- 1Department of Epidemiology, West Kazakhstan Marat Ospanov Medical University, Aktobe, Kazakhstan
- 2Drug Discovery and Development Industry, School of Pharmacy, Taipei Medical University, Taipei, Taiwan
- 3Department of Surgery No. 2, West Kazakhstan Marat Ospanov Medical University, Aktobe, Kazakhstan
- 4Stem Cells Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
- 5Department of Pharmacology, Medical School, Shiraz University of Medical Sciences, Shiraz, Iran
- 6Department of Languages, West Kazakhstan Marat Ospanov Medical University, Aktobe, Kazakhstan
- 7Department of Pharmaceutical Technology, Avicenna Tajik State Medical University, Dushanbe, Tajikistan
- 8Department of Public Health and Hygiene, Astana Medical University, Astana, Kazakhstan
- 9School of General Medicine-2, Asfendiyarov Kazakh National Medical University, Almaty, Kazakhstan
- 10Department of Microbiology, Virology and Immunology, West Kazakhstan Marat Ospanov Medical University, Aktobe, Kazakhstan
- 11Department for Scientific Work, West Kazakhstan Marat Ospanov Medical University, Aktobe, Kazakhstan
- 12Department of General Medical Practice No. 2, West Kazakhstan Marat Ospanov Medical University, Aktobe, Kazakhstan
- 13Department of Natural Sciences, West Kazakhstan Marat Ospanov Medical University, Aktobe, Kazakhstan
Microplastics (MPs) are increasingly implicated in cancer biology through effects on gene expression, stress responses, and treatment susceptibility; however, causal links remain provisional. We systematically screened PubMed and Google Scholar (through September 2025) to identify cancer-related genes reported to be altered by MP exposure and then evaluated microRNAs (miRNAs) with anticancer activity that may target those genes. Mature miRNA sequences were retrieved from RNAcentral and assessed against MP-altered genes using RNAhybrid for target-site prediction and minimum free-energy (mfe) hybridization. MPs were reported to modulate genes across multiple tumor types—including breast, gastric, liver, lung, colorectal, cervical, pancreatic, and skin. In silico analyses identified candidate miRNAs with favorable mfe values for these targets, including miR-483-3p, miR-365, miR-331-3p, miR-138-5p, miR-760, miR-1-3p, miR-665, miR-490-3p, miR-370-3p, miR-520a, miR-638, miR-559, miR-532-3p, miR-593-5p, and miR-29b. These interactions suggest putative avenues to counter MP-associated oncogenic programs and therapy resistance. Because mfe predictions do not establish functional regulation, all findings should be interpreted as hypothesis-generating. Priorities for validation include reporter assays, gene/protein modulation, phenotypic rescue, and in vivo testing in MP-exposed models. Collectively, our results nominate miRNAs as candidate tools to interrogate and potentially mitigate MP-associated carcinogenic mechanisms.
1 Introduction
Microplastics (MPs) are conventionally defined as plastic particles <5 mm (5,000 µm) in diameter, while nanoplastics (NPs) are generally <1 µm. For the purposes of this review, we focused on the range of 1–5,000 μm, consistent with commonly reported experimental studies (Hale et al., 2020). Among these, human health is one of the most critical concerns. Studies have confirmed the presence of MPs in the human body worldwide, where they can accumulate in various types of cells (Vethaak and Legler, 2021). This accumulation may lead to several adverse health outcomes, including gut microbiota disruption and respiratory disorders (Winiarska et al., 2024).
Microplastics (MPs < 5 mm) have been detected in human tissues and may perturb cancer-related pathways including oxidative stress, lipid metabolism, inflammation, and drug transport. Studies report that MPs can upregulate efflux transporters (ABCB1/ABCG2) and alter chemotherapeutic susceptibility (Rosellini et al., 2023), enhance metastatic features in breast cancer (Park et al., 2023), promote therapy resistance via ASGR2 in gastric cancer (Kim et al., 2022), and aggravate radiation-induced intestinal injury (Chen Y. et al., 2024). Despite these observations, mechanisms remain poorly defined.
One of the most concerning potential health impacts of MPs is cancer. Previous studies have suggested MPs as possible contributors to carcinogenic processes, but the evidence remains preliminary and largely associative (Kumar et al., 2024). In addition to initiating tumorigenesis through mechanisms such as DNA damage (Hu et al., 2022), may also influence the response of cancer cells to anti-cancer therapies, potentially contributing to drug resistance (Kim et al., 2022). These findings underscore the need to explore novel strategies for cancer treatment.
Therefore, scientists have attempted to develop various kinds of anti-cancer therapeutic agents in recent years (Sun et al., 2023). Recent research has focused on developing innovative anticancer strategies, including advanced drug platforms (Kaliyev et al., 2024), stem-cell–based therapies (Kaliyev et al., 2024), and public education initiatives (Barani, 2024). Among these, microRNAs (miRNAs) have emerged as promising anti-cancer agents.
miRNAs are ∼22-nucleotide non-coding RNAs that guide Argonaute complexes to complementary mRNA regions, leading to mRNA degradation or translational repression. Each miRNA regulates many targets, allowing broad control of oncogenic networks involving proliferation, apoptosis, invasion, and drug resistance (Szczepanek et al., 2022). These regulatory properties make miRNAs attractive therapeutic candidates for MP-associated cancers. miRNAs possess several advantageous properties, including the ability to regulate cancer-related pathways, modulate drug sensitivity, deliver therapeutic molecules, and enable personalized treatment approaches (Szczepanek et al., 2022), making them strong candidates for treating MP-associated cancers. However, there is still a lack of comprehensive understanding regarding the potential of miRNAs to treat MP- associated tumors and the molecular mechanisms through which they exert anti-cancer effects. Most previous studies have focused on how MPs alter miRNA expression and function (Chen T. et al., 2024).
Therefore, the present study aims to investigate the therapeutic potential of miRNAs in MP-associated cancers through an in silico analysis. Additionally, this review explores possible molecular mechanisms underlying the anti-cancer activity of miRNAs and offers insights for future in-vivo and in-vitro research to further clarify their role in treating MP-associated malignancies.
2 Materials and methods
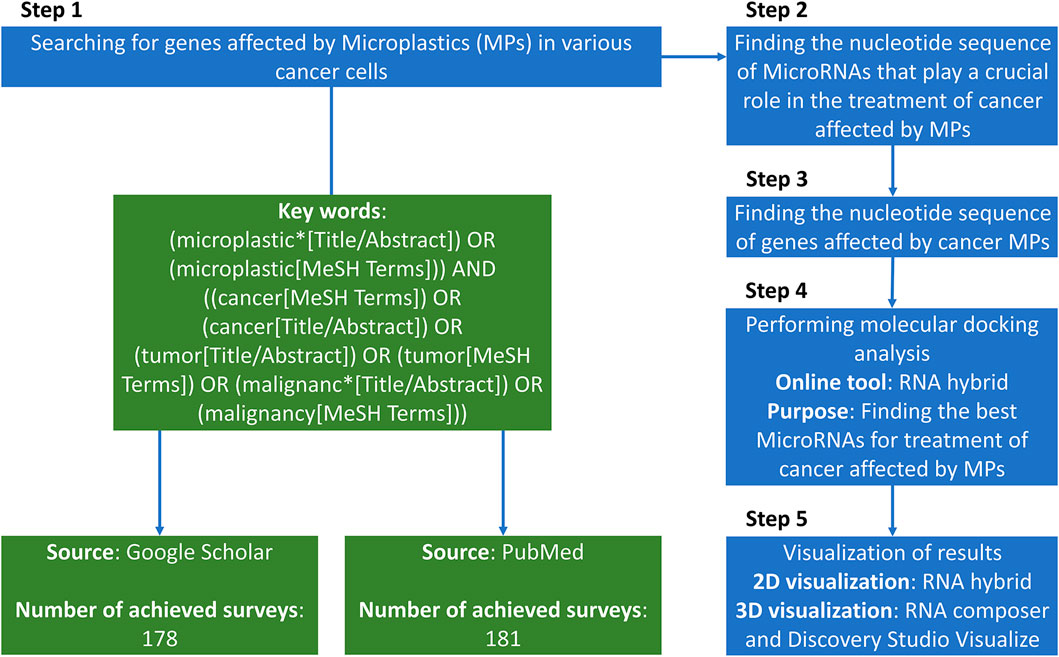
2.1 Literature identification and selection
We queried PubMed and Google Scholar from database inception to 30 September 2025 (Figure 1). Example search string (PubMed): (microplastic* OR nanoplastic* OR “plastic-related”) AND (cancer OR tumor OR carcinoma OR leukemia) AND (gene OR transcript* OR “drug resistance” OR efflux OR MAPK OR ABCB1 OR ABCG2) and for miRNAs: (microRNA OR miRNA) AND (anticancer OR tumor suppress* OR apoptosis OR chemosensit*) AND (breast OR gastric OR liver OR lung OR colorectal OR cervical OR pancreatic OR melanoma).
Inclusion criteria: (i) primary in vitro/in vivo/clinical studies in human cancer models or human tissues reporting MP exposure (or plastic-related compounds) and gene/protein/pathway changes; (ii) peer-reviewed; (iii) English. Exclusion: reviews, editorials, non-human non-cancer models, studies reporting expression changes without cancer relevance, or miRNAs lacking anticancer functional evidence. Two reviewers independently screened titles/abstracts and full texts; disagreements were resolved by a third reviewer.
2.2 Sequence sources and target selection
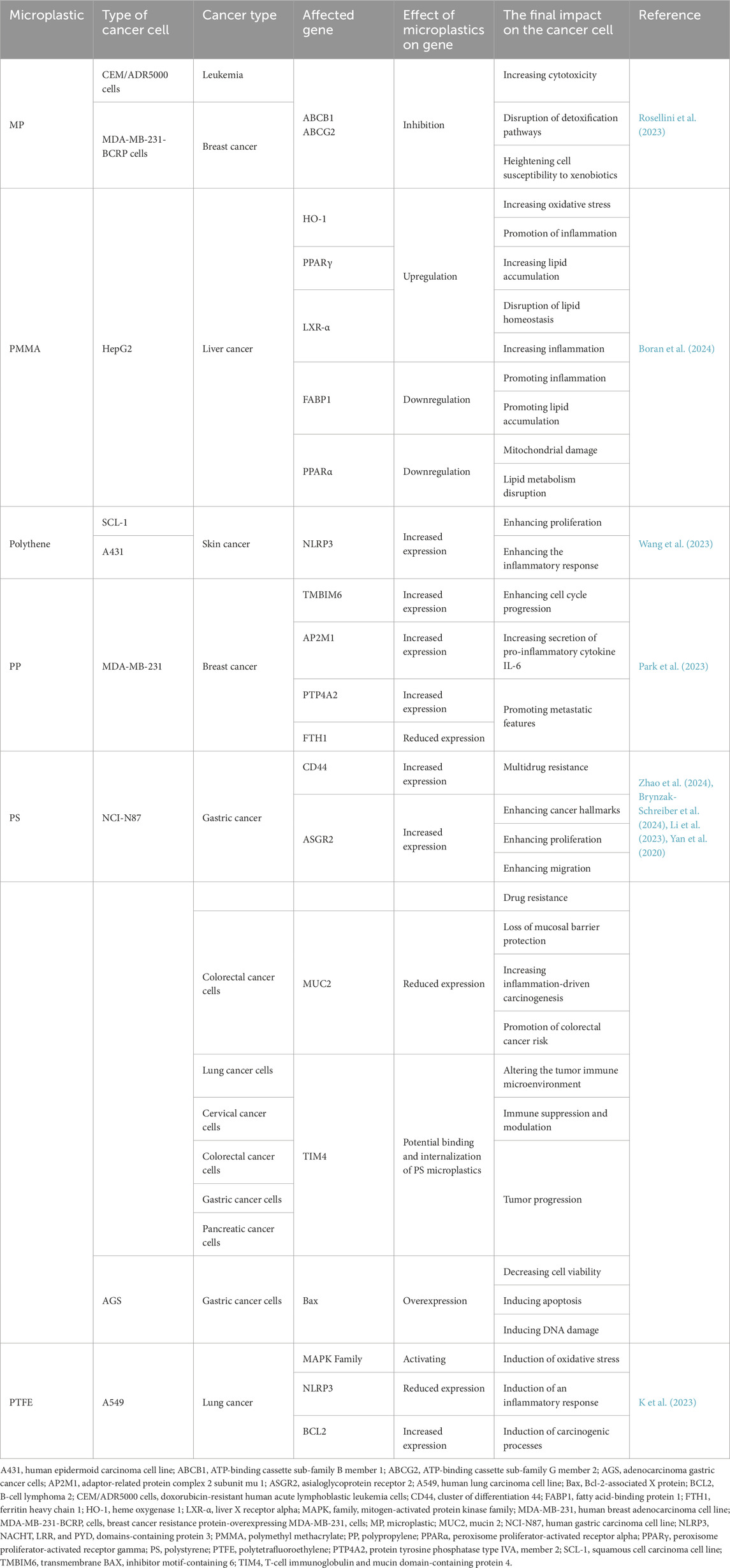
Mature miRNA sequences were retrieved from RNAcentral; human gene sequences (mRNA/UTR/coding region where available) were obtained from NCBI Gene/RefSeq. We evaluated genes previously reported as MP-altered in cancer contexts (Tables 1–3). (Chen T. et al., 2024).
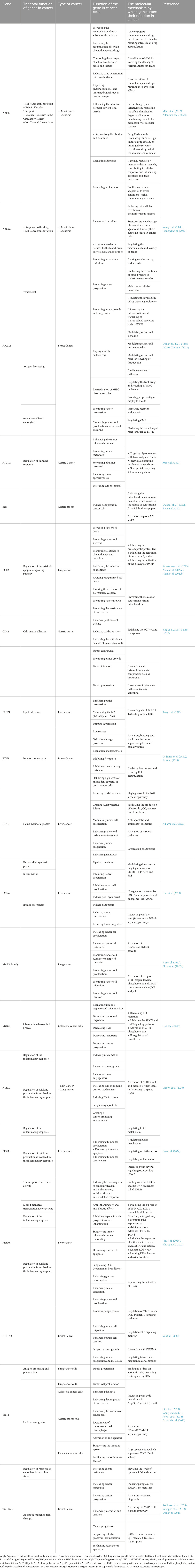
Table 2. Detailed information about gene function and molecular mechanisms that are affected by MPs, based on previous studies.
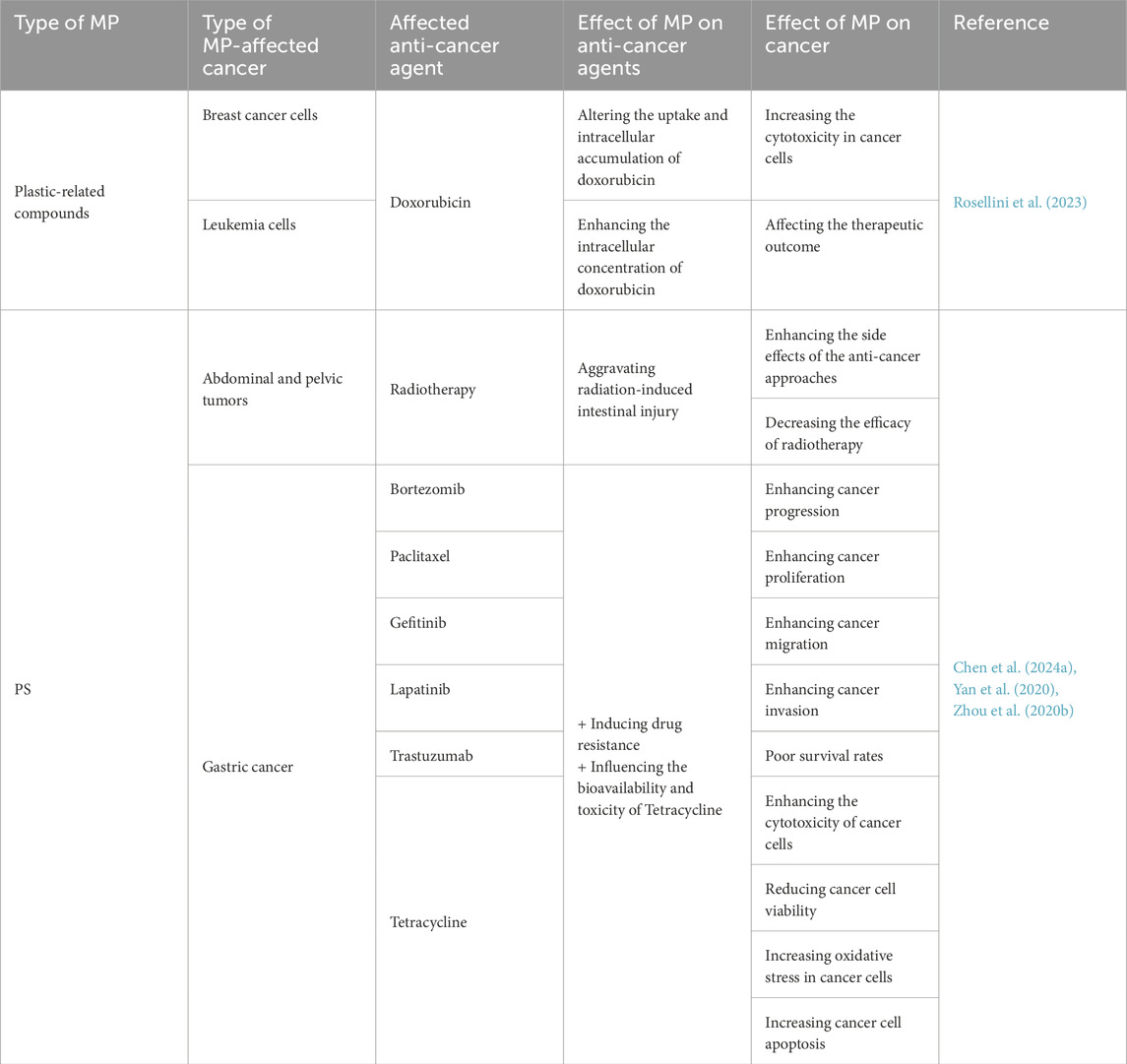
Table 3. Various effects of MPs on the anti-cancer agents and the final effect caused by these impacts on cancer cells.
2.3 In silico hybridization
We used RNAhybrid to predict target-site hybridization and minimum free energy (mfe). Default parameters were applied unless noted; where possible we scanned 3′UTRs preferentially and considered coding sequence if 3′UTR data were unavailable. For each gene, we screened multiple miRNAs with anticancer evidence and recorded the lowest mfe site per miRNA-gene pair. Lower (more negative) mfe was interpreted as more stable predicted binding. No single mfe threshold was used for exclusion; instead, candidates were prioritized by relative mfe within gene-specific comparisons (Chen T. et al., 2024; Jain et al., 2021). MFE criteria included: RNAhybrid minimum-free-energy (mfe) values were interpreted as strong (≤−20 kcal mol−1), suggestive (−15 to −19.9), and borderline (>−15). Pairs above −20 were retained only if supported by prior functional evidence and are flagged as low-priority hypothese.
2.4 Visualization and networks
Two-dimensional pairing diagrams were exported from RNAhybrid. 3D miRNA cartoons were rendered in Discovery Studio Visualizer for illustrative purposes only. Cytoscape was used to depict miRNA–gene–pathway relationships (Figure 2). Representative 2D pairing plots, 3D illustrations, and network diagrams are shown in Figure 3.
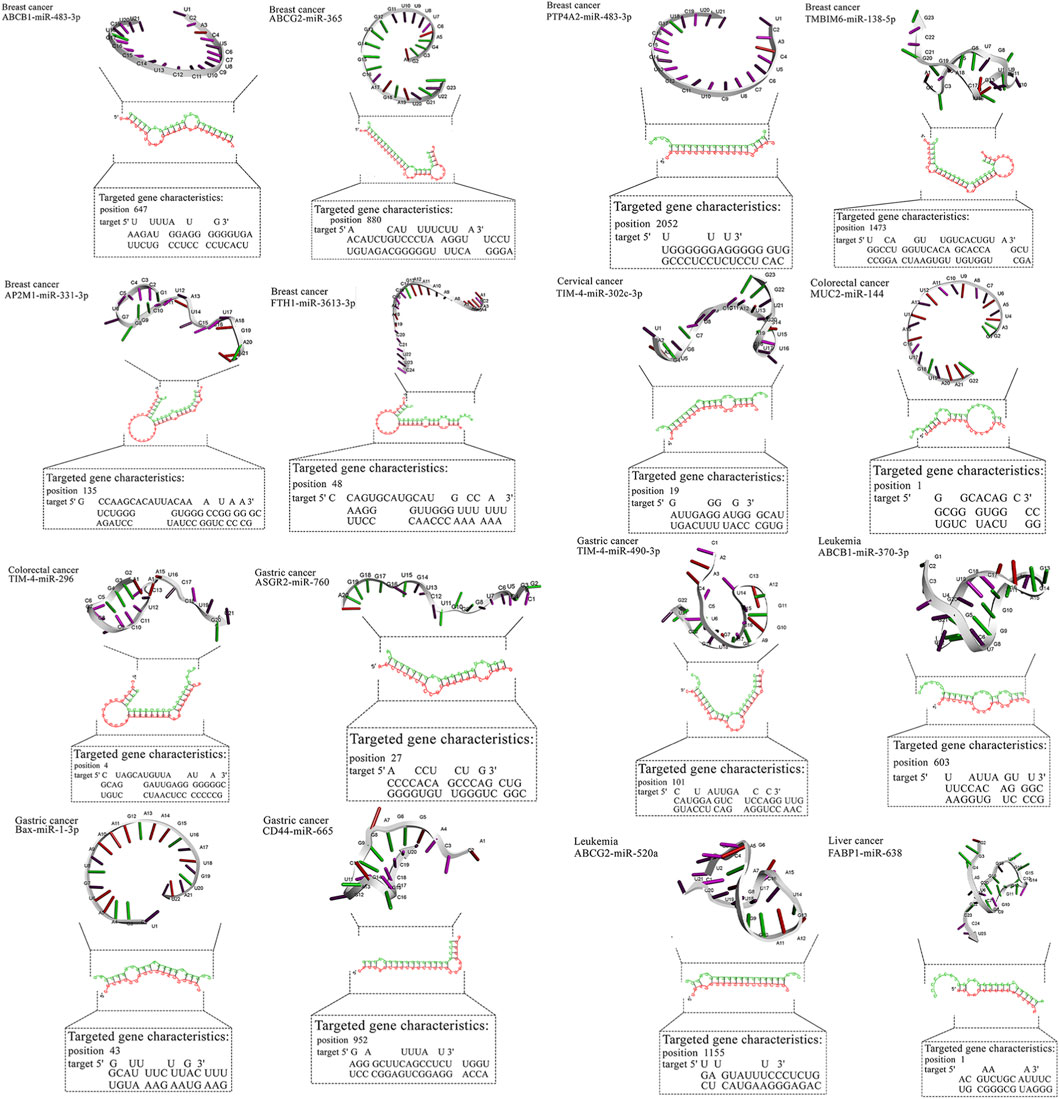
Figure 2. Detailed data about molecular interactions between microRNAs with anti-cancer activity and genes affected by MPs. The 3D structure at the top of the figure represents microRNA with the highest binding affinity toward the gene affected by MPs. The data about the targeted gene is depicted in the lower part of the figure. ABCB1, ATP Binding Cassette Subfamily B Member 1; ABCG2, ATP Binding Cassette Subfamily G Member 2; AP2M1, Adaptor Related Protein Complex 2 Subunit Mu 1; ASGR2, Asialoglycoprotein Receptor 2; Bax, BCL2 Associated X, Apoptosis Regulator; BCL2, B-cell CLL/lymphoma 2; BRAF, B-Raf Proto-Oncogene, Serine/Threonine Kinase; c-jun, Jun Proto-Oncogene, AP-1 Transcription Factor Subunit; CD44, CD44 Molecule (Indian Blood Group); ERK, Extracellular Signal-Regulated Kinase; FABP1, Fatty Acid Binding Protein 1; FTH1, Ferritin Heavy Chain 1; HO-1, Heme Oxygenase 1; JNK, c-Jun N-terminal Kinase; KRAS, KRAS Proto-Oncogene, GTPase; LXR-α, Liver X Receptor Alpha; MAP2K1, Mitogen-Activated Protein Kinase Kinase 1; MAP2K4, Mitogen-Activated Protein Kinase Kinase 4; MAPK14, Mitogen-Activated Protein Kinase 14; MUC2, Mucin 2; NLRP3, NLR Family Pyrin Domain Containing 3; NLRP3, NLR Family Pyrin Domain Containing 3; PPARα, Peroxisome Proliferator-Activated Receptor Alpha; PPARγ, Peroxisome Proliferator-Activated Receptor Gamma; PTP4A2, Protein Tyrosine Phosphatase Type IVA, Member 2; TMBIM6, Transmembrane BAX Inhibitor Motif Containing 6; TIM4, T-cell Immunoglobulin and Mucin-domain Containing-4; TIM4, T-cell Immunoglobulin and Mucin-domain Containing-4; TIM4, T-cell Immunoglobulin and Mucin-domain Containing-4; TIM4, T-cell Immunoglobulin and Mucin-domain Containing-4; TIM4, T-cell Immunoglobulin and Mucin-domain Containing-4.
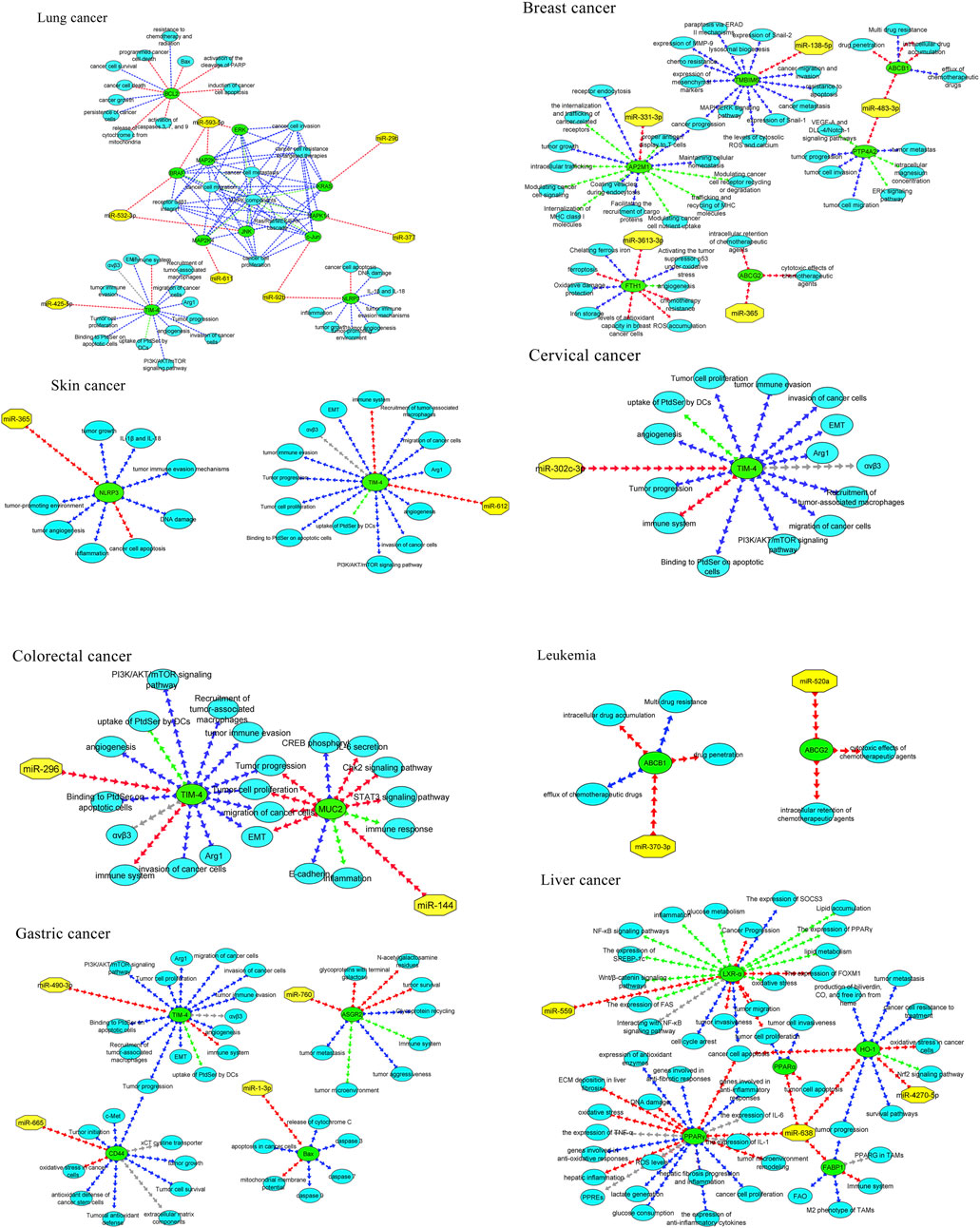
Figure 3. MicroRNAs with the in-silico capability to suppress genes in MPs-based cancers and their possible targeted molecular pathways. The blue, red, green, and gray arrows represent activation (or upregulation), inhibition (or downregulation), regulation, and interaction, respectively. Moreover, yellow hexagons, blue ovals, and green ovals demonstrate microRNAs, targeted genes, and pathways affected by targeted genes, respectively. ABCB1, ATP Binding Cassette Subfamily B Member 1; ABCG2, ATP Binding Cassette Subfamily G Member 2; AP2M1, Adaptor Related Protein Complex 2 Subunit Mu 1; ASGR2, Asialoglycoprotein Receptor 2; Bax, BCL2 Associated X, Apoptosis Regulator; BCL2, B-cell CLL/lymphoma 2; BRAF, B-Raf Proto-Oncogene, Serine/Threonine Kinase; c-jun, Jun Proto-Oncogene, AP-1 Transcription Factor Subunit; CD44, CD44 Molecule (Indian Blood Group); ERK, Extracellular Signal-Regulated Kinase; FABP1, Fatty Acid Binding Protein 1; FTH1, Ferritin Heavy Chain 1; HO-1, Heme Oxygenase 1; JNK, c-Jun N-terminal Kinase; KRAS, KRAS Proto-Oncogene, GTPase; LXR-α, Liver X Receptor Alpha; MAP2K1, Mitogen-Activated Protein Kinase Kinase 1; MAP2K4, Mitogen-Activated Protein Kinase Kinase 4; MAPK14, Mitogen-Activated Protein Kinase 14; MUC2, Mucin 2; NLRP3, NLR Family Pyrin Domain Containing 3; NLRP3, NLR Family Pyrin Domain Containing 3; PPARα, Peroxisome Proliferator-Activated Receptor Alpha; PPARγ, Peroxisome Proliferator-Activated Receptor Gamma; PTP4A2, Protein Tyrosine Phosphatase Type IVA, Member 2; TMBIM6, Transmembrane BAX Inhibitor Motif Containing 6; TIM4, T-cell Immunoglobulin and Mucin-domain Containing-4; TIM4, T-cell Immunoglobulin and Mucin-domain Containing-4; TIM4, T-cell Immunoglobulin and Mucin-domain Containing-4; TIM4, T-cell Immunoglobulin and Mucin-domain Containing-4; TIM4, T-cell Immunoglobulin and Mucin-domain Containing-4.
2.5 Interpretive caveats
mfe predictions do not prove targeting; they require orthogonal validation (reporter assays with wild-type/mutant sites, miRNA gain/loss, protein readouts, and phenotypic rescue under MP exposure).
3 Results
3.1 MPs modulate cancer-relevant genes across tumor types
Across studies, MPs were linked to changes in efflux transporters (ABCB1, ABCG2), stress and survival mediators (TMBIM6, HO-1), immune modulators (TIM4), metabolic regulators (PPARα/γ, LXR-α, FABP1), and ECM/adhesion factors (CD44). In breast cancer models, polypropylene increased AP2M1, PTP4A2, and TMBIM6 while reducing FTH1, a pattern consistent with enhanced trafficking/ER-stress signaling and diminished iron-mediated tumor suppressive functions. In gastric cancer, polystyrene exposure associated with higher CD44 and ASGR2, aligning with stemness/adhesion and glycoprotein handling. In liver cancer, PMMA upregulated HO-1 and downregulated PPARα/FABP1, suggesting oxidative stress and lipid dysregulation. Lung cancer models exposed to PTFE implicated MAPK cascade genes and BCL2, consistent with proliferation and apoptosis evasion. Collectively, these reports converge on MP-associated activation of survival and resistance programs with tumor- and polymer-specific nuances. An overview of MP-altered genes by tumor type is summarized in Figure 3 (Table 1).
3.2 Functional roles of MP-altered genes
Table 2 maps each gene to cancer functions and mechanisms. Notable axes include: (i) drug efflux (ABCB1/ABCG2) → reduced intracellular drug levels; (ii) endocytosis/trafficking (AP2M1) → receptor signaling and nutrient uptake; (iii) immune modulation (TIM4) → efferocytosis and immune tolerance; (iv) metabolism/oxidative balance (PPARs, LXR-α, FABP1, HO-1) → growth and stress adaptation; (v) MAPK signaling → proliferation/migration; and (vi) cell death control (BAX↑, BCL2↑) → apoptosis set-point shifts. These roles provide mechanistic context for potential miRNA interventions (Table 2).
3.3 Impact of MPs on anticancer therapies
Reports indicate both sensitization and resistance, but the weight of evidence suggests attenuation of therapy efficacy in several contexts (e.g., altered uptake/efflux; MAPK-mediated survival; inflammation-mediated radioprotection). The table summarizes polymer/tumor-specific patterns and implicated agents (e.g., doxorubicin, taxanes, HER2/EGFR inhibitors), emphasizing the need to measure MP exposure in preclinical efficacy studies (Table 3).
3.4 miRNAs as therapeutic candidates
We collated anticancer miRNAs with functional evidence (apoptosis, invasion, chemosensitization). This pool served as input to the in silico screen. Where multiple candidates mapped to one target, prioritization was by relative mfe plus prior functional plausibility. Examples of anticancer miRNAs selected for screening are illustrated in Figure 3 (Supplementary Table S1).
3.5 Predicted miRNA–gene interactions
For breast cancer, miR-483-3p and miR-138-5p showed favorable predictions against ABCB1/PTP4A2 and TMBIM6, respectively, while miR-365 and miR-331-3p mapped to ABCG2/AP2M1. In gastric cancer, miR-665 and miR-760 targeted CD44 and ASGR2; in liver cancer, miR-638 showed broad predictions (e.g., HO-1, PPARα/γ). Lung cancer candidates clustered on MAPK nodes (miR-532-3p, miR-593-5p, miR-29b). These pairings nominate tractable validation sets (reporter assays ± MP exposure, phenotypic readouts). Representative RNAhybrid pairing diagrams and 3D cartoons for top-ranked pairs appear in Figure 3, and the integrated miRNA–gene–pathway network is provided in Figure 3 (Table 4).
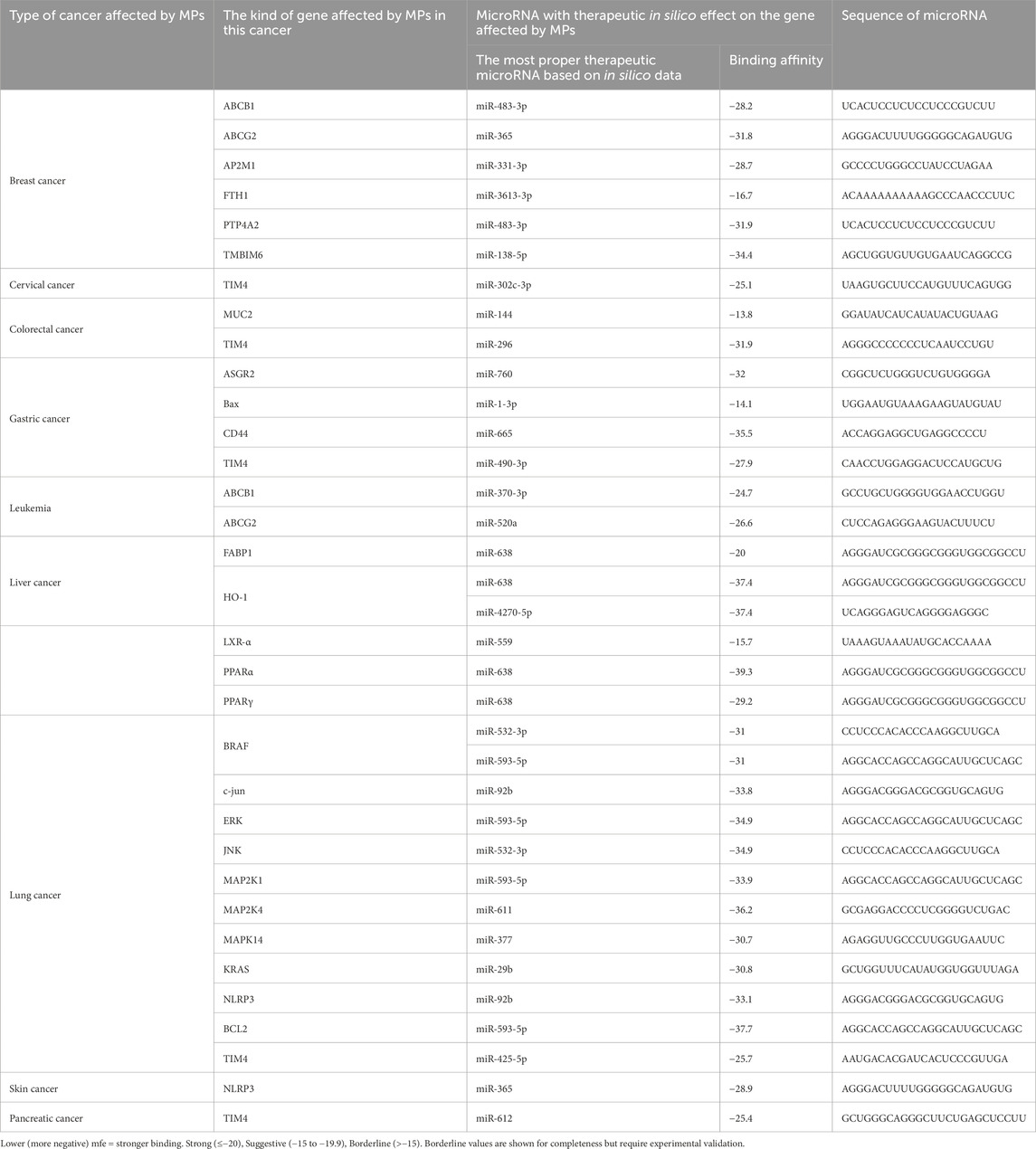
Table 4. Detailed information about the binding affinity of microRNAs and genes in cancer affected by MPs.
Most high-affinity interactions showed mfe ≤ −20 kcal mol−1 (e.g., TMBIM6/miR-138-5p, CD44/miR-665, PPARα/miR-638, MAP2K4/miR-611). Moderate (−15 to −19.9) values such as FTH1/miR-3613-3p were retained due to prior experimental evidence of anticancer function. Borderline pairs (e.g., BAX/miR-1-3p, −14.1) were listed for transparency but are considered exploratory. Representative hybridization structures are illustrated in Figure 3.
4 Discussion
Our results are consistent with prior evidence that MP exposure modulates canonical cancer pathways. Enhanced efflux and drug resistance observed experimentally (Rosellini et al., 2023) parallel our identification of ABCB1/ABCG2 as MP-responsive genes and their suppression by miR-483-3p and miR-365. Polypropylene-induced metastasis (Park et al., 2023) corresponds to increased AP2M1/PTP4A2/TMBIM6, predicted to be inhibited by miR-331-3p and miR-138-5p. Upregulation of ASGR2 in gastric cancer (Kim et al., 2022) matches our predicted targeting by miR-760. Similarly, activation of MAPK and BCL2 signaling (Jain et al., 2021; Ramkumar et al., 2023; Zhou Z. et al., 2020) is reflected in our miR-532-3p/miR-593-5p predictions. These consistencies strengthen the biological plausibility of our computational findings while emphasizing the need for experimental validation.
Besides, MPs affect some genes in cancer cells and suppress their effects. For instance, Polymethyl methacrylate (PMMA) inhibits liver cancer cells by downregulating FABP1 and PPAR alpha. According to prior surveys, MPs can downregulate specific genes in tumor cells, such as BCAS3, PHF19, and PRKCD, whose expression has been suppressed by microplastics in previous studies (Chen et al., 2025).
Moreover, our study demonstrates that MPs can affect some genes in breast cancer. ABCB1 is one of these genes that encodes P-glycoprotein (P-gp), an ATP-binding cassette (ABC) efflux transporter involved in multidrug resistance (MDR) in breast cancer, which expels chemotherapeutic drugs from cells, reduces drug efficacy, and contributes to treatment failure (Miao et al., 2017). It regulates ion channels, affecting apoptosis, proliferation, and other cancer-related processes (Altamura et al., 2022).
ABCG2 is another gene affected by MPs, and it encodes Breast Cancer Resistance Protein (BCRP), another ABC transporter involved in MDR by exporting chemotherapeutic agents, such as mitoxantrone and doxorubicin, out of cancer cells, reducing drug efficacy (Zhang et al., 2022). It also influences drug bioavailability and toxicity, acting as a barrier in the blood-brain barrier, liver, and intestines, with alterations linked to treatment failure in cancers with high ABCG2 expression (Wang et al., 2020; Franczyk et al., 2022).
AP2M1, a key component of the clathrin adaptor protein complex, facilitates receptor-mediated endocytosis in cancer cells, enhancing nutrient and growth factor uptake, which supports tumor growth and survival (Shin et al., 2021; Liu et al., 2021; Münz, 2020). Its overexpression is associated with aggressive cancer phenotypes and chemoresistance (Liu et al., 2023). Notably, this gene is also influenced by MPs in breast cancer.
Other genes affected by MPs in breast cancer include FTH1, PTP4A2, and TMBIM6. FTH1, a tumor suppressor, regulates iron storage and oxidative stress protection in cancer cells and stabilizes p53 under stress conditions. Silencing FTH1 leads to increased tumor growth, migration, and chemoresistance, along with upregulation of oncogenes like c-MYC and G9a (Di Sanzo et al., 2020; Ali et al., 2021). PTP4A2, upregulated in breast cancer, promotes cancer progression through various pathways (Chouleur et al., 2024). TMBIM6, a key regulator of stress responses, is linked to increased chemoresistance, cancer progression, and metastasis in breast cancer, as well as reduced patient survivalTMBIM6, a key regulator of stress responses, is linked to increased chemoresistance, cancer progression, and metastasis in breast cancer, as well as reduced patient survival (Robinson et al., 2025).
The other effect on MPs on genes in cancer cells is their effect on cervical cancer cells. Based on prior surveys, TIM4, along with TIM3, plays an essential role in the degradation of dying tumor cells via autophagy, reducing antigen presentation and impairing cytotoxic T lymphocyte (CTL) responses, creating immune tolerance, and weakening the antitumor immune response (Junjappa et al., 2019).
Furthermore, the present study demonstrates that two genes in colorectal cancer (CRC) cells are affected by MPs: MUC2 and TIM4. MUC2 is a protective barrier in epithelial cells, playing a role in cell differentiation and maintaining the balance of adhesion. Altered MUC2 expression impacts the progression of CRC by influencing cellular proliferation, apoptosis, and epithelial integrity (Iranmanesh et al., 2021). Notably, the silencing of MUC2 increases IL-6 secretion, which activates the STAT3 signaling pathway, promoting tumor cell migration, epithelial-mesenchymal transition (EMT), and metastasis. MUC2 downregulation leads to the activation of STAT3 and Chk2, suppression of CREB phosphorylation, and loss of E-cadherin, facilitating cancer progression and metastasis (Hsu et al., 2017). Moreover, TIM4 acts as an oncogene through different mechanisms, including supporting tumor cell proliferation, migration, invasion, and immune evasion, and contributing to tumor immune tolerance by impairing antigen presentation and cytotoxic T cell responses (Liu et al., 2020).
According to our findings, ASGR2, Bax, CD44, and TIM4 are genes impacted by MPs in gastric cancer cells. ASGR2 enhances tumor survival and metastasis, with higher levels linked to poor prognosis in gastric cancer (Xue et al., 2021). CD44 regulates cell adhesion, motility, and survival, promoting gastric cancer progression through tumor growth, invasion, and metastasis (Jang et al., 2011). It also supports tumor survival by enhancing antioxidant defenses and reducing oxidative stress (Zavros, 2017). TIM4 is overexpressed in gastric cancer tissues, correlating with increased angiogenesis, tumor growth, and poorer patient survival outcomes (Wang et al., 2021). In contrast, Bax induces apoptosis in gastric cancer cells through the mitochondrial pathway, promoting pro-apoptotic signaling, mitochondrial membrane collapse, and subsequent caspase activation (Shabani et al., 2020; Shen et al., 2023).
The other genes affected by MPs in cancer cells are ABCB1 and ABCG2 in Leukemia. ABCB1 is an efflux transporter that helps pump chemotherapy drugs out of cells, and its activation contributes to MDR. In AML, overexpression of ABCB1 has been linked to poor treatment outcomes ABCB1 (Sucha et al., 2022). ABCG2 functions as an efflux transporter that can extrude a wide variety of chemotherapy drugs out of the cells, thereby reducing their effectiveness and contributing to drug resistance in leukemia cells. Moreover, the overexpression of ABCG2 in leukemia cells is associated with poor clinical outcomes, including reduced complete remission rates and overall survival (Damiani and Tiribelli, 2023).
In addition, FABP1, HO-1, LXR-α, PPAR-alpha (PPARα), and PPARγ have been affected by MPs in liver cancer cells. FABP1 supports tumor progression by maintaining the M2 phenotype of tumor-associated macrophages (TAMs), which is associated with immune suppression and cancer progression. FABP1 deficiency in TAMs reduced tumor growth, invasion, and migration in vitro, highlighting its role in enhancing cancer cell proliferation and aggressiveness (Tang et al., 2023). HO-1 promotes cancer cell survival by suppressing pro-apoptotic pathways, regulating mitochondrial oxidative stress, activating the transcription of antioxidant and detoxifying genes, and enhancing the cell’s ability to counteract oxidative damage and resist apoptosis (Alharbi et al., 2022). LXR-α plays a crucial role in regulating lipid metabolism, inflammation, and immune responses in HCC. Its activation inhibits tumor cell proliferation by inducing cell cycle arrest and apoptosis, and reduces tumor invasiveness and migration (Han et al., 2023).
PPARα plays a significant role in the development and progression of liver cancer by controlling lipid metabolism, glucose regulation, and inflammation in the liver cells (Pan et al., 2024). PPARγ is a protective factor in liver cancer by some mechanisms, including inhibiting hepatic fibrosis progression and inflammation, suppressing tumor microenvironment remodeling, and promoting apoptosis and senescence in hepatocellular carcinoma (HCC) cells (Ishtiaq et al., 2022).
Additionally, genes involved in the MAPK signaling pathway include BRAF, c-Jun, ERK, JNK, MAP2K1, MAP2K4, MAPK14, and KRAS, which are genes affected by MPs in lung cancer cells. These genes are crucial for cell proliferation and survival, and their activation leads to the uncontrolled growth of cancer cells (Pradhan et al., 2019). Other genes, such as NLRP3, BCL2, and TIM4, are also affected by MPs in the mentioned cancer. The activation of NLRP3 creates chronic inflammation that promotes tumors by inducing DNA damage, enhancing angiogenesis, and suppressing apoptosis in cancer cells (Tang et al., 2020). The BCL2 gene contributes to the resistance of small cell lung cancer (SCLC) to Aurora kinase B (AURKB) inhibitors. It suppresses apoptosis and DNA damage caused by these inhibitors, allowing cells to avoid programmed cell death even under therapeutic stress (Ramkumar et al., 2023). TIM4 acts as an oncogene by supporting tumor cell proliferation, migration, invasion, and immune evasion, and also contributes to tumor immune tolerance by impairing antigen presentation and cytotoxic T cell responses (Liu et al., 2020).
Additionally, MPs affect NLRP3 in skin cancer cells. NLRP3 enhances inflammation, stimulates angiogenesis, and promotes the proliferation and migration of tumor cells (Ciazyn et al., 2020). Additionally, our study revealed that TIM4 is another gene in pancreatic cancer cells that MPs can influence. TIM4 is crucial in creating an immune-suppressive environment, enabling tumor cells to evade immune detection. It also supports tumor progression by reducing the effectiveness of immune cells, such as macrophages and T cells, in targeting cancer cells (Shi et al., 2021).
MPs may also influence the efficacy of cancer therapies. They can alter the metabolism and bioavailability of therapeutic drugs by interfering with their absorption and distribution in the body. This could lead to either reduced or enhanced drug activity, depending on the interactions between the MPs and the drugs (Deng et al., 2025). Interestingly, our study also shows that MPs can reduce and enhance the anti-cancer drug activity. However, according to the prior surveys and our study, MPs can deteriorate the impacts of anti-cancer drugs (Zhao et al., 2024) and exert this resistance against various anticancer agents.
The present study highlights the potential of certain microRNAs as candidate therapeutics in breast cancer models; however, these predictions are hypothesis-generating and require validation in experimental and clinical contexts (Zhao et al., 2019). Our in silico analysis highlights microRNAs targeting genes influenced by MPs in breast cancer cells, aligning with prior findings on their anti-breast cancer capabilities (Menbari et al., 2020). Specific microRNAs, including miR-483-3p, miR-365, miR-331-3p, and miR-138-5p, demonstrated strong binding affinity for genes such as ABCB1, ABCG2, AP2M1, PTP4A2, and TMBIM6, marking them as promising candidates for microRNA therapy. These microRNAs also target various molecular pathways, enhancing their anti-cancer effects (Liu et al., 2019; Zhao et al., 2020; Rasoolnezhad et al., 2021; Shen et al., 2020). In-silico analysis also revealed miR-3613-3p’s potential to target FTH1, a gene involved in iron homeostasis and oxidative stress regulation in cancer cells (Di Sanzo et al., 2020), making it a key therapeutic candidate.
In addition to breast cancer, microRNAs also exhibit anti-cancer effects in cervical cancer (Hasanzadeh et al., 2019; Ding et al., 2021), colorectal cancer (Cheng et al., 2020), gastric cancer (Spitz and Gavathiotis, 2022), leukemia (Gil-Kulik et al., 2024; Xiao et al., 2024), liver cancer (K et al., 2020; Ramalingam et al., 2024), lung cancer (Pradhan et al., 2019; Naeli et al., 2020), pancreatic cancer (Li et al., 2021; Javadrashid et al., 2021), and skin cancer (Mohamm et al., 2021). Our study corroborates these findings, with in silico analyses revealing specific microRNAs that interact with key genes associated with tumorigenesis in these cancers. For instance, miR-302c-3p targets TIM4 in cervical cancer, miR-144 targets MUC2 in colorectal cancer, miR-706 and miR-665 target ASGR2 and CD44 in gastric cancer, and miR-532-3p and miR-593-5p target MAPK genes in lung cancer. These microRNAs demonstrate their therapeutic potential by influencing various molecular pathways, as shown in Figure 4. Overall, the study reinforces the growing potential of microRNAs as targeted therapies across multiple malignancies.
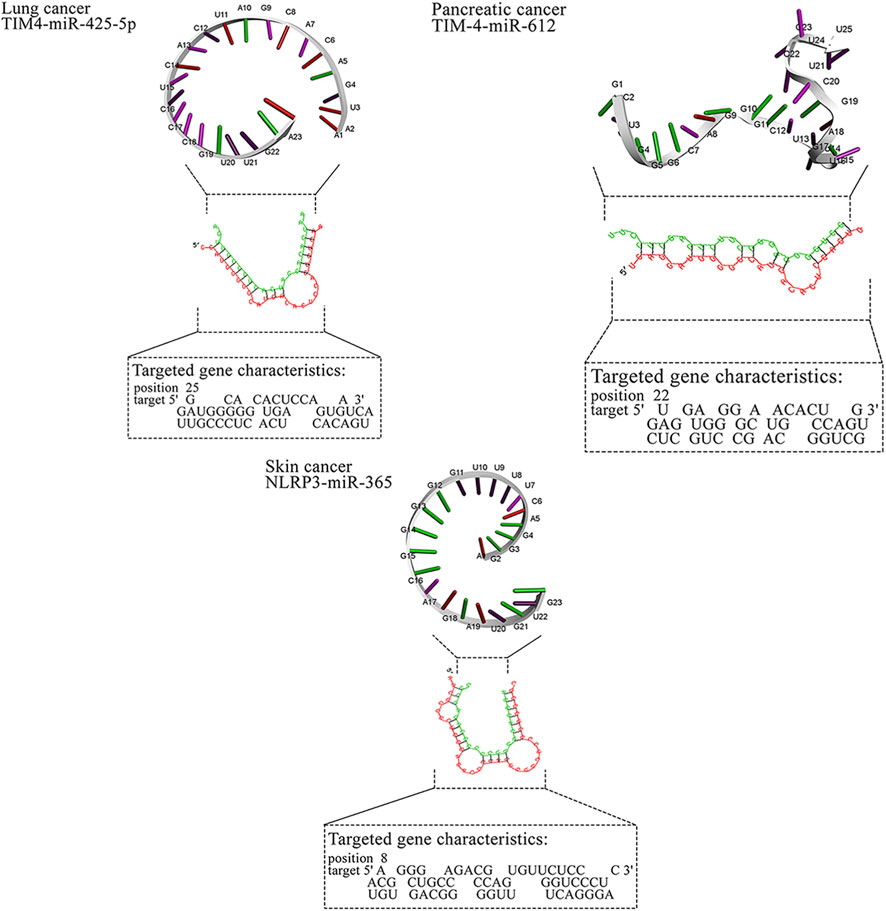
Figure 4. MicroRNAs with the in silico capability to suppress genes in MPs-based cancers and their possible targeted molecular pathways. The blue, red, green, and gray arrows represent activation (or upregulation), inhibition (or downregulation), regulation, and interaction, respectively. Moreover, yellow hexagons, blue ovals, and green ovals demonstrate microRNAs, targeted genes, and pathways affected by targeted genes, respectively. ABCB1, ATP Binding Cassette Subfamily B Member 1; ABCG2, ATP Binding Cassette Subfamily G Member 2; AP2M1, Adaptor Related Protein Complex 2 Subunit Mu 1; ASGR2, Asialoglycoprotein Receptor 2; Bax, BCL2 Associated X, Apoptosis Regulator; BCL2, B-cell CLL/lymphoma 2; BRAF, B-Raf Proto-Oncogene, Serine/Threonine Kinase; c-jun, Jun Proto-Oncogene, AP-1 Transcription Factor Subunit; CD44, CD44 Molecule (Indian Blood Group); ERK, Extracellular Signal-Regulated Kinase; FABP1, Fatty Acid Binding Protein 1; FTH1, Ferritin Heavy Chain 1; HO-1, Heme Oxygenase 1; JNK, c-Jun N-terminal Kinase; KRAS, KRAS Proto-Oncogene, GTPase; LXR-α, Liver X Receptor Alpha; MAP2K1, Mitogen-Activated Protein Kinase Kinase 1; MAP2K4, Mitogen-Activated Protein Kinase Kinase 4; MAPK14, Mitogen-Activated Protein Kinase 14; MUC2, Mucin 2; NLRP3, NLR Family Pyrin Domain Containing 3; NLRP3, NLR Family Pyrin Domain Containing 3; PPARα, Peroxisome Proliferator-Activated Receptor Alpha; PPARγ, Peroxisome Proliferator-Activated Receptor Gamma; PTP4A2, Protein Tyrosine Phosphatase Type IVA, Member 2; TMBIM6, Transmembrane BAX Inhibitor Motif Containing 6; TIM4, T-cell Immunoglobulin and Mucin-domain Containing-4; TIM4, T-cell Immunoglobulin and Mucin-domain Containing-4; TIM4, T-cell Immunoglobulin and Mucin-domain Containing-4; TIM4, T-cell Immunoglobulin and Mucin-domain Containing-4; TIM4, T-cell Immunoglobulin and Mucin-domain Containing-4.
4.1 Limitations
First, studies linking MPs to gene changes are heterogeneous in polymer type, size, dose, and exposure model; many use surrogates (e.g., plastic-related compounds) rather than standardized particles. Second, mfe predictions do not demonstrate binding or regulation; off-targeting and RNA context effects are likely. Third, we did not perform a quantitative meta-analysis due to heterogeneity and incomplete reporting.
4.2 Future work
We propose a tiered pipeline: 1. verify MP-induced gene changes under standardized exposures; 2. validate miRNA targeting (luciferase wild-type/mutant, protein knockdown, rescue); 3. evaluate phenotypes (viability, invasion, efflux, radiosensitization) with and without MPs; 4. test delivery and safety in vivo.
5 Conclusion
MP exposure has been reported to perturb cancer-relevant genes and therapy responses across tumor types. Our in silico analyses nominate miRNAs that may counter these MP-associated programs. These findings are hypothesis-generating and require rigorous experimental and translational validation.
Author contributions
AB: Writing – review and editing, Visualization, Data curation, Formal Analysis. AZ: Investigation, Conceptualization, Methodology, Visualization, Formal Analysis, Writing – original draft. NM: Investigation, Writing – review and editing, Validation, Data curation. NT: Writing – review and editing, Supervision, Resources, Project administration. KZ: Formal Analysis, Writing – review and editing, Visualization, Data curation. RSS: Writing – review and editing, Supervision, Project administration. RS: Writing – review and editing, Data curation. GY: Investigation, Writing – review and editing. AK: Writing – review and editing, Methodology, Validation. AU: Writing – review and editing, Data curation, Project administration. ANZ: Resources, Investigation, Writing – review and editing. AT: Project administration, Supervision, Methodology, Conceptualization, Writing – review and editing, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by the grant financing on scientific programs of the Ministry of Science and Higher Education of the Republic of Kazakhstan «Investigation of Microplastic Contamination in Packaged Foods and Water in Kazakhstan and Its Impact on Cancer Cell Proliferation: An In Vitro Study» (IRN AP26100885).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2025.1699693/full#supplementary-material
References
Alam, S., Mohammad, T., Padder, R. A., Hassan, M. I., and Husain, M. (2022a). Thymoquinone and quercetin induce enhanced apoptosis in non-small cell lung cancer in combination through the Bax/Bcl2 cascade. J. Cell Biochem. 123 (2), 259–274. doi:10.1002/jcb.30162
Alam, M., Alam, S., Shamsi, A., Adnan, M., Elasbali, A. M., Al-Soud, W. A., et al. (2022b). Bax/Bcl-2 Cascade is regulated by the EGFR pathway: therapeutic targeting of non-small cell lung cancer. Front. Oncol. 12, 869672. doi:10.3389/fonc.2022.869672
Alharbi, K. S., Almalki, W. H., Albratty, M., Meraya, A. M., Najmi, A., Vyas, G., et al. (2022). The therapeutic role of nutraceuticals targeting the Nrf2/HO-1 signaling pathway in liver cancer. J. Food Biochem. 46 (10), e14357. doi:10.1111/jfbc.14357
Ali, A., Shafarin, J., Abu Jabal, R., Aljabi, N., Hamad, M., Sualeh, M. J., et al. (2021). Ferritin heavy chain (FTH1) exerts significant antigrowth effects in breast cancer cells by inhibiting the expression of c-MYC. FEBS Open Bio 11 (11), 3101–3114. doi:10.1002/2211-5463.13303
Altamura, C., Gavazzo, P., Pusch, M., and Desaphy, J. F. (2022). Ion channel involvement in tumor drug resistance. J. Pers. Med. 12 (2), 210. doi:10.3390/jpm12020210
Astuti, Y., Raymant, M., Quaranta, V., Clarke, K., Abudula, M., Smith, O., et al. (2024). Efferocytosis reprograms the tumor microenvironment to promote pancreatic cancer liver metastasis. Nat. Cancer 5 (5), 774–790. doi:10.1038/s43018-024-00731-2
Barani, M. (2024). The role of environmental education in improving human health: literature review. West Kazakhstan Med. J. 66 (4), 373–386. doi:10.18502/wkmj.v66i4.17769
Boran, T., Zengin, O. S., Seker, Z., Akyildiz, A. G., Kara, M., Oztas, E., et al. (2024). An evaluation of a hepatotoxicity risk induced by the microplastic polymethyl methacrylate (PMMA) using HepG2/THP-1 co-culture model. Environ. Sci. Pollut. Res. Int. 31 (20), 28890–28904. doi:10.1007/s11356-024-33086-3
Brynzak-Schreiber, E., Schogl, E., Bapp, C., Cseh, K., Kopatz, V., Jakupec, M. A., et al. (2024). Microplastics role in cell migration and distribution during cancer cell division. Chemosphere 353, 141463. doi:10.1016/j.chemosphere.2024.141463
Caronni, N., Piperno, G. M., Simoncello, F., Romano, O., Vodret, S., Yanagihashi, Y., et al. (2021). TIM4 expression by dendritic cells mediates uptake of tumor-associated antigens and anti-tumor responses. Nat. Commun. 12 (1), 2237. doi:10.1038/s41467-021-22535-z
Chen, Y., Zeng, Q., Luo, Y., Song, M., He, X., Sheng, H., et al. (2024a). Polystyrene microplastics aggravate radiation-induced intestinal injury in mice. Ecotoxicol. Environ. Saf. 283, 116834. doi:10.1016/j.ecoenv.2024.116834
Chen, T., Lin, Q., Gong, C., Zhao, H., and Peng, R. (2024b). Research progress on micro (nano)Plastics exposure-induced miRNA-Mediated biotoxicity. Toxics 12 (7), 475. doi:10.3390/toxics12070475
Chen, Y., Zhang, Z., Ji, K., Zhang, Q., Qian, L., and Yang, C. (2025). Role of microplastics in the tumor microenvironment (review). Oncol. Lett. 29 (4), 193. doi:10.3892/ol.2025.14939
Cheng, B., Zhang, Y., Wu, Z. W., Cui, Z. C., and Li, W. L. (2020). MiR-144 inhibits colorectal cancer cell migration and invasion by regulating PBX3. Eur. Rev. Med. Pharmacol. Sci. 24 (18), 9361–9369. doi:10.26355/eurrev_202009_23019
Chouleur, T., Emanuelli, A., Souleyreau, W., Derieppe, M. A., Leboucq, T., Hardy, S., et al. (2024). PTP4A2 promotes glioblastoma progression and macrophage polarization under microenvironmental pressure. Cancer Res. Commun. 4 (7), 1702–1714. doi:10.1158/2767-9764.CRC-23-0334
Ciazynska, M., Bednarski, I. A., Wodz, K., Narbutt, J., and Lesiak, A. (2020). NLRP1 and NLRP3 inflammasomes as a new approach to skin carcinogenesis. Oncol. Lett. 19 (3), 1649–1656. doi:10.3892/ol.2020.11284
Damiani, D., and Tiribelli, M. (2023). ABCG2 in acute myeloid leukemia: old and new perspectives. Int. J. Mol. Sci. 24 (8), 7147. doi:10.3390/ijms24087147
Deng, X., Gui, Y., and Zhao, L. (2025). The micro(nano)plastics perspective: exploring cancer development and therapy. Mol. Cancer 24 (1), 30. doi:10.1186/s12943-025-02230-z
Di Sanzo, M., Quaresima, B., Biamonte, F., Palmieri, C., and Faniello, M. C. (2020). FTH1 pseudogenes in cancer and cell metabolism. Cells 9 (12), 2554. doi:10.3390/cells9122554
Ding, H. M., Zhang, H., Wang, J., Zhou, J. H., Shen, F. R., Ji, R. N., et al. (2021). miR-302c-3p and miR-520a-3p suppress the proliferation of cervical carcinoma cells by targeting CXCL8. Mol. Med. Rep. 23 (5), 322–10. doi:10.3892/mmr.2021.11961
Franczyk, B., Rysz, J., and Gluba-Brzozka, A. (2022). Pharmacogenetics of drugs used in the treatment of cancers. Genes (Basel) 13 (2), 311. doi:10.3390/genes13020311
Gil-Kulik, P., Kluz, N., Przywara, D., Petniak, A., Wasilewska, M., Fraczek-Chudzik, N., et al. (2024). Potential use of exosomal non-coding MicroRNAs in leukemia therapy: a systematic review. Cancers (Basel) 16 (23), 3948. doi:10.3390/cancers16233948
Hale, R. C., Seeley, M. E., La Guardia, M. J., Mai, L., and Zeng, E. Y. (2020). A global perspective on microplastics. J. Geophys. Research-Oceans 125 (1), e2018JC014719. doi:10.1029/2018JC014719
Han, N., Yuan, M., Yan, L., and Tang, H. (2023). Emerging insights into liver X receptor alpha in the tumorigenesis and therapeutics of human cancers. Biomolecules 13 (8), 1184. doi:10.3390/biom13081184
Hasanzadeh, M., Movahedi, M., Rejali, M., Maleki, F., Moetamani-Ahmadi, M., Seifi, S., et al. (2019). The potential prognostic and therapeutic application of tissue and circulating microRNAs in cervical cancer. J. Cell Physiol. 234 (2), 1289–1294. doi:10.1002/jcp.27160
Hsu, H. P., Lai, M. D., Lee, J. C., Yen, M. C., Weng, T. Y., Chen, W. C., et al. (2017). Mucin 2 silencing promotes colon cancer metastasis through interleukin-6 signaling. Sci. Rep. 7 (1), 5823. doi:10.1038/s41598-017-04952-7
Hu, X., Yu, Q., Gatheru Waigi, M., Ling, W., Qin, C., Wang, J., et al. (2022). Microplastics-sorbed phenanthrene and its derivatives are highly bioaccessible and may induce human cancer risks. Environ. Int. 168, 107459. doi:10.1016/j.envint.2022.107459
Iranmanesh, H., Majd, A., Mojarad, E. N., Zali, M. R., and Hashemi, M. (2021). Investigating the relationship between the expression level of mucin gene cluster (MUC2, MUC5A, and MUC5B) and clinicopathological characterization of colorectal cancer. Galen. Med. J. 10, e2030. doi:10.31661/gmj.v10i0.2030
Ishtiaq, S. M., Arshad, M. I., and Khan, J. A. (2022). PPARγ signaling in hepatocarcinogenesis: mechanistic insights for cellular reprogramming and therapeutic implications. Pharmacol. Ther. 240, 108298. doi:10.1016/j.pharmthera.2022.108298
Jain, A. S., Prasad, A., Pradeep, S., Dharmashekar, C., Achar, R. R., Ekaterina, S., et al. (2021). Everything old is new again: drug repurposing approach for non-small cell lung cancer targeting MAPK signaling pathway. Front. Oncol. 11, 741326. doi:10.3389/fonc.2021.741326
Jang, B. I., Li, Y., Graham, D. Y., and Cen, P. (2011). The role of CD44 in the pathogenesis, diagnosis, and therapy of gastric cancer. Gut Liver 5 (4), 397–405. doi:10.5009/gnl.2011.5.4.397
Javadrashid, D., Mohammadzadeh, R., Baghbanzadeh, A., Safaee, S., Amini, M., Lotfi, Z., et al. (2021). Simultaneous microRNA-612 restoration and 5-FU treatment inhibit the growth and migration of human PANC-1 pancreatic cancer cells. EXCLI J. 20, 160–173. doi:10.17179/excli2020-2900
Jia, S., Yang, Y., Zhu, Y., Yang, W., Ling, L., Wei, Y., et al. (2024). Association of FTH1-Expressing circulating tumor cells with efficacy of neoadjuvant chemotherapy for patients with breast cancer: a prospective cohort study. Oncologist 29 (1), e25–e37. doi:10.1093/oncolo/oyad195
Junjappa, R. P., Kim, H. K., Park, S. Y., Bhattarai, K. R., Kim, K. W., Soh, J. W., et al. (2019). Expression of TMBIM6 in cancers: the involvement of Sp1 and PKC. Cancers (Basel) 11 (7), 974. doi:10.3390/cancers11070974
Karbasforooshan, H., Hayes, A. W., Mohammadzadeh, N., Zirak, M. R., and Karimi, G. (2020). The possible role of sirtuins and microRNAs in hepatocellular carcinoma therapy. Cell Cycle 19 (23), 3209–3221. doi:10.1080/15384101.2020.1843813
K, C. P., Maharjan, A., Acharya, M., Lee, D., Kusma, S., Gautam, R., et al. (2023). Polytetrafluorethylene microplastic particles mediated oxidative stress, inflammation, and intracellular signaling pathway alteration in human derived cell lines. Sci. Total Environ. 897, 165295. doi:10.1016/j.scitotenv.2023.165295
Kaliyev, A. A., Mussin, N. M., and Tamadon, A. (2024). The importance of mesenchymal stromal/stem cell therapy for cancer. West Kazakhstan Med. J., 106–110. doi:10.18502/wkmj.v66i2.16452
Kim, H., Zaheer, J., Choi, E. J., and Kim, J. S. (2022). Enhanced ASGR2 by microplastic exposure leads to resistance to therapy in gastric cancer. Theranostics 12 (7), 3217–3236. doi:10.7150/thno.73226
Kumar, N., Lamba, M., Pachar, A. K., Yadav, S., and Acharya, A. (2024). Microplastics - a growing concern as carcinogens in cancer etiology: emphasis on biochemical and molecular mechanisms. Cell Biochem. Biophysics 82 (4), 3109–3121. doi:10.1007/s12013-024-01436-0
Li, X., Jiang, W., Gan, Y., and Zhou, W. (2021). The application of exosomal MicroRNAs in the treatment of pancreatic cancer and its research progress. Pancreas 50 (1), 12–16. doi:10.1097/MPA.0000000000001713
Li, S., Keenan, J. I., Shaw, I. C., and Frizelle, F. A. (2023). Could microplastics be a driver for early onset colorectal cancer? Cancers (Basel) 15 (13), 3323. doi:10.3390/cancers15133323
Liu, F., Zhuang, L., Wu, R. X., and Li, D. Y. (2019). miR-365 inhibits cell invasion and migration of triple negative breast cancer through ADAM10. J. Buon 24 (5), 1905–1912.
Liu, W., Xu, L., Liang, X., Liu, X., Zhao, Y., Ma, C., et al. (2020). Tim-4 in health and disease: friend or foe? Front. Immunol. 11, 537. doi:10.3389/fimmu.2020.00537
Liu, Q., Bautista-Gomez, J., Higgins, D. A., Yu, J., and Xiong, Y. (2021). Dysregulation of the AP2M1 phosphorylation cycle by LRRK2 impairs endocytosis and leads to dopaminergic neurodegeneration. Sci. Signal 14 (693), eabg3555. doi:10.1126/scisignal.abg3555
Liu, X., Zhao, X., Yang, J., Wang, H., Piao, Y., and Wang, L. (2023). High expression of AP2M1 correlates with worse prognosis by regulating immune microenvironment and drug resistance to R-CHOP in diffuse large B cell lymphoma. Eur. J. Haematol. 110 (2), 198–208. doi:10.1111/ejh.13895
Menbari, M. N., Rahimi, K., Ahmadi, A., Mohammadi-Yeganeh, S., Elyasi, A., Darvishi, N., et al. (2020). miR-483-3p suppresses the proliferation and progression of human triple negative breast cancer cells by targeting the HDAC8>oncogene. J. Cell Physiol. 235 (3), 2631–2642. doi:10.1002/jcp.29167
Miao, L., Guo, S., Lin, C. M., Liu, Q., and Huang, L. (2017). Nanoformulations for combination or cascade anticancer therapy. Adv. Drug Deliv. Rev. 115, 3–22. doi:10.1016/j.addr.2017.06.003
Mohammadi, M., Spotin, A., Mahami-Oskouei, M., Shanehbandi, D., Ahmadpour, E., Casulli, A., et al. (2021). MicroRNA-365 promotes apoptosis in human melanoma cell A375 treated with hydatid cyst fluid of Echinococcus granulosus sensu stricto. Microb. Pathog. 153, 104804. doi:10.1016/j.micpath.2021.104804
C. Münz (2020). Autophagy proteins influence endocytosis for MHC restricted antigen presentation (Elsevier).
Naeli, P., Yousefi, F., Ghasemi, Y., Savardashtaki, A., and Mirzaei, H. (2020). The role of MicroRNAs in lung cancer: implications for diagnosis and therapy. Curr. Mol. Med. 20 (2), 90–101. doi:10.2174/1566524019666191001113511
Pan, Y., Li, Y., Fan, H., Cui, H., Chen, Z., Wang, Y., et al. (2024). Roles of the peroxisome proliferator-activated receptors (PPARs) in the pathogenesis of hepatocellular carcinoma (HCC). Biomed. Pharmacother. 177, 117089. doi:10.1016/j.biopha.2024.117089
Park, J. H., Hong, S., Kim, O. H., Kim, C. H., Kim, J., Kim, J. W., et al. (2023). Polypropylene microplastics promote metastatic features in human breast cancer. Sci. Rep. 13 (1), 6252. doi:10.1038/s41598-023-33393-8
Pradhan, R., Singhvi, G., Dubey, S. K., Gupta, G., and Dua, K. (2019). MAPK pathway: a potential target for the treatment of non-small-cell lung carcinoma. Future Med. Chem. 11 (8), 793–795. doi:10.4155/fmc-2018-0468
Ramalingam, P. S., Elangovan, S., Mekala, J. R., and Arumugam, S. (2024). Liver X receptors (LXRs) in cancer-an Eagle's view on molecular insights and therapeutic opportunities. Front. Cell Dev. Biol. 12, 1386102. doi:10.3389/fcell.2024.1386102
Ramkumar, K., Tanimoto, A., Della Corte, C. M., Stewart, C. A., Wang, Q., Shen, L., et al. (2023). Targeting BCL2 overcomes resistance and augments response to Aurora kinase B inhibition by AZD2811 in small cell lung cancer. Clin. Cancer Res. 29 (16), 3237–3249. doi:10.1158/1078-0432.CCR-23-0375
Rasoolnezhad, M., Safaralizadeh, R., Hosseinpourfeizi, M. A., Banan-Khojasteh, S. M., and Baradaran, B. (2021). MiRNA-138-5p: a strong tumor suppressor targeting PD-L-1 inhibits proliferation and motility of breast cancer cells and induces apoptosis. Eur. J. Pharmacol. 896, 173933. doi:10.1016/j.ejphar.2021.173933
Robinson, K. S., Sennhenn, P., Yuan, D. S., Liu, H., Taddei, D., Qian, Y., et al. (2025). TMBIM6/BI-1 is an intracellular environmental regulator that induces paraptosis in cancer via ROS and Calcium-activated ERAD II pathways. Oncogene 44 (8), 494–512. doi:10.1038/s41388-024-03222-x
Rosellini, M., Turunen, P., and Efferth, T. (2023). Impact of plastic-related compounds on P-Glycoprotein and breast cancer resistance protein in vitro. Molecules 28 (6), 2710. doi:10.3390/molecules28062710
Shabani, F., Mahdavi, M., Imani, M., Hosseinpour-Feizi, M. A., and Gheibi, N. (2020). Calprotectin (S100A8/S100A9)-induced cytotoxicity and apoptosis in human gastric cancer AGS cells: alteration in expression levels of Bax, Bcl-2, and ERK2. Hum. Exp. Toxicol. 39 (8), 1031–1045. doi:10.1177/0960327120909530
Shen, S., Zhang, S., Liu, P., Wang, J., and Du, H. (2020). Potential role of microRNAs in the treatment and diagnosis of cervical cancer. Cancer Genet. 248-249, 25–30. doi:10.1016/j.cancergen.2020.09.003
Shen, J., Yang, H., Qiao, X., Chen, Y., Zheng, L., Lin, J., et al. (2023). The E3 ubiquitin ligase TRIM17 promotes gastric cancer survival and progression via controlling BAX stability and antagonizing apoptosis. Cell Death Differ. 30 (10), 2322–2335. doi:10.1038/s41418-023-01221-1
Shi, B., Chu, J., Huang, T., Wang, X., Li, Q., Gao, Q., et al. (2021). The scavenger receptor MARCO expressed by tumor-associated macrophages are highly associated with poor pancreatic cancer prognosis. Front. Oncol. 11, 771488. doi:10.3389/fonc.2021.771488
Shin, J., Nile, A., and Oh, J. W. (2021). Role of adaptin protein complexes in intracellular trafficking and their impact on diseases. Bioengineered 12 (1), 8259–8278. doi:10.1080/21655979.2021.1982846
Shin, Y., Choi, H. Y., Kwak, Y., Yang, G. M., Jeong, Y., Jeon, T. I., et al. (2023). TMBIM6-mediated miR-181a expression regulates breast cancer cell migration and invasion via the MAPK/ERK signaling pathway. J. Cancer 14 (4), 554–572. doi:10.7150/jca.81600
Spitz, A. Z., and Gavathiotis, E. (2022). Physiological and pharmacological modulation of BAX. Trends Pharmacol. Sci. 43 (3), 206–220. doi:10.1016/j.tips.2021.11.001
Sucha, S., Sorf, A., Svoren, M., Vagiannis, D., Ahmed, F., Visek, B., et al. (2022). ABCB1 as a potential beneficial target of midostaurin in acute myeloid leukemia. Biomed. Pharmacother. 150, 112962. doi:10.1016/j.biopha.2022.112962
Sun, L., Liu, H., Ye, Y., Lei, Y., Islam, R., Tan, S., et al. (2023). Smart nanoparticles for cancer therapy. Signal Transduct. Target Ther. 8 (1), 418. doi:10.1038/s41392-023-01642-x
Szczepanek, J., Skorupa, M., and Tretyn, A. (2022). MicroRNA as a potential therapeutic molecule in cancer. Cells 11 (6), 1008. doi:10.3390/cells11061008
Tang, D., Liu, H., Zhao, Y., Qian, D., Luo, S., Patz, E. F., et al. (2020). Genetic variants of BIRC3 and NRG1 in the NLRP3 inflammasome pathway are associated with non-small cell lung cancer survival. Am. J. Cancer Res. 10 (8), 2582–2595.
Tang, W., Sun, G., Ji, G. W., Feng, T., Zhang, Q., Cao, H., et al. (2023). Single-cell RNA-sequencing atlas reveals an FABP1-dependent immunosuppressive environment in hepatocellular carcinoma. J. Immunother. Cancer 11 (11), e007030. doi:10.1136/jitc-2023-007030
Vethaak, A. D., and Legler, J. (2021). Microplastics and human health. Science. 371 (6530), 672–674. doi:10.1126/science.abe5041
Wang, Y., Fang, Z., Hong, M., Yang, D., and Xie, W. (2020). Long-noncoding RNAs (lncRNAs) in drug metabolism and disposition, implications in cancer chemo-resistance. Acta Pharm. Sin. B 10 (1), 105–112. doi:10.1016/j.apsb.2019.09.011
Wang, W., Min, K., Chen, G., Zhang, H., Deng, J., Lv, M., et al. (2021). Use of bioinformatic database analysis and specimen verification to identify novel biomarkers predicting gastric cancer metastasis. J. Cancer 12 (19), 5967–5976. doi:10.7150/jca.58768
Wang, Y., Xu, X., and Jiang, G. (2023). Microplastics exposure promotes the proliferation of skin cancer cells but inhibits the growth of normal skin cells by regulating the inflammatory process. Ecotoxicol. Environ. Saf. 267, 115636. doi:10.1016/j.ecoenv.2023.115636
Winiarska, E., Jutel, M., and Zemelka-Wiacek, M. (2024). The potential impact of nano- and microplastics on human health: understanding human health risks. Environ. Res. 251 (Pt 2), 118535. doi:10.1016/j.envres.2024.118535
Xiao, J., Wan, F., Tian, L., and Li, Y. (2024). Tumor suppressor miR-520a inhibits cell growth by negatively regulating PI3K/AKT signaling pathway in acute myeloid leukemia. Adv. Clin. Exp. Med. 33 (7), 729–738. doi:10.17219/acem/171299
Xue, S., Ma, M., Bei, S., Li, F., Wu, C., Li, H., et al. (2021). Identification and validation of the immune regulator CXCR4 as a novel promising target for gastric cancer. Front. Immunol. 12, 702615. doi:10.3389/fimmu.2021.702615
Yan, X., Zhang, Y., Lu, Y., He, L., Qu, J., Zhou, C., et al. (2020). The complex toxicity of tetracycline with polystyrene spheres on gastric cancer cells. Int. J. Environ. Res. Public Health 17 (8), 2808. doi:10.3390/ijerph17082808
Yu, M., Lin, C., and Wei, M. (2023). A pan-cancer analysis of oncogenic protein tyrosine phosphatase subfamily PTP4As. J. Holist. Integr. Pharm. 4 (2), 185–198. doi:10.1016/j.jhip.2023.07.001
Zavros, Y. (2017). Initiation and maintenance of gastric cancer: a focus on CD44 variant isoforms and cancer stem cells. Cell Mol. Gastroenterol. Hepatol. 4 (1), 55–63. doi:10.1016/j.jcmgh.2017.03.003
Zhang, Y. S., Yang, C., Han, L., Liu, L., and Liu, Y. J. (2022). Expression of BCRP/ABCG2 protein in invasive breast cancer and response to neoadjuvant chemotherapy. Oncol. Res. Treat. 45 (3), 94–101. doi:10.1159/000520871
Zhao, J., Li, D., and Fang, L. (2019). MiR-128-3p suppresses breast cancer cellular progression via targeting LIMK1. Biomed. Pharmacother. 115, 108947. doi:10.1016/j.biopha.2019.108947
Zhao, M., Zhang, M., Tao, Z., Cao, J., Wang, L., and Hu, X. (2020). miR-331-3p suppresses cell proliferation in TNBC cells by downregulating NRP2. Technol. Cancer Res. Treat. 19, 1533033820905824. doi:10.1177/1533033820905824
Zhao, J., Zhang, H., Shi, L., Jia, Y., and Sheng, H. (2024). Detection and quantification of microplastics in various types of human tumor tissues. Ecotoxicol. Environ. Saf. 283, 116818. doi:10.1016/j.ecoenv.2024.116818
Zhou, Z., Zhou, Q., Wu, X., Xu, S., Hu, X., Tao, X., et al. (2020a). VCAM-1 secreted from cancer-associated fibroblasts enhances the growth and invasion of lung cancer cells through AKT and MAPK signaling. Cancer Lett. 473, 62–73. doi:10.1016/j.canlet.2019.12.039
Keywords: microplastics, microRNAs, cancer, therapy resistance, in silico, RNAhybrid
Citation: Baspakova A, Zare A, Mussin NM, Tanideh N, Zhilisbayeva KR, Safarzoda Sharoffidin R, Suleimenova R, Yelgondina G, Kaliyeva AE, Umbetova AA, Zinaliyeva A and Tamadon A (2025) In-silico pharmacological insights into the therapeutic potential of microRNAs for microplastic-associated cancers. Front. Cell Dev. Biol. 13:1699693. doi: 10.3389/fcell.2025.1699693
Received: 05 September 2025; Accepted: 29 October 2025;
Published: 25 November 2025.
Edited by:
Angelo Sparaneo, IRCCS Casa Sollievo della Sofferenza Hospital, ItalyReviewed by:
Sevgi Marakli, Yıldız Technical University, TürkiyeZixuan Gou, Peking University, China
Copyright © 2025 Baspakova, Zare, Mussin, Tanideh, Zhilisbayeva, Safarzoda Sharoffidin, Suleimenova, Yelgondina, Kaliyeva, Umbetova, Zinaliyeva and Tamadon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amin Tamadon, YW1pbnRhbWFkZG9uQHlhaG9vLmNvbQ==
†These authors share first authorship
 Akmaral Baspakova
Akmaral Baspakova Afshin Zare
Afshin Zare Nadiar M. Mussin
Nadiar M. Mussin Nader Tanideh
Nader Tanideh Kulyash R. Zhilisbayeva6
Kulyash R. Zhilisbayeva6 Amin Tamadon
Amin Tamadon