- 1Department of Animal Production, College of Food and Agricultural Sciences, King Saud University, Riyadh, Saudi Arabia
- 2Department of Comparative Medicine, King Faisal Specialist Hospital & Research Centre, Riyadh, Saudi Arabia
- 3Department of Laboratory Medicine, Faculty of Applied Medical Sciences, Umm Al-Qura University, Makkah, Saudi Arabia
The rho-associated coiled-coil-containing proteins (ROCKs or rho kinase) are effectors of the small rho-GTPase rhoA, which acts as a signaling molecule to regulate a variety of cellular processes, including cell proliferation, adhesion, polarity, cytokinesis, and survival. Owing to the multifunctionality of these kinases, an increasing number of studies focus on understanding the pleiotropic effects of the ROCK signaling pathway in the coordination and control of growth (proliferation, initiation, and progression), development (morphology and differentiation), and survival in many cell types. There is growing evidence that ROCKs actively phosphorylate several actin-binding proteins and intermediate filament proteins during oocyte cytokinesis, the preimplantation embryos as well as the stem cell development and differentiation. In this review, we focus on the participation of ROCK proteins in oocyte maturation, blastocyst formation, and stem cell development with a special focus on the selective targeting of ROCK isoforms, ROCK1, and ROCK2. The selective switching of cell fate through ROCK inhibition would provide a novel paradigm for in vitro oocyte maturation, experimental embryology, and clinical applications.
Introduction
Rho-associated coiled-coil-containing protein serine/threonine kinases (ROCKs) are the most recognized and studied downstream effectors of the small GTPase RhoA, which regulates a plethora of cellular events, particularly actin-mediated cellular activities (Schwartz, 2004). With a primary role in the spatiotemporal organization of actin cytoskeletal-related cellular events, ROCK proteins act as the key that enables the interaction of actin cytoskeleton with molecular motors, that convert signals into forces essential for the regulation of cellular functions such as contraction, adhesion, migration, proliferation, and apoptosis (Riento and Ridley, 2003; Jaffe and Hall, 2005; Ohgushi et al., 2010). Ever since the discovery of the pleiotropic nature of ROCK, it has sparked the interest of various research and medical fields, including the pharmaceutical (Hahmann and Schroeter, 2009; Feng et al., 2015; Honjo and Tanihara, 2018; Knipe et al., 2018; Zhou, 2018), neuroregeneration (Wang et al., 2015), stem cell, and regenerative medicine (Krawetz et al., 2011; Li et al., 2015; Vernardis et al., 2017), cancer research (Street and Bryan, 2011; Kümper et al., 2016; Wei et al., 2016), and reproductive and developmental biology fields (Kawagishi et al., 2004; Laeno et al., 2013; Duan et al., 2014b; Miranda-Rodríguez et al., 2017; Tukur et al., 2020).
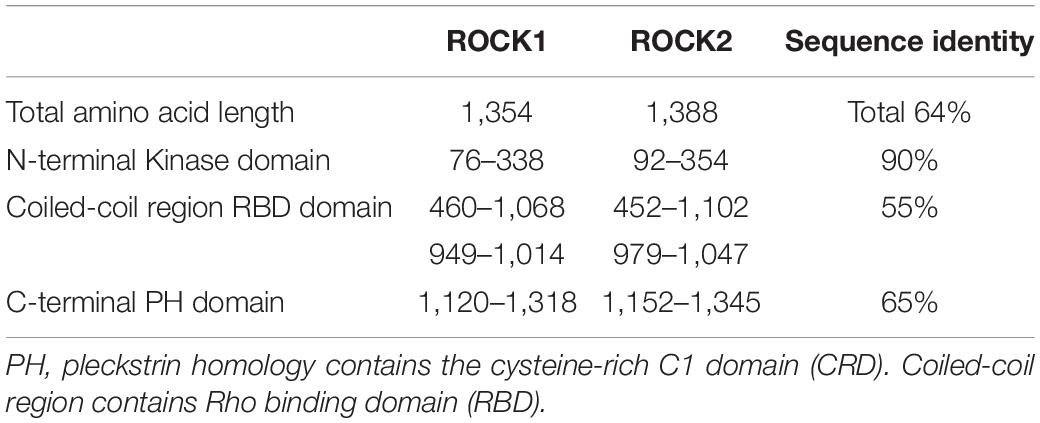
Structurally, ROCK consist of four domains: an amino (N-terminal) domain, a kinase domain, a central coiled-coil domain (known as the rho-binding domain, RBD), and a C-terminal domain. ROCK exists in two isoforms, ROCK1 and ROCK2, encoded by separate but closely related genes, ROCK1 and ROCK2, respectively (Newell-Litwa et al., 2015; Rath et al., 2016). ROCK1 and ROCK2 are homologous and share about 64% amino acid sequence identity (Table 1). They differ in the activation mechanisms; both can be activated by binding of RhoGTP to the Rho binding domain (RBD). Meanwhile, ROCK1 can be activated through cleavage by caspase-3, while ROCK2 can be activated by granzyme B and caspase-2. Autophosphorylation of ROCK1 at Ser1333 and of ROCK2 at Ser1366 reflects the activation status of the kinases (Ishizaki et al., 1996; Nakagawa et al., 1996). Despite their structural similarity and similar action on the same downstream substrates, studies in murine models showed that ROCK1 and ROCK2 are expressed in different cells, tissue locations, and stages of development (Zhang et al., 2014; Zanin-Zhorov et al., 2016; Lu et al., 2020). They have different cellular localizations; ROCK1 possesses cytosolic localization and association with centrosomes (Chevrier et al., 2002), and the plasma membrane (Stroeken et al., 2006). In contrast, ROCK2 can be found in the cytoplasm and nucleus, associated with the centrosome, and co-localized with actin and vimentin filaments in different cell types (Leung et al., 1995, 1996; Sin et al., 1998; Katoh et al., 2001; Kawabata et al., 2004; Ma et al., 2006; Tanaka et al., 2006). Therefore, neither compensates for the loss of the other (Zhu et al., 2011). Specifically, ROCK2 is associated with blastocyst development in swine (Zhang et al., 2014).
General Mechanism of Rock Regulation and Function
For a detailed description of the general mechanism of ROCK, the reader is referred to the reviews of Amin et al. (2013) and Hartmann et al. (2015). Soluble hormones, cytokines, integrins, and growth factors bind to cell surface receptors to stimulate cellular responses. These stimuli trigger G-protein-coupled receptors to activate Rho-GTPases. This activation or deactivation depends on the coupling of Rho with the guanine nucleotide exchange factors (GTP and GDP), the molecular switch that regulates Rho-ROCK signaling (Dvorsky et al., 2004; Mott and Owen, 2015; Bagci et al., 2019). In the active form, Rho is bonded with GTP. Rho inactivation occurs when Rho-GTPase hydrolyzes GTP into GDP, switching Rho from GTP bounded (active) state to GDP bounded (inactive) form (Müller et al., 2020; Figure 1). Rho signaling is positively regulated through Rho guanine nucleotide exchange factors (RhoGEFs) that promote and catalyze the release of bound GDP for GTP (Cook et al., 2014). Meanwhile, Rho GTPase-activating proteins (RhoGAPs) are negatively regulating Rho signaling through stimulating the hydrolysis of Rho GTPases (Figure 1; Moon and Zheng, 2003). Additionally, Rho guanine nucleotide-dissociation inhibitors (Rho GDIs) can bind to and prevent the dissociation of GDP (Olofsson, 1999). If activated, Rho isoforms including RhoA, RhoB, and RhoC bind to and activate rho kinase (Sit and Manser, 2011).
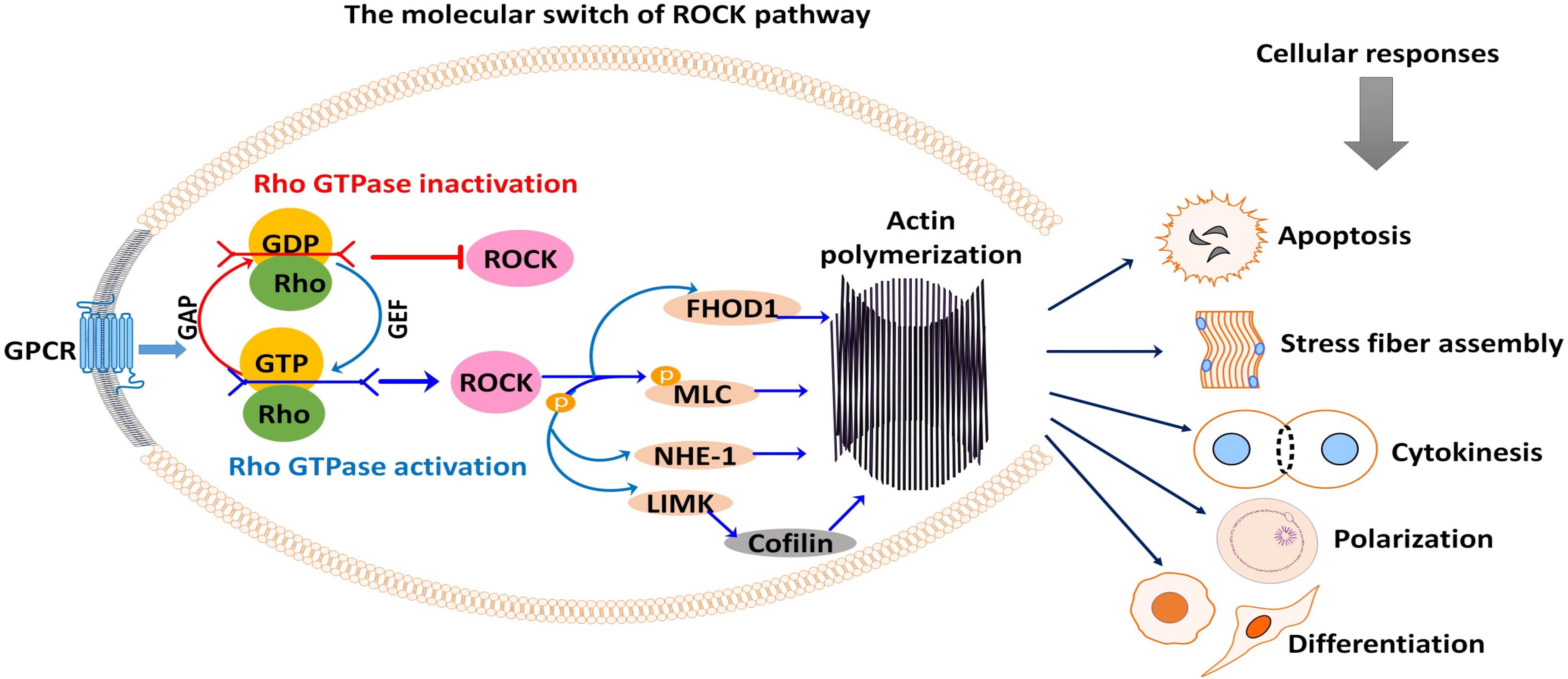
Figure 1. Rho-associated coiled-coil-containing protein serine/threonine kinase (ROCK) signaling activation and subsequent phosphorylation (activation/inhibition) of several downstream substrates are responsible for many cellular events. Signals from G-protein-coupled receptor (GPCR) stimulate the coupling of Rho with GEF or GAP, which switches ROCK on or off. As shown, ROCK downstream substrates are mostly involved in actin-mediated cellular activities such as apoptosis, stress fiber formation, cytokinesis, cell polarization, cell adhesion, and cell differentiation. GAP, GTPase-activating protein; GEF, guanine nucleotide exchange factor; GDP, guanosine diphosphate; GTP, guanosine triphosphate; FHOD1, formin homology 2 domain-containing 1; MLC, myosin light chain; NHE-1, Na+/H+ exchanger type 1; LIMK, LIM kinase.
Corroborating previous studies, the regulation of cell mechanics from stress fiber assembly, cell polarization, cell shape, and focal adhesion are mediated via RhoA GTPase–ROCK pathways. Recent findings by Lachowski et al. (2020) identified ROCK as a pivotal player in the transmission of signals through which the G-protein-coupled estrogen receptor (GPER) regulates actin polymerization rate and modulates cell polarization in fibroblasts.
Having identified the multifunctionality of ROCK, the contribution of the Rho/ROCK signaling was deeply explored, and several substrates linking ROCK to a variety of cellular activities were identified. After several works, rho kinase became a target molecule for cellular therapy, regenerative medicine, and stem cell research. A recent and noteworthy finding is the critical role of ROCK in Na+/H+ exchanger type 1 (NHE-1)-induced cell death. NHE-1 was previously known to regulate intermediate filament structure and cell shape. Recently, Wakabayashi et al. (2019) showed that the overexpression of NHE1 activated and sustained the ROCK pathway causing loss of human-induced pluripotent stem cells (iPSCs). This finding helped to elucidate the riddle behind the loss of iPSCs during cell passage after their dissociation.
Downstream Targets of Rock
Activated ROCK phosphorylates various downstream substrates, including the myosin-binding subunit (MBS) of myosin phosphatase, myosin light chain (MLC), ezrin–radixin–moesin (ERM) proteins, LIM kinase, Na+/H+ exchanger type 1 (NHE-1), intermediate filaments vimentin (VIM) and glial fibrillary acidic protein (GFAP), formin homology 2 domain-containing 1 (FHOD1), and phosphatase and tensin homology (PTEN) (Schwartz, 2004; Schofield and Bernard, 2013; Hartmann et al., 2015). Through these substrates, ROCK mediates a wide range of cellular responses that involve the actin cytoskeleton, including cytoskeletal remodeling, actin–myosin contraction, actin filament stabilization, and microtubule dynamics (Figure 1). These cellular responses are entirely related to the cell cycle and meiotic maturation of oocytes, fate, and development of preimplantation embryos, and stem cell differentiation (Laeno et al., 2013; Duan et al., 2014b). Thus, ROCK has become a unique multifaceted protein of interest in oocyte, embryo, and stem cell research.
ROCK phosphorylates MLC and MBS to coordinate actinomyosin-mediated cellular contraction. Primarily, MLC phosphorylation causes cellular contraction. To further enhance this, MBS phosphorylation functionally inhibits MLC dephosphorylation by blocking myosin phosphatase activity, leading to an increase in MLC phosphorylation (Amano et al., 1996). This enables a conformational change that allows myosin II to assemble into bipolar filaments that bind to actin. Actin-activated ATPase activity increases, and eventually, actin stress fibers are formed. Focal adhesions stimulate actin–myosin interaction that induces tension, cellular contraction, stress fiber assembly, cell motility, proliferation, cytokinesis, cell adhesion, survival, and gene expression (Riento and Ridley, 2003). In particular, the ROCK pathway plays a pivotal role in cytoskeleton machinery required for cytokinesis, morphology, furrowing, and cell division; Rho-kinase stimulates polar relaxation, allowing the spindle to push and extend the cell sides permitting anaphase elongation. Moreover, Rho-kinase stimulates myosin II recruitment to the equatorial cortex, where it begins to contract in a broad zone permitting the contractile actin ring to assemble, and the cytokinetic furrow ingresses (Hickson et al., 2006). LIM kinase (LIMK) phosphorylates cofilin to prevent its binding with F-actin, inhibiting actin depolymerization and filament severing. Cofilin in turn promotes actin polymerization and stability. Recent works by Zhang et al. (2019) and Morales-Quinones et al. (2020) have clearly explained how LIMK implicates cytoskeletal organization and muscle contraction through the Rho pathway. The disruption of LIMK activities resulted in the loss of muscle contraction and vasoconstriction reducing cofilin phosphorylation and stiffening of arterial muscle (Morales-Quinones et al., 2020). Besides, the phosphorylation of ERM family proteins facilitates the linkage between actin and the plasma membrane. Furthermore, NHE-1 promotes both actin–membrane interactions and the function of the intermediate filament protein vimentin to regulate intermediate filament structure. The ROCK–cofilin–actin pathway is essential for meiotic development and cytokinesis during mouse oocyte maturation (Duan et al., 2014b). Results suggest that the ROCK/MLC/actomyosin, as well as ROCK/LIMK/cofilin pathways, regulate meiotic spindle migration and cytokinesis during bovine oocyte maturation (Lee et al., 2015). Besides, during cytokinesis, ROCK phosphorylates the intermediate filaments VIM and GFAP at the cleavage furrow, which is crucial for normal cell division. Slight distortion in the normal regulation of VIM and GFAP assembly/disassembly is associated with cytokinetic failure, aneuploidy, and bi-nucleation, which can result in cellular senescence (Matsuyama et al., 2013; de Pablo et al., 2019).
Localization of Rho Kinase in Oocyte and Embryo
The distribution and function of ROCK in oocyte and embryo varies as development progresses suggesting a spatiotemporal role of ROCK at different stages of oocyte and embryo development (Figure 2). Northern blot analysis of Rho kinase protein, real-time PCR (rtPCR) analysis of Rho-kinase mRNA, and inhibition of ROCK has been employed to show the presence and involvement of Rho-kinase in the oocyte and embryo of rabbits (Stankova et al., 2014), mice (Duan et al., 2014a,b), cows (Hwang et al., 2013; Lee et al., 2015), felines (Arayatham et al., 2017), swine (Zhang et al., 2014), and camels (Tukur et al., 2020). In the bovine oocyte, ROCK was localized around the nucleus at the germinal vesicle (GV) stage but spread to the rest of the cytoplasm in later developmental stages (Lee et al., 2015). In the mouse oocyte, upon resumption of meiosis I, ROCK mRNA is localized around the spindles, colocalizing with cytoplasmic actin and mitochondria (Duan et al., 2014b), while it accumulated around the chromosomes at anaphase. At metaphase II, ROCK was again detected around the spindle. The localization pattern of ROCK was similar to that of cytoplasmic actin, which colocalizes with the centrosome in the cleavage furrow during mitosis (Riento and Ridley, 2003). Both ROCK 1 and ROCK 2 are very close in structure, function, and distribution; however, a distinction is observed in the amount and concentration of their mRNA and protein as development progresses (Zhang et al., 2014). A significant increase in ROCK1 mRNA and protein was clearly observed from the eight-cell and morula stages in swine, which then decreased significantly when blastocysts were formed (Zhang et al., 2014). An abundance of ROCK2 was observed at the blastocyst stage, indicating distinct roles for ROCK1 and ROCK2 at different stages of oocyte and embryo development. In porcine oocytes, ROCK1 accumulates in the ooplasm and after fertilization localizes in the cytoplasm from the two-cell stage to the blastocyst, while ROCK2 is found throughout the cytoplasm of the GV oocyte, and around the meiotic spindle at the MI and MII stage. In porcine preimplantation embryos, ROCK2 was detected in both the cytoplasm and the nucleus (Zhang et al., 2014).
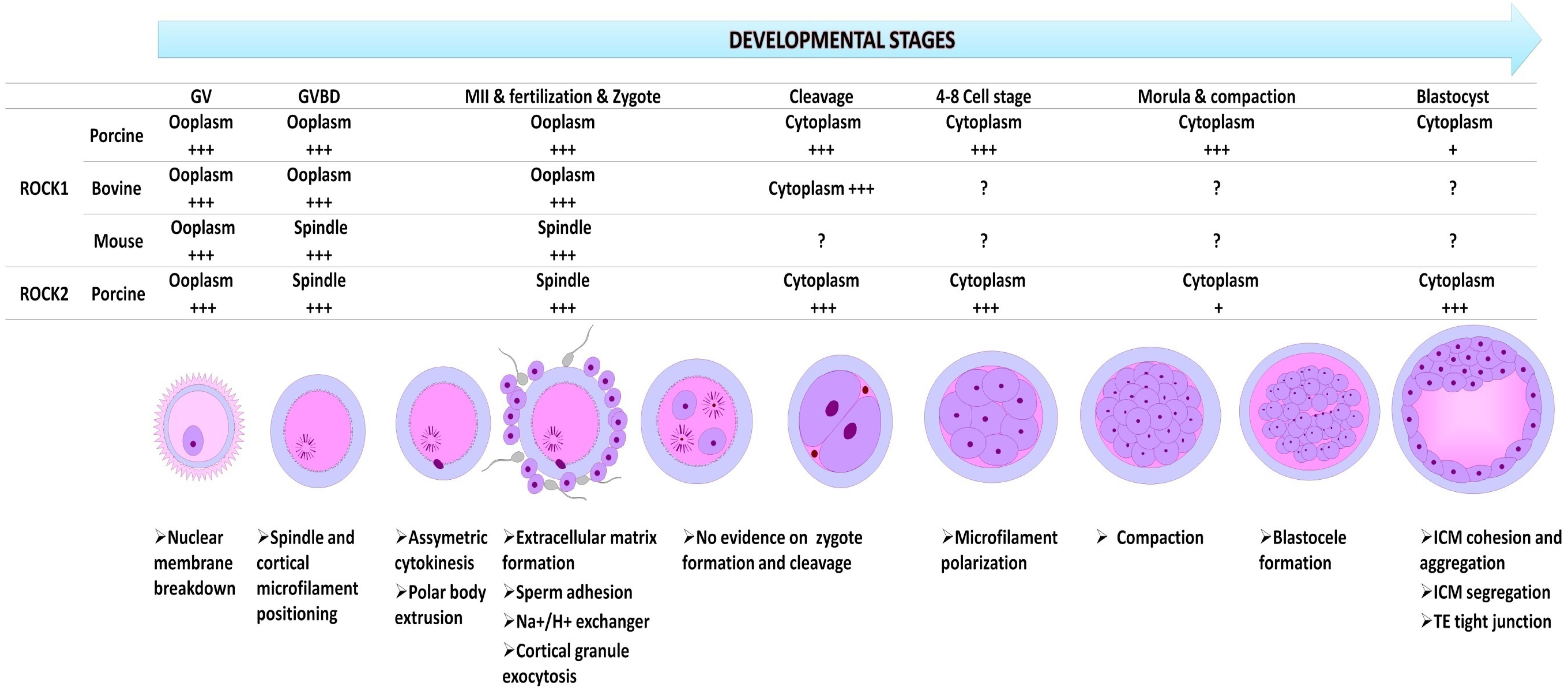
Figure 2. The relative spatial and temporal expression of ROCK1 and ROCK2 in oocytes and preimplantation embryos in different species, and their functions at every developmental stage. GV, germinal vesicle stage; Germinal vesicle breakdown at metaphase I (GVBD; MI); MII, metaphase 2; +++, richly expressed and predominantly distributed; +, very low expression; ?, no data available.
Rho Kinase and Oocyte Maturation
Oocyte maturation is a complex event that involves several crucial intercellular, intracellular, and molecular processes that lead to the generation of a mature and competent oocyte that can be fertilized by sperm and support early embryogenesis. Alterations in the normal choreography of this complex event have been associated with poorly developed oocytes, aneuploidy, miscarriage, infertility, and birth defects (Cimadomo et al., 2018; Bennabi et al., 2020). One unique feature of this event is asymmetric cytokinesis, without which successful oocyte maturation and fertilization cannot be achieved. Asymmetric cytokinesis depends on oocyte polarization, which is mediated by spindles that are assembled asymmetrically against the oocyte cortex, causing the expulsion of half of the chromosomes into tiny polar bodies. Spindle and cortical positioning and reorganization are crucial for polar body formation, as well as ensuring that the size of the polar body is appropriate, preventing loss of maternal cytoplasmic resources in the oocyte necessary for early embryo development (Barrett and Albertini, 2007; Clift and Schuh, 2013; Sun and Kim, 2013; Liu et al., 2018; Roeles and Tsiavaliaris, 2019). Through this process, the oocyte maintains a haploid chromosome number, as the other three genome copies are deposited into the polar bodies. These processes rely heavily on actin organization (Liu et al., 2018; Roeles and Tsiavaliaris, 2019; Santella et al., 2020).
Various factors that promote actin filament formation, migration, and assembly during the entire process of oocyte maturation have been exhaustively studied (Namgoong and Kim, 2016; Roeles and Tsiavaliaris, 2019). ROCK is among the kinases that regulate cytokinesis, control actin-binding proteins, and regulate Rho-mediated cellular activities, which include actin cytoskeletal organization, actin fiber formation, and actin dynamics in muscle cells and oocytes (Jaffe and Hall, 2005; Sit and Manser, 2011; Wang et al., 2017).
ROCK acts via the modulation of actin-binding proteins, including cofilin, profilin, FHOD1, tubulin, and FLNA during oocyte maturation (Wang et al., 2017). Particularly, ROCK inhibition decreased cofilin phosphorylation and caused defects in spindle migration, asymmetry, and polar body extrusion in mouse oocytes (Wang et al., 2017). This indicates a major role in cytoskeletal dynamics, spindle organization, and ultimately oocyte meiotic maturation, which is dependent on spindle positioning. During oocyte maturation (Figure 2), the germinal vesicle (GV), which is the nucleus of the oocyte must rupture, a phenomenon referred to as germinal vesicle breakdown (GVBD). The oocyte then re-initiates meiosis I and completes its development into a mature gamete. ROCK has been shown to be involved in the process of GVBD in bovine, mouse, porcine, and camel oocytes. Interestingly, this approach can be employed positively to control spontaneous meiotic resumption in immature oocyte in vitro maturation (IVM). However, prolonged inhibition of ROCK can have profound negative effects on oocyte and embryo development. In the camel, the prolonged inhibition resulted in either abnormal polar bodies or polar body failure (Tukur et al., 2020). Duan et al. (2014b) found that prolonged inhibition of ROCK activity during mouse oocyte maturation interfered with meiotic spindle migration and polar body extrusion. A similar phenotype was observed in bovine (Lee et al., 2015) and porcine (Suzuki et al., 2011) oocytes after ROCK inhibition. An improvement in fertilization and cleavage rate was observed, however, when ROCK was suppressed in vitrified feline oocytes (Arayatham et al., 2017). This could be attributed to the variation in the dose of ROCK inhibitor molecule that was used in the experiment or to the efficacy of specific dose on oocytes of different species. The dose of 10 μM of the ROCK inhibitor is commonly and widely reported.
Importantly, the ROCK pathway regulates the preparation of the oocyte to receive the sperm for fertilization on different levels (Rangel-Mata et al., 2007; Yodoi et al., 2009). Prostaglandin E2 signaling in cumulus cells negatively regulates Rho/ROCK signaling in cumulus cells to facilitate the fertilization process (Yodoi et al., 2009). ROCK/actomyosin pathway facilitates the surface accumulation of integrin and fibronectin fibril formation in the extracellular matrix of cumulus cells, which is a key event for interfering sperm penetration (Yodoi et al., 2009; Niringiyumukiza et al., 2018). Rho-kinase helps actin filament organization, cytoskeleton remodeling, and the action of Na+–H+ exchanger that is required for internal pH regulation and cortical granule exocytosis (Rangel-Mata et al., 2007). The ROCK–actomyosin pathway is therefore an essential requirement for oocyte fertilization by the sperm.
Many studies have focused on understanding the role of actin-binding proteins and have identified the regulatory pathways involved in oocyte development. It has been established that ROCK acts directly on MLC by direct phosphorylation (Lee et al., 2015; Liu et al., 2018), which increases cellular contraction, facilitating the interaction of myosin with F-actin. Further investigation revealed low levels of cortical actin, phosphorylated cofilin, and phosphorylated MLC when ROCK activity was interrupted (Lee et al., 2015). The LIMK is also directly phosphorylated by ROCK and regulates actin polymerization and microtubule dynamics via phosphorylation and inactivation of cofilin (Arayatham et al., 2017). In mice, LIMK was detected after GV breakdown, and it increased gradually from metaphase I to metaphase II, localizing to the microtubule organizing center (MTOC) of the spindle pole in MII oocytes.
Rock and Blastocyst Development
There is increasing evidence that Rho-kinase is required for early mammalian embryonic development (Laeno et al., 2013) up to the blastocyst stage in mice (Kawagishi et al., 2004) and swine (Zhang et al., 2014). Rho-kinase mRNA has been detected in abundance at all stages of preimplantation and development (Kawagishi et al., 2004), promoting cleavage and blastocyst formation. Clayton et al. (1999) showed that inhibition of Rho-kinase with Clostridium botulinum C3-transferase disturbed intercellular flattening at compaction and prevented cytocortical microfilament polarization of eight-cell blastomeres. Additionally, treatment of porcine four-cell stage embryos and morula with Rho-kinase inhibitor Y-27632 resulted in defective morula compaction and cavitation (Son et al., 2010; Kwon et al., 2016). Similarly, the two-cell embryo treated with Y-27632 resulted in morula formation but inhibited the blastocoel formation and failed to develop blastocysts (Kawagishi et al., 2004; Kono et al., 2014). This was a result of inhibition of compaction and adhesion required for proper morphogenesis (Laeno et al., 2013). Moreover, Y-27632 reduced the rate of re-expansion of blastocysts when chemically collapsed by cytochalasin D suggesting that ROCK is involved in blastocyst formation (Kawagishi et al., 2004). Moreover, ROCK inhibition increased tight junction permeability of the trophectoderm through suppression of tight junction genes (such as CXADR, OCLN, TJP1, and CDH1) (Kwon et al., 2016) and reduced expression of trophectoderm-specific gene, CDX2 (Kono et al., 2014).
Surprisingly, inhibition of ROCK in cryopreserved vitrified (an ultra-rapid transition of liquid matter into an amorphous glass-like phase without ice crystal formation with the aid of liquid nitrogen) feline oocytes during IVM did not affect oocyte maturation and embryo developmental competence in terms of formation of the morula and blastocyst. Accordingly, the suppression of ROCK improves the revivability of feline (vitrified), human, and bovine blastocysts (Hochi et al., 2010; Huang et al., 2016; Arayatham et al., 2017). These reports suggest a positive effect of Rho-kinase inhibition on post-thawed embryos, rather than on fresh embryos. In fresh embryos, several reports (Kawagishi et al., 2004; Duan et al., 2014a) showed that the absence of ROCK significantly hinders oocyte and embryonic development.
Roles of Rock in Embryonic Tissue Segregation and Stem Cell Differentiation
The blastocyst consists of the trophectoderm (TE), and the pluripotent inner cell mass (ICM) expands by the compaction of the ICM, which adopts an ovoidal shape. This process and morphology are crucial for normal embryogenesis. ROCK is required for cohesion of ICM cells, and the formation of segregated tissues called primitive endoderm (PrE) and epiblast (Epi), in the ICM of the mouse blastocyst (Laeno et al., 2013). ROCK inhibition with 20 μM Y-27632 caused dramatic changes in ICM cells and mouse embryonic stem cell line morphology and prevented cell aggregation and colony formation; they became flattened and spread as a monolayer on the culture dish, resulting in increased surface area of cultured cells, and this effect was mediated through inhibition of F-actin and microtubules (Laeno et al., 2013).
Rho-kinase, as an indispensable enzyme implicated in a plethora of cellular activities and functions, exhibited somewhat related yet distinct roles in different cell types. For instance, in stem cell differentiation, an investigation by Li et al. (2015) established in their study on bone marrow mesenchymal stem cells (BMSCs) that inhibition of ROCK with Y-27632 facilitated the differentiation of BMSCs into keratinocyte-like cells, and promoted the proliferation and survival of human primary keratinocytes. Likewise, suppression of ROCK with 20–50 μM Y-27632 promoted the differentiation of mouse embryonic stem cells into neurons (Kamishibahara et al., 2016) by activating the extracellular signal-regulated kinase signaling pathway. Similarly, the multipotency of rat sagittal suture mesenchymal stem cells showed a ROCK-dependent osteogenic differentiation under mechanical tension (Li et al., 2020). Furthermore, the ROCK pathway mediates the mechanical cues critical for neural stem cell differentiation (Kang et al., 2020).
Suppression of ROCK activity regulates the stem cells’ self-renewal and differentiation into the three germ layer derivatives. This suppression could be used as a tool for regular pluripotent stem cell culture and transportation/shipment and sharing cells between researchers to avoid geographic and logistic barriers (Kurosawa, 2012; Rivera et al., 2020; Ye et al., 2020). Recently, ROCK inhibitor fasudil showed improvement in the establishment of cloned embryo-derived embryonic stem cells through exerting an anti-apoptotic pathway (So et al., 2020a). It is, therefore, worthwhile to investigate how the modulation of Rho-kinase signaling can influence stem cell activities such as apoptosis (Joo et al., 2012; Li et al., 2015) and secretory activities (Zonderland et al., 2020). Particularly, mesenchymal stromal cell (MSC)-derived stanniocalcin-1 is critically regulated with ROCK signaling, owing to its importance in the anti-inflammatory, anti-apoptosis, and promoting angiogenesis associated with MSC therapy (Zonderland et al., 2020). Supplementing cell culture with ROCK inhibitor Y-27632 has proven to be a simple, efficient, and versatile approach for obtaining an abundance of embryonic stem cell-like cells at high purity suitable for use in regenerative medicine and therapeutics. Chapman et al. (2014) showed that long-term inhibition of Rho-kinase with Y-27632 improved the long-term proliferation of human keratinocytes, in addition to blocking and rescuing cells from aging. Joo et al. (2012) made a similar observation during the differentiation and proliferation of endothelial cells derived from an embryonic stem cell (ESC)-derived Flk1+ mesodermal precursor cells. Recently, Kim et al. (2020) found that ROCK inhibitor Y-27632 together with Matrigel improved the isolation, proliferation rate, and differentiation potential of urine-derived stem cells in humans. Furthermore, ROCK inhibitors have been used to promote proliferation, viability, and differentiation of mesenchymal stem cells (Baharvand et al., 2010; Mellott et al., 2014; Nakamura et al., 2014; Li et al., 2015).
Recent investigations in regenerative medicine and therapeutic studies have demonstrated the importance of rho kinase regulation in cell viability, proliferation, regeneration, and differentiation. The works of Yang et al. (2020) revealed that the addition of Y-27632 in the culture medium enhanced the viability of stem cells from human exfoliated deciduous teeth (SHED) and their differentiation into neuron-like cells. A corroborative result was reported by Baek et al. (2019) indicating the potential of rho kinase regulation.
Survival of cryopreserved human pluripotent stem cells (hPSCs) has been improved following the manipulation of Rho-kinase. Temporal inhibition of ROCK signaling represents a proven approach to prevent cell death and reinitiate cell proliferation after freezing or thawing pluripotent stem cells (So et al., 2020b). It has been suggested that ROCK inhibition increases the survival of hESCs because of continued and enhanced cell–cell interactions, reduced dissociation-induced apoptosis, and aided in the maintenance of pluripotent characteristics, and promoted cell propagation. Shi and Wei (2007) and Okumura et al. (2016) showed that cell death by apoptosis is mediated through the ROCK pathway; ROCK inhibition suppressed MLC phosphorylation, slowed cytokinesis, and prevented differentiation and cell death.
Rock Inhibitors in Oocytes, Embryos, and Stem Cells
There is compelling evidence for the roles of ROCK signaling in oocyte meiotic and cytoplasmic maturation (Liu et al., 2018; Tukur et al., 2020), including those in embryo cleavage, blastocysts, and segregation of ICM (Kawagishi et al., 2004; Laeno et al., 2013). Moreover, ROCK signaling is pivotal in pluripotent and embryonic stem cells, where it is implicated in proliferation, differentiation, and survival (Zhang et al., 2011; Kamishibahara et al., 2016; Vernardis et al., 2017). In serum-free medium supplemented with the ROCK inhibitor, the survival of dissociated human embryonic stem cells was significantly improved. The inhibition of ROCK is not only useful in preventing and protecting hPSCs from apoptosis but also ensures that the phenotype of stem cells is maintained (Watanabe et al., 2007). This indicates that the ROCK inhibitors are a promising approach in stem cell research for the development of stem cell bioprocesses (Vernardis et al., 2017), and it may be worth exploring ROCK by extensively focusing on its modulation/inhibition in the control of cell growth, differentiation, fate, and development.
These studies established a rationale for the use of ROCK inhibitors as molecular tools to study the biological functions of ROCK and ROCK signaling in cellular events (Liao et al., 2007; Feng et al., 2015). Consequently, the biotechnology industry has developed several isoform-selective and non-selective ROCK inhibitors to determine the role of ROCK in a variety of cells and tissues (Liao et al., 2007; Defert and Boland, 2017; Saadeldin et al., 2020). Notably, ROCK inhibitors including fasudil and Y-27632 have been widely used in oocyte, embryo, and stem cell research (Laeno et al., 2013; Duan et al., 2014a; Defert and Boland, 2017; So et al., 2020b; Tukur et al., 2020). Interestingly, the selective efficacy of ROCK inhibitors is both dose and time dependent. For instance, Y27632, at a dose of 0.3 μM, blocks ROCK2, while ROCK1 appears sensitive and can be blocked at a lower dose of 0.22 μM (Liao et al., 2007). Paradoxically, SLx-2119 blocks ROCK1 at a concentration of 25 nM, while ROCK2 can be inhibited at lower concentrations of 5 nM (Shifrin et al., 2005). Moreover, the inhibitors H-1152P and ROKα inhibitor BF showed differential efficacy at different dosages (Liao et al., 2007). It was shown that Y27632 has time-dependent efficacy, especially when applied to dynamic meiotic oocytes; it exerted harmful effects when applied for 2, 4, 6, or 24 h during meiosis I, and beneficial effects when applied for 6 or 24 h after the first polar body extrusion (Tukur et al., 2020). Conversely, XD-4000 is a selective inhibitor for ROCK2. Several other inhibitors are under investigation for clinical purposes, although the most commonly used ROCK inhibitors in the fields of stem cell and developmental biology are fasudil and Y-27632, which act with similar efficiency (So et al., 2020b).
Interestingly, a photoactivatable small-molecule inhibitor was developed to be light-controlled for spatiotemporal control of ROCK activities in live embryos (Morckel et al., 2012). Researchers developed this caged Rockout (cRO) by attaching a 6-nitropiperonyloxymethyl (NPOM) moiety onto the indole nitrogen of Rockout (RO), which is degraded by exposure to UV light, thus restoring ROCK inhibitory activity. Adopting this cRO approach could facilitate fantastic discoveries in regenerative medicine, stem cell or tissue engineering, and organ culture, or other contexts that are affected by ROCK signaling (Zhang et al., 2009; Morckel et al., 2012). The weakness of ROCK inhibitors lies in their inability to distinguish between ROCK isoforms; hence, this approach may not be sufficient to understand the specific role of ROCK and the ROCK signaling pathways in different cells. A more reliable method will be a combination of this approach and genetic knockout models (Thumkeo et al., 2003; Rikitake et al., 2005).
Conclusion and Future Directions
The spatial and temporal pleiotropic effects of ROCK isoforms control critical steps in oocyte meiosis, early embryonic stem cell lineage differentiation, and other pluripotent stem cell development; however, selective inhibitors of ROCK1 and ROCK2 isoforms are in the early stages of development. Selective ROCK inhibitors will be required to distinguish ROCK isoforms and to understand the critical roles of the ROCK pathway in cellular differentiation and elucidate their distinctive roles in oocyte, embryo, and pluripotent stem cell development. The most reliable method will likely be a combination of this approach and the use of gene knockout and/or knockdown animal models. Moreover, approaches such as cumulus-specific, oocyte-specific, and/or blastocyst-specific disruption of ROCK signaling molecules will be useful to address the physiological significance of Rho-ROCK signaling in the early steps of fertilization and embryonic development. ROCK targeting might be used for modulating the early developmental stages to control pregnancy such as novel targeted contraceptive drugs.
Author Contributions
IS and HT conceptualized the study. IS, HT, RA, and RS wrote and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Deputyship for Research and Innovation, Ministry of Education, Saudi Arabia, through the project number IFKSURP-14.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the project number IFKSURP-14.
References
Amano, M., Ito, M., Kimura, K., Fukata, Y., Chihara, K., Nakano, T., et al. (1996). Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271, 20246–20249. doi: 10.1074/jbc.271.34.20246
Amin, E., Dubey, B. N., Zhang, S. C., Gremer, L., Dvorsky, R., Moll, J. M., et al. (2013). Rho-kinase: regulation, (dys)function, and inhibition. Biol. Chem. 394, 1399–1410. doi: 10.1515/hsz-2013-0181
Arayatham, S., Tiptanavattana, N., and Tharasanit, T. (2017). Effects of vitrification and a Rho-associated coiled-coil containing protein kinase 1 inhibitor on the meiotic and developmental competence of feline oocytes. J. Reprod. Dev. 63, 511–517. doi: 10.1262/jrd.2017-004
Baek, S.-K., Cho, Y.-S., Kim, I.-S., Jeon, S.-B., Moon, D.-K., Hwangbo, C., et al. (2019). A Rho-associated coiled-coil containing kinase inhibitor, Y-27632, improves viability of dissociated single cells, efficiency of colony formation, and cryopreservation in porcine pluripotent stem cells. Cell. Reprogram. 21, 37–50. doi: 10.1089/cell.2018.0020
Bagci, H., Sriskandarajah, N., Robert, A., Boulais, J., Elkholi, I. E., Tran, V., et al. (2019). Mapping the proximity interaction network of the Rho-family GTPases reveals signalling pathways and regulatory mechanisms. Nat. Cell Biol. 22, 120–134. doi: 10.1038/s41556-019-0438-7
Baharvand, H., Salekdeh, G. H., Taei, A., and Mollamohammadi, S. (2010). An efficient and easy-to-use cryopreservation protocol for human ES and iPS cells. Nat. Protocol. 5, 588–594. doi: 10.1038/nprot.2009.247
Barrett, S. L., and Albertini, D. F. (2007). Allocation of gamma-tubulin between oocyte cortex and meiotic spindle influences asymmetric cytokinesis in the mouse oocyte1. Biol. Reprod. 76, 949–957. doi: 10.1095/biolreprod.106.057141
Bennabi, I., Crozet, F., Nikalayevich, E., Chaigne, A., Letort, G., Manil-Ségalen, M., et al. (2020). Artificially decreasing cortical tension generates aneuploidy in mouse oocytes. Nat. Commun. 11:1649.
Chapman, S., Mcdermott, D. H., Shen, K., Jang, M. K., and Mcbride, A. A. (2014). The effect of Rho kinase inhibition on long-term keratinocyte proliferation is rapid and conditional. Stem Cell Res. Ther. 5:60. doi: 10.1186/scrt449
Chevrier, V. R., Piel, M., Collomb, N., Saoudi, Y., Frank, R., Paintrand, M., et al. (2002). The Rho-associated protein kinase p160ROCK is required for centrosome positioning. J. Cell Biol. 157, 807–817. doi: 10.1083/jcb.200203034
Cimadomo, D., Fabozzi, G., Vaiarelli, A., Ubaldi, N., Ubaldi, F. M., and Rienzi, L. (2018). Impact of maternal age on oocyte and embryo competence. Front. Endocrinol. 9:327. doi: 10.3389/fendo.2018.00327
Clayton, L., Hall, A., and Johnson, M. H. (1999). A role for Rho-like GTPases in the polarisation of mouse eight-cell blastomeres. Dev. Biol. 205, 322–331. doi: 10.1006/dbio.1998.9117
Clift, D., and Schuh, M. (2013). Restarting life: fertilization and the transition from meiosis to mitosis. Nat. Rev. Mol. Cell Biol. 14, 549–562. doi: 10.1038/nrm3643
Cook, D. R., Rossman, K. L., and Der, C. J. (2014). Rho guanine nucleotide exchange factors: regulators of Rho GTPase activity in development and disease. Oncogene 33, 4021–4035. doi: 10.1038/onc.2013.362
de Pablo, Y., Marasek, P., Pozo-Rodrigalvarez, A., Wilhelmsson, U., Inagaki, M., Pekna, M., et al. (2019). Vimentin phosphorylation is required for normal cell division of immature astrocytes. Cells 8:1016. doi: 10.3390/cells8091016
Defert, O., and Boland, S. (2017). Rho kinase inhibitors: a patent review (2014 - 2016). Expert Opin. Ther. Pat. 27, 507–515. doi: 10.1080/13543776.2017.1272579
Duan, X., Chen, K.-L., Zhang, Y., Cui, X.-S., Kim, N.-H., and Sun, S.-C. (2014a). ROCK inhibition prevents early mouse embryo development. Histochem. Cell Biol. 142, 227–233. doi: 10.1007/s00418-014-1201-6
Duan, X., Liu, J., Dai, X. X., Liu, H. L., Cui, X. S., Kim, N. H., et al. (2014b). Rho-GTPase effector ROCK phosphorylates cofilin in actin-meditated cytokinesis during mouse oocyte meiosis. Biol. Reprod. 90:37.
Dvorsky, R., Blumenstein, L., Vetter, I. R., and Ahmadian, M. R. (2004). Structural Insights into the interaction of ROCKI with the switch regions of RhoA. J. Biol. Chem. 279, 7098–7104. doi: 10.1074/jbc.m311911200
Feng, Y., Lograsso, P. V., Defert, O., and Li, R. (2015). Rho Kinase (ROCK) Inhibitors and their therapeutic potential. J. Med. Chem. 59, 2269–2300. doi: 10.1021/acs.jmedchem.5b00683
Hahmann, C., and Schroeter, T. (2009). Rho-kinase inhibitors as therapeutics: from pan inhibition to isoform selectivity. Cell. Mol. Life Sci. 67, 171–177. doi: 10.1007/s00018-009-0189-x
Hartmann, S., Ridley, A. J., and Lutz, S. (2015). The function of Rho-associated kinases ROCK1 and ROCK2 in the pathogenesis of cardiovascular disease. Front. Pharmacol. 6:276. doi: 10.3389/fphar.2015.00276
Hickson, G. R. X., Echard, A., and O’farrell, P. H. (2006). Rho-kinase controls cell shape changes during cytokinesis. Curr. Biol. 16, 359–370. doi: 10.1016/j.cub.2005.12.043
Hochi, S., Abdalla, H., Hara, H., Shimoda, M., Morita, H., Kuwayama, M., et al. (2010). Stimulatory effect of Rho-associated coiled-coil kinase (ROCK) inhibitor on revivability of in vitro-produced bovine blastocysts after vitrification. Theriogenology 73, 1139–1145. doi: 10.1016/j.theriogenology.2010.01.015
Honjo, M., and Tanihara, H. (2018). Impact of the clinical use of ROCK inhibitor on the pathogenesis and treatment of glaucoma. Jap. J. Ophthalmol. 62, 109–126. doi: 10.1007/s10384-018-0566-9
Huang, S., Ding, C., Mai, Q., Xu, Y., and Zhou, C. (2016). Inhibition of Rho-associated protein kinase increases the ratio of formation of blastocysts from single human blastomeres. Mol. Med. Rep. 13, 2046–2052. doi: 10.3892/mmr.2016.4766
Hwang, I.-S., Hara, H., Chung, H.-J., Hirabayashi, M., and Hochi, S. (2013). Rescue of vitrified-warmed bovine oocytes with Rho-associated coiled-coil kinase inhibitor. Biol. Reprod. 89:26.
Ishizaki, T., Maekawa, M., Fujisawa, K., Okawa, K., Iwamatsu, A., Fujita, A., et al. (1996). The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 15, 1885–1893. doi: 10.1002/j.1460-2075.1996.tb00539.x
Jaffe, A. B., and Hall, A. (2005). RHO GTPASES: biochemistry and biology. Ann. Rev. Cell Dev. Biol. 21, 247–269.
Joo, H. J., Choi, D.-K., Lim, J. S., Park, J.-S., Lee, S.-H., Song, S., et al. (2012). ROCK suppression promotes differentiation and expansion of endothelial cells from embryonic stem cell–derived Flk1+ mesodermal precursor cells. Blood 120, 2733–2744. doi: 10.1182/blood-2012-04-421610
Kamishibahara, Y., Kawaguchi, H., and Shimizu, N. (2016). Rho kinase inhibitor Y-27632 promotes neuronal differentiation in mouse embryonic stem cells via phosphatidylinositol 3-kinase. Neurosci. Lett. 615, 44–49. doi: 10.1016/j.neulet.2016.01.022
Kang, P. H., Schaffer, D. V., Kumar, S., and Discher, D. (2020). Angiomotin links ROCK and YAP signaling in mechanosensitive differentiation of neural stem cells. Mol. Biol. Cell 31, 386–396. doi: 10.1091/mbc.e19-11-0602
Katoh, K., Kano, Y., Amano, M., Onishi, H., Kaibuchi, K., and Fujiwara, K. (2001). Rho-kinase–mediated contraction of isolated stress fibers. J. Cell Biol. 153, 569–584. doi: 10.1083/jcb.153.3.569
Kawabata, S., Usukura, J., Morone, N., Ito, M., Iwamatsu, A., Kaibuchi, K., et al. (2004). Interaction of Rho-kinase with myosin II at stress fibres. Gene. Cell. 9, 653–660. doi: 10.1111/j.1356-9597.2004.00749.x
Kawagishi, R., Tahara, M., Sawada, K., Ikebuchi, Y., Morishige, K., Sakata, M., et al. (2004). Rho-kinase is involved in mouse blastocyst cavity formation. Biochem. Biophys. Res. Commun. 319, 643–648. doi: 10.1016/j.bbrc.2004.05.040
Kim, K., Gil, M., Dayem, A. A., Choi, S., Kang, G.-H., Yang, G.-M., et al. (2020). Improved Isolation and culture of urine-derived stem cells (USCs) and enhanced production of immune cells from the USC-derived induced pluripotent stem cells. J. Clin. Med. 9:827. doi: 10.3390/jcm9030827
Knipe, R. S., Probst, C. K., Lagares, D., Franklin, A., Spinney, J. J., Brazee, P. L., et al. (2018). The Rho kinase isoforms ROCK1 and ROCK2 each contribute to the development of experimental pulmonary fibrosis. Am. J. Resp. Cell Mol. Biol. 58, 471–481. doi: 10.1165/rcmb.2017-0075oc
Kono, K., Tamashiro, D. A. A., and Alarcon, V. B. (2014). Inhibition of RHO–ROCK signaling enhances ICM and suppresses TE characteristics through activation of hippo signaling in the mouse blastocyst. Dev. Biol. 394, 142–155. doi: 10.1016/j.ydbio.2014.06.023
Krawetz, R. J., Taiani, J., Greene, A., Kelly, G. M., and Rancourt, D. E. (2011). Inhibition of Rho kinase regulates specification of early differentiation events in P19 embryonal carcinoma stem cells. PLoS One 6:e26484. doi: 10.1371/journal.pone.0026484
Kümper, S., Mardakheh, F. K., Mccarthy, A., Yeo, M., Stamp, G. W., Paul, A., et al. (2016). Rho-associated kinase (ROCK) function is essential for cell cycle progression, senescence and tumorigenesis. eLife 5:e12994.
Kurosawa, H. (2012). Application of Rho-associated protein kinase (ROCK) inhibitor to human pluripotent stem cells. J. Biosci. Bioeng. 114, 577–581. doi: 10.1016/j.jbiosc.2012.07.013
Kwon, J., Kim, N.-H., and Choi, I. (2016). ROCK activity regulates functional tight junction assembly during blastocyst formation in porcine parthenogenetic embryos. Peer J. 4:e1914. doi: 10.7717/peerj.1914
Lachowski, D., Cortes, E., Matellan, C., Rice, A., Lee, D. A., Thorpe, S. D., et al. (2020). G protein-coupled estrogen receptor regulates actin cytoskeleton dynamics to impair cell polarization. Front. Cell Dev. Biol. 8:592628. doi: 10.3389/fcell.2020.592628
Laeno, A. M., Tamashiro, D. A., and Alarcon, V. B. (2013). Rho-associated kinase activity is required for proper morphogenesis of the inner cell mass in the mouse blastocyst. Biol. Reprod. 89:122.
Lee, S. R., Xu, Y. N., Jo, Y. J., Namgoong, S., and Kim, N. H. (2015). The Rho-GTPase effector ROCK regulates meiotic maturation of the bovine oocyte via myosin light chain phosphorylation and cofilin phosphorylation. Mol. Reprod. Dev. 82, 849–858. doi: 10.1002/mrd.22524
Leung, T., Chen, X. Q., Manser, E., and Lim, L. (1996). The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol. Cell. Biol. 16, 5313–5327. doi: 10.1128/mcb.16.10.5313
Leung, T., Manser, E., Tan, L., and Lim, L. (1995). A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J. Biol. Chem. 270, 29051–29054. doi: 10.1074/jbc.270.49.29051
Li, W., Zhao, J., Wang, J., Sun, L., Xu, H., Sun, W., et al. (2020). ROCK-TAZ signaling axis regulates mechanical tension-induced osteogenic differentiation of rat cranial sagittal suture mesenchymal stem cells. J. Cell. Physiol. 235, 5972–5984. doi: 10.1002/jcp.29522
Li, Z., Han, S., Wang, X., Han, F., Zhu, X., Zheng, Z., et al. (2015). Rho kinase inhibitor Y-27632 promotes the differentiation of human bone marrow mesenchymal stem cells into keratinocyte-like cells in xeno-free conditioned medium. Stem Cell Res. Ther. 6:17.
Liao, J. K., Seto, M., and Noma, K. (2007). Rho kinase (ROCK) inhibitors. J. Cardiovasc. Pharmacol. 50, 17–24. doi: 10.1097/fjc.0b013e318070d1bd
Liu, J., Wang, Q.-C., Duan, X., Cui, X.-S., Kim, N.-H., Zhang, Y., et al. (2018). Profilin 1 plays feedback role in actin-mediated polar body extrusion in mouse oocytes. Reprod. Fertil. Dev. 30:752. doi: 10.1071/rd17354
Lu, W., Wen, J., and Chen, Z. (2020). Distinct roles of ROCK1 and ROCK2 on the cerebral ischemia injury and subsequently neurodegenerative changes. Pharmacology 105, 3–8. doi: 10.1159/000502914
Ma, Z., Kanai, M., Kawamura, K., Kaibuchi, K., Ye, K., and Fukasawa, K. (2006). Interaction between ROCK II and Nucleophosmin/B23 in the regulation of centrosome duplication. Mol. Cell. Biol. 26, 9016–9034. doi: 10.1128/mcb.01383-06
Matsuyama, M., Tanaka, H., Inoko, A., Goto, H., Yonemura, S., Kobori, K., et al. (2013). Defect of mitotic vimentin phosphorylation causes microophthalmia and cataract via aneuploidy and senescence in lens epithelial cells. J. Biol. Chem. 288, 35626–35635. doi: 10.1074/jbc.m113.514737
Mellott, A. J., Godsey, M. E., Shinogle, H. E., Moore, D. S., Forrest, M. L., and Detamore, M. S. (2014). Improving viability and transfection efficiency with human umbilical cord wharton’s jelly cells through use of a ROCK inhibitor. Cell. Reprogram. 16, 91–97. doi: 10.1089/cell.2013.0069
Miranda-Rodríguez, J. R., Salas-Vidal, E., Lomelí, H., Zurita, M., and Schnabel, D. (2017). RhoA/ROCK pathway activity is essential for the correct localization of the germ plasm mRNAs in zebrafish embryos. Dev. Biol. 421, 27–42. doi: 10.1016/j.ydbio.2016.11.002
Moon, S. Y., and Zheng, Y. (2003). Rho GTPase-activating proteins in cell regulation. Trends. Cell Biol. 13, 13–22. doi: 10.1016/s0962-8924(02)00004-1
Morales-Quinones, M., Ramirez-Perez, F. I., Foote, C. A., Ghiarone, T., Ferreira-Santos, L., Bloksgaard, M., et al. (2020). LIMK (LIM Kinase) inhibition prevents vasoconstriction- and hypertension-induced arterial stiffening and remodeling. Hypertension 76, 393–403. doi: 10.1161/hypertensionaha.120.15203
Morckel, A. R., Lusic, H., Farzana, L., Yoder, J. A., Deiters, A., and Nascone-Yoder, N. M. (2012). A photoactivatable small-molecule inhibitor for light-controlled spatiotemporal regulation of Rho kinase in live embryos. Development 139, 437–442. doi: 10.1242/dev.072165
Mott, H. R., and Owen, D. (2015). Structures of Ras superfamily effector complexes: what have we learnt in two decades? Crit. Rev. Biochem. Mol. Biol. 50, 85–133. doi: 10.3109/10409238.2014.999191
Müller, P. M., Rademacher, J., Bagshaw, R. D., Wortmann, C., Barth, C., Van Unen, J., et al. (2020). Systems analysis of RhoGEF and RhoGAP regulatory proteins reveals spatially organized RAC1 signalling from integrin adhesions. Nat. Cell Biol. 22, 498–511. doi: 10.1038/s41556-020-0488-x
Nakagawa, O., Fujisawa, K., Ishizaki, T., Saito, Y., Nakao, K., and Narumiya, S. (1996). ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 392, 189–193. doi: 10.1016/0014-5793(96)00811-3
Nakamura, K., Yoshimura, A., Kaneko, T., Sato, K., and Hara, Y. (2014). ROCK inhibitor Y-27632 maintains the proliferation of confluent human mesenchymal stem cells. J. Period. Res. 49, 363–370. doi: 10.1111/jre.12114
Namgoong, S., and Kim, N.-H. (2016). Roles of actin binding proteins in mammalian oocyte maturation and beyond. Cell Cycle 15, 1830–1843. doi: 10.1080/15384101.2016.1181239
Newell-Litwa, K. A., Badoual, M., Asmussen, H., Patel, H., Whitmore, L., and Horwitz, A. R. (2015). ROCK1 and 2 differentially regulate actomyosin organization to drive cell and synaptic polarity. J. Cell Biol. 210, 225–242. doi: 10.1083/jcb.201504046
Niringiyumukiza, J. D., Cai, H., and Xiang, W. (2018). Prostaglandin E2 involvement in mammalian female fertility: ovulation, fertilization, embryo development and early implantation. Reprod. Biol. Endocrinol. 16:43.
Ohgushi, M., Matsumura, M., Eiraku, M., Murakami, K., Aramaki, T., Nishiyama, A., et al. (2010). Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell 7, 225–239. doi: 10.1016/j.stem.2010.06.018
Okumura, N., Fujii, K., Kagami, T., Makiko, N., Kitahara, M., Kinoshita, S., et al. (2016). Activation of the Rho/Rho kinase signaling pathway is involved in cell death of corneal endothelium. Invest. Opthalmol. Vis. Sci. 57:6843. doi: 10.1167/iovs.16-20123
Olofsson, B. (1999). Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell. Signal. 11, 545–554. doi: 10.1016/s0898-6568(98)00063-1
Rangel-Mata, F., Mendez-Marquez, R., Martinez-Cadena, G., Lopez-Godinez, J., Nishigaki, T., Darszon, A., et al. (2007). Rho, Rho-kinase, and the actin cytoskeleton regulate the Na+ -H+ exchanger in sea urchin eggs. Biochem. Biophys. Res. Commun. 352, 264–269. doi: 10.1016/j.bbrc.2006.11.015
Rath, N., Morton, J. P., Julian, L., Helbig, L., Kadir, S., Mcghee, E. J., et al. (2016). ROCK signaling promotes collagen remodeling to facilitate invasive pancreatic ductal adenocarcinoma tumor cell growth. EMBO Mol. Med. 9, 198–218. doi: 10.15252/emmm.201606743
Riento, K., and Ridley, A. J. (2003). ROCKs: multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 4, 446–456. doi: 10.1038/nrm1128
Rikitake, Y., Oyama, N., Wang, C.-Y. C., Noma, K., Satoh, M., Kim, H.-H., et al. (2005). Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1 +/− haploinsufficient mice. Circulation 112, 2959–2965. doi: 10.1161/circulationaha.105.584623
Rivera, T., Zhao, Y., Ni, Y., and Wang, J. (2020). Human-induced pluripotent stem cell culture methods under cGMP conditions. Curr. Prot. Stem Cell Biol. 54:e117.
Roeles, J., and Tsiavaliaris, G. (2019). Actin-microtubule interplay coordinates spindle assembly in human oocytes. Nat. Commun. 10:4651.
Saadeldin, I. M., Swelum, A. A.-A., Elsafadi, M., Mahmood, A., Osama, A., Shikshaky, H., et al. (2020). Thermotolerance and plasticity of camel somatic cells exposed to acute and chronic heat stress. J. Adv. Res. 22, 105–118. doi: 10.1016/j.jare.2019.11.009
Santella, L., Limatola, N., and Chun, J. T. (2020). Cellular and molecular aspects of oocyte maturation and fertilization: a perspective from the actin cytoskeleton. Zoological Lett. 6:5.
Schofield, A. V., and Bernard, O. (2013). Rho-associated coiled-coil kinase (ROCK) signaling and disease. Crit. Rev. Biochem. Mol. Biol. 48, 301–316. doi: 10.3109/10409238.2013.786671
Schwartz, M. (2004). Rho signalling at a glance. J. Cell Sci. 117, 5457–5458. doi: 10.1242/jcs.01582
Shi, J., and Wei, L. (2007). Rho kinase in the regulation of cell death and survival. Archiv. Immunol. Therap. Exp. 55, 61–75. doi: 10.1007/s00005-007-0009-7
Shifrin, V., Annand, R. R., Flusberg, D., Mcgonigle, S., Wong, E., Paradise, E., et al. (2005). Effects of SLx-2119, a novel small molecule inhibitor of Rho-associated kinase ROCK (ROK), on growth of human tumor xenografts in nude mice. Proc. Amer. Assoc. Cancer Res. 46:668.
Sin, W. C., Chen, X. Q., Leung, T., and Lim, L. (1998). RhoA-binding kinase alpha translocation is facilitated by the collapse of the vimentin intermediate filament network. Mol. Cell. Biol. 18, 6325–6339. doi: 10.1128/mcb.18.11.6325
Sit, S. T., and Manser, E. (2011). Rho GTPases and their role in organizing the actin cytoskeleton. J. Cell Sci. 124, 679–683. doi: 10.1242/jcs.064964
So, S., Karagozlu, M. Z., Lee, Y., and Kang, E. (2020a). Fasudil increases the establishment of somatic cell nuclear transfer embryonic stem cells in mouse. J. Anim. Reprod. Biotechnol. 35, 21–27. doi: 10.12750/jarb.35.1.21
So, S., Lee, Y., Choi, J., Kang, S., Lee, J.-Y., Hwang, J., et al. (2020b). The Rho-associated kinase inhibitor fasudil can replace Y-27632 for use in human pluripotent stem cell research. PLoS One 15:e0233057. doi: 10.1371/journal.pone.0233057
Son, M.-J., Park, J.-M., Min, S.-H., Park, H.-D., and Koo, D.-B. (2010). Rho-associated kinase is involved in preimplantation development and embryonic compaction in pigs. J. Embryo Trans. 25, 103–110.
Stankova, V., Tsikolia, N., and Viebahn, C. (2014). Rho kinase activity controls directional cell movements during primitive streak formation in the rabbit embryo. Development 142, 92–98. doi: 10.1242/dev.111583
Street, C. A., and Bryan, B. A. (2011). Rho kinase proteins–pleiotropic modulators of cell survival and apoptosis. Anticancer Res. 31, 3645–3657.
Stroeken, P. J. M., Alvarez, B., Rheenen, J. V., Wijnands, Y. M., Geerts, D., Jalink, K., et al. (2006). Integrin cytoplasmic domain-associated protein-1 (ICAP-1) interacts with the ROCK-I kinase at the plasma membrane. J. Cell. Physiol. 208, 620–628. doi: 10.1002/jcp.20699
Sun, S.-C., and Kim, N.-H. (2013). Molecular mechanisms of asymmetric division in oocytes. Microsc. Microanal. 19, 883–897. doi: 10.1017/s1431927613001566
Suzuki, H., Koyama, K.-I., Kabashima, K., Fang, J., and Matsuzaki, M. (2011). Temporary inhibition of germinal vesicle breakdown by rho kinase inhibitor Y-27632 is detrimental to oocyte maturation. J. Mammal. Ova Res. 28, 126–130. doi: 10.1274/jmor.28.126
Tanaka, T., Nishimura, D., Wu, R.-C., Amano, M., Iso, T., Kedes, L., et al. (2006). Nuclear Rho kinase, ROCK2, targets p300 acetyltransferase. J. Biol. Chem. 281, 15320–15329. doi: 10.1074/jbc.m510954200
Thumkeo, D., Keel, J., Ishizaki, T., Hirose, M., Nonomura, K., Oshima, H., et al. (2003). Targeted disruption of the mouse Rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol. Cell. Biol. 23, 5043–5055. doi: 10.1128/mcb.23.14.5043-5055.2003
Tukur, H. A., Aljumaah, R. S., Swelum, A. A.-A., Alowaimer, A. N., Abdelrahman, M., and Saadeldin, I. M. (2020). Effects of short-term inhibition of Rho kinase on dromedary camel oocyte in vitro maturation. Animals 10:750. doi: 10.3390/ani10050750
Vernardis, S. I., Terzoudis, K., Panoskaltsis, N., and Mantalaris, A. (2017). Human embryonic and induced pluripotent stem cells maintain phenotype but alter their metabolism after exposure to ROCK inhibitor. Sci. Rep. 7:42138.
Wakabayashi, S., Morihara, H., Yokoe, S., Nakagawa, T., Moriwaki, K., Tomoda, K., et al. (2019). Overexpression of Na+/H+ exchanger 1 specifically induces cell death in human iPS cells via sustained activation of the Rho kinase ROCK. J. Biol. Chem. 294, 19577–19588. doi: 10.1074/jbc.ra119.010329
Wang, H., Guo, J., Lin, Z., Namgoong, S., Oh, J. S., and Kim, N. H. (2017). Filamin A is required for spindle migration and asymmetric division in mouse oocytes. FASEB J. 31, 3677–3688. doi: 10.1096/fj.201700056r
Wang, P., Yang, Y., Shao, Q., and Zhou, W. (2015). Selective inhibition of ROCK kinase isoforms to promote neuroregeneration after brain surgery. Med. Chem. Res. 25, 40–50. doi: 10.1007/s00044-015-1463-0
Watanabe, K., Ueno, M., Kamiya, D., Nishiyama, A., Matsumura, M., Wataya, T., et al. (2007). A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 25, 681–686. doi: 10.1038/nbt1310
Wei, L., Surma, M., Shi, S., Lambert-Cheatham, N., and Shi, J. (2016). Novel insights into the roles of Rho Kinase in cancer. Arch. Immunol. Ther. Exp. 64, 259–278. doi: 10.1007/s00005-015-0382-6
Yang, S., Xin, C., Zhang, B., Zhang, H., and Hao, Y. (2020). Synergistic effects of Rho kinase inhibitor Y-27632 and Noggin overexpression on the proliferation and neuron-like cell differentiation of stem cells derived from human exfoliated deciduous teeth. IUBMB Life 72, 665–676. doi: 10.1002/iub.2208
Ye, Y., Wang, X., Ma, C., Chen, X., Liang, H., and Zhang, W. (2020). Transporting ESCs in FBS at ambient temperature. Stem Cell Res. 49:102009. doi: 10.1016/j.scr.2020.102009
Yodoi, R., Tamba, S., Morimoto, K., Segi-Nishida, E., Nishihara, M., Ichikawa, A., et al. (2009). RhoA/Rho kinase signaling in the cumulus mediates extracellular matrix assembly. Endocrinology 150, 3345–3352. doi: 10.1210/en.2008-1449
Zanin-Zhorov, A., Flynn, R., Waksal, S. D., and Blazar, B. R. (2016). Isoform-specific targeting of ROCK proteins in immune cells. Small GTPases 7, 173–177. doi: 10.1080/21541248.2016.1181698
Zhang, C., Chen, F., Liu, X., Han, X., Hu, Y., Su, X., et al. (2019). Gliotoxin induces cofilin phosphorylation to promote actin cytoskeleton dynamics and internalization of aspergillus fumigatus into type II human pneumocyte cells. Front. Microbiol. 10:1345. doi: 10.3389/fmicb.2019.01345
Zhang, J., Yang, P. L., and Gray, N. S. (2009). Targeting cancer with small molecule kinase inhibitors. Nat. Rev. Cancer 9, 28–39. doi: 10.1038/nrc2559
Zhang, J. Y., Dong, H. S., Oqani, R. K., Lin, T., Kang, J. W., and Jin, D. I. (2014). Distinct roles of ROCK1 and ROCK2 during development of porcine preimplantation embryos. Reproduction 148, 99–107. doi: 10.1530/rep-13-0556
Zhang, P., Wu, X., Hu, C., Wang, P., and Li, X. (2011). Rho kinase inhibitor Y-27632 and accutase dramatically increase mouse embryonic stem cell derivation. In Vitro Cell. Dev. Biol. Anim. 48, 30–36. doi: 10.1007/s11626-011-9471-y
Zhou, Y. (2018). Most good, least harm: isoform-specific targeting of ROCK in pulmonary fibrosis. Am. J. Resp. Cell Mol. Biol. 58, 421–422. doi: 10.1165/rcmb.2017-0426ed
Zhu, M., Liu, P. Y., Kasahara, D. I., Williams, A. S., Verbout, N. G., Halayko, A. J., et al. (2011). Role of Rho kinase isoforms in murine allergic airway responses. Eur. Resp. J. 38, 841–850. doi: 10.1183/09031936.00125010
Keywords: rho kinase, oocyte, stem cells, differentiation, actin
Citation: Saadeldin IM, Tukur HA, Aljumaah RS and Sindi RA (2021) Rocking the Boat: The Decisive Roles of Rho Kinases During Oocyte, Blastocyst, and Stem Cell Development. Front. Cell Dev. Biol. 8:616762. doi: 10.3389/fcell.2020.616762
Received: 13 October 2020; Accepted: 07 December 2020;
Published: 11 January 2021.
Edited by:
Andrea Erika Münsterberg, University of East Anglia, United KingdomReviewed by:
Sachin Kotak, Indian Institute of Science (IISc), IndiaJames C.-G. Hombría, Andalusian Center for Development Biology (CABD), Spain
Copyright © 2021 Saadeldin, Tukur, Aljumaah and Sindi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Islam M. Saadeldin, aXNhYWRlbGRpbkBrc3UuZWR1LnNh
 Islam M. Saadeldin
Islam M. Saadeldin Hammed A. Tukur
Hammed A. Tukur Riyadh S. Aljumaah
Riyadh S. Aljumaah Ramya A. Sindi
Ramya A. Sindi