- 1Department of Dermatology, Xiangya Hospital, Central South University, Changsha, China
- 2National Engineering Research Center of Personalized Diagnostic and Therapeutic Technology, Changsha, Hunan, China
- 3Furong Laboratory, Changsha, Hunan, China
- 4Hunan Key Laboratory of Skin Cancer and Psoriasis, Hunan Engineering Research Center of Skin Health and Disease, Xiangya Hospital, Central South University, Changsha, China
- 5National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, Hunan, China
- 6Department of Plastic and Cosmetic Surgery, Xiangya Hospital, Central South University, Changsha, China
Background: Azvudine has been approved in China for the treatment of COVID-19 patients. Previous studies have suggested a correlation between high levels of lactate dehydrogenase (LDH) and the severity of COVID-19. However, the impact of LDH levels in COVID-19 patients receiving Azvudine treatment remains unclear.
Methods: In this retrospective cohort study, we analyzed the data of 351 hospitalized COVID-19 patients who were consecutively treated with Azvudine, with or without high LDH levels. The clinical features, treatment strategies and prognosis data were collected and analyzed.
Results: Among the 351 hospitalized patients with COVID-19 treated with Azvudine (119 with high-LDH levels), the median age was 69 years (range 58–78), and 213 (60.7%) were male. Common symptoms included cough (86.0%), expectoration (73.5%), fever (69.8%), polypnea (47.6%) and poor appetite (46.4%). Patients with high LDH levels exhibited significantly elevated leucocyte and neutrophil counts, elevated level of myocardial enzymes, as well as higher levels of inflammatory markers such as interleukin-6, interleukin-10, procalcitonin, C reactive protein, ferritin, and prolonged erythrocyte sedimentation rate upon admission. COVID-19 patients with high-LDH levels had higher rates of corticosteroid therapy, non-invasive and invasive mechanical ventilation, worsened and death (2.5% vs. 0%). The Cox proportional hazard model demonstrated that high LDH levels (adjusted hazard ratio = 5.27; 95% confidence interval: 1.19, 14.50) were associated with a more unfavorable composite disease progression outcome among COVID-19 patients treated with Azvudine, after accounting for potential confounding variables.
Conclusion: High-LDH levels predict a worse composite disease progression outcome in COVID-19 patients treated with Azvudine.
Introduction
Coronavirus disease 2019 (COVID-19), caused by the infection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has emerged as a significant global public health threat in recent years (The, 2020; The, 2023). Azvudine, a nucleoside analog that inhibits HIV-1 RNA-dependent RNA polymerase (Yu and Chang, 2020), has shown promise in combating COVID-19. Zhang et al. discovered during the 2021 COVID-19 outbreak that oral administration of Azvudine effectively inhibits SARS-CoV-2 replication, preserves thymus immune function, and provides rapid treatment for COVID-19 patients (Zhang et al., 2021). In July 2022, the China National Medical Products Administration and the National Health Commission of China approved Azvudine for the treatment of adult patients with mild COVID-19 (Yu and Chang, 2022). Several subsequent clinical trials, including our own previous study, demonstrated the effectiveness of oral Azvudine in curing COVID-19 patients (Ren et al., 2020; Zhang et al., 2021; Yu and Chang, 2022; Deng et al., 2023; Shen et al., 2023; Sun et al., 2023). However, the association between inflammatory biomarkers and prognosis in COVID-19 patients undergoing Azvudine treatment remains unclear.Lactate dehydrogenase (LDH), a cytoplasmic glycolytic enzyme found in almost every tissue, is commonly used as an indicator of tissue damage (Jurisic et al., 2015). Numerous studies have shown that elevated LDH levels are positively associated with the severity of COVID-19 (Bartziokas and Kostikas, 2021; Szarpak et al., 2021; Alonso-Bernáldez et al., 2023; Ergenc et al., 2023). However, it is still uncertain whether LDH can predict the prognosis in COVID-19 patients receiving Azvudine treatment. In the present retrospective study, we reviewed the clinical data of 351 adult patients with positive RT-PCR for SARS-CoV-2 infection who were treated with Azvudine. We compared the clinical characteristics, laboratory markers and short-term prognosis, including mortality, between patients with high-LDH levels and those without. The aim of this study was to investigate the role of high-LDH levels as a predictive marker of response to Azvudine treatment in COVID-19 patients.
Methods
Study design
We conducted a single-center, retrospective cohort study of hospitalized adult patients with positive RT-PCR for SARS-CoV-2 infection, who were given Azvudine in Xiangya Hospital, during the period from December 5, 2022 to January 26, 2023. We excluded patients who were younger than 18 years; those without LDH test results; those have a low LDH (<50U/L) or those who did not receive Azvudine treatment. This study was approved by the institutional review board of Xiangya Hospital, Central South University (202002024), and individual patient-informed consent was not required for this retrospective cohort study using anonymized data.
Data source
The electronic health records of COVID-19 patients were retrieved from the inpatient system of Xiangya Hospital. These comprehensive records include various details such as demographic information, admission records, diagnoses, prescribed medications, drug dispensing records, procedures, laboratory tests, and dates of discharge or death. The health records were then linked with anonymized vaccination records provided by the Department of Immunization, Center for Disease Control and Prevention of Hunan Province using unique identification numbers (China Identity Card).
Definition of conditions
LDH levels were assessed using the lactate dehydrogenase substrate method (Beckman AU5800). The values of LDH were collected as continuous variables and analyzed in binary form. Patients were categorized into two groups based on their LDH levels. The normal-LDH group included patients with LDH values ranging from 50 to 250 U/L, while the high-LDH group included patients with LDH values greater than 250 U/L. Severe COVID-19 patients were defined as having respiratory rate ≥30 times per minute, or oxygen saturation ≤ 93%, or PaO2/FiO2 ≤300 mmHg, or lung infiltrates >50% on admission.
Statistical analysis
Descriptive statistics were conducted to summarize all variables in the study. Categorical variables were compared using the Fisher exact test or χ2 test, while continuous variables were compared using the t test or the Mann-Whitney U test, as appropriate, to evaluate the study outcome. Continuous variables were presented as mean (SD) or median [interquartile range (IQR)] values, while categorical variables were presented as proportions. Disease progression outcome was depicted using the Kaplan-Meier method and compared between patients with normal LDH levels or with high LDH levels using the log-rank test. Multivariate Cox regression models were used to determine the independent risk factors for disease progression during hospitalization. The adjusted hazard ratio (aHR) was calculated to assess the risk factors. Statistical analysis was conducted using SPSS version 25.0 (IBM) and R version 4.2.1., and statistical charts were generated using Excel 2016 (Microsoft). The significance level was set at P < 0.05 for all statistical analyses.
Results
Baseline characteristics of COVID-19 patients
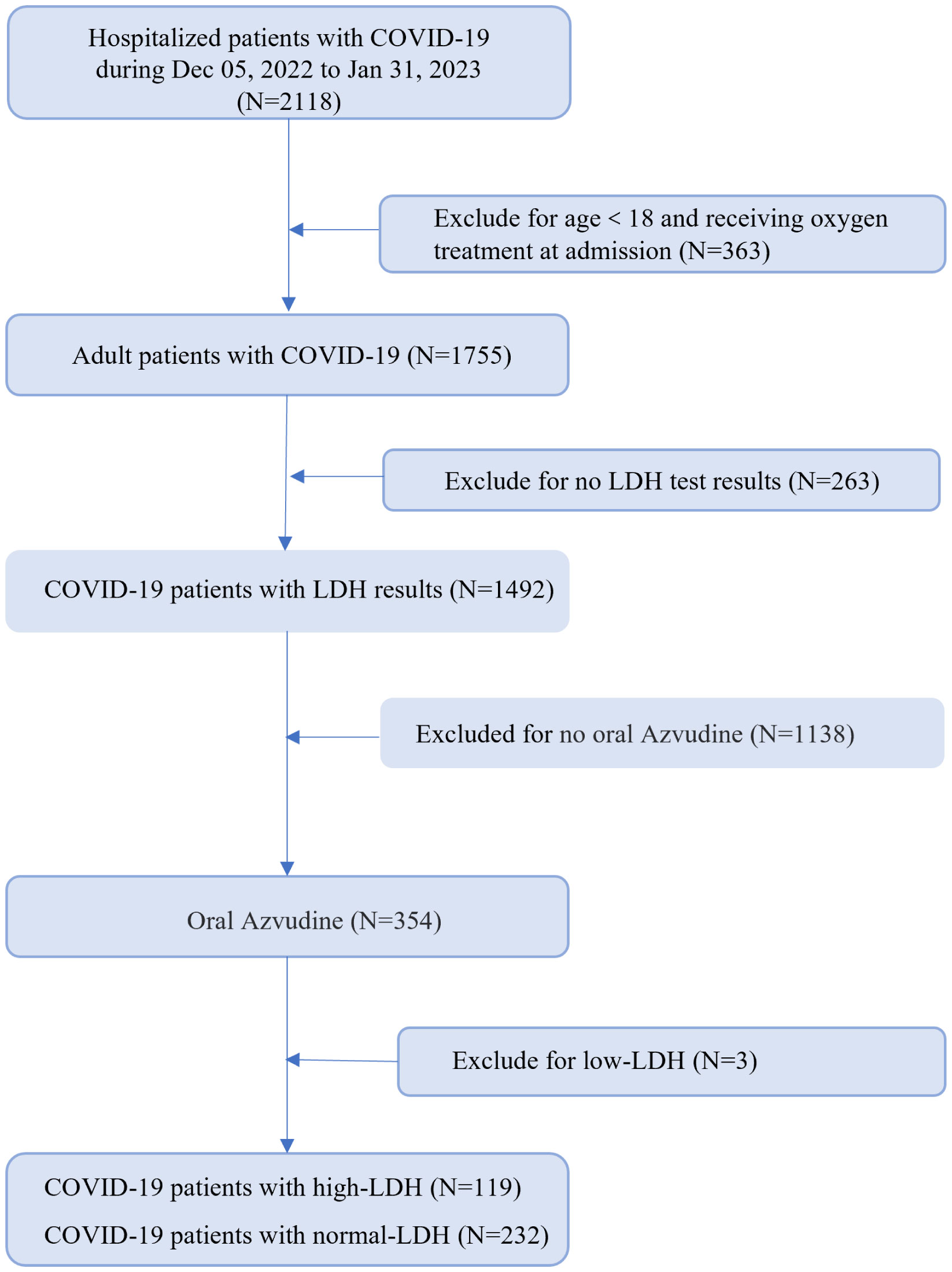
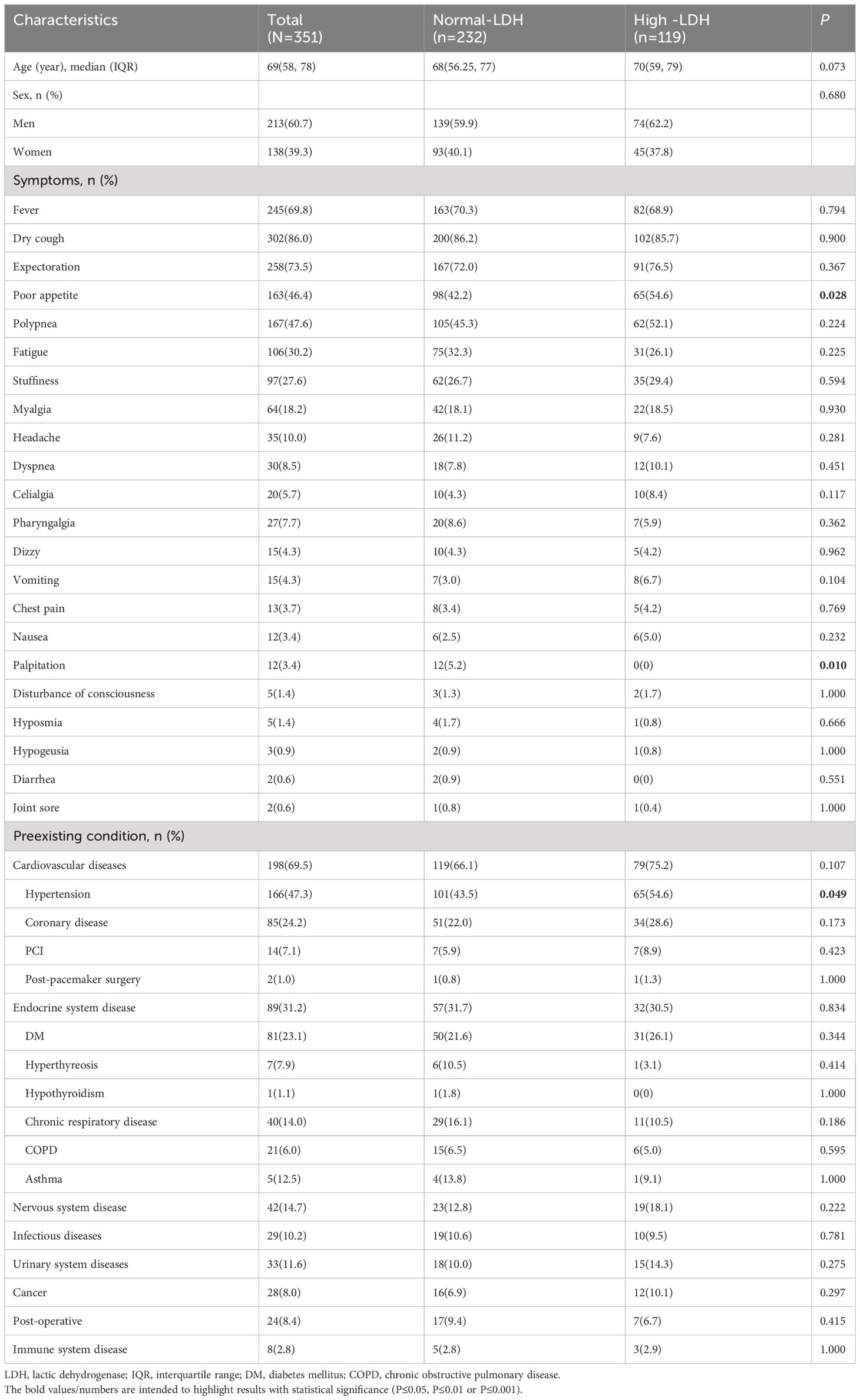
We collected and analyzed information from 351 hospitalized patients with COVID-19. All patients underwent LDH testing, received Azvudine treatment, and were followed up for a period of 30 days. More than half of the patients (51.3%) had high LDH levels (Figure 1). The demographic and clinical characteristics of the patients on admission were summarized in Table 1. The median age was 69 years (IQR, 58–78), and 213 (60.7%) were male. Common symptoms at the onset of illness were dry cough (302, 86.0%), expectoration (258, 73.5%), fever (245, 69.8%), polypnea (167, 47.6%) and poor appetite (163, 46.4%). Comorbidities were present in more than half of the patients. Cardiovascular diseases were the most common comorbidity, with 198 patients (69.5%) having this condition. Other comorbidities included endocrine system disease (89, 31.2%), nervous system disease (42, 14.7%), infectious diseases (29, 10.0%), urinary system diseases (33, 11.6%), cancer (28, 8.0%), post-operative conditions (24, 8.4%), and immune system disease (8, 2.0%). There were no significant differences in gender and age between the normal-LDH and high-LDH groups of patients. Regarding COVID-19-related clinical symptoms, patients with high-LDH were more likely to experience poor appetite (54.6% vs. 42.2%, P = 0.028) and palpitation (5.2% vs. 0.0%, P = 0.010) compared to those with normal-LDH levels. However, there were no significant differences in the proportions of other clinical symptoms between the two groups. In terms of preexisting conditions, the high-LDH group had a higher prevalence of hypertension compared to the normal-LDH group (54.6% vs. 43.5%, P = 0.049). However, there were no significant differences in the prevalence of other diseases such as coronary disease, Percutaneous Coronary Intervention, or post-pacemaker surgery between the two groups.
Laboratory findings on admission
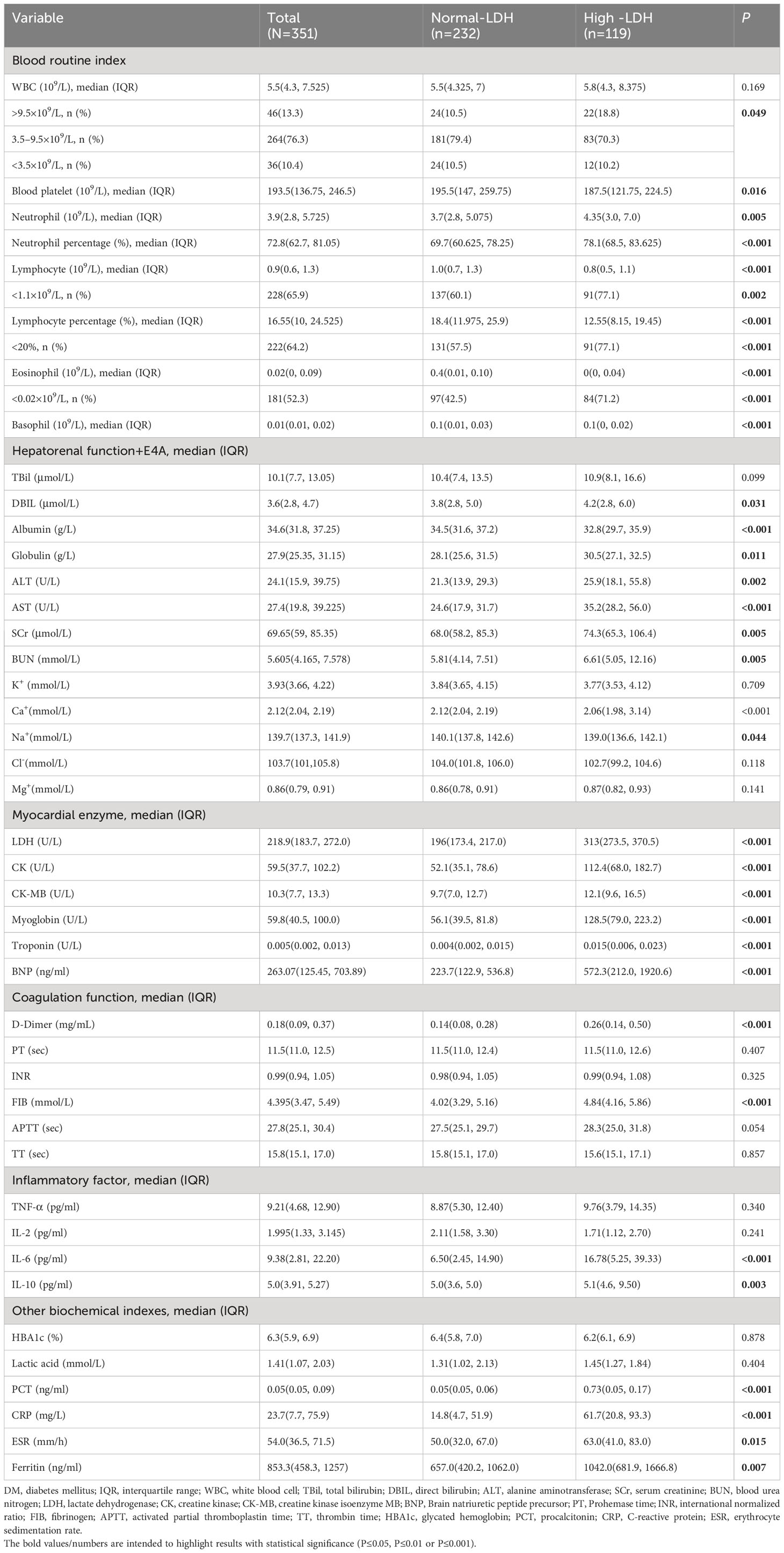
The laboratory findings on admission of the COVID-19 patients with or without high-LDH levels were presented in Table 2. The majority of patients (76.3%) had a normal leukocyte count, while 13.1% had an increased leukocyte count and 10.4% had a decreased leukocyte count. Patients with high LDH levels had a higher median neutrophil count and neutrophil percentage, but a lower lymphocyte count, lymphocyte percentage, eosinophil count, and basophil count. Remarkably, patients with high LDH levels also showed significantly higher serum concentrations of interleukin-6 (IL-6), interleukin-10 (IL-10), procalcitonin (PCT), C-reactive protein (CRP), and ferritin. Additionally, they exhibited a prolonged erythrocyte sedimentation rate (ESR), indicating a more pronounced inflammatory response in individuals with high LDH levels. These compelling findings provide valuable insights into the association between LDH and markers of inflammation, suggesting that LDH may serve as an indicator of heightened systemic inflammation in patients with COVID-19. Additionally, patients with elevated LDH levels exhibited higher concentrations of organ function markers, including direct bilirubin (DBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum creatinine (SCr), blood urea nitrogen (BUN), creatine kinase (CK), creatine kinase MB isoenzyme (CK-MB), myoglobin, troponin, and B-type natriuretic peptide (BNP), compared to patients with normal LDH levels. Regarding coagulation function markers, patients with high LDH levels demonstrated increased levels of D-dimer and fibrinogen (FIB) compared to those with normal LDH levels.
Analysis of severity, treatment and prognosis of patients with COVID-19
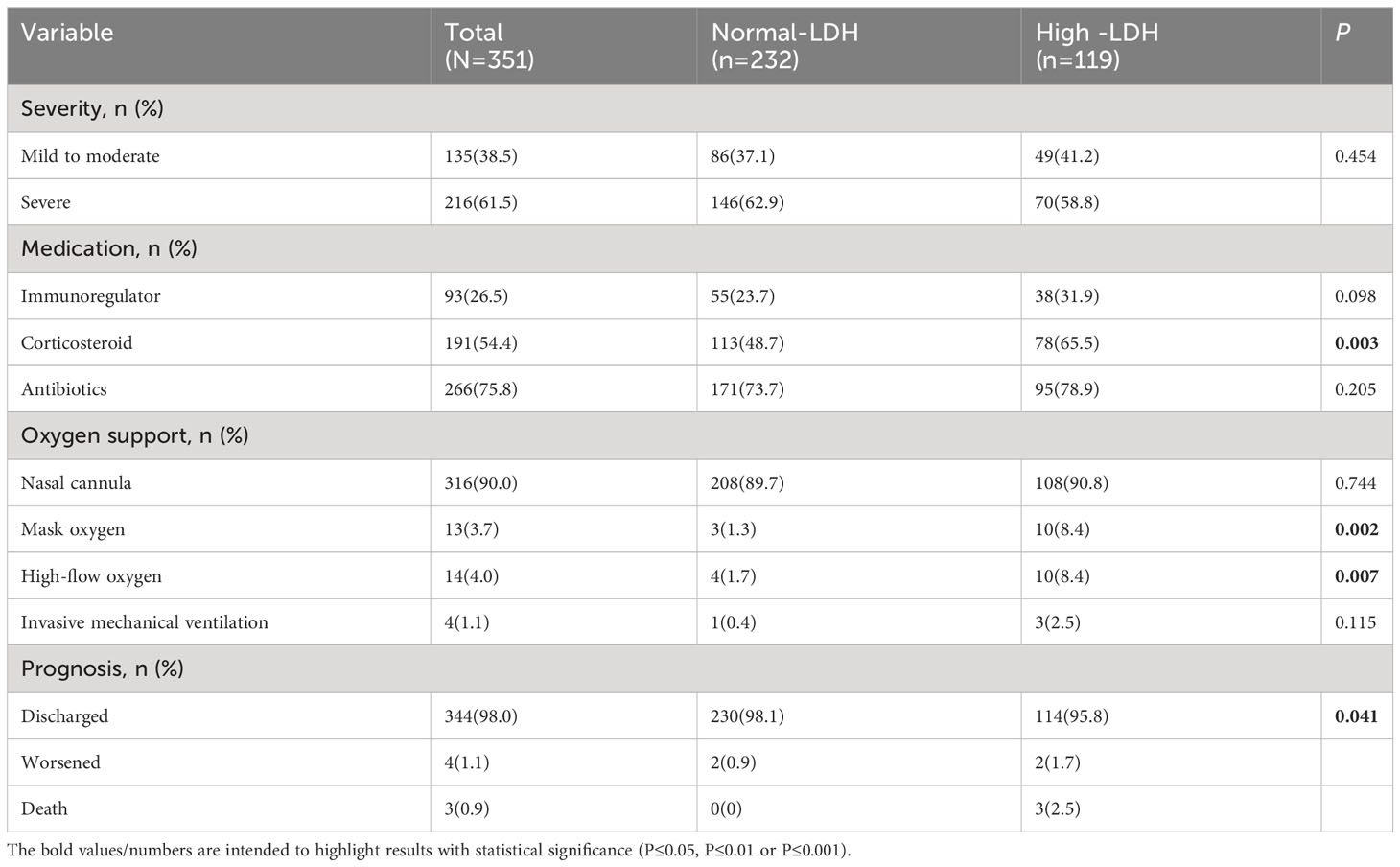
We compared the severity, treatment, and short-term prognosis between COVID-19 patients with normal lactate dehydrogenase (LDH) levels and those with high LDH levels, as shown in Table 3. While no significant difference in severity was observed between the two groups, it was evident that patients with high LDH levels had a higher utilization of corticosteroid therapy (65.5% vs. 48.7%, P = 0.003), mask oxygen support (8.4% vs. 1.3%, P = 0.002), and high-flow oxygen support (8.4% vs. 1.7%, P = 0.007). Furthermore, patients with high LDH levels exhibited a higher rate of deterioration and fatality (1.7% vs. 0.9%, 2.5% vs. 0, P = 0.041) compared to those with normal LDH levels.
Analysis of the association between high-LDH and composite disease progression outcome in COVID-19 patients
The Kaplan-Meier survival curve and Cox proportional hazard model were used to further assess the association between high LDH levels and the composite disease progression outcome of COVID-19. The results showed that COVID-19 patients with high LDH levels had a significantly higher cumulative incidence of exacerbation of disease progression than those with normal LDH levels (P < 0.001) (Figure 2). More importantly, high LDH levels were found to be an independent risk factor for the composite disease progression outcome in COVID-19 patients treated with Azvudine, after adjusting for age and sex only (aHR = 4.68; 95% CI: 1.78, 12.34; P = 0.002), or adjusting for steroid and antibiotics (aHR = 4.98; 95% CI: 1.88, 13.16; P = 0.001), or additionally adjusting for age, sex, severity, time from symptom onset to admission, preexisting conditions, steroid, antibiotics, and vaccine (aHR = 5.27; 95% CI: 1.19, 14.50; P = 0.001) (Table 4).
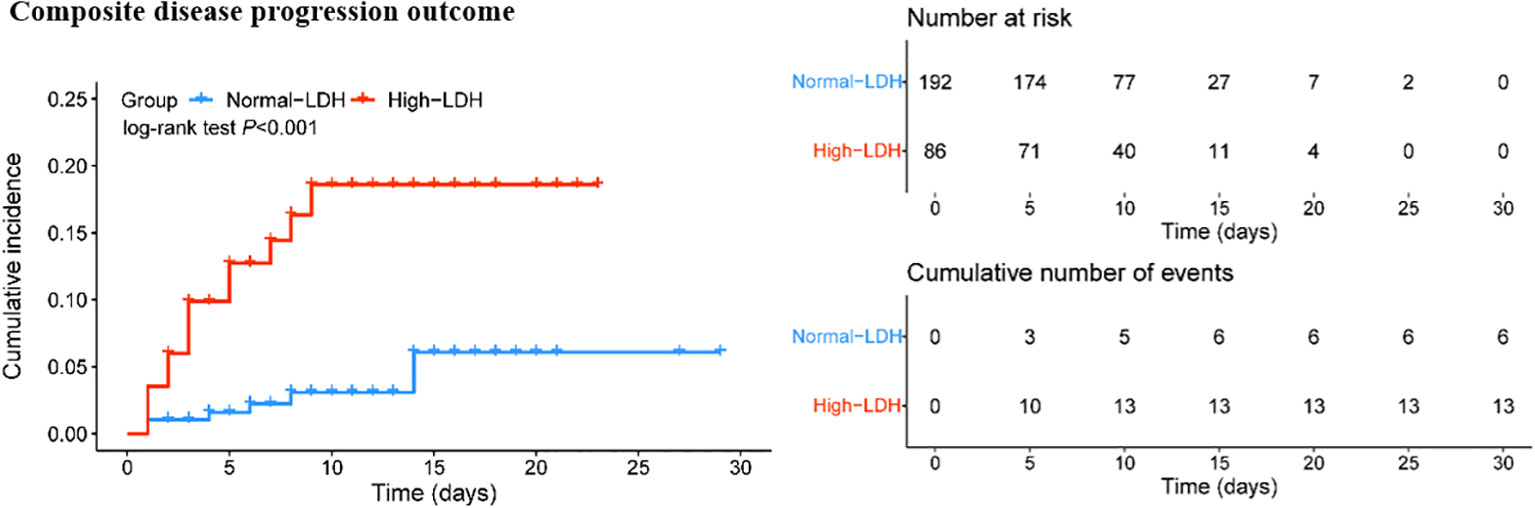
Figure 2 Cumulative incidence of composite disease progression outcome for high LDH levels and normal LDH levels.
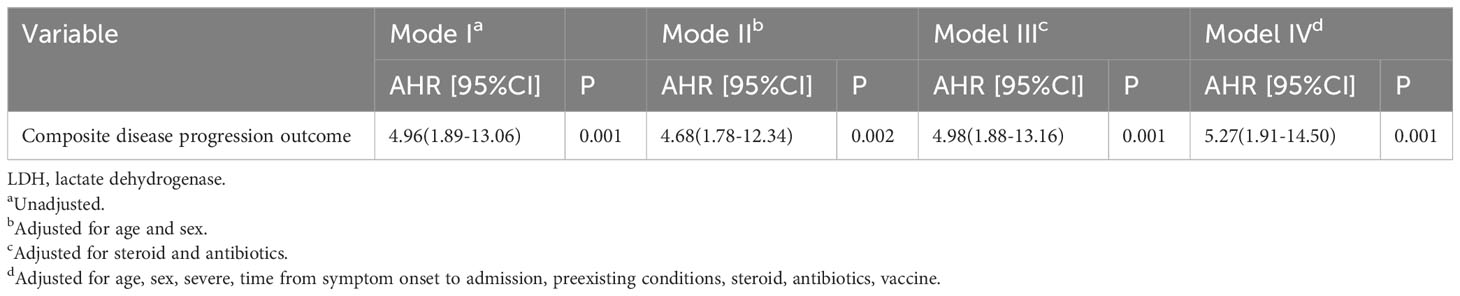
Table 4 Associations of high-LDH with fatality of COVID-19 patients treated with Azvudine in Cox proportion hazard models.
Discussion
The global outbreak of COVID-19 has caused significant fear and concern (Makević et al., 2022; Huang et al., 2023).. In response, scientists and researchers have developed vaccines to prevent infection and new drugs to treat infected individuals. Azvudine, as the first domestic oral antiviral agent approved in China, has been reported to shorten the time of nucleic acid negative conversion and cure patients with both common and severe COVID-19 (Ren et al., 2020; Yu and Chang, 2022; Shen et al., 2023).
In this retrospective cohort study, we found that COVID-19 patients treated with Azvudine had a relatively high proportion (33.9%) of high LDH levels, and demonstrated that high LDH levels was associated with alterations in laboratory markers. Moreover, individuals with high LDH levels upon SARS-CoV-2 infection require more intensive supportive treatment at the time of diagnosis and generally have a poorer prognosis compared to those with normal LDH levels.
Elevated LDH levels are indicative of tissue or cellular damage, making it a common marker for tissue injury. LDH elevation is also commonly observed during viral infections including MERS-CoV (Alsolamy, 2015; Al Ghamdi et al., 2016), H7N9 (Shi et al., 2013), and H5N1 (Oner et al., 2006). Similarly, in many severe cases of COVID-19, increased LDH activity has also been observed, serving as a marker of disease severity (Sidhwani et al., 2023). Previous studies have shown that after SARS-CoV-2 infection, there is an increase in white blood cell count, especially neutrophil count, leading to excessive cytokine production, cytokine storm, and systemic organ damage (Fialek et al., 2022). In our study, laboratory examination results upon hospital admission showed that COVID-19 patients in the high LDH group had higher neutrophil counts and increased proportions of white blood cells compared to the low LDH group. This may explain why patients with high LDH are more susceptible to pathogen infection due to weakened immune function following viral infection. We also found that patients with high LDH levels often exhibited decreased lymphocyte and eosinophil counts, indicating a possible correlation between lymphocyte damage and LDH release. Previous research has shown that inflammatory cytokines can increase LDH release from cells. For example, TNF-alpha can increase LDH release from Raji cells (Jurisic et al., 2004; Oner et al., 2006; Fialek et al., 2022; Sidhwani et al., 2023). The study conducted by Khalid et al. (2023) demonstrated a significant increase in the levels of IL-6, CRP, and PCT in patients with severe and critical conditions following SARS-CoV-2 infection. In our study, we further observed elevated serum concentrations of IL-6, IL-10, CRP, and PCT in patients with high LDH levels, suggesting a potential association between inflammatory cytokine release and LDH. Compared to the normal LDH group, patients with high LDH levels also demonstrated elevated levels of organ function markers such as DBIL, ALT, AST, SCr, BUN, CK, CK-MB, and BNP. In terms of coagulation function markers, patients in the high LDH group showed higher levels of D-dimer and FIB. Overall, our study results suggest that patients with high LDH levels may be at risk of a heightened inflammatory state and multi-organ dysfunction, which aligns with the Shi et al.’ conclusions (Shi et al., 2013).
Although there were no significant differences in disease severity between patients with or without high LDH levels, patients with high LDH levels tended to receive more immunoregulator and corticosteroid treatment and mechanical ventilation. The Cox regression model indicated that high LDH levels was an independent predictor for the composite disease progression outcome of COVID-19 patients treated with Azvudine, even after adjusting for potential confounders. These results suggest that high LDH levels may be a candidate biomarker for worse prognosis in patients treated with Azvudine.
To our best knowledge, this is the first study to report an association between high LDH levels and outcomes in patients with COVID-19 treated with Azvudine. However, several limitations deserve attention. Firstly, the LDH isoenzymes or LDH subunits were not tested due to limited resources. LDH isoenzyme or LDH subunits analysis in the future may help identify the source of increased LDH. Secondly, due to the massive number of patients and the lack of medical resources, the interval from illness onset to hospital admission was more than 5 days for most patients, which could have implications for disease progression and outcomes. Nevertheless, patients with high LDH levels had a similar interval from illness onset to hospital admission compared to those without normal LDH levels. Thirdly, although the data were consecutively collected and adjusted for a large number of confounders, we could not exclude the possibility of selection bias or confounding by indication due to the nature of retrospective cohort design.
In conclusion, we clarified the correlation between LDH levels and the prognosis of COVID-19 patients treated with Azvudine, and found that high LDH levels were associated with poorer short-term outcomes in COVID-19 patients treated with Azvudine. Therefore, stronger personal prophylactic strategies are advised for patients with high LDH levels, and more intensive surveillance and treatment should be considered when they are infected with SARS-CoV-2, especially for geriatric patients or those with preexisting comorbidities.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Xiangya Hospital, Central South University (202002024). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This study was approved by the institutional review board of Xiangya Hospital, Central South University (202002024), and individual patient-informed consent was not required for this retrospective cohort study using anonymized data.
Author contributions
Conception and design: GD, WZ, WC. Acquisition of data: MM, YS, YD. Interpretation of data, statistical analysis and manuscript writing: GD, WZ, WC. Revision of manuscript and administrative, technical, or material support: GD, WZ, WC.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 82102803, 82272849 to GD), Natural Science Foundation of Hunan Province (Grant Nos. 2021JJ40976 to GD).
Acknowledgments
We thank all the hospital staff members for their efforts in collecting the information that used in this study. We thank staffs from the Center for Disease Control and Prevention of Hunan Province for the linkage of vaccination data. We also thank the patients who participated in this study and their families.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the Centers for Disease Control and Prevention or the institutions with which the authors are affiliated.
References
Al Ghamdi, M., Alghamdi, K. M., Ghandoora, Y., Alzahrani, A., Salah, F., Alsulami, A., et al. (2016). Treatment outcomes for patients with Middle Eastern Respiratory Syndrome Coronavirus (MERS CoV) infection at a coronavirus referral center in the Kingdom of Saudi Arabia. BMC Infect. Dis. 16, 174. doi: 10.1186/s12879-016-1492-4
Alonso-Bernáldez, M., Cuevas-Sierra, A., Micó, V., Higuera-Gómez, A., Ramos-Lopez, O., Daimiel, L., et al. (2023). An interplay between oxidative stress (Lactate dehydrogenase) and inflammation (Anisocytosis) mediates COVID-19 severity defined by routine clinical markers. Antioxid (Basel Switzerland) 12 (2), 234. doi: 10.3390/antiox12020234
Alsolamy, S. (2015). Middle East respiratory syndrome: knowledge to date. Crit. Care Med. 43 (6), 1283–1290. doi: 10.1097/CCM.0000000000000966
Bartziokas, K., Kostikas, K. (2021). Lactate dehydrogenase, COVID-19 and mortality. Med clinica 156 (1), 37. doi: 10.1016/j.medcli.2020.07.043
Deng, G., Li, D., Sun, Y., Jin, L., Zhou, Q., Xiao, C., et al. (2023). Real-world effectiveness of Azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: A retrospective cohort study. J. Med. Virol. 95 (4), e28756. doi: 10.1002/jmv.28756
Ergenc, I., Capar, E., Erturk, S. B., Bahramzade, G., Atalah, F., Kocakaya, D., et al. (2023). Diagnostic performance of lactate dehydrogenase (LDH) isoenzymes levels for the severity of COVID-19. J. Med. Biochem. 42 (1), 16–26. doi: 10.5937/jomb0-37234
Fialek, B., Pruc, M., Smereka, J., Jas, R., Rahnama-Hezavah, M., Denegri, A., et al. (2022). Diagnostic value of lactate dehydrogenase in COVID-19: A systematic review and meta-analysis. Cardiol. J. 29 (5), 751–758. doi: 10.5603/CJ.a2022.0056
Huang, S., Gao, Z., Wang, S. (2023). China's COVID-19 reopening measures-warriors and weapons. Lancet (London England) 401 (10377), 643–644. doi: 10.1016/S0140-6736(23)00213-1
Jurisic, V., Bumbasirevic, V., Konjevic, G., Djuricic, B., Spuzic, I. (2004). TNF-alpha induces changes in LDH isotype profile following triggering of apoptosis in PBL of non-Hodgkin's lymphomas. Ann. Hematol. 83 (2), 84–91. doi: 10.1007/s00277-003-0731-0
Jurisic, V., Radenkovic, S., Konjevic, G. (2015). The actual role of LDH as tumor marker, biochemical and clinical aspects. Adv. Exp. Med. Biol. 867, 115–124. doi: 10.1007/978-94-017-7215-0_8
Khalid, A., Aqeel, R. F., Nawaz, A., Ahmad, J., Fatima, S. T., Shahid, S., et al. (2023). Immune-inflammatory markers & clinical characteristics for outcomes in hospitalized SARS-CoV-2 infected patients of Pakistan: a retrospective analysis. Hematol. (Amsterdam Netherlands) 28 (1), 2199629. doi: 10.1080/16078454.2023.2199629
Makević, A., Ilić, A., Pantović-Stefanović, M., Murić, N., Djordjević, N., Jurišić, V. (2022). Anxiety in patients treated in a temporary hospital in Belgrade, Serbia, during the first epidemic wave of COVID-19. Int. J. Disaster Risk Reduct. IJDRR 77, 103086. doi: 10.1016/j.ijdrr.2022.103086
Oner, A. F., Bay, A., Arslan, S., Akdeniz, H., Sahin, H. A., Cesur, Y., et al. (2006). Avian influenza A (H5N1) infection in eastern Turkey in 2006. N. Engl. J. Med. 355 (21), 2179–2185. doi: 10.1056/NEJMoa060601
Ren, Z., Luo, H., Yu, Z., Song, J., Liang, L., Wang, L., et al. (2020). A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv. Sci. (Weinheim Baden-Wurttemberg Germany) 7 (19), e2001435. doi: 10.1002/advs.202001435
Shen, M., Xiao, C., Sun, Y., Li, D., Wu, P., Jin, L., et al. (2023). Real-world effectiveness of Azvudine in hospitalized patients with COVID-19: a retrospective cohort study. medRxiv 2023.01.23.23284899. doi: 10.1101/2023.01.23.23284899
Shi, J., Xie, J., He, Z., Hu, Y., He, Y., Huang, Q., et al. (2013). A detailed epidemiological and clinical description of 6 human cases of avian-origin influenza A (H7N9) virus infection in Shanghai. PloS One 8 (10), e77651. doi: 10.1371/journal.pone.0077651
Sidhwani, S. K., Mirza, T., Khatoon, A., Shaikh, F., Khan, R., Shaikh, O. A., et al. (2023). Inflammatory markers and COVID-19 disease progression. J. Infect. Public Health 16 (9), 1386–1391. doi: 10.1016/j.jiph.2023.06.018
Sun, Y., Jin, L., Dian, Y., Shen, M., Zeng, F., Chen, X., et al. (2023). Oral Azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study. EClinicalMedicine 59, 101981. doi: 10.1016/j.eclinm.2023.101981
Szarpak, L., Ruetzler, K., Safiejko, K., Hampel, M., Pruc, M., Kanczuga-Koda, L., et al. (2021). Lactate dehydrogenase level as a COVID-19 severity marker. Am. J. Emerg. Med. 45, 638–639. doi: 10.1016/j.ajem.2020.11.025
The, L. (2020). Facing up to long COVID. Lancet (London England) 396 (10266), 1861. doi: 10.1016/s0140-6736(20)32662-3
The, L. (2023). The COVID-19 pandemic in 2023: far from over. Lancet (London England) 401 (10371), 79. doi: 10.1016/s0140-6736(23)00050-8
Yu, B., Chang, J. (2020). Azvudine (FNC): a promising clinical candidate for COVID-19 treatment. Signal Transduct. Target Ther. 5 (1), 236. doi: 10.1038/s41392-020-00351-z
Yu, B., Chang, J. (2022). The first Chinese oral anti-COVID-19 drug Azvudine launched. Innovation (Cambridge (Mass)) 3 (6), 100321. doi: 10.1016/j.xinn.2022.100321
Keywords: azvudine, lactate dehydrogenase, COVID-19, prognosis, SARS-CoV-2
Citation: Mao M, Dian Y, Sun Y, Chen W, Zhu W and Deng G (2023) Lactate dehydrogenase predicts disease progression outcome in COVID-19 patients treated with Azvudine. Front. Cell. Infect. Microbiol. 13:1237277. doi: 10.3389/fcimb.2023.1237277
Received: 09 June 2023; Accepted: 04 October 2023;
Published: 18 October 2023.
Edited by:
Sreekanth Gopinathan Pillai, Indian Institute of Chemical Technology (CSIR), IndiaReviewed by:
Sanjeev Kumar, International Centre for Genetic Engineering and Biotechnology, IndiaLukasz Szarpak, Maria Sklodowska-Curie Medical Academy, Poland
Anson S. Maroky, Annamalai University, India
Copyright © 2023 Mao, Dian, Sun, Chen, Zhu and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangtong Deng, ZGVuZ2d1YW5ndG9uZ0BvdXRsb29rLmNvbQ==; Wu Zhu, emh1d3U3MEBob3RtYWlsLmNvbQ==; Wangqing Chen, bGFuY2hlbjIwMDhAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Manyun Mao1,2,3,4,5†
Manyun Mao1,2,3,4,5† Wangqing Chen
Wangqing Chen Wu Zhu
Wu Zhu Guangtong Deng
Guangtong Deng