- Division of Molecular Biology and Human Genetics, Faculty of Medicine and Health Sciences, DST-NRF Centre of Excellence for Biomedical Tuberculosis Research, South African Medical Research Council Centre for Tuberculosis Research, Stellenbosch University, Cape Town, South Africa
Despite recent advances in tuberculosis (TB) drug development and availability, successful antibiotic treatment is challenged by the parallel development of antimicrobial resistance. As a result, new approaches toward improving TB treatment have been proposed in an attempt to reduce the high TB morbidity and mortality rates. Host-directed therapies (HDTs), designed to modulate host immune components, provide an alternative approach for improving treatment outcome in both non-communicable and infectious diseases. Many candidate immunotherapeutics, designed to target regulatory myeloid immune components in cancer, have so far proven to be of value as repurposed HDT in TB. Several of these studies do however lack detailed description of the mechanism or host pathway affected by TB HDT treatment. In this review, we present an argument for greater appreciation of the role of regulatory myeloid cells, such as myeloid-derived suppressor cells (MDSC), as potential targets for the development of candidate TB HDT compounds. We discuss the role of MDSC in the context of Mycobacterium tuberculosis infection and disease, focussing primarily on their specific cellular functions and highlight the impact of HDTs on MDSC frequency and function.
Introduction
The global TB health concern is exacerbated by the emergence of drug-resistant Mycobacterium tuberculosis (Mtb) strains. Other considerations, such as the substantial economic burden imposed by the length of TB treatment and the associated drug toxicity, favor the development of novel TB drugs (Islam et al., 2017). Surprisingly, the current pipeline for the development of new antibiotic compounds against Mtb remains slim. TB therapeutic research is now focused on the establishment of novel treatment strategies, such as host-directed therapies (HDTs), as an adjunctive approach to the current treatment regimen. HDTs aimed at modulating host immune homeostasis to ensure eradication of the invading pathogen, whilst simultaneously limiting tissue pathology, appears most promising. Similar HDT approaches correcting aberrant host pathways by way of targeting immune checkpoints, have shown huge success in cancer treatment plans. While immunotherapeutics has placed much emphasis on active enhancement of adaptive immune cell function through direct targeting of T-cell checkpoints, myeloid cells have recently emerged as equally attractive immune targets (Burga et al., 2013). Regulatory myeloid cells, such as myeloid-derived suppressor cells (MDSC), constitute a key innate immune checkpoint that impedes protective immunity in cancer (Young et al., 1987; Gabrilovich and Nagaraj, 2009). Common signaling pathways and similarities in immune regulation in malignancy and infectious disease, support the idea that cancer immunotherapeutic discoveries, can guide TB HDT strategies focused on pharmacological modulation of regulatory myeloid cells. We discuss the unfavorable role of regulatory myeloid cells in oncology, efforts to target MDSC in cancer clinical trials, knowledge on their negative contribution to Mtb control and highlight TB HDT compounds with potential to manipulate MDSC.
Regulatory Myeloid Cells in Tuberculosis: Myeloid-Derived Suppressor Cells
While the role of immunosuppressive regulatory T-cells have been demonstrated (Singh et al., 2012; Larson et al., 2013), the involvement of regulatory myeloid cells in TB, is not yet fully appreciated. In this regard, one of the mechanisms accounting for inadequate T-cell responses, is through defective engagement of innate immunity (Daker et al., 2015). Therefore, identification of new targets that regulate innate immune cell function and promote optimal activity of protective anti-TB immune responses, are likely to contribute to development of effective HDT targets.
Myeloid cells are the first responders to Mtb challenge during pulmonary infection and are critically involved in the induction of adaptive immunity, containment of bacilli and orchestration of inflammation. The key contribution of innate immunity in the initiation and regulation of adaptive immunity has led to the design of immunotherapies modulating innate cells, aimed at controlling diseases such as cancer (Qin et al., 2015). While MDSC are considered crucial in curbing inflammation-induced pathology, chronic or excess inflammation results in accumulation of MDSC (Ostrand-Rosenberg and Sinha, 2009). Overabundant MDSC, in turn, produce inflammatory mediators which recruit additional MDSC, thereby exacerbating inflammation (Cheng et al., 2008; Sinha et al., 2008). MDSC have also gained attention in the TB field due to their host immunosuppressive potential and ability to harbor Mtb bacilli (Knaul et al., 2014). MDSC frequencies are significantly expanded in the blood of TB patients, but decrease in number following successful TB chemotherapy (du Plessis et al., 2013). Several lines of evidence demonstrate the detrimental effect of MDSC on anti-TB immunity, including T-cell activation, proliferation, trafficking, regulatory T-cell induction and T-cell cytokine responses (du Plessis et al., 2013; Obregón-Henao et al., 2013; Knaul et al., 2014; Daker et al., 2015). MDSC may also impair phagocyte responses through production of IL-10 and TGF-β, inhibiting DC and macrophage function, and polarizing these cells toward a Th2 phenotypic response, as shown in tumor biology (Knaul et al., 2014). Such impairments are likely to affect Mtb control mechanisms, as well as the initiation and maintenance of effective adaptive immunity. MDSC are not only capable of regulating the intensity of T-cell responses to particular antigens, but also determine the numbers and activity of other immuno-regulatory cells. Given this immuno-modulatory capacity, MDSC should be considered as potential targets for fine-tuning the host response to Mtb. The major value of MDSC-immunotherapeutic strategies, is that these agents may be combined with traditional TB treatment and other HDT options to improve and optimize pathogen clearance.
Pharmacological Targeting of Regulatory Myeloid Cells
The phenotypic and functional diversity of cellular subsets present within the myeloid compartment remains underappreciated and poorly investigated in the context of TB. The complexity of innate phagocytes in the lungs of TB patients is particularly striking, suggesting that detailed characterization is imperative to understanding the mechanisms of TB susceptibility or protection (Silver et al., 2016). MDSC, purposed to regulate inflammation, have gained attention due to their central role in prevention of host anti-tumor immunity and subsequent immune escape (Lesokhin et al., 2012). MDSC not only support tumor cell metastasis, proliferation and angiogenesis, but also create an immunosuppressive environment for cancer cell evasion of host immunity and chemotherapy-induced senescence, thereby promoting disease and treatment resistance (Gabrilovich et al., 2012; Bronte et al., 2016; Kumar et al., 2016). An area of intense research in the oncology field, is the identification and targeting of MDSC mechanisms and molecules supporting tumor escape. Reversal of MDSC function, inhibition of MDSC recruitment or depletion of MDSC numbers, have all shown promise in enhancing the activity of cancer vaccines and therapies in preclinical models, with growing evidence from clinical trials (Di Mitri et al., 2015; Draghiciu et al., 2015a).
Strategies for Reversing MDSC Impact On Anti-TB Immunity
Regulatory myeloid cells such as MDSC have been successfully depleted with anti-Gr1+ antibodies in murine cancer models, with an associated reduction in tumor burden (Condamine et al., 2014). The Gr1+ antigen is, however, not present in humans, and is also a non-specific granulocyte marker, making this MDSC depletion strategy highly contentious (Xing et al., 2016). These findings do, however, suggest that depletion of MDSC is an effective immunotherapeutic approach. Other MDSC depletion strategies have shown greater success as cancer immunotherapy at pre-clinical and clinical trial level, and should be considered as repurposed treatment options for TB (Draghiciu et al., 2015a). MDSC targeting strategies can be categorized as approaches to (1) block MDSC inducing factors, (2) reverse MDSC functionality, and (3) differentiate MDSC into non-suppressive cells (Figure 1 and Table 1). There is, however, considerable cross-talk and overlap between these pathways, with numerous feedback mechanisms necessitating investigations in each stage of the TB disease spectrum.
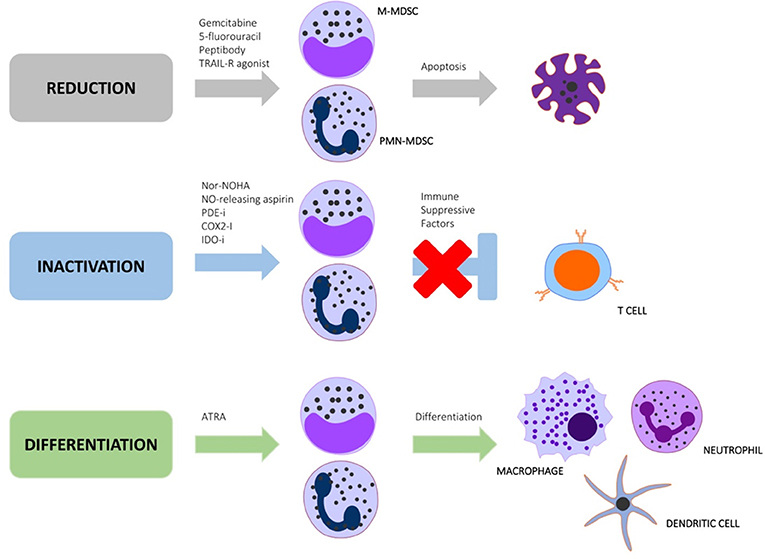
Figure 1. Immunotherapeutic strategies aimed at targeting regulatory myeloid cell pathways to reduce, inactivate or differentiate MDSC.
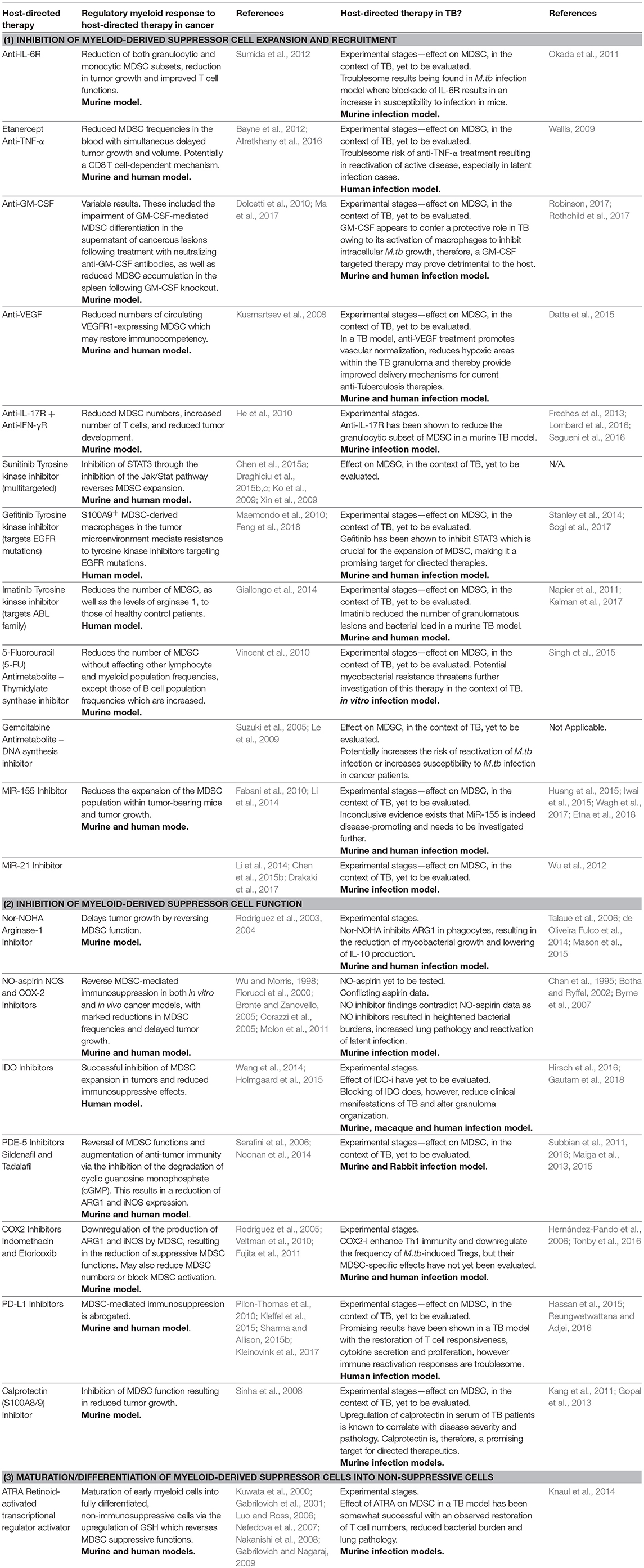
Table 1. Agents affecting regulatory myeloid cell pathways have been tested as immunotherapeutics in cancer, some of which have also shown promise when evaluated in TB.
Inhibition of MDSC Expansion and Recruitment
Cytokines
In cancer, mediators known to enhance the expansion of MDSC include interleukin-6 (IL-6), tumor necrosis alpha (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF), cyclooxygenase-2 (COX2), prostaglandins, stem-cell factor (SCF), macrophage colony-stimulating factor (M-CSF), vascular-endothelial growth factor (VEGF), IL-1β and IL-17 (Shipp et al., 2016). A recent study has shown that blocking of the IL-6 receptor (IL-6R) or TGF-β in tumor-bearing mice, decreases both monocytic and granulocytic MDSC subsets, alongside a reduction in tumor growth and improvement in T-cell function (Sumida et al., 2012). In contrast, results with GM-CSF treatment have been variable (Ma et al., 2017). Mice deficient in IL-17R and IFN-γR, also demonstrated lower MDSC levels and increased T-cells that were associated with a reduction in tumor development (He et al., 2010).
In TB, some of these cytokines are also being investigated as potential targets in HDT approaches, but none have investigated the effect on MDSC specifically. Instead, adjunctive cytokine treatment as intervention in TB has, however, largely focused on supplementation of mediators which activate macrophages to promote mycobacterial killing or blockade of pro-inflammatory cytokine signaling to limit lung damage (Condos et al., 1997, 2003; Vogt and Nathan, 2011). The majority of studies report on IFN-γ treatment of TB and demonstrate a reduction in pro-inflammatory cytokine production, enhancement of TB-specific CD4 T-cell responses, enhanced sputum conversion and reduced radiological involvement, although some inconsistent outcomes have been reported (Park et al., 2007; Dawson et al., 2009). IFN-γ treatment has also shown promise as HDT in a small study on Cuban patients infected with non-tuberculous mycobacterial lung disease, such as M. avium (Suárez-Méndez et al., 2004). This suggests that some HDTs may also be beneficial in the treatment of NTM. It was shown that IFN- γ improved the extent and clearance rate of pulmonary and radiological symptoms of these patients by the month 18 time point (Suárez-Méndez et al., 2004). Pre-clinical data on anti-TNF-α, anti-VEGF and IL-6R blockers as TB HDT in TB animal models and case studies of severe pulmonary TB patients have been encouraging (Okada et al., 2011; Datta et al., 2015). It remains crucial to appreciate the complex role of cytokines in TB immune regulation, necessitating in depth characterization of the optimal cytokine dose and timing to determine the cytokine's effect on MDSC induction, and resultantly on disease modulation.
Enzymes and Transcription Factors
In cancer, several of the cytokine mediators targeted by TB HDTs, trigger activation of the transcription factor, signal transducer and activator of transcription 3 (STAT3), which activates the signaling pathway mediating tumor-MDSC induction (Gao et al., 2007). In cancer, STAT3 is critically involved in MDSC expansion by stimulating expression of immature myeloid cell (IMC) genes involved in MDSC development (Tartour et al., 2011; Sansone and Bromberg, 2012; Table 1, Figure 1). STAT3 is phosphorylated by the tyrosine kinase Janus kinase/signal transducer and activator of transcription (Jak/Stat) pathway (Nicolas et al., 2012). Agents targeting these kinases in cancer patients are being investigated in an attempt to reverse MDSC expansion. For example, the tyrosine kinase inhibitor (TKI) sunitinib, blocks MDSC expansion in cancer patients and tumor bearing mice (Chen et al., 2015a; Draghiciu et al., 2015b,c). Furthermore, STAT3 overexpression in myeloid cells trigger expansion of MDSC and upregulate S100A9, which directs MDSC accumulation (Cheng et al., 2008; Wu et al., 2011). In TB, protein kinase inhibitors have also emerged as attractive candidates in the development of antimicrobial drugs. The TKI, gefitinib, a FDA-approved compound for the treatment of non-small-cell lung carcinoma, has recently been tested in human TB due to its ability to inhibit the epidermal growth factor receptor (EGFR) and activate autophagy to restrict bacterial growth. Gefitinib demonstrated in vitro and in vivo efficacy against Mtb infection, although the involvement of MDSC has not been considered (Stanley et al., 2014; Sogi et al., 2017). The TKI, imatinib, has also shown therapeutic efficacy in TB mouse models, leading to the initiation of a pre-clinical study on Mtb infection in a non-human primate model, but again, the role of MDSC was not investigated (Napier et al., 2011; Kalman et al., 2017). Inhibitors of protein kinase R are also being screened in TB mouse models, whereas other anti-cancer kinase inhibitors such as sunitinib, malate and curcumin analogs with known effects on tumor-derived MDSC induction, remains to be tested in TB.
Cytotoxic Agents
In cancer, selected cytotoxic cancer agents have also been used to deplete MDSC through a yet undefined mechanism(s). For example, 5-fluorouracil (5-FU) and gemcitabine treatment reduces the number of MDSC without affecting the frequency of T-cells, dendritic cells, NKT- or NK-cells, yet increases the B-cell population (Le et al., 2009; Vincent et al., 2010). In TB, the effect of 5-FU on host immunity, more specifically, MDSC, has not been tested, and although its direct bactericidal action against Mtb has been reported, mycobacterial resistance to 5-FU continues to be a concern (Singh et al., 2015). Efforts evaluating 5-FU as TB HDT has been directed to investigation on inhibitors of phosphodiesterase-5 (PDE-5), which has a known effect on MDSC function (see section Phosphodiesterase Inhibitors).
Micro-RNA
With antisense technology improving, targeting of microRNAs (MiR) by immunotherapeutics is increasingly considered (Li et al., 2014). In cancer, MiR-155 and MiR-21 are critically required for the expansion of MDSC in tumor-bearing mice and to facilitate tumor growth; miR 93-106b cluster regulate expression of PD-L1 on MDSC; while a specific role for miR-142-3p has recently been suggested in cancer through preventing differentiation of myeloid cells (Li et al., 2014; Chen et al., 2015b). The role of MiR in TB continues to be unraveled, as reports emerge of Mtb facilitating expression of MiR-155 to disrupt the process of autophagy (Wagh et al., 2017; Etna et al., 2018). Although MiR-155 is responsive to TB therapy, it remains inconclusive if its expression is disease promoting, as MiR-155 knockout mice are susceptible to Mtb infection (Iwai et al., 2015; Wagh et al., 2017). MiR-21 is also upregulated following Mtb infection, reportedly to escape the host immune response by downregulating the genes for TNF-α and IL-6 (Wu et al., 2012). Also in TB, HDT manipulation of other miR, controlling key myeloid functions, has also been proposed. Although not yet tested in clinical trials, Mtb induced expression of miR-142-3p targets an actin-binding protein leading to reduced phagocytosis in primary human macrophages (Bettencourt et al., 2013). MiR-106b-5p was also specifically upregulated in human macrophages following Mtb infection to reduce function by lowering cathepsinS expression and favoring Mtb survival (Pires et al., 2017). Greater understanding of MiR function and regulation, also in the context of regulatory myeloid cells is, however, required before therapeutic targeting of host MiR becomes a reality. This is likely to be accompanied by the requirement of cell-specific delivery techniques, to avoid potential off-target immunological effects (Iannaccone et al., 2014).
Inhibition of MDSC Function
Enzyme Inhibitors
Arginase-1
In cancer, a critical mechanism whereby MDSC induce lymphocyte suppression is by local depletion of essential amino acids required for T-cell proliferation. Tumor-MDSC highly express the enzyme arginase-1 (ARG1) which catabolizes l-arginine to urea and ornithine (Wu and Morris, 1998; Bogdan, 2001), thereby inhibiting T-cell proliferation through decreased CD3-theta chain expression (Rodriguez et al., 2003, 2004). In lung cancer, treatment with the arginase inhibitor Nω-hydroxy-L-arginine (nor-NOHA) delays tumor growth by reversing MDSC function (Rodriguez et al., 2004). In TB, Nor-NOHA inhibition of ARG1 in phagocytes has also shown promise, through its reduction of mycobacterial growth and lowering of IL-10 production in vitro (Talaue et al., 2006; de Oliveira Fulco et al., 2014).
iNOS
In cancer, MDSC also generate oxidative stress by increasing levels of reactive oxygen species (ROS) and inducible nitric oxide (iNOS) with resultant immunosuppressive effects (Bronte and Zanovello, 2005; Youn et al., 2008). ROS and iNOS activity also steers the production of harmful peroxynitrites, H202 and NO (Schmielau and Finn, 2001; Youn et al., 2008; Table 1, Figure 1). Although NO is crucial, in TB, to mycobacterial control, nitrogen and oxygen intermediates suppress T-cell function by nitration of the T-cell receptor (Nagaraj et al., 2007), induction of T-cell apoptosis (Mannick et al., 1999), reduction of MHC expression(Harari and Liao, 2004) and reduction of CD3-theta chain expression (Schmielau and Finn, 2001). NOS inhibitors have been shown to reverse MDSC-mediated immunosuppression in both in vitro and in vivo cancer models (Wu and Morris, 1998; Bronte and Zanovello, 2005). For example, nitro-aspirin (NO-aspirin), a new molecule in which aspirin is covalently linked to a NO-group, suppresses the production of ROS and provides feedback inhibition to iNOS (Fiorucci et al., 2000). In vitro and in vivo models of NO-aspirin treatment have also been shown to reduce MDSC numbers, reverse MDSC induced inhibition of T-cell responses by reducing CCL2 chemokine nitration and delay tumor growth (Molon et al., 2011). NO-aspirin also inhibits the COX-2 enzyme, another MDSC inducer (Corazzi et al., 2005). In TB, information on aspirin as HDT has been conflicting, but the effect of NO-aspirin remains to be investigated (Byrne et al., 2007). In spite of this, findings from murine TB emphasize NO as vital component in innate immune control of Mtb, and argues against the use of NO inhibitors as these result in heightened bacterial burden, increased lung pathology and mortality (Chan et al., 1995) and reactivation of latent Mtb infection (Botha and Ryffel, 2002).
Phosphodiesterase Inhibitors
Phosphodiesterase-5 (PDE-5) inhibitors (PDE-5-i), such as sildenafil and tadalafil, have good safety profiles as these have been used for decades to treat pulmonary hypertension, cardiac hypertrophy and erectile dysfunction. PDE-5-i were shown to reverse MDSC function in cancer patients and augment anti-tumor immunity by inhibiting the degradation of cyclic guanosine monophosphate (cGMP), leading to reduction in ARG1 and iNOS expression (Serafini et al., 2006; Noonan et al., 2014). Promising pre-clinical findings demonstrate that treatment with PDE-5-i improve T-cell responses, delay tumor growth and abrogate Treg proliferation in several cancer types (Serafini et al., 2006). This has resulted in a number of clinical trials on PDE-5-i in cancer, including a study evaluating whether treatment of oropharyngeal carcinoma patients with tadalafil could enhance T-cell tumor infiltration (NCT00843635); whether tadalafil can improve responses to dexamethasone chemotherapy (NCT01374217), if sildenafil treatment improves the outcome of non-small cell lung carcinoma (NCT00752115), or if tadalafil in combination with a novel vaccine and gemcitabine chemotherapy or radiation therapy, improves cancer outcome (NCT01342224).
In TB, the severe side effects associated with thalidomide, has led to the consideration of analogs, such as PDE-i, with similar potential to inhibit TNF-α (Aragon-Ching et al., 2007). PDE-i have been shown to decrease TB disease severity, reduce lung pathology and bacillary load in mouse models (Subbian et al., 2011, 2016; Maiga et al., 2013, 2015). The effect of PDE-i on immune cell phenotypes and function during human Mtb infection and TB disease remains poorly defined, but future and ongoing trials could cast more light on the impact of PDE-i on TB host immune responses, and necessitate evaluation of the effect on MDSC (NCT02968927).
Cyclooxygenase Inhibitors
Prostaglandin-E2 (PGE-2) has both pro-inflammatory and immune-suppressive properties and is synthesized by cyclo-oxygenase-2 (COX2). MDSC highly express the PGE-2 receptor, E-prostanoid 4, which, upon binding, induces ARG1 (Rodriguez et al., 2005). In cancer, Treatment of MDSC with COX inhibitors (COX2-i) have shown to down-regulate their production of ARG1 and iNOS, thereby reducing MDSC suppressive function (Veltman et al., 2010). COX2-i may thus target MDSC on multiple levels, either by reducing their numbers or by blocking their activation (Rodriguez et al., 2005; Fujita et al., 2011). In TB, COX2-i also significantly improved host immunity to Mtb in animal models by enhancing Th1 immunity (Hernández-Pando et al., 2006). In vitro treatment of TB patients' blood samples with the COX2-i, indomethacin, a nonsteroidal anti-inflammatory drug, downregulated the frequency of Mtb-induced Tregs and impaired T-cell proliferation and antigen-specific cytokine responses (Tonby et al., 2016). Although this study did not consider the effect on regulatory myeloid cells, an ongoing trial evaluating the impact of the COX2-i, etoricoxib, on immune-mediated host clearance of Mtb, will allow for assessment of MDSC functionality (NCT02503839).
Indoleamine 2,3-Dioxygenase Inhibitors
Monocytic MDSC also express high levels of the enzyme indoleamine 2,3-dioxygenase (IDO) that mediates immunosuppression through a mechanism involving regulatory T-cells (Tregs) (Holmgaard et al., 2015). In cancer, inhibition of IDO successfully blocks expansion of MDSC in tumors, as well as reducing the immunosuppressive effects of MDSC in IDO deficient mice (Yang, 2009; Wang et al., 2014; Holmgaard et al., 2015). Mtb is also a potent inducer of IDO (Hirsch et al., 2016). In TB, even though the mycobacterial burden in IDO-deficient mice is comparable to those of wild type mice (Blumenthal et al., 2012), a recent report demonstrated that blocking of IDO decreases both the clinical manifestations of TB as well as microbial and pathological correlates in macaques by altering granuloma organization (Gautam et al., 2018). Additionally, IDO inhibitors may reduce the number of MDSC in the lungs of TB patients, however this remains to be tested.
Checkpoint Inhibitors
In cancer, tumor-derived MDSC highly express programmed death ligand-1 (PD-L1), which engages the PD-1 receptor on T-cells, resulting in an exhausted phenotype (Jiang et al., 2015; O'Donnell et al., 2017). Blocking of PD-L1 abrogated MDSC-induced immune suppression in a murine melanoma model (Kleffel et al., 2015; Kleinovink et al., 2017).
In TB, the use of a PD-L1 checkpoint inhibitor has shown some promise in an in vitro model through its restoration of T-cell responsiveness to TB antigens, cytokine secretion and proliferation (Sharma and Allison, 2015a). Similarly, TB treatment has been shown to reduce expression of the genes responsible for PD-L1 expression on T-cells and natural killer cells (Hassan et al., 2015). However, by reactivating the immune system through treatment with this checkpoint inhibitor, two cancer patients have developed active TB disease (Reungwetwattana and Adjei, 2016). This highlights the complexities associated with modulation of immune regulatory molecules, such as PD-L1, which are upregulated on multiple immune cell subsets in a range of disease conditions (Shen et al., 2016). Even so, inhibition of PD-L1 on immune regulatory cells such as MDSC in TB, merits further investigations.
In cancer, the calcium-binding pro-inflammatory alarmin, calprotectin (S100A8/9), is highly expressed on murine tumor-derived MDSC and contributes to the induction of MDSC (Sinha et al., 2008). Interference with S100A8/A9 signaling inhibits MDSC function, leading to decreased tumor growth (Sinha et al., 2008). In TB patients, serum abundance of S100A8/9 correlates with disease severity while also mediating neutrophilic inflammation and lung pathology in Mtb-infected experimental animals (Kang et al., 2011; Gopal et al., 2013). Given the association of S100A8/9 with MDSC, current TB treatment strategies and lung inflammation may thus benefit from targeting this molecule, with the expectation that it could limit immunosuppression and neutrophilic influx.
Maturation/Differentiation of MDSC in Non-Suppressive Cells
Differentiation of MDSC into mature myeloid cells without immunosuppressive functionalities is another promising immunotherapeutic strategy. Various studies have shown enhanced MDSC levels in the bone marrow and spleen of vitamin-A deficient mice (Kuwata et al., 2000; Walkley et al., 2002).
All-Trans Retinoic Acid
All-trans retinoic acid (ATRA) is a vitamin-A metabolite that activates retinoid-activated transcriptional regulators (Nakanishi et al., 2008). These factors activate target genes resulting in the maturation of early tumor-associated myeloid cells into fully differentiated, non-immunosuppressive cells (Nefedova et al., 2007). In cancer, ATRA treatment also up-regulates glutathione (GSH), a ROS scavenger, thereby reversing MDSC immunosuppressive function (Nefedova et al., 2007). Furthermore, ATRA treatment of MDSC led to their differentiation into mature DC, granulocytes and monocytes, through their upregulation of differentiation markers such as HLA-DR (Lathers et al., 2004; Gabrilovich and Nagaraj, 2009). On the other hand, ATRA also increases expression of the transcription factor FoxP3, thereby inducing the development of Tregs which are considered to be detrimental to anti-tumor and anti-TB immunity alike (Ma et al., 2014). Despite this potential deleterious side effect, several clinical trials are evaluating ATRA as treatment option to modulate MDSC in cancer patients (NCT00601796; NCT00618891).
In TB, ATRA and other retinoic acids have shown to enhance anti-mycobacterial immune functions in phagocytes upon in vitro Mtb infection, which was dependent on its ability to reduce total cellular cholesterol and increase lysosomal acidification (Crowle and Ross, 1989; Wheelwright et al., 2014). Although vitamin-A deficiency strongly predicts risk of incident TB among HHC of TB patients, pre-clinical trials of vitamin-A supplementation has not been promising, likely due to the complex metabolic route required for conversion of vitamin-A to the active ingredient, ATRA (Lawson et al., 2010; Visser et al., 2011). The therapeutic impact of ATRA on MDSC has been evaluated in a TB mouse model, demonstrating that MDSC ablation restores T-cell numbers, reduces Mtb burden and decrease lung pathology (Knaul et al., 2014). More recently it was also shown that ATRA augments autophagy of Mtb in human and murine alveolar macrophages(Coleman et al., 2018) Further experiments are however required to fully establish the impact of ATRA on MDSC function in TB patients when employed as adjunctive therapy.
Targeting MDSC in Non-Tuburculous Intracellular Infections
In addition to their involvement in Mtb infections, MDSC have versatile roles in other intracellular pathogenic infections (Dorhoi and Du Plessis, 2018). Their effect during these infections are governed by the pathogen species and the course of infection. Targeting of MDSC in other intracellular infections could thus involve either the reduction or expansion of their suppressive capacity and/or frequency, depending on their beneficial or detrimental impact on disease outcome. For example, MDSC are increased in both clinical and experimental viral infections such as HIV, SIV, and LP-BM5, contributing to pathogen survival through TH1 immunosuppression as observed by the correlation with viral load and CD4 T-cell count (Gama et al., 2012; Vollbrecht et al., 2012; Garg and Spector, 2014; O'Connor et al., 2016). Similar detrimental effects have been reported for cytomegalovirus (CMV) infections (Garg et al., 2017), with MDSC impairing viral clearance (Daley-Bauer et al., 2012). MDSC accumulation has been reported also for various non-tuberculous intracellular bacterial infections. Staphylococcus aureus infections are sustained by MDSC promoting an immunosuppressive environment and impairing macrophage responsiveness (Heim et al., 2015; Tebartz et al., 2015), whereas MDSC frequencies in Francisella tularensis infection correlate with the extent of tissue pathology, loss of pulmonary function and host mortality (Periasamy et al., 2016). Above mentioned depletion strategies might thus be applicable to a broad range of intracellular bacterial infections, although the timing of administration might require careful consideration.
Conclusion
Tumors escape immune attack by a variety of mechanisms, often complementary in their ability to induce immunosuppression. Molecular interventions targeting innate immune cell regulatory pathways are making great advances in the immune-oncology field. Immune based cancer therapies are now also being recognized for their ability to potentiate anti-pathogen immunity when used in combination with classical treatment approaches. As for cancer, TB is described as a chronic inflammatory disease, characterized by a dysregulated immune profile. Data from cancer research suggest that MDSC can also be disarmed at pre-determined time points to redefine the outcome of disease. Therapeutic targeting of regulatory myeloid cells, such as MDSC and their molecular drivers, are, therefore, considered to be an exciting new strategy to help ameliorate TB via more effective and less-toxic strategies. Nevertheless, amongst the several approaches of TB HDT being sought, targeting of MDSC have not been explored, or their mechanistic involvement in the success of selected HDT, not appreciated. There is, however, a pressing need to study key signaling pathways and intermediates involved in the induction and function of regulatory myeloid cells, to allow pre-clinical screening of re-purposed drugs showing promise in oncology trials. For instance, the impact of metabolic pathways on MDSC function has only recently been recognized and requires investigation in the TB context. It will also be important to identify markers specific to MDSC, in particular, the predominant monocytic subset, to advance identification and development of suitable MDSC-targeting TB immunotherapies. We propose that MDSC remain an under investigated regulatory myeloid cell population that holds great promise in the TB HDT field.
Author Contributions
GW and NDP conceptualized the manuscript. NDP, LAK, and VL designed and drafted the manuscript with input from GW.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge the financial support from the European & Developing Countries Clinical Trials Partnership (EDCTP; CDF1546) and National Institute of Health (NIH) International Collaborations in Infectious Disease Research (ICIDR): Biology and Biosignatures of anti-TB Treatment Response (5U01IA115619/03).
References
Aragon-Ching, J. B., Li, H., Gardner, E. R., and Figg, W. D. (2007). Thalidomide analogues as anticancer drugs. Recent Pat. Anticancer Drug Discov. 2, 167–174. doi: 10.2174/157489207780832478
Atretkhany, K.-S. N., Nosenko, M. A., Gogoleva, V. S., Zvartsev, R. V., Qin, Z., Nedospasov, S. A., et al. (2016). TNF neutralization results in the delay of transplantable tumor growth and reduced MDSC accumulation. Front. Immunol. 7:147. doi: 10.3389/fimmu.2016.00147
Bayne, L. J., Beatty, G. L., Jhala, N., Clark, C. E., Rhim, A. D., Stanger, B. Z., et al. (2012). Tumor-derived granulocyte-macrophage colony stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell 21, 822–835. doi: 10.1016/j.ccr.2012.04.025
Bettencourt, P., Marion, S., Pires, D., Santos, L. F., Lastrucci, C., Carmo, N., et al. (2013). Actin-binding protein regulation by microRNAs as a novel microbial strategy to modulate phagocytosis by host cells: the case of N-Wasp and miR-142-3p. Front. Cell. Infect. Microbiol. 3:19. doi: 10.3389/fcimb.2013.00019
Blumenthal, A., Nagalingam, G., Huch, J. H., Walker, L., Guillemin, G. J., Smythe, G. A., et al. (2012). M. tuberculosis induces potent activation of IDO-1, but this is not essential for the immunological control of infection. PLoS ONE 7:e37314. doi: 10.1371/journal.pone.0037314
Bogdan, C. (2001). Nitric oxide and the immune response. Nat. Immunol. 2, 907–916. doi: 10.1038/ni1001-907
Botha, T., and Ryffel, B. (2002). Reactivation of latent tuberculosis by an inhibitor of inducible nitric oxide synthase in an aerosol murine model. Immunology 107, 350–357. doi: 10.1046/j.1365-2567.2002.01511.x
Bronte, V., Brandau, S., Chen, S.-H., Colombo, M. P., Frey, A. B., Greten, T. F., et al. (2016). Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 7:12150. doi: 10.1038/ncomms12150
Bronte, V., and Zanovello, P. (2005). Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 5, 641–654. doi: 10.1038/nri1668
Burga, R. A., Thorn, M., Nguyen, C. T., Licata, L., Espat, N. J., Junghans, R. P., et al. (2013). Targeting myeloid-derived suppressor cells and the PD-1/PD-L1 axis to enhance immunotherapy with anti-CEA designer T cells for the treatment of colorectal liver metastases. J. Clin. Oncol. 31, 3079–3079. doi: 10.1200/jco.2013.31.15_suppl.3079
Byrne, S. T., Denkin, S. M., and Zhang, Y. (2007). Aspirin antagonism in isoniazid treatment of tuberculosis in mice. Antimicrob. Agents Chemother. 51, 794–795. doi: 10.1128/AAC.01145-06
Chan, J., Tanaka, K., Carroll, D., Flynn, J., and Bloom, B. R. (1995). Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium Tuberculosis. Infect. Immun. 63, 736–740.
Chen, H.-M., Ma, G., Gildener-Leapman, N., Eisenstein, S., Coakley, B. A., Ozao, J., et al. (2015a). Myeloid-derived suppressor cells as an immune parameter in patients with concurrent sunitinib and stereotactic body radiotherapy. Clin. Cancer Res. 21, 4073–4085. doi: 10.1158/1078-0432.CCR-14-2742
Chen, J., Xu, T., and Chen, C. (2015b). The critical roles of miR-21 in anti-cancer effects of curcumin. Ann. Transl. Med. 3:330. doi: 10.3978/j.issn.2305-5839.2015.09.20
Cheng, P., Corzo, C. A., Luetteke, N., Yu, B., Nagaraj, S., Bui, M. M., et al. (2008). Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med. 205, 2235–2249. doi: 10.1084/jem.20080132
Coleman, M. M., Basdeo, S. A., Coleman, A. M., Ní Cheallaigh, C., Peral de Castro, C., McLaughlin, A. M., et al. (2018). All-trans retinoic acid augments autophagy during intracellular bacterial infection. Am. J. Respir. Cell Mol. Biol. doi: 10.1165/rcmb.2017-0382OC. [Epub ahead of print].
Condamine, T., Kumar, V., Ramachandran, I. R., Youn, J.-I., Celis, E., Finnberg, N., et al. (2014). ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis. J. Clin. Invest. 124, 2626–2639. doi: 10.1172/JCI74056
Condos, R., Raju, B., Canova, A., Zhao, B.-Y., Weiden, M., Rom, W. N., et al. (2003). Recombinant gamma interferon stimulates signal transduction and gene expression in alveolar macrophages in vitro and in tuberculosis patients. Infect. Immun. 71, 2058–2064. doi: 10.1128/IAI.71.4.2058-2064.2003
Condos, R., Rom, W. N., and Schluger, N. W. (1997). Treatment of multidrug-resistant pulmonary tuberculosis with interferon-gamma via aerosol. Lancet Lond. Engl. 349, 1513–1515. doi: 10.1016/S0140-6736(96)12273-X
Corazzi, T., Leone, M., Maucci, R., Corazzi, L., and Gresele, P. (2005). Direct and irreversible inhibition of cyclooxygenase-1 by nitroaspirin (NCX 4016). J. Pharmacol. Exp. Ther. 315, 1331–1337. doi: 10.1124/jpet.105.089896
Crowle, A. J., and Ross, E. J. (1989). Inhibition by retinoic acid of multiplication of virulent tubercle bacilli in cultured human macrophages. Infect. Immun. 57, 840–844.
Daker, S. E., Sacchi, A., Tempestilli, M., Carducci, C., Goletti, D., Vanini, V., et al. (2015). Granulocytic myeloid derived suppressor cells expansion during active pulmonary tuberculosis is associated with high nitric oxide plasma level. PLoS ONE 10:e0123772. doi: 10.1371/journal.pone.0123772
Daley-Bauer, L. P., Wynn, G. M., and Mocarski, E. S. (2012). Cytomegalovirus impairs antiviral CD8+ T cell immunity by recruiting inflammatory monocytes. Immunity 37, 122–133. doi: 10.1016/j.immuni.2012.04.014
Datta, M., Via, L. E., Kamoun, W. S., Liu, C., Chen, W., Seano, G., et al. (2015). Anti-vascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery. Proc. Natl. Acad. Sci. U.S.A. 112, 1827–1832. doi: 10.1073/pnas.1424563112
Dawson, R., Condos, R., Tse, D., Huie, M. L., Ress, S., Tseng, C.-H., et al. (2009). Immunomodulation with recombinant interferon-gamma1b in pulmonary tuberculosis. PLoS ONE 4:e6984. doi: 10.1371/journal.pone.0006984
de Oliveira Fulco, T., Andrade, P. R., de Mattos Barbosa, M. G., Pinto, T. G. T., Ferreira, P. F., Ferreira, H., et al. (2014). Effect of apoptotic cell recognition on macrophage polarization and mycobacterial persistence. Infect. Immun. 82, 3968–3978. doi: 10.1128/IAI.02194-14
Di Mitri, D., Toso, A., and Alimonti, A. (2015). Molecular pathways: targeting tumor-infiltrating myeloid-derived suppressor cells for cancer therapy. Clin. Cancer Res. 21, 3108–3112. doi: 10.1158/1078-0432.CCR-14-2261
Dolcetti, L., Peranzoni, E., Ugel, S., Marigo, I., Fernandez Gomez, A., Mesa, C., et al. (2010). Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur. J. Immunol. 40, 22–35. doi: 10.1002/eji.200939903
Dorhoi, A., and Du Plessis, N. (2018). Monocytic myeloid-derived suppressor cells in chronic infections. Front. Immunol. 8:1895. doi: 10.3389/fimmu.2017.01895
Draghiciu, O., Boerma, A., Hoogeboom, B. N., Nijman, H. W., and Daemen, T. (2015c). A rationally designed combined treatment with an alphavirus-based cancer vaccine, sunitinib and low-dose tumor irradiation completely blocks tumor development. Oncoimmunology 4:e1029699. doi: 10.1080/2162402X.2015.1029699
Draghiciu, O., Lubbers, J., Nijman, H. W., and Daemen, T. (2015a). Myeloid derived suppressor cells—an overview of combat strategies to increase immunotherapy efficacy. OncoImmunology 4:e954829. doi: 10.4161/21624011.2014.954829
Draghiciu, O., Nijman, H. W., Hoogeboom, B. N., Meijerhof, T., and Daemen, T. (2015b). Sunitinib depletes myeloid-derived suppressor cells and synergizes with a cancer vaccine to enhance antigen-specific immune responses and tumor eradication. Oncoimmunology 4:e989764. doi: 10.4161/2162402X.2014.989764
Drakaki, A., Koutsioumpa, M., O'Brien, N. A., Vorvis, C., Iliopoulos, D., and Slamon, D. J. (2017). A chemically-modified miR-21 inhibitor (ADM-21) as a novel potential therapy in bladder cancer. J. Clin. Oncol. 35, 335–335. doi: 10.1200/JCO.2017.35.6_suppl.335
du Plessis, N., Loebenberg, L., Kriel, M., von Groote-Bidlingmaier, F., Ribechini, E., Loxton, A. G., et al. (2013). Increased frequency of myeloid-derived suppressor cells during active tuberculosis and after recent Mycobacterium Tuberculosis infection suppresses T-cell function. Am. J. Respir. Crit. Care Med. 188, 724–732. doi: 10.1164/rccm.201302-0249OC
Etna, M. P., Sinigaglia, A., Grassi, A., Giacomini, E., Romagnoli, A., Pardini, M., et al. (2018). Mycobacterium tuberculosis-induced miR-155 subverts autophagy by targeting ATG3 in human dendritic cells. PLoS Pathog. 14:e1006790. doi: 10.1371/journal.ppat.1006790
Fabani, M. M., Abreu-Goodger, C., Williams, D., Lyons, P. A., Torres, A. G., Smith, K. G. C., et al. (2010). Efficient inhibition of miR-155 function in vivo by peptide nucleic acids. Nucleic Acids Res. 38, 4466–4475. doi: 10.1093/nar/gkq160
Feng, P.-H., Yu, C.-T., Chen, K.-Y., Luo, C.-S., Wu, S. M., Liu, C.-Y., et al. (2018). S100A9+ MDSC and TAM-mediated EGFR-TKI resistance in lung adenocarcinoma: the role of RELB. Oncotarget 9, 7631–7643. doi: 10.18632/oncotarget.24146
Fiorucci, S., Santucci, L., Cirino, G., Mencarelli, A., Familiari, L., Soldato, P. D., et al. (2000). IL-1 beta converting enzyme is a target for nitric oxide-releasing aspirin: new insights in the antiinflammatory mechanism of nitric oxide-releasing nonsteroidal antiinflammatory drugs. J. Immunol. 165, 5245–5254.
Freches, D., Korf, H., Denis, O., Havaux, X., Huygen, K., and Romano, M. (2013). Mice genetically inactivated in interleukin-17A receptor are defective in long-term control of Mycobacterium Tuberculosis infection. Immunology 140, 220–231. doi: 10.1111/imm.12130
Fujita, M., Kohanbash, G., Fellows-Mayle, W., Hamilton, R. L., Komohara, Y., Decker, S. A., et al. (2011). COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 71, 2664–2674. doi: 10.1158/0008-5472.CAN-10-3055
Gabrilovich, D. I., and Nagaraj, S. (2009). Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174. doi: 10.1038/nri2506
Gabrilovich, D. I., Ostrand-Rosenberg, S., and Bronte, V. (2012). Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268. doi: 10.1038/nri3175
Gabrilovich, D. I., Velders, M. P., Sotomayor, E. M., and Kast, W. M. (2001). Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J. Immunol. 166, 5398–5406. doi: 10.4049/jimmunol.166.9.5398
Gama, L., Shirk, E. N., Russell, J. N., Carvalho, K. I., Li, M., Queen, S. E., et al. (2012). Expansion of a subset of CD14highCD16negCCR2low/neg monocytes functionally similar to myeloid-derived suppressor cells during SIV and HIV infection. J. Leuk. Bio. 91, 803–816. doi: 10.1189/jlb.1111579
Gao, S. P., Mark, K. G., Leslie, K., Pao, W., Motoi, N., Gerald, W. L., et al. (2007). Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J. Clin. Invest. 117, 3846–3856. doi: 10.1172/JCI31871
Garg, A., and Spector, S. A. (2014). HIV type 1 gp120-induced expansion of myeloid derived suppressor cells is dependent on interleukin 6 and suppresses immunity. J. Infect. Dis. 209, 441–451. doi: 10.1093/infdis/jit469
Garg, A., Trout, R., and Spector, S. A. (2017). Human immunodeficiency virus type-1 myeloid derived suppressor cells inhibit cytomegalovirus inflammation through interleukin-27 and B7-H4. Sci. Rep. 7:44485. doi: 10.1038/srep44485
Gautam, U. S., Foreman, T. W., Bucsan, A. N., Veatch, A. V., Alvarez, X., Adekambi, T., et al. (2018). In vivo inhibition of tryptophan catabolism reorganizes the tuberculoma and augments immune-mediated control of Mycobacterium Tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 115, E62–E71. doi: 10.1073/pnas.1711373114
Giallongo, C., Parrinello, N., Tibullo, D., La Cava, P., Romano, A., Chiarenza, A., et al. (2014). Myeloid derived suppressor cells (MDSCs) are increased and exert immunosuppressive activity together with polymorphonuclear leukocytes (PMNs) in chronic myeloid leukemia patients. PLoS ONE 9:e101848. doi: 10.1371/journal.pone.0101848
Gopal, R., Monin, L., Torres, D., Slight, S., Mehra, S., McKenna, K. C., et al. (2013). S100A8/A9 proteins mediate neutrophilic inflammation and lung pathology during tuberculosis. Am. J. Respir. Crit. Care Med. 188, 1137–1146. doi: 10.1164/rccm.201304-0803OC
Harari, O., and Liao, J. K. (2004). Inhibition of MHC II gene transcription by nitric oxide and antioxidants. Curr. Pharm. Des. 10, 893–898. doi: 10.2174/1381612043452893
Hassan, S. S., Akram, M., King, E. C., Dockrell, H. M., and Cliff, J. M. (2015). PD-1, PD-L1 and PD-L2 gene expression on T-cells and natural killer cells declines in conjunction with a reduction in PD-1 protein during the intensive phase of tuberculosis treatment. PLoS ONE 10:e0137646. doi: 10.1371/journal.pone.0137646
He, D., Li, H., Yusuf, N., Elmets, C. A., Li, J., Mountz, J. D., et al. (2010). IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J. Immunol. 184, 2281–2288. doi: 10.4049/jimmunol.0902574
Heim, C. E., Vidlak, D., Scherr, T. D., Hartman, C. W., Garvin, K. L., and Kielian, T. (2015). IL-12 promotes myeloid-derived suppressor cell recruitment and bacterial persistence during Staphylococcus aureus orthopedic implant infection. J. Immunol. 194, 3861–3872. doi: 10.4049/jimmunol.1402689
Hernández-Pando, R., Orozco-Esteves, H., Maldonado, H. A., Aguilar-León, D., Vilchis-Landeros, M. M., Mata-Espinosa, D. A., et al. (2006). A combination of a transforming growth factor-β antagonist and an inhibitor of cyclooxygenase is an effective treatment for murine pulmonary tuberculosis. Clin. Exp. Immunol. 144, 264–272. doi: 10.1111/j.1365-2249.2006.03049.x
Hirsch, C. S., Rojas, R., Wu, M., and Toossi, Z. (2016). Mycobacterium Tuberculosis induces expansion of Foxp3 Positive CD4 T-cells with a regulatory profile in tuberculin non-sensitized healthy subjects: implications for effective immunization against TB. J. Clin. Cell. Immunol. 7:428. doi: 10.4172/2155-9899.1000428
Holmgaard, R. B., Zamarin, D., Li, Y., Gasmi, B., Munn, D. H., Allison, J. P., et al. (2015). Tumor-expressed IDO recruits and activates MDSCs in a Treg-dependent manner. Cell Rep. 13, 412–424. doi: 10.1016/j.celrep.2015.08.077
Huang, J., Jiao, J., Xu, W., Zhao, H., Zhang, C., Shi, Y., et al. (2015). MiR-155 is upregulated in patients with active tuberculosis and inhibits apoptosis of monocytes by targeting FOXO3. Mol. Med. Rep. 12, 7102–7108. doi: 10.3892/mmr.2015.4250
Iannaccone, M., Dorhoi, A., and Kaufmann, S. H. E. (2014). Host-directed therapy of tuberculosis: what is in it for microRNA? Expert Opin. Ther. Targets 18, 491–494. doi: 10.1517/14728222.2014.897696
Islam, M. M., Hameed, H. M. A., Mugweru, J., Chhotaray, C., Wang, C., Tan, Y., et al. (2017). Drug resistance mechanisms and novel drug targets for tuberculosis therapy. J. Genet. Genomics 44, 21–37. doi: 10.1016/j.jgg.2016.10.002
Iwai, H., Funatogawa, K., Matsumura, K., Kato-Miyazawa, M., Kirikae, F., Kiga, K., et al. (2015). MicroRNA-155 knockout mice are susceptible to Mycobacterium Tuberculosis infection. Tuberc. Edinb. Scotl. 95, 246–250. doi: 10.1016/j.tube.2015.03.006
Jiang, Y., Li, Y., and Zhu, B. (2015). T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 6:e1792. doi: 10.1038/cddis.2015.162
Kalman, D., Bisson, G., Gumbo, T., and Kaushal, D. (2017). Development of Gleevec for TB and TB/HIV. Grantome.
Kang, D. D., Lin, Y., Moreno, J.-R., Randall, T. D., and Khader, S. A. (2011). Profiling early lung immune responses in the mouse model of tuberculosis. PLoS ONE 6:e16161. doi: 10.1371/journal.pone.0016161
Kleffel, S., Posch, C., Barthel, S. R., Mueller, H., Schlapbach, C., Guenova, E., et al. (2015). Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell 162, 1242–1256. doi: 10.1016/j.cell.2015.08.052
Kleinovink, J. W., Marijt, K. A., Schoonderwoerd, M. J. A., van Hall, T., Ossendorp, F., and Fransen, M. F. (2017). PD-L1 expression on malignant cells is no prerequisite for checkpoint therapy. Oncoimmunology 6:e1294299. doi: 10.1080/2162402X.2017.1294299
Knaul, J. K., Jörg, S., Oberbeck-Mueller, D., Heinemann, E., Scheuermann, L., Brinkmann, V., et al. (2014). Lung-residing myeloid-derived suppressors display dual functionality in murine pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 190, 1053–1066. doi: 10.1164/rccm.201405-0828OC
Ko, J. S., Zea, A. H., Rini, B. I., Ireland, J. L., Elson, P., Cohen, P., et al. (2009). Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 15, 2148–2157. doi: 10.1158/1078-0432.CCR-08-1332
Kumar, V., Patel, S., Tcyganov, E., and Gabrilovich, D. I. (2016). The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 37, 208–220. doi: 10.1016/j.it.2016.01.004
Kusmartsev, S., Eruslanov, E., Kübler, H., Tseng, T., Sakai, Y., Su, Z., et al. (2008). Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J. Immunol. 181, 346–353. doi: 10.4049/jimmunol.181.1.346
Kuwata, T., Wang, I. M., Tamura, T., Ponnamperuma, R. M., Levine, R., Holmes, K. L., et al. (2000). Vitamin A deficiency in mice causes a systemic expansion of myeloid cells. Blood 95, 3349–3356.
Larson, R. P., Shafiani, S., and Urdahl, K. B. (2013). Foxp3(+) regulatory T cells in tuberculosis. Adv. Exp. Med. Biol. 783, 165–180. doi: 10.1007/978-1-4614-6111-1_9
Lathers, D. M. R., Clark, J. I., Achille, N. J., and Young, M. R. I. (2004). Phase 1B study to improve immune responses in head and neck cancer patients using escalating doses of 25-hydroxyvitamin D3. Cancer Immunol. Immunother. CII 53, 422–430. doi: 10.1007/s00262-003-0459-7
Lawson, L., Thacher, T. D., Yassin, M. A., Onuoha, N. A., Usman, A., Emenyonu, N. E., et al. (2010). Randomized controlled trial of zinc and vitamin A as co-adjuvants for the treatment of pulmonary tuberculosis. Trop. Med. Int. Health TM IH 15, 1481–1490. doi: 10.1111/j.1365-3156.2010.02638.x
Le, H. K., Graham, L., Cha, E., Morales, J. K., Manjili, M. H., and Bear, H. D. (2009). Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int. Immunopharmacol. 9, 900–909. doi: 10.1016/j.intimp.2009.03.015
Lesokhin, A. M., Hohl, T. M., Kitano, S., Cortez, C., Hirschhorn-Cymerman, D., Avogadri, F., et al. (2012). Monocytic CCR2+ myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res. 72, 876–886. doi: 10.1158/0008-5472.CAN-11-1792
Li, L., Zhang, J., Diao, W., Wang, D., Wei, Y., Zhang, C.-Y., et al. (2014). MicroRNA-155 and MicroRNA-21 promote the expansion of functional myeloid-derived suppressor cells. J. Immunol. 192, 1034–1043. doi: 10.4049/jimmunol.1301309
Lombard, R., Doz, E., Carreras, F., Epardaud, M., Le Vern, Y., Buzoni-Gatel, D., et al. (2016). IL-17RA in non-hematopoietic cells controls CXCL-1 and 5 critical to recruit neutrophils to the lung of mycobacteria-infected mice during the adaptive immune response. PLoS ONE 11:e0149455. doi: 10.1371/journal.pone.0149455
Luo, X. M., and Ross, A. C. (2006). Retinoic acid exerts dual regulatory actions on the expression and nuclear localization of interferon regulatory factor-1. Exp. Biol. Med. Maywood NJ. 231, 619–631. doi: 10.1177/153537020623100517
Ma, J., Liu, Y., Li, Y., Gu, J., Liu, J., Tang, J., et al. (2014). Differential role of all-trans retinoic acid in promoting the development of CD4+ and CD8+ regulatory T cells. J. Leukoc. Biol. 95, 275–283. doi: 10.1189/jlb.0513297
Ma, N., Liu, Q., Hou, L., Wang, Y., and Liu, Z. (2017). MDSCs are involved in the protumorigenic potentials of GM-CSF in colitis-associated cancer. Int. J. Immunopathol. Pharmacol. 30, 152–162. doi: 10.1177/0394632017711055
Maemondo, M., Inoue, A., Kobayashi, K., Sugawara, S., Oizumi, S., Isobe, H., et al. (2010). Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 362, 2380–2388. doi: 10.1056/NEJMoa0909530
Maiga, M., Ammerman, N. C., Maiga, M. C., Tounkara, A., Siddiqui, S., Polis, M., et al. (2013). Adjuvant host-directed therapy with types 3 and 5 but not type 4 phosphodiesterase inhibitors shortens the duration of tuberculosis treatment. J. Infect. Dis. 208, 512–519. doi: 10.1093/infdis/jit187
Maiga, M. C., Ahidjo, B. A., Maiga, M., and Bishai, W. R. (2015). Roflumilast, a type 4 phosphodiesterase inhibitor, shows promising adjunctive, host-directed therapeutic activity in a mouse model of tuberculosis. Antimicrob. Agents Chemother. 59, 7888–7890. doi: 10.1128/AAC.02145-15
Mannick, J. B., Hausladen, A., Liu, L., Hess, D. T., Zeng, M., Miao, Q. X., et al. (1999). Fas-induced caspase denitrosylation. Science 284, 651–654. doi: 10.1126/science.284.5414.651
Mason, C., Poretta, E., Bishai, W., Ali, J., and Zea, A. (2015). “cAMP-induced arginase and their role in Mycobacterium tuberculosis growth: Emerging of new adjunctive therapies,” in Front. Immunol. Conference Abstract: IMMUNOCOLOMBIA2015 - 11th Congress of the Latin American Association of Immunology - 10o. Congreso de la Asociación Colombiana de Alergia, Asma e Inmunología. doi: 10.3389/conf.fimmu.2015.05.00302
Molon, B., Ugel, S., Del Pozzo, F., Soldani, C., Zilio, S., Avella, D., et al. (2011). Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J. Exp. Med. 208, 1949–1962. doi: 10.1084/jem.20101956
Nagaraj, S., Gupta, K., Pisarev, V., Kinarsky, L., Sherman, S., Kang, L., et al. (2007). Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med. 13, 828–835. doi: 10.1038/nm1609
Nakanishi, M., Tomaru, Y., Miura, H., Hayashizaki, Y., and Suzuki, M. (2008). Identification of transcriptional regulatory cascades in retinoic acid-induced growth arrest of HepG2 cells. Nucleic Acids Res. 36, 3443–3454. doi: 10.1093/nar/gkn066
Napier, R. J., Rafi, W., Cheruvu, M., Powell, K. R., Zaunbrecher, M. A., Bornmann, W., et al. (2011). Imatinib-sensitive tyrosine kinases regulate mycobacterial pathogenesis and represent therapeutic targets against tuberculosis. Cell Host Microbe. 10, 475–485. doi: 10.1016/j.chom.2011.09.010
Nefedova, Y., Fishman, M., Sherman, S., Wang, X., Beg, A. A., and Gabrilovich, D. I. (2007). Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res. 67, 11021–11028. doi: 10.1158/0008-5472.CAN-07-2593
Nicolas, C. S., Peineau, S., Amici, M., Csaba, Z., Fafouri, A., Javalet, C., et al. (2012). The JAK/STAT pathway is involved in synaptic plasticity. Neuron 73, 374–390. doi: 10.1016/j.neuron.2011.11.024
Noonan, K. A., Ghosh, N., Rudraraju, L., Bui, M., and Borrello, I. (2014). Targeting immune suppression with PDE5 inhibition in end-stage multiple myeloma. Cancer Immunol. Res. 2, 725–731. doi: 10.1158/2326-6066.CIR-13-0213
Obregón-Henao, A., Henao-Tamayo, M., Orme, I. M., and Ordway, D. J. (2013). Gr1intCD11b+ myeloid-derived suppressor cells in Mycobacterium Tuberculosis infection. PLoS ONE 8:e80669. doi: 10.1371/journal.pone.0080669
O'Connor, M. A., Vella, J. L., and Green, W. R. (2016). Reciprocal relationship of T regulatory cells and monocytic myeloid-derived suppressor cells in LP-BM5 murine retrovirus-induced immunodeficiency. J. Gen. Virol. 97, 509–522. doi: 10.1099/jgv.0.000260
O'Donnell, J. S., Long, G. V., Scolyer, R. A., Teng, M. W. L., and Smyth, M. J. (2017). Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat. Rev. 52, 71–81. doi: 10.1016/j.ctrv.2016.11.007
Okada, M., Kita, Y., Kanamaru, N., Hashimoto, S., Uchiyama, Y., Mihara, M., et al. (2011). Anti-IL-6 receptor antibody causes less promotion of tuberculosis infection than anti-TNF-α antibody in mice. Clin. Dev. Immunol. 2011:404929. doi: 10.1155/2011/404929
Ostrand-Rosenberg, S., and Sinha, P. (2009). Myeloid-derived suppressor cells: linking inflammation and cancer. J. Immunol. 182, 4499–4506. doi: 10.4049/jimmunol.0802740
Park, S.-K., Cho, S., Lee, I.-H., Jeon, D.-S., Hong, S.-H., Smego, R. A., et al. (2007). Subcutaneously administered interferon-gamma for the treatment of multidrug-resistant pulmonary tuberculosis. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 11, 434–440. doi: 10.1016/j.ijid.2006.12.004
Periasamy, S., Avram, D., McCabe, A., MacNamara, K. C., Sellati, T. J., and Harton, J. A. (2016). An immature myeloid/myeloid-suppressor cell response associated with necrotizing inflammation mediates lethal pulmonary tularemia. PLoS Pathog. 12:e1005517. doi: 10.1371/journal.ppat.1005517
Pilon-Thomas, S., Mackay, A., Vohra, N., and Mulé, J. J. (2010). Blockade of programmed death ligand 1 enhances the therapeutic efficacy of combination immunotherapy against melanoma. J. Immunol. Baltim. Md 1950 184, 3442–3449. doi: 10.4049/jimmunol.0904114
Pires, D., Bernard, E. M., Pombo, J. P., Carmo, N., Fialho, C., Gutierrez, M. G., et al. (2017). Mycobacterium Tuberculosis modulates miR-106b-5p to control cathepsin S expression resulting in higher pathogen survival and poor T-cell activation. Front. Immunol. 8:1819. doi: 10.3389/fimmu.2017.01819
Qin, H., Wei, G., Gwak, D., Dong, Z., Xiong, A., and Kwak, L. W. (2015). Targeting tumor-associated myeloid cells for cancer immunotherapy. Oncoimmunology 4:e983961. doi: 10.4161/2162402X.2014.983761
Reungwetwattana, T., and Adjei, A. A. (2016). Anti–PD-1 antibody treatment and the development of acute pulmonary tuberculosis. J. Thorac. Oncol. 11, 2048–2050. doi: 10.1016/j.jtho.2016.10.008
Robinson, R. T. (2017). T Cell production of GM-CSF protects the host during experimental tuberculosis. MBio 8:8652. doi: 10.1128/mBio.02087-17
Rodriguez, P. C., Hernandez, C. P., Quiceno, D., Dubinett, S. M., Zabaleta, J., Ochoa, J. B., et al. (2005). Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J. Exp. Med. 202, 931–939. doi: 10.1084/jem.20050715
Rodriguez, P. C., Quiceno, D. G., Zabaleta, J., Ortiz, B., Zea, A. H., Piazuelo, M. B., et al. (2004). Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 64, 5839–5849. doi: 10.1158/0008-5472.CAN-04-0465
Rodriguez, P. C., Zea, A. H., DeSalvo, J., Culotta, K. S., Zabaleta, J., Quiceno, D. G., et al. (2003). L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J. Immunol. 171, 1232–1239.
Rothchild, A. C., Stowell, B., Goyal, G., Nunes-Alves, C., Yang, Q., Papavinasasundaram, K., et al. (2017). Role of granulocyte-macrophage colony-stimulating factor production by T cells during Mycobacterium tuberculosis infection. MBio 8:e01514-17. doi: 10.1128/mBio.01514-17
Sansone, P., and Bromberg, J. (2012). Targeting the interleukin-6/Jak/stat pathway in human malignancies. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 30, 1005–1014. doi: 10.1200/JCO.2010.31.8907
Schmielau, J., and Finn, O. J. (2001). Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 61, 4756–4760.
Segueni, N., Tritto, E., Bourigault, M.-L., Rose, S., Erard, F., Bert, M. L., et al. (2016). Controlled Mycobacterium tuberculosis infection in mice under treatment with anti-IL-17A or IL-17F antibodies, in contrast to TNFa neutralization. Sci. Rep. 6:36923. doi: 10.1038/srep36923
Serafini, P., Meckel, K., Kelso, M., Noonan, K., Califano, J., Koch, W., et al. (2006). Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J. Exp. Med. 203, 2691–2702. doi: 10.1084/jem.20061104
Sharma, P., and Allison, J. P. (2015a). Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214. doi: 10.1016/j.cell.2015.03.030
Sharma, P., and Allison, J. P. (2015b). The future of immune checkpoint therapy. Science 348, 56–61. doi: 10.1126/science.aaa8172
Shen, L., Gao, Y., Liu, Y., Zhang, B., Liu, Q., Wu, J., et al. (2016). PD-1/PD-L pathway inhibits M.tb-specific CD4+ T-cell functions and phagocytosis of macrophages in active tuberculosis. Sci. Rep. 6:38362. doi: 10.1038/srep38362
Shipp, C., Speigl, L., Janssen, N., Martens, A., and Pawelec, G. (2016). A clinical and biological perspective of human myeloid-derived suppressor cells in cancer. Cell. Mol. Life Sci. CMLS 73, 4043–4061. doi: 10.1007/s00018-016-2278-y
Silver, R. F., Myers, A. J., Jarvela, J., Flynn, J., Rutledge, T., Bonfield, T., et al. (2016). Diversity of human and macaque airway immune cells at baseline and during tuberculosis infection. Am. J. Respir. Cell Mol. Biol. 55, 899–908. doi: 10.1165/rcmb.2016-0122OC
Singh, A., Dey, A. B., Mohan, A., Sharma, P. K., and Mitra, D. K. (2012). Foxp3+ regulatory T cells among tuberculosis patients: impact on prognosis and restoration of antigen specific IFN-γ producing T cells. PLoS ONE 7:e44728. doi: 10.1371/journal.pone.0044728
Singh, V., Brecik, M., Mukherjee, R., Evans, J. C., Svetlíková, Z., Blaško, J., et al. (2015). The complex mechanism of antimycobacterial action of 5-fluorouracil. Chem. Biol. 22, 63–75. doi: 10.1016/j.chembiol.2014.11.006
Sinha, P., Okoro, C., Foell, D., Freeze, H. H., Ostrand-Rosenberg, S., and Srikrishna, G. (2008). Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J. Immunol. 181, 4666–4675. doi: 10.4049/jimmunol.181.7.4666
Sogi, K. M., Lien, K. A., Johnson, J. R., Krogan, N. J., and Stanley, S. A. (2017). The tyrosine kinase inhibitor gefitinib restricts mycobacterium tuberculosis growth through increased lysosomal biogenesis and modulation of cytokine signaling. ACS Infect. Dis. 3, 564–574. doi: 10.1021/acsinfecdis.7b00046
Stanley, S. A., Barczak, A. K., Silvis, M. R., Luo, S. S., Sogi, K., Vokes, M., et al. (2014). Identification of host-targeted small molecules that restrict intracellular Mycobacterium tuberculosis growth. PLoS Pathog. 10:e1003946. doi: 10.1371/journal.ppat.1003946
Suárez-Méndez, R., García-García, I., Fernández-Olivera, N., Valdés-Quintana, M., Milanés-Virelles, M. T., Carbonell, D., et al. (2004). Adjuvant interferon gamma in patients with drug - resistant pulmonary tuberculosis: a pilot study. BMC Infect. Dis. 4:44. doi: 10.1186/1471-2334-4-44
Subbian, S., Tsenova, L., Holloway, J., Peixoto, B., O'Brien, P., Dartois, V., et al. (2016). Adjunctive phosphodiesterase-4 inhibitor therapy improves antibiotic response to pulmonary tuberculosis in a rabbit model. EBioMed. 4, 104–114. doi: 10.1016/j.ebiom.2016.01.015
Subbian, S., Tsenova, L., O'Brien, P., Yang, G., Koo, M.-S., Peixoto, B., et al. (2011). Phosphodiesterase-4 inhibition alters gene expression and improves isoniazid-mediated clearance of Mycobacterium Tuberculosis in rabbit lungs. PLoS Pathog. 7:e1002262. doi: 10.1371/journal.ppat.1002262
Sumida, K., Wakita, D., Narita, Y., Masuko, K., Terada, S., Watanabe, K., et al. (2012). Anti-IL-6 receptor mAb eliminates myeloid-derived suppressor cells and inhibits tumor growth by enhancing T-cell responses. Eur. J. Immunol. 42, 2060–2072. doi: 10.1002/eji.201142335
Suzuki, E., Kapoor, V., Jassar, A. S., Kaiser, L. R., and Albelda, S. M. (2005). Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 11, 6713–6721. doi: 10.1158/1078-0432.CCR-05-0883
Talaue, M. T., Venketaraman, V., Hazbón, M. H., Peteroy-Kelly, M., Seth, A., Colangeli, R., et al. (2006). Arginine homeostasis in J774.1 macrophages in the context of Mycobacterium Bovis BCG infection. J. Bacteriol. 188, 4830–4840. doi: 10.1128/JB.01687-05
Tartour, E., Pere, H., Maillere, B., Terme, M., Merillon, N., Taieb, J., et al. (2011). Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev. 30, 83–95. doi: 10.1007/s10555-011-9281-4
Tebartz, C., Horst, S. A., Sparwasser, T., Huehn, J., Beineke, A., Peters, G., et al. (2015). A major role for myeloid-derived suppressor cells and a minor role for regulatory T cells in immunosuppression during Staphylococcus aureus infection. J. Immunol. 194, 1100–1111. doi: 10.4049/jimmunol.1400196
Tonby, K., Wergeland, I., Lieske, N. V., Kvale, D., Tasken, K., and Dyrhol-Riise, A. M. (2016). The COX- inhibitor indomethacin reduces Th1 effector and T regulatory cells in vitro in Mycobacterium Tuberculosis infection. BMC Infect. Dis. 16:599. doi: 10.1186/s12879-016-1938-8
Veltman, J. D., Lambers, M. E. H., van Nimwegen, M., Hendriks, R. W., Hoogsteden, H. C., Aerts, J. G. J. V., et al. (2010). COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer 10:464. doi: 10.1186/1471-2407-10-464
Vincent, J., Mignot, G., Chalmin, F., Ladoire, S., Bruchard, M., Chevriaux, A., et al. (2010). 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 70, 3052–3061. doi: 10.1158/0008-5472.CAN-09-3690
Visser, M. E., Grewal, H. M., Swart, E. C., Dhansay, M. A., Walzl, G., Swanevelder, S., et al. (2011). The effect of vitamin A and zinc supplementation on treatment outcomes in pulmonary tuberculosis: a randomized controlled trial. Am. J. Clin. Nutr. 93, 93–100. doi: 10.3945/ajcn.110.001784
Vogt, G., and Nathan, C. (2011). In vitro differentiation of human macrophages with enhanced antimycobacterial activity. J. Clin. Invest. 121, 3889–3901. doi: 10.1172/JCI57235
Vollbrecht, T., Stirner, R., Tufman, A., Roider, J., Huber, R. M., Bogner, J. R., et al. (2012). Chronic progressive HIV-1 infection is associated with elevated levels of myeloid-derived suppressor cells. Aids. 26, F31–F37. doi: 10.1097/QAD.0b013e328354b43f
Wagh, V., Urhekar, A., and Modi, D. (2017). Levels of microRNA miR-16 and miR-155 are altered in serum of patients with tuberculosis and associate with responses to therapy. Tuberc. Edinb. Scotl. 102, 24–30. doi: 10.1016/j.tube.2016.10.007
Walkley, C. R., Yuan, Y.-D., Chandraratna, R. A., and McArthur, G.A. (2002). Retinoic acid receptor antagonism in vivo expands the numbers of precursor cells during granulopoiesis. Leukemia 16, 1763–1772. doi: 10.1038/sj.leu.2402625
Wallis, R. S. (2009). Infectious complications of tumor necrosis factor blockade. Curr. Opin. Infect. Dis. 22, 403–409. doi: 10.1097/QCO.0b013e32832dda55
Wang, X.-F., Wang, H.-S., Wang, H., Zhang, F., Wang, K.-F., Guo, Q., et al. (2014). The role of indoleamine 2,3-dioxygenase (IDO) in immune tolerance: focus on macrophage polarization of THP-1 cells. Cell. Immunol. 289, 42–48. doi: 10.1016/j.cellimm.2014.02.005
Wheelwright, M., Kim, E. W., Inkeles, M. S., De Leon, A., Pellegrini, M., Krutzik, S. R., et al. (2014). All-trans retinoic acid triggered antimicrobial activity against Mycobacterium Tuberculosis is dependent on NPC2. J. Immunol. 192, 2280–2290. doi: 10.4049/jimmunol.1301686
Wu, G., and Morris, S. M. (1998). Arginine metabolism: nitric oxide and beyond. Biochem. J. 336 (Pt 1), 1–17. doi: 10.1042/bj3360001
Wu, W.-Y., Li, J., Wu, Z.-S., Zhang, C.-L., and Meng, X.-L. (2011). STAT3 activation in monocytes accelerates liver cancer progression. BMC Cancer 11:506. doi: 10.1186/1471-2407-11-506
Wu, Z., Lu, H., Sheng, J., and Li, L. (2012). Inductive microRNA-21 impairs anti-mycobacterial responses by targeting IL-12 and Bcl-2. FEBS Lett. 586, 2459–2467. doi: 10.1016/j.febslet.2012.06.004
Xin, H., Zhang, C., Herrmann, A., Du, Y., Figlin, R., and Yu, H. (2009). Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 69, 2506–2513. doi: 10.1158/0008-5472.CAN-08-4323
Xing, Y.-F., Zhou, Y.-Q., Ma, G.-W., Feng, D.-Y., Cai, X.-R., and Li, X. (2016). Issues with anti-Gr1 antibody-mediated myeloid-derived suppressor cell depletion. Ann. Rheum. Dis. 75:e49. doi: 10.1136/annrheumdis-2016-209786
Yang, L. (2009). Indoleamine 2,3 Dioxygenase (IDO) as a Mediator of Myeloid Derived Suppressor Cell Function in Breast Cancer. Baltimore, MD: Maryland Univ Baltimore County Baltimore Md).
Youn, J.-I., Nagaraj, S., Collazo, M., and Gabrilovich, D. I. (2008). Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 181, 5791–5802. doi: 10.4049/jimmunol.181.8.5791
Keywords: regulatory myeloid cells, myeloid-derived suppressor cells, Mycobacterium tuberculosis, host-directed therapy, immunotherapy
Citation: du Plessis N, Kotze LA, Leukes V and Walzl G (2018) Translational Potential of Therapeutics Targeting Regulatory Myeloid Cells in Tuberculosis. Front. Cell. Infect. Microbiol. 8:332. doi: 10.3389/fcimb.2018.00332
Received: 26 March 2018; Accepted: 28 August 2018;
Published: 21 September 2018.
Edited by:
Frank Brombacher, International Centre for Genetic Engineering and Biotechnology (ICGEB), South AfricaReviewed by:
Elsa Anes, Universidade de Lisboa, PortugalTracey L. Bonfield, Case Western Reserve University, United States
Copyright © 2018 du Plessis, Kotze, Leukes and Walzl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nelita du Plessis, bmVsaXRhQHN1bi5hYy56YQ==
 Nelita du Plessis
Nelita du Plessis Leigh A. Kotze
Leigh A. Kotze Vinzeigh Leukes
Vinzeigh Leukes Gerhard Walzl
Gerhard Walzl