Abstract
Background:
Chemotherapy alone exhibits suboptimal efficacy in patients with treatment-naïve advanced gastric cancer (GC). Randomized controlled trials (RCTs) have demonstrated that combining Programmed Cell Death Protein-1 (PD-1) blockade with chemotherapy significantly improves overall survival (OS) compared to chemotherapy alone. However, the efficacy of PD-1 inhibitors in patients with low Programmed Cell Death-Ligand 1 (PD-L1) expression remains unclear.
Methods:
Electronic databases were searched for RCTs comparing PD-1/PD-L1 inhibitors plus chemotherapy to placebo plus chemotherapy or chemotherapy alone in treatment-naïve advanced gastric or gastroesophageal junction adenocarcinoma patients. Individual patient-level data (IPD) for overall survival (OS) and progression-free survival (PFS) were reconstructed. The KMSubtraction algorithm was employed to derive IPD for the PD-L1-low subgroup. Treatment effects in PD-L1-high and PD-L1-low subgroups were evaluated using Cox proportional hazards models with shared frailty to account for between-study heterogeneity. Interaction tests were performed to assess differences in treatment effects between these subgroups.
Results:
Nine RCTs were included in the qualitative analysis. A combined positive score (CPS) of 5 was selected as the cutoff for analysis, with CheckMate 649 and ORIENT-16 trials included. In the CPS<5 subgroup, OS (CheckMate 649: HR = 0.97, 95% CI 0.81–1.17, P = 0.758; ORIENT-16: HR = 0.94, 95% CI 0.68–1.31, P = 0.725) and PFS (CheckMate 649: HR = 0.95, 95% CI 0.79–1.14, P = 0.580; ORIENT-16: HR = 0.73, 95% CI 0.52–1.01, P = 0.055) did not significantly differ between patients receiving PD-1 blockade plus chemotherapy and those receiving chemotherapy alone. Pooled analysis of reconstructed OS IPD from CheckMate 649 and ORIENT-16 (N = 2,231) revealed that PD-1 blockade significantly improved OS in the CPS≥5 subgroup (HR = 0.69, 95% CI 0.60–0.79, P < 0.001), but not in the CPS<5 subgroup (HR = 0.96, 95% CI 0.82–1.13, P = 0.643). Interaction tests showed a significantly attenuated treatment effect on OS in the CPS<5 subgroup compared to the CPS≥5 subgroup (Pinteraction = 0.002). Similar findings were observed in the pooled analysis of PFS data (Pinteraction = 0.011).
Conclusion:
The addition of PD-1 inhibitors to first-line chemotherapy provides minimal benefit in patients with CPS<5. Therefore, PD-1 inhibitors should be individualized for this patient subset.
Introduction
Gastric cancer (GC) is the fifth most common cancer worldwide, with an estimated 1.1 million new cases and 769,000 deaths in 2020 (Sung et al., 2021). For decades, fluoropyrimidine and platinum-based doublet chemotherapy has been the most frequently used first-line treatment for patients with HER-2-negative advanced GC (Alsina et al., 2023). During this timeframe, the median overall survival (OS) was unsatisfactory, with a median OS less than 12 months (Wagner et al., 2017). Recently, the advent of immune checkpoint inhibitors has significantly transformed the treatment for advanced GC (Zhang et al., 2022; Wang et al., 2023). The ATTRACTION-02 study found that the Programmed Cell Death Protein-1 (PD-1) inhibitor nivolumab resulted in prolonged OS in patients with advanced GC refractory to standard treatment (Kang et al., 2017). In the first-line setting, the CheckMate 649 study has demonstrated that the addition of chemotherapy significantly improved OS and progression-free survival (PFS) in patients with treatment-naïve advanced GC, leading to the US Food and Drug Administration (FDA)’s approval of nivolumab plus chemotherapy for the first-line treatment of advanced GC (Janjigian et al., 2021; FDA, 2025). Additionally, several randomized clinical trials (RCTs) have shown the superior efficacy of anti-PD-1 therapy plus chemotherapy versus chemotherapy alone in patients with treatment-naïve advanced GC (Kang et al., 2022; Moehler et al., 2023; Rha et al., 2023; Xu et al., 2021).
Despite the partial success of anti-PD-1–chemotherapy combinations in first-line advanced GC, 40%–50% of patients still do not respond and median OS improvements remain modest (about 2–3 months), highlighting the urgent need for effective biomarkers to guide patient selection, with PD-L1 CPS being more predictive than TPS. (Janjigian et al., 2021; Kang et al., 2022; Rha et al., 2023; Havel et al., 2019; Wang and Xu, 2023; Martin and Märkl, 2019; Kulangara et al., 2019). Although the CheckMate 649 trial revealed that patients with high PD-L1 expression (CPS≥5) benefitted better from the addition of nivolumab to chemotherapy than those with low PD-L1 expression (CPS<5) (FDA, 2025). However, the FDA’s approval of nivolumab plus chemotherapy for first-line advanced gastric cancer did not mandate PD-L1 testing (FDA, 2025), meaning many patients with low PD-L1 expression (CPS<5) may receive a treatment not specifically suited for them. Given the strong correlation between PD-1 blockade efficacy and PD-L1 expression levels shown in CheckMate 649 and other studies, identifying patients most likely to benefit from this combination therapy is crucial. Moreover, most clinical trials have not reported detailed outcomes or Kaplan-Meier (KM) survival curves for the PD-L1-low subgroup. This data gap makes it difficult to draw clear conclusions about the potential benefits or lack thereof for patients with low PD-L1 expression. Without reliable evidence for this subgroup, clinicians face significant uncertainties in decision-making, especially regarding whether to use PD-1 blockade plus chemotherapy for patients with CPS<5. This missing data also hampers the development of more personalized treatment strategies and may lead to unnecessary treatment-related toxicities and costs for patients unlikely to benefit.
To address this issue, we first searched for and collected published RCTs studying PD-1 blockade plus chemotherapy versus chemotherapy alone in treatment-naïve advanced GC, reconstructed individual patient-level data (IPD) from the previously reported KM curves of the all-randomized population and the PD-L1-high subgroup, and further derived the IPD of the PD-L1-low subgroup based on the reconstructed IPD. We then performed a pooled analysis of these data and assessed whether the treatment effect of PD-1 blockade plus chemotherapy versus chemotherapy alone differed between PD-L1-high and PD-L1-low subgroups. The pipeline of analyses performed in this study is shown in Figure 1.
FIGURE 1
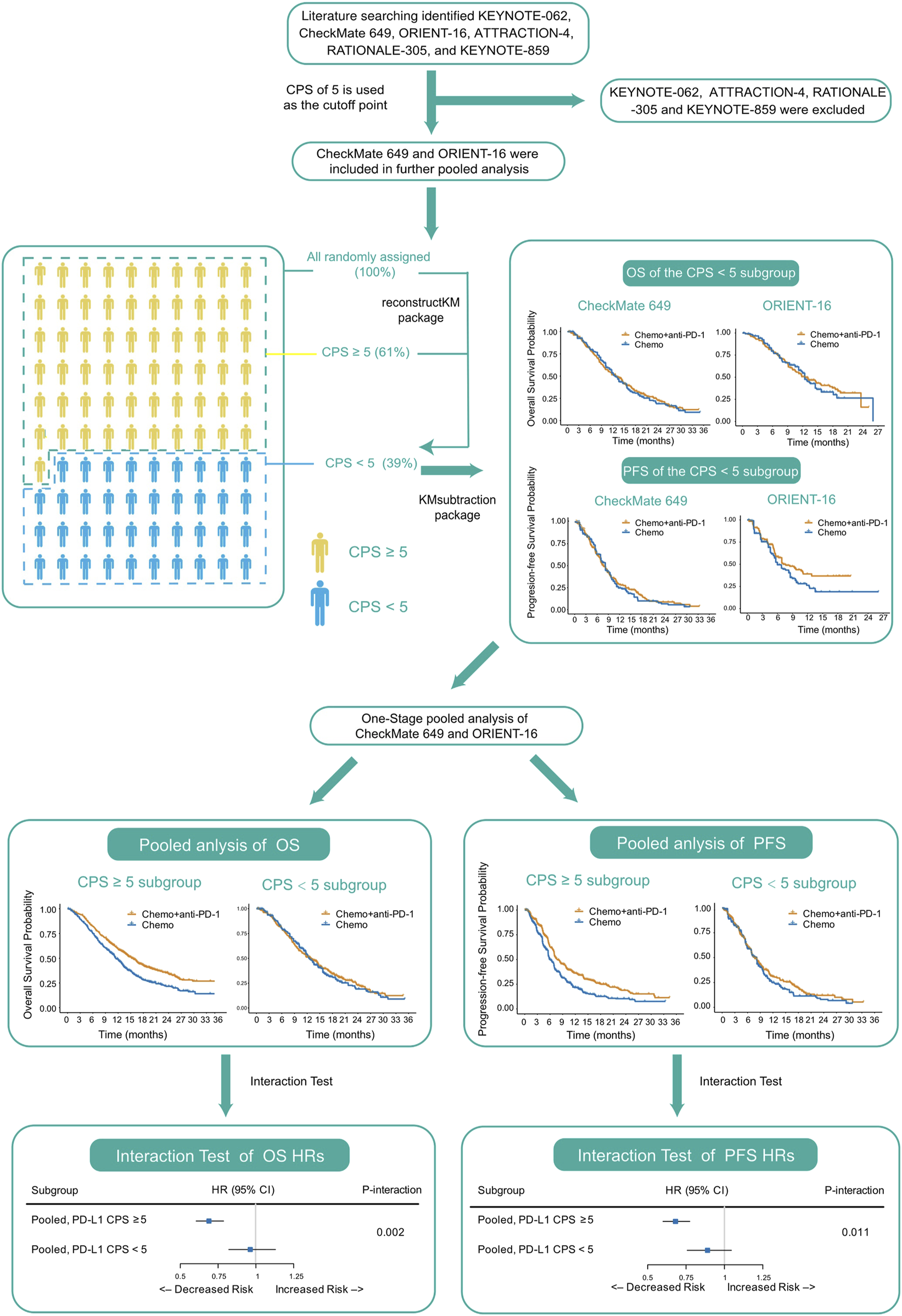
Pipeline of analyses performed in this study. Abbreviations: CPS, Combined Positive Score; OS, overall survival; PFS, progression-free survival; HR, hazard ratio.
Methods
Study selection
The PubMed database was searched for RCTs published between January 1, 2000, and March 1, 2023, using the following search string: ((((first-line) OR (previously untreated)) AND (nivolumab OR pembrolizumab OR toripalimab OR camrelizumab OR tislelizumab OR sintilimab OR serplulimab OR cemiplimab OR atezolizumab OR avelumab OR durvalumab OR (PD-1) OR (PD-L1))) AND (((gastric) OR (gastroesophageal junction)) OR (gastroesophageal junction))) AND ((“2000/01/01”[Date - Publication]: “2023/03/01”[Date - Publication]))). The American Society of Clinical Oncology and European Society for Medical Oncology Congress websites were also searched for publications to identify results from clinical trials that have not yet been published in peer-reviewed journals using the same search string. RCTs investigating the efficacy of PD-1/PD-L1 inhibitors plus chemotherapy versus placebo plus chemotherapy or chemotherapy alone in patients with treatment-naïve advanced gastric or gastro-esophageal junction adenocarcinoma were included. Retrospective studies, single-arm phase I and II trials, and neoadjuvant or adjuvant setting trials were excluded.
Reconstruction of IPD
DigitizeIt software version 2.2 (http://www.digitizeit.de/) was used to scan published OS and PFS KM plots from eligible trials. Risk tables and event numbers were manually curated. Subsequently, to solve the inverted KM equations, these data were input into an algorithm based on iterative numerical methods, as implemented in the R package reconstructKM (Guyot et al., 2012).
For trials lacking KM curves for the population with low PD-L1 expression, the R package KMSubtraction was used to retrieve survival data. KMSubtraction is a workflow used to derive unreported subgroup survival data from known subgroups (Zhao et al., 2022a). For this study, it was used to derive data for subgroups with low PD-L1 expression from the data of all-comers and subgroups with high PD-L1 expression. Minimal-cost bipartite matching was used as the primary matching algorithm.
Quality assessment of data reconstruction and matching
The quality of reconstruction was evaluated before performing a pooled analysis. Reconstructed KM curves for all-comers and subgroups with high PD-L1 expression were compared with the original published KM curves. KM curves were evaluated according to OS and PFS hazard ratios (HRs) and median OS and PFS. The accuracy of the reconstructed data was further validated by comparing KMSubtraction-derived KM curves and HRs for subgroups with low PD-L1 expression to the original published HRs. Empirical cumulative distribution plots and Bland-Altman plots were used to demonstrate discrepancies in the follow-up time between matched pairs to evaluate the effectiveness of matching (Zhao et al., 2022a). The KM curves of the matched cohorts were also plotted. The limits of error for KMSubtraction were determined by conducting Monte Carlo simulations with 10,000 iterations.
Pooled analysis of OS and PFS
One-stage pooled analyses using reconstructed and derived IPD were conducted to elucidate whether the treatment effect of PD-1 blockade plus chemotherapy versus chemotherapy alone differed between the PD-L1-high and PD-L1-low subgroups. In all analyses, the primary outcome was prespecified as OS and the secondary outcome was PFS. Between-study heterogeneity was accounted by incorporating a random-effects term using the shared-frailty model. The gamma-distributed frailty was also used. HRs and the corresponding 95% confidence intervals (CIs) were computed using a Cox proportional hazards regression model.
All analyses were conducted in R, version 4.1.0, using the survival, ggplot2, survminer, reconstructKM, KMSubtraction, and frailtyEM packages. Statistical significance was set at a two-sided P < 0.05.
Results
Overview of included trials
Six RCTs met the selection criteria and were included in the analysis: KEYNOTE-062 (Shitara et al., 2020), CheckMate 649 (Janjigian et al., 2021), ORIENT-16 (Xu et al., 2021), Attraction-4 (Kang et al., 2022), RATIONALE-305 (Moehler et al., 2023), and KEYNOTE-859 (Rha et al., 2023) (Supplementary Figure S1; Table 1). Dako 22C3 was the most commonly used PD-L1 IHC assay (n = 3 [KEYNOTE-062, KEYNOTE-859, and ORIENT-16]), followed by the 28-8 (n = 2 [CheckMate 649 and ATTRACTION-4]) and SP263 (n = 1 [RATIONALE-305]) assays. Except for KEYNOTE-062, the other five trials achieved positive results that PD-1 blockade plus chemotherapy showed superior efficacy to chemotherapy alone for patients with treatment-naïve advanced GC. Among them, CheckMate 649 reported OS and PFS KM curves for the all-randomized population and the CPS≥1 and CPS≥5 subgroups, but not for the CPS<1 or CPS<5 subgroups; however, it reported OS HRs for the CPS<1, CPS<5, and CPS<10 subgroups, and the results showed no significant benefits of PD-1 blockade plus chemotherapy in terms of OS in all three subgroups. ORIENT-16 reported OS and PFS KM curves for the all-randomized population and the CPS≥5 subgroup, but not for the CPS<5 subgroup. This trial also did not report OS or PFS HRs for the CPS<5 subgroup. ATTRACTION-4 only reported OS and PFS KM curves for the all-randomized population; OS and PFS HRs were reported for the TPS≥1%, and <1% subgroups, but the treatment effect did not differ between these subgroups. RATIONALE-305 released only OS KM curves and OS HRs for the SP263 assay-based PD-L1-positive subgroup. KEYNOTE-859 only reported OS and PFS KM curves for the all-randomized population and also reported OS HRs for the CPS<1 and CPS<10 subgroups, both of which showed no significant benefits from PD-1 blockade plus chemotherapy. Ultimately, a CPS of 5 was determined to be the sole cutoff with sufficient KM curves to allow pooled analysis, and CheckMate 649 and ORIENT-16 were finally included.
TABLE 1
| Trial | Population | Treatment | N | PD-L1 assay | Reported outcomes (as KM plots) |
|---|---|---|---|---|---|
| CheckMate 649 | Global (Asian, 22.5%) | Nivolumab + FOLFOX/XELOX | 789 | IHC 28–8 | OS (1) All patients (2) PD-L1 CPS ≥1 (3) PD-L1 CPS ≥5 PFS (1) All patients (2) PD-L1 CPS ≥1 (3) PD-L1 CPS ≥5 |
| FOLFOX/XELOX | 792 | ||||
| ORIENT-16 | Chinese | Sintilimab + XELOX | 327 | IHC 22C3 | OS (1) All patients (2) PD-L1 CPS ≥5 PFS (1) All patients (2) PD-L1 CPS ≥5 |
| Placebo + XELOX | 323 | ||||
| KEYNOTE-062 | Global (Asian, 24.7%) | Pembrolizumab + PF | 257 | IHC 22C3 | OS (1) PD-L1 CPS ≥1 (2) PD-L1 CPS ≥10 PFS (1) PD-L1 CPS ≥1 (2) PD-L1 CPS ≥10 |
| Placebo + PF | 250 | ||||
| ATTRACTION-4 | Asian | Nivolumab + SOX/XELOX | 362 | IHC 28–8 | OS: All patients PFS: All patients |
| Placebo + SOX/XELOX | 362 | ||||
| RATIONALE 305 | Global (Asian, 73.8%) | Tislelizumab + PF/XELOX | 274 | IHC SP263 | OS: PD-L1 vCPS ≥5 PFS: PD-L1 vCPS ≥5 |
| Placebo + PF/XELOX | 272 | ||||
| KEYNOTE-859 | Global (Asian, 33.2%) | Pembrolizumab + PF/XELOX | 790 | IHC 22C3 | OS: All patients PFS: All patients |
| Placebo + PF/XELOX | 789 |
Summary of the published randomized trials included in the analysis.
Abbreviations: CPS, combined positive score; HR, hazard ratio; IHC, immunohistochemistry; KM, Kaplan-Meier; OS, overall survival; PD-1, Programmed Cell Death-Protein 1; PD-L1, Programmed Cell Death-Ligand 1; PFS, progression-free survival; vCPS, SP263 assay-based PD-L1; FOLFOX, 5-fluorouracil/leucovorin/oxaliplatin; XELOX, capecitabine/oxaliplatin; SOX, S-1/oxaliplatin.
Quality assessment of data reconstruction and matching
The reconstructed IPD resulted in OS and PFS HRs and median OS and PFS that were similar to those of the originally reported curves of the all-randomized population and the CPS≥5 subgroup in both CheckMate 649 and ORIENT-16 (Supplementary Table S1). Matched pairs on empirical cumulative distributions and Bland-Altman plots (with means of absolute differences in follow-up time approximating 0) had few discrepancies for the derived IPD of the CPS<5 subgroup. Furthermore, a near-complete overlap (with HRs and log-rank tests approximating 1) was observed in the KM plots between the matched cohorts (Supplementary Figures S2–5). For each implementation of KMSubtraction, small and negligible limits of error between the reconstructed unmatched plots and original unreported plots were demonstrated via simulations from 10,000 Monte Carlo iterations (mean |ln(HR)| = 0.017 for both treatment arms in CheckMate 649; mean |ln(HR)| = 0.020 and 0.021 for the PD-1 blockade plus chemotherapy and chemotherapy alone arms, respectively, in ORIENT-16).
OS in the CPS<5 subgroup in CheckMate 649 and ORIENT-16
In the CheckMate 649 study in the CPS<5 subgroup, there was no significant difference in OS between patients treated with nivolumab plus chemotherapy and those treated with chemotherapy alone (HR = 0.97, 95% CI, 0.81–1.17, P = 0.758), with a median OS of 12.8 (95% CI, 10.8–14.6) versus 12.6 (95% CI, 11.7–13.6) months, respectively (Figure 2A). Similarly, in the ORIENT-16 study in the subgroup of CPS<5, the OS did not improve significantly in patients treated with the PD-1 inhibitor sintilimab in combination with chemotherapy compared to those treated with chemotherapy alone (HR = 0.94, 95% CI, 0.68–1.31, P = 0.725), with a median OS of 12.4 (95% CI, 10.5–17.4) versus 12.4 (95% CI, 11.2–14.5) months, respectively (Figure 2B).
FIGURE 2
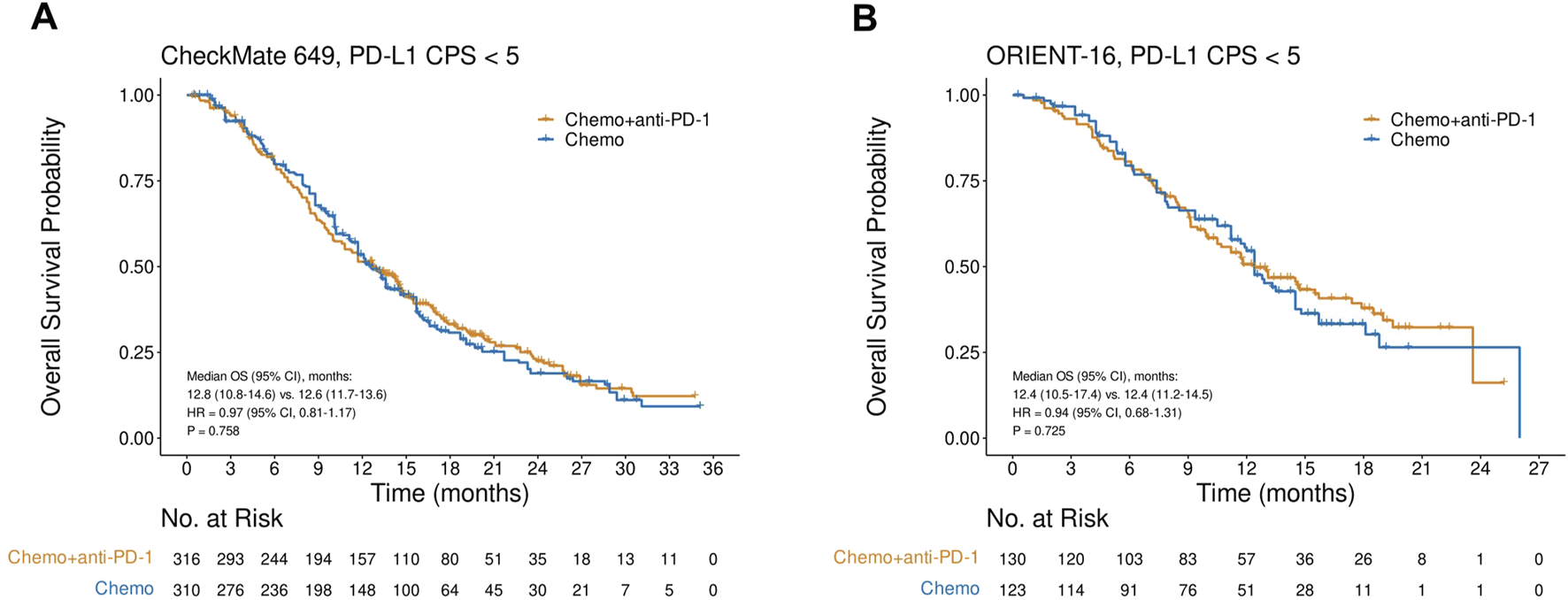
Kaplan-Meier plots for overall survival in the PD-L1 CPS<5 subgroups in CheckMate 649 (A) and ORIENT-16 (B) based on reconstructed individual patient-level data. Abbreviations: CPS, Combined Positive Score; OS, overall survival; HR, hazard ratio; Chemo, chemotherapy; Chemo + anti-PD-1, chemotherapy plus PD-1 blockade.
PFS in the CPS<5 subgroup in CheckMate 649 and ORIENT-16
In CheckMate 649, the CPS<5 subgroup gained no significant PFS improvements with the addition of nivolumab to chemotherapy (HR = 0.95, 95% CI, 0.79–1.14, P = 0.580), and the median PFS in the nivolumab plus chemotherapy arm and chemotherapy-only arm were 7.7 (95% CI, 7.0–8.7) and 8.2 (95% CI, 7.1–8.9) months, respectively (Figure 3A). Similarly, in the CPS<5 subgroup in the ORIENT-16 study, there was no significant difference in PFS between patients treated with sintilimab plus chemotherapy and those treated with chemotherapy alone (HR = 0.73, 95% CI, 0.52–1.01, P = 0.055). The median PFS was 7.2 (95% CI, 5.7–12.8) months in the sintilimab plus chemotherapy arm and 6.0 (95% CI, 5.4–8.6) months in the chemotherapy-only arm (Figure 3B).
FIGURE 3
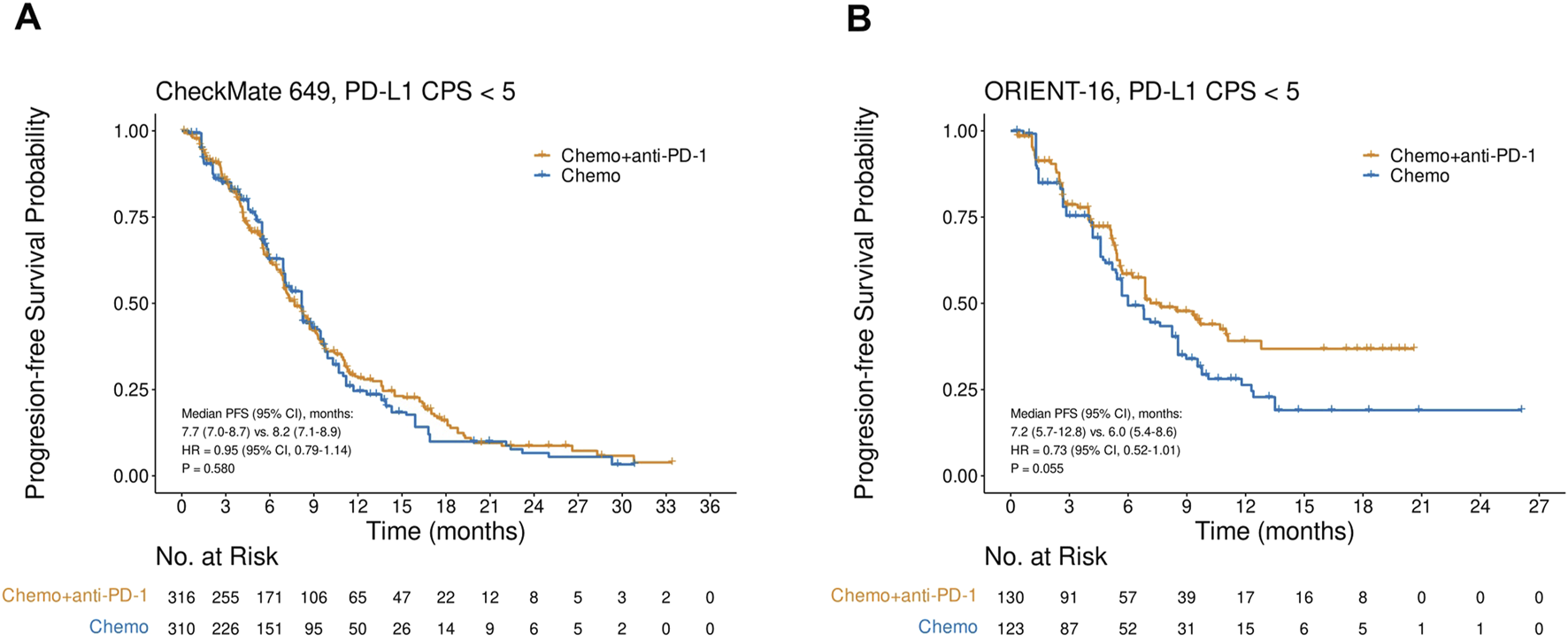
Kaplan-Meier plots for progression-free survival in the PD-L1 CPS<5 subgroups in (A) CheckMate 649 and (B) ORIENT-16 based on reconstructed individual patient-level data. Abbreviations: CPS, Combined Positive Score; PFS, progression-free survival; HR, hazard ratio; Chemo, chemotherapy; Chemo + anti-PD-1, chemotherapy plus PD-1 blockade.
Pooled analysis of CheckMate 649 and ORIENT-16 based on PD-L1 status
Pooled analysis of the reconstructed OS IPD from CheckMate 649 and ORIENT-16 showed that the addition of PD-1 blockade to chemotherapy significantly improved patient OS in the CPS≥5 subgroup (HR = 0.69, 95% CI, 0.60–0.79, P < 0.001), with a median OS of 15.2 (95% CI, 14.0–16.6) versus 11.5 (95% CI, 11.0–12.7) months, respectively (Figure 4A). In contrast, in the pooled CPS<5 subgroup, there was no significant difference in OS between patients treated with PD-1 blockade plus chemotherapy and those treated with chemotherapy alone (HR = 0.96, 95% CI, 0.82–1.13, P = 0.643, Figure 4B). The interaction test revealed that the treatment effect on OS significantly attenuated in the CPS<5 subgroup than in the CPS≥5 subgroup (Pinteraction = 0.002, Figure 4C). Similarly, in the pooled analysis of the reconstructed PFS IPD from CheckMate 649 and ORIENT-16, the addition of PD-1 blockade to chemotherapy significantly improved patient OS in the CPS≥5 subgroup (HR = 0.68, 95% CI, 0.60–0.77, P < 0.001, Figure 5A), but not in the CPS<5 subgroup (HR = 0.89, 95% CI, 0.76–1.05, P = 0.157, Figure 5B). The interaction test showed that the treatment effect on PFS was significantly less prominent in the CPS<5 subgroup than in the CPS≥5 subgroup (Pinteraction = 0.011, Figure 5C).
FIGURE 4
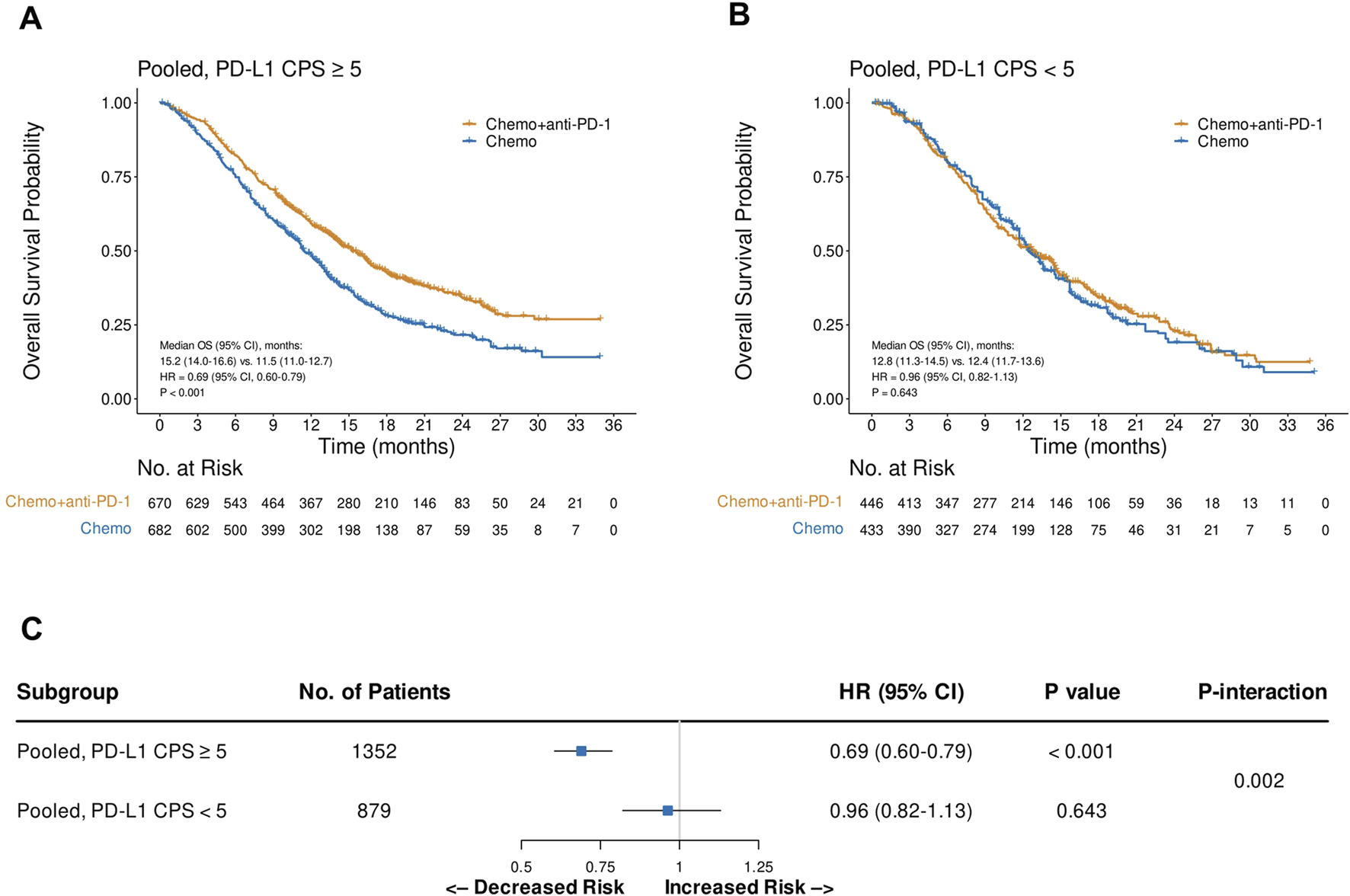
Kaplan-Meier plots for overall survival in the one-stage pooled analysis of CheckMate 649 and ORIENT-16 in the PD-L1 CPS≥5 (A) and CPS<5 (B) subgroups, and the interaction test results regarding the difference in treatment effects between the CPS≥5 and CPS<5 subgroups (C). Abbreviations: CPS, Combined Positive Score; OS, overall survival; HR, hazard ratio; Chemo, chemotherapy; Chemo + anti-PD-1, chemotherapy plus PD-1 blockade.
FIGURE 5
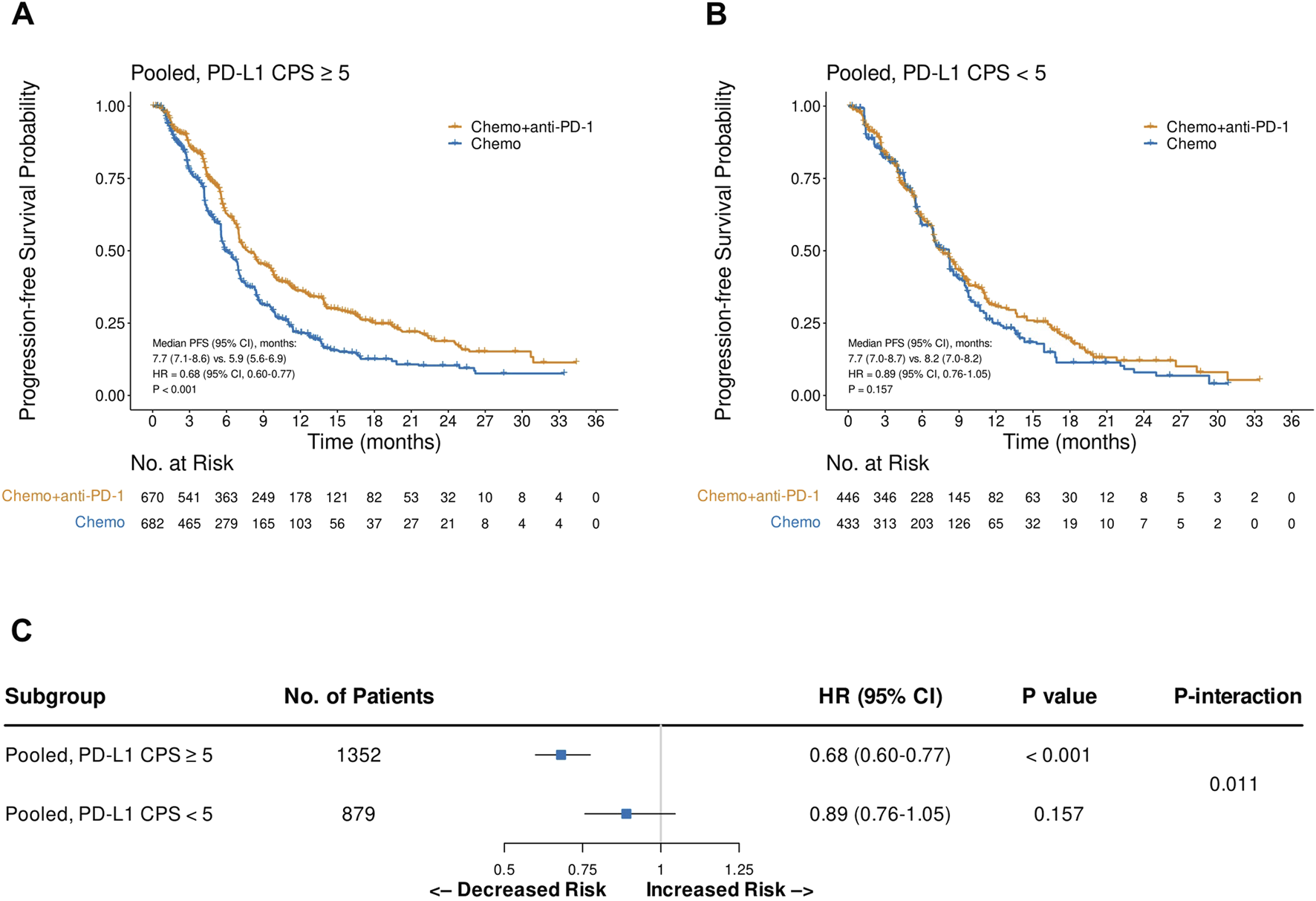
Kaplan-Meier plots for progression-free survival in the one-stage pooled analysis of CheckMate 649 and ORIENT-16 in the PD-L1 CPS≥5 (A) and CPS<5 (B) subgroups, and the interaction test results regarding the difference in treatment effects between the CPS≥5 and CPS<5 subgroups (C). Abbreviations: CPS, Combined Positive Score; PFS, progression-free survival; HR, hazard ratio; Chemo, chemotherapy; Chemo + anti-PD-1, chemotherapy plus PD-1 blockade.
Discussion
Recently, multiple RCTs have shown that PD-1 blockade plus chemotherapy outperforms chemotherapy alone as a first-line therapy for patients with advanced GC, especially for those with high PD-L1 expression levels (Janjigian et al., 2021; Kang et al., 2022; Moehler et al., 2023; Rha et al., 2023; Xu et al., 2021; Shitara et al., 2020). However, most RCTs did not report survival data or KM curves for patients with low PD-L1 expression; therefore, it remains unclear whether the treatment effect in the all-randomized population is largely driven by that in the CPS≥5 subgroup (Yoon et al., 2022). Therefore, we reconstructed the IPD of the CPS≥5 and CPS<5 subgroups from two large-scale RCTs: CheckMate 649 and ORIENT-16. The reconstructed data revealed that, compared to the CPS≥5 subgroup, the treatment effect in the CPS<5 subgroup was significantly less pronounced in terms of overall survival (OS) and progression-free survival (PFS).
These findings provide robust evidence for the utility of the PD-L1 CPS as a biomarker for predicting the efficacy of PD-1 blockade plus chemotherapy in patients with treatment-naïve advanced GC. Therefore, it is recommended that the PD-L1 CPS be routinely tested prior to first-line therapy for patients with advanced GC. For patients with CPS≥5, PD-1 blockade in combination with chemotherapy should be considered the standard first-line treatment. In contrast, for those with a CPS<5, the lack of efficacy of adding PD-1 blockade to chemotherapy should be part of the informed discussion of treatment options with patients, Decisions regarding the use of anti-PD-1 therapy should be made on a case-by-case basis, taking into account each patient’s specific circumstances, such as socioeconomic status, alternative therapeutic options, and predisposition to immune-related adverse events.
In the subgroup analyses of CheckMate 649 and KEYNOTE-859, PD-1 blockade plus chemotherapy did not improve OS compared to chemotherapy alone in patients with CPS<1 (HR = 0.95 and 0.92, respectively) (Janjigian et al., 2021; Rha et al., 2023). Therefore, determining whether patients with CPS 1–4 still benefit from PD-1 blockade plus chemotherapy is of interest. In a recent study, Zhao et al. reconstructed IPD from CheckMate 649 and found that the addition of nivolumab to chemotherapy did not improve OS and PFS in patients with CPS 1–4 (HR = 0.95 and 0.96, respectively) (Zhao et al., 2022b), further suggesting that neither the CPS<1 nor CPS 1–4 subgroups benefit from this combination treatment. Subgroup analysis of CheckMate 649 showed that high microsatellite instability and high tumor mutational burdens were associated with greater survival benefits from PD-1 blockade plus chemotherapy versus chemotherapy alone (Janjigian et al., 2021; Lei et al., 2022); and whether the incorporation of these features with PD-L1 expression can further improve patient selection is worthy of further investigation (Chen et al., 2023). These results have important implications for clinical practice, suggesting that for patients with CPS<5, PD-1 blockade plus chemotherapy may not be the optimal choice. Clinical decisions should take into account individual patient characteristics, such as certain tumor biology features, including microsatellite instability status and tumor mutational burden levels, as well as features of the immune microenvironment, to more precisely select appropriate treatment strategies.
Our findings highlight the urgent need to need to develop efficacious therapeutics to improve the survival of patients with PD-L1-low advanced GC. Some combination regimens have shown promising preliminary results. For instance, a recent phase 2 study investigated the efficacy of regorafenib, a potent inhibitor of angiogenic and oncogenic kinases, in combination with the PD-1 inhibitor camrelizumab and chemotherapy in patients with treatment-naïve advanced GC, which revealed a favorable objective response rate of 61.5% in the CPS<1 subgroup (Peng et al., 2021). The upregulation of DKK1 impairs CD8+ T cell functions and promotes MDSC-mediated immunosuppression (Shi et al., 2022). In a recent phase 2 study, the combination of a DKK1 inhibitor with the PD-1 inhibitor tislelizumab and first-line chemotherapy yielded a remarkable objective response rate of 100% in patients with DKK1-high and PD-L1-low GC (Klempner et al., 2022). These regimens warrant further confirmatory investigation, and future research should explore the role of these biomarkers in patient selection and develop more personalized treatment options for patients with PD-L1-low expression.
This study has some limitations. First, we analyzed the reconstructed IPD rather than the original IPD. However, the method used for IPD reconstruction has been validated in previous studies with excellent accuracy and reproducibility (Wang et al., 2021). Second, PD-L1 CPS was not a prespecified stratification factor in either CheckMate 649 or ORIENT-16; therefore, there may be confounding factors between the treatment arms in both trials. However, the findings regarding the treatment effect in the CPS<5 subgroup were consistent with those of the CheckMate 649 and ORIENT-16. Moreover, different IHC assays (28–8 and Dako 22C3) were used to assess PD-L1 expression in CheckMate 649 and ORIENT-16, which may have led to between-heterogeneity and posed challenges to the pooled analysis. However, good concordance in identifying PD-L1-high and PD-L1-low cases between the 28-8 and Dako 22C3 assays was reported in a previous GC study (Ahn and Kim, 2021), suggesting the potential interchangeability of these two PD-L1 assays (Wang et al., 2022).
In summary, this pooled analysis of two large-scale RCTs in treatment-naïve advanced GC, CheckMate 649 and ORIENT-16, demonstrated that the efficacy of PD-1 blockade plus chemotherapy versus chemotherapy alone significantly attenuated in the CPS<5 subgroup than in the CPS≥5 subgroup. Since the added value of anti-PD-1 therapy to first-line chemotherapy is minimal in patients with a CPS<5, the use of PD-1 inhibitors should be individualized for this patient subset. In addition, major efforts should be made to develop highly efficacious therapeutics beyond the anti-PD-1-chemotherapy combination to improve the survival outcomes of these patients.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
WZ: Writing – review and editing, Conceptualization, Writing – original draft, Validation, Project administration, Methodology, Visualization, Formal Analysis, Data curation. Z-ZC: Visualization, Resources, Project administration, Methodology, Writing – review and editing, Data curation. ZF: Project administration, Validation, Writing – review and editing, Data curation, Investigation. XZ: Project administration, Writing – review and editing, Validation, Resources, Conceptualization, Supervision. Y-TC: Project administration, Validation, Methodology, Conceptualization, Investigation, Writing – review and editing, Funding acquisition, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Yangfan Special Project of the Shanghai Qimingxing Program (22YF1459200), the Postdoctoral Fellowship Program of China Postdoctoral Science Foundation (GZC20230961), and the National Natural Science Foundation of China (82303866).
Acknowledgments
We thank Pei-Hua Cao (Department of Biostatistics, School of Public Health, Southern Medical University, Guangzhou, China) for his suggestions on the data analysis.
Conflict of interest
Author XZ was employed by Shenyang Kingmed Diagnostics Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2025.1636288/full#supplementary-material
Abbreviations
CPS, Combined Positive Score; GC, gastric cancer; HR, hazard ratio; IHC, immunohistochemistry; IPD, individual patient-level data; KM, Kaplan-Meier; OS, overall survival; PD-1, Programmed Cell Death-Protein 1; PD-L1, Programmed Cell Death-Ligand 1; PFS, progression-free survival; RCT, randomized controlled trial; TPS, tumor proportion score.
References
1
Ahn S. Kim K. M. (2021). PD-L1 expression in gastric cancer: interchangeability of 22C3 and 28–8 pharmDx assays for responses to immunotherapy. Mod. Pathol.34, 1719–1727. 10.1038/s41379-021-00823-9
2
Alsina M. Arrazubi V. Diez M. Tabernero J. (2023). Current developments in gastric cancer: from molecular profiling to treatment strategy. Nat. Rev. Gastroenterol. Hepatol.20, 155–170. 10.1038/s41575-022-00703-w
3
Chen Y. X. Wang Z. X. Jin Y. Zhao Q. Liu Z. X. Zuo Z. X. et al (2023). An immunogenic and oncogenic feature-based classification for chemotherapy plus PD-1 blockade in advanced esophageal squamous cell carcinoma. Cancer Cell41, 919–932.e5. 10.1016/j.ccell.2023.03.016
4
FDA (2025). FDA approves first immunotherapy for initial treatment of gastric cancer. Available online at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-immunotherapy-initial-treatment-gastric-cancer.
5
Guyot P. Ades A. E. Ouwens M. J. Welton N. J. (2012). Enhanced secondary analysis of survival data: reconstructing the data from published kaplan-meier survival curves. BMC Med. Res. Methodol.12, 9. 10.1186/1471-2288-12-9
6
Havel J. J. Chowell D. Chan T. A. (2019). The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer19, 133–150. 10.1038/s41568-019-0116-x
7
Janjigian Y. Y. Shitara K. Moehler M. Garrido M. Salman P. Shen L. et al (2021). First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (checkMate 649): a randomised, open-label, phase 3 trial. Lancet398, 27–40. 10.1016/S0140-6736(21)00797-2
8
Kang Y. K. Boku N. Satoh T. Ryu M. H. Chao Y. Kato K. et al (2017). Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet390, 2461–2471. 10.1016/S0140-6736(17)31827-5
9
Kang Y. K. Chen L. T. Ryu M. H. Oh D. Y. Oh S. C. Chung H. C. et al (2022). Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.23, 234–247. 10.1016/S1470-2045(21)00692-6
10
Klempner S. J. Chao J. Uronis H. E. Sirard C. A. Kagey M. Baum J. et al (2022). DKN-01 and tislelizumab ± chemotherapy as a first-line (1L) and second-line (2L) investigational therapy in advanced gastroesophageal adenocarcinoma (GEA): distinguish trial. J. Clin. Oncol.40 (4_Suppl. l), 292. 10.1200/JCO.2022.40.4_suppl.292
11
Kulangara K. Zhang N. Corigliano E. Guerrero L. Waldroup S. Jaiswal D. et al (2019). Clinical utility of the combined positive score for programmed death Ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch. Pathol. Lab. Med.143 (3), 330–337. 10.5858/arpa.2018-0043-OA
12
Lei M. Janjigian Y. Y. Ajani J. A. Moehler M. Wang X. Shen L. et al (2022). Abstract CT023: nivolumab (NIVO) plus chemotherapy (Chemo) vs chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): Checkmate 649 biomarker analyses. Cancer Res.82 (12_Suppl. ment), CT023. 10.1158/1538-7445.AM2022-CT023
13
Martin B. Märkl B. (2019). Immunologic biomarkers and biomarkers for immunotherapies in gastrointestinal cancer. Visc. Med.35, 3–10. 10.1159/000496565
14
Moehler M. H. Kato K. Arkenau H.-T. Oh D.-Y. Tabernero J. Cruz-Correa M. et al (2023). Rationale 305: phase 3 study of tislelizumab plus chemotherapy vs placebo plus chemotherapy as first-line treatment (1L) of advanced gastric or gastroesophageal junction adenocarcinoma (GC/GEJC). J. Clin. Oncol.41 (4_Suppl. l), 286. 10.1200/JCO.2023.41.4_suppl.286
15
Peng Z. Wei J. Wang F. Ying J. Deng Y. Gu K. et al (2021). Camrelizumab combined with chemotherapy followed by camrelizumab plus apatinib as first-line therapy for advanced gastric or gastroesophageal junction adenocarcinoma. Clin. Cancer Res.27, 3069–3078. 10.1158/1078-0432.CCR-20-4691
16
Rha S. Y. Oh D. Y. Yañez P. Bai Y. Ryu M. H. Lee J. et al (2023). Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol.24 (11), 1181–1195. 10.1016/S1470-2045(23)00515-6
17
Shi T. Zhang Y. Wang Y. Song X. Wang H. Zhou X. et al (2022). DKK1 promotes tumor immune evasion and impedes anti-PD-1 treatment by inducing immunosuppressive macrophages in gastric cancer. Cancer Immunol. Res.10, 1506–1524. 10.1158/2326-6066.CIR-22-0218
18
Shitara K. Van Cutsem E. Bang Y. J. Fuchs C. Wyrwicz L. Lee K. W. et al (2020). Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol.6, 1571–1580. 10.1001/jamaoncol.2020.3370
19
Sung H. Ferlay J. Siegel R. L. Laversanne M. Soerjomataram I. Jemal A. et al (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71, 209–249. 10.3322/caac.21660
20
Wagner A. D. Syn N. L. Moehler M. Grothe W. Yong W. P. Tai B. C. et al (2017). Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev.8, CD004064. 10.1002/14651858.CD004064.pub4
21
Wang Z. X. Cui C. Yao J. Zhang Y. Li M. Feng J. et al (2022). Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): a multi-center phase 3 trial. Cancer Cell40, 277–288.e3. 10.1016/j.ccell.2022.02.007
22
Wang Z. X. Pan Y. Q. Li X. Tsubata T. Xu R. H. (2023). Immunotherapy in gastrointestinal cancers: advances, challenges, and countermeasures. Sci. Bull. (Beijing)68, 763–766. 10.1016/j.scib.2023.03.036
23
Wang Z. X. Wu H. X. Xie L. Lin W. H. Liang F. Li J. et al (2021). Exploration of modified progression-free survival as a novel surrogate endpoint for overall survival in immuno-oncology trials. J. Immunother. Cancer9, e002114. 10.1136/jitc-2020-002114
24
Wang Z.-X. Xu R.-H. (2023). Chemo-immunotherapy in advanced esophageal squamous cell carcinoma: present and future. Holist. Integ Oncol.2, 5. 10.1007/s44178-023-00028-x
25
Xu J. Jiang H. Pan Y. Gu K. Cang S. Han L. et al (2021). LBA53 sintilimab plus chemotherapy (Chemo) versus chemo as first-line treatment for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT-16): first results of a randomized, double-blind, phase III study. Ann. Oncol.32, S1331. 10.1016/j.annonc.2021.08.2133
26
Yoon H. H. Jin Z. Kour O. Kankeu Fonkoua L. A. Shitara K. Gibson M. K. et al (2022). Association of PD-L1 expression and other variables with benefit from immune checkpoint inhibition in advanced gastroesophageal cancer: systematic review and meta-analysis of 17 phase 3 randomized clinical trials. JAMA Oncol.8, 1456–1465. 10.1001/jamaoncol.2022.3707
27
Zhang Y. Ye G. Yang Q. Zheng B. Zhang G. Hu Y. et al (2022). Landscape of exitrons in gastric cancer. EBiomedicine84, 104272. 10.1016/j.ebiom.2022.104272
28
Zhao J. J. Syn N. L. Tan B. K. J. Yap D. W. T. Teo C. B. Chan Y. H. et al (2022a). KMSubtraction: reconstruction of unreported subgroup survival data utilizing published Kaplan-Meier survival curves. BMC Med. Res. Methodol.22, 93. 10.1186/s12874-022-01567-z
29
Zhao J. J. Yap D. W. T. Chan Y. H. Tan B. K. J. Teo C. B. Syn N. L. et al (2022b). Low programmed death-ligand 1-expressing subgroup outcomes of first-line immune checkpoint inhibitors in gastric or esophageal adenocarcinoma. J. Clin. Oncol.40, 392–402. 10.1200/JCO.21.01862
Summary
Keywords
advanced gastric cancer, programmed cell death Protein-1 blockade, chemotherapy, programmed cell death-ligand 1 expression, combined positive score, randomized controlled trials
Citation
Zhou W, Cai Z-Z, Fan Z, Zheng X and Chen Y-T (2025) PD-L1 expression predicts the efficacy of PD-1 blockade plus chemotherapy versus chemotherapy alone in treatment-naïve advanced or metastatic gastric cancer: a pooled analysis of reconstructed individual patient-level data from two randomized trials. Front. Cell Dev. Biol. 13:1636288. doi: 10.3389/fcell.2025.1636288
Received
27 May 2025
Accepted
23 June 2025
Published
03 July 2025
Volume
13 - 2025
Edited by
Xuexin Li, Karolinska Institutet (KI), Sweden
Reviewed by
Min Liang, Fifth Affiliated Hospital of Guangzhou Medical University, China
Fei Liu, Center for National Cancer, Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Updates
Copyright
© 2025 Zhou, Cai, Fan, Zheng and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Tong Chen, amber04@126.com; Xu Zheng, zhengzhv@163.com
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.