- Department of Molecular Cell Biology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Suwon, South Korea
Antimicrobial resistance (AMR) in pathogens is the result of indiscriminate use of antibiotics and consequent metabolic/genetic modulation to evolve survival strategies and clonal-selection in AMR strains. As an alternative to antibiotic treatment, antivirulence strategies are being developed, not only to combat bacterial pathogenesis, but also to avoid emerging antibiotic resistance. Vibrio vulnificus is a foodborne pathogen that causes gastroenteritis, necrotizing wound infections, and sepsis with a high rate of mortality. Here, we developed an inhibitor-screening reporter platform to target HlyU, a master transcriptional regulator of virulence factors in V. vulnificus by assessing rtxA1 transcription under its control. The inhibitor-screening platform includes wild type and ΔhlyU mutant strains of V. vulnificus harboring the reporter construct PrtxA1::luxCDABE for desired luminescence signal detection and control background luminescence, respectively. Using the inhibitor-screening platform, we identified a small molecule, fursultiamine hydrochloride (FTH), that inhibits the transcription of the highly invasive repeat-in-toxin (rtxA1) and hemolysin (vvhA) along with other HlyU regulated virulence genes. FTH has no cytotoxic effects on either host cells or pathogen at the tested concentrations. FTH rescues host cells from the necrotic cell-death induced by RtxA1 and decreases the hemolytic activity under in vitro conditions. The most important point is that FTH treatment does not induce the antivirulence resistance. Current study validated the antivirulence strategy targeting the HlyU virulence transcription factor and toxin-network of V. vulnificus and demonstrated that FTH, exhibits a potential to inhibit the pathogenesis of deadly, opportunistic human pathogen, V. vulnificus without inducing AMR.
Introduction
Vibrio vulnificus is an opportunistic, Gram-negative, halophilic pathogen (Starks et al., 2000). It causes necrotizing wound infections, cellulitis, gastroenteritis, and devastating septicemia, with mortality rates up to 50%, especially in immunocompromised subjects (Linkous and Oliver, 1999; Jones and Oliver, 2009), which is one of the highest among foodborne diseases (Mead et al., 1999). V. vulnificus easily evades the host innate immune system to quickly propagate in the bloodstream, which causes death within a few days of infection. This fast rate of disease progression is attributed to the presence of capsular polysaccharide and repeat-in-toxin (RtxA1), which are both suggested to be inhibitory factors of phagocytosis (Tamplin et al., 1985; Lo et al., 2011). The source of V. vulnificus infection is usually raw and undercooked seafood (Gulig et al., 2005). Third or fourth generation cephalosporins, tetracycline (Chiang and Chuang, 2003; Lee et al., 2014), and quinolones can be used to kill V. vulnificus during infection (Tang et al., 2002; Wong et al., 2015). However, recently, emergence of antimicrobial resistance (AMR) in various Vibrio species, including V. parahaemolyticus and V. vulnificus, has been reported in several countries (Baker-Austin et al., 2009; Elmahdi et al., 2016; Siboni et al., 2016; Baker-Austin and Oliver, 2018). Thus, V. vulnificus, an antimicrobial resistant, deadly, opportunistic human pathogen is prevalent in a spatiotemporal manner in estuaries and is considered to be an environmental and clinical burden, posing a major health concern among the foodborne infectious diseases (Siboni et al., 2016; Heng et al., 2017).
AMR in general, is the mixed response of a pathogen's offensive and defensive survival strategies against the stress imposed by antimicrobial agents. Consequently, AMR strains continue to emerge through the clonal selection of mutationally altered variants during antibiotic treatment (Blair et al., 2015). Moreover, antibiotic treatment kills bacterial pathogens in the bloodstream, leading to the release of cytotoxins, and lipopolysaccharide (LPS) (Jackson and Kropp, 1992; Prins et al., 1994). This in turn can cause a hyper-immune response, toxic anaphylactic shock, and fatality for patients (Opal, 2010). Therefore, virulence specific therapeutics are being evolved as an alternative approach to circumvent both AMR and pathogenesis. Antivirulence therapeutic strategy may prove advantageous because (a) it may not induce a pathogen to develop AMR (b) being non-antibiotic in nature, it may not disturb the native gut-microbiota, and (c) it acts solely by inhibiting virulence factors without threatening general physiology and survival of pathogen (Cegelski et al., 2008).
In vivo-induced antigen technology (IVIAT) in V. vulnificus has shown that virulence regulator HlyU is preferentially induced during infection conditions (Kim et al., 2003). Among the virulence factors contributing toward V. vulnificus pathogenesis, toxins are considered to be major players in the progression of pathogenesis and evasion of the host innate immune system (Lee et al., 2004, 2008a,b; Kim et al., 2008; Liu and Crosa, 2012; Letchumanan et al., 2017). HlyU is a master transcriptional regulator of virulence in V. vulnificus, that has evolved from the ArsR/SmtB family (Busenlehner et al., 2003; Saha and Chakrabarti, 2006). It positively regulates the expression of the major pore-forming toxins (PFT) in V. vulnificus, RtxA1, and hemolysin (VvhA) (Liu and Crosa, 2012). HlyU regulates the transcription of rtxA1 by direct binding to the upstream regulatory region of rtxA1 promoter (Liu et al., 2009, 2011; Liu and Crosa, 2012). RtxA1-deficient strains are defective in host infection and increase the LD50 in mouse models by 100-fold (Kim et al., 2008; Shao et al., 2011). Microarray analysis revealed that hlyU positively regulates the rtxA1 gene expression (Liu et al., 2007). HlyU has been reported to bind to the AT-rich upstream regulatory region at −417 to −376 bp of the rtx operon transcription start site, which codes for the major pore-forming toxin (PFT), RtxA1 during V. vulnificus infection (Liu et al., 2009, 2011; Liu and Crosa, 2012). Another key gene in the cytotoxin regulatory circuit is hns, which codes for a global repressor, H-NS. H-NS binds to five sites in the upstream regulatory region of rtxA1 promoter (PrtxA1) and forms a bridge within DNA. As a result, it obstructs the movement or exclude the entry of RNA polymerase leading to the repression of rtxA1 (Liu et al., 2009; Liu and Crosa, 2012) under non-pathogenic or free-living conditions. HlyU acts as anti-repressor of H-NS and binds to PrtxA1 with higher affinity than H-NS; thereby, de-repressing rtxA1 gene expression (Liu et al., 2009). The expression of hlyU is regulated by quorum sensing master regulator, SmcR, a LuxR homolog of V. harveyi (Shao et al., 2011; Liu and Crosa, 2012). Therefore, identification of small molecules to inhibit the HlyU-controlled expression of virulence factors during host-pathogen interactions appears to be a robust strategy to tackle V. vulnificus virulence. In a previous study, a small molecule, resveratrol was identified using a host cell viability assay. Resveratrol inhibits the expression of rtxA1 gene, whereas the cognate upstream regulator (HlyU) and VvhA were not found to be inhibited (Kim et al., 2010). To the best of our knowledge, there are no reports identifying a small molecule inhibitor targeting HlyU transcription factor and its cytotoxins to inhibit V. vulnificus virulence.
In this study, we targeted HlyU-regulated virulence factors by employing an antivirulence approach. We designed an inhibitor-screening platform composed of wild type and a ΔhlyU deletion mutant of V. vulnificus harboring luxCDABE under the PrtxA1 promoter. By screening 1840 small molecules comprising natural compounds and the FDA-approved Prestwick Library, we identified a non-toxic small molecule, f ursultiamine hydrochloride (FTH). We showed that FTH inhibits HlyU-regulated toxin genes (rtxA1 and vvhA) at the transcriptional level without affecting the expression of hns, which acts as repressor of rtxA1 (Liu et al., 2009). FTH does not inhibit the transcription of hlyU, which we tested using qRT-PCR and a transcriptional fusion of PhlyU_hlyU-luxCDABE. The luxCDABE reporter-gene tag and its comparative expression under HlyU-regulated PrtxA1 and the non-specific synthetic PEM7 promoter in wild type cells and a ΔhlyU mutant revalidated the specificity of the reporter system targeting HlyU. Furthermore, treatment of FTH in wild type V. vulnificus harboring PrtxA1 or the synthetic PEM7 promoter showed a specific inhibition of luminescence with the PrtxA1 promoter, but not with the PEM7 construct, demonstrating that FTH specifically targets native chromosomal HlyU under ambient bacterial growth conditions. Being a thiamine derivative, FTH neither affects bacterial viability at the tested concentrations nor poses any toxicity to host cells under in vitro conditions. FTH also rescues HeLa cells from cytoskeleton destabilization and subsequent necrotic cell death induced by RtxA1 under in vitro conditions. The current study demonstrates that FTH can effectively inhibit the HlyU-regulated virulence factors RtxA1, VvhA, and plpA2 at the transcriptional level, and thus significantly reduced the virulence of V. vulnificus by disarming its powerful pore-forming toxins and its ability to kill host cells.
Material and Methods
Bacterial Strains and Cell Culture Conditions
The strains and plasmids used in this study are listed in Table S1. Vibrio vulnificus MO6-24/O wild type (Wright et al., 1990) (hereafter termed as WT V. vulnificus), deletion mutants ΔhlyU (ZW141) and ΔrtxA1 (MW064) of V. vulnificus MO6-24/O were used in this study (Lee et al., 2007; Jang et al., 2017). V. vulnificus was revived on a Vibrio sp. selective medium, Thiosulfate citrate bile salts sucrose—Oxoid (TCBS) agar plate. A single colony from TCBS-agar plate was inoculated in Luria-Bertani medium supplemented with 2% sodium chloride (LBS) broth and incubated at 37°C under orbital shaking culture conditions. The growth of V. vulnificus was measured by optical density at 600 nm (OD600) and enumerated by determining the bacterial colony forming units (CFU) on LBS-agar (1.5% agar) plate. The antibiotic concentration used for the selection and/or growth of recombinant and mutant strains was 2 μg/ml and 300 μg/ml for chloramphenicol (Cm) and kanamycin (Km), respectively. E. coli DH5α was grown in LB medium (broth/agar) with the appropriate concentrations of antibiotics (ampicillin, Amp: 100 μg/ml and chloramphenicol, Cm: 33 μg/ml) to select recombinant strains harboring plasmids with corresponding antibiotic resistance genes. Unless stated otherwise, HeLa cells were cultured at 37°C with 5% CO2 in Dulbecco's Modified Eagle's Medium (DMEM) with high glucose, containing 10% fetal bovine serum (FBS) for routine culturing.
Construction of Inhibitor-Screening Reporter Strain, Non-specific Promoter-Driven Luminescent Strain, and HlyU::luxCDABE Transcriptional Fusion Strain in V. vulnificus
The promoter-less empty backbone plasmid pBBRMCS2::luxCDABE (Lenz et al., 2004) was used to construct the reporter strain and other genetically engineered strains. The backbone plasmid, pBBRMCS2::luxCDABE was kindly provided by Prof. Sang Ho Choi (Seoul National University, Korea). V. vulnificus genomic DNA was extracted using G-Spin genomic DNA extraction kit (Intron Biotech, Korea). High-fidelity Taq DNA polymerase was used for PCR amplification. The restriction endonuclease enzymes were purchased from New England Biolab (NEB, USA). Plasmid extraction (Intron Biotech, Korea) and gel purification (Cosmogenetech LaboPass Gel Extraction kit, Korea) were performed as per the manufacturers' protocols. All recombinant DNA techniques were performed according to the Standard Laboratory Manual (Sambrook et al., 1989). Briefly, the 754 bp promoter region of rtxA1 (PrtxA1) in V. vulnificus was PCR-amplified from genomic DNA using specific primers (Table S2). The gel-purified PrtxA1 PCR product and pBBRMC2::luxCDABE plasmid were restriction digested with SacI and SpeI, and ligated and cloned at the same sites of the promoter-less pBBRMC2::luxCDABE vector to achieve the pBBRMC2_PrtxA1::luxCDABE reporter plasmid.
To make a transcriptional fusion of hlyU with luxCDABE, the hlyU ORF along with its 128 bp native promoter was amplified using specific primer pairs (Table S2). The resulting 425 bp PCR product and the vector pBBRMCS2::luxCDABE were restriction digested with SacI and BamHI. The restriction-digested vector and inserts were gel-purified and ligated at the same sites of the promoter-less pBBRMCS2::luxCDABE plasmid to generate the transcriptional fusion plasmid pBBRMCS2_PhlyU_hlyU-luxCDABE. A 150 bp DNA fragment containing the 47 bp PEM7 synthetic promoter was amplified from pGEN-luxCDABE (Lane et al., 2007) using the specific primer pairs shown in Table S2. The PEM7 DNA fragment and the pBBRMCS2::luxCDABE vector were restriction digested with SacI and BamHI. The 150 bp DNA fragment containing the PEM7 promoter possessed an internal BamH1 site, resulting in an 89 bp digested PEM7 fragment, containing the complete synthetic promoter. The SacI-BamHI digested vector and the 89 bp PEM7 promoter fragment were ligated and cloned directionally to obtain the pBBRMCS2_PEM7::luxCDABE plasmid.
The putative clones obtained were confirmed by plasmid isolation, followed by restriction digestion and analytical agarose gel electrophoresis. Both strands of all cloned DNA fragments were sequenced by the di-deoxy method, and the accuracy of sequences was ascertained by BLAST and peak analysis of nucleotides. The recombinant plasmids and the empty vector backbone (pBBRMCS2::luxCDABE) were electroporated into WT and hlyU deletion mutant, ΔhlyU (Table S1) of V. vulnificus as described elsewhere (Klevanskaa et al., 2014), with slight modification. Briefly, 50 ml cultures were grown from single colonies picked from TCBS agar. The cells were allowed to grow up to OD600 0.8 equivalent cells. Cells were harvested by centrifugation at 4000 rpm for 15 min at 4°C. All the steps, buffers, media, and cuvettes during the electrocompetent cell preparation were maintained at 4°C or on ice. The cell pellet was washed with 50 ml cold 1 mM Tris-Cl buffer (pH 6.0) supplemented with 200 mM sucrose. After washing, the cells were resuspended in 1000 μl cold GYT medium (10% glycerol, 0.125% w/v yeast extract, and 0.25% w/v tryptone). An aliquot of 100 μl of electrocompetent cells was added to a chilled electroporation cuvette with a 0.1 cm electrode gap. Approximately 700 ng of plasmid DNA was added to 100 μl of electrocompetent cells just before the electroporation pulse. The cells were electroporated using the BIORAD Gene Pulser X-cell instrument at 750 V, 25 μF capacitance, and 200 Ω resistance. The electroporated cells were recovered in 900 μl LB for 1 h and plated on LBS-plates supplemented with 2 μg/ml Cm. By this approach, V. vulnificus WT strain harboring pBBRMC2_PrtxA1::luxCDABE (hereafter termed as WT reporter strain) under the regulation of native chromosomal hlyU was created for screening the chemical libraries. The V. vulnificus ΔhlyU reporter strain harboring pBBRMC2_PrtxA1::luxCDABE was used as a control to assess the background signal.
Screening of Chemical Libraries
Overnight cultures of WT and ΔhlyU (control) reporter strains were sub-cultured with 1% inoculum and supplemented with 2 μg/ml chloramphenicol in LBS for primary chemical screening. A total number of 1,840 small molecules (consisting of 800 natural product compounds and 1,040 Food and Drug Administration [FDA]-approved chemicals from the Prestwick Library) were screened. The final concentration of tested chemicals for screening was 20 μM. The luminescence and bacterial growth (OD600) of samples were recorded in multi-well plates after 6 h using a microplate reader (Tecan Infinite M200, Switzerland). The primary hits were identified on the basis of reduction in the luminescence signal per unit OD600 relative to the untreated or vehicle (DMSO 0.2% v/v)-treated control. The hits were re-checked in a concentration-dependent manner, and were sorted for further analysis. The selected chemicals were purchased from Prestwick Chemicals, and stocks of 10 mM or 100 mM were prepared in DMSO or water.
Quantitative Real-Time PCR
Overnight cultures from freshly streaked single colonies of WT or ΔhlyU V. vulnificus were diluted to 2 × 106 CFU/ml in 3 ml fresh LBS and incubated at 37°C, 220 rpm in 14 ml polypropylene culture tubes. WT V. vulnificus was treated with the small molecule inhibitors or with DMSO (as a control) and was grown up to OD600 ~1.8–2.0. RNA isolation from V. vulnificus reporter strains was carried out with the similar bacterial cells input and incubated for 9 h in 48-well plates with or without FTH. The treated cells were harvested by centrifugation at 5000 × g for 5 min. The cell pellets devoid of supernatant were immediately treated with RNA-Protect reagent (QIAGEN). Total RNA was isolated using the QIAGEN RNeasy Mini kit as per the manufacturer's instructions. A total of 1 μg of RNA from both the control and treated samples was subjected to DNase I treatment (amplification grade; Sigma) for 15 min at room temperature. The reaction was stopped by adding stop buffer and DNaseI was heat inactivated by incubating the samples at 70°C for 10 min. First-strand cDNA synthesis was performed using the Ecodry Premix (random hexamer) kit (Takara Bio, USA). DNase I-treated RNA samples were subjected to target gene PCR amplification to check for genomic DNA contamination. The primers (Table S2) used for quantitative RT-PCR (qRT-PCR) ranged from 19 to 21 bp in length with annealing temperatures of 55 ± 2°C, and the amplified product size of 200 bp. Each qRT-PCR reaction consisted of 300 nM forward and reverse primers, 100 ng cDNA, and 1X Taq universal SYBR Green supermix (Bio-Rad) containing dNTPs and Taq polymerase with its buffer. Expression of the rtxA1, vvhA, hlyU, and hns genes was normalized with the endogenous gyrB gene (Table S2). Relative gene expression was analyzed using the 2−ΔΔCT method (Schmittgen and Livak, 2008). Three independent qRT-PCR experiments were performed for the analysis. Statistical significance was calculated by student's t-test (p < 0.05).
Host Cell Viability Assay
A host cell viability assay as a measure of cell proliferation was performed to evaluate the cytotoxicity of the FTH small molecule using the EZ Cytox cell viability assay kit (DoGen, Korea). HeLa cells were seeded at a density of 2 × 104 cells per well in 100 μl of culture medium using 96-well plates. After 24 h of incubation, FTH was added at concentrations ranging from 1 to 2048 μM at two-fold increments. After 48 h of drug incubation, EZ Cytox kit solution was added to each well and plates were incubated for 2 h. The absorbance of dye was measured spectrophotometrically at A450 using a multi-plate reader (Tecan Infinite M200, Switzerland). The mammalian cell culture media was used as blank. The IC50 was calculated using GraphPad Prism 6.01. The data were plotted, transformed to log values, and then fitted onto a non-linear curve after normalization. FTH untreated cells were considered to be 100% viable.
Hemolysis Assay
Overnight cultures of WT V. vulnificus were inoculated in fresh LBS with or without FTH inhibitor (10–60 μM), and incubated for 3 h wherein ΔhlyU served as a positive control of hemolysis inhibition. The cell free culture supernatants were withdrawn and mixed with 1% human red blood cells (hRBCs) and were incubated at 37°C for 1 h under shaking culture conditions. The cell debris was removed by centrifugation at 1,500 rpm (0.2 × g). The hemolysis was visualized using absorption spectra (500–650 nm) with 1:1 dilution of supernatant to hRBCs. To calculate the hemolytic unit, the culture supernatants were diluted by 1/2, 1/4, and 1/8 with PBS and the remaining method was followed as described above. The hemolytic unit was expressed as the reciprocal of the dilution factor showing 50% hemolysis absorbance at 540 nm (A540) as described by Lee et al. (2013).
Cytoskeleton Staining and Cellular Phenotype
HeLa cells (3 × 104) were cultured in 20 mm glass-bottom cell culture confocal discs for 48 h. The cells were replenished with fresh DMEM media without FBS before starting the experiment. HeLa cells were infected with 20 moi of WT V. vulnificus with or without the simultaneous addition of FTH (100 and 200 μM) and incubated for 1 h. HeLa cells were infected with 20 moi of ΔhlyU and ΔrtxA1 strains of V. vulnificus (Table S1) as controls. Following incubation, the cells were washed with PBS, fixed with 4% paraformaldehyde (in PBS), and permeabilized with 0.1% Triton X-100 (in PBS). The cells were stained with 1 unit of rhodamine phalloidin (Invitrogen, USA) for 1 h in the dark (Kim et al., 2008). The cells were then washed with PBS and counterstained with 4′,6′-diamino-2-phenylindole (DAPI) for 5 min, followed by thorough PBS washing. The stained cells were imaged at 400 × magnification using a laser scanning confocal microscope (LSM 710, Zeiss). The frequency of cytoskeleton-destabilized rounded cells was obtained by counting 1000 cells in various microscopic fields of each sample. A control experiment was performed to assess the effect of FTH on WT V. vulnificus in DMEM medium. The LBS grown log phase culture of WT V. vulnificus cells was washed with PBS and 106 equivalent bacterial cells were adjusted by measuring the optical density. The washed cells (106) were incubated with 0, 100, and 200 μM FTH for an hour in DMEM medium. After incubation, the cells were harvested, washed with PBS, diluted, and plated on LBS agar plates to enumerate the CFU.
Assessment of Laboratory-Induced Adaptive Evolution of Antimicrobial Resistance vs. Antivirulence Resistance
Fluoroquinolone antibiotics are one of the current successful therapeutic options for treatment of V. vulnificus infection. Norfloxacin was taken as a representative of the flouroquinolone antibiotics and compared with the antivirulent FTH for the adaptive evolution of their corresponding resistance against WT V. vulnificus. Norfloxacin was tested at the concentration of 0.155 μg/ml while FTH was tested at two different concentrations (40 and 60 μM), to evaluate antivirulence resistance. The norfloxacin concentration was selected by taking the average of a range of MIC90 values (0.063–0.25 μg/ml) for various V. vulnificus strains, as reported elsewhere (Morris et al., 1985). WT V. vulnificus culture was grown in LBS overnight and 1% inoculum was used for norfloxacin or FTH exposure and subsequent transfers for around 2 weeks. The treated and untreated V. vulnificus cultures were continuously transferred to observe the adaptive evolution of antimicrobial and antivirulence resistance in response to sustained antibiotic or antivirulence chemical pressure on WT V. vulnificus. In the case of norfloxacin-treated samples, the total input inoculum (1%) was centrifuged and resuspended in a new tube containing fresh LBS supplemented with 0.155 μg/ml norfloxacin. In the case of untreated or FTH-treated samples, 1% of 18 h grown culture was sub-cultured for next transfer. The sub-culturing and transfers were continued until bacterial growth was visible in norfloxacin treated culture. The MIC assay was performed according to CLSI guidelines (2012) by the microbroth dilution method in Mueller Hinton Broth (MHB) (Wiegand et al., 2008).
Results
HlyU-Regulated Transcription of Virulence Factors and the Construction of Inhibitor-Screening Reporter
HlyU acts as master virulence transcription factor controlling an array of virulence genes including highly invasive toxins (Liu et al., 2007). Therefore, it is conceivable that HlyU is an important target for antivirulence therapeutics. An overview of the HlyU upstream and downstream regulatory cascades are depicted in (Figure 1I). Based on the hlyU regulatory cascades (Figure 1I), the scorable inhibitor-screening reporter platform was developed in V. vulnificus to identify inhibitors of the HlyU-regulated transcriptional network (Figure 1II). The reporter plasmid pBBRMC2_PrtxA1::luxCDABE was introduced into WT and ΔhlyU V. vulnificus strains (Figure 1II, Table S1). The reporter system was validated by measuring its luminescence at different time points, and the WT reporter strain showed maximum luminescence at 6 h of incubation in microtitre plates when inoculated with 1% overnight-grown culture (Figure S1). The ΔhlyU reporter strain luminescence was one-third of the WT reporter at the same time point. The ΔhlyU reporter strain therefore served as a background control and facilitated in identifying potential hits during the primary inhibitor screening (Figure 1II).
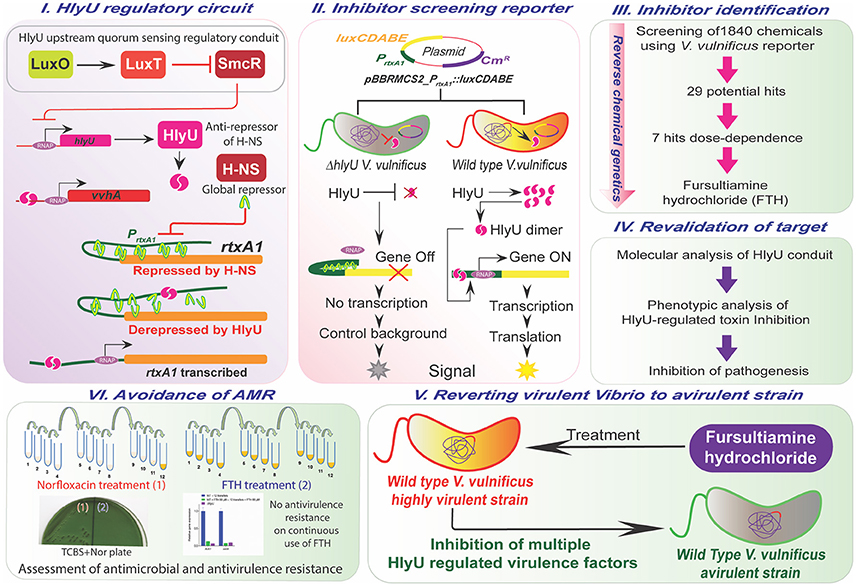
Figure 1. Overview of the study. The upstream and downstream regulatory network of HlyU (Panel I), the design of the reporter platform (Panel II), the workflow for the identification, validation, and assessment of antivirulence characteristics of small molecule inhibitor, FTH targeting HlyU in V. vulnificus (Panels III–V), and adaptive evolution demonstrating the avoidance of antimicrobial and antivirulence resistance development during antivirulence approach (Panel VI).
Screening of Chemicals for Identifying Inhibitor Hits
The primary screening was performed using the chemicals in the libraries at 20 μM concentration in the WT inhibitor-screening reporter strain (Figure 1III). The initial hits were grouped into three categories based on percent inhibition of WT reporter luminescence per unit OD600. Chemicals showing less than 30% inhibition were considered “mild antivirulence hits,” while chemicals showing more than 30% inhibition were categorized as “potential antivirulence hits” (29 chemicals) and were selected for further scrutiny. Chemicals showing 100% inhibition of luminescence signal due to cessation of bacterial growth were not the focus of this study (Figure 2A). Further evaluation of “potential antivirulence hits” based on the dose-dependent decrease in luminescence per unit OD600 narrowed the focus to seven molecules, namely zuclopenthixol hydrochloride (ZH), benzamil hydrochloride (BH), omeprazole (OME), fursultiamine hydrochloride (FTH), vatalanib (VAT), GBR 12909 dihydrochloride (GBR), and mebhydroline 1,5-naphtalenedisulfonate (MND). These chemicals showed a concentration-dependent inhibition of the luminescence signal in the WT reporter strain (Figure 2B). These seven chemicals were then subjected to careful examination regarding their effects on WT V. vulnificus growth pattern. The selected chemical treatments were compared with the DMSO placebo treatment in WT V. vulnificus, and growth patterns were monitored at OD600 every hour for 8 h. VAT, GBR, and MND were excluded because they affected V. vulnificus growth profile in a dose-dependent manner (data not shown). The remaining four chemicals were assessed for their effects on rtxA1 mRNA expression levels in WT V. vulnificus at 20 μM concentration that was used for primary screening. RtxA1 gene expression test was considered the first key step toward finding a true inhibitor, since the reporter system was designed with the promoter of rtxA1, which is directly regulated by HlyU. The molecular analysis for relative rtxA1 gene expression showed that only FTH significantly decreased rtxA1 transcription (i.e., by at least 1.5-fold) when compared to the corresponding DMSO-treated control. The rtxA1 mRNA level was negligible in ΔhlyU, which confirmed that HlyU is the key regulator of the major cytotoxin RtxA1 in V. vulnificus (Figure 2C). Moreover, the varying concentrations of FTH (20–60 μM and 100–200 μM) did not inhibit the growth, as also represented by the CFU counts of WT V. vulnificus (Figures S2, S3). One of the most important parameters for evaluating a potent candidate as a therapeutic agent is its toxicity to host cell or safety. FTH cytotoxicity was therefore evaluated using HeLa cells. FTH showed negligible toxicity toward the host cells since the IC50 value of FTH was found to be 893.1 μM (Figure 2D). The FTH concentration tested against V. vulnificus is 60–200 μM which is significantly lower than the IC50 concentration for the host cells. Therefore, FTH appeared to have the properties suitable for being developed as an antivirulence agent because it did not pose any toxicity toward host or pathogen in the range of concentrations used to inhibit the major cytotoxins RtxA1 and VvhA. FTH is the synthetic counterpart and active form of allithiamine, which occurs naturally in garlic. The disulfide bond in FTH is considered to be essential for its biological activity in treating thiamine deficiency (Lonsdale, 2004) (Figure 2E).
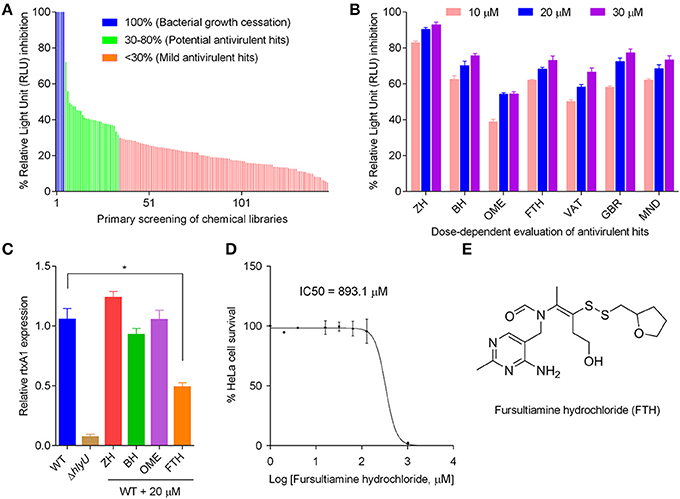
Figure 2. Steps to identify the antivirulence agent fursultiamine hydrochloride (FTH). (A) Primary screening of small molecule libraries (1,840 chemicals at 20 μM) using V. vulnificus reporter. A total of 150 chemicals were shown as representative for inhibition of relative light units (RLU) per unit optical density (OD600) after 6 h of incubation. The RLU/OD600 ratio and the percent inhibition of normalized luminescence signal allowed subdivision of chemicals into four groups. Complete (100%) RLU inhibition was observed with chemicals that allowed no or minimal growth of bacteria (“bacterial growth cessation”), and these were excluded from further analysis. The group showing more than 30% RLU inhibition is labeled “potential antivirulence hits.” (B) Dose-dependent response of selected chemicals from the group labeled “potential antivirulence hits” using V. vulnificus reporter. ZH, zuclopenthixol hydrochloride; BH, benzamil hydrochloride; OME, omeprazole; FTH, fursultiamine hydrochloride; VAT, vatalanib; GBR, GBR 12909 dihydrochloride; and MND, mebhydroline 1,5-naphtalenedisulfonate. (C) Quantitative RT-PCR of rtxA1 gene of WT V. vulnificus following the treatment with ZH, BH, OME, and FTH (20 μM), which were selected because these chemicals did not inhibit V. vulnificus growth. (D) HeLa cell proliferation as a measure of the in vitro cytotoxicity of FTH using EZ-Cytox cell viability kit. A450 values were measured after 48 h, and IC50 values were calculated with GraphPad prism 6.01. (E) Structure of fursultiamine hydrochloride.
Verification of the Transcriptional Conduits of HlyU by FTH
Although the rationale for the design and development of the reporter strain was based on known transcriptional conduits of HlyU (Figures 1I,II), it has not yet been confirmed whether FTH works by targeting HlyU transcriptional regulator. Therefore, the effect of FTH on the transcription of rtxA1 and other known genes involved in its regulatory cascade such as vvhA and hns (Figure 1) were tested using qRT-PCR (Figure 3). As expected, FTH inhibited the transcription of rtxA1 and vvhA genes in a dose-dependent manner (Figures 3A,B). HlyU regulates another virulence determinant phospholipase A2 (plpA2) in V. vulnificus which has been recently reported (Jang et al., 2017). The phospholipase A2 is essential for host cell lysis and necrotic epithelial cell death. FTH significantly (P < 0.05) decreased the plpA2 gene expression in V. vulnificus (Figure S4). The result indicates that FTH targets HlyU function and thereby repress the various virulence determining genes along with toxin encoding genes network.

Figure 3. Concentration-dependent expression of genes in the HlyU regulatory network in response to FTH treatment of wild type V. vulnificus. The relative expression of genes in response to varying concentrations of FTH is depicted as follows: (A) rtxA1, encoding the RtxA1 toxin; (B) vvhA, encoding hemolysin; (C) hlyU, encoding HlyU transcriptional regulator, and (D) hns, encoding a global repressor. Respective DMSO controls showed no difference in gene expression of WT sample. (E) A transcriptional fusion of PhlyU-hlyU::luxCDABE gene showing the level of luminescence in wild type and ΔhlyU V. vulnificus; (F) Revalidation of hlyU transcription with varying concentration of FTH depicts the unchanged level of hlyU transcript in wild type V. vulnificus harboring the transcriptional fusion PhlyU-hlyU::luxCDABE. The percent RLU per unit OD600 was calculated based on the RLU values shown in (E).
There was no significant change in the transcription of hlyU upon FTH treatment (Figure 3C). Similarly, FTH did not show a significant modulatory effect on the transcription of the hns gene when compared to the untreated control (Figure 3D). H-NS is a known repressor of rtxA1 and vvhA toxin encoding genes, thus the alteration in hns gene expression may also change toxin gene expression. The consistent hlyU transcript level following FTH treatment was further confirmed using a transcriptional fusion of hlyU with the promoter-less luxCDABE reporter (PhlyU_hlyU::luxCDABE) under its native PhlyU promoter in WT V. vulnificus (Figures 3E,F). Varying concentrations of FTH added to WT V. vulnificus possessing the transcriptional fusion construct showed no inhibition in luminescence per unit OD600, suggesting unchanged transcriptional levels of the hlyU gene. The constant hlyU transcript levels observed with and without FTH treatment by both qRT-PCR (Figure 3C) and by transcriptional fusion studies (Figure 3F) supports the hypothesis that FTH does not target upstream regulatory genes in the HlyU transcriptional regulatory network (Figure 1I). In addition, the effect of FTH on the HlyU-regulated gene expression in V. vulnificus reporter, ΔhlyU knockout reporter and ΔhlyU complemented strains was evaluated, wherein ΔhlyU harboring the reporter plasmid served as negative control. The FTH concentration dependent inhibition of rtxA1 and vvhA gene-expression in the ΔhlyU complemented strain reproducibly confirmed that FTH operates through HlyU transcriptional regulator without disturbing the gene expression of both hlyU and hns regulators (Figure S6).
In Vivo Assessment for Specific Targeting of HlyU Using FTH
To affirm that the inhibition of the RtxA1 and VvhA cytotoxins by FTH occurs specifically through HlyU, an 89 bp synthetic promoter (PEM7) was cloned into the promoter-less luxCDABE-containing pBBRMCS2 plasmid and electroporated into WT and ΔhlyU V. vulnificus. WT V. vulnificus containing PEM7::luxCDABE was used as a non-specific control to assess the specificity of FTH toward HlyU, as HlyU drives the reporter gene expression under the PrtxA1 promoter (Figure S5A). In a comparative analysis of WT and ΔhlyU reporter strains, ΔhlyU reporter strain showed only one third the luminescence of the WT reporter strain, re-emphasizing the direct role of HlyU in the regulation of the rtxA1 toxin gene (Figure S5B). In a comparative assessment of the effect of FTH on the promoter activities of PrtxA1 (specific) vs. PEM7 (non-specific) in the presence of native chromosomal HlyU, the wild type reporter strain displayed specific inhibition of luminescence with PrtxA1 in response to varying concentrations of FTH, but no inhibition was observed with the synthetic promoter PEM7 tagged with luxCDABE. This suggests that FTH specifically targets the HlyU transcriptional regulator, and attenuates the expression of the RtxA1 toxin under ambient bacterial growth conditions (Figure S5C).
Inhibition of the Hemolysis Activity of V. vulnificus by FTH
The reduction in rtxA1 and vvhA gene expression upon FTH treatment was further verified by phenotypic assessment of WT V. vulnificus on human RBCs (hRBCs) by evaluating the hemolytic activity as hemolytic unit since both RtxA1 and VvhA contribute to hemolytic activity by forming pores in the cell membranes of hRBCs (Kim et al., 2008). The culture supernatant of ΔhlyU strains was used as a positive control of hemolysis inhibition. The culture supernatant of WT V. vulnificus grown for 3 h in the absence and presence of FTH were treated to hRBCs for hemolytic activity assessment. The inhibition of the hemolytic activity was visualized by the reduction of signature hemolytic peaks in the absorption spectra of FTH treated samples in concentration-dependent manner (Figures 4A,B). The hemolysis of hRBC at 60 μM FTH treatment was found to be negligible and was comparable to the hemolytic units of ΔhlyU strain (Figure 4C). These results suggest that the rtxA1 and vvhA gene expression and activity is positively controlled by HlyU. It is noteworthy that the hemolysin activity promotes the early dissemination and growth of bacteria in vivo, aiding their spread from the small intestine to other organs and contributing to the development of early pathogenesis during V. vulnificus infection (Jeong and Satchell, 2012). Therefore, FTH mediated targeting of the master virulence transcriptional regulator, HlyU, inhibited the production of hemolysin and RtxA1 at the transcriptional level and is expected to reduce the virulence and inhibit the disease progression of V. vulnificus.

Figure 4. The effect of FTH on the hemolytic activity of V. vulnificus. Untreated and FTH-treated (10, 20, 40, and 60 μM) 3 h culture supernatants of WT V. vulnificus were used for hemolysis visualization and hemolytic unit calculation (see method for details). (A) Visualization of hemolysis by WT V. vulnificus in the absence and presence of FTH, and (B) the absorption spectra showing the characteristic hemolysis peaks in various treatments. (C) Quantitative estimation of hemolysis inhibition activity of FTH expressed as hemolytic unit.
Rescuing Host Cytoskeletal Destabilization Using FTH
It has been reported that the expression of rtxA1 toxin of V. vulnificus dramatically increase by contact to host cells in a time-dependent manner. Our results showed transcriptional repression of the rtxA1 gene by FTH treatment, and thus, it is necessary to further confirm the effect of FTH by assessing the phenotype of the RtxA1 toxin on the host cells. Cytoskeletal destabilization is the hallmark of the RtxA1 toxin, along with plasma membrane blebbing and consequent necrotic cell death by cytolytic activity (Kim et al., 2008). Therefore, RtxA1 level can be estimated by the RtxA1-mediated destabilization of cytoskeleton, which results in the distortion of cell shape tending toward cell rounding. In this study, we monitored the cell rounding by staining the F-actin component of the cytoskeleton in HeLa cells using rhodamine phalloidin. WT V. vulnificus triggered more than 95% cell rounding while the FTH treatment (200 μM) merely affected 25 ± 5% host cells compared to 11 ± 4% round-cells in ΔhlyU and ΔrtxA1 mutant controls (Figures 5A,B). We also checked that FTH (100 and 200 μM) has insignificant effect on bacterial CFU of WT V. vulnificus in DMEM after an hour of treatment, using the same experimental conditions (Figure S3). FTH-mediated significant (75%) protection of host cells from RtxA1-induced cytoskeletal destabilization and cell-rounding is well represented by the transcriptional inhibition of rtxA1, confirming that FTH plays a role in suppressing the HlyU-regulated gene expression of rtxA1.

Figure 5. Rescue of cytoskeletal destabilization by FTH treatment using a human cell line. (A) HeLa cells were treated with WT V. vulnificus with and without FTH (100 and 200 μM), along with ΔhlyU, ΔrtxA1 and PBS controls. V. vulnificus control strains (ΔhlyU and ΔrtxA1) lacking toxins show a discrete cytoskeleton network (red) spread throughout the cytoplasm, while the WT V. vulnificus possessing the RtxA1 toxin totally destabilized the cytoskeletal network and cellular morphology. The presence of FTH (200 μM) with WT V. vulnificus rescued the cytoskeletal destabilization and protected cells from rounding in a concentration-dependent manner. Scale bar is equal to 20 micron. (B) Frequency of cytoskeletal destabilization and consequent rounding of HeLa cells as a measure of RtxA1 activity. A total of 1,000 cells were counted in random microscopic fields for each sample, and percentages were calculated based on round vs. intact shaped cells. FTH treatment inhibited RtxA1 at the transcriptional level. Thus, the cytoskeleton was found to be intact in the FTH-treated wild type sample (normal cell shape at 200 μM) but not in the untreated WT sample (rounded cells).
Avoidance of Antivirulence Resistance in V. vulnificus by FTH
Antimicrobial resistance that arises in various bacterial pathogens during antibiotic-mediated treatment is caused by adaptive evolution and clonal selection due to sustained antibiotic pressure. AMR in V. vulnificus appears to be rare. However, a few examples of V. vulnificus AMR have been reported recently (Elmahdi et al., 2016). To better understand AMR emergence, norfloxacin was chosen to test laboratory AMR development at a concentration of 0.155 μg/ml. This concentration was adopted by taking the average of a range of reported minimum inhibitory concentration (MIC90) values for six Vibrio strains (0.063–0.25 μg/ml) (Morris et al., 1985). To examine the adaptive evolution of AMR in the laboratory, wild type V. vulnificus was repeatedly transferred with or without 0.155 μg/ml norfloxacin, or with 40 or 60 μM FTH, along with untreated controls. A clumped-particulate suspension, likely V. vulnificus, appeared at the eleventh transfer. At the twelfth transfer, a visible turbidity appeared in tubes containing V. vulnificus treated with the norfloxacin (0.155 μg/ml) (Figure 6A). The adapted cultures were preserved at twelfth transfer for further analysis. Norfloxacin resistance and the preliminary identity of the adaptively evolved norfloxacin resistant (NorR) strain, along with the untreated cultures, were verified by MIC assessment (Figure 6B) and growth on Vibrio-specific TCBS agar plates (Figure 6C). This was followed by streaking, growth, and colony phenotype observation on TCBS agar plates containing norfloxacin (0.155 μg/ml) (Figures 6C I,II). The norfloxacin-adapted NorR strains could grow on TCBS norfloxacin plates (0.155 μg/ml) but the untreated or FTH-adapted strain did not produce any colonies, even in the inoculation zone (Figure 6C II). The identity of the evolved NorR strain, the FTH-treated strain, and the −80°C stock cultures were further verified by PCR amplification and sequencing analysis using 16S rRNA gene-specific primers for V. vulnificus MO6-24/O (Table S2). Sequence analysis of the amplified 16S rRNA genes demonstrated that the evolved norfloxacin-resistant (NorR) V. vulnificus strain and the FTH-treated strain originated from the same V. vulnificus strain (MO6-24/O).
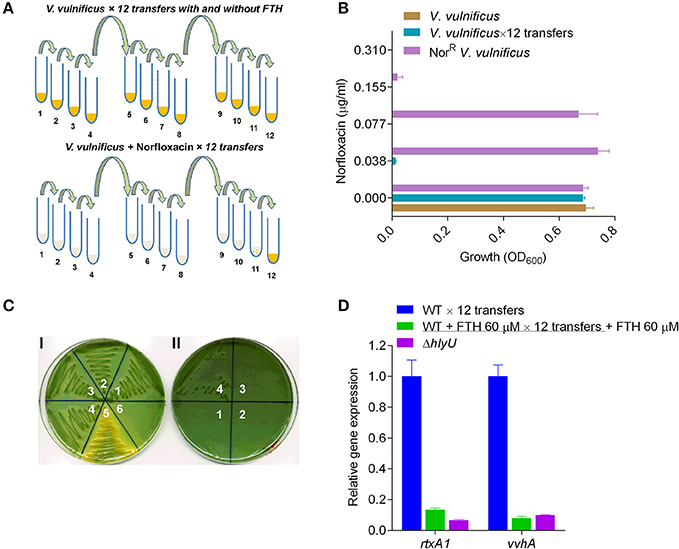
Figure 6. Laboratory-induced adaptive evolution of antimicrobial vs. antivirulence resistance in V. vulnificus. (A) Schematic diagram showing laboratory-induced adaptive evolution by continuous transferring of V. vulnificus exposed to norfloxacin (0.155 μg/ml) or FTH (40 and 60 μM). The numbers below the tubes represent the number of transfers. Yellow color in tubes denotes the growth. (B) Evaluation of norfloxacin MIC against non-transferred V. vulnificus; untreated V. vulnificus transferred 12 times (V. vulnificus × 12 transfers) and adaptively evolved, norfloxacin-resistant V. vulnificus (NorR V. vulnificus). For MIC calculations, an initial bacterial inoculum of 1 × 105 CFU/ml was used for NorR V. vulnificus, V. vulnificus, and V. vulnificus × 12 transfers. NorR V. vulnificus adapted to grow at 0.077 μg/ml, in contrast to the WT V. vulnificus control strain without any treatment. This data showed that V. vulnificus can evolve and acquire resistance in order to survive sublethal antibacterial concentrations in a very short amount of time. (C) Verification of identity of the adaptively evolved, putative NorR V. vulnificus strain. (I) Various numbers (1-6) on TCBS agar plate represent the original stock wild type V. vulnificus (1), the untreated V. vulnificus × 12 transfers (2), FTH-treated V. vulnificus (3), the adaptively evolved NorR V. vulnificus (4), a sugar-fermenting V. alginolyticus strain acts as a positive control of histochemical plate for sugar fermenting Vibrio (5), and E. coli DH5α as negative control (6). All the Vibrio strains (but not E. coli DH5α) grew on the TCBS agar plates. (II) Reconfirmation of norfloxacin resistance of adaptively evolved NorR strain on TCBS agar plate supplemented with 0.155 μg/ml norfloxacin. Growth of the adaptively evolved NorR strain (4), on TCBS-norfloxacin plate while the controls (1–3) did not show any sign of growth confirms its identity and its evolved norfloxacin resistance in Vibrio species. (D) The antivirulence resistance of FTH-exposed strains were analyzed by gene expression of rtxA1 and vvhA to verify resistance against FTH. The qRT-PCR results showed the same level of rtxA1 and vvhA transcriptional inhibition in the FTH-exposed strain and in the original stock culture of V. vulnificus.
We also tested the development of antivirulence resistance in FTH-exposed strains by analyzing rtxA1 gene expression of WT V. vulnificus treated with 60 μM FTH. The qRT-PCR results showed the same level of rtxA1 transcriptional inhibition in the FTH-adapted strain as in the original stock culture of V. vulnificus (Figure 6D). These results suggest that an antivirulence approach can be used to avoid the development of both antimicrobial and antivirulence resistance (Figures 6C,D). Specifically, drug resistance acquired through short-term acclimatization (by metabolic modulation) or through the long-term acquisition and accumulation of mutations is unlikely when using an antivirulence agent of nutraceutical nature.
Discussion
Antibiotics are certainly one of the most celebrated achievements of human history, and their precise, targeted use has saved millions of patients from deadly infectious diseases. However, the indiscriminate, excessive, and untargeted use of antibiotics has enhanced the rate of AMR emergence. V. vulnificus is an emerging, antibiotic-resistant, opportunistic pathogen. Antibiotics such as the quinolones and tetracycline are successfully employed in the treatment of V. vulnificus infections. However, the emergence of a higher rate of AMR (Baker-Austin et al., 2009; Baker-Austin and Oliver, 2018) and a lower rate of discovery of new antibiotics not only worsen the prophylaxis and treatment regime but also push back the mankind to the pre-antibiotic era to reconsider alternative approaches to treat AMR infections. Bacteriophages are being used as a specific and targeted alternative therapy to address the AMR strains both in environmental and clinical setting (Cooper et al., 2016; Letchumanan et al., 2016; Yen et al., 2017). However, the “phage-resistance” can also occur at a very high rate which may cause the debility of phage and consequent survival and persistence of bacterial pathogen during treatment (Aminov et al., 2017; Yen et al., 2017). Similarly, probiotics such as Streptomyces sp. is known to reduce the Vibrio sp. load in aquaculture. Nevertheless, the accumulation of the off-flavor compounds and lateral gene transfer of antibiotics resistance gene are the current limitations associated with probiotics application (Tan et al., 2016). Thus, as an alternative to antibiotics, bacteriophages and probiotics treatment, another anticipated evolution-proof antivirulence approach is being developed to treat AMR bacterial infections. The disarmament of early virulence factors, such as cytotoxins, in V. vulnificus at the transcriptional level using an antivirulent small molecule represents an attractive strategy to inhibit both pathogenesis and antibiotic resistance (Figure 1). Early virulence factors responsible for the establishment and dissemination of pathogenesis include RtxA1 and hemolysin (VvhA), which are highly invasive pore-forming toxins (PFTs). Cytotoxins play essential roles in establishing pathogenesis in the host by (a) supporting pathogen entry into the host cells (b) destabilizing the cytoskeletal network of host cells to inhibit phagocytosis (Lo et al., 2011), and (c) evading the innate immune system for rapid dissemination into the bloodstream (Song et al., 2016). The HlyU transcriptional regulator de-represses the expression of cytotoxins; HlyU can therefore be targeted using small molecules to inhibit V. vulnificus pathogenesis by inhibiting expression of key cytotoxins.
The reporter platform for the inhibitor-screening was developed using reverse chemical genetics in the target microbe, V. vulnificus, based on the HlyU regulatory cascade (Figures 1I,II). Specifically, the native chromosomal HlyU binds to the PrtxA1 promoter tagged with a promoter-less luxCDABE in the reporter plasmid. This plasmid-based reporter system showed a background signal even in the absence of HlyU, presumably due to low leaky expression and multiple copy number. The background luminescence in ΔhlyU V. vulnificus was therefore used as the control background signal (Figure S1). By screening chemicals using the luminescence reporter system, we identified fursultiamine hydrochloride (FTH) as a potent small molecule inhibitor (Figure 2) that decreased the expression of cytotoxins under the control of HlyU (Figures 1III,IV). FTH is a vitamin B1 derivative or synthetic thiamine analog, originally used to treat thiamine deficiency (Lonsdale, 2004). There is a single report of an inhibitor of rtxA1 transcription (Kim et al., 2010). In this report, resveratrol was identified from a cell-viability assay as a modulator of host-microbe interactions in terms of adhesion, motility, and consequent cytotoxicity (Kim et al., 2010), but its specific regulatory cascade has not been identified. However, in the current study, we identified FTH as an inhibitor of HlyU transcriptional regulator from rtxA1 transcription targeted—reporter platform, and validated its inhibition activity by checking the expression of HlyU downstream genes and global repressor genes (Figure 3). In addition, we further verified that the transcription of hlyU remained unchanged by FTH by examining a transcriptional fusion of hlyU (with its native promoter) to luxCDABE (Figures 3E,F). The results with transcriptional fusion indicated that FTH-mediated inhibition of luminescence in the WT V. vulnificus reporter strain was not due to the inhibition of any known or unknown upstream gene/regulator (Figure 1I).
The revalidation of FTH target using forward chemical genetics showed that FTH targeted HlyU, wherein the specific PrtxA1 promoter and a non-specific, synthetic PEM7 promoter were used to drive the expression of luxCDABE reporter operon. The specific inhibition of luminescence by FTH was concentration-dependent and only occurred with the PrtxA1 (and not the PEM7) promoter. Thus, several lines of experimental evidence distinctly suggest that FTH inhibits the expression of virulence factors through HlyU (Figure S5). Although FTH targets HlyU transcriptional regulator, the precise inhibitory mode of action of FTH on HlyU protein function remains unexplored and should be considered for future study. Hemolytic activity due to RtxA1 and VvhA was reduced upon FTH treatment and was comparable to the activity of ΔhlyU V. vulnificus (Figure 4). This finding is consistent with the transcriptional data inhibiting the expression of these toxins (Figure 3). RtxA1, or multifunctional autoprocessing repeats-in-toxin (MARTX) is known to be secreted through type I secretion system (Kim et al., 2013). FTH significantly reduced the cytoskeleton destabilization activity of RtxA1 on host cells (Figure 5). Cytoskeleton is crucial for phagocytosis (Swanson and Baer, 1995) and RtxA1 is known to inhibit phagocytosis which eventually helps V. vulnificus survival by evading host innate immune system (Lo et al., 2011). Taken together, the treatment of antivirulence FTH molecule targeting the HlyU, disarmed major potent pore forming toxins (RtxA1 and VvhA) and transformed them into a strain with reduced virulence (Figure 1V; Figures 4, 5).
The development of evolution proof antivirulence drug against pathogenic bacteria is an emerging challenge which can be alleviated by designing virulence factor specific genetically engineered screening platform and the careful examination of the small molecule leads for characteristics of an antivirulence drug, which has elegantly been opined and explained (Allen et al., 2014). In this context, the laboratory adaptive evolution of V. vulnificus against norfloxacin treatment demonstrates the rapid and enormous capability of this pathogen to acclimatize against antibiotic stress (Figure 6). Interestingly, continuous exposure of V. vulnificus to the antivirulence compound FTH did not cause the development of antivirulence resistance (Figure 1VI; Figure 6) presumably due to its nontoxicity to both host and pathogen and nutraceutical nature. However, there is a frequent trade-off between pharmaceutical and nutraceutical molecules for potency and toxicity levels. To support the in vitro data, we attempted to validate the FTH efficacy as an antivirulent drug by establishing a simple yet strong immune system possessing Galleria mellonella (greater wax moth) infection model (Loh et al., 2013) for the first time, for V. vulnificus (Figure S7). FTH showed marginally higher protection (~30% survival) of the wax moth larvae against V. vulnificus infections than that of FTH untreated infection group (10% survival) (Figure S8). Unfortunately, we found that the FTH-mediated protection data is inconsistent with a high level of variability in 3 independent experiments presumably due to in vivo drug instability in wax moth larvae as reported earlier in mice model (Fung et al., 2013). The instability of FTH may be alleviated by generating a wide range of FTH derivatives by protecting its antivirulence activities in future studies.
Despite the in vivo instability issue, FTH has several advantages as an antivirulence agent, such as (a) enhanced bioavailability due to its reduced absorption barrier (b) a high IC50 that poses no toxicity to the host (c) effective targeting of virulence transcription factor, HlyU (d) transcriptional inhibition of highly invasive cytotoxins, and (e) avoidance of AMR development during antivirulence strategy to tackle V. vulnificus infections presumably due to its nutraceutical nature and low selection pressure (Figures 1V,VI). In conclusion, FTH is a potential antivirulence agent that may be useful in combating V. vulnificus pathogenesis by inhibiting the transcriptional network of cytotoxins without causing the emergence of antimicrobial and antivirulence resistance. Our results also suggest that an antivirulence strategy targeting the expression of virulence factors regulated by HlyU might be a promising approach for the treatment of infectious disease caused by V. vulnificus.
Author Contributions
SI and AC performed the experiments. SI and AC wrote the manuscript with the support of KK. The experiments were designed and performed mainly under the supervision of AC and KK.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer YM and handling editor declared their shared affiliation.
Acknowledgments
The Vibrio vulnificus strains (WT, ΔhlyU and ΔrtxA1) and backbone plasmid (pBBRMCS2::luxCDABE) were kindly provided by Prof. Sang Ho Choi (Seoul National University, Seoul). We acknowledge Prof. S. H. Choi for fruitful discussion and suggestions during the manuscript preparation. We also thank Prof. Joon Haeng Rhee (Chonnam National University, Gwangju) for providing the RtxA1 antibody. This work was supported by the grants of National Research Foundation of Korea to AC (2014R1A1A22060303) and KK (2017M3A9E4078553).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2018.00152/full#supplementary-material
References
Allen, R. C., Popat, R., Diggle, S. P., and Brown, S. P. (2014). Targeting virulence: can we make evolution-proof drugs? Nat. Rev. Microbiol. 12, 300–308. doi: 10.1038/nrmicro3232
Aminov, R., Caplin, J., Chanishvili, N., Coffey, A., Cooper, I., De Vos, D., et al. (2017). Application of bacteriophages. Microbiol. Aust. 38, 63–66. doi: 10.1071/MA17029
Baker-Austin, C., McArthur, J. V., Lindell, A. H., Wright, M. S., Tuckfield, R. C., Gooch, J., et al. (2009). Multi-site analysis reveals widespread antibiotic resistance in the marine pathogen Vibrio vulnificus. Microb. Ecol. 57, 151–159. doi: 10.1007/s00248-008-9413-8
Baker-Austin, C., and Oliver, J. D. (2018). Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ. Microbiol. 20, 423–430. doi: 10.1111/1462-2920.13955
Blair, J. M., Webber, M. A., Baylay, A. J., Ogbolu, D. O., and Piddock, L. J. (2015). Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 13, 42–51. doi: 10.1038/nrmicro3380
Busenlehner, L. S., Pennella, M. A., and Giedroc, D. P. (2003). The SmtB/ArsR family of metalloregulatory transcriptional repressors: structural insights into prokaryotic metal resistance. FEMS Microbiol. Rev. 27, 131–143. doi: 10.1016/S0168-6445(03)00054-8
Cegelski, L., Marshall, G. R., Eldridge, G. R., and Hultgren, S. J. (2008). The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 6, 17–27. doi: 10.1038/nrmicro1818
Chiang, S. R., and Chuang, Y. C. (2003). Vibrio vulnificus infection: clinical manifestations, pathogenesis, and antimicrobial therapy. J. Microbiol. Immunol. Infect. 36, 81–88.
Cooper, C. J., Khan Mirzaei, M., and Nilsson, A. S. (2016). Adapting drug approval pathways for bacteriophage-based therapeutics. Front. Microbiol. 7:1209. doi: 10.3389/fmicb.2016.01209
Elmahdi, S., DaSilva, L. V., and Parveen, S. (2016). Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: a review. Food Microbiol. 57, 128–134. doi: 10.1016/j.fm.2016.02.008
Fung, E., Sugianto, P., Hsu, J., Damoiseaux, R., Ganz, T., and Nemeth, E. (2013). High-throughput screening of small molecules identifies hepcidin antagonists. Mol. Pharmacol. 83, 681–690. doi: 10.1124/mol.112.083428.
Gulig, P. A., Bourdage, K. L., and Starks, A. M. (2005). Molecular pathogenesis of Vibrio vulnificus. J. Microbiol. 43 Spec No, 118–131.
Heng, S. P., Letchumanan, V., Deng, C. Y., Ab Mutalib, N. S., Khan, T. M., Chuah, L. H., et al. (2017). Vibrio vulnificus: an environmental and clinical burden. Front. Microbiol. 8:997. doi: 10.3389/fmicb.2017.00997
Jackson, J. J., and Kropp, H. (1992). beta-Lactam antibiotic-induced release of free endotoxin: in vitro comparison of penicillin-binding protein (PBP) 2-specific imipenem and PBP 3-specific ceftazidime. J. Infect. Dis. 165, 1033–1041. doi: 10.1093/infdis/165.6.1033
Jang, K. K., Lee, Z. W., Kim, B., Jung, Y. H., Han, H. J., Kim, M. H., et al. (2017). Identification and characterization of Vibrio vulnificus plpA encoding a phospholipase A2 essential for pathogenesis. J. Biol. Chem. 292, 17129–17143. doi: 10.1074/jbc.M117.791657
Jeong, H. G., and Satchell, K. J. (2012). Additive function of Vibrio vulnificus MARTX(Vv) and VvhA cytolysins promotes rapid growth and epithelial tissue necrosis during intestinal infection. PLoS Pathog. 8:e1002581. doi: 10.1371/journal.ppat.1002581
Jones, M. K., and Oliver, J. D. (2009). Vibrio vulnificus: disease and pathogenesis. Infect. Immun. 77, 1723–1733. doi: 10.1128/IAI.01046-08
Kim, J. R., Cha, M. H., Oh, D. R., Oh, W. K., Rhee, J. H., and Kim, Y. R. (2010). Resveratrol modulates RTX toxin-induced cytotoxicity through interference in adhesion and toxin production. Eur. J. Pharmacol. 642, 163–168. doi: 10.1016/j.ejphar.2010.05.037
Kim, Y. R., Lee, S. E., Kim, B., Choy, H., and Rhee, J. H. (2013). A dual regulatory role of cyclic adenosine monophosphate receptor protein in various virulence traits of Vibrio vulnificus. Microbiol. Immunol. 57, 273–280. doi: 10.1111/1348-0421.12031
Kim, Y. R., Lee, S. E., Kim, C. M., Kim, S. Y., Shin, E. K., Shin, D. H., et al. (2003). Characterization and pathogenic significance of Vibrio vulnificus antigens preferentially expressed in septicemic patients. Infect. Immun. 71, 5461–5471. doi: 10.1128/IAI.71.10.5461-5471.2003
Kim, Y. R., Lee, S. E., Kook, H., Yeom, J. A., Na, H. S., Kim, S. Y., et al. (2008). Vibrio vulnificus RTX toxin kills host cells only after contact of the bacteria with host cells. Cell. Microbiol. 10, 848–862. doi: 10.1111/j.1462-5822.2007.01088.x
Klevanskaa, K., Bier, N., Stingl, K., Strauch, E., and Hertwig, S. (2014). PVv3, a new shuttle vector for gene expression in Vibrio vulnificus. Appl. Environ. Microbiol. 80, 1477–1481. doi: 10.1128/AEM.03720-13
Lane, M. C., Alteri, C. J., Smith, S. N., and Mobley, H. L. (2007). Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc. Natl. Acad. Sci. U.S.A. 104, 16669–16674. doi: 10.1073/pnas.0607898104
Lee, B. C., Choi, S. H., and Kim, T. S. (2008a). Vibrio vulnificus RTX toxin plays an important role in the apoptotic death of human intestinal epithelial cells exposed to Vibrio vulnificus. Microbes Infect. 10, 1504–1513. doi: 10.1016/j.micinf.2008.09.006
Lee, B. C., Lee, J. H., Kim, M. W., Kim, B. S., Oh, M. H., Kim, K. S., et al. (2008b). Vibrio vulnificus rtxE is important for virulence, and its expression is induced by exposure to host cells. Infect. Immun. 76, 1509–1517. doi: 10.1128/IAI.01503-07
Lee, H. J., Kim, J. A., Lee, M. A., Park, S. J., and Lee, K. H. (2013). Regulation of haemolysin (VvhA) production by ferric uptake regulator (Fur) in Vibrio vulnificus: repression of vvhA transcription by Fur and proteolysis of VvhA by Fur-repressive exoproteases. Mol. Microbiol. 88, 813–826. doi: 10.1111/mmi.12224
Lee, J. H., Kim, M. W., Kim, B. S., Kim, S. M., Lee, B. C., Kim, T. S., et al. (2007). Identification and characterization of the Vibrio vulnificus rtxA essential for cytotoxicity in vitro and virulence in mice. J. Microbiol. 45, 146–152.
Lee, S. E., Ryu, P. Y., Kim, S. Y., Kim, Y. R., Koh, J. T., Kim, O. J., et al. (2004). Production of Vibrio vulnificus hemolysin in vivo and its pathogenic significance. Biochem. Biophys. Res. Commun. 324, 86–91. doi: 10.1016/j.bbrc.2004.09.020
Lee, Y. C., Hor, L. I., Chiu, H. Y., Lee, J. W., and Shieh, S. J. (2014). Prognostic factor of mortality and its clinical implications in patients with necrotizing fasciitis caused by Vibrio vulnificus. Eur. J. Clin. Microbiol. Infect. Dis. 33, 1011–1018. doi: 10.1007/s10096-013-2039-x
Lenz, D. H., Mok, K. C., Lilley, B. N., Kulkarni, R. V., Wingreen, N. S., and Bassler, B. L. (2004). The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 118, 69–82. doi: 10.1016/j.cell.2004.06.009
Letchumanan, V., Chan, K. G., Khan, T. M., Bukhari, S. I., Ab Mutalib, N. S., Goh, B. H., et al. (2017). Bile sensing: the activation of Vibrio parahaemolyticus virulence. Front. Microbiol. 8:728. doi: 10.3389/fmicb.2017.00728
Letchumanan, V., Chan, K. G., Pusparajah, P., Saokaew, S., Duangjai, A., Goh, B. H., et al. (2016). Insights into bacteriophage application in controlling Vibrio species. Front. Microbiol. 7:1114. doi: 10.3389/fmicb.2016.01114
Linkous, D. A., and Oliver, J. D. (1999). Pathogenesis of Vibrio vulnificus. FEMS Microbiol. Lett. 174, 207–214. doi: 10.1111/j.1574-6968.1999.tb13570.x
Liu, M., Alice, A. F., Naka, H., and Crosa, J. H. (2007). The HlyU protein is a positive regulator of rtxA1, a gene responsible for cytotoxicity and virulence in the human pathogen Vibrio vulnificus. Infect. Immun. 75, 3282–3289. doi: 10.1128/IAI.00045-07
Liu, M., and Crosa, J. H. (2012). The regulator HlyU, the repeat-in-toxin gene rtxA1, and their roles in the pathogenesis of Vibrio vulnificus infections. Microbiol. Open 1, 502–513. doi: 10.1002/mbo3.48
Liu, M., Naka, H., and Crosa, J. H. (2009). HlyU acts as an H-NS antirepressor in the regulation of the RTX toxin gene essential for the virulence of the human pathogen Vibrio vulnificus CMCP6. Mol. Microbiol. 72, 491–505. doi: 10.1111/j.1365-2958.2009.06664.x
Liu, M., Rose, M., and Crosa, J. H. (2011). Homodimerization and binding of specific domains to the target DNA are essential requirements for HlyU to regulate expression of the virulence gene rtxA1, encoding the repeat-in-toxin protein in the human pathogen Vibrio vulnificus. J. Bacteriol. 193, 6895–6901. doi: 10.1128/JB.05950-11
Lo, H. R., Lin, J. H., Chen, Y. H., Chen, C. L., Shao, C. P., Lai, Y. C., et al. (2011). RTX toxin enhances the survival of Vibrio vulnificus during infection by protecting the organism from phagocytosis. J. Infect. Dis. 203, 1866–1874. doi: 10.1093/infdis/jir070
Loh, J. M., Adenwalla, N., Wiles, S., and Proft, T. (2013). Galleria mellonella larvae as an infection model for group A Streptococcus. Virulence 4, 419–428. doi: 10.4161/viru.24930
Lonsdale, D. (2004). Thiamine tetrahydrofurfuryl disulfide: a little known therapeutic agent. Med. Sci. Monit. 10, RA199–RA203.
Mead, P. S., Slutsker, L., Dietz, V., McCaig, L. F., Bresee, J. S., Shapiro, C., et al. (1999). Food-related illness and death in the United States. Emerg. Infect. Dis. 5, 607–625. doi: 10.3201/eid0505.990502
Morris, J. G., Tenney, J. H., and Drusano, G. L. (1985). In vitro susceptibility of pathogenic Vibrio species to norfloxacin and six other antimicrobial agents. Antimicrob. Agents Chemother. 28, 442–445. doi: 10.1128/AAC.28.3.442
Opal, S. M. (2010). Endotoxins and other sepsis triggers. Contrib. Nephrol. 167, 14–24. doi: 10.1159/000315915
Prins, J. M., van Deventer, S. J., Kuijper, E. J., and Speelman, P. (1994). Clinical relevance of antibiotic-induced endotoxin release. Antimicrob. Agents Chemother. 38, 1211–1218. doi: 10.1128/AAC.38.6.1211
Saha, R. P., and Chakrabarti, P. (2006). Molecular modeling and characterization of Vibrio cholerae transcription regulator HlyU. BMC Struct. Biol. 6:24. doi: 10.1186/1472-6807-6-24
Sambrook, J., Fritsch, F. E., and Maniatis, T. (1989). Molecular Cloning: a Laboratory Manual. New York, NY: Cold Spring Harbour Laboratory.
Schmittgen, T. D., and Livak, K. J. (2008). Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108. doi: 10.1038/nprot.2008.73
Shao, C. P., Lo, H. R., Lin, J. H., and Hor, L. I. (2011). Regulation of cytotoxicity by quorum-sensing signaling in Vibrio vulnificus is mediated by SmcR, a repressor of hlyU. J. Bacteriol. 193, 2557–2565. doi: 10.1128/JB.01259-10
Siboni, N., Balaraju, V., Carney, R., Labbate, M., and Seymour, J. R. (2016). Spatiotemporal dynamics of Vibrio spp. within the Sydney harbour estuary. Front. Microbiol. 7:460. doi: 10.3389/fmicb.2016.00460
Song, E. J., Lee, S. J., Lim, H. S., Kim, J. S., Jang, K. K., Choi, S. H., et al. (2016). Vibrio vulnificus VvhA induces autophagy-related cell death through the lipid raft-dependent c-Src/NOX signaling pathway. Sci. Rep. 6:27080. doi: 10.1038/srep27080
Starks, A. M., Schoeb, T. R., Tamplin, M. L., Parveen, S., Doyle, T. J., Bomeisl, P. E., et al. (2000). Pathogenesis of infection by clinical and environmental strains of Vibrio vulnificus in iron-dextran-treated mice. Infect. Immun. 68, 5785–5793. doi: 10.1128/IAI.68.10.5785-5793.2000
Swanson, J. A., and Baer, S. C. (1995). Phagocytosis by zippers and triggers. Trends Cell Biol. 5, 89–93. doi: 10.1016/S0962-8924(00)88956-4
Tamplin, M. L., Specter, S., Rodrick, G. E., and Friedman, H. (1985). Vibrio vulnificus resists phagocytosis in the absence of serum opsonins. Infect. Immun. 49, 715–718.
Tan, L. T., Chan, K. G., Lee, L. H., and Goh, B. H. (2016). Streptomyces bacteria as potential probiotics in aquaculture. Front. Microbiol. 7:79. doi: 10.3389/fmicb.2016.00079
Tang, H. J., Chang, M. C., Ko, W. C., Huang, K. Y., Lee, C. L., and Chuang, Y. C. (2002). In vitro and in vivo activities of newer fluoroquinolones against Vibrio vulnificus. Antimicrob. Agents Chemother. 46, 3580–3584. doi: 10.1128/AAC.46.11.3580-3584.2002
Wiegand, I., Hilpert, K., and Hancock, R. E. (2008). Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 3, 163–175. doi: 10.1038/nprot.2007.521
Wong, K. C., Brown, A. M., Luscombe, G. M., Wong, S. J., and Mendis, K. (2015). Antibiotic use for Vibrio infections: important insights from surveillance data. BMC Infect. Dis. 15:226. doi: 10.1186/s12879-015-0959-z
Wright, A. C., Simpson, L. M., Oliver, J. D., and Morris, J. G. Jr. (1990). Phenotypic evaluation of acapsular transposon mutants of Vibrio vulnificus. Infect. Immun. 58, 1769–1773.
Keywords: Vibrio vulnificus, rtxA1, hemolysin, hlyU, drug identification and repositioning, fursultiamine hydrochloride
Citation: Imdad S, Chaurasia AK and Kim KK (2018) Identification and Validation of an Antivirulence Agent Targeting HlyU-Regulated Virulence in Vibrio vulnificus. Front. Cell. Infect. Microbiol. 8:152. doi: 10.3389/fcimb.2018.00152
Received: 26 January 2018; Accepted: 23 April 2018;
Published: 11 May 2018.
Edited by:
Yinduo Ji, University of Minnesota, United StatesReviewed by:
Yusuke Minato, University of Minnesota, United StatesLearn-Han Lee, Monash University Malaysia, Malaysia
Copyright © 2018 Imdad, Chaurasia and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akhilesh Kumar Chaurasia, Y2hhdXJhc2lhQHNra3UuZWR1
Kyeong Kyu Kim, a3llb25na3l1QHNra3UuZWR1
 Saba Imdad
Saba Imdad Akhilesh Kumar Chaurasia
Akhilesh Kumar Chaurasia Kyeong Kyu Kim
Kyeong Kyu Kim