rao P, Karam JA, Tannir NM, and Tang C. Radiation therapy for the management of renal medullary carcinoma: A multi-case study. Clin Genitourin Cancer. (2024) 22:102065. doi: 10.1016/j.clgc.2024.102065
10. Zaitsev A, Chelushkin M, Dyikanov D, Cheremushkin I, Shpak B, Nomie K, et al. Precise reconstruction of the TME using bulk RNA-seq and a machine learning algorithm trained on artificial transcriptomes. Cancer Cell. (2022) 40:879–894 e816. doi: 10.1016/j.ccell.2022.07.006
11. Radtke AJ, Postovalova E, Varlamova A, Bagaev A, Sorokina M, Kudryashova O, et al. Multi-omic profiling of follicular lymphoma reveals changes in tissue architecture and enhanced stromal remodeling in high-risk patients. Cancer Cell. (2024) 42:444–463 e410. doi: 10.1016/j.ccell.2024.02.001
12. Gubbiotti MA, McCutcheon IE, Rao P, Genovese G, Wang L, Tarasov A, et al. A novel case of glial transdifferentiation in renal medullary carcinoma brain metastasis. Acta Neuropathol Commun. (2025) 13:12 doi: 10.1186/s40478-025-01929-w
13. Poggio F, Tagliamento M, Pirrone C, Soldato D, Conte B, Molinelli C, et al. Update on the management of breast cancer during pregnancy. Cancers (Basel). (2020) 12(12):3616. doi: 10.3390/cancers12123616
14. Girardelli S, Bonomo B, Papale M, di Loreto E, Grossi E, Scarfone G, et al. Weekly paclitaxel for pregnancy associated breast cancer. Clin Breast Cancer. (2024) 24:199–203. doi: 10.1016/j.clbc.2023.11.007
15. Wilson NR, Wiele AJ, Surasi DS, Rao P, Sircar K, Tamboli P, et al. Efficacy and safety of gemcitabine plus doxorubicin in patients with renal medullary carcinoma. Clin Genitourin Cancer. (2021) 19:e401–8. doi: 10.1016/j.clgc.2021.08.007
16. Shah AY, Karam JA, Malouf GG, Rao P, Lim ZD, Jonasch E, et al. Management and outcomes of patients with renal medullary carcinoma: a multicentre collaborative study. BJU Int. (2017) 120:782–92. doi: 10.1111/bju.13705
17. Msaouel P, Walker CL, Genovese G, and Tannir NM. Molecular hallmarks of renal medullary carcinoma: more to c-MYC than meets the eye. Mol Cell Oncol. (2020) 7:1777060. doi: 10.1080/23723556.2020.1777060
18. Cajaiba MM, Dyer LM, Geller JI, Jennings LJ, George D, Kirschmann D, et al. The classification of pediatric and young adult renal cell carcinomas registered on the children’s oncology group (COG) protocol AREN03B2 after focused genetic testing. Cancer. (2018) 124:3381–9. doi: 10.1002/cncr.31578
19. Triarico S, Rivetti S, Capozza MA, Romano A, Maurizi P, Mastrangelo S, et al. Transplacental passage and fetal effects of antineoplastic treatment during pregnancy. Cancers (Basel). (2022) 14(13):3103. doi: 10.3390/cancers14133103
20. Calsteren KV, Verbesselt R, Devlieger R, De Catte L, Chai DC, Van Bree R, et al. Transplacental transfer of paclitaxel, docetaxel, carboplatin, and trastuzumab in a baboon model. Int J Gynecol Cancer. (2010) 20:1456–64. doi: 10.1111/IGC.0b013e3181fb18c8
21. Zhang J, Dai Q, Park D, and Deng X. Targeting DNA replication stress for cancer therapy. Genes (Basel). (2016) 7(8):51. doi: 10.3390/genes7080051
22. Nickoloff JA. Targeting replication stress response pathways to enhance genotoxic chemo- and radiotherapy. Molecules. (2022) 27(15):4736. doi: 10.3390/molecules27154736
23. Wiesweg M, Aydin S, Koeninger A, Stein A, Schara U, van Roye C, et al. Administration of gemcitabine for metastatic adenocarcinoma during pregnancy: A case report and review of the literature. AJP Rep. (2014) 4:17–22. doi: 10.1055/s-0034-1368091
24. Kim JH, Kim HS, Sung CW, Kim KJ, Kim CH, and Lee KY. Docetaxel, gemcitabine, and cisplatin administered for non-small cell lung cancer during the first and second trimester of an unrecognized pregnancy. Lung Cancer. (2008) 59:270–3. doi: 10.1016/j.lungcan.2007.06.017
25. Salani R, Billingsley CC, and Crafton SM. Cancer and pregnancy: an overview for obstetricians and gynecologists. Am J Obstet Gynecol. (2014) 211:7–14. doi: 10.1016/j.ajog.2013.12.002
26. Esposito S, Tenconi R, Preti V, Groppali E, and Principi N. Chemotherapy against cancer during pregnancy: A systematic review on neonatal outcomes. Med (Baltimore). (2016) 95:e4899. doi: 10.1097/MD.0000000000004899
27. Van Calsteren K, Verbesselt R, Beijnen J, Devlieger R, De Catte L, Chai DC, et al. Transplacental transfer of anthracyclines, vinblastine, and 4-hydroxy-cyclophosphamide in a baboon model. Gynecol Oncol. (2010) 119:594–600. doi: 10.1016/j.ygyno.2010.08.019
Keywords: renal cell carcinoma unclassified with medullary phenotype, pregnancy, SMARCB1 loss, chemotherapy, case report
Citation: Kontoyiannis PD, Kuykendal A, Tang C, Cheng JP, Chan B, Thomas SS, Rao P, Lim B and Msaouel P (2025) Case Report: Successful delivery following chemotherapy in a pregnant patient with metastatic SMARCB1-deficient renal medullary carcinoma. Front. Oncol. 15:1606647. doi: 10.3389/fonc.2025.1606647
Received: 06 April 2025; Accepted: 21 July 2025;
Published: 29 August 2025.
Edited by:
John Peter Sfakianos, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Konstantinos Ferentinos, German Oncology Center, CyprusPiergiuseppe Colombo, Humanitas University, Italy
Copyright © 2025 Kontoyiannis, Kuykendal, Tang, Cheng, Chan, Thomas, Rao, Lim and Msaouel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pavlos Msaouel, cG1zYW91ZWxAbWRhbmRlcnNvbi5vcmc=
 Panayiotis D. Kontoyiannis
Panayiotis D. Kontoyiannis Adam Kuykendal2
Adam Kuykendal2 Chad Tang
Chad Tang Pavlos Msaouel
Pavlos Msaouel