- 1Anesthesiology, St Jude Children’s Research Hospital, Memphis, TN, United States
- 2Department of Cardiothoracic Anesthesiology, Cleveland Clinic Foundation, Cleveland, OH, United States
- 3Department of Biostatistics, St Jude Children’s Research Hospital, Memphis, TN, United States
- 4Bone Marrow Transplant and Cellular Therapy, St Jude Children’s Research Hospital, Memphis, TN, United States
- 5Global Pediatric Medicine, St Jude Children’s Research Hospital, Memphis, TN, United States
Background: During hematopoietic stem and progenitor cell collection from the bone marrow under anesthesia, pediatric donors are exposed to potential complications including hypotension, pain, blood transfusion, endotracheal intubation risks, prone positioning injuries, and postoperative nausea and vomiting (PONV). We evaluated the overall incidence and severity of adverse events to identify opportunities to improve perioperative outcomes for this unique population.
Methods: With institutional review board approval, all donors under 18 years of age who had bone marrow harvest under general anesthesia between 2010 and 2024 at our institution were included in this retrospective study. Autologous donors and donors whose cells were collected by apheresis without anesthesia were excluded.
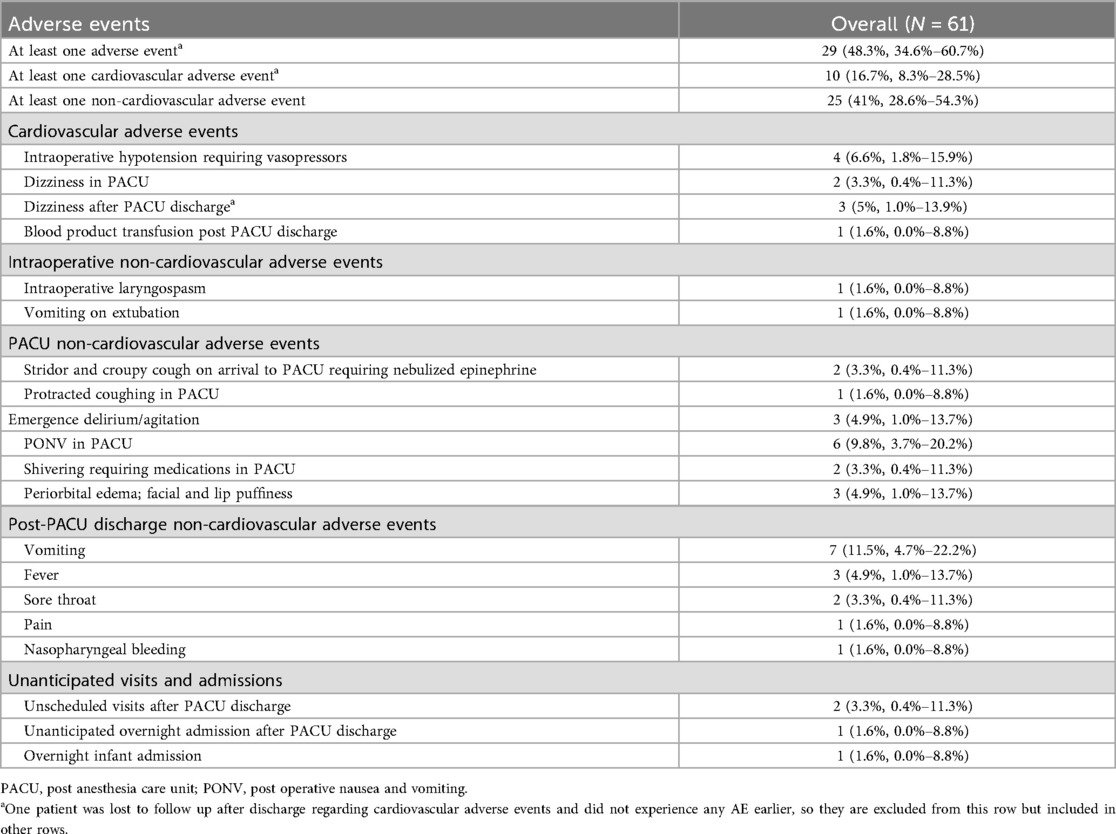
Results: The study included 61 donors with a mean age of 9.62 years, mean donor/recipient weight ratio of 1.57, mean harvest volume of 14.2 mL/kg donor weight, and mean fasting duration for clear liquids and solids of 9.31 and 11.3 h, respectively. Twenty-nine (47.5%) experienced at least 1 adverse event. 10 (16.4%) donors experienced at least 1 cardiovascular adverse event. Out of ten episodes of significant hypotension events, 4 donors required vasopressors intraoperatively, 2 experienced dizziness in the post-anesthesia care unit (PACU), and 4 experienced symptomatic hypotension after discharge from the PACU. One infant required blood product transfusion. Six donors (9.8%) experienced post-operative nausea vomiting (PONV), and 7 others (11.5%) experienced post-discharge vomiting. There was one overnight admission, 1 readmission, and 2 unanticipated visits.
Conclusions: This single institution study highlights improvement opportunities for the perioperative care of pediatric bone marrow donors. We propose strategies to optimize preoperative fasting, intraoperative analgesia, and antiemetic prophylaxis and recommend a procedure-specific intravenous fluid replacement calculator, and admission and discharge criteria for bone marrow donors.
1 Introduction
Pediatric bone marrow donors undergo a procedure that provides the donor with no immediate physical benefits but may result in some long-term psychological and physical benefits (1, 2). Donors may experience postoperative pain, cardiovascular adverse events (AEs) associated with rapid, high-volume blood loss, and other complications associated with anesthesia, endotracheal intubation, prone positioning, and blood product transfusion. Younger donors and those with more severe illness may experience severe AEs and require blood product transfusion (3, 4). In addition, as hematopoietic stem cell harvest is an outpatient procedure, health care providers rely heavily on caregivers to seek support for AEs that occur after discharge. We conducted this study on perioperative outcomes for pediatric bone marrow donors in response to reports of AEs at our institution. These AEs included hypotension during anesthesia that required intervention and a cardiovascular event that occurred in patient housing after hospital discharge. Our aim was to understand the overall incidence and severity of AEs, identify improvement opportunities, and improve perioperative outcomes for this unique population.
2 Methods
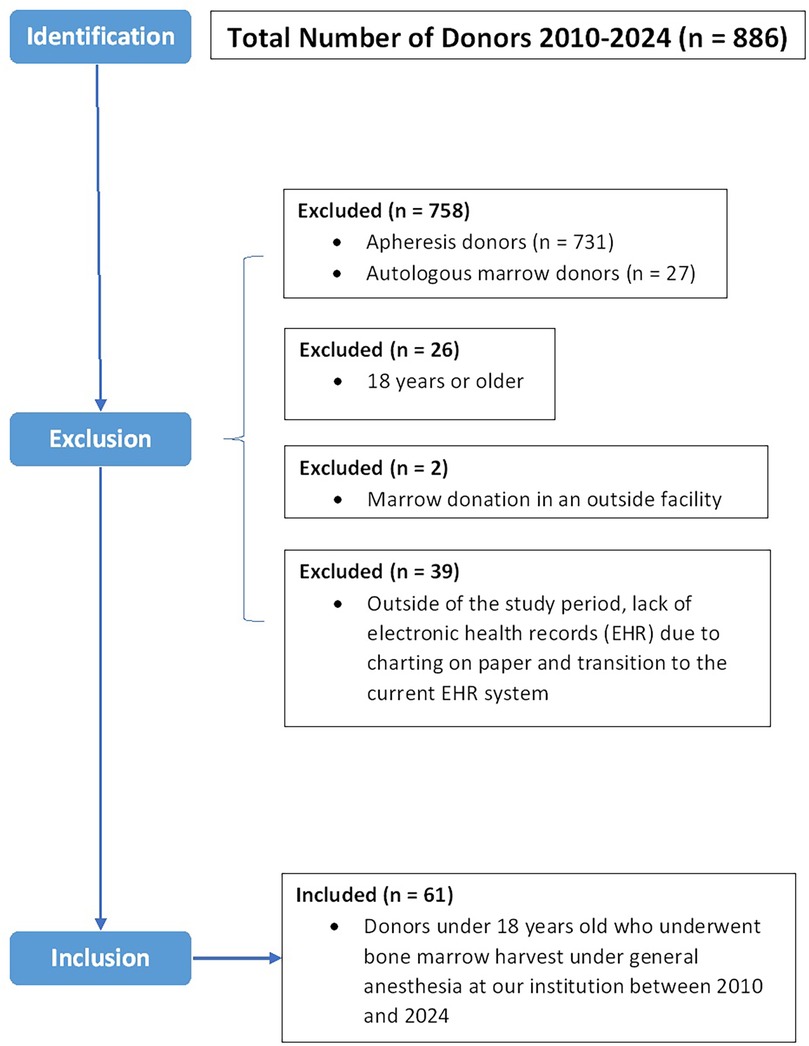
This retrospective study was approved by the Institutional Review Board (IRB) at St. Jude Children's Research Hospital (IRB number 20-0505, reference 007980). All donors under 18 years of age who underwent a bone marrow harvest procedure under general anesthesia between 2010 and 2024 at our institution, with an electronic health record were included. This timeline was chosen to maximize the sample size in view of rarity of these procedures. Donors without electronic health record, autologous donors and donors whose cells were collected by apheresis were excluded (Figure 1).
This was an outpatient procedure. Anesthetic management was at the discretion of the patient's anesthesia care team. No major differences in the anesthetic technique were anticipated during the study period. Patients were discharged from the post-anesthesia care unit (PACU) to their caregivers in hospital housing with the option to return for an unscheduled visit to the acute care clinic if needed. There were no procedure-specific discharge criteria. Patients returned the next morning for a scheduled laboratory test appointment. There were no perioperative intravenous (IV) fluid or blood product administration protocols for these procedures during the study period. The protocols for bone marrow collection for autologous or allogeneic hematopoietic progenitor cell transplantation were based on the institutional guidelines (5). During the study period, our fasting policy involved fasting from clear liquids for 2 h, solids (including non-human milk) for 8 h, and breast milk for 3 h prior to anesthesia. This changed to 1 h for clear liquids and 6 h for non-human milk in August 2021.
Pre-anesthesia data included donor demographics and comorbidities, along with preanesthetic clinical parameters such as donors’ and recipients’ ages and weights. Resolved comorbidities, defined as clinical conditions experienced by the patients in the past that were resolved at the time of admission for the procedure, were excluded. Intraoperative data included anesthetic technique, clinical parameters, medications, IV fluid and blood products, bone marrow harvest volume, and AEs. Immediate postoperative data included length of PACU stay, pain scores, clinical parameters, postoperative nausea and vomiting (PONV), IV fluid and blood products, and AEs. Data from after PACU discharge, which were obtained from documentation on the scheduled postoperative day 1 visit, included clinical parameters, IV fluid and blood products, and AEs attributable to the procedure.
Clinical parameters including heart rate, blood pressure, oxygen saturation, and temperature obtained during the preoperative phase on the day of the procedure, as well as hemoglobin and hematocrit levels obtained on the day before anesthesia, were considered baseline. Donors’ heights and weights were obtained on the day of or the day before anesthesia. The recipient weight from a date within a week of anesthesia was used to calculate the donor to recipient (D/R) weight ratio.
Hypotension was defined as a decrease in non-invasive blood pressure of greater than 20% of the baseline. In addition, severe hypotension was defined as episodes resulting in symptoms including dizziness or syncope or requiring vasopressors.
The primary and secondary outcome measures were cardiovascular and non cardiovascular adverse events respectively. Any clinical unforeseen events that affected the patient and required or involved additional monitoring, escalation of care, disability or death, were considered as adverse events. Unscheduled visits or readmissions within 24 h of anesthesia, overnight admissions, intensive care unit admission, or calls to either the rapid response team or the Harvey team (code blue) were considered AEs. Severe hypotension requiring vasopressors, symptomatic hypotension in PACU or after PACU discharge presenting with dizziness or syncope, cardiac arrests and other unanticipated cardiovascular events requiring escalation of care were categorized as cardiovascular adverse events. Transient intraoperative hypotension that responded to IV fluids, asymptomatic postoperative hypotension, and other near-miss events were excluded from the AE analysis.
2.1 Statistical analysis
Baseline, intraoperative, PACU, and post-PACU discharge variables were analyzed by calculating mean and standard deviation (SD) (continuous variables) or count and percentage (categorical variables). AE occurrence was summarized by count, percentage and the exact binomial 95% confidence interval of proportion. Asymptomatic hypotension and hypotension not requiring vasopressors were analyzed separately from other AEs.
The univariable associations between prespecified covariates and the outcomes of occurrence of at least one monitored AE or cardiovascular AE, excluding mild to moderate hypotension within 24 h of operation, were analyzed by logistic regression. One patient was excluded from association analysis due to lost follow up during the post PACU-discharge period. The analysis of IV fluid used was restricted to patients who received the corresponding infusion. These results are summarized by odds ratios, Wald test 95% confidence intervals, and Wald test p-values. No adjustments were made for multiple comparisons, and these analyses are intended to be exploratory.
3 Results
There were 64 healthy donors during the study period. After excluding 3 donors older than 18 years of age, we included 61 donors in our study.
Table 1 describes the age, sex, race, and body mass index of donors, as well as the D/R weight ratio, marrow harvest volume, donor fasting duration, and baseline donor hemoglobin and hematocrit data. The youngest donor was 7 months old. Three donors were less than 2 years old and 11 were 2 to 5 years old. Nine donors (14.8%) had ongoing comorbidities. Notably, 1 donor had ongoing rhinorrhea and occasional dry cough, 1 had a neurodevelopmental delay, and 2 had well-controlled reactive airway disease. Three donors had a history of snoring at night without sleep studies. One donor with snoring was also treated with levothyroxine and spironolactone for hypothyroidism and lower extremity edema of unknown etiology. The mean fasting duration was 11.3 h (SD, 1.5 h) for solids and 9.31 h (SD, 3.73 h) for clear liquids.
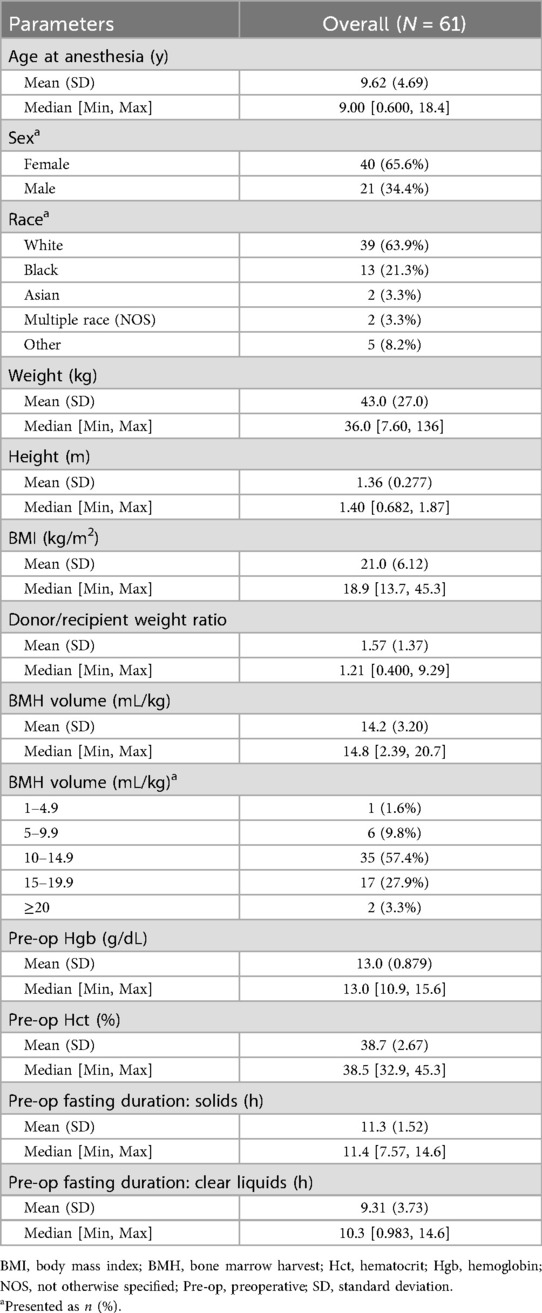
Table 1. Donor demographics, preoperative donor clinical parameters, hematopoietic stem cell volume, and donor to recipient ratios.
Forty patients (65.7%) had a D/R weight ratio of <1.6 (Figure 2). Twenty-four patients (39.4%) had a D/R weight ratio of <1. Nineteen donors (31.2%) had a marrow harvest volume of >15 mL/kg donor weight, with 2 (3.3%) having harvest volumes >20 mL/kg. All donors with harvest volumes of >20 mL/kg had a D/R weight ratio of <1. Approximately 53% of the donors with a harvest volume of 15–19.9 mL/kg donor weight had a D/R weight ratio of >1. The preoperative mean hemoglobin and hematocrit levels were 13.0 g/dL (SD, 0.88 g/dL) and 38.7% (SD, 2.67%), respectively.
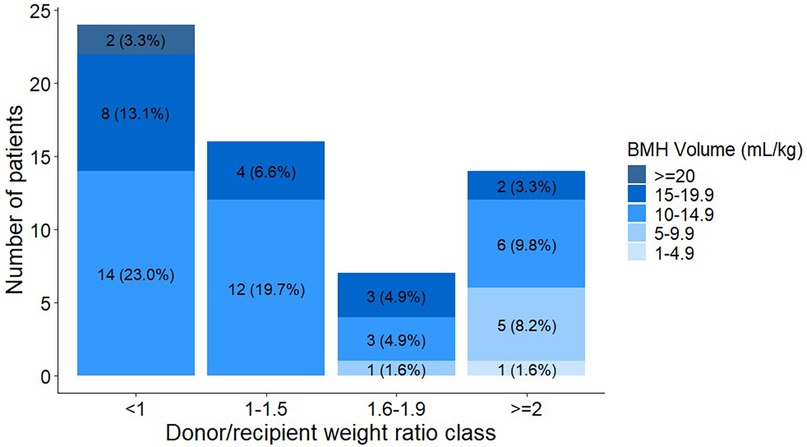
Figure 2. The bone marrow harvest volume from the intraoperative phase is shown for each donor/recipient weight ratio category. Bar plots show the number and percentage of donors (N = 61). BMH, bone marrow harvest.
The details of anesthetic technique are described in Table 2. The median intraoperative crystalloid and colloid volumes were 27.5 mL/kg (range, 6.63–67 mL/kg) and 8.88 mL/kg (range, 2.15–26.2 mL/kg). In the PACU, the median crystalloid and colloid volumes administered were 6.2 mL/kg (range, 0.63–1,000 mL/kg), and 7.8 mL/kg (range, 5.5–10.1 mL/kg) respectively. After PACU discharge, crystalloids and packed red blood cells (PRBCs) were administered to 1 patient (1.6%) each, both of whom required admission. The median decreases in hemoglobin and hematocrit levels from the preoperative to the postoperative phase were 3 g/dL (range, 0.8–4.6 g/dL) and 9% (range, 2%–14.6%), respectively (Figure 3).
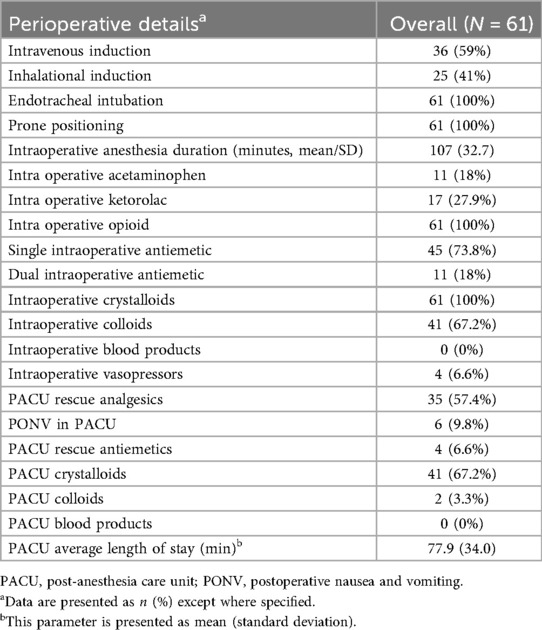
Table 2. Summary of perioperative anesthesia, analgesia, antiemetics, fluids, and blood products used.
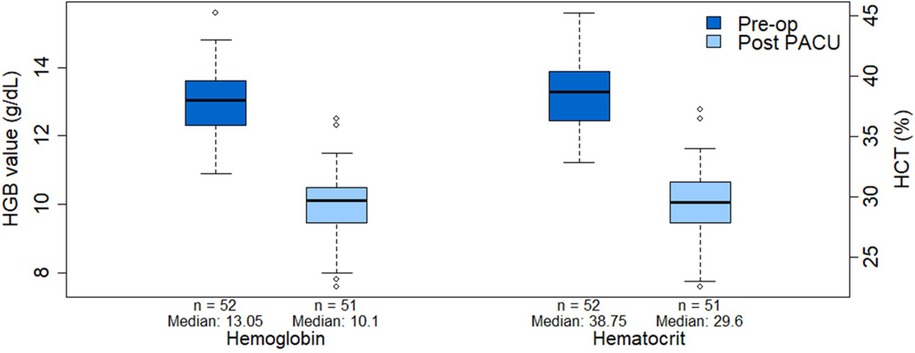
Figure 3. Comparison of hemoglobin and hematocrit values between the preoperative and post-PACU periods. HCT, hematocrit; HGB, hemoglobin; Pre-op, preoperative; PACU, post-anesthesia care unit.
The average length of stay in the PACU was 77.9 min. The mean highest pain score on arrival at the PACU was 3.59 (SD, 3.07). Thirty-five patients (57.4%) received additional analgesics in the PACU. Six patients (9.8%) experienced nausea or vomiting, and 4 patients (6.6%) received an additional antiemetic in the PACU.
AEs are listed in Table 3. Overall, 29 donors (48.3%) experienced at least 1 AE. One patient was lost to follow up after discharge. One of 3 donors who experienced dizziness after discharge from the PACU had 2 syncopal episodes requiring a code team call to hospital housing and emergent inpatient admission for fluid resuscitation. Another donor with dizziness had an unscheduled visit and received IV fluids in the acute care clinic. The third donor did not make a visit to the acute care clinic and chose to rest at housing. One donor required an inpatient admission and PRBC transfusion after discharge from the PACU.
During the intraoperative period, 52 donors (85.2%) experienced hypotension, 4 (7.69%) of whom required vasopressors. The mean volume per kilogram of crystalloids administered to donors who required vasopressors was higher than that for those who did not (45.7 vs. 26.5 mL/kg, respectively). We observed no clinically relevant differences in fasting duration, D/R weight ratio, or marrow harvest volumes between groups with and without hypotension during the intraoperative period. We could not perform statistical analysis due to the high event rate and limited sample size. Twenty-three donors (37.7%) experienced hypotension in the PACU. No differences in intraoperative crystalloid and colloid volumes were observed between groups with and without PACU hypotension.
Two (3.3%), 16 (26.2%), and 12 (19.7%) donors experienced at least one non-cardiovascular AE in the intraoperative period, PACU, and post-discharge period, respectively (Table 3).
The most frequent non-cardiovascular AE was nausea/vomiting (N/V). Six donors (9.8%) experienced N/V in the PACU. Of these 6 patients, 4 were female. Four of the 6 donors received 1 antiemetic during anesthesia, 1 received none, and 1 received 2 antiemetics. Four of these 6 donors had a PONV risk score of 2 and received ondansetron. The other 2 donors had a risk score of 3, with 1 receiving 2 antiemetics and the other receiving none. They had an average marrow harvest volume of 14.3 mL/kg and average intraoperative crystalloid and colloid infusion volumes of 26.3 mL/kg and 7.9 mL/kg, respectively. The fasting durations for solids and clear liquids were notably long among these 6 donors, with an average of 635 min and 480 min, respectively. No patients with N/V received intraoperative vasopressors, indicating the absence of significant hypotension. Within 24 h after PACU discharge, 7 donors (11.5%) experienced post-discharge N/V. Of these 7 donors, 6 were female. Their average fasting durations for solids and clear liquids were 696 and 529 min, respectively. Five donors had a PONV risk score of 3 or higher. Among these donors, 2 received dual antiemetic prophylaxis intraoperatively, 1 received 1 antiemetic, and 2 received none. Two donors with a risk score of 2 received 1 antiemetic each. One of these donors also experienced PONV.
There were no deaths or cardiac arrests within the first 24 h after anesthesia.
Four donors (6.6%) experienced at least 1 escalation of care event. One patient (1.6%) was readmitted to the hospital due to 2 syncopal episodes and received crystalloids. After a discussion between Anesthesiology and Transplantation Services staff about marrow blood volume loss and the risk of hemodynamic instability, the 7-month-old patient was admitted as an inpatient after the procedure and was discharged on postoperative day 1. This infant received PRBC transfusion prior to hospital discharge. Two patients (3.3%) had an unscheduled visit to the acute care clinic after discharge from the hospital; one for dizziness on standing and vomiting and another for continued N/V, sore throat, and low energy.
We found no statistically significant association between the occurrence of overall AEs or cardiovascular AEs and any variables including age, preoperative fasting duration, D/R weight ratio, stem cell harvest volume, duration of anesthesia, and intraoperative fluid volume (Supplementary Tables S1, S2).
4 Discussion
We have reviewed our experience with the perioperative care of pediatric donors undergoing bone marrow harvest at our institution, identified adverse outcomes and improvement opportunities, and proposed strategies to mitigate AEs. These quality improvement strategies are based on our experience and include the implementation of a procedure specific IV fluid volume calculator and post operative admission and discharge criteria, methods to reduce preoperative clear liquid fasting duration, and intraoperative analgesic and antiemetic optimization.
Although only a few pediatric donors underwent bone marrow harvest at our institution over the last 15 years, we focused on this group because of their potential exposure to adverse outcomes despite the lack of either immediate physical benefits or the ability to consent. Although the sample size was small, it represents a relatively large sample in view of its rarity.
Previous studies evaluating pediatric donor experiences have primarily analyzed psychosocial distress and post-traumatic growth, harvesting techniques and volumes, harvesting in the context of gene therapy, factors influencing allogeneic transfusion, and the role of granulocyte colony-stimulating factor priming (2, 6–9). Some immediate and long-term somatic effects of pediatric marrow donation have been evaluated (1, 4). Previous adult studies have evaluated opioid consumption reduction in donors through regional anesthetic techniques, outcomes of preharvest autologous blood collection and transfusion in donors, and factors associated with pre- and post-harvest anemia (10–12). No recommendations have been made with respect to optimal perioperative fluid volumes, pain management, prevention of N/V, or discharge criteria for pediatric hematopoietic stem cell donors.
Although younger children have a higher risk of complications during anesthesia, children and infants of any age can be hematopoietic stem cell donors if the 5 conditions outlined by the American Academy of Pediatrics Committee on Bioethics are met (13). In alignment with a previous study, approximately 15% of our donors were less than 5 years old (14).
We noted fasting durations that significantly exceeded guidelines. The fasting guidelines for clear liquids until August 2021 at our institution was 2 h based on the American Society of Anesthesiologists guidelines (15). Although the fasting recommendation for clear liquids prior to anesthesia was changed to 1 h in August 2021, we did not observe a significant reduction in fasting times for clear liquids in this population after the policy change. At our institution, bone marrow harvests are typically scheduled with a start time of 7:30 am in the operating room. Children may prefer not to drink clear liquids early in the morning, and caregivers may not be aware of the advantages of administering clear liquids up to 1 h before anesthesia. These factors may have contributed to the long fasting durations observed in our donors. Although we were unable to investigate the possible association between fasting duration and hypotension or PONV, previous studies have reported increased hunger and thirst, reduced feeling of wellbeing, and hypotension at induction due to prolonged fasting times (16–18). Therefore, we recommend the administration of clear liquids 1 h prior to anesthesia and the start of IV fluid administration in the preoperative holding area to compensate for the fasting-associated fluid deficit (Figure 4).
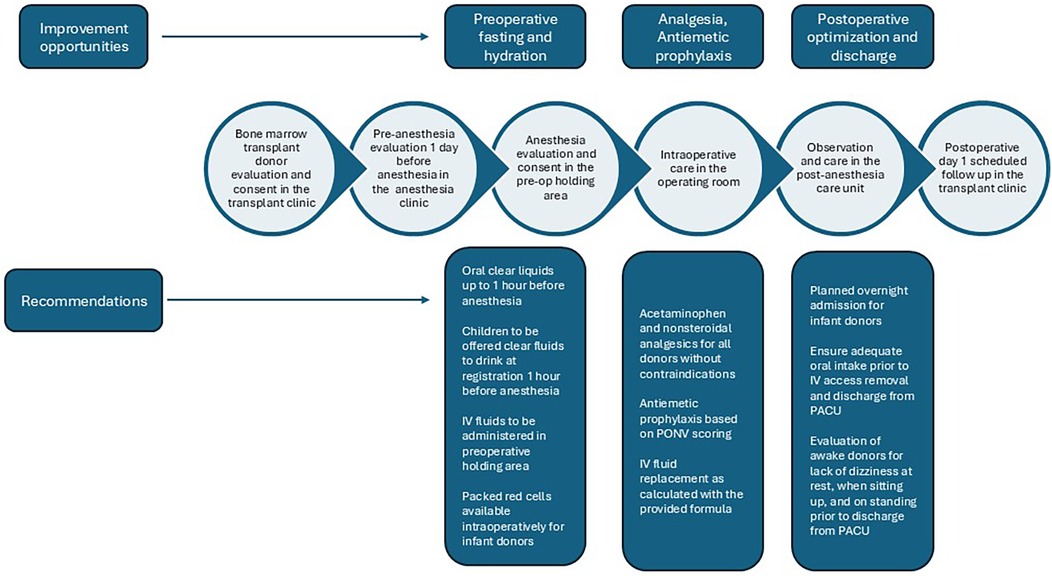
Figure 4. Improvement opportunities and recommendations for the perioperative care of pediatric bone marrow donors. IV, intravenous; PACU, post-anesthesia care unit; PONV, postoperative nausea and vomiting.
Although 60 patients (98.4%) received opioid analgesics during anesthesia, few received multimodal pain management with additional acetaminophen or a nonsteroidal analgesic. Although the average PACU pain scores were <4, more than half of the donors required rescue analgesia in the PACU. Acetaminophen and other nonsteroidals are administered sparingly at our institution due to their potential to mask fever, exacerbate thrombocytopenia, or trigger acute renal dysfunction in pediatric oncology populations. This practice may have influenced the observed underutilization of simple analgesics during the bone marrow harvest procedure, even when such precautions were not indicated. Here, we recommend intraoperative use of simple analgesics in addition to opioids (Figure 4).
Although bone marrow harvesting is not identified as a procedure associated with a high risk of N/V, we observed a higher than anticipated incidence of N/V in our study. Most donors received a single prophylactic antiemetic, which is usually a 5HT3 antagonist. Dexamethasone was the second antiemetic administered to those who received dual prophylaxis. We noted an inconsistent practice of tailoring antiemetics to PONV risk factors (19). Due to the small sample size, we were unable to identify statistically significant risk factors for N/V such as prolonged fasting duration and hypotension. We recommend the administration of antiemetics based on PONV risk scores to reduce N/V rates among donors (Figure 4). Other interventions, including reducing fasting durations and optimizing intraoperative IV fluid volumes, may also help reduce N/V.
We observed a high incidence of mild to moderate hypotension that responded to IV fluids, as well as hypotension events that were symptomatic or required vasopressors. Our institutional policy acknowledges the risks associated with high bone marrow harvest volumes and requires volumes to be limited to 15 mL/kg donor weight. In exceptional circumstances requiring higher volumes, the risks are discussed with the family, reviewed by the Transplant Quality Manager, and approved by the Marrow Facility Medical Director and the Transplant Program Director. Therefore, although nearly 40% of the donors in our study weighed less than the recipients, the marrow harvest volume exceeded 15 mL/kg in only approximately 31% of the donors. However, the speed of marrow aspiration may have caused substantial transient blood loss that contributed to intraoperative hypotension.
The average intraoperative crystalloid volume administered was twice the mean harvest volume, with nearly two-thirds of the donors receiving colloids intraoperatively at a 1:1 ratio to marrow harvest volume. Although fluid replacement volumes seemed appropriate based on the ratio of blood loss to volume replacement, approximately 85% and 38% of the donors experienced transient intraoperative and postoperative hypotension, respectively. In addition, about 15% of the donors had intraoperative hypotension requiring vasopressors and postoperative hypotension with symptoms including dizziness and syncope. This may have been due to prolonged fasting durations, but the association was not statistically significant. Our subgroup analysis showed that clinically relevant higher volumes of crystalloids were administered to hypotensive donors who required vasopressors than to those who did not. This may reflect an appropriate escalation of interventions, with volume resuscitation used before pharmacological management with vasopressors. Statistical significance could not be established between vasopressor use and fasting duration due to the small sample size. To reduce the incidence of intraoperative and postoperative hypotension, we recommend oral hydration until 1 h prior to anesthesia, with children being offered a clear liquid drink when they arrive at registration. Given our study results, we propose the use of an IV fluid replacement volume calculator based on the child's fasting fluid deficit and maintenance requirements, marrow harvest volume, and the average IV colloid usage. We also recommend that preoperative replacement of fasting deficits in children with vascular access be performed in the holding area. The following formula may be used to calculate the minimum IV fluid replacement volume:
[*30 mL/kg is two times the average marrow harvest volume; **10 mL/kg is the average colloid administered based on our study results]
Pediatric bone marrow donors are typically discharged from the PACU to be cared for by their caregivers after the procedure. At the time of discharge, donors are usually sleepy and easy to rouse and exit in a wheelchair, stroller, or hospital-provided cart. Therefore, symptoms such as dizziness that indicate inadequate cardiovascular optimization may be missed. Patients are not routinely evaluated for postural hypotension in the PACU before discharge. An infant in our study required overnight admission and blood product transfusion. Given previous reports of higher AEs and transfusion requirements in infants, we recommend admitting infant donors overnight for observation after bone marrow harvest. The high incidence of unanticipated hospital visits and readmission among our donors indicates the need to ensure adequate oral intake and identify clinical signs of dizziness at rest or standing indicating significant hypotension prior to PACU discharge. We recommend longer postoperative observation times to enable the evaluation of a fully awake patient for dizziness at rest, sitting up, and standing, along with the establishment of adequate liquid oral intake without N/V prior to discharge.
5 Limitations
The main limitations of our study are its retrospective nature and the small sample size. Ours being a single institution study, the conclusions would benefit from prospective and external validation. In particular, we acknowledge that some of our variables such as fasting times, intra operative fluid management, analgesic and antiemetic use, and criteria for admission and discharge, may be different from other institutions affecting outcomes Although we identified clinically relevant concerns such as prolonged fasting durations and inconsistent antiemetic and analgesic administration, we were unable to establish statistical significance between these observations and the occurrence of AEs. Inferential analyses were limited by potentially increased type-1 error due to exploring multiple comparisons and limited power due to the modest sample size and event rates. Therefore, our recommendations are based on the logical interpretation of clinically relevant results. The retrospective nature of our study may have contributed to the underreporting of minor but important complications. We did not report on the long-term physical and psychological outcomes for these donors.
6 Conclusions
Pediatric bone marrow donors are a unique group of patients who are unable to provide informed consent, and these children undergo a procedure that involves the risks of anesthesia, intubation, prone positioning, significant blood loss, and IV fluid and blood product therapy without physical benefit to themselves and with only potential long-term psychological benefits. Our study reports a high incidence of AEs in this patient population and highlights many opportunities for care improvement. Based on our experience, we propose quality improvement strategies to optimize preoperative fasting, administer simple analgesics in addition to opioids to all donors, use PONV scoring to select an antiemetic prophylactic regimen, use a procedure-specific IV fluid replacement calculator, admit all infant donors overnight, and follow discharge criteria for non-infant donors. Future studies would benefit from a larger, multicenter sample, use of multivariable models to control for confounders, and standardization of perioperative management protocols.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: raw data can be made available on request. Requests to access these datasets should be directed to Kavitha Raghavan.a2F2aXRoYS5yYWdoYXZhbkBzdGp1ZGUub3Jn.
Ethics statement
The studies involving humans were approved by Institutional research board, St Jude Children's Research Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
KR: Conceptualization, Supervision, Writing – review & editing, Investigation, Methodology, Software, Project administration, Funding acquisition, Resources, Data curation, Validation, Formal analysis, Visualization, Writing – original draft. BS: Writing – original draft, Data curation, Writing – review & editing. YB: Writing – original draft, Formal analysis, Data curation, Writing – review & editing. SS: Data curation, Formal analysis, Writing – review & editing, Writing – original draft. BT: Supervision, Writing – review & editing, Methodology, Funding acquisition, Writing – original draft, Data curation. DA: Resources, Supervision, Writing – review & editing, Writing – original draft, Methodology, Validation, Investigation, Conceptualization, Data curation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
This work was, in part, supported by American Lebanese Syrian Associated Charities (ALSAC). Biostatistics support was provided by the Biostatistics Shared Resource (BSR) of St. Jude Children's Research Hospital and the St. Jude Comprehensive Cancer Center (National Cancer Institute grant P30 CA021765).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanes.2025.1713946/full#supplementary-material
References
1. van Walraven SM, Straathof LM, Switzer GE, Lankester A, Korthof ET, Brand A, et al. Immediate and long-term somatic effects, and health-related quality of life of BM donation during early childhood. A single-center report in 210 pediatric donors. Bone Marrow Transplant. (2013) 48(1):40–5. doi: 10.1038/bmt.2012.102
2. Klippenstein ADW, Piotrowski CC, Winkler J, West CH. Growth in the face of overwhelming pressure: a narrative review of sibling donor experiences in pediatric hematopoietic stem cell transplant. J Child Health Care. (2023) 27(1):60–77. doi: 10.1177/13674935211043680
3. Witt V, Peters C. Collection of HSC in children. In: Carreras E, Dufour C, Mohty M, Kröger N, editors. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies. Cham (CH): Springer (2019). p. 123–6. Copyright 2019, EBMT and the Author(s).
4. Paciaroni K, Alfieri C, Isgrò A, De Angelis G, Ribersani M, Marziali M, et al. One single bone marrow harvesting from donors under 3 years of age: assessing safety and efficacy of the procedure. Bone Marrow Transplant. (2019) 54(7):1121–3. doi: 10.1038/s41409-018-0415-y
5. Hematopoietic Progenitor Cell Collection: Bone Marrow Harvest. Department of Bone Marrow Transplantation and Cellular Therapy. St. Jude Children’s Research Hospital. (2024).
6. Tucci F, Frittoli M, Barzaghi F, Calbi V, Migliavacca M, Ferrua F, et al. Bone marrow harvesting from paediatric patients undergoing haematopoietic stem cell gene therapy. Bone Marrow Transplant. (2019) 54(12):1995–2003. doi: 10.1038/s41409-019-0573-6
7. Witt V, Pichler H, Fritsch G, Peters C. Multiple small versus few large amount aspirations for bone marrow harvesting in autologous and allogeneic bone marrow transplantation. Transfus Apher Sci. (2016) 55(2):221–4. doi: 10.1016/j.transci.2016.07.017
8. Yabe M, Morimoto T, Shimizu T, Koike T, Takakura H, Ohtsubo K, et al. Feasibility of marrow harvesting from pediatric sibling donors without hematopoietic growth factors and allotransfusion. Bone Marrow Transplant. (2014) 49(7):921–6. doi: 10.1038/bmt.2014.73
9. Furey A, Rastogi S, Prince R, Jin Z, Smilow E, Briamonte C, et al. Bone marrow harvest in pediatric sibling donors: role of granulocyte colony-stimulating factor priming and CD34+ cell dose. Biol Blood Marrow Transplant. (2018) 24(2):324–9. doi: 10.1016/j.bbmt.2017.10.031
10. Farhadfar N, Murthy HS, Logan BR, Sees JA, Ayas M, Battiwalla M, et al. Impact of autologous blood transfusion after bone marrow harvest on unrelated donor’s health and outcome: a CIBMTR analysis. Bone Marrow Transplant. (2020) 55(11):2121–31. doi: 10.1038/s41409-020-0911-8
11. Getta BM, Tong D, Deren S, Huang G, Hogg M, Collins D, et al. Pre- and post-bone marrow harvest anaemia is associated with lower CD34+ stem cell collection, high harvest volume and female gender. Intern Med J. (2020) 50(3):299–306. doi: 10.1111/imj.14419
12. McCoy NC, Hay EL, Romeo DA, Doty JW, Wolf BJ, Hudspeth MP. Decreased opioid consumption in bone marrow harvest patients using quadratus lumborum blocks in a standardized protocol. Front Med. (2022) 9:862309. doi: 10.3389/fmed.2022.862309
13. Asking children to donate bone marrow: 5 must-meet conditions. (2019). Available online at: https://www.ama-assn.org/delivering-care/ethics/asking-children-donate-bone-marrow-5-must-meet-conditions (Accessed May 07, 2024).
14. AlAnazi A, Nadeem A, Siddiqui K, AlAhmari A, Ghemlas I, AlJefri A, et al. Can the bone marrow harvest volume be reduced safely in hematopoietic stem cell transplantation with pediatric sibling donors? Blood Res. (2023) 58(1):28–35. doi: 10.5045/br.2023.2022167
15. Joshi GP, Abdelmalak BB, Weigel WA, Harbell MW, Kuo CI, Soriano SG, et al. 2023 American society of anesthesiologists practice guidelines for preoperative fasting: carbohydrate-containing clear liquids with or without protein, chewing gum, and pediatric fasting duration-A modular update of the 2017 American society of anesthesiologists practice guidelines for preoperative fasting. Anesthesiology. (2023) 138(2):132–51. doi: 10.1097/ALN.0000000000004381
16. Aroonpruksakul N, Punchuklang W, Kasikan K, Laotaweesuk N, Phoson P, Khongrod R, et al. The actual duration of preoperative fasting in pediatric patients, and its effects on hunger and thirst: a prospective observational study. Transl Pediatr. (2023) 12(2):146–54. doi: 10.21037/tp-22-358
17. Al-Robeye AM, Barnard AN, Bew S. Thirsty work: exploring children’s experiences of preoperative fasting. Paediatr Anaesth. (2020) 30(1):43–9. doi: 10.1111/pan.13759
18. Simpao AF, Wu L, Nelson O, Gálvez JA, Tan JM, Wasey JO, et al. Preoperative fluid fasting times and postinduction low blood pressure in children: a retrospective analysis. Anesthesiology. (2020) 133(3):523–33. doi: 10.1097/ALN.0000000000003343
Keywords: hematopoietic stem cells, general anesthesia, child, hypotension, fasting, postoperative nausea and vomiting, patient discharge
Citation: Raghavan KC, Sweeney B, Bi Y, Selukar S, Triplett B and Anghelescu D (2025) An institutional review of perioperative outcomes in pediatric bone marrow donors. A retrospective study. Front. Anesthesiol. 4:1713946. doi: 10.3389/fanes.2025.1713946
Received: 26 September 2025; Accepted: 6 November 2025;
Published: 25 November 2025.
Edited by:
José Eduardo Guimarães Pereira, Hospital Central do Exercito, BrazilReviewed by:
Carlos Darcy Alves Bersot, Federal University of São Paulo, BrazilMarcelo Zylberberg, Federal University of Rio de Janeiro, Brazil
Copyright: © 2025 Raghavan, Sweeney, Bi, Selukar, Triplett and Anghelescu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kavitha C. Raghavan, a2F2aXRoYS5yYWdoYXZhbkBzdGp1ZGUub3Jn
 Kavitha C. Raghavan
Kavitha C. Raghavan Brandy Sweeney2
Brandy Sweeney2 Doralina Anghelescu
Doralina Anghelescu