- Consejo Nacional de Investigaciones Cientificas y Técnicas, Argentina
The acceptance of Darwin's theory of evolution by natural selection is not complete and it has been pointed out its limitation to explain the complex processes that constitute the transformation of species. It is necessary to discuss the explaining power of the dominant paradigm. It is common that new discoveries bring about contradictions that are intended to be overcome by adjusting results to the dominant reductionist paradigm using all sorts of gradations and combinations that are admitted for each case. In addition to the discussion on the validity of natural selection, modern findings represent a challenge to the interpretation of the observations with the Darwinian view of competition and struggle for life as theoretical basis. New holistic interpretations are emerging related to the Net of Life, in which the interconnection of ecosystems constitutes a dynamic and self-regulating biosphere: viruses are recognized as a macroorganism with a huge collection of genes, most unknown that constitute the major planet's gene pool. They play a fundamental role in evolution since their sequences are capable of integrating into the genomes in an “infective” way and become an essential part of multicellular organisms. They have content with “biological sense” i.e., they appear as part of normal life processes and have a serious role as carrier elements of complex genetic information. Antibiotics are cell signals with main effects on general metabolism and transcription on bacterial cells and communities. The hologenome theory considers an organism and all of its associated symbiotic microbes (parasites, mutualists, synergists, amensalists) as a result of symbiopoiesis. Microbes, helmints, that are normally understood as parasites are cohabitants and they have cohabited with their host and drive the evolution and existence of the partners. Each organism is the result of integration of complex systems. The eukaryotic organism is the result of combination of bacterial, virus, and eukaryotic DNA and it is the result of the interaction of its own genome with the genome of its microbiota, and their metabolism are intertwined (as a “superorganism”) along evolution. The darwinian paradigm had its origin in the free market theories and concepts of Malthus and Spencer. Then, nature was explained on the basis of market theories moving away from an accurate explanation of natural phenomena. It is necessary to acknowledge the limitations of the dominant dogma. These new interpretations about biological processes, molecules, roles of viruses in nature, and microbial interactions are remarkable points to be considered in order to construct a solid theory adjusted to the facts and with less speculations and tortuous semantic traps.
“I do not write for those who examine
new books quickly, often
with the intention of finding in them their ideas
preconceived, but for the few who read,
who meditate deeply, who love
study of nature and are capable of
even sacrificing their own interests,
for the knowledge of a new truth.”J. B. Lamarck (1744–1829)
Introduction
While the Modern Synthetic Theory is the most widely accepted evolutionary theory, many authors consider that it is necessary to evaluate its explanatory power and a self-criticism of orthodoxy from the inability to explain the phenomena and discoveries daily observed (Ehrlich and Birch, 1967; Goldsmith, 1989; Margulis and Sagan, 1995; Kampis, 1997; Sandín, 1997; Abdalla, 2006).
This dominant paradigm based on a conception of the transmission of strictly Mendelian characters has as basic tenets: (1) Evolution is a gradual process of substitution of alleles within a population. The source of variability in these alleles would be the point mutations or micromutations. (2) The genetic material is only the raw material. What drives the evolutionary process is natural selection (Mayr, 1966; Dobzhansky et al., 1977; Sandín, 1997).
However, with the data provided by different areas of biology, this theoretical framework based on natural selection appears weak to explain the complex evolutionary processes. At least, it is necessary to discuss the explaining power of the dominant paradigm. It is common that new discoveries bring about contradictions that are intended to be overcome by adjusting results to the dominant reductionist paradigm using all sorts of gradations and combinations that are admitted for each case (Sandín, 1997; Forterre, 2010). Nowadays there are new interpretations about biological processes, new approaches and perspectives that are remarkable points to be considered in order to construct a solid theory more adjusted to the facts, and with less speculations and tortuous semantic traps.
The present work is a humble contribution to that discussion with the intention of enriching it by providing new perspectives in evolution related to complex systems.
The Kidnapping of Biology
Darwinism grew out of the Malthusian concepts and vision that disease and food shortages act as regulators of the population favoring the fittest in a continuous struggle for life. Darwin wrote his book “On the Origin of Species by Means of Natural Selection, or the maintenance of favored races in the struggle for existence” (1859) based on Malthus theory and then in the expressions of Herbert Spencer: “As more individuals are produced which may survive, there must be necessarily a struggle for existence (…) is the doctrine of Malthus applied with multiplied force to the nature” (Darwin, 1869). And elsewhere he writes: “I call this principle which preserves all small variation, it is useful, natural selection mark your faculty with man's selection. But the expression used by Herbert Spencer that the fittest survive is more accurate.” In words of Sandín: “The idea expressed more forcefully in the work of Darwin is the extrapolation of the activities of ranchers and farmers to the phenomena of nature” (Sandín, 1997).
It is not the purpose of this paper to review the historical injustice done to those scientists who built the evolutionary scientific basis and began the studies of evolutionary mechanisms. But, it is important to briefly remember some facts. Darwin was not the “inventor” of evolution, neither the idea of evolutionary process was in the “air” before him. On the contrary, it was in a much more solid basis.
Jean Baptiste Pierre-Antoine de Monet, Chevallier de Lamarck, published the more structured Theory of Evolution in 1809 in his book Philosophie Zoologique. He was a disciple of Georges-Louis Leclerc, comte de Buffon and Professor of the Natural History Museum. In 1800, he gave a lecture exposing a coherent theory on the transformation and laid the foundations of epigenesis and organism-environment interaction derived from the mechanism of adaptation. Buffon was the author of an encyclopedia on nature, in 44 volumes (only 36 of them were published in life), the “Histoire naturelle, générale et particulière,” where he mentioned that the species observed were transformed and links between organisms. Frederic Gerard in “Theorie de l'evolution des formes organiques,” (1841–1849) exhibited a clear distinction between micro and macroevolution based on thorough paleontological studies. To these works are added those from Agassiz, Geoffroy Saint Hilaire (stating “teratologies” abrupt morphological changes that occur during the development), von Zittel, von Baer, Tremaux, who developed the “allopatric speciation” and “punctuated equilibrium long before Darwin and Gould” (Wilkins and Nelson, 2008), and others. The idea of evolution was known and studied among naturalists. Later, after receiving a letter from Wallace set forth the concept of natural selection independently, Darwin published his famous book “From Origin of Species by Means of Natural Selection, or the conservation of Favoured Races in the Struggle for Life.”
The idea of natural selection was noted by many philosophers and scientists before Darwin, from the ancient Greek philosophers Empedocles and Aristóteles (third and fourth centuries BC) to Edward Blyth (1810–1873) and Wallace. From 1835 to 1837, Blyth published some articles in The British Magazine of Natural History (Vols. 8, 9, and 10) dealing with natural selection, adaptive radiation, and the struggle for life. It is known that Darwin recieved copies of this magazine while in Peru in 1835 during his voyage on the Beagle. In 1750, the concept of natural selection was noted by Pierre-Louis Moreau de Maupertuis in his “Essay on Cosmology.” Also, it was defined by Denis Diderot (1713–1784), William Charles Wells (in an essay from 1813, “Two Essays … with Some Observations on the Causes of the Differences of color and form Between the white and black races of men. By the Late WC Wells … with a Memoir of His Life, written by himself”), Patrick Matthew (1790–1874) as well as by James Cowles Prichard and William Lawrence.
In the midst of industrial revolution, Darwin observed the growth of misery and poverty. He was influenced and linked to laissez faire policies, propelled by Adam Smith, who proposed the lowest state intervention (it was postulated, among others things, to stop creating schools) so as to “naturally” remove the homeless through a free competition. Gertrude Himmelfarb noted that Darwinism was a biological justification of the status of the victorian society as the “fittest”: “The theory of natural selection, it is said, could only have originated in England, because only laissez faire England provided the atomistic, egotistic mentality necessary to its conception. Only there could Darwin have blandly assumed that the basic unit was the individual, the basic instinct selfinterest, and the basic activity struggle. Spengler, describing the Origin as: “the application of economics to biology,” said that it reeked of the atmosphere of the English factory … natural selection arose … in England because it was a perfect expression of Victorian “greed-philosophy” of the capitalist ethic and Manchester economics” (1962, p. 418). In that place and time, there was a social predisposition for that kind of evolution theory.
History often tells us that Darwin found rejection in society of the time and among the church hierarchy. However, Darwin found great support among the most influential scientists and their ideas were welcomed by the X-club. This elite society of the time consisted of a group made among others by Joseph Dalton Hooker, Thomas Henry Huxley, John Lubbock, Herbert Spencer, who propelled Darwinian ideas and had remarkable power to control the Royal Society (Barton, 1998).
It is well known the discussion between Huxley, defender of Darwin, and the Bishop of Oxford, Wilberforce. While the church defended the fixity, that species did not change, they also questioned the weaknesses of Darwinian proposal, which assumed the transformation of species as a fact but without proof that it occurs by the proposed mechanisms. Wilberforce was right on some points: the first question, in the course of human history there was no evidence of any new species development. Secondly, the selective pressures, although it is true that they have an effect, they do not cause a change of species. Finally, the phenomenon of hybrid sterility was a strong evidence in favor of the fixity of species. Thus, this well known dispute raises a dichotomy that is useful to both dogmas nowadays. It is stating that any challenge to the Darwinian “science” is a “creationist” attack and avoids a scientific discussion about the weaknesses of Darwinism and a recognition that this is also a dogma. The really important issue is not the creationist critics, because this is faith, but the scientific criticisms and what we can do to build a more scientific evolutionary theory.
Since the inception of darwinist natural selection (the cornerstone of the dominant theory), the acclamation was not a unanimous reaction. Among the scientific criticisms received, we can mention those of Charles Darwin1, Adam Sedgwick, Aldous Huxley, Karl von Baer, Louis Agassiz, Richard Owen, Cherles Lyell, Richard Lewontin, St. George Mivart, Albert von Kölliker, Clémence Royer, Robert Peters, etc. This was, not because there was a naive resistant to the science from creationists, but Darwin's theory had huge gaps in its “pure state” and did not explain the complexities observed in organisms and it did not fit the fossil record available at the time (and less to the present). Therefore, from the beginning, this was a theory scientifically problematic (Abdalla, 2006).
The Discussion about Natural Selection
In Darwin's work, natural selection takes many forms and nuances. Darwin postulates this “mechanism” generator of new species in a scenario of continued competition. It also takes other definitions as a determinant of character preservation, general process, survival of the fittest, agent, power, cause of extinction, strength. The definitions given for this invisible arm is also varied and their use to explain it all leads to acquire a stunning conceptual flexibility (Cervantes, 2011a).
In words of Futuyma: “Natural selection is the only mechanism known to cause the evolution of adaptations, so many biologists would simply define an adaptation as a characteristic that has evolved by natural selection” and “any consistent difference in fitness among phenotypically different classes of biological entities” (Futuyma, 2009). We cannot define it as a mechanism, given that in a mechanism there are elements known and arranged to ensure a predictable performance. Furthermore, as natural selection would be the generator of species and the insurer of the survival of the fittest, it must also generate morphological novelties (Cervantes, 2011a).
For Dawkins “there is of course no ‘architect.’ The DNA instructions have been assembled by natural selection.” However, “Natural selection is not an external force or agent, and certainly not a purposeful one. It is a name for statistical differences in reproductive success among genes, organisms, or populations, and nothing more.” (Dawkins, 1976). But, “natural selection, i.e., survival and differential reproduction of organisms, is the main controlling agent of evolutionary change (Dobzhansky et al., 1977).
Also, Natural selection is at one and the same time a blind and creative process (Dobzhansky, 1973). The idea of selection implies a teleological residue. Selection implies intention since this term refers to a deliberate action of men. If we consider that natural selection is a process, we are allowed to associate it with any natural phenomenon, and we would be allocated for this purpose or intentional phenomenon. The phenomenon (evolution) is confused with the concept that seeks to explain (the selection) (Cervantes, 2011a,b).
Schluter (2009), who did not define natural selection, writes: “The main question today is how selection leads to speciation (…) what are the mechanisms of natural selection (…).” It is assumed that mechanisms of natural selection (that is not a mechanism) are not known. Even though it is generally accepted that natural selection could not only generate all species but also “drive” the evolution, i.e., the generation of new structures, the cause of appearance of the existing body systems.
At the same time, natural selection is a statistical difference, cause of adaptations, process, mechanism, the assembler of DNA, the agent that acts over DNA, the result of the adaptations (reproductive success once adapted to the environment), the difference in fitness, the result of that difference, the differential survival of entities. For Cervantes, it is a semantic ghost. A concept that is many things at the same time is probably nothing (Cervantes, 2011a). We can agree that everybody understands natural selection as survival and differential reproduction of organisms. However, the term refers both the causes as the effects and takes lot of nuances along literature. This indefiniteness made everything seem to be explained but nothing is explained actually. Everything leads us to confirm the existence of pliable natural selection with the existence of living organisms (survivors) and that they are adapted to their environment. That leaves us still at the starting point of evolutionary research.
Linguistic traps of Darwinism began in Darwin's work but continued through time and spread more confusion. During 70 s there was a discussion about the tautological nature of natural selection. Initially, natural selection claimed that in nature not only a few survive, but also that the fittest survive. That is, those that survive are the fittest to survive, because survival means that not all of them do it, surviving means ability to survive and they survive precisely because they are the fittest. It's a circular reasoning that does not represent any advance in knowledge. What any evolutionary theory should prove is what the laws of evolution are and do not say that the fittest survive. Peters argues that given its inability to make predictions it cannot be called a scientific theory (Peters, 1976). Natural selection is currently used to explain relationships among organisms, without being used in the context of the evolutionary process, i.e., major organizational, morphological, physiological changes and the origin of species. The core of the problem is that, despite the defenses that can be done in favor of natural selection, it does not add any knowledge or information to contribute to the explanation of the process.
From the point of view of the renowned philosopher and epistemologist Karl Popper the criterion of demarcation, i.e., a rule that defines when a theory is scientific or not, is its falsifiability. If a proposition is not falsifiable it is not scientific, and his rebuttal is determined by experimentation, the scientific method. As a tautology, natural selection is not falsifiable, and then, with this criterion, it is not a scientific theory (Popper, 1963).
For Ehrlich and Birch, in agreement with Popper, Darwinism “cannot be refuted by any possible observations and it is thus outside empirical science” (…). It is “an evolutionary dogma accepted by most of us as part of our training” (Ehrlich and Birch, 1967). A concept that was very vague from the beginning, in a text with little scientific rigor and a lot of ambiguity, was sustained over time and forced to fit the new discoveries.
Taking natural selection as correct, it can also lead to inconsistencies in the theory (Bouchard and Rosenberg, 2004). With knowledge of the complexity of the microbial world (natural selection arises from the observation of domestic animals) and the complexity revealed by genetics until today, the excessive eagerness to believe in natural selection is striking.
New Findings, Old Paradigm
Besides the semantic problems, another questionable aspect of the dominant theory is the important place occupied by random mutation. Mutation is not a solid explanation neither at levels of generation of new structures that constitutes the evolution nor in the generation of new species (Bernhard, 1967; Schützemberger, 1967). In Bacteria, mutation rates are subject to complex regulation that we are now just beginning to understand (Wright, 2000). Furthermore, bacterial populations tend to have low mutation rates which give stability to their genomes and avoid lethal mutations (Martinez et al., 2009a).
Darwinian reductionism in which everything is reduced to the sum of the parts leads to determinism according to which if we know the parts we can understand the whole. In this regard, it is believed that the complexity of life can be explained by the mechanical interaction of the fundamental molecules, mainly nucleic acids (DNA and RNA). For Abdalla, one of the facets of the potential crisis of paradigm in biology is related to this reflection on the complexity. The neo-Darwinian paradigm eventually leads to a reductionist approach that believes life is a result of localized phenomena in the DNA molecule, subjected to random changes and natural selection (Abdalla, 2006).
Throughout these decades several mechanisms and biological processes have been described that are difficult to frame within the Synthetic Theory: the mobile elements, repeat DNA sequences, the homeotic genes, regulatory sequences, the implication of endogens virus in the regulation and control of embryonic development, morphogenetic fields with incredible precision in the spatial and temporal process of the formation (Harrison, 1937; Weiss, 1939; Child, 1941). A lot of processes control cell functioning and self-regulate each other conforming complex networks, molecular memory, gene-gene communication, and multitasking of eukaryotic genomes (Ball, 2001; Mattick and Gagen, 2001). The evidence provided by evolutionary ontogeny, those provided by the fossil record, the “Evo-Devo,” the morphological novelties, horizontal transfer, the integration of genomes, the presence of a high percentage of bacterial and viral genes in eukaryotic genomes, the response to the environment and epigenetic phenomena, self-organizing systems are some of the aspects that constitute a body of knowledge that points out the limitations of the theory of competition, natural selection, and random mutations.
The evidence shows that genetic moving elements through changes in location and duplication, chromosomal rearrangements, cause changes in gene expression and regulation. These sequences also are a constituent part of the structures. For example, more than one gene sequence expressed in 37 human tissues have been identified as belonging to endogenous retroviruses (Johnson and Coffin, 1999; Mattick and Gagen, 2001; Vitali et al., 2003; Mallet et al., 2004; Hamilton, 2006). Furthermore, with the new discoveries, it is necessary to redefine gene, that is far away to be the gene in which it is sustained the Darwinist theory (Gerstein, 2007; Ledford, 2008; Buchanan et al., 2009). It is doubt the existance of the common ancestor (and the known domains Bacteria, Eukaria, and Archaea needs to be redefined Boyer et al., 2010).
The idea of natural selection is powerful because of being so simple. The embryological and genomic remodeling observed in evolution (Gilbert et al., 1996) seem not at all explained by the survival of the fittest (the less fit can also survive) and with that warlike scenario in which even the genes competes and where living beings are used by their own genes (Sandín, 1997).
Maybe, it is time “to resynthesize biology, put organism back into its environment; connect it again to its evolutionary past” (Woese, 2004).
Other Perspectives
Since there are basic facts of evolution that are the most difficult to “fit” in the framework of conventional theory, it is necesary to evaluate the explanatory power of the central dogma. The study of the dominant paradigm shortcomings in the light of the continuous discoveries involve sociological, biological, and epistemological aspects leading to a kind of Kuhnian revolution which other sciences such as physics have already experienced. On the contrary, in many reports the continuous discoveries are adjusted to the paradigm that the results contradict.
The Darwinian perspective does not take into account that reductionism leads to study living things, or partial aspects of them as if they were independent entities. Also it is common to refer that natural selection acts at “different levels,” and each character, molecule or process is explained (or assumed to be explained) by action of this strength/mechanism/differential reproduction/etc. Organisms clearly do not exist as isolated organisms but in terms of its environment consisting of living and non-living forms at different levels between which there are interconnections and interdependencies. Living organisms are in intense exchanges with their environment and are capable of self-organization forming a dynamic ecosystem. The interconnection of ecosystems constitutes a dynamic and self-regulating biosphere: the Net of Life (Sandín, 1997; Maturana and Varela, 1999). Even when these concepts appear to be well studied, it persists the intention to explain everything by a selfishness and warfare view (Nedelcu et al., 2011; Vannier-Santos and Lenzi, 2011) that does not take into account that organisms evolved intertwined and the coexistence is the result of what we are studying. As one example, Nedelcu et al. (2011) deal with the problem of altruism. The authors conclude that “active death in single-celled organisms is a maladaptive trait maintained as a byproduct of selection on pro-survival functions, but that could—under conditions in which kin/group selection can act—be co-opted into an altruistic trait” (Nedelcu et al., 2011). In this case, even when the authors are assuming that it is necessary a new paradigm, they just create new tortuous semantic traps and metaphors to explain by economical terms the phenomenon studied. A new theoretical basis is necessary a that takes into account the integrative and associative process that are observed in nature and the evolution of organism in association with their partners and the environment instead of maintaining economic prejudices and speculations that organisms live and exist thanks to cost-benefits and selfish transactions.
In the times of the origin of the theoretical basis of population genetics (basis of the “Theory Modern Synthetic”) the existing genetic knowledge about the processes and mechanisms were very limited. Although the concept of transmission characters according to Mendelian inheritance type was a simplification of some processes today we know that they are really much more complex (Buchanan et al., 2009). Evolution of life is a process of complex systems integrating to other systems, integrating higher levels (Kauffman, 1993; Margulis and Sagan, 1995; Johnson and Coffin, 1999; Doolittle, 2000; Gupta, 2000; Davidson and Erwin, 2006). The components of basic units of bacteria that would have all the processes and mechanisms of cellular life appear to have been preserved with very few changes along the evolutionary process. Viruses, by chromosomal integration mechanism, which would, either individually or through their combination, introduce new sequences responsible for controlling embryonic development of new tissues and organs, as well as regulating its operation. It seems that association and cooperation have been underrated in the biology that only sees a battle for life in nature.
Since the inception of natural selection and the Darwinian view of nature, the definition of life is skewed. Nowadays, the discover of giant viruses, mimivirus, the description of amoebae as genitors of new microorganisms, the attempt to understand the evolutionary history of eukaryotic Nucleocytoplasmic Large DNA Viruses (NCLDV), focuses the attention on the fundamental question of the definition of life (Raoult, 2010a).
The concept of autopoiesis was introduced by Humberto Maturana and Francisco Varela (Varela et al., 1974). It considers a living system as a dynamic composite entity, a unity as a closed network of productions of components in away through their interactions in composition and decomposition, the components: (1) recursively constituted the same network of production that produced them, and (2) specify the extension of the network and constitute operational boundaries that separate it as a dynamic unity in a space defined by elements of the kind of those that compose it. It is an autopoietic system (Maturana, 2002). The word autopoiesis connotes the organization of living systems as closed networks of molecular production. Living systems exist only as long as their autopoietic organization is conserved. “Autopoiesis is the actual manner of being as the organization that constitutes living systems as singular entities in the molecular space” (Maturana, 2002).
Structural changes in the living system are foreign to the characterization of an observer and external or internal, but they occur contingent on structural meeting with the environment. In forming a lineage of living beings, what defines the lineage is the maintenance of autopoiesis over generations. For Maturana, biodiversity is the result of the formation and transformation of lineages in a continuous phylogenetic coderiva. That is, during the continuity of a lineage of living beings, an ontogenic phenotype is conserved in a reproductive sequence. This occurs in a systemic dynamics and not in a genetic one. The systemic genotype may change but the lineage may be kept. The new lineage will emerge, depending on the conditions that are systemic to this effect, as a variant of the original whenever the new ontogenic phenotype is preserved systemically (Maturana and Mpodozis, 1999). A system, facing a profound environmental change, may respond with a structural quantum leap or collapsing (general theory of systems). Organisms arise due to a structural dynamics independent of them. Nothing happens during this diversification that can be called selective force or pressure. An observer may notice a differential survival of different kinds of organisms that constitute a population (we can remember the dubious experiment of the peppered moth), but the observer cannot affirm that what led to this survival differential was a selection. This historic result from the phylogenetic deriva is the consequence of a systemic process in which there is no “pressure.” To the extent that living beings are autopoietic systems that exist in ontogenic structural coderiva and breed in conditions of conservation of organization and adapt or die, producing lineages and ontogenetic phenotypic variations, are spontaneous and inevitable processes (Maturana and Mpodozis, 1999).
To this holistic new perspective, it is possible to enumerate different topics in which the results interpreted within the Darwinist preconceptions arise other interpretations that allow a better assessment of the facts observed.
Antibiotics
Antibiotics, which are the main molecules used by microorgamisms as weapons in the Darwinian view, are now re-studied as molecular signals (Linares et al., 2006; Fajardo and Martínez, 2008; Jayaraman, 2009).
It is known that subminimal inhibitory concentrations of antibiotics could produce subtle changes in bacterial physiology. The behavior of the bacterial population is an integrated response to different cell-to-cell signals (Martinez et al., 2009a). At concentrations found naturally in the environment where the organism lives as producer, the main effects are general metabolism, changing patterns of transcription in a dose-dependent (Tsui et al., 2004; Yim et al., 2006a,b; Fajardo and Martínez, 2008; Martinez et al., 2009a). Also, some of these changes are antibiotic-specific. Antibiotics that inhibit bacterial topoisomerases might, at low concentrations, trigger SOS response or enhance RNA stability and produce changes in DNA supercoiling (Linares et al., 2006), responses that are beneficial for the microorgamisms involved (Linares et al., 2006; Martinez et al., 2009a).
Under the traditional view, the generation of resistance to antibiotics used in clinical medicine would be an evolutionary strategy of pathogenic microorganisms, calling these resistance mechanisms as pathogenicity or virulence factors. The limited interpretation of antibiotics as weapons results in misinterpretation of resistance mechanisms as specific shields that confer the protection against the weapons. This is the implicit belief of many reports related to this topic but the mechanisms involved in this resistance are more complex and they are being elucidated.
Alternative functional roles for resistance elements are now being proposed. Firstly, the presence of an antibiotic resistance gene does not necessarily imply that its original role was to help resist the action of the antibiotic (Martinez et al., 2009a). Also, the incidence of bacteria carrying multidrug resistance (MDR) pumps is not limited to environments with a high antibiotic load. Pumps that extrude antibiotics instead of being “bacterial strategies” against humans appear to have the function of detoxification of intracellular antibiotics rather than resistance to external ones (Martinez et al., 2009b). Furthermore, it is necessary to remark that some of these MDR pumps can efflux signal compounds indicating that signaling networks may be important in triggering antibiotic resistance (Martinez et al., 2009a). In many cases, the expressions of MDR pumps are related in regulating Quorum Sensing homeostasis (Martinez et al., 2009b).
Microbial cell-signaling is a result of an integrated system, interrelated ecosystem that it is far away of be weapons against their neighbors. Horizontal gene tansfer (HGT) is related to developing of competence and these both processes could be triggered by agressive and stressing conditions. High (toxic and stressing) concentrations of antibiotics (that are rarely found in nature) are the consequence of human activity. This artificial selection results in the disseminationan of resistance by HGT (for example, the spread of integrons). This process (that the traditional dogma could called “exaptation”) is a result of the antropogenic activity and the complex mechanisms involved demonstrate that these interrelated process change against a stressful condition to restore the homeostasis of the whole system.
Antibiotics are produced in normal conditions in nature by microorganisms that are in a physiological state similar to what in the laboratory is called stationary phase. At these levels these molecules are signals that mantain the homeostasis. This state (so-called “stress”) triggers a signal and those genes related to resistance genes are activated, acting as extrusion of the signal molecules. In the case of other mechanism of resistence that involves enzymatic modification of antibiotics, implies the synthesis of enzymes that have a metabolic function primarily as precursors phosphorylate “antibiotic.”
Bacteriocins
Bacteriocins are defined as antimicrobial proteinaceous compounds synthesized ribosomally by bacteria (Diep and Nes, 2002). Even though authors report that the ecological function of these peptides is not yet fully understood, they decide that bacteriocins represent an important component of the warfare in nature (Riley, 1998; Gillor et al., 2008; Desriac et al., 2010). However, this is a limited interpretation since it is a directional result of an experiment that is searching for inhibition. And this function, in great amount, is assumed as the ecological function.
Bacteriocins are defined only by a partial and forced effect while their role in the microbial ecology context is forgotten. But, a look to the reports related to bacteriocins reflect their function as signals in a more complex context that the darwinian view of bacterial compounds as weapons in the fight between microbial competitors for colonizing of the same niche (Riley, 1998; Riley and Wertz, 2002; Desriac et al., 2010). The evaluation, and screening of bacteriocins is achieved in a “five stars restaurant” of a broth in lab, and we obtain great amount of bacteriocins that are used to inhibit the growth of another strain. Subclass IIa bacteriocins, for example, recognize mannose phosphotransferase system in the membrane of producer and “target” strains. Different strains display different expression levels of a man-PTS gene that corresponded to the variation in bacteriocin sensitivity (Kjos et al., 2009). These peptides act as signals among cells of a species and could interact with their environment, that is, abiotic and biotic factors around (Perry et al., 2009a,b). The bacteria and other organisms respond and send other signal to the bacteriocin producer. As with antibiotics, the search for bacteriocins were performed in order to obtain inhibitors, and many researchers tend to consider that microbes use them with the same function (Cotter et al., 2005; Papagianni and Anastasiadou, 2009). This is allegorical, because the obsessive behavior to produce profitability replaces the study of the phenomenon itself. As the essence of Darwinism was born in liberal trade and prejudices are so ingrained, it is easy to fall into confusion and move an ideology and human behavior to nature.
Viruses
A key aspect is the role of viruses in evolution (Rohwer et al., 2009). Viruses have acquired a new interpretation based on their capacity to insert genomes in cells and they are recognized as a macroorganism with a huge collection of genes, most unknown that constitute the major planet's gene pool. The continuing sequenciation of phages and virus is a way to the unknown (Rosario et al., 2009). Continued virus gene rearrangements derived from virus particles have formed a mosaic gene that underlies the creation of new structures and the generation of new species (Tristem et al., 1995; Johnson and Coffin, 1999; Tristem, 2000; Casjens, 2003; Johnson, 2008).
Genomes of all living organisms are mosaic of genes. Eukariotyc genome has genes from bacterial, archaeal and viral origins. Similarly, organelles like mitochondria do not have a single common ancestor but likely have numerous ancestors, including proto-Rickettsiales, proto-Rhizobiales, and proto-Alphaproteobacteria, as well as current alphaproteobacterial species (Georgiades and Raoult, 2011). Lateral Gene Transfer among intracellular bacteria allows the gene exchange between phylogenetically very different bacteria (Saisongkorh et al., 2010). The representation of the evolutionary pathway as a tree leading to a single common ancestor is incorrect and obsolete. Raoult suggests that the evolution of species looks much more like a rhizome (Raoult, 2010b). The evolutionary history of intracellular bacteria Rickettsia felis and mitochondria from Reclinomonas americana, Homo sapiens, Pediculus humanus, and Saccharomyces cerevisiae were represented in the form of a rhizome (Georgiades and Raoult, 2011; Merhej et al., 2011). It was also affirmed that “the tree of life is not sufficient to explain the chimeric structure of current genomes, and the theory of a single common ancestor and a top-down tree does not reflect our current state of knowledge” (Georgiades and Raoult, 2011). The integration of complex systems (von Bertalanffy, 1950) is an alternative to build a strong theoretical framework more adjust to facts and recent discoveries, since the recent comprehension of genome complexity it is not possible to be explained by a tautology of natural selection and random mutation.
Organisms arise from the integration of complex systems into one another. In these processes viruses play a fundamental role since their sequences are capable of integrating into the genomes in an “infective” way and become an essential part of multicellular organisms. There is evidence that viral sequences in the genome of complex organisms have content with “biological sense” i.e., appear as part of normal life processes, and have a serious role of carrier elements of complex genetic information (Sandín, 1997; Mattick and Gagen, 2001; Vitali et al., 2003; Mallet et al., 2004; Hamilton, 2006; Hunter, 2008; Forterre, 2010). The simultaneous sequence integration in several individuals (i.e., the integration of a complex system within another) changes radically not only the process and the identity of character-creating agent, but also the meaning of this process. These sequences are involved in regulating gene expression or codifying very similar proteins in different animal groups (Medstrand and Mag, 1998; Mi et al., 2000; Villareal and De Filippis, 2000; Jamain et al., 2001). In addition, there are clear differences between the endogenous retroviral populations (ERVs) of reptiles, birds, and mammals (Tristem et al., 1995) and between primate specific (Johnson and Coffin, 1999), which implies specificity in functional sequences.
A comparative study of virome, the viral community associated with human hosts, from cystic fibrosis and non-cystic fibrosis individuals host have revealed that disease and and non-diseased states are defined by metabolism and not by taxonomy. The non-diseased airway virome contains a set of shared core metabolic functions, which deviate strongly in the face of chronic disease (Willner et al., 2009). This represents that integration of viruses goes beyond the genetic record level but also at individual levels with great importance in metabolic processes and the adaptation of the host.
The presence of viral genome in a big percentage in prokaryotes and eukaryotes and their essential roles is a common phenomenon that highlights the great evolutionary importance. For instance, the action and expression of a gene derived from an ERV allows the formation of placenta in mammals (Mallet et al., 2004). The virus and ERVs are implicated in the most of the adaptative mutations in the last 500 millions of years. Retrotransposons have been identified involved in the regulation of genes related to the histocompatibility (McDonald, 1995), with expression in tissues of different tetra1-alphaglobulins human (Kim et al., 1989) as well as in other mammals and invertebrates (Dnig and Lipshitz, 1994).
In bacterial cells, viruses are related to the generation of microcompartiments (Yeates et al., 2007) where they have regulatory and structural functions. Organelles as carboxisoma consist of thousands of protein subunits assembled in a viral-like structure or scaffold (Kerfeld et al., 2005) and genes that codify it are present in both autotrophic and heterotrophic bacteria. They are also found in bacteria considered pathogenic. The insertive nature of virus fits these observations. These findings would not be so “mysterious” if one could think from another perspective. However, the only interest seems to be, again, developing a strategy to fight against bacteria (Yeates et al., 2007). Integrative capacities of the virus added to their great genetic diversity of this extraordinary gene pool (Bruüssow and Hendrix, 2002) (>1030 tailed phages in the biosphere) constitutes an opportunity to strengthen the observation of its role in the evolutionary process. The authors remark that “micro-compartments could have evolved by divergent evolution with bacteria ‘capturing’ a virus and using both its genes and structural features for its own ends.” Under this teleological explanation it seems that selfishness of nucleotide sequences and bacteria (which are sometimes selfish, sometimes exploited, sometimes exploitative) lead them in some remote past to capture and exploit virus. But scientific evidence can ensure that the demonstrated ability of the virus to insert itself into chromosomes (integrating complex systems) is what allowed the structural, morphological change, in this case is the appearance of a carboxisoma. The structural changes that imply evolution and the mechanisms are viral insertion that permits an evolutionary quantum leap.
Microbiome and Hologenome
Microbiome is the collective genome of our indigenous microbes (microbiota). The term also applies as a synonym of microbiota since “biome” refers to “ecosystems” in ecology (Lederberg and McCray, 2001; Dominguez-Bello and Blaser, 2008). Gut microbiome is taxonomically complex, it constitutes an ecologically dynamic community and it influences development, maturation, regulation (stimulation and suppression) of the immune system (Mazmanian et al., 2005; Smits et al., 2005; Hattori and Taylor, 2009; Mai and Draganov, 2009; Kau et al., 2011). Microorganisms have also been implicated in vitamin production, digestion, energy homeostasis, integrity of intestinal barrier, and angiogenesis in the human body (Dominguez-Bello and Blaser, 2008; Kau et al., 2011; Rosenberg and Zilber-Rosenberg, 2011; Slonczewski and Foster, 2011). Works with gnotobiotic mice (also known as germ-free mice, i.e., mice that are born in aseptic conditions and reared in a sterile or microbially controlled laboratory environment) demonstrate that the painstaking separation of a mammal from its associated microbiome results in an underdeveloped immune system, longer digestion times, and lower metabolic rates than those that have been normally colonized (Wostmann, 1981). Alterations of this microbiome could potentially affect human health and promote disease state or disbiosis (Rogler, 2010).
Either by cell number or by genome size the microbiota outnumbers their host. The hologenome theory considers that the holobiont, an organism and all of its associated symbiotic microbes, including parasites, mutualists, synergists, and amensalists as a result of symbiopoiesis, or codevelopment of the host and symbiont (Margulis and Fester, 1991; Rohwer et al., 2009; Gilbert et al., 2010; Rosenberg and Zilber-Rosenberg, 2011). This evolutionary approach that considers any organism as a result of integration with microorganisms has many implications and it is related to the Bioma Depletion Theory (also called “hygiene hypothesis”) that considers that humans (and all mammals) and their microbiome evolved as a “superorganism” (Kinross et al., 2008; Rook, 2009). The immune system can be seen as having evolved as an interface with symbiotic organisms more than as a defense against invading organisms. The widely appreciated medical care in combination with technology, increased the occurence of allergic disorders, autoimmune diseases, and left us an over-reactive immune response caused by a loss and separation of our partners, our microbiome that normally interact with our immune system (Figure 1) (Garn and Renz, 2007; Kau et al., 2011). These partners involve not only the commensal bacteria, but metazoans “parasites” and millions of virus. Bacteria comprising the microbiome have mobile elements that include plasmids, transposons, integrons, bacteriophages (Jones, 2010) that constitute the mobilome (Siefert, 2009). This genetic pool and the HGT within the microbiome is a key factor of the microbiome activity and constitute the dynamic response to the environment leading to the adaptation of the holobiont. It fuels the adaptive potential of the whole holobiont (Figure 1). The metabolism of microbiome and the host are intertwined constituting an integrated organism. In multicellular eukaryotes, transposition, genome reorganizations, retrovirus extrusion, or insertion, etc., must be taking place in the germ line to result in a structural or metabolic change. Somatic cells have an intragenomic dynamics in response to environmental conditions.
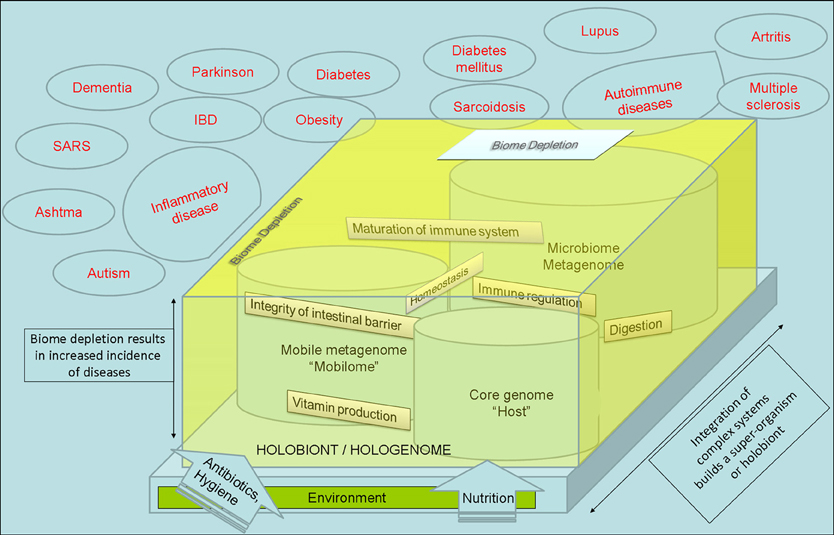
Figure 1. The Integration of Complex Systems considers that any superorganism or holobiont is the result of integration of pre-existing systems. Mobile elements or “mobilome” respond to the environmental factors with dynamic movement between genomes that constitutes a key mechanism for metabolic and structural changes on microbiome. The metabolism of microbiome and the host are intertwined constituting an integrated organism. The medical care, use of antibiotics, technology, and western way of life, resulted in a change and lost of our microbiome and an increased occurence of autoimmune and metabolic diseases that are related with an immune disbalance.
Vannier-Santos and Lenzi (2011) explain that taking into account that organisms identified as “parasites” are almost the 80% of known species and considering that all the theoretical explanation obtained are based in just a little part of the total organisms that exist (Windsor, 1998), we can refer to parasites as cohabitants, since the association drives the evolution and existence of the organisms (Vannier-Santos and Lenzi, 2011). Microbes, helmints, that normally are understood as parasites have cohabited with their host and they are even greater than the host. If nature is a continuous battle, bacteria and parasites should have won a long time ago. Considering that Life exists as a net, as a process (Maturana and Varela, 1999) it is possible to say that no organism is a free-living specie in sensu stricto.
The host and its symbiotic microbiota with its hologenome, acts in cooperation (that becomes cooperation a priority instead of competition) and suggests that it should be considered a unit of selection in evolution (Zilber Rosenberg and Rosenberg, 2008). Even when the authors remark that the theory is in agreement with Darwinism, the hologenome theory represents a holistic approach that considers each specie or organism as a result of an integration and this is a mechanism that is observed at every level of nature: integration of virus, endosymbiotic relationships, and holobionts. This paradigm (like symbiogenesis from Merenchovzky and Margulis) contrasts the observable facts in nature against the individualistic, selfish, and economist conception of Darwinism.
The hologenome theory and these holistic approaches are in agreement with the autopoiesis concept of Maturana and Varela (Varela et al., 1974; Maturana and Varela, 1999; Maturana, 2002) and it could be interpreted as a continuity of the Lynn Margulis endosymbiotic theory (Margulis and Fester, 1991): the existence of each organism is the consequence of integration of pre-existing organisms. The genome of each organism is the result of combination of bacterial, virus, and eukaryotic DNA. Finally, the organism is the result of the interaction of their own genome with the genome of the microbiota (the hologenome), and their metabolism was and are intertwined (as a “superorganism”) along evolution (Zilber Rosenberg and Rosenberg, 2008; Gazla and Carracedo, 2009; Kau et al., 2011; Tilg and Kaser, 2011; Vannier-Santos and Lenzi, 2011) (Figure 1).
Conclusion
We cannot ignore that competition exists, but giving it a creative sense, as an evolutionary engine is an overestimation. Darwin based his theory on economic thoughts of liberal trade put forward by Adam Smith and also the theories of Malthus and Spencer (Weikart, 2009). To this, he added the projection of social and cultural values and worldview of their own time on Nature. Thus, an economic system (and an ideology) was projected on nature. Everything is understood according to cost-benefit and organisms are in a warfare where they are exploiting each other, they produce “weapons,” they have social dilemmas and cooperation is a consequence of a “mafia strategy” (Dawkins, 1976; Nogueira et al., 2009).
Natural selection is a linguistic trap. It has many definitions and nuances along the literature and just adds more confusion to the interpretations of facts. It appears that many biologists seem to be unaware that in their anti-creationism, they have replaced one dogma for another, the dogma of the all-powerful natural selection to which they cling with so much faith.
In order to fit the continuous discoveries innumerable metaphors were created, based mainly on economic relations of society (Ball, 2011). Nowadays, the abuse of “personification” (for example, speaking of selfish genes) and metaphors to explain the components and phenomena of nature are common (Ball, 2011). Many hypotheses, concepts and terms that were purely speculative became unquestioned concepts which were welded to the scientific language. They were used systematically to explain everything. The abuse of terms such as competition between proteins and between genes, the selection pressure, fitness, cost-benefit ratios, arsenal, weapons, war, exploitation, self-serving punishment, coercive strategies, mafia, policing (Boyd, 2006; Cant and Johnstone, 2006; Lehmann and Keller, 2006), the destruction of others, the “problem” of altruism, and many others expressions that attempt to explain the relationships between organisms, denote a continuation of a theory with an important ideological basis and a lot of subjective and moral categorizations. Even when a metaphor clearly based on market could be used to explain a relationship between organisms, it assumes as true that life follows the capitalism rules. They are antropocentric projections of dogmas and social economic models.
The data shows us that integration of complex systems into other complex systems as a result of a property of life: autopoiesis (Varela et al., 1974) is a priority instead of competition as the engine of evolution, stressing the importance of self-organization and symbiosis. Integration is a pattern that it is observed at every level: virus and phages “living” in an intracellular state, where they participate actively in the metabolism and in the plasticity of the genome, bacteria forming complex populations, bacteria living inside eucariotic hosts while existing metabolic and genomic exchanges, bacteria and “parasites” have cohabited for thousands of years with their host/cohabitant and co-evolving constituting an holobiont with deep and complex metabolic intertwined. Integration, partnership, symbiosis, viral insertion, etc., are mechanisms that cause evolutionary steps. A change in the approach and the appraisement of these processes will have no need for twisted excuses to explain the “strange phenomenon” of cooperation.
Viruses and bacteria share the double condition of pathogen and the basic unit of life. They have been fundamental in the origin of complex living beings. Their “negative” aspect would be the result of some factor breaking the natural balance of its activities (release of endogenous virus particles, expression of virulence genes) (Gabus et al., 2001; Kho et al., 2004; Seifarth et al., 2005).
Holistic perspectives are emerging strongly based on experimental data but a stride is still necessary to remove of our biological language, many metaphors and prejudices based on market theories that do not reflect what actually occurs in nature. Gaining a comprehensive understanding of the human being as an organism resulting from the integration of systems and understanding the processes of life within the framework of Systems Theory (von Bertalanffy, 1950) can make a better approach to the pathologies that result from the imbalance of our biome.
The presented new interpretations of different facts and discoveries are just a few examples that could be enumerated, but only a deeper interdisciplinary work can go further in the development of a new perspective on the theoretical foundations of evolutionary theory. Autopoiesis, symbiopoiesis, and evolution of biological systems by integration of complex systems are emergent theories that take into account facts and biological properties instead of economical transactions and are plausible explanations to understand biological diversity and evolutionary process. This could make possible more accurate interpretations of biological processes as well as a new perception and attitude toward nature. It is necessary that biology allow the emergence of other points of view and alternative analysis, otherwise it is a dogmatic discipline of unique thinking and with a great deal of faith.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
To Dr. Máximo Sandín for having read this work.
Footnotes
- ^“I admit … that in the earlier editions of my Origin of Species I probably attributed too much to the action of natural descent of the survival of the fittest.” —Charles Darwin, The Descent of Man, Vol. 1 (1871 1st ed.), p. 152.
References
Ball, P. (2001). Ideas for a new biology. Nature. http://www.fractal.org/Life-Science-Technology/Publications/Ideas-for-a-new-biology.htm. Last access April 18, 2012.
Ball, P. (2011). A metaphor too far. Nature. doi: 10.1038/news.2011.115. http://www.nature.com/news/2011/110223/full/news.2011.115.html. Last access April 18, 2012.
Barton, R. (1998). Huxley, Lubbock, and half a dozen others: professionals and gentlemen in the formation of the X-club, 1851–1864. Isis 89, 410–444.
Bernhard, R. (1967). Heresy in the halls of biology: mathematicians question darwinism. Sci. Res. 2, 59–66.
Bouchard, F., and Rosenberg, A. (2004). Fitness, probability and the principles of natural selection. Br. J. Philos. Sci. 55, 693–712.
Boyer, M., Madoui, M. A., Gimenez, G., La Scola, B., and Raoult, D. (2010). Phylogenetic and phyletic studies of informational genes in genomes highlight existence of a 4 domain of life including giant viruses. PLoS One 5:e15530. doi: 10.1371/journal.pone.0015530
Buchanan, A. V., Sholtis, S., Richtsmeier, J., and Weiss, K. M. (2009). What are genes “for” or where are traits “from”? What is the question? Bioessays 31, 198–208.
Cant, M., and Johnstone, R. (2006). Self-serving punishment and the evolution of cooperation. J. Evol. Biol. 19, 1383–1385.
Casjens, S. (2003). Prophages and bacterial genomics: what have we learned so far? Mol. Microbiol. 49, 277–300.
Cervantes, E. (2011a). Charles Darwin, o el origen de la máquina incapaz de distinguir. Digital CSIC. http://digital.csic.es/handle/10261/35958
Cervantes, E. (2011b). Economía semántica para la manipulación del conocimiento: la palabra Evolución y su uso como trampa en “On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life”. Digital CSIC. http://digital.csic.es/handle/10261/31352
Child, C. M. (1941). Patterns and Problems of Development. Chicago, IL: University of Chicago Press.
Cotter, P. D., Hill, C., and Ross, R. P. (2005). Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3, 777–788.
Darwin, C. (1869). On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life, 5th Edn. London: John Murray.
Davidson, E. H., and Erwin, D. H. (2006). Gene regulatory networks and the evolution of animal body plans. Science 311, 796–800.
Desriac, F., Defer, D., Bourgougnon, N., Brillet, B., Le Chevalier, P., and Fleury, Y. (2010). Bacteriocin as weapons in the marine animal-associated bacteria warfare: Inventory and potential applications as an aquaculture probiotic. Mar. Drugs 8, 1153–1577.
Diep, I. B., and Nes, I. F. (2002). Ribosomally synthesized antibacterial peptides in Gram positive bacteria. Curr. Drug Targets 3, 107–122.
Dnig, D., and Lipshitz, H. D. (1994). Spatially regulated expression of retrovirus-like transposons during Drosophila melanogaster embryogenesis. Genet. Res. 64, 167–181.
Dobzhansky, T. (1973). Nothing in biology makes sense except in the light of evolution. Am. Biol. Teach. 35, 125–129.
Dobzhansky, T., Ayala, F., Stebbins, G., and Valentine, J. (1977). Evolution. San Francisco, CA: W.H. Freeman.
Dominguez-Bello, M., and Blaser, M. (2008). Do you have a probiotic in your future? Microbes Infect. 10, 1072–1076.
Ehrlich, P., and Birch, L. (1967). Evolutionary history and population biology. Nature 214, 349–352.
Fajardo, A., and Martínez, J. L. (2008). Antibiotics as signals that trigger specific bacterial responses. Curr. Opin. Microbiol. 11, 161–167.
Forterre, P. (2010). Giant viruses: conflicts in revisiting the virus concepts. Intervirology 53, 362–378.
Gabus, C., Derrington, E., Leblanc, P., Chnaiderman, J., Dormont, D., Swietnicki, V., Morillas, M., Surewicz, M., Marc, D., Nandi, P., and Darlix, J. (2001). The prion protein has DNA strand transfer properties similar to retroviral nucleocapsid protein. J. Mol. Biol. 307, 1011–1021.
Garn, H., and Renz, H. (2007). Epidemiological and immunological evidence for the hygiene hypothesis. Immunobiology 212, 441–452.
Gazla, I. N., and Carracedo, M. C. (2009). Effect of intracellular Wolbachia on interspecific crosses between Drosophila melanogaster and Drosophila simulans. Genet. Mol. Res. 8, 861–869.
Gerstein, M. (2007). What is a gene, post-ENCODE? History and updated definition. Genome Res. 17, 669–681.
Georgiades, K., and Raoult, D. (2011). The rhizome of Reclinomonas americana, Homo sapiens, Pediculus humanus and Saccharomyces cerevisiae mitochondria. Biol. Direct 6, 55.
Gilbert, S., McDonald, E., Boyle, N., Buttino, N., Gyi, L., Mai, M., Prakash, N., and Robinson, J. (2010). Symbiosis as a source of selectable epigenetic variation: taking the heat for the big guy. Philos. Trans. R. Soc. B Biol. Sci. 365, 671–678.
Gilbert, S. F., Opitz, J. M., and Raffs, R. A. (1996). Resynthesizing evolutionary and developmental biology. Dev. Biol. 173, 357–372.
Gillor, O., Etzion, A., and Riley, M. A. (2008). The dual role of bacteriocins as anti and probiotics. Appl. Microbiol. Biotechnol. 81, 591–606.
Gupta, R. S. (2000). The natural evolutionary relationships among prokaryotes. Crit. Rev. Microbiol. 26, 111–131.
Hattori, M., and Taylor, T. (2009). The human intestinal microbiome: a new frontier of human biology. DNA Res. 16, 1–12.
Hunter, P. (2008). Not so simple after all. A renaissance of research into prokaryotic evolution and cell structure. EMBO Rep. 9, 224–226.
Jamain, S., Girondot, M., Leroy, P., Clergue, M., Quach, H., Fellous, M., and Bourgeron, T. (2001). Transduction of the human gene FAM8A1 by endogenous retrovirus during primate evolution. Genomics 78, 38–45.
Jayaraman, R. (2009). Antibiotic resistance: an overview of mechanisms and a paradigm shift. Curr. Sci. 96, 1475–1484.
Johnson, W., and Coffin, J. (1999). Constructing primate phylogenies from ancient retrovirus sequences. PNAS 96, 10254–10260.
Kampis, G. (1997). “Evolution as its own cause and effect,” in Evolutionary Systems, eds S. Salthe and G. van de Vijver (Kluwer, Dordrecht), 255–266.
Kau, A., Ahern, P., Griffin, N., Goodman, A., and Gordon, J. (2011). Human nutrition, the gut microbiome and the immune system. Nature 474, 327–336.
Kerfeld, C., Sawaya, M., Tanaka, S., Nguyen, C., Phillips, M., Beeby, M., and Yeates, T. (2005). Protein structures forming the shell of primitive bacterial organelles. Science 309, 936–938.
Kho, A., Zhao, Q., Cai, Z., Butte, A., Pomeroy, S., Rowitch, D., and Kohane, I. (2004). Conserved mechanisms across development and tumorigenesis revealed by a mouse development perspective of human cancers. Genes Dev. 18, 629–640.
Kim, J., Yu, C., Bailey, A., Hardison, R., and Shen, C. (1989). Unique sequence organization and erythroid cell-specific nuclear factor-binding of mammalian theta-1 globin promoters. Nucleic Acids Res. 17, 5687–5700.
Kinross, J., von Roon, A., Holmes, E., Darzi, A., and Nicholson, J. (2008). The human gut microbiome: implications for future health care. Curr. Gastroenterol. Rep. 10, 396–403.
Kjos, M., Nes, I. F., and Diep, D. B. (2009). Class II one-peptide bacteriocins target a phylogenetically defined subgroup of mannose phosphotransferase systems on sensitive cells. Microbiology 155, 2949–2961.
Lederberg, J., and McCray, A. (2001). “Ome Sweet” Omics-A Genealogical Treasury of Words. Scientist 15, 8.
Lehmann, L., and Keller, L. (2006). The evolution of cooperation and altruism-a general framework and a classification of models. Evol. Biol. 19, 1365–1376.
Linares, J., Gustafsson, I., Baquero, F., and Martinez, J. (2006). Antibiotics as intermicrobial signaling agents instead of weapons. PNAS 103, 19484–19489.
Mai, V., and Draganov, P. (2009). Recent advances and remaining gaps in our knowledge of associations between gut microbiota and human health. World J. Gastroenterol. 7, 81–85.
Mallet, F., Bouton, O., Prud'homme, S., Cheynet, V., Oriol, G., Bonnaud, B., Lucotte, G., Duret, L., and Mandrand, B. (2004). The endogenous retroviral locus ERVWE1 is a bona fide gene involved in hominoid placental physiology. PNAS 101, 1731–1736.
Margulis, L., and Fester, R. (1991). Symbiosis as a Source of Evolutionary Innovation: Speciation and Morphogenesis. Boston, MA: MIT Press.
Martinez, J., Fajardo, A., Garmendia, L., Hernández, A., Linares, J., Martínez-Solano, L., and Sánchez, M. (2009a). A global view of antibiotic resistance. FEMS Microbiol. Rev. 33, 44–65.
Martinez, J., Sanchez, M., Martinez-Solano, L., Hernandez, A., Garmendia, L., Fajardo, A., Garmendia, L., Fajardo, A., and Alvarez-Ortega, C. (2009b). Functional role of bacterial multidrug efflux pumps in microbial natural ecosystems. FEMS Microbiol. Rev. 33, 430–449.
Mattick, J., and Gagen, M. (2001). The evolution of controlled multitasked gene networks: the role of introns and other noncoding RNAs in the development of complex organisms. Mol. Biol. Evol. 18, 1611–1630.
Maturana, H. (2002). Autopoiesis, structural coupling and cognition: a history of these and other notions in the biology of cognition. Cybern. Hum. Knowing 9, 3–4.
Maturana, H., and Mpodozis, J. (1999). De l'origin des spèces par voie de la dèrive naturelle. Eds. Presses Universitaries de Lyon.
Maturana, H., and Varela, F. (1999). The Tree of Knowledge. The Biological Basis of Human Knowledge (3rd Edn.). Madrid: Debate.
Mazmanian, S., Liu, C., Tzianabos, A., and Kasper, D. (2005). An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122, 107–118.
McDonald, J. (1995). Transposable elements: possible catalysts of organismic evolution. Trends Ecol. Evol. 10, 123–126.
Medstrand, P., and Mag, D. L. (1998). Human-specific integrations of the HERV-K endogenous retrovirus family. J. Virol. 72, 9782–9787.
Merhej, V., Notredame, C., Royer-Carenzi, M., Pontarotti, P., and Raoult, D. (2011). The rhizome of life: the sympatric Rickettsia felis paradigm demonstrates the random transfer of DNA sequences. Mol. Biol. Evol. 28, 3213–3223.
Mi, S., Lee, X., Li, X., Veldman, G., Finnerty, H., Racie, L., Lavallie, E., Tang, X., Edouard, P., Howes, S., Keith, J., and Mccoy, J. (2000). Syncitin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403, 785–789.
Nedelcu, A., Driscoll, W., Durand, P., Herron, M., and Rashidi, A. (2011). On the paradigm of altruistic suicide in the unicellular world. Evolution 65, 3–20.
Nogueira, T., Rankin, D. J., Touchon, M., Taddei, F., Brown, S. P., and Rocha, E. P. (2009). Horizontal gene transfer of the secretome drives the evolution of bacterial cooperation and virulence. Curr. Biol. 20, 1683–1691.
Papagianni, M., and Anastasiadou, S. (2009). Pediocins: the bacteriocins of Pediococci. Sources, production, properties and applications. Microb. Cell Fact. 8, 3.
Perry, J. A., Cvitkovitch, D. G., and Lévesque, C. M. (2009a). Cell death in Streptococcus mutans biofilms: a link between CSP and extracellular DNA. FEMS Microbiol. Lett. 299, 261–266.
Perry, J. A., Jones, M. B., Peterson, S. N., Cvitkovitch, D. G., and Lévesque, C. M. (2009b). Peptide alarmone signalling triggers an auto-active bacteriocin necessary for genetic competence. Mol. Microbiol. 72, 905–917.
Popper, K. (1963). Conjectures and Refutations: The Growth of Scientific Knowledge. Barcelona: Paidós, 1981.
Raoult, D. (2010a). Giant viruses from amoeba in a post-Darwinist viral world. Intervirology 53, 251–253.
Riley, M. A., and Wertz, J. E. (2002). Bacteriocins: evolution, ecology, and application. Annu. Rev. Microbiol. 56, 117–137.
Rogler, G. (2010). The importance of gut microbiota in mediating the effect of NOD2 defects in inflammatory bowel disease. Gut 59, 153–154.
Rohwer, F., Prangishvili, D., and Lindell, D. (2009). Roles of viruses in the environment. Environ. Microbiol. 11, 2771–2774.
Rook, G. (2009). Review series on helminths, immune modulation and the hygiene hypothesis: the broader implications of the hygiene hypothesis. Immunology 126, 3–11.
Rosario, K., Nilsson, C., Lim, Y., Ruan, Y., and Breitbart, M. (2009). Metagenomic analysis of viruses in reclaimed water. Environ. Microbiol. 11, 2806–2820.
Rosenberg, E., and Zilber-Rosenberg, I. (2011). Symbiosis and development: the hologenome concept. Birth Defects Res. C Embryo Today 93, 56–66.
Saisongkorh, W., Robert, C., La Scola, B., Raoult, D., and Rolain, J. M. (2010). Evidence of transfer by conjugation of type IV secretion system genes between Bartonella species and Rhizobium radiobacter in amoeba. PLoS One 13:5. doi: 10.1371/journal.pone.0012666
Schützemberger, M. (1967). “Algorythms and the neo-darwinian theory of evolution,” in Mathematical Challenges to the Neo-Darwinian Interpretation of Evolution, eds P. S. Moorhead and M. M. Kaplan (Filadelfia: Wistar Institute Press), 73–80.
Seifarth, W., Frank, O., Zeilfelder, U., Spiess, B., Greenwood, A. D., Hehlmann, R., and Leib-Mösch, C. (2005). Comprehensive analysis of human endogenous retrovirus transcriptional activity in human tissues with a retrovirus-specific microarray. J. Virol. 79, 341–352.
Siefert, J. (2009). “Defining microbiome,” in Horizontal Gene Transfer Genomes in Flux, eds M. B. Gogarten, J. P. Gogarten, and L. Olendzenski, Vol. 532, (New York, NY: Humana Press), 13–27.
Slonczewski, J., and Foster, J. (2011) Microbiology: An Evolving Science. New York, NY: W. W. Norton and Co.
Smits, H. H., Engering, A., van der Kleij, D., de Jong, E., Schipper, K., van Capel, T., Zaat, B., Yazdanbakhsh, M., Wierenga, E., van Kooyk, Y., and Kapsenberg, M. (2005). Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J. Allergy Clin. Immunol. 115, 1260–1267.
Tilg, H., and Kaser, A. (2011). Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Invest. 121, 2126–2131.
Tristem, M. (2000). Identification and characterization of novel human endogenous retrovirus families by phylogenetic screening of the human genome mapping project database. J. Virol. 74, 3715–3730.
Tristem, M., Myles, T., and Hill, T. (1995). A highly divergent retroviral sequence in the tuatara (Sphenodon). Virology 210, 1.
Tsui, W., Yim, G., Wang, H., McClure, J., Surette, M., and Davies, J. (2004). Dual effects of MLS antibiotics: transcriptional modulation and interactions on the ribosome. Chem. Biol. 11, 1307–1316.
Vannier-Santos, M., and Lenzi, H. (2011). Parasites or cohabitants: cruel omnipresent usurpers or creative “éminences grises”? J. Parasitol. Res. doi: 10.1155/2011/214174. [Epub ahead of print].
Varela, F., Maturana, H., and Uribe, R. (1974). Autopoiesis: the organization of living systems, its characterization and a model. Biosystems 5, 187–196.
Villareal, L., and De Filippis, V. (2000). A hypothesisi for DNA viruses as the origin of eukaryotic replication proteins. J. Virol. 74, 7079–7084.
Vitali, P., Royo, H., Seitz, H., Bachellerie, J. P., Hüttenhofer, A., and Cavaillé, J. (2003). Identification of 13 novel human modification guide RNAs. Nucleic Acids Res. 31, 6543–6551.
Weikart, R. (2009). Was Darwin or Spencer the father of laissez-faire social darwinism? J. Econ. Behav. Organ. 71, 20–28.
Wilkins, J. S., and Nelson, G. J. (2008). Trémaux on species: a theory of allopatric speciation (and punctuated equilibrium) before Wagner. Hist. Philos. Life Sci. 30, 179–206.
Willner, D., Furlan, M., Haynes, M., Schmieder, R., Angly, F. E., Silva, J., Tammadoni, S., Nosrat, B., Conrad, D., and Rohwer, F. (2009). Metagenomic analysis of respiratory tract DNA viral communities in cystic fibrosis and non-cystic fibrosis individuals. PLoS One 4:e7370. doi: 10.1371/journal.pone.0007370
Wright, B. E. (2000). A biochemical mechanism for nonrandom mutations and evolution. J. Bacteriol. 182, 2993–3001.
Yeates, T., Tsai, Y., Tanaka, S., Sawaya, M., and Kerfeld, C. (2007). Self-assembly in the carboxysome: a viral capsid-like protein shell in bacterial cells. Biochem. Soc. Trans. 35, 508–511.
Yim, G., de la Cruz, F., Spiegelman, G., and Davies, J. (2006a). Transcription modulation of Salmonella enterica serovar Typhimurium promoters by sub-MIC levels of rifampin. J. Bacteriol. 188, 7988–7891.
Yim, G., Wang, H., and Davies, J. (2006b). The truth about antibiotics. Int. J. Med. Microbiol. 296, 163–170.
Keywords: Darwinism, natural selection, evolution, paradigm, virus, hologenome, autopoiesis
Citation: Salvucci E (2012) Selfishness, warfare, and economics; or integration, cooperation, and biology. Front. Cell. Inf. Microbio. 2:54. doi: 10.3389/fcimb.2012.00054
Received: 10 October 2011; Accepted: 06 April 2012;
Published online: 01 May 2012.
Edited by:
Didier Raoult, Université de la Méditerranée, FranceReviewed by:
Kalliopi Georgiades, Unité de Recherche en maladies infectieuses tropicales emergentes, FranceJean-Marc Rolain, Centre National de la Recherche Scientifique, France
Fernando Baquero, Ramón y Cajal Institute for Health Research, Spain
Copyright: © 2012 Salvucci. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Emiliano Salvucci, Consejo Nacional de Investigaciones Cientificas y Técnicas, Argentina. e-mail:c2FsdnVjY2kuZW1pbGlhbm9AZ21haWwuY29t
