- 1Laboratory of Gynecologic Oncology, Fujian Maternity and Child Health Hospital, College of Clinical Medicine for Obstetrics and Gynecology and Pediatrics, Fujian Medical University, Fuzhou, Fujian, China
- 2Fujian Key Laboratory of Women and Children’s Critical Diseases Research, Fujian Maternity and Child Health Hospital (Fujian Women and Children’s Hospital), Fuzhou, Fujian, China
- 3Fujian Clinical Research Center for Gynecological Oncology, Fujian Maternity and Child Health Hospital (Fujian Obstetrics and Gynecology Hospital), Fuzhou, Fujian, China
- 4The State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Institute of Diagnostics and Vaccine Development in Infectious Diseases, School of Public Health, Xiamen University, Xiamen, Fujian, China
- 5Fujian Provincial Cervical Disease Diagnosis and Treatment Health Center, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian, China
- 6Department of Clinical Laboratory, Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian, China
Background: Postmenopausal estrogen deficiency disrupts vaginal microecological balance. This cross-sectional study investigates the epidemiology of vaginal infections and alterations in microbiota composition, enzymes, and metabolites among premenopausal and postmenopausal gynecologic outpatients.
Methods: The study analyzed the vaginal microecology data from 27,346 women who underwent examinations at Fujian Maternity and Child Health Hospital between 2018 and 2023. Parameters including vaginal cleanliness, bacterial density, and diversity were systematically evaluated. Additionally, a total of 20 participants (10 premenopausal and 10 postmenopausal women) were enrolled for nontargeted LC-MS metabolomic analysis through stratified random sampling.
Results: The population comprised 22,525 (82.4%) premenopausal women (18–44 years), 3,456 (12.6%) transitioning women (45–55 years), and 1,365 (5.0%) postmenopausal women (>55 years). In mixed infections, BV + VVC co-infections predominated (1264/2766, 45.7%). Postmenopausal women showed significantly higher BV prevalence (22.8% vs. 17.9%, P < 0.001) and AV (24.8% vs. 4.6%, P < 0.001), but lower rates of VVC (1.2% vs. 8.2%, P < 0.001). In postmenopausal women, BV-associated biomarkers (including clue cells and sialidase activity) and inflammatory markers (such as pus cells and leukocyte esterase activity) were concurrently elevated. Metabolomic analysis identified elevated chenodeoxycholic acid glycine conjugate levels alongside reduced O-phosphothreonine, morpholine, and diethanolamine.
Conclusion: Age significantly influences vaginal microecology, altering infection epidemiology, microbiota, enzymes, and metabolites. Accounting for these age-related estrogen changes in clinical interventions is critical for effective management.
1 Introduction
Vaginal microecology consists of the vaginal microbiota, immune environment, anatomy, and cervicovaginal fluid enriched with metabolites, enzymes, and cytokines (Brotman et al., 2018; Ye and Qi, 2024). The vaginal microbiota plays a pivotal role in preventing pathogen colonization and maintaining gynecologic and reproductive health (Witkin and Linhares, 2017). Vaginal microecology is dynamic and influenced by various endogenous and exogenous factors, including age, diet, behavior, health, ethnicity, sexual habits, and gynecological or urinary health (Kaur et al., 2020). Fluctuations in estrogen levels during different reproductive stages (puberty, menstruation, pregnancy, and menopause) significantly alter the vaginal microecology (Kaur et al., 2020). Despite the high prevalence of vaginal microbiota disorders in postmenopausal women, this health concern remains inadequately addressed.
In postmenopausal women, diminished estrogen levels induce vaginal epithelial atrophy accompanied by a pronounced reduction or complete depletion of glycogen reserves. This hormonal decline directly compromises Lactobacillus dominance, elevates vaginal pH, and promotes enterobacterial colonization (Petricevic et al., 2013). A comparative cohort study of 70 pre- and postmenopausal women (Yoshikata et al., 2022) revealed significantly reduced Lactobacillus abundance, heightened microbial diversity, and increased vaginal pH in postmenopausal women. However, these findings should be interpreted cautiously due to the small sample size and lack of assessments for postmenopausal vaginal infections.
Vaginal infections encompass bacterial vaginosis (BV), aerobic vaginitis (AV), vulvovaginal candidiasis (VVC), trichomonas vaginitis (TV), and cytolytic vaginosis (CV). These conditions collectively account for up to 50% of gynecologic consultations and impose substantial economic burdens on healthcare systems (Mitra et al., 2016). BV is characterized by vaginal microbiota dysbiosis, featuring elevated levels of Gardnerella vaginalis (GV), Atopobium, Prevotella, Sneathia, Peptostreptococcus, Megasphaera, and BV-associated bacteria (BVAB1–BVAB3), alongside reduced Lactobacilli spp (Muzny et al., 2019). Diagnosis relies on the Nugent score (microscopic bacterial morphotypes) or the Amsel criteria (requiring ≥3 of the following: thin discharge, elevated pH, amine odor, or clue cells in wet mount microscopy) (Cauci et al., 2002). CV results from Lactobacilli overgrowth, inducing vaginal squamous epithelial cell lysis and symptomatic manifestations (Xu et al., 2019). VVC, caused by Candida species, is characterized by white, viscous discharge resembling tofu residue and intense vulvar itching (Gonçalves et al., 2016). TV, caused by Trichomonas vaginalis, is confirmed through nucleic acid amplification tests (NAATs). This pathogen colonizes both genital and urinary tracts, provoking urethritis or cystitis (Schwebke et al., 2024). AV arises from diminished Lactobacilli and proliferation of aerobic bacteria. The heightened microbial diversity and pathogenic complexity associated with AV frequently lead to mixed infections (Ma et al., 2022).
Hormonal or environmental shifts in cervicovaginal microbial communities alter the metabolome. For instance, non-Lactobacillus dominant communities, particularly in high-grade dysplasia, perturb amino acid and nucleotide metabolisms (Nelson et al., 2015; Ilhan et al., 2019). Investigating microbe-metabolite crosstalk in the reproductive tract is crucial for understanding inflammation, adverse pregnancy outcomes, tumorigenesis, and for identifying diagnostic and therapeutic targets (Li et al., 2020). Nevertheless, the relevance of cervicovaginal metabolites, particularly those associated with the age or menopause, remains insufficiently elucidated.
Existing epidemiological evidence demonstrates that hormonal status—a key determinant of women’s health trajectories throughout life stages—profoundly influences vaginal ecosystem dynamics (Brotman et al., 2018). Therefore, we constructed a cohort of 27,346 individuals in the for clinical vaginal microecology analysis, supplemented by untargeted metabolomic analysis of 20 vaginal discharge specimens. We aimed to investigate the epidemiology of age-stratified vaginal infections in pre- and postmenopausal women through a hospital-based cross-sectional study, and characterize microbiota composition, enzymes, and metabolites alterations.
2 Materials and methods
2.1 Study population
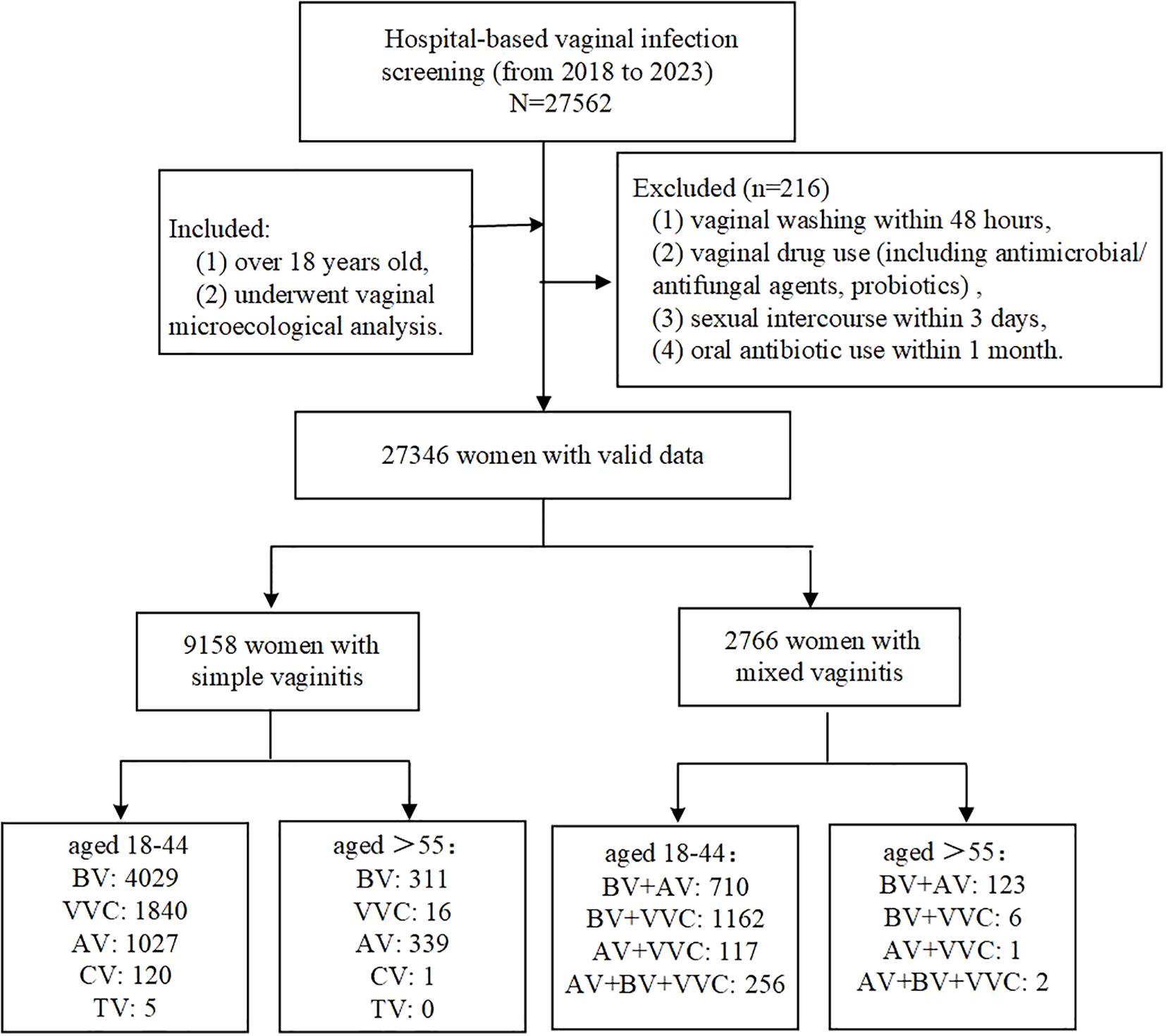
This study enrolled 27,346 participants from Fujian Maternity and Child Health Hospital, affiliated with Fujian Medical University, between April 2018 and July 2023 (Figure 1). Participants met the inclusion criteria if they were (1) over 18 years old, (2) underwent vaginal microecological analysis, with the cohort comprising symptomatic women (e.g., vaginitis), asymptomatic individuals undergoing routine gynecological screening, and pre-procedural evaluation for assisted reproductive technology (ART) or gynecological surgeries. Exclusion criteria included: (1) vaginal washing within 48 hours, (2) vaginal drug use (including antimicrobial/antifungal agents, probiotics), (3) sexual intercourse within 3 days, and (4) oral antibiotic use within 1 month. Participants were stratified into premenopausal (18–44 years), menopausal transition (45–55 years), and postmenopausal (>55 years) groups based on age-defined reproductive status (Wang et al., 2021; Menopause Study Group et al., 2023). Based on the above-described premenopausal and postmenopausal populations, a total of 20 participants (10 premenopausal and 10 postmenopausal women) were enrolled via stratified random sampling for non-targeted LC-MS metabolomic analysis. This study was approved by the Ethics Committee of Fujian Maternity and Child Health Hospital (2020KY148). All experiments followed relevant regulations and were conducted under Ethics Committee supervision.
2.2 Sample collection
Sterile cotton swabs collected vaginal discharge from the upper third of the vaginal wall with 10–15 seconds of rotation. Samples were treated using the pre-treatment solution of the Unit-700 vaginal microecological analyzer (Unit-700, Shtars, China). The swab was immersed in the pre-treatment solution, and the tube walls were gently squeezed to ensure thorough dissolution of secretions into the liquid. Automated detection was then performed using the Unit-700 vaginal microecological analyzer coupled with the Comet 800 microscopic examination and analysis system.
2.3 Vaginal microecology analysis
2.3.1 Microscopic examination of brine wet film
1. Slice preparation: The collected vaginal secretion sample was applied directly and evenly onto a slide that was dripping with normal saline.
2. Microscopic examination: the presence or absence of Trichomonas and Candida hyphae were observed under low magnification, while the number of leukocytes, epithelial cells, bacilli and cocci was observed under high magnification to identify the presence of Candida bacteria and spores.
2.3.2 Evaluation criteria
Vaginal PH value was determined by color strips. Vaginal pH: normal, pH ≤ 4.5; abnormal, pH > 4.5. Vaginal cleanliness was classified according to the National Clinical Laboratory Practice Guidelines (18th edition): grades I–II were considered normal, while grades III–IV were classified as abnormal. The bacterial diversity was graded as follows (Cooperative Group of Infectious Disease Chinese Chinese Society of Obstetrics and Gynecology Chinese Medical Association, 2016): level I (+) was defined as 1–3 bacterial species identified per oil immersion microscope field of view; level II (++), 4–6 species; level III (+++), 7–9 species; and level IV (++++), ≥10 species. Normal microbiota status was defined as diversity and density scores between ++ and +++, with deviations from this range indicating dysbiosis. Sialidase colorless is normal (-), red or purple is positive (+). LE colorless is normal (-), and green or blue is positive (+). H2O2>2 mmol/L is red or purple, negative (-), H2O2<2 mmol/L is positive (+), blue.
Diagnostic evaluations were performed based on the Vaginal Microecology Evaluation System (version 2016) (Cooperative Group of Infectious Disease Chinese Chinese Society of Obstetrics and Gynecology Chinese Medical Association, 2016), as follows: (1) Normal vaginal microecology: density and diversity grades were II–III, the dominant bacteria were Lactobacillus, no pathogenic microorganisms were detected, vaginal pH value was 3.8–4.5, H2O2 was positive, leukocyte esterase and sialidase were negative; (2) BV: Nugent score ≥7 points; (3) VVC: fungal spores or pseudohyphae could be found microscopically under oil; (4) AV: according to the clinical manifestations and microscopic Donders score ≥3 points; (5) Trichomoniasis: a large number of white blood cells and active Trichomoniasis under the microscope; (6) Mixed vaginitis: two or more types of vaginitis existing at the same time. All laboratory procedures were conducted according to the manufacturer’s instructions.
2.4 Metabolite extraction
The samples were thawed slowly at 4°C, and metabolites were extracted using 300 μL of methanol: acetonitrile (2:1, v:v) at –20°C for 2 h. The samples were then centrifuged at 4000 g for 20 min at 4°C. After centrifugation, 300 μL of supernatant was dried with a vacuum concentration meter and then redissolved in 150 μL methanol:H2O (1:1, v:v). Then, the samples were centrifuged at 4000 g for 30 min at 4°C. Ten microliters of supernatant was mixed as a quality control (QC) sample.
2.5 Data processing and metabolite identification
Vaginal secretion samples were eluted from swabs using methanol and subjected to nontargeted liquid chromatography-mass spectrometry (LC-MS)-based metabolomics conducted by BI TREE (Shanghai, China). Briefly, LC-MS was performed using ultra-performance liquid chromatography (Waters 2D UPLC; Waters, Milford, USA) and high-resolution mass spectrometry (Thermo Fisher Scientific, Waltham, USA), and sSamples were analyzed in positive and negative ion modes. Features in the LC–MS metabolomics raw data were aligned, and peak areas were determined using XCMSonline (https://xcmsonline.scripps.edu/). Statistical analysis was performed by an online tool called MetaboAnalyst 4.0 (http://www.metaboanalyst.ca). Hierarchical clustering was used to detect the classification ability and concentration levels of metabolites. Log transmission and autoscaling were used on metabolomics data to make the data obey a normal distribution for drawing a heatmap. The Kruskal–Wallis test of the original data was used for pairwise comparisons. Chemometrics analysis of principal component analysis (PCA) was applied to reveal the global metabolic difference of different groups and evaluate the stability and credibility.
2.6 Statistical analysis
The data were calculated using the IBM SPSS statistical package version 22.0 (IBM, Corporation, Armonk, USA). Categorical variables were expressed as numbers (N) and percentages (%), and continuous variables were expressed as medians. Comparisons between premenopausal and postmenopausal women were performed using the chi-squared test and Fisher’s exact test for proportions. The significance level was set at a two-tailed p-value < 0.05.
3 Results
3.1 Characteristics of the study population
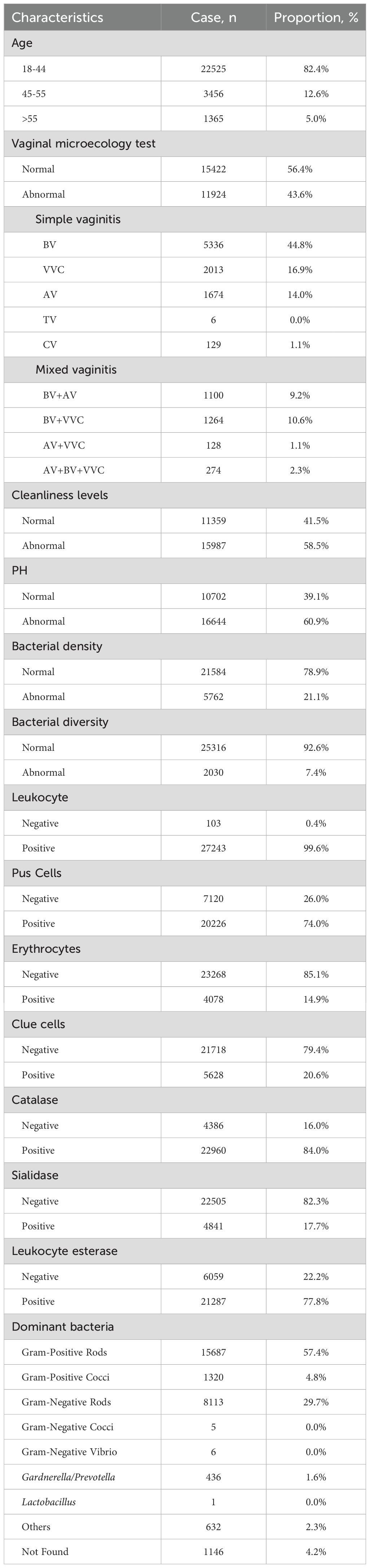
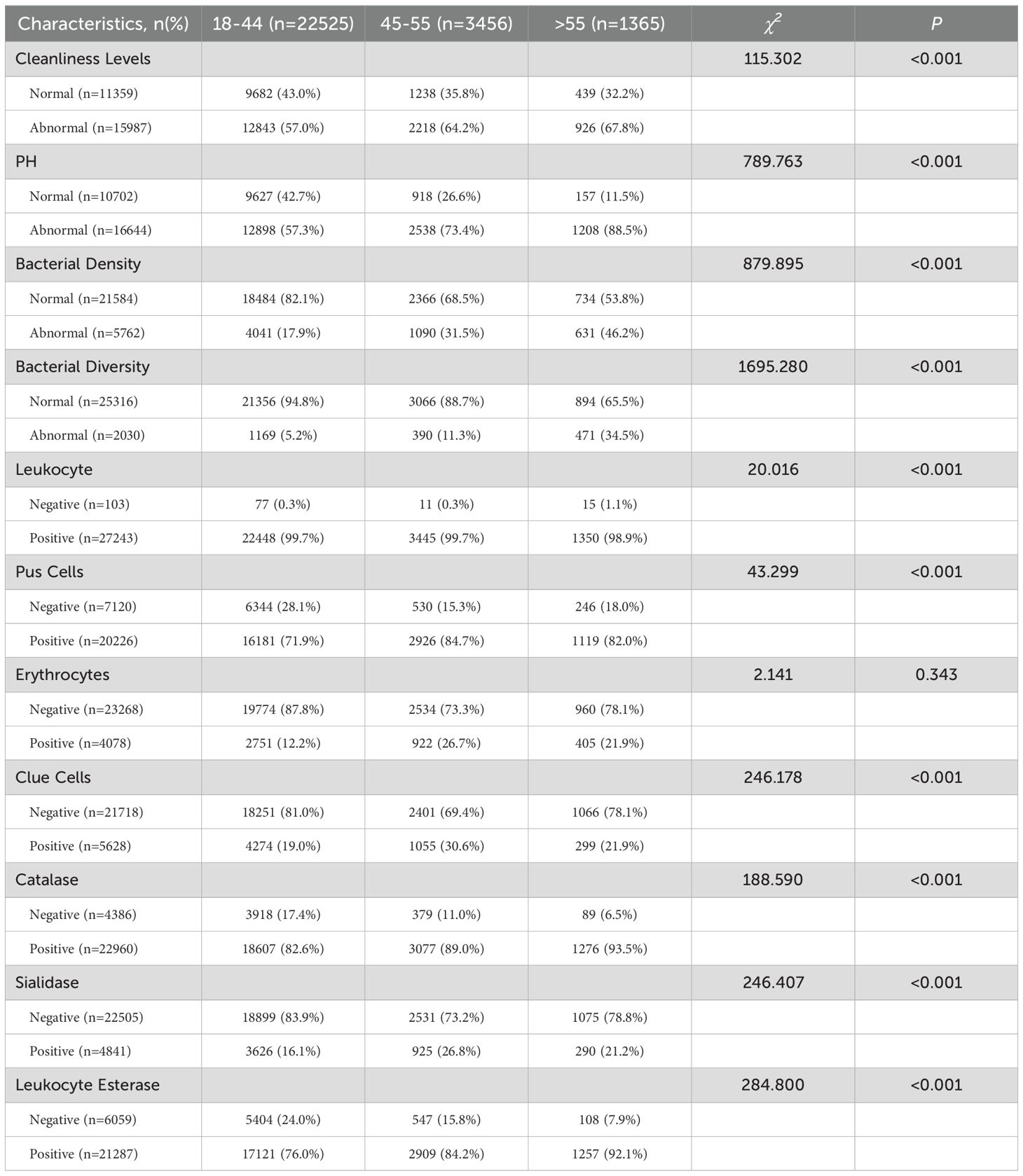
Demographic and clinical characteristics of the cohort (N=27,346) are summarized in Table 1. The population comprised 22,525 (82.4%) premenopausal women (18–44 years), 3,456 (12.6%) transitioning women (45–55 years), and 1,365 (5.0%) postmenopausal women (>55 years). Vaginal microbiological profiles indicated that 15,422 women (56.4%) had normal vaginal microbiology, while 9,158 (33.5%) presented with simple infections and 2,766 (10.1%) had mixed infections.
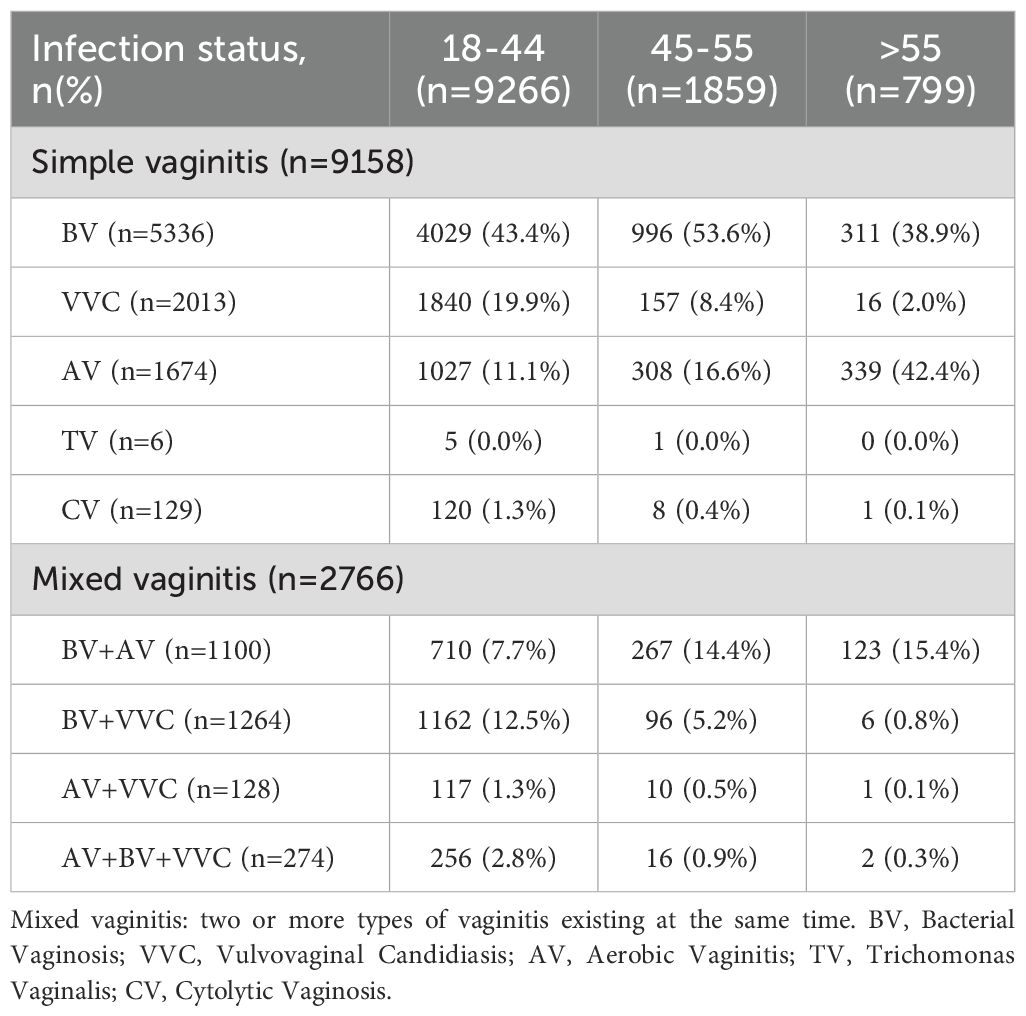
Among single infections (Table 2), BV (5336/9158, 58.3%) was most prevalent, followed by VVC (2013/9158, 22.0%), AV (1674/9158, 18.3%), CV (129/9158, 1.4%), and TV (6/9158, 0.0%). In mixed infections, BV + VVC co-infections predominated (1264/2766, 45.7%). Age-stratified analysis demonstrated BV prevalence peaked in the 18 - 44 (43.4%) and 45 - 55 (53.6%) cohorts, whereas AV incidence was highest in the >55-year-old group (42.4%).
3.2 Vaginal infections in pre- and postmenopausal women
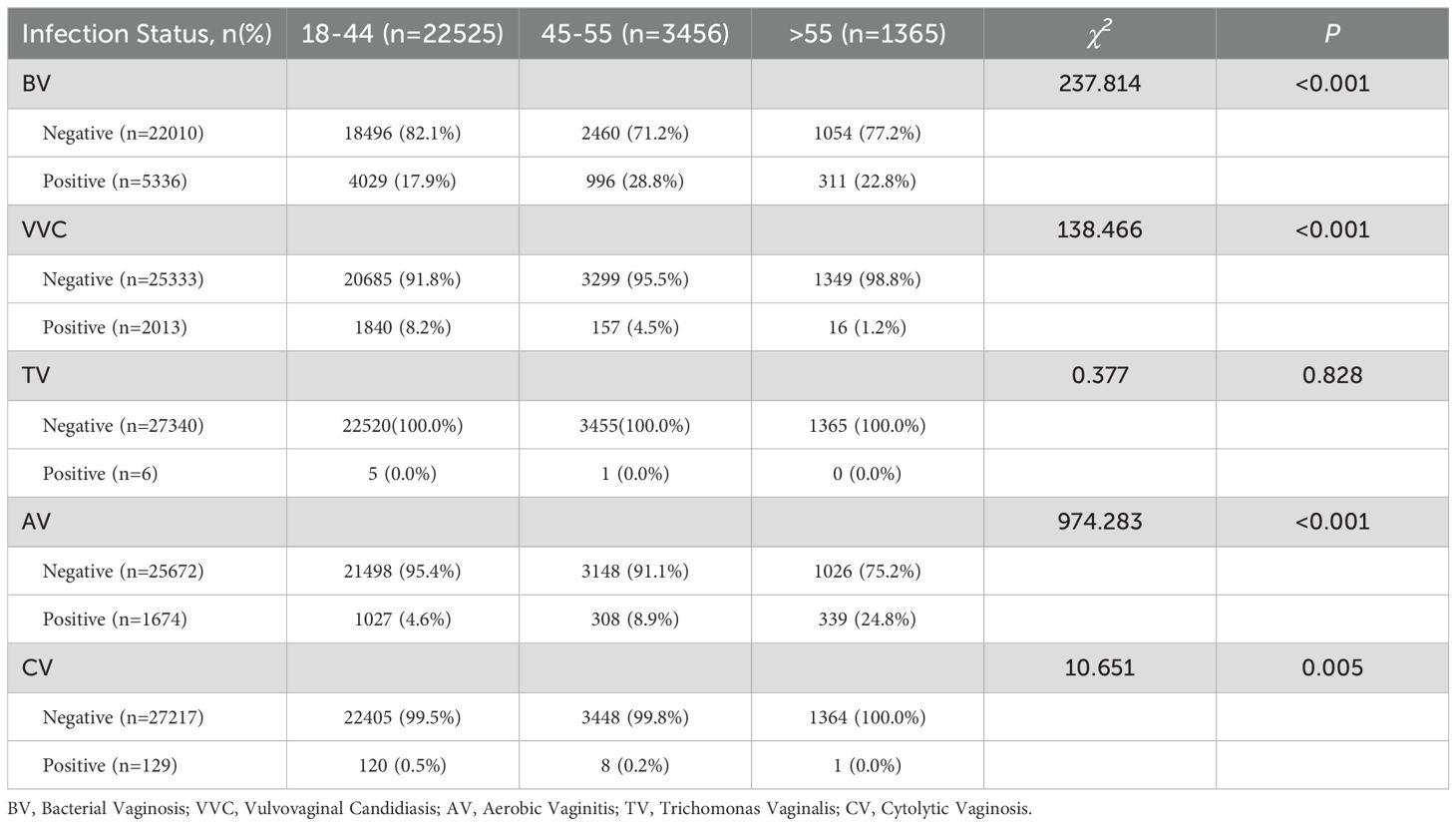
To assess age-related differences in vaginitis infections, chi-square tests were performed for five infection types across groups (Table 3). Postmenopausal women showed significantly higher BV prevalence (22.8% vs. 17.9%, P < 0.001) and AV (24.8% vs. 4.6%, P < 0.001), but lower rates of VVC (1.2% vs. 8.2%, P < 0.001). Although CV exhibited intergroup differences (P = 0.005), interpretation requires caution due to limited sample sizes. No significant association was observed for TV (P=0.828).
3.3 Vaginal microenvironment in pre- and postmenopausal women
Further comparative analysis of vaginal microenvironment parameters revealed that menopausal status (age > 55 years) significantly impacted these parameters (Table 4). Postmenopausal women demonstrated significantly elevated abnormality rates in bacterial density (χ² = 879.895, P<0.001) and diversity (χ² = 1695.280, P<0.001). BV-associated biomarkers, including clue cells (χ² = 246.178, P < 0.001) and sialidase activity (χ² = 246.407, P< 0.001), were markedly increased. Concurrently, inflammatory markers such as pus cells (χ² = 43.299, P < 0.001) and leukocyte esterase activity (χ² = 284.800, P < 0.001) showed marked elevation.
3.4 Metabolite differences in pre- and postmenopausal women
A comprehensive metabolomic landscape was obtained using LC-MS-based analysis of vaginal discharge samples. The PCA model visualization results (Figure 2A) revealed distinct compositional differences in vaginal metabolites between pre- and postmenopausal women. Screening thresholds for differential metabolites were defined as fold change (FC) > 2 or < 0.5 with P < 0.05. Metabolite profiling identified 219 downregulated metabolites and 1 upregulated metabolite in the postmenopausal vaginal microenvironment (Figure 2B). Figure 2C shows the first 10 differential metabolites. Levels of nine metabolites, such as O-Phosphothreonine, Morpholine, and Diethanolamine, were decreased in postmenopausal women. Conversely, chenodeoxycholic acid glycine conjugate levels were moderately increased in postmenopausal women. The KEGG analysis showed that significantly differential pathways included Central carbon metabolism in cancer, Nucleotide metabolism, ABC transporters, Alcoholism, etc (Figure 2D). These findings collectively illustrate dynamic metabolic shifts associated with the menopause.
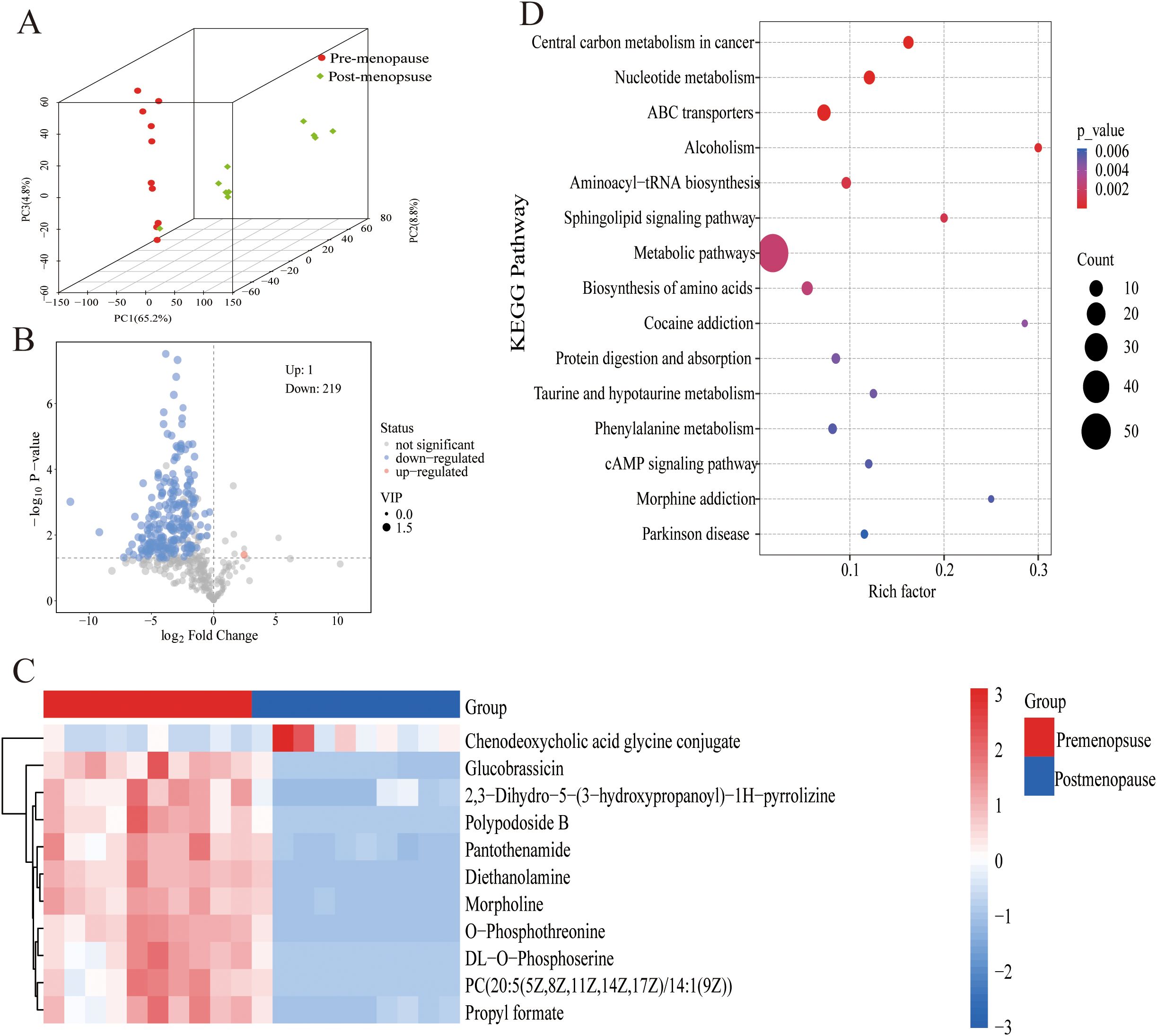
Figure 2. Metabolite differences in pre- and postmenopausal women. (A) Significantly regulated metabolites between pre-menopause and post-menopause groups. (B) The DEGs between pre-menopause and post-menopause groups. (C) Significantly regulated metabolites between pre-menopause and post-menopause groups were shown with heat maps. (D) Functional enrichment analysis of DEGs.
4 Discussion
Age-related decline in estrogen affects the vaginal microecology. Our findings reveal a postmenopausal shift characterized by elevated incidences of BV and AV, contrasted with reduced prevalence of VVC. Metabolomic profiling further indicates a generalized downregulation of vaginal metabolic activity postmenopause, with henodeoxycholic acid glycine conjugate emerging as a prominently upregulated metabolite.
Our research revealed an increased incidence of BV among postmenopausal women, a phenomenon warranting further investigation. BV is characterized by the depletion or significant reduction of Lactobacillus, accompanied by a marked proliferation of anaerobic microbes (Zhu et al., 2024). Gardnerella vaginalis, an anaerobic pathogen, has been identified as the primary etiological agent of BV. Gardnerella possesses several virulence factors that can contribute to its pathogenic phenotype, including a hemolysin, mucus-degrading sialidases, resistance factors, and biofilm formation (Qin and Xiao, 2022). Gardnerella-derived biofilms have been strongly implicated in BV recurrence (Li et al., 2024). Gardnerella sialidases cleave terminal sialic acid residues off of human glycans (Chen et al., 2024). By desialylation, sialidases not only alter the function of sialic acid-containing glycoconjugates but also play a vital role in the attachment, colonization and spread of many other vaginal pathogens.
Liu Zhaohui’s study employed metagenomic detection to investigate the vaginal microbiota composition in postmenopausal and postpartum women. The study revealed that the dominance of Lactobacillus decreased, whereas microbial diversity increased significantly in postmenopausal and postpartum women (Li et al., 2024). In the vaginal microbiota of premenopausal women, Lactobacillus species constituted approximately 71.98%, and pathogenic flora constituted approximately 16.87%. In contrast, postmenopausal women exhibited reduced proportions of Lactobacillus (10.08%) and elevated pathogenic flora (26.78%) (Yoshikata et al., 2022). Postmenopausal hormonal depletion diminishes vaginal epithelial glycogen levels, thereby limiting metabolic substrates essential for Lactobacillus proliferation. Furthermore, Lactobacillus suppresses pathogen colonization through competitive exclusion, inhibition of epithelial adhesion, and secretion of antimicrobial compounds (e.g., bacteriocins, lactic acid) and immunomodulatory agents. However, further metagenomics techniques are needed to identify changes in the proportion of different Lactobacillus species in the postmenopausal vaginal microenvironment.
VVC susceptibility exhibits an age-dependent decline, consistent with our observations. This phenomenon is mechanistically linked to elevated vaginal pH and diminished glycogen levels in postmenopausal women, which collectively restrict Candida proliferation (Zhao et al., 2022).
For menopausal-associated BV and VVC, therapeutic interventions frequently require combination therapy (Dothard et al., 2023; Li et al., 2024). Estrogen therapy has demonstrated clinical efficacy in addressing menopause-associated VVC and BV by restoring vaginal epithelial integrity and glycogen reserves (Sookkhee et al., 2024). Gliniewicz et al. conducted a comparative analysis of vaginal microbiota profiles, contrasting premenopausal women with postmenopausal individuals receiving hormone replacement therapy (HRT). By measuring 16s rRNA gene copies, the researchers determined that postmenopausal women receiving HRT were most often dominated by species of Lactobacillus, and had similar bacterial counts to premenopausal women (Gliniewicz et al., 2019). Furthermore, emerging as a complementary therapeutic modality, probiotic supplementation shows considerable potential for correcting vaginal dysbiosis. Clinical trials report that probiotic-treated patients exhibit microbiota shifts favoring Lactobacillus colonization (Burton et al., 2003).
The metabolites produced within the female reproductive tract play a pivotal role in the pathogenesis of female genital tract inflammation, and may serve as biological markers for disease severity, diagnosis, and prognosis. Metabolites are primarily amino acids, carbohydrates, and lipids, and are closely associated with physiological processes. We conducted a metabolomic sequencing of vaginal secretions from ten premenopausal and ten postmenopausal women, and observed differential expression levels of ten metabolites. The carbohydrates include polypodoside B, while the amino acids encompass glucobrassicin, pantothenamide and O-phosphothreonine. The lipids comprise chenodeoxycholic acid. Additionally, the presence of acid glycine conjugate and PC(20:5(5Z,8Z,11Z,14Z,17Z)/14:1(9Z)) propyl formate was identified. These metabolites exhibited differential expression in the aforementioned metabolic differences. In the aforementioned metabolic profile, only the lipid chenodeoxycholic acid glycine conjugate demonstrated an increase in concentration in the metabolic profile of the premenopausal female subjects.
The vaginal microenvironment shares many similarities with the intestinal microenvironment in that the flora interacts with the vaginal/intestinal microenvironment, the vagina/intestinal tract provides the microorganisms with the nutrients needed for their own reproduction, while at the same time the microorganisms produce a large number of metabolites as a result of their interactions with the host. There has been a great deal of research on Chenodeoxycholic acid in the gut microecology (Cai et al., 2022), Chenodeoxycholic acid (CDCA), a primary bile acid, significantly regulates local immunity via several mechanisms. First, CDCA activates the farnesoid X receptor (FXR), which suppresses pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β, thereby attenuating inflammation. Second, CDCA facilitates the development of RORγ+ regulatory T cells (Tregs), which are crucial for maintaining immune balance and suppressing inflammation. Third, CDCA modulates glucagon-like peptide-1 (GLP-1) expression and secretion. GLP-1 not only governs glucose metabolism but also has immunomodulatory functions (Rau et al., 2016; Fiorucci et al., 2018).
The advantage of this study lies in the large sample size and the utilization of metabolomics research methods. Several study limitations should be noted. First, our hospital-based study may not fully represent community populations, future multicenter studies incorporating community-based sampling strategies are warranted to validate the generalizability of these findings. However, this hospital-based study design ensured standardization of diagnostic procedures. Secondly, the retrospective data resulted in incomplete documentation of key covariates, including Symptoms and signs of vaginitis, oral contraceptive use, HRT, and hygiene practices (Daher et al., 2022). Future studies should incorporate prospective longitudinal tracking of these variables—including detailed contraceptive/HRT exposure histories, standardized symptom assessments, and hygiene behavior monitoring—with stratified analyses to elucidate their mechanistic roles.
Vaginitis in premenopausal and postmenopausal women differs significantly, as do their metabolic mechanisms. Consequently, clinical management of postmenopausal vaginal infections demands specialized approaches. Beyond pathogen-directed antimicrobial therapy, restoration of vaginal microecological balance through adjunctive interventions such as topical estrogen or probiotic supplementation should be prioritized to address the hypoestrogenic microenvironment characteristic of postmenopausal women.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The study was approved by the Ethics Committee of Fujian Maternity and Child Health Hospital (2020KY148). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because the study was retrospective in nature and involved the use of de-identified patient data. All patient information was anonymized to ensure privacy and confidentiality, and the study was conducted in accordance with institutional and ethical guidelines.
Author contributions
WYL: Investigation, Formal Analysis, Writing – original draft, Writing – review & editing. LYW: Formal Analysis, Writing – original draft, Writing – review & editing. BHD: Investigation, Writing – review & editing. YHZ: Data curation, Methodology, Writing – review & editing. YZ: Supervision, Data curation, Writing – review & editing. LW: Investigation, Methodology, Writing – review & editing. JS: Methodology, Writing – review & editing. YFL: Methodology, Writing – review & editing. MJZ: Project administration, Supervision, Data curation, Writing – review & editing. PMS: Funding acquisition, Data curation, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (grant no. 82271658), Fujian Health Young and Middle-aged Scientific Research Major Project (grant no. 2021ZQNZD011), Fujian Provincial Natural Science Foundation of China (grant no.2023J011218), the Startup Fund for scientific research, Fujian Medical University (grant no.2023QH2047).
Acknowledgments
We would like to thank the participants for their patience and kindness.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AV, Aerobic vaginitis; BV, Bacterial vaginosis; CST, Community state type; CV, Cytolytic vaginosis; GV, Gardnerella vaginalis; HRT, Hormone replacement therapy; LE, Leukocyte esterase; TV, Trichomonas vaginitis; VVC, Vulvovaginal candidiasis.
References
Brotman, R. M., Shardell, M. D., Gajer, P., Fadrosh, D., Chang, K., Silver, M. I., et al. (2018). Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 25, 1321–1330. doi: 10.1097/GME.0000000000001236
Burton, J. P., Cadieux, P. A., and Reid, G. (2003). Improved understanding of the bacterial vaginal microbiota of women before and after probiotic instillation. Appl. Environ. Microbiol. 69, 97–101. doi: 10.1128/AEM.69.1.97-101.2003
Cai, J., Sun, L., and Gonzalez, F. J. (2022). Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 30, 289–300. doi: 10.1016/j.chom.2022.02.004
Cauci, S., Driussi, S., De Santo, D., Penacchioni, P., Iannicelli, T., Lanzafame, P., et al. (2002). Prevalence of bacterial vaginosis and vaginal flora changes in peri- and postmenopausal women. J. Clin. Microbiol. 40, 2147–2152. doi: 10.1128/JCM.40.6.2147-2152.2002
Chen, L., Li, J., and Xiao, B. (2024). The role of sialidases in the pathogenesis of bacterial vaginosis and their use as a promising pharmacological target in bacterial vaginosis. Front. Cell Infect. Microbiol. 14. doi: 10.3389/fcimb.2024.1367233
Cooperative Group of Infectious Disease Chinese Chinese Society of Obstetrics and Gynecology Chinese Medical Association (2016). Expert consensus on the clinical application of vaginal microecology test. Zhonghua Fu Chan Ke Za Zhi 51, 721–723. doi: 10.3760/cma.j.issn.0529-567X.2016.10.001
Daher, A., Albaini, O., Siff, L., Farah, S., and Jallad, K. (2022). Intimate hygiene practices and reproductive tract infections: A systematic review. Gynecol Obstet Clin. Med. 2, 129–135. doi: 10.1016/j.gocm.2022.06.001
Dothard, M. I., Allard, S. M., and Gilbert, J. A. (2023). The effects of hormone replacement therapy on the microbiomes of postmenopausal women. Climacteric. 26, 182–192. doi: 10.1080/13697137.2023.2173568
Fiorucci, S., Biagioli, M., Zampella, A., and Distrutti, E. (2018). Bile acids activated receptors regulate innate immunity. Front. Immunol. 9. doi: 10.3389/fimmu.2018.01853
Gliniewicz, K., Schneider, G. M., Ridenhour, B. J., Williams, C. J., Song, Y., Farage, M. A., et al. (2019). Comparison of the vaginal microbiomes of premenopausal and postmenopausal women. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.00193
Gonçalves, B., Ferreira, C., Alves, C. T., Henriques, M., Azeredo, J., and Silva, S. (2016). Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 42, 905–927. doi: 10.3109/1040841X.2015.1091805
Ilhan, Z. E., Łaniewski, P., Thomas, N., Roe, D. J., Chase, D. M., and Herbst-Kralovetz, M. M. (2019). Deciphering the complex interplay between microbiota, HPV, inflammation and cancer through cervicovaginal metabolic profiling. EBioMedicine. 44, 675–690. doi: 10.1016/j.ebiom.2019.04.028
Kaur, H., Merchant, M., Haque, M. M., and Mande, S. S. (2020). Crosstalk between female gonadal hormones and vaginal microbiota across various phases of women’s gynecological lifecycle. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.00551
Li, H., Zang, Y., Wang, C., Li, H., Fan, A., Han, C., et al. (2020). The interaction between microorganisms, metabolites, and immune system in the female genital tract microenvironment. Front. Cell Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.609488
Li, M., Zeng, Z., Wang, X., Liu, Y., Wei, H., Liu, J., et al. (2024). Mechanisms of S. agalactiae promoting G. vaginalis biofilm formation leading to recurrence of BV. NPJ Biofilms Microbiomes. 10, 138. doi: 10.1038/s41522-024-00601-w
Li, X., Zhang, Z., Bai, H., and Liu, Z. (2024). Analysis of vaginal microbiota during postpartum and postmenopausal periods based on metagenomics. BMC Microbiol. 24, 501. doi: 10.1186/s12866-024-03648-z
Ma, X., Wu, M., Wang, C., Li, H., Fan, A., Wang, Y., et al. (2022). The pathogenesis of prevalent aerobic bacteria in aerobic vaginitis and adverse pregnancy outcomes: a narrative review. Reprod. Health 19, 21. doi: 10.1186/s12978-021-01292-8
Menopause Study Group, Chinese Society of Obstetrics and Gynecology, and Chinese Medical Association (2023). Chinese guidelines for menopause management and menopausal hormone therapy. Zhonghua Fu Chan Ke Za Zhi 58, 4–21. doi: 10.3760/cma.j.cn112141-20221118-00706
Mitra, A., MacIntyre, D. A., Marchesi, J. R., Lee, Y. S., Bennett, P. R., and Kyrgiou, M. (2016). The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome. 4, 58. doi: 10.1186/s40168-016-0203-0
Muzny, C. A., Taylor, C. M., Swords, W. E., Tamhane, A., Chattopadhyay, D., Cerca, N., et al. (2019). An updated conceptual model on the pathogenesis of bacterial vaginosis. J. Infect. Dis. 220, 1399–1405. doi: 10.1093/infdis/jiz342
Nelson, T. M., Borgogna, J. L., Brotman, R. M., Ravel, J., Walk, S. T., and Yeoman, C. J. (2015). Vaginal biogenic amines: biomarkers of bacterial vaginosis or precursors to vaginal dysbiosis? Front. Physiol. 6. doi: 10.3389/fphys.2015.00253
Petricevic, L., Domig, K. J., Nierscher, F. J., Sandhofer, M. J., Krondorfer, I., Kneifel, W., et al. (2013). Differences in the vaginal lactobacilli of postmenopausal women and influence of rectal lactobacilli. Climacteric. 16, 356–361. doi: 10.3109/13697137.2012.725788
Qin, H. and Xiao, B. (2022). Research progress on the correlation between gardnerella typing and bacterial vaginosis. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.858155
Rau, M., Stieger, B., Monte, M. J., Schmitt, J., Jahn, D., Frey-Wagner, I., et al. (2016). Alterations in enterohepatic fgf15 signaling and changes in bile acid composition depend on localization of murine intestinal inflammation. Inflammation Bowel Dis. 22, 2382–2389. doi: 10.1097/MIB.0000000000000879
Schwebke, J. R., Nyirjesy, P., Dsouza, M., and Getman, D. (2024). Vaginitis and risk of sexually transmitted infections: results of a multi-center U.S. clinical study using STI nucleic acid amplification testing. J. Clin. Microbiol. 62, e0081624. doi: 10.1128/jcm.00816-24
Sookkhee, S., Khamnoi, P., Sastraruji, T., Boonkum, S., Wikan, N., and Nimlamool, W. (2024). Synergistic Inhibition of Synbiotic Cultures among Lactobacilli and Plant Extracts against Vaginal Discharge Causing Candida albicans. Nutrients. 16, 1372. doi: 10.3390/nu16091372
Wang, M., Kartsonaki, C., Guo, Y., Lv, J., Gan, W., Chen, Z., et al. (2021). Factors related to age at natural menopause in China: results from the China Kadoorie Biobank. Menopause. 28, 1130–1142. doi: 10.1097/GME.0000000000001829
Witkin, S. S. and Linhares, I. M. (2017). Why do lactobacilli dominate the human vaginal microbiota? BJOG. 124, 606–611. doi: 10.1111/1471-0528.14390
Xu, H., Zhang, X., Yao, W., Sun, Y., and Zhang, Y. (2019). Characterization of the vaginal microbiome during cytolytic vaginosis using high-throughput sequencing. J. Clin. Lab. Anal. 33, e22653. doi: 10.1002/jcla.22653
Ye, J. and Qi, X. (2024). Vaginal microecology and its role in human papillomavirus infection and human papillomavirus associated cervical lesions. APMIS. 132, 928–947. doi: 10.1111/apm.13356
Yoshikata, R., Yamaguchi, M., Mase, Y., Tatsuzuki, A., Myint, K. Z. Y., and Ohta, H. (2022). Age-related changes, influencing factors, and crosstalk between vaginal and gut microbiota: A cross-sectional comparative study of pre- and postmenopausal women. J. Womens Health (Larchmt). 31, 1763–1772. doi: 10.1089/jwh.2022.0114
Zhao, T., Xiao, X., Xiao, L., Wu, X., and Yuan, T. (2022). Bacterial vaginosis, vulvovaginal candidiasis, and trichomonal vaginitis in reproductive-age women in Yunnan, China: a descriptive study. J. Obstet Gynaecol. 42, 3187–3192. doi: 10.1080/01443615.2022.2109134
Keywords: vaginal microecology, vaginal infection, menopause, microbiota, metabolites
Citation: Lin W, Wang L, Dong B, Zhang Y, Zhang Y, Wang L, Shen J, Lu Y, Zheng M and Sun P (2025) Age-related vaginal microecology and infection epidemiology among premenopausal and postmenopausal gynecologic outpatients: a cross-sectional study. Front. Cell. Infect. Microbiol. 15:1569667. doi: 10.3389/fcimb.2025.1569667
Received: 01 February 2025; Accepted: 15 July 2025;
Published: 04 August 2025.
Edited by:
Claudio Foschi, University of Bologna, ItalyReviewed by:
Leslie Thian Lung Than, Putra Malaysia University, MalaysiaMingzhu Li, Peking University People’s Hospital, China
Copyright © 2025 Lin, Wang, Dong, Zhang, Zhang, Wang, Shen, Lu, Zheng and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengming Sun, Zm1zdW4xOTc1QGZqbXUuZWR1LmNu; Meijin Zheng, emhlbmdtZWlqaW5AZmpzZnkuY29t
†These authors have contributed equally to this work
‡ORCID: Pengming Sun, orcid.org/0000-0002-5072-6091
 Wenyu Lin
Wenyu Lin Liying Wang
Liying Wang Binhua Dong
Binhua Dong Yuhang Zhang4
Yuhang Zhang4 Liang Wang
Liang Wang Pengming Sun
Pengming Sun