- 1Dipartimento di Medicina Sperimentale, Sez. Fisiologia Umana e Funzioni Biologiche Integrate “F. Bottazzi”, Università degli Studi della Campania “Luigi Vanvitelli”, Naples, Italy
- 2Laboratoire LR11ES41 Génétique Biodiversité et Valorisation des Bio-ressourcés, Institut Supérieur de Biotechnologie de Monastir, Université de Monastir, Monastir, Tunisia
This paper confirms the damaging effects produced by MP and Cd on testicular activity in the rat. Oral treatment with both chemicals resulted in testicular damage, documented by biomolecular and histological alterations, particularly by impaired morphometric parameters, increased apoptosis, reduced testosterone synthesis, and downregulation of the steroidogenic enzyme 3β-HSD. We also demonstrated, for the first time, that both MP and Cd can affect the protein level of PTMA, a small peptide that regulates germ cell proliferation and differentiation. Interestingly, the cytoarchitecture of testicular cells was also altered by the treatments, as evidenced by the impaired expression and localization of DAAM1 and PREP, two proteins involved in actin- and microtubule-associated processes, respectively, during germ cells differentiation into spermatozoa, impairing normal spermatogenesis. Finally, we showed that the effect of simultaneous treatment with MP and Cd were more severe than those produced by MP alone and less harmful than those of Cd alone. This could be due to the different ways of exposure of the two substances to rats (in drinking water for Cd and in oral gavage for MP), since being the first contact in the animals’ gastrointestinal tract, MP can adsorb Cd, reducing its bioavailability through the Trojan-horse effect.
1 Introduction
Reproductive activity is one of the main attributes of living beings, as it is fundamental to species survival and genetic variability. To successfully achieve it, the production of good quality gametes, capable of fertilizing or being fertilized, is of primary importance (Serrano et al., 2022). Male gamete differentiation occurs during spermatogenesis, a genetic and molecular program during which immature GC undergo several stages of cell division (mitotic and meiotic) and then, by intimate biochemical and morphological modification, to produce mature SPZ (Dunleavy et al., 2019). The entire progression is regulated not only by a sophisticated network of autocrine/paracrine/endocrine factors, but also by modulating the expression of several exclusive testicular genes (Venditti et al., 2020a; Miyaso et al., 2022).
With the aim of expanding knowledge about the mechanisms of spermatogenesis and human reproduction, we have previously characterized some proteins, such as PTMA (Venditti and Minucci, 2017; Minucci and Venditti, 2022; Venditti et al., 2022), and two cytoskeleton-related proteins, DAAM1 (Minucci and Venditti, 2022; Pariante et al., 2016a; Venditti et al., 2020b; c; Venditti et al., 2021a) and PREP (Santillo et al., 2019; Venditti and Minucci, 2019; Venditti et al., 2020b; Venditti et al., 2021a; Minucci and Venditti, 2022), in the testis, suggesting their involvement in the proliferative and differentiative stages of gametogenesis. In particular, PTMA, one of the most acidic mammalian polypeptides (3.5 isoelectric point), is an anti-apoptotic factor associated with post-meiotic GC progression and SPZ interaction with the oocyte (Venditti and Minucci, 2017). DAAM1 is a formin that promotes the nucleation of unbranched actin filaments (Pariante et al., 2016a; Venditti et al., 2018; Venditti et al., 2020b; Venditti et al., 2020c; Venditti et al., 2021a; Minucci and Venditti, 2022), while PREP is a serine protease associated with microtubules (Santillo et al., 2019; Venditti et al., 2019; Venditti and Minucci, 2019; Venditti et al., 2020b; Venditti et al., 2021a; Minucci and Venditti, 2022).
It is well known that since spermatogenesis is a very complex mechanism, even the slightest “mistake” can cause problems in its proper functioning, resulting in reduced sperm quality, and thus infertility (Vander Borght and Wyns, 2018). Indeed, many studies have described a progressive decline in fertility rates over the past 50 years, ranging from the 7%–8% observed in the early 1960s to the 15% we are witnessing today (Levine et al., 2017; Levine et al., 2022). Although in many cases, the causes of male infertility can be attributed to well-known factors (genetic abnormalities, lifestyle), for most cases the etiology is still unknown (idiopathic infertility) (Serrano et al., 2022). It should be considered that, among the factors affecting sperm quality, exposure to environmental pollutants is undoubtedly one of the main causes since, although male infertility is a worldwide health problem, its incidence is higher in more technologically advanced and developed countries (Marić et al., 2021; Selvaraju et al., 2021).
Among the excess of hazardous substances released into the environment, MP can affect human, animal, and plant health. These particles result from the degradation of plastic objects into fragments less 5 mm in size, which can be widely distributed throughout environment compartments. MP can be easily ingested by a variety of organisms, especially in the aquatic environment, entering the food web, therefore, the non-occupational population is inevitably, and often unawares, continuously exposed to MP (EFSA, 2016; Lehner et al., 2019). Scientific knowledge about the health risks posed by MP pollution on wildlife and humans is growing but, at the same time, remains extremely limited, specially when considering critical biological functions, such as reproduction. MP have recently been shown to affect mammalian testicular physiology by inducing inflammation, oxidative stress, impairment of the SE cytoarchitecture and BTB integrity, ultimately, leading to abnormal differentiation of mature gametes (Hou et al., 2021; Jin et al., 2021; 2022; Li et al., 2021; Wei et al., 2021; Ilechukwu et al., 2022; Wen et al., 2022).
Another aspect to be considered is that MP inevitably coexist with other chemicals in the environment and, due to their high surface area/volume ratio and hydrophobicity, can adsorb and transport them, thanks to a phenomenon called the “Trojan horse” effect; this influences their behavior once ingested, altering the uptake of chemicals and their distribution in tissues, with synergistic and/or antagonistic effects (He et al., 2021).
This paper focuses on the effects of MP and/or Cd on several parameters related to rat testicular activity, such as steroidogenesis, apoptosis, and, for a deeper comprehension of their effects, the analysis was also extended to the expression and localization of PTMA, DAAM1 and PREP. Indeed, many works reporting the effects of environmental pollutants on reproductive physiology have shown that one of the main targets of these substances is exactly the cytoskeleton of testicular cells (Wang et al., 2020a; Wang et al., 2020b; Belgacem et al., 2022), and we recently demonstrated that DAAM1 and PREP are specific targets of Cd toxicity (Chemek et al., 2018; Venditti et al., 2020d; 2021b), however; to our knowledge, no paper has studied the impact of MP, alone or given in combination with Cd, on DAAM1 and PREP. Regarding PTMA, we recently showed its significant role, as anti-apoptotic factor, in regulating cell cycle progression occurring during normal and/or pathological cell differentiation of mammalian testis (Venditti et al., 2022); but, to date, no evidence demonstrated whether PTMA may be a target of environmental pollutants, as MP and/or Cd.
Finally, this is because much evidence emphasizes the importance of using in vivo and in vitro models to predict reproductive fitness in humans exposed to environmental pollutants, as well as to better comprehend the mechanisms that regulate cellular and molecular events occurring during spermatogenesis (Li et al., 2016; Gao et al., 2021; Wang et al., 2022a; Wang et al., 2022b).
2 Materials and methods
2.1 Animals, experimental design, and sample collection
Thirty-two-two-months-old male Wistar rats, weighting 222 ± 18.97 g, were housed individually in stainless steel cages under controlled conditions of temperature (22° ± 2°C), light (hours light/dark schedule), and humidity (55% ± 20%). The animals had free access to food and water ad libitum. The rats, randomly divided into four groups (n = 8 each), were treated as follows: 1) control, which received daily oral gavage with ultrapure water; 2) 0.1 mg pristine polystyrene MP-treated (#PS/Q-R-KM491; GmbH, Berlin, Germany); 3) Cd + MP-treated (50 mg CdCl2/L + 0.1 mg MP); 4) Cd-treated (50 mg CdCl2/L in drinking water; Sigma-Aldrich, Milan, Italy). MP solution was prepared by diluting l mg of MP in 5 mL of MiliporeMili-Q water and processed by ultrasonic vibration, 0.5 mL of the resulting solution was administered by oral gavage once daily (0.1 mg/day corresponding to 1.5 × 106 particles/day). The concentration of MP was chosen in accordance with Deng et al. (2017); Haddadi et al. (2022), while that used for Cd was the same as previously utilized in our studies (Chemek et al., 2018; Venditti et al., 2020d; Venditti et al., 2021b; Venditti et al., 2021c). The two separate exposure modes (drinking water for Cd and oral gavage for MP) were chosen to avoid early adsorption of Cd by MP.
Animals were treated for 30 days and weighted every 5 days. At 31st day, blood was obtained by cardiac puncture in heparinized tubes, then the animals were euthanized with 4% chloral hydrate (i.p. 10 ml/kg). Blood was centrifuged at 3,500 rpm for 15 min at 4°C, and plasma was collected and stored at −80°C for T assay. From each rat, the left testis was immersed in 10% formol buffer for histological studies, and the right testis was stored at −80°C for biomolecular studies. The rats were housed in accordance with the EEC 609/86 Directives regulating the welfare of experimental animals. The experimental protocol was approved by the ethics committee of Institute of Biotechnology, University of Monastir, (Ref: CER-SVS/ISBM022/2020).
2.2 Serum T concentration
Serum T level was quantified using the ELISA kit for T (#DE1559; Demeditec Diagnostics GmbH). The detection limit for T was defined at 0.083 ng/ml, and the optical density was read at 450 nm. An ELISA reader (#RT-2100C; Rayto Life and Analytical Sciences Co., Shenzhen, China) automatically calculated the concentration.
2.3 Histology and TUNEL assay
Fixed testes were dehydrated in increasing concentrations of ethanol before paraffin embedding. Serial sections five-μm thick were stained with hematoxylin/eosin. For histopathological evaluation, 30 seminiferous tubules/animal, for a total of 240 tubules per group, were counted under light microscope (Leica DM 2500, Leica Microsystems, Wetzlar, Germany). Photographs were taken using the Leica DFC320 R2 digital Camera (Leica Microsystems, Wetzlar, Germany).
Apoptotic cells were investigated by TUNEL-assay using the DeadEnd™ Fluorometric TUNEL System (#G3250; Promega Corp., Madison, WI, United States) following manufacturer’s protocol. The sections were then counterstained with mounting medium with DAPI (#ab104139; Abcam, Cambridge, United Kingdom) to mark the cell nuclei. The sections were observed and acquired with the optical microscope (Leica DM 5000 B + CTR 5000, Leica Microsystems, Wetzlar, Germany) with UV lamp and saved with IM 1000 software. For the count of the percentage of TUNEL positive cells, 30 fields/samples were considered and analyzed, for a total of 240 per group.
2.4 RT-PCR analysis
Total RNA was extracted from testicular samples using RNA-Xpress Reagent (#MB601; HiMedia Laboratories GmbH; Einhausen, Germany) and processed as described in Venditti et al. (2021b). For details on the used primers, see Supplementary Table S1. The relative amount of the Daam1 and Prep mRNAs were calculated from the values of Daam1/Act and Prep/Act ratio values and represented as OD units. All the RT-PCR were performed in triplicate.
2.5 Protein extraction and WB analysis
Proteins were extracted from the testis according to Ergoli et al. (2020). Lowry assay was used to measure protein concentration (Lowry et al., 1951). For each lane, 40 μg of proteins were separated SDS–PAGE by at nine or 15% and transferred to Hybond-P PVDF membranes (#GE10600023; Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). For details on the used antibodyes, see Supplementary Table S2. All signals, including those used to obtain the Bax/Bcl-2 ratio, were quantified by densitometric analysis using ImageJ software (version 1.53 g) and adjusted with respect to ß-Actin levels. All the WBs were performed in triplicate.
2.6 IF analysis
For IF staining, testis sections were processed according to Venditti and Minucci (2022). For details on the used antibodies, see Supplementary Table S2. The cells’ nuclei were marked with mounting medium with DAPI. The sections were observed and acquired under the optical microscope (LeicaDM5000 B+ CTR 5000) with a UV lamp and saved with IM 1000 software (version 4.7.0). Densitometric analysis of the IF signal was performed with ImageJ doftware and 30 fields/samples, for a total of 240 per group, were considered.
2.7 Statistical analysis
Data are reported as mean ± SEM. Differences between the groups were considered statistically significant at p < 0.05. Analyses were performed using one-way ANOVA; Tukey’s post hoc t-test was applied when appropriate with Prism 5.0, GraphPad Software (San Diego, CA, United States).
3 Results
3.1 Histological study
Representative images of rat testis sections are shown Figure 1. The control testis exhibited a regular SE, with GC in all different stages of differentiation and the tubular lumens filled with mature SPZ (rhombus), as well as a normal interstitial compartment, with the presence of regular LC and blood vessels. Abnormal seminiferous tubules, with general disorganization of the epithelium, were evident in all treated groups, as indicated by loss of contact and the presence of abundant empty spaces between cells (white triangle) other than desquamation of GC from the basement membrane (white arrow) and congestion (black arrow).
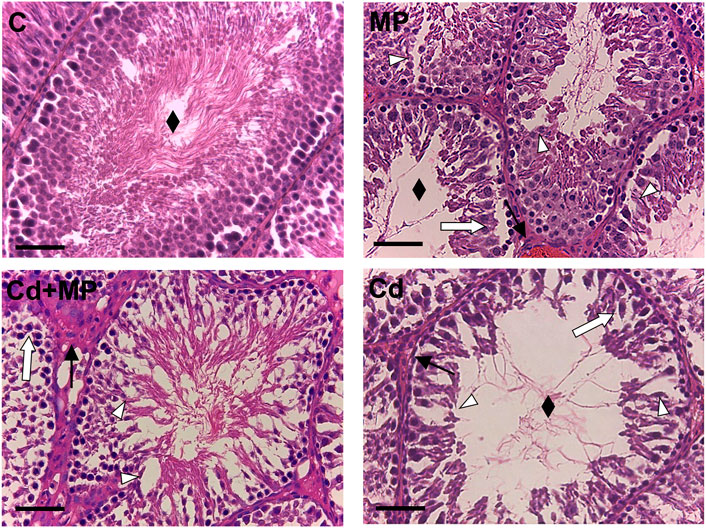
FIGURE 1. Hematoxylin-eosin staining of control, MP and/or Cd-treated rat testis. Rhombus: tubules lumen; White arrow: GC desquamation; Triangle: space between GC; Black arrow: dilatation of blood vessels. Scale bars represent 40 µm.
The histology results were confirmed by the analysis of morphometric parameters (Table 1), which showed that tubule diameter, epithelium thickness, and the percentage of tubular lumens occupied by SPZ were significantly lower in the MP (p < 0.05), Cd + MP (p < 0.01) and Cd (p < 0.001) groups as compared to the control.
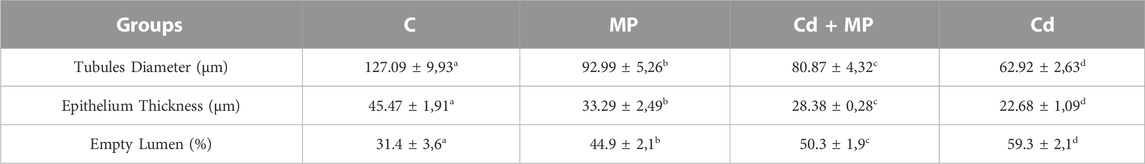
TABLE 1. Effect MP and/or Cd on testicular morphometric parameters. Values are expressed as mean ± SEM from six animals in each group. a vs. b: p < 0.05; a vs. c: p < 0.01; a vs. d: p < 0.001; b vs. c: p < 0.05; b vs. d: p < 0.01; c vs. d: p < 0.01.
Interestingly, the results showed that in the Cd-treated group, all the above parameters had the lowest values (Table 1).
3.2 Effect of MP and/or Cd testicular steroidogenesis
To assess the effects of MP and/or Cd on testicular steroidogenesis, serum T level, as well as 3β-HSD protein level and localization were evaluated (Figure 2). Data revealed that T concentration was significantly decreased in MP (p < 0.05), Cd + MP (p < 0.01) and Cd (p < 0.001) compared with controls (Figure 2A).
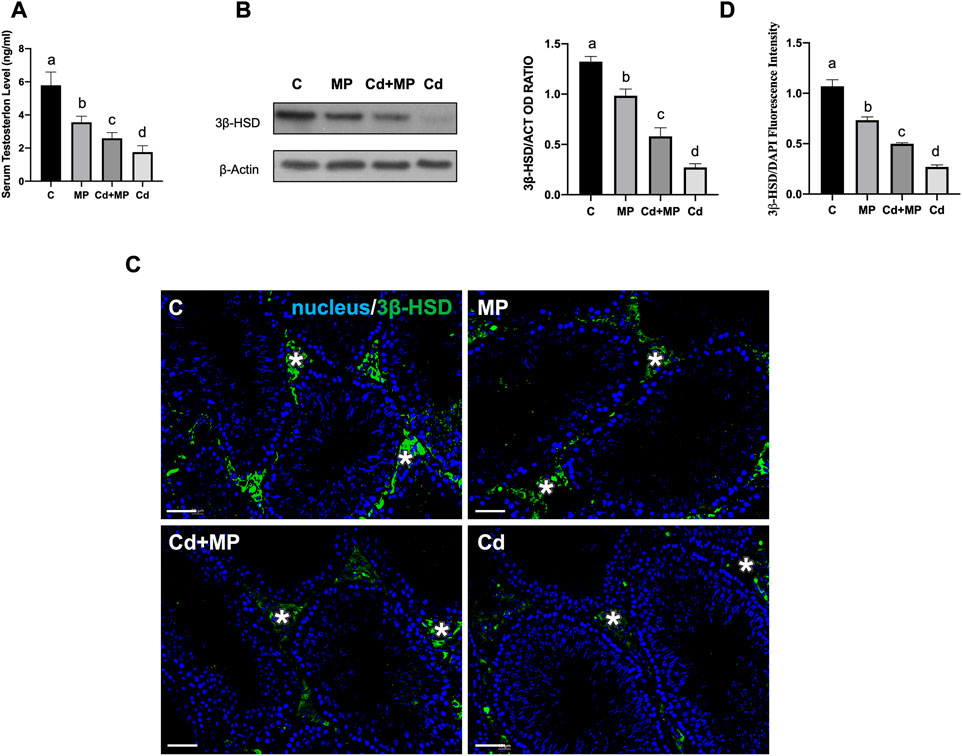
FIGURE 2. Steroidogenesis analysis of control, MP and/or Cd-treated rat testis. (A) Serum T level in animals treated with MP and/or Cd. (B) WB analysis showing the expression of 3β-HSD (42 kDa) in the testes of animals treated with MP and/or Cd. Histogram shows its relative protein levels. Data were normalized with ß-Actin and reported as OD ratio. (C) IF analysis of 3β-HSD (green) in testes of animals treated with MP and/or Cd. Slides were counterstained with DAPI-fluorescent nuclear staining (blue). Scale bars represent 20 μm. Asterisk: LC. (D) Histogram showing the quantification of 3β-HSD fluorescence signal intensity, using ImageJ. All the values are expressed as means ± SEM from eight animals in each group. a vs. b p < 0.05; a vs. c p < 0.01; a vs. d p < 0.001; b vs. c p < 0.05; b vs. d p < 0.01; c vs. d p < 0.01. Each experiment has been performed in triplicate.
WB analysis performed on 3β-HSD, an enzyme involved in T biosynthesis, showed a decrease in its level in MP (p < 0.05), Cd + MP (p < 0.01) and Cd (p < 0.001) as compared to the controls (Figure 2B).
The impairment of T production was additionally confirmed by IF staining of 3β-HSD, shown in Figure 2C. For all groups analyzed, the signal specifically localized in the interstitial LC (asterisks, Figure 2C), but was fainter in the testis of treated animals. Fluorescence intensity analysis showed a similar, statistically significant trend to that observed for protein level (Figure 2D).
For all the considered parameters, the values were lower in the Cd group.
3.3 Effect of MP and/or Cd on apoptosis
Figure 3 shows the effect of MP administration, alone or together with Cd, on the apoptotic rate of germ and somatic cells. WB analysis revealed an increase in Bax/Bcl-2 ratio and Caspase-3 protein level in the MP (p < 0.05), Cd + MP (p < 0.01) and Cd (p < 0.001) groups as compared to the control (Figure 3A).
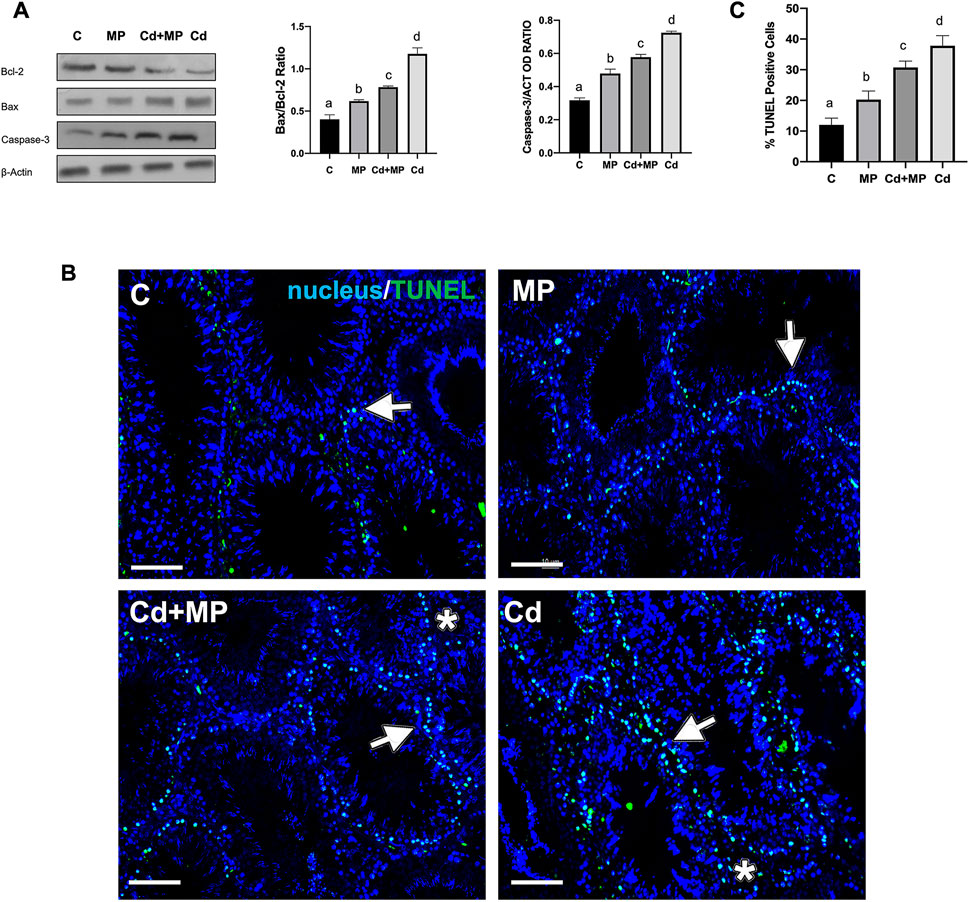
FIGURE 3. Apoptosis rate analysis of control, MP and/or Cd-treated rat testis. (A) WB analysis showing the expression of Bcl-2 (26 kDa); Bax (23 kDa) and Caspase-3 (31 kDa) in the testes of animals treated with MP and/or Cd. Histograms show the Bax/Bcl-2 ratio and Caspase-3 relative protein levels, respectively. Data were normalized with ß-Actin and reported as OD ratio. (B) Determination of apoptotic cells through the detection of TUNEL-positive cells (green) in the testes of animals treated with MP and/or Cd. Slides were counterstained with DAPI-fluorescent nuclear staining (blue). Scale bars represent 20 μm. Arrows: SPG; Asterisks: LC. (C) Histogram showing the % of TUNEL positive cells. All the values are expressed as means ± SEM from eight animals in each group. a vs. b p < 0.05; a vs. c p < 0.01; a vs. d p < 0.001; b vs. c p < 0.05; b vs. d p < 0.01; c vs. d p < 0.01. Each experiment has been performed in triplicate.
In support of these data, a TUNEL assay was performed on all samples (Figure 2B). Data showed dispersed apoptotic cells in the control group, mainly SPG (arrows), and scattered LC (asterisks). As expected, in MP (p < 0.05) Cd + MP (p < 0.01) and Cd (p < 0.001) groups, the number of apoptotic cells increased dramatically, as compared to the control (Figures 2B,C). However, Cd treatment provoked more apoptosis than MP (p < 0.05) and Cd + MP (p < 0.01).
3.4 Effect of MPs and/or Cd on PTMA
WB analysis showed that PTMA protein level decreased in MP (p < 0.05), Cd + MP (p < 0.01) and Cd (p < 0.001) as compared to the controls (Figure 4A).
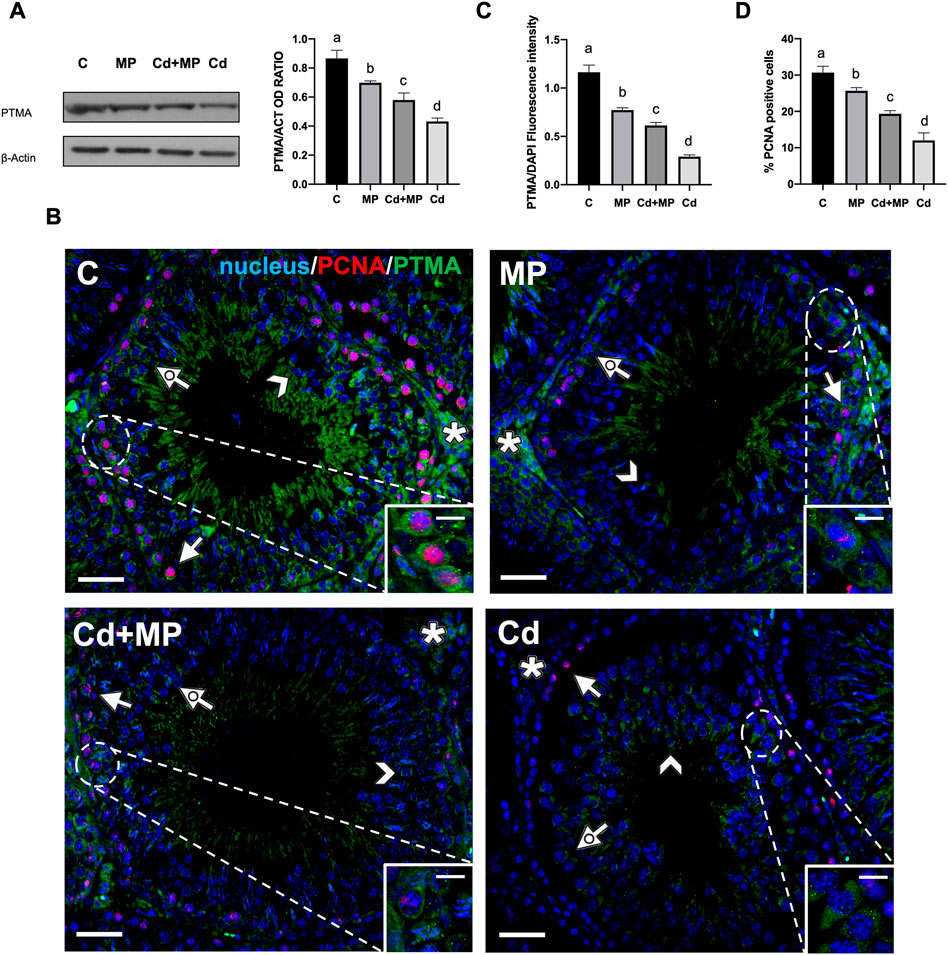
FIGURE 4. WB and IF analysis of PTMA and PCNA of control, MP and/or Cd-treated rat testis. (A) WB analysis showing the expression of PTMA (15 kDa) in the testes of animals treated with MP and/or Cd. Histogram shows its relative protein level. Data were normalized with ß-Actin and reported as OD ratio. (B) IF analysis of PTMA (green) and PCNA (red) in the testes of animals treated with MP and/or Cd. Slides were counterstained with DAPI-fluorescent nuclear staining (blue). Scale bars represent 20 μmand 10 μm in the insets. Arrow: SPG; dotted arrow: SPC; arrowhead: SPT; asterisk: LC. (C) Histogram showing the fluorescence signal intensity of PTMA. Data were normalized with DAPI signal. (D) Histogram showing the % of PCNA positive cells. All the values are expressed as means ± SEM from eight animals in each group. a vs. b p < 0.05; a vs. c p < 0.01; a vs. d p < 0.001; b vs. c p < 0.05; b vs. d p < 0.01; c vs. d p < 0.01. Each experiment has been performed in triplicate.
The observed decrease was further confirmed by an IF analysis of PTMA, performed together with PCNA, a commonly used proliferation marker, on all samples (Figure 4B). In the control group, PTMA mainly localized in the perinuclear cytoplasm in SPG (arrows and insets), SPC (dotted arrows) and SPT (arrowheads). In addition, a positive signal was also observed in the interstitial LC (asterisks). In all treated groups, PTMA maintained its localization, but the signal intensity decreased significantly in MP (p < 0.05), Cd + MP (p < 0.01) and Cd (p < 0.001) as compared to the controls (Figure 4C).
As for PCNA, it was localized in the nucleus of proliferating cells as the layer of SPG at basal tubules in all groups (Figure 4B). However, the number of PCNA-positive cells showed an opposite trend to that observed for thef TUNEL-positive cells described in the previous paragraph, as it was lower in MP (p < 0.05), Cd + MP (p < 0.01) and Cd (p < 0.001) than in controls (Figure 4D). Again, Cd had the worst effects, as compared to MP and Cd + MP.
3.5 Effect of MP and/or Cd on DAAM1
RT-PCR and WB analyses were performed on DAAM1 (Figures 5A,B). Although it is less sensitive than real-time qPCR, semiquantitative RT-PCR is a specific technique that allows us to appreciate changes in the expression level of target genes.
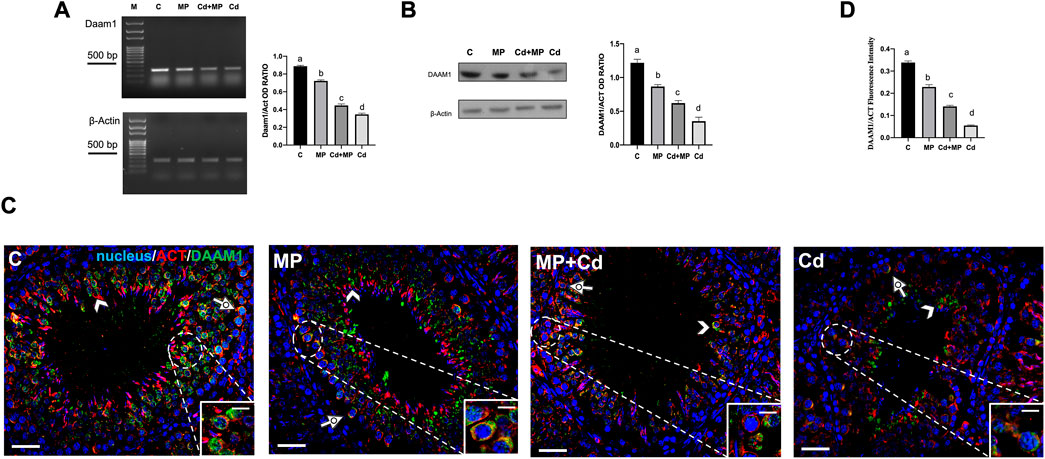
FIGURE 5. DAAM1 expression and localization in control, MP and/or Cd-treated rat testis. (A) Agarose gel electrophoresis of RT-PCR products showing the expression of Daam1 (380 bp), in testes of animals treated with MP and/or Cd. M: represents base-pairs marker (Solis BioDyne, Tartu, Estonia). Histogram shows the relative mRNA levels of Daam1. Data were normalized with ß-actin and reported as OD ratio. (B) WB analysis showing the protein levels of DAAM1 (112 kDa) in testes of animals treated with MP and/or Cd. Histogram shows its relative protein level. Data were normalized with ß-Actin and reported as OD ratio. (C) IF analysis of DAAM1 (green) and ß-Actin (red) in testes of animals treated with MP and/or Cd. Slides were counterstained with DAPI-fluorescent nuclear staining (blue). Scale bars represent 20 μm and 10 μm in the insets. Dotted arrows: SPC; arrowheads: SPT. (D) Histogram showing the quantification of DAAM1 fluorescence signal intensity; data were normalized with ß-Actin signal. All the values are expressed as means ± SEM from eight animals in each group. a vs. b p < 0.05; a vs. c p < 0.01; a vs. d p < 0.001; b vs. c p < 0.05; b vs. d p < 0.01; c vs. d p < 0.01. Each experiment has been performed in triplicate.
Analysis of Daam1 mRNA levels showed that its expression was downregulated by treatments. Specifically, the level of Daam1 decreased significantly in MP (p < 0.05), Cd + MP (p < 0.01) and Cd (p < 0.001) as compared to the controls (Figure 5A). WB analysis revealed a similar trend (Figure 5B). In fact, exposure to MP (p < 0.05), Cd + MP (p < 0.01) and Cd (p < 0.001) reduced the level of DAAM1 protein as compared to the control, similar to what was described previously.
To localize DAAM1 in the gonads of rats treated with MP and/or Cd, IF double staining was made along with its cytoskeletal partner, actin, (Figure 5C). In sections of control testis, DAAM1 localized in the perinuclear cytoplasm of SPC (dotted arrows, inset), and SPT (arrowheads), colocalizing with ß-actin, as evidenced by the yellow-orange intermediate staining. In the GC of treated animals, a drastic decrease of DAAM1 staining intensity was observed, with red signal predominating, however, the formin and ß-actin continued to colocalize in the perinuclear zone of SPC (dotted arrows). Fluorescence intensity analysis showed a comparable statistically significant trend, as previously specified for the protein level (Figure 5D).
Interestingly, also in this case, the effects of Cd on DAAM1 were the worst induced.
3.6 Effect of MP and/or Cd on PREP
Figure 6 shows the effects of MP and or Cd treatments on PREP expression and localization.
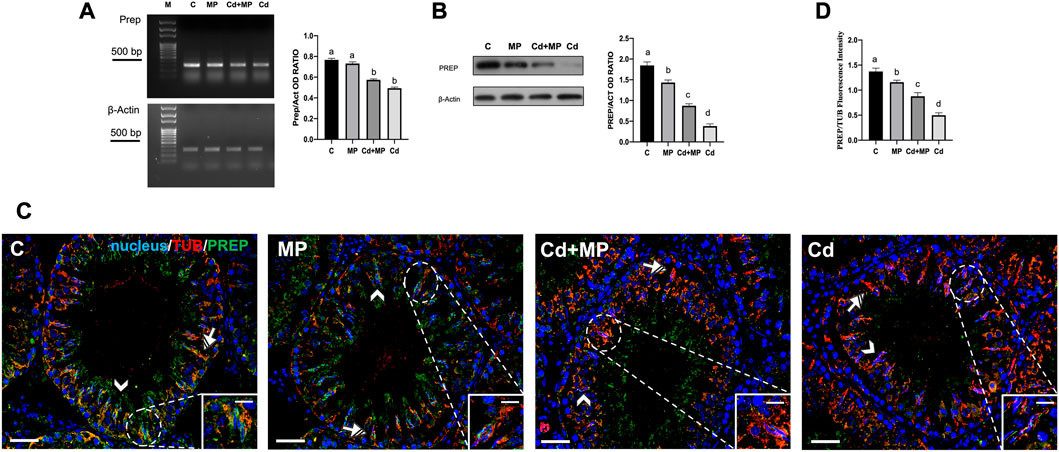
FIGURE 6. PREP expression and localization in control, MP and/or Cd-treated rat testis. (A) Agarose gel electrophoresis of RT-PCR products showing the expression of PREP (392 bp), in testes of animals treated with MP and/or Cd. M: represents base-pairs marker (Solis BioDyne, Tartu, Estonia). Histogram shows the relative mRNA levels of Prep. Data were normalized with β-actin and reported as OD ratio. (B) WB analysis showing the protein levels of PREP (80 kDa) in testes of animals treated with MP and/or Cd. Histogram shows its relative protein level. Data were normalized with β-Actin and reported as OD ratio. (C) IF analysis of PREP (green) and α-Tubulin (red) in testes of animals treated with MP and/or Cd. Slides were counterstained with DAPI-fluorescent nuclear staining (blue). Scale bars represent 20 μm and 10 μm in the insets. Arrowheads: SPT; striped arrows: SC. (D) Histogram showing the quantification of PREP fluorescence signal intensity, data were normalized with α-Tubulin signal. All thje values are expressed as means ± SEM from eight animals in each group. a vs. b p < 0.05; a vs. c p < 0.01; a vs. c p < 0.001; b vs. c p < 0.05; b vs. d p < 0.01; c vs. d p < 0.01. Each experiment has been performed in triplicate.
In contrast to what was observed for Daam1, the mRNA level of Prep was downregulated only by Cd treatment, as no differences were observed between the MP and control, and Cd + MP and Cd groups (Figure 6A). PREP protein level decreased in all experimental groups, as the values in the MP (p < 0.05), Cd + MP (p < 0.01) and Cd (p < 0.001) groups were lower than those in the control (Figure 6B).
Immunolocalization of PREP showed that in the control it localized predominantly within the SPT (arrowheads) and the cytoplasmic extensions of SC, where colocalization with tubulin was evidenced by the yellow-orange intermediate color (striped arrows; insets). In sections from animals exposed to MP and or Cd, the distribution pattern of PREP was equivalento to that of the control and, but a decrease in staining was evident in GC (arrowheads) and in the SC cytoplasm (striped arrows). Finally, the analysis of the fluorescence intensity exhibited a similar, statistically significant trend to that above described.
From the analysis of the all the data, it is evident that the treatment with Cd alone produced the worst effects on PREP.
4 Discussion
In this paper we evaluated, for the first time, the combined effect of MP and Cd on the rat testis. We used 60-days-old rats, that, at this time-point, cannot be defined adult yet but in period of the late puberty/early adulthood. However, it has been well recognized that rats are sexually mature at this stage, as the first wave of the spermatogenic cycle is completed, and they can produce mature SPZ. In fact, the two signs of sexual maturity are the presence of complete spermiogenesis in the seminiferous tubules and mature SPZ in the epididymis (Picut et al., 2018).
Currently, especially in the most industrialized states, there is a critical impairment in the quantity and quality of SPZ, implying that the discharge of many pollutants into the environment is a major contributor to reduced fertility (Selvaraju et al., 2021). Furthermore, according to WHO guidelines (WHO, 2021), the seminogram, the analysis of macroscopic (volume, pH, viscosity) and microscopic (concentration, morphology, vitality, and motility of SPZ) characteristics is, to date, the best and most routinely used test for the assessment of male fertility; on the other hand, this test is not the most accurate, because seminal parameters within the proposed reference range do not promise fertility, nor do values outside these limits automatically indicate male infertility (Serrano et al., 2022). Thus, more detailed analyses of the molecular mechanism(s) of testis and SPZ physiology are mandatory not only to expand knowledge of spermatogenesis but also, by identifying new molecular markers, to improve the standard assessment of male fertility through routine semen analysis. Since many pollutants exert their harmful effects through proteins and signaling pathways that are also involved in the regulation of the testicular activity, the use of in vivo and in vitro treated-models may be useful not only to manage reproductive dysfunction, but also to better elucidate the mechanisms that support spermatogenesis under physiological conditions (Gao et al., 2021).
In this paper, the combined, injurious effect of MP and Cd on the rat testis was evaluated.
It is well known that, in the Wistar rats’ spermatogenesis lasts about 63 days, including SPZ maturation during epididymal transit (Picut et al., 2018). Although to observe a more accurate impact of the used pollutants on sperm quality, the treatment should have lasted at least 63 days, in this paper, in any case, alterations were observed in all the cell types that compose the SE, and in the mature SPZ. Thus, it can be assumed that all cells, even those that have already started mitotic and meiotic divisions, as well as spermiogenesis, are susceptible to MP and/or Cd effects, independently of the duration of treatment.
Here, in support of data shown in other papers, we confirm that both MP (Jin et al., 2022; Wei et al., 2022; Wen et al., 2022) and Cd (Zhu et al., 2020; Venditti et al., 2021c; Kechiche et al., 2021; Ali et al., 2022) can act as EDCs. Indeed, the presence of scattered apoptotic LC and altered steroidogenesis, evidenced by the decreased serum T concentration and 3β-HSD protein level, result in impaired central and local steroid availability and production. It should be noted that T, acting as a survival factor, controls the entire spermatogenetic process, preventing GC entry into apoptosis, therefore, its reduction may be an additional cause of induced apoptosis (Huhtaniemi, 2018; Venditti et al., 2021d). This last point was also confirmed by the increase in the Bax/Bcl-2 ratio and caspase-3 levels, as well as the decrease in the number of PCNA positive cells, indexes of the pro-apoptotic state and reduced proliferation, respectively.
In addition, our results showed, for the first time, that PTMA is a target protein of the harmful effects of MP and Cd. PTMA is a peculiar small polypeptide that, due to its unfolded structure, can interact with several molecules, participating in different biological pathways and activities, such as cell cycle regulation (Karachaliou et al., 2020). Indeed, PTMA has been demonstrated to act as an anti-apoptotic factor, inhibiting apoptosome formation (Zhang et al., 2014), or, through its association with the c-myc oncogene, promoting cell cycle progression (Boán et al., 2001). Therefore, the increase in apoptosis observed following MP and Cd treatments could also be related to the reduction in PTMA levels. It should also be mentioned, regarding spermatogenesis, that previous works have reported the specific association of PTMA with male gametes differentiation and, in particular, with acrosome biogenesis and early fertilization events (Ferrara et al., 2010; 2013; Pariante et al., 2016b; Venditti and Minucci, 2017). Thus, MP- and/or Cd-induced reduction of its protein levels could consequently result in impaired gamete quality and function.
One of the most important features of the spermatogenic process is its dynamics, characterized by proliferative and differentiative phases, during which round SPG intimately change their morphology and shape to produce mature SPZ. This is possible mainly due to the cytoskeleton of somatic and germ cells, as well as the hundreds of associated proteins that regulate the appropriate organization and activity of cytoskeletal elements (Li et al., 2017).
First, we confirmed that Cd can have a negative impact on the activity of many transcription factors (Venditti et al., 2021b), as the downregulation of DAAM1 and PREP expression was also observed here. Second, the results obtained showed that MP can also have an influence on DAAM1 and PREP; however, whereas for DAAM1 MP alone were able to decrease its mRNA level, for PREP such decrease was observed only for the protein. Therefore, given the role of these two proteins in the cytoskeletal remodeling that occurs during GC differentiation, we hypothesize that the observed impaired histological features, such as loss of contact and the presence of abundant empty spaces between cells, as well as the damaged morphometric parameters, may be due to a reduced expression of DAAM1 and PREP induced by MP and Cd treatment.
Interestingly, regarding PREP, in our previous work we found an increase of its expression induced by Cd (Venditti et al., 2020d). This could be justified with differences in the experimental design and in the age of the rats. Indeed, previously, rats were not directly exposed to Cd, but their mother were, during gestation and lactation (Venditti et al., 2020d), whereas, here, adult male rats were treated. Considering that effects of Cd on the function of the hypothalamus-pituitary-testis axis is age-dependent (Lafuente et al., 2000; 2001), and that PREP expression and activity changes in the rat brain during embryonal and post-natal development (Agirregoitia et al., 2010; Hannula et al., 2011), the divergent results may be attributed to the described varied Cd action and the changes of PREP levels during rats’ development.
Finally, the combined effects of Cd + MP were more intense than those induced by MP, but better than those induced by Cd alone. This antagonistic effect has been previously demonstrated in many experimental models and conditions, as MP reduced Cd toxicity in plants (Lian et al., 2020; Wang et al., 2022b; Zhang et al., 2022), fish (Wang S. et al., 2022; Yang et al., 2022), and mice (Wang et al., 2022d). Considering that the two substances were administered separately (oral gavage for MP and drinking water for Cd) and that the rates of adsorption/release of heavy metals by MP depend on pH values (Hildebrandt et al., 2021; Chen et al., 2022), we hypothesized that MP, by exploiting a sort of “positive-Trojan horse effect”, may reduce the bioavailability of Cd, explaining the minor consequences observed by concomitant treatment with MP-Cd as compared with Cd alone. Therefore, current studies focus on the use of “pre-adsorbed” MP with Cd to evaluate an effective “Trojan horse” effect on the rat testis.
5 Conclusion
In this paper, we further documented the combined effects of MP and Cd on rat testis physiology. Particular attention was given to their impact on SE histology, hormonal milieu, apoptosis, and, for the first time, on the expression of the two cytoskeleton-associated proteins DAAM1 and PREP. Our data confirm that cytoskeleton elements are a major target of environmental pollutants, resulting, ultimately, in impaired testicular activity.
Interestingly, because MP and Cd were administered to rats contemporaneously, but through two separate ways, the observed alterations were less than for Cd alone, probably due to reduced bioavailability of this heavy metal. Finally, this work emphasizes the role of proteins that regulate cytoskeletal dynamics in the proper progression of spermatogenesis.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by The experimental procedure was approved by the Ethics Committee for Research in life science and health of the Higher Institute of Biotechnology of Monastir (CER-SVS/ISBM-protocol 022/2020) and was carried out accordingly to the UNESCO Recommendation Concerning Science and Scientific Research (1974, 2017).
Author contributions
IM and SM: conceptualization and design of the study. MV and MB: formal analysis, investigation and methodology. MV: figure preparation. MV and MB: writing—original draft preparation. IM and SM: writing—review and editing. SM: funding acquisition and supervision. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was supported by the Italian Ministry and Research (Grant PRIN to SM 2020).
Acknowledgments
We want to thank the project Erasmus + KA107 (Napoli-Monastir) of the Università della Campania “Luigi Vanvitelli”, and the Ministry of Higher Education and Scientific Research of Tunisia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2023.1145702/full#supplementary-material
Abbreviations
3β-HSD, 3β-Hydroxysteroid dehydrogenase; Bax, Bcl-2 Associated X; Bcl-2, B-cell lymphoma 2; BSA, bovine serum albumin; BTB, blood-testis barrier; Cd, cadmium; DAAM1, dishevelled-associated activator of morphogenesis 1; DAPI, 4′,6-diamidino-2-phenylindole; mEDCs, endocrine disrupting chemicals; GC, germ cells; IF, immunofluorescence; LC, Leydig cells; MP, microplastics; OD, optical density; PCNA, proliferating cell nuclear antigen; PREP, prolyl endopeptidase; PTMA, prothymosin α; SC, Sertoli cells; SE, seminiferous epithelium; SPG, spermatogonia; SPC, spermatocytes; SPT, spermatids; SPZ, spermatozoa; T, testosterone; TUNEL, Terminal Transferase dUTP Nick End Labeling; WB, Western blotting.
References
Agirregoitia, N., Bizet, P., Agirregoitia, E., Boutelet, I., Peralta, L., Vaudry, H., et al. (2010). Prolyl endopeptidase mRNA expression in the central nervous system during rat development. J. Chem. Neuroanat. 40, 53–62. doi:10.1016/j.jchemneu.2010.03.002
Ali, W., Ma, Y., Zhu, J., Zou, H., and Liu, Z. (2022). Mechanisms of cadmium-induced testicular injury: A risk to male fertility. Cells 11, 3601. doi:10.3390/cells11223601
Belgacem, H., Venditti, M., Ben Salah-Abbès, J., Minucci, S., and Abbès, S. (2022). Potential protective effect of lactic acid bacteria against zearalenone causing reprotoxicity in male mice. Toxicon 209, 56–65. doi:10.1016/j.toxicon.2022.02.011
Boán, F., Viñas, A., Buceta, M., Domínguez, F., Sánchez, L., Gómez-Márquez, J., et al. (2001). Prothymosin alpha, a mammalian c-myc-regulated acidic nuclear protein, provokes the decondensation of human chromosomes in vitro. Cytogenet. Cell Genet. 93, 171–174. doi:10.1159/000056980
Chemek, M., Venditti, M., Boughamoura, S., Mimouna, S. B., Messaoudi, I., and Minucci, S. (2018). Involvement of testicular DAAM1 expression in zinc protection against cadmium-induced male rat reproductive toxicity. J. Cell. Physiol. 233, 630–640. doi:10.1002/jcp.25923
Chen, X. J., Ma, J. J., Yu, R. L., Hu, G. R., and Yan, Y. (2022). Bioaccessibility of microplastic-associated heavy metals using an in vitro digestion model and its implications for human health risk assessment. Environ. Sci. Pollut. Res. Int. 29, 76983–76991. doi:10.1007/s11356-022-20983-8
Deng, Y., Zhang, Y., Lemos, B., and Ren, H. (2017). Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 7, 46687. doi:10.1038/srep46687
Dunleavy, J. E. M., O'Bryan, M. K., Stanton, P. G., and O'Donnell, L. (2019). The cytoskeleton in spermatogenesis. Reproduction 157, R53–R72. doi:10.1530/REP-18-0457
EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) (2016). Statement on the presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 14 (6).
Ergoli, M., Venditti, M., Piccillo, E., Minucci, S., and Politano, L. (2020). Study of expression of genes potentially responsible for reduced fitness in patients with myotonic dystrophy type 1 and identification of new biomarkers of testicular function. Mol. Reprod. Dev. 87, 45–52. doi:10.1002/mrd.23307
Ferrara, D., Izzo, G., Pariante, P., Donizetti, A., d'Istria, M., Aniello, F., et al. (2010). Expression of prothymosin alpha in meiotic and post-meiotic germ cells during the first wave of rat spermatogenesis. J. Cell. Physiol. 224, 362–368. doi:10.1002/jcp.22131
Ferrara, D., Pariante, P., Di Matteo, L., Serino, I., Oko, R., and Minucci, S. (2013). First evidence of prothymosin α localization in the acrosome of mammalian male gametes. J. Cell. Physiol. 228, 1629–1637. doi:10.1002/jcp.24332
Gao, S., Wu, X., Wang, L., Bu, T., Perrotta, A., Guaglianone, G., et al. (2021). Signaling proteins that regulate spermatogenesis are the emerging target of toxicant-induced male reproductive dysfunction. Front. Endocrinol. (Lausanne) 12, 800327. doi:10.3389/fendo.2021.800327
Haddadi, A., Kessabi, K., Boughammoura, S., Ben Rhouma, M., Mlouka, R., Banni, M., et al. (2022). Exposure to microplastics leads to a defective ovarian function and change in cytoskeleton protein expression in rat. Environ. Sci. Pollut. Res. Int. 29, 34594–34606. doi:10.1007/s11356-021-18218-3
Hannula, M. J., Männistö, P. T., and Myöhänen, T. T. (2011). Sequential expression, activity and nuclear localization of prolyl oligopeptidase protein in the developing rat brain. Dev. Neurosci. 33, 38–47. doi:10.1159/000322082
He, J., Yang, X., and Liu, H. (2021). Enhanced toxicity of triphenyl phosphate to zebrafish in the presence of micro- and nano-plastics. Sci. Total. Environ. 756, 143986. doi:10.1016/j.scitotenv.2020.143986
Hildebrandt, L., Nack, F. L., Zimmermann, T., and Pröfrock, D. (2021). Microplastics as a Trojan horse for trace metals. J. Hazard. Mat. Lett. 2, 100035. doi:10.1016/j.hazl.2021.100035
Hou, B., Wang, F., Liu, T., and Wang, Z. (2021). Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice. J. Hazard. Mat. 405, 124028. doi:10.1016/j.jhazmat.2020.124028
Huhtaniemi, I. (2018). Mechanisms in Endocrinology: Hormonal regulation of spermatogenesis: Mutant mice challenging old paradigms. Eur. J. Endocrinol. 179, R143–R150. doi:10.1530/EJE-18-0396
Ilechukwu, I., Ehigiator, B. E., Ben, I. O., Okonkwo, C. J., Olorunfemi, O. S., Modo, U. E., et al. (2022). Chronic toxic effects of polystyrene microplastics on reproductive parameters of male rats. Environ. Anal. Health. Toxicol. 37, e2022015–0. doi:10.5620/eaht.2022015
Jin, H., Ma, T., Sha, X., Liu, Z., Zhou, Y., Meng, X., et al. (2021). Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mat. 401, 123430. doi:10.1016/j.jhazmat.2020.123430
Jin, H., Yan, M., Pan, C., Liu, Z., Sha, X., Jiang, C., et al. (2022). Chronic exposure to polystyrene microplastics induced male reproductive toxicity and decreased testosterone levels via the LH-mediated LHR/cAMP/PKA/StAR pathway. Part. Fibre. Toxicol. 19, 13. doi:10.1186/s12989-022-00453-2
Karachaliou, C. E., Kalbacher, H., Voelter, W., Tsitsilonis, O. E., and Livaniou, E. (2020). In vitro immunodetection of prothymosin alpha in normal and pathological conditions. Curr. Med. Chem. 27, 4840–4854. doi:10.2174/0929867326666190807145212
Kechiche, S., Venditti, M., Knani, L., Jabłońska, K., Dzięgiel, P., Messaoudi, I., et al. (2021). First evidence of the protective role of melatonin in counteracting cadmium toxicity in the rat ovary via the mTOR pathway. Environ. Pollut. 270, 116056. doi:10.1016/j.envpol.2020.116056
Lafuente, A., Márquez, N., Pérez-Lorenzo, M., Pazo, D., and Esquifino, A. I. (2001). Cadmium effects on hypothalamic-pituitary-testicular axis in male rats. Exp. Biol. Med. 226, 605–611. doi:10.1177/153537020122600615
Lafuente, A., Márquez, N., Pérez-Lorenzo, M., Pazo, D., and Esquifino, A. (2000). Pubertal and postpubertal cadmium exposure differentially affects the hypothalamic–pituitary–testicular axis function in the rat. Food Chem. Toxicol. 38, 913–923. doi:10.1016/S0278-6915(00)00077-6
Lehner, R., Weder, C., Petri-Fink, A., and Rothen-Rutishauser, B. (2019). Emergence of nanoplastic in the environment and possible impact on human health. Environ. Sci. Technol. 53, 1748–1765. doi:10.1021/acs.est.8b05512
Levine, H., Jørgensen, N., Martino-Andrade, A., Mendiola, J., Weksler-Derri, D., Jolles, M., et al. (2022). Temporal trends in sperm count: A systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries. Hum. Reprod. Update dmac035, dmac035. doi:10.1093/humupd/dmac035
Levine, H., Jørgensen, N., Martino-Andrade, A., Mendiola, J., Weksler-Derri, D., Mindlis, I., et al. (2017). Temporal trends in sperm count: A systematic review and meta-regression analysis. Hum. Reprod. Update 23, 646–659. doi:10.1093/humupd/dmx022
Li, L., Gao, Y., Chen, H., Jesus, T., Tang, E., Li, N., et al. (2017). Cell polarity, cell adhesion, and spermatogenesis: Role of cytoskeletons. F1000Research 6, 1565. doi:10.12688/f1000research.11421.1
Li, N., Mruk, D. D., Lee, W. M., Wong, C. K., and Cheng, C. Y. (2016). Is toxicant-induced Sertoli cell injury in vitro a useful model to study molecular mechanisms in spermatogenesis? Semin. Cell. Dev. Biol. 59, 141–156. doi:10.1016/j.semcdb.2016.01.003
Li, S., Wang, Q., Yu, H., Yang, L., Sun, Y., Xu, N., et al. (2021). Polystyrene microplastics induce blood-testis barrier disruption regulated by the MAPK-Nrf2 signaling pathway in rats. Environ. Sci. Pollut. Res. Int. 28, 47921–47931. doi:10.1007/s11356-021-13911-9
Lian, J., Wu, J., Zeb, A., Zheng, S., Ma, T., Peng, F., et al. (2020). Do polystyrene nanoplastics affect the toxicity of cadmium to wheat (Triticum aestivum L.)? Environ. Pollut. 263, 114498. doi:10.1016/j.envpol.2020.114498
Lowry, O., Rosenbrough, N., Farr, A., and Randall, R. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275. doi:10.1016/S0021-9258(19)52451-6
Marić, T., Fučić, A., and Aghayanian, A. (2021). Environmental and occupational exposures associated with male infertility. Arh. Hig. Rada. Toksikol. 72, 101–113. doi:10.2478/aiht-2021-72-3510
Minucci, S., and Venditti, M. (2022). New insight on the in vitro effects of melatonin in preserving human sperm quality. Int. J. Mol. Sci. 23, 5128. doi:10.3390/ijms23095128
Miyaso, H., Ogawa, Y., and Itoh, M. (2022). Microenvironment for spermatogenesis and sperm maturation. Histochem. Cell. Biol. 157, 273–285. doi:10.1007/s00418-021-02071-z
Pariante, P., Dotolo, R., Venditti, M., Ferrara, D., Donizetti, A., Aniello, F., et al. (2016a). First evidence of DAAM1 localization during the post-natal development of rat testis and in mammalian sperm. J. Cell. Physiol. 231, 2172–2184. doi:10.1002/jcp.25330
Pariante, P., Dotolo, R., Venditti, M., Ferrara, D., Donizetti, A., Aniello, F., et al. (2016b). Prothymosin alpha expression and localization during the spermatogenesis of Danio rerio. Zygote 24, 583–593. doi:10.1017/S0967199415000568
Picut, C. A., Ziejewski, M. K., and Stanislaus, D. (2018). Comparative aspects of pre- and postnatal development of the male reproductive system. Birth Defects Res. 110, 190–227. doi:10.1002/bdr2.1133
Santillo, A., Venditti, M., Minucci, S., Chieffi Baccari, G., Falvo, S., Rosati, L., et al. (2019). D-Asp upregulates PREP and GluA2/3 expressions and induces p-ERK1/2 and p-Akt in rat testis. Reproduction 158, 357–367. doi:10.1530/REP-19-0204
Selvaraju, V., Baskaran, S., Agarwal, A., and Henkel, R. (2021). Environmental contaminants and male infertility: Effects and mechanisms. Andrologia 53, e13646. doi:10.1111/and.13646
Serrano, R., Garcia-Marin, L. J., and Bragado, M. J. (2022). Sperm phosphoproteome: Unraveling male infertility. Biol. (Basel) 11, 659. doi:10.3390/biology11050659
Vander Borght, M., and Wyns, C. (2018). Fertility and infertility: Definition and epidemiology. Clin. Biochem. 62, 2–10. doi:10.1016/j.clinbiochem.2018.03.012
Venditti, M., Aniello, F., Santillo, A., and Minucci, S. (2019). Study on PREP localization in mouse seminal vesicles and its possible involvement during regulated exocytosis. Zygote 27, 160–165. doi:10.1017/S0967199419000194
Venditti, M., Arcaniolo, D., De Sio, M., and Minucci, S. (2022). First evidence of the expression and localization of prothymosin α in human testis and its involvement in testicular cancers. Biomolecules 12, 1210. doi:10.3390/biom12091210
Venditti, M., Arcaniolo, D., De Sio, M., and Minucci, S. (2021a). Preliminary investigation on the involvement of cytoskeleton-related proteins, DAAM1 and PREP, in human testicular disorders. Int. J. Mol. Sci. 22, 8094. doi:10.3390/ijms22158094
Venditti, M., Ben Rhouma, M., Romano, M. Z., Messaoudi, I., Reiter, R. J., and Minucci, S. (2021b). Altered expression of DAAM1 and PREP induced by cadmium toxicity is counteracted by melatonin in the rat testis. Genes (Basel) 12, 1016. doi:10.3390/genes12071016
Venditti, M., Ben Rhouma, M., Romano, M. Z., Messaoudi, I., Reiter, R. J., and Minucci, S. (2021c). Evidence of melatonin ameliorative effects on the blood-testis barrier and sperm quality alterations induced by cadmium in the rat testis. Ecotoxicol. Environ. Saf. 226, 112878. doi:10.1016/j.ecoenv.2021.112878
Venditti, M., Chemek, M., Minucci, S., and Messaoudi, I. (2020d). Cadmium-induced toxicity increases prolyl endopeptidase (PREP) expression in the rat testis. Mol. Reprod. Dev. 87, 565–573. doi:10.1002/mrd.23345
Venditti, M., Donizetti, A., Aniello, F., and Minucci, S. (2020a). EH domain binding protein 1-like 1 (EHBP1L1), a protein with calponin homology domain, is expressed in the rat testis. Zygote 28, 441–446. doi:10.1017/S0967199420000301
Venditti, M., Fasano, C., Minucci, S., Serino, I., Sinisi, A. A., Dale, B., et al. (2020b). DAAM1 and PREP are involved in human spermatogenesis. Reprod. Fertil. Dev. 32, 484–494. doi:10.1071/RD19172
Venditti, M., Fasano, C., Santillo, A., Aniello, F., and Minucci, S. (2018). First evidence of DAAM1 localization in mouse seminal vesicles and its possible involvement during regulated exocytosis. C. R. Biol. 341, 228–234. doi:10.1016/j.crvi.2018.03.001
Venditti, M., and Minucci, S. (2022). Differential expression and localization of EHBP1L1 during the first wave of rat spermatogenesis suggest its involvement in acrosome biogenesis. Biomedicines 10, 181. doi:10.3390/biomedicines10010181
Venditti, M., and Minucci, S. (2017). Prothymosin alpha expression in the vertebrate testis: A comparative review. Zygote 25, 760–770. doi:10.1017/S096719941700065X
Venditti, M., and Minucci, S. (2019). Subcellular localization of prolyl endopeptidase during the first wave of rat spermatogenesis and in rat and human sperm. J. Histochem. Cytochem. 67, 229–243. doi:10.1369/0022155418810064
Venditti, M., Romano, M. Z., Aniello, F., and Minucci, S. (2021d). Preliminary investigation on the ameliorative role exerted by D-aspartic acid in counteracting ethane dimethane sulfonate (EDS) toxicity in the rat testis. Toxic. Rat Testis Animals (Basel) 11, 133. doi:10.3390/ani11010133
Venditti, M., Santillo, A., Falvo, S., Di Fiore, M. M., Chieffi Baccari, G., and Minucci, S. (2020c). D-aspartate upregulates DAAM1 protein levels in the rat testis and induces its localization in spermatogonia nucleus. Biomolecules 10, 677. doi:10.3390/biom10050677
Wang, L., Bu, T., Wu, X., Gao, S., Li, X., De Jesus, A. B., et al. (2022a). Cell-cell interaction-mediated signaling in the testis induces reproductive dysfunction—lesson from the toxicant/pharmaceutical models. Cells 11, 591. doi:10.3390/cells11040591
Wang, L., Lin, B., Wu, L., Pan, P., Liu, B., and Li, R. (2022b). Antagonistic effect of polystyrene nanoplastics on cadmium toxicity to maize (Zea mays L.). Chemosphere 307 (1), 135714. doi:10.1016/j.chemosphere.2022.135714
Wang, L., Xu, M., Chen, J., Zhang, X., Wang, Q., Wang, Y., et al. (2022d). Distinct adverse outcomes and lipid profiles of erythrocytes upon single and combined exposure to cadmium and microplastics. Chemosphere 307 (2), 135942. doi:10.1016/j.chemosphere.2022.135942
Wang, L., Yan, M., Wu, S., Mao, B., Wong, C. K. C., Ge, R., et al. (2020b). Microtubule cytoskeleton and spermatogenesis-lesson from studies of toxicant models. Toxicol. Sci. 177, 305–315. doi:10.1093/toxsci/kfaa109
Wang, L., Yan, M., Wu, S., Wu, X., Bu, T., Wong, C. K. C., et al. (2020a). Actin binding proteins, actin cytoskeleton and spermatogenesis - lesson from toxicant models. Reprod. Toxicol. 96, 76–89. doi:10.1016/j.reprotox.2020.05.017
Wang, S., Xie, S., Wang, Z., Zhang, C., Pan, Z., Sun, D., et al. (2022c). Single and combined effects of microplastics and cadmium on the cadmium accumulation and biochemical and immunity of Channa argus. Biol. Trace. Elem. Res. 200, 3377–3387. doi:10.1007/s12011-021-02917-6
Wei, Y., Zhou, Y., Long, C., Wu, H., Hong, Y., Fu, Y., et al. (2021). Polystyrene microplastics disrupt the blood-testis barrier integrity through ROS-Mediated imbalance of mTORC1 and mTORC2. Environ. Pollut. 289, 117904. doi:10.1016/j.envpol.2021.117904
Wei, Z., Wang, Y., Wang, S., Xie, J., Han, Q., and Chen, M. (2022). Comparing the effects of polystyrene microplastics exposure on reproduction and fertility in male and female mice. Toxicology 465, 153059. doi:10.1016/j.tox.2021.153059
Wen, S., Chen, Y., Tang, Y., Zhao, Y., Liu, S., You, T., et al. (2022). Male reproductive toxicity of polystyrene microplastics: Study on the endoplasmic reticulum stress signaling pathway. Food Chem. Toxicol. 172, 113577. doi:10.1016/j.fct.2022.113577
World Health Organization (2021). WHO laboratory manual for the examination and processing of human semen. 6th ed. Geneva, Switzerland: WHO Press.
Yang, H., Zhu, Z., Xie, Y., Zheng, C., Zhou, Z., Zhu, T., et al. (2022). Comparison of the combined toxicity of polystyrene microplastics and different concentrations of cadmium in zebrafish. Aquat. Toxicol. 250, 106259. doi:10.1016/j.aquatox.2022.106259
Zhang, M., Cui, F., Lu, S., Lu, H., Jiang, T., Chen, J., et al. (2014). Increased expression of prothymosin-α, independently or combined with TP53, correlates with poor prognosis in colorectal cancer. Int. J. Clin. Exp. Pathol. 7, 4867–4876.
Zhang, Z., Li, Y., Qiu, T., Duan, C., Chen, L., Zhao, S., et al. (2022). Microplastics addition reduced the toxicity and uptake of cadmium to Brassica chinensis L. Sci. Total. Environ. 852, 158353. doi:10.1016/j.scitotenv.2022.158353
Keywords: microplastic, cadmium, endocrine disrupters, prothymosin alpha, cytoskeleton, DAAM1, PREP, testis
Citation: Venditti M, Ben Hadj Hassine M, Messaoudi I and Minucci S (2023) The simultaneous administration of microplastics and cadmium alters rat testicular activity and changes the expression of PTMA, DAAM1 and PREP. Front. Cell Dev. Biol. 11:1145702. doi: 10.3389/fcell.2023.1145702
Received: 16 January 2023; Accepted: 27 February 2023;
Published: 09 March 2023.
Edited by:
Mengcheng Luo, Wuhan University, ChinaReviewed by:
Maria Maddalena Di Fiore, University of Campania Luigi Vanvitelli, ItalyAtsushi P. Kimura, Hokkaido University, Japan
Copyright © 2023 Venditti, Ben Hadj Hassine, Messaoudi and Minucci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimo Venditti, bWFzc2ltby52ZW5kaXR0aUB1bmljYW1wYW5pYS5pdA==; Sergio Minucci, c2VyZ2lvLm1pbnVjY2lAdW5pY2FtcGFuaWEuaXQ=
 Massimo Venditti
Massimo Venditti Majida Ben Hadj Hassine2
Majida Ben Hadj Hassine2 Sergio Minucci
Sergio Minucci