- 1Faculty of Data Science, City University of Macau, Macau, China
- 2Shenzhen Key Laboratory of Metabolic Health, Center for Energy Metabolism and Reproduction, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
- 3Reproductive Medicine Centre, Shenzhen Hengsheng Hospital, Shenzhen, China
- 4Reproductive Center of Shenzhen Armed Police Hospital, Shenzhen, China
- 5Reproductive Medicine Department, Shenzhen Luohu People’s Hospital, Shenzhen, China
- 6Faculty of Pharmaceutical Sciences, Shenzhen University of Advanced Technology, Shenzhen, China
- 7Sino-European Center of Biomedicine and Health, Shenzhen, China
Introduction: Letrozole monotherapy, while effective for ovulation induction, may compromise endometrial receptivity in frozen embryo transfer (FET) cycles due to estrogen suppression. This study aimed to compare the FET outcomes and analyze the molecular mechanisms of endometrial receptivity among low-dose letrozole plus HMG (LeH) with letrozole monotherapy (Le) and natural cycles (NC).
Methods: This retrospective cohort study included 5,673 infertile patients undergoing FET with one of the following protocols: LeH (n = 2,997), Le (n = 1,762), or NC (n = 914). Endometrial receptivity was assessed via serum hormone assays, scanning electron microscopy (SEM) of pinopodes on post-ovulation days 3 (D3; pre-FET) and 5 (D5; estimated implantation window), and proteomic analyses of endometrial tissue, uterine fluid, and serum on D3.
Results: Clinical outcomes revealed that the LeH group had significantly higher implantation, clinical pregnancy and live birth rates compared to the Le and NC groups, especially among older women. Notably, the Le group was associated with thinner endometrium, lower estradiol levels, reduced vascularization flow index (VFI), and a lower proportion of receptive-phase endometria (28% vs. 60% in NC). In contrast, the LeH group maintained normal endometrial parameters, and resulted in a high proportion of fully developed pinopodes (84%). Proteomic profiling revealed that the Le group adversely affected processes related to cell adhesion and inflammatory regulation, while the LeH group reversed these alterations. It activated pathways important for embryo implantation and promoted an anti-inflammatory environment.
Discussion: These results suggest that the LeH regimen may mitigate letrozole-induced endometrial impairment and enhances FET outcomes through structural, molecular, and immunological mechanisms, offering a promising approach for optimized endometrial preparation.
1 Introduction
In recent years, the popularity of frozen embryo transfer (FET) has increased substantially (De Geyter et al., 2020; Sunderam et al., 2020) owing to its lower cancellation rates and greater scheduling flexibility (Zhang et al., 2019). The neutral effect of FET on reproductive outcomes has further led to the widespread adoption of a “freeze-all” strategy in many assisted reproductive technology (ART) centers (Cobo et al., 2024; Melo et al., 2022). Within FET cycles, the adequacy of endometrial preparation is a critical determinant of success (Vinsonneau et al., 2022; Groenewoud et al., 2013), and optimizing ovulation induction protocols to enhance endometrial receptivity remains a central challenge (Xia et al., 2023).
Letrozole (Le), a third-generation aromatase inhibitor, is widely used for ovulation induction, particularly in patients with polycystic ovary syndrome (PCOS) (Bhatnagar, 2007; Castillo and Kol, 2024). Its ability to reduce estrogen levels and promote monofollicular development (Li et al., 2022) minimizes negative impacts on cervical mucus and the endometrium (Chen et al., 2023), with meta-analyses confirming significant improvements in live birth and pregnancy rates (Franik et al., 2018). However, the very mechanism that makes letrozole effective—systemic estrogen suppression—may also impair endometrial proliferation and receptivity, potentially compromising its clinical benefits in FET cycles.
Human menopausal gonadotropin (HMG), which contains both FSH and LH, is another common agent used for ovulation induction (Quaas and Legro, 2019). Although it poses risks such as ovarian hyperstimulation syndrome (OHSS) and multiple pregnancies in PCOS patients (Tummon et al., 2005; Diamond et al., 2015; Colucci et al., 2000), studies have found no significant differences in neonatal outcomes between HMG and hormone replacement treatment (HRT) cycles (Zhang Y. et al., 2023). Notably, heterogeneity in clinical pregnancy rates have been observed across different therapeutic combinations of letrozole and HMG, potentially due to variations in the dosage of letrozole and/or HMG (Xi et al., 2015; Lin et al., 2019; Zhang et al., 2021). Irrespective of these differences, these combination strategies consistently mitigate the risks of OHSS and excessive multifollicular development (Zhang et al., 2021), thereby furnishing a promising therapeutic avenue for optimizing the trade-off between efficacy and safety (Xi et al., 2015).
In our ART center, we have observed that letrozole-based cycles often present an inverse relationship between endometrial thickness and estradiol levels: adequate endometrial thickness (
Based on these observations, we hypothesized that supplementing letrozole with low-dose HMG could mitigate the endometrial thinning associated with letrozole monotherapy, thereby improving endometrial receptivity and overall FET outcomes. This study aims to investigate whether the LeH regimen can refine endometrial preparation and enhance the efficiency of FET cycles by counteracting the potential negative effects of letrozole on the endometrium. Through a combination of clinical, ultrastructural, and molecular analyses, we seek to provide comprehensive evidence supporting the use of LeH as a superior regimen for ovulation induction in FET.
2 Materials and methods
2.1 Study design and participants
This retrospective cohort study analyzed data from 5,673 infertile patients undergoing FET between 2015 and 2022 at the Reproductive Center of Shenzhen Armed Police Hospital. Participants were divided into three groups based on the endometrial preparation protocol: the LeH regimen

Figure 1. The flowchart of participants. HMG, Human Menopausal Gonadotropin; SEM, Scanning Electron Microscopy.
2.2 Endometrial preparation protocols
LeH Group: Participants received ovarian ultrasonography on the third menstrual cycle day. If no follicles
Le Group: Letrozole (2.5–5 mg/day) was given for 3–5 days if no follicle
NC Group: Ovarian ultrasonography began on the 10th menstrual cycle day, continuing until follicles reached
For all groups, HCG (10,000 U, Livzon, China) was administered if LH levels were
2.3 Ethical approval
This study received approval from the Ethics Committee of the Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences (approval number: SIAT-IRB-180215-H0201) and was executed in accordance with their directives. All participants provided written informed consent (2016-1.0 version) and were enrolled in alignment with the Declaration of Helsinki.
2.4 Inclusion and exclusion criteria
Inclusion criteria comprised women under 35 years of age with a regular menstrual cycle (26–37 days) and at least one high-quality embryo available for transfer. Those in a fresh cycle were required to have an endometrial thickness
2.5 Sample collection
Blood, uterine fluid, and endometrial tissue samples were collected from 33 participants on D3 and D5. Serum was isolated and stored at
2.6 Serum hormone detection by RIA
Serum hormone levels were measured using a commercial iodine-[125I] RIA kit (Beijing North Biotechnology Institute, China) for estradiol, testosterone, progesterone, LH, FSH, and prolactin. All samples were assayed at least in triplicate. The intra-assay and inter-assay coefficients of variation were
2.7 Histological and ultrastructural examination
Endometrial tissues were sectioned and stained with H&E for histological evaluation Xiao et al. (2025). Scanning electron microscopy (SEM) was performed to assess the ultrastructural morphology of the endometrium, with particular attention to pinopode development Zhao et al. (2021). For each sample, multiple representative fields were examined to ensure a consistent assessment.
2.8 Protein array analysis
Relative expression levels of 440 human cytokines in serum, uterine fluid, and endometrial tissue extracts collected on D3 were quantified using the G-Series Human Cytokine Antibody Array 440 (RayBiotech, China) Wang Q. et al. (2024).
2.9 Bioinformatics analysis
Fold-change (FC) and adjusted
2.10 Statistical analysis
Data were analyzed using GraphPad Prism 9.0 and SPSS 19.0, represented as mean
3 Results
3.1 The LeH regimen improves clinical pregnancy outcomes in FET cycles
Based on reproductive physiology, patients were divided into three age groups for analysis. The clinical data revealed that the LeH group had significantly higher rates of embryo implantation, clinical pregnancy and live birth rate than the other groups, with the Le group outperforming the NC group. Notably, as age increased, these rates declined in all groups, but the LeH group’s effectiveness became more pronounced (Table 1).
3.2 The LeH regimen mitigates letrozole-induced endometrial inadequacy
Our initial clinical observation indicated that letrozole monotherapy might compromise endometrial preparedness. To test this, we first compared endocrine and ultrasonographic parameters on the day of HCG administration. Consistent with letrozole’s mechanism of action, the Le group exhibited significantly reduced endometrial thickness, estradiol levels, and vascularization flow index (VFI) compared to the NC group (Table 2). Importantly, the LeH regimen prevented this decline, showing comparable endometrial thickness and VFI to the NC group, and a trend toward higher estradiol levels (Table 2), suggesting that HMG co-administration counteracts letrozole’s anti-estrogenic effects on the endometrium.
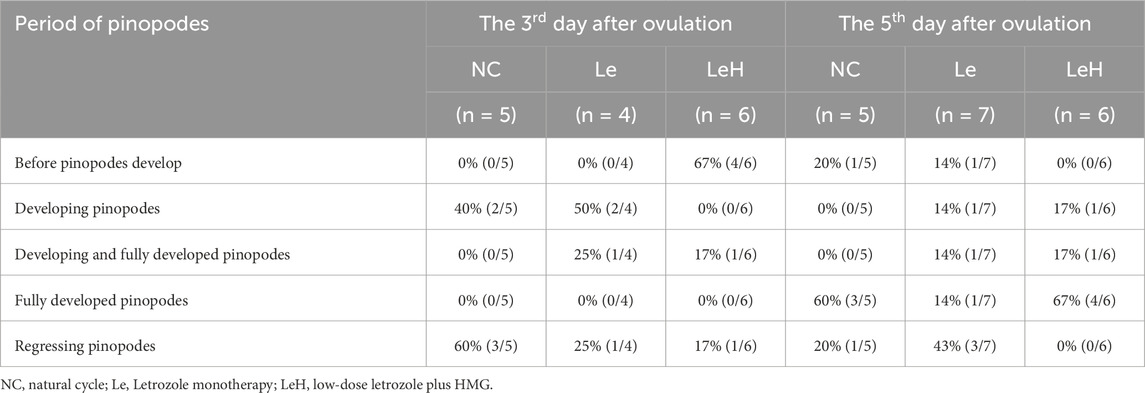
While no differences were observed in endometrial morphology (Supplementary Figure S1), ultrastructural analysis revealed profound differences. On D5 (the presumed window of implantation), a significantly higher proportion of endometrial samples in the LeH group (84%) exhibited a fully developed receptive phenotype with mature pinopodes, compared to the Le (28%) and NC (60%) groups (Figure 2A; Supplementary Figure S2; Table 3).

Figure 2. Integrated assessment of endometrial receptivity in Le/LeH/NC groups. Ultrastructural dynamics of pinopodes analyzed by SEM on D3 (pre-FET, n = 15) and D5 (implantation window, n = 18) post-ovulation. Yellow arrowheads: microvilli; red arrowheads: pinopodes. Scale bars: 20
In addition, serum hormone analysis indicated that the Le group had significantly lower estradiol levels on D3 relative to both the NC and LeH groups, with no significant differences observed in other hormones (Supplementary Figure S3). Collectively, these results demonstrate that the LeH regimen effectively promotes optimal ultrastructural maturation of the endometrium, facilitating a receptive state for embryo implantation.
3.3 LeH promotes endometrial receptivity by upregulating critical signaling pathways
To elucidate the molecular mechanisms by which the LeH regimen enhances endometrial receptivity, we performed protein microarray analysis on endometrial tissues collected on D3 (Supplementary Figures S4, S5). Compared to the NC group, the Le group exhibited a predominant downregulation of DEPs, whereas the LeH group showed primarily upregulation of DEPs (Figure 4A).
Bioinformatic analysis (GO and KEGG) indicated that letrozole monotherapy (Le vs. NC) significantly downregulated pathways critical for implantation, including Cytokine–cytokine receptor interaction, Cell–cell adhesion, inflammatory processes (e.g., Rheumatoid arthritis, Leukocyte activation), and pathways related to endometrial development (e.g., Morphogenesis of an epithelial fold, Tube morphogenesis) (Figure 2B). In contrast, the LeH regimen markedly upregulated these same categories of pathways (Figures 2C,D). Notably, LeH restored the expression of 29 DEPs that were downregulated in the Le group (Figure 4D) and significantly enriched pathways supportive of endometrial development (Figure 2D).
Further enrichment analysis across the three groups revealed that pathways such as Cell adhesion molecules and Positive regulation of response to external stimulus—which were downregulated in the Le group—were upregulated under the LeH regimen (Figures 2E–G). Sankey analysis identified key DEPs within these pathways, including CD6, CDH1, NCAM1, and SDC3 in cell adhesion, and BMPR2, CCL24, IL21, IL6R, KDR, LAG3, PGC, TLR4, VEGF, and XCL1 in the response to external stimulus (Figure 2H). PPI network analysis demonstrated functional connectivity among these DEPs across comparison groups (Figures 2I–K). Together, these data indicate that the LeH regimen enhances endometrial receptivity by activating a synergistic network of pathways essential for embryo attachment and implantation.
3.4 LeH fosters an anti-inflammatory uterine cavity microenvironment
The uterine fluid, which engages in direct contact with the embryo, plays a crucial role in implantation success. Protein microarray analysis of uterine fluid revealed that, compared to the NC group, the LeH regimen was associated with significant downregulation of processes related to inflammatory responses, including Response to bacterium and Immune effector process (Figures 3A–C; Figures 4B,E; Supplementary Figures S6, S7). Common pathway enrichment analysis further indicated downregulation of inflammatory processes such as Hepatitis in both LeH vs. NC and Le vs. NC comparisons; however, the extent of downregulation was markedly less pronounced in the LeH group (Figures 3D–F). These findings suggest that the LeH regimen promotes an anti-inflammatory and immune-tolerant microenvironment within the uterine fluid, which may protect the embryo from adverse maternal immune reactions.
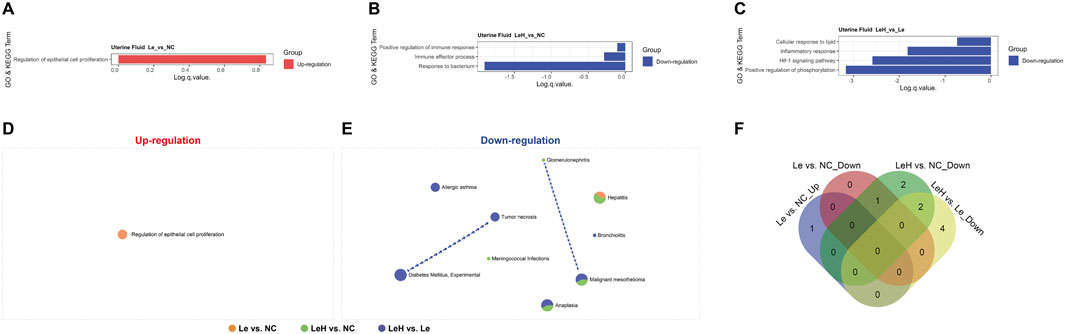
Figure 3. Proteomic profiling of preimplantation uterine fluid. Uterine fluid from Le (n = 4), LeH (n = 6), and NC (n = 4) groups collected on D3 (pre-FET) was analyzed by 440-protein arrays. GO and KEGG enrichment analysis (A–C). Metascape analysis of up- and downregulated DEPs (D, E). Venn diagram of shared pathways (F).

Figure 4. Multi-sample comparison of shared DEPs in endometrial tissues, serum, and uterine fluid at D3 pre-FET. Differential protein expression analysis quantified up- and downregulated proteins across groups and evaluated shared DEPs. This comprehensive assessment was applied to endometrial tissues (A,D), uterine fluid (B,E), and serum (C,F), harvested on day 3 (D3, pre-FET) post-ovulation. Overlapping DEPs are indicated, with identical DEPs shared across sample types represented by the same color.
3.5 LeH reduces systemic inflammation, supporting endometrial preparedness
To assess the systemic effects of the LeH regimen, we conducted serum protein microarray analysis. The results indicated a widespread downregulation of DEPs in both the Le and LeH groups compared to the NC group (Figure 4C; Supplementary Figures S8, S9). A total of 20 DEPs were commonly downregulated in the Le and LeH groups relative to NC. Among these, IL21, AFP, and GRO
However, the LeH group demonstrated a more pronounced and targeted downregulation of key pathways, most notably Cytokine–cytokine receptor interaction and Inflammatory response (Figures 5A–F). Interaction network analysis highlighted central roles for cytokines such as CCL11, CXCL1, CXCL5, IL1B, and IL4 in this systemic anti-inflammatory effect (Figures 5G–J). These results suggest that the LeH regimen may enhance endometrial receptivity not only locally but also systemically, through attenuation of pro-inflammatory signals in the circulation.

Figure 5. Integrated proteomic profiling of D3 serum with focused pathway analysis of serum DEPs. Proteomic analysis employed a 440-target protein array (11 sub-arrays) on serum samples from groups Le (n = 4), LeH (n = 6), and NC (n = 5), harvested on day 3 (D3, pre-FET) post-ovulation. Bioinformatic analysis included GO and KEGG enrichment bar charts for inter-group comparisons in serum (A–C). Metascape enrichment analysis identified upregulated (D) and downregulated (E) DEPs in serum, followed by a Venn diagram of shared signaling pathways (F). A Sankey diagram visualized relationships between comparison groups, DEPs, and key signaling pathways (G). Finally, STRING-generated protein–protein interaction networks of DEPs within these key pathways are shown for serum comparisons: Le vs. NC (downregulated DEPs) (H), LeH vs. NC (downregulated DEPs) (I), and LeH vs. Le (downregulated DEPs) (J).
4 Discussion
This study provides comprehensive clinical and mechanistic evidence supporting the superiority of the LeH regimen in preparing the endometrium for FET. Our findings confirm the initial hypothesis that co-administration of HMG can counteract the negative impact of letrozole monotherapy on endometrial receptivity, ultimately leading to improved pregnancy outcomes. The LeH regimen enhances endometrial receptivity through a multi-faceted mechanism involving structural normalization, molecular pathway activation, and the creation of a supportive local and systemic immune environment (Figure 6).

Figure 6. Diagram illustrating the LeH regimen for optimized endometrial preparation in frozen embryo transfer (FET). Compared to NC, the Le group exhibited lower estrogen levels during proliferative/early-secretory phases and fewer patients with receptive-state pinopodes at implantation—potentially due to suppressed endometrial preparation pathways. Conversely, the LeH group showed elevated proliferative-phase estrogen levels, increased receptive pinopodes, and ameliorated systemic inflammation. This enhancement likely arises from LeH-mediated upregulation of endometrial preparation pathways and establishment of an embryo-supportive anti-inflammatory uterine milieu.
The ultrastructural observation that the LeH group yielded a significantly higher proportion of endometria with fully developed pinopodes on D5 is a pivotal finding. Although the status of pinopodes as a definitive marker of endometrial receptivity has been debated (Aunapuu et al., 2018; Quinn et al., 2007; Quinn and Casper, 2009; Jin et al., 2017; Qiong et al., 2017), they remain a widely recognized morphological indicator (Lazim et al., 2023; Zhou and Zhou, 2025; Salmasi et al., 2024) whose development is tightly regulated by hormonal cues (Matson et al., 2017; Quinn et al., 2020). The enhanced pinopode development in the LeH group, together with its superior clinical outcomes, suggests that the LeH regimen more effectively establishes the hormonal milieu necessary for a receptive endometrial phenotype. Although our retrospective data featured uneven group sizes, age stratification was employed to control for this potential source of bias.
At the molecular level, our protein array data offer a clear mechanistic explanation for the clinical results. Le group predominantly suppressed the expression of proteins and pathways critical for implantation, including those involved in cell adhesion (Pathare and Hinduja, 2022; Grund and Grümmer, 2018), cytokine signaling, and inflammatory responses (Robertson et al., 2018; Granot et al., 2012). This suppression likely underlies the suboptimal receptivity associated with letrozole monotherapy. Crucially, the LeH regimen not only reversed this suppressive effect but actively upregulated these essential pathways. The restoration of “Cell adhesion molecules” and “P. regulation of response to external stimulus” pathways is particularly significant, as these processes are fundamental to embryo attachment and stromal decidualization. The identification of key proteins like CDH1, NCAM1, VEGF, and the novel candidate NECTIN4 provides specific therapeutic targets and underscores LeH’s ability to activate a synergistic receptivity network, although the causal relationship requires future validation.
The hormonal profile observed in the LeH group provides a foundational explanation for its benefits (Shaw et al., 2010). The significantly higher estradiol levels on the day of HCG administration, attributable to HMG supplementation, are critical for overcoming the systemic estrogen suppression caused by letrozole. Estradiol is a master regulator of endometrial proliferation, angiogenesis, and the expression of implantation mediators (Robertson et al., 2018; Granot et al., 2012). Thus, by ensuring adequate estrogenic priming, the LeH regimen creates a permissive environment for the subsequent molecular and structural changes that define receptivity, without compromising ovulation or luteal phase progesterone function.
Beyond the endometrium itself, our study reveals that the LeH regimen favorably modulates the uterine cavity (Sang et al., 2020; Zhang et al., 2017) and systemic environments. The anti-inflammatory signature observed in the uterine fluid of the LeH group—characterized by the downregulation of immune effector processes—suggests a shift towards immune tolerance, which is vital for protecting the semi-allogeneic embryo (Clark et al., 2015; Pantos et al., 2021). Furthermore, the pronounced downregulation of systemic inflammatory pathways, such as “Cytokine-cytokine receptor interaction,” indicates that LeH benefits the endometrium not only directly but also remotely by reducing a potentially harmful circulating inflammatory state, which is known to be detrimental to implantation and pregnancy maintenance (Li et al., 2024; Zhang H. et al., 2023).
5 Conclusion
In conclusion, our results demonstrate that the LeH regimen represents an optimized approach for endometrial preparation in FET cycles. It successfully mitigates the principal drawback of letrozole—impaired endometrial receptivity due to hypoestrogenism—by providing supplemental gonadotropin support. The regimen acts through a coordinated hierarchy of effects: (1) ensuring sufficient estradiol levels for endometrial proliferation and priming; (2) upregulating key molecular pathways governing embryo adhesion and implantation; (3) fostering a receptive endometrial ultrastructure; and (4) inducing a protective anti-inflammatory environment both locally within the uterus and systemically. Future prospective randomized studies are warranted to confirm these findings and to refine patient selection criteria for this promising protocol.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZX: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing – original draft, Writing – review and editing. FW: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review and editing. YY: Project administration, Writing – review and editing, Formal Analysis, Investigation, Methodology, Software, Writing – original draft. TX: Methodology, Software, Writing – review and editing. JC: Formal Analysis, Methodology, Writing – review and editing. ML: Data curation, Methodology, Writing – review and editing. HL: Data curation, Formal Analysis, Writing – review and editing. CC: Conceptualization, Methodology, Software, Supervision, Writing – review and editing. XF: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review and editing. JZ: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This research was supported by the following funding sources: National Key R&D Program of China (Grant No. 2024YFA1803001); Shenzhen Medical Research Foundation (Grant No. B2404004); National Natural Science Foundation of China (Grant Nos. 32571489 and 82301907); Basic and Applied Basic Research Foundation of Guangdong Province (Grant Nos. 2024A1515010059 and 2024A1515030279); Shenzhen Science and Technology Innovation Program (Grant Nos. JCYJ20220818101218040, JCYJ20220818103608017, JCYJ20220818103607015, JCYJ20230807140805011, and JCYJ20220818102811025); Shenzhen Key Laboratory of Metabolic Health (Grant No. ZDSYS20210427152400001); and Sino-European Center of Biomedicine and Health.
Acknowledgements
We thank the patients who participated in this study and the clinical staff for their support in sample collection and data management.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcell.2025.1725350/full#supplementary-material
References
Aunapuu, M., Kibur, P., Jãrveots, T., and Arend, A. (2018). Changes in morphology and presence of pinopodes in endometrial cells during the luteal phase in women with infertility problems: a pilot study. Med. Kaunas. 54, 74. doi:10.3390/medicina54050074
Bhatnagar, A. S. (2007). The early days of letrozole. Breast Cancer Res. Treat. 105 (Suppl. 1), 3–5. doi:10.1007/s10549-007-9699-0
Bülow, N., and Macklon, N. (2025). The role of letrozole in in vitro fertilization treatment: new remedy or old mirage? Fertil. Steril. 123, 41–49. doi:10.1016/j.fertnstert.2024.10.040
Castillo, J., and Kol, S. (2024). Ideal frozen embryo transfer regime. Curr. Opin. Obstetrics Gynecol. 36, 148–154. doi:10.1097/GCO.0000000000000943
Chen, L., Jiang, S., Xi, Q., Li, W., Lyu, Q., and Kuang, Y. (2023). Optimal lead follicle size in letrozole human menopausal gonadotrophin intrauterine insemination cycles with and without spontaneous lh surge. Reprod. Biomed. Online 46, 566–576. doi:10.1016/j.rbmo.2022.11.003
Clark, I. A., James, E. L., Iyadurai, L., and Holmes, E. A. (2015). “Mental imagery in psychopathology: from the lab to the clinic,” in Clinical perspectives on autobiographical memory. Wellcome trust–funded monographs and book chapters. Editors L. A. Watson, and D. Berntsen (Cambridge, UK: Cambridge University Press). doi:10.1017/CBO9781107707080.010
Cobo, A., Coello, A., De Los Santos, M. J., Remohi, J., and Bellver, J. (2024). Embryo long-term storage does not affect assisted reproductive technologies outcome: analysis of 58,001 vitrified blastocysts over 11 years. Am. J. Obstetrics Gynecol. 231, 238.e1–238.e11. doi:10.1016/j.ajog.2024.03.033
Colucci, W. S., Elkayam, U., Horton, D. P., Abraham, W. T., Bourge, R. C., Johnson, A. D., et al. (2000). Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide study group. N. Engl. J. Med. 343, 246–253. doi:10.1056/NEJM200007273430403
De Geyter, C., Calhaz-Jorge, C., Kupka, M. S., Wyns, C., Mocanu, E., Motrenko, T., et al. (2020). Art in Europe, 2015: results generated from European registries by eshre. Hum. Reprod. Open 2020, hoz038. doi:10.1093/hropen/hoz038
Diamond, M. P., Legro, R. S., Coutifaris, C., Alvero, R., Robinson, R. D., Casson, P., et al. (2015). Letrozole, gonadotropin, or clomiphene for unexplained infertility. N. Engl. J. Med. 373, 1230–1240. doi:10.1056/NEJMoa1414827
Franik, S., Eltrop, S. M., Kremer, J. A., Kiesel, L., and Farquhar, C. (2018). Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 5, CD010287. doi:10.1002/14651858.CD010287.pub3
Granot, I., Gnainsky, Y., and Dekel, N. (2012). Endometrial inflammation and effect on implantation improvement and pregnancy outcome. Reproduction 144, 661–668. doi:10.1530/REP-12-0217
Groenewoud, E. R., Cantineau, A. E., Kollen, B. J., Macklon, N. S., and Cohlen, B. J. (2013). What is the optimal means of preparing the endometrium in frozen-thawed embryo transfer cycles? A systematic review and meta-analysis. Hum. Reprod. Update 19, 458–470. doi:10.1093/humupd/dmt030
Grund, S. and, and Grümmer, R. (2018). Direct cell -cell interactions in the endometrium and in endometrial pathophysiology. Int. J. Mol. Sci. 19, 2460. doi:10.3390/ijms19082460
Jin, X. Y., Zhao, L. J., Luo, D. H., Liu, L., Dai, Y. D., Hu, X. X., et al. (2017). Pinopode score around the time of implantation is predictive of successful implantation following frozen embryo transfer in hormone replacement cycles. Hum. Reprod. 32, 2394–2403. doi:10.1093/humrep/dex312
Lazim, N., Elias, M. H., Sutaji, Z., Abdul Karim, A. K., Abu, M. A., Ugusman, A., et al. (2023). Expression of HOXA10 gene in women with endometriosis: a systematic review. Int. J. Mol. Sci. 24, 12869. doi:10.3390/ijms241612869
Li, H. Q., Pan, X. L., Su, N. J., Lu, X. P., Chen, J. Q., and Chen, X. W. (2022). Retrospective analysis: the application of human menopausal gonadotropin combined with letrozole for iui in patients undergoing artificial insemination by husband due to unexplained or mild Male factors. Front. Endocrinol. 13, 1038433. doi:10.3389/fendo.2022.1038433
Li, X., Luan, T., Wei, Y., Zhang, J., Zhou, L., Zhao, C., et al. (2024). Association between the systemic immune-inflammation index and gnrh antagonist protocol ivf outcomes: a cohort study. Reprod. Biomed. Online 48, 103776. doi:10.1016/j.rbmo.2023.103776
Lin, J., Wang, N., Huang, J., Cai, R., Fan, Y., Kuang, Y., et al. (2019). Pregnancy and neonatal outcomes of hmg stimulation with or without letrozole in endometrial preparation for frozen-thawed embryo transfer in ovulatory women: a large retrospective cohort study. Drug Des. Dev. Ther. 13, 3867–3877. doi:10.2147/DDDT.S212235
Matson, B. C., Pierce, S. L., Espenschied, S. T., Holle, E., Sweatt, I. H., Davis, E. S., et al. (2017). Adrenomedullin improves fertility and promotes pinopodes and cell junctions in the peri-implantation endometrium. Biol. Reproduction 97, 466–477. doi:10.1093/biolre/iox101
Melo, P., Wood, S., Petsas, G., Chung, Y., Easter, C., Price, M. J., et al. (2022). The effect of frozen embryo transfer regimen on the association between serum progesterone and live birth: a multicentre prospective cohort study (Profet). Hum. Reprod. Open 2022, hoac054. doi:10.1093/hropen/hoac054
Pantos, K., Simopoulou, M., Maziotis, E., Rapani, A., Grigoriadis, S., Tsioulou, P., et al. (2021). Introducing intrauterine antibiotic infusion as a novel approach in effectively treating chronic endometritis and restoring reproductive dynamics: a randomized pilot study. Sci. Rep. 11, 15581. doi:10.1038/s41598-021-95072-w
Pathare, A. D. S., and Hinduja, I. (2022). Endometrial expression of cell adhesion genes in recurrent implantation failure patients in ongoing ivf cycle. Reprod. Sci. 29, 513–523. doi:10.1007/s43032-021-00708-x
Qiong, Z., Jie, H., Yonggang, W., Bin, X., Jing, Z., and Yanping, L. (2017). Clinical validation of pinopode as a marker of endometrial receptivity: a randomized controlled trial. Fertil. Steril. 108, 513–517.e2. doi:10.1016/j.fertnstert.2017.07.006
Quaas, A. M., and Legro, R. S. (2019). Pharmacology of medications used for ovarian stimulation. Best Pract. and Res. Clin. Endocrinol. and Metabolism 33, 21–33. doi:10.1016/j.beem.2018.10.002
Quinn, C. E., and Casper, R. F. (2009). Pinopodes: a questionable role in endometrial receptivity. Hum. Reprod. Update 15, 229–236. doi:10.1093/humupd/dmn052
Quinn, C., Ryan, E., Claessens, E. A., Greenblatt, E., Hawrylyshyn, P., Cruickshank, B., et al. (2007). The presence of pinopodes in the human endometrium does not delineate the implantation window. Fertil. Steril. 87, 1015–1021. doi:10.1016/j.fertnstert.2006.08.101
Quinn, K. E., Matson, B. C., Wetendorf, M., and Caron, K. M. (2020). Pinopodes: recent advancements, current perspectives, and future directions. Mol. Cell. Endocrinol. 501, 110644. doi:10.1016/j.mce.2019.110644
Robertson, S. A., Care, A. S., and Moldenhauer, L. M. (2018). Regulatory t cells in embryo implantation and the immune response to pregnancy. J. Clin. Investigation 128, 4224–4235. doi:10.1172/JCI122182
Salmasi, S., Heidar, M. S., Khaksary Mahabady, M., Rashidi, B., and Mirzaei, H. (2024). Micrornas, endometrial receptivity and molecular pathways. Reproductive Biol. Endocrinol. 22, 139. doi:10.1186/s12958-024-01304-9
Sang, Y., Li, Y., Xu, L., Li, D., and Du, M. (2020). Regulatory mechanisms of endometrial decidualization and pregnancy-related diseases. Acta Biochimica Biophysica Sinica 52, 105–115. doi:10.1093/abbs/gmz146
Shaw, N. D., Histed, S. N., Srouji, S. S., Yang, J., Lee, H., and Hall, J. E. (2010). Estrogen negative feedback on gonadotropin secretion: evidence for a direct pituitary effect in women. J. Clin. Endocrinol. Metabolism 95, 1955–1961. doi:10.1210/jc.2009-2108
Sunderam, S., Kissin, D. M., Zhang, Y., Jewett, A., Boulet, S. L., Warner, L., et al. (2020). Assisted reproductive technology surveillance – united States, 2017. MMWR Surveill. Summ. 69, 1–20. doi:10.15585/mmwr.ss6909a1
Tummon, I., Gavrilova-Jordan, L., Allemand, M. C., and Session, D. (2005). Polycystic ovaries and ovarian hyperstimulation syndrome: a systematic review. Acta Obstetricia Gynecol. Scand. 84, 611–616. doi:10.1111/j.0001-6349.2005.00788.x
Vinsonneau, L., Labrosse, J., Porcu-Buisson, G., Chevalier, N., Galey, J., Ahdad, N., et al. (2022). Impact of endometrial preparation on early pregnancy loss and live birth rate after frozen embryo transfer: a large multicenter cohort study (14,421 frozen cycles). Hum. Reprod. Open 2022, hoac007. doi:10.1093/hropen/hoac007
Wang, M., Zhong, B., Li, M., Wang, Y., Yang, H., and Du, K. (2021). Identification of potential core genes and pathways predicting pathogenesis in head and neck squamous cell carcinoma. Biosci. Rep. 41, BSR20210805. doi:10.1042/bsr20204148
Wang, H., Xu, Y. H., and Guo, Y. (2024a). Novel prognostic marker tgfbi affects the migration and invasion function of ovarian cancer cells and activates the integrin αvβ3-pi3k-akt signaling pathway. J. Ovarian Res. 17, 50. doi:10.1186/s13048-024-01377-5
Wang, Q., Chi, J., Zeng, W., Xu, F., Li, X., Wang, Z., et al. (2024b). Discovery of crucial cytokines associated with deep vein thrombus formation by protein array analysis. BMC Cardiovasc. Disord. 24, 374. doi:10.1186/s12872-024-04030-7
Xi, W., Liu, S., Mao, H., Yang, Y., Xue, X., and Lu, X. (2015). Use of letrozole and clomiphene citrate combined with gonadotropins in clomiphene-resistant infertile women with polycystic ovary syndrome: a prospective study. Drug Des. Dev. Ther. 9, 6001–6008. doi:10.2147/DDDT.S83259
Xia, T. T., Zeng, K. F., and Peng, Q. M. (2023). Comparison of three ovulation induction therapies for patients with polycystic ovary syndrome and infertility. J. Clin. Pharmacol. 63, 1371–1376. doi:10.1002/jcph.2318
Xiao, Z., Chen, J., Fan, X., Zhao, W., Chu, C., and Zhang, J. V. (2025). The impact of chemokine-like receptor 1 gene knockout on lipopolysaccharide-induced epididymo-orchitis in mice. J. Interferon and Cytokine Res. 45, 1–11. doi:10.1089/jir.2024.0152
Yang, Y. L., Ren, L. R., Sun, L. F., Huang, C., Xiao, T. X., Wang, B. B., et al. (2016). The role of gpr1 signaling in mice corpus luteum. J. Endocrinol. 230, 55–65. doi:10.1530/JOE-15-0521
Zhang, Y., Wang, Q., Wang, H., and Duan, E. (2017). Uterine fluid in pregnancy: a biological and clinical outlook. Trends Mol. Med. 23, 604–614. doi:10.1016/j.molmed.2017.05.002
Zhang, J., Liu, H., Wang, Y., Mao, X., Chen, Q., Fan, Y., et al. (2019). Letrozole use during frozen embryo transfer cycles in women with polycystic ovary syndrome. Fertil. Steril. 112, 371–377. doi:10.1016/j.fertnstert.2019.04.014
Zhang, X., Zheng, A., Yang, J., Feng, T., Zhang, Y., Hao, Y., et al. (2021). Application of pulsed rhythmic drug administration to ovulation induction therapy in pcos patients with clomiphene-resistance: a retrospective research. Reprod. Sci. 28, 3193–3199. doi:10.1007/s43032-021-00639-7
Zhang, H., Li, X., Zhang, F., Li, F., Jin, H., Su, Y., et al. (2023a). Serum c-reactive protein levels are associated with clinical pregnancy rate after in vitro fertilization among normal-weight women. Front. Endocrinol. 14, 934766. doi:10.3389/fendo.2023.934766
Zhang, Y., Fu, X., Gao, S., Gao, S., Gao, S., Ma, J., et al. (2023b). Letrozole use in vitrified single-blastocyst transfer cycles is associated with lower risk of large for gestational age infants in patients with polycystic ovary syndrome. J. Assisted Reproduction Genet. 40, 2885–2894. doi:10.1007/s10815-023-02956-z
Zhang, X., Xu, Y., Shi, L., Chen, X., Hu, M., Zhang, M., et al. (2024). Fgf6 inhibits oral squamous cell carcinoma progression by regulating pi3k/akt and mapk pathways. Sci. Rep. 14, 26877. doi:10.1038/s41598-024-78552-7
Zhao, Y., He, D., Zeng, H., Luo, J., Yang, S., Chen, J., et al. (2021). Expression and significance of mir-30d-5p and socs1 in patients with recurrent implantation failure during implantation window. Reproductive Biol. Endocrinol. 19, 138. doi:10.1186/s12958-021-00820-2
Zhou, L., and Zhou, L. (2025). The mechanisms and therapeutic effects of granulocyte colony-stimulating factor in reproduction. Reproductive Biol. Endocrinol. 23, 78. doi:10.1186/s12958-025-01414-y
Keywords: infertility, endometrial receptivity, implantation, pinopode, frozen embryo transfer, letrozole, human menopausalgonadotropin, proteomics
Citation: Xiao Z, Wang F, Yang Y, Xiao T, Chen J, Li M, Liao H, Chu C, Fan X and Zhang JV (2025) Low-dose letrozole-HMG regimen reverses letrozole-induced endometrial impairment and improves frozen embryo transfer outcomes. Front. Cell Dev. Biol. 13:1725350. doi: 10.3389/fcell.2025.1725350
Received: 15 October 2025; Accepted: 25 November 2025;
Published: 16 December 2025.
Edited by:
Antonio Diez Juan, Vitrolife, SwedenReviewed by:
Sepide Goharitaban, Tabriz University of Medical Sciences, IranJunfeng Li, Luohe Central Hospital, China
Copyright © 2025 Xiao, Wang, Yang, Xiao, Chen, Li, Liao, Chu, Fan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian V. Zhang, amlhbi56aGFuZ0BzaWF0LmFjLmNu; Xiujun Fan, eGl1anVuLmZhbkBnbWFpbC5jb20=; Chiawei Chu, Y3djaHVAY2l0eXUubW8=
†These authors have contributed equally to this work
 Zhonglin Xiao
Zhonglin Xiao Feng Wang3,4†
Feng Wang3,4† Yali Yang
Yali Yang Jie Chen
Jie Chen Xiujun Fan
Xiujun Fan Jian V. Zhang
Jian V. Zhang