- 1Department of Respiratory and Critical Care Medicine, Fuzhou University Affiliated Provincial Hospital, Fujian Provincial Hospital, Shengli Clinical Medical College of Fujian Medical University, Fuzhou, China
- 2Department of Critical Care Medicine, Fuzhou University Affiliated Provincial Hospital, Fujian Provincial Hospital, Shengli Clinical Medical College of Fujian Medical University, Fuzhou, China
- 3Shengli Clinical Medical College of Fujian Medical University; Department of Emergency, Fujian Provincial Hospital; Fuzhou University Affiliated Provincial Hospital; Fujian Provincial Key Laboratory of Emergency Medicine, Fuzhou, China
- 4The United Innovation of Mengchao Hepatobiliary Technology Key Laboratory of Fujian Province, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou, China
- 5Department of Anesthesiology, Shengli Clinical Medical College of Fujian Fuzhou University Affiliated Provincial Hospital, Fuzhou, China
Introduction: Given the crucial role of paracrine signaling in the therapeutic function of adipose tissue-derived mesenchymal stem cells (ADSCs) for skin wound repair, this study aimed to evaluate the efficacy of ADSC-conditioned medium (ACM) in enhancing type 2 diabetic (T2D) wound healing.
Methods: The effect of ACM on the viability and angiogenesis of human umbilical vein endothelial cells (HUVECs) was first evaluated using the CCK-8 assay and q-PCR analysis, respectively. Next, a T2D rat model was established through the combination of a high-fat diet and streptozotocin (STZ). Following the establishment of full-thickness skin defects in T2D rats, ACM or serum-free cultured medium was daily injected around the wound edges for 7 days. Afterward, the skin wound healing rate was analyzed, and the skin tissues were assessed by histopathological examination. The mRNA levels of TNF-α, IL-1β, IL-6, COX-2, IL-12, and IFN-γ were evaluated by q-PCR analysis. Additionally, transcriptome sequencing and immunohistochemistry were performed to reveal the potential mechanisms of ACM in T2D skin wound healing.
Results: ACM significantly enhanced HUVEC proliferation and angiogenesis while upregulating the expression of EGF, bFGF, VEGF, and KDR. In T2D rats, ACM accelerated wound closure and suppressed pro-inflammatory mediators (TNF-α, IL-1β, IL-6, COX-2, IL-12, and IFN-γ). Notably, transcriptome analysis revealed ACM-mediated downregulation of TNF and chemokine signaling pathways.
Discussion: ACM promotes diabetic wound healing through dual mechanisms: (1) stimulating vascularization by inducing growth factor expression and (2) modulating the inflammatory microenvironment by inhibiting TNF/chemokine cascades. These findings position ACM as a promising cell-free therapy for impaired wound healing in diabetes.
1 Introduction
Skin wound healing is a complex process involving multiple stages, such as hemostasis, inflammation, angiogenesis, and remodeling, which requires the coordinated effort of various cell types and signaling pathways (Freedman et al., 2023; Martin and Nunan, 2015). There are several factors, such as ischemia, diabetes, age, nutrition, hormones, obesity, infection, smoking, alcoholism, and radiation and chemotherapy, which can influence one or more stages of this process, resulting in improper or impaired wound healing (Roux et al., 2025). In particular, delayed wound healing in diabetic patients is increasing globally due to the lack of effective intervention strategies and the widespread prevalence of diabetes (De Souza et al., 2023).
Given their excellent immunoregulation, multidirectional differentiation ability, and paracrine function, adipose-derived mesenchymal stem cells (ADSCs) have emerged as a novel and promising strategy for treating diabetic wounds in both preclinical and clinical studies (Yan et al., 2024; Carstens et al., 2021; Huerta et al., 2023). However, the effectiveness of ADSCs in repairing diabetic wounds is limited by their low engraftment efficiency, which could be partially attributed to the stark contrast between optimized in vitro culture conditions and the harsh pathological microenvironment of chronic wound sites (Yu et al., 2023). Therefore, further research is needed to improve the efficacy of ADSC therapy for diabetic wound healing. Recently, increasing evidence has suggested that the paracrine function of ADSCs plays a leading role in skin wound regeneration (Ma et al., 2023; Ren et al., 2024; Wei et al., 2024; Wang et al., 2024). In particular, instead of mesenchymal stem cells (MSCs), using MSC-conditioned medium or secretome also provides a therapeutic potential for reducing irradiated skin injuries (Lin et al., 2023) and scar fibrosis (Zhang et al., 2021; Wang et al., 2024). More importantly, this cell-free strategy effectively avoids the potential limitation of low cell engraftment of MSCs for wound healing.
In this study, we investigated whether ADSC-conditioned medium (ACM) can be used for accelerating diabetic wound healing in rats. To achieve this purpose, we evaluated the therapeutic effect of ACM on skin wounds both in vitro and in vivo and the potential mechanism of ACM in diabetic wound healing. The results suggest that ACM may offer a promising strategy to promote diabetic wound recovery.
2 Materials and methods
2.1 Animals
Twenty adult male Sprague–Dawley (SD) rats (weighing 180 g–200 g) were obtained from the Shanghai Slack Laboratory Animal Center (license number: SCXK hu 2022-0004). All rats were housed in a standard specific pathogen-free (SPF) barrier environment at 20 °C–26 °C and 40%–70% humidity under a 12 h/12 h light–dark cycle. All animal experiments were approved by the Experimental Animal Ethics Center of Mengchao Hepatobiliary Hospital of Fujian Medical University (MCHH-AEC-2022-08). All experiments were designed and reported in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines 2.0.
2.2 Preparation of ADSC-conditioned medium
The ADSCs were isolated and cultured according to previously published methods (Liao et al., 2017). In brief, adipose tissues were obtained from the inguinal region of male SD rats (n = 5) and washed with PBS solution. The tissues were then cut into small fragments and digested with 0.1% type I collagenase, followed by neutralization with α-MEM containing 10% FBS. Subsequently, the cells were cultured at a density of 1 × 106 cells/mL in T-75 plates. ADSCs at passage 3 were collected and cultured at a density of 2 × 106 cells per 10 cm plate. After overnight cell adhesion, the cultured ADSCs were washed with PBS solution to remove residual serum and then replaced with serum-free medium (YOCON, China) for 48 h. After incubation, the conditioned medium (10 mL/2 × 106 ADSCs) was collected and centrifuged at 3,000 g for 5 min to remove any cell debris. Furthermore, the ADSC-conditioned medium was concentrated using ultrafiltration with a tangential flow filtration capsule (Pall, United States) containing a 3-kDa molecular weight cut-off membrane, following the manufacturer’s instructions. Finally, the concentration of the ADSC-conditioned medium was analyzed using a BCA assay kit (TransGen Biotech, China) and stored at −80 °C. A concentration of 100 ng/mL ADSC-conditioned medium was used in the present study.
2.3 HUVEC culture
The human umbilical vein endothelial cell (HUVEC) line was obtained from the National Institutes for Food and Drug Control (Beijing, China) and cultured with RPMI 1640 containing 10% FBS supplemented with 2% FBS, VEGF, IGF-1, and EGF at 37 °C/5% CO2. For the tubule formation assay, 96-well plates were pre-coated with Matrigel (Corning, 10 mg/mL, 1:50 dilution in EGM-2) for 1 h at 37 °C to simulate the basement membrane matrix, as standardized in angiogenesis assays.
2.4 Cell viability assay
HUVECs were cultured at a density of 1 × 104 cells per well in 96-well plates. After overnight cell adhesion, the cell supernatants were removed and replaced with 100 μL ADSC-conditioned medium, while the cells treated with serum-free medium were used as the negative control. After incubation for 24 h or 48 h, cell viability was evaluated using a CCK-8 assay kit (TransGen Biotech, China), according to the manufacturer’s instructions.
2.5 Quantitative real-time PCR analysis
Total RNA was collected using a TRIzol reagent kit (TransGen Biotech, China) following the manufacturer’s instructions. Afterward, mRNA was reverse-transcribed into cDNA using a cDNA synthesis kit (Roche, Germany). The quantitative real-time PCR analysis was performed in an ABI StepOnePlus Real-time PCR System (Carlsbad, United States), and the PCR conditions were as follows: 95 °C for 15 s, 60 °C for 30 s, and 70 °C for 30 s, for a total of 40 cycles. The primer sequences are listed in Table 1. The 2-△△Ct formula was used to analyze the relative gene expression.
2.6 Diabetic skin-injured model and ACM treatment
The type 2 diabetic (T2D) model was established using a previously described method (Liao et al., 2017). In brief, the SD rats (n = 10) were fed with a high-fat diet (HFD) containing 66.5% normal chow, 20% sucrose, 10% lard, 2% cholesterol, and 1.5% cholate. After being fed with the HFD for 4 weeks, all rats were administered 25 mg/kg of streptozotocin (STZ) by intraperitoneal injection twice/week for 2 weeks. Rats treated with STZ and exhibiting a non-fasting blood glucose level ≥11.1 mmol/L were considered successful in establishing the T2D model. Next, the T2D rats were anesthetized with 40 mg/kg of pentobarbital sodium, and a full-thickness skin defect of 1 cm in diameter was created using a previously described method (Hur et al., 2017). Subsequently, the rats were randomly (random table method) divided into T2D skin-injured model and ACM groups (n = 5/group) and housed separately. Rats in the ACM group were treated daily with 100 μL ACM administered intradermally around the wound edges for 7 days, while model rats received an equal volume of serum-free medium. Normal rats (n = 5) were used as the negative control. On the 5th day after the last ACM treatment (the 12th day in total), all rats were euthanized with 100 mg/kg of pentobarbital sodium, and the wounds were harvested for further evaluation. All animals were selected at random for outcome assessment.
2.7 Histological examination
Tissues were collected and fixed in 4% paraformaldehyde for 24 h and then paraffin-embedded and sectioned into slices. Tissue sections were evaluated with hematoxylin and eosin (HE) staining and Masson staining, respectively. Finally, a double-blind histological examination was performed using an ortho-microscope (Zeiss, Germany) by two expert pathologists.
2.8 RNA sequencing
Total RNA from skin tissues was subjected to polyA-selected RNA-sequencing on the Illumina HiSeq X10 platform in a blinded manner. Using the DESeq2 package, RNA-seq analysis was carried out to determine the different gene expression (DEG) among three groups: normal vs. model and ACM vs. model. False discovery rate (FDR) of < 0.05 and fold change of ≥ 2 or ≤ 2 were the principles for DEG screening. Gene Ontology (GO) analysis was used to analyze the gene functions of the DEGs, and the Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis was used to target the DEGs’ enrichment pathway.
2.9 Immunohistochemistry
The skin wound sections were drenched in a citrate antigen retrieval solution (Beyotime Institute of Biotechnology, China) and heat-treated in a pressure cooker for 2 min, naturally cooled to RT, and washed with PBS buffer three times. Following incubation with 3% H2O2 for 10 min, the sections were blocked with 5% BSA for 30 min. The sections were then incubated overnight at 4 °C with primary antibodies against TNF-α, NF-κB, p-NF-κB, MAPK, p-MAPK, CXCL1, CXCL2, and CXCL8 at a dilution ratio of 1:200 . After washing three times with PBS, the sections were incubated with the secondary antibody at RT for another 2 h, followed by staining with DAB. The samples were observed using an ortho-microscope (Zeiss, Germany) in a blinded manner by the assessors.
2.10 Statistical analysis
All quantitative data were expressed as the mean ± standard deviation. GraphPad Prism version 9.0 (GraphPad Software, United States) was used for statistical analysis. The ANOVA was used to evaluate the significant differences among three independent groups, while the two-tailed paired sample Student’s t-tests were used to evaluate the significant differences between two groups. p < 0.05 was considered a statistically significant difference.
3 Results
3.1 ACM promotes HUVEC angiogenesis and proliferation in vitro
Flow cytometry analysis showed that ADSCs typically expressed CD73 and CD90 while lacking expression of CD45, CD19, and CD34 (Supplementary Figure S1), suggesting the successful isolation and culture of ADSCs in this study. The impaired skin wound healing in diabetic individuals is largely attributed to diabetic angiopathy, which is characterized by the dysfunction and impairment of the arteries throughout the body (Jin et al., 2022). We, therefore, investigated the effect of ACM on the angiogenesis and proliferation of vascular cells in vitro. After incubation with ACM for 24 or 48 h, the viability of HUVECs was significantly increased (Figure 1A), suggesting that ACM promoted HUVEC proliferation. After 2 days of continuous ACM incubation, HUVECs showed vascular-like morphological changes (Figure 1B), and the expression of genes associated with angiogenesis, including EGF, bFGF, VEGF, and KDR, was significantly upregulated after ACM treatment (Figure 1C), implying that ACM promotes HUVEC angiogenesis in vitro.

Figure 1. ACM promotes vascular cell proliferation and angiogenesis. (A) ACM promotes HUVEC proliferation. (B) Representative images of HUVECs after ACM treatment (scale bar, 100 μm). (C) Relative mRNA expression of EGF, bFGF, VEGF, and KDR in HUVECs after ACM treatment.
3.2 ACM accelerates T2D skin wound healing
Based on the pro-angiogenic potential of ACM in vascular cells, we established a T2D skin wound model to further evaluate its therapeutic effects. As shown in Figures 2A, B, the skin wound healing rate of T2D rats was significantly improved by the continuous ACM treatment for 7 days compared to that of the model group. Moreover, increased tissue regeneration and decreased inflammatory infiltration were also observed in the ACM group compared to those in the model group (Figure 2C). Given the excellent performance of ACM on angiogenesis in vitro, we also investigated the beneficial effect of ACM on angiopathy in vivo. We found that the number of CD31+ cells and the expression of FGF-2 and VEGF were markedly increased in the ACM group compared to those in the model group (Figures 2D, E), suggesting that ACM could also improve angiopathy in vivo. Therefore, these data suggest that ACM accelerates T2D skin wound healing.
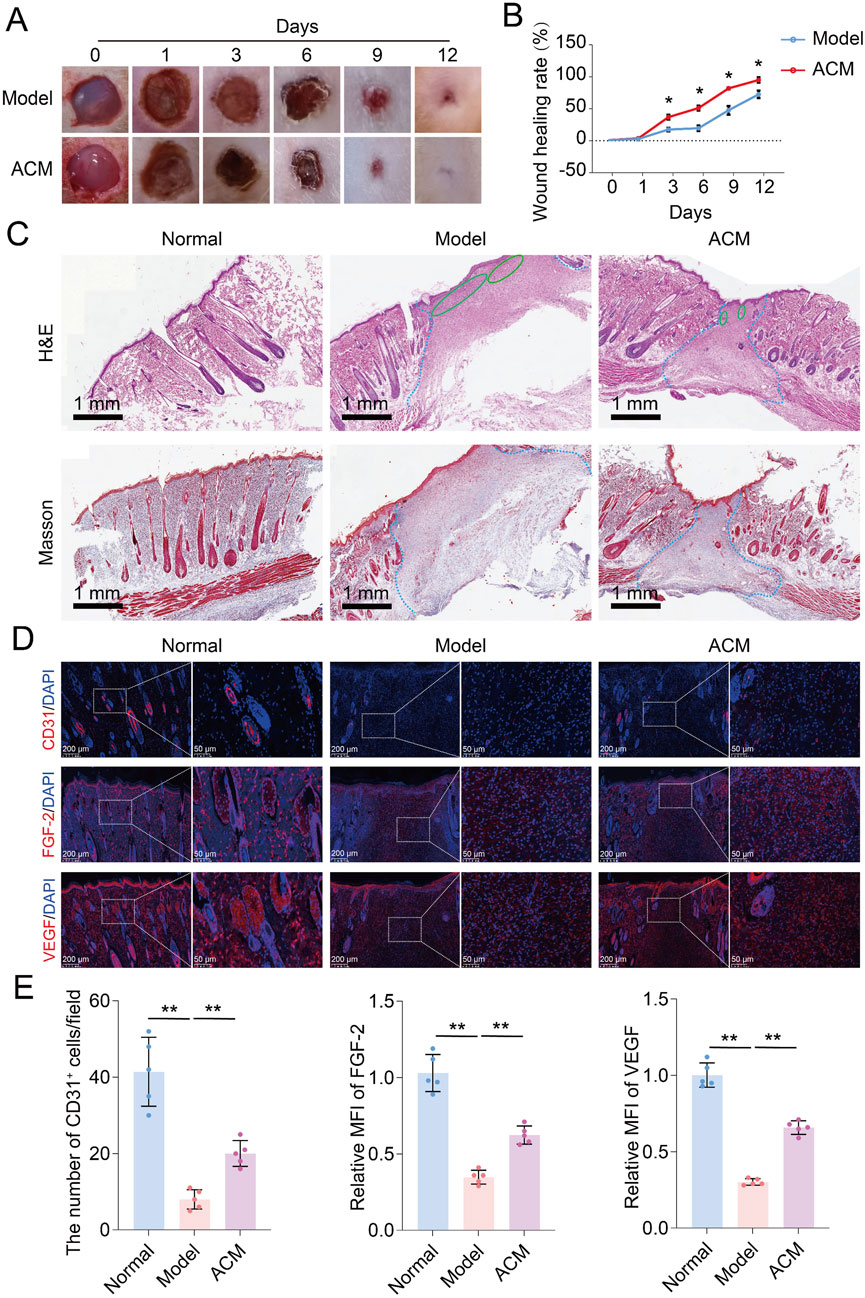
Figure 2. ACM accelerates T2D skin wound healing in rats. (A) General observation of skin wounds after ACM treatment. (B) Skin wound healing rate after ACM treatment. (C) Histopathological changes in skin wounds after ACM treatment by HE and Masson staining, respectively (scale bar, 1 mm). The area within the blue dotted line represents the damaged region. The green oval shape represents the area of inflammation. (D) Immunofluorescence expression of CD31, FGF-2, and VEGF in skin wounds after ACM treatment. Scale bar = 200 μm and 50 μm, respectively. (E) Number of CD31+ cells and the relative MFI of FGF-2 and VEGF expression in skin wounds after ACM treatment.
3.3 ACM inhibits T2D skin wound inflammation
Given that excessive inflammation is a typical characteristic of skin wounds (Huang et al., 2022), we further analyzed the inflammatory genes in T2D skin wound tissues. As shown in Figure 3, the mRNA expression levels of TNF-α, IL-1β, IL-6, COX-2, IL-12, and IFN-γ were significantly increased in T2D skin wounds compared to those in normal skin tissues, indicating excessive inflammation. However, these levels were effectively decreased after ACM treatment compared to those in the model groups, suggesting that ACM could inhibit this excessive inflammation. Moreover, we found that ACM treatment reduced inflammatory cell infiltration. This included a reduction in both the CD3+ T cells and F4/80+ macrophages (Supplementary Figure S3). Furthermore, the expression of TNF-α, IL-1β, and IL-6 was significantly decreased in the ACM-treated groups (Figure 4). Taken together, these data suggest that ACM mitigates the inflammatory response in skin wounds of diabetic rats.
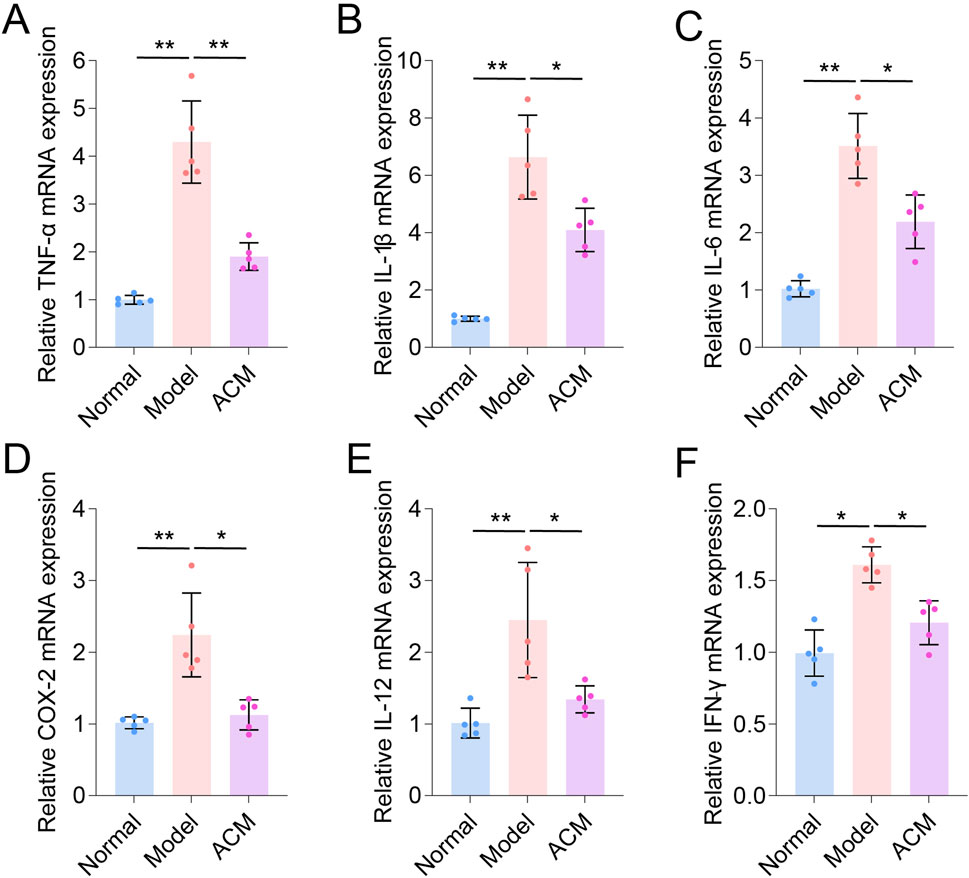
Figure 3. ACM inhibits the mRNA level of inflammatory factors in T2D skin wounds. The relative mRNA expression of TNF-α (A), IL-1β (B), IL-6 (C), COX-2 (D), IL-12 (E), and IFN-γ (F) in skin wounds.
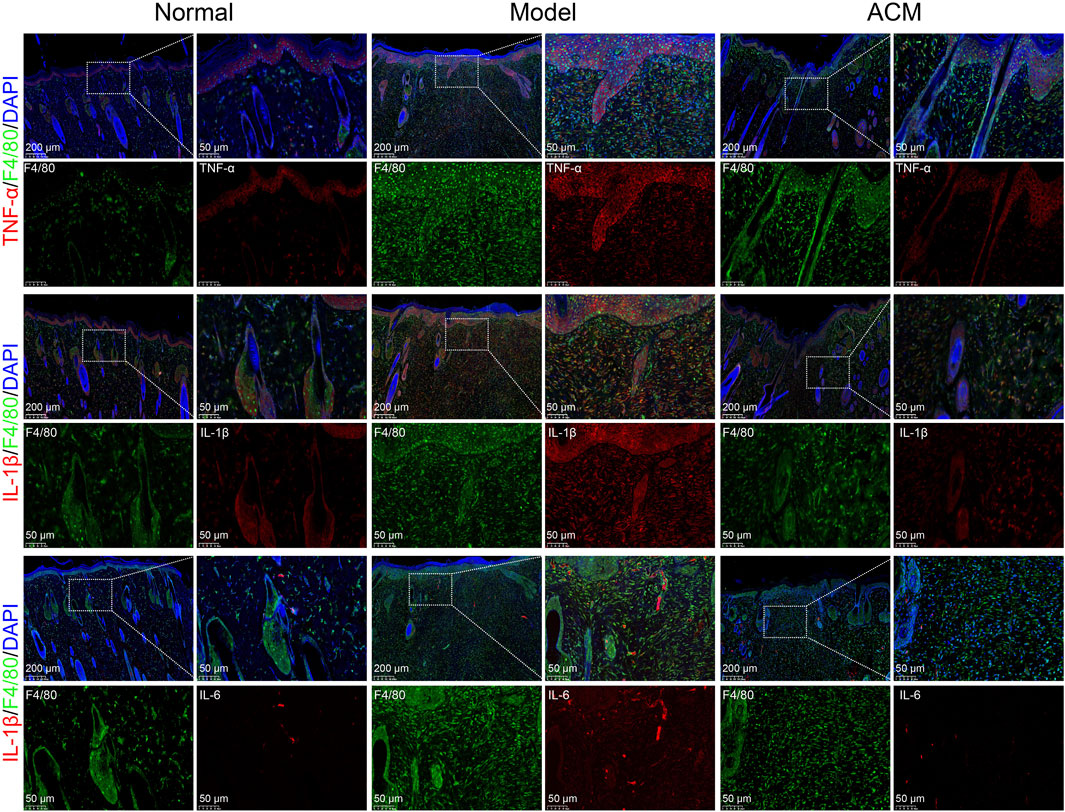
Figure 4. ACM inhibits the expression of inflammatory factors in macrophages. The immunofluorescence expression of TNF-α, IL-1β, and IL-6 in F4/80+ macrophages in skin wounds after ACM treatment. Scale bar = 200 μm and 50 μm, respectively.
3.4 ACM promotes T2D skin wound healing by targeting the TNF and chemokine signaling pathway
The potential molecular mechanism of ACM in accelerating T2D skin wound healing was further explored by RNA sequencing. Volcano plot analysis revealed differential expression of 10,655 genes was different between the normal and model groups (normal vs. model) and 5,287 genes between the ACM and model groups (ACM vs. model); a total of 4,269 genes were common to both comparisons (Figures 5A–C). GO annotation and pathway enrichment analysis showed that the upregulation of TNF and chemokine signaling was observed in the model group (compared with the normal group), while downregulation of TNF and chemokine signaling was clearly observed in the ACM group compared with the model groups (Figures 5D, E), suggesting that the potential molecular mechanism of ACM in T2D skin wound healing is by targeting the TNF and chemokine signaling pathway.
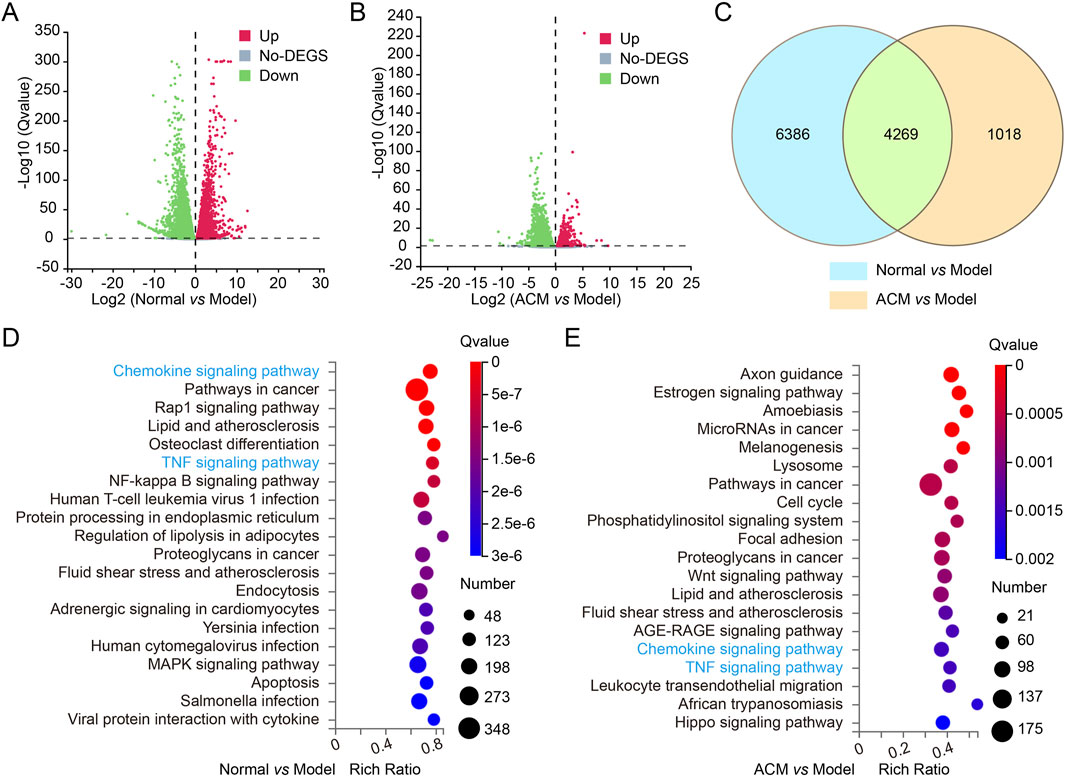
Figure 5. Transcriptome sequencing analysis of T2D skin wound tissues in rats. The volcano plot for differential gene expression of normal vs. model groups (A) and ADSC vs. model (B) groups. The gray pixel represents a gene where the difference in expression is not significant, while red and green pixels represent those that are significant. (C) Venn diagrams exhibiting the number of identified genes and the overlay of these identified genes. GO annotation and pathway enrichment analysis in normal vs. model groups (D) and ADSC vs. model (E) groups.
To confirm the RNA sequencing results, we further evaluated the protein expression of the main regulators in TNF and chemokine signaling. As shown in Figure 6, the TNF signaling-related proteins, including TNF-α, NF-κB, p-NF-κB, MAPK, and p-MAPK, and the chemokine signaling-related proteins, including CXCL1, CXCL2, and CXCL8, were all downregulated by ACM treatment in T2D skin wounds, suggesting that ACM accelerated T2D skin wound healing via the downregulation of TNF and chemokine signaling.
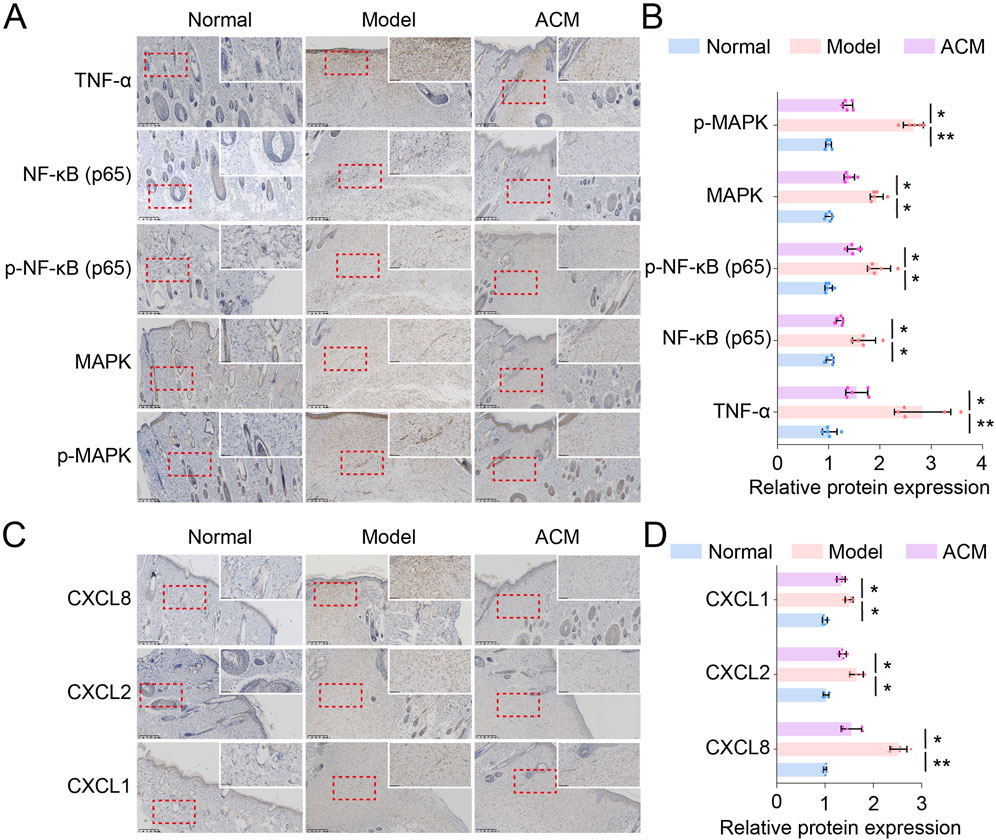
Figure 6. ACM downregulates TNF and chemokine signaling in T2D skin wound tissues. (A) Representative images of TNF-α, NF-κB, p-NF-κB, MAPK, and p-MAPK expression in skin tissues. Scale bars = 200 μm. (B) Relative expressions of TNF-α, NF-κB, p-NF-κB, MAPK, and p-MAP . (C) Representative images of CXCL1, CXCL2, and CXCL8 expression in skin tissues. Scale bars = 200 μm. (D) Relative expressions of CXCL1, CXCL2, and CXCL8.
4 Discussion
Angiogenesis is an essential part of skin wound regeneration, and it is also prone to being impaired by the diabetes status (Fan et al., 2024), excessive inflammation (Guo et al., 2023), oxidative stress, and other chronic wound conditions (De Wolde et al., 2021). Given the excellent performance of MSCs in promoting vasculogenesis through paracrine factors (e.g., VEGF, EGF, and bFGF) (Guillamat-Prats, 2021), MSC secretome or conditioned medium provides a new strategy for accelerating angiogenesis of skin wounds (Hade et al., 2022). Adipose tissue-derived ACM was assessed in this study to confirm its beneficial effects on angiogenesis, owing to the abundant availability and easy accessibility of adipose tissues (Bunnell, 2021). As expected, ACM effectively promoted HUVEC proliferation and angiogenesis. In particular, ACM also upregulated the expression of VEGF, EGF, bFGF, and KDR in HUVECs. Therefore, these data suggest that ACM contributes to cutaneous wound regeneration.
Considering that T2D accounts for more than 90% of diabetes cases (Edlitz and Segal, 2022), a T2D skin wound injury rat model was used to assess the therapeutic effect of ACM on skin wounds. Significantly, we found that ACM could promote the skin wound healing rate. It is well-known that excessive inflammation is caused by the crosstalk of various immune cells, including neutrophils (Zhu et al., 2021), macrophages (Lv et al., 2023), and lymphocytes (Baltzis et al., 2014), which is also characterized by the high expression of various pro-inflammatory factors, including TNF-α, IL-1β, IL-6, COX-2, IL-12, and IFN-γ (Quagliariello et al., 2021; Acosta et al., 2008; Schürmann et al., 2014). In this study, we proved that these pro-inflammatory factors were highly expressed in skin wounds, which means that excessive inflammation occurred in T2D rats. More importantly, we found that ACM could reduce the excessive inflammation. In particular, the transcriptome sequencing data further confirmed that ACM-accelerated T2D skin wound healing is closely related to the downregulation of TNF and the chemokine signaling pathway. Taken together, ACM provides a new promising strategy for accelerating T2D skin wound healing, which is partly through the TNF and chemokine signaling pathway.
It was previously shown that ADSC secretome reduces scar formation in skin wound healing (An et al., 2021) by inhibiting TGF-β1 and collagen expression (Wang et al., 2024). Paracrine cytokines and extracellular vesicles (e.g., exosomes) have been reported to be the major factors in the biological effects of ADSCs on wound healing (Wang et al., 2024). In this study, we further demonstrated that ACM accelerated diabetic skin wound healing through its anti-inflammatory functions. Given that MSC-conditioned medium or secretome has a complex composition, including extracellular vesicles (containing various types of lipids, proteins, and nucleic acids) and effector molecules (e.g., PGE2 and IDO) (Kota et al., 2017; Maughon et al., 2022; Zhao et al., 2023), the enhancement of ACM in skin wound regeneration may involve multiple targets and pathways. Further studies should focus on the different components and targets of ACM in the therapeutic role in T2D skin wound repair to verify more detailed mechanisms. Recently, Yin et al. reported that ADSC exosomes promote diabetic wound healing by regulating macrophage polarization (Yin and Shen, 2024) and epidermal autophagy (Ren et al., 2024). Therefore, exosomes of ACM would play a key role in accelerating diabetic wound healing. Furthermore, before proceeding with further clinical trials or applications, it is crucial to ensure strict control over the large-scale production, stability, and quality considerations related to ACM production.
In this study, we showed that ACM could enhance vascular proliferation and angiogenesis, promote skin wound healing in type 2 diabetes, and inhibit the inflammatory response. The mechanism may involve the downregulation of the TNF and chemokine pathways.
5 Conclusion
In conclusion, our study demonstrates that ACM can significantly accelerate the healing of diabetic skin wounds by promoting vascular remodeling and suppressing inflammation through the TNF and chemokine pathways.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The animal study was approved by the Animal Ethics Committee of Mengchao Hepatobiliary Hospital of Fujian Medical University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
LH: Funding acquisition, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review and editing. ZL: Investigation, Methodology, Visualization, Writing – review and editing. HL: Formal analysis, Resources, Writing – review and editing. XL: Methodology, Writing – review and editing. NL: Methodology, Supervision, Writing – review and editing. XW: Funding acquisition, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Scientific Foundation of Fujian Health Department (grant no. 2020QNB006), the Startup Fund for Scientific Research, Fujian Medical University (grant no. 2020QH1139), the Natural Science Foundation of Fujian Province (grant nos. 2024J011028 and 2024J011219), and the Natural Science Foundation of China (grant no. 82271238).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2025.1659444/full#supplementary-material
References
Acosta, J. B., del Barco, D. G., Vera, D. C., Savigne, W., Lopez-Saura, P., Guillen Nieto, G., et al. (2008). The pro-inflammatory environment in recalcitrant diabetic foot wounds. Int. Wound. J. 5, 530–539. doi:10.1111/j.1742-481X.2008.00457.x
An, Y. H., Kim, D. H., Lee, E. J., Lee, D., Park, M. J., Ko, J., et al. (2021). High-efficient production of adipose-derived stem cell (ADSC) secretome through maturation process and its non-scarring wound healing applications. Front. Bioeng. Biotechnol. 9, 681501. doi:10.3389/fbioe.2021.681501
Baltzis, D., Eleftheriadou, I., and Veves, A. (2014). Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Adv. Ther. 31, 817–836. doi:10.1007/s12325-014-0140-x
Bunnell, B. A. (2021). Adipose tissue-derived mesenchymal stem cells. stem cells 10, 3433. doi:10.3390/cells10123433
Carstens, M. H., Quintana, F. J., Calderwood, S. T., Sevilla, J. P., Ríos, A. B., Rivera, C. M., et al. (2021). Treatment of chronic diabetic foot ulcers with adipose-derived stromal vascular fraction cell injections: safety and evidence of efficacy at 1 year. Stem Cells Transl. Med. 10, 1138–1147. doi:10.1002/sctm.20-0497
De Souza, A., Santo, G. E., Amaral, G. O., Sousa, K. S. J., Parisi, J. R., Achilles, R. B., et al. (2023). Electrospun skin dressings for diabetic wound treatment: a systematic review. J. Diabetes Metab. Disord. 23, 49–71. doi:10.1007/s40200-023-01324-z
De Wolde, S. D., Hulskes, R. H., Weenink, R. P., Hollmann, M. W., and Van Hulst, R. A. (2021). The effects of hyperbaric oxygenation on oxidative stress, inflammation and angiogenesis. Biomolecules 11, 1210. doi:10.3390/biom11081210
Edlitz, Y., and Segal, E. (2022). Prediction of type 2 diabetes mellitus onset using logistic regression-based scorecards. Elife 11, e71862. doi:10.7554/eLife.71862
Fan, R., Zhao, J., Yi, L., Yuan, J., McCarthy, A., Li, B., et al. (2024). Anti-inflammatory peptide-conjugated silk fibroin/cryogel hybrid dual fiber scaffold with hierarchical structure promotes healing of chronic wounds. Adv. Mat. 36, e2307328. doi:10.1002/adma.202307328
Freedman, B. R., Hwang, C., Talbot, S., Hibler, B., Matoori, S., and Mooney, D. J. (2023). Breakthrough treatments for accelerated wound healing. Sci. Adv. 9, eade7007. doi:10.1126/sciadv.ade7007
Guillamat-Prats, R. (2021). The role of MSC in wound healing, scarring and regeneration. Cells 10, 1729. doi:10.3390/cells10071729
Guo, Y., Ma, M., Liu, Z., Lv, L., Pan, X., Liu, Q., et al. (2023). Chronic poor healing wounds of post cesarean scar diverticulum: altered angiogenesis and immunobiology. J. Reprod. Immunol. 157, 103929. doi:10.1016/j.jri.2023.103929
Hade, M. D., Suire, C. N., Mossell, J., and Suo, Z. (2022). Extracellular vesicles: emerging frontiers in wound healing. Med. Res. Rev. 42, 2102–2125. doi:10.1002/med.21918
Huang, C., Dong, L., Zhao, B., Lu, Y., Huang, S., Yuan, Z., et al. (2022). Anti-inflammatory hydrogel dressings and skin wound healing. Clin. Transl. Med. 12, e1094. doi:10.1002/ctm2.1094
Huerta, C. T., Voza, F. A., Ortiz, Y. Y., Liu, Z. J., and Velazquez, O. C. (2023). Mesenchymal stem cell-based therapy for non-healing wounds due to chronic limb-threatening ischemia: a review of preclinical and clinical studies. Front. Cardiovasc. Med. 10, 1113982. doi:10.3389/fcvm.2023.1113982
Hur, W., Lee, H. Y., Min, H. S., Wufuer, M., Lee, C. W., Hur, J. A., et al. (2017). Regeneration of full-thickness skin defects by differentiated adipose-derived stem cells into fibroblast-like cells by fibroblast-conditioned medium. Stem Cell Res. Ther. 8, 92. doi:10.1186/s13287-017-0520-7
Jin, W., Chen, X., Kong, L., and Huang, C. (2022). Gene therapy targeting inflammatory pericytes corrects angiopathy during diabetic wound healing. Front. Immunol. 13, 960925. doi:10.3389/fimmu.2022.960925
Kota, D. J., Prabhakara, K. S., Toledano-Furman, N., Bhattarai, D., Chen, Q., DiCarlo, B., et al. (2017). Prostaglandin E2 indicates therapeutic efficacy of mesenchymal stem cells in experimental traumatic brain injury. Stem Cells 35, 1416–1430. doi:10.1002/stem.2603
Liao, N., Zheng, Y., Xie, H., Zhao, B., Zeng, Y., Liu, X., et al. (2017). Adipose tissue-derived stem cells ameliorate hyperglycemia, insulin resistance and liver fibrosis in the type 2 diabetic rats. Stem Cell Res. Ther. 8, 286. doi:10.1186/s13287-017-0743-7
Lin, Z., Shibuya, Y., Imai, Y., Oshima, J., Sasaki, M., Sasaki, K., et al. (2023). Therapeutic potential of adipose-derived stem cell-conditioned medium and extracellular vesicles in an in vitro radiation-induced skin injury model. Int. J. Mol. Sci. 24, 17214. doi:10.3390/ijms242417214
Lv, D., Cao, X., Zhong, L., Dong, Y., Xu, Z., Rong, Y., et al. (2023). Targeting phenylpyruvate restrains excessive NLRP3 inflammasome activation and pathological inflammation in diabetic wound healing. Cell Rep. Med. 4, 101129. doi:10.1016/j.xcrm.2023.101129
Ma, H., Siu, W. S., and Leung, P. C. (2023). The potential of MSC-Based cell-free therapy in wound healing-a thorough literature review. Int. J. Mol. Sci. 24, 9356. doi:10.3390/ijms24119356
Martin, P., and Nunan, R. (2015). Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 173, 370–378. doi:10.1111/bjd.13954
Maughon, T. S., Shen, X., Huang, D., Michael, A. O. A., Shockey, W. A., Andrews, S. H., et al. (2022). Metabolomics and cytokine profiling of mesenchymal stromal cells identify markers predictive of T-cell suppression. Cytotherapy 24, 137–148. doi:10.1016/j.jcyt.2021.08.002
Quagliariello, V., De Laurentiis, M., Rea, D., Barbieri, A., Monti, M. G., Carbone, A., et al. (2021). The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc. Diabetol. 20, 150. doi:10.1186/s12933-021-01346-y
Ren, H., Su, P., Zhao, F., Zhang, Q., Huang, X., He, C., et al. (2024). Adipose mesenchymal stem cell-derived exosomes promote skin wound healing in diabetic mice by regulating epidermal autophagy. Burns Trauma 12, tkae001. doi:10.1093/burnst/tkae001
Roux, S., Marchès, A., Galiacy, S., Merbahi, N., and Simon, M. (2025). Biological solutions activated by cold plasma at atmospheric pressure: a new therapeutic approach for skin wound healing. Biomed. Pharmacother. 186, 118001. doi:10.1016/j.biopha.2025.118001
Schürmann, C., Goren, I., Linke, A., Pfeilschifter, J., and Frank, S. (2014). Deregulated unfolded protein response in chronic wounds of diabetic Ob/ob mice: a potential connection to inflammatory and angiogenic disorders in diabetes-impaired wound healing. Biochem. Biophys. Res. Commun. 446, 195–200. doi:10.1016/j.bbrc.2014.02.085
Wang, K., Yang, Z., Zhang, B., Gong, S., and Wu, Y. (2024). Adipose-derived stem cell exosomes facilitate diabetic wound healing: mechanisms and potential applications. Int. J. Nanomedicine. 19, 6015–6033. doi:10.2147/IJN.S466034
Wang, M., Zhao, J., Li, J., Meng, M., and Zhu, M. (2024). Insights into the role of adipose-derived stem cells and secretome: potential biology and clinical applications in hypertrophic scarring. Stem Cell Res. Ther. 15, 137. doi:10.1186/s13287-024-03749-6
Wei, J. T., He, T., Shen, K., Xu, Z. G., Han, J. T., and Yang, X. K. (2024). Adipose stem cell-derived exosomes in the treatment of wound healing in preclinical animal models: a meta-analysis. Burns Trauma 12, tkae025. doi:10.1093/burnst/tkae025
Yan, D., Song, Y., Zhang, B., Cao, G., Zhou, H., Li, H., et al. (2024). Progress and application of adipose-derived stem cells in the treatment of diabetes and its complications. Stem Cell Res. Ther. 15, 3. doi:10.1186/s13287-023-03620-0
Yin, D., and Shen, G. (2024). Exosomes from adipose-derived stem cells regulate macrophage polarization and accelerate diabetic wound healing via the circ-Rps5/miR-124-3p axis. Immun. Inflamm. Dis. 12, e1274. doi:10.1002/iid3.1274
Yu, S., Yu, S., Liu, H., Liao, N., and Liu, X. (2023). Enhancing mesenchymal stem cell survival and homing capability to improve cell engraftment efficacy for liver diseases. Stem Cell Res. Ther. 14, 235. doi:10.1186/s13287-023-03476-4
Zhang, C., Wang, T., Zhang, L., Chen, P., Tang, S., Chen, A., et al. (2021). Combination of lyophilized adipose-derived stem cell concentrated conditioned medium and polysaccharide hydrogel in the inhibition of hypertrophic scarring. Stem Cell Res. Ther. 12, 23. doi:10.1186/s13287-020-02061-3
Zhao, H., Li, Z., Wang, Y., Zhou, K., Li, H., Bi, S., et al. (2023). Bioengineered MSC-derived exosomes in skin wound repair and regeneration. Front. Cell Dev. Biol. 11, 1029671. doi:10.3389/fcell.2023.1029671
Keywords: adipose tissue-derived mesenchymal stem cells, conditioned medium, type 2 diabetes, skin wound, regeneration
Citation: Huang L, Lin Z, Liu H, Lin X, Liao N and Wu X (2025) Mesenchymal stem cell-conditioned medium accelerates type 2 diabetic wound healing by targeting TNF and chemokine signaling. Front. Cell Dev. Biol. 13:1659444. doi: 10.3389/fcell.2025.1659444
Received: 07 July 2025; Accepted: 02 September 2025;
Published: 24 September 2025.
Edited by:
Mustapha Najimi, Institute of Experimental and Clinical Research- UCLouvain, BelgiumReviewed by:
Giacomina Brunetti, University of Bari Aldo Moro, ItalyAhmet Emin Topal, Bahçeşehir University, Türkiye
Copyright © 2025 Huang, Lin, Liu, Lin, Liao and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naishun Liao, bGlhb25zMDQ2QDE2My5jb20=; Xiaodan Wu, d3hpYW9kYW5Ac2luYS5jb20=
†These authors have contributed equally to this work
 Long Huang
Long Huang Zhongbao Lin2†
Zhongbao Lin2† Naishun Liao
Naishun Liao Xiaodan Wu
Xiaodan Wu