- 1Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 2Diagnostic Microbiology and Antimicrobial Resistance Laboratory, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 3Infection Control Center, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 4Department of Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 5Clinical Medicine Research Center, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 6Department of Microbiology and Immunology, College of Medicine, National Cheng Kung University, Tainan, Taiwan
This prospective study aimed to investigate the clinical and microbiological characteristics of different Aeromonas species. Clinical isolates of Aeromonas species between 2016 to 2018 were collected in a university hospital in southern Taiwan. The species was determined by rpoD or gyrB sequencing. A total of 222 Aeromonas isolates from 160 patients in 164 episodes were identified. The crude in-hospital mortality was 17.2%. The most frequently isolated species was Aeromonas veronii (30.6%), followed by A. caviae (24.8%), A. hydrophila (23%), and A. dhakensis (16.7%). The major clinical manifestations were primary bacteremia (31.1%), skin and soft tissue infection (22.6%), and biliary tract infection (18.3%). The most common underlying diseases were malignancy (45.1%), diabetes mellitus (27.4%), and liver cirrhosis or chronic hepatitis (26.2%). A. hydrophila and A. dhakensis predominated in the skin and soft tissue infection (p<0.0001), whereas A. vernoii and A. caviae prevailed in primary bacteremia and biliary tract infections (p=0.012). Pneumonia, malignancy, and ascF-ascG genotype were independent factors associated with mortality. Ertapenem susceptibility was decreased in A. sobria (42.9%), A. veronii (66.7%), A. dhakensis (73%), and A. hydrophila (84.3%). Cefotaxime resistance was found in 30.9% of A. caviae and 18.9% of A. dhakensis isolates, much more prevalent than the other species. The metallo-β-lactamase blaCphA was almost invariably present in A. dhakensis, A. hydrophila, and A. veronii (100%, 100% and 89.9%, respectively). Amp-C β-lactamases such as blaMOX and blaAQU-1 were identified in all A. caviae and 91.9% of A. dhakensis isolates. Cefepime, fluoroquinolones and tigecycline showed good in vitro activity against aeromonads.
Introduction
The Aeromonas species are Gram-negative, rod-shaped bacteria that inhabited soil and aquatic environment ubiquitously, from fresh and brackish water, seawater, groundwater, sewage to drinking water. In addition, they were also found in fish and seafood, dairy, meats, and vegetables intended for human consumption (Fernández-Bravo and Figueras, 2020). They cause a wide spectrum of diseases in humans, notably acute gastroenteritis, septicemia, and soft tissue infections, as well as hepatobiliary tract infections, peritonitis, respiratory tract infections, urogenital tract infections, indwelling-device related infections, ocular infections, meningitis, and hemolytic uremic syndrome (Janda and Abbott, 2010).
The pathogenicity of aeromonads is complex owing to their multiple virulence factors acting collectively or separately, including structural components like flagella, adhesins, lipopolysaccharide and capsule, extracellular enzymes like lipases, proteases, elastases, and hemolytic enzymes that cause cell and tissue damage, enterotoxins that induce diarrhea, and most importantly the type III secretion system (T3SS) that injects toxins directly into host cells. The T3SS is composed of thorn-shaped or syringe structure, effector proteins that are injected, and chaperones that assist and protect structural and effector proteins during transport (Tomás, 2012; Rasmussen-Ivey et al., 2016; Gonçalves Pessoa et al., 2019). AscV serves as an indicator for the presence of the type III secretion machinery. AscF-AscG serves as translocation apparatus (Vilches et al., 2004; Chacón et al., 2004; Burr and Frey, 2007). AexT is an effector protein possessing ADP-ribosyltransferase and GTPase acting protein activities and is homologous to the Pseudomonas aeruginosa effector ExoT/ExoS (Braun et al., 2002; Tomás, 2012). The cytotoxic enterotoxin Act provokes the degeneration of intestinal epithelium and leads to bloody diarrhea, while the cytotonic enterotoxins, including heat-stable type Ast and heat-labile type Alt, cause non-bloody diarrhea (Gonçalves Pessoa et al., 2019).
Southern Taiwan locates in a subtropical area and is an Aeromonas-prevalent region, with an incidence of Aeromonas bacteremia of 76 per million inhabitants per year, much higher than that in western countries with an annual incidence of merely up to 1.5 per million (Wu et al., 2014). Historically, A. hydrophila had been the most common species isolated in bacteremia in Southern Taiwan (Ko and Chuang, 1995; Ko et al., 2000; Tang et al., 2014), but recent advances in molecular studies based on 16s RNA (Martínez-Murcia et al., 1992), housekeeping genes (Yáñez et al., 2003; Soler et al., 2004; Martínez-Murcia et al., 2011), and genome sequencing (Colston et al., 2014), had led to the reclassification of aeromonads. As a result, the reported prevalence of the most predominant clinical species of Aeromonas has changed over the years, with most (96.5%) of the aeromonads associated with clinical cases identified as A. caviae (29.9%), A. dhakensis (26.3%), A. veronii (24.8%), and A. hydrophila (15.5%) (Fernández-Bravo and Figueras, 2020). Besides, concordance was low between phylogenetic identification and the commercial identification systems, with incorrect identification at species level (Lamy et al., 2010). For example, it could be difficult to separate A. veronii biovar sobria from A. hydrophila using conventional biochemical tests. A. veronii biovar sobria shares common phenotypes with A. sobria sensu stricto and was often reported mistakenly as A. sobria (Janda and Abbott, 2010). A. dhakensis was mistaken as A. hydrophila for decades and is often misidentified as A. hydrophila, A. veronii, or A. caviae by commercial phenotypic tests (Chen et al., 2016). Since 16s RNA is highly conserved in aeromonads, housekeeping genes like gyrB (subunit B of DNA gyrase) and rpoD (sigma factor S70) offer less mean sequence similarity values and hence higher resolution than the 16s RNA gene (Yáñez et al., 2003; Soler et al., 2004; Martínez-Murcia et al., 2011).
In this prospective study, we investigated patients with clinical isolates of Aeromonas species determined by DNA sequence matching of rpoD or gyrB between 2016 to 2018 in a medical center in Southern Taiwan. The demographic factors, clinical outcome, drugs susceptibility of Aeromonas isolates, and the prevalence of genes responsible for drug resistance and virulence were analyzed. The study aimed to provide a better understanding of the association between clinical spectrum and different Aeromonas species determined by molecular typing.
Methods
Patients
Aeromonas isolates in National Cheng Kung University Hospital, a university-affiliated medical center with approximately 1200 beds located in Tainan, Taiwan, were collected from January 2016 to December 2018. The study was ethically approved by The Institutional Review Board of National Cheng Kung University Hospital (IRB no. A-ER-104-352). Medical chart records were reviewed retrospectively, and information collected included underlying diseases, sites from which specimens were obtained for culture, infectious diseases caused by Aeromonas species, and clinical outcomes. The requirement for informed consent was waived by the Institution Review Board.
Species Identification
A total of 222 isolates were available for analysis and stored at -70°C until use. The Aeromonas isolates were identified by the MALDI-TOF MS V2.0 (bioMérieux, Marcy-l’Étoile, France), and species identification of each Aeromonas isolates was determined based on the partial sequences of rpoD (and gyrB, if necessary) (Yáñez et al., 2003; Soler et al., 2004). The sequences amplified were compared with reference sequences from the GenBank database using BLAST (http://www.ncbi.nlm.nih.gov/BLAST/). Isolates with a dissimilarity value of ≤1% were considered the same species.
Detection of Resistance Genes and Virulence Factors
Genes contributing to antibiotic resistance and virulence were detected by polymerase chain reaction (PCR) using previously described primers. Resistance genes included AmpC β-lactamases blaAQU-1 (Wu et al., 2013) and blaMOX-like (Wu et al., 2015), metallo-β-lactamases (MBL) blaCphA (Wu et al., 2012), blaKPC, blaIMP, blaVIM, blaNDM, blaOXA-23-like and blaOXA-48-like, and extended-spectrum β-lactamases blaTEM, blaPER, blaSHV, and blaCTX-M (Wu et al., 2011). Virulence factors included the polar flagellum (fla), collagenase (col), lipase (lip), elastase (ela), aerolysin (aerA), hemolysin (hlyA), heat-stable enterotoxin (ast), heat-labile enterotoxin (alt), cytotoxic enterotoxin (act), and three components of T3SS, ascV, ascF-ascG, and aexT.
Antimicrobial Drug Susceptibility Testing
The antimicrobial drug susceptibility testing was determined by the disk diffusion test and interpreted following the Clinical and Laboratory Standards Institute (CLSI) recommendations for A. hydrophila complex (Clinical and Laboratory Standards Institute, 2016). The criteria for tigecycline susceptibility followed the U. S. Food and Drug Administration criteria for Enterobacteriaceae.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation (S.D.) and compared using the Wilcoxon Rank Sum test or the Student’s independent t-test, as appropriate. Categorical variables were compared using the Chi-square test or Fisher’s exact test if the expected counts were less than five. A p-value < 0.05 was considered statistically significant. Those variables with a P-value < 0.05 in the univariate analyses were put into a multivariate logistic regression model to adjust for confounding. Statistical analyses were conducted using the statistical package SPSS for Windows (version 22.0, SPSS, Chicago, IL, USA).
Results
Patient Characteristics
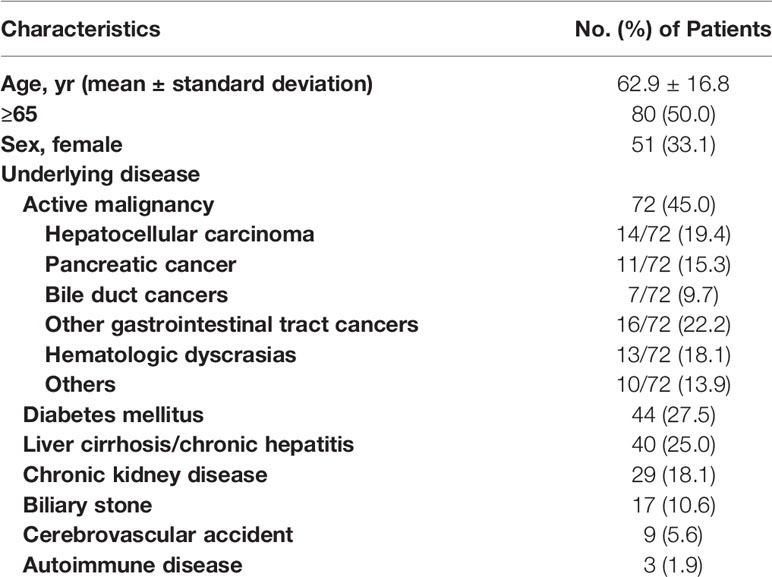
During the study period, a total of 222 Aeromonas isolates were obtained from 160 patients. Four patients had recurrent episodes of Aeromonas infection at least 180 days apart within the study period, yielding a total of 164 episodes. The demographic data and clinical characteristics of the patients are summarized in Table 1. The mean age was 62.9 (S.D. 16.8) years, ranging from 4 months to 93 years. Male patients outnumbered female patients (109/160, 68.1%). The major underlying diseases were active malignancy (72/160, 45.0%), followed by diabetes mellitus (44/160, 27.5%), liver cirrhosis or chronic hepatitis (40/160, 25.0%), and chronic kidney diseases including those receiving renal replacement therapy (29/160, 18.1%). The most common type of cancer in patients with active malignancies was hepatocellular carcinoma (14/72, 19.4%), followed by pancreatic cancer (11/72, 15.3%). Most of the patients in these episodes (145/164, 88.4%) were hospitalized. Ninety-six (58.5%) of the episodes were polymicrobial infections mixed with other bacteria. Seventy-one (43.3%) of the episodes presented with bacteremia. The crude in-hospital mortality was 17.2% (28/163, one missing due to transference to another hospital). As shown in Figure 1, the most common clinical manifestations were primary bacteremia (51/164, 31.1%), skin and soft tissue infection (SSTI, 37/164, 22.6%), and biliary tract infection (BTI, 30/164, 18.3%). Biliary tract infection was associated with biliary stones (p=0.001) and pancreatobiliary cancers (including pancreatic cancer, cholangiocarcinoma, and ampullary cancer, p<0.0001), but not with liver cirrhosis/chronic hepatitis (p=0.17) or hepatocellular carcinoma (p=0.72).
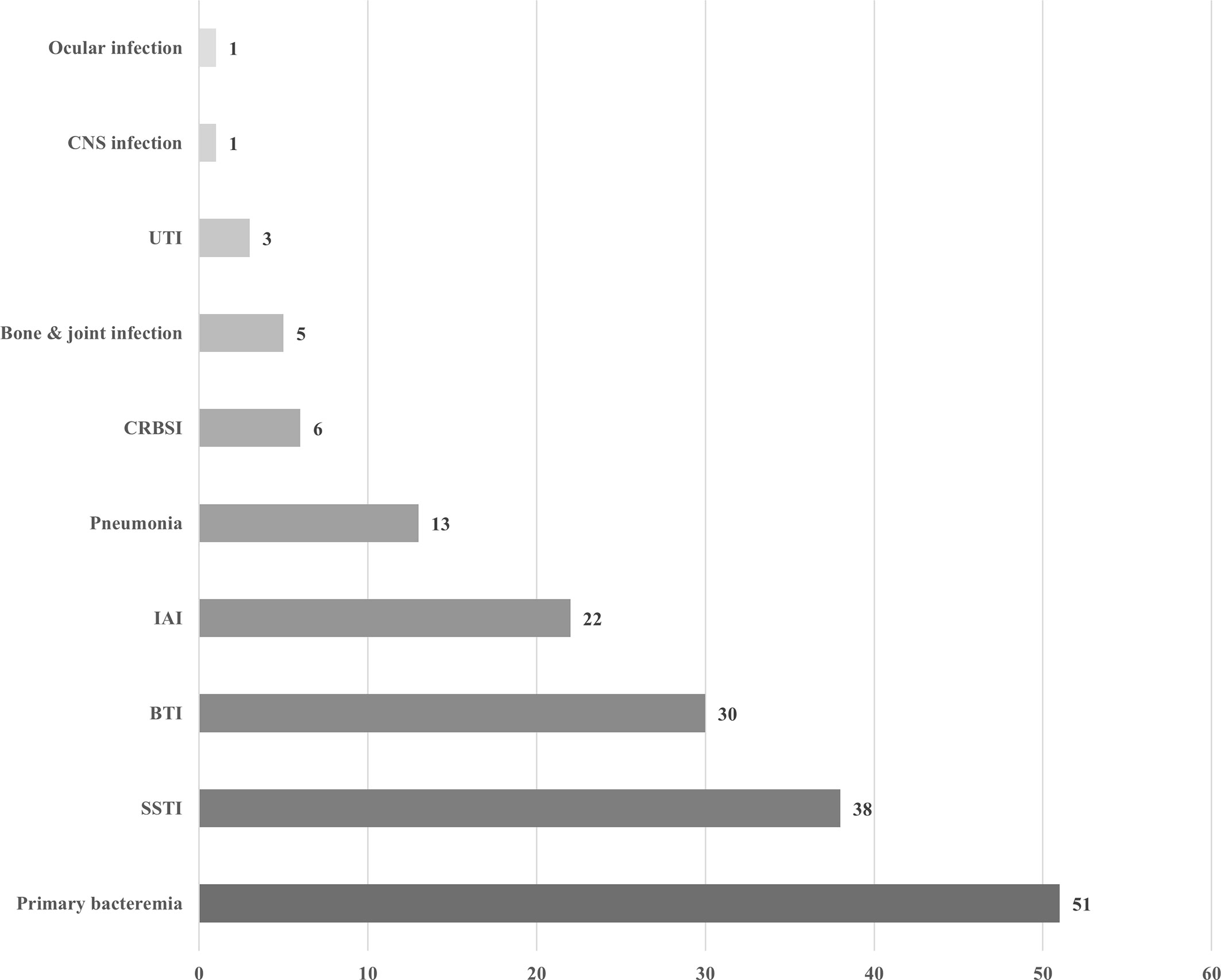
Figure 1 Clinical manifestations of 164 episodes of Aeromonas infection. UTI, urinary tract infection; CRBSI, catheter-related bloodstream infection; IAI, intra-abdominal infection; BTI, biliary tract infection; SSTI, skin and soft tissue infection.
Comparison of the rpoD Sequencing and the MALDI-TOF MS System for Identification of Aeromonas Species
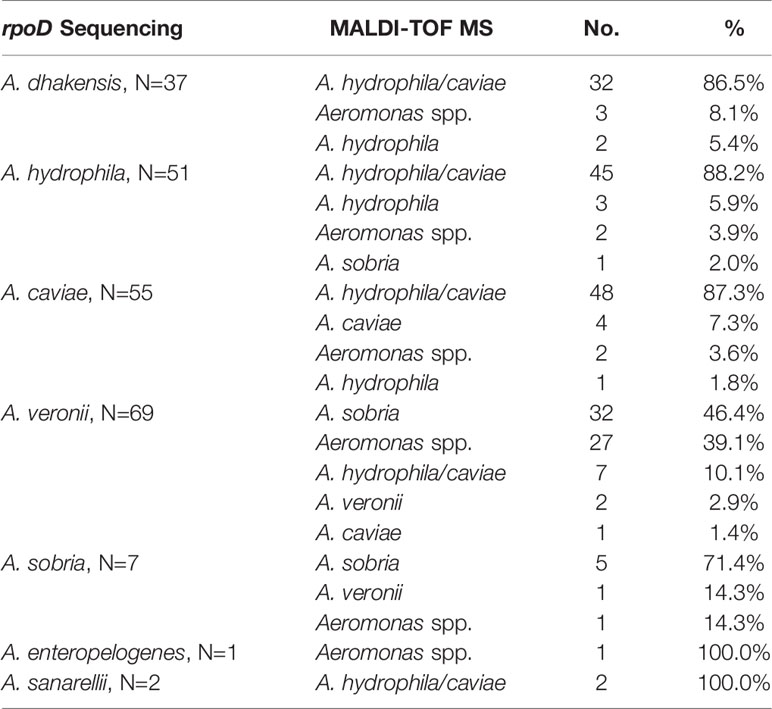
As shown in Table 2, the most common species isolated was Aeromonas veronii (69/222, 31.1%), followed by A. caviae (55/222, 24.8%), A. hydrophila (51/222, 23.0%), and A. dhakensis (37/222, 16.7%). The MALDI-TOF MS system correctly identified all isolates at the same genus level with molecular methods, but only 48.2% (107/222) achieved at the same species level. Most of the A. caviae (48/55, 87.3%) and A. hydrophila (45/51, 88.2%) isolates were identified as A. hydrophila/caviae using MALDI-TOF MS system. The A. veronii isolates had a mere 2.9% concordance at the species level between molecular typing and MALDI-TOF MS, and nearly half of them were as A. sobria. The MALDI-TOF MS 2.0 version system is unable to identify A. dhakensis due to no corresponding data in the database.
The Difference in Clinical Characteristics, Virulence Genes, Resistance Genes, and Antimicrobial Susceptibility Between Species
Two patients had two different Aeromonas species isolated from the same specimen, and another two had two different Aeromonas species isolated from two consecutive blood cultures. All were omitted in the following analysis. A. veronii was the most common species isolated in bacteremic patients (28/69, 40.6%), followed by A. caviae (19/69, 27.5%), A. hydrophila (13/69, 18.8%), and A. dhakensis (6/69, 8.7%). A. hydrophila (16/36, 44.4%) and A. dhakensis (12/36, 33.3%) predominated in the skin and soft tissue infection (p<0.0001), whereas A. veronii (13/29, 44.8%) and A. caviae (10/29, 34.5%) prevailed in biliary tract infections (p=0.011). A. caviae preponderates in pneumonia (8/13, 61.5%, p=0.017), while A. dhakensis (7/22, 31.8%) and A. caviae (6/22, 27.3%) dominated in intra-abdominal infections (p=0.012). Patients with hematological malignancies were infected by exclusively A. veronii and A. hydrophila (8/13, 61.5% and 5/13, 38.5%, respectively, p=0.014).
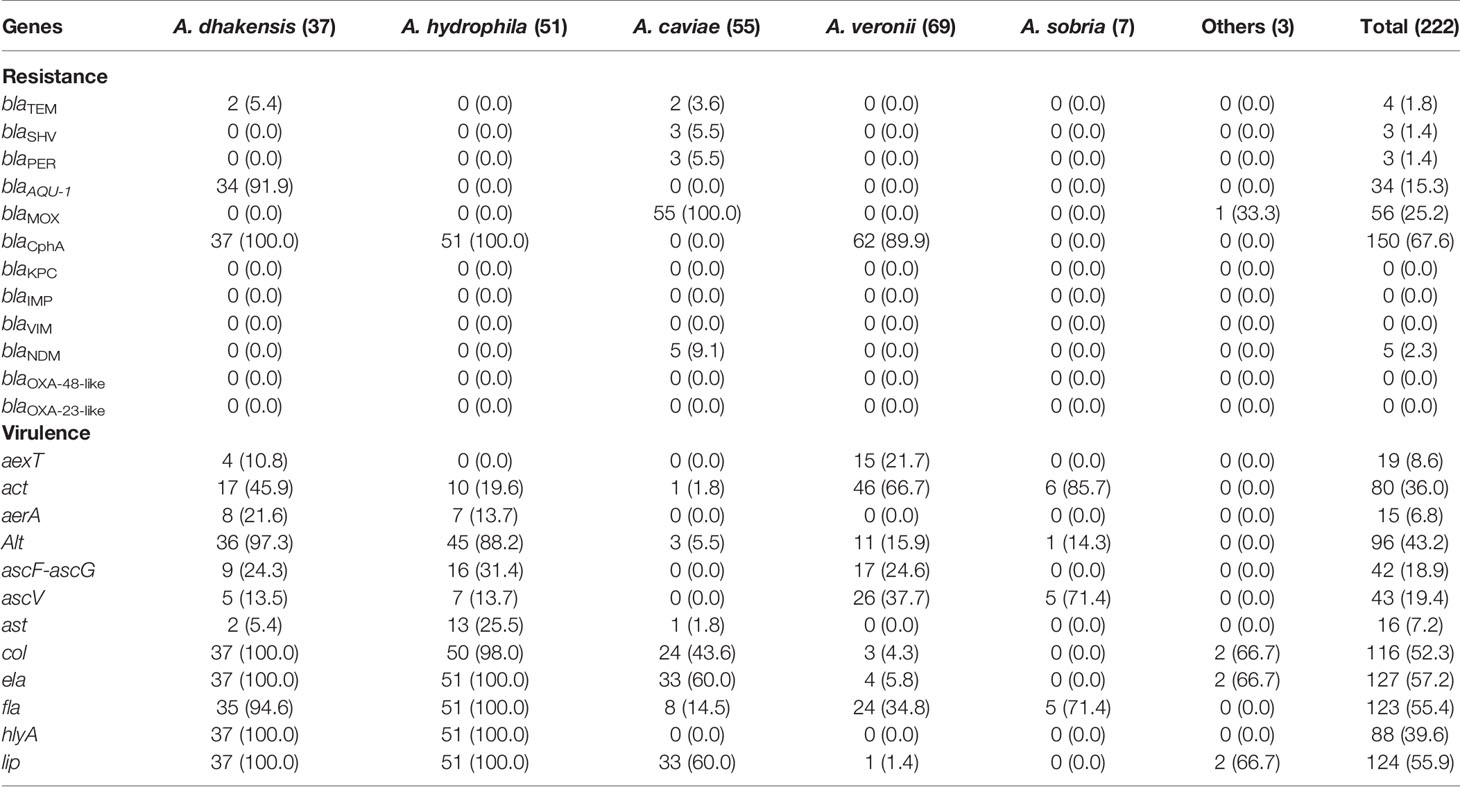
As for virulence genes, which were shown in Table 3, both A. dhakensis and A. hydrophila almost invariably carried col, ela, fla, hlyA, lip, and alt (88.2 to 100%). The difference between them was that A. dhakensis carried act and aexT more often and possessed ast less frequently without reaching statistical significance. A. veronii and A. sobria both possessed act and ascV more often (p<0.0001). ascF-ascG was found in A. hydrophila (31.4%), A. dhakensis (24.3%), and A. veronii (24.6%).
Regarding resistance genes, the AmpC β-lactamase gene blaAQU-1 was exclusive for A. dhakensis isolates (34/37, 91.9%), and blaMOX was present in all A. caviae isolates. The metallo-β-lactamase (MBL) gene blaCphA was present in all A. dhakensis and A. hydrophila isolates and most of the A. veronii (62/69, 81.6%) isolates, but not in A. caviae or A. sobria. 9.1% (5/55) of A. caviae isolates carried New Delhi Metallo-beta-lactamase (blaNDM). 14.5% (8/55) of A. caviae and 10.8% (4/37) of A. dhakensis isolates also carried extended-spectrum β-lactamase (ESBL) genes (ex. blaTEM, blaSHV, blaPER, and blaCTX-M). None of the 222 isolates possess other metallo-β-lactamases such as blaKPC, blaIMP, blaVIM, blaOXA-23-like, and blaOXA-48-like.
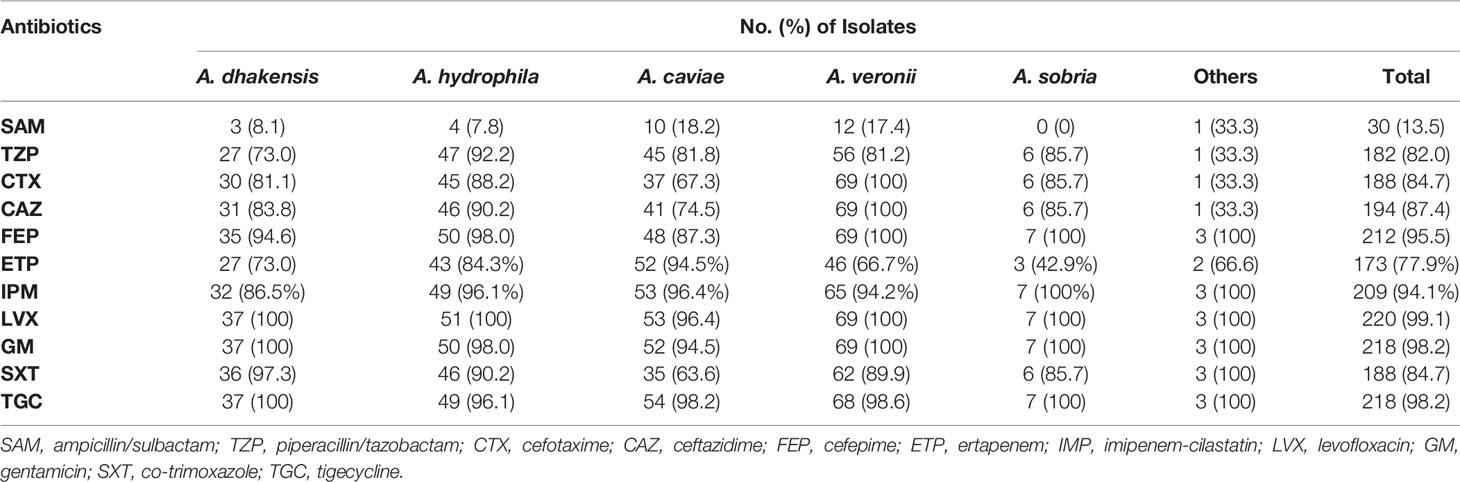
The antimicrobial susceptibility test was conducted for 220 isolates, and the results were shown in Table 4. Both A. dhakensis and A. hydrophila showed reduced susceptibility to cefotaxime and ertapenem (81.1% and 88.2% for cefotaxime and 73% and 84.3% for ertapenem, respectively). The ertapenem susceptibility was decreased in A. veronii and A. sobria (66.7% and 42.9%, respectively). A. caviae was less susceptible to cefotaxime (67.3%). A. sanarellii carried blaMOX and displayed 100% resistance to third-generation cephalosporins such as cefotaxime and ceftazidime, as well as reduced susceptibility to ertapenem (50%). Nearly 90% of Aeromonas isolates were susceptible to cefepime, tigecycline, and levofloxacin.
Risk Factors for Mortality
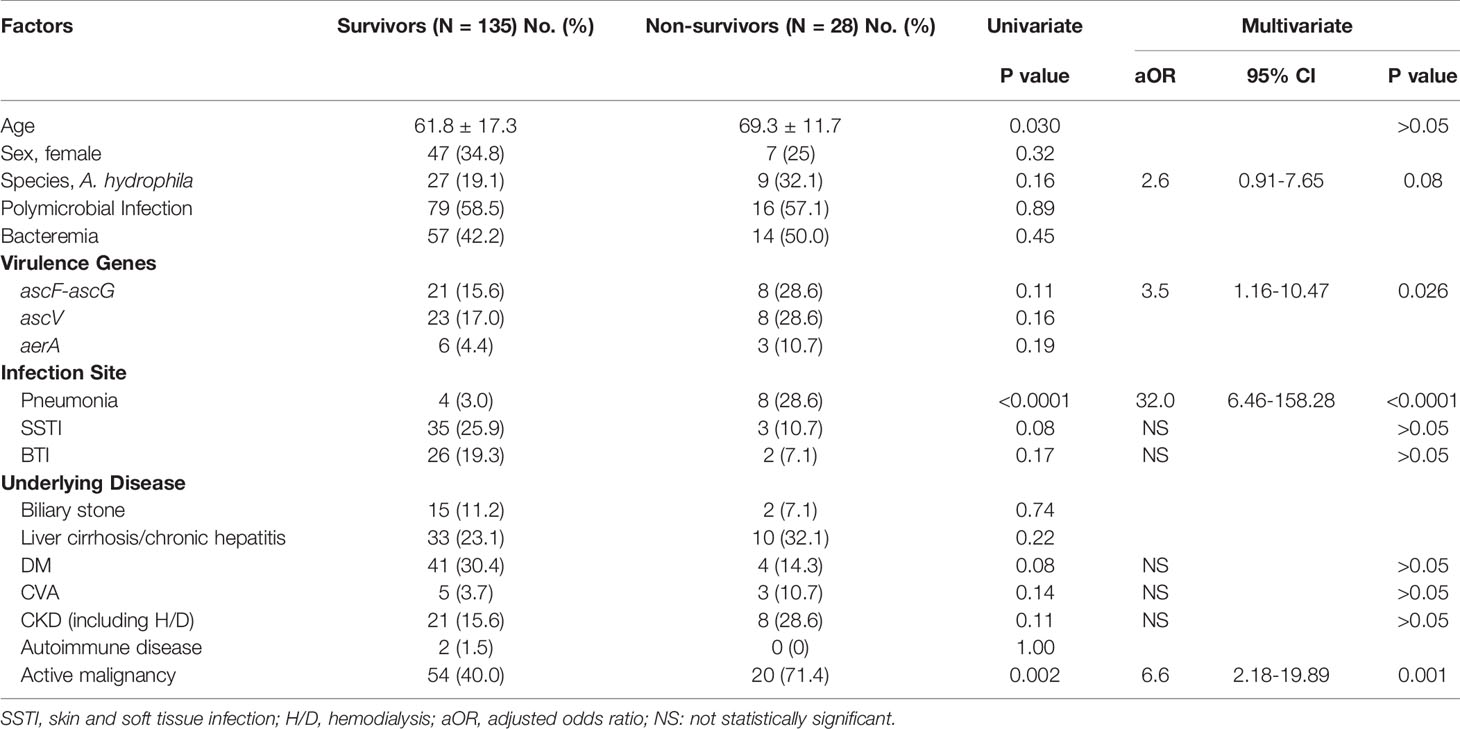
As shown in Table 5, non-survivors were older (p=0.030), tended to have pneumonia (p < 0.0001), and malignancy (p=0.002) when compared with non-survivors in univariate analysis. In univariate analysis, non-survivors were older (p=0.030), tended to have pneumonia (p<0.0001), and malignancy (p=0.002) when compared with non-survivors. There was no significant difference in mortality between different species or cancer types. In multivariate logistic regression analysis, independent risk factors associated with mortality were pneumonia (adjusted odds ratio (aOR)=32.0, p<0.0001), malignancy (aOR=6.6, p=0.001), and ascF-ascG carriage (aOR=3.5, p=0.026).
Discussion
The prevalence of human infections caused by A. veronii and A. dhakensis might be underestimated since both would be misidentified as A. hydrophila or A. sobria by the phenotype-based identification system or even MALDI-TOF MS as shown in the present study. A. dhakensis was found to be the most common Aeromonas species isolated from wound cultures, more virulent than A. hydrophila ex vivo and in animal models (Chen et al., 2014), as well as harboring the highest 14-day sepsis-related mortality rate among monomicrobial Aeromonas bacteremia (Wu et al., 2015). A. dhakensis was found to be the dominant aeromonad in Singapore and Malaysia, accounting for 45-50% of all Aeromonas species identified (Puthucheary et al., 2012; Khor et al., 2018). In Australia, A. dhakensis was the most prevalent aeromonad in clinical and water samples, especially in wounds (Aravena-Román et al., 2011). T3SSs are found in many Gram-negative bacterial pathogens including Pseudomonas, Yersinia, Salmonella, Shigella, as well as enteropathogenic and enterohemorrhagic Escherichia coli (Wu et al., 2007). The T3SS of Aeromonas is similar to that of Yersinia (Vilches et al., 2004), with at least 21 effector proteins (Rangel et al., 2019) that exhibit cytotoxicity, induce apoptosis, reduce phagocytosis, and trigger cytokines/chemokines production (Yu et al., 2004; Burr et al., 2005; Sierra et al., 2010). Strains of A. salmonicida and A. hydrophila with mutations in the T3SS apparatus were shown to be less virulent than non-mutated strains (Vilches et al., 2004; Yu et al., 2004; Burr et al., 2005). Our previous research demonstrated that ascF-ascG was mainly present in A. hydrophila, A. dhakensis, and A. veronii (50%, 14.3%, and 1%, respectively) (Wu et al., 2019), and ascV was previously more common in A. hydrophila comparing with A. dhakensis (92.3% vs 51.4%, p=0.017) (Chen et al., 2014), but there were shreds of evidence demonstrating an association between the presence of ascV, aexT or ascF-ascG genes and the development of extraintestinal infections or bacteremia among patients with Aeromonas isolates (Wu et al., 2007). The present study illustrated that ascV carriage was similar between A. hydrophila and A. dhakensis (13.7% vs 13.5%), and A. veronii possessed ascF-ascG gene more often than previously reported. The ascF-ascG gene was independently correlated to crude in-hospital mortality in the present study, a correlation that had not yet been elucidated in other studies.
The distribution of the AmpC β-lactamases and MBL genes were found to be species-specific in a previous study conducted in our hospital, with all A. dhakensis, A. caviae, and A. hydrophila isolates carrying blaAQU-1, blaMOX, and blaCepH, respectively (Wu et al., 2015). Consistent with this finding, the present study demonstrated increased resistance to third-generation cephalosporins among the three aeromonads harboring genes encoding AmpC β-lactamases. In the present study, blaAQU-1 was found exclusively but not universally in 91.9% of the A. dhakensis isolates. Reduced susceptibility to cefepime was found among ESBL genes-carrying A. caviae and A. dhakensis isolates, but 2% of A. hydrophila isolates also exhibited cefepime resistance without identifiable ESBL genes in the present study. Resistance to ertapenem was quite high among aeromonads carrying the MBL gene blaCphA, such as A. dhakensis, A. hydrophila, and A. veronii, and resistance to imipenem could be found in the aforementioned aeromonads, as well as blaNDM-carrying A. caviae. A. caviae was found to carry blaNDM on the chromosome from water seepage samples in New Dehli in 2010 (Walsh et al., 2011). Clinicians should be aware of the emergence of blaNDM in A. caviae. Moreover, 57.1% of A. sobria isolates showed intermediate susceptibility to ertapenem without carrying blaCphA or other carbapenemases tested in the present study. Other carbapenemases, such as the class D penicillinase AmpS, had been discovered in A. sobria (Walsh et al., 1995a; Walsh et al., 1995b). The two A. sanarellii isolates, one of them carrying blaMOX gene, displayed non-susceptibility to cefotaxime and piperacillin/tazobactam, and one of them was resistant to ertapenem and tetracycline. Other AmpC β-lactamases and MBL or porin alterations not examined in this study may contribute to the drug resistance.
Aeromonas infection had been linked to patients with liver cirrhosis or cancer with poorer outcomes in Taiwan (Ko and Chuang, 1995; Ko et al., 2000; Wang et al., 2009), an island that had been endemic with hepatitis B (Chan et al., 2004). The present study demonstrated that the proportion of patients with active malignancy had surpassed liver cirrhosis as the most common underlying disease in patients with Aeromonas infection, possibly attributed to the mass vaccination program of hepatitis B vaccine since 1984, thereby reducing the carrier rate by 85% (Chan et al., 2004). Hepatocellular carcinoma (HCC), which was linked to liver cirrhosis and chronic hepatitis B and C infection, was the most common cancer type in the present study, accounting for 19.4% of all patients with active malignancies, and had the highest crude in-hospital mortality rate (6/14, 42.9%) among all comorbidities. Liver cirrhosis confers susceptibility to infection by immune dysfunction including reduced secretory IgA and bile acid, and alteration in the gut microbiome, making the host susceptible to infections originating from the gut (Bajaj et al., 2021). On the other hand, chemotherapies directed against malignancies confer susceptibility to food-borne infections by disrupting the gut mucosal barrier, and the following neutropenia predisposes the host to opportunistic infections (National Comprehensive Cancer Network, 2020). The risk of infection in patients with HCC and pancreatobiliary cancers may also be increased due to hepatobiliary obstruction caused by tumors, as a sequela of hepatobiliary reconstruction surgery (National Comprehensive Cancer Network, 2020), and resistance to bile salts of aeromonads (Want and Millership, 1990). Chao et al. discovered that patients with cancer are associated with higher mortality in Aeromonas bacteremia, pneumonia, and biliary tract infection (Chao et al., 2013a; Chao et al., 2013b; Tang et al., 2014). In contrast, the outcome of skin and soft tissue infection attributed to Aeromonas was associated with diabetes mellitus but not immune status (Chao et al., 2013c).
In the present study, patients with hematologic malignancies were infected by A. veronii and A. hydrophila exclusively, and the crude in-hospital mortality was 30.8% (4/13). In another tertiary medical center in Southern Taiwan, Tsai et al. found that 35.6% of 41 patients with hematologic dyscrasias succumbed to Aeromonas bacteremia within 14 days of onset, with a remarkably high resistant rate to imipenem (35.6%) (Tsai et al., 2006). Patients with hematological malignancies were particularly vulnerable to opportunistic infection owing to the frequent leukopenia due to marrow infiltration of malignant cells or dysfunctional marrow, the severe mucosal damage, and prolonged neutropenia following higher-intensity chemotherapies, and a shift in enteric microbial flora accompanied by severe illness and antimicrobial usage (National Comprehensive Cancer Network, 2020).
Biliary tract infection with aeromonads was associated with biliary stone and pancreatobiliary cancer in our study, agreeing with other studies in southern Taiwan and Japan (Chao et al., 2013a; Kitagawa et al., 2020). Pancreatic cancer was the second most common cancer in our study, and the sum of patients with pancreatobiliary cancers outnumbered patients with HCC. In Japan, pancreatobiliary cancer, liver cirrhosis, and obstructive biliary disease contributed equally to comorbidity in patients with Aeromonas bacteremia, and 57.9% of bacteremia originated from biliary tract infection (Kitagawa et al., 2020). The incidence of pancreatobiliary cancers was around 20.93 per 100,000 inhabitants per year in Taiwan, far way behind the incidence of breast cancer, colorectal cancer, lung cancer, prostate cancer, and hepatocellular carcinoma (119.71, 70.05, 65.05, 56.72, and 36.62 per 100,000 inhabitants per year, respectively) (Health Promotion Administration, Ministry of Health and Welfare, 2020). This phenomenon may be attributed to the preponderance and even possible carcinogenesis of aeromonads in patients with pancreatobiliary cancers, or simply an institutional bias since our hospital was renowned for the treatment of pancreatic cancer (Su et al., 2020). Overexpression of the p38 MAPK pathway is observed in the pancreatic cancer cells and hepatocellular cells (Dhillon et al., 2007). Our previous research (Chen et al., 2018) have discovered that A. dhakensis infection causes p38 mitogen-activated protein kinase (MAPK) pathway activation in Caenorhabditis elegans model. We presume that Aeromonas species living in the hepatobiliary tract trigger the development of cancers by activating p38 MAPK pathway. Further studies are warranted to clarify the causality between Aeromonas and pancreatobiliary cancer. Half (8/16) of the patients with biliary stones were infected with A. caviae and half (6/12) of those with pancreatic cancer with A. veronii. Since A. caviae and A. veronii carry blaMOX and blaCphA, respectively, cefepime and fluoroquinolone are drugs of choice in these patients.
Three-fourths of the patients with Aeromonas pneumonia died in the hospital. Pneumonia was the most significant factor associated with crude in-hospital mortality in the present study, yet it was caused by A. caviae, the least virulent species with the highest resistance to third-generation cephalosporins and therefore most healthcare-associated among the commonly encountered aeromonads (Wu et al., 2015). A study conducted in another hospital located in the same city addressing 84 patients with Aeromonas pneumonia showed that the in-hospital mortality was merely 28.6% (Chao et al., 2013c). Since the majority (10/13, 76.9%) of these patients had a polymicrobial infection, the high mortality in the present may reflect the complex comorbidities and prolonged hospitalization, not the virulence of Aeromonas itself.
This study had several limitations. First, clinical information was collected retrospectively. Selection bias may be present since there could be more severe patients and more patients with rarer malignancies such as pancreatobiliary carcinomas were referred to the study hospital. Second, this study was conducted in a single medical center, and a multicenter study is warranted for a more comprehensive understanding of the epidemiology of clinical infections caused by aeromonads in other areas. Finally, a severity score was not available in this study, which may contribute to the risk of mortality in the outcome analysis.
Conclusion
A. veronii, A. caviae, A. hydrophila, and A. dhakensis were the most frequently isolated species in Aeromonas infections. Infection with ascF-ascG Aeromonas and underlying malignancies were associated with mortality. Cefepime, fluoroquinolones, and tigecycline are the drugs of choice for Aeromonas infections, especially for skin and soft tissue infections and biliary tract infections in patients with underlying pancreaticobiliary cancers.
Authors Contributions
P-LC conceived and designed the experiments. S-LS performed all the experiments. Y-WC analyzed the data and drafted the paper. C-WL, N-YL, and C-ST provided technical help on data analysis. C-CL, W-CK, C-LL, L-SS, and M-CL critically commented on the analysis. P-LC reviewed and edited the paper. All authors contributed to the article and approved the submitted version.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Funding
The study was partially supported by research grants from the Ministry of Science and Technology, Taiwan (MOST 110-2628-B-006-028), and National Cheng Kung University Hospital (NCKUH-10902001 and NCKUH-110040027).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aravena-Román, M., Harnett, G. B., Riley, T. V., Inglis, T. J. J., Chang, B. J. (2011). Aeromonas Aquariorum is Widely Distributed in Clinical and Environmental Specimens and can be Misidentified as Aeromonas Hydrophila. J. Clin. Microbiol. 49, 3006–3008. doi: 10.1128/JCM.00472-11
Bajaj, J. S., Kamath, P. S., Reddy, K. R. (2021). The Evolving Challenge of Infections in Cirrhosis. N. Engl. J. Med. 384, 2317–2330. doi: 10.1056/NEJMra2021808
Braun, M., Stuber, K., Schlatter, Y., Wahli, T., Kuhnert, P., Frey, J. (2002). Characterization of an ADP-Ribosyltransferase Toxin (AexT) From Aeromonas Salmonicida Subsp. Salmonicida. J. Bacteriol. 184, 1851–1858. doi: 10.1128/JB.184.7.1851-1858.2002
Burr, S. E., Frey, J. (2007). Analysis of Type III Effector Genes in Typical and Atypical Aeromonas Salmonicida. J. Fish Dis. 30, 711–714. doi: 10.1111/j.1365-2761.2007.00859.x
Burr, S. E., Pugovkin, D., Wahli, T., Segner, H., Frey, J. (2005). Attenuated Virulence of an Aeromonas Salmonicida Subsp. Salmonicida Type III Secretion Mutant in a Rainbow Trout Model. Microbiol. Read. Engl. 151, 2111–2118. doi: 10.1099/mic.0.27926-0
Chacón, M. R., Soler, L., Groisman, E. A., Guarro, J., Figueras, M. J. (2004). Type III Secretion System Genes in Clinical Aeromonas Isolates. J. Clin. Microbiol. 42, 1285–1287. doi: 10.1128/JCM.42.3.1285-1287.2004
Chan, C. Y., Lee, S. D., Lo, K. J. (2004). Legend of Hepatitis B Vaccination: The Taiwan Experience. J. Gastroenterol. Hepatol. 19, 121–126. doi: 10.1111/j.1440-1746.2004.03153.x
Chao, C. M., Lai, C. C., Tang, H. J., Ko, W. C., Hsueh, P. R. (2013a). Biliary Tract Infections Caused by Aeromonas Species. Eur. J. Clin. Microbiol. Infect. Dis. 32, 245–251. doi: 10.1007/s10096-012-1736-1
Chao, C. M., Lai, C. C., Tang, H. J., Ko, W. C., Hsueh, P. R. (2013b). Skin and Soft-Tissue Infections Caused by Aeromonas Species. Eur. J. Clin. Microbiol. Infect. Dis. 32, 543–547. doi: 10.1007/s10096-012-1771-y
Chao, C. M., Lai, C. C., Tsai, H. Y., Wu, C. J., Tang, H. J., Ko, W. C., et al. (2013c). Pneumonia Caused by Aeromonas Species in Taiwan 2004-2011. Eur. J. Clin. Microbiol. Infect. Dis. 32, 1069–1075. doi: 10.1007/s10096-013-1852-6
Chen, P. L., Lamy, B., Ko, W. C. (2016). Aeromonas Dhakensis, an Increasingly Recognized Human Pathogen. Front. Microbiol. 7, 793. doi: 10.3389/fmicb.2016.00793
Chen, P. L., Wu, C. J., Chen, C. S., Tsai, P. J., Tang, H. J., Ko, W. C. (2014). A Comparative Study of Clinical Aeromonas Dhakensis and Aeromonas Hydrophila Isolates in Southern Taiwan: A. Dhakensis is More Predominant and Virulent. Clin. Microbiol. Infect. 20, O428–O434. doi: 10.1111/1469-0691.12456
Chen, Y. W., Ko, W. C., Chen, C. S., Chen, P. L. (2018). RIOK-1 Is a Suppressor of the p38 MAPK Innate Immune Pathway in Caenorhabditis elegans. Front Immunol 9, 774. doi: 10.3389/fimmu.2018.00774
Clinical and Laboratory Standards Institute (2016). Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria. 3rd (Wayne, PA: Clinical and Laboratory Standards Institute).
Colston, S. M., Fullmer, M. S., Beka, L., Lamy, B., Gogarten, J. P., Graf, J. (2014). Bioinformatic Genome Comparisons for Taxonomic and Phylogenetic Assignments Using Aeromonas as a Test Case. mBio 5, e02136. doi: 10.1128/mBio.02136-14
Dhillon, A., Hagan, S., Rath, O., et al (2007). MAP Kinase Signalling Pathways in Cancer. Oncogene 26, 3279–90. doi: 10.1038/sj.onc.1210421
Fernández-Bravo, A., Figueras, M. J. (2020). An Update on the Genus Aeromonas: Taxonomy, Epidemiology, and Pathogenicity. Microorganisms 8:129. doi: 10.3390/microorganisms8010129
Gonçalves Pessoa, R. B., de Oliveira, W. F., Marques, D. S. C., Dos Santos Correia, M. T., de Carvalho, E. V. M. M., Coelho, L. C. B. B. (2019). The Genus Aeromonas: A General Approach. Microb. Pathog. 130, 81–94. doi: 10.1016/j.micpath.2019.02.036
Health Promotion Administration, Ministry of Health and Welfare (2020) The Cancer Registry Annual Report in Taiwan 2018. Available at: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=269&pid=13498 (Accessed April 7, 2021).
Janda, J. M., Abbott, S. L. (2010). The Genus Aeromonas: Taxonomy, Pathogenicity, and Infection. Clin. Microbiol. Rev. 23, 35–73. doi: 10.1128/CMR.00039-09
Khor, W. C., Puah, S. M., Koh, T. H., Tan, J. A. M. A., Puthucheary, S. D., Chua, K. H. (2018). Comparison of Clinical Isolates of Aeromonas From Singapore and Malaysia With Regard to Molecular Identification, Virulence, and Antimicrobial Profiles. Microb. Drug Resist. Larchmt. N. 24, 469–478. doi: 10.1089/mdr.2017.0083
Kitagawa, H., Ohge, H., Yu, L., Kayama, S., Hara, T., Kashiyama, S., et al. (2020). Aeromonas Dhakensis is Not a Rare Cause of Aeromonas Bacteremia in Hiroshima, Japan. J. Infect. Chemother. Off. J. Jpn. Soc Chemother. 26, 316–320. doi: 10.1016/j.jiac.2019.08.020
Ko, W. C., Chuang, Y. C. (1995). Aeromonas Bacteremia: Review of 59 Episodes. Clin. Infect. Dis. 20, 1298–1304. doi: 10.1093/clinids/20.5.1298
Ko, W. C., Lee, H. C., Chuang, Y. C., Liu, C. C., Wu, J. J. (2000). Clinical Features and Therapeutic Implications of 104 Episodes of Monomicrobial Aeromonas Bacteraemia. J. Infect. 40, 267–273. doi: 10.1053/jinf.2000.0654
Lamy, B., Laurent, F., Verdier, I., Decousser, J.-W., Lecaillon, E., Marchandin, H., et al. (2010). Accuracy of 6 Commercial Systems for Identifying Clinical Aeromonas Isolates. Diagn. Microbiol. Infect. Dis. 67, 9–14. doi: 10.1016/j.diagmicrobio.2009.12.012
Martínez-Murcia, A. J., Benlloch, S., Collins, M. D. (1992). Phylogenetic Interrelationships of Members of the Genera Aeromonas and Plesiomonas as Determined by 16S Ribosomal DNA Sequencing: Lack of Congruence With Results of DNA-DNA Hybridizations. Int. J. Syst. Bacteriol. 42, 412–421. doi: 10.1099/00207713-42-3-412
Martínez-Murcia, A. J., Monera, A., Saavedra, M. J., Oncina, R., Lopez-Alvarez, M., Lara, E., et al. (2011). Multilocus Phylogenetic Analysis of the Genus Aeromonas. Syst. Appl. Microbiol. 34, 189–199. doi: 10.1016/j.syapm.2010.11.014
National Comprehensive Cancer Network (2020) Prevention and Treatment of Cancer-Related Infections (Version 2.2020). Available at: https://www.nccn.org/professionals/physician_gls/pdf/infections.pdf (Accessed June 15, 2021).
Puthucheary, S. D., Puah, S. M., Chua, K. H. (2012). Molecular Characterization of Clinical Isolates of Aeromonas Species From Malaysia. PloS One 7, e30205. doi: 10.1371/journal.pone.0030205
Rangel, L. T., Marden, J., Colston, S., Setubal, J. C., Graf, J., Gogarten, J. P. (2019). Identification and Characterization of Putative Aeromonas Spp. T3SS Effectors. PloS One 14, e0214035. doi: 10.1371/journal.pone.0214035
Rasmussen-Ivey, C. R., Figueras, M. J., McGarey, D., Liles, M. R. (2016). Virulence Factors of Aeromonas Hydrophila: In the Wake of Reclassification. Front. Microbiol. 7:1337. doi: 10.3389/fmicb.2016.01337
Sierra, J. C., Suarez, G., Sha, J., Baze, W. B., Foltz, S. M., Chopra, A. K. (2010). Unraveling the Mechanism of Action of a New Type III Secretion System Effector AexU From Aeromonas Hydrophila. Microb. Pathog. 49, 122–134. doi: 10.1016/j.micpath.2010.05.011
Soler, L., Yáñez, M. A., Chacón, M. R., Aguilera-Arreola, M. G., Catalán, V., Figueras, M. J., et al. (2004). Phylogenetic Analysis of the Genus Aeromonas Based on Two Housekeeping Genes. Int. J. Syst. Evol. Microbiol. 54, 1511–1519. doi: 10.1099/ijs.0.03048-0
Su, Y. Y., Chiang, N. J., Tsai, H. J., Yen, C. J., Shan, Y. S., Chen, L. T. (2020). The Impact of Liposomal Irinotecan on the Treatment of Advanced Pancreatic Adenocarcinoma: Real-World Experience in a Taiwanese Cohort. Sci. Rep. 10, 7420. doi: 10.1038/s41598-020-64421-6
Tang, H. J., Lai, C. C., Lin, H. L., Chao, C. M. (2014). Clinical Manifestations of Bacteremia Caused by Aeromonas Species in Southern Taiwan. PloS One 9, e91642. doi: 10.1371/journal.pone.0091642
Tomás, J. M. (2012). The Main Aeromonas Pathogenic Factors. ISRN Microbiol. 2012, 1–22. doi: 10.5402/2012/256261
Tsai, M. S., Kuo, C. Y., Wang, M. C., Wu, H. C., Chien, C. C., Liu, J. W. (2006). Clinical Features and Risk Factors for Mortality in Aeromonas Bacteremic Adults With Hematologic Malignancies. J. Microbiol. Immunol. Infect. 39, 150–154.
Vilches, S., Urgell, C., Merino, S., Chacón, M. R., Soler, L., Castro-Escarpulli, G., et al. (2004). Complete Type III Secretion System of a Mesophilic Aeromonas Hydrophila Strain. Appl. Environ. Microbiol. 70, 6914–6919. doi: 10.1128/AEM.70.11.6914-6919.2004
Walsh, T. R., Hall, L., MacGowan, A. P., Bennett, P. M. (1995a). Sequence Analysis of Two Chromosomally Mediated Inducible β-Lactamases From Aeromonas Sobria, Strain 163a, One a Class D Penicillinase, the Other an AmpC Cephalosporinase. J. Antimicrob. Chemother. 36, 41–52. doi: 10.1093/jac/36.1.41
Walsh, T. R., Payne, D. J., MacGowan, A. P., Bennett, P. M. (1995b). A Clinical Isolate of Aeromonas Sobria With Three Chromosomally Mediated Inducible β-Lactamases: A Cephalosporinase, a Penicillinase and a Third Enzyme, Displaying Carbapenemase Activity. J. Antimicrob. Chemother. 35, 271–279. doi: 10.1093/jac/35.2.271
Walsh, T. R., Weeks, J., Livermore, D. M., Toleman, M. A. (2011). Dissemination of NDM-1 Positive Bacteria in the New Delhi Environment and its Implications for Human Health: An Environmental Point Prevalence Study. Lancet Infect. Dis. 11, 355–362. doi: 10.1016/S1473-3099(11)70059-7
Wang, C. Y., Chi, C. Y., Ho, M. W., Ho, C. M., Lin, P. C., Wang, J. H. (2009). Clinical Presentations, Prognostic Factors, and Mortality in Patients With Aeromonas Sobria Complex Bacteremia in a Teaching Hospital: A 5-Year Experience. J. Microbiol. Immunol. Infect. 42, 510–515.
Want, S. V., Millership, S. E. (1990). Effects of Incorporating Ampicillin, Bile Salts and Carbohydrates in Media on the Recognition and Selection of Aeromonas Spp. From Faeces. J. Med. Microbiol. 32, 49–54. doi: 10.1099/00222615-32-1-49
Wu, C. J., Chen, P. L., Hsueh, P. R., Chang, M. C., Tsai, P. J., Shih, H. I., et al. (2015). Clinical Implications of Species Identification in Monomicrobial Aeromonas Bacteremia. PloS One 10, e0117821. doi: 10.1371/journal.pone.0117821
Wu, C. J., Chen, P. L., Tang, H. J., Chen, H. M., Tseng, F. C., Shih, H. I., et al. (2014). Incidence of Aeromonas Bacteremia in Southern Taiwan: Vibrio and Salmonella Bacteremia as Comparators. J. Microbiol. Immunol. Infect. 47, 145–148. doi: 10.1016/j.jmii.2012.08.019
Wu, C. J., Chen, P. L., Wu, J. J., Yan, J. J., Lee, C. C., Lee, H. C., et al. (2012). Distribution and Phenotypic and Genotypic Detection of a Metallo-β-Lactamase, CphA, Among Bacteraemic Aeromonas Isolates. J. Med. Microbiol. 61, 712–719. doi: 10.1099/jmm.0.038323-0
Wu, C. J., Chuang, Y. C., Lee, M. F., Lee, C. C., Lee, H. C., Lee, N. Y., et al. (2011). Bacteremia Due to Extended-Spectrum-β-Lactamase-Producing Aeromonas Spp. At a Medical Center in Southern Taiwan. Antimicrob. Agents Chemother. 55, 5813–5818. doi: 10.1128/AAC.00634-11
Wu, C. J., Ko, W. C., Lee, N. Y., Su, S. L., Li, C. W., Li, M. C., et al. (2019). Aeromonas Isolates From Fish and Patients in Tainan City, Taiwan: Genotypic and Phenotypic Characteristics. Appl. Environ. Microbiol. 85, 1–12. doi: 10.1128/AEM.01360-19
Wu, C. J., Wang, H. C., Chen, P. L., Chang, M. C., Sunny Sun, H., Chou, P. H., et al. (2013). AQU-1, a Chromosomal Class C β-Lactamase, Among Clinical Aeromonas Dhakensis Isolates: Distribution and Clinical Significance. Int. J. Antimicrob. Agents 42, 456–461. doi: 10.1016/j.ijantimicag.2013.08.002
Wu, C. J., Wu, J. J., Yan, J. J., Lee, H. C., Lee, N. Y., Chang, C. M., et al. (2007). Clinical Significance and Distribution of Putative Virulence Markers of 116 Consecutive Clinical Aeromonas Isolates in Southern Taiwan. J. Infect. 54, 151–158. doi: 10.1016/j.jinf.2006.04.002
Yáñez, M. A., Catalán, V., Apráiz, D., Figueras, M. J., Martínez-Murcia, A. J. (2003). Phylogenetic Analysis of Members of the Genus Aeromonas Based on gyrB Gene Sequences. Int. J. Syst. Evol. Microbiol. 53, 875–883. doi: 10.1099/ijs.0.02443-0
Keywords: Aeromonas, identification, rpoD sequencing, ascF-ascG, type 3 secretion system, antimicrobial resistance, virulence, pancreaticobiliary cancers
Citation: Chen Y-W, Su S-L, Li C-W, Tsai C-S, Lo C-L, Syue L-S, Li M-C, Lee C-C, Lee N-Y, Ko W-C and Chen P-L (2021) Pancreaticobiliary Cancers and Aeromonas Isolates Carrying Type Ⅲ Secretion System Genes ascF-ascG Are Associated With Increased Mortality: An Analysis of 164 Aeromonas Infection Episodes in Southern Taiwan. Front. Cell. Infect. Microbiol. 11:749269. doi: 10.3389/fcimb.2021.749269
Received: 31 July 2021; Accepted: 05 October 2021;
Published: 19 October 2021.
Edited by:
Yang Zhang, University of Pennsylvania, United StatesReviewed by:
Yu Zhou, Institut Pasteur of Shanghai (CAS), ChinaHao Zhang, University of Pennsylvania, United States
Copyright © 2021 Chen, Su, Li, Tsai, Lo, Syue, Li, Lee, Lee, Ko and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Po-Lin Chen, Y3BsaW5AbWFpbC5uY2t1LmVkdS50dw==
 Ying-Wen Chen
Ying-Wen Chen Shu-Li Su2
Shu-Li Su2 Ching-Lung Lo
Ching-Lung Lo Ling-Shan Syue
Ling-Shan Syue Min-Chi Li
Min-Chi Li Ching-Chi Lee
Ching-Chi Lee Nan-Yao Lee
Nan-Yao Lee Wen-Chien Ko
Wen-Chien Ko Po-Lin Chen
Po-Lin Chen