Abstract
Background:
Allograft pathologies, such as valvular, coronary artery, or aortic disease, may occur early and late after cardiac transplantation. Cardiac surgery after heart transplantation (CASH) may be an option to improve quality of life and allograft function and prolong survival. Experience with CASH, however, has been limited to single-center reports.
Methods:
We performed a retrospective, multicenter study of heart transplant recipients with CASH between January 1984 and December 2020. In this study, 60 high-volume cardiac transplant centers were invited to participate.
Results:
Data were available from 19 centers in North America (n = 7), South America (n = 1), and Europe (n = 11), with a total of 110 patients. A median of 3 (IQR 2–8.5) operations was reported by each center; five centers included ≥ 10 patients. Indications for CASH were valvular disease (n = 62), coronary artery disease (CAD) (n = 16), constrictive pericarditis (n = 17), aortic pathology (n = 13), and myxoma (n = 2). The median age at CASH was 57.7 (47.8–63.1) years, with a median time from transplant to CASH of 4.4 (1–9.6) years. Reoperation within the first year after transplantation was performed in 24.5%. In-hospital mortality was 9.1% (n = 10). 1-year survival was 86.2% and median follow-up was 8.2 (3.8–14.6) years. The most frequent perioperative complications were acute kidney injury and bleeding revision in 18 and 9.1%, respectively.
Conclusion:
Cardiac surgery after heart transplantation has low in-hospital mortality and postoperative complications in carefully selected patients. The incidence and type of CASH vary between international centers. Risk factors for the worse outcome are higher European System for Cardiac Operative Risk Evaluation (EuroSCORE II) and postoperative renal failure.
Introduction
Heart transplantation (HTX) confers excellent long-term survival in select patients with symptomatic end-stage heart failure. Cardiac surgery after heart transplantation (CASH) is a rarely used approach to improve allograft function and quality of life, and prolong survival (1), but has been described only in case reports and single-center experiences (2, 3). Although symptomatic tricuspid regurgitation is known as the most common cause for CASH (2), other valvular diseases, aortic pathology, and cardiac allograft vasculopathy (CAV) can occur in the HTX population (1–3). Coronary artery bypass grafting (CABG) is a safe surgical therapy for CAV, in selected cases, with acceptable long-term outcomes (1, 2). Furthermore, transcatheter and minimally invasive strategies to treat allograft pathologies have been reported with excellent short-term outcomes (4–9).
In this retrospective, multicenter, cohort study, we evaluated the safety of CASH and risk factors for subsequent morbidity and mortality.
Materials and Methods
The study cohort was comprised of heart transplant recipients who underwent CASH between January 1984 and December 2020. CASH includes cardiac and aortic transcatheter interventions but does not include retransplantation, pacemaker/defibrillator placement, or reoperation due to bleeding complications. In total, 60 high-volume cardiac transplant centers performing more than 15 HTXs per year were invited to participate in the study. Each participating center obtained ethics approval from its institutional review board (Vienna ethics committee reference number: 1894/2017) and a data use agreement was executed with each center.
Relevant data on demographics, medical history, medications, surgeries, cardiac testing, outcomes, and complications were collected from the patients’ medical records and were de-identified by each center’s study coordinator. A password-protected, validated Excel file was sent to the primary investigator for statistical analysis. The European System for Cardiac Operative Risk Evaluation (EuroSCORE II) (10, 11), a validated scoring system that predicts the risk of in-hospital mortality after major cardiac surgery, was calculated.
The inverse Kaplan–Meier method was used to quantify median follow-up (12). To evaluate the effect of selected clinical factors on in-hospital mortality, we used univariate logistic regression models accounting for the center as a random effect. Survival after reoperation was described using the Kaplan–Meier method. Two-sided p < 0.05 were considered statistically significant. Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, United States).
Results
Study Population
Data from 110 patients were submitted by 19 centers (the United States-6; Spain-3; Austria-2; Germany-2; Argentina-1; Canada-1; Croatia-; France-1; Italy-1; and Slovakia-1) with a median of 3 (interquartile range [IQR] 2–8.5) patients per center; five centers reported ≥ 10 patients. The incidence of CASH was 0.86% (lowest 0.17%, highest 2.46%; and total number of HTX: 14,185). The median age at CASH was 57.7 (IQR 47.8–63.1) years with a median interval between HTX and CASH of 4.4 (IQR 1–9.6) years. Indications for surgery was valvular disease (n = 62), coronary artery disease (CAD) (n = 16), constrictive pericarditis (n = 17), aortic disease (n = 13), and myxoma (n = 2). Five patients (4.5%) had infectious etiology, including two fungal constrictive pericarditis, one aortic and one tricuspid valve endocarditis, and one infectious aortic pseudoaneurysm at the suture line. In 27 (24.5%) patients, CASH was performed in the first year, including 10 in the first 30 days. Thirty-six patients (32.7%) had an urgent indication for surgery. The surgical approaches were redo-sternotomy (n = 104), thoracotomy (n = 4), and transcatheter (TAVR; n = 2). Patient characteristics are provided in Table 1.
TABLE 1
| N | 110 |
| Sex (male), n | 90 (82%) |
| Age at HTX, IQR, y | 51.8 (40.7–57.5) |
| Donor age, IQR, y | 42 (28–49) |
| EuroSCORE II, IQR | 4.7 (2.9–8.2) |
| LVEF < 50%, n | 13 (11.8%) |
| Diabetes, n | 27 (24.5%), 14 NIDDM/13 IDDM |
| Creatinine pre-op (mg/dL), IQR | 1.6 (1.3–2.2) |
| CKD (GFR < 60 mL/min), n | 59 (53.6%), 5 dialysis dependent |
| CPB-time, IQR, min | 109 (91.5–165) |
| Cross-clamp time, IQR, min | 65 (46–94.5) |
| ICU stay, IQR, d | 3.5 (1–6.3) |
| In-hospital stay, IQR, d | 15 (10–24.5) |
| Perioperative complication, n | |
| Acute kidney injury | 20 (18.2%) |
| Bleeding revision | 10 (9.1%) |
| Pneumonia | 8 (7.3%) |
| Wound infection | 5 (4.5%) |
| Need for pacemaker | 5 (4.5%) |
| Allograft failure | 4 (3.6%) |
| Stroke | 2 (1.8%) |
| In-hospital mortality, n | 10 (9.1%) |
| 1-year survival (SE) | 86.2% (3.3) |
| Follow-up, IQR, y | 8.2 (3.8–14.6) |
Patient Characteristics, including postoperative details.
CKD, chronic kidney disease; CPB-time, cardiopulmonary bypass time; LVEF, left ventricular ejection fraction; GFR, glomerular filtration rate; HTX, heart transplantation; ICU, intensive care unit; IDDM, insulin-dependent diabetes mellitus; NIDDM, non-insulin-dependent diabetes mellitus; SE, standard error.
Outcome
The most common postoperative complication was acute kidney injury (Table 1). A permanent pacemaker was needed due to atrioventricular block in five patients, all after tricuspid valve surgery. Postoperative graft failure resulted in early death after tricuspid valve surgery in two patients and after pericardiectomy in one patient. In another patient, graft function recovered with postoperative extracorporeal membrane oxygenation (ECMO) support after total aortic arch replacement, aortic valve replacement, and CABG. Causes of death after discharge were cardiac (n = 14), infectious (n = 10), malignancy (n = 9), neurological complications (n = 3), and other (n = 8).
Overall survival was 86.2 ± 3.3 and 76.7 ± 4.2% after 1 and 3 years, respectively (Figure 1A). The 3-year survival stratified by indication for CASH is shown in Figure 1B. In-hospital mortality was 9.1% (n = 10), with systemic infection (n = 6), graft failure (n = 3), and bleeding (n = 1) as causes of death. Patients with urgent (compared with elective) CASH had worse in-hospital, 1-, and 3-year survival (Kaplan–Meier estimate: 83.3, 80.4, and 63.6% vs. 94.6, 89.1, and 82.8%, respectively; Figure 1C). In-hospital mortality was higher in patients with postoperative acute kidney injury (n = 20, 19.1%; 35.0 vs. 3.5% for urgent vs. elective, respectively). In univariate logistic regression analysis, postoperative acute kidney injury (odds ratio [OR] 14.7 [95% confidence interval [CI] 3.3–65.6], p = 0.0006) and higher EuroSCORE II (OR for 2-fold increase 2.0 [1.0–3.7], p = 0.04) were statistically significantly associated with in-hospital mortality. Higher in-hospital mortality was associated with urgent indication for surgery (OR 3.5 [0.9–13.6], p = 0.07), as well as older age at the time of reoperation (OR for 10-year increase 1.8 [0.9–3.6], p = 0.08). However, these effects were not statistically significant. Heart transplant recipient sex (p = 0.32), time since HTX (p = 0.89), and baseline serum creatinine (p = 0.55) were not statistically significantly associated with in-hospital mortality in univariate logistic regression models (Supplementary Table 1).
FIGURE 1
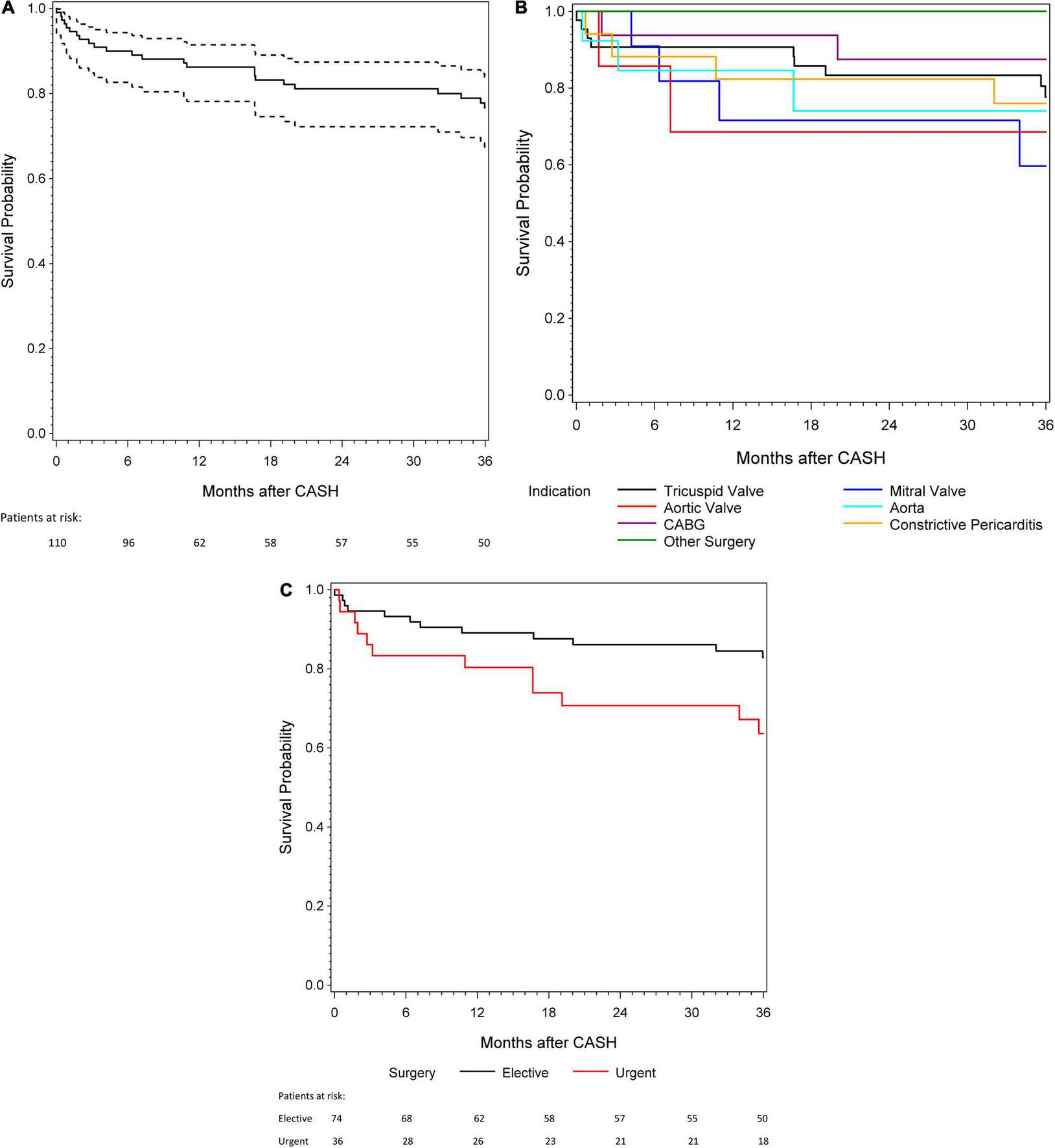
(A) A 3-year survival for patients with cardiac surgery after heart transplantation (CASH). (B) A 3-year survival according to the different indications for CASH. (C) A 3-year survival in patients with urgent and elective indication for CASH.
Valvular Disease
Tricuspid Valve Surgery
Tricuspid valve surgery was the most common CASH (n = 48) at 14 centers (Table 2): 7 centers with one patient, 4 centers with two, and 4 centers with more than two (n = 3, 8, 10, and 12). Of these 48 patients, 5 had tricuspid valve surgery in combination with surgical procedures involving the mitral valve (n = 3), aortic valve (n = 1), and aorta (n = 1).
TABLE 2
| N | 43 |
| Sex (male), n | 34 (79.1%) |
| Age at CASH, y | 52.0 (35.4–63.4) |
| EuroSCORE II | 4.4 (2.9–6.3) |
| LVEF < 50%, n | 7 (16.3%) |
| Creatinine pre-op (mg/dL) | 1.7 (1.5–2.3) |
| CKD (GFR < 60 mL/min), n | 21 (48.8%), 2 dialysis dependent |
| Time to CASH, y | 3.8 (0.9–9.8) |
| Urgent operation, n | 14 (32.6%) |
| Indication, n | |
| Biopsy induced | 22 |
| Annular dilation | 8 |
| Degenerative | 4 |
| Pacemaker lead | 1 |
| Endocarditis | 1 |
| Unknown cause | 7 |
| Operation, n | |
| Repair | 22 (51.2%) |
| biological valve/mechanical valve | 19 (44.2%)/2 (4.7%) |
| Access (sternotomy), n | 42; thoracotomy n = 1 |
| ICU stay, d | 3.5 (2–8) |
| Complications, n | |
| Renal replacement therapy | 11 (26.2%) |
| AVB, PM-implant | 5 (11.9%) |
| Bleeding revision | 5 (11.9%) |
| Right heart failure | 2 (4.7%) |
| Pneumonia | 4 (9.3%) |
| Wound infection | 1 |
| Dissection at cannulation site | 1 |
| Recurrent severe regurgitation | 1 (biological valve replacement) |
| In-hospital mortality, n | 4 (9.3%) |
| 1-year survival (SE) | 90.7% (9.3) |
Tricuspid valve surgery.
AVB, atrioventricular block; CASH, cardiac surgery after heart transplantation; CKD, chronic kidney disease; LVEF, left ventricular ejection fraction; GFR, glomerular filtration rate; ICU, intensive care unit; PM-implant, pacemaker implantation; SE, standard error.
For isolated tricuspid valve surgeries, the indication was severe, symptomatic tricuspid regurgitation. Biopsy-induced tricuspid regurgitation was the most common indication for surgery. Twelve patients (27.9%) underwent CASH in the first year post-transplant, most within the first 90 days (n = 9).
Major complications were comprised of intraoperative aortic dissection at the cannulation site, which required hemiarch replacement in one patient. Two patients developed intraoperative right heart failure, and both died perioperatively. One patient underwent biological tricuspid valve replacement 4 months after repair due to severe recurrent regurgitation. Four early deaths occurred on postoperative days (PODs) 0, 12, 26, and 34.
Mitral Valve Surgery
Five centers reported 12 patients who had mitral valve surgery (Table 3). One patient with concomitant mitral and aortic valve replacement and tricuspid valve reconstruction is described in the aortic valve surgery section. Three patients had tricuspid valve surgery (2 repairs; 1 replacement) as a concomitant procedure. The median age at CASH was 61.5 (IQR 51.6–62.6) years, and the time to CASH was 7.2 (IQR 3.1–10.1) years. One procedure was performed on postoperative day (POD) 2 due to severe mitral regurgitation with a ruptured cord in the anterior leaflet. Surgical access was usually sternotomy, except for one patient who had a thoracotomy. The median postoperative intensive care unit (ICU) stay was 5 (IQR 1–8) days and the in-hospital stay was 15 (IQR 10–21.8) days. No early perioperative (in-hospital) or surgery-related deaths occurred. Postoperative complications were acute kidney injury and reintubation in one patient. Furthermore, 1-year survival was 71.6%, and death was not related to the mitral valve surgery.
TABLE 3
| Pt | Sex | Age | Euro SCORE II | LVEF < 50% | Creatinine | GFR < 60 ml/min | Time to CASH, y | Urgency | Indication | Pathology | Operation | Mortality | Follow-up, y |
| 1 | m | 47.8 | 10.27 | − | 1.3 | − | 2 days | Urgent | Ruptured chord | Regurgitation | Biological valve | − | 5.7 |
| 2 | f | 66.6 | 6.93 | n.a. | 2.7 | yes | 0.6 | Elective | Annular dilation | Regurgitation | Repair | − | 1.2 |
| 3 | m | 65.1 | 9.53 | yes | 1.6 | yes | 3 | Urgent | Degenerative | Regurgitation | Mechanical valve | Cardiac | 2.8 |
| 4 | f | 53.1 | 10.43 | − | 4 | dialysis | 3.2 | Urgent | Degenerative | Regurgitation | Mechanical valve | Other | 0.9 |
| 5 | m | 61.6 | 2.89 | − | 1.1 | − | 5.1 | Elective | Degenerative | Regurgitation | Mechanical valve | Other | 14.4 |
| 6 | m | 63.7 | 3.05 | − | 1.3 | − | 7.2 | Elective | Degenerative | Regurgitation | Mechanical valve | Other | 12.3 |
| 7 | m | 61.5 | 4.8 | − | 2.5 | yes | 7.8 | Elective | Degenerative | Regurgitation | Repair | Infection | 0.4 |
| 8 | f | 50.2 | 3.42 | yes | n.a. | − | 9.6 | Elective | Degenerative | Both | Mechanical valve | − | 0.7 |
| 9 | m | 61.5 | 8.22 | n.a. | 3.4 | yes | 10.6 | Elective | Degenerative | Stenosis | Mechanical valve | MOF | 5.1 |
| 10 | m | 61.5 | 5.67 | − | 1.2 | − | 10.8 | Urgent | Degenerative | Regurgitation | Mechanical valve | Other | 9.3 |
| 11 | m | 26.3 | 3.46 | − | 0.7 | − | 13.4 | Elective | Degenerative | Regurgitation | Repair | n.a. | 0.5 |
| med | 61.5 (51.6–62.6) | 5.7 (3.4–8.9) | 1.5 (1.2–2.7) | 7.2 (3.1–10.1) |
Mitral valve surgery.
CASH, cardiac surgery after heart transplantation; Creatinine, Creatinine pre-op (mg/dL); LVEF, left ventricular ejection fraction; GFR, glomerular filtration rate; ICU, intensive care unit; n.a., not available; MOF, multi-organ failure; pt, patient.
Aortic Valve Surgery
Cardiac surgery after heart transplantation due to aortic valve disease was performed in 7 patients at six centers (Table 4). The median age and allograft age at CASH were 61.3 (IQR 56.9–68.7) and 59.2 (IQR 45.8–70.6) years, respectively. One patient underwent CASH in the first year (POD 20, unknown cause of aortic regurgitation), and the median time to CASH was 7.1 (IQR 3.7–10.6) years. Surgical access was sternotomy in all but two patients who had transcatheter aortic valve replacement (TAVR). The postoperative ICU stay was 4 days (IQR 1.8–6.3), and the in-hospital stay was 18 days (11–34). Two patients had a complicated postoperative course requiring surgical revision (bleeding) and died 52 and 219 days after CASH, respectively; all the others are still alive. Four patients underwent aortic valve surgery due to aortic disease (aneurysm/dissection) and are described in the aortic surgery section.
TABLE 4
| Pt | Sex | Age | Euro SCORE II | Creatinine | Time to CASH, y | Urgency | Pathology | Operation | Concomitant procedure | Complications | Mortality | Follow-up, y |
| 1 | m | 54.4 | 16.5 | 1.4 | 0.1 | Urgent | Regurgitation | Mechanical valve | CABG | Bleeding, RRT, stroke, pneumonia | MOF | 0.1 |
| 2 | m | 52.9 | 6.7 | 1.2 | 2.0 | Urgent | Endocarditis | Biological valve | − | 7.7 | ||
| 3 | m | 59.3 | 2.7 | n.a. | 5.3 | Elective | Regurgitation | Mechanical valve | − | 0.5 | ||
| 4 | m | 62.3 | 15.8 | 4.4 | 7.1 | Urgent | Stenosis | Mechanical valve | Mitral + tricuspid | RRT | − | 0.9 |
| 5 | m | 61.3 | 2.8 | 1.7 | 9.7 | Elective | Stenosis | TAVR, femoral | − | 3.8 | ||
| 6 | m | 75.1 | 7.0 | 2.1 | 11.4 | Elective | Stenosis | Biological valve | Bleeding | Bleeding | 0.6 | |
| 7 | m | 83.2 | 5.1 | 1.0 | 22.9 | Elective | Stenosis | TAVR, apical | − | 2.5 | ||
| med | 61.3 (56.9–68.7) | 6.7 (4.0–11.4) | 1.5 (1.3–2.0) | 7.1 (3.7–10.6) |
Aortic valve surgery.
CABG, coronary artery bypass graft; CASH, cardiac surgery after heart transplantation; ICU, intensive care unit; n.a not available; MOF, multi-organ failure; MV, mitral valve; pt, patient; RRT, renal replacement therapy.
Aortic Surgery
Aortic surgery due to ascending aortic pathologies (dissection, aneurysm, or pseudoaneurysm) was performed in 13 patients at 11 centers (Table 5). The median age at CASH was 57.5 (IQR 50.3–61.1) years and the time to CASH was 3.9 (IQR 0.8–7.6) years. Four patients had surgery within the first year (PODs 20, 50, 110, and 235). Allograft function was preserved in all patients. The median preoperative serum creatinine was 1.1 (1.0–1.7) mg/dl. Two patients had reduced kidney function preoperatively, including one patient who was dialysis-dependent.
TABLE 5
| Pt | Sex | Age | Euro SCORE II | Time to CASH, y | Urgency | Indication | Operation | Concomitant Procedure | Complications | Mortality | Follow-up, y |
| 1 | m | 58.6 | 9.4 | 20 days | Urgent | Dissection | Bentall, mechanical | − | 8.5 | ||
| 2 | m | 57.1 | 21.2 | 0.1 | Urgent | Pseudoaneurysm | Supracommissural replacement | TV repair | infection | MOF | 0.3 |
| 3 | m | 61.5 | 10.4 | 0.3 | Urgent | Pseudoaneurysm | Supracommissural replacement | − | 2.6 | ||
| 4 | m | 39.8 | 7.1 | 0.8 | Elective | Pseudoaneurysm | Supracommissural replacement | − | 5.4 | ||
| 5 | m | 58.0 | 13.1 | 1.3 | Elective | Aneurysm | Total arch replacement | CABG, aortic valve, stentgraft | ECMO | − | 0.9 |
| 6 | m | 61.1 | 28.3 | 2.4 | Urgent | Pseudoaneurysm | Supracommissural replacement | Bleeding | Bleeding | 21 days | |
| 7 | f | 53.8 | 9.1 | 3.9 | Urgent | Dissection | Supracommissural replacement | Cardiac | 5.7 | ||
| 8 | m | 50.3 | 23.3 | 5.1 | Urgent | Dissection | Supracommissural replacement | CABG, aortic valve | − | 4.0 | |
| 9 | m | 57.5 | 8.7 | 7.1 | Urgent | Aneurysm | Hemiarch replacement | CABG | − | 0.3 | |
| 10 | m | 48.7 | 3.8 | 7.6 | Elective | Pseudoaneurysm | Supracommissural replacement | Stroke | 3.9 | ||
| 11 | f | 61.6 | 6.7 | 7.7 | Elective | Aneurysm | Supracommissural replacement | Cardiac | 4.2 | ||
| 12 | f | 32.7 | 13.8 | 10.0 | Urgent | Aneurysm | Bentall, biological | CABG | − | 1.1 | |
| 13 | m | 72.3 | 31.3 | 18.1 | Urgent | Dissection | Supracommissural replacement | Malignancy | 1.4 | ||
| med | 57.5 (50.3–61.1) | 10.4 (8.7–21.2) | 3.9 (0.8–7.6) |
Aortic surgery.
CABG, coronary artery bypass graft; CASH, cardiac surgery after heart transplantation; ICU, intensive care unit; MOF, multi-organ failure; pt, patient; TV, tricuspid valve.
The median postoperative ICU stay was 5 (IQR 4–6) days, and the in-hospital stay was 18 (IQR 14–24) days. One patient required temporary ECMO due to postoperative stunning and allograft function subsequently recovered. Early postoperative death (PODs 14 and 97) occurred in two patients after a complicated clinical course. The 1-year survival was 84.6%.
Coronary Artery Bypass Grafting Surgery
Sixteen patients (87.5% male) from eight centers who had CAD as the indication for CABG surgery are described in Table 6. The median age and allograft age at CASH were 60.3 (IQR 54.6–63.3) and 45.8 (IQR 36.9–54.3) years, respectively. The time to CASH was 8.8 (IQR 5.9–9.8) years. Allograft function, as measured by the left ventricular ejection fraction (LVEF), was severely impaired (< 30%) in two patients, preserved (≥ 50%) in seven patients, and data were not available in seven patients. Eleven patients had reduced kidney function preoperatively, including a patient who was dialysis-dependent.
TABLE 6
| Pt | Sex | Age | Euro SCORE II | Time to CASH, y | Urgency | Indication | Operation | OP-details | Complications | Mortality | Follow-up, y | PCI/HTX after CASH |
| 1 | m | 63.0 | 6.8 | 3 days | Urgent | Donor stenosis | Vein RCA | RVAD 13d | − | 12.3 | − | |
| 2 | m | 51.8 | 2.0 | 3.6 | Elective | CAV | LIMA LAD | Cardiac | 5.9 | PCI | ||
| 3 | f | 62.4 | 11.5 | 4.0 | Urgent | Iatrogenic dissection | Vein LAD + CX | Sepsis | MOF | 0.2 | PCI | |
| 4 | m | 59.5 | 3.7 | 5.4 | Urgent | CAV | LIMA LAD, Vein CX | − | 3.8 | − | ||
| 5 | m | 52.9 | 2.7 | 6.1 | Elective | CAV | LIMA CX, RIMA DG | Cardiac | 9.1 | − | ||
| 6 | m | 57.4 | 4.6 | 7.8 | Elective | CAV | Vein LAD + CX | Tumor | 7.1 | PCI | ||
| 7 | m | 65.6 | 5.5 | 7.8 | Elective | CAV | LIMA LAD, RIMA RCA, Vein CX | − | 16.6 | PCI | ||
| 8 | m | 62.4 | 4.0 | 8.5 | Elective | CAV | LIMA LAD, RIMA CX | Tumor | 7.1 | − | ||
| 9 | m | 71.4 | 3.7 | 9.0 | Elective | CAV | LIMA LAD, RIMA RCA (T-graft) | Tumor | 8.5 | − | ||
| 10 | m | 64.3 | 8.7 | 9.3 | Elective | CAV | LIMA LAD, RIMA DG, Radial CX | Sternal infection | Cardiac | 7.6 | − | |
| 11 | m | 55.2 | 2.7 | 9.3 | Elective | CAV | LIMA LAD | off-pump | − | 12.3 | PCI | |
| 12 | f | 59.6 | 2.5 | 9.6 | Elective | CAV | LIMA LAD, Vein CX | − | 11.8 | PCI, Re-HTX | ||
| 13 | m | 60.9 | 2.8 | 10.4 | Elective | CAV | LIMA LAD | MID-CAB | Cardiac | 13.3 | PCI | |
| 14 | m | 38.4 | 8.1 | 10.6 | Elective | CAV | LIMA LAD | MID-CAB | RRT | Infection | 1.7 | − |
| 15 | m | 48.3 | 2.7 | 12.0 | Elective | CAV | LIMA LAD + DG, RIMA CX, Vein RCA | − | 19.2 | PCI | ||
| 16 | m | 69.8 | 29.5 | 16.2 | Urgent | CAV | LIMA LAD, Vein CX + DG + RCA | − | 3.9 | − | ||
| med | 60.3 (54.6–63.3) | 3.9 (2.7–7.1) | 8.8 (5.9–9.8) |
Coronary artery bypass graft surgery.
CABG, coronary artery bypass graft; CAV, cardiac allograft vasculopathy; CASH, cardiac surgery after heart transplantation; CX, circumflex artery; DG, diagonal branch; ICU, intensive care unit; LIMA, left internal mammary artery; n.a., not available; MID-CAB, minimally invasive direct coronary artery bypass; MOF, multi-organ failure; PCI, percutaneous coronary intervention; pt, patient; re-HTX, cardiac retransplantation; RIMA, right internal mammary artery; RVAD.
One patient had percutaneous coronary intervention (PCI) in the allograft prior to CABG surgery. Four patients had diabetes mellitus, including two patients who were insulin-dependent. Coronary angiography was performed in all of the planned CABG surgeries, and computed tomography (CT) was available in only half the patients.
The indications for CASH were CAV (n = 14), iatrogenic left main stem dissection following routine coronary angiography (n = 1), and right coronary artery stenosis unknown at the time of HTX (CABG performed on POD 3). Surgical access was median re-sternotomy in all but two patients who underwent minimally invasive direct coronary artery bypass (MIDCAB). The left internal mammary artery was used as the bypass graft in 81.3% of procedures, and the right internal mammary artery in 37.5%. Saphenous vein grafts were used in half of the patients, radial artery only in one patient.
The median postoperative ICU stay was 2.5 (IQR 1–3.8) days and the in-hospital stay 12.5 (IQR 11–20.8) days. The 1-year survival was 93.8% with only one CASH-related death (POD 59, after iatrogenic left main stem dissection). Due to progression of CAV, half of the patients subsequently had PCI with drug-eluting stents and one patient underwent cardiac retransplantation.
In six additional patients, CABG surgery was a concomitant procedure, and the patients are described in the aortic valve surgery (n = 2), aortic surgery (n = 3), and constrictive pericarditis (n = 1) sections.
Pericardiectomy
Five centers reported 17 patients who had pericardiectomy due to constrictive pericarditis (Table 7). Three centers reported more than one case each (n = 4, 5, and 6). The median age at CASH was 56.3 (IQR 54.7–63.5) years and the time to CASH was 1.7 (0.7–3.1) years. Five patients underwent pericardiectomy in the first year. One patient was on vasopressor support with an urgent indication for surgery. Median preoperative serum creatinine was 1.6 (1.5–2.2) mg/dl. Kidney function was reduced preoperatively [glomerular filtration rate (GFR) < 60 ml/min] in 58.8% of patients, and none were dialysis-dependent. Access was via a median re-sternotomy for extensive pericardiectomy. The median ICU stay was 1.5 (IQR 1–2.5) days and in-hospital stay was 15 (IQR 8–19) days. In one patient, intraoperative injury of the left anterior descending artery resulted in anastomosis of a vein bypass graft. Reintubation was required for pneumonia in one patient, and another patient had a deep sternal infection. One patient had fatal postoperative right heart failure. Overall 1-year survival was 82.4%, and two early deaths were reported (PODs 21 and 83).
TABLE 7
| Pt | Sex | Age | Euro SCORE II | Time to CASH, y | Urgency | Complications | Mortality | Follow-up, y |
| 1 | m | 61.5 | 2.1 | 0.2 | Urgent | n.a. | 2.7 | |
| 2 | m | 63.5 | 4.3 | 0.2 | Elective | Cardiac | 0.9 | |
| 3 | m | 55.1 | 4.7 | 0.3 | Elective | − | 9.1 | |
| 4 | m | 57.4 | 4.7 | 0.5 | Elective | − | 1.5 | |
| 5 | m | 65.7 | 7.7 | 0.7 | Elective | RRT | − | 6.9 |
| 6 | f | 47.5 | 3.4 | 1.0 | Elective | Infection | − | 8.2 |
| 7 | m | 63.6 | 5.2 | 1.5 | Elective | Malignancy | 3.5 | |
| 8 | m | 45.7 | 2.7 | 1.6 | Elective | n.a. | 4.4 | |
| 9 | m | 64.8 | 3.1 | 1.7 | Elective | Infection | 3.5 | |
| 10 | m | 56.3 | 4.7 | 2.6 | Elective | − | 0.2 | |
| 11 | m | 31.8 | 2.7 | 3.0 | Elective | Infection | 4.2 | |
| 12 | m | 26.3 | 4.7 | 3.1 | Elective | − | 9.1 | |
| 13 | m | 61.6 | 2.9 | 3.1 | Elective | n.a. | 3.5 | |
| 14 | m | 55.9 | 2.7 | 3.5 | Elective | Right heart failure | Cardiac | 0.1 |
| 15 | f | 54.7 | 4.6 | 4.3 | Elective | Infection, RRT | MOF | 0.2 |
| 16 | m | 69.4 | 4.7 | 8.3 | Urgent | Malignancy | 14.1 | |
| 17 | m | 55.5 | 2.7 | 17.3 | Elective | − | 3.7 | |
| med | 56.3 (54.7–63.5) | 4.3 (2.7–4.7) | 1.7 (0.7–3.1) |
Pericardiectomy.
CASH, cardiac surgery after heart transplantation; FU, follow-up; ICU, intensive care unit; n.a., not available; MOF, multi-organ failure; pat, patient; RRT, renal replacement therapy.
Other Operations
Left atrial myxoma resection was performed 1.7 and 14.3 years after HTX (Table 8). Both had an uneventful postoperative course and no recurrent disease.
TABLE 8
| Patient 1 | Patient 2 | Patient 3 | |
| Indication | Pulmonary valve regurgitation | Myxoma | Myxoma |
| CASH | Biological valve | Resection | Resection |
| Sex | Male | Female | Male |
| Age | 36.1 | 68 | 28.5 |
| EuroSCORE II | 7.8 | 9.9 | 2.7 |
| Time to CASH, y | 7 days | 1.7 | 14.3 |
| Urgency | Urgent | Elective | Elective |
| Complications | Bleeding, pneumonia | ||
| Cause of death | − | − | − |
| Follow-up, y | 3.8 | 0.7 | 5 |
Other operations.
CASH, cardiac surgery after heart transplantation.
One patient underwent biological pulmonary valve replacement due to regurgitation (valve injury at the time of procurement/implantation) on POD 7. After a complicated postoperative course with surgical revision due to bleeding and pneumonia, the patient is alive 3.8 years after CASH without prosthesis degeneration.
Discussion
Our multicenter study describes the largest cohort of patients with CASH worldwide. We demonstrate that CASH is an acceptable therapy for different cardiovascular pathologies early and late after HTX. Overall in-hospital and 1-year mortality after CASH were acceptable (9.1 and 13.8%, respectively). Urgent indication for CASH, such as endocarditis, infected pseudoaneurysm, aortic dissection, and iatrogenic complications, was strikingly, but not statistically significantly, associated with higher in-hospital mortality. These indications are also associated with high mortality in the general heart surgery population, and in previous reports on CASH (2, 3). Postoperative acute kidney injury requiring renal replacement therapy was the most common complication after CASH and was associated with higher in-hospital mortality. Age at time of CASH and higher EuroSCORE II were also associated with higher in-hospital mortality. However, the effect of age was not statistically significant.
The incidence of postoperative systemic infection and deep sternal wound infection was low (13, 14). Reduction or discontinuation of mammalian target of rapamycin (mTOR) inhibitors several weeks to months prior to elective CASH may be considered due to reports of impaired wound healing (15).
Severe tricuspid regurgitation is the most common reason for CASH (16). HTX-specific tricuspid valve pathologies are biopsy-induced injury to the chordae (leaflets), ischemic injury to the papillary muscle as a consequence of CAV or rejection, and distortion of the valvular apparatus [biatrial implantation technique (2, 17–24). Endocarditis is rare, but is more common than in the general population due to increased risk arising from immunosuppression and frequent central venous access, such as endomyocardial biopsies (25).
Indications for surgery must be carefully considered, especially in patients with ventricular dysfunction and/or pulmonary hypertension because they are at risk of right ventricular failure after CASH. Potential underlying disease processes, such as CAV or acute rejection must be ruled out prior to CASH.
Tricuspid valve repair with annuloplasty should only be performed in HTX patients with annular dilation; valve replacement is recommended in complex valvular pathologies or residual regurgitation after repair (21). Besides the risks associated with life-long anticoagulation, mechanical valve replacement rules out the right-sided endomyocardial biopsy. Interventional edge-to-edge repair of the tricuspid valve using the MitraClip system has been reported with perioperative success (26), but long-term data are lacking.
Mitral valve surgery after HTX has been described rarely. Pathology can be related to annular dilation or degeneration of the leaflets or papillary muscles, typically as a consequence of CAV or acute rejection, often accompanied by ventricular dysfunction (27–29). Iatrogenic injury after left-sided endomyocardial biopsy may lead to acute mitral regurgitation. Mitral stenosis has been described in dialysis-dependent HTX patients in association with hyperparathyroidism (30). Mitral valve replacement may be preferred over repair due to complex valvular pathologies in patients with HTX, and to achieve shorter cardiopulmonary bypass times in patients with ventricular dysfunction. In three of our patients, concomitant tricuspid valve repair or replacement was performed without perioperative complications, but postprocedural death occurred 0.5, 0.9, and 2.8 years after CASH.
Minimally invasive CASH via thoracotomy confers the advantage of avoiding resternotomy. Transcatheter interventions may be reasonable for patients at high surgical risk with appropriate valvular pathology, but the atrial and atrio-ventricular anatomy can be challenging due to distortions after HTX (9).
Cardiac surgery after heart transplantation due to symptomatic degenerative aortic stenosis in the allograft may occur more often with the acceptance of marginal donor hearts. Single-center case reports have reported acceptable perioperative outcomes (16, 31–33), and case reports on TAVR in HTX patients with high surgical risk have presented data on favorable short-term outcomes (4–8); however, long-term data, as well as data are data on the durability of biological and mechanical valves, are lacking. Aortic valve endocarditis after HTX is extremely rare (34). Our cohort included a patient with biological valve replacement due to endocarditis and 7.7 years of follow-up without valvular degeneration.
Aortic surgery was the most heterogeneous group in our series. Case reports have described successful CASH for ascending aortic aneurysm, dissection, or pseudoaneurysm of the aortic anastomosis (1–3, 35). Aortic pathologies after HTX typically arise at the site of aortic anastomosis due to flow turbulence (donor/recipient aortic size mismatch), infection, or hypertension (36). Due to urgency, the precise preoperative planning of surgery is limited in acute type A dissection and infected pseudoaneurysm. Our data are in line with previously published data (1). Two patients with urgent surgery due to infected pseudoaneurysm had early surgery-related deaths, highlighting the high risk of mortality associated with this rare disease (36).
Surgical revascularization for CAV is safe, with acceptable long-term results, in HTX patients with acceptable coronary anatomy (type A lesion, Stanford Classification), and elective indication for surgery (1–3, 37). This approach is generally limited, however, by the diffuse nature of CAV (37, 38) and inexorable disease progression in most patients (3, 39), which may necessitate additional interventions (3, 40). The patency of arterial grafts is superior to vein grafts in CASH, with patency of the internal thoracic artery reported up to 20 years (3, 41). Though the left internal thoracic artery was used in most of our patients, the radial artery had good mid-term results in a published case series and may be an adjunct graft in patients with CABG prior to HTX (3).
Constrictive pericarditis after HTX is rare and is typically associated with recurrent pericardial effusions, allograft rejection, or biopsy-related complications (42–44). As most of the participating centers did not report any cases of constrictive pericarditis requiring pericardiectomy, we cannot draw conclusions as to whether patients were undiagnosed at some centers and whether surgical and/or treatment strategies differed at the three centers that reported cases of constrictive pericarditis.
This study is limited by its retrospective nature, the small numbers for each procedure type, and the fact that participation was by invitation only—not all eligible heart transplant centers reported data. It may be of interest, however, for multinational heart transplant registries to begin collecting data on CASH, given the growing population of heart transplant recipients worldwide with improved long-term survival and the increasing use of minimally invasive surgical and transcatheter approaches.
We conclude that CASH is generally safe, with low in-hospital mortality and postoperative complications in carefully selected patients. Nevertheless, it is rarely performed, with differences in practice between heart transplant centers worldwide. Higher EuroSCORE II and postoperative acute kidney injury are associated with higher in-hospital mortality.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
JG and AA-Z designed the study and wrote the manuscript. JG organized the database. AK performed the statistical analysis. All authors contributed to manuscript revision and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.879612/full#supplementary-material
Abbreviations
- BiVAD
biventricular assist device
- CABG
coronary artery bypass grafting
- CAV
cardiac allograft vasculopathy
- CASH
cardiac surgery after heart transplantation
- CI
confidence interval
- ECMO
extracorporeal membrane oxygenation
- EuroSCORE II
European System for Cardiac Operative Risk Evaluation
- GFR
glomerular filtration rate
- HTX
heart transplantation
- IQR
interquartile range
- LVEF
left ventricular ejection fraction
- OR
odds ratio
- PCI
percutaneous coronary intervention
- POD
postoperative day
- TAVR
transcatheter aortic valve replacement.
References
1.
HolmesTRJanszPCSprattPMacdonaldPSDhitalKHaywardCet alCardiac surgery is successful in heart transplant recipients.Heart Lung Circ. (2014) 23:703–10. 10.1016/j.hlc.2014.03.003
2.
GoerlerHSimonAWarneckeGMeyerALKuehnCHaverichAet alCardiac surgery late after heart transplantation: a safe and effective treatment option.J Thorac Cardiovasc Surg. (2010) 140:433–9. 10.1016/j.jtcvs.2010.02.033
3.
GoeklerJZuckermannAOsorioEBrkicFFUyanik-UenalKLauferGet alCardiac surgery after heart transplantation: elective operation or last exit strategy?Transplant Direct. (2017) 3:e209. 10.1097/TXD.0000000000000725
4.
ZanuttiniDArmelliniIBiscegliaTSpedicatoLBernardiGMuzziRet alTranscatheter aortic valve implantation for degenerative aortic valve regurgitation long after heart transplantation.Ann Thorac Surg. (2013) 96:1864–6. 10.1016/j.athoracsur.2013.03.040
5.
BruschiGDe MarcoFOregliaJColomboPMoreoADe ChiaraBet alTranscatheter aortic valve implantation after heart transplantation.Ann Thorac Surg. (2010) 90:e66–8. 10.1016/j.athoracsur.2010.08.021
6.
ChandolaRCusimanoROstenMHorlickE. Postcardiac transplant transcatheter core valve implantation for aortic insufficiency secondary to impella device placement.Ann Thorac Surg. (2012) 93:e155–7. 10.1016/j.athoracsur.2011.12.025
7.
De PraetereHCiarkaADuboisCHerijgersP. Transapical transcatheter aortic valve implantation in a heart transplant recipient with severely depressed left ventricular function.Interact Cardiovasc Thorac Surg. (2013) 16:906–8. 10.1093/icvts/ivt048
8.
AhmadKTerkelsenCJTerpKAMathiassenONNorgaardBLAndersenHRet alTranscatheter aortic valve implantation in a young heart transplant recipient crossing the traditional boundaries.J Thorac Dis. (2016) 8:E711–4. 10.21037/jtd.2016.07.61
9.
FerraroPBiondi-ZoccaiGGiordanoA. Transcatheter mitral valve repair with mitraclip for significant mitral regurgitation long after heart transplantion.Catheter Cardiovasc Interv. (2016) 88:144–9. 10.1002/ccd.26153
10.
NashefSARoquesFSharplesLDNilssonJSmithCGoldstoneARet alEuroSCORE II.Eur J Cardiothorac Surg. (2012) 41:734–44discussion744–5. 10.1093/ejcts/ezs043
11.
Garcia-ValentinAMestresCABernabeuEBahamondeJAMartinIRuedaCet alValidation and quality measurements for EuroSCORE and EuroSCORE II in the Spanish cardiac surgical population: a prospective, multicentre study.Eur J Cardiothorac Surg. (2016) 49:399–405. 10.1093/ejcts/ezv090
12.
SchemperMSmithTL. A note on quantifying follow-up in studies of failure time.Control Clin Trials. (1996) 17:343–6. 10.1016/0197-2456(96)00075-x
13.
FilsoufiFRahmanianPBCastilloJGPinneySBroumandSRAdamsDH. Incidence, treatment strategies and outcome of deep sternal wound infection after orthotopic heart transplantation.J Heart Lung Transplant. (2007) 26:1084–90. 10.1016/j.healun.2007.07.036
14.
ZuckermannABartenMJ. Surgical wound complications after heart transplantation.Transpl Int. (2011) 24:627–36. 10.1111/j.1432-2277.2011.01247.x
15.
NashanBCitterioF. Wound healing complications and the use of mammalian target of rapamycin inhibitors in kidney transplantation: a critical review of the literature.Transplantation. (2012) 94:547–61. 10.1097/TP.0b013e3182551021
16.
KpodonuJMassadMGGehaAS. Surgical considerations in the correction of valve dysfunction following heart transplantation.Clin Transplant. (2005) 19:694–7. 10.1111/j.1399-0012.2004.00316.x
17.
LocaliRFMatsuokaPKCherboTGabrielEABuffoloE. [Should biatrial heart transplantation still be performed?: a meta-analysis].Arq Bras Cardiol. (2010) 94:829–40. 10.1590/s0066-782x2010000600018
18.
SunJPNiuJBanburyMKZhouLTaylorDOStarlingRCet alInfluence of different implantation techniques on long-term survival after orthotopic heart transplantation: an echocardiographic study.J Heart Lung Transplant. (2007) 26:1243–8. 10.1016/j.healun.2007.09.016
19.
LowerRRShumwayNE. Studies on orthotopic homotransplantation of the canine heart.Surg Forum. (1960) 11:18–9.
20.
CrumbleyAJIIIVan BakelAB. Tricuspid valve repair for biopsy-induced regurgitation after cardiac transplantation.Ann Thorac Surg. (1994) 58:1156–60. 10.1016/0003-4975(94)90478-2
21.
FilsoufiFSalzbergSPAndersonCACouperGSCohnLHAdamsDH. Optimal surgical management of severe tricuspid regurgitation in cardiac transplant patients.J Heart Lung Transplant. (2006) 25:289–93. 10.1016/j.healun.2005.09.013
22.
WongRCAbrahamsZHannaMPangraceJGonzalez-StawinskiGStarlingRet alTricuspid regurgitation after cardiac transplantation: an old problem revisited.J Heart Lung Transplant. (2008) 27:247–52. 10.1016/j.healun.2007.12.011
23.
BergerYHar ZahavYKassifYKoganAKupersteinRFreimarkDet alTricuspid valve regurgitation after orthotopic heart transplantation: prevalence and etiology.J Transplant. (2012) 2012:120702. 10.1155/2012/120702
24.
KwonMHSheminRJ. Tricuspid valve regurgitation after heart transplantation.Ann Cardiothorac Surg. (2017) 6:270–4. 10.21037/acs.2017.04.02
25.
Sherman-WeberSAxelrodPSuhBRubinSBeltramoDManacchioJet alInfective endocarditis following orthotopic heart transplantation: 10 cases and a review of the literature.Transpl Infect Dis. (2004) 6:165–70. 10.1111/j.1399-3062.2004.00074.x
26.
MisumidaNSteidleyDEEleidMF. Edge-to-edge tricuspid valve repair for severe tricuspid regurgitation 20 years after cardiac transplantation.ESC Heart Fail. (2020) 7:4320–5. 10.1002/ehf2.12992
27.
CaveroMAPulponLARubioJABurgosRLozanoIMoreuJet alMitral valve replacement in a heart transplant recipient with iatrogenic mitral regurgitation.Ann Thorac Surg. (1996) 61:1530–2. 10.1016/0003-4975(95)01178-1
28.
WijburgERBalkAHvan HerwerdenLA. Double valve repair in a transplanted heart.J Thorac Cardiovasc Surg. (1998) 115:250–1. 10.1016/s0022-5223(98)70469-6
29.
WigfieldCHLewisAParryGDarkJH. Mitral valve dysfunction and repair following orthotopic heart transplantation: a case report.Transplant Proc. (2008) 40:1796–7. 10.1016/j.transproceed.2007.10.010
30.
DepaceNLRohrerAHKotlerMNBrezinJHParryWR. Rapidly progressing, massive mitral annular calcification. Occurrence in a patient with chronic renal failure.Arch Intern Med. (1981) 141:1663–5.
31.
JoyceDLRussellSDConteJVCattaneoSM. Aortic valve replacement in a diseased bicuspid valve eleven years after transplantation.Interact Cardiovasc Thorac Surg. (2009) 8:594–5. 10.1510/icvts.2008.194050
32.
GoenenMJJacquetLDe KockMVan DyckMSchoevardtsJCChalantCH. Aortic valve replacement thirty-one months after orthotopic heart transplantation.J Heart Lung Transplant. (1991) 10:604–7.
33.
FianeAESvennevigJLFroysakerT. Aortic valve replacement four years after cardiac transplantation.Eur Heart J. (1993) 14:1140–2. 10.1093/eurheartj/14.8.1140
34.
UnicDStarcevicBSicajaMBaricDRudezIBiocicSet alAortic valve endocarditis in a transplanted heart after urethral instrumentation.Ann Thorac Surg. (2013) 96:e61–2. 10.1016/j.athoracsur.2013.03.113
35.
PozziMHannaSSebbagLObadiaJF. Donor aortic dissection in a heart transplantation recipient.Interact Cardiovasc Thorac Surg. (2018) 27:790–1. 10.1093/icvts/ivy153
36.
ViganoMRinaldiMD’ArminiAMPederzolliCMinzioniGGrandeAM. The spectrum of aortic complications after heart transplantation.Ann Thorac Surg. (1999) 68:105–11. 10.1016/s0003-4975(99)00471-3
37.
GaoSZAldermanELSchroederJSSilvermanJFHuntSA. Accelerated coronary vascular disease in the heart transplant patient: coronary arteriographic findings.J Am Coll Cardiol. (1988) 12:334–40. 10.1016/0735-1097(88)90402-0
38.
MusciMPasicMMeyerRLoebeMWellnhoferEWengYet alCoronary artery bypass grafting after orthotopic heart transplantation.Eur J Cardiothorac Surg. (1999) 16:163–8. 10.1016/s1010-7940(99)00207-9
39.
BhamaJKNguyenDQScolieriSTeutebergJJToyodaYKormosRLet alSurgical revascularization for cardiac allograft vasculopathy: is it still an option?J Thorac Cardiovasc Surg. (2009) 137:1488–92. 10.1016/j.jtcvs.2009.02.026
40.
Prada-DelgadoOEstevez-LoureiroRLopez-SainzAGargallo-FernandezPPaniagua-MartinMJMarzoa-RivasRet alPercutaneous coronary interventions and bypass surgery in patients with cardiac allograft vasculopathy: a single-center experience.Transplant Proc. (2012) 44:2657–9. 10.1016/j.transproceed.2012.09.043
41.
LopesRDMehtaRHHafleyGEWilliamsJBMackMJPetersonEDet alProject of Ex Vivo vein graft engineering via transfection IVI. Relationship between vein graft failure and subsequent clinical outcomes after coronary artery bypass surgery.Circulation. (2012) 125:749–56. 10.1161/CIRCULATIONAHA.111.040311
42.
Tchana-SatoVAncionAAnsartFDefraigneJO. Constrictive pericarditis after heart transplantation: a case report.Eur Heart J Case Rep. (2020) 4:1–6. 10.1093/ehjcr/ytaa240
43.
UmerAKhalidNChhabraLMemonSSpodickDH. Constrictive pericarditis complicating cardiac transplantation.J Cardiothorac Surg. (2015) 10:109. 10.1186/s13019-015-0314-x
44.
KumarREntrikinDWNtimWOCarrJJKincaidEHHinesMHet alConstrictive pericarditis after cardiac transplantation: a case report and literature review.J Heart Lung Transplant. (2008) 27:1158–61. 10.1016/j.healun.2008.07.010
Summary
Keywords
cardiac transplantation, heart transplantation, cardiac surgery, heart failure, cardiac retransplantation
Citation
Gökler J, Aliabadi-Zuckermann AZ, Kaider A, Ambardekar AV, Antretter H, Artemiou P, Bertolotti AM, Boeken U, Brossa V, Copeland H, Generosa Crespo-Leiro M, Eixerés-Esteve A, Epailly E, Farag M, Hulman M, Khush KK, Masetti M, Patel J, Ross HJ, Rudež I, Silvestry S, Suarez SM, Vest A and Zuckermann AO (2022) Indications, Complications, and Outcomes of Cardiac Surgery After Heart Transplantation: Results From the Cash Study. Front. Cardiovasc. Med. 10:879612. doi: 10.3389/fcvm.2022.879612
Received
19 February 2022
Accepted
20 April 2022
Published
09 June 2022
Volume
10 - 2022
Edited by
Peter Moritz Becher, University Heart and Vascular Center, University Medical Center Hamburg-Eppendorf, Germany
Reviewed by
Suguru Ohira, Westchester Medical Center, United States; Dan Nistor, Târgu Mureş Emergency Institute for Cardiovascular Diseases and Transplantation (IUBCVT), Romania
Updates
Copyright
© 2022 Gökler, Aliabadi-Zuckermann, Kaider, Ambardekar, Antretter, Artemiou, Bertolotti, Boeken, Brossa, Copeland, Generosa Crespo-Leiro, Eixerés-Esteve, Epailly, Farag, Hulman, Khush, Masetti, Patel, Ross, Rudež, Silvestry, Suarez, Vest and Zuckermann.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johannes Gökler, johannes.goekler@meduniwien.ac.at
This article was submitted to Heart Failure and Transplantation, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.