- 1Division of Cardiology, Department of Internal Medicine, Tongji Medical College, Tongji Hospital, Huazhong University of Science and Technology, Wuhan, China
- 2Hubei Key Laboratory of Genetics and Molecular Mechanisms of Cardiological Disorders, Wuhan, China
- 3Division of Cardiology, Panzhihua Central Hospital, Panzhihua, China
Background: Common variants may contribute to the variation of prognosis of heart failure (HF) among individual patients, but no systematical analysis was conducted using transcriptomic and whole exome sequencing (WES) data. We aimed to construct a genetic risk score (GRS) and estimate its potential as a predictive tool for HF-related mortality risk alone and in combination with traditional risk factors (TRFs).
Methods and Results: We reanalyzed the transcriptomic data of 177 failing hearts and 136 healthy donors. Differentially expressed genes (fold change >1.5 or <0.68 and adjusted P < 0.05) were selected for prognosis analysis using our whole exome sequencing and follow-up data with 998 HF patients. Statistically significant variants in these genes were prepared for GRS construction. Traditional risk variables were in combination with GRS for the construct of the composite risk score. Kaplan–Meier curves and receiver operating characteristic (ROC) analysis were used to assess the effect of GRS and the composite risk score on the prognosis of HF and discriminant power, respectively. We found 157 upregulated and 173 downregulated genes. In these genes, 31 variants that were associated with the prognosis of HF were finally identified to develop GRS. Compared with individuals with low risk score, patients with medium- and high-risk score showed 2.78 (95%CI = 1.82–4.24, P = 2 × 10−6) and 6.54 (95%CI = 4.42–9.71, P = 6 × 10−21) -fold mortality risk, respectively. The composite risk score combining GRS and TRF predicted mortality risk with an HR = 5.41 (95% CI = 2.72–10.64, P = 1 × 10−6) for medium vs. low risk and HR = 22.72 (95% CI = 11.9–43.48, P = 5 × 10−21) for high vs. low risk. The discriminant power of GRS is excellent with a C statistic of 0.739, which is comparable to that of TRF (C statistic = 0.791). The combination of GRS and TRF could significantly increase the predictive ability (C statistic = 0.853).
Conclusions: The 31-SNP GRS could well distinguish those HF patients with poor prognosis from those with better prognosis and provide clinician with reference for the intensive therapy, especially when combined with TRF.
Clinical Trial Registration: https://www.clinicaltrials.gov/, identifier: NCT03461107.
Introduction
Heart failure (HF) is the final pathway of many cardiovascular problems with high morbidity and mortality (1, 2). Along with growing aging population and HF-related risk factors (e.g., hypertension, obesity, diabetes), the incidence and prevalence of HF have continuously increased (3–5). Despite effective drug treatment including β-blockers and inhibitors of the renin-angiotensin-aldosterone system, the prognosis of HF has still remained unoptimistic (4, 6).
The clinical course and prognosis of HF patients showed significantly variable among different subgroups of patients (5, 7). In view of this, a substantial amount of studies were carried out to develop the prognostic multivariable models for mortality risk stratification of HF (5, 8–12). There have been three validated and commonly used scores in chronic HF including the MECKI score, the Seattle HF Risk Model, and the MAGGIC Risk score (13–15). In these models, plenty of variables such as baseline characteristics, medical history, demographics physical exam, laboratory values, and biological markers were taken into account to develop the risk score (11, 16). Importantly, they all displayed an excellent discrimination with C statistic beyond 0.7 and could provide an accurate prediction for survival of HF (9, 13, 17). However, all these models only paid attention to conventional risk factors and ignored the importance of genetic factors in the progression of HF (1, 2). A growing body of evidence has demonstrated that hereditary factor played a vital role in the prognosis of HF (18–21). But these investigations just focused on a single variant, most of which had only modest or small effect on the mortality risk prediction of HF. Thus, it is essential to evaluate the cumulative effects of multiple loci on the mortality risk of HF and develop an HF-related genetic risk score (GRS), which could combine with traditional risk factors for the assessment of the composite risk score.
Therefore, we aim to construct a GRS for the prognosis of HF and evaluate a composite risk score comprised of both GRS and traditional risk factors in its ability to predict the mortality risk of HF.
Methods
Study Subjects for Whole Exome Sequencing
The study protocol conforms to the ethical guidelines of the 1,975 Declaration of Helsinki as reflected in the a priori approval by the Review Board of Tongji College of Medicine. Written informed consents were obtained from all patients before enrollment. This study is based on data from two previous studies (22, 23). Details about HF population, whole exome sequencing (WES), and bioinformatics workflow, data processing, and quality control have been described previously (22). Among our population, there are 704 patients with an LVEF value <40%, 160 patients with an LVEF value = 40–49%, and 134 patients with LVEF > 50%. The diagnosis and exclusion criteria of chronic HF have been described previously in detail (19). The composite of heart transplantation and cardiovascular death were defined as the primary end points.
Transcriptomic Analysis and Gene Selection
Cordero et al. have conducted RNA-sequencing of 177 failing hearts and 136 healthy donor controls (23). Related data are available in GEO (accession number GSE57338). As we all know, differentially expressed genes are more likely to play a vital role in the process of HF. So we used GEO2R to compare HF and control groups to identify genes that are differentially expressed across experimental conditions. Genes with fold change (FC)>1.5 or <0.68 and adjusted P < 0.05 [adjusted by FDR (false discovery rate)] were selected as candidate genes for further analysis, which could also reduce the chance of overfitting the prediction model compared with involving all genes.
Genetic Risk Score
Common single nucleotide polymorphisms (SNPs) with minor allele frequency (MAF)>0.05 in the candidate genes were extracted from our WES data. Kaplan–Meier curves were performed to evaluate the effect of above common SNPs on the prognosis of HF. Statistically significant variants were further analyzed using Cox proportional hazard to assess hazard ratios (HRs) with 95% confidence intervals (CI) for each SNP. Variants in strong linkage disequilibrium (LD) with each other (r2 > 0.9) were analyzed using our WES data, and only one SNP was selected as tagged SNP for the construction of GRS. Genotypes with higher mortality risk for HF were given a weighted score of 1* hazard ratio (HR), while the rest were given a weighted score of 1. For each patient, the sum of the weighted scores from above SNPs were calculated and used to predict major clinical events-free survival.
Composite Risk Score Construction
All traditional HF mortality-related variables were entered into multivariable Cox proportional hazards models together with the GRS to evaluate its independent relationship to the mortality risk of HF. The GRS was divided into thirds, and groups of low, moderate, and high risk were created with subjects in the low genetic risk of GRS as the reference. Similarly, all the continuous variables were divided into thirds and into groups of low, moderate, and high risk. The corresponding beta coefficients for each variable were then used to create a weighted composite score consisting of those variables showing a significant association with the prognosis of HF. The beta coefficients from each category were used for the continuous variables categorized. The composite risk score was divided into thirds and further into groups of low, moderate, and high risk and then analyzed using Cox proportional hazards models.
Statistical Analysis
Statistical analyses were performed with Statistical Package for the Social Sciences (SPSS), version 13.0, and R version 3.5.0. Data were presented as mean ± standard deviation (SD) for continuous variables and median [interquartile range (IQR)] or numbers (percentages) for categorical or dichotomous variables. Linkage disequilibrium was calculated using Haploview version 4.1. Kaplan–Meier curves and the Cox proportional hazards regression model were used to assess the association of GRS and the composite risk score with the prognosis of HF. Statistical significance were compared by either unpaired or paired, two-tailed Student's t-test or one-way ANOVA followed by Bonferroni's post-hoc test, where appropriate.
Traditional risk factors for mortality risk of HF were defined as age, gender, hypertension, diabetes, smoking, LVEF, hemoglobin, NT-proBNP (logarithmic transformation of NT-proBNP is used in order to minimize the effect of extreme values), serum creatinine, potassium, sodium, systolic blood pressure, and diastolic blood pressure. Receiver operating characteristic (ROC) curve analysis with MedCalc 11.5 (http://www.medcalc.be/) was performed to compare the discriminant power of traditional risk factors, GRS, and the composite risk score. All comparisons were two-sided, and P < 0.05 was considered as significant.
Results
Subjects Characteristics
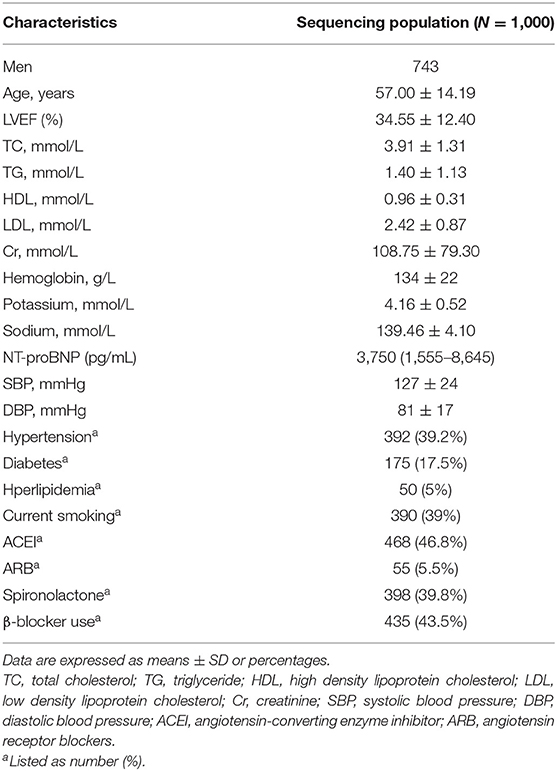
A total of 1,000 chronic HF patients (787 patients with dilated cardiomyopathy and 213 patients with ischemic cardiomyopathy) were recruited, in which we completed the follow-up with 998 patients finally. During the follow-up, 260 primary endpoint events occurred. Detailed characteristics of the participants are listed in Table 1.
Differential Gene Expression Analysis
Through analyzing the transcriptomic data from GEO (accession number GSE57338), we found 157 upregulated and 173 downregulated genes with adjusted P < 0.05 when the threshold of FC was set at >1.5 and <0.68 (Supplementary Table 1). The FDR (false discovery rate), which could reduce the false positive rate, was used for the adjustment of the p-value. The overview of the comparison of the differential gene expression between HF and control groups is shown in Figure 1.
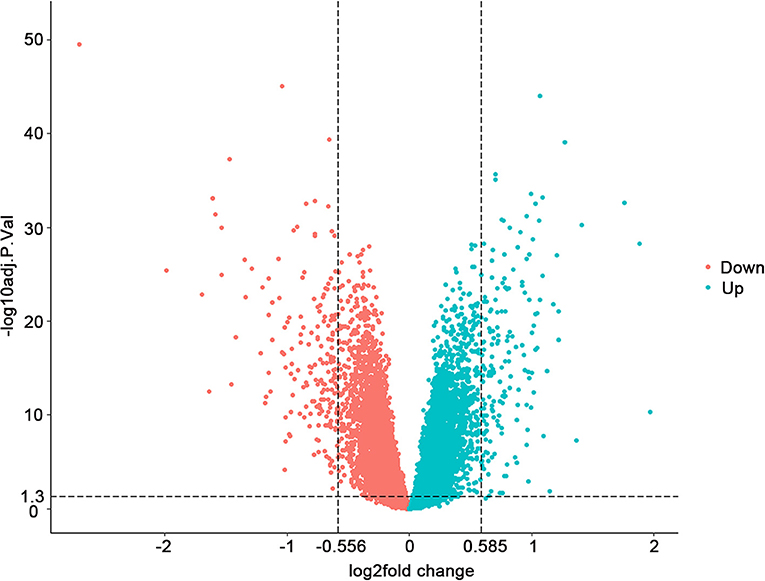
Figure 1. Differential gene expression between 177 failing hearts and 136 healthy donor controls. Volcano plots depicting the extent (x-axis) and significance (y-axis) of differential gene expression between failing and healthy heart samples. Fold change represents failing vs. control hearts.
SNP Prognosis Analysis
A total of 582 common SNPs in the above selected 330 differential expression genes were found from our WES data. Subsequently, we performed Kaplan–Meier curve analysis for 582 variants using our follow-up data. A total of 37 and 6 SNPs were associated with the prognosis of HF in the dominant (Supplementary Table 2) and recessive models (Supplementary Table 3), respectively. Given that rs420137, rs436743, rs370434, rs420054, rs404435, rs3003174, rs402388, rs2501176, and rs2932988 were in strong LD (r2 > 0.9) with each other, we selected rs420137 as the tagged SNP for further GRS development. Similarly, rs741143, rs3210140, and rs653521 were, respectively, chosen as tagged SNPs for their LD with other SNPs (Supplementary Figure 1). Although rs2297224 showed statistical significance in both the dominant and recessive models, we regarded it as a recessive model since it has a smaller P-value and higher HR. Finally, 27 SNPs in the dominant model (Table 2) and 4 SNPs in the recessive model (Table 3) were prepared to develop the GRS.
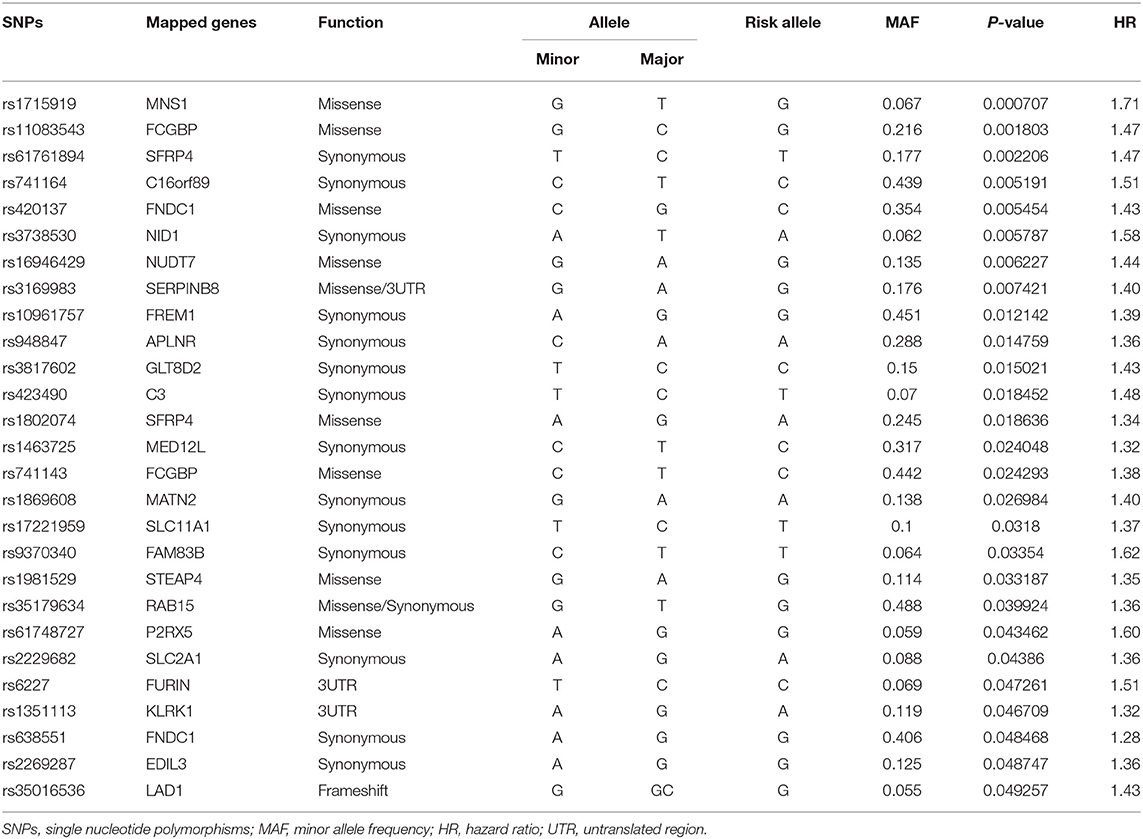
Table 2. Statistically significant variants using Cox proportional hazard analysis in dominant model.
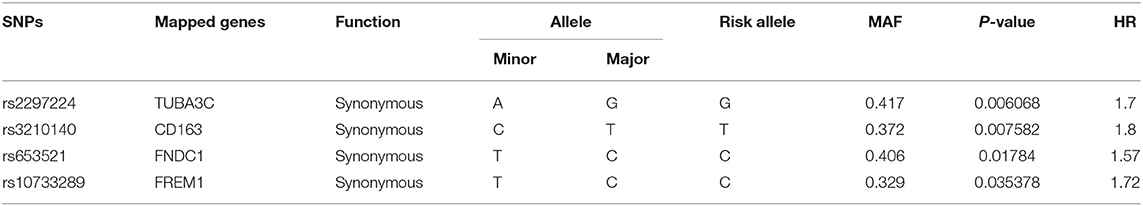
Table 3. Statistically significant variants using Cox proportional hazard analysis in recessive model.
GRS
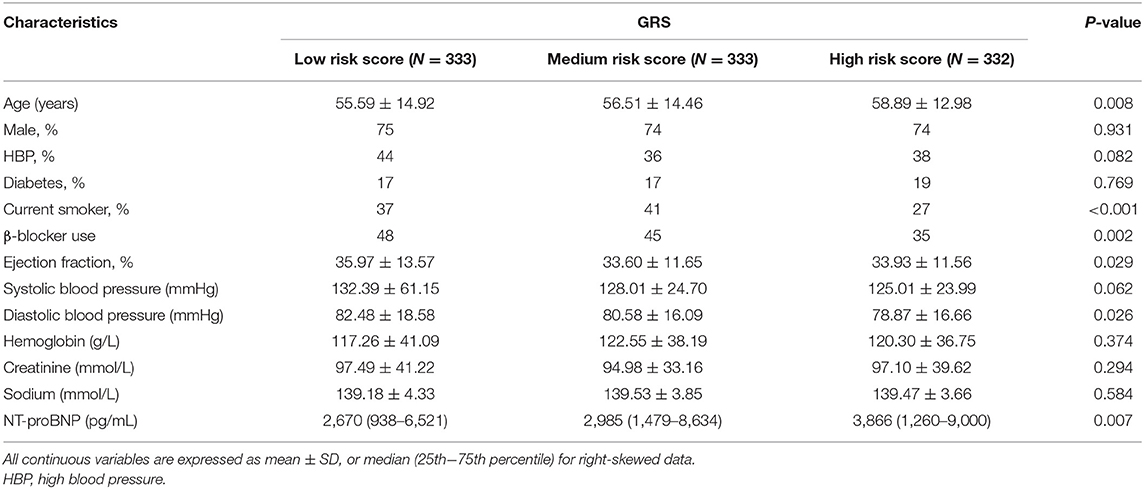
To evaluate the cumulative effects of above 31 SNPs, GRS for each individual was calculated. As shown in Figure 2, the GRS conformed to a bell-shaped distribution, ranging from 34.82 to 42.23 points with a median value of 38.78. We divided the scores into thirds of low (34.82–38.20), medium (38.21–39.26), and high (39.27–42.23) risk from the overall GRS. These accounted for 33.4, 33.4, and 33.2% of chronic HF patients and 11.5, 29.1, and 59.4% of primary endpoint events, respectively. The baseline characteristics of the participants in the low-, medium-, and high-risk groups are listed in Table 4.
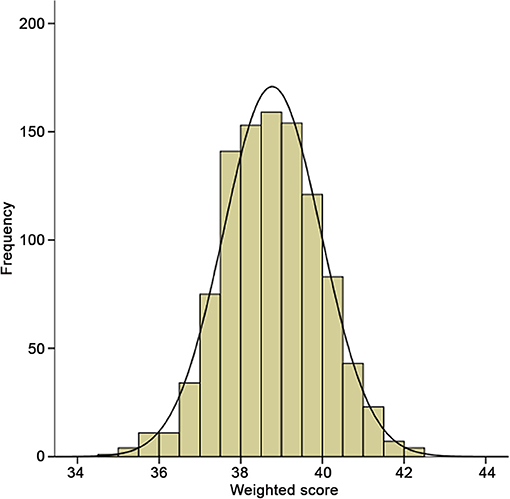
Figure 2. Distribution of integer risk score for all 998 HF patients. The distribution shows a nearly bell-shaped curve, ranging from 34.82 to 42.23 points with a median value of 38.78.
Furthermore, we conducted prognosis analysis using the Cox proportional hazards regression model. As shown in Figure 3A, compared with the low-risk group, medium- and high-risk groups were associated with poorer prognosis of HF (HR = 2.78, 95% CI = 1.82–4.24, P = 2 × 10−6 for medium vs. low risk group; HR = 6.54, 95% CI = 4.42–9.71, P = 6 × 10−21 for high vs. low risk group) (Table 5). The statistical significance in multivariate analysis remained after adjusting for traditional risk factors including age, gender, hypertension, diabetes, hyperlipidemia, smoking status, and β-blocker use (HR = 2.38, 95% CI = 1.55–3.66, P = 7 × 10−5 for medium vs. low risk group; HR = 5.43, 95% CI = 3.65–8.06, P = 6 × 10−17 for high vs. low risk group) (Table 5).
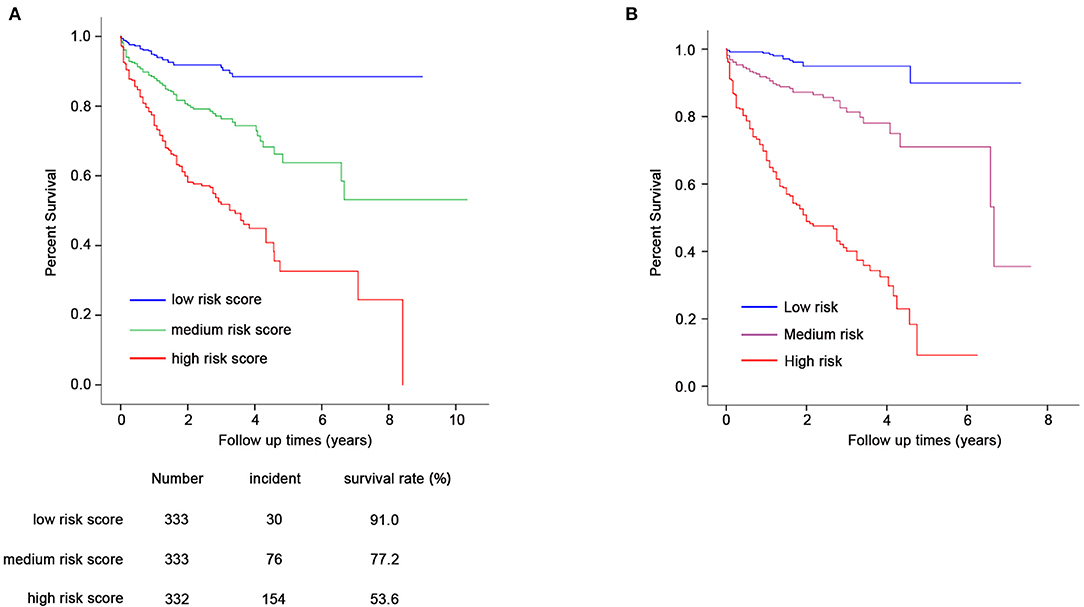
Figure 3. Prognostic analysis for GRS and composite risk score (A,B). Cox proportional hazards regression model was used for prognosis analysis. (A) Compared with the low-risk group (N = 333), medium (N = 333), and high-risk groups (N = 332) showed increased HF-related mortality risk (HR = 2.78, 95% CI = 1.82–4.24, P = 2 × 10−6 for medium- vs. low-risk group; HR = 6.54, 95% CI = 4.42–9.71, P = 6 × 10−21 for high- vs. low-risk group). The statistical significance remains after adjustment for age, gender, hypertension, diabetes, hyperlipidemia, smoking status, and β-blocker use. (B) Composite risk score with medium and high risk showed significantly increased mortality risk of HF (HR = 5.41, 95% CI = 2.72–10.64, P = 1 × 10−6 for medium vs. low risk; HR = 22.72, 95% CI = 11.90–43.48, P = 5 × 10−21 for high vs. low risk).
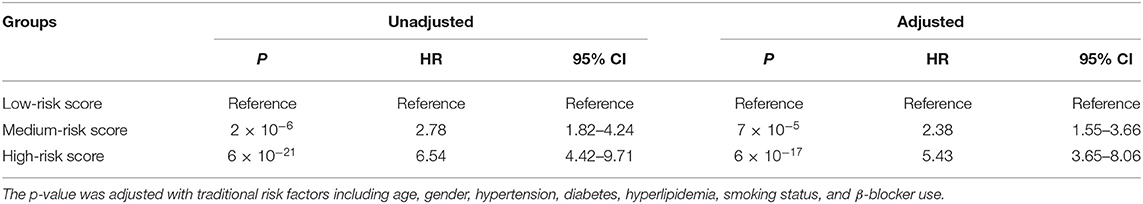
Table 5. Prognosis analysis for groups with different risk score using Cox proportional hazards regression model.
Composite Risk Score
Traditional risk variables were in combination with GRS for the evaluation of the composite effect. After multivariable Cox proportional hazards analysis with all HF mortality-related traditional risk factors and GRS, there remained 10 variables that showed significant association with the prognosis of HF (Table 6). As shown in Table 6, all continuous and categorical variables have respective beta coefficients, which were weighted for composite risk score construction. The low, medium, and high risk of the composite risk score accounted for 5.1, 23.9, and 71.0% of primary endpoint events, respectively.
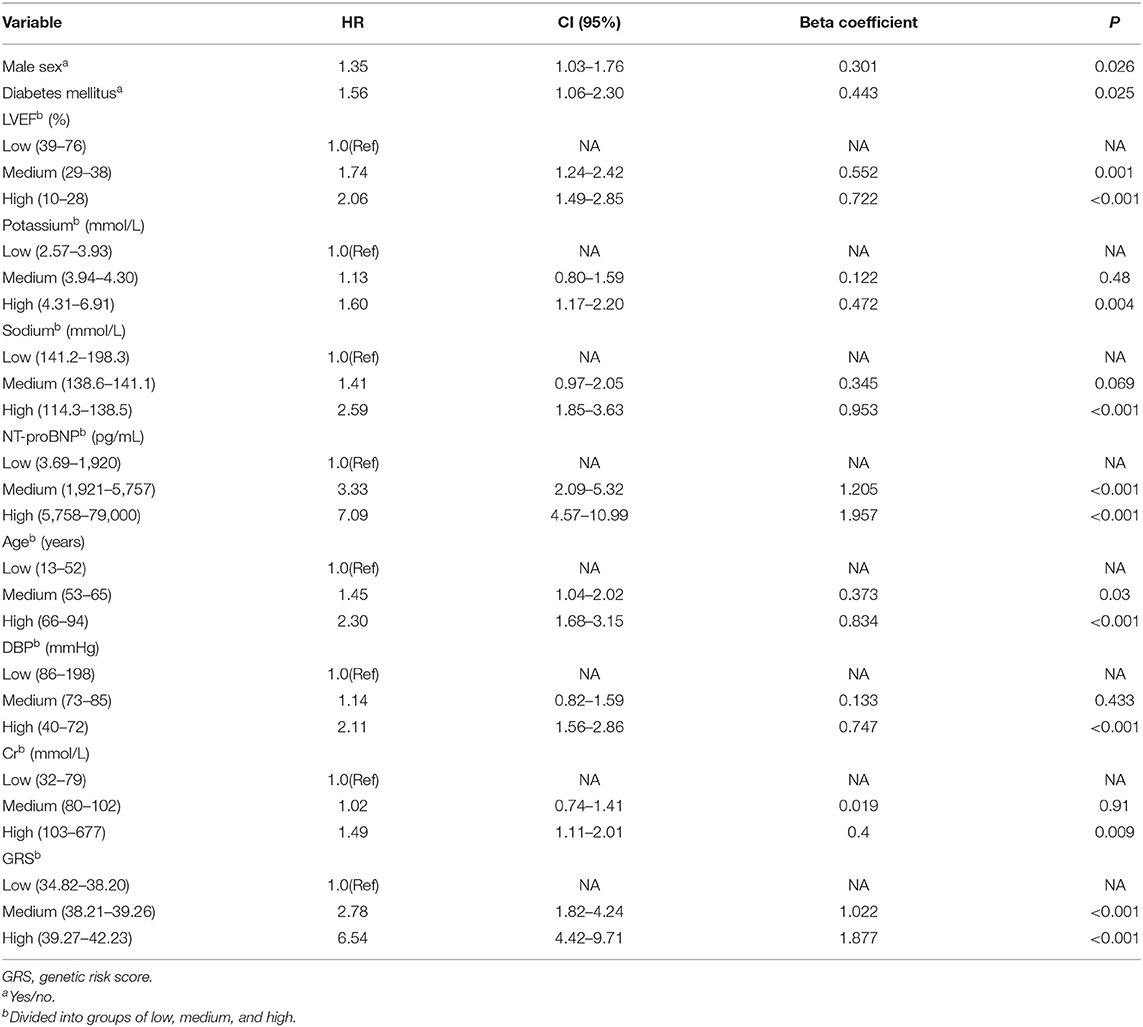
Table 6. Cox regression analysis of association between HF-related mortality risk and continuous variables categorized into groups of low, medium, and high.
Prognostic analysis using the Cox proportional hazards regression model showed that the composite risk scores with medium and high risk were significantly associated with increased mortality risk of HF when compared with low risk (HR = 5.41, 95% CI = 2.72–10.64, P = 1 × 10−6 for medium vs. low risk; HR = 22.72, 95% CI = 11.90–43.48, P = 5 × 10−21 for high vs. low risk) (Table 7, Figure 3B).
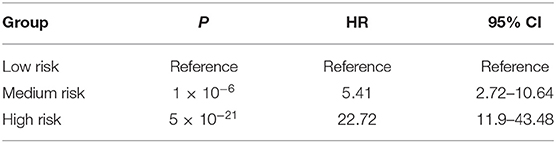
Table 7. Prognostic analysis for composite risk score using Cox proportional hazards regression model.
Discriminative Power Analysis
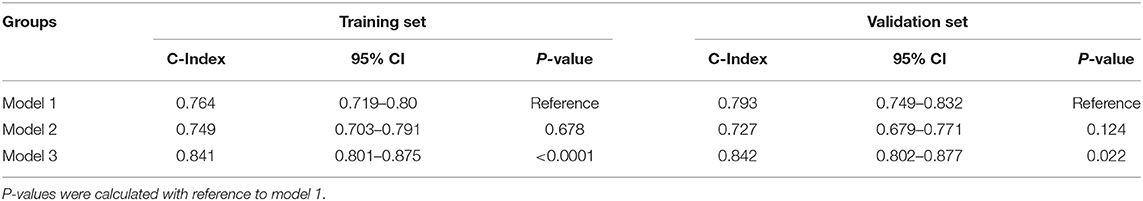
We assessed the discriminative power of the three models: model 1, nine traditional risk factors (TRFs) only; model 2, GRS; model 3, composite risk score. The average AUCs for models 1, 2, and 3 were 0.791 (95% CI = 0.761–0.819), 0.739 (95% CI = 0.707–0.770), and 0.853 (95% CI = 0.826–0.877), respectively. Their true prediction rates reached up to 79.3, 75.4, and 83.5%, respectively. The ROC curves for the three models are shown in Figure 4A. There was no statistically significant difference between models 1 and 2 (P = 0.06). However, the composite risk score could significantly improve the discriminative power when compared with TRF or GRS alone (P < 0.0001 for model 3 vs. model 1; and P < 0.0001 for model 3 vs. model 2) (Figure 4B). In order to avoid overfitting, we conducted cross-validations. The population was randomly divided into two groups, including the training set (449 patients) and the validation set (449 patients). As shown in Table 8, the composite risk score was superior to both TRF and GRS in discriminative power in the training and validation sets (training set: P < 0.0001 for model 3 vs. model 1, and P < 0.0001 for model 3 vs. model 2; validation set: P = 0.022 for model 3 vs. model 1, and P < 0.0001 for model 3 vs. model 2), which is consistent with the results from the total population. Besides, the discriminative power showed no difference between models 1 and 2 (Table 8).
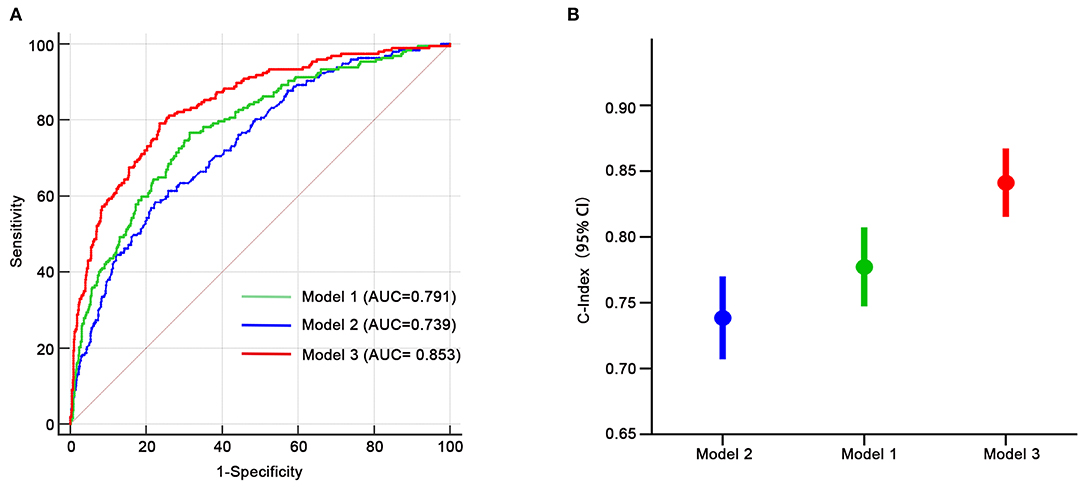
Figure 4. Receiver-operating characteristic curves for HF-related mortality risk. (A,B) Model 1, only age, gender, diabetes, LVEF, log-transformed NT-proBNP, serum creatinine, sodium, potassium, diastolic blood pressure; model 2, only GRS; model 3, composite risk score. AUC, area under the curve.
Discussion
Our results indicated that medium- and high-risk score groups were associated with 2.78- and 6.54-fold higher mortality risk when compared with the low-risk score group (HR = 2.78, 95% CI = 1.82–4.24, P = 2 × 10−6 for medium- vs. low-risk group; HR = 6.54, 95% CI = 4.42–9.71, P = 6 × 10−21 for high- vs. low-risk group). Furthermore, we combined GRS and traditional risk factors to construct the composite risk score, which could more significantly distinguish individuals with different mortality risk (HR = 5.41, 95% CI = 2.72–10.64, P = 1 × 10−6 for medium vs. low risk; HR = 22.72, 95% CI = 11.90–43.48, P = 5 × 10−21 for high vs. low risk). Besides, we compared the discriminative power of traditional risk factors, GRS, and combined models for HF using ROC curve analysis. The data showed that GRS and TRF were comparable in the discriminative power (P = 0.06), both with a high c statistic beyond 0.7. The combination of TRF and GRS could significantly increase the ability of prediction for survival of HF with c statistic reaching up to 0.853.
Heart failure has been a serious social problem with high mortality (9, 14, 24). Despite advanced drug and device therapies, 5-year mortality rates remained <40% (25, 26). Up to now, a series of HF-related traditional risk factors have been used to construct the prognostic multivariable models for mortality risk stratification (6, 14, 27–31). They all had a well discrimination power with C statistic beyond 0.7 (9, 13, 17). Besides, the prognostic value of circulating microRNAs on the mortality risk of HF has also been investigated recently (32, 33). Importantly, plenty of studies on the association between genetic variants and the prognosis of HF have shed light on the variable mortality risk of individual patients. Based on these, our study was carried out to comprehensively construct a GRS and composite risk score for HF prognosis.
First, our investigation was based on the data from transcriptomic analysis of 313 human heart samples and WES of 998 HF patients, which could comprehensively assess the SNPs associated with HF-related mortality risk.
Second, our GRS was constructed with a total of 31 SNPs, which represented the largest GRS study for the prognosis of HF (18, 22). Furthermore, our GRS achieved greater risk discrimination than the previously published genomic risk score (22). For example, the medium- and high-risk score groups have 2.78- and 6.54-fold HR, respectively, for the prognosis of HF in comparison with the low-risk score group. Importantly, the prediction ability was independent of traditional risk factors. Notably, the composite risk score could dramatically improve the discrimination ability with the mortality risk of high and medium risk reaching up to 22.72- and 5.41-fold, respectively, when compared with the low-risk group. The risk stratification for HF patients could help identify those patients in need of more intensive treatment and also help target appropriate populations for trials of new therapies.
Third, the discriminative power of GRS was displayed excellently, which was comparable to the traditional prediction models with nine known risk factors at present. And the GRS added substantial prognostic power to the traditional risk model with a c-index of 0.853. These suggested that the combination of genetic and traditional risk factors could well discriminate the risk mortality for individual patients, which represented a promising direction in the future.
The main limitation of our study was the single-center study with only one cohort. Although the results were statistically significant, additional larger studies would help confirm our findings.
Conclusions
In conclusion, we found a total of 31 SNPs associated with HF-related mortality risk by using large-scale prognosis analysis. GRS, derived from the 31 SNPs, was significantly associated with the prognosis of HF and displayed excellent discrimination ability for mortality risk of HF. Moreover, the combination of GRS and conventional risk factors could substantially improve the discrimination power. The results indicated that our GRS could identify individuals with increased HF-related mortality risk and provide clinician with reference for the intensive therapy, especially when combined with traditional risk factors. Future strategies for prognostic assessment of HF should include an individualized assessment in which traditional risk factors are combined with an evaluation of GRS as well.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by Review Board of Tongji College of Medicine. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
DH and SH designed experiments. DH, SL, and YS analyzed data. DH and LX wrote the manuscript. DH performed experiments and analyzed data. YW and DW reviewed the manuscript. Sample preparation and protocol were carried out by DH. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
Funding
This work was supported by grants from the National Key R&D Program of China (No. 2017YFC0909400), the National Program on Key Basic Research Project (973 Program, No. 2012CB518004), the National Nature Science Foundation Key project (Nos. 91439203 and 81790624), and the National Precision Medicine Project (No. SQ2017YFSF090157).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the staff of the Division of Cardiology and follow-up nurses for their help in the recruitment and follow-up of heart failure patients.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.634966/full#supplementary-material
Abbreviations
HF, heart failure; WES, whole exome sequencing; GRS, genetic risk score; TRF, traditional risk factors; ROC, receiver operating characteristics; SNPs, single nucleotide polymorphisms; MAF, allele frequency; LD, linkage disequilibrium.
References
1. Komajda M, Charron P, Tesson F. Genetic aspects of heart failure. Eur J Heart Fail. (1999) 1:121–6. doi: 10.1016/S1388-9842(99)00026-4
2. Li X, Zhang P. Genetic determinants of myocardial dysfunction. J Med Genet. (2017) 54:1–10. doi: 10.1136/jmedgenet-2016-104308
3. Khan SS, Ning H, Shah SJ, Yancy CW, Carnethon M, Berry JD, et al. 10-Year Risk Equations for Incident Heart Failure in the General Population. J Am Coll Cardiol. (2019) 73:2388–97. doi: 10.1016/j.jacc.2019.02.057
4. Rossignol P, Hernandez AF, Solomon SD, Zannad F. Heart failure drug treatment. Lancet. (2019) 393:1034–44. doi: 10.1016/S0140-6736(18)31808-7
5. Vazquez R, Bayes-Genis A, Cygankiewicz I, Pascual-Figal D, Grigorian-Shamagian L, Pavon R, et al. The MUSIC Risk score: a simple method for predicting mortality in ambulatory patients with chronic heart failure. Eur Heart J. (2009) 30:1088–96. doi: 10.1093/eurheartj/ehp032
6. Jones NR, Roalfe AK, Adoki I, Hobbs FD, Taylor CJ. Survival of patients with chronic heart failure in the community: a systematic review and meta-analysis. Eur J Heart Fail. (2019) 21:1306–25. doi: 10.1186/s13643-018-0810-x
7. Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJ, Swedberg KB, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. (2006) 27:65–75. doi: 10.1093/eurheartj/ehi555
8. Yap J, Lim FY, Chia SY, Allen JC, Jaufeerally FR, Macdonald MR, et al. Prediction of survival in Asian patients hospitalized with heart failure: validation of the OPTIMIZE-HF risk score. J Card Fail. (2019) 25:571–5. doi: 10.1016/j.cardfail.2019.02.016
9. Sartipy U, Dahlström U, Edner M, Lund LH. Predicting survival in heart failure: validation of the MAGGIC heart failure risk score in 51,043 patients from the Swedish heart failure registry. Eur J Heart Fail. (2014) 16:173–9. doi: 10.1111/ejhf.32
10. Ersbøll M, Valeur N, Mogensen UM, Andersen MJ, Møller JE, Velazquez EJ, et al. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol. (2013) 61:2365–73. doi: 10.1016/j.jacc.2013.02.061
11. Xanthopoulos A, Tryposkiadis K, Giamouzis G, Konstantinou D, Giannakoulas G, Karvounis H, et al. Larissa heart failure risk score: a proposed simple score for risk stratification in chronic heart failure. Eur J Heart Fail. (2018) 20:614–6. doi: 10.1002/ejhf.1132
12. Win S, Hussain I, Hebl VB, Dunlay SM, Redfield MM. Inpatient mortality risk scores and postdischarge events in hospitalized heart failure patients: a community-based study. Circ Heart Fail. (2017) 10:e003926. doi: 10.1161/CIRCHEARTFAILURE.117.003926
13. Agostoni P, Corrà U, Cattadori G, Veglia F, La Gioia R, Scardovi AB, et al. Metabolic exercise test data combined with cardiac and kidney indexes, the MECKI score: a multiparametric approach to heart failure prognosis. Int J Cardiol. (2013) 167:2710–8. doi: 10.1016/j.ijcard.2012.06.113
14. Kalogeropoulos AP, Georgiopoulou VV, Giamouzis G, Smith AL, Agha SA, Waheed S, et al. Utility of the seattle heart failure model in patients with advanced heart failure. J Am Coll Cardiol. (2009) 53:334–42. doi: 10.1016/j.jacc.2008.10.023
15. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Køber L, Squire IB, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. (2013) 34:1404–13. doi: 10.1093/eurheartj/ehs337
16. O'Connor C, Fiuzat M, Mulder H, Coles A, Ahmad T, Ezekowitz JA, et al. Clinical factors related to morbidity and mortality in high-risk heart failure patients: the GUIDE-IT predictive model and risk score. Eur J Heart Fail. (2019) 21:770–8. doi: 10.1002/ejhf.1450
17. Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, et al. The seattle heart failure model: prediction of survival in heart failure. Circulation. (2006) 113:1424–33. doi: 10.1161/CIRCULATIONAHA.105.584102
18. Agra RM, Gago-Dominguez M, Paradela-Dobarro B, Torres-Español M, Alvarez L, Fernandez-Trasancos A, et al. Obesity-related genetic determinants of heart failure prognosis. Cardiovasc Drugs Ther. (2019) 33:415–24. doi: 10.1007/s10557-019-06888-8
19. Hu D, Huang J, Hu S, Zhang Y, Li S, Sun Y, et al. A common variant of RIP3 promoter region is associated with poor prognosis in heart failure patients by influencing SOX17 binding. J Cell Mol Med. (2019) 23:5317–28. doi: 10.1111/jcmm.14408
20. Angermann CE, Kaspar M, Marx A, Kittel-Schneider S, Menhofer D, Störk S, et al. A functional variant of the neuropeptide S receptor-1 gene modulates clinical outcomes and healthcare utilization in patients with systolic heart failure: results from the interdisciplinary network heart failure (INH) study. Eur J Heart Fail. (2017) 19:314–23. doi: 10.1002/ejhf.706
21. Dorsheimer L, Assmus B, Rasper T, Ortmann CA, Ecke A, Abou-El-Ardat K, et al. Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA Cardiol. (2019) 4:25–33. doi: 10.1001/jamacardio.2018.3965
22. Li S, Sun Y, Hu S, Hu D, Li C, Xiao L, et al. Genetic risk scores to predict the prognosis of chronic heart failure patients in Chinese Han. J Cell Mol Med. (2019) 24:285–93. doi: 10.1111/jcmm.14722
23. Cordero P, Parikh VN, Chin ET, Erbilgin A, Gloudemans MJ, Shang C, et al. Pathologic gene network rewiring implicates PPP1R3A as a central regulator in pressure overload heart failure. Nat Commun. (2019) 10:2760. doi: 10.1038/s41467-019-10591-5
24. Rosenberg J, Schou M, Gustafsson F, Badskjaer J, Hildebrandt P. Prognostic threshold levels of NT-proBNP testing in primary care. Eur Heart J. (2009) 30:66–73. doi: 10.1093/eurheartj/ehn525
25. Wang JJ-C, Rau C, Avetisyan R, Ren S, Romay MC, Stolin G, et al. Genetic dissection of cardiac remodeling in an isoproterenol-induced heart failure mouse model. PLoS Genet. (2016) 12:e1006038. doi: 10.1371/journal.pgen.1006038
26. Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. (2002) 347:1397–402. doi: 10.1056/NEJMoa020265
27. Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, et al. Discrimination and calibration of clinical prediction models: users' guides to the medical literature. JAMA. (2017) 318:1377–84. doi: 10.1001/jama.2017.12126
28. Collier TJ, Pocock SJ, McMurray JJ, Zannad F, Krum H, van Veldhuisen DJ, et al. The impact of eplerenone at different levels of risk in patients with systolic heart failure and mild symptoms: insight from a novel risk score for prognosis derived from the EMPHASIS-HF trial. Eur Heart J. (2013) 34:2823–9. doi: 10.1093/eurheartj/eht247
29. Feola M, Testa M, Lombardo E, Piccolo S, Avogadri E, Leto L. The prediction of one-year mortality in elderly congestive heart failure patients: a clinical score. Int J Cardiol. (2013) 168:2895–6. doi: 10.1016/j.ijcard.2013.03.170
30. Gardner RS, Chong KS, Morton JJ, McDonagh TA. A change in N-terminal pro-brain natriuretic peptide is predictive of outcome in patients with advanced heart failure. Eur J Heart Fail. (2007) 9:266–71. doi: 10.1016/j.ejheart.2006.07.002
31. Jackson CE, Castagno D, Maggioni AP, Køber L, Squire IB, Swedberg K, et al. Differing prognostic value of pulse pressure in patients with heart failure with reduced or preserved ejection fraction: results from the MAGGIC individual patient meta-analysis. Eur Heart J. (2015) 36:1106–14. doi: 10.1093/eurheartj/ehu490
32. Bayés-Genis A, Lanfear DE, Ronde MW de, Lupón J, Leenders JJ, Liu Z, et al. Prognostic value of circulating microRNAs on heart failure-related morbidity and mortality in two large diverse cohorts of general heart failure patients. Eur J Heart Fail. (2018) 20:67–75. doi: 10.1002/ejhf.984
Keywords: genetic risk score, traditional risk factors, prediction, heart failure, prognosis
Citation: Hu D, Xiao L, Li S, Hu S, Sun Y, Wang Y and Wang DW (2021) Prediction of HF-Related Mortality Risk Using Genetic Risk Score Alone and in Combination With Traditional Risk Factors. Front. Cardiovasc. Med. 8:634966. doi: 10.3389/fcvm.2021.634966
Received: 29 November 2020; Accepted: 01 March 2021;
Published: 26 April 2021.
Edited by:
Vinicius Tragante, University Medical Center Utrecht, NetherlandsReviewed by:
Zhuozhong Wang, The Second Affiliated Hospital of Harbin Medical University, ChinaPaulo M. Dourado, University of São Paulo, Brazil
Michal Mokry, University Medical Center Utrecht, Netherlands
Copyright © 2021 Hu, Xiao, Li, Hu, Sun, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dao Wen Wang, ZHd3YW5nQHRqaC50am11LmVkdS5jbg==
†These authors have contributed equally to this work
 Dong Hu
Dong Hu Lei Xiao
Lei Xiao Shiyang Li3
Shiyang Li3 Dao Wen Wang
Dao Wen Wang