- Department of Urology, Tulane University Health Sciences Center, New Orleans, LA, United States
Telomere shortening is considered as a marker of cellular senescence and it is regulated by various signaling pathways. Sperm telomere appears to play important role in its longevity and function. Antioxidant intake has been known to prevent the shortening of telomere. In the management of male infertility, antioxidants are commonly used to counterbalance the seminal oxidative stress. It is important to understand how antioxidants treatment may modulate telomere signaling in sperm. In the current study, we have identified 377 sperm proteins regulated by antioxidants based on data mining of published literature. Bioinformatic analysis revealed involvement of 399 upstream regulators and 806 master regulators associated with differentially expressed sperm proteins. Furthermore, upstream regulator analysis indicated activation of kinases (EGFR and MAPK3) and transcription factors (CCNE1, H2AX, MYC, RB1, and TP53). Hence, it is evident that antioxidant supplementation activates molecules associated with telomere function in sperm. The outcome of this in silico study suggests that antioxidant therapy has beneficial effects on certain transcription factors and kinases associated with sperm telomere maintenance and associated signaling pathways that may play an important role in the management of male factor infertility.
Introduction
Telomere length (structures with non-coding hexanucleotide “TTAGGG” repeats) at the end of each chromosome determines its stability and genomic integrity. In human somatic (diploid) cells, telomere length is about 5 to 15 kb (Cross et al., 1989), whereas in germ cells (haploid) it is 10–15 kb (Samassekou et al., 2010; Ozturk, 2015). Telomere protects the chromosomal DNA from damage and is considered as a marker of cellular senescence (Bernadotte et al., 2016). Thus, telomere length maintenance is essential for normal cellular processes. Any abnormality in telomere length has been linked to age-related diseases as well as cancer (Stanley and Armanios, 2015).
In general, decrease in telomere length or telomere shortening adversely affects the functional characteristics of chromosomal DNA. Limited number of studies have focused on the role of sperm telomeres in reproduction and male infertility (Santana et al., 2019; Tahamtan et al., 2019; Darmishonnejad et al., 2020), of which few suggest that sperm telomere length (STL) is associated with sperm quality and DNA integrity (Ferlin et al., 2013; Cariati et al., 2016; Rocca et al., 2016; Darmishonnejad et al., 2020). Telomeres are highly rich in guanine and susceptible to oxidative damage (Coluzzi et al., 2014). In vitro studies suggest that oxidative stress accelerates telomere shortening and disrupts telomerase activity (Epel et al., 2004; Richter and von Zglinicki, 2007).
Increased oxidative stress associated with leukocytospermia is one of the prominent causes of male infertility and has deleterious effect on sperm DNA (Agarwal et al., 2019b). Moreover, sperm with poor chromatin protamination status are vulnerable to such oxidative attack (De Iuliis et al., 2009). Rocca et al. (2016) reported that protamination status of sperm chromatin is linked with STL (Rocca et al., 2016). Hence, defective chromatin packaging can increase the exposure of DNA to reactive oxygen species (ROS) resulting in telomere dysregulation in mature sperm.
Antioxidants that counterbalance the increased levels of seminal ROS are widely used in the management of oxidative stress-mediated male infertility (Agarwal et al., 2021a,b). Use of antioxidants in treatment of male infertility have shown to improve semen parameters (Keskes-Ammar et al., 2003; Smits et al., 2019; Arafa et al., 2020). Furthermore, antioxidant supplementation activates the molecular mechanism(s) associated with free radical scavenging in idiopathic infertile men and has positive beneficial effect on fertility associated sperm proteins (Agarwal et al., 2019a). In a cross-sectional study of children and adolescents, dietary antioxidants have been reported to reduce shortening of leukocyte telomere length (García-Calzón et al., 2015). However, the role of antioxidants in modulating sperm telomere signaling and maintenance is unknown. Therefore, the aim of this study is to review and conduct in silico analysis of omics data of sperm in patients subjected to antioxidant treatment to understand the effect of antioxidants on pathways involved in regulating STL in infertile men.
Methods
A comprehensive literature search was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The articles were retrieved (Figure 1) from PubMed database on July 4, 2021 using the following string of keywords “(antioxidant∗ and sperm∗ and male infertility) and (proteomic∗ OR genomic∗ OR transcriptomic∗)”. Preliminary screening was carried out based on the following inclusion criteria: (a) studies conducted in humans, (b) involved antioxidant supplementation/treatment, and (c) reported laboratory evaluation of male infertility. Reviews, meta-analysis and studies not reporting clinical data were excluded. After preliminary screening, all the original studies were evaluated based on PICO (Population, Intervention, Control, and Outcome) guidelines (Supplementary Table 1).
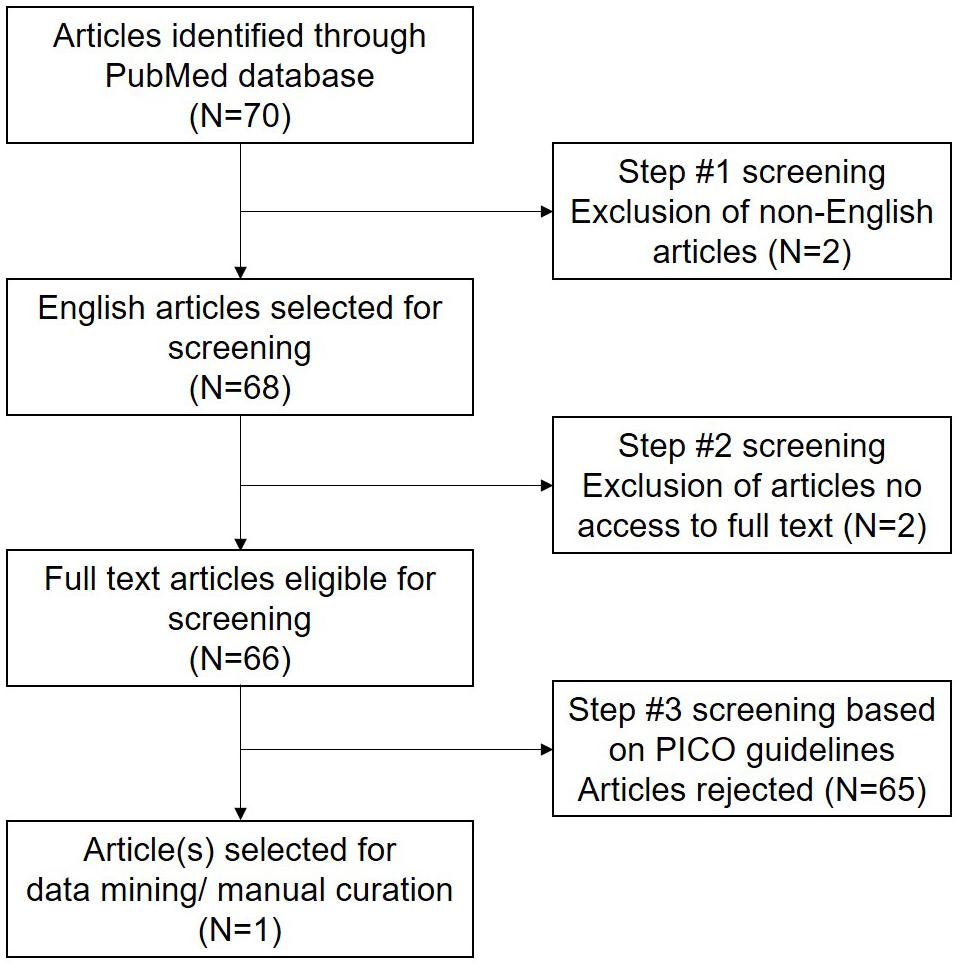
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) workflow reporting the literature search strategy.
Extensive data mining was carried out based on computational and manual approaches. The article (n = 1) in compliance with PICO guidelines was thoroughly searched for differentially expressed biomolecules reported in spermatozoa of infertile men. These annotated and curated biomolecules list containing gene/protein symbols with their respective expression values were saved as Microsoft Excel file. For further downstream analysis, this list was uploaded to ingenuity pathway analysis (IPA) software. Initially core analysis was conducted, and then casual network analysis was carried out to identify antioxidant activated kinases and transcription factors in sperm (Krämer et al., 2014). In-depth analysis was performed to identify those activated kinases and transcription factors that were either directly involved or linked with the molecules regulating telomere signaling pathway. Molecular Interaction Search Tool (MIST) was used to display interaction between the transcription factors and kinases associated with telomere signaling and maintenance pathway (Hu et al., 2018).
Results and Discussion
Antioxidants are widely used in the treatment of male infertility. A recent global survey reported that 85.6% of physicians involved in the management of male infertility prescribe antioxidants as a part of their treatment regime (Agarwal et al., 2021a). Apart from improving the semen parameters, antioxidant intake increases the sperm DNA integrity without any side effects/complications (Zini et al., 2009; Majzoub et al., 2017; Arafa et al., 2020). Besides these benefits, antioxidants can delay the reduction of telomere length of somatic cells (Prasad et al., 2017). At subcellular level, antioxidants modulate proteins associated with CREM (cAMP responsive element modulator) signaling, mitochondrial function and protein oxidation (Agarwal et al., 2019a). They are also reported to activate antioxidant defense mechanism in sperm (Agarwal et al., 2019a). It is essential to understand the effect of antioxidant supplementation on mechanisms/pathways associated with sperm telomere. In the current study, we have used data mining and manual curation techniques to identify the molecules (sperm proteins) altered post-antioxidant treatment. For the first time, using an in silico approach this study sheds light on the beneficial role of antioxidants in regulating telomere signaling and maintenance pathways of sperm.
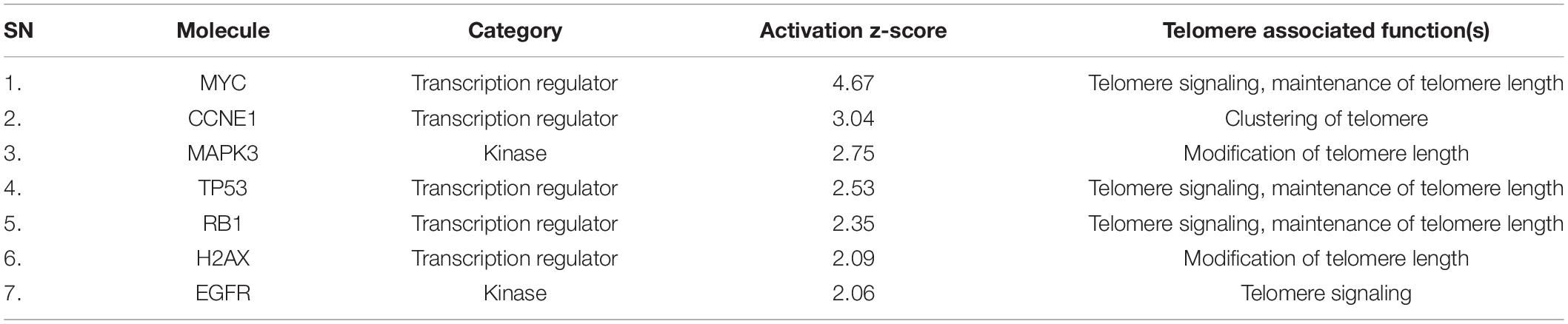
Availability of different data mining strategies and accessibility to omics data made the researchers to reinvestigate the curated data with bioinformatic tools (Zhang and Chen, 2011; Alanis-Lobato, 2015). Such analysis led to the discovery of several existing and missing pathways linked to human diseases (Fechete et al., 2011; Narasimhan et al., 2014; Kharrat et al., 2019). Kothandaraman et al. (2016) used the data mining technique to identify genes associated with pathogenesis of idiopathic male infertility (Kothandaraman et al., 2016). In the current study, data mining and manual curation resulted in identification of 377 differentially expressed proteins in sperm following antioxidant therapy (Supplementary Table 2). Upstream regulator analysis (URA) revealed a total of 399 and 806 upstream regulators and master regulators, respectively. Upstream regulator analysis is an unique feature available in IPA to identify upstream regulators associated with differentially expressed genes/proteins (Li et al., 2015). Sperm proteomic studies have employed URA to identify regulatory molecules associated with reproductive function (Agarwal et al., 2019a; Panner Selvam et al., 2019). Figure 2 shows the distribution of 73 upstream regulators and 338 master regulators either activated (Z-score ≥ 2) or inhibited (Z-score ≤ –2) in our dataset. It is important to emphasize that none of the inhibited regulators were found to be involved in telomere function. Therefore, it clearly indicates that antioxidant supplementation has no negative effect on STL.
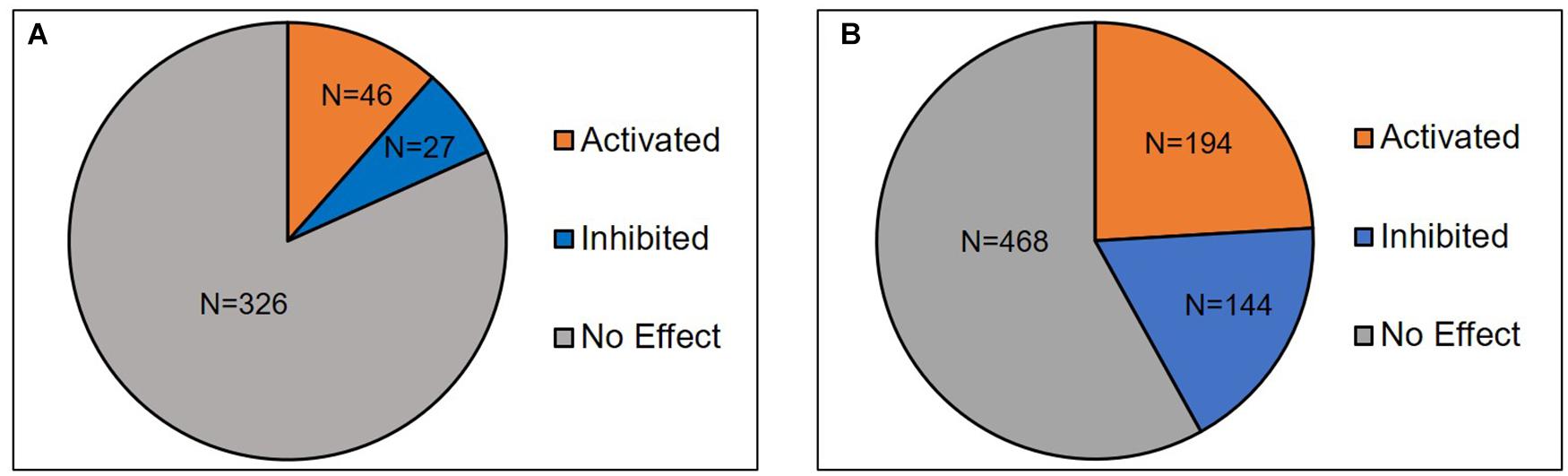
Figure 2. Distribution of (A) upstream regulators (n = 399) and (B) master regulators (n = 806) identified in the sperm post-antioxidant therapy.
In-depth analysis revealed activation of kinases (EGFR: epidermal growth factor receptor and MAPK3: mitogen-activated protein kinase 3) associated with telomere function (Table 1). Epidermal growth factor receptor signaling pathway plays a pivotal role in regulation of telomere length via inhibiting telomerase activity (Maida et al., 2002; Tian et al., 2002; Augustine et al., 2017), whereas MAPK3/ERK2 pathway regulates telomeric repeat-binding factor 2 (TRF-2) to maintain telomere stability in a cell (Picco et al., 2016). In addition to kinases, using computational analysis we have also identified transcription factors (CCNE1: cyclin E1, H2AX: H2A.X variant histone, MYC: MYC proto-oncogene, RB1: RB transcriptional corepressor 1 and TP53: tumor protein p53) linked to the maintenance of telomere in sperm (Table 1). CCNE1 is mainly responsible for telomere stability (Martinerie et al., 2014), while absence of H2AX is linked to genomic instability (Celeste et al., 2002; Fernandez-Capetillo et al., 2003). Similarly, MYC regulates telomerase (Wang et al., 1998), particularly c-MYC interacts with TRF1/PIN2 (proteinase Inhibitor 2) leading to extension of telomere repeats (Kim and Chen, 2007).
Expression of RB1 proteins controls telomere length (García-Cao et al., 2002), while TP53 directly binds with chromosomal DNA and increases the stability of telomere (Tutton and Lieberman, 2017). Altered expression of these kinases and transcription factors may contribute toward telomere dysfunction in sperm of infertile men. Furthermore, MIST analysis displayed the interaction type (protein-protein or genetic) between the molecules (EGFR, MAPK3, CCNE1, H2AX, MYC, RB1, and TP53) and their abundance in the testis (Figure 3). New findings of this study clearly show that antioxidant supplementation activates the transcription regulators and kinases involved in sperm telomere signaling and maintenance pathway that may improve their longevity and function. Future clinical trials evaluating the STL post-antioxidant supplementation are warranted in infertile men to confirm its role in maintaining telomere integrity and sperm function. Such studies may provide more insight on the use of STL as a new prognostic or therapeutic marker of antioxidant effectiveness in the management of male infertility.
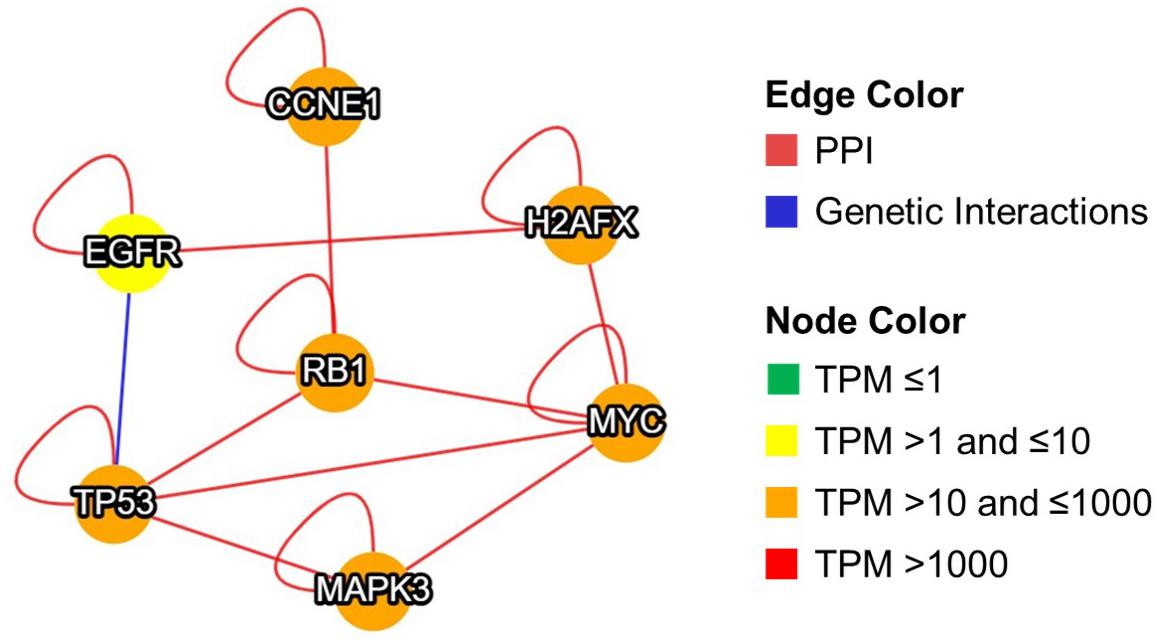
Figure 3. Interaction between transcription regulators and kinases involved in telomere signaling and maintenance pathway in sperm after antioxidant treatment. Graphical representation of network developed using MIST. PPI: protein-protein interaction, TPM: transcripts per million, MYC: MYC proto-oncogene, CCNE1: cyclin E1, MAPK3: mitogen-activated protein kinase 3, TP53: tumor protein p53, RB1: RB transcriptional corepressor 1, H2AX/H2AFX: H2A.X variant histone, EGFR: epidermal growth factor receptor.
Conclusion
For the first time, using bioinformatic approach, our results demonstrate that antioxidant therapy has positive effect on transcription factors and kinases associated with telomere function in sperm. Altered expression of EGFR, MAPK3, CCNE1, H2AX, MYC, RB1, and TP53 can serve as biomarkers for telomere dysfunction in sperm of infertile men, and opens new approaches to target improved therapies.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
MP conceived the idea and study design and conducted bioinformatic analysis. MP, SB, and SS wrote this article, reviewed, and approved the submitted version. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are thankful to Department of Urology, Tulane University School of Medicine for supporting this research.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2021.768510/full#supplementary-material
References
Agarwal, A., Finelli, R., Selvam, M. K. P., Leisegang, K., Majzoub, A., Tadros, N., et al. (2021a). A Global Survey of Reproductive Specialists to Determine the Clinical Utility of Oxidative Stress Testing and Antioxidant Use in Male Infertility. World J. Mens Health 39, 470–488. doi: 10.5534/wjmh.210025
Agarwal, A., Leisegang, K., Majzoub, A., Henkel, R., Finelli, R., Panner Selvam, M. K., et al. (2021b). “Utility of Antioxidants in the Treatment of Male Infertility: Clinical Guidelines Based on a Systematic Review and Analysis of Evidence.”. World J. Mens Health 39, 233–290. doi: 10.5534/wjmh.200196
Agarwal, A., Parekh, N., Panner Selvam, M. K., Henkel, R., Shah, R., Homa, S. T., et al. (2019b). Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Mens Health 37, 296–312. doi: 10.5534/wjmh.190055
Agarwal, A., Panner Selvam, M. K., Samanta, L., Vij, S. C., Parekh, N., Sabanegh, E., et al. (2019a). Effect of Antioxidant Supplementation on the Sperm Proteome of Idiopathic Infertile Men. Antioxidants 8:antiox8100488. doi: 10.3390/antiox8100488
Alanis-Lobato, G. (2015). “Mining protein interactomes to improve their reliability and support the advancement of network medicine.”. Front. Genet. 6:296. doi: 10.3389/fgene.2015.00296
Arafa, M., Agarwal, A., Majzoub, A., Panner Selvam, M. K., Baskaran, S., Henkel, R., et al. (2020). Efficacy of Antioxidant Supplementation on Conventional and Advanced Sperm Function Tests in Patients with Idiopathic Male Infertility. Antioxidants 9:antiox9030219. doi: 10.3390/antiox9030219
Augustine, T., Maitra, R., and Goel, S. (2017). “Telomere length regulation through epidermal growth factor receptor signaling in cancer.”. Genes Cancer 8, 550–558. doi: 10.18632/genesandcancer.140
Bernadotte, A., Mikhelson, V. M., and Spivak, I. M. (2016). “Markers of cellular senescence. Telomere shortening as a marker of cellular senescence.”. Aging 8, 3–11. doi: 10.18632/aging.100871
Cariati, F., Jaroudi, S., Alfarawati, S., Raberi, A., Alviggi, C., Pivonello, R., et al. (2016). “Investigation of sperm telomere length as a potential marker of paternal genome integrity and semen quality.”. Reprod. Biomed. Online 33, 404–411. doi: 10.1016/j.rbmo.2016.06.006
Celeste, A., Petersen, S., Romanienko, P. J., Fernandez-Capetillo, O., Chen, H. T., Sedelnikova, O. A., et al. (2002). “Genomic instability in mice lacking histone H2AX.”. Science 296, 922–927. doi: 10.1126/science.1069398
Coluzzi, E., Colamartino, M., Cozzi, R., Leone, S., Meneghini, C., O’Callaghan, N., et al. (2014). “Oxidative stress induces persistent telomeric DNA damage responsible for nuclear morphology change in mammalian cells.”. PLoS One 9:e110963. doi: 10.1371/journal.pone.0110963
Cross, S. H., Robin, C., Allshire, Stewart, J., and McKay et al. (1989). “Cloning of human telomeres by complementation in yeast.”. Nature 338, 771–774.
Darmishonnejad, Z., Zarei-Kheirabadi, F., Tavalaee, M., Zarei-Kheirabadi, M., Zohrabi, D., and Nasr-Esfahani, M. H. (2020). “Relationship between sperm telomere length and sperm quality in infertile men.”. Andrologia 52:e13546. doi: 10.1111/and.13546
De Iuliis, G. N., Thomson, L. K., Mitchell, L. A., Finnie, J. M., Koppers, A. J., Hedges, A., et al. (2009). “DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-2’-deoxyguanosine, a marker of oxidative stress.”. Biol. Reprod. 81, 517–524. doi: 10.1095/biolreprod.109.076836
Epel, E. S., Blackburn, E. H., Lin, J., Dhabhar, F. S., Adler, N. E., Morrow, J. D., et al. (2004). “Accelerated telomere shortening in response to life stress.”. Proc. Natl. Acad. Sci. U S A. 101, 17312–17315. doi: 10.1073/pnas.0407162101
Fechete, R., Heinzel, A., Perco, P., Mönks, K., Söllner, J., Stelzer, G., et al. (2011). “Mapping of molecular pathways, biomarkers and drug targets for diabetic nephropathy.“. Proteomics Clin. Appl. 5, 354–366. doi: 10.1002/prca.201000136
Ferlin, A., Rampazzo, E., Rocca, M. S., Keppel, S., Frigo, A. C., De Rossi, A., et al. (2013). “In young men sperm telomere length is related to sperm number and parental age.”. Hum. Reprod. 28, 3370–3376. doi: 10.1093/humrep/det392
Fernandez-Capetillo, O., Liebe, B., Scherthan, H., and Nussenzweig, A. (2003). “H2AX regulates meiotic telomere clustering.”. J. Cell Biol. 163, 15–20. doi: 10.1083/jcb.200305124
García-Calzón, S., Moleres, A., Martínez-González, M. A., Martínez, J. A., Zalba, G., and Marti, A. (2015). “Dietary total antioxidant capacity is associated with leukocyte telomere length in a children and adolescent population.”. Clin. Nutr. 34, 694–699. doi: 10.1016/j.clnu.2014.07.015
García-Cao, M., Gonzalo, S., Dean, D., and Blasco, M. A. (2002). “A role for the Rb family of proteins in controlling telomere length.”. Nat. Genet. 32, 415–419. doi: 10.1038/ng1011
Hu, Y., Vinayagam, A., Nand, A., Comjean, A., Chung, V., Hao, T., et al. (2018). Molecular Interaction Search Tool (MIST): an integrated resource for mining gene and protein interaction data. Nucleic Acids Res. 46, D567–D574. doi: 10.1093/nar/gkx1116
Keskes-Ammar, L., Feki-Chakroun, N., Rebai, T., Sahnoun, Z., Ghozzi, H., Hammami, S., et al. (2003). “Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men.”. Arch. Androl. 49, 83–94. doi: 10.1080/01485010390129269
Kharrat, N., Assidi, M., Abu-Elmagd, M., Pushparaj, P. N., Alkhaldy, A., Arfaoui, L., et al. (2019). Data mining analysis of human gut microbiota links Fusobacterium spp. with colorectal cancer onset. Bioinformation 15, 372–379. doi: 10.6026/97320630015372
Kim, H., and Chen, J. (2007). “c-Myc interacts with TRF1/PIN2 and regulates telomere length.”. Biochem. Biophys. Res. Commun. 362, 842–847. doi: 10.1016/j.bbrc.2007.08.064
Kothandaraman, N., Agarwal, A., Abu-Elmagd, M., and Al-Qahtani, M. H. (2016). “Pathogenic landscape of idiopathic male infertility: new insight towards its regulatory networks.”. NPJ Genom. Med. 1:16023. doi: 10.1038/npjgenmed.2016.23
Krämer, A., Green, J., Pollard, J. Jr., and Tugendreich, S. (2014). Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30, 523–530. doi: 10.1093/bioinformatics/btt703
Li, X., Long, J., He, T., Belshaw, R., and Scott, J. (2015). “Integrated genomic approaches identify major pathways and upstream regulators in late onset Alzheimer’s disease.”. Sci. Rep. 5:12393. doi: 10.1038/srep12393
Maida, Y., Kyo, S., Kanaya, T., Wang, Z., Yatabe, N., Tanaka, M., et al. (2002). “Direct activation of telomerase by EGF through Ets-mediated transactivation of TERT via MAP kinase signaling pathway.”. Oncogene 21, 4071–4079. doi: 10.1038/sj.onc.1205509
Majzoub, A., Agarwal, A., and Esteves, S. C. (2017). “Antioxidants for elevated sperm DNA fragmentation: a mini review.”. Transl. Androl. Urol. 6(Suppl. 4), S649–S653. doi: 10.21037/tau.2017.07.09
Martinerie, L., Manterola, M., Chung, S. S., Panigrahi, S. K., Weisbach, M., Vasileva, A., et al. (2014). “Mammalian E-type cyclins control chromosome pairing, telomere stability and CDK2 localization in male meiosis.”. PLoS Genet. 10:e1004165. doi: 10.1371/journal.pgen.1004165
Narasimhan, K., Govindasamy, M., Gauthaman, K., Kamal, M. A., Abuzenadeh, A. M., Al-Qahtani, M., et al. (2014). “Diabetes of the brain: computational approaches and interventional strategies.”. CNS Neurol. Disord. Drug Targets 13, 408–417. doi: 10.2174/18715273113126660156
Ozturk, S. (2015). “Telomerase activity and telomere length in male germ cells.”. Biol. Reprod. 92:53. doi: 10.1095/biolreprod.114.124008
Panner Selvam, M. K., Agarwal, A., and Pushparaj, P. N. (2019). Altered Molecular Pathways in the Proteome of Cryopreserved Sperm in Testicular Cancer Patients before Treatment. Int. J. Mol. Sci. 20:ijms20030677. doi: 10.3390/ijms20030677
Picco, V., Coste, I., Giraud-Panis, M. J., Renno, T., Gilson, E., and Pagès, G. (2016). “ERK1/2/MAPK pathway-dependent regulation of the telomeric factor TRF2.”. Oncotarget 7, 46615–46627. doi: 10.18632/oncotarget.10316
Prasad, K. N., Wu, M., and Bondy, S. C. (2017). “Telomere shortening during aging: Attenuation by antioxidants and anti-inflammatory agents.”. Mech. Ageing Dev. 164, 61–66. doi: 10.1016/j.mad.2017.04.004
Richter, T., and von Zglinicki, T. (2007). “A continuous correlation between oxidative stress and telomere shortening in fibroblasts.”. Exp. Gerontol. 42, 1039–1042. doi: 10.1016/j.exger.2007.08.005
Rocca, M. S., Speltra, E., Menegazzo, M., Garolla, A., Foresta, C., and Ferlin, A. (2016). “Sperm telomere length as a parameter of sperm quality in normozoospermic men.”. Hum. Reprod. 31, 1158–1163. doi: 10.1093/humrep/dew061
Samassekou, O., Gadji, M., Drouin, R., and Yan, J. (2010). “Sizing the ends: normal length of human telomeres.”. Ann. Anat. 192, 284–291. doi: 10.1016/j.aanat.2010.07.005
Santana, V. P., Miranda-Furtado, C. L., Pedroso, D. C. C., Eiras, M. C., Vasconcelos, M. A. C., and Ramos, E. S. (2019). The relationship among sperm global DNA methylation, telomere length, and DNA fragmentation in varicocele: a cross-sectional study of 20 cases. Syst. Biol. Reprod. Med. 65, 95–104. doi: 10.1080/19396368.2018.1557762
Smits, R. M., Mackenzie-Proctor, R., Yazdani, A., Stankiewicz, M. T., Jordan, V., and Showell, M. G. (2019). “Antioxidants for male subfertility.”. Cochrane Database Syst. Rev. 3:Cd007411. doi: 10.1002/14651858.CD007411.pub4
Stanley, S. E., and Armanios, M. (2015). “The short and long telomere syndromes: paired paradigms for molecular medicine.”. Curr. Opin. Genet. Dev. 33, 1–9. doi: 10.1016/j.gde.2015.06.004
Tahamtan, S., Tavalaee, M., Izadi, T., Barikrow, N., Zakeri, Z., Lockshin, R. A., et al. (2019). “Reduced sperm telomere length in individuals with varicocele is associated with reduced genomic integrity.”. Sci. Rep. 9:4336. doi: 10.1038/s41598-019-40707-2
Tian, X. X., Pang, J. C., Zheng, J., Chen, J., To, S. S., and Ng, H. K. (2002). “Antisense epidermal growth factor receptor RNA transfection in human glioblastoma cells down-regulates telomerase activity and telomere length.”. Br. J. Cancer 86, 1328–1332. doi: 10.1038/sj.bjc.6600244
Tutton, S., and Lieberman, P. M. (2017). “A role for p53 in telomere protection.”. Mol. Cell Oncol. 4:e1143078. doi: 10.1080/23723556.2016.1143078
Wang, J., Xie, L. Y., Allan, S., Beach, D., and Hannon, G. J. (1998). “Myc activates telomerase.”. Genes Dev. 12, 1769–1774. doi: 10.1101/gad.12.12.1769
Zhang, F., and Chen, J. Y. (2011). “Data mining methods in Omics-based biomarker discovery.”. Methods Mol. Biol. 719, 511–526. doi: 10.1007/978-1-61779-027-0_24
Keywords: antioxidants, bioinformatics, data mining, male infertility, sperm telomere, upstream regulators
Citation: Panner Selvam MK, Baskaran S and Sikka SC (2021) Telomere Signaling and Maintenance Pathways in Spermatozoa of Infertile Men Treated With Antioxidants: An in silico Approach Using Bioinformatic Analysis. Front. Cell Dev. Biol. 9:768510. doi: 10.3389/fcell.2021.768510
Received: 31 August 2021; Accepted: 23 September 2021;
Published: 11 October 2021.
Edited by:
Souvik Dey, Jadavpur University, IndiaReviewed by:
Ahmed T. Alahmar, University of Babylon, IraqArlindo A. Moura, Federal University of Ceara, Brazil
Copyright © 2021 Panner Selvam, Baskaran and Sikka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manesh Kumar Panner Selvam, bXBhbm5lcnNlbHZhbUB0dWxhbmUuZWR1; Suresh C. Sikka, c3Npa2thQHR1bGFuZS5lZHU=
 Manesh Kumar Panner Selvam
Manesh Kumar Panner Selvam Saradha Baskaran
Saradha Baskaran