- Department of Biology, University of Colorado Colorado Springs, Colorado Springs, CO, United States
Apples are a popular and globally important crop. The fruits are eaten fresh, pressed for juice, fermented as cider, processed into sauce, dried, and more. There are thousands of different cultivars, a small subset of which are grown on a commercial scale. Genetic analysis has shown that, as a group, domestic apples have a complicated genetic background, with contributions from multiple wild species. By contrast, most of the highly produced commercialized modern cultivars share a narrow range of genetic diversity. However, as apples are outcrossing, propagated vegetatively, and long-lived, wild and heirloom varieties can be maintained and are valuable sources of genetic diversity for desirable traits. Apples are also amenable to genetic transformation, and work in this area has resulted in improved resistance to diseases and a commercialized non-browning variety, the Arctic™ Apple. Traditional breeding, breeding guided by modern genetic knowledge, and biotechnology all contribute to the overall process of apple cultivar development and represent an important example of how many approaches can be used in crop improvement. As global biosafety regulations continue to develop and change, countries will be tasked with developing guidelines for both the creation and import of apple trees and apple products.
1 Introduction
Malus x domestica Borkh. (domestic apple, hereafter apple) is a staple fruit crop grown in many regions of the world. In 2023, over 661,000 ha of apple trees were harvested, mostly in China, India, and Russia, with over 90 countries reporting output (FAO, 2023).
In general, apples are considered either dessert or cider, with the latter being used for the brewing of fermented alcoholic cider. Dessert apples are intended for the fresh fruit market, and are also processed into juice, vinegar, applesauce, dried fruit, and more (Downing, 1989; Guine et al., 2021). Dessert apple juice can be consumed fresh, or fermented into cider, but this approach is aimed at meeting rising consumer as cider cultivars are less commonly grown than dessert varieties (Soomro et al., 2022). By contrast, traditional cider apples are not meant for fresh consumption and are sometimes referred to as “spitters” due to their high phenolic content and rather unpalatable fruits (Marks et al., 2007).
2 Genetics and domestication
Domestic apple was one of the earlier plant genomes to be sequenced, with the first genome version published in 2010 (Velasco et al., 2010). Apples were domesticated in Asia, and genetic analysis of wild and domestic varieties shows evidence of breeding with local wild species in both Asia and Europe as varieties were developed (Cornille et al., 2014; Chen et al., 2023). Many countries have bred locally important varieties of apples, and there is interest in preserving these heritage varieties (see Supplementary Table S1 and examples therein).
Of the over 7,000 domestic apple cultivars, nearly all commercial production is from just a handful of varieties, which share a lot of common genetic heritage (Noiton and Alspach, 1996; Forsline et al., 2003; Pereira-Lorenzo et al., 2018). This lack of diversity in existing elite cultivars adds to the challenge of apple improvement. In general, domestic apple trees are outcrossing, and varieties of interest are maintained by vegetative propagation, often involving grafting a long-established practice (Cornille et al., 2014; Pereira-Lorenzo et al., 2018). As these trees can also be long lived, very old varieties can still be found in modern times. In addition, many wild varieties of Malus are compatible with domestic trees, providing an important resource for key traits (Cornille et al., 2014).
2.1 Select apple traits of interest
Like crops in general, apple trees are vulnerable to pathogens, which cause significant economic losses. These include the fungus Venturia inaequalis which causes apple scab, Erwinia amylovora, causing fire blight, Penicillium expansum leading to postharvest disease, and more (MacHardy, 1996; Malnoy et al., 2012; Luciano-Rosario et al., 2020). In general, highly produced commercial cultivars exhibit little to no resistance to those three diseases, which are managed through cultivation practices (Gessler et al., 2006; Holb, 2007; Kellerhals et al., 2012; Luciano-Rosario et al., 2020). Similarly, post-harvest losses from fungal growth can be considerable, with little genetic resistance, fungicides re the typical control method (Gupta and Saxena, 2023). There is great interest in identifying or developing disease resistant cultivars.
In addition to disease resistance, interesting marketable fruit traits are sought after, such as red fleshed fruits, tiny fruits, large sized fruits, or early ripening (Janick et al., 1996; Volz et al., 2009). Other preferred characteristics of fresh market apples such as fruit color, pattern, and texture are variable by region and across time (Janick et al., 1996). As apples intended for the fresh fruit market can undergo extensive storage to allow year-round sales of this seasonal fruit, longer shelf-life is a valuable trait (Janick et al., 1996). Consumers are also interested in obtaining nutritious foods (Rahman et al., 2021). Apples are high in a variety of compounds, including ascorbic acid, commonly known as vitamin C (overviewed in (Planchon et al., 2004)). Analysis of vitamin C content of apple indicates both genes and the environment factor into vitamin levels, with older cultivars tending to have much higher levels on average than newer varieties (Planchon et al., 2004).
2.2 Approaches to cultivar development
Given the great diversity of apple cultivars as a group and the presence of genetically compatible wild relatives, there are abundant genetic resources available for potentially obtaining apples with desired genetically encoded traits. Apple germplasm collections in countries around the world are valuable resources for conserving and documenting this diversity (Supplementary Table S1). Phenotype screening of collections of wild and domestic apple have identified resistance to fire blight, apple scab, and other diseases, and some individuals even exhibit multiple resistance (Luby et al., 2002; Volk et al., 2008). An addition, extensive research identifying candidate genes or quantitative trait loci (QTLs) for fire blight and apple scab resistance now makes it possible to perform marker assisted seeding selection (MASS) in apple (Ru et al., 2015). Thus, trees and progeny can now be genotyped to track traits genetically. The long juvenile period and lack of self-compatibility of apples in general means is time-consuming to integrate traits and develop cultivars using naturally occurring plant maturation (Pereira-Lorenzo et al., 2018). This approach works well, and breeding efforts started in the 1940s have successfully bred in resistance to apple scab from wild M. floribunda into domestic apple (Crosby et al., 1992). Similar efforts to create additional scab-resistant apple cultivars have yielded numerous new varieties, but overall the market success of scab resistant cultivars developed to date has been low (Gessler et al., 2006).
Genetic engineering represents a second and faster option for changing domestic apple. Transformation of domestic cultivars occurred in the 1980s and wild apple is a more recent development (James et al., 1989; Zhang et al., 2021). A variety of genetic engineering approaches have been used in apple, including cisgenesis, transgenesis, RNA interference (RNAi), Viral Induced Gene Silencing (VIGS), and Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR, see Table 1 and citations therein). The same apple scab resistance gene introgressed by traditional breeding with M. floribunda into domestic apple was transformed directly into Gala apples, maintaining the cultivar background and obtaining resistance in this single generation (Belfanti et al., 2004). Resistance to both apple scab and fire blight occurred with transfer of the Zea maize (corn) Leaf colour (Lc) gene, but the positive gravitropism of the branches meant the trees would be commercially unsuitable for fruit production (Flachowsky et al., 2010a).
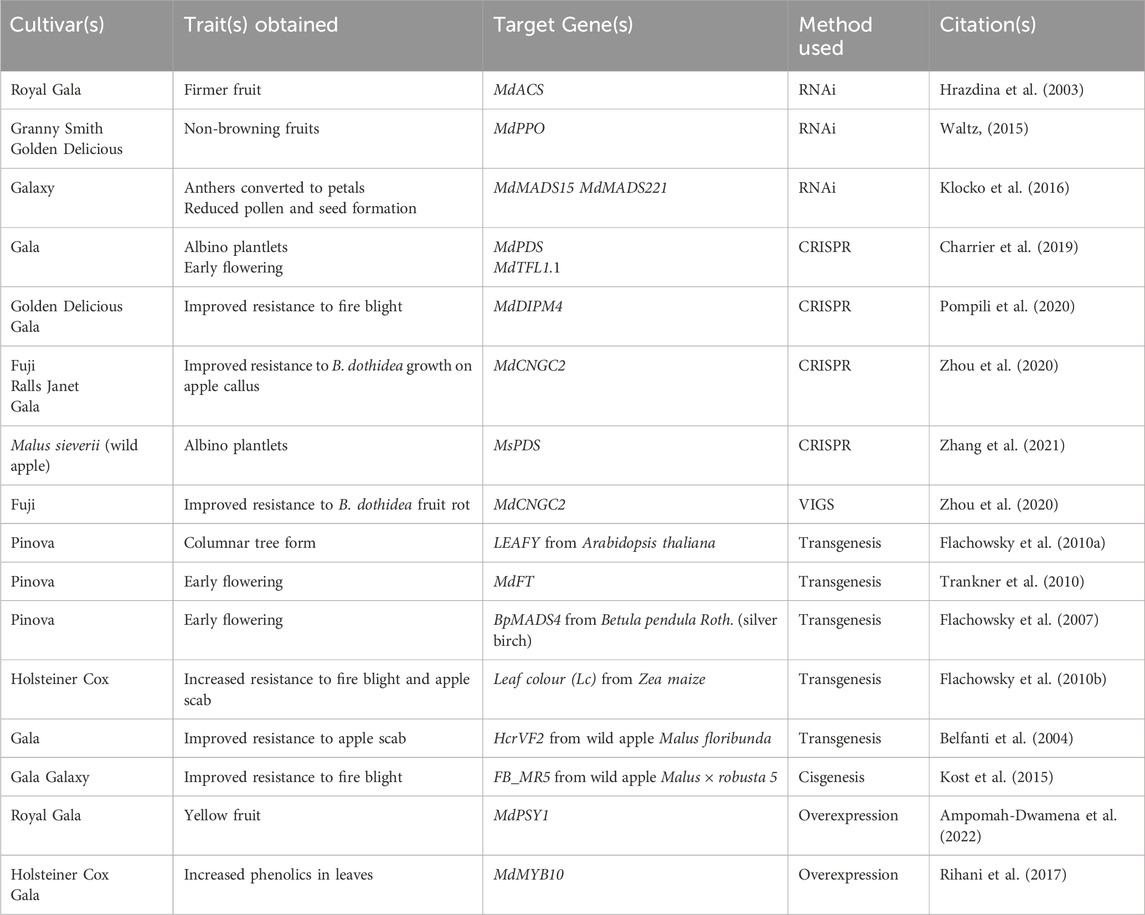
Table 1. Examples of apple traits obtained by genetic engineering. Unless indicated, cultivars are domestic apple.
Given the long juvenile period of apple trees, one goal of genetic engineering was to produce precocious flowering, which could then be used to shorten generation times and obtain accelerated breeding. This approach required some optimization, as it was challenging to obtain trees that flowered, but not too early. Transgenic addition of LEAFY (LFY) from Arabidopsis thaliana (Arabidopsis) led to a more compact and columnar tree form, but no early flowering (Flachowsky et al., 2010b). Overexpression of one FLOWERING LOCUS T homolog from domestic apple (MdFT1) led to very early flowering with blooms observed while trees were still undergoing in vitro cultivation (Trankner et al., 2010). Use of BpMADS4 from Betula pendula Roth. (silver birch) led to the creation of several events, one of which was used to rapidly breed fire blight resistance from an ornamental cultivar of apple to a fruit-bearing cultivar, with five generations occurring in just 7 years (Flachowsky et al., 2007; Schlatholter et al., 2018). This same BpMADS4 event was also used for introgression of resistance to blue mold from wild M. sieversii to Gala (Luo et al., 2020). Individuals with or without the BpMADS4 gene can be identified by DNA testing, allowing for genotyping in each generation (Luo et al., 2020).
In general, genetic engineering approaches are most straightforward with single genes of large influence, but it is possible to target multiple genes at once in apple with RNAi or CRISPR (Klocko et al., 2016; Jacobson et al., 2023). Unlike their conventionally bred counterparts, engineered trees are subject to many regulations, and are challenging to bring to the commercial market.
2.3 Regulatory considerations
Apples and apple products are globally produced and traded, with apples grown in over 90 countries (Nations, 2025). Nearly all apples are conventional crops, produced without genetic engineering, but there is potential for engineered apples to become more prevalent. Currently, non-browning apples obtained by RNAi are grown in Canada and the United States (Waltz, 2015; Duford, 2024). One challenge to wider usage of engineered apples is that regulations regarding definitions and guidelines for genetically engineered crops and crop products are still under development and vary greatly by country and region. Some countries, such as the United States, have guidelines but until recently had no labeling requirements for GMO crops and products. Starting in mid-2025, the United States will now require labeling of foods with ingredients produced by recombinant DNA technology, including Arctic™ apple (Becker, 2023). By contrast, Europe has implemented labeling and monitoring of GMOs, which also included gene-edited crops (Ruffell, 2018). However, a recent decision by the Council of the European Union updated the guidelines to better encompass current plant breeding and modification techniques, which opens up the possibility for future new crop adoptions (Union, 2025). How apple trees such as the fire-blight resistant individuals with the resistance gene introgressed from compatible relatives via genetically accelerated breeding, but which lack transgenes, would be regulated remains to be seen. It is possible that consumers may be in favor of trees produced with these new technologies. There is growing interest in food produced fewer pesticides or fungicides, and if apples produced by new technologies meet consumer needs and desires then regulations may adapt to meet market demand (Rahman et al., 2021).
As science moves quickly, some recent innovations in plant gene targeting offer potential options for achieving transgene-free genome-edited apple trees. It is now possible perform transient CRISPR editing in apple, or to use excision to remove transgenes after edits (Malnoy et al., 2016; Osakabe et al., 2018; Dalla Costa et al., 2020). These approaches, while not highly efficient, are likely to be faster than using breeding to separate transgenes from edits and would allow for maintaining the overall genetic background of the cultivar used. It is possible that if the targeted changes are small and the transgenes are not stably integrated, apple trees produced with this approach could get approval in the European Union or elsewhere. As the field of biotech crops continues to develop, both in terms of science and regulations, it will be interesting to see what is possible, and which possibilities reach consumers. Both growers and consumers may also be a significant influence in the marketability of engineered apples. Transgenic virus-resistant Carica papaya (papaya) trees were created and are credited with saving the Hawaiian papaya industry, which at the time was mostly small farmers (Gonsalves, 2006). More recently, the engineered “PinkGlow” Ananas comosus (pineapple) was released in the United States as a novelty item and is popular with consumers (Jay, 2024). Perhaps as more engineered fruits enter the global market apples could be part of the portfolio.
Author contributions
AK: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2025.1617110/full#supplementary-material
References
Ampomah-Dwamena, C., Tomes, S., Thrimawithana, A. H., Elborough, C., Bhargava, N., Rebstock, R., et al. (2022). Overexpression of PSY1 increases fruit skin and flesh carotenoid content and reveals associated transcription factors in apple (Malus × domestica). Front. Plant Sci. 13, 967143. doi:10.3389/fpls.2022.967143
Becker, K. (2023). National bioengineered food disclosure standard; list of bioengineered foods. D. o. Agriculture. Fed. Regist. 88.
Belfanti, E., Silfverberg-Dilworth, E., Tartarini, S., Patocchi, A., Barbieri, M., Zhu, J., et al. (2004). The HcrVf2 gene from a wild apple confers scab resistance to a transgenic cultivated variety. Proc. Natl. Acad. Sci. U. S. A. 101 (3), 886–890. doi:10.1073/pnas.0304808101
Charrier, A., Vergne, E., Dousset, N., Richer, A., Petiteau, A., and Chevreau, E. (2019). Efficient targeted mutagenesis in apple and first time edition of pear using the CRISPR-cas9 system. Front. Plant Sci. 10, 40. doi:10.3389/fpls.2019.00040
Chen, X., Cornille, A., An, N., Xing, L., Ma, J., Zhao, C., et al. (2023). The East Asian wild apples, Malus baccata (L.) Borkh and Malus hupehensis (Pamp.) Rehder., are additional contributors to the genomes of cultivated European and Chinese varieties. Mol. Ecol. 32, 5125–5139. doi:10.1111/mec.16485
Cornille, A., Giraud, T., Smulders, M. J., Roldan-Ruiz, I., and Gladieux, P. (2014). The domestication and evolutionary ecology of apples. Trends Genet. 30 (2), 57–65. doi:10.1016/j.tig.2013.10.002
Crosby, J. A., Janick, J., Pecknold, P. C., Korban, S. S., O'Conner, P. A., Ries, S. M., et al. (1992). Breeding apples for scab resistance: 1945-1990. Acta Hort. 317, 43–70. doi:10.17660/actahortic.1992.317.5
Dalla Costa, L., Piazza, S., Pompili, V., Salvagnin, U., Cestaro, A., Moffa, L., et al. (2020). Strategies to produce T-DNA free CRISPRed fruit trees via Agrobacterium tumefaciens stable gene transfer. Sci. Rep. 10 (1), 20155. doi:10.1038/s41598-020-77110-1
Duford, M. J. (2024). Arctic apples. Available online at: https://homefortheharvest.com/arctic-apples/.
Flachowsky, H., Hattasch, C., Hofer, M., Peil, A., and Hanke, M. V. (2010a). Overexpression of LEAFY in apple leads to a columnar phenotype with shorter internodes. Planta 231 (2), 251–263. doi:10.1007/s00425-009-1041-0
Flachowsky, H., Peil, A., Sopanen, T., Elo, A., and Hanke, V. (2007). Overexpression of BpMADS4 from silver birch (Betula pendula Roth.) induces early-flowering in apple (Malus x domestica Borkh.). Plant Breed. 126, 137–145. doi:10.1111/j.1439-0523.2007.01344.x
Flachowsky, H., Szankowski, I., Fischer, T. C., Richter, K., Peil, A., Hofer, M., et al. (2010b). Transgenic apple plants overexpressing the Lc gene of maize show an altered growth habit and increased resistance to apple scab and fire blight. Planta 231 (3), 623–635. doi:10.1007/s00425-009-1074-4
Forsline, P. L., Aldwinckle, H. S., Dickson, E. E., Luby, J. J., and Hokanson, S. C. (2003). Collection, maintenance, characterization, and utilization of wild apples of central Asia. Wild apple and fruit trees of central Asia. J. Janick, Hortic. Rev. 29, 1–62. doi:10.1002/9780470650868.ch1
Gessler, C., Patocchi, A., Sansavini, S., Tartarini, S., and Gianfranceschi, L. (2006a). Venturia inaequalis resistance in apple. Crit. Rev. Plant Sci. 25, 473–503. doi:10.1080/07352680601015975
Gonsalves, D. (2006). Transgenic papaya: development, release, impact and challenges. Adv. Virus Res. 67, 317–354. doi:10.1016/s0065-3527(06)67009-7
Guine, R. P. F., Barroca, M. J., Coldea, T. E., Bartkiene, E., and Anjos, O. (2021). Apple fermented products: an overview of technology, properties and Health effects. Processes 9, 223. doi:10.3390/pr9020223
Gupta, S., and Saxena, S. (2023). Endophytes: saviour of apples from post-harvest fungal pathogens. Biol. Control 182, 105234. doi:10.1016/j.biocontrol.2023.105234
Holb, I. J. (2007). Classification of apple cultivar reactions to scab in integrated and organic production systems. Can. J. Plant Pathology 29, 251–260. doi:10.1080/07060660709507467
Hrazdina, G., Kiss, E., Galli, Z., Rosenfield, C. L., Norelli, J. L., and Aldwinckle, H. S. (2003). Downregulation of ethylene production in 'Royal Gala' apples. Acta Hortic. 628, 239–251. doi:10.17660/actahortic.2003.628.29
Jacobson, S., Bondarchuk, N., Nguyen, T. A., Canada, A., McCord, L., Artlip, T. S., et al. (2023). Apple CRISPR-cas9-A recipe for successful targeting of AGAMOUS-like genes in domestic apple. Plants (Basel) 12 (21), 3693. doi:10.3390/plants12213693
James, D. J., Passey, A. J., Barbara, D. J., and Bevan, M. (1989). Genetic transformation of apple (Malus pumila Mill.) using a disarmed Ti-binary vector. Plant Cell. Rep. 7 (8), 658–661. doi:10.1007/bf00272054
Janick, J., Cummins, J. N., Brown, S. K., and Hemmat, M. (1996). Apples. Fruit breed, volume 1: tree and tropical fruits. John Wiley and Sons, Inc.
Kellerhals, M., Szalatnay, D., Hunziker, K., Duffy, B., Nybom, H., Ahmadi-Afzadi, M., et al. (2012). European pome fruit genetic resources evaluated for disease resistance. Trees 26, 179–189. doi:10.1007/s00468-011-0660-9
Klocko, A. L., Borejsza-Wysocka, E., Brunner, A. M., Shevchenko, O., Aldwinckle, H., and Strauss, S. H. (2016). Transgenic suppression of AGAMOUS genes in apple reduces fertility and increases floral attractiveness. PLoS One 11 (8), e0159421. doi:10.1371/journal.pone.0159421
Kost, T. D., Gessler, C., Jansch, M., Flachowsky, H., Patocchi, A., and Broggini, G. A. (2015). Development of the first cisgenic apple with increased resistance to fire blight. PLoS One 10 (12), e0143980. doi:10.1371/journal.pone.0143980
Luby, J. J., Alspach, P. A., Bus, V., and Oraguzie, N. C. (2002). Field resistance to fire blight in a diverse apple (Malus sp.) germplasm collection. J. Am. Soc. Hortic. Sci. 127, 245–253. doi:10.21273/jashs.127.2.245
Luciano-Rosario, D., Keller, N. P., and Jurick, W. M. (2020). Penicillium expansum: biology, omics, and management tools for a global postharvest pathogen causing blue mould of pome fruit. Mol. Plant Pathol. 21 (11), 1391–1404. doi:10.1111/mpp.12990
Luo, F., Norelli, J. L., Howard, N. P., Wisniewski, M., Flachowsky, H., Hanke, M. V., et al. (2020). Introgressing blue mold resistance into elite apple germplasm by rapid cycle breeding and foreground and background DNA-informed selection. Tree Genet. and Genomes 16, 28. doi:10.1007/s11295-020-1419-5
MacHardy, W. E. (1996). Apple scab: biology, epidemiology, and management. St. Paul, MN: The American Phytopathological Society Press.
Malnoy, M., Martens, S., Norelli, J. L., Barny, M. A., Sundin, G. W., Smits, T. H., et al. (2012). Fire blight: applied genomic insights of the pathogen and host. Annu. Rev. Phytopathology 50, 475–494. doi:10.1146/annurev-phyto-081211-172931
Malnoy, M., Viola, R., Jung, M. H., Koo, O. J., Kim, S., Kim, J. S., et al. (2016). DNA-free genetically edited grapevine and apple protoplast using CRISPR/Cas9 ribonucleoproteins. Front. Plant Sci. 7, 1904. doi:10.3389/fpls.2016.01904
Marks, S. C., Mullen, W., and Crozier, A. (2007). Flavonoid and hydroxycinnamate profiles of English apple ciders. J. Agric. Food Chem. 55, 8723–8730. doi:10.1021/jf071155u
Noiton, D. A. M., and Alspach, P. A. (1996). Founding clones, inbreeding, coancestry, and status number of modern apple cultivars. J. Am. Soc. Hortic. Sci. 121, 773–782. doi:10.21273/jashs.121.5.773
Osakabe, Y., Liang, Z., Ren, C., Nishitani, C., Osakabe, K., Wada, M., et al. (2018). CRISPR–Cas9-mediated genome editing in apple and grapevine. Nat. Protoc. 13, 2844–2863. doi:10.1038/s41596-018-0067-9
Pereira-Lorenzo, S., Fischer, M., Ramos-Cabrer, A. M., and Castro, I. (2018). “Apple (Malus spp.) breeding: present and future,” in Advances in plant breeding strategies: fruits, 3. Springer International Publishing., 3–29. doi:10.1007/978-3-319-91944-7_1
Planchon, V., Lateur, M., Dupont, P., and Lognay, G. (2004). Ascorbic acid level of Belgian apple genetic resources. Sci. Hortic. 100, 51–61. doi:10.1016/j.scienta.2003.08.003
Pompili, V., Dalla Costa, L., Piazza, S., Pindo, M., and Malnoy, M. (2020). Reduced fire blight susceptibility in apple cultivars using a high-efficiency CRISPR/Cas9-FLP/FRT-based gene editing system. Plant Biotechnol. J. 18 (3), 845–858. doi:10.1111/pbi.13253
Rahman, S. M. E., Mele, M. A., Lee, Y. T., and Islam, M. Z. (2021). Consumer preference, quality, and safety of organic and conventional fresh fruits, vegetables, and cereals. Foods 10 (1), 105. doi:10.3390/foods10010105
Rihani, K. A. L., Jacobsen, H. J., Hofmann, T., Schwab, W., and Hassan, F. (2017). Metabolic engineering of apple by overexpression of the MdMyb10 gene. J. Genet. Eng. Biotechnol. 15 (1), 263–273. doi:10.1016/j.jgeb.2017.01.001
Ru, S., Main, D., Evans, K., and Peace, C. (2015). Current applications, challenges, and perspectives of marker-assisted seedling selection in Rosaceae tree fruit breeding. Tree Genet. and Genomes 11, 8. doi:10.1007/s11295-015-0834-5
Ruffell, D. (2018). The EU Court of Justice extends the GMO Directive to gene-edited organisms. FEBS Lett. 592, 3653–3657. doi:10.1002/1873-3468.13293
Schlatholter, I., Jansch, M., Flachowsky, H., Broggini, G. A. L., Hanke, M. V., and Patocchi, A. (2018). Generation of advanced fire blight-resistant apple (Malus × domestica) selections of the fifth generation within 7 years of applying the early flowering approach. Planta 247 (6), 1475–1488. doi:10.1007/s00425-018-2876-z
Soomro, T., Watts, S., Migicovsky, Z., and Myles, S. (2022). Cider and dessert apples: what is the difference? Plants People Planet 4, 593–598. doi:10.1002/ppp3.10284
Trankner, C., Lehmann, S., Hoenicka, H., Hanke, M. V., Fladung, M., Lenhardt, D., et al. (2010). Over-expression of an FT-homologous gene of apple induces early flowering in annual and perennial plants. Planta 232 (6), 1309–1324. doi:10.1007/s00425-010-1254-2
Union, C. o. t. E. (2025). New genomic techniques: Council agrees negotiating mandate. Available online at: https://www.consilium.europa.eu/en/press/press-releases/2025/03/14/new-genomic-techniques-councilagrees-negotiating-mandate.
Velasco, R., Zharkikh, A., Affourtit, J., Dhingra, A., Cestaro, A., Kalyanaraman, A., et al. (2010). The genome of the domesticated apple (Malus × domestica Borkh.). Nat. Genet. 42 (10), 833–839. doi:10.1038/ng.654
Volk, G. M., Richards, C. M., Reilley, A. A., Henk, A. D., Reeves, P. A., Forsline, P. L., et al. (2008). Genetic diversity and disease resistance of wild Malus orientalis from Turkey and southern Russia. J. Am. Soc. Hortic. Sci. 133, 383–389. doi:10.21273/jashs.133.3.383
Volz, R. K., Oraguzie, N. C., Whitworth, C. J., How, N., Chagné, D., Carlisle, C. M., et al. (2009). Breeding for red flesh colour in apple: progress and challenges. Leuven, Belgium: International Society for Horticultural Science ISHS.
Waltz, E. (2015). Nonbrowning GM apple cleared for market. Nat. Biotechnol. 33 (4), 326–327. doi:10.1038/nbt0415-326c
Zhang, Y., Zhou, P., Bozorov, T. A., and Zhang, D. (2021). Application of CRISPR/Cas9 technology in wild apple (Malus sieverii) for paired sites gene editing. Plant Methods 17 (1), 79. doi:10.1186/s13007-021-00769-8
Keywords: apple, biotechnology, germplast, breeding, GMO, apple scab, fire blight
Citation: Klocko AL (2025) Mini review: Apple improvement, traditional approaches, biotechnology options, and regulatory considerations. Front. Bioeng. Biotechnol. 13:1617110. doi: 10.3389/fbioe.2025.1617110
Received: 23 April 2025; Accepted: 28 May 2025;
Published: 04 June 2025.
Edited by:
Clara Rubinstein, Institute for Scientific Cooperation in Environment and Health (ICCAS), ArgentinaCopyright © 2025 Klocko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amy L. Klocko, YWtsb2NrbzJAdWNjcy5lZHU=
 Amy L. Klocko
Amy L. Klocko