- 1Department of Thoracic Surgery, The Affiliated Hospital of Zunyi Medical University, Zunyi, China
- 2Department of Cardiac Surgery, The Affiliated Hospital of Guizhou Medical University, Guiyang, China
- 3Department of Biochemistry, Zunyi Medical University, Zunyi, China
Background: The expression of progestin and adipoQ receptor 3 (PAQR3) is generally downregulated in multiple tumors, which is associated with tumor progression and poor prognosis.
Methods: The clinical value of PAQR3 was analyzed using various databases and in 60 patients with non-small cell lung cancer (NSCLC). In addition, cell counting kit-8 (CCK-8), colony formation, and flow cytometry assays were used to evaluate the effect of PAQR3 on the growth of NSCLC cells in vitro. Gene set enrichment analysis (GSEA) was performed to investigate the possible mechanism through which PAQR3 is involved in the progression of lung cancer. Furthermore, western blotting was employed to verify the relevant mechanism.
Results: The expression of PAQR3 was decreased in 60 NSCLC patients and was related to the histological subtype, lymph node metastasis, tumor size, and diagnosis of NSCLC. Patients with lung adenocarcinoma with increased PAQR3 expression tended to have a better prognosis. Besides, PAQR3 inhibited proliferation, clone formation, and cycle transition in NSCLC cells, but induced apoptosis. The results of GSEA showed that PAQR3 regulated the progression of lung cancer by affecting cell cycle, DNA replication, and the p53 signaling pathway. We confirmed that PAQR3 overexpression inhibited the expression of NF-κB, while it increased the expression of p53, phospho-p53, and Bax. On the contrary, PAQR3 inhibition played an opposite role in these proteins.
Conclusion: PAQR3 inhibited the growth of NSCLC cells through the NF-κB/P53/Bax signaling pathway and might be a new target for diagnosis and treatment.
Introduction
Non-small cell lung cancer (NSCLC) is the commonest type of lung cancer and accounts for approximately 85% of lung cancer cases (Florczuk et al., 2017; Dai et al., 2018; Siegel et al., 2020). Its common tissue subtypes include adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Lung cancer is not only the main cause of death among men with tumors, but also a common disease associated with cancer-related deaths among female patients (Siegel et al., 2020). The increasing awareness of cancer and improvements in diagnostic and treatment techniques, such as surgery, chemotherapy, and radiotherapy, have provided some survival benefits to NSCLC patients, although the 5-year overall survival (OS) remains low. In recent years, the emergence of targeted therapy has resulted in revolutionary changes in the treatment of lung cancer. For instance, targeted treatment of NSCLC patients with EGFR mutations could improve their survival time (Ghafoor et al., 2018; Hsu et al., 2019), and targeted therapy for anaplastic lymphoma kinase positive disease could also improve the prognosis of patients with metastatic NSCLC (McCoach et al., 2017). However, the efficacy is far from satisfactory. Therefore, it is essential to explore new therapeutic targets to improve the prognosis of NSCLC patients.
Progestin and adipoQ receptor 3 (PAQR3) is a newly discovered tumor suppressor (Lei et al., 2020). The expression level of PAQR3 in colon cancer tissues is significantly lower than that in normal colon tissues, and this change is closely associated with the malignant degree of colon cancer (Wang et al., 2012). In human breast cancer, the expression of PAQR3 is decreased and negatively correlated with that of HER2. PAQR3 inhibits the proliferation, clone formation, migration, and invasion of breast cancer cells (Li et al., 2015). The expression of PAQR3 is decreased and closely associated with serum alpha-fetoprotein (AFP), clinical stage, tumor size, and survival time in hepatocellular carcinoma. Consistently, it inhibits tumor proliferation and migration (Wu et al., 2014). In addition, the expression of PAQR3 in esophageal cancer tissues is lower than that in normal esophageal tissues, and is significantly correlated with tumor size, lymph node metastasis, and local recurrence among esophageal cancer patients. In addition, it inhibits the proliferation, cell cycle, clone formation, migration, and invasion of esophageal cancer cells, as confirmed in a nude mouse xenograft model (Zhou et al., 2017; Bai et al., 2017, 2018). In addition, PAQR3 expression is decreased in some other tumors such as osteosarcoma, gastric cancer, and breast cancer, and the functions in these tumor tissues are consistent with those mentioned above, and include the inhibition of the growth and migration of tumor cells (Ling et al., 2014; Li et al., 2015; Ma et al., 2015; Zhao et al., 2017). In summary, PAQR3 played a role in the occurrence and development of all reported cancers as a tumor suppressor gene, and no opposite effect has been found.
At present, PAQR3 expression has been found to be decreased in lung cancer tissues and is significantly correlated with the pathological classification, degree of differentiation, TNM stage, and lymph node metastasis in patients with lung cancer. Kaplan–Meier survival analysis showed that the positive expression rate of PAQR3 protein was positively associated with survival time and the 5-year survival rate (Liang et al., 2017). Li et al. (2018) found that PAQR3 inhibits cell proliferation and cell cycle transition, while promoting apoptosis in NSCLC cells through the PI3K/AKT signaling pathway. The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) databases contain a large number of high-throughput sequencing results, while Oncomine, Timer, and Ualcan database results are derived from TCGA and GEO data. We used the database in advance to demonstrate that PAQR3 expression is increased in NSCLC tissues. This is contrary to the results reported by Liang et al. (2017). In addition, the clinical value and biological function of PAQR3 in NSCLC have not been fully elucidated, particularly in regulating the occurrence and development of NSCLC. Therefore, in the present study, we analyzed the expression of PAQR3 and its clinical value in NSCLC, and explored its function in the progression of NSCLC to provide a new target for molecular diagnosis and treatment of lung cancer.
Materials and Methods
Oncomine, Timer, and Ualcan Databases
The screening criteria for the Oncomine database1 were: (1) gene: PAQR3; (2) analysis type: cancer vs. normal analysis; (3) data type: mRNA; (4) p < 0.01; and (5) foldchange: 1.5. The expression of PAQR3 in different types of cancer was analyzed in the gene expression module of the Timer database2. Moreover, its expression in NSCLC and normal samples was analyzed in the Ualcan database3, and the correlations between the expression level and clinicopathological features (race, weight, smoking history, cancer stage, grade, etc.) of NSCLC patients were analyzed. The clinical data are detailed in Supplxsementary Table S1.
PrognoScan and Kaplan-Meier Plotter Databases
The data in the PrognoScan database4 mainly originate from the RNA data in the GEO database. The data in the Kaplan–Meier Plotter database5 comes from the RNA data of the TCGA and GEO databases. Using the PrognoScan database, we analyzed the relationship between the expression level of PAQR3 and the prognosis of patients. The screening criterion was COX p < 0.05 which means statistically significant. For the Kaplan-Meier Plotter database, we used the median value of PAQR3 expression as the grouping standard to analyze the relationship between PAQR3 expression level and the prognoses of lung adenocarcinoma (LUAD) and lung squamous carcinoma (LUSC) patients.
Analysis of Biological Functions Related to PAQR3
The Cancer Cell Line Encyclopedia (CCLE) database contains more than 1000 databases of human cancer cells. In the present study, PAQR3 co-expressed genes were screened using the screening criteria of r (r > 0.4 or < −0.4) and p < 0.001 through the mRNA expression data of lung cancer cells downloaded from the CCLE database, and analysis of Gene Ontology (GO), analysis of the Kyoto Encyclopedia of Genes and Genomes (KEGG), and gene set enrichment analysis (GSEA) were performed. GO enrichment and KEGG pathway analyses were performed using the clusterProfiler software package on the R platform. GSEA is a method used to reveal mRNA level data via basic knowledge (Zhou et al., 2020). Gene expression data from the CCLE were divided into high and low expression groups according to the median level of PAQR3 mRNA expression, and GSEA was used to explore the influence of different levels of PAQR3 on each gene and to analyze the mechanism underlying the involvement of PAQR3 in the progression of lung cancer. The genome was sequenced 1000 times per analysis. In addition, the level of PAQR3 was used as a phenotypic marker. The nominal p-value (NOM p) and the normalized enrichment score were used to classify enrichment pathways in each phenotype (Guo et al., 2020).
Clinical Samples
Cancerous tissues and normal tissues located 5 cm adjacent to cancerous tissues were collected from 60 NSCLC patients who underwent surgery at the Department of Thoracic Surgery of Zunyi Medical University from December 2017 to January 2019. None of the patients was treated with neoadjuvant chemotherapy, radiotherapy, immunosuppressant therapy, biotherapy, or targeted therapy before surgery. Their clinical data are shown in Supplementary Table S2. The study protocol was approved by the Ethics Committee of The Affiliated Hospital of Zunyi Medical University (approval no. KLLY-2018-095). All 60 patients provided written informed consent prior to undergoing surgery.
Cell Culture
A549 and H1299 NSCLC cells were purchased from the cell bank of the typical Culture Preservation Committee of the Chinese Academy of Sciences. RPMI-1640 complete culture medium was used and incubated routinely in 5% CO2 at 37°C.
Quantitative Reverse Transcription Polymerase Chain Reaction
Total RNA was extracted from NSCLC tissues according to the manufacturer’s instructions (Invitrogen, United States). cDNA was synthesized using a reverse transcription kit (Takara, Japan). The expression of PAQR3 mRNA was detected by RT-qPCR. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. The primer sequences were: PAQR3: 5′-TGTCGAAGATGGATGGCATTAGA-3′ (forward), 5′-ACCTGACGCCAGTAGTATTACACACA-3′ (reverse) and GAPDH: 5′-AACGGATTTGGTCGTATTG-3′ (forward), 5′-GGAAGATGGTG ATGGGATT-3′ (reverse). These experiments were performed in triplicate.
Western Blot Assay
Total proteins were extracted from tissues and cells and quantified using the bicinchoninic acid method according to the manufacturer’s instructions (Solarbio, China) and separated by electrophoresis. Extracted proteins were then resolved via 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electro-transferred onto a polyvinylidene difluoride (PVDF) membrane. The membranes were blocked with 5% skimmed milk on a shaker for 1 h. The primary antibodies used in the present study were 1:300 anti-PAQR3 (Abcam, Cambridge, United Kingdom), 1:1000 anti-NF-κB (HuaBio, China), 1:2000 anti-p53, 1:3000 anti-Bax (Proteintech, China), and 1:1000 anti-p-p53 (CST, United States). A 1:3000 anti-GAPDH (Bioss, China) and 1: 2000 anti-β-tubulin (HuaBio, China) were used as controls. Images were obtained using Image LabTM Software (ChemiDocTM XRS+, Bio-Rad Laboratories Inc., Hercules, CA, United States). These experiments were performed in triplicate.
Generation of Stable Cells
The cDNA sequence of PAQR3 (NM_001040202.2) was obtained from NCBI. Lentiviral-based Lv201CT and psi-LVRU6GP vector (containing puromycin and enhanced green fluorescent protein) were constructed by GeneCopoeia, Inc (Rockville, MD, United States). Lentiviral ORF cDNA clones for PAQR3 (EX-E2253-Lv201 with N-eGFP) and empty vector control plasmid (EX-NEG-Lv201) were obtained from GeneCopoeia. Specifically, an shRNA with a target sequence (LPP-HSH003695-LVRU6GP) in the PAQR3 coding DNA sequence and one non-targeting shRNA as negative control (LPP-CSHCTR001-LVRU6GP) were used in this experiment (referred to as Si-PAQR3 and Si-NC, respectively). The sequence of the interference control group was ACAGAAGCGATTGTTGATC, and the sequence of the interference PAQR3 group was GCATTAGATTATGCAGGAATT. The stable overexpression or knockdown model of PAQR3 in NSCLC cells (A549 and H1299 cells) was performed using an EndoFectin transfection (GeneCopoeia, Inc.) following the manufacturer’s protocol. Stable NSCLC cells were selected in a medium containing puromycin (Solaibo, Beijing, China), 2 μmol/L of A549, and 4 μmol/L of H1299 for 14 days. After 2–3 passages in the presence of puromycin, the cultured cells were evaluated to verify that the PAQR3 overexpression or knockdown model was constructed successfully and used for experiments.
Cell Counting Kit-8 Assay
The cell counting kit-8 (CCK-8) assay was used to evaluate the effect of PAQR3 on the proliferation of A549 and H1299 cells. The A549 and H1299 cells in the logarithmic growth phase from the overexpression control group (Vector) and PAQR3 overexpression group (PAQR3-OV), or from the knockdown control group (Si-NC) and PAQR3 knockdown group (Si-PAQR3) were seeded in 96-well culture plates. The number of cells was 2.5 × 103 cells/well, and wells were set up in multiples of two. According to the operation of the kit, 10 μl/hole of CCK-8 was added and incubated at 37°C and with 5% CO2 for 3 h. The optical density was measured at 450 nm using a microplate reader (Bio-Rad, United States). These experiments were performed in triplicate.
Clone Formation Assay
Stable cells in the logarithmic growth phase were seeded in a 6-well culture plate containing medium at 1500 cells/well. The cells were grouped as described above and incubated at 37°C for 2 to 3 weeks. The culture was terminated at the right time, fixed with 4% paraformaldehyde (Solaibo, Beijing, China) for 10 min, stained with crystal violet (Sigma-Aldrich, China) for 30 min, and colonies containing more than 10 cells were observed under a microscope. These experiments were performed in triplicate.
Cell Cycle and Apoptosis
Stable A549 and H1299 cells (4 × 105 cells/well) were plated in cell culture dishes (60 mm × 60 mm). For the cell cycle analysis, cells were collected by trypsinization after 2 days, washed once with phosphate-buffered saline (PBS), and analyzed according to the instructions stipulated in the cell cycle kit (ABP Biosciences, China). For cell apoptosis analysis, cells were collected after transfection by trypsinization (without ethylenediaminetetraacetic acid), washed once with PBS, added to 500 μL of 1 × binding buffer, and stained with 1 μL of Annexin V-PE and 5 μL of 7-AAD (Nanjing Kaiji, China). Flow cytometry was used for subsequent detection. These experiments were performed in triplicate.
Statistical Analysis
The differences in expression between cancerous and normal tissues were calculated using the chi-squared test. Cell proliferation, number of clones, cycle, and apoptosis were analyzed using the Student t-test or one-way analysis of variance. We performed receiver operating characteristic (ROC) curve analysis on PAQR3 mRNA expression in normal and cancer tissues to evaluate whether PAQR3 has a diagnostic reference value for NSCLC (Wang et al., 2018). In this evaluation, the comprehensive statistic of the area under the curve (AUC) was used for quantitative analysis. The closer the AUC was to 1, the better was the diagnostic effect. All statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, United States), and differences were considered statistically significant at P < 0.05. The data are representative of three independent experiments and presented as the mean ± standard deviation. The data were plotted using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, United States).
Results
PAQR3 Was Abnormally Expressed in NSCLC Tissues
In the Oncomine and Timer databases, we found abnormal expression of PAQR3 in various tumors. It was increased in gastric cancer, head and neck tumors, lung cancer, and some other tumors, while it was decreased in breast cancer, melanoma, prostate cancer, and other tumors (Supplementary Figure S1A). Similar results were found in the Timer database (Supplementary Figure S1B). However, to date, numerous studies have reported that PAQR3 is a newly discovered tumor suppressor. Therefore, the expression of PAQR3 mRNA and protein was further verified in the Ualcan database and clinical NSCLC samples. In the Ualcan database, we found that the expression of PAQR3 was increased in LUAD and LUSC (Figures 1A,B). Among 60 collected NSCLC samples, the expression of PAQR3 mRNA in most cancer tissues was decreased significantly (Figure 1C). The expression of PAQR3 mRNA was decreased, while the difference was not significant in the 30 LUAD samples (Figure 1D). In addition, for the other 30 LUSC samples, it was also significantly decreased (Figure 1E). In addition, compared with the paired normal tissues, the expression of PAQR3 protein in 75% (45 cases) of samples was significantly lower (Figure 1F). The quantization diagram is presented in the Supplementary Figure S2.
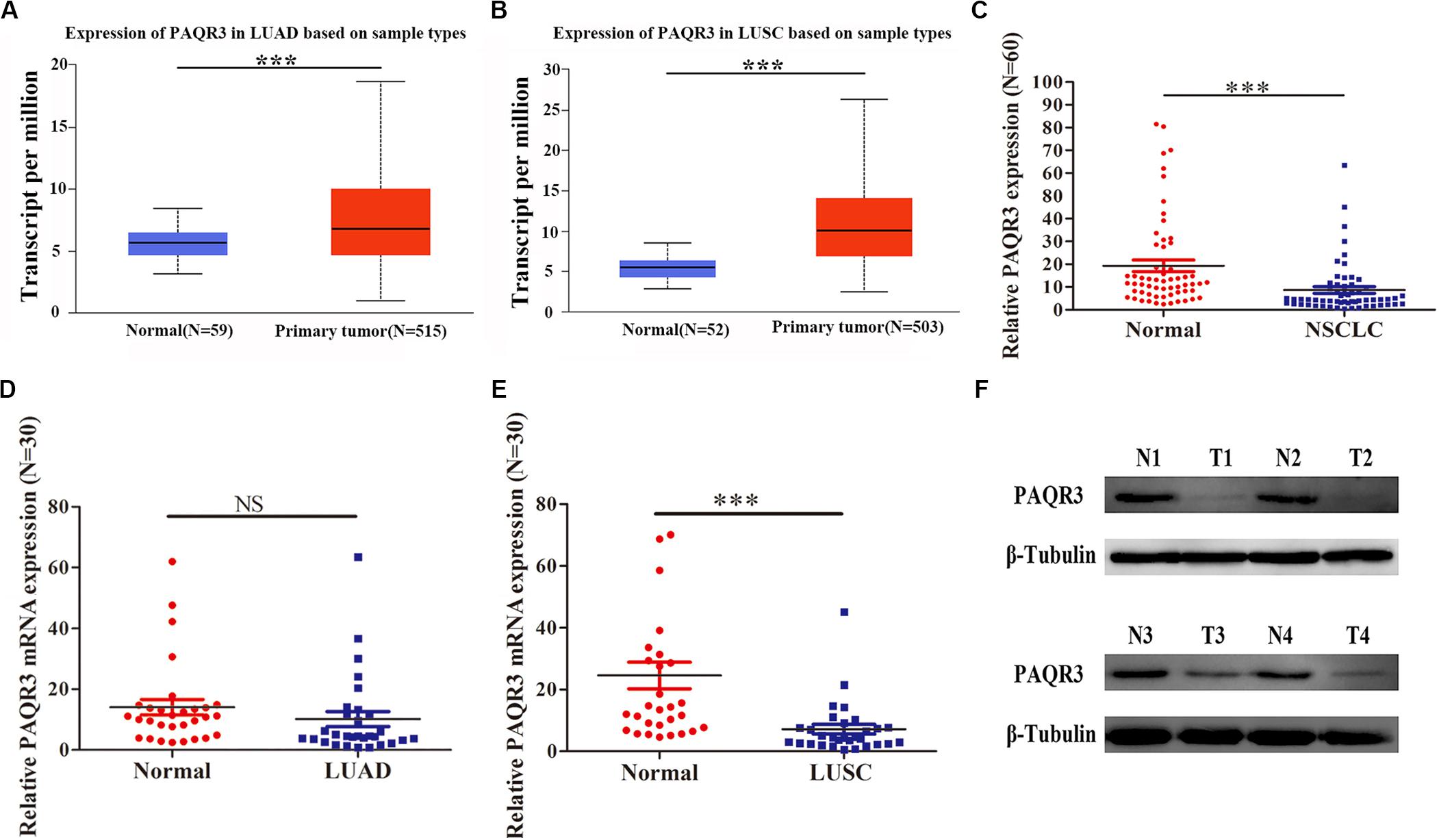
Figure 1. Expression of PAQR3 in Ualcan database and 60 clinical NSCLC samples. (A,B) PAQR3 mRNA level in LUAD and LUSC from Ualcan Database. (C–F) Expression of PAQR3 in 60 collected NSCLC samples. Note: Normal, normal lung tissue; NSCLC, non-small cell lung cancer; LUAD, lung adenocarcinoma; LUSC, lung squamous carcinoma; ***P < 0.001; NS, no statistical significance; vs. Normal.
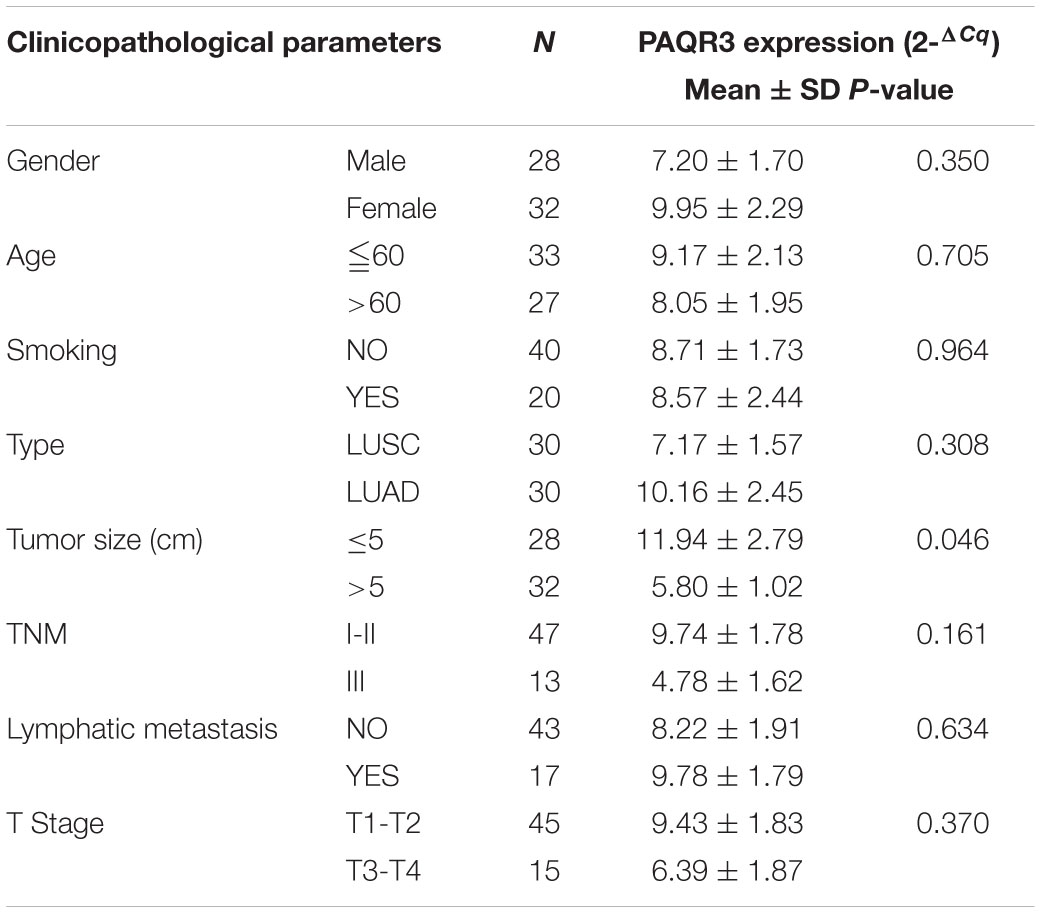
PAQR3 Expression Was Correlated With Histological Subtype, Lymph Node Metastasis, and Tumor Size Among NSCLC Patients
In the Ualcan database, we found that the level of PAQR3 mRNA was significantly associated with race, smoking history, clinical stage, histological subtype, and lymph node metastasis among LUSC patients (Figures 2A–E). Among LUAD patients, it was significantly associated with histological subtype and lymph node metastasis (Figures 2F,G). However, in the 60 clinical samples, the level of PAQR3 mRNA was only significantly correlated with tumor size (P < 0.05) (Table 1).
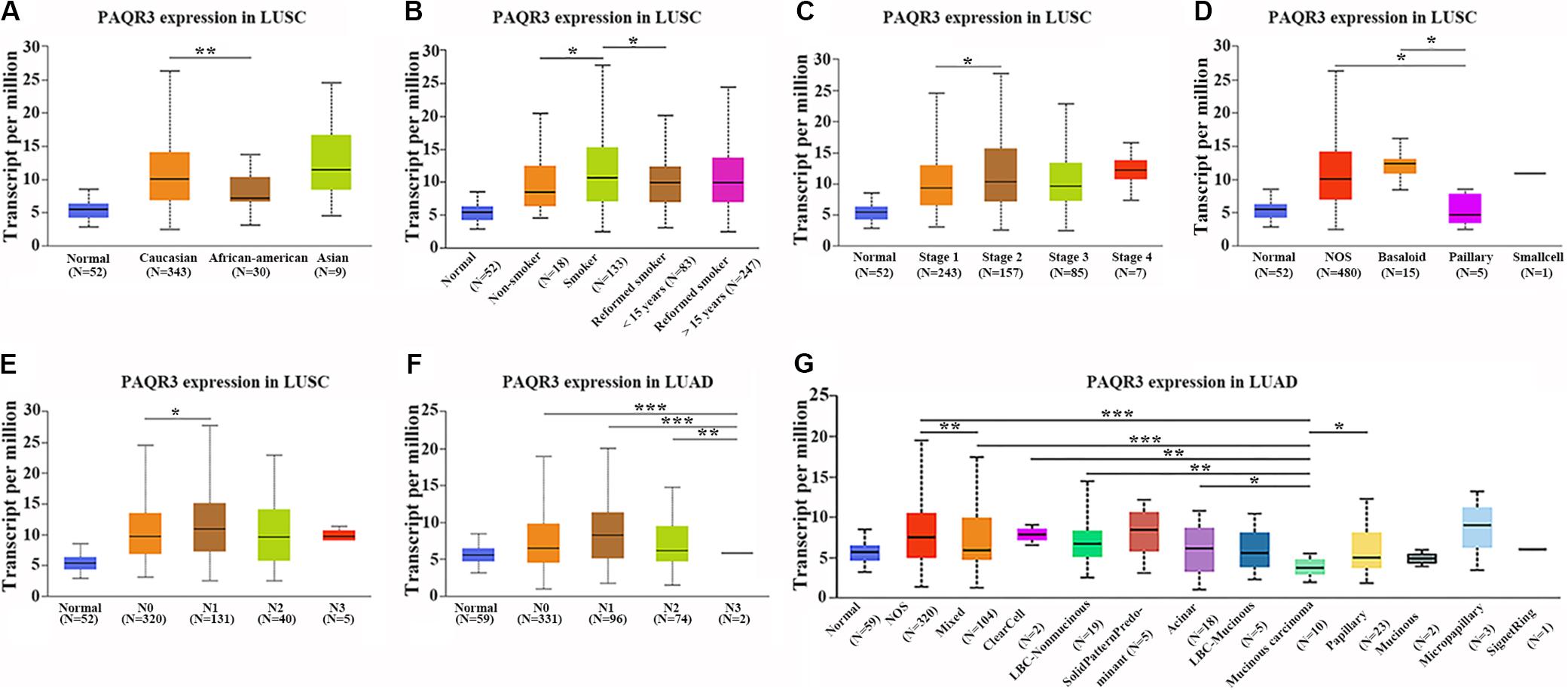
Figure 2. Abnormal expression of PAQR3 mRNA according to the clinicopathological features of NSCLC patients in the Ualcan database. (A–E) The clinicopathological features of LUSC patients were race, smoking, clinical stage, histological subtype, and lymph node metastasis, respectively. (F,G) Lymph node metastasis and histological subtypes of LUAD patients. NSCLC, non-small cell lung cancer; LUAD, lung adenocarcinoma; LUSC, lung squamous carcinoma; *P < 0.05; **P < 0.01; ***P < 0.001.
PAQR3 Is a Potential Diagnostic and Prognostic Marker of NSCLC
The role of PAQR3 in the diagnosis and prognosis of NSCLC patients was further analyzed. In 60 NSCLC tissues, ROC analysis revealed that the level of PAQR3 was predictive of NSCLC (Figures 3A–C). In the PrognoScan database, the GSE31210 dataset revealed that LUAD patients with elevated PAQR3 levels tended to have a better prognosis (Cox P < 0.05) (Supplementary Figure S3). Moreover, the results of the Kaplan–Meier Plotter database were similar to those of the PrognoScan database (Figures 3D–F). Increased PAQR3 expression also predicted better prognosis in LUSC patients, although the difference was not significant.
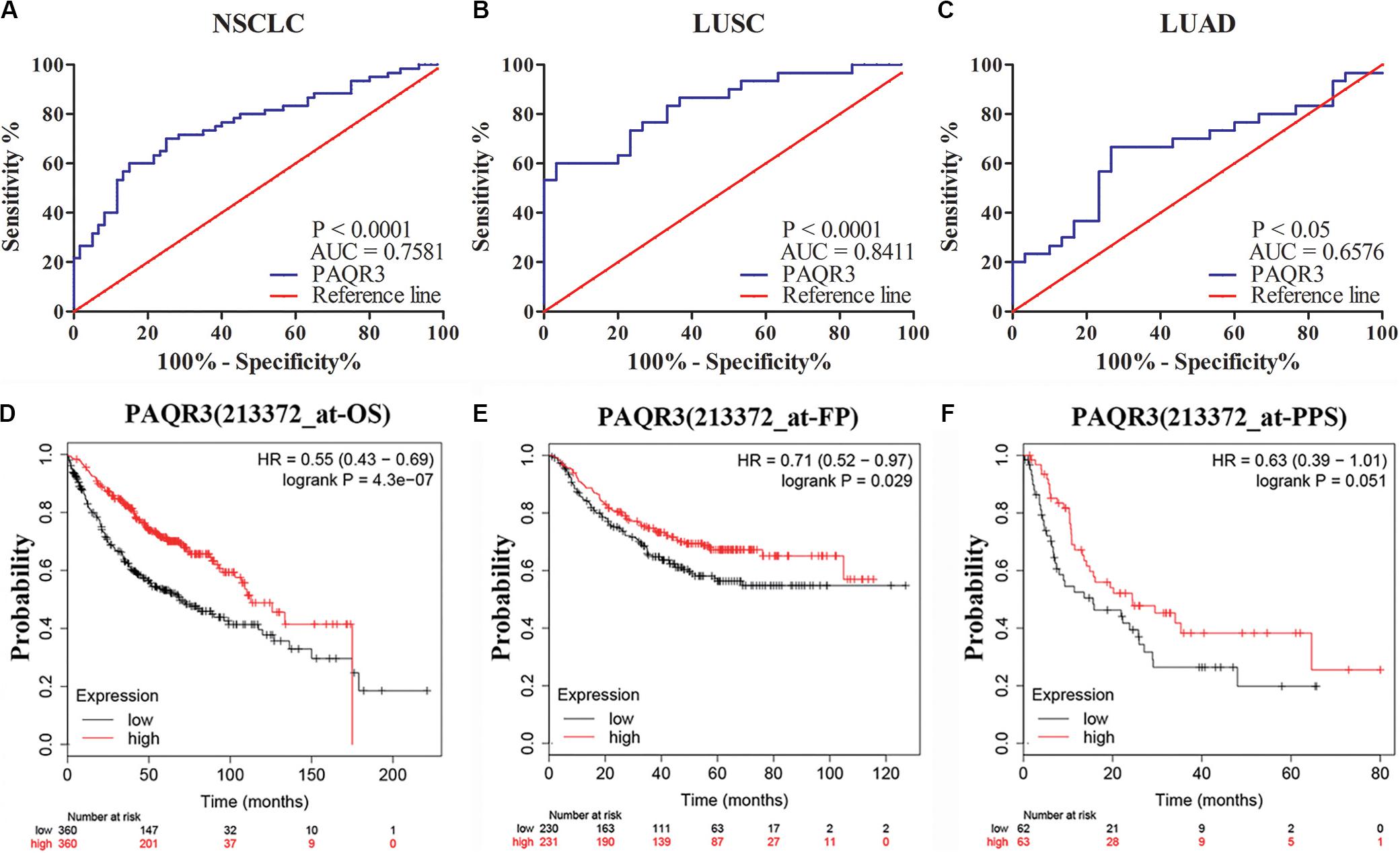
Figure 3. PAQR3 was a potential diagnostic and prognostic marker in NSCLC. (A) NSCLC; (B) LUAD; (C) LUSC; (D) OS; (E) FP; (F) PPS. NSCLC, non-small cell lung cancer; LUAD, lung adenocarcinoma; LUSC, lung squamous carcinoma; AUC, area under the curve; OS, overall survival; FP, first progression; PPS, post-progression survival.
PAQR3 Inhibits the Growth of NSCLC Cells
Here, we used lentivirus to transfect NSCLC A549 and H1299 cells to construct PAQR3-overexpressed and PAQR3-interfered stable cells and found that the levels of PAQR3 mRNA and protein were significantly increased or decreased, respectively (Figures 4A,B). The results of the CCK-8 assay showed that increased PAQR3 expression significantly inhibited the proliferation and clone number, while decreased PAQR3 expression had the opposite effect (Figures 4C–F). In addition, flow cytometry analysis showed that overexpression of PAQR3 significantly induced the transition from the G1 to S phase and promoted apoptosis of NSCLC cells (Figures 5A,B), whereas interfering with PAQR3 yielded opposite outcomes (Figures 5C,D).
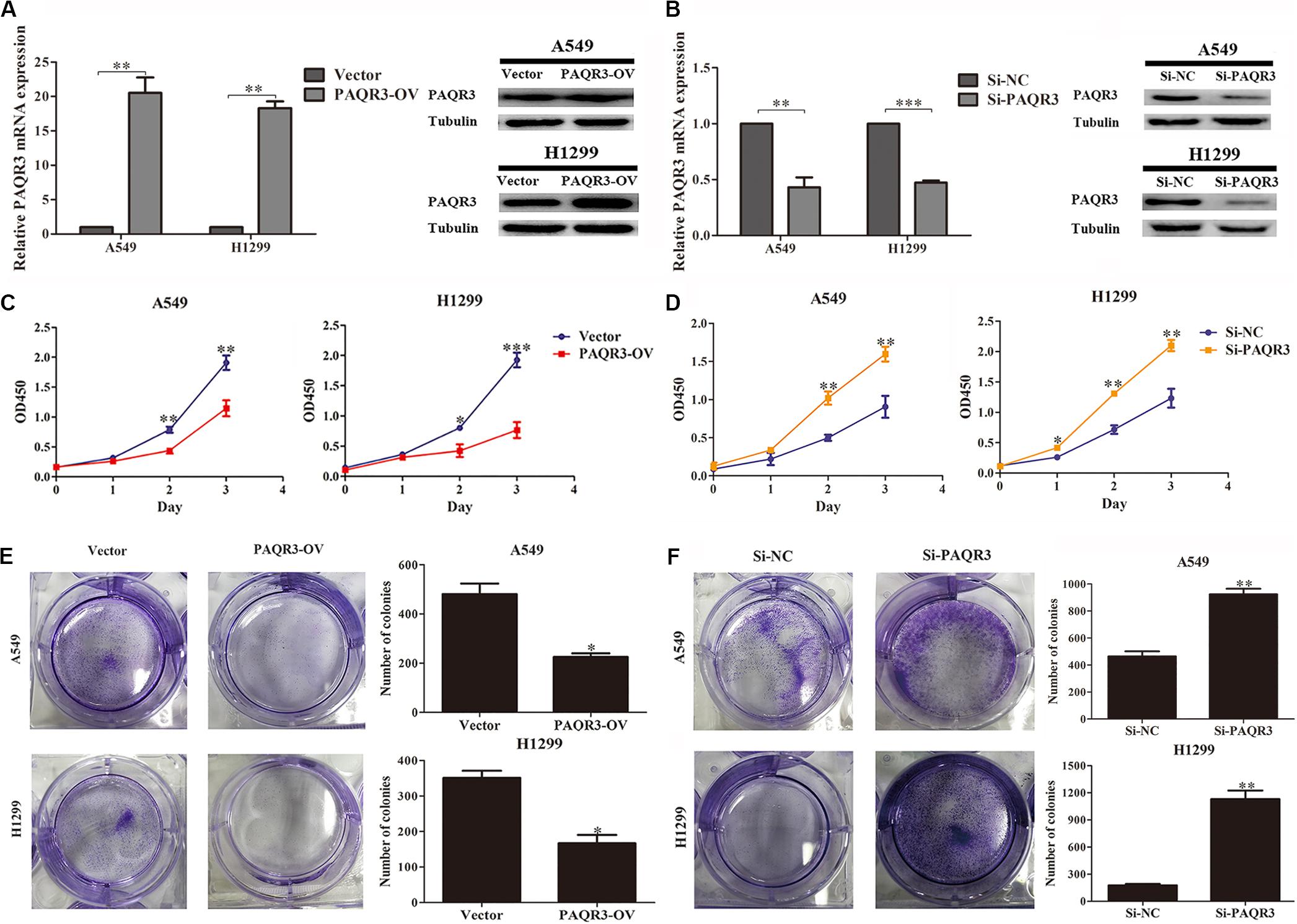
Figure 4. PAQR3 inhibited the proliferation of NSCLC cells. (A,B) The levels of PAQR3 mRNA and protein in PAQR3 overexpressed/interfered cells. (C,D) The effect of PAQR3 overexpression/inhibition on the proliferation of NSCLC cells were detected by CCK-8 assay. (E,F) The effect of PAQR3 overexpression/inhibition on the clone formation of NSCLC cells were assessed via clone formation assay. NSCLC, non-small cell lung cancer; Vector, overexpression control group; PAQR3-OV, PAQR3 overexpression group; Si-NC, knockdown control group; Si-PAQR3, PAQR3 knockdown group; *P < 0.05; **P < 0.01; ***P < 0.001; vs. relative control group.
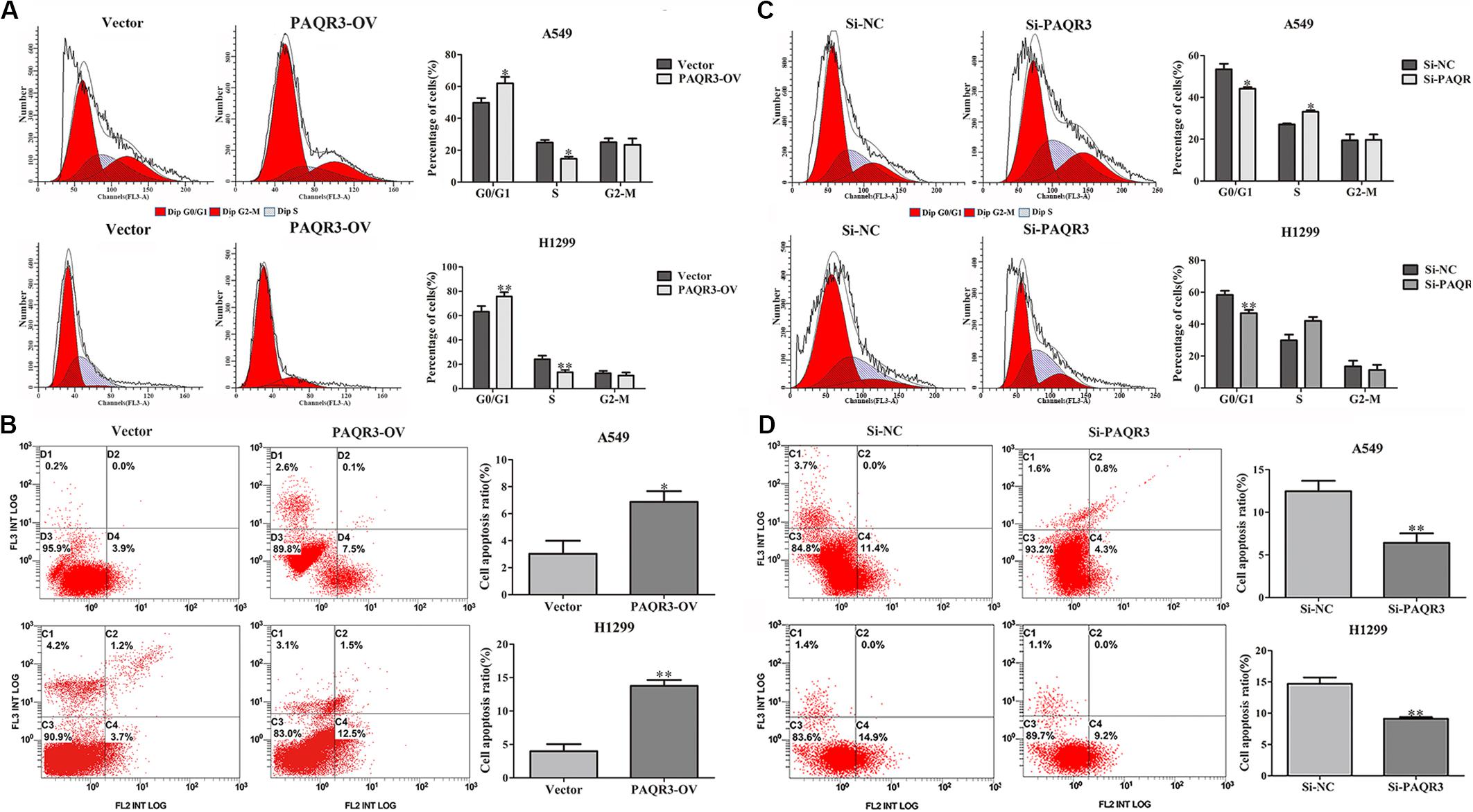
Figure 5. PAQR3 inhibited the cell cycle, but promoted apoptosis of NSCLC cells. (A,B) The effect of PAQR3 overexpression on cell cycle and apoptosis of NSCLC were detected by flow cytometry. (C,D) The effect of PAQR3 interference on cell cycle and apoptosis of NSCLC were detected by flow cytometry. NSCLC, non-small cell lung cancer; Vector, overexpression control group; PAQR3-OV, PAQR3 overexpression group; Si-NC, knockdown control group; Si-PAQR3, PAQR3 knockdown group; *P < 0.05; **P < 0.01; vs. relative control group.
GO and KEGG Enrichment Analyses Revealed Pathways Regulated by PAQR3 in NSCLC
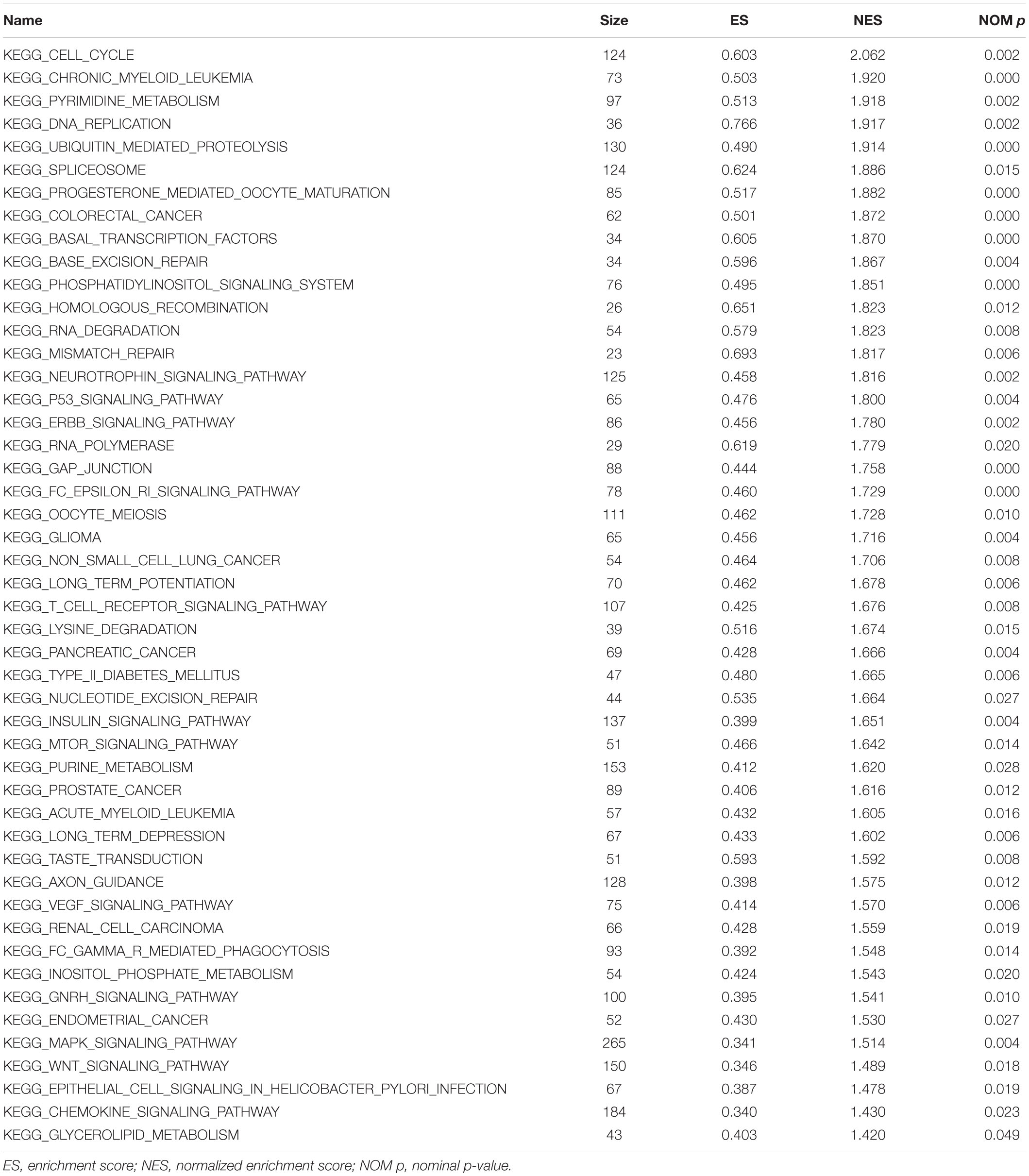
The data were downloaded from the CCLE database, and 2015 co-expressed genes were screened (Supplementary Table S3). Among them, 1673 positively- and 342 negatively related genes were included. Figure 6 shows the top 20 positively- and negatively related PAQR3 co-expressed genes. GO annotations showed that PAQR3 co-expressed genes were mainly involved in DNA replication, DNA binding, RNA transport, etc (Figures 7A–C and Supplementary Table S4). In addition, KEGG analysis showed that they were mainly involved in the regulation of the cell cycle, DNA replication, Fanconi anemia pathway, and some other processes (Figure 7D). Second, it was revealed that increased PAQR3 expression was associated with cell cycle, DNA replication, homologous recombination, and the p53 signaling pathway through GSEA (NOM p < 0.05) (Figure 8 and Table 2). Therefore, this could suggest that PAQR3 might regulate the progress of NSCLC through the cell cycle, DNA replication, homologous recombination, and p53 signaling pathway.
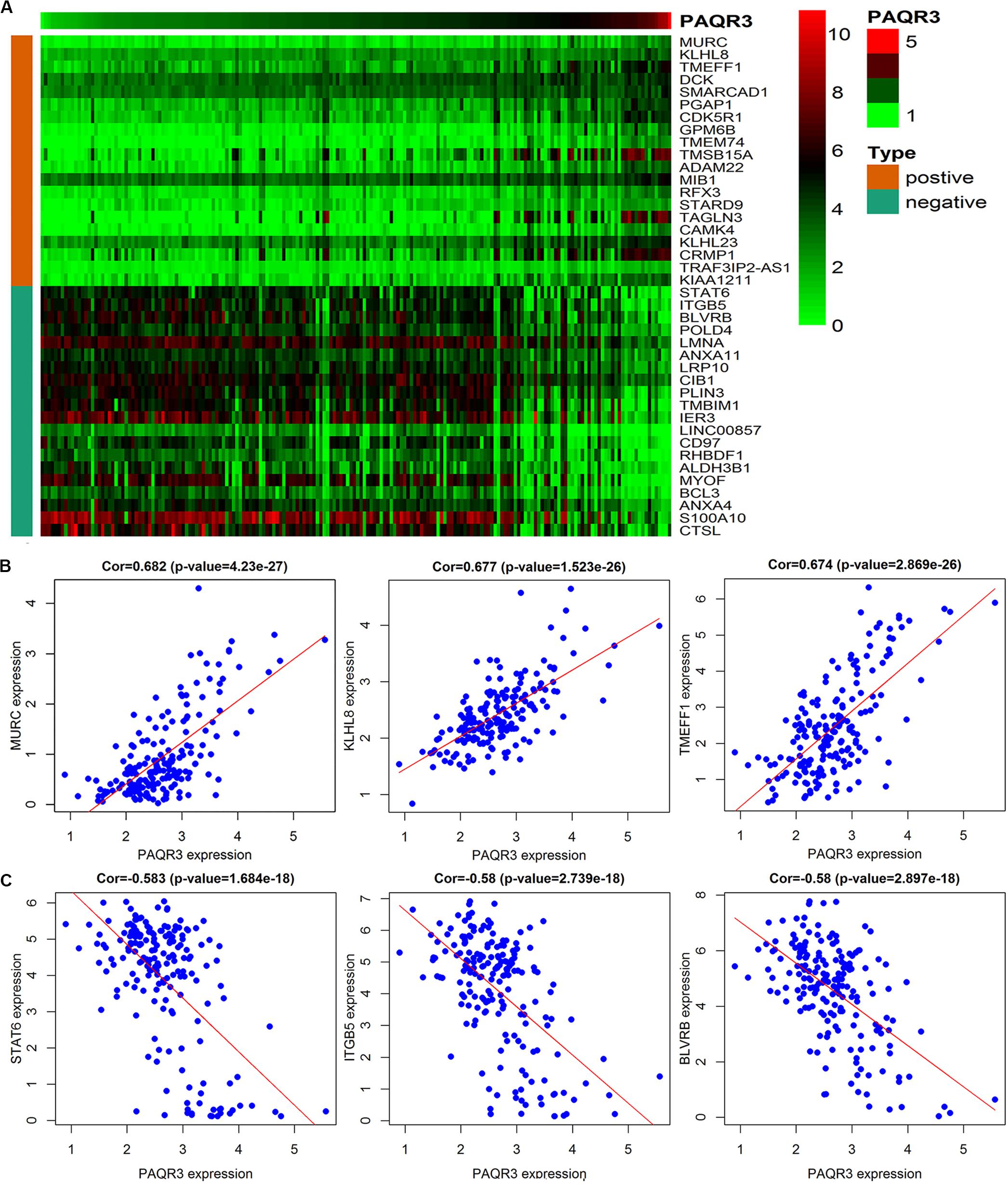
Figure 6. The top 20 positive and negative genes related to PAQR3. (A) The heat map of the top 20 positively and negatively related genes; (B,C) The top 3 positively and negatively related genes. positive, positively related genes; negative, negatively related genes; Cor, correlation coefficient.
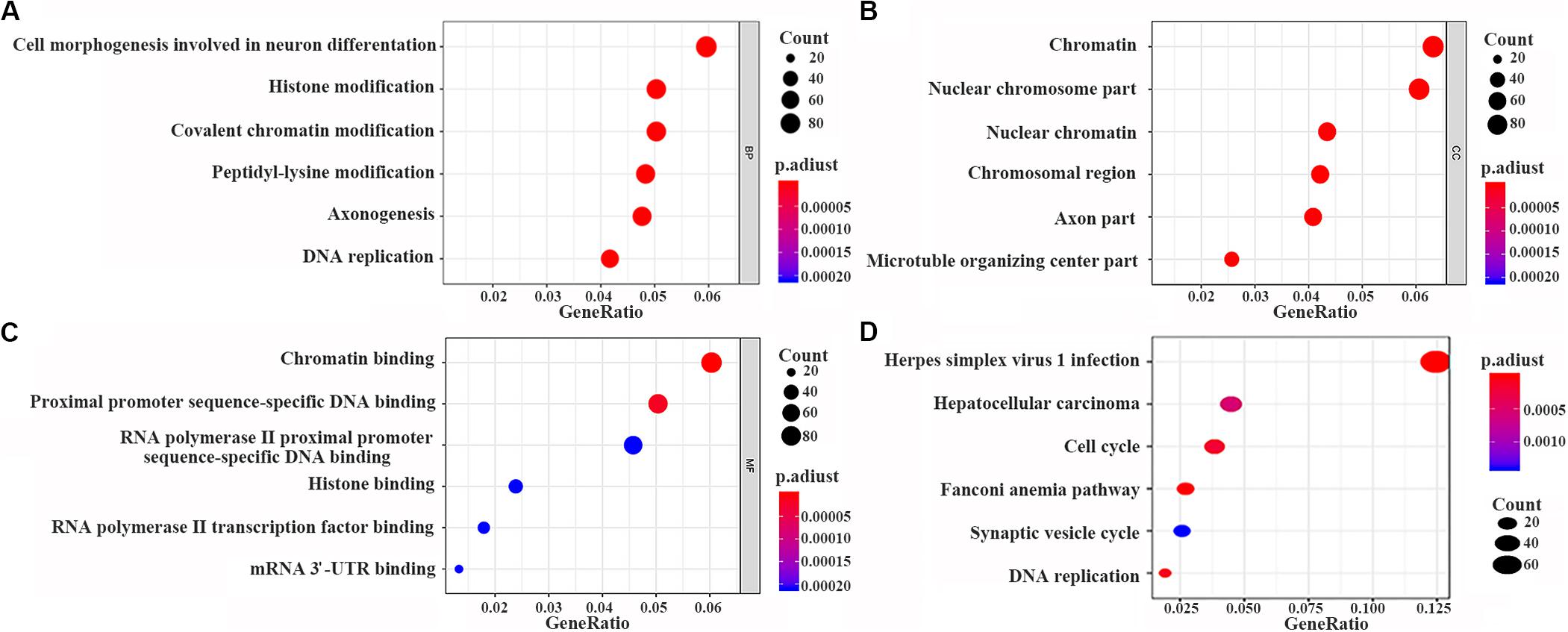
Figure 7. PAQR3 co-expressed genes analyzed by GO and KEGG with clusterProfiler software package on the R platform. (A) BP; (B) CC; (C) MF; (D) KEGG. BP, biological process; MF, molecular function; CC, cellular component; GO, gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
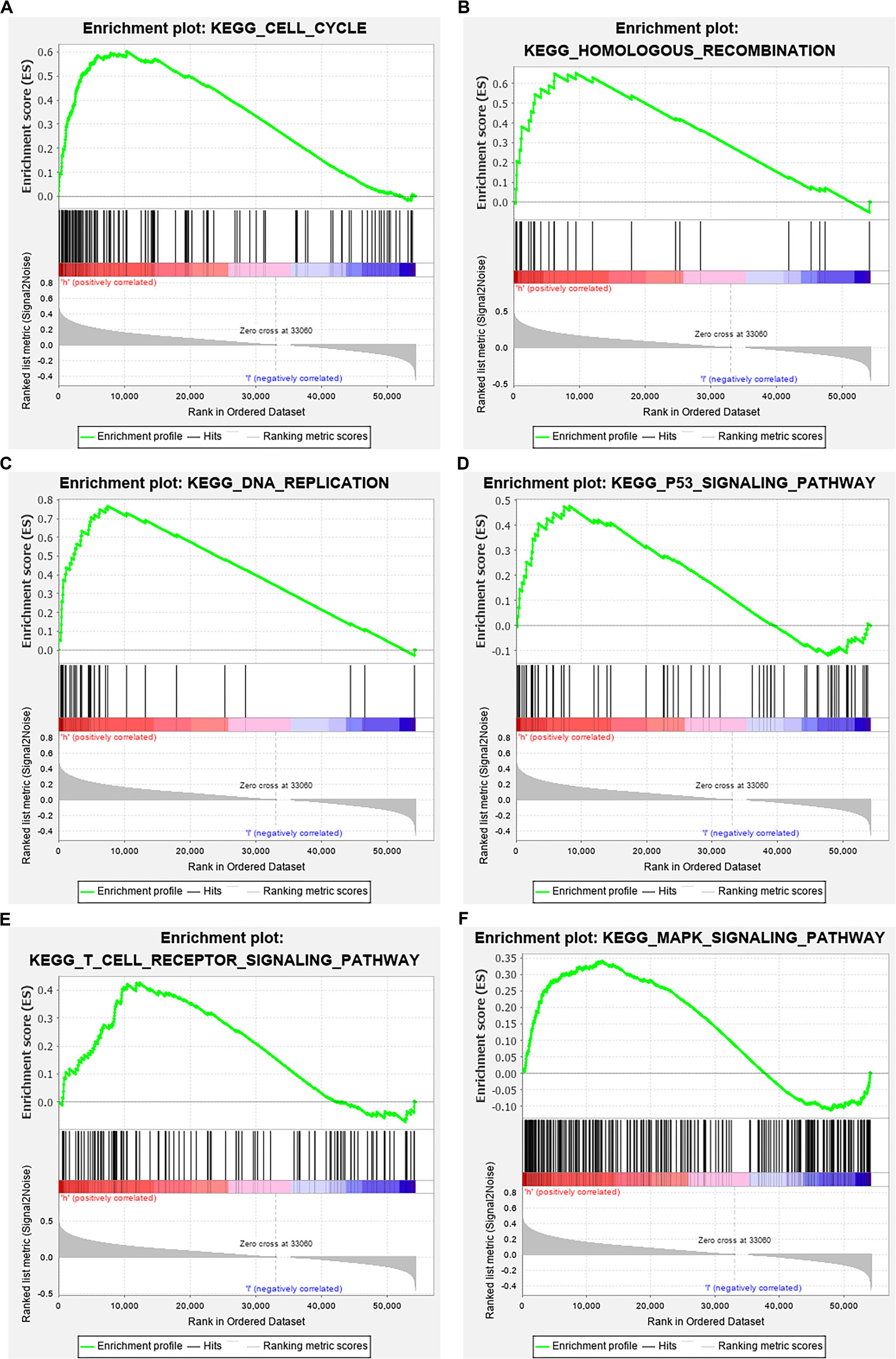
Figure 8. GSEA analysis revealed related signaling pathways enriched in PAQR3 high expression. (A) Cell cycle; (B) Homologous recombination; (C) DNA replication; (D) p53 signaling pathway; (E) T cell receptor signaling pathway; (F) MAPK signaling pathway. GSEA, gene set enrichment analysis.
PAQR3 Inhibited the Growth of NSCLC Through the NF-κB/p53/Bax Signaling Pathway
The effect of PAQR3 on the p53 signaling pathway was analyzed. In the stable transfection model of A549 and H1299 cells with overexpressed or silenced PAQR3, we found that overexpression of PAQR3 inhibited the expression of NF-κB protein and increased the expression of p53, p-p53, and Bax protein in A549 cells. Overexpression of PAQR3 inhibited the expression of NF-κB protein and increased the expression of Bax protein in H1299 cells, while no expression of p53 protein and p53 phosphorylation was detected (Figure 9A). However, interfering with the expression of PAQR3 in A549 cells promoted the expression of NF-κB protein and inhibited the expression of p53 protein, p53 phosphorylation, and Bax protein. Interfering with the expression of PAQR3 in H1299 cells enhanced the expression of NF-κB protein and inhibited the expression of Bax protein (Figure 9B).
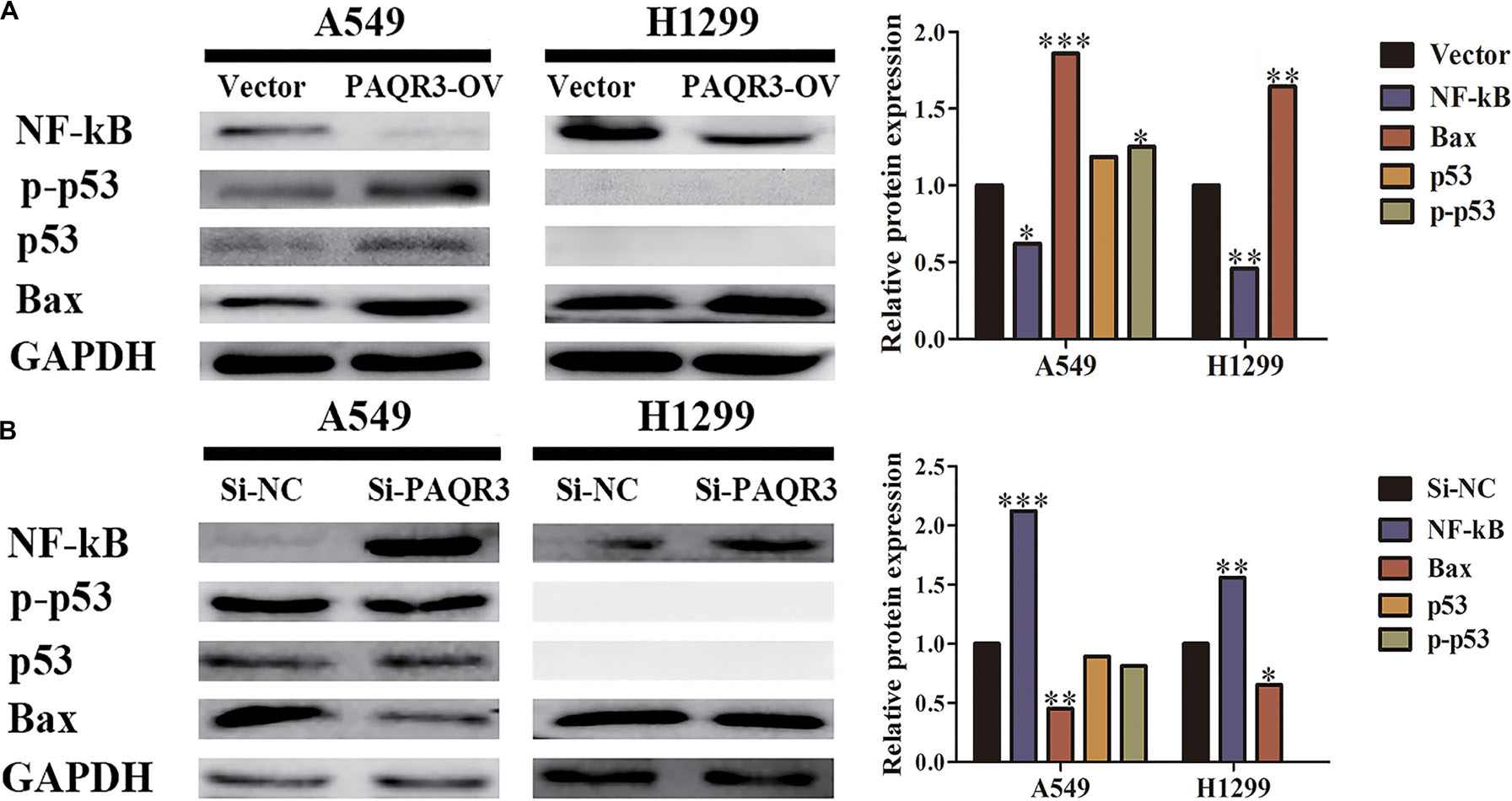
Figure 9. The effect of PAQR3 on NF-κB/p53/Bax signaling pathway was detected by western blotting. (A) Overexpression of PAQR3 inhibited the expression of NF-κB, but increased those of p53, phosphorylated p53, and Bax. (B) Knockdown of PAQR3 increased the expression of NF-κB protein, but inhibited those of p53, phosphorylated p53 and Bax. Vector, overexpression control group; PAQR3-OV, PAQR3 overexpression group; Si-NC, knockdown control group; Si-PAQR3, PAQR3 knockdown group; *P < 0.05; **P < 0.01; ***P < 0.001; vs. relative control group.
Discussion
A large number of studies have found that differentially expressed genes were potential biomarkers for prognosis in NSCLC patients. For instance, Han et al. found that the level of tripartite motif containing 47 (TRIM47) was increased in cancer tissues and closely related to poor prognosis in NSCLC. In addition, multivariate Cox regression analysis also showed that TRIM47 was an independent prognostic factor (Han et al., 2017). Liu et al. (2016) found that the levels of DEK in the cancer tissues were significantly upregulated and correlated with the pathological stage, disease-free survival, and OS time in NSCLC. The Cox proportional hazard regression model showed that DEK expression was an independent risk factor that affected prognosis (Liu et al., 2016). In addition, Schmidt et al. (2015) found that the level of PD-L1 in NSCLC was associated with tumor classification, tumor enlargement, positive lymph node, and prognosis of patients who received adjuvant therapy.
At present, the function of PAQR3 in cancers appears to be controversial. The level of PAQR3 was decreased both in tissues and cells of esophageal cancer, and overexpression of PAQR3 significantly inhibited the proliferation, clone formation, and invasion of esophageal cancer cells and blocked the transition from the G1 phase to the S phase. Moreover, increased PAQR3 expression inhibited the growth of ECA-109 cell xenograft tumors (Zhou et al., 2017; Bai et al., 2017, 2018). Compared with adjacent tissues, the expression of PAQR3 in laryngeal squamous cell carcinoma (LSCC) tissues was significantly downregulated, and overexpression of PAQR3 inhibited the proliferation and invasion of LSCC cells (Wu et al., 2016). In osteosarcoma, the expression of PAQR3 was decreased and was associated with metastasis in patients. In addition, overexpression of PAQR3 inhibited the proliferation, migration, and invasion of osteosarcoma MG-63 cells by promoting the phosphorylation of ERK (Ma et al., 2015). In the present study, we found that the level of PAQR3 mRNA in NSCLC was increased in cancer tissues analyzed using data from the Oncomine, Timer, and Ualcan databases, which were inconsistent with the conclusions reported by Liang et al. (2017) and Li et al. (2018). Therefore, we further determined the expression of PAQR3 in 60 clinical tissues by RT-qPCR and western blotting. We found that the levels of PAQR3 mRNA and protein were both decreased in most NSCLC patients. Furthermore, the level of PAQR3 mRNA was correlated with the histological subtype, lymph node metastasis, and tumor size, and was vital in the diagnosis of both NSCLC and its subtypes. In the databases related to prognosis, higher levels of PAQR3 predicted better prognosis for patients with LUAD. Therefore, we concluded that PAQR3 acted as a tumor suppressor in the progression of NSCLC and was expected to be a potential marker for prognosis and diagnosis in NSCLC.
p53 is an important tumor suppressor that plays vital roles in cell growth, cell cycle, apoptosis, angiogenesis, and genomic stability (Hiyama et al., 1998; Robles and Harris, 2001; Xu et al., 2014; Katifelis et al., 2018; Pfaff et al., 2018). In the process of tumorigenesis and development, increased p53 expression blocked the transformation of the cell cycle and promoted apoptosis. Through GSEA analysis, we found that increased PAQR3 expression was significantly correlated with the cell cycle, DNA replication, p53 signaling pathway, and some other processes of lung cancer. NF-κB and Bax proteins are the upstream and downstream targets of p53, respectively, and play significant roles in cell growth (Barone et al., 2012; Liang and Zhang, 2016; Sun et al., 2018; Chen W. et al., 2019). Moreover, Chen L. et al. (2019) found that PAQR3 regulated the phosphorylation of FoxO1 through the NF-κB signaling pathway in insulin-resistant HepG2 cells. In the present study, we found that increased PAQR3 expression in A549 cells inhibited NF-κB (P65) protein expression and increased p53 protein, p53 phosphorylation, and Bax protein expression. Increased PAQR3 expression in H1299 cells inhibited NF-κB expression and promoted Bax protein expression, while p53 protein and p53 phosphorylation expression were not affected. Opposite results would be present when PAQR3 was knocked down. Both A549 and H1299 cells are LUAD cells, the difference being that p53 protein is absent in H1299 cells. Through GSEA and model construction, we found that NF-κB, Bax, and p53 were changed in A549 cells, while the expression levels of p53 protein and p53 phosphorylation protein in H1299 cells had no effect. Only the NF-κB and Bax proteins changed, which were expected results. These results suggest that PAQR3 acts as a tumor suppressor in the occurrence and development of NSCLC through the NF-kB/p53/Bax signaling pathway.
Compared with previous studies, our research is more comprehensive. Using various databases, we found that the expression of PAQR3 was increased in LUSC and LUAD tissues, and verified the expression of PAQR3 in clinical NSCLC tissues by RT-qPCR and western blotting. At the cellular level, a new clone formation assay was added to verify the effect of PAQR3 on the growth of NSCLC cells. In addition, we used GSEA to evaluate whether PAQR3 may regulate the progression of lung cancer through the p53 signaling pathway and confirmed our prediction in model cells. However, our study still had several shortcomings, which warrant that we further supplement the relevant results of PAQR3 in nude mice. In summary, the expressions of PAQR3 mRNA and protein were downregulated and was significantly related to tumor size, histological subtype, and lymph node metastasis in NSCLC patients. Moreover, it could be a positive biomarker for prognosis among patients with LUAD. In addition, PAQR3 inhibited the growth through the NF-κB/p53/Bax signaling pathway to participate in regulating the progression of NSCLC.
Data Availability Statement
All datasets presented in this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Affiliated Hospital of Zunyi Medical University (KLLY-2018-095). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
GX, H-LL, and CC designed the study. QG and X-XK performed the experiments. S-XF, W-LG, Y-XS, and CC supervised the project. CC and X-XK analyzed the data. QG drafted the manuscript, which was corrected by GX and H-LL. All authors contributed to completing this study, read, and approved the final manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (grant no. 81960532) and the Guizhou Provincial Health and Construction Commission (grant no. gzwjkj2019-1-143).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2020.581919/full#supplementary-material
Footnotes
- ^ https://www.oncomine.org/resource/login
- ^ https://cistrome.shinyapps.io/timer/
- ^ http://ualcan.path.uab.edu
- ^ http://www.abren.net/PrognoScan/
- ^ http://kmplot.com/analysis/
References
Bai, G., Chu, J., Eli, M., Bao, Y., and Wen, H. (2017). PAQR3 overexpression suppresses the aggressive phenotype of esophageal squamous cell carcinoma cells via inhibition of ERK signaling. Biomed. Pharmacother. 94, 813–819. doi: 10.1016/j.biopha.2017.07.154
Bai, G., Yang, M., Zheng, C., Zhang, L., and Eli, M. (2018). Suppressor PAQR3 associated with the clinical significance and prognosis in esophageal squamous cell carcinoma. Oncol. Lett. 15, 5703–5711. doi: 10.3892/ol.2018.8004
Barone, E., Cenini, G., Sultana, R., Di Domenico, F., Fiorini, A., Perluigi, M., et al. (2012). Lack of p53 decreases basal oxidative stress levels in the brain through upregulation of thioredoxin-1, biliverdin reductase-A, manganese superoxide dismutase, and nuclear factor kappa-B. Antioxid. Redox Signal. 16, 1407–1420. doi: 10.1089/ars.2011.4124
Chen, L., Sun, X., Xiao, H., Xu, F., Yang, Y., Lin, Z., et al. (2019). PAQR3 regulates phosphorylation of FoxO1 in insulin-resistant HepG2 cells via NF-κB signaling pathway. Exp. Cell Res. 381, 301–310. doi: 10.1016/j.yexcr.2019.04.031
Chen, W., Cai, G., Liao, Z., Lin, K., Li, G., and Li, Y. (2019). miRNA-766 induces apoptosis of human colon cancer cells through the p53/Bax signaling pathway by MDM4. Exp. Ther. Med. 17, 4100–4108. doi: 10.3892/etm.2019.7436
Dai, L., Smith, C. D., Foroozesh, M., Miele, L., and Qin, Z. (2018). The sphingosine kinase 2 inhibitor ABC294640 displays anti-non-small cell lung cancer activities in vitro and in vivo. Int. J. Cancer 142, 2153–2162. doi: 10.1002/ijc.31234
Florczuk, M., Szpechcinski, A., and Chorostowska-Wynimko, J. (2017). miRNAs as biomarkers and therapeutic targets in non-small cell lung cancer: current perspectives. Target. Oncol. 12, 179–200. doi: 10.1007/s11523-017-0478-5
Ghafoor, Q., Baijal, S., Taniere, P., O’Sullivan, B., Evans, M., and Middleton, G. (2018). Epidermal growth factor receptor (EGFR) kinase inhibitors and non-small cell lung cancer (NSCLC)-advances in molecular diagnostic techniques to facilitate targeted therapy. Pathol. Oncol. Res. 24, 723–731. doi: 10.1007/s12253-017-0377-1
Guo, Q., Ke, X. X., Liu, Z., Gao, W. L., Fang, S. X., Chen, C., et al. (2020). Evaluation of the prognostic value of STEAP1 in lung adenocarcinoma and insights into its potential molecular pathways via bioinformatic analysis. Front. Genet. 11:242. doi: 10.3389/fgene.2020.00242
Han, Y., Tian, H., Chen, P., and Lin, Q. (2017). TRIM47 overexpression is a poor prognostic factor and contributes to carcinogenesis in non-small cell lung carcinoma. Oncotarget 8, 22730–22740. doi: 10.18632/oncotarget.15188
Hiyama, H., Iavarone, A., and Reeves, S. A. (1998). Regulation of the cdk inhibitor p21 gene during cell cycle progression is under the control of the transcription factor E2F. Oncogene 16, 1513–1523. doi: 10.1038/sj.onc.1201667
Hsu, P. C., Jablons, D. M., Yang, C. T., and You, L. (2019). Epidermal growth factor receptor (EGFR) pathway, yes-associated protein (YAP) and the regulation of programmed death-ligand 1 (PD-L1) in non-small cell lung cancer (NSCLC). Int. J. Mol. Sci 20:3821. doi: 10.3390/ijms20153821
Katifelis, H., Lyberopoulou, A., Mukha, I., Vityuk, N., Grodzyuk, G., Theodoropoulos, G. E., et al. (2018). Ag/Au bimetallic nanoparticles induce apoptosis in human cancer cell lines via P53, CASPASE-3 and BAX/BCL-2 pathways. Artif. Cells Nanomed. Biotechnol. 46, S389–S398.
Lei, L., Ling, Z. N., Chen, X. L., Hong, L. L., and Ling, Z. Q. (2020). Characterization of the Golgi scaffold protein PAQR3, and its role in tumor suppression and metabolic pathway compartmentalization. Cancer Manag. Res. 12, 353–362. doi: 10.2147/CMAR.S210919
Li, X., Li, M., Chen, D., Shi, G., and Zhao, H. (2018). PAQR3 inhibits proliferation via suppressing PI3K/AKT signaling pathway in non-small cell lung cancer. Arch. Med. Sci. 14, 1289–1297. doi: 10.5114/aoms.2017.72220
Li, Z., Ling, Z. Q., Guo, W., Lu, X. X., Pan, Y., Wang, Z., et al. (2015). PAQR3 expression is downregulated in human breast cancers and correlated with HER2 expression. Oncotarget 6, 12357–12368. doi: 10.18632/oncotarget.3657
Liang, L., and Zhang, Z. (2016). Gambogic acid inhibits malignant melanoma cell proliferation through mitochondrial p66shc/ROS-p53/Bax-mediated apoptosis. Cell. Physiol. Biochem. 38, 1618–1630. doi: 10.1159/000443102
Liang, X., Sun, B., Han, J., Zhao, X., and Liu, Z. (2017). Expression and clinical significance of progesterone and adiponectin receptor family member 3 in lung cancer. Zhongguo Fei Ai Za Zhi 20, 259–263. doi: 10.3779/j.issn.1009-3419.2017.04.06
Ling, Z. Q., Guo, W., Lu, X. X., Zhu, X., Hong, L. L., Wang, Z., et al. (2014). A Golgi-specific protein PAQR3 is closely associated with the progression, metastasis and prognosis of human gastric cancers. Ann. Oncol. 25, 1363–1372. doi: 10.1093/annonc/mdu168
Liu, X., Qi, D., Qi, J., Mao, Z., Li, X., Zhang, J., et al. (2016). Significance of DEK overexpression for the prognostic evaluation of non-small cell lung carcinoma. Oncol. Rep. 35, 155–162. doi: 10.3892/or.2015.4365
Ma, Z., Wang, Y., Piao, T., Li, Z., Zhang, H., Liu, Z., et al. (2015). The tumor suppressor role of PAQR3 in osteosarcoma. Tumour Biol. 36, 3319–3324. doi: 10.1007/s13277-014-2964-z
McCoach, C. E., Blumenthal, G. M., Zhang, L., Myers, A., Tang, S., Sridhara, R., et al. (2017). Exploratory analysis of the association of depth of response and survival in patients with metastatic non-small-cell lung cancer treated with a targeted therapy or immunotherapy. Ann. Oncol. 28, 2707–2714. doi: 10.1093/annonc/mdx414
Pfaff, M. J., Mukhopadhyay, S., Hoofnagle, M., Chabasse, C., and Sarkar, R. (2018). Tumor suppressor protein p53 negatively regulates ischemia-induced angiogenesis and arteriogenesis. J. Vasc. Surg. 68, 222S–233S. doi: 10.1016/j.jvs.2018.02.055
Robles, A. I., and Harris, C. C. (2001). p53-mediated apoptosis and genomic instability diseases. Acta Oncol. 40, 696–701. doi: 10.1080/02841860152619106
Schmidt, L. H., Kümmel, A., Görlich, D., Mohr, M., Bröckling, S., Mikesch, J. H., et al. (2015). PD-1 and PD-L1 expression in NSCLC indicate a favorable prognosis in defined subgroups. PLoS One 10:e0136023. doi: 10.1371/journal.pone.0136023
Siegel, R. L., Miller, K. D., and Jemal, A. (2020). Cancer statistics, 2020. CA Cancer J. Clin. 70, 7–30. doi: 10.3322/caac.21590
Sun, Q. M., Hu, B., Fu, P. Y., Tang, W. G., Zhang, X., Zhan, H., et al. (2018). Long non-coding RNA 00607 as a tumor suppressor by modulating NF-κB p65/p53 signaling axis in hepatocellular carcinoma. Carcinogenesis 39, 1438–1446. doi: 10.1093/carcin/bgy113
Wang, M., Kong, W., He, B., Li, Z., Song, H., Shi, P., et al. (2018). Vitamin D and the promoter methylation of its metabolic pathway genes in association with the risk and prognosis of tuberculosis. Clin. Epigenetics 10, 118. doi: 10.1186/s13148-018-0552-6
Wang, X., Li, X., Fan, F., Jiao, S., Wang, L., Zhu, L., et al. (2012). PAQR3 plays a suppressive role in the tumorigenesis of colorectal cancers. Carcinogenesis 33, 2228–2235. doi: 10.1093/carcin/bgs245
Wu, H. G., Zhang, W. J., Ding, Q., Peng, G., Zou, Z. W., Liu, T., et al. (2014). Identification of PAQR3 as a new candidate tumor suppressor in hepatocellular carcinoma. Oncol. Rep. 32, 2687–2695. doi: 10.3892/or.2014.3532
Wu, Q., Zhuang, K., and Li, H. (2016). PAQR3 plays a suppressive role in laryngeal squamous cell carcinoma. Tumour Biol. 37, 561–565. doi: 10.1007/s13277-015-3770-y
Xu, B., Wang, W., Guo, H., Sun, Z., Wei, Z., Zhang, X., et al. (2014). Oxidative stress preferentially induces a subtype of micronuclei and mediates the genomic instability caused by p53 dysfunction. Mutat. Res. 770, 1–8. doi: 10.1016/j.mrfmmm.2014.08.004
Zhao, C., Li, Y., Chen, G., Wang, F., Shen, Z., and Zhou, R. (2017). Overexpression of miR-15b-5p promotes gastric cancer metastasis by regulating PAQR3. Oncol. Rep. 38, 352–358. doi: 10.3892/or.2017.5673
Zhou, F., Wang, S., and Wang, J. (2017). PAQR3 inhibits the proliferation and tumorigenesis in esophageal cancer cells. Oncol. Res. 25, 663–671. doi: 10.3727/096504016X14761384026719
Keywords: non-small cell lung cancer, PAQR3, prognosis, GSEA, tumor growth
Citation: Guo Q, Ke X-X, Fang S-X, Gao W-L, Song Y-X, Chen C, Lu H-L and Xu G (2020) PAQR3 Inhibits Non-small Cell Lung Cancer Growth by Regulating the NF-κB/p53/Bax Axis. Front. Cell Dev. Biol. 8:581919. doi: 10.3389/fcell.2020.581919
Received: 10 July 2020; Accepted: 14 September 2020;
Published: 06 October 2020.
Edited by:
Rebecca Ann Wingert, University of Notre Dame, United StatesReviewed by:
Jun Wang, Texas Heart Institute, United StatesDaniela Carlisi, University of Palermo, Italy
Copyright © 2020 Guo, Ke, Fang, Gao, Song, Chen, Lu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Xu, eGdsaGwzMzNAMTYzLmNvbQ==; Hong-Ling Lu, bF9ob25nbGluZzJAeWFob28xNjMuY29t; bF9ob25nbGluZzJAMTYzLmNvbQ==; Cheng Chen, MjkyMTcwMzZAcXEuY29t
†These authors have contributed equally to this work
 Qiang Guo
Qiang Guo Xi-Xian Ke
Xi-Xian Ke Shi-Xu Fang
Shi-Xu Fang Wei-Long Gao
Wei-Long Gao Yong-Xiang Song
Yong-Xiang Song Cheng Chen1*
Cheng Chen1* Hong-Ling Lu
Hong-Ling Lu Gang Xu
Gang Xu