- 1Department of Cell and Molecular Biology, Tulane University, New Orleans, LA, United States
- 2Division of Hematology-Oncology, Department of Internal Medicine, Medical School, University of Michigan, Ann Arbor, MI, United States
- 3Tulane Brain Institute, Tulane University, New Orleans, LA, United States
Angiogenesis is a highly coordinated process involving the control of various endothelial cell behaviors. Mechanisms for transcription factor involvement in the regulation of endothelial cell dynamics and angiogenesis have become better understood, however much remains unknown, especially the role of non-DNA binding transcriptional cofactors. Here, we show that Zmiz1, a transcription cofactor, is enriched in the endothelium and critical for embryonic vascular development, postnatal retinal angiogenesis, and pathological angiogenesis in a model of oxygen-induced retinopathy (OIR). In mice, endothelial cell-specific deletion of Zmiz1 during embryogenesis led to lethality due to abnormal angiogenesis and vascular defects. Inducible endothelial cell-specific ablation of Zmiz1 postnatally resulted in impaired retinal vascular outgrowth, decreased vascular density, and increased vessel regression. In addition, angiogenic sprouting in the superficial and deep layers of the retina was markedly reduced. Correspondingly, vascular sprouting in fibrin bead assays was significantly reduced in the absence of Zmiz1, while further in vitro and in vivo evidence also suggested deficits in EC migration. In agreement with the defective sprouting angiogenesis phenotype, gene expression analysis of isolated retinal endothelial cells revealed downregulation of tip-cell enriched genes upon inactivation of Zmiz1. Lastly, our study suggested that endothelial Zmiz1 is critical for intraretinal revascularization following hypoxia exposure in the OIR model. Taken together, these findings begin to define the previously unspecified role of endothelial Zmiz1 in physiological and pathological angiogenesis.
Introduction
Vasculogenesis and angiogenesis are key processes required for the initial formation and subsequent expansion of pre-existing blood vessels, respectively. Development of a functional vasculature is critical for tissue growth, regeneration, and homeostasis, while impaired vascularization is associated with various disease processes, including stroke, vascular malformations, retinopathy, and cancer (Greenberg, 1998; Carmeliet, 2003; Crawford et al., 2009; Sherwood et al., 1971). The angiogenic generation of new blood vessels is a multi-step regulatory process intimately involving endothelial cells (ECs). For instance, vascular sprouting requires specialized ECs called tip cells that respond to both chemo-attractant and repulsive extracellular signals to guide the growth and morphogenesis of the vasculature. Functional behaviors of ECs, such as these, are regulated by transcription factors (TFs) and transcription cofactors that mediate cell-specific gene expression changes during different phases of blood vessel growth (Hamik et al., 2006; De Val and Black, 2009). However, the transcription cofactors that modulate TF involvement in the EC transcriptional program have largely remained elusive.
Zmiz1 is a transcription cofactor belonging to the protein inhibitor of activated STAT (PIAS) protein family (Liu et al., 1998). As a transcriptional cofactor, Zmiz1 does not bind DNA directly, but instead interacts with DNA-binding TFs. Zmiz1 has been shown to regulate the transcriptional activity of multiple TFs, including P53, Androgen receptor, Smad3/4 and Notch1 (Lee et al., 2007; Li et al., 2011; Li et al., 2006). Similar to the diversity of its co-partners, Zmiz1 has been shown to have roles in various developmental processes and diseases. Zmiz1 is associated with Notch-dependent T-cell development and leukemogenesis (Pinnell et al., 2015) and numerous studies point to Zmiz1 involvement in erythropoiesis (Castillo-Castellanos et al., 2021), osteosarcoma (Zhou et al., 2022), diabetes (Alghamdi et al., 2022), multiple sclerosis (Fewings et al., 2017) and in a range of neurodevelopmental disorders (Carapito et al., 2019; Lu et al., 2022; Phetthong et al., 2021). In terms of vascular development, global deletion of Zmiz1 in mice leads to lethality at embryonic day (E) 10.5 due to cardiovascular defects. Overall, the embryos are severely underdeveloped and display abnormal blood vessel development (Beliakoff et al., 2008). Further, microarray analysis of VEGF-deficient ECs displayed downregulation of Zmiz1 in the gene ontology cluster associated with blood vessel development suggesting a functional role in the developing endothelium (Domigan et al., 2015). Related, our group recently showed that Zmiz1 transcriptionally regulates lymphatic EC gene expression (K C et al., 2024). However, the exact function of Zmiz1 in blood vessel development and angiogenesis remains unclear.
Utilizing the murine retina, a well-established model for studying the different processes of angiogenesis (Uemura et al., 2006; Stahl et al., 2010), we investigated the role of Zmiz1 in angiogenesis. In the current study, we report that loss of endothelial Zmiz1 during embryogenesis was lethal due to vascular defects. Additionally, we demonstrated that Zmiz1 is a fundamental regulator of postnatal retinal vascular growth, including a specific and crucial function in sprouting angiogenesis. We further provided evidence of Zmiz1 involvement in the regulation of tip-cell gene expression during the vascular sprouting process. Lastly, in an oxygen-induced retinopathy (OIR) mouse model, Zmiz1 was implicated in pathological neovascularization.
Materials and methods
Mice and breeding
All mice were housed in individual ventilated cages, in a temperature-controlled room with a 12 h light/dark cycle. All animal protocols in this study were approved and performed in accordance with Tulane University’s Institutional Animal Care and Use Committee policies and adhere to ethical standards. In our studies the following transgenic mouse lines were used: Zmiz1fl/fl, Tie2-Cre, and Cdh5-CreERT2 (Pinnell et al., 2015; Kisanuki et al., 2001; Wang et al., 2010). All mice were maintained in a mixed genetic background. Zmiz1fl/fl mice were crossed with both Tie2-Cre and Cdh5-CreERT2 lines for embryonic and postnatal studies respectively. Littermate controls of both sexes were used in all experiments. Genotyping was performed as previously detailed using genomic DNA (Huang et al., 2020).
Tamoxifen treatment
To induce Cre activity, 100 μg of tamoxifen (Sigma, T5648) was administered orally to newborn pups born from Zmiz1fl/fl;Cdh5-CreERT2 and Zmiz1fl/fl mating pairs from P1-P3 for early deletion or from P5-P7 for late deletion. For P42 and P60 mice, 2 mg of tamoxifen was injected intraperitoneally daily from P28-P31.
Embryo dissection, processing, and immunofluorescent analysis
Embryos were collected from pregnant females at indicated time points following timed mating, designating embryonic day 0.5 (E0.5) as noon on the day a vaginal plug was observed. Embryos with yolk sacs were dissected and imaged by brightfield in 1X PBS, followed by overnight fixation in 4% PFA at 4 °C. Next, both yolk sacs and embryos were incubated in PBST buffer (PBS with 0.5% Triton X-100) overnight at 4 °C and then transferred into CAS-Block (Life technologies, 008120) for 4 h at room temperature. Both yolk sac tissues and whole embryos were incubated in primary antibody to rat anti-PECAM-1(BD Pharmingen, 553370) diluted in CAS-Block overnight at 4 °C. Following primary antibody incubation, both yolk sacs and embryos were incubated overnight at 4 °C with secondary antibodies to chicken anti-rat Alexa Fluor 488 (Life technologies, A21470) diluted in CAS-Block. Lastly, both yolk sacs and embryos were washed in PBST buffer before imaging. The amniotic membrane was used to collect genomic DNA for the purpose of genotyping embryos.
Retina dissection, processing, and immunofluorescent analysis
Eyes were collected at P7, P10, P12, P42 and P60 for analysis. Retinas were dissected from eyes and whole-mount retina staining was performed as previously described (Crist et al., 2018); P60 retinas were incubated with IB4-488 for 3 h at room temperature. Primary antibodies used for whole mount retina immunofluorescent staining: IB4-488 (1:250, Invitrogen, 121411), IB4-594 (1:250, Invitrogen, 12143), SMA-Cy3 (1:250, Sigma Aldrich, C6198), ERG-488 (1:250, Abcam, Ab196374), ERG-647 (1:250, Abcam, Ab196149), COLLAGEN IV (1:100, Millipore, AB756P), NG2 (1:100, Millipore, AB5320), Ki67-488 (1:250, Cell signaling, 11882), CL-CASPASE3 (1:100, Cell signaling, 9661), ESM-1 (1:100, R&D systems, AF1999), CXCR4 (1:100, R&D systems, MAB 21651), and ZMIZ1 (1:100, Santa Cruz, Sc-376825).
Analysis of retinal vasculature
Quantifications of retinal vasculature were performed on high-resolution confocal images using Nikon NIS-elements and Angiotool analysis software (Zudaire et al., 2011). Radial outgrowth was determined as the distance between the vascular front and the central optic nerve in each leaflet of the retina and averaged. Whole retinal images were used to measure the vascular density and the number of branching points and COLLAGEN IV sleeves (IB4 negative and COLLAGEN IV positive). Quantification of sprouts was performed at the vascular front in 20X images where white arrowheads indicate cellular protrusions identified as angiogenic sprouts.
Isolation of murine retina and lung endothelial cells
Retinal ECs were isolated from P7 pups as previously described (Patel et al., 2022). Briefly, isolation of ECs from the retinas was performed using the Miltenyi Neural tissue Dissociation Kit (P) with minor modifications (Miltenyi, 130-092-628). For each “biological” sample, 8-10 retinas were pooled and digested in enzyme mix 1 and enzyme mix 2 to obtain a single cell suspension. Cells were incubated with CD31 conjugated dynabeads for 30 min at 4 °C. Following the incubation, CD31+ cells were separated from the cell mixture via magnetic activated cell sorting. Next, RNA was isolated from the cells and used for downstream RNA-sequencing. Lung ECs were isolated from P7 pups as previously described (Crist et al., 2018). Lung ECs were isolated from both control and Zmiz1iECKO mice and RNA was extracted for downstream assays.
Cell culture and siRNA transfection
TeloHAECs (ATCC, CRL-4052) were cultured in EBM-2 media (Lonza, CC-3156) supplemented with EGM-2 bullet kit (Lonza, CC-3162) according to manufacturer’s instructions. All cells were maintained at 37 °C and 5% CO2. TeloHAECs were seeded in a 6-well plate overnight. The following day, cells were transfected with 20 μM of either pooled control-siRNAs (Dharmacon, D-001810-01-05) or Zmiz1-siRNAs (Dharmacon, L-007034-00-0005) using Lipofectamine3000 (Thermofisher, L3000015) following manufacturer’s instructions and were utilized for experiments 48 h post transfection. Zmiz1-siRNAs were purchased in the SMARTpool format, which consists of 4 different Zmiz1-targeted siRNAs in one reagent.
RNA extraction and quantitative RT-PCR
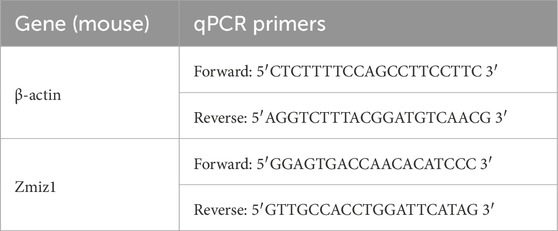
RNA was extracted from TeloHAECs and isolated retina and lung ECs using the GeneJET RNA Purification Kit (Thermofisher, K0732). Nanodrop spectrophotometer measurements of RNA 260/280 absorbance ratios for RNA samples ranged from 1.90–2.09. First strand cDNA synthesis was performed using the iScript Reverse Transcription Supermix kit (Biorad, 1708840). Quantitative RT-PCR was carried out using the PerfeCTa SYBR Green Fastmix (Quantabio, 95071) and gene-specific primers (Table 1) on CFX96 system (Biorad). RT negative controls were included within the sample groups. Relative gene expression was determined using the ΔΔCt method and normalized against β-actin.
Scratch assay
Scratch assays were performed on confluent control-siRNA and Zmiz1-siRNA treated TeloHAECs. A horizontal and vertical wound was generated in each well using a 200 μL pipet tip followed by three washes with PBS. Phase contrast images were taken right after the scratch (0 h) and 12 h after incubation at 37 °C and 5% CO2. The percentage of wound closure was determined using the ImageJ software.
Fibrin gel bead assay
Fibrin bead sprouting assay was performed as previously described (Pauleikhoff et al., 2023). Briefly, control and Zmiz1 siRNA treated teloHAECs were coated on cytodex beads and embedded into a fibrin gel. Human lung fibroblasts (NHLF) (Lonza, CC-2512) were cultured on the gels and media was replaced every other day (as previously published, fibroblasts are commonly used and necessary for efficient sprouting in the fibrin gel bead assays) (Nakatsu et al., 2007). Sprouting activity was monitored over the next few days and on day 5 the beads were imaged. Approximately 15 beads per condition were quantified for the number of sprouts per bead from 4 independent experiments. Sprout imaging and analysis were performed as described above.
RNA sequencing and differential gene expression analysis
RNA sequencing and gene expression analysis was performed as previously described (Patel et al., 2022). Briefly, Total RNA extracted from isolated retinal ECs were quantified and verified before library preparation for sequencing. Library preparation methodology was based on mRNA polyA selection. Sequenced reads were aligned to the mouse (mm10) reference genome in the Basespace sequence hub (Illumina). The mm10 reference genome was used because our data was collected in 2019 before the release of the mm39 reference genome in 2020. The aligned reads were used to quantify mRNA expression to determine differentially expressed genes; we utilized the BaseSpace app developed by Illumina, which uses Trimmed Mean of M-values (TMM) for read counts normalization before performing differential gene expression within the pipeline. Gene ontology (GO) analysis of differentially expressed genes was performed using graphical gene-set enrichment tool for plants and animals—Shiny Go version 0.76 (Ge et al., 2020) —with a false discovery rate (FDR) set at 0.05. Sequencing data were deposited in the Gene Expression Omnibus (GEO) database: accession number GSE242406.
OIR model
OIR studies were performed similar to previous work (Patel et al., 2022). Neonatal pups and their nursing mother were exposed to 75% oxygen from P7-P12 in a designed chamber (Biospherix, ProOx110); from P12-P17 they were moved to room air (Scott and Fruttiger, 2010). Tamoxifen (100 μg) was administered to the pups orally from P12-P14 to assess neovascularization at P17 and from P7-P9 to assess the vaso-obliteration phase at P12. Retinas were immunofluorescently labeled with Isolectin-IB4 as discussed above and vascular loss and neovascularization were quantified using an automated OIR retinal image analysis software (Xiao et al., 2017). C57BL/6J mice were subjected to either normal room oxygen (nOIR) or the OIR schedule. At P12 and P17, retinas were collected for RNA after the retinal pigment epithelium was removed. RNA isolation and quantitative RT-PCR for Zmiz1 and β-actin was then performed as described above.
Statistical analysis
Data analysis was performed using Graphpad Prism 10.3. All values are presented as bar graphs with error bars representing mean ± standard error of the mean (s.e.m). Statistical significance was determined by the two-tailed unpaired t-test between two conditions with a p-value less than or equal to 0.05 was considered statistically significant.
Results
Zmiz1 is expressed in the endothelium of various tissues
Given the unexplored, yet potential role of Zmiz1 in vascular development and function, we first surveyed mouse EC databases to evaluate Zmiz1 expression in various tissues. Analysis of organ-specific EC transcriptomic data at P7 revealed highest expression of Zmiz1 mRNA in brain ECs and comparable expression between kidney, liver, and lung ECs (Sabbagh et al., 2018) (Supplementary Figure S1A). Evaluation of single cell transcriptomic data generated using cells isolated from an adult mouse brain via fluorescence-activated cell sorting (FACS) showed a mean expression of 2.28 for Zmiz1 in ECs25 (Supplementary Figure S1B). Analysis of another single-cell transcriptomic data set using isolated brain ECs from P7 mice allowed for assessment of Zmiz1 in different EC subtypes (arterial, venous, and capillary ECs) (Sabbagh et al., 2018). Zmiz1 expression was detected across the different EC cell types at relatively similar levels (Supplementary Figure S1C). Furthermore, bulk RNA sequencing of murine retinal ECs at different developmental stages showed the presence of Zmiz1 transcripts during early postnatal development, with highest expression levels between postnatal days (P) 6–15, and a decrease from P21-P50 (Jeong et al., 2017) (Supplementary Figure S1D). In addition, utilizing immunofluorescence analysis, we demonstrated that ZMIZ1 protein was highly enriched throughout the blood vessels of the developing P7 retina, including the arteries, veins, and capillaries (Supplementary Figure S1E). Collectively, these studies established the presence of Zmiz1 in the endothelial lineage of postnatal and adult mouse tissues. Further, examination of human EC databases has shown ZMIZ1 expression in both vascular and lymphatic endothelial cells (Khan et al., 2019).
Early endothelial cell-specific ablation of Zmiz1 results in vascular defects and embryonic lethality
Previous work indicated that Zmiz1 null mice die embryonically due to defects in angiogenesis (Beliakoff et al., 2008). To further investigate the endothelial role for Zmiz1 in embryonic vascular development, conditional Zmiz1 flox mice (Zmiz1fl/fl) were crossed to mice expressing Cre recombinase under the vascular Tie2-promoter (Tie2-Cre) (Figure 1A), thereby achieving constitutive Zmiz1-EC deletion during embryogenesis. Homozygous deletion of Zmiz1 resulted in embryonic lethality as no viable Zmiz1fl/fl; Tie2-Cre (referred to as Zmiz1cECKO; constitutive EC-knockout) pups were born (Figure 1B). Conversely, the other control genotyped pups (Zmiz1fl/+, Zmiz1fl/fl and Zmiz1fl/+;Tie2-Cre) were born at normal Mendelian ratios. In timed mating experiments, E12.5 Zmiz1cECKO embryos displayed growth retardation and were significantly reduced in size as compared to the littermate Zmiz1fl/fl controls (Figures 1C,D). Between E12.5 and E13.5, mutant embryos appeared pale with a lack of blood flow in the embryo, displayed an absence of blood-filled yolk sac vessels, and/or exhibited localized hemorrhages (Figure 1C). By E14.5 all the mutant embryos were necrotic and being reabsorbed (data not shown). PECAM-1 antibody staining of blood vessels in Zmiz1cECKO embryos at E12.5 revealed defects in the vascular patterning of the cranial and trunk vasculature (Figure 1E). In Zmiz1cECKO embryos, the cranial blood vessels were not well defined and failed to form larger caliber vessels, while the dorsal vasculature in the trunk, including intersomitic vessels, were truncated and disorganized. Analysis of yolk sacs at E12.5 revealed the lack of large and distinctive blood vessels in Zmiz1cECKO embryos in comparison to littermate controls (Figures 1C-F). Taken together, these data showed that Zmiz1 is critical for embryonic vascular development and further indicated a major contributing role of defective angiogenesis as a cause for embryonic lethality in Zmiz1 null mice.
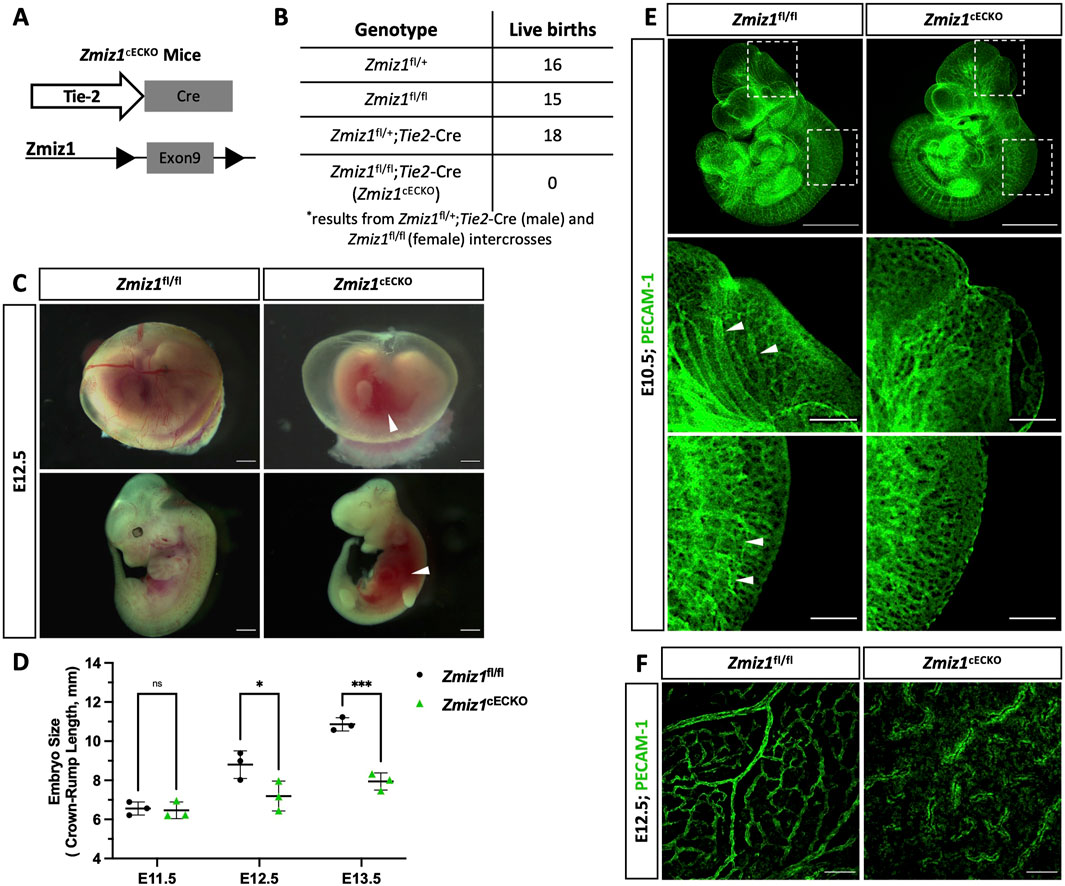
Figure 1. Endothelial Zmiz1 is essential for angiogenesis during embryonic development. (A) Strategy for Zmiz1 exon 9 deletion specifically in the ECs during embryogenesis using the Tie2-Cre mouse model (Zmiz1 constitutive endothelial cell knockout; Zmiz1cECKO). (B) Genotypic analysis of the progeny generated by crossing Zmiz1fl/+;Tie2-Cre males with Zmiz1fl/fl females at postnatal day (P) 21. All genotypes, except Zmiz1fl/fl;Tie2-Cre, were observed at normal Mendelian ratios. (C) Brightfield images showing gross morphology of the intact yolk sacs and whole embryos at embryonic day (E) 12.5. White arrowheads indicate areas of hemorrhage. Scale bars: 1 mm. (D) Quantification of embryo size as indicated by crown-rump length from E11.5- E13.5. (E) Whole-mount PECAM-1 immunofluorescent staining of E11.5 Zmiz1fl/fl and Zmiz1cECKO embryos. Scale bars 1 mm. Higher magnification of embryos shows vessel detail in the cranial and tail region (white arrowheads indicate well-organized and connected vessels in cranial and tail region). Scale bars: 100 μm. (F) PECAM-1 staining in E12.5 Zmiz1fl/fl and Zmiz1cECKO yolk sacs. Note the absence of distinct, organized vessels within the yolk sac of the mutants. Scale bars: 100 μm. Error bars represent mean ± s.e.m; two-tailed unpaired t-test. ns (not significant; P > 0.05), *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Endothelial-Zmiz1 is required for postnatal retinal angiogenesis but not vascular maintenance
To elucidate the role of Zmiz1 in the endothelium during physiological postnatal angiogenesis, we generated inducible EC-specific Zmiz1 knockout mice. This was accomplished by crossing the Zmiz1fl/fl line with mice carrying tamoxifen-inducible Cre recombinase under control of the EC-specific Cdh5 promoter (Cdh5(PAC)-iCreERT2) (Wang et al., 2010). Cre-mediated inactivation of Zmiz1 in ECs (Zmiz1fl/fl;Cdh5-CreERT2, referred to as Zmiz1iECKO; Zmiz1-inducible EC knockout mice) was induced by daily oral administration of tamoxifen from P1-P3 for early induction (Figure 2A). Tamoxifen treatment resulted in an approximately 80% reduction of Zmiz1 mRNA levels in isolated lung ECs (iLECs) of Zmiz1iECKO pups compared to littermate controls (Zmiz1fl/fl) at P7 (Figure 2B). Retinal blood vessels were analyzed at P7 or P10 following early induction, or at P12 following late induction. Whole-mount retinas immunofluorescently labeled for the retinal endothelial cell marker, isolectinB4 (IB4), showed a significant reduction of vascular outgrowth, density, and branching in Zmiz1iECKO as compared to littermate controls at P7 (Figures 2C–F). Moreover, impaired retinal angiogenesis was also observed in the heterozygote mice (Zmiz1fl/wt-iECKO), which exhibited decreased vascular outgrowth and density in comparison to the P7 controls (Supplementary Figure S2). However, the vascular defects were not as severe as those observed in the homozygous Zmiz1iECKO mutants. Interestingly, Zmiz1iECKO mice also exhibited defects in the overall number of retinal arteries and veins at P7. Immunofluorescent antibody staining of retinas with IB4 and alpha-Smooth muscle actin (α-SMA) revealed decreased numbers of main arteries and veins in Zmiz1 mutant retinas as compared to Zmiz1fl/fl mice (Figures 2G–I; Supplementary Figure S2).
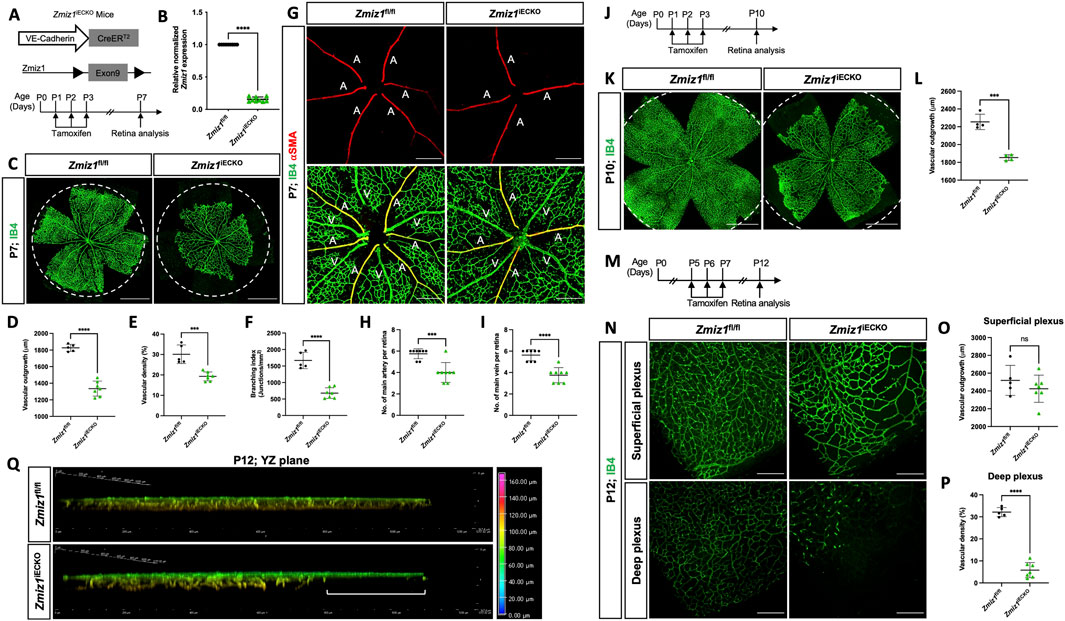
Figure 2. Zmiz1 EC deficiency leads to impaired retinal vascular morphogenesis. (A) Schematic illustration of the VE-Cadherin (Cdh5)-CreERT2 transgene and Cre-mediated recombination of Zmiz1 floxed (fl) exon 9 to generate EC-specific deletion of Zmiz1 (Zmiz1 induced endothelial cell knockout; Zmiz1iECKO). Time points utilized within the study for tamoxifen administration and retinal analysis are noted. (B) Relative Zmiz1 expression levels in isolated lung ECs from Zmiz1fl/fl and Zmiz1iECKO mice at P7 as determined via qRT-PCR (n = 3). (C) Whole-mount retinas stained for isolectin-B4 (IB4). Dotted white circle represents outgrowth in Zmiz1fl/fl retina. Scale bar, 1,000 μm. (D–F) Quantification of indicated morphometric parameters analyzed within Zmiz1fl/fl (n = 5) and Zmiz1iECKO (n = 7) retinas at P7. (G) Whole-mount retinas stained for IB4 and alpha-SMOOTH MUSCLE ACTIN (α-SMA). Scale bar, 250 μm. A, artery; V, vein. (H,I) Quantification of main arteries and veins within Zmiz1fl/fl (n = 8) and Zmiz1iECKO (n = 8) retinas at P7. (J) Schematic showing tamoxifen administration in pups for early induction studies and analyzed at P10. (K) Representative images of whole-mount retinas stained with IB4 at P10. Dotted white circle represents outgrowth in Zmiz1fl/fl retina. Scale bars 1,000 μm. (L) Quantification of vascular outgrowth in Zmiz1fl/fl (n = 4) and Zmiz1iECKO (n = 4) retinas at P10. (M) Schematic showing tamoxifen administration in pups for late induction studies. (N) Representative images of IB4 vessels in superficial and deep layers at P12. Scale bars: 250 μm. (O,P) Quantification of vascular outgrowth and deep vascular plexus density in Zmiz1fl/fl (n = 5) and Zmiz1iECKO (n = 7) retinas at P12. (Q) 3D vertical view in the YZ plane with depth coding of Zmiz1fl/fl and Zmiz1iECKO retinas at P12. Note disorganized sprouts and the absence of vertical sprouting in the periphery of the Zmiz1iECKO retinas (white bracket). Error bars represent mean ± s.e.m; two-tailed unpaired t-test. ns (not significant; P > 0.05), *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
We also point out that though Zmiz1fl/fl mice treated with tamoxifen served as our controls throughout, we also assessed the retina vasculature of Zmiz1fl/fl;Cdh5-CreERT2 pups that were not subjected to tamoxifen. In this case, we found that there were no differences in multiple vascular parameters assessed when comparing Zmiz1fl/fl and Zmiz1fl/fl;Cdh5-CreERT2 mice without tamoxifen: vascular density, branchpoints and the number of major arteries and veins (Supplementary Figures S3A,C–F). However, there was a slight increase in vascular outgrowth in Zmiz1fl/fl retinas (Supplementary Figure S3B), while the outgrowth in Zmiz1fl/fl;Cdh5-CreERT2 retinas was still similar to Zmiz1fl/fl mice subjected to tamoxifen (Figure 2D). Taken together, these control experiments indicated that there were no substantial vascular defects associated with potential leaking of Cre-recombinase in our studies (Supplementary Figure S3).
To further assess vascular morphology at later stages, P10 retinas from early deletion of Zmiz1 (P1-P3) were analyzed (Figure 2J). At this time point, the vasculature in the retina has typically reached the peripheral edge. However, at P10, vascular outgrowth was strongly impaired in Zmiz1iECKO mice versus littermate controls (Figures 2K,L and data not shown). Thus, collectively, the early Zmiz1 deletion data demonstrated that endothelium-specific loss of Zmiz1 in postnatal mice leads to significant deficits in angiogenesis and an overall reduced vascular presence.
These initial analyses primarily focused on the retina vasculature in a two-dimensional fashion (i.e., the superficial vessels). Consequently, we evaluated the angiogenic role of Zmiz1 in three-dimensional space by analyzing deep vascular plexus formation in the retina—superficial capillaries begin sprouting vertically around P7 to give rise to the deep vascular plexus in the outer plexiform layer. Introduction of tamoxifen from P5-P7, when many of the vessels have differentiated and patterned properly but the vasculature is still remodeling and migrating to the periphery (Figure 2M), resulted in Zmiz1iECKO retinas with a relatively normal patterned superficial vascular plexus that reached the periphery at P12 (Figures 2N,O). However, the vascularization of the deeper vascular plexus was significantly affected, as notably fewer vessels were observed in comparison to Zmiz1fl/fl mice (Figures 2N–Q). Specifically, compared to control retinas, deep layer vessels were absent at the periphery in Zmiz1 mutants, while the vessels that did invade more centrally appeared disorganized (Figures 2P,Q). Therefore, these findings indicated that Zmiz1 is also important for the perpendicular, angiogenic sprouting events required to populate the deeper layer of the retina.
Based on the findings above, Zmiz1 is essential during periods of active retinal angiogenesis, which is consistent with its expression levels in ECs during early mouse retina development (Jeong et al., 2017) (Supplementary Figure S1D). However, the role of Zmiz1 in vascular maintenance during adulthood was unknown. To address this, we induced deletion of Zmiz1 in adult mice from P28-P31 and analyzed retinas at P42 and P60 (Supplementary Figure S4A). Interestingly, Zmiz1iECKO mice did not display any obvious vascular patterning or morphological defects (Supplementary Figure S4B). Evaluation of vascular density in both the superficial and deep vascular plexi revealed no significant difference in control and Zmiz1iECKO mice (Supplementary Figures S4C–G). Thus, these results suggested that Zmiz1 is not required for maintenance of vascular patterning and morphology during adulthood, which notedly corresponds to a continuous decrease in Zmiz1 endothelial cell expression from P15-P50 in the retina (Jeong et al., 2017) (Supplementary Figure S1D).
Loss of Zmiz1 leads to increased retinal vessel regression
During postnatal retinal angiogenesis, the growth of initial blood vessels to the formation of a mature vascular network involves various cellular processes, such as EC migration, proliferation, apoptosis, vessel regression, and vessel remodeling. To assess whether some of these processes were defective and contributed to the vascular phenotypes in Zmiz1iECKO retinas, we first analyzed EC proliferation and apoptosis. EC proliferation was assessed by co-staining for ETS-related gene (ERG; EC-specific nuclei marker) and the proliferation marker Ki67. We observed no significant difference in the rate of EC proliferation between Zmiz1iECKO and control retinas at P7 (Figures 3A,B). Similarly, co-staining for CLEAVED CASPASE 3 (a key protease in apoptosis) and ERG revealed that EC apoptosis at P7, which is normally low, was unaffected by loss of Zmiz1 (Figures 3C,D). Next, we assessed blood vessel regression by co-immunolabeling for IB4 and the basement matrix component COLLAGEN IV (COL IV). During vascular remodeling, COL IV sleeves devoid of ECs (COL IV positive; IB4 negative) are observed as vessels regress, and alterations in this process are typically associated with unstable, defective vascular remodeling. We identified a significant increase in the number of COL IV sleeves within Zmiz1iECKO retinas compared to the controls, indicative of increased vessel regression and decreased vascular stability (Figures 3E,F). However, when we assessed this at P10, we did not observe differences in vessel regression between Zmiz1 control and mutant mice (Supplementary Figures S5E,F). In addition, we examined pericyte vessel coverage since EC-pericyte interactions are also known to affect the angiogenesis process (Egginton et al., 1996; Chiaverina et al., 2019). However, immunofluorescent antibody staining for pericyte marker NEURON-GLIAL ANTIGEN 2 (NG2) and IB4 revealed no noticeable differences in pericyte coverage between the control and Zmiz1iECKO retinas at P7 (Figures 3G,H). Moreover, we did not identify any changes in NG2 coverage or rates of EC proliferation and death at P10 when comparing Zmiz1f/f and Zmiz1iECKO retinas (Supplementary Figure S5). These data established that EC proliferation, EC apoptosis, and pericyte coverage are unchanged in the absence of Zmiz1. Conversely, loss of Zmiz1 in ECs resulted in substantial increases in vessel regression at P7 indicating an overall contribution of Zmiz1 to vascular stability during angiogenic remodeling of the retinal vascular network.
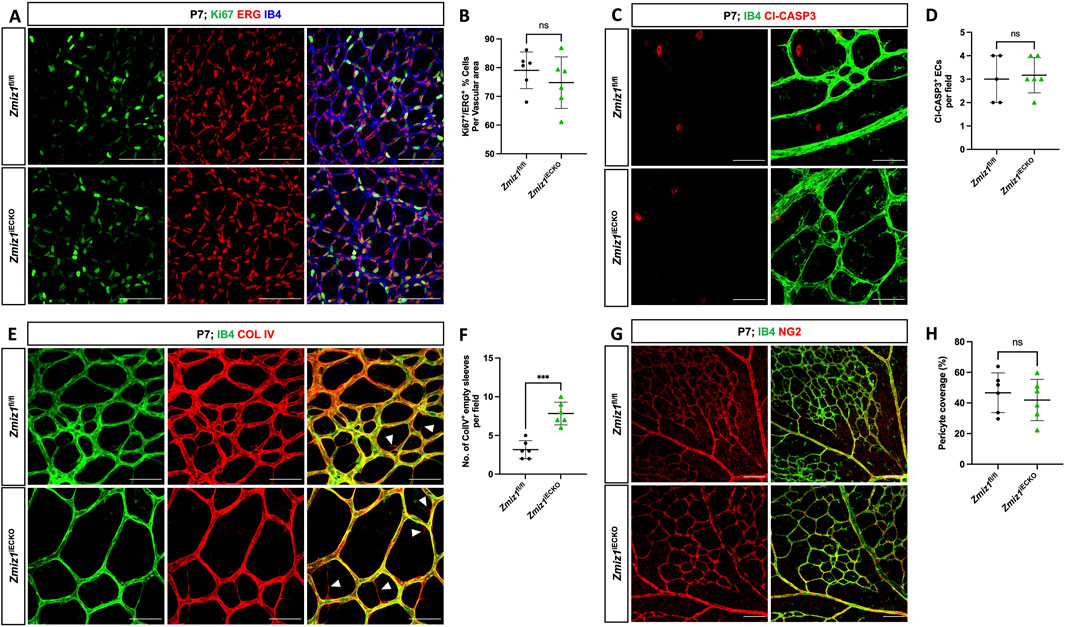
Figure 3. Deletion of Zmiz1 in ECs results in increased retinal vascular regression. (A) Representative images of P7 retinas stained with Ki67, ERG, and IB4. Scale bars: 100 μm. (B) Quantification of Ki67+ proliferating ECs (ERG+) in Zmiz1fl/fl (n = 6) and Zmiz1iECKO (n = 6) mice at P7. (C) Representative images of P7 retinas stained with IB4 and CLEAVED CASPASE 3 (Cl-CASP3). Scale bars: 50 μm. (D) Quantification of Cl-CASP3+ apoptotic ECs in Zmiz1fl/fl (n = 6) and Zmiz1iECKO (n = 6) mice at P7. (E) Representative images of P7 retinas stained with IB4 and COLLAGEN IV (COL IV; white arrowheads indicate empty COL IV sleeves devoid of ECs). Scale bars 50 μm. (F) Quantification of empty COL IV sleeves in Zmiz1fl/fl (n = 6) and Zmiz1iECKO (n = 6) retinas at P7. (G) Representative images of P7 retinas stained with IB4 and NEURAL/GLIAL ANTIGEN 2 (NG2). Scale bars 100 μm. (H) Quantification of pericyte coverage in Zmiz1fl/fl (n = 6) and Zmiz1iECKO (n = 6) retinas at P7. Error bars represent mean ± s.e.m; two-tailed unpaired t-test. ns (not significant; P > 0.05), ***P < 0.001.
Zmiz1 mutants exhibit defective sprouting angiogenesis
During initial observations of the retinal vasculature (Figure 2), we noticed an obvious difference in the sprouting angiogenic blood vessels at the vascular front between Zmiz1 control and mutant retinas. Specifically, there appeared to be fewer vessels sprouting toward the retina periphery in Zmiz1iECKO retinas. Indeed, quantification confirmed a significant decrease in the number of P7 vascular sprouts in Zmiz1 mutant retinas compared to controls (Figures 4A,B; Supplementary Figure S6). This reduction was notable because deficits in sprouting angiogenesis regularly result in retinal vascular phenotypes like those we observed in the Zmiz1iECKO retinas (Par et al., 2019; Arribas et al., 2024). Therefore, to further evaluate the role of Zmiz1 in sprouting angiogenesis, we performed the fibrin bead assay with immortalized human aortic ECs (telomerase human aortic EC; TeloHAEC). Beads were coated with equal numbers of TeloHAECs treated with either scramble-control siRNA or Zmiz1-targeted siRNA and allowed to sprout for 120 h. Control siRNA treated TeloHAECs showed robust sprout formation, whereas cells subjected to Zmiz1 siRNA displayed a significant reduction in the length and number of sprouting vessels (Figures 4C,D). Quantitative polymerase chain reaction (qPCR) verified that Zmiz1 expression was appreciably reduced in Zmiz1 siRNA TeloHAECs (Figure 4E).
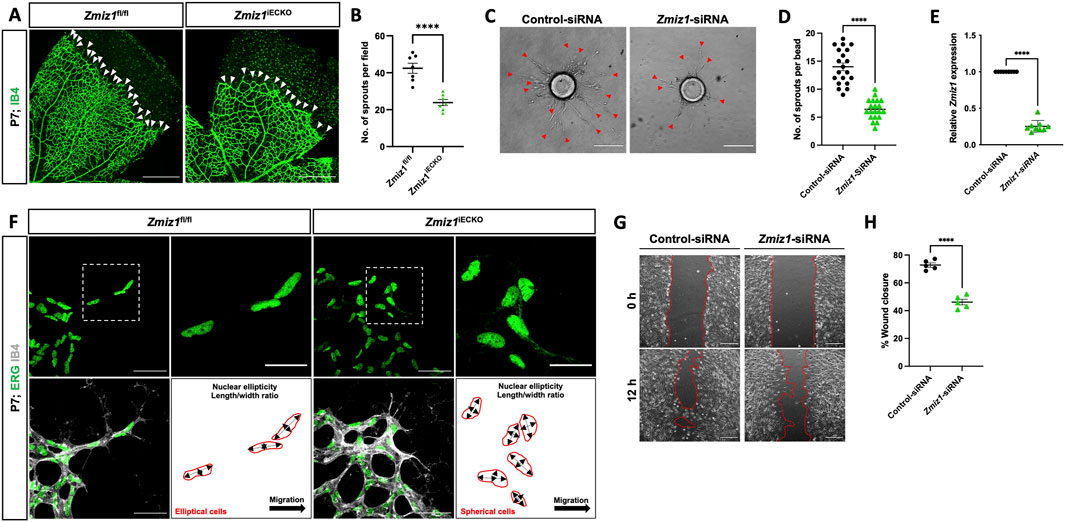
Figure 4. Zmiz1 is required for sprouting angiogenesis and EC migration. (A) Images of IB4 immunolabeled retinas at the vascular front in P7 Zmiz1fl/fl and Zmiz1iECKO mice (white arrowheads mark the leading-edge vascular sprouts). Scale bar, 250 μm. (B) Quantification of the number of sprouts within Zmiz1fl/fl (n = 7) and Zmiz1iECKO (n = 8) retinas at P7 (quantifications represent the average number of sprouts within each leaflet). (C) Representative images of bead assays embedded in 3D fibrinogen gel at 120 h following control and Zmiz1 siRNA treatments (red arrowheads mark sprouts emanating from the bead). Scale bar, 100 μm. (D) Quantification of the number of sprouts per bead (n = 20). (E) qRT-PCR analysis of control and Zmiz1 siRNA treated TeloHAECs for Zmiz1 mRNA levels normalized to GAPDH transcripts (n = 3). (F) Close-up images of retinas stained for IB4 and ETS related gene (ERG) to mark EC nuclei at the vascular front within P7 Zmiz1fl/fl (n = 7) and Zmiz1iECKO (n = 8) retinas. Cell-shape schematics indicating nuclear ellipticity within each group are represented. Scale bar, 50 μm and 25 μm for insets, which highlight sprouts. (G) Images of scratch wound assays performed on TeloHAEC confluent monolayers following control and Zmiz1 siRNA treatments. Images at 0 and 12 h following the scratch are depicted. Scale bar, 200 μm. (H) Quantification of the percentage of wound closure in TeloHAECs subjected to the various siRNA treatments in G (n = 5). Error bars represent mean ± s.e.m; two-tailed unpaired t-test: ****P < 0.0001.
Previous reports noted defective migration of neuronal cells upon loss of Zmiz1 function in vivo (Carapito et al., 2019). This led us to explore the possible role of Zmiz1 in the regulation of tip-cell behavior and migration during sprout formation/elongation—tip cells are at the leading end of the sprouting periphery vessels and direct vessel growth and migration. In this context, we first analyzed the nuclei shape of tip-cell-associated ECs at the vascular front; elliptical nuclei are often connected with directed migration, while spherical-shaped nuclei are linked to non-migratory cells (Kim et al., 2019). In control mice, ERG-positive nuclei of tip ECs were predominately elliptical in shape and directed toward the avascular area where the retinal vessels typically migrate. (Figure 4F). However, in Zmiz1iECKO mice, the nuclei of ECs at the leading edge were more spherical, including those in the comparatively shorter sprouts, suggesting an impaired cell migration phenotype (Figure 4F). To obtain a better grasp on Zmiz1 function in EC migration, cell migration assays (scratch assay) were carried out with TeloHAECs. Under normal culture conditions, Zmiz1 siRNA TeloHAECs showed a decreased repopulation of cells into the “wound” area in comparison to control siRNA-treated ECs (Figures 4G,H) further suggesting defective migratory properties in the absence of Zmiz1.
Zmiz1 inactivation leads to gene expression changes in the endothelium, including reduced expression of tip-cell-associated genes
To further define the role of Zmiz1 in the regulation of EC behavior, we performed transcriptional profiling of isolated retinal ECs (iRECs) at P7 (Figure 5A). RNA-Sequencing (RNA-seq) analysis of three biological replicates of pooled iRECs from control and Zmiz1iECKO mice revealed 854 upregulated and 1,530 downregulated genes following the loss of Zmiz1 (Figure 5B). Gene ontology (GO) analysis unveiled upregulated genes enriched for biological processes such as immune response and T-cell activation (Figure 5C), which is relevant to previous work demonstrating an important role for Zmiz1 in T cell development and leukemogenesis (Pinnell et al., 2015). Importantly, downregulated genes, representing targets that are likely positively regulated by Zmiz1, were enriched for GO terms associated with developmental processes such as angiogenesis, blood vessel morphogenesis and vascular development (Figure 5D). These transcriptional findings were in alignment with our phenotypic embryonic and retinal studies (Figures 1–4). Given our overall findings connecting Zmiz1 function to pro-angiogenic growth, especially at the retinal vascular front, we examined the iREC RNA-seq data for indications that Zmiz1 affects transcriptional regulation of tip-cell-associated genes involved in retinal angiogenesis. Analysis revealed that several notably well-characterized genes either enriched in tip-cell expression and/or involved in tip-cell formation and function, such as Apln (Del Toro et al., 2010), Angpt2 (Gale et al., 2002), Esm1 (Rocha et al., 2014), and Cxcr4 (Strasser et al., 2010) were markedly downregulated in Zmiz1 mutant retinas (Figure 5E). Furthermore, immunofluorescent antibody stains for ESM1 and CXCR4 confirmed that expression of these tip-cell markers was notably reduced at the vascular front in Zmiz1 deficient retinas, while controls showed typical, strong expression in ECs at the leading edge (Figures 5F,G). Together, the iREC RNA-seq data and expression analysis provide further evidence that Zmiz1 is a critical tip-cell regulator required for proper retinal sprouting angiogenesis.
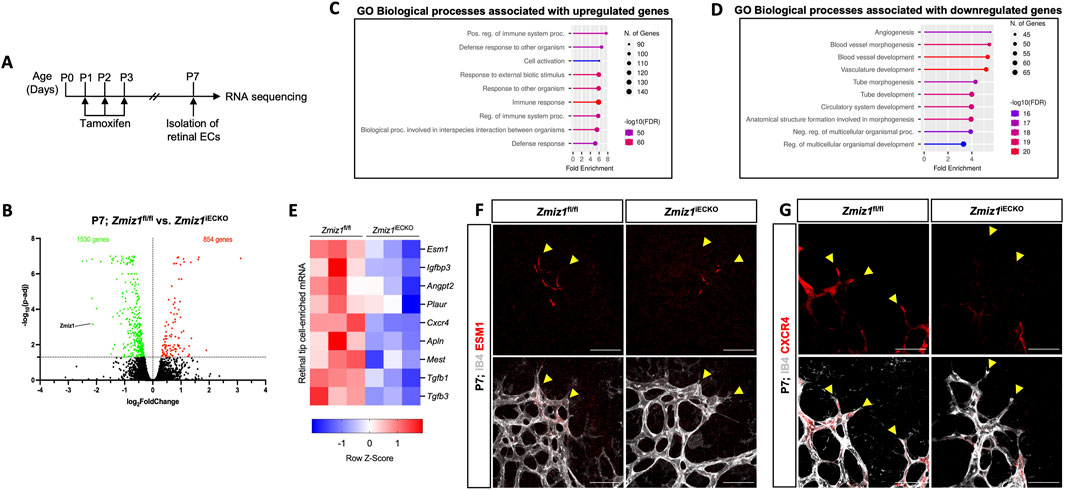
Figure 5. Expression of retinal endothelial tip-cell markers is downregulated upon Zmiz1 genetic ablation. (A) Illustration depicting the strategy used for RNA-seq analysis on isolated retinal ECs (iRECs). (B) MA plot of differentially expressed genes between Zmiz1fl/fl and Zmiz1iECKO iRECs at P7. Statistically significant upregulated and downregulated genes are depicted as red and green dots, respectively. Zmiz1 is highlighted and confirms reduced expression in Zmiz1iECKO iRECs. (C,D) Gene ontology (GO) analysis of biological process terms enriched in upregulated (C) and downregulated (D) genes of Zmiz1iECKO iRECs (false discovery rate (FDR) are shown). (E) Clustered heat map of gene count Z scores for retinal tip-cell enriched mRNAs. Columns represent pools of different biological samples (8-10 retinas/pool). We note that in our analyses, the floxed conditions are normalized to the iECKO conditions. (F,G) Close-up views of Zmiz1fl/fl and Zmiz1iECKO retinal tip-cell regions immunofluorescently labeled for IB4 and the tip-cell enriched markers ESM1 (F) and CXCR4 (G). Scale bars, 25 μm. Notice overall reduced and sometimes absent expression of ESM1 and CXCR4 in the tip-cells (yellow arrowheads) of Zmiz1 retinas.
Loss of Zmiz1 in an OIR retinopathy model leads to reduced neovascularization
Zmiz1 has been linked to various pathological features and diseases, such as leukemia (Pinnell et al., 2015), osteosarcoma (Zhou et al., 2022), diabetes (Alghamdi et al., 2022), erythropoiesis (Castillo-Castellanos et al., 2021), and several neurodevelopmental disorders (Carapito et al., 2019; Lu et al., 2022; Phetthong et al., 2021). To investigate whether Zmiz1 might have a role in pathological angiogenesis, we performed oxygen-induced retinopathy (OIR) studies, which are designed to emulate conditions similar to ocular retinopathies. Both Zmiz1 control and mutant pups were housed in a hyperoxia chamber (75% oxygen) from P7 to P12 to allow blood vessel regression and death. At P12 they were transferred to normal, room oxygen levels to create a hypoxic environment that promotes neovascularization (Figure 6A). Pups were induced with tamoxifen for 3 consecutive days once placed in a normal oxygen environment (P12-14) and retinas were harvested and analyzed at P17. Compared to the Zmiz1fl/fl mice, Zmiz1 deficient retinas exhibited significantly larger areas of vaso-obliteration (areas devoid of vasculature) at P17 suggesting that neoangiogenesis was defective (Figures 6B–D). Accordingly, Zmiz1iECKO retinas displayed significantly fewer regions with neovascular tufts—areas of active growth and revascularization that occur in the OIR retinal model as the blood vessel network undergoes a corrective remodeling phase—than Zmiz1fl/fl retinas (Figures 6B–D). Additionally, we surveyed a previous RNA-seq data set associated with the OIR model to ascertain if Zmiz1 expression changes during the neovascularization stage (Pauleikhoff et al., 2023). Quantification of Zmiz1 mRNA showed that there is no statistical difference in expression between OIR and non-OIR treated C57BL6 mice at P14 or P17 (Supplementary Figure S7A–C) indicating that Zmiz1 expression is not upregulated in response to hypoxia and the generation of new blood vessels. To validate this finding, we carried out similar experiments and confirmed by qPCR that the mRNA levels of Zmiz1 are not statistically different between OIR and non-OIR retinas in C57BL6 mice at P17 (Supplementary Figure S7D). Further, there were no significant differences in Zmiz1 expression between OIR and non-OIR subjected retinas at P12 when the vaso-obliteration process is complete (Supplementary Figure S7E).
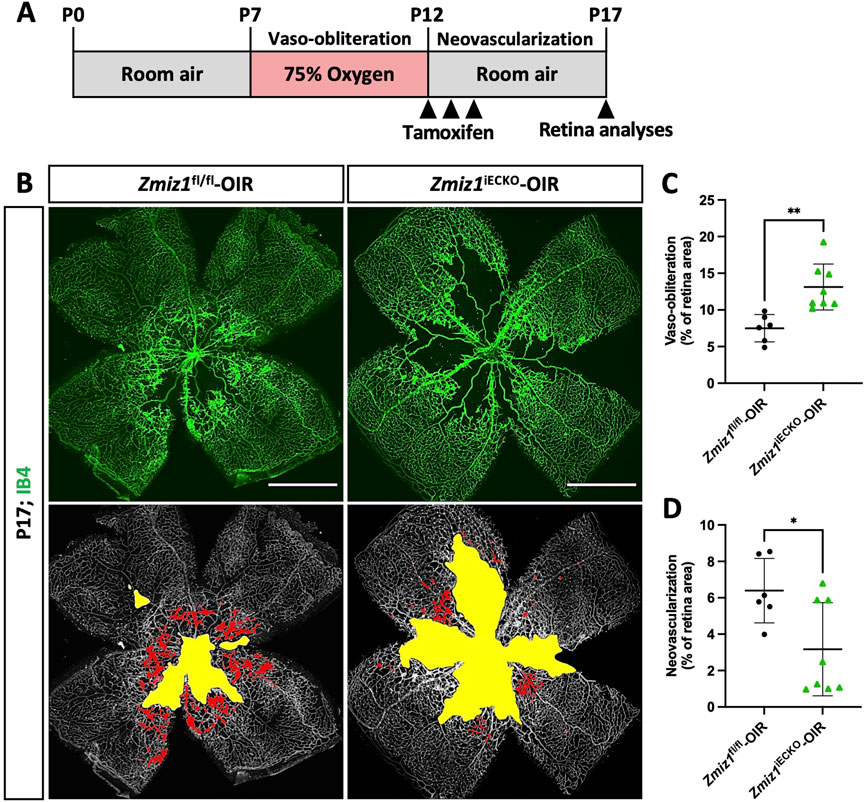
Figure 6. Loss of endothelial Zmiz1 impairs pathological angiogenesis. (A) Schematic summary of the OIR strategy implemented to assess Zmiz1 in pathological neovascularization. (B) Representative whole-mount images of the vasculature immunolabeled with IB4 in Zmiz1fl/fl-OIR (n = 6) and Zmiz1iECKO-OIR (n = 8) P17 retinas. Yellow space demarcates the avascular area, while the red markings highlight the neovascular tufts within each retina. Scale bars: 1,000 μm. (C,D) Quantification of the areas of vaso-obliteration (avascular space) and retinal neovascularization in Zmiz1fl/fl-OIR (n = 6) and Zmiz1iECKO-OIR (n = 8) P17 mice. Error bars represent mean ± s.e.m; two-tailed unpaired t-test. ns (not significant; P > 0.05), *P < 0.05, **P < 0.01.
Lastly, to assess whether Zmiz1 might have a role during the vaso-obliteration phase (P7-12), we induced deletion of Zmiz1 from P7-P9 and examined the avascular areas at P12. Interestingly, we did not observe any differences in the overall sizes of the avascular zones between Zmiz1fl/fl and Zmiz1iECKO retinas (Supplementary Figure S8). Taken together, these experimental results demonstrated that Zmiz1 is required for hypoxia-regulated angiogenesis during the revascularization process in a retinopathy setting but is not important during the vaso-obliteration step.
Discussion
The process of new blood vessel formation is vital for organismal growth and survival. Our study highlighted an important role of Zmiz1 during embryonic and postnatal retinal vascular development. We showed that constitutive loss of endothelial Zmiz1 during embryogenesis resulted in vascular defects and embryonic lethality. Using inducible EC-specific Cre mice, we demonstrated that angiogenesis was disrupted in Zmiz1-deficient ECs both in physiological and pathological settings, providing evidence of Zmiz1 as an important regulator of the developing vasculature. Specifically, early postnatal deletion of endothelial Zmiz1 resulted in delayed retinal outgrowth of superficial vascular plexus both at P7 and P10. In addition, late induction of endothelial Zmiz1 deletion severely impaired perpendicular vascular sprouting resulting in reduced densities of vascular plexus in the deep layer at P12. The disruption in vascular outgrowth and morphology in Zmiz1 mutants is potentially attributed to the reduction in tip cell numbers, subsequent decreased sprout formation and reduced expression of crucial tip-cell genes. We also observed that Zmiz1 is critical for intraretinal vascularization following ischemic injury in the OIR retina. Taken together, our studies begin to provide important clues that highlight the complex role of Zmiz1 in angiogenic development.
Although our work provides new information detailing the role of Zmiz1 in angiogenesis, a full appreciation of the transcriptional mechanisms by which Zmiz1 regulates blood vessel growth via angiogenesis will require additional investigation. However, transcriptional profiling of Zmiz1-deficient ECs in this study did uncover some noteworthy targets, including Apln (Del Toro et al., 2010), Angpt2 (Gale et al., 2002), Esm1 (Rocha et al., 2014) and Cxcr4 (Strasser et al., 2010), that are potentially and directly regulated by Zmiz1 (Figure 5). These genes are well-established to be associated with the acquisition of specialized tip/stalk-cell phenotypes in ECs during the sprouting process (Guo et al., 2024). Consequently, it is reasonable to infer that downregulation of their expression would lead to abnormal vascular development, such as that observed in our Zmiz1 mutant retinas. Future experiments focused on elucidating the precise actions of Zmiz1 on this subset of genes could offer valuable insights into the regulatory mechanisms that govern sprouting angiogenesis.
In a similar context, several prominent pathways, including VEGF-A and NOTCH signaling, are critical players regulating tip-cell and stalk-cell selection, whose ratio is critical during the angiogenic sprouting process in physiological and pathological conditions (Blanco and Gerhardt, 2013; Ubezio et al., 2016). It is plausible, for instance, that the defective patterning of arteries observed in Zmiz1iECKO mice is due to misregulation of the DLL4-NOTCH1 pathway as this signaling cascade is important for arteriogenesis during vascular development (Liu et al., 2003). In support of this possibility, previous work demonstrated a transcriptional partnership between Zmiz1 and NOTCH1 in the regulation of T-cell phenotypic contributions to Leukemia (Pinnell et al., 2015). Additionally, microarray analysis following inactivation of VEGF-A in cultured human umbilical vein ECs (HUVECs) revealed downregulation of Zmiz1 in the blood-vessel gene cluster (Domigan et al., 2015), which could also have an impact on tip/stalk-cell mediated growth of the vasculature. Therefore, several studies indicate a potential link between VEGF-A, NOTCH1 and Zmiz1 in regulation of sprouting angiogenesis. However, Zmiz1 may influence angiogenesis through mechanisms independent of the VEGFA and NOTCH signaling pathways, further underscoring the existing gap in our understanding of its transcriptional contributions to angiogenesis.
Our characterization of endothelial deficient mice demonstrated that the loss of Zmiz1 in ECs influenced early vascular development, as evidenced by the aberrant embryonic vasculature, and inhibited vascular expansion and impaired perpendicular branching of retinal vasculature. However, the inactivation of Zmiz1 in mature vasculature with quiescent ECs had no obvious effect on overall blood vessel integrity and remodeling. These findings suggest a more defined role of Zmiz1 in early embryonic and postnatal life, which will be essential to unraveling its molecular regulatory mechanisms. Interestingly, gene set enrichment analysis (GSEA) of gene sets identified during different retinal developmental stages revealed higher enrichment of genes associated with NOTCH signaling pathway at P6 when sprouting angiogenesis is highly prevalent in the superficial vasculature (Jeong et al., 2017). Thus, considering the connection of Zmiz1 to NOTCH signaling and the similar developmental timing, we speculate that Zmiz1 interacts with key putative TFs regulating EC behavior during the early phase of vascular development.
This study also revealed that Zmiz1 may have an important role during pathophysiological angiogenesis, as loss of Zmiz1 resulted in the reduced ability to revascularize the retina in the OIR experiments. This finding may be relevant given the building evidence linking alterations in Zmiz1 to various disease settings. For example, several patients with a Zmiz1 variant-associated syndromic neurodevelopment disorder also displayed congenital cardiovascular malformations. Although these abnormalities were not described in detail, it is tempting to postulate that they could be related to defects in the endothelial lineage. Thus, future assessment and specifics of these cardiovascular issues will be especially interesting, as our preliminary data suggest that defective Zmiz1 function in the endothelium could have a causal role in the cardiac phenotypes of these patients.
In the field of vascular biology, many TFs that are important for EC differentiation and maturation have been identified, however their mechanisms of regulation are not fully defined. This study introduces a transcription cofactor with a previously unknown specific role in vascular development and function that may be involved in regulating the activity of vascular transcription factors, including those described above. Thus, our study begins to delineate an important role of endothelial Zmiz1 in physiological and pathological angiogenesis.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/geo/, GSE242406.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. The animal study was approved by Tulane University Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
NP: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review and editing. SB: Data curation, Methodology, Formal analysis, Validation, Writing – review and editing. AdB: Data curation, Methodology, Formal analysis, Validation, Writing – review and editing. RKC: Data curation, Investigation, Methodology, Writing – original draft, Writing – review and editing. AvB: Investigation, Methodology, Writing – original draft, Writing – review and editing. KC: Investigation, Methodology, Writing – original draft, Writing – review and editing. MC: Writing – review and editing, Resources. SM: Conceptualization, Resources, Supervision, Writing – original draft, Writing – review and editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Tulane University Committee of Research (COR) Fellowship, NIH-R01 HL139713 (SMM), NIH-R01 HL163196 (SMM) and NIH/NIAID-R01 AI136941 (MYC).
Acknowledgments
We would like to thank Jovanny Zabaleta and Jone Garai at The Louisiana Cancer Research Consortium (LCRC) Translational Genomics Core Center for their continued support of our RNA-Seq experiments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2025.1570587/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Summary of EC-specific Zmiz1 expression within various tissues. (A) Analysis of Zmiz1 expression levels in ECs and total tissue of P7 brain, kidney, liver, lung, and adult brain using the Vascular Endothelial Cell Trans-omics Resource Database (VECTRDB). (B) Zmiz1 expression in each cell type isolated from an adult mouse brain can be visualized within the violin plots. (C) Analysis of Zmiz1 expression levels in P7 isolated brain ECs within subtypes using single cell RNA-seq data in VECTRDB. (D) Expression levels of Zmiz1 in murine retinal endothelial cells during postnatal development determined using the available bulk RNA sequencing data. (E) Representative images of P7 retinas stained with IB4 and Zmiz1 (A, artery; V, vein). Scale bars: 200 μm.
SUPPLEMENTARY FIGURE S2 | Heterozygous loss of Zmiz1 in ECs leads to defects in radial outgrowth and vascular density. (A) Representative images of whole-mount retinas stained with IB4 and α-SMA at P7. Scale bars: 1,000 μm. (B,C) Quantification of vascular outgrowth and vascular density in Zmiz1fl/wt (n = 8) and Zmiz1fl/wt;iECKO (n = 16) retinas at P7. Error bars represent mean ± s.e.m; two-tailed unpaired t-test. ns (not significant; P > 0.05), ***P < 0.001, ****P < 0.0001.
SUPPLEMENTARY FIGURE S3 | Zmiz1f/f and Zmiz1fl/fl; Cdh5-CreERT2 retinas without tamoxifen exhibit similar vascular parameters. (A) Representative images of whole-mount P7 retinas stained for IB4 (green) and alpha-SMOOTH MUSCLE ACTIN (α-SMA; red). Dotted white circle represents outgrowth in Zmiz1fl/fl retina. Scale bar, 1,000 μm. A, artery; V, vein. (B-E) Quantification of noted morphometric vascular parameters of Zmiz1fl/fl (n = 6) and Zmiz1iECKO (n = 5) retinas not subjected to tamoxifen. No significant differences were observed, except in vascular outgrowth. However, it is noted that the vascular outgrowth here in Zmiz1iECKO retinas is similar to Zmiz1f/f retinas with tamoxifen (Figure 2D). Error bars represent mean ± s.e.m; two-tailed unpaired t-test. ns (not significant; P > 0.05), *P = 0.0383.
SUPPLEMENTARY FIGURE S4 | Endothelial Zmiz1 is dispensable for vascular remodeling and maintenance during adulthood. (A) Schematic showing tamoxifen administration in 4 weeks old mice and analysis at P42 and P60. (B) Representative images of whole-mount retinas stained with IB4 at P42 and P60. Scale bars 1,000 μm. (C) Representative images of IB4+ vessels in superficial and deep layers of Zmiz1fl/fl and Zmiz1iECKO retinas at P42. Scale bars: 200 μm. (D,E) Quantification of superficial and deep vascular plexus density in Zmiz1fl/fl (n = 6) and Zmiz1iECKO (n = 8) retinas at P42. (F,G) Quantification of superficial and deep vascular plexus density in Zmiz1fl/fl (n = 7) and Zmiz1iECKO (n = 6) retinas at P60. Error bars represent mean ± s.e.m; two-tailed unpaired t-test. ns (not significant; P > 0.05).
SUPPLEMENTARY FIGURE S5 | No changes in retinal vascular regression, pericyte coverage and EC proliferation and death are observed in Zmiz1 mutant mice at P10. (A) Representative images of P10 retinas stained with Ki67, ERG, and IB4. Scale bars: 100 μm. (B) Quantification of Ki67+ proliferating ECs (ERG+) in Zmiz1fl/fl (n = 3) and Zmiz1iECKO (n = 7) mice at P10. (C) Representative images of P10 retinas stained with IB4 and CLEAVED CASPASE 3 (Cl-CASP3). Scale bars: 50 μm. (D) Quantification of Cl-CASP3+ apoptotic ECs in Zmiz1fl/fl (n = 3) and Zmiz1iECKO (n = 7) mice at P10. (E) Representative images of P10 retinas stained with IB4 and COLLAGEN IV (COL IV; white arrowheads indicate empty COL IV sleeves devoid of ECs). Scale bars 50 μm. (F) Quantification of empty COL IV sleeves in Zmiz1fl/fl (n = 3) and Zmiz1iECKO (n = 7) retinas at P10. (G) Representative images of P10 retinas stained with IB4 and NEURAL/GLIAL ANTIGEN 2 (NG2). Scale bars 100 μm. (H) Quantification of pericyte coverage in Zmiz1fl/fl (n = 3) and Zmiz1iECKO (n = 7) retinas at P10. Error bars represent mean ± s.e.m; two-tailed unpaired t-test. ns (not significant; P > 0.05).
SUPPLEMENTARY FIGURE S6 | Whole-mount retinas from Figure 4. (A) Whole-mount Zmiz1fl/fl and Zmiz1iECKO P7 retinas from Figure 4A. IB4 (green) at P7.
SUPPLEMENTARY FIGURE S7 | Expression of Zmiz1 is unchanged during the neovascularization phase in the OIR model. (A) Schematic summary of the OIR model with RNA-seq analysis time points noted by black and blue arrow heads. Black arrows indicate time points from prior public data sets and blue arrows indicate time points from the current studies. (B,C) Quantifications of Zmiz1 normal read counts obtained from publicly available RNA-seq data comparing P14 (B) and P17 (C) C57BL/6J retinas subjected to non-OIR (nOIR) and OIR environments (Pauleikhoff et al., 2023). Note no changes in Zmiz1 expression levels during the neovascularization phase. nOIR_P14 (n = 7), OIR_P14 (n = 6), nOIR_P17 (n = 6) and OIR_P17 (n = 8) retinas. (D,E) Relative Zmiz1 expression levels from non-OIR (nOIR) and OIR subjected C57BL/6J retinas at P12 (D) and P17 (E), as determined by qRT-PCR and normalized to β-actin. nOIR_P12 (n = 4), OIR_P12 (n = 5), nOIR_P17 (n = 4) and OIR_P17 (n = 5) retinas. Error bars represent mean ± s.e.m; two-tailed unpaired t-test. ns (not significant).
SUPPLEMENTARY FIGURE S8 | Loss of endothelial Zmiz1 has no additional impact on the vasculature during the vaso-obliteration stage in the OIR model. (A) Schematic summary of the strategy implemented to assess Zmiz1 contributions during the vaso-obliteration phase in the OIR model. (B) Representative whole-mount images of the vasculature immunolabeled with IB4 in Zmiz1fl/fl-OIR (n = 8) and Zmiz1iECKO-OIR (n = 9) P12 retinas. Yellow space demarcates the avascular area, while red markings indicate neovascular tufts within each retina (note the absence of tufts since this is prior to the neovascularization stage). Scale bars: 1,000 μm. (C,D) Quantification of the areas of vaso-obliteration (avascular space) and retinal neovascularization in Zmiz1fl/fl-OIR (n = 8) and Zmiz1iECKO-OIR (n = 8) P12 mice. Error bars represent mean ± s.e.m; two-tailed unpaired t-test. ns, not significant (P > 0.05).
References
Alghamdi, T. A., Krentz, N. A. J., Smith, N., Spigelman, A. F., Rajesh, V., Jha, A., et al. (2022). Zmiz1 is required for mature β-cell function and mass expansion upon high fat feeding. doi:10.1101/2022.05.18.492530
Arribas, V., Onetti, Y., Ramiro-Pareta, M., Villacampa, P., Beck, H., Alberola, M., et al. (2024). Endothelial TDP-43 controls sprouting angiogenesis and vascular barrier integrity, and its deletion triggers neuroinflammation. JCI Insight 9 (5), e177819. doi:10.1172/jci.insight.177819
Beliakoff, J., Lee, J., Ueno, H., Aiyer, A., Weissman, I. L., Barsh, G. S., et al. (2008). The PIAS-like protein Zimp10 is essential for embryonic viability and proper vascular development. Mol. Cell Biol. 28 (1), 282–292. doi:10.1128/MCB.00771-07
Blanco, R., and Gerhardt, H. (2013). VEGF and notch in tip and stalk cell selection. Cold Spring Harb. Perspect. Med. 3 (1), a006569. doi:10.1101/cshperspect.a006569
Carapito, R., Ivanova, E. L., Morlon, A., Meng, L., Molitor, A., Erdmann, E., et al. (2019). ZMIZ1 variants cause a syndromic neurodevelopmental disorder. Am. J. Hum. Genet. 104 (2), 319–330. doi:10.1016/j.ajhg.2018.12.007
Carmeliet, P. (2003). Angiogenesis in health and disease. Nat. Med. 9 (6), 653–660. doi:10.1038/nm0603-653
Castillo-Castellanos, F., Ramírez, L., and Lomelí, H. (2021). zmiz1a zebrafish mutants have defective erythropoiesis, altered expression of autophagy genes, and a deficient response to vitamin D. Life Sci. 284, 119900. doi:10.1016/j.lfs.2021.119900
Chiaverina, G., Di Blasio, L., Monica, V., Accardo, M., Palmiero, M., Peracino, B., et al. (2019). Dynamic interplay between pericytes and endothelial cells during sprouting angiogenesis. Cells 8 (9), 1109. doi:10.3390/cells8091109
Crawford, T., Alfaro Iii, D., Kerrison, J., and Jablon, E. (2009). Diabetic retinopathy and angiogenesis. Curr. Diabetes Rev. 5 (1), 8–13. doi:10.2174/157339909787314149
Crist, A. M., Lee, A. R., Patel, N. R., Westhoff, D. E., and Meadows, S. M. (2018). Vascular deficiency of Smad4 causes arteriovenous malformations: a mouse model of hereditary hemorrhagic telangiectasia. Angiogenesis 21 (2), 363–380. doi:10.1007/s10456-018-9602-0
De Val, S., and Black, B. L. (2009). Transcriptional control of endothelial cell development. Dev. Cell 16 (2), 180–195. doi:10.1016/j.devcel.2009.01.014
Del Toro, R., Prahst, C., Mathivet, T., Siegfried, G., Kaminker, J. S., Larrivee, B., et al. (2010). Identification and functional analysis of endothelial tip cell–enriched genes. Blood 116 (19), 4025–4033. doi:10.1182/blood-2010-02-270819
Domigan, C. K., Warren, C. M., Antanesian, V., Happel, K., Ziyad, S., Lee, S., et al. (2015). Autocrine VEGF maintains endothelial survival through regulation of metabolism and autophagy. J. Cell Sci. 128 (12), 2236–2248. doi:10.1242/jcs.163774
Egginton, S., Hudlicka, O., Brown, M. D., Graciotti, L., and Granata, A. L. (1996). In VivoPericyte–Endothelial cell interaction during angiogenesis in adult cardiac and skeletal muscle. Microvasc. Res. 51 (2), 213–228. doi:10.1006/mvre.1996.0022
Fewings, N. L., Gatt, P. N., McKay, F. C., Parnell, G. P., Schibeci, S. D., Edwards, J., et al. (2017). The autoimmune risk gene ZMIZ1 is a vitamin D responsive marker of a molecular phenotype of multiple sclerosis. J. Autoimmun. 78, 57–69. doi:10.1016/j.jaut.2016.12.006
Gale, N. W., Thurston, G., Hackett, S. F., Renard, R., Wang, Q., McClain, J., et al. (2002). Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev. Cell 3 (3), 411–423. doi:10.1016/s1534-5807(02)00217-4
Ge, S. X., Jung, D., and Yao, R. (2020). ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics 36 (8), 2628–2629. doi:10.1093/bioinformatics/btz931
Greenberg, D. A. (1998). Angiogenesis and stroke. Drug News Perspect. 11 (5), 265–270. doi:10.1358/dnp.1998.11.5.657287
Guo, Y., Zhang, S., Wang, D., Heng, B. C., and Deng, X. (2024). Role of cell rearrangement and related signaling pathways in the dynamic process of tip cell selection. Cell Commun. Signal 22 (1), 24. doi:10.1186/s12964-023-01364-1
Hamik, A., Wang, B., and Jain, M. K. (2006). Transcriptional regulators of angiogenesis. Arterioscler. Thromb. Vasc. Biol. 26 (9), 1936–1947. doi:10.1161/01.ATV.0000232542.42968.e3
Huang, K., Crist, A. M., Patel, N. R., Blanks, A., Carter, K., Cleaver, O., et al. (2020). Annexin A3 is necessary for parallel artery-vein alignment in the mouse retina. Dev. Dyn. 249 (5), 666–678. doi:10.1002/dvdy.154
Jeong, H. W., Hernández-Rodríguez, B., Kim, J., Kim, K. P., Enriquez-Gasca, R., Yoon, J., et al. (2017). Transcriptional regulation of endothelial cell behavior during sprouting angiogenesis. Nat. Commun. 8 (1), 726. doi:10.1038/s41467-017-00738-7
Khan, S., Taverna, F., Rohlenova, K., Treps, L., Geldhof, V., de Rooij, L., et al. (2019). EndoDB: a database of endothelial cell transcriptomics data. Nucleic Acids Res. 47 (D1), D736–D744. doi:10.1093/nar/gky997
Kim, Y. H., Choi, J., Yang, M. J., Hong, S. P., Lee, C. K., Kubota, Y., et al. (2019). A MST1–FOXO1 cascade establishes endothelial tip cell polarity and facilitates sprouting angiogenesis. Nat. Commun. 10 (1), 838. doi:10.1038/s41467-019-08773-2
Kisanuki, Y. Y., Hammer, R. E., Miyazaki, J., Williams, S. C., Richardson, J. A., and Yanagisawa, M. (2001). Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 230 (2), 230–242. doi:10.1006/dbio.2000.0106
K C, R., Patel, N. R., Shenoy, A., Scallan, J. P., Chiang, M. Y., Galazo, M. J., et al. (2024). Zmiz1 is a novel regulator of lymphatic endothelial cell gene expression and function. PLOS ONE 19 (5), e0302926. doi:10.1371/journal.pone.0302926
Lee, J., Beliakoff, J., and Sun, Z. (2007). The novel PIAS-like protein hZimp10 is a transcriptional co-activator of the p53 tumor suppressor. Nucleic Acids Res. 35 (13), 4523–4534. doi:10.1093/nar/gkm476
Li, X., Thyssen, G., Beliakoff, J., and Sun, Z. (2006). The novel PIAS-like protein hZimp10 enhances smad transcriptional activity. J. Biol. Chem. 281 (33), 23748–23756. doi:10.1074/jbc.M508365200
Li, X., Zhu, C., Tu, W. H., Yang, N., Qin, H., and Sun, Z. (2011). ZMIZ1 preferably enhances the transcriptional activity of androgen receptor with short polyglutamine tract. PLoS ONE 6 (9), e25040. doi:10.1371/journal.pone.0025040
Liu, B., Liao, J., Rao, X., Kushner, S. A., Chung, C. D., Chang, D. D., et al. (1998). Inhibition of Stat1-mediated gene activation by PIAS1. Proc. Natl. Acad. Sci. 95 (18), 10626–10631. doi:10.1073/pnas.95.18.10626
Liu, Z. J., Shirakawa, T., Li, Y., Soma, A., Oka, M., Dotto, G. P., et al. (2003). Regulation of Notch1 and Dll4 by vascular endothelial growth factor in Arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol. Cell Biol. 23 (1), 14–25. doi:10.1128/MCB.23.1.14-25.2003
Lu, G., Ma, L., Xu, P., Xian, B., Wu, L., Ding, J., et al. (2022). A de novo ZMIZ1 Pathogenic Variant for Neurodevelopmental Disorder With Dysmorphic Facies and Distal Skeletal Anomalies. Front. Genet. 13, 840577. doi:10.3389/fgene.2022.840577
Nakatsu, M. N., Davis, J., and Hughes, C. C. W. (2007). Optimized fibrin gel bead assay for the study of angiogenesis. J. Vis. Exp. 3, 186. doi:10.3791/186
Park, H., Yamamoto, H., Mohn, L., Ambühl, L., Kanai, K., Schmidt, I., et al. (2019). Integrin-linked kinase controls retinal angiogenesis and is linked to Wnt signaling and exudative vitreoretinopathy. Nat. Commun. 10 (1), 5243. doi:10.1038/s41467-019-13220-3
Patel, N. R., K, C. R., Blanks, A., Li, Y., Prieto, M. C., and Meadows, S. M. (2022). Endothelial cell polarity and extracellular matrix composition require functional ATP6AP2 during developmental and pathological angiogenesis. JCI Insight 7 (19), e154379. doi:10.1172/jci.insight.154379
Pauleikhoff, L., Boneva, S., Boeck, M., Schlecht, A., Schlunck, G., Agostini, H., et al. (2023). Transcriptional comparison of human and murine retinal neovascularization. Invest Ophthalmol. Vis. Sci. 64 (15), 46. doi:10.1167/iovs.64.15.46
Phetthong, T., Khongkrapan, A., Jinawath, N., Seo, G. H., and Wattanasirichaigoon, D. (2021). Compound heterozygote of point mutation and chromosomal microdeletion involving OTUD6B coinciding with ZMIZ1 variant in syndromic intellectual disability. Genes 12 (10), 1583. doi:10.3390/genes12101583
Pinnell, N., Yan, R., Cho, H. J., Keeley, T., Murai, M. J., Liu, Y., et al. (2015). The PIAS-like coactivator Zmiz1 is a direct and selective cofactor of Notch1 in T cell development and leukemia. Immunity 43 (5), 870–883. doi:10.1016/j.immuni.2015.10.007
Rocha, S. F., Schiller, M., Jing, D., Li, H., Butz, S., Vestweber, D., et al. (2014). Esm1 modulates endothelial tip cell behavior and vascular permeability by enhancing VEGF bioavailability. Circ. Res. 115 (6), 581–590. doi:10.1161/CIRCRESAHA.115.304718
Sabbagh, M. F., Heng, J. S., Luo, C., Castanon, R. G., Nery, J. R., Rattner, A., et al. (2018). Transcriptional and epigenomic landscapes of CNS and non-CNS vascular endothelial cells. eLife 7, e36187. doi:10.7554/eLife.36187
Scott, A., and Fruttiger, M. (2010). Oxygen-induced retinopathy: a model for vascular pathology in the retina. Eye 24 (3), 416–421. doi:10.1038/eye.2009.306
Sherwood, L. M., Parris, E. E., and Folkman, J. (1971). Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285 (21), 1182–1186. doi:10.1056/nejm197111182852108
Stahl, A., Connor, K. M., Sapieha, P., Chen, J., Dennison, R. J., Krah, N. M., et al. (2010). The mouse retina as an angiogenesis model. Investig. Opthalmology Vis. Sci. 51 (6), 2813–2826. doi:10.1167/iovs.10-5176
Strasser, G. A., Kaminker, J. S., and Tessier-Lavigne, M. (2010). Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood 115 (24), 5102–5110. doi:10.1182/blood-2009-07-230284
Ubezio, B., Blanco, R. A., Geudens, I., Stanchi, F., Mathivet, T., Jones, M. L., et al. (2016). Synchronization of endothelial Dll4-Notch dynamics switch blood vessels from branching to expansion. eLife 5, e12167. doi:10.7554/eLife.12167
Uemura, A., Kusuhara, S., Katsuta, H., and Nishikawa, S. I. (2006). Angiogenesis in the mouse retina: a model system for experimental manipulation. Exp. Cell Res. 312 (5), 676–683. doi:10.1016/j.yexcr.2005.10.030
Wang, Y., Nakayama, M., Pitulescu, M. E., Schmidt, T. S., Bochenek, M. L., Sakakibara, A., et al. (2010). Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465 (7297), 483–486. doi:10.1038/nature09002
Xiao, S., Bucher, F., Wu, Y., Rokem, A., Lee, C. S., Marra, K. V., et al. (2017). Fully automated, deep learning segmentation of oxygen-induced retinopathy images. JCI Insight 2 (24), e97585. doi:10.1172/jci.insight.97585
Zhou, Y., Jin, Q., Chang, J., Zhao, Z., and Sun, C. (2022). Long non-coding RNA ZMIZ1-AS1 promotes osteosarcoma progression by stabilization of ZMIZ1. Cell Biol. Toxicol. 38 (6), 1013–1026. doi:10.1007/s10565-021-09641-w
Keywords: Zmiz1, transcription, co-factor, angiogenesis, retina, retinopathy
Citation: Patel NR, Bavishi SA, Bartoletti AP, K C R, Blanks A, Carter K, Chiang MY and Meadows SM (2025) Endothelial Zmiz1 modulates developmental angiogenesis in the retina. Front. Cell Dev. Biol. 13:1570587. doi: 10.3389/fcell.2025.1570587
Received: 03 February 2025; Accepted: 15 September 2025;
Published: 01 October 2025.
Edited by:
Marie Kmita, Montreal Clinical Research Institute (IRCM), CanadaReviewed by:
Gladys Y.-P. Ko, Texas A and M University, United StatesRakesh Radhakrishnan, University of Minnesota Twin Cities, United States
Copyright © 2025 Patel, Bavishi, Bartoletti, K C, Blanks, Carter, Chiang and Meadows. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stryder M. Meadows, c21lYWRvd3NAdHVsYW5lLmVkdQ==
†ORCID: Shreya A. Bavishi, orcid.org/0000-0003-3161-8973; Adella P. Bartoletti, orcid.org/0009-0004-7547-7898; K. C. Rajan, orcid.org/0000-0003-1121-3661; Stryder M. Meadows, orcid.org/0000-0003-0968-7155
‡These authors have contributed equally to this work
 Nehal R. Patel
Nehal R. Patel Shreya A. Bavishi1†‡
Shreya A. Bavishi1†‡ Rajan K C
Rajan K C Mark Y. Chiang
Mark Y. Chiang Stryder M. Meadows
Stryder M. Meadows