- Department of Anesthesiology, School of Medicine, Yale University, New Haven, CT, United States
Background: Intraoperative hypotension (IOH) is associated with serious adverse outcomes after noncardiac surgery. Preoperative predictors of IOH remain poorly characterized. Intraoperative hemodynamic instability is strongly associated with IOH. The authors hypothesized that submaximal cardiopulmonary exercise testing (smCPET) measures of forced vital capacity (FVC) and gas-exchange derived pulmonary capacitance to peak oxygen uptake slope (GXCAP-VO2) would be associated with two measures of intraoperative hemodynamic instability: intraoperative vasopressor use and systolic average real variability (ARV), respectively.
Methods: This secondary analysis of a feasibility study included adults >60 years undergoing elective noncardiac surgery and completed preoperative smCPET. Multiple cardiopulmonary measures, including FVC, peak oxygen uptake (VO2) and GXCAP-VO2 slope were collected. The primary outcome of intraoperative vasopressor use, and secondary outcome of ARV were tested with multivariable logistic regression and generalized linear models to assess associations supported by decision boundary and mediation analysis.
Results: Among 101 participants, 54 had measured FVC (median 2.56 L) and 101 had measured GXCAP-VO2 slope (median 29.8). After adjustment, each standard deviation increase in FVC (0.89 L) was associated with halved odds of vasopressor use [2.47 L (SD 0.88) vs. 2.9 L (SD 0.86) adjusted Odds Ratio: 0.496 (95% CI: 0.25–1.01) p = 0.052]. Participants with FVC <2.18 L and surgery duration >152 min had the highest risk of vasopressor use. Systolic ARV was negatively associated with increasing surgical time (p < 0.001). For each 10-unit increase in GXCAP-VO2 slope, systolic ARV is expected to decrease by 9.8% [incidence rate ratio = 0.902, 95% CI (0.84, 0.97)].
Conclusion: Lower measured FVC and GXCAP-VO2 slope were associated with measures of intraoperative hemodynamic instability in older adults undergoing noncardiac surgery. Preoperative assessment of pulmonary function and cardiopulmonary reserve may identify patients at higher risk for intraoperative hemodynamic instability. These exploratory observations establish a foundation for future research on smCPET measures for the prediction of perioperative complications, recognizing intraoperative hemodynamic instability as a complex interplay of patient, anesthetic, and surgical factors.
1 Introduction
Intraoperative hypotension (IOH) is a significant risk factor for cardiovascular and renal morbidity, increased length of stay, 30-day perioperative mortality and occurs in >60% of surgical procedures (1–3). IOH has been recently defined as an intraoperative mean arterial pressure less than 65 mmHg of >15 cumulative minutes duration, although definitions vary (4–7). Identifying high risk patients may enable focused preventive strategies to reduce its occurrence since broad-based intraoperative blood pressure management strategies have not improved major vascular outcomes after noncardiac surgery (8). The causal link between intraoperative hemodynamic instability and IOH is strong (9). Intraoperative hemodynamic instability, as measured by average real variability (ARV), has been associated with myocardial injury, postoperative delirium, and postoperative acute kidney injury, although long-term ramifications are still unclear (10–13). Higher systolic ARV has been associated with major adverse cardiovascular events in hospitalized patients as well as intensive care-related mortality (14, 15). Understanding the mechanisms underlying this hemodynamic instability may facilitate the development of preoperative risk stratification for intraoperative hemodynamic instability.
The pathophysiology underlying intraoperative hemodynamic instability involves complex cardiopulmonary interactions, particularly under anesthesia and positive pressure ventilation. This cardiopulmonary coupling suggests that preoperative pulmonary function may identify risk for intraoperative hemodynamic compromise. Among potential measures, forced vital capacity (FVC), a routine pulmonary function test, has emerged as a promising predictor of perioperative outcomes (16, 17). Pulmonary spirometry measures correlate with adverse perioperative outcomes across various surgical populations and prehabilitative measures that improve lung mechanics, and enhance FVC, can reduce length of stay (18–20). Furthermore, FVC impairment is underdiagnosed, as spirometry studies show that 35.7% of participants with airflow obstruction had no previously known respiratory disease (21). FVC integrates multiple aspects of cardiopulmonary function, including respiratory muscle strength, and chest wall compliance. Lower FVC may impair intrathoracic pressure and venous return (22). These factors may be clinically silent, but unmasked during the hemodynamic challenges posed by general anesthesia, positive pressure ventilation and surgery. Impaired FVC often precedes clinically apparent cardiopulmonary dysfunction and may reflect subtle changes in chest wall mechanics, respiratory muscle strength, or cardiovascular coupling (23, 24). However, the precise relationship between FVC and IOH, as well as optimal cutoff values for risk stratification, requires further investigation.
Brief submaximal cardiopulmonary exercise testing (smCPET) has been previously validated to conventional CPET measures and quantitatively detects cardiopulmonary fitness in a variety of cardiac and pulmonary conditions (25–30). Prior reports have demonstrated its feasibility for preoperative evaluation (30). CPET measures have predicted a wide variety of early and late postoperative complications (31–34). Brief smCPET, which includes spirometry data, generates numerous conventional cardiopulmonary exercise testing measures such as peak oxygen uptake (VO2), metabolic equivalents (METs) and measures such as gas-exchange derived pulmonary capacitance (GXCAP). GXCAP, which closely correlates pulmonary vascular capacitance, is a sensitive measure for the diagnosis and prognosis of conditions complicated by pulmonary hypertension (29, 35, 36). Similarly, the GXCAP-VO2 slope coefficient may represent a combined marker of the relationship between pulmonary arterial capacitance and adequate left sided cardiac output (peak VO2). When lower GXCAP-VO2 slope is observed, this may be a marker of reduced ability to buffer intraoperative hemodynamic fluctuations and identify less efficient compensation to volume shifts and anesthetic-induced vasodilation (37).
Despite the theoretical framework linking pulmonary function, and pulmonary vascular capacitance to intraoperative hemodynamic instability, the specific relationships remain unexplored. Intraoperative hemodynamic instability [increased systolic average real variability (ARV) and/or vasopressor use] frequently precedes IOH. This study aimed to (1) determine if lower measured FVC is associated with increased intraoperative vasopressor use, suggesting that pulmonary mechanics play a role in intraoperative hemodynamic instability, and (2) examine if lower GXCAP-VO2 slope is associated with increasing systolic ARV. The authors hypothesized that lower GXCAP-VO2 slope, indicating impaired pulmonary vascular reserve, would be associated with increased systolic ARV.
2 Methods
2.1 Study design
This was a secondary analysis of the active open-label SHAPE feasibility clinical device study [IRB#2000033885; Clinical Trials.gov #NCT05743673 (38)], assessing the feasibility of preoperative smCPET in a high-volume preoperative clinic. This trial was registered prior to participant enrollment in study procedures.
2.2 Participants
This exploratory study used a convenience sample of 101 older adults from a single center, with only 54 having FVC data. This non-probability sampling limits generalizability beyond similar patient populations and clinical settings.
2.3 Inclusion and exclusion criteria
Participants were presenting for preoperative evaluation prior to elective noncardiac surgery. Inclusion criteria included age 60 years and older, with a Revised Cardiac Risk Index (RCRI) of ≤2, and subjective metabolic equivalents (METs) of ≥4, defined as the self-reported ability to reliably climb 2 flights of stairs. Exclusion criteria included severe or critical heart valve disease, reported exertional angina, severe ambulatory limitations, end-stage renal disease, severe peripheral vascular disease or neurological motor deficits. Participants under legal guardianship, non-English-speaking, or those without personal health care decision-making capacity were excluded.
2.4 Study endpoints
Primary endpoint
FVC was analyzed as a predictor of vasopressor use, while GXCAP-VO2 slope was analyzed separately as a predictor of systolic ARV. These were distinct analyses addressing different elements of hemodynamic instability. The primary endpoint was defined as the presence or absence of intraoperative vasopressor administration, a standard of care intervention for the treatment of IOH events.
Secondary endpoint
Average real variability (AVR) of systolic blood pressure was calculated across procedural time for each participant (see Figure 1). Systolic AVR reflects direct measurement of cardiac ejection force, myocardial contractility and balance of vascular resistance during the procedure, permitting a quantification of hemodynamic stability across the procedure time. Forty or greater intraoperative blood pressure (BP) measurements were accepted as the minimum for systolic ARV calculation. A weight-adjusted vasopressor equivalent per kg per unit time (vasopressor equivalent kg-1 min −1) was calculated for participants requiring treatment. Vasopressor equivalence was defined as norepinephrine equivalents: ephedrine (1:820), phenylephrine (1:10), vasopressin (5:1), or epinephrine (1:1)] through intermittent boluses or via continuous infusion (39, 40). An IOH event was calculated as the presence of MAP ≤65 mmHg for ≥15 min.
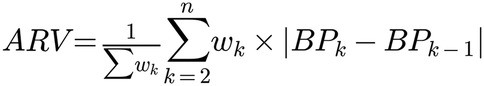
Figure 1. Average real variability (ARV) equation used in this investigation where: wk, weight assigned to measurement k; BPk, blood pressure measurement at time point k; BPk−1, blood pressure measurement at previous time point (k-1); n = total number of measurements; ││ = absolute value notation. ∑, summation notation.
2.5 Measurements
Demographic and clinical characteristics
After written informed consent, age, gender, body mass index, smoking status, baseline comorbidities and functional status were obtained prior to brief preoperative smCPET.
Cardiopulmonary exercise testing protocol
The FDA-approved Shape II® is a breath by breath exercise testing system that exploits brief submaximal exercise effort (2 min of baseline data, 3 min of graded exercise on a stationary stairstep, and 1 min of recovery data), followed by predictive analytics, to generate various measures of actual and predicted peak cardiopulmonary performance (see Supplementary Table S1 and Figure 3 for a comprehensive overview of study procedures). Prediction of peak performance values are generated using the oxygen utilization slope equation, and the device has been previously validated to conventional cardiopulmonary exercise testing measurements (25, 28, 41). After data collection, participants were instructed on task execution (5 min) and performed a 6-minute smCPET test session. Spirometry values (FVC, Forced expiratory volume, 1 s, % predicted), peak VO2, GXCAP, peak METs and the anaerobic threshold were extracted from the session data. Intraoperative data was collected after participant discharge and included surgical type (nonthoracic vs. thoracic), surgical time, and intraoperative blood pressure (systolic, diastolic, MAP), heart and pulse oximetry data points. Physiologic and Operative Severity Score for the enumeration of Mortality and Morbidity (POSSUM) operative severity was individually calculated from the preoperative and operative record of individual participants (42). Anesthetic management followed institutional protocols. Data on specific anesthetic agents, ventilation parameters and fluid management were not systematically collected.
2.6 Statistical analysis plan
Descriptive statistics were calculated for all variables. Continuous variables were summarized using means, standard deviations, medians, and interquartile ranges (IQR), while categorical variables were presented as counts and percentages.
Univariate analysis was performed to analyze the relationship between smCPET parameters and hemodynamic outcomes using univariate tests. For continuous variables, Pearson correlations coefficients (r) were calculated to assess linear relationships and alternatively with Spearman's rank correlation (ρ) for non-normally distributed data. Mann–Whitney U tests were used to compare continuous variables with non-normal distributions, Student's t test for normally distributed data and Chi square for categorical data.
Logistic regression models were constructed to examine the relationship between preoperative variables and binary outcomes (intraoperative vasopressor administration, IOH events) and generalized linear models for continuous data (Systolic ARV). Models were adjusted for relevant clinic and surgical factors, including surgical time, pre-admission comorbidities, and presented as adjusted odds ratios (ORadj) or incident rate ratios (IRR) with 95% confidence intervals. Predictive ability was evaluated using receiver operating characteristic (ROC) curve analysis. Bootstrap resampling (1,000 iterations) was performed to estimate 95% confidence intervals for the area under the curve (AUC), sensitivity, and specificity. For the primary endpoint, mediation analysis was performed to assess the influence of relationships between measured FVC, surgery time and operative severity (43). Decision boundary plot analysis was used to identify clinically relevant thresholds for key predictors of the primary and secondary endpoint. Quantile regression analysis (systolic ARV), or ROC analysis with Youden's J statistic (intraoperative vasopressor use) was used to identify optimal thresholds (44). Model fit was estimated using the coefficient of determination (R2) (McFadden's pseudo-R2) and likelihood ratios for linear and logistic regression models. To inform the relationship between measured FVC and intraoperative vasopressor use, a Bayesian logistic regression was performed as a sensitivity analysis, using weakly informative priors (normal distribution with mean 0 and SD 2.5). Bayes factors were calculated to quantify evidence strength. All statistical analysis was performed on R version 4.3.2 (R Foundation for Statistical Computing, Vienna Austria). A p-value of <0.05 was accepted for significance.
3 Results
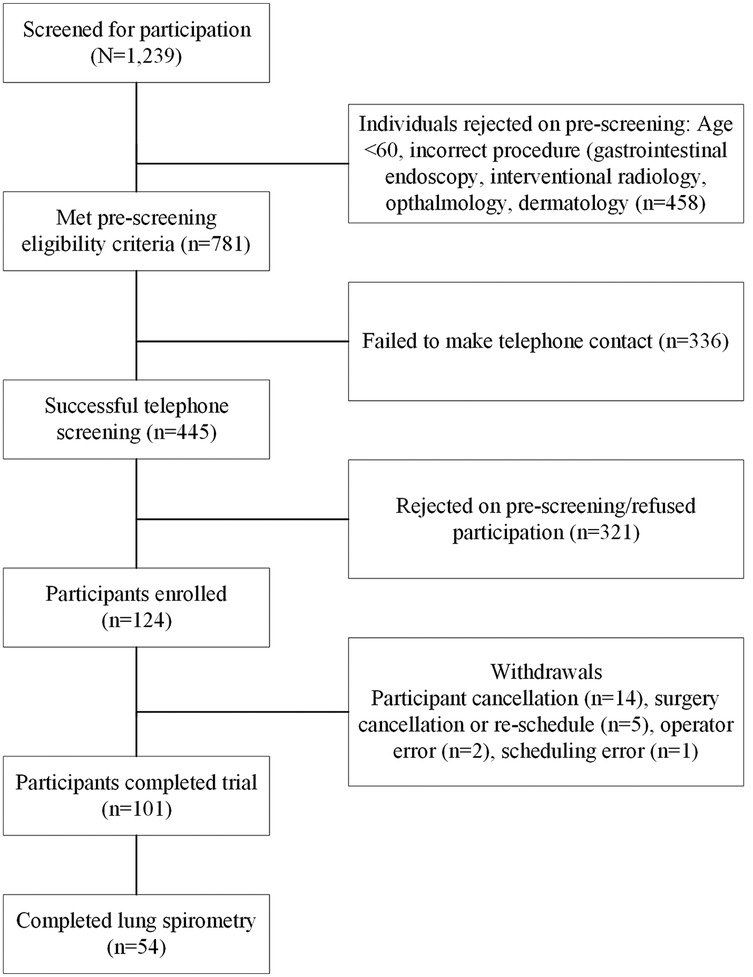
3.1 Participant baseline characteristics
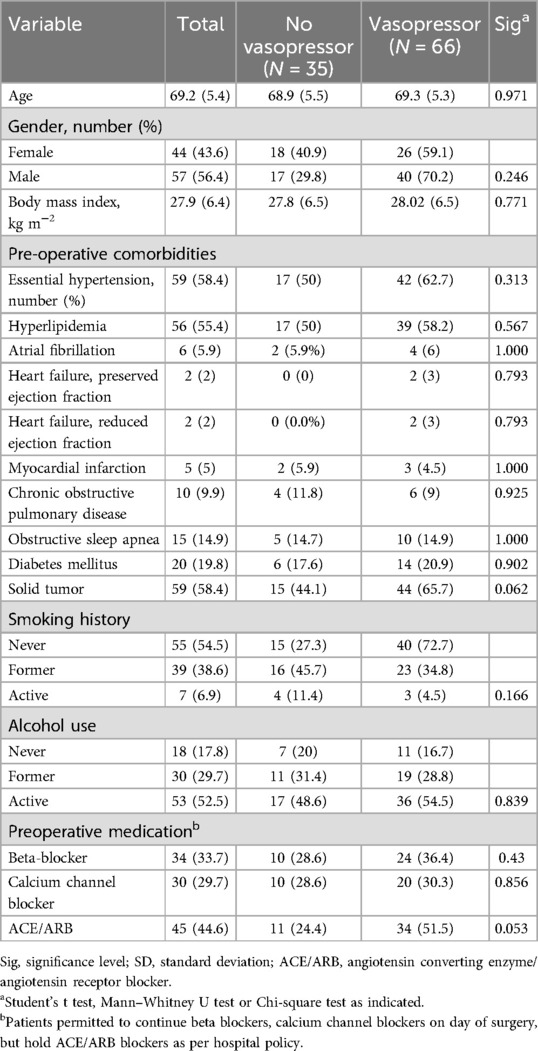
101 participants completed the study protocol (see Figure 2). 54 of 101 (53.4%) completed lung spirometry and 97 of 101 (96%) had calculated systolic ARV. Baseline characteristics of the cohort are presented in Table 1. Participants with intraoperative vasopressor requirements were not different in terms of age 68.9 vs. 69.3 years old, p = 0.971), gender (43.6 vs. 40.9% female, p = 0.246) but had a higher incidence of pre-admission history of solid tumor (3.3 vs. 18.2%, p = 0.062) and higher incidence of ACE/ARB use (51.5 vs. 24.4%, p = 0.053). Of 101 participants, 54 (53.4%) had measured FVC, median 2.56 [IQR: 2.03–3.15] and FVC % predicted, 74.5 [60–85.5]. All 101 participants had a GXCAP-VO2 slope with a median value of 29.8 [24.4–32.5].
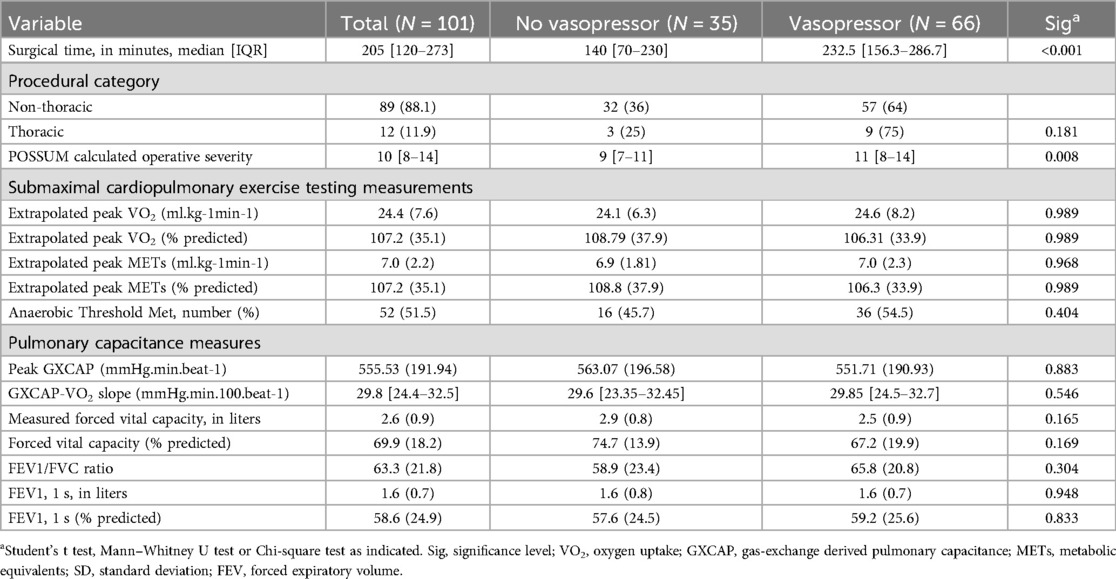
Median surgical time was higher in the vasopressor group (140 vs. 205 min, p < 0.001), with no differences between thoracic and nonthoracic cohorts (see Table 2). Of 101 participants, 99 (98%) were performed under general endotracheal anesthesia, with 2 performed as general anesthesia with a natural airway, with no differences between vasopressor or no vasopressor groups (p = 0.461). Regarding smCPET measures, mean peak VO2 (24.45 vs. 24.09 ml kg-1 min-1, p = 0.989) was not different between groups. No differences were observed in preoperative smCPET measures, but trends were observed in measured FVC (p = 0.165), and relative FVC (0.169).
3.2 Preliminary exploration of blood pressure response patterns
Mean cohort vasopressor equivalents kg-1 min-1 were 0.531 mcg/kg/min (±0.95) and 66 of 101 participants (65.3%) had intraoperative vasopressor administration, of which 22 of 66 (33.3%) participants received vasopressor infusions rather than intermittent bolus dosing. Among 101 participants, 11 (10.9%) had an IOH event. A median 2 recorded occurrences [IQR: 0–7] per procedure were spent with MAP <65 mmHg comprising a median 0.79% [IQR: 0–2.8] of procedure total time. Only 9 out 11 participants (81.8%) with IOH events received intraoperative vasopressor administration.
There was a strong positive relationship between surgery time and intraoperative vasopressor use, [median 248.6 vs. 156.1 min Odds Ratio (OR): 1.008 (95% CI: 1.003–1.012) p = 0.002], suggesting that for each minute increase in surgery time, the probability of vasopressor use increased by 0.75%. Increasing surgery time was not associated with vasopressor infusion implementation (p = 0.273). However, surgery time was the strongest predictor of intraoperative vasopressor use in the cohort [OR: 2.706 (95% CI: 1.567–4.794) p = 0.0016], with an AUC of 0.707 (sensitivity: 0.89, specificity: 0.36). Each SD increase (153 min) of surgery time nearly doubled the odds for vasopressor use. After preoperative smCPET measures were analyzed, only measured FVC predicted intraoperative vasopressor use [OR: 0.53 (95% CI: 0.28–1.01) p = 0.055]. Each SD decrease (0.9 L) in FVC halved the odds of intraoperative vasopressor use.
Systolic blood pressure AVR was 8.7 [IQR: 6.3–12.4] with a strong negative monotonic correlation between increasing surgical time and systolic ARV based on Pearson correlation coefficient (r = −0.568, p < 0.001) and Spearman's rho (−0.642, p < 0.001), where increasing surgical time resulted in lower systolic ARV (see Figure 3). Calculated POSSUM operative severity provided moderate discriminative ability for any intraoperative vasopressor use [OR: 1.862 (95% CI: 1.118–3.227) p = 0.016]. When examining systolic ARV and increasing vasopressor use (as vasopressor equivalents kg −1 min-1), a weak negative relationship was observed (Pearson's r = −0.235, p = 0.02; Spearman's rho = −0.27, p = 0.007). When excluding participants without vasopressor use, the correlation was no longer significant (Pearson r = −0.164, p = 0.198; Spearman rho = −0.010, p = 0.938). A mediation analysis to determine whether surgery time mediates the relationship between operative severity (POSSUM operative severity score) and intraoperative vasopressor use. Operative severity significantly predicted vasopressor use [path c; B = 0.16, SE = 0.07, z = 2.40, p = .016, OR = 1.17, 95% CI (1.03, 1.33)]. In the second step, operative severity significantly predicted surgery time (path a; B = 19.78, SE = 2.99, t = 6.61, p < .001), indicating that higher operative severity was associated with longer surgeries. When both POSSUM operative severity and surgery time were included in the model predicting vasopressor use, surgery time significantly predicted vasopressor use [path b; B = 0.007, SE = 0.003, z = 2.42, p = .015, OR = 1.01, 95% CI (1.00, 1.01)], while the effect of operative severity became non-significant [path c’; B = 0.05, SE = 0.08, z = 0.59, p = .552, OR = 1.05, 95% CI (0.90, 1.22)]. The indirect effect (a × b = 0.13) accounted for 83.2% of the total effect, suggesting that surgery time substantially mediates the relationship between operative severity and vasopressor use.
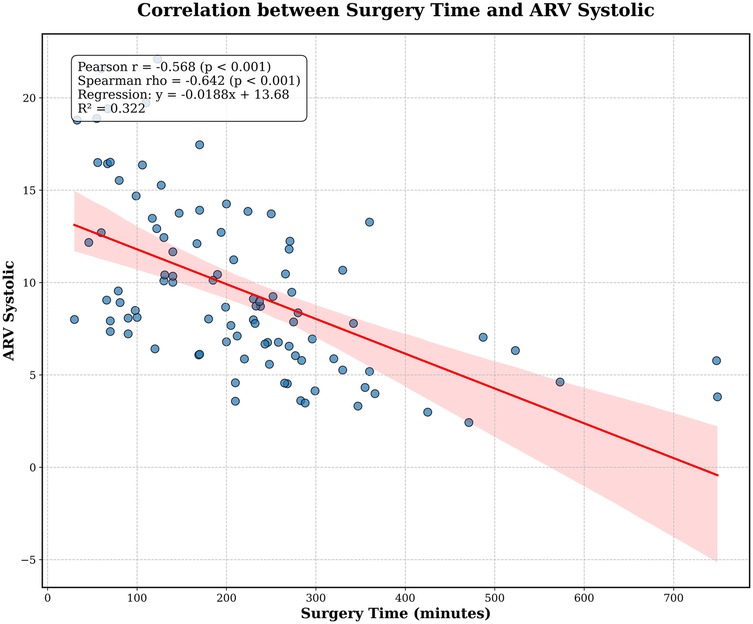
Figure 3. Correlation between increasing surgery time and systolic average real variability (ARV), notable for the strong negative monotonic correlation with decreasing systolic ARV.
3.3 Primary endpoint analysis
54 (53.5%) of 101 participants had preoperative lung spirometry during preoperative smCPET. 34 (63%) of 54 had intraoperative vasopressor administration. Using receiver operating characteristic cure analysis, FVC alone demonstrated modest discriminative ability for predicting vasopressor use [2.47 (SD 0.88) vs. 2.9 (SD 0.86) Liters; AUC: 0.65, p = 0.064] with an optimal FVC threshold of 2.18 L. Sensitivity at the optimal threshold was 0.5 and specificity was 0.789 at this optimal threshold. An adjusted logistic regression model with surgical time, pre-admission solid tumor presence, ACE/ARB use, and measured FVC demonstrated that surgery time [263.6 min (SD 162.5) vs. 170.9 min (SD 108.4), ORadj: 1.007 (95% CI: 1.0 −1.013) p = 0.036] and measured FVC [2.47 L (SD 0.88) vs. 2.9 L (SD 0.86) ORadj: 0.496 (95% CI: 0.25–1.01) p = 0.052] most contributed to the model [AUC: 0.76 (95% CI: 0.59–0.88); sensitivity (0.91) and specificity (0.59) see Figure 4]. ACE use (p = 0.58) and solid tumor presence (p = 0.32) did not contribute to the final model. Decision boundary plot analysis (see Supplementary Figure S1) estimated that surgeries >152 min and measured FVC <2.18 L were associated with the highest risk for intraoperative vasopressor use. To enhance the veracity of these findings, a logistic regression model, constructed for measured FVC and adjusted for surgical time and calculated POSSUM operative severity, retained significance [measured FVC: ORadj: 0.5 (95% CI: 0.25–1.01) p = 0.054]. POSSUM operative severity (p = 0.99) did not contribute to the model. An interaction analysis demonstrated that POSSUM operative severity did not vary based on measured FVC (p = 0.31).
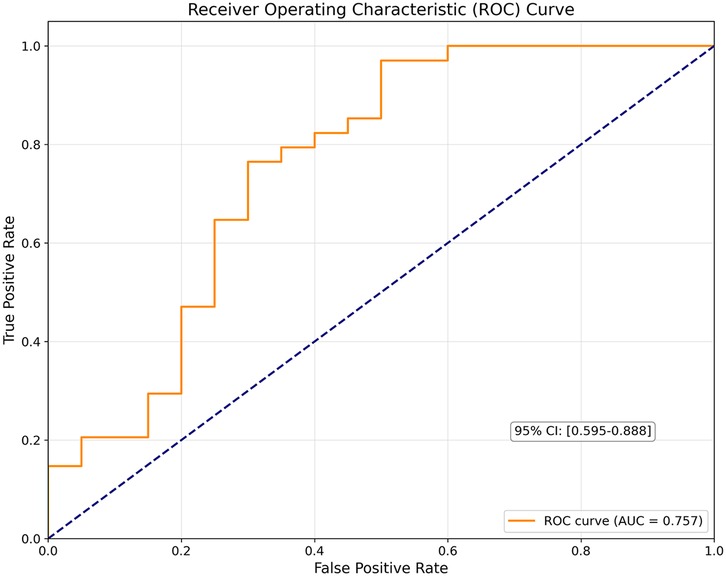
Figure 4. Receiver operating characteristic curve of the constructed multivariable logistic regression model (includes measured forced vital capacity and surgical time).
A Bayesian analysis was conducted to examine the association between forced vital capacity (FVC) and intraoperative vasopressor use. Using conjugate normal priors, the difference in mean FVC between groups was estimated through posterior distributions derived from three prior specifications: weakly informative N (0, 12), skeptical N (0, 0.32), and vague N (0, 102). The observed difference of 0.355 L (SE = 0.251) favoring the non-vasopressor group yielded posterior mean estimates ranging from 0.209 L (95% CI: −0.168, 0.586) under the skeptical prior to 0.355 L (95% CI: −0.136, 0.846) under the vague prior. Across all prior specifications, the probability that participants without vasopressors had higher FVC values ranged from 86.2% to 92.2%, with Bayes factors varying from 0.863 (skeptical prior, favoring null) to 14.621 (vague prior, strong evidence). The weakly informative prior analysis, considered most appropriate for this context, yielded a posterior mean difference of 0.334 L (95% CI: −0.142, 0.811), P (δ > 0) = 0.915, and BF = 1.598, indicating weak evidence against the null hypothesis (see Supplementary Figure 2). These findings suggest a clinically meaningful difference of approximately 12%–14% in mean FVC between groups, though the evidence remains limited, mainly related to the small sample size.
3.4 Secondary endpoint analysis
97 of 101 participants had measured GXCAP-VO2 slope and calculated systolic ARV. There was a moderate but significant negative correlation (Pearson) between GXCAP-VO2 slope and systolic ARV (r = −0.264, p = 0.009). The Spearman correlation was similar (ρ = −0.271, p = 0.007), suggesting a consistently negative monotonic relationship (see Supplementary Figure S2). A generalized linear model (GLM; gamma log link function) with a log link function was used to examine the relationship between systolic ARV and GXCAP-VO2 slope, adjusted for procedure category and surgery time. The model was significant, χ2 (3, N = 103) = 50.52, p < .001, with a modest McFadden's pseudo R2 of 0.092. A significant negative association between GXCAP-VO2 slope and systolic ARV was observed (b = −0.0103, SE = 0.0039, p = 0.008). For each 10-unit increase in GXCAP-VO2 slope, systolic ARV is expected to decrease by 9.8% [incidence rate ratio = 0.902, 95% CI (0.84, 0.97)]. Surgery time was also associated with systolic ARV (b = −0.0020, SE = 0.0003, p < .001), with an incidence rate ratio of 0.99 [95% CI (0.997, 0.999)], indicating that for each additional minute of surgery time, systolic ARV decreases by 0.20%. Using quantile linear regression, the optimal threshold for GXCAP-VO2 slope was estimated to be between 11.8 and 24.2, with <24 providing moderate sensitivity (0.5) and specificity (0.84) for intraoperative hemodynamic instability, as measured by systolic ARV (see Supplementary Figure S3).
4 Discussion
This study examined indirect measures of intraoperative hemodynamic instability in older adults undergoing elective noncardiac surgery and explored their association with preoperative smCPET measures. Key findings included: (1) surgical time and POSSUM operative severity were strongly associated with intraoperative vasopressor use; (2) among smCPET-derived measures, measured FVC was associated with vasopressor use; (3) participants with an FVC <2.18l and surgery >152 min had the highest risk for intraoperative vasopressor use; and (4) lower GXCAP-VO2 slope was associated with higher systolic ARV, with a 9.8% reduction in ARV per 10-unit decrease in observed GXCAP-VO2 slope.
4.1 Clinical implications: blood pressure response patterns
In our studied cohort, increasing surgical time and POSSUM operative severity were associated with intraoperative vasopressor use. POSSUM operative severity was associated with intraoperative vasopressor use but did not contribute to the constructed logistic regression model when surgical time was included, suggesting two possibilities: reduced sample size, or that operative severity correlates with longer, and likely more complex, surgeries, increasing the probability of vasopressor use. To assess this, a mediation analysis was performed, where the effect of operative severity on vasopressor use operates primarily through its effect on surgery time. Higher operative severity leads to longer surgeries, which in turn increased the likelihood of vasopressor use. In simpler terms, higher operative severity itself did not directly predict vasopressor use when controlling for surgery duration.
There was a strong negative correlation between increasing surgical time and systolic ARV, but not with increasing vasopressor equivalents kg-1 min-1. The induction of any surgery often involves significant adjustment and titration of anesthetic agents, suggesting a contribution to higher systolic ARV, whereas as surgery progresses, the anesthetic depth likely becomes more consistent. Secondly, physiological compensatory mechanisms may become more effective over time and increased ARV, due to excess stimulation from surgical exposure and closure, would be proportionally less in longer procedures when compared to the maintenance phase. Vasopressors are typically administered to achieve a targeted blood pressure threshold, rather than reduce systolic blood pressure variability, thus the weak correlation suggests that vasopressors may successfully achieve that goal, but may not reduce moment-to-moment systolic variability. Secondly, individual vasopressors may vary on their effects on systolic vs. diastolic pressure, and are routinely given reactively, e.g., administered after variability has occurred (45).
4.2 Clinical implications: FVC for the prediction of intraoperative vasopressor use
Measured FVC demonstrated near significance for association with increasing vasopressor administration. This was further confirmed by a Bayesian approach, where the consistency of directional effects across different prior specifications strengthens confidence in the observed association, and warrants further investigation with larger samples to confirm these preliminary findings. The physiological rationale for measured FVC as a risk predictor is compelling. Low FVC often reflects restrictive lung physiology, decreased lung compliance, reduced thoracic cavity volume, or impaired chest wall mechanics. These abnormalities contribute to decreased venous return during positive pressure ventilation. Higher airway pressures may be required to achieve adequate minute ventilation, leading to increased intrathoracic pressure, further impairing venous return and cardiac output. Additionally, chronic adaptation to restricted pulmonary function frequently presents with increased sympathetic tone and baseline vasoconstriction, which the sympatholytic action of volatile and intravenous anesthetics may unmask, creating higher probability of lower systemic blood pressure and further pushing the decision to initiate intraoperative vasopressor use. Low pulmonary reserve may also decrease oxygen delivery, causing tissue hypoxia and peripheral vasodilation.
Notably in our small cohort, absolute FVC was significant, while FVC % predicted was not, suggesting larger absolute lung volumes correlate with better thoracic capacity and venous return during mechanical ventilation. This might indicate that enhanced attention to tailoring mechanical ventilation, based on individual factors, may have a salutary effect on intraoperative vasopressor requirements. This is further supported by evidence that FVC correlates better than predicted body weight for determining appropriate tidal volumes (46). Alternatively, given the small sample size, and narrow range of BMI in the studied cohort, it is possible that relative FVC normalization, using these nomograms, diluted its independent predictive power in the multivariate logistic regression model, or that % predicted FVC shares variance with other predictors in the model, in ways that absolute FVC does not. Regardless, FVC may be a promising simplified measure of frailty and cardiopulmonary impairment, as lower spirometry values have been strongly associated with lower metabolic equivalents (47). This is further supported by measures of inspiratory muscle testing, specifically maximal inspiratory pressure (MIP) and maximal sniff nasal inspiratory pressure (SNIP), which have strong predictive value on survival in heart failure patients (48).
4.3 Clinical implications: GXCAP-VO2 slope and systolic ARV
Lower GXCAP-VO2 slope was associated with higher systolic ARV. This observation supports the supposition that it is a dynamic marker of the cardiopulmonary system's ability to accommodate increased blood flow during stress. Reduced pulmonary compliance, higher pulmonary vascular resistance, or reduced right ventricular-pulmonary artery (RV-PA) coupling, particularly in the setting of positive pressure ventilation, results in increasing RV afterload, more variable RV stroke volume and inconsistent left ventricular filling (49). RV-PA coupling represents optimal efficiency between RV contractility and PA afterload, when impaired, this mismatch may lead to compromised stroke volume and cardiac output. These effects are amplified under anesthesia due to drug-related peripheral vasodilation, sympatholytic actions, positive pressure ventilation, and surgical positioning. Normal compensatory mechanisms (increased sympathetic activation, enhanced baroreflex activity) are blunted, with combined effects result in higher beat to beat systolic pressure variation. This manifests as increased systolic ARV as the RV struggles to maintain consistent stroke volume, creating variable left ventricular filling. Overall, this suggests that GXCAP-VO2 slope represents optimal biventricular function, and possibly ventricular interdependence, as altered RV filling or pressure affects left ventricular geometry and function through septal displacement and pericardial restraints (37).
4.4 Summary
In summary, these observations challenge the intuitive assumption that longer surgeries lead to more hemodynamic instability. The reduced ARV during longer surgical procedures and the weak relationship with increasing vasopressor requirement suggest that other factors (anesthetic depth, fluid management, temperature control) may be more globally important for the reduction of intraoperative hemodynamic variability. Regarding smCPET measures, measured FVC and GXCAP-VO2 slope were the most compelling markers for intraoperative vasopressor use and systolic ARV, respectively. Lower measured FVC may suggest that underdiagnosed decreased lung compliance, reduced thoracic cavity volume, or impaired chest wall mechanics, may contribute more to IOH than previously thought, while lower GXCAP-VO2 slope may flag patients at higher risk for intraoperative hemodynamic instability. The clinical utility of FVC as a preoperative risk predictor is framed by several practical advantages. First, pulmonary function testing is widely available, inexpensive, and can be routinely performed in many preoperative evaluation clinics. Second, FVC measurement is standardized, objective, and reproducible. Third, unlike complex risk scores or specialized cardiac testing, FVC results are immediately available and easily interpreted by clinicians across specialties. Furthermore, FVC may capture aspects of impaired physiological reserve not readily apparent through conventional preoperative assessment. Our findings align with established literature demonstrating that frailty and limited exercise capacity predict poor surgical outcomes. Preoperative CPET assessment may identify frail patients who could benefit from prehabilitation programs before surgery. Future studies should investigate whether targeted CPET-based parameters can reduce intraoperative hemodynamic instability.
4.5 Limitations
Our study was not powered to definitively establish these associations, but rather to identify potential relationships for future targeted research. First, only 53.4% of participants had measured FVC, this data loss introduces high risk for potential selection bias, reduced statistical power and limits our ability to establish causal relationships. Mitigation of overfitting logistic regression models was attempted (using the 1:10 covariate to outcome rule), which may result in unmeasured confounders. Measured FVC had a modest AUC, suggesting limited standalone discriminative ability, which was expected, given the heterogeneity of patient factors and intraoperative care. Intraoperative vasopressor use reflects both patient physiology and clinician decision making, making it an imperfect surrogate for intraoperative hemodynamic instability. Only borderline statistical significance was observed, with wide confidence intervals, reducing our ability to detect true relationships. It would be challenging to separate the different effects of intraoperative interventions (operative severity, vasopressor selection) on both systolic ARV or intraoperative vasopressor use. Anesthetic techniques and procedural complexity have significant impact on intraoperative hemodynamic stability. This study did not capture specific anesthetic agents, doses or technique used, which represents a significant limitation. Systolic ARV measurements required a 40 measurement minimum, however, the differences between invasively obtained (arterial line) and noninvasive measurements present an unknown confounder to this endpoint. The binary classification of the primary endpoint oversimplifies clinical decision making, although it is likely superior to IOH event tabulation, given that IOH is usually aggressively treated in the intraoperative time period. The funding source's interest in CPET technology validation could theoretically bias interpretation toward positive findings; however, our reporting of borderline statistical significance and study limitations demonstrates transparent, unbiased analysis. Furthermore, focusing on linear relationships understates the complex interactions of intraoperative hemodynamics. Undoubtably, external validation is required to confirm the findings of this exploratory investigation.
4.6 Future directions
While promising, these findings are hypothesis-generating and require external validation before clinical implementation of measured FVC in preoperative risk assessment can be recommended. Prospective studies should focus on three areas: (1) validating the relationship between decreased ARV and surgical duration while identifying associated risk factors; (2) confirming the preliminary association between FVC and intraoperative vasopressor use; and (3) evaluating whether preoperative pulmonary optimization strategies can effectively reduce IOH.
4.7 Conclusions
The authors provide a theoretical framework and preliminary evidence for two quantitative physiological measures that may aid in identification of intraoperative hemodynamic instability risk; lower measured FVC for intraoperative vasopressor use and lower GXCAP-VO2 slope for higher systolic ARV. Participants with FVC <2.18 L undergoing surgeries ≥152 min faced highest risk for intraoperative vasopressor use, while each standard deviation increase in GXCAP-VO2 slope corresponded to 9.3% lower systolic ARV. These observations establish a foundation for future research on smCPET measures as predictors of perioperative complications, recognizing IOH as a complex interplay of patient, anesthetic, and surgical factors.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Yale University Institutional Review Board, Yale University, New Haven CT. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
DA: Conceptualization, Data curation, Investigation, Software, Writing – review & editing. ZC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Shape Medical Systems, Inc. (White Bear Lake, MN, USA) provided technical support, disposable mouthpieces, and the submaximal cardiopulmonary device used for this clinical investigation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanes.2025.1610632/full#supplementary-material
References
1. Wijnberge M, Schenk J, Bulle E, Vlaar AP, Maheshwari K, Hollmann MW, et al. Association of intraoperative hypotension with postoperative morbidity and mortality: systematic review and meta-analysis. BJS Open. (2021) 5(1):1–8. doi: 10.1093/bjsopen/zraa018
2. Shimada T, Cohen B, Shah K, Mosteller L, Bravo M, Ince I, et al. Associations between intraoperative and post-anesthesia care unit hypotension and surgical ward hypotension. J Clin Anesth. (2021) 75:110495. doi: 10.1016/j.jclinane.2021.110495
3. Turan A, Chang C, Cohen B, Saasouh W, Essber H, Yang D, et al. Incidence, severity, and detection of blood pressure perturbations after abdominal surgery: a prospective blinded observational study. Anesthesiology. (2019) 130(4):550–9. doi: 10.1097/ALN.0000000000002626
4. Bijker JB, van Klei WA, Kappen TH, van Wolfswinkel L, Moons KG, Kalkman CJ. Incidence of intraoperative hypotension as a function of the chosen definition: literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology. (2007) 107(2):213–20. doi: 10.1097/01.anes.0000270724.40897.8e
5. Saasouh W, Manafi N, Manzoor A, McKelvey G. Mitigating intraoperative hypotension: a review and update on recent advances. Adv Anesth. (2024) 42(1):67–84. doi: 10.1016/j.aan.2024.07.006
6. Christensen AL, Jacobs E, Maheshwari K, Xing F, Zhao X, Simon SE, et al. Development and evaluation of a risk-adjusted measure of intraoperative hypotension in patients having nonemergent, noncardiac surgery. Anesth Analg. (2021) 133(2):445–55. doi: 10.1213/ANE.0000000000005287
7. Karim HMR, Bansal V. Is research reporting intraoperative hypotension apt enough? Indian J Anaesth. (2024) 68(5):496–9. doi: 10.4103/ija.ija_209_24
8. Marcucci M, Painter TW, Conen D, Lomivorotov V, Sessler DI, Chan MTV, et al. Hypotension-avoidance versus hypertension-avoidance strategies in noncardiac surgery: an international randomized controlled trial. Ann Intern Med. (2023) 176(5):605–14. doi: 10.7326/M22-3157
9. Scott MJ, the AHIWG. Perioperative patients with hemodynamic instability: consensus recommendations of the anesthesia patient safety foundation. Anesth Analg. (2024) 138(4):713–24. doi: 10.1213/ANE.0000000000006789
10. Putowski Z, Czok M, Krzych ŁJ. The impact of intraoperative blood pressure variability on the risk of postoperative adverse outcomes in non-cardiac surgery: a systematic review. J Anesth. (2022) 36(2):316–22. doi: 10.1007/s00540-022-03035-w
11. Park S, Lee HC, Jung CW, Choi Y, Yoon HJ, Kim S, et al. Intraoperative arterial pressure variability and postoperative acute kidney injury. Clin J Am Soc Nephrol. (2020) 15(1):35–46. doi: 10.2215/CJN.06620619
12. Shen X, Tao H, Chen W, Sun J, Jin R, Zhang W, et al. Perioperative blood pressure variability as a risk factor for postoperative delirium in the patients receiving cardiac surgery. BMC Anesthesiol. (2024) 24(1):424. doi: 10.1186/s12871-024-02817-x
13. Hallqvist L, Mårtensson J, Granath F, Sahlén A, Bell M. Intraoperative hypotension is associated with myocardial damage in noncardiac surgery: an observational study. Eur J Anaesthesiol. (2016) 33(6):450–6. doi: 10.1097/EJA.0000000000000429
14. Harefa Wijaya IP, Muhadi Rumende CM, Nasution SA, Koesnoe S, et al. The association between 24-h blood pressure variability and major adverse cardiac events (MACE) in hospitalized patients with acute myocardial infarction: a retrospective cohort study. Egypt Heart J. (2021) 73(1):88. doi: 10.1186/s43044-021-00213-1
15. Hou C, Wang X, Li Y, Hei F. The relationship between short-term mean arterial pressure variability and mortality in critically ill patients. Front Cardiovasc Med. (2022) 9:870711. doi: 10.3389/fcvm.2022.870711
16. Geraci TC, Ng T. When is it safe to operate for lung cancer? Selection of fiscally responsible cardiopulmonary function tests for limited resection (wedge resection and segmentectomy), standard lobectomy, sleeve lobectomy, and pneumonectomy. Thorac Surg Clin. (2021) 31(3):255–63. doi: 10.1016/j.thorsurg.2021.04.006
17. Sato T, Watanabe A, Kondo H, Kanzaki M, Okubo K, Yokoi K, et al. Long-term results and predictors of survival after surgical resection of patients with lung cancer and interstitial lung diseases. J Thorac Cardiovasc Surg. (2015) 149(1):64. doi: 10.1016/j.jtcvs.2014.08.086
18. Gomes Neto M, Martinez BP, Reis HF, Carvalho VO. Pre- and postoperative inspiratory muscle training in patients undergoing cardiac surgery: systematic review and meta-analysis. Clin Rehabil. (2017) 31(4):454–64. doi: 10.1177/0269215516648754
19. Fujii M, Nishina D, Bessho R. Preoperative assessment of pulmonary function tests and outcomes after cardiac surgery. Heart Surg Forum. (2020) 23(2):E245–e9. doi: 10.1532/hsf.2791
20. Oh TK, Park IS, Na JE, S H. Value of preoperative spirometry test in predicting postoperative pulmonary complications in high-risk patients after laparoscopic abdominal surgery. PLoS One. (2018) 13(12):e0209347. doi: 10.1371/journal.pone.0209347
21. Chambers T, Gooneratne M, Singh R, Pang C, McDonnell G, Ricketts W. Making the case for spirometry as part of the perioperative multidisciplinary team assessment. Future Healthc J. (2022) 9(1):79–82. doi: 10.7861/fhj.2021-0116
22. Katz S, Arish N, Rokach A, Zaltzman Y, Marcus E-L. The effect of body position on pulmonary function: a systematic review. BMC Pulm Med. (2018) 18(1):159. doi: 10.1186/s12890-018-0723-4
23. Buono D, Arena MG, Borlaug R, Carbone BA, Canada S, Kirkman JM, et al. Exercise intolerance in patients with heart failure. JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73(17):2209–25. doi: 10.1016/j.jacc.2019.01.072
24. Huang YC, Lin TY, Wu HT, Chang PJ, Lo CY, Wang TY, et al. Cardiorespiratory coupling is associated with exercise capacity in patients with chronic obstructive pulmonary disease. BMC Pulm Med. (2021) 21(1):22. doi: 10.1186/s12890-021-01400-1
25. Baba R, Nagashima M, Goto M, Nagano Y, Yokota M, Tauchi N, et al. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Coll Cardiol. (1996) 28(6):1567–72. doi: 10.1016/S0735-1097(96)00412-3
26. Bernstein EJ, Mandl LA, Gordon JK, Spiera RF, Horn EM. Submaximal heart and pulmonary evaluation: a novel noninvasive test to identify pulmonary hypertension in patients with systemic sclerosis. Arthritis Care Res (Hoboken). (2013) 65(10):1713–8. doi: 10.1002/acr.22051
27. Khatri V, Neal JE, Burger CD, Lee AS. Prognostication in pulmonary arterial hypertension with submaximal exercise testing. Diseases. (2015) 3(1):15–23. doi: 10.3390/diseases3010015
28. Miller AD, Woods PR, Olson TP, Hulsebus ML, O'Malley KA, MacCarter D, et al. Validation of a simplified, portable cardiopulmonary gas exchange system for submaximal exercise testing. Open Sports Med J. (2010) 4:34–40. doi: 10.2174/1874387001004010034
29. Woods PR, Bailey KR, Wood CM, Johnson BD. Submaximal exercise gas exchange is an important prognostic tool to predict adverse outcomes in heart failure. Eur J Heart Fail. (2011) 13(3):303–10. doi: 10.1093/eurjhf/hfq187
30. Carr Z, Agarkov D, Li J, Charchaflieh J, Brenes-Bastos A, Freund J, et al. Implementation of brief submaximal cardiopulmonary testing in a high-volume pre-surgical evaluation clinic: a feasibility study. JMIR Perioper Med. (2025) 8:1–11. doi: 10.2196/65805
31. Prentis JM, Manas DM, Trenell MI, Hudson M, Jones DJ, Snowden CP. Submaximal cardiopulmonary exercise testing predicts 90-day survival after liver transplantation. Liver Transpl. (2012) 18(2):152–9. doi: 10.1002/lt.22426
32. Prentis JM, Trenell MI, Jones DJ, Lees T, Clarke M, Snowden CP. Submaximal exercise testing predicts perioperative hospitalization after aortic aneurysm repair. J Vasc Surg. (2012) 56(6):1564–70. doi: 10.1016/j.jvs.2012.05.097
33. Snowden CP, Prentis JM, Anderson HL, Roberts DR, Randles D, Renton M, et al. Submaximal cardiopulmonary exercise testing predicts complications and hospital length of stay in patients undergoing major elective surgery. Ann Surg. (2010) 251(3):535–41. doi: 10.1097/SLA.0b013e3181cf811d
34. Carlisle J, Swart M. Mid-term survival after abdominal aortic aneurysm surgery predicted by cardiopulmonary exercise testing. Br J Surg. (2007) 94(8):966–9. doi: 10.1002/bjs.5734
35. Taylor BJ, Olson TP, Chul Ho K, Maccarter D, Johnson BD. Use of noninvasive gas exchange to track pulmonary vascular responses to exercise in heart failure. Clin Med Insights Circ Respir Pulm Med. (2013) 7:53–60. doi: 10.4137/CCRPM.S12178
36. Moallem N, H M, Parikh R. Sub-maximal Exercise Testing in Interstitial Lung Disease: Assessing the Role of GXCAP to Detect Concomitant Pulmonary Hypertension. Washington, D.C.: American Thoracic Society (2023).
37. Naeije R, Badagliacca R. The overloaded right heart and ventricular interdependence. Cardiovasc Res. (2017) 113(12):1474–85. doi: 10.1093/cvr/cvx160
38. A feasibility study of the SHAPE™ test of aerobic fitness in older adults presenting for moderate to high-risk surgery (2023). Available online at: https://clinicaltrials.gov/study/NCT05743673
39. Saravanan S, Kocarev M, Wilson RC, Watkins E, Columb MO, Lyons G. Equivalent dose of ephedrine and phenylephrine in the prevention of post-spinal hypotension in caesarean section. Br J Anaesth. (2005) 96(1):95–9. doi: 10.1093/bja/aei265
40. Goradia S, Sardaneh AA, Narayan SW, Penm J, Patanwala AE. Vasopressor dose equivalence: a scoping review and suggested formula. J Crit Care. (2021) 61:233–40. doi: 10.1016/j.jcrc.2020.11.002
41. Hollenberg M, Tager IB. Oxygen uptake efficiency slope: an index of exercise performance and cardiopulmonary reserve requiring only submaximal exercise. J Am Coll Cardiol. (2000) 36(1):194–201. doi: 10.1016/S0735-1097(00)00691-4
42. Prytherch DR, Whiteley MS, Higgins B, Weaver PC, Prout WG, Powell SJ. POSSUM and portsmouth POSSUM for predicting mortality. Physiological and operative severity score for the enUmeration of mortality and morbidity. Br J Surg. (1998) 85(9):1217–20. doi: 10.1046/j.1365-2168.1998.00840.x
43. Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: r package for causal mediation analysis. J Stat Softw. (2014) 59(5):1–38. doi: 10.18637/jss.v059.i05
44. Koenker R, Bassett G. Regression quantiles. Econometrica. (1978) 46(1):33–50. doi: 10.2307/1913643
45. Overgaard CB, Džavík V. Inotropes and vasopressors. Circulation. (2008) 118(10):1047–56. doi: 10.1161/CIRCULATIONAHA.107.728840
46. Hoftman N, Eikermann E, Shin J, Buckley J, Navab K, Abtin F, et al. Utilizing forced vital capacity to predict low lung compliance and select intraoperative tidal volume during thoracic surgery. Anesth Analg. (2017) 125(6):1922–30. doi: 10.1213/ANE.0000000000001885
47. Eimer C, Urbaniak N, Dempfle A, Becher T, Schädler D, Weiler N, et al. Pulmonary function testing in preoperative high-risk patients. Perioper Med (Lond). (2024) 13(1):14. doi: 10.1186/s13741-024-00368-w
48. Meyer FJ, Borst MM, Zugck C, Kirschke A, Schellberg D, Kübler W, et al. Respiratory muscle dysfunction in congestive heart failure: clinical correlation and prognostic significance. Circulation. (2001) 103(17):2153–8. doi: 10.1161/01.CIR.103.17.2153
Keywords: intraoperative hypotension, intraoperative hemodynamic instability, preoperative risk stratification, submaximal cardiopulmonary exercise testing, CPET, anesthesiology
Citation: Agarkov D and Carr ZJ (2025) Preliminary insights into cardiopulmonary reserve and hemodynamic stability: exploring submaximal cardiopulmonary exercise testing parameters as potential predictors of intraoperative hemodynamic instability. Front. Anesthesiol. 4:1610632. doi: 10.3389/fanes.2025.1610632
Received: 12 April 2025; Accepted: 13 August 2025;
Published: 28 August 2025.
Edited by:
Alparslan Turan, Cleveland Clinic, United StatesReviewed by:
Habib Md Reazaul Karim, All India Institute of Medical Sciences, IndiaChristian Bohringer, UC Davis Medical Center, United States
Copyright: © 2025 Agarkov and Carr. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zyad J. Carr, enlhZC5jYXJyQHlhbGUuZWR1
 Daniel Agarkov
Daniel Agarkov Zyad J. Carr
Zyad J. Carr