- 1University of Pittsburgh School of Medicine (UPSOM), Pittsburgh, PA, United States
- 2Department of Anesthesiology and Perioperative Medicine (UPSOM), Pittsburgh, PA, United States
- 3Veterans Affairs Pittsburgh Healthcare System, Surgery Service Line, Pittsburgh, PA, United States
- 4Department of General Internal Medicine, University of Pittsburgh School of Medicine (UPSOM), Pittsburgh, PA, United States
We present cost-minimization and cost-benefit perspectives related to postoperative nausea/vomiting (PONV) strategies. Ongoing consensus guideline use (2003-2020) is characterized as high-frequency “€5,000 Kilogram of Cure” (related to high-cost hospital rescue/workup after “€3 Milligram of Prevention,” averaging €2,500/patient); this was compared to an innovative “5+ 3, €55 Gram of Prevention” technique, entailing 5-drug prophylaxis (palonosetron, perphenazine, aprepitant, diphenhydramine, dexamethasone), and 3-drug boosters (with these italicized agents). The €55 Gram of Prevention front-loaded cost increase (followed by a €500 “Gram of Cure” lower average cost, based on 90+% prophylactic efficacy) assumes lower associated PONV incidence/costs, during which downstream, in-hospital consensus-guided €5,000/patient Kilogram of Cure costs are strategically avoided via prevention beyond consensus-guided €3 Milligram of Prevention. Cost-minimization and cost-benefit tables are provided, incorporating existing cost outcomes from United States data sets. Comparative cost outcomes are adapted to a sovereign nation's internet postings of health economic “cost quadrants,” motivated by cost savings and outcome improvement via early prevention, as opposed to low-cost prevention and high-cost outcome variability. The 5 + 3 technique substitutes palonosetron (twice preventatively, 40 h apart) for ondansetron (q4 h, and risking rebound PONV), rendering the 5 + 3 technique as (i) cost-minimizing, and (ii) showing an incremental cost-benefit ratio that would favor preventative policy acceptance. The 5 + 3 technique representing €55 Gram of Prevention appears to obviate €5,000 Kilogram of Cure in 50% of patients represented by 2003–2020 consensus guidelines, with the consensus-guided approach being harmed by the enticements of low-cost and short-duration prophylactic and rescue ondansetron, carrying costly rebound (vs. breakthrough) PONV risks that have been previously reported.
1 Introduction
1.1 Five-drug antiemetic prophylaxis, instead of consensus-guided PONV strategies
We recently published a case series (1), and (in this journal) a perspective statement (2), regarding routine 5-drug multimodal antiemetic prophylaxis (5-MMAEPPx) comprised of palonosetron, perphenazine, aprepitant, diphenhydramine, and dexamethasone, beyond existing (3) consensus guidelines (CG), for meaningful prevention and management of postoperative nausea/vomiting (PONV). In these publications (1, 2), PONV was frequently driven by the opioid-sparing analgesic maneuver entailing intrathecal morphine (ITM) dosed via a newly-calculated algorithm (4), for 5-MMAEPPx patients, along with strategic avoidance of high abuse-liability opioids (HALO, such as fentanyl, remifentanil, hydromorphone, and oxycodone). Based on this background, we will present cost-minimization and cost-benefit analyses, first outlining the following assumptions.
1.2 High abuse-liability opioids vs. less-hyperalgesic, lower abuse potential, longer-acting opioids: PONV risk differences
We first shall declare fentanyl, remifentanil, hydromorphone, and oxycodone as “usual” HALO agents, traditionally characterized as highly lipophilic. We shall next declare ITM, methadone, and buprenorphine as less abuse-liable (but not HALO); these could be characterized as less lipophilic. We assume that high PONV incidence after ITM (1), being double vs. HALO, is also applicable to longer-duration systemic opioids (i.e., methadone and buprenorphine). For methadone, we assume 62% PONV incidence (5) without 5-MMAEPPx, and for buprenorphine, we assume 42% PONV incidence (6) without 5-AEPPx, based on a secondary analysis of this (7) study. If methadone/buprenorphine is used for perioperative analgesia in lieu of HALO, then (as with ITM) methadone/buprenorphine is assumed (in these analyses) to cause at least twice as frequent PONV under CG prophylaxis. HALO is a term that we recently introduced in this journal (8).
As such, historical control PONV rates in this (1) report did not entail ITM/5-MMAEPPx, but rather CG-guided PONV prophylaxis, rescue with repeat ondansetron, and use of the listed HALO agents during and after surgery. In other words, we forecast enhanced/sustained PONV prophylaxis (5-MMAEPPx, and 3-drug booster-dosing, abbreviated hereafter as “5 + 3″) may (i) indirectly address the opioid epidemic originating from usual perioperative exposure to HALO, and (ii) render as reasonable a therapeutic substitution away from less-nauseating HALO to, instead, longer-duration, less-HALO (and less hyperalgesia) with likely more nausea [such as ITM (1), methadone (5), and buprenorphine (6)]. In doing do, we forecast achieving a primary objective of PONV prevention with 90+% success (1) throughout hospitalization, en route toward a co-primary objective of HALO avoidance (or at least HALO dose reduction), since higher pain scores (including pain from opioid-induced hyperalgesia from HALO) also contribute to PONV risks (8). Other PONV risks (beyond the scope of this discussion) which are generally accepted include the use of nitrous oxide, the use of neostigmine for the reversal of non-depolarizing neuromuscular blockade, and inflation of the upper gastrointestinal tract (with positive pressure ventilation via face mask or laryngeal mask airway).
PONV from longer-duration opioids appears to be more easily prevented (1, 2, 4, 8) when not limited to a 1-to-4-drug CG prophylaxis regimen (and reflexive ondansetron rescue) driven by reasonable but incomplete (3) risk factors. Not only may current CG and restricted risk factor considerations lead to poorer outcomes, but they also may escalate hospital costs (9). We will take the perspective of a theoretical health ministry of a sovereign nation that is reconsidering the tandem of HALO and CG-based PONV prevention and management (CG/HALO) from two viewpoints: (i) easily demonstrated cost-minimization and cost-benefit analyses, and (ii) possibly unnecessary cost-effectiveness analysis, based on a calculation of negative values of incremental cost-benefit ratios (ICBR) when applying 5 + 3 MMAEPPx instead of CG/HALO.
1.3 CG-PONV prophylaxis: “penny-wise but pound-foolish” unconscious bias, and pending statements regarding health equity and PONV prophylaxis?
CG may carry an unconscious bias of being “penny-wise” at the risk of being “pound-foolish”, having been created in an era (∼1996–2003) of cost-containment concerns related to patent-protected ondansetron [which lost patent protection in 2007 (10)]. Further, recent health equity (11, 12) and race/ethnicity (e.g., in the Chinese (13)) PONV-specific publications may render PONV prophylaxis as an emerging health equity issue. In that light, particularly addressing the tandem of CG/HALO, 5 + 3 MMAEPPx has the potential to be inclusive of all patients at any PONV risk, particularly since (i) all 5 + 3 MMAEPPx agents are off-patent, safe, easy, and fiscally responsible, (ii) attention is being directed to HALO avoidance in the process, and (iii) we presume ITM/methadone/buprenorphine are more emetogenic than HALO, warranting additional prophylaxis and booster dosing.
1.4 CG for PONV prophylaxis—promulgated “law of diminishing returns”?
Traditional CG may promulgate “diminishing returns” of additional antiemetics with differing mechanisms of action, after a finite threshold (e.g., four, with one of these four typically being propofol) is reached. The 5 + 3 MMAEPPx (1, 2, 8) strategy may have profound cost-saving implications, based on cost analyses from 2019 (9) that preceded the 2020 iteration of CG (3), with 5 + 3 likely evading historical “diminishing returns” observed after three “typical” CG antiemetics (ondansetron, droperidol, and dexamethasone, per Apfel et al., 2004) (14). We note that CG did not have the benefit of an included antihistamine as a fourth antiemetic, as does 5-MMAEPPx (1, 2, 8).
1.5 QTc intervals—not applicable to palonosetron
Another likely motivator for the inaugural CG (15) were QTc-interval concerns (on electrocardiography) posed by some antidopaminergics (e.g., droperidol, in higher doses [2.5–5 mg] than those used for PONV prophylaxis [0.625–1.25 mg] (14)), and by some 5-HT3 antagonists [but not palonosetron (16)].
1.6 “Ounce of prevention, and pound of cure”
Given “an ounce of prevention” being worth “a pound of cure” [in United States (US) weights and measures dialect], we will apply (i) Euros (€) as currency-of-choice, and (ii) the metric system of weights and measures (mg, grams, kg) instead of ounces and pounds. We will then incorporate data from 600,000 US inpatient hospital stays described in the aforementioned 2019 (9) report, which found that when ≥1 dose of HALO agents fentanyl, hydromorphone, or morphine are given for postoperative pain in-hospital, PONV follows in 44%–72% [with 2–5 day additional length of stay (LOS) burden, incurring an overall $2,000-$9,000 cost increment per patient]. These (9) authors concluded that PONV (along with respiratory depression from any opioid) is substantially more prevalent than previously reported.
We assume that PONV in routine care [44%–72% incidence after 1 + opioid dose(s)] is a significant hospital cost driver well-after operating room and recovery room/post-anesthesia care unit (PACU) time within the awareness of anesthesia personnel. Instead, we assume “ping-pong PONV,” triggered by usual opioid dosing (with HALO), then opioid-induced hyperalgesia and more HALO dosing, then ondansetron as a “penny-wise” antiemetic which is underappreciated in its “pound foolish” creation of rebound nausea (17) (Supplementary Figure Image S1). This cycle is assumed to lead to costly health care interventions (gastroenterology or advanced radiology consultations/procedures) and prolonged length of stay (as multiple factors contributing to the $2,000-$9,000 cost increment). Separately (and conversely), an enhanced recovery strategy (e.g., surgical/gynecological oncology) encompassing both aggressive PONV prevention, and initial non-opioid postoperative pain management, led to lower LOS (3–4 days) and hospital costs ($5,000–7,500/case) (18). Finally, we assume that PONV that “breaks through” the 5 + 3 MMAEPPx technique may command the advanced workup and its associated costs, while CG-guided “Penny-wise Milligrams of Prevention,” followed by ondansetron-centered rescue and rebound PONV risks, would continue cost escalation until the possibly-flawed CG-default was supplanted by the 5 + 3 MMAEPPx technique.
In the economic structure of a theoretical health ministry, revisiting cost-minimization (Table 1) and cost-benefit details (Table 2) may (i) more rapidly “turn the tide” against PONV by adopting more aggressive 5-MMAEPPx (1, 2), and (ii) further turn the tide by incorporating three postoperative boosters (palonosetron every 40 h, along with daily perphenazine and aprepitant), instead of standard practice of frequent ondansetron rescue [and its reported (16, 17) risks of rebound PONV].
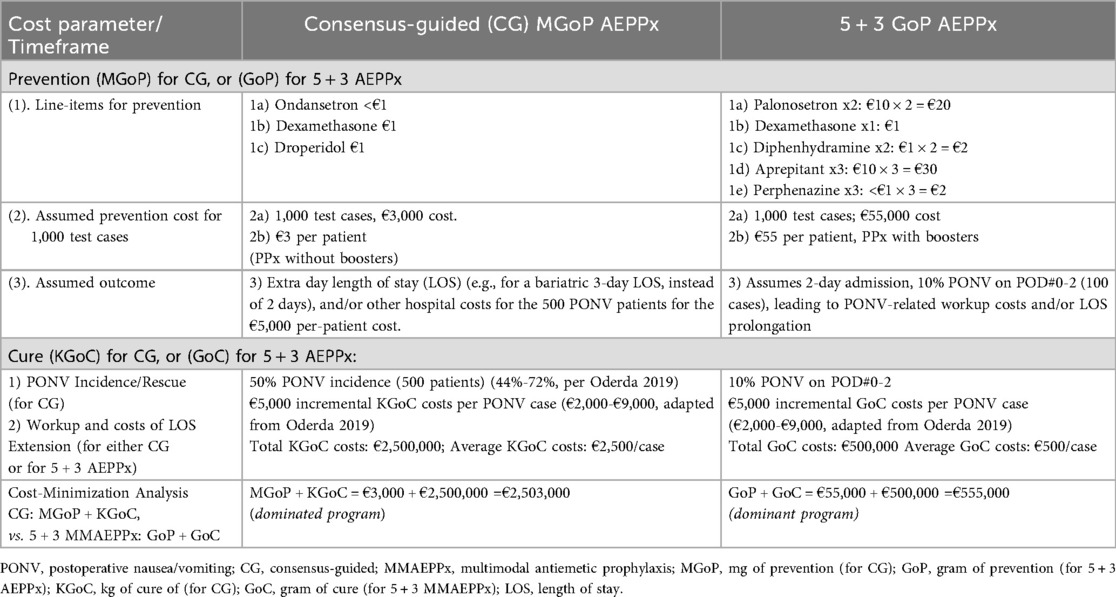
Table 1. Mixed-method cost minimization illustration, addressing PONV prophylaxis options achievable for a theoretical health ministry: comparison of consensus-guided versus 5 + 3 AEPPx).
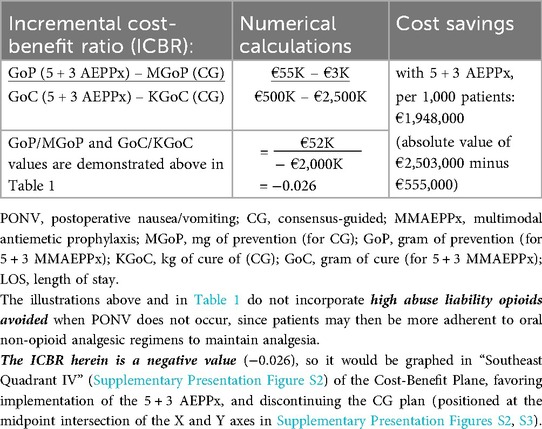
Table 2. Mixed-method cost-benefit analysis illustration per 1,000 patients per treatment, addressing PONV prophylaxis options achievable for a theoretical health ministry: comparison of consensus-guided versus 5 + 3 MMAEPPx.
2 Methods
2.1 Overview/cost minimization analysis
The cost-minimization analysis (Table 1) itemizes the comparative “Gram of Prevention” (GoP) costs of 5 + 3 MMAEPPx (€23 for prophylaxis, €32 for boosters) vs. CG “Milligram of Prevention” (MGoP, €3 for prophylaxis, and no boosters). Both groups are assumed to receive intraoperative propofol as a sixth and fourth antiemetic, respectively, neutralizing the propofol costs of the compared treatments. After these CG MGoP costs, the CG “Kilogram of Cure” (KGoC) costs assume an in-hospital €5,000 cost per symptomatic patient (or €2,500/patient overall) from the adapted $2,000-$9,000 cost range/case per Oderda et al.[2019] (9)). Therefore, €5,000/symptomatic patient for 50% PONV in CG patients [positioned within the 44%-72% PONV occurrence range reported in 2019 (9)], and the same €5,000/symptomatic patient (average €500/patient overall) in a conservative 10% of 5 + 3 MMAEPPx cases (1, 4, 8), encompasses KGoC costs of PONV workup and rescue, and/or additional hospital day [e.g., three day length of stay instead of two, after bariatric surgery (1, 4, 8)]. Both CG-based estimates (€5,000 KGoC/case, and 50% PONV incidence) are deliberately conservative, with both estimates positioned below or at the midpoints reported by Oderda et al. (9). Neither the costs of ITM, methadone, and/or buprenorphine, nor the costs of usual HALO drugs, are incorporated into this analysis.
2.2 Cost-benefit analysis
We then performed a cost-benefit analysis (Table 2), where GoP [for 5 + 3 MMAEPPx/ITM (vs. MGoP for CG/HALO)] above assumes the antecedent (first value listed) of the ICBR, while KGoC (hospitalization cost implications) assumes the ICBR consequent (i.e., latter value listed in the ratio, detailed further below and in Supplementary Content Presentation Figures S2, S3).
2.3 Cost-effectiveness “quadrants,” and sovereign nation health ministry implications favoring 5 + 3 MMAEPPx in “southeast quadrant IV.”
Near-verbatim transcription of figures and captions (from Health Canada) are shown, and are adapted to the theoretical sovereign nation health ministry perspective herein, found in Presentation Figures S2, S3. We paraphrase these (see captions for each Supplementary Content Presentation Figures S2, S3) specifically by addressing the quadrant positioning of 5 + 3 AEPPx (vs. CG) while in-hospital on subsequent days.
2.4 Basis of adding palonosetron to the previously-reported aprepitant/perphenazine daily antiemetic booster
As further background, we observed (4) that booster doses of perphenazine and aprepitant on POD#1 showed association of protection against POD#1 PONV after bariatric sleeve gastrectomy surgery, a procedure that is well-described (19) as emetogenic. However, we have not yet found the perphenazine-aprepitant booster to (8) to be similarly effective for surgery beyond sleeve gastrectomy, thus assuming in the described model herein that booster palonosetron every 40 h after the index preoperative dose would avoid ondansetron rebound PONV (17), as a supplement to daily booster perphenazine and aprepitant pre- and post-operatively (therefore comprising the complete 5 + 3 MMAEPPx strategy).
3 Results
When considering the economic objectives of the health ministry of the theoretical sovereign nation, we found that 5 + 3 MMAEPPx results in significantly better clinical and financial outcomes over the current CG-based approach for PONV prevention. While the initial costs (GoP vs. MGoP) would be higher (€23 for prophylaxis and €32 for boosters for 5 + 3 GoP, vs. €3 per CG prophylaxis and no boosters for MGoP, as per traditional practice), the novel 5 + 3 MMAEPPx would reduce the overall costs of PONV prevention and management (herein referred to, respectively, as the GoC or KGoC). This conclusion was reached based on assumptions of (i) €5,000 cost per patient PONV encounter in-hospital, and (ii) a CG-guided PONV incidence of 50%, with an overall cost savings of €1,948,000 per 1,000 patients (see below, and Table 2).
Table 1 presents the costs of CG-based PONV prophylaxis, compared with 5 + 3 MMAEPPx, detailing costs for both GoP and MGoP, and GoC and KGoC, as well as providing an estimate of cost minimization. Cost implications are further expanded in Table 2, where the ICBR is calculated to quantify the cost-benefit of 5 + 3 AEPPx strategy, vs. traditional CG-based PONV prophylaxis. The negative value for the ICBR, obtained in Table 2, is further explained and illustrated in Supplementary Presentation Figure S2, showing how 5 + 3 AEPPx would be positioned in “Southeast Quadrant IV” of the cost-effectiveness plane, a scenario where an intervention is more effective and less costly compared to the alterative. Supplementary Presentation Figure S3 further reinforces that 5 + 3 AEPPx (green dot) dominates current CG-based strategies (red dot) in both cost-savings and clinical outcomes.
4 Discussion
4.1 Three-drug PONV prophylaxis booster
Regarding the proposed three-drug booster (and rescue alternatives to ondansetron), such boosters could first entail replacing routine rescue ondansetron [and its risks of rebound nausea (16, 17)] with off-patent scheduled palonosetron (every 40 h), and next by proactively administering low-dose perphenazine (every 12–24 h in patients without Parkinson disease) and aprepitant (every 24 h), now that all three booster agents are unburdened (from a cost standpoint) by patent protection. In this booster context, sedating antihistamines could then be reserved for PONV rescue (e.g., IV diphenhydramine), or could also be used as an additional AEPPx booster (e.g., PO dimenhydrinate).
4.2 Is ondansetron-induced rebound PONV creating a long-overlooked cost?
It seems possible that unwanted outcomes from some potential CG biases have paradoxically elevated care costs by emphasizing ondansetron rescue [with rebound (16, 17) PONV as a potential side effect], serving as an impetus for in-hospital further workup as a cost driver. These events would also seem to inhibit CG innovations toward the 5 + 3 MMAEPPx strategy. This may be related to CG having an apparent “hard stop” after four prophylaxis drugs. We acknowledge the “fourth” CG intervention being preferential propofol over volatile agents (with additional CG guidance regarding avoided volatile agent and avoided opioid as further, non-counted interventions). We counter-propose diphenhydramine and dexamethasone as the “fourth and fifth” drugs in 5 + 3 MMAEPPx, allowing intraoperative propofol to be a “bonus sixth” drug in 5 + 3 MMAEPPx, providing useful delayed benefits into POD#1 (1).
4.3 Is the model sufficiently robust across the entire range of per-patient PONV costs?
In Supplementary Table S1, we inserted the outcomes of the analyses from Tables 1, 2 herein, into an Excel® (Microsoft®, Redmond, WA, United States) spreadsheet, and computed scenarios involving the adapted €2,000 to €9,000 cost-per-case of PONV, showing that the 5 + 3 MMAEPPx dominated the CG strategy in each scenario. Finally, we created a “best cost/worst cost” cure scenario of €2,000 cost of cure for CG-routed PONV, vs. €9,000 cost of cure for “5 + 3” MMAEPPx, addressing the question of “what if suppressing PONV actually increases costs of care downstream?” Even this best cost/worst cost latter scenario showed “5 + 3” dominance in the cost minimization (€48,000 savings) and cost-benefit analyses (−0.5 incremental cost-benefit ratio (Supplementary Table S1, sustaining with its negative value its position in Southeast Quadrant IV, Supplementary Presentation Figures S2, S3).
5 Conclusion
By utilizing all five of the listed off-patent antiemetics, as previously reported (1, 2, 4, 8), the described 5 + 3 AEPPx regimen aligns well with the pharmacoeconomic objectives of the theoretical sovereign nation's health ministry. The integration of 3-drug booster prophylaxis, as described, introduces a novel strategy for sustained PONV prevention in-hospital, with likely additional length-of-stay and patient satisfaction benefits superimposed on cost minimization. The described cost-conscious maneuvers could not only allow for better PONV protection against less-hyperalgesic opioids such as methadone and/or buprenorphine elevated to more routine use, which like ITM are more emetogenic per dose, but also (based on presumed less-frequent dosing than with usual HALO agents in routine care, related to opioid-induced hyperalgesia) address perioperative origins of new, persistent opioid use up to and including the opioid epidemic. There may be value in the collective of anesthesia personnel (i) looking beyond the PACU for true PONV outcomes and costs, as reported by Oderda et al.[2019 (9)], and (ii) re-evaluating the clinical impact of after-PACU ondansetron-induced rebound PONV as reported by Apfel et al.[2012 (17)] in the setting of (now inexpensive) palonosetron (16). If antiemetic strategies serendipitously prepare us better for addressing new, persisting opioid use by first separating HALO agents from ITM/methadone/buprenorphine, with latter non-HALO agents being likely more emetogenic, it may be reasonable to not only consider ondansetron less expensive than palonosetron, but also reconsider palonosetron as now “favorably inexpensive” (with ondansetron simply being “cheap”), including for addressing long-term opioid risks originating from surgery and anesthesia encounters.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
BW: Writing – original draft, Writing – review & editing, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. LL: Investigation, Writing – review & editing, Validation, Conceptualization, Resources, Methodology. KS: Conceptualization, Resources, Visualization, Investigation, Methodology, Writing – review & editing, Supervision, Software.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanes.2025.1610320/full#supplementary-material
Supplementary Figure S1 | Author-proposed “vicious cycle” perpetuation of both opioid escalation and postoperative nausea/vomiting after care entry into the anesthesia/surgery processes in the upper left hand corner. The reader is guided from there by the positioned arrows.
References
1. Williams BA, Holder-Murray JM, Nettrour JF, Ibinson JW, DeRenzo JS, Dalessandro C, et al. Aim for zero: prevention of postoperative nausea and vomiting using an off-patent five drug multimodal approach. Br J Anaesth. (2023) 131(1):e1–4. doi: 10.1016/j.bja.2023.01.005
2. Williams BA, Schumacher CA, Choragudi R, Garbelotti KE, Ludden JM, Hall DE. Historical perspectives supporting the ambitious anesthetist aiming for zero nausea/vomiting: should one trust every consensus statement every time? Front Anesthesiol. (2025) 3(1525030):1–7. doi: 10.3389/fanes.2024.1525030
3. Gan TJ, Belani KG, Bergese S, Chung F, Diemunsch P, Habib AS, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. (2020) 131:411–48. doi: 10.1213/ANE.0000000000004833
4. Williams BA, Hall DE, Dalessandro C, Garbelotti KE, Ludden JM. Patient-centered intrathecal morphine dose-response in major abdominal surgeries when augmented by innovative five-drug antiemetic prophylaxis. Front Anesthesiol. (2025) 4:1521409. doi: 10.3389/fanes.2025.1521409
5. Kharasch ED, Brunt LM, Blood J, Komen H. Intraoperative methadone in next-day discharge outpatient surgery: a randomized, double-blinded, dose-finding pilot study. Anesthesiology. (2023) 139:405–19. doi: 10.1097/aln.0000000000004663
6. Schumacher CA, Choragudi RV, Mikolic JM, Boudreaux-Kelly MY, Williams BA. A multi-variable, Veteran-centered study of PONV risk factors in separate regional vs. general anesthesia contexts. Front Anesthesiol. (2025) 4:1631506. doi: 10.3389/fanes.2025.1631506
7. Williams BA, Ibinson JW, Mangione MP, Modrak RT, Tonarelli EJ, Rakesh H, et al. Research priorities regarding multimodal peripheral nerve blocks for postoperative analgesia and anesthesia based on hospital quality data extracted from over 1,300 cases (2011–2014). Pain Med. (2015) 16:7–12. doi: 10.1111/pme.12609
8. Williams BA, Choragudi R, Schumacher CA, Garbelotti KE, Ezaru CS, Boudreaux-Kelly MY, et al. Upgrading intrathecal morphine for postoperative pain mitigation in abdominal surgery: an exploratory multiple regression analysis of observational data addressing coadministered spinal magnesium sulfate, en route to both enhanced systemic opioid sparing and opioid avoidance. Front Anesthesiol. (2025) 4:1592643. doi: 10.3389/fanes.2025.1592643
9. Oderda GM, Senagore AJ, Morland K, Iqbal SU, Kugel M, Liu S, et al. Opioid-related respiratory and gastrointestinal adverse events in patients with acute postoperative pain: prevalence, predictors, and burden. J Pain Pall Care Pharmacother. (2019) 33:82–97. doi: 10.1080/15360288.2019.1668902
10. Glass PS, White PF. Practice guidelines for the management of postoperative nausea and vomiting: past, present, and future. Anesth Analg. (2007) 105:1528–9. doi: 10.1213/01.ane.0000295854.53423.8A
11. Goldson KV, Brennan E, Burton BN, Faloye AO, Habermann EB, Hanson KT, et al. Does management of postoperative nausea and vomiting differ by patient demographics? An evaluation of perioperative anesthetic management—an observational study. Anesthesiology. (2025) 42:704–15. doi: 10.1097/ALN.0000000000005367
12. White RS, Andreae MH, Lui B, Ma X, Tangel VE, Turnbull ZA, et al. Antiemetic administration and its association with race: a retrospective cohort study. Anesthesiology. (2023) 138(6):587–601. doi: 10.1097/ALN.0000000000004549
13. Zhang Q, Ye X, Shi S, Zhou S, Ma D, Ouyang W, et al. Pyridoxine prevents postoperative nausea and vomiting in gynecological laparoscopic surgery: a double-blind randomized controlled trial. Anesthesiology. (2025) 42:655–65. doi: 10.1097/ALN.0000000000005354
14. Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. (2004) 350:2441–51. doi: 10.1056/NEJMoa032196
15. Gan TJ, Meyer T, Apfel CC, Chung F, Davis PJ, Eubanks S, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. (2003) 97:62–71. doi: 10.1213/01.ane.0000068580.00245.95
16. Apfel CC, Jukar-Rao S. Is palonosetron also effective for opioid-induced and post-discharge nausea and vomiting? Br J Anaesth. (2012) 108:371–3. doi: 10.1093/bja/aer516
17. Apfel CC, Philip BK, Cakmakkaya OS, Shilling A, Shi YY, Leslie JB, et al. Who is at risk for postdischarge nausea and vomiting after ambulatory surgery? Anesthesiology. (2012) 117:475–86. doi: 10.1097/ALN.0b013e318267ef31
18. Nelson G, Kalogera E, Dowdy SC. Enhanced recovery pathways in gynecologic oncology. Gynecol Oncol. (2014) 135:586–94. doi: 10.1016/j.ygyno.2014.10.006
Keywords: palonosetron, perphenazine, aprepitant, diphenhydramine, dexamethasone, postoperative nausea and vomiting
Citation: Williams BA, La Colla L and Smith KJ (2025) The ambitious anesthetist aiming for zero nausea/vomiting: can a €55 gram of prevention obviate a €5,000 kilogram of cure? A cost-minimization and cost-benefit perspective. Front. Anesthesiol. 4:1610320. doi: 10.3389/fanes.2025.1610320
Received: 11 April 2025; Accepted: 26 August 2025;
Published: 29 September 2025.
Edited by:
Thomas Schricker, McGill University, CanadaReviewed by:
Christian Bohringer, UC Davis Medical Center, United StatesCopyright: © 2025 Williams, La Colla and Smith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brian A. Williams, d2lsbGlhbXNiYUBhbmVzLnVwbWMuZWR1; d2lsbGlhbXNiYS51cG1jQGdtYWlsLmNvbQ==
 Brian A. Williams
Brian A. Williams Luca La Colla1,2
Luca La Colla1,2