- 1Department of Cardiology, Rabin Medical Center, Petah Tikva, Israel
- 2Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
- 3Davidoff Cancer Center, Rabin Medical Center, Petah Tikva, Israel
To the best of our knowledge, this is the first published report of anti-immunoglobulin-like transcript 3 (ILT3)-induced myocarditis. A 48-year old female patient with refractory acute myeloid leukemia who was given a single dose of anti-ILT3 monotherapy presented with fever, hypotension, chest pain, and elevated cardiac biomarkers. Systolic bi-ventricular function was in normal limits. The patient was promptly treated with pulse dose steroids with a rapid hemodynamic and clinical improvement and declining levels of cardiac biomarkers. The diagnosis of acute myocarditis was confirmed using cardiac magnetic resonance imaging applying the revised Lake Lewis criteria. While larger-scale data are needed in order to assess the incidence, management and prognosis of anti-ILT-3 induced myocarditis, we believe a high level of suspicion for adverse non-target cardiac effects is required in patients receiving this novel class of drugs.
Introduction
A 48-year-old female patient, BRCA-1 carrier, was diagnosed with triple negative breast carcinoma on age 44 and treated with neoadjuvant chemotherapy (adriamycin, carboplatin, and paclitaxel) followed by bilateral mastectomy and radiotherapy. As part of a clinical research protocol, she was also given pembrolizumab, an immune checkpoint inhibitor (ICI), both as neoadjuvant and maintenance therapy for another 12-months. On age 47, the patient presented with tri-lineage cytopenia and was diagnosed with myelodysplastic syndrome with excess of blasts. Cytogenetics revealed a monosomal complex karyotype with deletion of chromosomes 5 and 7 with no molecular aberration. The patient underwent allogeneic hematopoietic cell transplantation from a matched sibling with myeloablative treosulfan-based conditioning, with full donor chimerism on day 30. Due to an increased risk for relapse, maintenance therapy with azacytidine and venetoclax was initiated on day 60, however on day 180 the patient relapsed with overt-transformation to acute myeloid leukemia. The patient was then treated with salvage chemotherapy (FLAG-IDA protocol) combined with venetoclax for 7 days, yet unfortunately she did not respond. Finally, the patient was recruited to a clinical immunotherapy trial in a different hospital.
Twelve-days after her first monotherapy treatment with humanized IgG4 anti immunoglobulin-like transcript 3 (ILT3) [MK-0482, MERCK (MSD)] (75 mg), the patient presented to the Hemato-Oncology Ambulatory Care Unit in the Davidoff Cancer Center, Rabin Medical Center (Israel) with fever and malaise (patient's presentation and management time-line is presented in Figure 1). Physical examination was unremarkable and vital signs were in normal range except for systemic fever (temperature 101.3 °F (38.5°C). Laboratory analysis demonstrated 2.1 K/micl leukocytes (normal-range values 4.5–11 K/micl) with 0.2 K/micl neutrophils (normal-range values 1.8–7.7 K/micl), hemoglobin 8.5 g/dL and platelets 12 K/micl. C-reactive protein was 17.3 mg/dL (normal-range values 0.0–0.5 mg/dL). Chest x-ray was normal and 12-leads electrocardiogram (ECG) revealed sinus rhythm with a T-wave inversion in aVL. On a working diagnosis of neutropenic fever, blood cultures were collected and empirical antibiotic (Meropenem) was initiated. COVID-19 status was negative. On day 2, the patient began complaining of a constant chest pain which exacerbated with breathing and was not relieved by oral analgesics. She denied any shortness of breath, palpitations or muscle pain. Blood pressure was 83/52 mmHg, pulse 107/min, oxygen saturation was 96% on room air, respiratory rate 20 breaths per minute. Repeated ECG was unchanged. Lungs were clear to auscultation bilaterally and heart sounds were rapid with no apparent new murmurs. The patient did not show signs of volume overload or pulmonary congestion. Neurologic examination was unremarkable. Troponin T level and NT pro-BNP level were 671 ng/L (normal-range values 0–14 ng/L) and 5,885 pg/ml (normal-range values below 125 pg/ml), respectively. Echocardiography demonstrated a lower limit of normal left ventricular systolic function (LVEF 55%) similar to her prior routine echocardiogram study performed 3 months earlier. Given her clinical presentation and recent novel immunotherapy treatment, the leading diagnosis was immunotherapy-induced acute myocarditis, and decisions were made to monitor her in the cardiac unit and to immediately initiate therapy with empirical pulse dose steroids (methylprednisolone 1 g for 3 days). Cardiac computed tomography revealed normal coronary arteries and ruled out acute pulmonary embolism. On day 3, the patient reported an improvement in her wellbeing and the amelioration of her chest pain. Blood pressure stabilized (103/85 mmHg) and laboratory analysis showed declining levels of cardiac biomarkers (Troponin T 224 ng/L and NT pro-BNP 5,815 pg/ml). The diagnosis of acute myocarditis was confirmed using cardiac magnetic resonance imaging (Figure 2) based on the updated Lake Louis criteria demonstrating subepicardial and mid-wall late gadolinium enhancement in the basal infero- and antero-lateral segments with increased values of native T1 time and T2 time revealing extensive diffuse myocardial edema. After the completion of 3 days of pulse steroid therapy, the patient was switched to oral high-dose (1 mg/Kg) prednisone treatment. Elaborated diagnostic work-up for infectious etiology which included blood cultures, inspiratory viral PCR panel and bacterial, rickettsial and viral serologies was unrevealing.
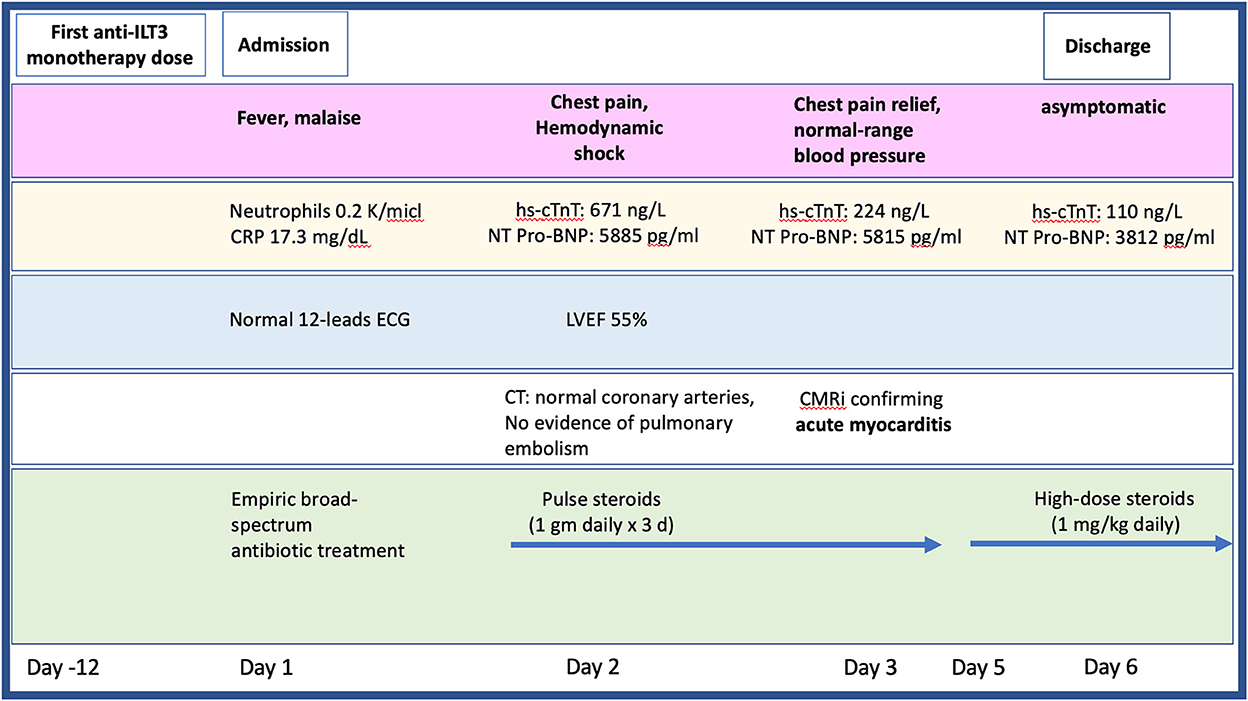
Figure 1. Timeline presenting the patient's clinical presentation, diagnostic findings, and treatment approach during hospitalization. CMR, cardiac magnetic resonance imaging; CT, computed tomography; ECG, electrocardiogram; hs-cTnT, high sensitive troponin; LVEF, left ventricular ejection fraction; NT pro-BNP, N-terminal pro-brain natriuretic peptide.
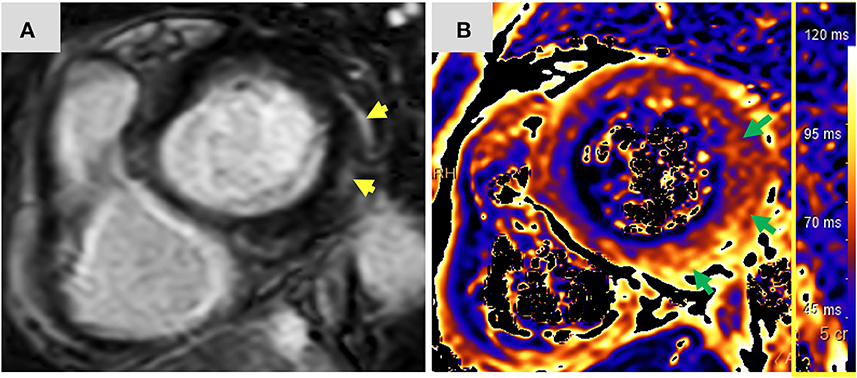
Figure 2. Magnetic resonance imaging findings: Short axis view (A) of late gadolinium enhancement illustrates sub-epicardial and mid-wall late gadolinium enhancement in the basal infero-lateral and antero-lateral segments (yellow arrows). The corresponding short axis view of T2 mapping (B) illustrates extensive diffuse interstitial myocardial edema (green arrows). Diffuse T2 value was 56.2 ms and focal T2 value in the infero-lateral segment was 61 ms (normal limit 55 ms). The presence of myocardial injury and extensive edema support the diagnosis of acute myocarditis according to the 2018 updated Lake Louise criteria. ms, miliseconds.
The patient was discharged home after a 5-day hospitalization with a favorable functional status. Her Troponin T and NT-proBNP levels at discharge were 110 ng/l and 3,812 pg/ml, respectively. A decision was made to discontinue investigational drug therapy.
Discussion
To the best of our knowledge, this is the first published report of anti-ILT3 monotherapy-induced acute myocarditis using the MK-0482 monoclonal antibody. ILT3, gene name LILRB4, is an inhibitory receptor expressed on myeloid-derived suppressor cells which is associated with immune tolerance within the tumor microenvironment. Anti-ILT3 were shown to abrogate myeloid immunosuppression and enable tumor killing (1). Moreover, antagonism of the ILT3 receptor may enhance the efficacy of immune checkpoint inhibitors (ICI) (2). Several phase 1 and phase 2 trials using these new class of drugs are currently ongoing worldwide in both solid and non-solid cancers. Data regarding both the efficiency and safety profile of these immunotherapy agents are still scarce, yet as recently presented in the American Society of Clinical Oncology annual meeting, the combined use of MK-0482 and pembrolizumab was associated with 2 cases of myositis, one of which was fatal (3). While cases of anti-ILT-3-induced myocarditis were not reported in this cohort, it is important to note that the co-existence of myositis and myocarditis is well-established with the use of ICIs (4). More data are needed in order to determine whether this clinical association also exists with the novel class of anti-ILT3 drugs.
Similarities between ICI-induced myocarditis and our presented case of anti-ILT-3 induced myocarditis are the short period of time between the first given dose of the antibody and the development of myocarditis [a median start of onset of 34 days of ICI-induced myocarditis (5–8)], the rise in troponin T which is evident in 94% of patients with ICI induced myocarditis (5, 9) and the presentation of acute myocarditis with normal left ventricular systolic function [found in 51% of patients with ICI-myocarditis (5–8)].
Due to the lack of published evidence regarding the treatment of anti-ILT-3-induced myocarditis and due to the similarities in the mechanism of action and clinical presentation to these two treatment modalities, we based our management strategy on current published scientific literature and society-guideline recommendations for ICI-induced myocarditis (8, 10, 11). Endomyocardial biopsy was not performed due to patient's severe thrombocytopenia, yet cardiac magnetic resonance imaging allowed for a definite diagnosis of acute myocarditis with extensive diffuse edema (12). The patient was hemodynamically monitored and cardiac biomarkers' levels were regularly followed. Prompt treatment (< 24 h from chest pain presentation) with high-dose steroids was given based on the recently published cardio-oncology society guidelines (8), as this was previously shown to improve patient's MACE and mortality (13). Due to patient's rapid hemodynamic improvement and declining biomarkers' levels, we did not use immunosuppressive drugs other than high-dose steroids, which provided both clinical and laboratory improvement. Notably, it is critical to underscore the importance of a good collaboration between the hemato-oncology and cardio-oncology providers, which allowed for the patient's rapid evaluation, diagnosis and therapy initiation.
While larger-scale data are needed in order to assess the incidence, management and prognosis of anti-ILT-3 induced myocarditis, we believe a high level of suspicion for adverse non-target cardiac effects is required in patients receiving this novel class of drugs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to b3NuYXRpdEBjbGFsaXQub3JnLmls.
Ethics statement
Written informed consent was obtained from the participant for the publication of this case report and any potentially identifiable images or data included in this article.
Author contributions
OI drafted the manuscript. AS, AH, MY, IN, PR, RK, and LS have reviewed and commented on the final draft. All authors contributed to the article and approved the submitted version.
Acknowledgments
We wish to deeply thank the patient for being an inspiration for us all.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Brandish PE, Palmieri A, Ayanoglu G, Baker J, Bueno R, Byford A, et al. Antibodies to ILT3 abrogate myeloid immunosuppression and enable tumor killing. BioRxiv. (2021) 2021:2021.12.18.472552. doi: 10.1101/2021.12.18.472552
2. Singh L, Muise E, Bhattacharya A, Grein J, Javaid S, Stivers P, et al. ILT3 (LILRB4) promotes the immunosuppressive function of tumor-educated human monocytic myeloid-derived suppressor cells. Mol Cancer Res. (2020) 19:molcanres.0622.2020. doi: 10.1158/1541-7786.MCR-20-0622
3. Gutierrez M, Spreafico A, Wang D, Golan T, Renouf D, Voskoboynik M, et al. Phase 1 first-in-human study of anti–ILT3 mAb MK-0482 as monotherapy and in combination with pembrolizumab in advanced solid tumors: dose escalation results. J Clin Oncol. (2022) 40(16_suppl):2505. doi: 10.1200/JCO.2022.40.16_suppl.2505
4. Pathak R, Katel A, Massarelli E, Villaflor VM, Sun V, Salgia R. Immune checkpoint inhibitor-induced myocarditis with myositis/myasthenia gravis overlap syndrome: a systematic review of cases. Oncologist. (2021) 26:1052–61. doi: 10.1002/onco.13931
5. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. (2018) 71:1755–64. doi: 10.1016/j.jacc.2018.02.037
6. Lehmann LH, Cautela J, Palaskas N, Baik AH, Meijers WC, Allenbach Y, et al. Clinical strategy for the diagnosis and treatment of immune checkpoint inhibitor-associated myocarditis: a narrative review. J Am Med Assoc Cardiol. (2021) 6:1329–37. doi: 10.1001/jamacardio.2021.2241
7. Agrawal N, Khunger A, Vachhani P, Colvin TA, Hattoum A, Spangenthal E, et al. Cardiac toxicity associated with immune checkpoint inhibitors: case series and review of the literature. Case Rep Oncol. (2019) 12:260–76. doi: 10.1159/000498985
8. Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur Heart J. (2022) 43:4229–361. doi: 10.1093/eurheartj/ehac244
9. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2022) 24:4–131. doi: 10.1093/eurheartj/ehab670
10. Thuny F, Alexandre J, Salem JE, Mirabel M, Dolladille C, Cohen-Solal A, et al. Management of immune checkpoint inhibitor-induced myocarditis: the French Working Group's plea for a pragmatic approach. JACC CardioOncol. (2021) 3:157–61. doi: 10.1016/j.jaccao.2020.12.001
11. Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. (2020) 9:e013757. doi: 10.1161/JAHA.119.013757
12. Bonaca MP, Olenchock BA, Salem JE, Wiviott SD, Ederhy S, Cohen A, et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. (2019) 140:80–91. doi: 10.1161/CIRCULATIONAHA.118.034497
Keywords: case report, anti-ILT-3 induced myocarditis, immunotherapy, acute myocarditis, cardio-oncology
Citation: Itzhaki Ben Zadok O, Shiyovich A, Hamdan A, Yeshurun M, Nardi Agmon I, Raanani P, Kornowski R and Shargian L (2022) Anti-immunoglobulin-like transcript 3 induced acute myocarditis—A case report. Front. Cardiovasc. Med. 9:1035569. doi: 10.3389/fcvm.2022.1035569
Received: 02 September 2022; Accepted: 17 November 2022;
Published: 08 December 2022.
Edited by:
Carlo Gabriele Tocchetti, University of Naples Federico II, ItalyReviewed by:
Nikhil Agrawal, University of Texas Health Science Center at Houston, United StatesShui Lian Yu, Guangzhou Medical University, China
Copyright © 2022 Itzhaki Ben Zadok, Shiyovich, Hamdan, Yeshurun, Nardi Agmon, Raanani, Kornowski and Shargian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Osnat Itzhaki Ben Zadok, b3NuYXQuaXR6aGFraUBnbWFpbC5jb20=
 Osnat Itzhaki Ben Zadok
Osnat Itzhaki Ben Zadok Arthur Shiyovich1,2
Arthur Shiyovich1,2