- 1Department of Pharmacology, School of Medicine, University of California, Genome and Biomedical Sciences Facility, Davis, CA, United States
- 2Molecular Horizons and School of Chemistry and Molecular Bioscience, University of Wollongong, Wollongong, NSW, Australia
- 3Illawarra Health and Medical Research Institute, Wollongong, NSW, Australia
- 4Monash Institute of Pharmaceutical Science (ATMCF), Monash University, Parkville, VIC, Australia
- 5Department of Plant Sciences, PRB Building, University of California, Davis, CA, Australia
Fungal infections have become an increasing threat as a result of growing numbers of susceptible hosts and diminishing effectiveness of antifungal drugs due to multi-drug resistance. This reality underscores the need to develop novel drugs with unique mechanisms of action. We recently identified 5-(N,N-hexamethylene)amiloride (HMA), an inhibitor of human Na+/H+ exchanger isoform 1, as a promising scaffold for antifungal drug development. In this work, we carried out susceptibility testing of 45 6-substituted HMA and amiloride analogs against a panel of pathogenic fungi. A series of 6-(2-benzofuran)amiloride and HMA analogs that showed up to a 16-fold increase in activity against Cryptococcus neoformans were identified. Hits from these series showed broad-spectrum activity against both basidiomycete and ascomycete fungal pathogens, including multidrug-resistant clinical isolates.
Introduction
Global estimates suggest that diseases caused by fungal pathogens affect over 1 billion people and kill approximately 1.7 million annually (Bongomin et al., 2017; Kainz et al., 2020). The severity of fungal diseases varies from asymptomatic in healthy hosts to disseminated, life-threatening infections in individuals that are immunosuppressed (Bongomin et al., 2017; Colombo et al., 2017). Over 90% of all reported fungal-related deaths are caused by Cryptococcus, Candida, Aspergillus, Histoplasma and Pneumocystis (Pfaller and Diekema, 2010). For the fungal species that are prevalent in the environment, such as Cryptococcus, Histoplasma, and Coccidioides, spores/desiccated yeast cells are inhaled and settle in the lungs where the infection can be asymptomatic to mild, but in susceptible hosts dissemination to other organs can result in death (Ellis and Pfeiffer, 1990; Woods, 2002; Eisenman et al., 2007; Brown et al., 2013).
The Cryptococcus spp. complex includes at least seven distinct species that can cause life-threatening disease and in countries where HIV infection is prevalent, cryptococcal meningitis is the most common form of adult meningitis (Zuger et al., 1986; Limper et al., 2017; Rajasingham et al., 2017). Rhodotorula mucilagenosa, a common environmental basidiomycete, is considered an emerging pathogen (Pfaller and Diekema, 2004; Wirth and Goldani, 2012). Most cases of R. mucilagenosa infections are bloodstream infections linked to central venous catheter use in susceptible hosts (Tuon et al., 2007; De Almeida et al., 2008; Wirth and Goldani, 2012; Falces-Romero et al., 2018; Kitazawa et al., 2018).
Candida albicans is the primary cause of 9.5% of all bloodstream infections in hospitals across the United States (Wisplinghoff et al., 2004). Candida auris was relatively unknown a decade ago but is today regarded as an emerging fungal pathogen that causes significant healthcare-associated outbreaks of bloodstream infections with high rates of mortality (Lockhart and Guarner, 2019). Although Candida albicans tends to be the most prevalent cause of candidiasis in humans, the last two decades has seen increases in infections caused by non-C. albicans Candida (NCAC) species. C. glabrata, C. parapsilosis, C. tropicalis and C. krusei are among the NCAC species that have emerged as important opportunistic fungal pathogens that are evolving to be more virulent and drug-resistant (Pfaller and Diekema, 2004; Wisplinghoff et al., 2004; Silva et al., 2012). Fluconazole-resistance among these Candida spp. is worrisome as fluconazole is the most commonly used antifungal agent for prophylaxis and treatment of Candida infections in resource-poor nations (Africa and Abrantes, 2016). Of particular concern is the high proportion of C. auris isolates that are resistant to three commonly used classes of antifungals: azoles, echinocandins and polyenes (Du et al., 2020; Frias-De-Leon et al., 2020). This multi-drug resistance creates significant challenges in clinical practice requiring the close monitoring of patients for treatment failure (Lockhart and Guarner, 2019; Hata et al., 2020).
Management of fungal diseases has become increasingly challenging due to the growing number of susceptible hosts and diminishing effectiveness of antifungal drugs. Indeed, the most pervasive and drug-resistant infections are now untreatable using first-line antifungals (Smith et al., 2015; Mpoza et al., 2018). This reality underscores the need to develop novel antifungal therapeutics with unique mechanisms of action able to effectively treat emerging resistant strains. While recent attempts at de novo antifungal drug discovery have produced only marginal success, drug repurposing (or re-positioning) provides an alternative approach to identify new indications for existing drugs (Kim et al., 2020; Wall and Lopez-Ribot, 2020).
In a recent study we examined whether amiloride, a K+-sparing diuretic, could be repurposed for the treatment of fungal infections (Vu et al., 2021). Amiloride, a WHO essential medicine, is a pyrazine acylguanidine originally developed as an inhibitor of renal epithelial Na+ channels (ENaCs) (Benos, 1982). We found that while amiloride has little antifungal activity, the 5-substituted analog, 5-(N,N-hexamethylene)amiloride, (HMA) shows modest minimum inhibitory concentrations (MICs) against isolates of Cryptococcus spp., and moderate synergy with several azole antifungals (Vu et al., 2021). Structure activity relationship (SAR) analysis revealed that hydrophobic substitutions on the 5-amino group of amiloride produced improvements in antifungal activity (Vu et al., 2021). HMA possesses nanomolar activity against Na+/H+ exchangers (NHEs) but minimal inhibitory activity toward ENaC, thus decreasing the clinical risk of ENaC-mediated hyperkalemia (Li et al., 1985; Kleyman and Cragoe, 1988). Collectively, our results suggested that HMA could serve as a starting point for antifungal drug development, where further optimization could produce new analogs with higher potency. Here, we investigated a library of 6-heteroaryl substituted HMA and amiloride analogs to determine whether further improvement in antifungal activity could be obtained from this scaffold. Compounds with substitutions at other positions around the pyrazine core of amiloride and HMA were also investigated.
Material and methods
Strains and media
KN99 is a common Cryptococcus neoformans serotype A laboratory strain derived from H99 (Nielsen et al., 2003). The Candida isolates and the Rhodotorula isolate were provided by Dr. G.R. Thompson, University of California, Davis. Drug-resistance of isolates was confirmed by the Fungus Testing Laboratory (San Antonia, Texas) and provided to us through Dr. G.M. Thompson. Strains were recovered from -80°C frozen stocks, grown in YPD (1% yeast extract, 2% bacto-peptone, and 2% dextrose) at 30°C and maintained on solid media containing 2% bacto-agar.
Amiloride and HMA analogs
Amiloride.HCl was sourced from Sigma-Aldrich. Amiloride and HMA analogs were synthesized as previously described (Matthews et al., 2011; Buckley et al., 2018; Buckley et al., 2019).
Antifungal activity testing by CLSI criteria
Susceptibility assays were carried out to determine MICs and MFCs according to the Clinical and Laboratory Standards Institute (CLSI). In vitro testing was carried out in RPMI 1640 medium containing L-glutamine, without sodium bicarbonate and buffered to pH 7.0 with MOPS in 96-well plates (96-well cell culture cluster, flat-bottom, Costar). Inoculum of C. neoformans (100 µL) was prepared in accordance with the CLSI standard (M27-A3), added to the 96-well plates and incubated for 48 h at 35 °C without shaking. Readings were taken by visual inspection of the opacity of wells. The minimum inhibitory concentration (MIC) was defined as the lowest drug concentration in a well at which 100% reduction in optical density was observed compared to the no-drug control well. The MIC was determined using concentrations from 2 µg/mL to 64 µg/mL. The minimum fungicidal concentrations (MFC) were determined by transferring the contents of the well identified as the MIC above and plated onto an YPD agar plate. The absence of colony forming units (CFUs) confirmed that the MFC was equivalent to the MIC.
Statistical analysis
The MIC and MFC values reported in Tables 1, 2, S1, S2 are the result of at least 3 replicates.
Results and discussion
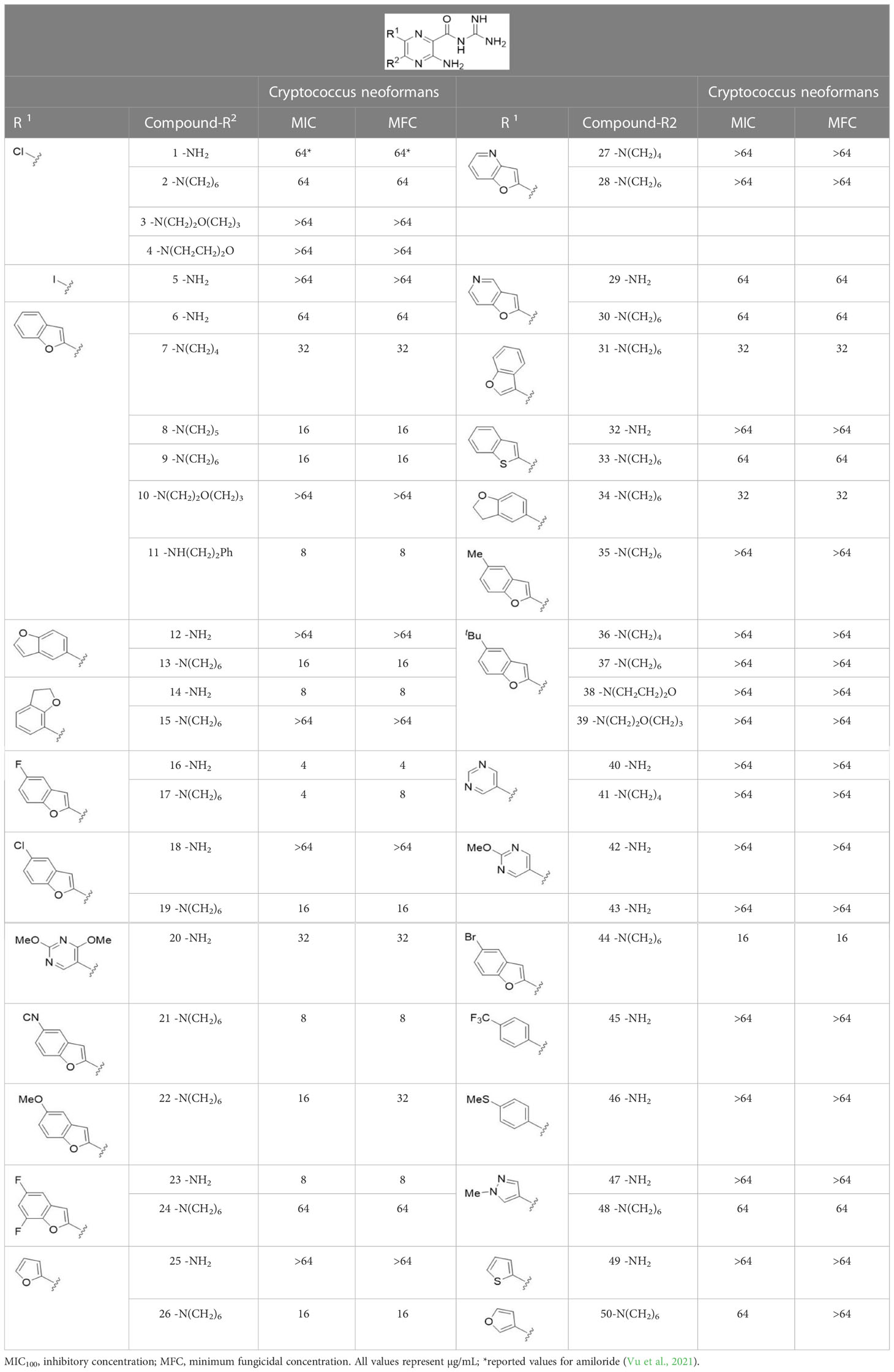
The antifungal activity of amiloride and HMA analogs carrying heteroaryl substitutions at the 5 and/or 6 position of the pyrazine ring were evaluated. Detailed physiochemical properties of HMA and 6-substituted match pairs have been reported in our recent work (Buckley et al., 2021a). A total of 64 analogs were examined by susceptibility assays against a strain of Cryptococcus neoformans (KN99) using the microbroth dilution method (Tables 1, S1). Screening of 45 6-(hetero)aryl substituted amiloride and HMA analogs reported previously revealed that antifungal activity was generally restricted to compounds bearing bicyclic heterocycles at the pyrazine 6-position (Buckley et al., 2018; Buckley et al., 2019; Buckley et al., 2021b; Hards et al., 2022). Consistent antifungal effects were seen for a series of 6-(2-benzofuran) analogs, with the HMA analog 9 and 5-piperidine 8 both showing MIC and MFC values of 16 µg/mL. Removal of the 5-azepane ring as in the matching amiloride analog 6 decreased activity, as did truncation of the amine at the 5-position, pyrrolidine 7, or incorporation of a polar O atom as in (1,4-oxazapane 10).
Substitution at the 5-azepane with a phenylethylamine 11 was favorable, producing a 2-fold increase in activity (MIC and MFC 8 µg/mL). Replacement of the ring O with S (2-benzothiophenes 12 and 13) did not improve activity. Introduction of a methyl substituent at the 5-position of the benzofuran ring increased activity by 8-fold (14 MIC and MFC 8 µg/mL) relative to the unsubstituted 2-benzofuran parent. Remarkably, this improvement was specific to the amiloride series, with no activity seen for the matching HMA analog 15 (MIC and MFC >64 µg/mL). 5-tBu substitution produced the largest increase in activity in both series (16 and 17), lowering MIC and MFC by up to 16-fold (4 µg/mL). A drop in activity was seen for the 5-fluorinated amiloride analog 18, while no change was seen for the matching HMA analog 19. Larger halogens slightly increased activity, with 5-Cl 20 producing 2-fold lower MIC and MFC values for the amiloride analog (32 µg/mL) and 8-fold higher activity for 5-Br HMA analog 21 (8 µg/mL). This trend did not extend to 5-CN substitution, where no improvement in activity was seen with HMA analog 22. An 8-fold improvement was seen for 5-MeO amiloride 23 (MIC and MFC 8 µg/mL) while an 8-fold drop in activity was observed for the matching HMA analog 24 (MIC 64 µg/mL and MFC 64 µg/mL).
5,7-Difluorination as in amiloride 25 and HMA 26 did not improve activity for either series. Similarly, improvements were not seen for a series of 4-furopyridine 27 and 28 or 5-furopyridine analogs 29 and 30, indicating sensitivity to a polar N atom at these positions. Altering the connectivity of the benzofuran 31 and 34 or equivalent 2,3-dihydrobenzofurans 32, 33 and 35 did not improve activity. Furthermore, activity was poor or absent for a diverse selection of analogs bearing 5- and 6-membered (hetero)aryl groups at the pyrazine 6-position (36-43, 45-50), underscoring the necessity of the 6-(2-benzofuran) motif for antifungal activity.
One exception to this trend was seen for the 4-CF3phenyl HMA analog 44 (MIC and MFC 16 µg/mL), which showed equivalent activity to the 2-benzofuran HMA analog 9. In addition, no antifungal activity was seen for a separate series of amiloride analogs bearing a variety of secondary alkyl amines at the pyrazine 5-position (Table S1), in keeping with our earlier observations with 5-glycinyl analogs of amiloride (Matthews et al., 2011).
Further testing of 13 active analogs against the Cn isolate confirmed their antifungal and fungicidal activity (Table S2). 5-tBu analogs 16 and 17 showed the highest activity against Cn (MIC and MFC 4 µg/mL. Phenylethylamine 11 and 5-Br benzofuran HMA analog 21 had average MICs of 7 µg/mL against Cn while the remaining 10 compounds displayed MICs ≥ 8 µg/mL.
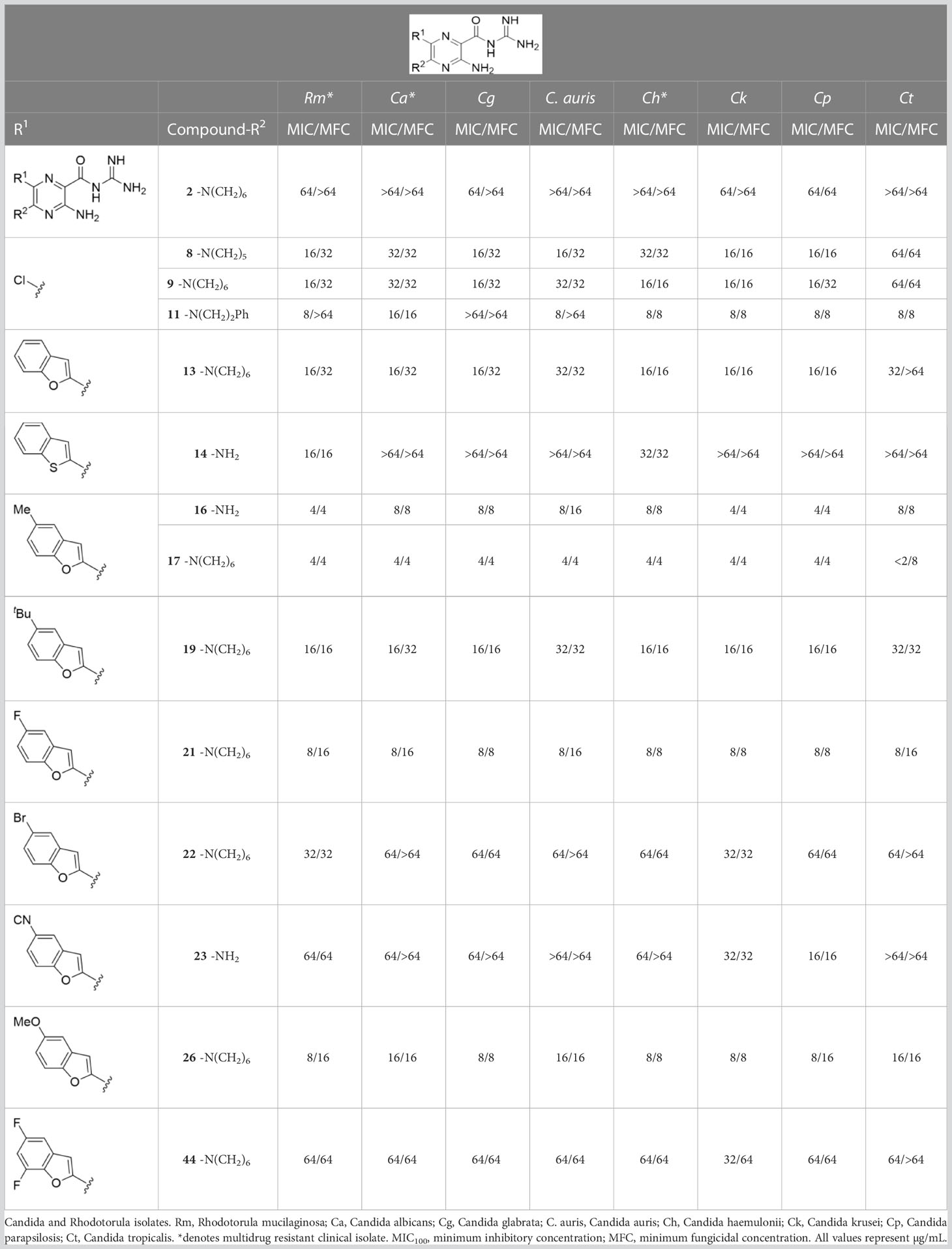
Analogs with MICs and MFCs ≤ 16 µg/mL against Cn were examined against a panel of 7 Candida isolates, including multi-drug resistant Candida auris and Candida haemulonii strains, along with the drug-resistant basidiomycete isolate, Rhodotorula mucilaginosa. Susceptibility assays revealed that the 5-tBu compounds 16 and 17, 5-Br benzofuran HMA 21 and 5,7-difluoro benzofuran HMA analog 26 were active against all fungal isolates, with MICs ranging from < 2 µg/mL to 16 µg/mL (Table 2). Phenylethylamine 11 inhibited growth of all isolates with the exception of C. glabrata (MICs ≤ 16 µg/mL), suggesting broad antifungal activity against both basidiomycetes and ascomycetes (Table 2). Broad spectrum activity was not seen for 4-CF3 phenyl analog 44, demonstrating the superiority of the 2-benzofuran group at the 6-position.
Conclusion
We previously questioned whether HMA could elicit its antifungal effects via inhibition of the fungal homolog, the endosomal Na+/H+ exchanger Nhx1 (Vu et al., 2021). We found HMA to be similarly potent in S. cerevisiae nhx1Δ and C. neoformans nhx1Δ strains relative to wild type controls, suggesting Nhx1 inhibition is likely not responsible for antifungal activity (Vu et al., 2021). This conclusion was supported in this work by the absence of antifungal activity for 6-pyrimidine HMA analog 37, a compound reported as a nM inhibitor of human NHE1 (Buckley et al., 2021a). However, we cannot rule out potential inherent differences in activity/sensitivity of fungal and human Na+/H+ exchangers that could lead to differential effects of analog #37. The modest antifungal activity of HMA coupled with its poor stability in vivo preclude its advancement as a viable candidate for animal studies (Buckley et al., 2021a).
In summary, the 6-(2-benzofuran) class of amiloride and HMA analogs described here represent progress toward lead compounds suitable for further investigation. For example, HMA analog 9 showed 2 to 3-fold higher activity against a range of drug-resistant pathogenic fungi (MIC and MFCs 16-32 µg/mL). This analog does not show K+-sparing or diuretic activity and features a more favorable pharmacokinetic profile relative to HMA in mice and rat, supporting its future evaluation in animal models of fungal infection (Buckley et al., 2021a). Future studies will investigate synergy of these compounds with standard-of-care antifungals.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
KV performed the susceptibility testing. KV & BB performed data analysis. BB & RB & MK provided library of compounds. AG supervised study. KV, EB, BB and AG wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by the Will W. Lester Endowment of the University of California awarded to EB. MK was supported by Australian National Health and Medical Research Council (NHMRC) Project Grant (APP1100432). BB acknowledges salary support from the Illawarra Cancer Careers.
Acknowledgments
We are grateful to Dr. J. Heitman and Dr. G.R. Thompson for providing fungal strains.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1101568/full#supplementary-material
Supplementary Table 1 | Antifungal activity of 5-substituted amiloride analogs against Cryptococcus neoformans isolates. MIC, minimum inhibitory concentration; MFC, minimum fungicidal concentration. All values represent µg/mL.
Supplementary Table 2 | Susceptibility of Cryptococcus neoformans to 14 amiloride and HMA analogs. MIC, minimum inhibitory concentration; MFC, minimum fungicidal concentration. All values represent µg/mL.
References
Africa, C. W., Abrantes, P. M. (2016). Candida antifungal drug resistance in sub-Saharan African populations: A systematic review. F1000Res 5 2832. doi: 10.12688/f1000research.10327.2
Benos, D. J. (1982). Amiloride: A molecular probe of sodium transport in tissues and cells. Am. J. Physiol. 242 (3), C131–C145. doi: 10.1152/ajpcell.1982.242.3.C131
Bongomin, F., Gago, S., Oladele, R. O., Denning, D. W. (2017). Global and multi-national prevalence of fungal diseases-estimate precision. J. Fungi (Basel) 3 (4). doi: 10.3390/jof3040057
Brown, J., Benedict, K., Park, B. J., Thompson, G. R., 3rd (2013). Coccidioidomycosis: epidemiology. Clin. Epidemiol. 5, 185–197. doi: 10.2147/CLEP.S34434
Buckley, B. J., Aboelela, A., Majed, H., Bujaroski, R. S., White, K. L., Powell, A. K., et al. (2021a). Systematic evaluation of structure-property relationships and pharmacokinetics in 6-(hetero)aryl-substituted matched pair analogs of amiloride and 5-(N,N-hexamethylene)amiloride. Bioorg Med. Chem. 37, 116116. doi: 10.1016/j.bmc.2021.116116
Buckley, B. J., Aboelela, A., Minaei, E., Jiang, L. X., Xu, Z., Ali, U., et al. (2018). 6-substituted hexamethylene amiloride (HMA) derivatives as potent and selective inhibitors of the human urokinase plasminogen activator for use in cancer. J. Med. Chem. 61 (18), 8299–8320. doi: 10.1021/acs.jmedchem.8b00838
Buckley, B. J., Kumar, A., Aboelela, A., Bujaroski, R. S., Li, X., Majed, H., et al. (2021b). Screening of 5- and 6-substituted amiloride libraries identifies dual-uPA/NHE1 active and single target-selective inhibitors. Int. J. Mol. Sci. 22 (6). doi: 10.3390/ijms22062999
Buckley, B. J., Majed, H., Aboelela, A., Minaei, E., Jiang, L., Fildes, K., et al. (2019). 6-substituted amiloride derivatives as inhibitors of the urokinase-type plasminogen activator for use in metastatic disease. Bioorg Med. Chem. Lett. 29 (24), 126753. doi: 10.1016/j.bmcl.2019.126753
Colombo, A. L., de Almeida Junior, J. N., Slavin, M. A., Chen, S. C., Sorrell, T. C. (2017). Candida and invasive mould diseases in non-neutropenic critically ill patients and patients with haematological cancer. Lancet Infect. Dis. 17 (11), e344–e356. doi: 10.1016/S1473-3099(17)30304-3
De Almeida, G. M., Costa, S. F., Melhem, M., Motta, A. L., Szeszs, M. W., Miyashita, F., et al. (2008). Rhodotorula spp. isolated from blood cultures: clinical and microbiological aspects. Med. Mycol 46 (6), 547–556. doi: 10.1080/13693780801972490
Du, H., Bing, J., Hu, T., Ennis, C. L., Nobile, C. J., Huang, G. (2020). Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PloS Pathog. 16 (10), e1008921. doi: 10.1371/journal.ppat.1008921
Eisenman, H. C., Casadevall, A., McClelland, E. E. (2007). New insights on the pathogenesis of invasive cryptococcus neoformans infection. Curr. Infect. Dis. Rep. 9 (6), 457–464. doi: 10.1007/s11908-007-0070-8
Ellis, D. H., Pfeiffer, T. J. (1990). Ecology, life cycle, and infectious propagule of cryptococcus neoformans. Lancet 336 (8720), 923–925.
Falces-Romero, I., Cendejas-Bueno, E., Romero-Gomez, M. P., Garcia-Rodriguez, J. (2018). Isolation of rhodotorula mucilaginosa from blood cultures in a tertiary care hospital. Mycoses 61 (1), 35–39. doi: 10.1111/myc.12703
Frias-De-Leon, M. G., Hernandez-Castro, R., Vite-Garin, T., Arenas, R., Bonifaz, A., Castanon-Olivares, L., et al. (2020). Antifungal resistance in candida auris: Molecular determinants. Antibiotics (Basel) 9 (9). doi: 10.3390/antibiotics9090568
Hards, K., Cheung, C. Y., Waller, N., Adolph, C., Keighley, L., Tee, Z. S., et al. (2022). An amiloride derivative is active against the F1Fo-ATP synthase and cytochrome bd oxidase of mycobacterium tuberculosis. Commun. Biol. 5 (1). doi: 10.1038/s42003-022-03110-8
Hata, D. J., Humphries, R., Lockhart, S. R., College of American Pathologists Microbiology, C. (2020). Candida auris: An emerging yeast pathogen posing distinct challenges for laboratory diagnostics, treatment, and infection prevention. Arch. Pathol. Lab. Med. 144 (1), 107–114. doi: 10.5858/arpa.2018-0508-RA
Kainz, K., Bauer, M. A., Madeo, F., Carmona-Gutierrez, D. (2020). Fungal infections in humans: the silent crisis. Microbial Cell 7 (6), 143–145. doi: 10.15698/mic2020.06.718
Kim, J. H., Cheng, L. W., Chan, K. L., Tam, C. C., Mahoney, N., Friedman, M., et al. (2020). Antifungal drug repurposing. Antibiotics (Basel) 9 (11). doi: 10.3390/antibiotics9110812
Kitazawa, T., Ishigaki, S., Seo, K., Yoshino, Y., Ota, Y. (2018). Catheter-related bloodstream infection due to rhodotorula mucilaginosa with normal serum (1–>3)-beta-D-glucan level. J. Mycol Med. 28 (2), 393–395. doi: 10.1016/j.mycmed.2018.04.001
Kleyman, T. R., Cragoe, E. J., Jr (1988). Amiloride and its analogs as tools in the study of ion transport. J. Membr Biol. 105 (1), 1–21. doi: 10.1007/BF01871102
Li, J. H., Cragoe, E. J., Jr., Lindemann, B. (1985). Structure-activity relationship of amiloride analogs as blockers of epithelial Na channels: I. pyrazine-ring modifications. J. Membr Biol. 83 (1-2), 45–56. doi: 10.1007/BF01868737
Limper, A. H., Adenis, A., Le, T., Harrison, T. S. (2017). Fungal infections in HIV/AIDS. Lancet Infect. Dis. 17 (11), e334–e343. doi: 10.1016/S1473-3099(17)30303-1
Lockhart, S. R., Guarner, J. (2019). Emerging and reemerging fungal infections. Semin. Diagn. Pathol. 36 (3), 177–181. doi: 10.1053/j.semdp.2019.04.010
Matthews, H., Ranson, M., Tyndall, J. D., Kelso, M. J. (2011). Synthesis and preliminary evaluation of amiloride analogs as inhibitors of the urokinase-type plasminogen activator (uPA). Bioorg Med. Chem. Lett. 21 (22), 6760–6766. doi: 10.1016/j.bmcl.2011.09.044
Mpoza, E., Rhein, J., Abassi, M. (2018). Emerging fluconazole resistance: Implications for the management of cryptococcal meningitis. Med. Mycol Case Rep. 19, 30–32. doi: 10.1016/j.mmcr.2017.11.004
Nielsen, K., Cox, G. M., Wang, P., Toffaletti, D. L., Perfect, J. R., Heitman, J. (2003). Sexual cycle of cryptococcus neoformans var. grubii and virulence of congenic a and alpha isolates. Infect. Immun. 71 (9), 4831–4841. doi: 10.1128/IAI.71.9.4831-4841.2003
Pfaller, M. A., Diekema, D. J. (2004). Rare and emerging opportunistic fungal pathogens: Concern for resistance beyond candida albicans and aspergillus fumigatus. J. Clin. Microbiol. 42 (10), 4419–4431. doi: 10.1128/JCM.42.10.4419-4431.2004
Pfaller, M. A., Diekema, D. J. (2010). Epidemiology of invasive mycoses in north America. Crit. Rev. Microbiol. 36 (1), 1–53. doi: 10.3109/10408410903241444
Rajasingham, R., Smith, R. M., Park, B. J., Jarvis, J. N., Govender, N. P., Chiller, T. M., et al. (2017). Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect. Dis. 17 (8), 873–881. doi: 10.1016/S1473-3099(17)30243-8
Silva, S., Negri, M., Henriques, M., Oliveira, R., Williams, D. W., Azeredo, J. (2012). Candida glabrata, candida parapsilosis and candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 36 (2), 288–305. doi: 10.1111/j.1574-6976.2011.00278.x
Smith, K. D., Achan, B., Hullsiek, K. H., McDonald, T. R., Okagaki, L. H., Alhadab, A. A., et al. (2015). Increased antifungal drug resistance in clinical isolates of cryptococcus neoformans in Uganda. Antimicrob. Agents Chemother. 59 (12), 7197–7204. doi: 10.1128/AAC.01299-15
Tuon, F. F., de Almeida, G. M., Costa, S. F. (2007). Central venous catheter-associated fungemia due to rhodotorula spp. –a systematic review. Med. Mycol 45 (5), 441–447. doi: 10.1080/13693780701381289
Vu, K., Blumwald, E., Gelli, A. (2021). The antifungal activity of HMA, an amiloride analog and inhibitor of Na(+)/H(+) exchangers. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.673035
Wall, G., Lopez-Ribot, J. L. (2020). Screening repurposing libraries for identification of drugs with novel antifungal activity. Antimicrob. Agents Chemother. 64 (9). doi: 10.1128/AAC.00924-20
Wirth, F., Goldani, L. Z. (2012). Epidemiology of rhodotorula: an emerging pathogen. Interdiscip Perspect. Infect. Dis. 2012, 465717. doi: 10.1155/2012/465717
Wisplinghoff, H., Bischoff, T., Tallent, S. M., Seifert, H., Wenzel, R. P., Edmond, M. B. (2004). Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39 (3), 309–317. doi: 10.1086/421946
Woods, J. P. (2002). Histoplasma capsulatum molecular genetics, pathogenesis, and responsiveness to its environment. Fungal Genet. Biol. 35 (2), 81–97. doi: 10.1006/fgbi.2001.1311
Keywords: amiloride, HMA, analogs, antifungal activity, Cryptococcus neoformans, MIC, MFC, Candida spp.
Citation: Vu K, Buckley BJ, Bujaroski RS, Blumwald E, Kelso MJ and Gelli A (2023) Antifungal activity of 6-substituted amiloride and hexamethylene amiloride (HMA) analogs. Front. Cell. Infect. Microbiol. 13:1101568. doi: 10.3389/fcimb.2023.1101568
Received: 18 November 2022; Accepted: 20 January 2023;
Published: 16 February 2023.
Edited by:
Ibrahim Bitar, Charles University, CzechiaReviewed by:
Nour Fattouh, Saint George University of Beirut, LebanonLukasz Kozubowski, Clemson University, United States
Copyright © 2023 Vu, Buckley, Bujaroski, Blumwald, Kelso and Gelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angie Gelli, YWNnZWxsaUB1Y2RhdmlzLmVkdQ==
 Kiem Vu
Kiem Vu Benjamin J. Buckley
Benjamin J. Buckley Richard S. Bujaroski
Richard S. Bujaroski Eduardo Blumwald
Eduardo Blumwald Michael J. Kelso2,3
Michael J. Kelso2,3 Angie Gelli
Angie Gelli