- 1School of Graduate Studies, Postgraduate Centre, Management and Science University, Shah Alam, Selangor, Malaysia
- 2Department of Basic Medical Sciences, College of Medicine, Ajman University, Ajman, United Arab Emirates
- 3Department of Anatomy and Physiology, School of Basic Medical Sciences, Faculty of Medicine, Universiti Sultan Zainal Abidin, Kuala Terengganu, Terengganu, Malaysia
- 4Faculty of Health and Life Sciences, Management and Science University, Shah Alam, Selangor, Malaysia
Major depressive disorder (MDD) is a multifactorial condition shaped by neurobiological, psychological, and environmental influences. Recent evidence highlights the gut–brain axis (GBA), a bidirectional communication system linking the gastrointestinal tract and central nervous system, as an important contributor to MDD pathogenesis via microbiota-mediated mechanisms. This narrative review synthesizes findings from preclinical and clinical studies published in the last decade, with emphasis on mechanistic insights from animal models and translational data from human cohorts. Key pathways include the microbial regulation of neurotransmitter production, immune modulation, vagus nerve signalling, and the metabolism of short-chain fatty acids (SCFAs). Dysbiosis in MDD is frequently characterized by reductions in butyrate-producing genera and elevations in pro-inflammatory taxa which have been linked to neuroinflammation, impaired neurotransmitter synthesis, and hypothalamic-pituitary-adrenal (HPA) axis dysregulation. Interventions such as probiotics, prebiotics, synbiotics, and psychobiotics show promise in alleviating depressive symptoms by modulating the gut microbiota. Emerging evidence also supports the beneficial roles of postbiotics, non-viable microbial products with immunomodulatory and neuroactive potential. Overall, microbial modulation offers a novel adjunctive strategy for depression management, particularly in treatment-resistant cases or to reduce the side effects of conventional drugs. However, heterogeneity in study design, small sample sizes, and limited causal evidence underscore the need for rigorous, large-scale trials. Future directions should prioritize identification of microbial biomarkers, optimization of strain-specific and dose–response data, and integration of gut-targeted approaches into personalized mental healthcare.
1 Introduction
Major depressive disorder (MDD) is a prevalent and disabling mental health condition, characterized by persistent low mood, anhedonia, cognitive impairments, and disruptions in sleep, appetite, and energy regulation lasting for a minimum of 2 weeks (Otte et al., 2016). With an estimated global prevalence affecting over 320 million individuals, MDD represents a leading cause of disability worldwide. The World Health Organization (WHO) anticipates it will become the foremost contributor to the global burden of disease in the near future (Nik Ramli et al., 2024; Zainal Abidin et al., 2023). Unlike transient experiences of sadness or grief, depression is marked by chronicity and functional impairment, often persisting long after triggering events have passed and significantly affecting quality of life and social participation (Belmaker and Agam, 2008).
The pathogenesis of depression is multifactorial, resulting from the intricate interaction of biological, psychological, and environmental influences. Neurobiological research has highlighted structural and functional changes in brain regions involved in emotion and cognition, particularly the prefrontal cortex (PFC), hippocampus, and amygdala (Sun et al., 2023). Neuroimaging consistently reveals reduced volume and hypoactivity in these areas among individuals with depression, impairing executive functioning and memory (Zhang et al., 2018). Concurrently, hyperactivation of the hypothalamic-pituitary-adrenal (HPA) axis and elevated cortisol levels contribute to neuronal atrophy and symptom exacerbation (Jesulola et al., 2018; Rafiee Sardooi et al., 2020). In addition to monoaminergic deficits, newer evidence implicates dysregulation in glutamate and gamma-aminobutyric acid (GABA) pathways both vital to synaptic plasticity and emotional stability (Sarawagi et al., 2021).
Moreover, psychosocial stressors, such as trauma, chronic stress, and limited social support, further intensify vulnerability to depression, particularly among individuals with genetic predispositions. This gene-environment interaction reinforces the individualized and dynamic nature of MDD’s etiology and progression (Belmaker and Agam, 2008).
While traditional treatments target central nervous system (CNS) dysfunction using pharmacological and psychotherapeutic modalities, growing attention has turned toward peripheral contributors to mental health, most notably the gut microbiota. The gut-brain axis (GBA), a bidirectional communication system connecting the gastrointestinal tract with the CNS, has emerged as a critical regulator of mood, cognition, and behavior (Carabotti et al., 2015; Morys et al., 2024).
Emerging research suggests that gut microbiota composition shaped by diet, environment, age, sex, and genetic factors can significantly impact neurophysiological and immune pathways relevant to depression (Ullah et al., 2024). Germ-free animal models have demonstrated altered brain structure, behavior, and emotional regulation in the absence of a functional microbiome, reinforcing its developmental importance (Nakhal et al., 2024). Certain microbial species contribute directly to the synthesis of key neurotransmitters, including serotonin and GABA, while also regulating inflammation via cytokine signaling processes increasingly recognized as central to MDD pathophysiology (Foster and McVey Neufeld, 2013; Irum et al., 2023).
Crucially, the gut microbiota is more amenable to modulation than central neural pathways. Interventions such as dietary adjustments, probiotic and prebiotic supplementation, and fecal microbiota transplantation (FMT) represent accessible, non-invasive strategies for restoring microbial balance and potentially mitigating depressive symptoms (Zhang et al., 2025). These emerging approaches offer promising adjuncts or alternatives to conventional antidepressant therapy and underscore the need for more integrative mental healthcare frameworks.
Thus, in this review, we examine the mechanisms linking gut microbiota dysbiosis to MDD and evaluate the therapeutic potential of microbiota-targeted strategies including probiotics, prebiotics, synbiotics, dietary interventions, FMT, and gut-focused pharmacological agents. We also discuss the translational potential of these findings for health promotion and behavioral health interventions, particularly in the context of education, prevention, and community-level mental health strategies.
1.1 Search and selection
This review is a narrative synthesis, not a systematic review. The literature was mainly found through PubMed indexed articles published between 2013 and 2025 using the terms “gut microbiota,” “major depressive disorder,” psychobiotics,” “probiotics,” “prebiotics,” “synbiotics,” and “postbiotics.” Only publications in English were assessed. Preclinical data was separated from clinical evidence based on study designs, with randomised controlled trials and cohort studies involving humans labelled clinical studies, while animal models and in vitro studies were deemed preclinical.
2 Gut microbiota system
The gut microbiota plays a fundamental role in maintaining human health, influencing a wide range of physiological processes including hormone production, immune regulation, metabolism, and communication between the gut and the brain. Central to this dynamic interaction is the GBA, a complex bidirectional communication network that links the gastrointestinal tract and the CNS via neural, endocrine, immune, and metabolic pathways (Ullah et al., 2024).
This sophisticated system facilitates constant crosstalk between the gut microbiota and the brain, allowing microbial signals to influence cognition, emotion, and behavior. Conversely, psychological stress and neural activity can impact gut function and microbiota composition, illustrating the deeply interconnected nature of this axis. Dysbiosis (e.g., reduced Faecalibacterium, increased Eggerthella) has been linked to systemic inflammation, immune dysregulation, and neuropsychiatric conditions, including MDD, with implications for drug metabolism and treatment response (Foster and McVey Neufeld, 2013; Cheng et al., 2024).
The gut microbiome is a diverse ecosystem composed of bacteria, viruses, archaea, and eukaryotic microorganisms, with a collective gene pool that vastly exceeds that of the human host. It harbors approximately 1,000 bacterial species and 7,000 strains. Among the dominant phyla are Firmicutes (e.g., Lactobacillus, Eubacterium, Clostridium), Bacteroidetes (e.g., Bacteroides, Prevotella), Actinobacteria, Proteobacteria, and Verrucomicrobia, all of which play essential roles in metabolic and neuroimmune homeostasis (Ullah et al., 2024).
The gut microbiota communicates with the enteric nervous system (ENS) often referred to as the “second brain” and the CNS by producing key signaling molecules such as cytokines, SCFAs, and neurotransmitter precursors. These biochemical messengers can influence blood-brain barrier (BBB) integrity, modulate inflammatory pathways, and alter the synthesis of mood-related neurotransmitters such as serotonin and GABA (Ullah et al., 2024).
Disruptions in the GBA due to gut dysbiosis can impair these communication mechanisms, leading to increased systemic inflammation and altered neurotransmitter production, which have been implicated in the onset and severity of depressive symptoms (Foster et al., 2021). A growing body of research, including findings by Kumar et al. (2023) has identified specific alterations in microbial genera associated with depression. Consistent reductions have been observed in beneficial taxa such as Clostridia, Bacteroides, Alistipes, Roseburia, Coprococcus, Dialister, Faecalibacterium, and Butyricicoccus. These bacteria are often involved in the production of anti-inflammatory SCFAs and the regulation of neurotransmitter pathways. Conversely, increased levels of potentially pathogenic or pro-inflammatory genera such as Proteobacteria, Actinobacteria, Clostridium, Streptococcus, and Oscillibacter have been noted in individuals with depression. Among these findings, Firmicutes and Actinobacteria appear to exhibit consistent patterns, with a notable decline in Firmicutes (particularly SCFA-producing species like Faecalibacterium) and a proliferation of certain Actinobacteria (e.g., Eggerthella) in depressed populations. Interestingly, some genera like Bacteroides and Clostridium show variability, with their abundance fluctuating based on factors such as individual genetics, diet, and environmental exposures, as well as study design and population demographics. Table 1 summarized the classification of the bacteria genera towards human and animal studies.

Table 1. A summary of the bacterial genera and their roles in short chain fatty acid (SCFA) levels and classification towards human and animal studies.
Collectively, these data suggest that microbial signatures associated with MDD are not only diverse but also context-dependent, highlighting the importance of personalized approaches when examining microbiota-based interventions for mental health. Additionally, research has shown that exposure to specific microbial strains can protect against stress-induced behavioral changes and systemic immune alterations, offering compelling evidence for the potential of microbe-based therapies in treating stress-related disorders (Nakhal et al., 2024).
SCFAs, including acetate, propionate, and butyrate, are metabolites produced by a healthy gut microbiota during the fermentation of dietary fibers and resistant starch (Silva et al., 2020). These SCFAs play a crucial role in immune modulation (Cheng et al., 2024). Butyrate, in particular, is thought to influence the gut-brain axis, potentially by enhancing colonic serotonin production, a key neurotransmitter involved in mood and behavior regulation. Additionally, animal studies suggest that butyrate may exert antidepressant-like effects by stimulating the production of brain-derived neurotrophic factor (BDNF), a protein essential for neuronal development and survival (Mansuy-Aubert and Ravussin, 2023).
Moreover, SCFAs are vital for reducing inflammation, maintaining intestinal barrier integrity, and regulating central nervous system function. However, microbial imbalance, or dysbiosis, can reduce SCFA production, diminishing their anti-inflammatory effects and promoting inflammation. Research indicates that restoring SCFA levels through dietary interventions or probiotic supplementation can enhance immune function and alleviate depressive symptoms. These findings highlight the therapeutic potential of modulating gut microbiota for treating depression (Mansuy-Aubert and Ravussin, 2023).
Given the urgent need for novel treatments and preventative strategies for mental health (Viana, 2024). Understanding the influence of the gut microbiota on emotional and cognitive functions, neurotransmitter synthesis, neuroinflammation, and gut barrier integrity is critical (Singh et al., 2024). This complex relationship involves bidirectional communication between the gut and the brain, with brain regions such as the insula and anterior cingulate cortex playing key roles in regulating both gut function and psychological responses (Kano and Fukudo, 2025). Despite variations in gut microbiota composition across studies, all evidence consistently demonstrates significant alterations in the gut microbiota of individuals with depression, suggesting that the gut microbiota may be a promising target for both the prevention and treatment of this disorder (Xiong et al., 2023). A comprehensive understanding of the gut microbiota’s influence on psychiatric conditions is vital for developing innovative and effective therapeutic strategies, emphasizing the need for an integrated approach to mental healthcare (Singh et al., 2024).
The gut microbiota is integral to the synthesis and regulation of neurotransmitters such as serotonin, dopamine, and glutamate, which are critical for neurological and immunological functions in the brain. This complex microbial community is diverse, with certain species potentially promoting mental wellbeing, while others may contribute to the development and progression of mental disorders (Xiong et al., 2023). Studies have revealed significant alterations in the gut microbiota composition of individuals with depression compared to healthy controls. Depressed patients showed reduced levels of Dialister and Coprococcus species, alongside elevated levels of Prevotella, Klebsiella, Streptococcus, and Clostridium XI, as well as decreased levels of Bacteroidetes. Animal studies supported these findings, showing that fecal microbiota transplants from depressed individuals induced depression-like behaviors in mice, while transplants from healthy rats prevented depression in susceptible rats. These results suggest that gut microbiota dysbiosis plays a significant role in depression by influencing protein expression along the gut–brain axis. While microbial composition varies across studies, certain families (e.g., Paraprevotella-positive and Streptococcaceae and Gemella-negative) and genera (e.g., Prevotella, Klebsiella, and Clostridium) have been consistently associated with depression (Xiong et al., 2023). Emerging evidence suggests that gut microbiome dysbiosis plays a crucial role in the neurovascular pathophysiology of glymphatic system dysfunction and cerebral small vessel disease, which may exacerbate neuroinflammation and impair the BBB function, both of which are critical processes in the development and progression of depression (Che Mohd Nassir et al., 2024).
3 Current evidence linking the gut microbiota to depression
3.1 Microbiota composition and diversity
Microbial diversity serves as a key indicator of gut ecosystem health, which is typically measured as alpha diversity (the richness and evenness of a sample) and the beta diversity (variance across groups). Less microbial diversity has been linked to many disease states, including MDD, in which patients frequently have less alpha diversity than healthy controls (Shen et al., 2021; Liu et al., 2023). This brings up the question of how much of the microbial change is due to antidepressant medication as opposed to the disease itself. The human gastrointestinal tract harbors a complex ecosystem of billions of microorganisms, collectively referred to as the gut microbiota, predominantly composed of bacteria. This microbial community plays a vital role in various physiological processes, including maintaining intestinal barrier integrity, regulating energy metabolism, defending against pathogens, and modulating immune function (Noh et al., 2021). While overall diversity and phylum-level analyses may not consistently reveal significant differences, studies comparing the gut microbiota of individuals with depression to healthy controls have identified specific bacterial taxa associated with this mental health condition (Gao et al., 2023).
MDD is often linked to a decrease in microbial diversity, characterized by lower levels of beneficial bacteria such as Bacteroides and Faecalibacterium in individuals with depression. Fluctuations in the relative abundance of Bacteroides have been observed in depressed individuals, with medication-free individuals exhibiting higher levels, suggesting a potential relationship between this bacterium and depressive symptoms. Faecalibacterium, a key producer of the anti-inflammatory SCFA butyrate, is significantly reduced in individuals with depression. This decline in beneficial microorganisms may contribute to the inflammatory processes observed in depression (Gao et al., 2023). Consistent findings across studies have revealed lower abundances of several beneficial bacterial genera, including Butyricicoccus, Coprococcus, Faecalibacterium, Fusicatenibacter, Eubacterium ventriosum group, Romboutsia, and Subdoligranulum in individuals with depression compared to healthy controls. Conversely, higher abundances of Eggerthella, Enterococcus, Escherichia, Flavonifractor, Holdemania, Lachnoclostridium, Paraprevotella, Rothia, and Streptococcus have been observed in individuals with depression. Several hypotheses have been proposed to explain these associations, primarily focusing on altered pro-inflammatory and anti-inflammatory signaling along the gut-brain axis (Gao et al., 2023).
3.1.1 Preclinical evidence
Research in animal models indicates that gut microbiota has a profound effect on stress responses, anxiety-like behaviors, depressive behaviors, and social behaviors. Neurological immunological, and endocrine pathways are mechanisms that could support the gut-brain link. Microbes can produce neuroactive compounds and affect host neurotransmitter signaling. Zhou et al. (2023) indicated that chronic unpredictable mild stress in animal models produces changes in depressive behaviours as well as composition of gut microbiota can regulate the physiological features of depression (Zhou et al., 2023). Similarly, studies involving animals have demonstrated that changes in gut microbiome can affect the action of antidepressants, and researchers have shown that certain antidepressants influence both the microbiome and the metabolism related to depression (Chevalier et al., 2020; Zhang W. et al., 2021).
3.1.2 Clinical evidence
In a clinical study of 90 young people in the United States, investigators compared gut microbiomes between 43 patients with MDD and healthy 47 controls. There were significant differences in gut microbiota community composition between groups across several taxonomic levels. On a phylum level, MDD patients showed lower levels of Firmicutes and higher levels of Bacteroidetes, with similar trends found in the class which are Clostridia and Bacteroidia and order levels as well (Liu et al., 2020). Ling et al. conducted a case-control study and determined participants who had experienced depression and whose faecal microbiota were significantly different than healthy control groups, confirming the notion that depressives’ symptoms are associated with specific gut microbiota profiles (Ling et al., 2022). The literature suggests there is often a correlation between depressive disorders and diminishing microbial diversity. Liu L. et al. (2022) also detected a negative association between microbial alpha-diversity and the severity of the depression symptoms measured in their study, suggesting people with more variety in their gut bacteria reported lower depressive symptoms (Liu L. et al., 2022). Also, Zhu J. et al. (2021) determined patients with anxiety and depression, had substantially less alpha diversity than healthy controls, demonstrating the link between diminished gut microbiota diversity and mental health disorders (Zhu J. et al., 2021).
3.2 Prebiotics, synbiotics, postbiotics, and probiotics
A review examined the role of probiotics, prebiotics, and postbiotics in alleviating depressive symptoms through modulation of the GBA (Luqman et al., 2024). In adults, the gut microbiota, primarily residing in the colon, can weigh up to approximately 1 kg. The dominant phyla within this complex ecosystem include Bacteroidetes and Firmicutes, while Actinobacteria, Proteobacteria, and Verrucomicrobia are present in smaller proportions. Additionally, the gut microbiome includes methanogenic archaea, eukaryotes (mainly yeast), and numerous bacteriophages (Bonaz et al., 2018). Probiotic consumption can help restore the balance of the gut microbiota (Alli et al., 2022). These beneficial microorganisms can positively influence the immune system, which is crucial for mood regulation. Depression and anxiety are often associated with dysregulated immune responses, characterized by increased inflammation. Probiotics may help mitigate this imbalance by stimulating anti-inflammatory responses and reducing the production of pro-inflammatory molecules (Rahmannia et al., 2024).
Prebiotics are non-digestible compounds, such as fructooligosaccharides, galacto-oligosaccharides, and xylooligosaccharides, that serve as food for gut microbes, thereby altering the gut microbiome composition in a manner that benefits the host (Bakshi et al., 2024; Bindels et al., 2015). They selectively stimulate the growth and/or activity of specific beneficial bacteria within the intestine, promoting improved host health (Bindels et al., 2015; Bevilacqua et al., 2024). Several studies have demonstrated the potential of prebiotics to influence stress, anxiety, and depression, possibly through a reduction in perceived stress associated with changes in Bifidobacterium spp. or other gut microbiota taxa (Bevilacqua et al., 2024).
Synbiotics synergistically combine probiotics and prebiotics (Alli et al., 2022). The primary objective of synbiotic supplementation is to enhance gut health by introducing beneficial bacteria (probiotics) and providing essential nutrients (prebiotics) to support their survival, growth, and activity within the digestive system. Meanwhile, postbiotics refer to substances formed as a result of formerly living microorganisms or inactivated microorganisms. A postbiotic may be composed of non-living, whole cells or structural elements of bacteria (e.g., cell walls). A postbiotic must be derived from a well-defined organism or mixture of organisms, with known genomic sequences, and produced using a reproducible technical approach to biomass production and inactivation (Vinderola et al., 2022). While probiotics are living microorganisms, postbiotics are composed of non-living microbial cells or their components, such as SCFAs, peptides, and polysaccharides. These substances can influence brain function and mood modulation through several potential mechanisms. Recent evidence suggests that some postbiotics may be superior and safer alternatives to probiotics for alleviating depressive symptoms. While research on postbiotics-based interventions for depression is still in its early stages, the evidence supporting their anti-depressive potential is promising (Liu et al., 2023).
Postbiotics can act on the immune system to reduce pro-inflammatory cytokines and increase anti-inflammatory responses. The reduction of systemic inflammation is crucial because chronic inflammation has been linked to the pathophysiology of depression (Chudzik et al., 2021). Postbiotics, such as SCFAs, have been shown to enhance the expression of tight junction proteins, improving gut barrier integrity and reducing intestinal permeability (Zhang et al., 2025). Additionally, postbiotics help preserve gut barrier function, preventing the translocation of lipopolysaccharides (LPS) into circulation, where they can lead to systemic inflammation and depressive symptoms (Varesi et al., 2023).
Probiotic compositions typically include diverse strains of Lactobacillus and Bifidobacterium, which have been shown to reduce inflammation and modulate immune responses by inhibiting the production of the cytokine IL-8 in human colon epithelial cells and decreasing intestinal permeability, thereby preventing endotoxemia (Alli et al., 2022). Prebiotics, such as dietary fiber, promote the growth of Lactobacilli and Bifidobacteria (Carlson et al., 2018). Animal studies have shown that supplementation with Bifidobacterium infantis can alleviate stress and depression in rats, while a 28-day course of Lactobacillus rhamnosus supplementation has been shown to reduce depressive symptoms in humans (Alli et al., 2022). Diets high in glucose, fructose, and sucrose can significantly alter the gut microbiota composition, marked by a dramatic increase in Bifidobacterium and a substantial decrease in Bacteroides. Studies in mice fed a fructose-rich diet have shown significant increases in Coprococcus, Ruminococcus, and Clostridium, while simultaneously observing a reduction in the Clostridiaceae family (Cryan et al., 2019). Figure 1 demonstrates the connection between gut microbiota and depression, highlighting how microbial imbalance contributes to neuroinflammation and neurotransmitter dysregulation. Also, to enhance clarity and evidence synthesis, support claims are presented in Table 2.
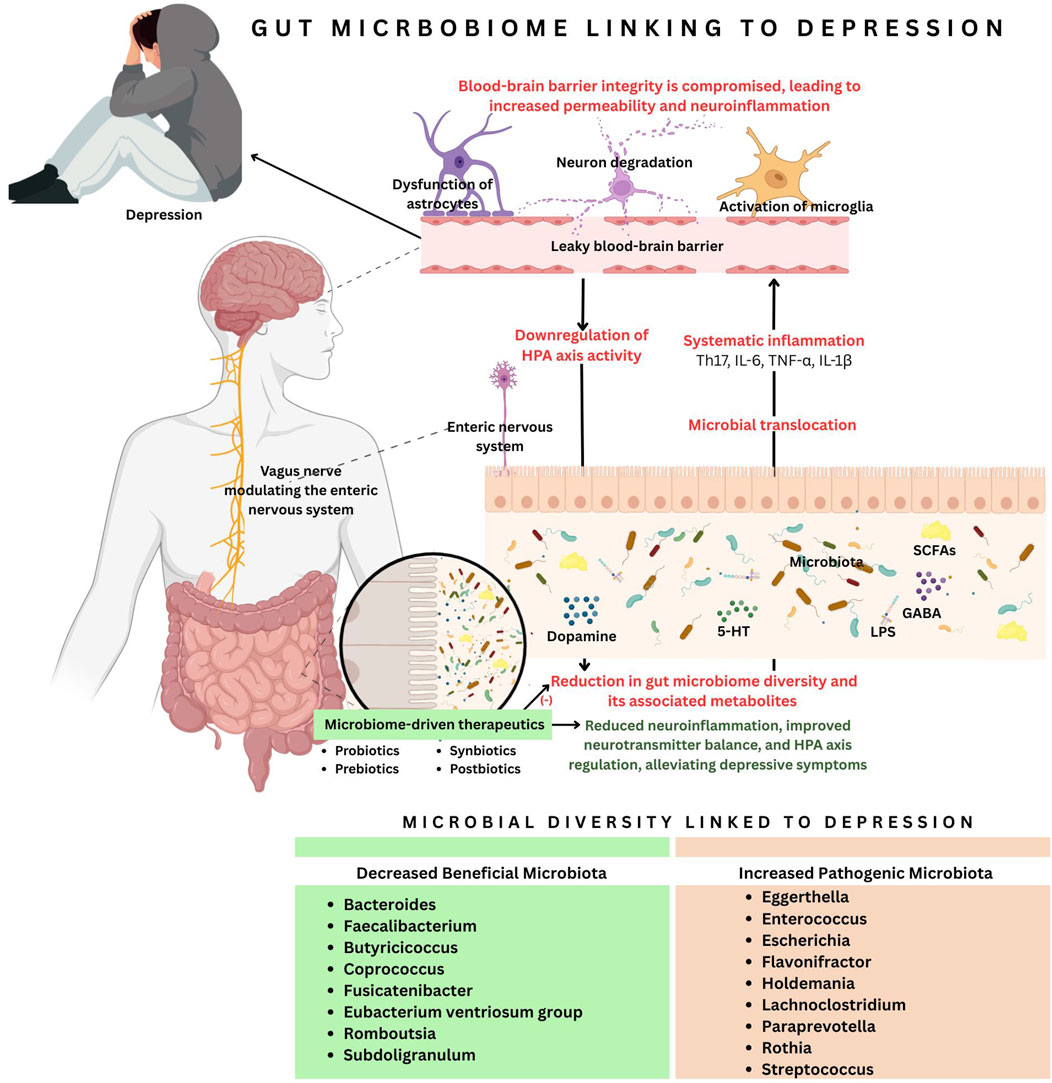
Figure 1. The connection between gut microbiota and depression highlights how microbial imbalance contributes to neuroinflammation and neurotransmitter dysregulation. A reduction in beneficial microbiota and an increase in pathogenic bacteria lead to microbial translocation, systemic inflammation (Th17, IL-6, TNF-α, IL-1β), and a leaky blood-brain barrier (BBB). This triggers neuron degeneration, microglial activation, and astrocyte dysfunction, resulting in reduced dopamine, serotonin, and gamma-aminobutyric acid (GABA) levels. Additionally, gut dysbiosis downregulates the hypothalamic-pituitary axis (HPA) and disrupts vagus nerve stimulation (VNS). Microbiota-driven therapeutics, such as probiotics, prebiotics, synbiotics and postbiotics, are a potential intervention to restore microbial imbalance and alleviate depressive symptoms.
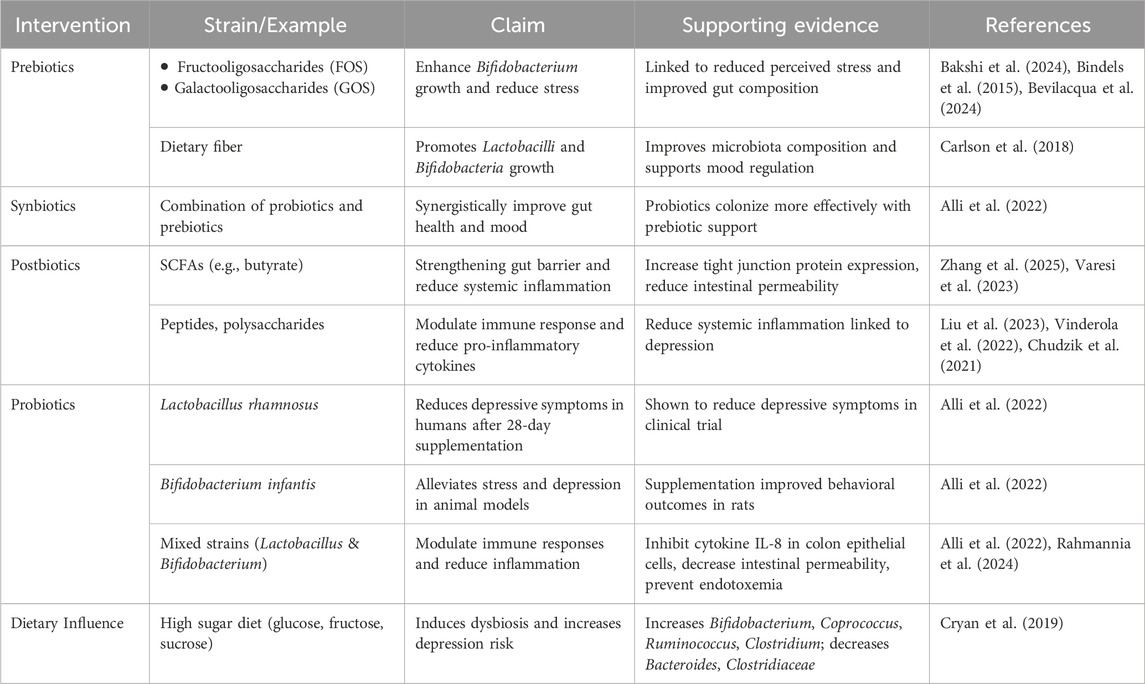
Table 2. Expanded support claims evidence: Prebiotics strains, Synbiotics strains, Postbiotics strains, Probiotics strains and Diet.
4 How depression alters the gut microbiota
Depression alters the composition and activity of the gut microbiota through several interconnected pathways, particularly inflammation, neurotransmitter modulation, and stress responses. Depressive states promote pro-inflammatory conditions that favour gut microorganisms associated with inflammation while inhibiting species that exert anti-inflammatory effects (Liu et al., 2024). Research on human depression has shown that depressive moods are linked to an increase in Bacteroides (pro-inflammatory) and a decrease in Clostridium species (SCFA producers), which affects butyrate metabolism (Liu et al., 2023). A lack of butyrate contributes to intestinal barrier dysfunction, leading to endotoxemia and systemic inflammation, which are further exacerbated by the depressive state (Liu et al., 2024). Additionally, there is an increase in Eggerthella and Ruminococcaceae species, which are associated with impaired glutamate metabolism, and a decrease in Coprococcus and Dialister species, which are involved in dopamine production (Radjabzadeh et al., 2022).
Furthermore, stress hormone signalling plays a crucial role in gut microbiota alterations. Microbial changes may occur early in MDD and could be part of its onset. Over time, pathogenic shifts in the gut microbiota contribute to dysbiosis by altering the gut environment (Liu et al., 2023). The activation of the HPA axis in depression increases cortisol production, which in turn affects gastrointestinal motility and secretion (Averina et al., 2024). These stress hormones also alter the gut environment, favouring certain pathogenic bacteria while disrupting beneficial SCFA-producing species (Bevilacqua et al., 2024; Madison and Kiecolt-Glaser, 2019). As a result, intestinal permeability (also known as “leaky gut”) increases, facilitating bacterial translocation (Kumar et al., 2023; Madison and Kiecolt-Glaser, 2019).
Dysbiosis in depression is also linked to neurotransmitter disturbances. Specific microbiota associated with depression shows reductions in neurotransmitter synthesis. For example, the depletion of Coprococcus results in a diminished supply of dopamine precursors, and strains like Coprococcus comes and Coprococcus catus are involved in the synthesis of DOPAC, a dopamine metabolite (Hamamah et al., 2022). Additionally, reductions in Subdoligranulum have been correlated with disruptions in GABA production (Radjabzadeh et al., 2022). Likewise, Bellach et al. (2024) noted that depletion of Subdoligranulum and other genera, such as Coprococcus and Faecalibacterium, may potentially serve as transdiagnostic markers of psychopathologies (Bellach et al., 2024). Also, Park et al. (2023) found that dysbiosis with Subdoligranulum associated with depressive symptoms, providing further support for these findings. They suggested that certain bacterial taxa, including Subdoligranulum, participate in neurotransmitter biosynthesis including GABA synthesis (Park et al., 2023). This implies that the presence of these gut microbes is significant for emotional regulation. Inhibited tryptophan metabolism also negatively affects serotonin production. Serotonin (5-HT) has been implicated in the development and treatment of depression, and its levels are regulated by the gut microbiota (Irum et al., 2023).
Alterations in microbial metabolites further contribute to depression-related changes in the gut. Depression has been shown to alter the gut metabolome by reducing SCFA synthesis, with 40%–60% less butyrate present in MDD patients (Liu et al., 2023; Liu et al., 2024). Additionally, the accumulation of neurotoxic metabolites such as p-cresol sulphate contributes to gut microbiota alterations in depression. p-Cresol has been shown to inactivate dopamine beta-hydroxylase, an enzyme necessary for converting dopamine into norepinephrine. This disruption can lead to neurotransmitter imbalances, which affect mood regulation and cognitive functions (Flynn et al., 2025).
Further research indicates that depressed individuals often exhibit an overabundance of bacteria responsible for producing phosphatidylcholines, contributing to gut dysbiosis and systemic toxicity that impair normal brain functioning (Xie et al., 2024). Disrupted bile acid metabolism has also been associated with depression. Secondary bile acids, modulated by the gut microbiota, are negatively correlated with the severity of depressive symptoms, suggesting that higher levels of bile acids may have a protective effect against depression (Liu et al., 2023).
Studies on depression treatments also demonstrate the influence of gut microbiota. For example, FMT from depressed individuals to germ-free rats successfully transmits depression traits, providing evidence for a causal relationship between the gut microbiota and depression (Irum et al., 2023). Conversely, supplementation with Clostridium butyricum, a butyrate-producing bacterium, has shown antidepressant properties by restoring gut microbial balance and reducing inflammation (Liu et al., 2024). These alterations in the gut microbiota create a self-perpetuating cycle, in which the conditions of depression worsen gut dysbiosis, neuroinflammation, and neurotransmitter imbalances. Figure 2 illustrates the difference between a healthy brain and a depressed brain and emphasizes the role of the GBA.
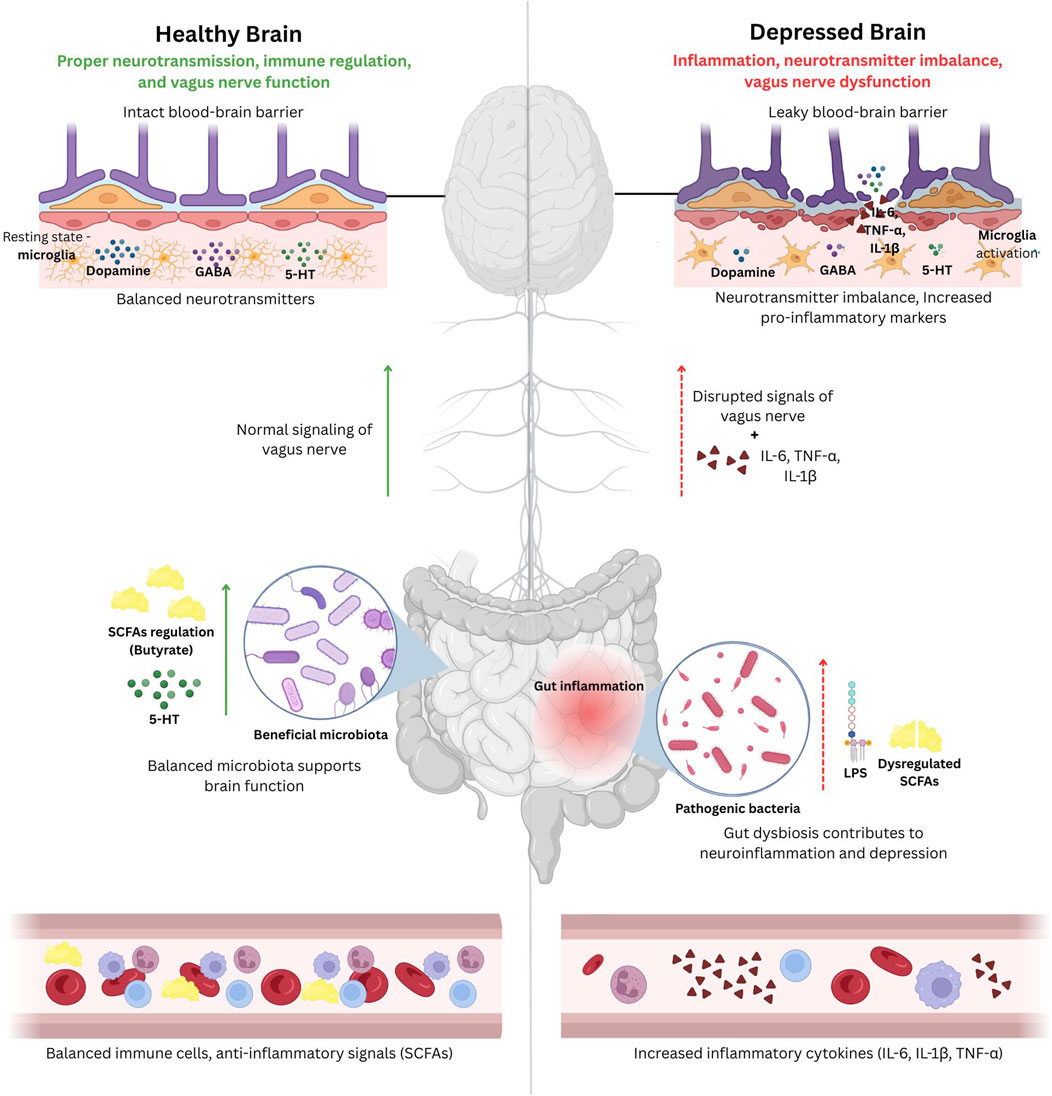
Figure 2. A schematic illustration of the difference between a healthy brain and a depressed brain and emphasizing the role of the gut-brain axis (GBA). The healthy brain (on the left) can maintain proper neurotransmission, regulation of the immune system, and vagus nerve regulation, all of which is supported by an intact blood-brain barrier, proper levels of neurotransmitters (dopamine, gamma-aminobutyric acid (GABA), serotonin) and ant-inflammatory signals (SCFAs produced by gut bacteria), and beneficial gut bacteria. Whereas, the depressed brain (on the right), will exhibit neuroinflammation, neurotransmitter imbalance, and vagus nerve dysfunction due to leaky blood-brain barrier, activation of microglia, increased levels of pro-inflammatory cytokines such as interleukins (IL-6, IL-1β) and tumor necrosis factor alpha (TNF-α), and gut dysbiosis leading to systemic inflammation. This demonstrates the impact of gut health on depression.
5 Mechanism of action
5.1 Neuroimmune modulation
Neuroinflammation, a hallmark of many neurological disorders, involves the activation of microglia and astrocytes in response to infections, trauma, and protein aggregation (e.g., amyloid-beta) (Zhang W. et al., 2023). These glial cells release pro-inflammatory cytokines and reactive oxygen species (ROS), contributing to neuronal damage. Conversely, neuroimmune regulation mediated by interactions between the nervous and immune systems helps maintain cytokine balance, protect the BBB, and limit microglial overactivation (Hein et al., 2020).
The GBA plays a central role in this process, with gut microbes producing SCFAs that modulate immune cells and neuroinflammation (Bostick et al., 2022). Gut dysbiosis disrupts this balance via increased lipopolysaccharide (LPS) translocation, triggering systemic inflammation and potentially altering blood-brain barrier permeability to psychoactive drugs (Clapp et al., 2017). This immune signaling, often driven by cytokines, can cross the BBB and promote neuroinflammation, a key factor in depression and other neuropsychiatric conditions (Medina-Rodriguez and Beurel, 2022).
Circadian rhythm disruption and chronic stress further exacerbate gut dysbiosis and neuroimmune dysfunction, impairing neurogenesis and increasing vulnerability to mood disorders (Ullah et al., 2024). Inflammatory depression, marked by elevated cytokines and altered gut microbiota (e.g., increased Bacteroides, decreased Clostridium), exemplifies this gut-immune-brain interaction (Liu et al., 2024; Zhang K. et al., 2023).
5.2 Neurotransmitter production
Neurotransmitters such as serotonin, dopamine, GABA, and glutamate are critical for brain function and are heavily influenced by gut microbiota. Although most serotonin (∼90%) is produced peripherally by enterochromaffin cells in the gut, its precursor tryptophan can cross the BBB for central synthesis (Chen et al., 2021). Gut microbes modulate tryptophan metabolism and influence neurotransmitter availability through several mechanisms, including direct precursor production, enzymatic catalysis, and signaling via microbial metabolites (Miri et al., 2023).
For example, spore-forming bacteria can enhance serotonin production by up-regulating TPH1 in enterochromaffin cells (Chen et al., 2021). Inflammatory pathways, particularly the kynurenine pathway activated by cytokines like IL-6, divert tryptophan from serotonin synthesis toward neurotoxic metabolites contributing to depression. Several bacteria (e.g., Streptococcus, Lactobacillus, Eubacterium coli) can also synthesize serotonin directly (Reyes-Martínez et al., 2023).
5.3 Vagus nerve pathways
The vagus nerve, the main conduit of gut-brain communication, is composed primarily of afferent fibers that relay sensory signals from the gut to the brain (Cao et al., 2024). These signals are processed in the nucleus tractus solitarii and influence emotion, behavior, and physiological homeostasis (Berthoud et al., 2021). Efferent vagal fibers transmit brain signals to the gut, regulating motility, secretion, inflammation, and even microbiota composition (Yu et al., 2020).
Gut-derived metabolites can stimulate vagal afferents, allowing microbial signals to influence brain activity (Jameson et al., 2024). This bidirectional feedback loop is integral to maintaining gut-brain homeostasis. Disruption through stress or dysbiosis can suppress vagal activity and activate the sympathetic nervous system, contributing to gastrointestinal and psychiatric disorders (Bonaz et al., 2018). Vagus nerve stimulation (VNS) has shown promise in treatment-resistant depression (Nahas et al., 2007). Clinical studies and neuroimaging suggest VNS modulates activity in mood-regulating brain regions such as the ventromedial prefrontal cortex, supporting its role in restoring emotional balance (Breit et al., 2018). Figure 3 illustrates the mechanism of action of GBA and its role in depression.
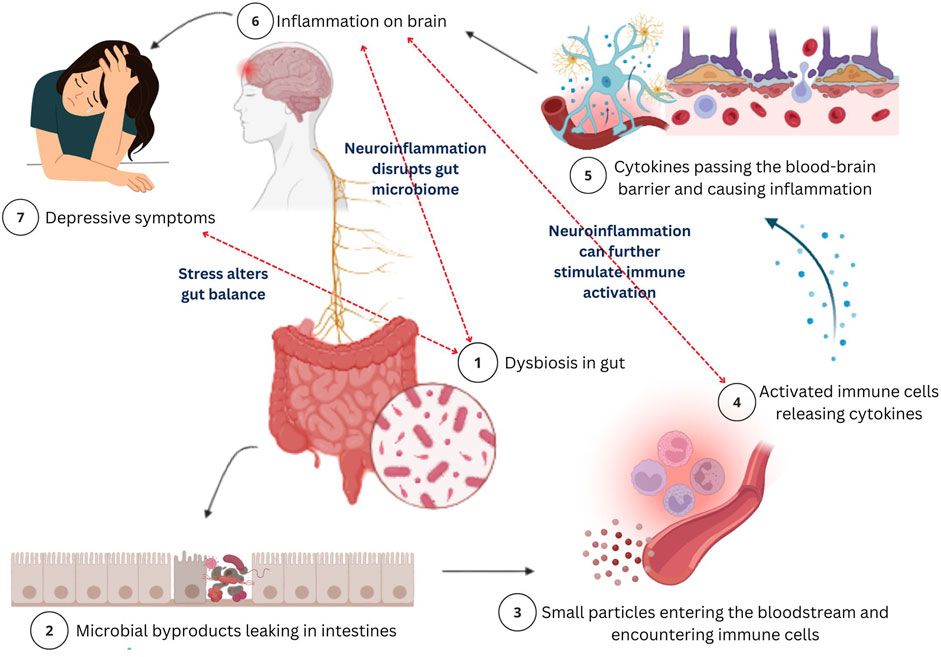
Figure 3. A schematic illustration of the mechanism of action of the gut-brain axis and its role in depression. Gut dysbiosis, an imbalance in gut bacteria (1) disrupts the normal gut function which leads to microbial byproducts leaking in intestines (2) and entering the bloodstream, allowing harmful particles to enter the bloodstream (3). These substances trigger an immune response, causing the release of cytokines (4). Further, these cytokines will travel to the brain by crossing the blood-brain barrier (BBB) (5) and causing inflammation (neuroinflammation) (6). This brain inflammation disrupts neurotransmitters and damages neurons, potentially leading to depression. The bidirectional nature is highlighted as neuroinflammation disrupts gut microbiota, exacerbating dysbiosis, while stress further alters gut balance, perpetuating this cycle of immune activation, inflammation, and depression. This process illustrates the connection between gut health and mental health, showing how gut bacteria imbalances can contribute to mood disorders through immune system dysfunction.
6 Specific microbial markers in depression
Mounting evidence has highlighted gut microbiota’s role in influencing neurochemical pathways involved in depression specifically through the modulation of neurotransmitters such as butyrate, GABA, glutamate, and serotonin (Radjabzadeh et al., 2022). Preclinical and clinical studies increasingly support the idea that microbial dysbiosis may contribute to the onset and progression of depression by disrupting these pathways (Radjabzadeh et al., 2022).
Recent research has identified specific microbial taxa associated with depressive symptoms, underscoring the intricate relationship between gut microbiota and mental health. A consistent finding across studies is the reduced abundance of butyrate-producing bacteria in individuals with MDD (Radjabzadeh et al., 2022; Modesto Lowe et al., 2023; Zhu L.-B. et al., 2021). Butyrate, a SCFA, plays a key role in maintaining intestinal barrier integrity, modulating inflammation, and supporting neurogenesis. It is commonly produced by Gram-positive anaerobic bacteria in the human colon through the fermentation of dietary fibers (Louis and Flint, 2009).
Butyrate can modulate the GBA via epigenetic mechanisms, such as upregulating cholinergic neuron expression, and can influence brain function by crossing the BBB and activating the vagus nerve and hypothalamus (Radjabzadeh et al., 2022). Several bacterial genera, including Faecalibacterium, Butyricimonas, Coprococcus, and Dialister, have been identified as key butyrate producers. Their depletion has been consistently associated with depressive symptoms (Singh et al., 2024; Radjabzadeh et al., 2022; Modesto Lowe et al., 2023). In particular:
1. Eggerthella and Actinomyces are increased in depression and correlate with lower levels of butyrate-producing bacteria (Suda and Matsuda, 2022).
2. Enterobacteriaceae, opportunistic pathogens enriched in depression, are often found alongside depleted levels of beneficial taxa such as Faecalibacterium (Gao et al., 2023).
3. Butyrate-producing microbes help maintain an anaerobic gut environment, which suppresses pathogenic colonization (e.g., Salmonella, E. coli) by increasing epithelial oxygen demand and preventing aerobic pathogen growth (Viana, 2024).
A large population-based study (Flemish Gut Flora Project, n = 1,054) and other investigations have shown that individuals with depression often exhibit reduced levels of Coprococcus and Dialister, alongside increased pro-inflammatory species such as Desulfovibrio (Modesto Lowe et al., 2023; Matsuzaki et al., 2024).
6.1 GABA-producing microbes
GABA is the brain’s primary inhibitory neurotransmitter and plays a critical role in mood regulation by counterbalancing excitatory signals like glutamate. Disruptions in GABA signaling are implicated in anxiety and depression. Certain gut bacteria can produce GABA, and their presence or absence may influence depressive symptoms via the gut-brain axis. For example:
Preclinical Evidence:
1. Lactobacillus rhamnosus has been shown to modulate GABA receptor expression in the brain and reduce depression-like behaviours in animal models (Liwinski et al., 2023).
2. Animal studies also support that GABA signalling can be regulated by gut microbiota through vagal nerve pathways, further reinforcing this gut–brain interaction (Radjabzadeh et al., 2022).
Clinical Evidence:
1. GABA-producing bacteria such as Parabacteroides, Eubacterium, Lactobacillus, Bifidobacterium, Bacteroides, and Blautia (mostly Bactroides fragilis) have been associated with depression in humans (Gao et al., 2023; Mhanna et al., 2024).
2. Some taxa, including Eggerthella, Subdoligranulum, Coprococcus, and Rumimococcaceae, have been associated with reduced GABA production and depression in human populations (Radjabzadeh et al., 2022).
6.2 Glutamate-producing microbes
Glutamate, the main excitatory neurotransmitter in the brain, is vital for synaptic plasticity, cognition, and emotional regulation. Its dysregulation has been implicated in the pathogenesis of depression. Gut microbes may influence glutamate production and associated neuroinflammatory pathways:
Preclinical Evidence:
1. Some strains of bacteria can alter neurotransmitter production and transport across and the blood-brain barrier, which can affect neuronal signalling (Radjabzadeh et al., 2022).
2. SCFAs such as butyrate have been associated with neuroprotective effects and antidepressant-like behaviours, primarily through their effects on glutamate release in experimental animals (Yang et al., 2023).
Clinical Evidence:
1. Individuals with MDD often exhibit a reduced presence of microbial genes involved in glutamate biosynthesis (Kovtun et al., 2022).
2. Species such as Faecalibacterium prausnitzii, Roseburia hominis, and Roseburia intestinalis which contribute to glutamate and SCFA metabolism are typically reduced in depression (Kovtun et al., 2022; Gruenbaum et al., 2024).
3. Increases in E. coli, Ruthenibacterium lactatiformans, Sellimonas, Eggerthella, Lachnoclostridium, and Hungatella have been associated with greater depressive symptomatology (Radjabzadeh et al., 2022; Averina et al., 2024).
6.3 Serotonin-producing microbes
Serotonin (5-HT), a key neurotransmitter in mood regulation, is predominantly synthesized (∼95%) in the gut. Its availability is tightly linked to gut microbiota, particularly through modulation of tryptophan metabolism and SCFA production (Lukić et al., 2022).
Preclinical Evidence:
1. Microbial metabolites such as phenolic and indolic compounds stimulate enteric serotonin production, which can influence central serotonin levels through the vagus nerve or systemic circulation (Margoob et al., 2024).
2. Turicibacter sanguinis can stimulate intestinal serotonin production and has been shown to absorb serotonin via a mechanism similar to the mammalian serotonin transporter (SERT). This uptake is inhibited by fluoxetine (an SSRI), suggesting functional relevance to antidepressant pathways (Hoffman and Margolis, 2020).
Clinical Evidence:
1. Lactobacillus and Bifidobacterium are known to enhance serotonin synthesis and have been linked to reduced depressive symptoms, highlighting their potential probiotic applications (Kosyra et al., 2024).
2. Individuals with depression have significantly altered gut microbiota composition, indicating that tryptophan-derived metabolites are impacted by gut microbiota diversity (Aleti et al., 2022).
7 Summary of microbial taxa associated with depression
Multiple microbial taxa have been implicated in depression through their roles in neurotransmitter production, inflammation regulation, and GBA (Table 3). These include:
• Butyrate-producing taxa: Faecalibacterium, Butyricimonas, Coprococcus, Subdoligranulum, E. ventriosum, Ruminococcus gauvreauii group.
• GABA-related taxa: L. rhamnosus, Bacteroides fragilis, Parabacteroides, Blautia.
• Glutamate-modulating taxa: F. prausnitzii, Roseburia spp., Sellimonas, Eggerthella, Hungatella.
• Serotonin-modulating taxa: Lactobacillus, Bifidobacterium, T. sanguinis.
• Pro-inflammatory taxa linked to depressive symptoms: Eggerthella, Desulfovibrio, Enterobacteriaceae, Lachnoclostridium.
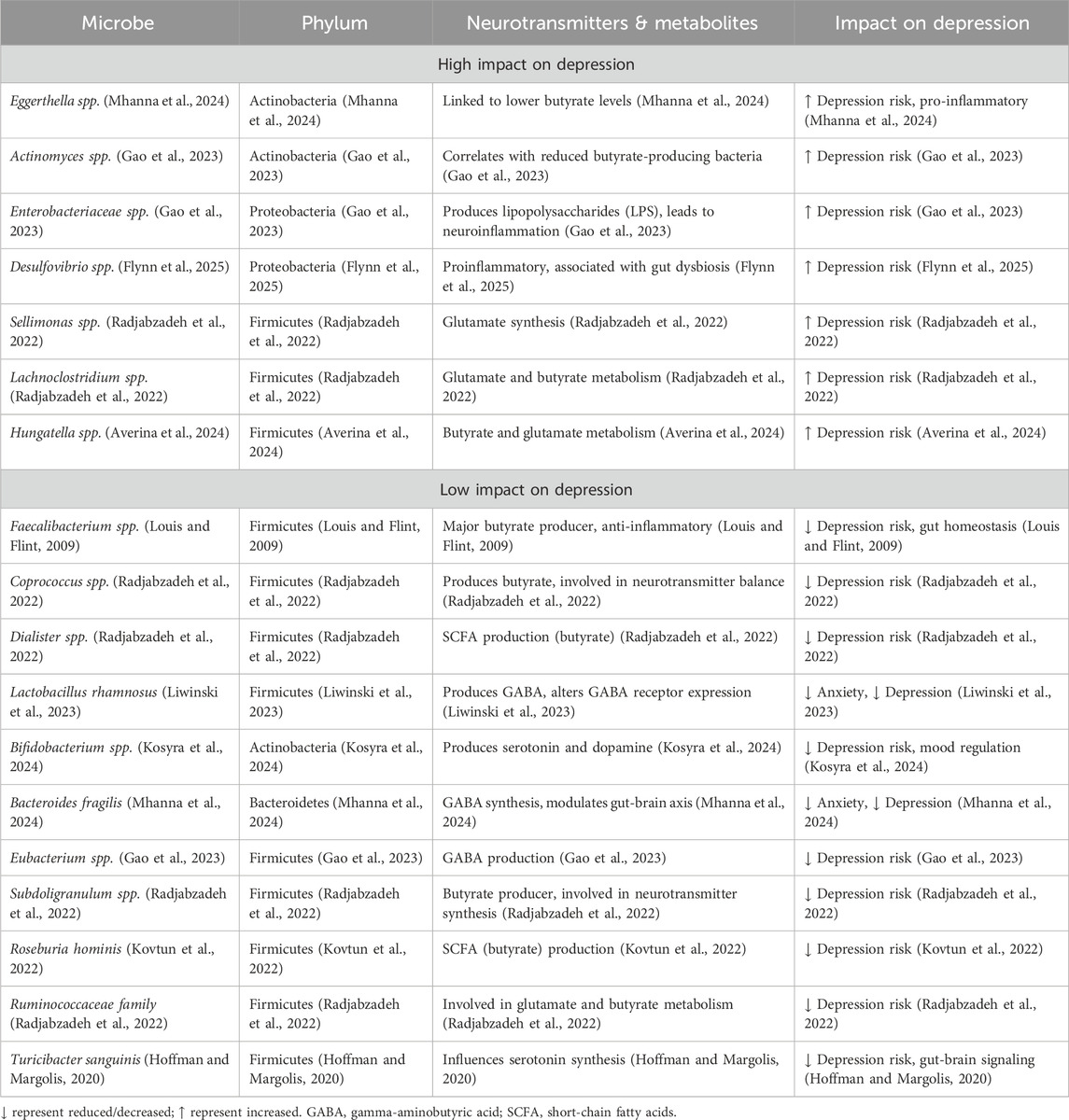
Table 3. A summary of the impact of different microbial taxa on depression through neurotransmitter and metabolite modulations.
These associations reflect the dynamic and multifactorial nature of gut-brain interactions and suggest potential biomarkers or therapeutic targets for microbiota-based interventions in depression.
8 Positive and negative impacts of psychotropic medications on gut microbiota
The interaction of psychotropic drugs and gut microbiota has increased attention in current research highlights, with conflicting findings between positive and negative for microbiota composition and health.
8.1 Antidepressant class
Selective Serotonin Reuptake Inhibitors (SSRIs), a widely used class of antidepressants, exemplify this negative uncertainty through their variable effects on the gut microbiota. Several studies indicates that SSRIs potentially reduce alpha diversity and affect healthy microbial communities which could cause dysbiosis and low-grade inflammation. Sertraline and fluoxetine, for an example, also possess antibacterial action, disrupting both Gram-positive and Gram-negative bacteria, where evidence suggests that these changes could worsen gut barrier dysfunction and immunological activation in certain cases (Hatamnejad et al., 2022). For instance, Zhang W. et al. (2021), noted that certain antidepressant like fluoxetine may alter intestinal microbiota and compromise gut microbiome function which can adversely impact in certain situations particularly when gut microbiome is in a vulnerable state (Zhang W. et al., 2021). Research also suggests that SSRIs may lead to dysbiosis and inflammation in the stomach due to their antibacterial capabilities, which potentially limit the beneficial microbial species (Ramsteijn et al., 2020; Desorcy-Scherer et al., 2024).
Alternatively, vortioxetine has gained prominence in the treatment of mood disorders due to its multimodal mode of action, which including serotonin reuptake inhibition and interactions with multiple serotonergic receptors. Vortioxetine’s action as a serotonin reuptake inhibitor and agonist at 5HT1A receptors is notable, as it acts as an antagonist at 5HT3 and 5HT7 receptors. Specifically, the 5HT7 receptor has been implicated in mood regulation and cognitive function. Such receptor-specific modulation is thought to facilitate neurogenesis and improve cognitive flexibility, which may address both emotional and cognition aspects of symptoms of depression. Vortioxetine’s broader physiological effects may extend to the GBA, which potentially supporting microbiome balance and overall health (Krupa et al., 2023). Besides that, Ye et al. (2021), also mentioned that certain genera such as Lachnospira and Roseburia, had a negative correlation to depression severity. This data demonstrates that Vortioxetine may induce a microbiome profile associated with better mental health outcomes. The GBA connecting the gut microbiota and the CNS is likely to be fundamental in mediating these effects (Ye et al., 2021).
8.2 Antipsychotic class
Antipsychotic drugs, particularly atypical antipsychotics including olanzapine and risperidone, are associated with serious metabolic health outcomes primarily though the alteration of metabolic pathways and altering gut microbiota. Evidence suggests that medication such as olanzapine drive the reduction of microbial richness in the gut, which correlates with adverse metabolic regulation outcomes, including weight gain and the risk for developing metabolic syndrome (Kamath et al., 2024; Pillinger et al., 2020; Jarari et al., 2025). Olanzapine, in particular, has demonstrated a substantial effect on consistent weight gain and the alteration of gut microbial profiles, causing a decrease in the Shannon’s index and beta diversity, subsequently affecting normal indexes of microbial diversity (Kamath et al., 2024).
Notably, lurasidone is an atypical antipsychotic, but has a different metabolic effect. Studies suggest lurasidone might help in developing better microbial diversity and has been called “gut neutral,” in contrast to the negative impacts of olanzapine and risperidone (Kamath et al., 2024; Collins et al., 2024). Preclinical studies suggest that Lurasidone enhances gut-derived metabolic profiles, making it a potentially promising treatment option for individuals at risk of metabolic side effects related to antipsychotic medications (Kamath et al., 2024; Munawar et al., 2021). Table 4 summarizes the classes of psychotropic medications, specifically contrasting antidepressants and antipsychotics, along with their mechanisms, microbiota impacts.
9 Demographic differences in gut microbiota and depression
Recent research has increasingly focused on the complex interplay between gut microbiota, demographic factors (such as sex and age), and MDD. Given the well-established sex-based disparities in the prevalence and presentation of MDD, emerging evidence suggests that differences in gut microbiota composition may partly explain these disparities.
Several studies have reported significant alterations in the gut microbiota of individuals with MDD, a condition known as dysbiosis. A comprehensive scoping review identified five studies reporting significant sex-specific differences in both alpha and beta diversity of gut microbiota among MDD patients compared to healthy controls (Niemela et al., 2024). Specifically, certain bacterial taxa appear to be more prevalent in women than in men. For example, female patients with MDD show higher relative abundances of specific bacteria compared to male patients under similar conditions (Niemela et al., 2024). In particular, women tend to have elevated levels of Actinobacteria, whereas men with MDD display lower levels of Bacteroidetes (Chen et al., 2018). Moreover, the severity of depressive symptoms has been linked to unique microbial signatures in each sex, suggesting that sex-specific biological processes may mediate the gut-brain connection in mood disorders (Niemela et al., 2024; Hu et al., 2023).
These findings underscore the importance of incorporating sex-stratified analyses in both the diagnosis and treatment of MDD. Gut microbial markers may hold potential as sex-specific diagnostic tools or treatment targets, highlighting the need for personalized approaches in mental health interventions (Niemela et al., 2024). In addition to sex differences, age-related changes in gut microbiota also appear to influence MDD pathogenesis. Notably, young adults (18–29 years) with MDD exhibit lower levels of Firmicutes and higher levels of Bacteroidetes, while middle-aged adults (30–59 years) demonstrate the opposite trend (Chen et al., 2020). In a study of young adults with depression, specific taxa such as Neisseria spp. and Prevotella nigrescens were found in significantly greater abundance among depressed individuals compared to healthy controls (Wingfield et al., 2021).
Furthermore, alterations were observed in the oral microbiome, with 21 bacterial species exhibiting significant differences in abundance, suggesting a broader microbial dysregulation involving both oral and gut environments (Wingfield et al., 2021). These microbial patterns may reflect underlying inflammatory mechanisms or contribute directly to the etiology of mood disorders (Kerff et al., 2024). In older adults, particularly those experiencing late-life depression, distinct microbial signatures have also been identified. Although taxonomic changes in this population are less well-defined, certain microbial alterations have been linked to both gastrointestinal symptoms and depressive mood (Miyaho et al., 2022). A common finding among elderly individuals is a decline in beneficial bacteria, such as Lactobacillus and Bifidobacterium, which has been associated with increased vulnerability to depression (Kumar et al., 2023). These age-related shifts may be influenced by cumulative health issues, diet, medication use, and systemic inflammation, all of which can affect gut-brain axis functioning (Zhang Q. et al., 2021).
Beyond age and sex, body mass index (BMI) and lifestyle-related factors such as ethnicity and urbanization also appear to shape gut microbiota composition. Higher BMI has been associated with reduced microbial diversity, a change that may contribute to both metabolic dysfunction and altered gut-brain communication (Cryan et al., 2019). Ethnicity, which often correlates with specific dietary and cultural practices, has also been linked to distinct gut microbial profiles, reflecting differences in environmental exposures and genetic backgrounds (Ren et al., 2023). Similarly, living environment urban versus rural plays a significant role. Urbanization is associated with reduced intra-individual gut microbiota diversity and increased variability between individuals, likely due to changes in diet, stress levels, antibiotic exposure, and environmental microbiota contact (Ren et al., 2023).
Taken together, these findings illustrate the critical role of demographic factors in shaping gut microbial ecosystems and influencing the onset, severity, and manifestation of depression. Recognizing and accounting for these variables is essential in developing more targeted, personalized strategies for mental health assessment and treatment.
10 Lifestyle and behavioral considerations in GBA modulation
Lifestyle factors including high-fibre diets, fermented foods, and prebiotics can promote microbiome diversity and may complement psychobiotic treatments by supporting mood and resilience to stress (Appleton, 2018; Aziz et al., 2024; Leeuwendaal et al., 2022). While behavioural frameworks and cultural tailing such as age, gender, ethnicity, surroundings) may impact adherence, these factors are less significant than the pharmacological modulation which is the focus of this research. Broader measures, such as microbiome-informed food policy or educational campaigns, may help to increase adoption, although they are only addressed here to offer context for psychobiotic therapies.
11 Research gaps and limitations
Nonetheless, despite progress we have barriers that inhibit GBA findings integrated into clinical practice. Human based studies often utilize small number of subjects or lack demographic diversity, and microbiota and targeted therapies are variable in strain, dosing and duration. This variability limits the comparability of studies, and we lack standardisation of microbial characterisation and classification of our intervention. Most studies have identified correlation rather than causation and we have unknown confounders, for example nutrient composition, treatment or regimens, sleep and socio-economic status.
Importantly, comprehensive strain-dose-duration-specific data are inconsistently recorded and hence cannot be provided systematically in this study. This omission emphasises are critical need that future research must fill. Large, well-controlled, and demographically varied cohorts, together with standardised methods and multi-omics techniques, are required to elucidate pathways and improve clinical translation.
12 Future directions and clinical translation
The next hurdle in the research of GBA is to turn foundational insights into functional therapeutics. Microbiome-based biomarkers could allow earlier identification and monitoring of potential therapies in depression, but repeatability is an issue due to changing effects of diet, medications and host physiology (Sochacka et al., 2024). Personalised microbiome-targeted therapeutics using custom probiotics, prebiotics, and synbiotics, are promising, but need to be thoroughly assessed on causative mechanisms, strain specific efficacy, and long-term safety (Ma et al., 2023). Regulatory conditions and quality assurance mechanisms are similarly even more important.
Psychobiotics with established mental health benefits are emerging as adjunct therapies to standard psychiatric treatments, but widespread application will require evidence of reliable efficacy and safety. The adoption of these ideas into clinical practice will require standardized methods of protocols, longitudinal assessments, and equitable availability.
Ultimately, improving clinical translation will require not just technical innovation but also collaborative, multidisciplinary work that links neurology with psychiatry. Gastrointestinal, dietic science and behavioural health. By bridging these disciplines, the field is ready to make advances with new, individualized solutions for depression prevention and management.
13 Conclusion
GBA represents a promising frontier in understanding and managing MDD. Emerging evidence underscores the role of gut microbiota in modulating mood through neuroimmune pathways, neurotransmitter production, and HPA axis regulation. Microbial imbalances are consistently linked to depressive symptoms, suggesting that restoring gut health may offer novel therapeutic opportunities. Microbiota-targeted strategies such as probiotics, prebiotics, and dietary interventions show potential as adjuncts to conventional treatments. However, progress is limited by small sample sizes, methodological inconsistencies, and a lack of causal evidence. Future research should focus on identifying reliable microbial biomarkers, leveraging advanced omics technologies, and developing personalized, evidence-based interventions. Integrating gut-focused approaches with existing therapies may enhance treatment outcomes, reduce resistance, and support holistic mental healthcare. Addressing regulatory, ethical, and implementation challenges will be essential to translating gut-brain research into effective, accessible clinical practice. Future research should prioritize human randomized controlled trials to validate microbial biomarkers (e.g., Coprococcus abundance) and explore pharmacomicrobiomics how gut microbes modulate drug pharmacokinetics and pharmacodynamics in depression.
Author contributions
ZZ: Conceptualization, Methodology, Visualization, Writing – original draft, Writing – review and editing, Project administration. ZH: Validation, Writing – review and editing. CC: Validation, Writing – review and editing. NS: Writing – review and editing. MC: Project administration, Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This APC was funded by an institutional grant from Management and Science University (MPG-001-022024-FHLS).
Acknowledgments
The authors would like to express their gratitude to Management and Science University for funding the article processing charge (APC). The authors also acknowledge that ChatGPT 4.0, an AI language model developed by OpenAI, was used to assist in refining certain limited sections of this manuscript, specifically for language editing and improvement. All AI-assisted content was carefully reviewed and thoroughly edited by the authors to ensure accuracy, scientific rigor, and compliance with academic writing standards. The authors assume full responsibility for the content of this manuscript, including any parts enhanced with AI tools, and remain accountable for any breach of publication ethics.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. In preparation of this work, we utilized AI-assisted tools, including ChatGPT 4.0, PaperPal, GeminiAI, and QuillBot. These tools were employed to support the initial drafting and refinement of specific sections of the manuscript and to enhance academic language. All AI-generated content underwent rigorous review and extensive editing by the authors to guarantee accuracy, scientific rigor, and compliance with academic writing standards. The authors bear full responsibility for the entire content of this manuscript, including any portions generated by AI tools, and are ac-countable for any violations of publication ethics.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Amrah, H., Saadah, O. I., Mosli, M., Annese, V., Al-Hindi, R. R., Edris, S., et al. (2023). Composition of the gut microbiota in patients with inflammatory bowel disease in Saudi Arabia: a pilot study. Saudi J. Gastroenterology 29 (2), 102–110. doi:10.4103/sjg.sjg_368_22
Aleti, G., Kohn, J., Troyer, E., Weldon, K., Huang, S., Tripathi, A., et al. (2022). Salivary bacterial signatures in depression-obesity comorbidity are associated with neurotransmitters and neuroactive dipeptides. BMC Microbiol. 22 (1), 75. doi:10.1186/s12866-022-02483-4
Alli, S. R., Gorbovskaya, I., Liu, J. C. W., Kolla, N. J., Brown, L., and Müller, D. J. (2022). The gut microbiome in depression and potential benefit of prebiotics, probiotics and synbiotics: a systematic review of clinical trials and observational studies. Int. J. Mol. Sci. 23 (9), 4494. doi:10.3390/ijms23094494
Angelova, I. Y., Kovtun, A. S., Averina, O. V., Koshenko, T. A., and Даниленко, В. Н. (2023). Unveiling the connection between microbiota and depressive disorder through machine learning. Int. J. Mol. Sci. 24 (22), 16459. doi:10.3390/ijms242216459
Appleton, J. (2018). The gut-brain axis: influence of microbiota on mood and mental health. Integr. Med. (Encinitas, Calif.) 17 (4), 28–32. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC6469458/
Averina, O. v., Poluektova, E. U., Zorkina, Y. A., Kovtun, A. S., and Danilenko, V. N. (2024). Human gut microbiota for diagnosis and treatment of depression. Int. J. Mol. Sci. 25 (11), 5782. doi:10.3390/ijms25115782
Aziz, T., Hussain, N., Hameed, Z., and Lin, L. (2024). Elucidating the role of diet in maintaining gut health to reduce the risk of obesity, cardiovascular and other age-related inflammatory diseases: recent challenges and future recommendations. Gut microbes 16 (1), 2297864. doi:10.1080/19490976.2023.2297864
Bakshi, I., Dey, S., Raut, A. J., Katta, S., and Sharma, P. (2024). Exploring the gut-brain axis: a comprehensive review of interactions between the gut microbiota and the central nervous system. Int. J. Multidiscip. Res. 6 (3), 19563. doi:10.36948/ijfmr.2024.v06i03.19563
Bellach, L., Kautzky-Willer, A., Heneis, K., Leutner, M., and Kautzky, A. (2024). The effects of caloric restriction and clinical psychological intervention on the interplay of gut microbial composition and stress in women. Nutrients 16 (16), 2584. doi:10.3390/nu16162584
Belmaker, R. H., and Agam, G. (2008). Major depressive disorder. N. Engl. J. Med. 358 (1), 55–68. doi:10.1056/NEJMra073096
Berthoud, H.-R., Albaugh, V. L., and Neuhuber, W. L. (2021). Gut-brain communication and obesity: understanding functions of the vagus nerve. J. Clin. Investigation 131 (10), e143770. doi:10.1172/JCI143770
Bevilacqua, A., Campaniello, D., Speranza, B., Racioppo, A., Sinigaglia, M., and Corbo, M. R. (2024). An update on prebiotics and on their health effects. Foods 13 (3), 446. doi:10.3390/foods13030446
Bindels, L. B., Delzenne, N. M., Cani, P. D., and Walter, J. (2015). Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterology and Hepatology 12 (5), 303–310. doi:10.1038/nrgastro.2015.47
Bonaz, B., Bazin, T., and Pellissier, S. (2018). The vagus nerve at the interface of the microbiota-gut-brain axis. Front. Neurosci. 12, 49. doi:10.3389/fnins.2018.00049
Bostick, J. W., Schonhoff, A. M., and Mazmanian, S. K. (2022). Gut microbiome-mediated regulation of neuroinflammation. Curr. Opin. Immunol. 76, 102177. doi:10.1016/j.coi.2022.102177
Breit, S., Kupferberg, A., Rogler, G., and Hasler, G. (2018). Vagus nerve as modulator of the brain–gut axis in psychiatric and inflammatory disorders. Front. Psychiatry 9, 44. doi:10.3389/fpsyt.2018.00044
Cao, Y., Li, R., and Bai, L. (2024). Vagal sensory pathway for the gut-brain communication. Seminars Cell and Dev. Biol. 156, 228–243. doi:10.1016/j.semcdb.2023.07.009
Carabotti, M., Scirocco, A., Antonietta Maselli, M., and Severi, C. (2015). The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 28, 203–209. Available online at: https://pubmed.ncbi.nlm.nih.gov/25830558/.
Carlson, J. L., Erickson, J. M., Lloyd, B. B., and Slavin, J. L. (2018). Health effects and sources of prebiotic dietary fiber. Curr. Dev. Nutr. 2 (3), nzy005. doi:10.1093/cdn/nzy005
Che Mohd Nassir, C. M. N., Che Ramli, M. D., Mohamad Ghazali, M., Jaffer, U., Abdul Hamid, H., Mehat, M. Z., et al. (2024). The microbiota–gut–brain axis: key mechanisms driving glymphopathy and cerebral small vessel disease. Life 15 (1), 3. doi:10.3390/life15010003
Chen, J., Zheng, P., Liu, Y., Zhong, X., Wang, H., Guo, Y., et al. (2018). Sex differences in gut microbiota in patients with major depressive disorder. Neuropsychiatric Dis. Treat. 14, 647–655. doi:10.2147/NDT.S159322
Chen, J.-J., He, S., Fang, L., Wang, B., Bai, S.-J., Xie, J., et al. (2020). Age-specific differential changes on gut microbiota composition in patients with major depressive disorder. Aging 12 (3), 2764–2776. doi:10.18632/aging.102775
Chen, Y., Xu, J., and Chen, Y. (2021). Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients 13 (6), 2099. doi:10.3390/nu13062099
Cheng, J., Hu, H., Ju, Y., Liu, J., Wang, M., Liu, B., et al. (2024). Gut microbiota-derived short-chain fatty acids and depression: deep insight into biological mechanisms and potential applications. General Psychiatry 37 (1), e101374. doi:10.1136/gpsych-2023-101374
Cheung, S. G., Goldenthal, A. R., Uhlemann, A.-C., Mann, J. J., Miller, J. M., and Sublette, M. E. (2019). Systematic review of gut microbiota and major depression. Front. Psychiatry 10, 34. doi:10.3389/fpsyt.2019.00034
Chevalier, G., Siopi, E., Guenin-Macé, L., Pascal, M., Laval, T., Rifflet, A., et al. (2020). Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat. Commun. 11 (1), 6363. doi:10.1038/s41467-020-19931-2
Choi, J., Kwon, H., Kim, Y., and Han, P. (2022). Extracellular vesicles from gram-positive and gram-negative probiotics remediate stress-induced depressive behavior in mice. Mol. Neurobiol. 59 (5), 2715–2728. doi:10.1007/s12035-021-02655-9
Chudzik, A., Orzyłowska, A., Rola, R., and Stanisz, G. J. (2021). Probiotics, prebiotics and postbiotics on mitigation of depression symptoms: modulation of the brain–gut–microbiome axis. Biomolecules 11 (7), 1000. doi:10.3390/biom11071000
Clapp, M., Aurora, N., Herrera, L., Bhatia, M., Wilen, E., and Wakefield, S. (2017). Gut microbiota’s effect on mental health: the gut-brain axis. Clin. Pract. 7 (4), 987. doi:10.4081/cp.2017.987
Collins, K., Kamath, S., Meola, T. R., Wignall, A., and Joyce, P. (2024). The oral bioavailability of lurasidone is impacted by changes to the gut microbiome: implications for antipsychotic therapy. doi:10.1101/2024.07.17.604016
Cryan, J. F., O’Riordan, K. J., Cowan, C. S. M., Sandhu, K. v., Bastiaanssen, T. F. S., Boehme, M., et al. (2019). The microbiota-gut-brain axis. Physiol. Rev. 99 (4), 1877–2013. doi:10.1152/physrev.00018.2018
Dawe, J., McCowan, L., Wilson, J., Okesene-Gafa, K., and Serlachius, A. (2020). Probiotics and maternal mental health: a randomised controlled trial among pregnant women with obesity. Sci. Rep. 10 (1), 1291. doi:10.1038/s41598-020-58129-w
Desorcy-Scherer, K., Zúñiga-Chaves, I., Reisner, M., Suen, G., and Hernandez, L. (2024). Investigating the influence of perinatal fluoxetine exposure on murine gut microbial communities during pregnancy and lactation. Sci. Rep. 14 (1), 13762. doi:10.1038/s41598-024-62224-7
Dong, Z., Xie, Q., Xu, F., Shen, X., Hao, Y., Li, J., et al. (2022). Neferine alleviates chronic stress-induced depression by regulating monoamine neurotransmitter secretion and gut microbiota structure. Front. Pharmacol. 13, 974949. doi:10.3389/fphar.2022.974949
Flynn, C. K., Adams, J. B., Krajmalnik-Brown, R., Khoruts, A., Sadowsky, M. J., Nirmalkar, K., et al. (2025). Review of elevated para-cresol in autism and possible impact on symptoms. Int. J. Mol. Sci. 26 (4), 1513. doi:10.3390/ijms26041513
Foster, J. A., and McVey Neufeld, K.-A. (2013). Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 36 (5), 305–312. doi:10.1016/j.tins.2013.01.005
Foster, J. A., Baker, G. B., and Dursun, S. M. (2021). The relationship between the gut microbiome-immune system-brain axis and major depressive disorder. Front. Neurology 12, 721126. doi:10.3389/fneur.2021.721126
Gao, M., Wang, J., Liu, P., Tu, H., Zhang, R., Zhang, Y., et al. (2023). Gut microbiota composition in depressive disorder: a systematic review, meta-analysis, and meta-regression. Transl. Psychiatry 13 (1), 379. doi:10.1038/s41398-023-02670-5
Gruenbaum, B. F., Merchant, K. S., Zlotnik, A., and Boyko, M. (2024). Gut microbiome modulation of glutamate dynamics: implications for brain health and neurotoxicity. Nutrients 16 (24), 4405. doi:10.3390/nu16244405
Guilherme, M. d. S., Valeri, F., Winter, J., Müller, M. B., Schwiertz, A., and Endres, K. (2022). Resilience and the gut microbiome: insights from chronically socially stressed wild-type mice. Microorganisms 10 (6), 1077. doi:10.3390/microorganisms10061077
Hamamah, S., Aghazarian, A., Nazaryan, A., Hajnal, A., and Covasa, M. (2022). Role of microbiota-gut-brain axis in regulating dopaminergic signaling. Biomedicines 10 (2), 436. doi:10.3390/biomedicines10020436
Hatamnejad, M. R., Ghavami, S. B., Shirvani, M., Ahmadabad, M. A., Shahrokh, S., Farmani, M., et al. (2022). Selective serotonin reuptake inhibitors and inflammatory bowel disease; beneficial or malpractice. Front. Immunol. 13, 980189. doi:10.3389/fimmu.2022.980189
Hein, Z. M., Kraiwattanapirom, N., Mukda, S., and Chetsawang, B. (2020). The induction of Neuron-Glial2 (NG2) expressing cells in methamphetamine toxicity-induced neuroinflammation in rat brain are averted by melatonin. J. Neuroimmunol. 344, 577232. doi:10.1016/j.jneuroim.2020.577232
Hoffman, J. M., and Margolis, K. G. (2020). Building community in the gut: a role for mucosal serotonin. Nat. Rev. Gastroenterology and Hepatology 17 (1), 6–8. doi:10.1038/s41575-019-0227-6
Hu, X., Li, Y., Wu, J., Zhang, H., Huang, Y., Tan, X., et al. (2023). Changes of gut microbiota reflect the severity of major depressive disorder: a cross sectional study. Transl. Psychiatry 13 (1), 137. doi:10.1038/s41398-023-02436-z
Huang, R., and Liu, Y. (2024). Efficacy of bifidobacterium-related preparations on depression: the first meta-analysis. Front. Psychiatry 15, 1463848. doi:10.3389/fpsyt.2024.1463848
Irum, N., Afzal, T., Faraz, M. H., Aslam, Z., and Rasheed, F. (2023). The role of gut microbiota in depression: an analysis of the gut-brain axis. Front. Behav. Neurosci. 17, 1185522. doi:10.3389/fnbeh.2023.1185522
Jameson, K. G., Kazmi, S. A., Ohara, T. E., Son, C., Yu, K. B., Mazdeyasnan, D., et al. (2024). Select microbial metabolites in the small intestinal lumen regulate vagal activity via receptor-mediated signaling. iScience 28 (2), 111699. doi:10.1016/j.isci.2024.111699
Jarari, A. M., Peela, J. R., Zakoko, A. M., Hawda, S. M., Rasoul, H. A. E., Peela, A. S. T., et al. (2025). The role of antipsychotic medications on metabolic and hematological parameters. Cureus 17, e82293. doi:10.7759/cureus.82293
Jesulola, E., Micalos, P., and Baguley, I. J. (2018). Understanding the pathophysiology of depression: from monoamines to the neurogenesis hypothesis model - are we there yet? Behav. Brain Res. 341, 79–90. doi:10.1016/j.bbr.2017.12.025
Kamath, S., Hunter, A., Collins, K., Wignall, A., and Joyce, P. (2024). The atypical antipsychotics lurasidone and olanzapine exert contrasting effects on the gut microbiome and metabolic function of rats. Br. J. Pharmacol. 181 (22), 4531–4545. doi:10.1111/bph.16507
Kano, M., and Fukudo, S. (2025). “Gut–brain interactions,” in Encyclopedia of the human brain (Elsevier), 312–333. doi:10.1016/B978-0-12-820480-1.00036-X
Kerff, F., Pasco, J. A., Williams, L. J., Jacka, F. N., Loughman, A., and Dawson, S. L. (2024). Associations between oral microbiota pathogens and elevated depressive and anxiety symptoms in men. doi:10.1101/2024.08.10.606819
Kosyra, K., Drabczyk, M., Marczyńska, Z., Zyśk, A., and Magda, I. (2024). Microbiota and depressive disorders – a review. J. Educ. Health Sport 60, 188–203. doi:10.12775/JEHS.2024.60.013
Kovtun, A. S., Averina, O. v., Angelova, I. Y., Yunes, R. A., Zorkina, Y. A., Morozova, A. Y., et al. (2022). Alterations of the composition and neurometabolic profile of human gut microbiota in major depressive disorder. Biomedicines 10 (9), 2162. doi:10.3390/biomedicines10092162
Krupa, A., Wojtasik-Bakalarz, K., and Siwek, M. (2023). Vortioxetine – pharmacological properties and use in mood disorders. The current state of knowledge. Psychiatr. Pol. 57 (6), 1109–1126. doi:10.12740/pp/onlinefirst/151570
Kumar, A., Pramanik, J., Goyal, N., Chauhan, D., Sivamaruthi, B. S., Prajapati, B. G., et al. (2023). Gut microbiota in anxiety and depression: unveiling the relationships and management options. Pharmaceuticals 16 (4), 565. doi:10.3390/ph16040565
Lee, S. M., Dong, T. S., Krause, B., Siddarth, P., Milillo, M. M., Lagishetty, V., et al. (2022). The intestinal microbiota as a predictor for antidepressant treatment outcome in geriatric depression: a prospective pilot study. Int. Psychogeriatrics 34 (1), 33–45. doi:10.1017/s1041610221000120
Leeuwendaal, N. K., Stanton, C., O'Toole, P. W., and Beresford, T. P. (2022). Fermented foods, health and the gut microbiome. Nutrients 14 (7), 1527. doi:10.3390/nu14071527
Li, Y., Li, J., Cheng, R., Liu, H., Zhao, Y., Liu, Y., et al. (2023). Alteration of the gut microbiome and correlated metabolism in a rat model of long-term depression. Front. Cell. Infect. Microbiol. 13, 1116277. doi:10.3389/fcimb.2023.1116277
Ling, Z., Cheng, Y., Chen, F., Yan, X., Liu, X., Shao, L., et al. (2022). Changes in fecal microbiota composition and the cytokine expression profile in school-aged children with depression: a case-control study. Front. Immunol. 13, 964910. doi:10.3389/fimmu.2022.964910
Liu, R. T., Rowan-Nash, A. D., Sheehan, A. E., Walsh, R. F. L., Sanzari, C. M., Korry, B. J., et al. (2020). Reductions in anti-inflammatory gut bacteria are associated with depression in a sample of young adults. Brain, Behav. Immun. 88, 308–324. doi:10.1016/j.bbi.2020.03.026
Liu, P., Gao, M., Liu, Z., Zhang, Y., Tu, H., Lei, L., et al. (2022a). Gut microbiome composition linked to inflammatory factors and cognitive functions in first-episode, drug-naive major depressive disorder patients. Front. Neurosci. 15, 800764. doi:10.3389/fnins.2021.800764
Liu, L., Wang, H., Zhang, H., Chen, X., Zhang, Y., Wu, J. Y., et al. (2022b). Toward a deeper understanding of gut microbiome in depression: the promise of clinical applicability. Adv. Sci. 9 (35), e2203707. doi:10.1002/advs.202203707
Liu, L., Wang, H., Chen, X., Zhang, Y., Zhang, H., and Xie, P. (2023). Gut microbiota and its metabolites in depression: from pathogenesis to treatment. EBioMedicine 90, 104527. doi:10.1016/j.ebiom.2023.104527
Liu, P., Liu, Z., Wang, J., Wang, J., Gao, M., Zhang, Y., et al. (2024). Immunoregulatory role of the gut microbiota in inflammatory depression. Nat. Commun. 15 (1), 3003. doi:10.1038/s41467-024-47273-w
Liwinski, T., Lang, U. E., Brühl, A. B., and Schneider, E. (2023). Exploring the therapeutic potential of gamma-aminobutyric acid in stress and depressive disorders through the gut–brain axis. Biomedicines 11 (12), 3128. doi:10.3390/biomedicines11123128
Louis, P., and Flint, H. J. (2009). Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 294 (1), 1–8. doi:10.1111/j.1574-6968.2009.01514.x
Lukić, I., Ivković, S., Mitić, M., and Adžić, M. (2022). Tryptophan metabolites in depression: modulation by gut microbiota. Front. Behav. Neurosci. 16, 987697. doi:10.3389/fnbeh.2022.987697
Luo, J., Chen, Y., Tang, G., Li, Z., Yang, X., Shang, X., et al. (2022). Gut microbiota composition reflects disease progression, severity and outcome, and dysfunctional immune responses in patients with hypertensive intracerebral hemorrhage. Front. Immunol. 13, 869846. doi:10.3389/fimmu.2022.869846
Luqman, A., He, M., Hassan, A., Ullah, M., Zhang, L., Rashid Khan, M., et al. (2024). Mood and microbes: a comprehensive review of intestinal microbiota’s impact on depression. Front. Psychiatry 15, 1295766. doi:10.3389/fpsyt.2024.1295766
Ma, K. K. Y., Anderson, J. K., and Burn, A. M. (2023). Review: school-Based interventions to improve mental health literacy and reduce mental health stigma - a systematic review. Child Adolesc. Ment. health 28 (2), 230–240. doi:10.1111/camh.12543
Madison, A., and Kiecolt-Glaser, J. K. (2019). Stress, depression, diet, and the gut microbiota: human–bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 28, 105–110. doi:10.1016/j.cobeha.2019.01.011
Maes, M., Vasupanrajit, A., Jirakran, K., Klomkliew, P., Chanchaem, P., Tunvirachaisakul, C., et al. (2022). Exploration of the gut microbiome in thai patients with major depressive disorder uncovered a specific bacterial profile with depletion of theruminococcusgenus as a putative biomarker. doi:10.1101/2022.11.06.22282014
Mansuy-Aubert, V., and Ravussin, Y. (2023). Short chain fatty acids: the messengers from down below. Front. Neurosci. 17, 1197759. doi:10.3389/fnins.2023.1197759
Margoob, M., Kouser, S., and Jan, N. (2024). “Serotonin: the link between gut Microbiome and brain,” in Serotonin - Neurotransmitter and hormone of brain, bowels and blood (London, United Kingdom: IntechOpen). doi:10.5772/intechopen.1003826
Matsuzaki, J., Kurokawa, S., Iwamoto, C., Miyaho, K., Takamiya, A., Ishii, C., et al. (2024). Intestinal metabolites predict treatment resistance of patients with depression and anxiety. Gut Pathog. 16 (1), 8. doi:10.1186/s13099-024-00601-3
McGuinness, A. J., Davis, J. A., Dawson, S. L., Loughman, A., Collier, F., O’Hely, M., et al. (2022). A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol. Psychiatry 27 (4), 1920–1935. doi:10.1038/s41380-022-01456-3
Medina-Rodriguez, E. M., and Beurel, E. (2022). Blood brain barrier and inflammation in depression. Neurobiol. Dis. 175, 105926. doi:10.1016/j.nbd.2022.105926
Mhanna, A., Martini, N., Hmaydoosh, G., Hamwi, G., Jarjanazi, M., Zaifah, G., et al. (2024). The correlation between gut microbiota and both neurotransmitters and mental disorders: a narrative review. Medicine 103 (5), e37114. doi:10.1097/MD.0000000000037114
Miri, S., Yeo, J., Abubaker, S., and Hammami, R. (2023). Neuromicrobiology, an emerging neurometabolic facet of the gut microbiome? Front. Microbiol. 14, 1098412. doi:10.3389/fmicb.2023.1098412
Miyaho, K., Sanada, K., Kurokawa, S., Tanaka, A., Tachibana, T., Ishii, C., et al. (2022). The potential impact of age on gut microbiota in patients with major depressive disorder: a secondary analysis of the prospective observational study. J. Personalized Med. 12 (11), 1827. doi:10.3390/jpm12111827
Modesto Lowe, V., Chaplin, M., and Sgambato, D. (2023). Major depressive disorder and the gut microbiome: what is the link? General Psychiatry 36 (1), e100973. doi:10.1136/gpsych-2022-100973
Morys, J., Małecki, A., and Nowacka-Chmielewska, M. (2024). Stress and the gut-brain axis: an inflammatory perspective. Front. Mol. Neurosci. 17, 1415567. doi:10.3389/fnmol.2024.1415567
Mottawea, W., Sultan, S., Landau, K., Bordenave, N., and Hammami, R. (2020). Evaluation of the prebiotic potential of a commercial synbiotic food ingredient on gut microbiota in an ex vivo model of the human Colon. Nutrients 12 (9), 2669. doi:10.3390/nu12092669
Munawar, N., Ahsan, K., Muhammad, K., Ahmad, A., Anwar, M. A., Shah, I., et al. (2021). Hidden role of gut microbiome dysbiosis in schizophrenia: antipsychotics or psychobiotics as therapeutics? Int. J. Mol. Sci. 22 (14), 7671. doi:10.3390/ijms22147671
Nahas, Z., Teneback, C., Chae, J.-H., Mu, Q., Molnar, C., Kozel, F. A., et al. (2007). Serial vagus nerve stimulation functional MRI in treatment-resistant depression. Neuropsychopharmacology 32 (8), 1649–1660. doi:10.1038/sj.npp.1301288
Nakhal, M. M., Yassin, L. K., Alyaqoubi, R., Saeed, S., Alderei, A., Alhammadi, A., et al. (2024). The microbiota–gut–brain axis and neurological disorders: a comprehensive review. Life 14 (10), 1234. doi:10.3390/life14101234
Nguyen, T. T., Kościółek, T., Maldonado, Y., Daly, R., Martin, A. S., McDonald, D., et al. (2019). Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophrenia Res. 204, 23–29. doi:10.1016/j.schres.2018.09.014
Niemela, L., Lamoury, G., Carroll, S., Morgia, M., Yeung, A., and Oh, B. (2024). Exploring gender differences in the relationship between gut microbiome and depression - a scoping review. Front. Psychiatry 15, 1361145. doi:10.3389/fpsyt.2024.1361145
Nik Ramli, N. N., Adzmi, N. A. S., Nasarudin, S. N. A. Z., Hashim, M. H., Abdul Mutalib, M., Mohamad Alwi, M. N., et al. (2024). Environmental enrichment preconditioning prevents chronic social defeat stress-induced memory impairment and hippocampal neurodegeneration. Asia Pac. J. Mol. Biol. Biotechnol., 29–38. doi:10.35118/apjmbb.2024.032.4.04
Noh, H., Jang, H.-H., Kim, G., Zouiouich, S., Cho, S.-Y., Kim, H.-J., et al. (2021). Taxonomic composition and diversity of the gut microbiota in relation to habitual dietary intake in Korean adults. Nutrients 13 (2), 366. doi:10.3390/nu13020366
Otte, C., Gold, S. M., Penninx, B. W., Pariante, C. M., Etkin, A., Fava, M., et al. (2016). Major depressive disorder. Nat. Rev. Dis. Prim. 2 (1), 16065. doi:10.1038/nrdp.2016.65
Park, S., Li, C., Wu, X., and Zhang, T. (2023). Gut microbiota alterations and their functional differences in depression according to enterotypes in Asian individuals. Int. J. Mol. Sci. 24 (17), 13329. doi:10.3390/ijms241713329
Pillinger, T., McCutcheon, R. A., Vano, L., Mizuno, Y., Arumuham, A., Hindley, G., et al. (2020). Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry 7 (1), 64–77. doi:10.1016/s2215-0366(19)30416-x
Prochazková, P., Roubalová, R., Dvořák, J., Kreisinger, J., Hill, M., Tlaskalová-Hogenová, H., et al. (2021). The intestinal microbiota and metabolites in patients with anorexia nervosa. Gut Microbes 13 (1), 1–25. doi:10.1080/19490976.2021.1902771
Radjabzadeh, D., Bosch, J. A., Uitterlinden, A. G., Zwinderman, A. H., Ikram, M. A., van Meurs, J. B. J., et al. (2022). Gut microbiome-wide association study of depressive symptoms. Nat. Commun. 13 (1), 7128. doi:10.1038/s41467-022-34502-3
Rafiee Sardooi, A., Reisi, P., and Yazdi, A. (2020). Protective effect of honey on learning and memory impairment, depression and neurodegeneration induced by chronic unpredictable mild stress. Physiology Pharmacol. 25 (1), 21–35. doi:10.32598/ppj.25.1.90
Rahmannia, M., Poudineh, M., Mirzaei, R., Aalipour, M. A., Shahidi Bonjar, A. H., Goudarzi, M., et al. (2024). Strain-specific effects of probiotics on depression and anxiety: a meta-analysis. Gut Pathog. 16 (1), 46. doi:10.1186/s13099-024-00634-8
Ramsteijn, A. S., Jašarević, E., Houwing, D. J., Bale, T. L., and Olivier, B. (2020). Antidepressant treatment with fluoxetine during pregnancy and lactation modulates the gut microbiome and metabolome in a rat model relevant to depression. Gut Microbes 11 (4), 735–753. doi:10.1080/19490976.2019.1705728
Ren, Y., Wu, J., Wang, Y., Zhang, L., Ren, J., Zhang, Z., et al. (2023). Lifestyle patterns influence the composition of the gut microbiome in a healthy Chinese population. Sci. Rep. 13 (1), 14425. doi:10.1038/s41598-023-41532-4
Reyes-Martínez, S., Segura-Real, L., Gómez-García, A. P., Tesoro-Cruz, E., Constantino-Jonapa, L. A., Amedei, A., et al. (2023). Neuroinflammation, microbiota-gut-brain axis, and depression: the vicious circle. J. Integr. Neurosci. 22 (3), 65. doi:10.31083/j.jin2203065
Sarawagi, A., Soni, N. D., and Patel, A. B. (2021). Glutamate and GABA homeostasis and neurometabolism in major depressive disorder. Front. Psychiatry 12, 637863. doi:10.3389/fpsyt.2021.637863
Scala, M., Tabone, M., Paolini, M., Salueña, A., Iturra, R. A., Ferreiro, V. R., et al. (2025). Unlocking the link between gut microbiota and psychopathological insights in anorexia nervosa: a systematic review. Eur. Eat. Disord. Rev. 33 (4), 700–718. doi:10.1002/erv.3179
Sharma, R., Gupta, D., Mehrotra, R., and Mago, P. (2021). Psychobiotics: the next-generation probiotics for the brain. Curr. Microbiol. 78 (2), 449–463. doi:10.1007/s00284-020-02289-5
Shen, Y., Yang, X., Li, G., Gao, J., and Liang, Y. (2021). The change of gut microbiota in MDD patients under SSRIs treatment. Sci. Rep. 11 (1), 14918. doi:10.1038/s41598-021-94481-1
Silva, Y. P., Bernardi, A., and Frozza, R. L. (2020). The role of short-chain fatty acids from gut Microbiota in gut-brain communication. Front. Endocrinol. 11, 25. doi:10.3389/fendo.2020.00025
Singh, J., Singh, A., Biswal, S., Zomuansangi, R., Lalbiaktluangi, C., Singh, B. P., et al. (2024). Microbiota-brain axis: exploring the role of gut microbiota in psychiatric disorders - a comprehensive review. Asian J. Psychiatry 97, 104068. doi:10.1016/j.ajp.2024.104068
Sochacka, K., Kotowska, A., and Lachowicz-Wiśniewska, S. (2024). The role of gut microbiota, nutrition, and physical activity in depression and obesity-interdependent Mechanisms/co-occurrence. Nutrients 16 (7), 1039. doi:10.3390/nu16071039
Suda, K., and Matsuda, K. (2022). How microbes affect depression: underlying mechanisms via the gut–brain axis and the modulating role of probiotics. Int. J. Mol. Sci. 23 (3), 1172. doi:10.3390/ijms23031172
Sun, S., Yu, H., Yu, R., and Wang, S. (2023). Functional connectivity between the amygdala and prefrontal cortex underlies processing of emotion ambiguity. Transl. psychiatry 13 (1), 334. doi:10.1038/s41398-023-02625-w
Ullah, H., Arbab, S., Tian, Y., Chen, Y., Liu, C., Li, Q., et al. (2024). Crosstalk between gut microbiota and host immune system and its response to traumatic injury. Front. Immunol. 15, 1413485. doi:10.3389/fimmu.2024.1413485
Valles-Colomer, M., Falony, G., Darzi, Y., Tigchelaar, E. F., Wang, J., Tito, R. Y., et al. (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4 (4), 623–632. doi:10.1038/s41564-018-0337-x
Varesi, A., Campagnoli, L. I. M., Chirumbolo, S., Candiano, B., Carrara, A., Ricevuti, G., et al. (2023). The brain-gut-microbiota interplay in depression: a key to design innovative therapeutic approaches. Pharmacol. Res. 192, 106799. doi:10.1016/j.phrs.2023.106799
Viana, T. R. X. (2024). Relationship of gut microbiota and mental health: the influence of the gut-brain axis. Int. J. Health Sci. 4 (25), 2–6. doi:10.22533/at.ed.1594252411039
Vinderola, G., Sanders, M. E., and Salminen, S. (2022). The concept of postbiotics. Foods 11 (8), 1077. doi:10.3390/foods11081077
Wingfield, B., Lapsley, C., McDowell, A., Miliotis, G., McLafferty, M., O’Neill, S. M., et al. (2021). Variations in the oral microbiome are associated with depression in young adults. Sci. Rep. 11 (1), 15009. doi:10.1038/s41598-021-94498-6
Xie, Z., Huang, J., Sun, G., He, S., Luo, Z., Zhang, L., et al. (2024). Integrated multi-omics analysis reveals gut microbiota dysbiosis and systemic disturbance in major depressive disorder. Psychiatry Res. 334, 115804. doi:10.1016/j.psychres.2024.115804
Xiong, R.-G., Li, J., Cheng, J., Zhou, D.-D., Wu, S.-X., Huang, S.-Y., et al. (2023). The role of gut microbiota in anxiety, depression, and other mental disorders as well as the protective effects of dietary components. Nutrients 15 (14), 3258. doi:10.3390/nu15143258
Yang, H., Li, M., Zhou, M., Xu, H., Huan, F., Liu, N., et al. (2021). Links between gut dysbiosis and neurotransmitter disturbance in chronic restraint stress-induced depressive behaviours: the role of inflammation. Inflammation 44 (6), 2448–2462. doi:10.1007/s10753-021-01514-y
Yang, Y., Mori, M., Wai, K. M., Jiang, T., Sugimura, Y., Munakata, W., et al. (2023). The association between gut microbiota and depression in the Japanese population. Microorganisms 11 (9), 2286. doi:10.3390/microorganisms11092286
Yao, H., Yang, H., Wang, Y., Xing, Q., Lin, Y., and Chai, Y. (2022). Gut microbiome and fecal metabolic alteration in systemic lupus erythematosus patients with depression. Front. Cell. Infect. Microbiol. 12, 1040211. doi:10.3389/fcimb.2022.1040211
Ye, X., Wang, D., Zhu, H., Wang, D., Li, J., Tang, Y., et al. (2021). Gut microbiota changes in patients with major depressive disorder treated with vortioxetine. Front. psychiatry 12, 641491. doi:10.3389/fpsyt.2021.641491
Yu, C. D., Xu, Q. J., and Chang, R. B. (2020). Vagal sensory neurons and gut-brain signaling. Curr. Opin. Neurobiol. 62, 133–140. doi:10.1016/j.conb.2020.03.006
Zainal Abidin, Z., Junit, J., Che Ramli, M. D., Seow, S. L. S., Cheng, P. G., Eshak, Z., et al. (2023). The antidepressant-like effect of NevGro® forte in chronic unpredictable mild stress (CUMS) model of depression in rats. Neurosci. Res. Notes 6 (4). doi:10.31117/neuroscirn.v6i4.261
Zhang, F. F., Peng, W., Sweeney, J. A., Jia, Z. Y., and Gong, Q. Y. (2018). Brain structure alterations in depression: psychoradiological evidence. CNS Neurosci. and Ther. 24 (11), 994–1003. doi:10.1111/cns.12835
Zhang, W., Qu, W., Wang, H., and Yan, H. (2021a). Antidepressants fluoxetine and amitriptyline induce alterations in intestinal microbiota and gut microbiome function in rats exposed to chronic unpredictable mild stress. Transl. Psychiatry 11 (1), 131. doi:10.1038/s41398-021-01254-5
Zhang, Q., Yun, Y., An, H., Zhao, W., Ma, T., Wang, Z., et al. (2021b). Gut microbiome composition associated with major depressive disorder and sleep quality. Front. Psychiatry 12, 645045. doi:10.3389/fpsyt.2021.645045
Zhang, Y., Zhang, R., Liu, P., Wang, J., Gao, M., Zhang, J., et al. (2022). Characteristics and mediating effect of gut microbiota with experience of childhood maltreatment in major depressive disorder. Front. Neurosci. 16, 926450. doi:10.3389/fnins.2022.926450
Zhang, W., Xiao, D., Mao, Q., and Xia, H. (2023a). Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 8 (1), 267. doi:10.1038/s41392-023-01486-5
Zhang, K., Liu, P., Liu, Z., Wang, J., Wang, J., Gao, M., et al. (2023b). Gut microbiota characteristics and its immunoregulatory role in inflammatory depression: joint clinical and animal data. doi:10.21203/rs.3.rs-3154268/v1
Zhang, J., He, J., Hu, J., Ji, Y., and Lou, Z. (2025). Exploring the role of gut microbiota in depression: pathogenesis and therapeutic insights. Asian J. Psychiatry 105, 104411. doi:10.1016/j.ajp.2025.104411
Zheng, P., Zeng, B., Zhou, C., Liu, M., Fang, Z., Xu, X., et al. (2016). Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 21 (6), 786–796. doi:10.1038/mp.2016.44
Zhou, C., Chen, Y., Xue, S., Shi, Q., Guo, L., Yu, H., et al. (2023). Rtms ameliorates depressive-like behaviors and regulates the gut microbiome and medium- and long-chain fatty acids in mice exposed to chronic unpredictable mild stress. CNS Neurosci. and Ther. 29 (11), 3549–3566. doi:10.1111/cns.14287
Zhu, J., Li, M., Shao, D., Ma, S., and Wei, W. (2021a). Altered fecal microbiota signatures in patients with anxiety and depression in the gastrointestinal cancer screening: a case-control study. Front. Psychiatry 12, 757139. doi:10.3389/fpsyt.2021.757139
Keywords: major depressive disorder, therapy, psychobiotics, postbiotics, gut microbiota
Citation: Zainal Abidin Z, Hein ZM, Che Mohd Nassir CMN, Shari N and Che Ramli MD (2025) Pharmacological modulation of the gut-brain axis: psychobiotics in focus for depression therapy. Front. Pharmacol. 16:1665419. doi: 10.3389/fphar.2025.1665419
Received: 14 July 2025; Accepted: 12 September 2025;
Published: 26 September 2025.
Edited by:
Magdalena Sowa-Kućma, University of Rzeszow, PolandReviewed by:
Xiaolei Zhu, Johns Hopkins University, United StatesMarcin Siwek, Jagiellonian University, Poland
Copyright © 2025 Zainal Abidin, Hein, Che Mohd Nassir, Shari and Che Ramli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Danial Che Ramli, bXVoZGRhbmlhbF9jaGVyYW1saUBtc3UuZWR1Lm15
 Zakirah Zainal Abidin
Zakirah Zainal Abidin Zaw Myo Hein
Zaw Myo Hein Che Mohd Nasril Che Mohd Nassir
Che Mohd Nasril Che Mohd Nassir Norshafarina Shari
Norshafarina Shari Muhammad Danial Che Ramli
Muhammad Danial Che Ramli