- 1Department of Gastrointestinal and Anal Diseases Surgery, The Affiliated Hospital of Shandong Second Medical University, Weifang, China
- 2Operating Room, The Affiliated Hospital of Shandong Second Medical University, Weifang, China
Emerging evidence underscores the critical role of Toll-like receptor 7 (TLR7)-mediated interferon (IFN) signaling in host defense against viral infections including SARS-CoV-2, through the modulation of both innate and adaptive immunity. However, the specific mechanisms by which TLR7 activation shapes SARS-CoV-2-specific immune responses, particularly via IRF-IFN pathways, remain incompletely elucidated. This review synthesizes current findings on how intrinsic TLR7-driven IFN signaling influences viral clearance, modulates adaptive immunity, and contributes to autoantibody production in COVID-19. A deeper understanding of these processes is essential for developing targeted therapeutic interventions and improved vaccines aimed at mitigating severe COVID-19 and preventing post-acute sequelae of SARS-CoV-2 infection (PASC).
1 Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, has emerged as a formidable global challenge, transcending immediate health crises to encompass multifaceted long-term implications (Zhu et al., 2020). The ongoing battle against SARS-CoV-2 infection has spurred in-depth exploration into the complex mechanisms of virus-host interactions, particularly focusing on host immunity. SARS-CoV-2 infection facilitates a cascade of inflammatory and immune responses by activating interferons (IFNs) and Toll-like receptors (TLRs), which are implicated in shaping the clinical outcomes of COVID-19, spanning asymptomatic carriage to acute respiratory distress syndrome (ARDS), multi-organ failure, and other severe manifestations (Paludan et al., 2021). As pattern recognition receptors (PRRs), TLRs function as a bridge linking innate immunity with adaptive immunity by recognizing conserved pathogen-associated molecular patterns (PAMPs) (Akira et al., 2006; Kawai and Akira, 2008). Among them, TLR2 and TLR4 are well documented TLRs contributing to the host defense against COVID-19 (Ashhurst et al., 2022; Sahanic et al., 2023; Habeichi et al., 2024). As a double-stranded RNA (dsRNA) sensor, TLR3 can also sense dsRNA intermediate during replication of single-stranded RNA (ssRNA) in host cells. Reduced expression of peripheral blood TLR3 is linked to an adverse outcome in severe COVID-19 patients, suggesting an underlying role of TLR3 in regulating IFN response during SARS-CoV-2 infection (Menezes et al., 2021). A number of studies have also demonstrated that other endosomal TLRs play important roles in the onset and progression of COVID-19, including TLR7, TLR8, and TLR9 (Fitzgerald and Kagan, 2020; Manik and Singh, 2022). Tlr7 is a gene located on the X chromosome. TLR7 is a crucial sensor in SARS-CoV-2 infection because it recognizes viral ssRNAs. This recognition triggers innate immune responses, including the production of type I IFNs, which are essential for controlling early viral replication and shaping adaptive immunity. Emerging evidence has supported the critical role of endosomal TLR7 and TLR7-mediated IFN signaling activation in SARS-CoV-2 infection, particularly regarding the modifying effects on the innate immune reaction initiation, cytokine storm, and systemic immune disorders in COVID-19. Understanding the role and mechanism of TLR7-mediated IFNs signaling activation in viral immunity is of utmost importance in exploring more effective strategy for the control of SARS-CoV-2 infection.
Long-COVID affects at least 10% of SARS-CoV-2 infections. Increasing studies have implicated that long-COVID is characteristic of a sustained proinflammatory immune profile, compromised humoral immunity, and diminished cellular immunity (Woodruff et al., 2022; Klein et al., 2023). Despite research progress during the past few years, the etiology and immunopathological mechanism underlying post-acute sequelae of SARS-CoV-2 infection (PASC) remain not fully understood due to the heterogeneous nature of the condition, which presents with a wide spectrum of symptoms and involves multiple organ systems. Furthermore, the underlying mechanisms may involve persistent viral reservoirs, autoimmune responses, and chronic inflammation, all of which are complex, interconnected, and difficult to disentangle in human studies. It has been well documented that the intrinsic TLR7 can induce a prominent type-I IFN response and robust antiviral immune responses by regulating adaptive immunity against SARS-CoV-2 infection (Rossmann et al., 2021; Miquel et al., 2023). Nonetheless, the underlying mechanism of TLR7 in regulating the adaptive immune response and its interaction with IFN signaling in COVID-19 and long-COVID remains not fully understood. The specific role of TLR7 in shaping the adaptive immune response to SARS-CoV-2, particularly its synergy with or antagonism of IFN signaling, remains unclear due to the complexity of the cytokine environment. This is compounded in long-COVID by the challenge of differentiating between ongoing viral persistence and virus-induced autoimmune pathology. Furthermore, most mechanistic insights come from murine models, which do not fully recapitulate the human immune response to the virus. This review is aimed to summarize currently published studies on the potential roles and mechanisms of TLR7 and IFN signaling in COVID-19. Elucidating the possible mechanisms behind innate and adaptive immune responses of TLR7-mediated IFN signaling activation in SARS-CoV-2 infection is promising for the exploration of more effective vaccines and therapeutic strategies particularly for severe COVID-19 and long-COVID.
2 Clinical evidence supporting the critical role of intrinsic TLR7 and TLR7 mutation in COVID-19
SARS-CoV-2 has a positive-sense, single-stranded, linear RNA genome. The ssRNA genome structure laid the foundation for the importance of nucleic acid-TLRs interactions. Many clinical cases have supported the importance of them in COVID patients, particularly TLR7. COVID-19 patients with the functional variants of TLR7 have an impaired type-I IFN response in the peripheral circulation, characterized by significant downregulation of IRF7, IFNβ1, IFNγ and IFN-stimulated gene 15 (ISG15) (Fallerini et al., 2021). The COVID-19 pandemic exhibits a male sex bias, with males being admitted to the intensive care unit (ICU) 2.84 times more frequently, and experiencing a 1.39 times higher mortality rate compared to females (Spiering and de Vries, 2021). Tlr7 gene has a gene-dose effect caused by X chromosome inactivation (XCI) escape. Recent studies have proposed that the gene-dose of Tlr7 may play a vital role in the sex-skewed severity observed in COVID-19 (Gómez-Carballa et al., 2022). Young men with severe COVID-19 exhibit the Tlr7 genetic variants, including the X-linked recessive TLR7 deficiency (van der Made et al., 2020; Asano et al., 2021). Approximately 1% of men under 60 years old with life-threatening COVID-19 exhibit X-linked recessive Tlr7 deficiency (Asano et al., 2021). In male COVID-19 patients, rare variants of Arg920Lys and Asp41Glu in Tlr7 gene have been identified, leading to functional impairment and the downregulation of cytokine-mediated signaling activation (Mantovani et al., 2022). Males with loss-of-function variants in TLR7 have an increased risk of life-threatening COVID-19 pneumonia. This suggests that gender disparities in SARS-CoV-2 outcomes result from the combined effects of multiple factors, including differences in immune responses and sex hormones (Vikse et al., 2020; Asano et al., 2021; Vadakedath et al., 2021; Naushad et al., 2023; Zovi et al., 2023). Besides, in the Korean population, no significant differences are observed in the genotype or allele frequencies of Tlr7 rs864058 polymorphism between female patients with COVID-19 and the healthy controls, supporting that the genetic polymorphisms of Tlr7 rs864058 is of low frequency and not associated with SARS-CoV-2 infection in Korean females (Zayed et al., 2023). Moreover, loss-of-function variants of Tlr7 have also been highlighted in modulating the long-term complications of post-COVID-19, such as severe long-term neurological deterioration, brain fog, and hypoxemic pneumonia (Bagci et al., 2023; Noor Eddin et al., 2023). In conclusion, Tlr7 gene mutation plays a pivotal role both in the acute antiviral response, disease severity, and the long-term immunity against COVID-19 in a sex-skewed manner. A notable phenomenon of XCI escape occurs in immune cells, with the escape of the Tlr7 gene in pDCs being particularly prominent. Escape frequencies are influenced by genetic background, environmental factors, and epigenetic regulation. A deeper examination of tissue-specific escape patterns, particularly in immune cell subsets, and the role of epigenetic modifiers such as XIST regulators or chromatin accessibility would provide a more robust framework. Furthermore, situating TLR7 within a broader X-chromosomal regulatory network, including interactions with other escapee genes like TLR8 or CXCR3 (Du et al., 2025; Sakkas et al., 2025), could reveal synergistic immunopathological pathways and improve the translational relevance of the proposed mechanism. In patients with systemic sclerosis (SSc), the frequency of TLR7 escape in pDCs is significantly increased, accompanied by aberrant overexpression of TLR7 and hyperactivation of IFN-I signaling, suggesting a link to autoimmune pathology (Gabriele et al., 2021; Spiering and de Vries, 2021; Du et al., 2025). X-inactive specific transcript (XIST), a key long non-coding RNA (lncRNA) responsible for initiating XCI, mediates chromosomal silencing by recruiting histone modifiers and DNA methyltransferases (Cerase et al., 2021; Rodermund et al., 2021). Its expression is dynamically regulated by culture medium composition, cellular differentiation status, and chromatin remodeling factors such as Chd8. Instability in XIST expression may lead to increased XCI escape. At the chromatin structural level, the Tlr7 gene dissociates from the suppressive region of the inactive X chromosome (Xi) upon escape and forms a transcriptionally active chromatin loop (Cloutier et al., 2022). Multiple immune-related genes on the X chromosome can cooperatively escape XCI, forming an immunoregulatory network. In addition to TLR7, TLR8 also frequently escapes in pDCs and acts synergistically with TLR7 to enhance the IFN-I response, thereby promoting fibrotic processes in diseases such as SSc (Du et al., 2025; Sakkas et al., 2025). As an adaptor protein in the TLR signaling pathway, escape of TASL further amplifies the downstream signaling of TLR7 (Scofield et al., 2025). Escaping XCI in immune cells, such as pDCs, could lead to biallelic expression of TLR7 and other X-linked immune regulators. This elevated gene dosage would potentially hypersensitize the innate immune system, thereby predisposing it to an exaggerated inflammatory response upon SARS-CoV-2 infection, elevating innate antiviral sensing, amplifying proinflammatory signaling and pushing the immune response toward a pathogenic cytokine storm. While potentially enhancing early virus control, this hyperactive response may predispose individuals to loss of immune balance, which accelerates cytokine-driven pathology and increases the risk of severe immunopathology in COVID-19. To unravel the impacts of gender bias and genetic variants of Tlr7 during SARS-CoV-2 infection not only provides insight into understanding the mechanism of TLR7 in antiviral immunity, but allows for potential therapeutic target for stimulation of TLR7-mediated IFN signaling activation during antiviral protection.
3 Role of TLR7 in COVID-19 by regulating inflammation and autoimmunity
3.1 Role of TLR7 and its interaction with other endosomal TLRs during the antiviral innate immunity
TLR7, predominantly expressed in sentinel immune cells such as monocytes, macrophages, and plasmacytoid dendritic cells (pDCs), plays a pivotal role in initiating type I IFN responses against viral infections. It is also functionally significant in B cells, where it promotes spontaneous germinal center formation, autoantibody production, and autoimmune activation (Soni et al., 2014; Cosgrove et al., 2023). TLR7 recognizes the ssRNAs of various viruses, including human immunodeficiency virus 1 (HIV-1), influenza virus, respiratory syncytial virus (RSV), and SARS-CoV-2, thereby activating downstream signaling pathways such as MyD88-dependent IFN production and inflammatory cytokine release (Fitzgerald and Kagan, 2020). While TLR7 has been extensively studied in the context of other viral infections, its role in SARS-CoV-2 immunobiology requires more precise contextualization. For instance, during HIV-1 infection, TLR7 synergizes with Fas/FasL to deplete CD4+ T cells via IFN regulatory factor 5 (IRF5) activation (Carmona-Pérez et al., 2023). In influenza, TLR7/MyD88 signaling promotes NF-κB activation and modulates T-cell responses, though its downregulation appears beneficial for viral control (Shi et al., 2020). Similarly, TLR7 and TLR8 collaborate in Th1-polarized antiviral responses against influenza and HIV (Heil et al., 2004). In RSV infection, TLR7 activation in pDCs and conventional DCs induces IFNβ through MyD88 and IFNAR pathways, while also facilitating IL-33 production in macrophages in coordination with TLR3 (Qi et al., 2015; Kim et al., 2019). Even in DNA viral infections such as EBV, TLR7 modulates IRF7 activation and interferon production, illustrating its broad functional versatility (Martin et al., 2007; Valente et al., 2012). However, a direct comparative framework linking these findings to SARS-CoV-2 remains inadequately developed. Transitions from general virology to COVID-19-specific mechanisms are often abrupt, and the relevance of other viral models (e.g., HIV, RSV, influenza) to SARS-CoV-2 pathogenesis is not systematically justified. A more integrated analysis should highlight both conserved and distinct roles of TLR7 across viruses, particularly within the unique immunopathological environment of SARS-CoV-2 infection.
Emerging evidence underscores the central role of TLR7 in SARS-CoV-2 infection. It mediates type I IFN and proinflammatory cytokine production (e.g., IL-6, TNF-α) via IRF5 activation in dendritic cells (Cords et al., 2023). Impaired TLR7 function in lung DCs and macrophages is linked to severe pulmonary immunopathology (Qin et al., 2021; Wu and Yang, 2021; Huang et al., 2023; Miquel et al., 2023). Additionally, SARS-CoV-2 skews macrophage polarization toward a proinflammatory phenotype and disrupts efferocytosis via coordinated TLR7/STING activation, exacerbating tissue damage and delaying repair (Salina et al., 2022; García-Nicolás et al., 2023). Although TLR7 deficiency in mice attenuates IFN and inflammatory mediator expression, suggesting a protective role for TLR7-dependent IFN responses, the precise mechanisms underlying its dual roles, antiviral protection versus hyperinflammation, remain unclear (Ghimire et al., 2025). The temporal dynamics of TLR7 activation critically influence immune outcomes. Early and transient induction supports controlled IFN-mediated antiviral responses, whereas delayed or persistent signaling often correlates with dysregulated inflammation and impaired viral clearance. However, the protective role of TLR7 against lethal SARS-CoV-2 infection in mice should be interpreted with caution due to the mouse model’s translational limitations and a body of contradictory evidence implicating TLR7 in hyperinflammation. Future studies employing non-human primate (NHP) COVID-19 models, such as rhesus macaques or cynomolgus monkeys, would be highly appropriate and scientifically advantageous for further investigating the role of TLR7 in SARS-CoV-2 infection and the immunopathology. NHPs share closer physiological, immunological, and genetic similarities with humans compared to murine models, particularly in terms of respiratory anatomy, immune cell subsets, Toll-like receptor expression patterns, and disease progression. This makes them especially relevant for studying nuanced and clinically translatable mechanisms of viral immunity, inflammatory regulation, and organ-specific responses, such as those involved in acute COVID-19 pneumonia or long-COVID.
Furthermore, while SARS-CoV-2 affects multiple extrapulmonary organs (e.g., heart, kidney, nervous system) in long-COVID, the tissue-specific regulation of TLR7 and its interactions with other endosomal TLRs remain poorly understood. Future studies should clarify how TLR7 signaling operates across different physiological contexts to reconcile its protective and pathogenic effects in COVID-19.
3.2 Dysregulation of adaptive immunity-mediated by TLR7 during SARS-CoV-2 infection
The intrinsic TLR7 has garnered significant attention for its potential therapeutic implications in various viral infections by managing adaptive immunity during the past few years. Influenza A virus leads to imbalanced proportions of Th1/Th2 and Th17/Treg by activating TLR7-IRF3 signaling (Zheng et al., 2021). The study by Carmona-Pérez L and the colleagues has implicated that the activation of TLR7/IRF-5 axis leads to reduced memory CD4+T cell in response to HIV-1 infection (Carmona-Pérez et al., 2023). A TLR7/9-IRF7 signaling axis in pDCs has also been documented in the regulation of CD4+ T cell response to HIV-1 infection (Chehimi et al., 2010). IRF5-deficient B cells exhibit diminished TLR7 and TLR9 expression and cytokine-induced IgG2a/c class-switch during polyoma virus infection, suggesting a TLR7/9-IRF5 axis in B cell-mediated antiviral immunity (Fang et al., 2012). Taken together, all these findings have strongly supported the critical role of TLR7 and the interaction with other endosomal TLRs in adaptive immunity against viral infections, including Influenza A, HIV-1 and polyoma viruses.
Emerging studies have implicated an uncoordinated adaptive immune response in COVID-19, while the potential molecular mechanism has not been fully understood. SARS-CoV-2 infection induces the dysregulation of T cell response, characterized by the expansion of regulatory T cell population, reduced T effector cell activation and enhanced SARS-CoV-2-specific T cell immunity (Rümke et al., 2023; Yin et al., 2024). An activated Th2- and Th17-associated immunity is observed following SARS-CoV-2 infection via the viral S protein (Jeong et al., 2023; Zhang et al., 2024). Prolonged antigen exposure leads to T cell exhaustion, impairing their ability to effectively recognize and eliminate SARS-CoV-2-infected cells. An imbalance of T-cell immunity has been confirmed in severe COVID-19, characterized by activated Th17 cells, incompetent memory T cells, and increased exhausted T cells (Das et al., 2023; Vazquez-Alejo et al., 2023). Besides, an increased CD8+ T cell exhaustion and abnormal NK-like CD16+CD8+ T cells has been observed in severe COVID-19 patients (Schreibing et al., 2022). Moreover, SARS-CoV-2 may hinder the robust memory B-cell and T-cell response upon re-exposure to viral reservoirs in the infected cells or tissues that harbor persistent viral genetic materials, such as DNAs or RNAs, allowing the virus to evade immune clearance and antiviral therapies. Nevertheless, SARS-CoV-2 vaccines can prevent from severe outcomes of COVID-19 by inducing a sufficient IFN response and a long-lasting and SARS-CoV-2-specific adaptive immune response mediated by CD4+ and CD8+ T cells (Zollner et al., 2021; Maringer et al., 2022; Moyon et al., 2022). Inadequate or prolonged IFN response contributes to the persistence of clinical symptoms and long-term complications of COVID-19 by feedback regulation of adaptive immunity (Elizalde-Díaz et al., 2022; Di Stadio et al., 2023). Taken together, the impaired immune recognition and immune killing by CD8+T cells, inhibited T-cell and B-cell responses, T cell exhaustion, imbalance of Th1/Th2 and Th17/Treg and the damaged IFN response contribute to the systemic inflammation and uncoordinated adaptive immunity in COVID-19 and long-COVID.
Some pharmacological agents (Imidazoquinoline, Thiazoquinolone, etc.) or adjuvants (Resiquimod R848, etc.) for COVID-19 vaccine acting on TLR7 or TLR8 are potentially used to combat COVID-19 by activating TLR7/8 signaling and inducing prominent type I IFN and Th1 antiviral responses, suggesting the potential role of endosomal TLR7/8 in regulating adaptive immunity in COVID-19 (Khalifa and Ghoneim, 2021). In the presence of TLR7/8 agonists, SARS-CoV-2 virus-like particles (VLPs) have been demonstrated to promote high neutralizing antibody titers, prominent type-I IFNs and differentiation of antigen-specific CD8+ T cells and Th1-biased CD4+ T cells (Salvi et al., 2021; Sebastião et al., 2023). T cell survival, activation and Th1 polarization are induced by SARS-CoV-2 subunit vaccines adding with TLR7/8 agonist as adjuvants (Yan et al., 2023; Yin et al., 2023). Therefore, Th1-mediated adaptive immune response in company with TLR7/8 stimulation is a critical determinant of SARS-CoV-2 vaccine efficacy. Besides, B cell-intrinsic TLR7 is involved in the regulation of B-cell immune response and the production of IgG2b/IgG2c neutralizing antibody in SARS-CoV-2 infection (Miquel et al., 2023). It has been reported that gain-of-function variants of TLR7 drive B cells activation in a B cell-intrinsic manner in systemic autoimmune diseases, while loss-of-function TLR7 variants confer higher risk of severe COVID-19 and poor prognosis (Cosgrove et al., 2023; Punnanitinont et al., 2023). Accordingly, TLR7-dependent immune dysfunction plays a vital role in the uncoordinated adaptive immune response in COVID-19 by regulating T-cell and B-cell immunity. Nonetheless, the molecular mechanism of TLR7 signaling in regulating SARS-CoV-2-specific cellular and humoral immunity remains elusive. Understanding the role of TLR7-medited immunity is essential for the elucidation of immunopathological mechanism of SARS-CoV-2 infection and the exploration of therapeutic interventions. Clinical trials or mechanically studies based on primates are warranted to investigate the underlying mechanism of endosomal TLR7 in mediating the adaptive immune response to SARS-CoV-2 infection in the future.
4 Mechanisms of TLR7 and the downstream IRF/IFN signaling pathways in regulating SARS-CoV-2 infection
4.1 TLRs-mediated IFN signaling activation in regulating autoimmunity
TLRs are vital for the initiation and activation of innate immunity, especially for anti-viral immunity. The cell-surface TLRs, such as TLR2 and TLR4, recognize viral protein particles of the virus. The endosomal TLRs of TLR3, TLR7, TLR8 and TLR9 are responsible for the sense the nucleic acid strains or intermediate nucleic acids inside the infected cells by viruses (Figure 1). Upon recognition by PAMPs, TLRs undergo a complex activation process characterized by homodimerization or heterodimerization of their ectodomains. This initial step is followed by the dimerization of their intracellular domains, leading to the recruitment of signaling adapters and subsequent activation (Sameer and Nissar, 2021). Distinct TLRs recruit different adaptors but share some downstream regulators in common, such as IRFs and NF-κB, which elicits IFNs and pro-inflammatory cytokine release (Lester and Li, 2014).
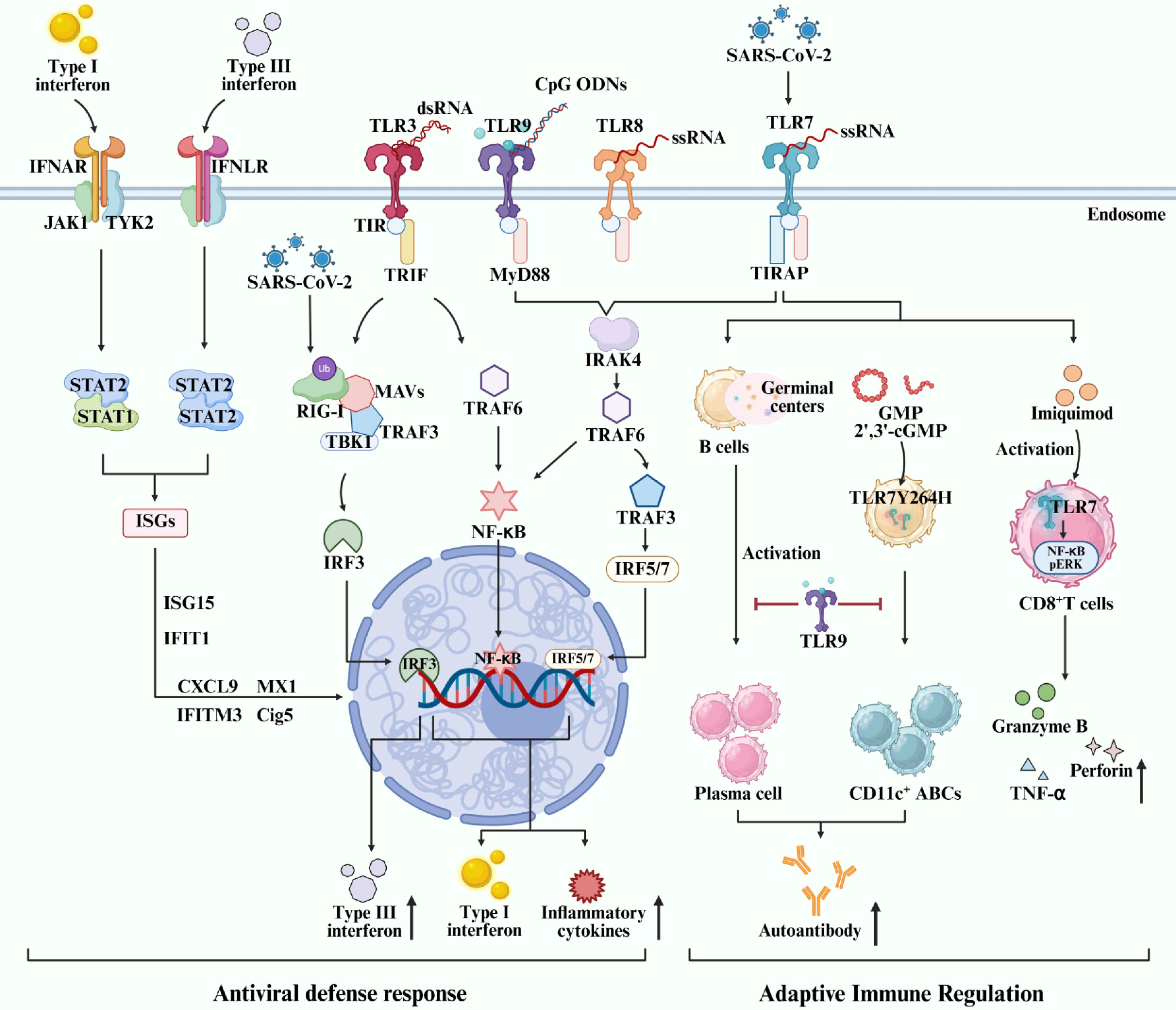
Figure 1. Role and molecular mechanism of endosomal TLRs-mediated IRFs/IFNs signaling activation in regulating autoimmune disorders and antiviral immunity.
4.1.1 TLRs-mediated IFN signaling activation in regulating antiviral immunity
During the last decade, the crosstalks between IRFs/IFN signaling and TLRs signaling have been implicated in various viral infections, such as Influenza A, SARS-CoV-2, and RSV. They induce or antagonize IFNs signaling depending on various factors. The membrane receptor for type I, II and III IFNs signal is IFN alpha receptor (IFNAR), IFN gamma receptor (IFNGR), and IFN lambda receptor (IFNLR), respectively. Activation of TLRs and the IFN signaling cascade leads to enhanced expression of antiviral interferon-stimulated genes (ISGs), ultimately contributing to the establishment of an antiviral state within the host cell. Multiple signaling pathways are engaged in antiviral defense, primarily mediated through two key adapter proteins: MyD88 and IL-1R resistance (TIR) domain-containing adapter-inducing IFN-β (TRIF) (Kosciuczuk et al., 2019; Gallucci et al., 2021; Ji et al., 2023). TLR activation recruits either the MyD88- or TRIF-dependent pathway. MyD88 initiates downstream NF-κB and mitogen-activated protein kinase (MAPK) signaling, leading to pro-inflammatory cytokine production. TRIF is selectively recruited to TLR3 and TLR4. And, TRIF activates TBK1, which phosphorylates IRF3/IRF7, inducing type I IFN production. Subsequent Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling is stimulated by IFNs to amplify antiviral gene expression. Additional pathways such as PI3K-Akt-mTOR further modulate immune and metabolic responses during infection. Two TNF receptor-associated factors (TRAFs), namely TRAF3 and TRAF6, can be activated by members of the IRAK family, leading to the production of type I IFN or pro-inflammatory cytokines, respectively (Häcker et al., 2006, 2011). Recent studies have suggested that type III IFN signaling play an important role in influenza virus infection by inducing a more sustained ISGs expression and a non-redundant front-line antiviral response to control viral replication and abundant inflammation through the IFNLR-related signaling in neutrophils and epithelial cells (Galani et al., 2017; Vlachiotis and Andreakos, 2019; Yadav et al., 2023). The heterodimerization of type III IFNLR is insufficient for JAK-STAT activation, while IFN-λ induces a JAK-STAT cascade and the ultimate activation of endogenous type III IFN pathway via JAK1 and TYK2 kinases (Figure 1) (Mesev et al., 2023). Type-I and -III IFNs are well documented to defend against SARS-CoV-2 infection, and type-II IFN has also been demonstrated to inhibit SARS-CoV-2 replication in human lung epithelial cells as well as ex vivo human lung tissues through IFNγ-induced IDO1-mediated antiviral response (Yang et al., 2024a). The interference with IFNs and IFN signaling activation is a primary strategy of SARS-CoV-2 to evade the immune recognition, surveillance and clearance of the immune system. The production of autoantibodies against IFNs and loss-of-function variants of type I IFN immunity genes can lead to impaired IFNs antiviral response and ultimately immune evasion of SARS-CoV-2, thereby aggravating COVID-19 or causing PASC (Znaidia et al., 2022; Yao et al., 2023). As a pattern recognition receptor, MDA5 can recognize the RNA of SARS-CoV-2 virus, particularly the dsRNA intermediates generated during viral replication. This recognition triggers downstream signaling via MAVS, leading to the activation of transcription factors IRF3, IRF5, and NF-κB, which subsequently induce the production of IFN-I and IFN-III and mediate antiviral immunity (Yin et al., 2021; Maiti, 2024). RIG-I specifically binds to the 3’ untranslated region (3’ UTR) of SARS-CoV-2 RNA through its helicase domain independently of its C-terminal domain. This mechanism is unique, as it does not stimulate RIG-I’s ATPase activity, thus avoiding excessive immune activation (Yamada et al., 2021). Transfection of RNA from SARS-CoV-2-infected cells into epithelial cells showed that a specific ∼200-nucleotide viral RNA fragment preferentially induces a RIG-I-dependent IFN-I response. This response was significantly reduced in RIG-I-knockout cells, confirming RIG-I as the key sensor for such small viral RNAs and its essential role in initiating innate immunity (Kouwaki et al., 2021). Mechanistically, SARS-CoV-2 antigens suppress IFN response by inhibiting JAK-STAT1 signaling activation (Minkoff and tenOever, 2023), disrupting the RIG-I-MAVS-TRAF3-TBK1 complex (Zheng et al., 2020), impeding RIG-I ubiquitination via tripartite motif 25 (TRIM25) (Wu et al., 2020; Khatun et al., 2023), and reducing IRF3 phosphorylation via Karyopherin alpha 2 (KPNA2) (Xia et al., 2020) (Figure 1). These actions collectively suppress the expression of antiviral IFNs and ISGs. Therefore, IFN-based therapy not only establishes a robust antiviral immune state but serves as a promising strategy to counteract SARS-CoV-2 immune evasion. Improving the therapeutic efficacy of IFN-based regimens by increasing the sensitivity of IFNs to SARS-CoV-2 and preventing IFN autoantibodies production as well as IFN gene modifications or editing, remains a key scientific issue to be addressed in the future. Viruses can thwart the endosomal TLRs sensing systems, thereby evading immune surveillance and immune clearance-mediated by TLRs sensing and the antiviral IFN signaling activation. Loss-of-function variants of TLR7 cause immune evasion of SARS-CoV-2 and life-threatening pneumonia among COVID-19 patients, characterized by reduced TLR7-related gene expression, downregulated cytokine-mediated signaling transduction, and subsequent impairment of type I and type II IFN responses (Solanich et al., 2021; Mantovani et al., 2022). Accordingly, TLRs-mediated IFN signaling activation is essential for the antiviral immunity in COVID-19.
4.1.2 TLRs-mediated IFN signaling activation in regulating autoimmune disorders
Currently published studies have extensively investigated the role of TLR7 signaling in humoral and cellular immune responses in autoimmune disorders, particularly regarding B cell-intrinsic TLR7 (Figure 1). B cell-intrinsic TLR7 but not TLR3 is required for the formation of germinal center and the production of autoantibodies (Browne, 2011; Soni et al., 2014). A TLR7 gain-of-function variant (TLR7Y264H) has been demonstrated to drive B cells activation and CD11c+ age-associated B cells accumulation in a B cell-intrinsic manner by selectively sensing guanosine and 2’,3’-cGMP in systemic lupus erythematosus (SLE) (Jackson et al., 2014). Accumulated studies have suggested B cell-intrinsic TLR9 attenuates systemic inflammation and autoimmunity by inhibiting antigen uptake and the processing of activated B cells, affinity maturation, generation of antigen-specific plasma cells, and serum IgG avidity (Jackson et al., 2014; Akkaya et al., 2018). Furthermore, the role of B cell-intrinsic TLR7 and TLR7 signaling in promoting severe SLE has also been well observed in TLR9-deficient mice, highlighting a cis regulatory interplay between the protective B cell-intrinsic TLR9 and the pathogenic B cell-intrinsic TLR7 in B cell immunity (Satterthwaite, 2021; Cosgrove et al., 2023). All these findings have suggested the opposed impact of B-cell intrinsic TLR7 and TLR9 in regulating adaptive immunity. Accordingly, B cell-directed TLR7 antagonism/TLR9 agonism may be a promising strategy to attenuate systemic inflammation and autoimmune disorders by controlling autoantibody production and aberrant adaptive immune response. However, the precise molecular mechanism remains unclear. Additionally, TLR7 is also essential for T cell response and autoimmune activation. The activation of TLR7 in the endosomes of CD8+ T cells by Imiquimod drives a remarkable cytokine transcript signature through the NF-κB and pERK signaling pathway in rheumatoid arthritis (RA) (Swain et al., 2022). Accordingly, TLR7 drives autoimmunity by activating both humoral and cellular immunity, making it a potential therapeutic target for autoimmune diseases. However, more future studies are warranted to elucidate the downstream signaling mechanism of TLR7-mediated IFN signaling activation in autoimmune diseases.
4.2 TLR7-mediated IFN signaling activation in SARS-CoV-2 infection
Activation of TLRs triggers the expression of IFNs and ISGs, forming a positive feedback loop in antiviral immunity. Currently available data has implicated cell-type specific distribution and activation of TLRs and IFN-related signaling molecules in defending against SARS-CoV-2 infection (Park and Iwasaki, 2020; Manik and Singh, 2022). However, the precise mechanism underlying TLR7 and the downstream IRF/IFN signaling pathways in regulating innate and adaptive immunity in COVID-19 has not been fully understood due to the complex and sometimes contradictory roles of IFN signaling, which can exhibit both protective antiviral and harmful pro-inflammatory effects in different stages of infection. Additionally, the extensive crosstalk between TLR7, other innate immune pathways, and adaptive immune cells creates a highly interconnected network that is difficult to dissect experimentally. TLR7 is a MyD88- and IRAK-4-dependent endosomal ssRNA sensor (Figure 1). Patients with inherited MyD88 or IRAK-4 deficiency are predisposed to COVID-19 pneumonia, suggesting the pivotal role of TLR7 signaling-related genes in the onset of severe COVID-19 (Garcia-Garcia et al., 2023). TLR7-MyD88-TIRAP signaling is crucial for the antiviral IFNs response to SARS-CoV-2 infection, whereas disrupting this signaling complex using various TLR7 antagonists and N-acetylcysteine leads to impaired viral RNA sensing and MyD88 signaling (van der Sluis et al., 2022). Most importantly, TLR7-mediated innate immunity plays a central role in the first-line antiviral defense against SARS-CoV-2 infection by activating IFN signaling pathway and enhancing the expression of IFNs and ISGs in immune cells (Jackson et al., 2023). IFN signaling pathway and ISGs play pivotal roles in the antiviral response in COVID-19 (Eskandarian Boroujeni et al., 2022; Song et al., 2023). Transcriptional activation of IFNs and ISGs is extensively observed in SARS-CoV-2-infected cells, such as ISG15, IFIT1, IFITM3, CXCL9, myxovirus resistance 1 (MX1), and Cig5 (Figure 1) (Skaug and Chen, 2010; Solimani et al., 2021; Unali et al., 2023). IRF3 and IRF7 are the main IRFs involved in the downstream of TLR signaling events, inducing the production of type I IFNs (Jefferies, 2019). Enhanced production of type I IFNs in DCs can be induced through the activation of TLR7-IRF5 signaling during SARS-CoV-2 infection (Figure 1) (Cords et al., 2023). A number of studies have implicated impaired type-I IFNs response due to autoantibodies against IFN-α and IFN-β, agonists of TLR7 or rare variants of key genes in TLR7-IRFs signaling is closely related to severe COVID-19 (Hadjadj et al., 2020; Zhou et al., 2020; Matuozzo et al., 2023). Higher risk of life-threatening COVID-19 is highly associated with anti-IFN-α autoantibody and loss-of-function variants of type I IFN immunity genes depending on TLR7. Therefore, the activation of IFN-related cascade due to dysregulated TLR7 ultimately leads to immune hyperactivation, particularly in severe COVID-19, but lacking well-known signaling mechanism. There might be intricate interplay between TLRs signaling and IRFs/IFNs signaling in regulating inflammation and antiviral immunity due to the obviously dysregulated TLRs and abnormal IFN signaling activation in COVID-19. Nonetheless, the precise signaling mechanism underlying diverse types of TLRs and IFNs underlying different viral infections is different, particularly the endosomal TLR7 and TLR7-mediated IRF/IFN signaling activation. Besides, IFN responses differ between diverse mice strains and between mice and humans. The role of TLR7-mediated IFN signaling activation in COVID-19 warrants to be investigated in more human clinical studies or studies using the SRAS-CoV-2-infected primates animal model. Furthermore, the association between innate immunity and B-intrinsic TLR7-mediated adaptive immune response and the production of antibody in SRAS-CoV-2 infection remains largely unknown. Elucidating the promising therapeutic target(s) in the TLR7-mediated IRF/IFN signaling pathway is critical for the development of therapeutic strategies or vaccines against SARS-CoV-2 infection that aim to modulate the host immune response for more effective viral clearance and prevention of severe COVID-19 or long-COVID.
5 SARS-CoV-2-specific adaptive immune dysregulation and the potential association with TLR7 in long-COVID
Currently, a great amount of people is suffering from long-COVID or PASC, whereas the potential biological factors driving post-COVID-19 pathology remain unclear. Some factors been well documented to contributing to PASC pathology, such as microbiome, coagulation, and abnormal neuroimmunity. Little is known of the precise molecular mechanisms underlying long COVID. The crosstalk between adaptive cellular immunity and humoral immunity contributes to long-COVID symptoms, such as sleep disturbance, fatigue, “brain fog”, and osteoarthralgia, by inducing long-sustained inflammation and uncoordinated adaptive immunity (Yin et al., 2024). The study by Yin K and the colleagues has suggested systemic inflammation and persistent T and B cell responses in long-COVID individuals (Yin et al., 2024). They reported elevated ratios of exhausted CD8+ T cells and inflamed tissues-infiltrated CD4+ T cells alongside with increased SARS-CoV-2 antibodies, suggesting SARS-CoV-2-specific immunity in 8 months post-infected long-COVID individuals. Furthermore, the attenuation of functional polarization of CD8+ T cells by blocking granzyme B and IFN-γ is demonstrated to be an effective approach to prevent from PASC and further complications (Wiech et al., 2022). Therefore, long-COVID manifests with sustained inflammation, impaired IFN response, and SARS-CoV-2-specific B and T cell responses. Maintaining the balance of adaptive immunity mediated by B and T cells is essential for controlling IFNs production and long-COVID progression. However, the underlying mechanism of IFN signaling activation in modulating adaptive immunity and its contribution to the establishment of long-term immunity against SARS-CoV-2 infection remain not fully understood. Identifying checkpoints in the upstream or downstream of IFN signaling involved in antiviral immunity is of great importance to explore novel therapeutic strategies or more effective vaccines to prevent PSAC.
Emerging studies has implicated the critical role of intrinsic TLR7 in adaptive immune response to SARS-CoV-2 and pathogen-like COVID-19 vaccines by regulating the activation of IFN signaling as discussed previously (Rossmann et al., 2021; Miquel et al., 2023; Yan et al., 2023; Yin et al., 2023). Little is known about the modifying effect of TLR7 in long-COVID. A previous study has implicated that a loss-of-function hemizygous variant (c.1386_1389dup) of TLR7 is positively associated with the long-term neurological complication of post-COVID-19 individuals, suggesting the potential role of TLR7 in long-COVID (Noor Eddin et al., 2023). Although the significant role of TLR7 and IRF-IFN signaling has also been implicated in antiviral response, the precise mechanism of TLR7-mediated IFN signaling activation in regulating the adaptive immunity against SARS-CoV-2 infection and long-COVID remains largely unknown. Whether and how TLR7-mediated IFN signaling activation contributes to the long-sustained inflammation and uncoordinated adaptive immunity in long-COVID is poorly defined. Long-COVID deeply affects the quality of life for affected individuals. Understanding the nuanced mechanisms underlying TLR7 and IRFs/IFNs signaling can offer valuable insights into the development of new targeted therapies for COVID-19 and highly effective vaccines to prevent from long-COVID.
6 Beneficial effects of vaccines and therapeutic strategies targeting TLR7 and IRFs/IFNs signaling in COVID-19
The beneficial effects of vaccines and therapeutic strategies targeting TLR7 and IRFs/IFNs signaling are paramount in combating COVID-19 pandemic and long-COVID. TLR7 activation triggers the innate immune response, leading to the production of IFNs and pro-inflammatory cytokines that help in combating with viral infections. Reversely, by enhancing IRF-mediated induction of IFNs, therapeutic strategies can bolster the antiviral defense mechanisms, dampen excessive inflammation, and promote viral clearance. Therefore, targeting TLR7 and IRFs-IFNs signaling pathways holds potentials in COVID-19 treatment, the establishment of long-term immunity to SARS-CoV-2 infection, and the exploration of highly effective antiviral vaccines.
Some currently available data has been gained from preclinical studies and early-phase clinical trials regarding TLR7-targeting drugs, IRF-targeting drugs and IFN-targeting drugs in COVID-19. Nonetheless, the efficacy and safety estimates for these drugs are still evolving. TLR7 agonists are being explored to boost antiviral immunity, while TLR7 antagonists aim to modulate inflammatory responses. Vesatolimod is a TLR7 agonist holding potentials in enhancing antiviral immunity (SenGupta et al., 2021; Usero et al., 2023). Vesatolimod activates TLR7 to induce the production of IFN-α/β and pro-inflammatory cytokines in immune cells, which enhances the body’s early recognition and response capacity against viruses and suppresses viral replication. Increased expression of ISGs and elevated cytokine release in patients following Vesatolimod treatment, suggesting it may enhance the immune response against SARS-CoV-2 through a similar mechanism (Riddler et al., 2021). But little is known about its therapeutic efficacy in COVID-19. Most importantly, the risk of exacerbating inflammation with TLR7 agonists should be treated seriously. TLR antagonist targeting TLR7/9 or agonist targeting TLR7/8 has also been focused on the regulation of autoimmunity, cancer and viral infections, while their potentials in modulating TLR7 signaling for COVID-19 is unclear yet (Khanmohammadi and Rezaei, 2021; Deshmukh et al., 2024; Wu et al., 2024). However, the primary off-target risk of these targeting agents is the induction of a systemic inflammatory response. The widespread release of IFN-α, TNF-α, and IL-6 can mimic severe viral infections or even cytokine release syndrome (CRS), leading to fever, fatigue, hypotension, and organ toxicity. TLR9 agonist play a crucial role in early antiviral immunity by recognizing viral nucleic acids, activating immune cells, and inducing the production of IFN-I and pro-inflammatory cytokines. However, excessive activation may trigger a cytokine storm, leading to immunopathological damage (Dyavar et al., 2021; Khanmohammadi and Rezaei, 2021). Although TLR9 is not a direct recognition receptor for SARS-CoV-2, its agonists can still indirectly participate in regulating the immune response in COVID-19 by mimicking viral nucleic acids or recognizing damage-associated molecular patterns (DAMPs) released during tissue injury (Khanmohammadi and Rezaei, 2021; Naqvi et al., 2022). TLR9 is widely expressed in various immune cells, such as B cells and plasmacytoid dendritic cells. Its agonists may non-specifically activate multiple cell types, potentially leading to systemic inflammation. For instance, TLR9 activation in B cells has been associated with autoimmune diseases including type 1 diabetes, suggesting a risk of exacerbating autoimmune pathology in conditions such as COVID-19 (Sha et al., 2021; Yang et al., 2024b). Furthermore, studies in obesity models have shown that B cell-specific deletion of TLR9 reduces adipose tissue inflammation, which conversely implies that TLR9 agonists might promote metabolic-related inflammatory pathways (Yang et al., 2024b). In severe COVID-19 patients, monocytes exhibit a state of TLR tolerance (Naqvi et al., 2022), wherein TLR9 agonists may fail to reverse this hypo-responsive condition and could potentially exacerbate immune exhaustion. Furthermore, the synergistic effects between TLR9 and TLR3 in conventional type 1 DCs (cDC1) are differentially regulated by the nuclear receptor co-repressor 1 (NCoR1) (Mishra et al., 2022). Notably, loss of NCoR1 enhances TLR9 responses while attenuating TLR3 signaling, indicating that cell type-specific epigenetic mechanisms may interfere with the targeting efficiency of TLR agonists. Clinical data regarding the efficacy and safety of IMO-9200 in COVID-19 are encouraged in the future. Nonetheless, the significant challenges, such as the potential for disrupting humoral immunity and the complex interplay between TLR7 and TLR9 in autoimmune contexts, should not be ignored. IRFs exert vital effects on the expression of IFNs, ISGs and other immune-related genes. Drugs targeting IRFs, such as inhibitors or modulators, could potentially influence the immune response to SARS-CoV-2 and modulate the host immune response to achieve a balance between viral clearance and immune-mediated tissue damage. Iberdomide (CC-220) is a cereblon-targeting drug that modulates the transcription factor IRF4, thereby playing a role in the context of viral infection (Ne et al., 2022). In clinical trials for SLE, iberdomide significantly reduced the type I IFN gene signature in patients with high baseline expression (Lipsky et al., 2022). Given that severe COVID-19 is also associated with aberrant type I IFN activation (Lipsky et al., 2022), it is hypothesized that iberdomide may mitigate COVID-19-related hyperinflammation through a similar mechanism. Furthermore, iberdomide modulates both innate and adaptive immune responses and remodels the tumor microenvironment, as evidenced in malignancies such as multiple myeloma (Van Oekelen et al., 2024). These immunomodulatory properties align with the need to counteract immune dysregulation observed in severe COVID-19, suggesting its potential applicability in managing COVID-19 immunopathology. GSK2831781, an inhibitor of IRF4, has been investigated due to its ability to modulate IRF-mediated immune response in T-cell-mediated inflammatory and autoimmune diseases, such as ulcerative colitis and psoriasis (Ellis et al., 2021; D’Haens et al., 2023). In the future, early-phase clinical trials may shed light on the efficacy and safety of these drugs in COVID-19 patients. IFNs are signaling proteins involved in antiviral defenses against SARS-CoV-2 by boosting the innate immunity and inhibiting viral replications. Clinical trials have evaluated the efficacy and safety profile of IFN-targeting drugs, whereas the results may vary depending on the specific formulation and patient population. Additionally, a number of factors, such as dosing, patient characteristics, and disease severity, can influence outcomes. Continued research and larger-scale clinical trials are warranted to further assess the potentials of these drugs in treating COVID-19. Overall, the combined approach of vaccination alongside therapeutic interventions targeting TLR7 and IRFs/IFNs signaling presents a multifaceted strategy to mitigate the impact of COVID-19. By harnessing the power of both preventive and treatment modalities, it would be more effective to control the spread of the virus, reduce disease burden, and safeguard public health.
7 Conclusions and future directions
Innate immunity is the first line to defend against SARS-CoV-2. The critical role of TLR7-mediated IFN signaling activation in innate immunity has been well documented in defending against various viral infections, including SARS-CoV-2 but lacking sufficient evidence for the signaling mechanism. The intrinsic TLR7 is not only essential for the initiation of innate immune response to SARS-CoV-2 infection but also the regulation of adaptive B cell- and T cell-mediated humoral and cellular immunity. The dual roles of TLR7 in antiviral defense and inflammatory pathology are shaped by the spatiotemporal context and magnitude of its activation. Under controlled conditions, transient TLR7 signaling in pDCs promotes type I IFN production, supporting effective viral control. However, sustained or excessive activation driven by high viral loads or self-RNA exposure triggers hyperinflammatory responses in macrophages and other myeloid cells, leading to pathological cytokine release. This shift is further amplified through synergistic crosstalk with other innate immune pathways (e.g., inflammasome activation, RIG-I/MDA5 sensing) and the failure of endogenous regulatory mechanisms, such as feedback inhibitors or tolerance induction. it exhibits extensive cross-talk with other nucleic acid-sensing pathways. For example, the co-activation of RIG-I enhances antiviral IFN responses but may also exacerbate inflammatory tissue damage if not properly regulated. In contrast, engagement of TLR3, which induces IRF3-driven IFN responses with less inflammatory output, may partially compensate for or modulate TLR7 hyperactivation. Feedback inhibitors also play a central role in shaping TLR7 signaling dynamics, such as IRAK-M, SOCS1, and A20, which attenuate TLR7-mediated NF-κB and IRF7 activation. Consequently, the functional outcome of TLR7 activation is determined by integrated signals from ligand availability, temporal dynamics, cellular microenvironment, concurrent PRR engagement, and the capacity of negative regulatory networks.
While the role of TLR7-mediated IRF/IFN signaling in the adaptive immune response to SARS-CoV-2, particularly in long-COVID, remains mechanistically unclear, its therapeutic potential warrants systematic investigation. To bridge key knowledge gaps, future studies should prioritize innovative approaches such as single-cell transcriptomics and spatial multi-omics within primate models to delineate cell-specific TLR7 responses and immune crosstalk. Clinical trials evaluating TLR7 agonists, IRF modulators, or IFN-based therapies should incorporate precise endpoints including viral persistence, T-cell memory quality, and autoantibody profiles, alongside integrative biomarkers such as TRS-based gene signatures or multiplex cytokine mapping. Furthermore, combining vaccination with targeted immunomodulation of the TLR7-IRF-IFN axis may offer a dual strategy to enhance viral clearance, regulate adaptive immunity, and mitigate long-COVID sequelae. Such focused efforts are essential to advance this pathway from mechanistic insight to therapeutic application.
Author contributions
JX: Writing – original draft, Supervision, Writing – review & editing. XW: Writing – original draft, Writing – review & editing. HW: Writing – original draft, Writing – review & editing. BQ: Writing – original draft. PG: Writing – original draft. BR: Project administration, Writing – review & editing, Supervision. SY: Writing – review & editing, Project administration, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Shandong National Natural Science Foundation, China (ZR2025MS1348) and National Natural Science Foundation of China (No.82003042).
Acknowledgments
The authors would like to thank all medical staff of the Affiliated Hospital of Shandong Second Medical University for their great help.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akira, S., Uematsu, S., and Takeuchi, O. (2006). Pathogen recognition and innate immunity. Cell 124, 783–801. doi: 10.1016/j.cell.2006.02.015
Akkaya, M., Akkaya, B., Kim, A. S., Miozzo, P., Sohn, H., Pena, M., et al. (2018). Toll-like receptor 9 antagonizes antibody affinity maturation. Nat. Immunol. 19, 255–266. doi: 10.1038/s41590-018-0052-z
Asano, T., Boisson, B., Onodi, F., Matuozzo, D., Moncada-Velez, M., Maglorius Renkilaraj, M. R. L., et al. (2021). X-linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci. Immunol. 6, eabl4348. doi: 10.1126/sciimmunol.abl4348
Ashhurst, A. S., Johansen, M. D., Maxwell, J. W. C., Stockdale, S., Ashley, C. L., Aggarwal, A., et al. (2022). Mucosal TLR2-activating protein-based vaccination induces potent pulmonary immunity and protection against SARS-CoV-2 in mice. Nat. Commun. 13, 6972. doi: 10.1038/s41467-022-34297-3
Bagci, G., Gundogdu, O., Pektas, A. N., Bagci, B., Avci, O., Gursoy, S., et al. (2023). The investigation of host genetic variants of toll-like receptor 7 and 8 in COVID-19. Nucleosides Nucleotides Nucleic Acids 42, 586–602. doi: 10.1080/15257770.2023.2176515
Browne, E. P. (2011). Toll-like receptor 7 controls the anti-retroviral germinal center response. PloS Pathog. 7, e1002293. doi: 10.1371/journal.ppat.1002293
Carmona-Pérez, L., Dagenais-Lussier, X., Mai, L. T., Stögerer, T., Swaminathan, S., Isnard, S., et al. (2023). The TLR7/IRF-5 axis sensitizes memory CD4+ T cells to Fas-mediated apoptosis during HIV-1 infection. JCI Insight 8, e167329. doi: 10.1172/jci.insight.167329
Cerase, A., Young, A. N., Ruiz, N. B., Buness, A., Sant, G. M., Arnold, M., et al. (2021). Chd8 regulates X chromosome inactivation in mouse through fine-tuning control of Xist expression. Commun. Biol. 4, 485. doi: 10.1038/s42003-021-01945-1
Chehimi, J., Papasavvas, E., Tomescu, C., Gekonge, B., Abdulhaqq, S., Raymond, A., et al. (2010). Inability of plasmacytoid dendritic cells to directly lyse HIV-infected autologous CD4+ T cells despite induction of tumor necrosis factor-related apoptosis-inducing ligand. J. Virol. 84, 2762–2773. doi: 10.1128/jvi.01350-09
Cloutier, M., Kumar, S., Buttigieg, E., Keller, L., Lee, B., Williams, A., et al. (2022). Preventing erosion of X-chromosome inactivation in human embryonic stem cells. Nat. Commun. 13, 2516. doi: 10.1038/s41467-022-30259-x
Cords, L., Woost, R., Kummer, S., Brehm, T. T., Kluge, S., Schmiedel, S., et al. (2023). Frequency of IRF5+ dendritic cells is associated with the TLR7-induced inflammatory cytokine response in SARS-CoV-2 infection. Cytokine 162, 156109. doi: 10.1016/j.cyto.2022.156109
Cosgrove, H. A., Gingras, S., Kim, M., Bastacky, S., Tilstra, J. S., and Shlomchik, M. J. (2023). B cell-intrinsic TLR7 expression drives severe lupus in TLR9-deficient mice. JCI Insight 8, e172219. doi: 10.1172/jci.insight.172219
D’Haens, G., Peyrin-Biroulet, L., Marks, D. J. B., Lisi, E., Liefaard, L., Beaton, A., et al. (2023). A randomised, double-blind, placebo-controlled study of the LAG-3-depleting monoclonal antibody GSK2831781 in patients with active ulcerative colitis. Aliment Pharmacol. Ther. 58, 283–296. doi: 10.1111/apt.17557
Das, S., Rai, G., Sood, V., Singh, P. K., Tyagi, A., Salhotra, R., et al. (2023). Incompetent memory immune response in severe COVID-19 patients under treatment. Heliyon 9, e20590. doi: 10.1016/j.heliyon.2023.e20590
Deshmukh, A., Pereira, A., Geraci, N., Tzvetkov, E., Przetak, M., Catalina, M. D., et al. (2024). Preclinical evidence for the glucocorticoid-sparing potential of a dual toll-like receptor 7/8 inhibitor in autoimmune diseases. J. Pharmacol. Exp. Ther. 388, 751–764. doi: 10.1124/jpet.123.001744
Di Stadio, A., Bernitsas, E., La Mantia, I., Brenner, M. J., Ralli, M., Vaira, L. A., et al. (2023). Targeting neuroinflammation to alleviate chronic olfactory dysfunction in long COVID: A role for investigating disease-modifying therapy (DMT)? Life (Basel) 13, 226. doi: 10.3390/life13010226
Du, Y., Faz-Lopez, B., Ah Kioon, M. D., Cenac, C., Pierides, M., Lakin, K. S., et al. (2025). Altered X-chromosome inactivation of the TLR7/8 locus and heterogeneity of pDCs in systemic sclerosis. J. Exp. Med. 222, e20231809. doi: 10.1084/jem.20231809
Dyavar, S. R., Singh, R., Emani, R., Pawar, G. P., Chaudhari, V. D., Podany, A. T., et al. (2021). Role of toll-like receptor 7/8 pathways in regulation of interferon response and inflammatory mediators during SARS-CoV2 infection and potential therapeutic options. BioMed. Pharmacother. 141, 111794. doi: 10.1016/j.biopha.2021.111794
Elizalde-Díaz, J. P., Miranda-Narváez, C. L., Martínez-Lazcano, J. C., and Martínez-Martínez, E. (2022). The relationship between chronic immune response and neurodegenerative damage in long COVID-19. Front. Immunol. 13. doi: 10.3389/fimmu.2022.1039427
Ellis, J., Marks, D. ,. J. B., Srinivasan, N., Barrett, C., Hopkins, T. G., Richards, A., et al. (2021). Depletion of LAG-3(+) T cells translated to pharmacology and improvement in psoriasis disease activity: A phase I randomized study of mAb GSK2831781. Clin. Pharmacol. Ther. 109, 1293–1303. doi: 10.1002/cpt.2091
Eskandarian Boroujeni, M., Sekrecka, A., Antonczyk, A., Hassani, S., Sekrecki, M., Nowicka, H., et al. (2022). Dysregulated interferon response and immune hyperactivation in severe COVID-19: targeting STATs as a novel therapeutic strategy. Front. Immunol. 13. doi: 10.3389/fimmu.2022.888897
Fallerini, C., Daga, S., Mantovani, S., Benetti, E., Picchiotti, N., Francisci, D., et al. (2021). Association of Toll-like receptor 7 variants with life-threatening COVID-19 disease in males: findings from a nested case-control study. Elife 10, e67569. doi: 10.7554/eLife.67569
Fang, C. M., Roy, S., Nielsen, E., Paul, M., Maul, R., Paun, A., et al. (2012). Unique contribution of IRF-5-Ikaros axis to the B-cell IgG2a response. Genes Immun. 13, 421–430. doi: 10.1038/gene.2012.10
Fitzgerald, K. A. and Kagan, J. C. (2020). Toll-like receptors and the control of immunity. Cell 180, 1044–1066. doi: 10.1016/j.cell.2020.02.041
Gabriele, L., Fragale, A., Romagnoli, G., Parlato, S., Lapenta, C., Santini, S. M., et al. (2021). Type I IFN-dependent antibody response at the basis of sex dimorphism in the outcome of COVID-19. Cytokine Growth Factor Rev. 58, 66–74. doi: 10.1016/j.cytogfr.2020.10.001
Galani, I. E., Triantafyllia, V., Eleminiadou, E. E., Koltsida, O., Stavropoulos, A., Manioudaki, M., et al. (2017). Interferon-lambda Mediates Non-redundant Front-Line Antiviral Protection against Influenza Virus Infection without Compromising Host Fitness. Immunity 46875–890 e876. doi: 10.1016/j.immuni.2017.04.025
Gallucci, S., Meka, S., and Gamero, A. M. (2021). Abnormalities of the type I interferon signaling pathway in lupus autoimmunity. Cytokine 146, 155633. doi: 10.1016/j.cyto.2021.155633
Garcia-Garcia, A., Perez de Diego, R., Flores, C., Rinchai, D., Sole-Violan, J., Deya-Martinez, A., et al. (2023). Humans with inherited MyD88 and IRAK-4 deficiencies are predisposed to hypoxemic COVID-19 pneumonia. J. Exp. Med. 220, e20220170. doi: 10.1084/jem.20220170
García-Nicolás, O., Godel, A., Zimmer, G., and Summerfield, A. (2023). Macrophage phagocytosis of SARS-CoV-2-infected cells mediates potent plasmacytoid dendritic cell activation. Cell Mol. Immunol. 20, 835–849. doi: 10.1038/s41423-023-01039-4
Ghimire, R., Shrestha, R., Amaradhi, R., Liu, L., More, S., Ganesh, T., et al. (2025). Toll-like receptor 7 (TLR7)-mediated antiviral response protects mice from lethal SARS-CoV-2 infection. J. Virol. 99, e0166824. doi: 10.1128/jvi.01668-24
Gómez-Carballa, A., Pardo-Seco, J., Pischedda, S., Rivero-Calle, I., Butler-Laporte, G., Richards, J. B., et al. (2022). Sex-biased expression of the TLR7 gene in severe COVID-19 patients: Insights from transcriptomics and epigenomics. Environ. Res. 215, 114288. doi: 10.1016/j.envres.2022.114288
Habeichi, N. J., Amin, G., Lakkis, B., Kataya, R., Mericskay, M., Booz, G. W., et al. (2024). Potential alternative receptors for SARS-coV-2-induced kidney damage: TLR-4, KIM-1/TIM-1, and CD147. Front. Biosci. (Landmark Ed) 29, 8. doi: 10.31083/j.fbl2901008
Häcker, H., Redecke, V., Blagoev, B., Kratchmarova, I., Hsu, L. C., Wang, G. G., et al. (2006). Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature 439, 204–207. doi: 10.1038/nature04369
Häcker, H., Tseng, P. H., and Karin, M. (2011). Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nat. Rev. Immunol. 11, 457–468. doi: 10.1038/nri2998
Hadjadj, J., Yatim, N., Barnabei, L., Corneau, A., Boussier, J., Smith, N., et al. (2020). Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369, 718–724. doi: 10.1126/science.abc6027
Heil, F., Hemmi, H., Hochrein, H., Ampenberger, F., Kirschning, C., Akira, S., et al. (2004). Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303, 1526–1529. doi: 10.1126/science.1093620
Huang, Z., Gao, Y., Han, Y., Yang, J., Yang, C., Li, S., et al. (2023). Revealing the roles of TLR7, a nucleic acid sensor for COVID-19 in pan-cancer. Biosaf Health. 5, 211–226. doi: 10.1016/j.bsheal.2023.05.004
Jackson, W. D., Giacomassi, C., Ward, S., Owen, A., Luis, T. C., Spear, S., et al. (2023). TLR7 activation at epithelial barriers promotes emergency myelopoiesis and lung antiviral immunity. Elife 12, e85647. doi: 10.7554/eLife.85647
Jackson, S. W., Scharping, N. E., Kolhatkar, N. S., Khim, S., Schwartz, M. A., Li, Q. Z., et al. (2014). Opposing impact of B cell-intrinsic TLR7 and TLR9 signals on autoantibody repertoire and systemic inflammation. J. Immunol. 192, 4525–4532. doi: 10.4049/jimmunol.1400098
Jefferies, C. A. (2019). Regulating IRFs in IFN driven disease. Front. Immunol. 10. doi: 10.3389/fimmu.2019.00325
Jeong, H. Y., Park, J. S., Woo, J. S., Lee, K. H., Choi, J. W., Kang, H. Y., et al. (2023). SARS-CoV-2 spike protein accelerates systemic sclerosis by increasing inflammatory cytokines, Th17 cells, and fibrosis. J. Inflammation (Lond) 20, 46. doi: 10.1186/s12950-023-00362-x
Ji, L., Li, T., Chen, H., Yang, Y., Lu, E., Liu, J., et al. (2023). The crucial regulatory role of type I interferon in inflammatory diseases. Cell Biosci. 13, 230. doi: 10.1186/s13578-023-01188-z
Kawai, T. and Akira, S. (2008). Toll-like receptor and RIG-I-like receptor signaling. Ann. N Y Acad. Sci. 1143, 1–20. doi: 10.1196/annals.1443.020
Khalifa, A. E. and Ghoneim, A. I. (2021). Potential value of pharmacological agents acting on toll-like receptor (TLR) 7 and/or TLR8 in COVID-19. Curr. Res. Pharmacol. Drug Discov. 2, 100068. doi: 10.1016/j.crphar.2021.100068
Khanmohammadi, S. and Rezaei, N. (2021). Role of Toll-like receptors in the pathogenesis of COVID-19. J. Med. Virol. 93, 2735–2739. doi: 10.1002/jmv.26826
Khatun, O., Sharma, M., Narayan, R., and Tripathi, S. (2023). SARS-CoV-2 ORF6 protein targets TRIM25 for proteasomal degradation to diminish K63-linked RIG-I ubiquitination and type-I interferon induction. Cell Mol. Life Sci. 80, 364. doi: 10.1007/s00018-023-05011-3
Kim, T. H., Oh, D. S., Jung, H. E., Chang, J., and Lee, H. K. (2019). Plasmacytoid Dendritic Cells Contribute to the Production of IFN-β via TLR7-MyD88-Dependent Pathway and CTL Priming during Respiratory Syncytial Virus Infection. Viruses 11, 730. doi: 10.3390/v11080730
Klein, J., Wood, J., Jaycox, J. R., Dhodapkar, R. M., Lu, P., Gehlhausen, J. R., et al. (2023). Distinguishing features of long COVID identified through immune profiling. Nature 623, 139–148. doi: 10.1038/s41586-023-06651-y
Kosciuczuk, E. M., Mehrotra, S., Saleiro, D., Kroczynska, B., Majchrzak-Kita, B., Lisowski, P., et al. (2019). Sirtuin 2-mediated deacetylation of cyclin-dependent kinase 9 promotes STAT1 signaling in type I interferon responses. J. Biol. Chem. 294, 827–837. doi: 10.1074/jbc.RA118.005956
Kouwaki, T., Nishimura, T., Wang, G., and Oshiumi, H. (2021). RIG-I-like receptor-mediated recognition of viral genomic RNA of severe acute respiratory syndrome coronavirus-2 and viral escape from the host innate immune responses. Front. Immunol. 12. doi: 10.3389/fimmu.2021.700926
Lester, S. N. and Li, K. (2014). Toll-like receptors in antiviral innate immunity. J. Mol. Biol. 426, 1246–1264. doi: 10.1016/j.jmb.2013.11.024
Lipsky, P. E., Vollenhoven, R. V., Dörner, T., Werth, V. P., Merrill, J. T., Furie, R., et al. (2022). Biological impact of iberdomide in patients with active systemic lupus erythematosus. Ann. Rheum. Dis. 81, 1136–1142. doi: 10.1136/annrheumdis-2022-222212
Maiti, A. K. (2024). MDA5 is a major determinant of developing symptoms in critically ill COVID-19 patients. Clin. Rev. Allergy Immunol. 67, 58–72. doi: 10.1007/s12016-024-09008-z
Manik, M. and Singh, R. K. (2022). Role of toll-like receptors in modulation of cytokine storm signaling in SARS-CoV-2-induced COVID-19. J. Med. Virol. 94, 869–877. doi: 10.1002/jmv.27405
Mantovani, S., Daga, S., Fallerini, C., Baldassarri, M., Benetti, E., Picchiotti, N., et al. (2022). Rare variants in Toll-like receptor 7 results in functional impairment and downregulation of cytokine-mediated signaling in COVID-19 patients. Genes Immun. 23, 51–56. doi: 10.1038/s41435-021-00157-1
Maringer, Y., Nelde, A., Schroeder, S. M., Schuhmacher, J., Hörber, S., Peter, A., et al. (2022). Durable spike-specific T cell responses after different COVID-19 vaccination regimens are not further enhanced by booster vaccination. Sci. Immunol. 7, eadd3899. doi: 10.1126/sciimmunol.add3899
Martin, H. J., Lee, J. M., Walls, D., and Hayward, S. D. (2007). Manipulation of the toll-like receptor 7 signaling pathway by Epstein-Barr virus. J. Virol. 81, 9748–9758. doi: 10.1128/jvi.01122-07
Matuozzo, D., Talouarn, E., Marchal, A., Zhang, P., Manry, J., Seeleuthner, Y., et al. (2023). Rare predicted loss-of-function variants of type I IFN immunity genes are associated with life-threatening COVID-19. Genome Med. 15, 22. doi: 10.1186/s13073-023-01173-8
Menezes, M. C. S., Veiga, A. D. M., Martins de Lima, T., Kunimi Kubo Ariga, S., Vieira Barbeiro, H., de Lucena Moreira, C., et al. (2021). Lower peripheral blood Toll-like receptor 3 expression is associated with an unfavorable outcome in severe COVID-19 patients. Sci. Rep. 11, 15223. doi: 10.1038/s41598-021-94624-4
Mesev, E. V., Guare, E. G., Ploss, A., and Toettcher, J. E. (2023). Synthetic heterodimers of type III interferon receptors require TYK2 for STAT activation. J. Interferon Cytokine Res. 43, 414–426. doi: 10.1089/jir.2023.0039
Minkoff, J. M. and tenOever, B. (2023). Innate immune evasion strategies of SARS-CoV-2. Nat. Rev. Microbiol. 21, 178–194. doi: 10.1038/s41579-022-00839-1
Miquel, C. H., Abbas, F., Cenac, C., Foret-Lucas, C., Guo, C., Ducatez, M., et al. (2023). B cell-intrinsic TLR7 signaling is required for neutralizing antibody responses to SARS-CoV-2 and pathogen-like COVID-19 vaccines. Eur. J. Immunol. 53, e2350437. doi: 10.1002/eji.202350437
Mishra, G. P., Jha, A., Ahad, A., Sen, K., Sen, A., Podder, S., et al. (2022). Epigenomics of conventional type-I dendritic cells depicted preferential control of TLR9 versus TLR3 response by NCoR1 through differential IRF3 activation. Cell Mol. Life Sci. 79, 429. doi: 10.1007/s00018-022-04424-w
Moyon, Q., Sterlin, D., Miyara, M., Anna, F., Mathian, A., Lhote, R., et al. (2022). BNT162b2 vaccine-induced humoral and cellular responses against SARS-CoV-2 variants in systemic lupus erythematosus. Ann. Rheum. Dis. 81, 575–583. doi: 10.1136/annrheumdis-2021-221097
Naqvi, I., Giroux, N., Olson, L., Morrison, S. A., Llanga, T., Akinade, T. O., et al. (2022). DAMPs/PAMPs induce monocytic TLR activation and tolerance in COVID-19 patients; nucleic acid binding scavengers can counteract such TLR agonists. Biomaterials 283, 121393. doi: 10.1016/j.biomaterials.2022.121393
Naushad, S. M., Mandadapu, G., Ramaiah, M. J., Almajhdi, F. N., and Hussain, T. (2023). The role of TLR7 agonists in modulating COVID-19 severity in subjects with loss-of-function TLR7 variants. Sci. Rep. 13, 13078. doi: 10.1038/s41598-023-40114-8
Ne, E., Crespo, R., Izquierdo-Lara, R., Rao, S., Koçer, S., Górska, A., et al. (2022). Catchet-MS identifies IKZF1-targeting thalidomide analogues as novel HIV-1 latency reversal agents. Nucleic Acids Res. 50, 5577–5598. doi: 10.1093/nar/gkac407
Noor Eddin, A., Al-Rimawi, M., Peer-Zada, F., Hundallah, K., and Alhashem, A. (2023). Novel TLR7 hemizygous variant in post-COVID-19 neurological deterioration: a case report with literature review. Front. Neurol. 14. doi: 10.3389/fneur.2023.1268035
Paludan, S. R., Pradeu, T., Masters, S. L., and Mogensen, T. H. (2021). Constitutive immune mechanisms: mediators of host defence and immune regulation. Nat. Rev. Immunol. 21, 137–150. doi: 10.1038/s41577-020-0391-5
Park, A. and Iwasaki, A. (2020). Type I and type III interferons - induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe 27, 870–878. doi: 10.1016/j.chom.2020.05.008
Punnanitinont, A., Kasperek, E. M., Zhu, C., Yu, G., Miecznikowski, J. C., and Kramer, J. M. (2023). TLR7 activation of age-associated B cells mediates disease in a mouse model of primary Sjogren’s disease. J. Leukoc. Biol. 115, 497–510. doi: 10.1093/jleuko/qiad135
Qi, F., Wang, D., Liu, J., Zeng, S., Xu, L., Hu, H., et al. (2015). Respiratory macrophages and dendritic cells mediate respiratory syncytial virus-induced IL-33 production in TLR3- or TLR7-dependent manner. Int. Immunopharmacol. 29, 408–415. doi: 10.1016/j.intimp.2015.10.022
Qin, Z., Liu, F., Blair, R., Wang, C., Yang, H., Mudd, J., et al. (2021). Endothelial cell infection and dysfunction, immune activation in severe COVID-19. Theranostics 11, 8076–8091. doi: 10.7150/thno.61810
Riddler, S. A., Para, M., Benson, C. A., Mills, A., Ramgopal, M., DeJesus, E., et al. (2021). Vesatolimod, a toll-like receptor 7 agonist, induces immune activation in virally suppressed adults living with human immunodeficiency virus-1. Clin. Infect. Dis. 72, e815–e824. doi: 10.1093/cid/ciaa1534
Rodermund, L., Coker, H., Oldenkamp, R., Wei, G., Bowness, J., Rajkumar, B., et al. (2021). Time-resolved structured illumination microscopy reveals key principles of Xist RNA spreading. Science 372, eabe7500. doi: 10.1126/science.abe7500
Rossmann, L., Bagola, K., Stephen, T., Gerards, A. L., Walber, B., Ullrich, A., et al. (2021). Distinct single-component adjuvants steer human DC-mediated T-cell polarization via Toll-like receptor signaling toward a potent antiviral immune response. Proc. Natl. Acad. Sci. U.S.A. 118, e2103651118. doi: 10.1073/pnas.2103651118
Rümke, L. W., Smit, W. L., Bossink, A., Limonard, G. J. M., Muilwijk, D., Haas, L. E. M., et al. (2023). Impaired SARS-CoV-2 specific T-cell response in patients with severe COVID-19. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1046639
Sahanic, S., Hilbe, R., Dünser, C., Tymoszuk, P., Löffler-Ragg, J., Rieder, D., et al. (2023). SARS-CoV-2 activates the TLR4/MyD88 pathway in human macrophages: A possible correlation with strong pro-inflammatory responses in severe COVID-19. Heliyon 9, e21893. doi: 10.1016/j.heliyon.2023.e21893
Sakkas, L. I., Bogdanos, D. P., and Chikanza, I. C. (2025). Sex bias in systemic sclerosis: from clinical to immunological differences. Clin. Rev. Allergy Immunol. 68, 51. doi: 10.1007/s12016-025-09062-1
Salina, A. C. G., Dos-Santos, D., Rodrigues, T. S., Fortes-Rocha, M., Freitas-Filho, E. G., Alzamora-Terrel, D. L., et al. (2022). Efferocytosis of SARS-CoV-2-infected dying cells impairs macrophage anti-inflammatory functions and clearance of apoptotic cells. Elife 11, e74443. doi: 10.7554/eLife.74443
Salvi, V., Nguyen, H. O., Sozio, F., Schioppa, T., Gaudenzi, C., Laffranchi, M., et al. (2021). SARS-CoV-2-associated ssRNAs activate inflammation and immunity via TLR7/8. JCI Insight 6, e150542. doi: 10.1172/jci.insight.150542
Sameer, A. S. and Nissar, S. (2021). Toll-like receptors (TLRs): structure, functions, signaling, and role of their polymorphisms in colorectal cancer susceptibility. BioMed. Res. Int. 2021, 1157023. doi: 10.1155/2021/1157023
Satterthwaite, A. B. (2021). TLR7 signaling in lupus B cells: new insights into synergizing factors and downstream signals. Curr. Rheumatol. Rep. 23, 80. doi: 10.1007/s11926-021-01047-1
Schreibing, F., Hannani, M. T., Kim, H., Nagai, J. S., Ticconi, F., Fewings, E., et al. (2022). Dissecting CD8+ T cell pathology of severe SARS-CoV-2 infection by single-cell immunoprofiling. Front. Immunol. 13. doi: 10.3389/fimmu.2022.1066176
Scofield, R. H., Wren, J. D., and Lewis, V. M. (2025). The toll like receptor 7 pathway and the sex bias of systemic lupus erythematosus. Front. Immunol. 16. doi: 10.3389/fimmu.2025.1479814
Sebastião, A. I., Mateus, D., Carrascal, M. A., Sousa, C., Cortes, L., Bachmann, M. F., et al. (2023). CuMV VLPs containing the RBM from SARS-coV-2 spike protein drive dendritic cell activation and th1 polarization. Pharmaceutics 15, 825. doi: 10.3390/pharmaceutics15030825
SenGupta, D., Brinson, C., DeJesus, E., Mills, A., Shalit, P., Guo, S., et al. (2021). The TLR7 agonist vesatolimod induced a modest delay in viral rebound in HIV controllers after cessation of antiretroviral therapy. Sci. Transl. Med. 13, eabg3071. doi: 10.1126/scitranslmed.abg3071
Sha, S., Pearson, J. A., Peng, J., Hu, Y., Huang, J., Xing, Y., et al. (2021). TLR9 deficiency in B cells promotes immune tolerance via interleukin-10 in a type 1 diabetes mouse model. Diabetes 70, 504–515. doi: 10.2337/db20-0373
Shi, Y., Xu, H., Xiao, Y., Liu, P., Pang, P., Wu, S., et al. (2020). Gegen qinlian decoction downregulates the TLR7 signalling pathway to control influenza A virus infection. BioMed. Pharmacother. 121, 109471. doi: 10.1016/j.biopha.2019.109471
Skaug, B. and Chen, Z. J. (2010). Emerging role of ISG15 in antiviral immunity. Cell 143, 187–190. doi: 10.1016/j.cell.2010.09.033
Solanich, X., Vargas-Parra, G., van der Made, C. I., Simons, A., Schuurs-Hoeijmakers, J., Antolí, A., et al. (2021). Genetic screening for TLR7 variants in young and previously healthy men with severe COVID-19. Front. Immunol. 12. doi: 10.3389/fimmu.2021.719115
Solimani, F., Meier, K., and Ghoreschi, K. (2021). Janus kinase signaling as risk factor and therapeutic target for severe SARS-CoV-2 infection. Eur. J. Immunol. 51, 1071–1075. doi: 10.1002/eji.202149173
Song, L., Wang, D., Abbas, G., Li, M., Cui, M., Wang, J., et al. (2023). The main protease of SARS-CoV-2 cleaves histone deacetylases and DCP1A, attenuating the immune defense of the interferon-stimulated genes. J. Biol. Chem. 299, 102990. doi: 10.1016/j.jbc.2023.102990
Soni, C., Wong, E. B., Domeier, P. P., Khan, T. N., Satoh, T., Akira, S., et al. (2014). B cell-intrinsic TLR7 signaling is essential for the development of spontaneous germinal centers. J. Immunol. 193, 4400–4414. doi: 10.4049/jimmunol.1401720
Spiering, A. E. and de Vries, T. J. (2021). Why females do better: the X chromosomal TLR7 gene-dose effect in COVID-19. Front. Immunol. 12. doi: 10.3389/fimmu.2021.756262
Swain, N., Tripathy, A., Padhan, P., Raghav, S. K., and Gupta, B. (2022). Toll-like receptor-7 activation in CD8+ T cells modulates inflammatory mediators in patients with rheumatoid arthritis. Rheumatol. Int. 42, 1235–1245. doi: 10.1007/s00296-021-05050-8
Unali, G., Crivicich, G., Pagani, I., Abou-Alezz, M., Folchini, F., Valeri, E., et al. (2023). Interferon-inducible phospholipids govern IFITM3-dependent endosomal antiviral immunity. EMBO J. 42, e112234. doi: 10.15252/embj.2022112234
Usero, L., Leal, L., Gómez, C. E., Miralles, L., Aurrecoechea, E., Esteban, I., et al. (2023). The combination of an mRNA immunogen, a TLR7 agonist and a PD1 blocking agent enhances in-vitro HIV T-cell immune responses. Vaccines (Basel) 11, 286. doi: 10.3390/vaccines11020286
Vadakedath, S., Kandi, V., Mohapatra, R. K., Pinnelli, V. B. K., Yegurla, R. R., Shahapur, P. R., et al. (2021). Immunological aspects and gender bias during respiratory viral infections including novel Coronavirus disease-19 (COVID-19): A scoping review. J. Med. Virol. 93, 5295–5309. doi: 10.1002/jmv.27081
Valente, R. M., Ehlers, E., Xu, D., Ahmad, H., Steadman, A., Blasnitz, L., et al. (2012). Toll-like receptor 7 stimulates the expression of Epstein-Barr virus latent membrane protein 1. PloS One 7, e43317. doi: 10.1371/journal.pone.0043317
van der Made, C. I., Simons, A., Schuurs-Hoeijmakers, J., van den Heuvel, G., Mantere, T., Kersten, S., et al. (2020). Presence of genetic variants among young men with severe COVID-19. Jama 324, 663–673. doi: 10.1001/jama.2020.13719
van der Sluis, R. M., Cham, L. B., Gris-Oliver, A., Gammelgaard, K. R., Pedersen, J. G., Idorn, M., et al. (2022). TLR2 and TLR7 mediate distinct immunopathological and antiviral plasmacytoid dendritic cell responses to SARS-CoV-2 infection. EMBO J. 41, e109622. doi: 10.15252/embj.2021109622
Van Oekelen, O., Amatangelo, M., Guo, M., Upadhyaya, B., Cribbs, A. P., Kelly, G., et al. (2024). Iberdomide increases innate and adaptive immune cell subsets in the bone marrow of patients with relapsed/refractory multiple myeloma. Cell Rep. Med. 5, 101584. doi: 10.1016/j.xcrm.2024.101584
Vazquez-Alejo, E., Tarancon-Diez, L., Espinar-Buitrago, M. S., Genebat, M., Calderon, A., Perez-Cabeza, G., et al. (2023). Persistent exhausted T-cell immunity after severe COVID-19: 6-month evaluation in a prospective observational study. J. Clin. Med. 12, 3539. doi: 10.3390/jcm12103539
Vikse, J., Lippi, G., and Henry, B. M. (2020). Do sex-specific immunobiological factors and differences in angiotensin converting enzyme 2 (ACE2) expression explain increased severity and mortality of COVID-19 in males? Diagnosis (Berl) 7, 385–386. doi: 10.1515/dx-2020-0054
Vlachiotis, S. and Andreakos, E. (2019). Lambda interferons in immunity and autoimmunity. J. Autoimmun. 104, 102319. doi: 10.1016/j.jaut.2019.102319
Wiech, M., Chroscicki, P., Swatler, J., Stepnik, D., De Biasi, S., Hampel, M., et al. (2022). Remodeling of T cell dynamics during long COVID is dependent on severity of SARS-coV-2 infection. Front. Immunol. 13. doi: 10.3389/fimmu.2022.886431
Woodruff, M. C., Ramonell, R. P., Haddad, N. S., Anam, F. A., Rudolph, M. E., Walker, T. A., et al. (2022). Dysregulated naive B cells and de novo autoreactivity in severe COVID-19. Nature 611, 139–147. doi: 10.1038/s41586-022-05273-0
Wu, Y., Ma, L., Zhuang, Z., Cai, S., Zhao, Z., Zhou, L., et al. (2020). Main protease of SARS-CoV-2 serves as a bifunctional molecule in restricting type I interferon antiviral signaling. Signal Transduct Target Ther. 5, 221. doi: 10.1038/s41392-020-00332-2
Wu, Y., Qin, J., Gu, Y., Zhao, G., Xu, P., Lin, S., et al. (2024). Radioresponsive delivery of toll-like receptor 7/8 agonist for tumor radioimmunotherapy enabled by core-cross-linked diselenide nanoparticles. ACS Nano 18, 2800–2814. doi: 10.1021/acsnano.3c05882
Wu, D. and Yang, X. O. (2021). Dysregulation of pulmonary responses in severe COVID-19. Viruses 13, 957. doi: 10.3390/v13060957
Xia, H., Cao, Z., Xie, X., Zhang, X., Chen, J. Y., Wang, H., et al. (2020). Evasion of type I interferon by SARS-coV-2. Cell Rep. 33, 108234. doi: 10.1016/j.celrep.2020.108234
Yadav, S., Varma, A., Muralidharan, A. O., Bhowmick, S., Mondal, S., and Mallick, A. I. (2023). The immune-adjunctive potential of recombinant LAB vector expressing murine IFNlambda3 (MuIFNlambda3) against Type A Influenza Virus (IAV) infection. Gut Pathog. 15, 53. doi: 10.1186/s13099-023-00578-5
Yamada, T., Sato, S., Sotoyama, Y., Orba, Y., Sawa, H., Yamauchi, H., et al. (2021). RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells. Nat. Immunol. 22, 820–828. doi: 10.1038/s41590-021-00942-0
Yan, W., Yu, W., Shen, L., Xiao, L., Qi, J., and Hu, T. (2023). A SARS-CoV-2 nanoparticle vaccine based on chemical conjugation of loxoribine and SpyCatcher/SpyTag. Int. J. Biol. Macromol. 253, 127159. doi: 10.1016/j.ijbiomac.2023.127159
Yang, D., Chan, J. F., Yoon, C., Luk, T. Y., Shuai, H., Hou, Y., et al. (2024a). Type-II IFN inhibits SARS-CoV-2 replication in human lung epithelial cells and ex vivo human lung tissues through indoleamine 2,3-dioxygenase-mediated pathways. J. Med. Virol. 96, e29472. doi: 10.1002/jmv.29472
Yang, X., Huang, J., Peng, J., Wang, P., Wong, F. S., Wang, R., et al. (2024b). Gut microbiota from B-cell-specific TLR9-deficient NOD mice promote IL-10(+) Breg cells and protect against T1D. Front. Immunol. 15. doi: 10.3389/fimmu.2024.1413177
Yao, T., Foo, C., Zheng, G., Huang, R., Li, Q., Shen, J., et al. (2023). Insight into the mechanisms of coronaviruses evading host innate immunity. Biochim. Biophys. Acta Mol. Basis Dis. 1869, 166671. doi: 10.1016/j.bbadis.2023.166671
Yin, Q., Luo, W., Mallajosyula, V., Bo, Y., Guo, J., Xie, J., et al. (2023). A TLR7-nanoparticle adjuvant promotes a broad immune response against heterologous strains of influenza and SARS-CoV-2. Nat. Mater. 22, 380–390. doi: 10.1038/s41563-022-01464-2
Yin, K., Peluso, M. J., Luo, X., Thomas, R., Shin, M. G., Neidleman, J., et al. (2024). Long COVID manifests with T cell dysregulation, inflammation and an uncoordinated adaptive immune response to SARS-CoV-2. Nat. Immunol. 25, 218–225. doi: 10.1038/s41590-023-01724-6
Yin, X., Riva, L., Pu, Y., Martin-Sancho, L., Kanamune, J., Yamamoto, Y., et al. (2021). MDA5 governs the innate immune response to SARS-coV-2 in lung epithelial cells. Cell Rep. 34, 108628. doi: 10.1016/j.celrep.2020.108628
Zayed, M., Kim, Y.-C., Lee, C.-S., and Jeong, B.-H. (2023). No association between SARS-coV-2 infection and the polymorphism of the toll-like receptor 7 (TLR7) gene in female population. Diagnostics 13, 3510. doi: 10.3390/diagnostics13233510
Zhang, T., Magazine, N., McGee, M. C., Carossino, M., Veggiani, G., Kousoulas, K. G., et al. (2024). Th2 and Th17-Associated Immunopathology Following SARS-CoV-2 Breakthrough Infection in Spike-Vaccinated ACE2-humanized Mice. J. Med. Virol. 96, e29408. doi: 10.1002/jmv.29408
Zheng, K., Wu, S. Z., Lv, Y. W., Pang, P., Deng, L., Xu, H. C., et al. (2021). Carvacrol inhibits the excessive immune response induced by influenza virus A via suppressing viral replication and TLR/RLR pattern recognition. J. Ethnopharmacol. 268, 113555. doi: 10.1016/j.jep.2020.113555
Zheng, Y., Zhuang, M. W., Han, L., Zhang, J., Nan, M. L., Zhan, P., et al. (2020). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) membrane (M) protein inhibits type I and III interferon production by targeting RIG-I/MDA-5 signaling. Signal Transduct Target Ther. 5, 299. doi: 10.1038/s41392-020-00438-7
Zhou, Q., Chen, V., Shannon, C. P., Wei, X. S., Xiang, X., Wang, X., et al. (2020). Interferon-alpha2b treatment for COVID-19. Front. Immunol. 11. doi: 10.3389/fimmu.2020.01061
Zhu, N., Zhang, D., Wang, W., Li, X., Yang, B., Song, J., et al. (2020). A novel coronavirus from patients with pneumonia in China 2019. N. Engl. J. Med. 382, 727–733. doi: 10.1056/NEJMoa2001017
Znaidia, M., Demeret, C., van der Werf, S., and Komarova, A. V. (2022). Characterization of SARS-coV-2 evasion: interferon pathway and therapeutic options. Viruses 14, 1247. doi: 10.3390/v14061247
Zollner, A., Watschinger, C., Rössler, A., Farcet, M. R., Penner, A., Böhm, V., et al. (2021). B and T cell response to SARS-CoV-2 vaccination in health care professionals with and without previous COVID-19. EBioMedicine 70, 103539. doi: 10.1016/j.ebiom.2021.103539
Keywords: toll-like Receptor 7, interferon, SARS-CoV-2, T cell immunity, B cell immunity, post-acute sequelae of SARS-CoV-2 infection
Citation: Xue J, Wang X, Wang H, Qiao B, Gao P, Ren B and Yan S (2025) Unraveled role of TLR7-mediated interferon signaling activation in COVID-19. Front. Cell. Infect. Microbiol. 15:1658249. doi: 10.3389/fcimb.2025.1658249
Received: 02 July 2025; Accepted: 23 September 2025;
Published: 08 October 2025.
Edited by:
Chiara Medaglia, Lab Manager of the Functional Genomics Research Center at the Fondazione Human Technopole, ItalyReviewed by:
Remo Castro Russo, Federal University of Minas Gerais, BrazilRoshan Ghimire, Oklahoma State University, United States
Copyright © 2025 Xue, Wang, Wang, Qiao, Gao, Ren and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shushan Yan, Znl5YW5zc0BzZHNtdS5lZHUuY24=; Bin Ren, cmVuYmluMDUzNkBzaW5hLmNvbQ==
†These authors have contributed equally to this work
 Junjian Xue
Junjian Xue Xiaoyin Wang1†
Xiaoyin Wang1† Shushan Yan
Shushan Yan