- Faculty of Medical Sciences, Pontificia Universidad Católica Argentina, Aires, Argentina
Prevention of neurodegenerative diseases is presently a major goal for our Society and melatonin, an unusual phylogenetically conserved molecule present in all aerobic organisms, merits consideration in this respect. Melatonin combines both chronobiotic and cytoprotective properties. As a chronobiotic, melatonin can modify phase and amplitude of biological rhythms. As a cytoprotective molecule, melatonin reverses the low degree inflammatory damage seen in neurodegenerative disorders and aging. Low levels of melatonin in blood characterizes advancing age. In experimental models of Alzheimer's disease (AD) and Parkinson's disease (PD) the neurodegeneration observed is prevented by melatonin. Melatonin also increased removal of toxic proteins by the brain glymphatic system. A limited number of clinical trials endorse melatonin's potentiality in AD and PD, particularly at an early stage of disease. Calculations derived from animal studies indicate cytoprotective melatonin doses in the 40–100 mg/day range. Hence, controlled studies employing melatonin doses in this range are urgently needed. The off-label use of melatonin is discussed.
Introduction
Symmetrical losses of neurons in the cognitive, motor, or sensory systems characterize neurodegenerative diseases showing prominent cognitive symptoms, like Alzheimer's disease (AD) and frontotemporal dementia, or predominantly motor symptoms like Parkinson's disease (PD), Huntington's disease or amyotrophic lateral sclerosis. Neuronal death in these entities can be ascribed to interrelated processes like free radical-mediated degeneration, mitochondrial dysfunction, low degree of inflammation and excitotoxicity (1, 2). Although the regular intake of antioxidants has been proposed for prevention of neurodegeneration, its effectiveness, however, has been questioned (3). In this context, the cytoprotection given by melatonin use deserves to be considered.
Melatonin, an unusual phylogenetically conserved compound present in all known aerobic phyla, has a promising significance as a cytoprotective molecule in addition to its chronobiotic properties (4). The pineal gland is the demonstrable source of melatonin in circulation, the decrease in plasma melatonin being one of the characteristics of advancing age in humans (5). The focus of this article is on the clinical use of melatonin in neurodegenerative diseases. The discussion of the basic biological data is restricted to its relevance for melatonin doses potentially employable in humans.
Basic Biology of Melatonin
“Chronobiotics” are defined as drugs displaying the capacity to synchronize or to increase the amplitude of the circadian rhythms, melatonin being the prototype (6, 7). Light-dark variation of melatonin synthesis defines the essential role of melatonin as a chronobiotic (8). Melatonin “opens the doors of sleep” by inhibiting the propensity to wakefulness derived from the suprachiasmatic nuclei (SCN) in late evening (9, 10). On the other hand, melatonin is the chemical code of darkness, an information crucial to the neuroendocrine system (11).
Although in mammals the circulating melatonin derives almost exclusively from the pineal gland (5), the methoxyindole is synthesized locally in most cells, tissues, and organs (12). Indeed, there is now strong evidence that melatonin is produced in every animal cell that has mitochondria (13, 14), melatonin being involved, among other functions, in the elimination of free radicals and the regulation of immune response to achieve cytoprotection (15).
MT1 and MT2 receptors, all belonging to the superfamily of membrane receptors associated with G proteins (G-protein coupled receptors, GPCR) are involved in the chronobiotic action of melatonin (16). MT1 and MT2 receptors have been identified in the SCN, hippocampus, thalamus, retina, vestibular nuclei and cerebral and cerebellar cortex (17).
More recently, another member, GPR50, was included in the melatonin receptor subfamily showing high sequence homology with MT1 and MT2 (18). However, GPR50 does not bind melatonin or any other known ligand. Rather, they form homo- and heteromers between each other and with other GPCRs (19).
Melatonin is not only generated and metabolized in the mitochondria but it was recently claimed that the neuroprotective effects of melatonin on the brain injury induced by ischemia/reperfusion were mediated by MT1 receptors located in the mitochondria but not in the membrane (20). This is remarkable because a GPCR like the MT1 is known as a cell-surface receptor that transmits extracellular signals into the cell.
Melatonin, an amphiphilic substance, can penetrate cell membranes. In the cytoplasm melatonin interacts with calmodulin and tubulin (21). Melatonin also enters the cell nucleus where the receptor sites were supposed to belong to the orphan receptor superfamily RZR/ROR (15). However, RZR/ROR demonstrably does not bind melatonin. Rather, melatonin may act indirectly via this transcription factor, e.g., by affecting the circadian accessory oscillator component RORα through sirtuin-1 (SIRT-1) activation (22).
The cytoprotective activity of melatonin exceeds that mediated via receptors. The amounts of melatonin found in almost every cell are much higher than those in circulation (12). Although the capacity of mitochondria to synthesize melatonin is now confirmed, intracellular melatonin does not get the extracellular space. Indeed, the doses of melatonin needed to change intracellular melatonin concentration are much higher than those employed as a chronobiotic (23, 24).
In cell cultures, physiologically relevant effects of melatonin are revealed at doses in the range of 10−8 to 10−9 M, these concentrations being enough for almost complete or total receptor saturation (25, 26). However, most studies on neuroprotective and anti-inflammatory effects in animals employ pharmacological doses, which clearly exceed the saturation of the receptor.
The focus in this review is on melatonin effects on neurodegeneration in animal studies as related to the possible human doses to be employed. It must be noted that cell line studies regarding AD and melatonin have delineated important melatonin mediated mechanisms in the line of prevention against AD as well. A comprehensive review on melatonin activity to reverse disrupted signaling mechanisms in neurodegeneration, including proteostasis dysfunction, disruption of autophagic integrity, and anomalies in the insulin, Notch, and Wnt/β-catenin signaling pathways has just been published (27).
In a way largely independent of receptors melatonin has antioxidant and scavenging effects (28). Melatonin has intrinsic free radical scavenging activity as well as is metabolized to compounds that display a higher antioxidant capacity. In addition, melatonin inhibits the synthesis of prooxidant enzymes and facilitates that of antioxidant enzymes. Melatonin exceeds that capacity of vitamin C and E to protect from oxidative damage (29). Melatonin also exerts cytoprotection in ischemia (independently of free radical scavenging) presumably via mitochondrial membrane stabilization (24).
Immunomodulation by melatonin includes proinflammatory and anti-inflammatory effects (30–32). The anti-inflammatory actions are of great medical interest since they are found in high-grade inflammation like brain injury, ischemia/reperfusion or sepsis, as well as in low-grade inflammation like aging or neurodegenerative processes.
The anti-inflammatory properties of melatonin are exerted by inhibiting the binding of nuclear factor κB (NF κB) to DNA (thus decreasing the synthesis of proinflammatory signals), by inhibiting cyclooxygenase (Cox) (21) mainly Cox-2 (33), and by suppressing the expression of the inducible nitric oxide synthase (iNOS) (34). Other signaling pathways involved include prevention of inflammasome NLRP3 activation, upregulation of nuclear factor erythroid 2-related factor 2 and inhibition of toll-like receptor-4 activation and high-mobility group box-1 signaling. The upregulation of SIRT-1 by melatonin appears to be of major importance. Collectively, these effects of melatonin are reflected in reduced levels of proinflammatory cytokines and increased production of anti-inflammatory cytokines (31).
The γ-aminobutyric acid (GABA)-ergic system can be involved in the neuroprotection mediated by melatonin. Indeed, melatonin exerts anti-excitatory and sedative effects (35, 36) and information exists that melatonin gives protection to neurons from the toxicity of the amyloid-β (Aβ) peptide via activation of GABAergic receptors (37). The up-regulation of GABA activity by melatonin could not be blocked by the melatonin receptor antagonist luzindole but it was impaired by the benzodiazepine antagonist flumazenil, suggesting an allosteric modulation of GABAA receptors by melatonin (38).
Melatonin displays also anti-excitotoxic activity. For example, melatonin prevents neuronal death induced by kainate, an ionotropic glutamate receptor agonist (39), and its administration protects hippocampal CA1 neurons from transient anterior ischemia (40), or from high doses of glucocorticoids (41). The lack of effects of luzindole or of the MT2 antagonist 4-phenyl-2-propionamidotetralin (4-P-PDOT) excludes the participation of melatonin receptors in melatonin anti-excitotoxic activity (42).
In addition to the animal models of AD and PD that are discussed in the present paper, melatonin has been shown to reduce neuronal damage due to the toxicity of cadmium (43, 44), hyperbaric hyperoxia (45, 46), toxicity by δ-aminolevulinic acid (47), γ radiation (48), focal ischemia (49), brain trauma (50, 51), and that resultant from several neurotoxins (52).
Melatonin Activity in Animal Models of AD
The extracellular deposits of Aß-formed senile plaques and the intracellular accumulation of neurofibrillary tangles due to hyperphosphorylation of tau protein are the pathological signatures of AD (1, 2). Aß promotes neuronal degeneration in AD neurons that have become vulnerable to age-related increases of oxidative stress and altered cellular energy metabolism. Hyperphosphorylated tau protein promotes the assembly of microtubules and is an important factor in stabilizing microtubules (1, 2).
The 39–43 amino acid residue Aß derives from the amyloid precursor protein (APP). Melatonin interferes with the maturation of APP in several cell lines (53).
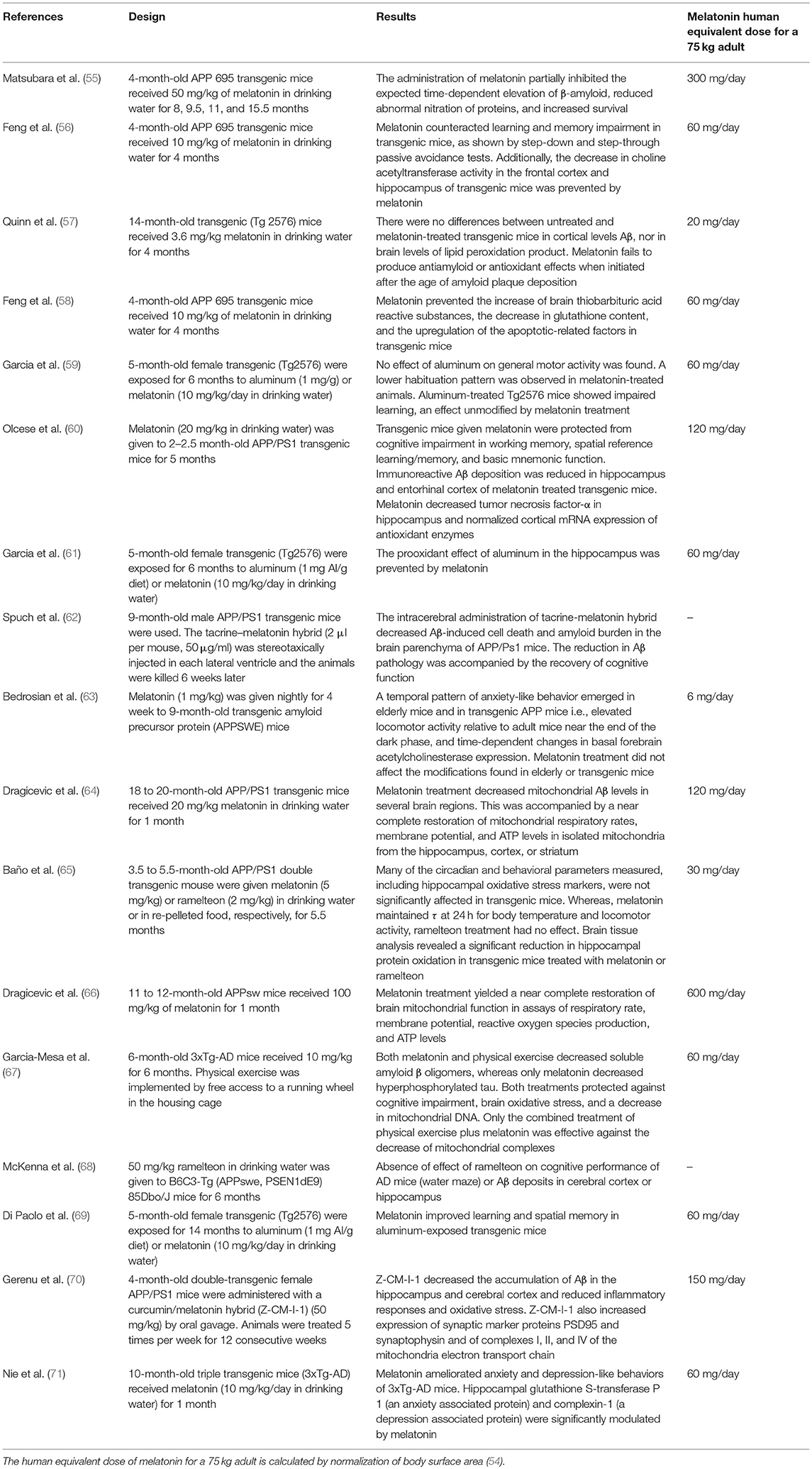
Table 1 summarizes the effect of melatonin in transgenic models of AD. The data indicate that melatonin modulates APP and Aß metabolism principally at the initial phases of the pathological process. From the doses of melatonin used in these different transgenic models, the human equivalent dose of melatonin for a 75 kg adult can be calculated by normalization of body surface area (54). Noteworthy, theoretical human equivalent doses calculated from Table 1 results ranged from 2- to 3-orders of magnitude greater than those employed in humans. However, a note of caution must be made since these studies include changes in the expression of genes with mutations characteristic of the hereditary form of AD, only responsible for 5% of AD cases. Senescence-accelerated OXYS rats appears to be a suitable non-transgenic model of sporadic AD (which accounts for 95% of AD patients) as characterized by the progressive age-related aggregation of Aβ and hyperphosphorylation of τ protein as well as mitochondrial dysfunction, loss of synapses, neuronal death, and concomitant cognitive decline (72). Remarkable, a very low dose of melatonin (0.04 mg daily p.o.) was effective to prevent all these changes (73). Additional studies are needed to solve the dose incongruences observed.
How melatonin inhibits generation of Aβ remains undefined. Melatonin may interact with Aß40 and Aß42 inhibiting the formation of progressive β-sheet and/or amyloid fibrils (74, 75). Such interaction appears to be independent on melatonin antioxidant properties (74).
Via blockage of formation of secondary sheets, melatonin may facilitate the peptide clearance induced by proteolytic degradation. Since GSK−3 is a common signaling pathway increasing Aß generation and tau hyperphosphorylation, melatonin could regulate APP and tau processing via protein kinase (PK) C activation (76, 77) and inhibition of GSK-3 pathway (78). Free radical are involved in Aß-induced neurotoxicity and cell death, melatonin effectively protecting cells against oxidative damage in vitro (79, 80) and in vivo (81–83).
In N2a and SH-SY5Y neuroblastoma cells exposed to wortmannin (84), calyculin A (85, 86), or okadaic acid (87–89) melatonin efficiently attenuated tau hyperphosphorylation via protein kinases and phosphatases, as well as antagonizes the oxidative stress given by these agents (90, 91). Regulation of PK A (92), PK C (93), Ca2+/calmodulin-dependent kinase II (94), and mitogen-activated protein kinase are other effects of melatonin unrelated to its antioxidant properties (95).
A crucial phenomenon for brain homeostasis is waste products' elimination by the glymphatic system. The term “glymphatic” describes active, lymphatic-like, water exchange movements in the brain extracellular space (ECS) driven by perivascular astrocytes, which contain aquaporin-4 (AQP4) located in their end feet (96). Since the elimination of Aβ peptide is strongly reduced in AQP4−/− mice (97), occurrence of an AQP4-driven glymphatic Aβ clearance seems feasible.
During sleep, the elimination of Aβ peptides increases considerably (98). Thus, the sleep disturbance found as a comorbidity in AD may contribute to the development and progression of the disease via a failure of Aβ clearance. Sleep deprivation disrupted apolipoprotein E clearance from brain ECS (99). That the glymphatic system participates in tau protein clearance was further indicated by the demonstration that AQP4 deficiency augmented the presence of extracellular tau and neuronal tangle formation in a murine model of traumatic brain injury (100).
An important recent observation by Pappolla et al. indicate that the administration of melatonin to AD transgenic mice augments the glymphatic clearance of Aβ (101). Relevant to this, melatonin is known to preserve slow wave sleep in patients (102). Indeed, glymphatic dysfunction has been related to various neurological disease in addition to AD, like stroke or traumatic brain injury (103).
The rise in the expression of proinflammatory cytokines triggered by microglial activation seems to play a role in the pathogenesis of AD (1, 2). Microglial release of proinflammatory cytokines induced by NF kB, Aß, and nitric oxide is effectively halted by melatonin (83). Binding of NF kB to DNA was also inhibited by melatonin (22, 31).
Clinical Application of Melatonin in AD
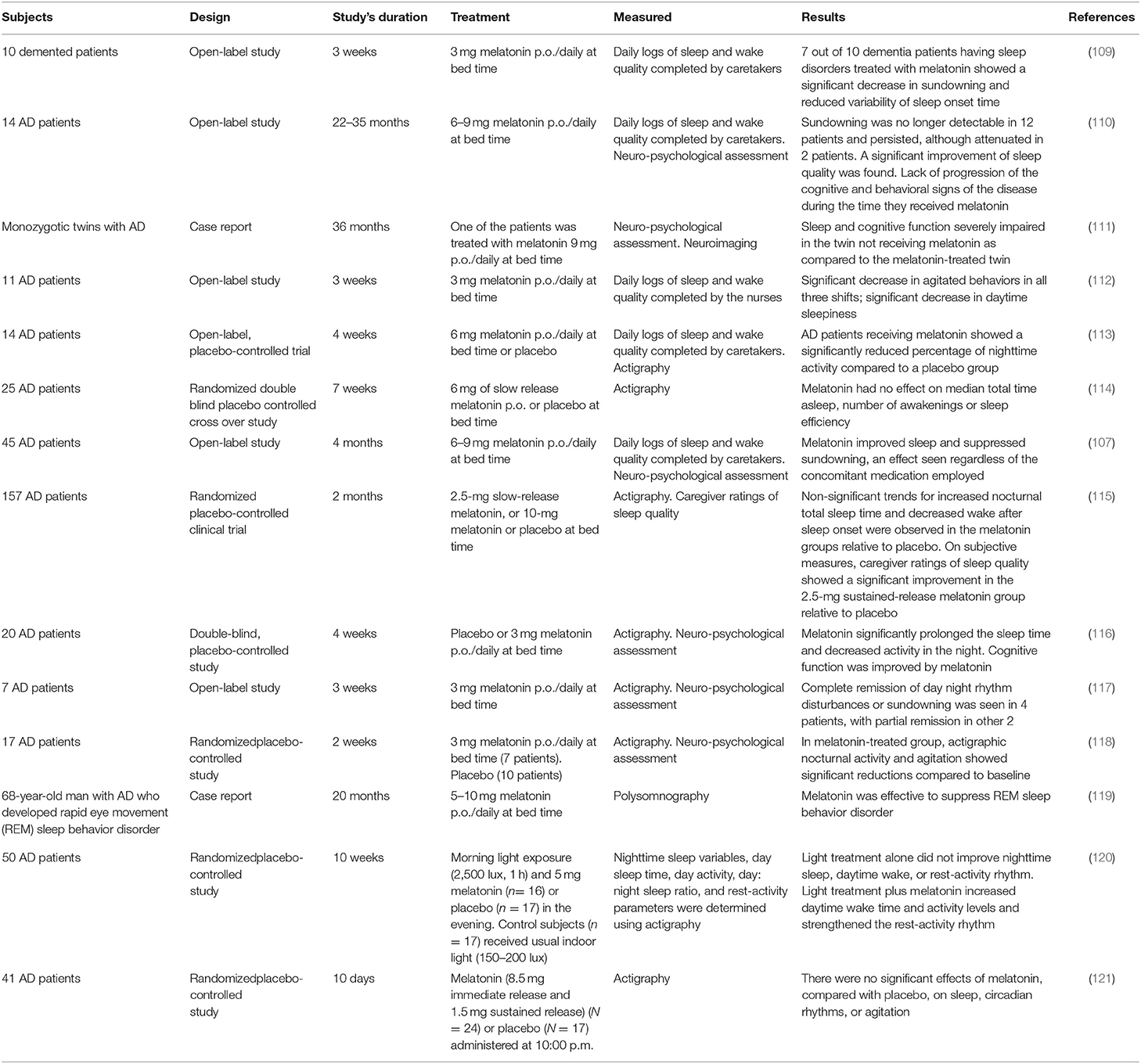
Cerebrospinal fluid (CSF) concentration of melatonin decreases even at the preclinical stages of AD (104). Circulating melatonin correlates negatively with neuropsychological evaluation in mild cognitive impairment (MCI) and AD patients (105). The relative deficiency of melatonin could be the cause or a consequence of neurodegeneration. In any event, the loss of melatonin aggravates the disease and causes early circadian disturbance as shown by “sundowning” (106). Sundowning comprises late afternoon or evening symptoms such as agitation, wandering, disorganized thinking, perceptual and emotional disturbances and reductions in attention. Chronotherapeutic interventions, such as timed administration of melatonin and exposure to bright light, relieve sundowning and improved sleep in AD patients (107, 108).
The irregular sleep/wake found in AD is effectively treated by melatonin (Table 2). A significant decrease in sundowning and reduced variability of sleep onset time were found in 7 out of 10 dementia patients with sleep disorders treated with 3 mg melatonin at bedtime for 3 weeks (109).
In another study including 14 AD patients treated with 6–9 mg/day for a period of 2–3 years, sleep quality improved (110). Sundowning was no longer detectable except for 2 patients. We also observed improvement of cognitive performance and reduction of amnesia after melatonin treatment. In a case report of monozygotic twins with AD followed for 36 months we reported a better sleep and cognitive function in the twin receiving melatonin (111).
The effectiveness of melatonin to improve sleep and alleviate sundowning were reported in open-label and placebo-controlled studies in AD patients (107, 112, 113, 115–120). Negative results were also published in fully developed AD patients treated with melatonin (114, 121). Indeed, large interindividual differences in sleep and agitation are common among AD patients.
A review of published results on the use of melatonin in AD (122) yielded seven reports (5 open studies, 2 case reports) (N = 89 patients) that supported a possible efficacy of melatonin in improving sleep, decreasing sundowning and improving cognitive deterioration. In six double blind, randomized placebo-controlled trials (N = 210 patients) sleep quality increased, sundowning decreased, and cognitive performance improved in 4 studies (N = 143) whereas there was absence of significant effects in 2 studies (N = 67) (122). Two meta-analyses supported the view that melatonin therapy is effective in improving sleep in patients with dementia (123, 124). In addition, the melatonergic agent ramelteon was effective in treating delirium of elderly patients in intensive care units (125).
Whether melatonin has any value in the treatment of fully developed AD remains undefined. It should be noted that the heterogeneity in pathology of the group examined is probably very high at this stage of disease. Therefore, information obtained at an earlier phase could be more valuable.
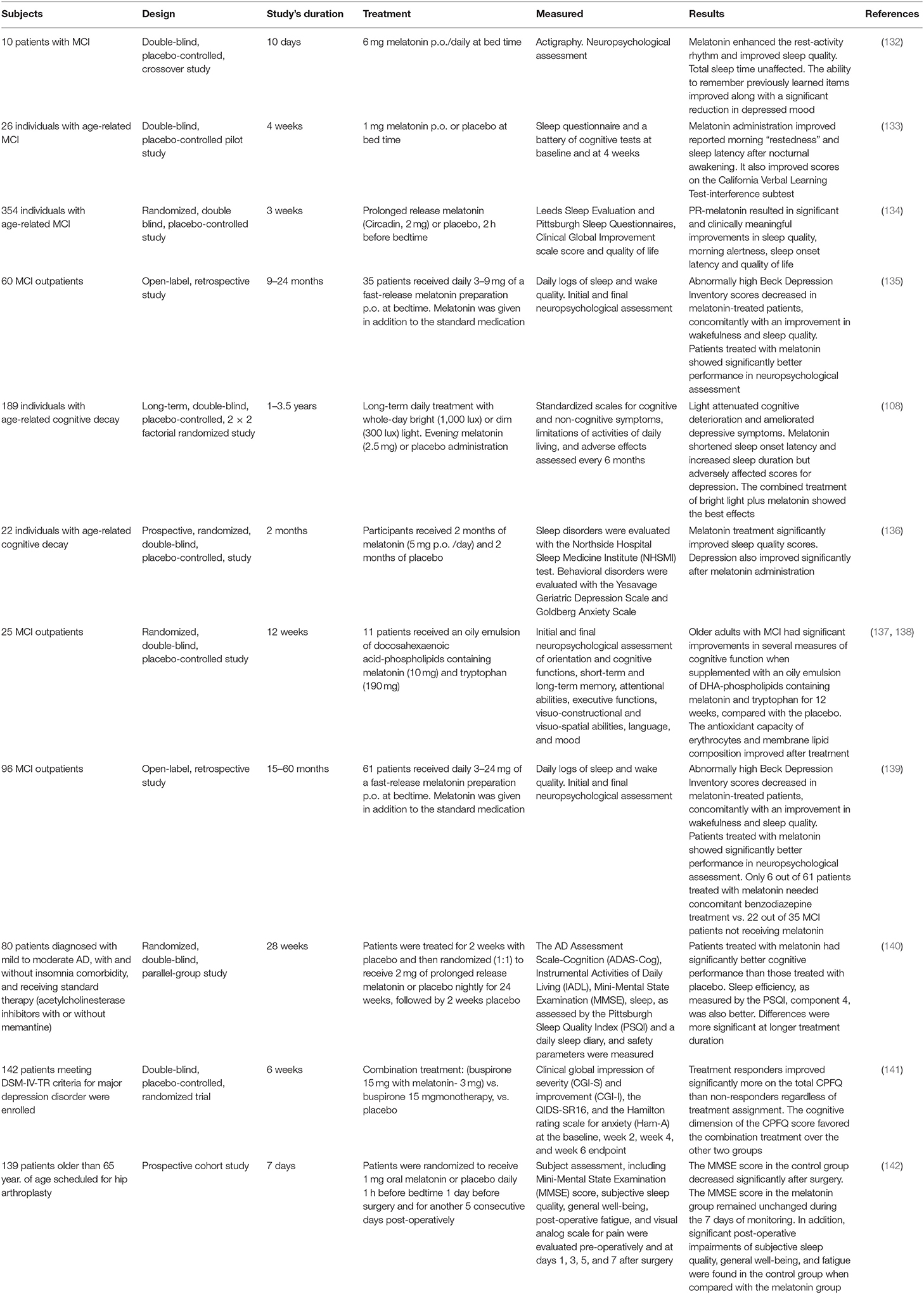
Patients with MCI have a deficit in cognitive functions with preservation of daily activities. MCI is a clinically important stage to identify and treat people at risk (126) because the estimate of the annual rate of conversion of MCI to dementia can be as high as 10–15%, In fact, the degenerative process in the brain of AD begins 20–30 years before the clinical onset of the disease (127–131).
As shown in Table 3, data published from MCI patients consistently showed that melatonin administration improves cognitive performance and sleep quality. For example, we reported a significant improvement of cognitive and depressive symptoms and sleep quality in 35 patients with MCI treated for up to 2 years with 3–9 mg/day of melatonin as an adjuvant (130). Significantly lower scores in Beck Depression Inventory and better performance in neuropsychological tests and in sleep and wakefulness subjective assessment were documented in 61 outpatients diagnosed with MCI and receiving 3–24 mg of melatonin daily for 15–60 months (134). Collectively, the results of Table 3 indicate that melatonin is an adjuvant drug useful for the treatment of MCI in a clinical setting.
The mechanisms that explain the therapeutic effect of melatonin in patients with MCI have not yet been defined. Promotion of slow-wave sleep in the elderly could be beneficial in MCI by increasing the functioning of the glymphatic system, or the secretion of growth hormone and neurotrophins, linked to the restorative phase of sleep.
The question of whether melatonin has a therapeutic value in the prevention or treatment of MCI deserves further analysis. Multicenter double-blind studies are needed to explore and further investigate the potential and utility of melatonin as a preventive drug against dementia. The doses of melatonin used should be re-evaluated in view of the equivalent human doses of melatonin derived from preclinical data, as indicated in Table 1. Unfortunately, of the 64 clinical trials related to melatonin in an initial state (recruitment and non-recruitment) listed in PubMed (ClinicalTrials.gov Search results 01/03/2019) none is directed to this query.
Studies on Melatonin Activity in Animal Models of PD
The progressive degeneration of neurons containing dopamine (DA) in the substantia nigra pars compacta (SNpc) characterizes PD (143). Since Lewy bodies are found not only in DA neurons but also in noradrenergic neurons of the brainstem, in serotonergic neurons of the raphe nuclei and in specific cholinergic neurons, PD is seen as a progressive disease affecting a variety of neurotransmitter systems. This explains the number of non-motor symptoms in PD, such as genitourinary, gastrointestinal, respiratory and cardiovascular disorders, anosmia and neuropsychiatric, visual, and sleep-related disorders. In fact, the non-motor preclinical phase of PD can cover more than 20 years, the relevance of neuroprotection being evident in this respect (144).
The inflammatory signature found in the pathogenesis of PD includes microglial activation, astrogliosis and lymphocytic infiltration (145). Several inflammatory mediators, e.g., NF-κB, interleukin (IL)-1, IL-6, Cox-2, tumor necrosis factor-α, iNOS, and interferon-γ are produced by glial cells (2).
PD and other Lewy body diseases are characterized by the aggregation of fibrillar α-synuclein (146). Mitochondrial dysfunction plays a role in this process since the folding and aggregation of proteins are promoted by free radicals (147, 148).
To develop animal models of altered brain DA function, 6-hydroxydopamine (6-OHDA), or the neurotoxin 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP), were injected into the nigrostriatal pathway of the rat (149).
Because of its potentiality to cause the disease in humans and in subhuman primates, MPTP is preferred among other neurotoxins to emulate parkinsonism in animal models. MPTP is selectively taken up by astrocytes and is metabolized into methyl 1-4 phenyl pyridinium (MPP+), this cation causing increased production of free radicals, depletion of ATP, and apoptosis. MPTP toxicity is selective to SNpc neurons and induced loss of striatal spines in non-human primates (150). Such striatal spine loss is a consistent neuropathologic finding in post-mortem PD human brains. Although the MPTP-treated monkey is considered the best experimental model of PD, a major drawback is the consistent lack of other neuronal loss besides the nigrostriatal dopaminergic system (151).
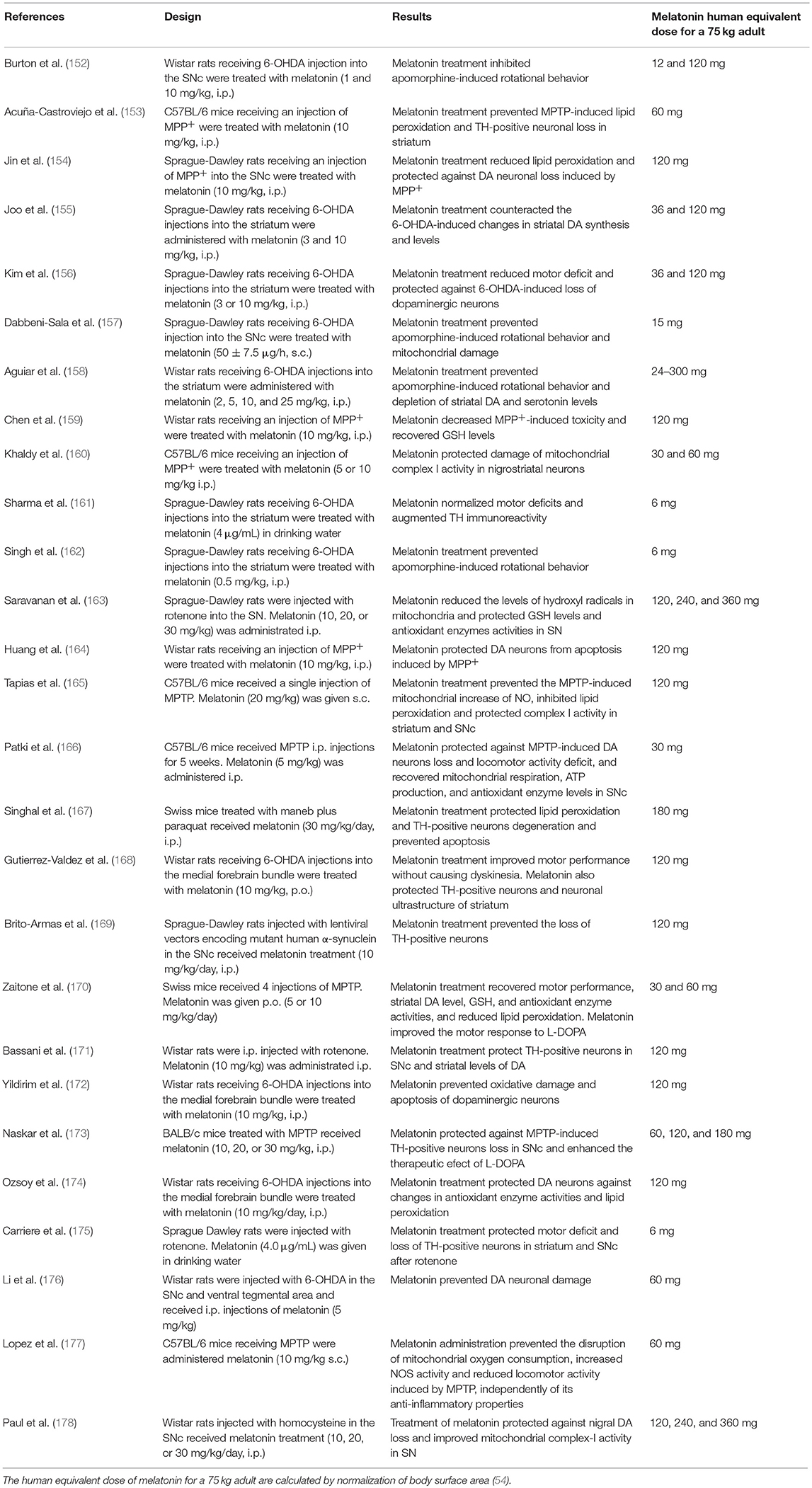
In Table 4 the in vivo effects of melatonin in several experimental models of PD are shown. Most experiments support the role of melatonin in prevention and treatment of experimental PD. As in the case of Table 1, the human equivalent doses of melatonin for a 75 kg adult, calculated by normalization of body surface area (54), are quoted for the sake of comparison with those employed in PD patients. Again, theoretical human equivalent doses derived from animal data are considerably greater than those employed in humans.
Most of the studies summarized in Table 4 include pretreatment with the methoxyindole and therefore they rely on the neuroprotective effect of melatonin preventing the death of dopaminergic neurons and consequently motor dysfunction (Table 4). In addition, some studies failed to observe motor benefits of melatonin treatment in animal models of PD regardless of protection of neurodegeneration. For example, Bassani et al. reported that the melatonin post-treatment for 28 days preserved tyrosine hydroxylase positive neurons and DA levels of rotenone-lesioned rats and improved the depressive-like behavior in the absence of significant improvement of motor deficit (171).
In PD patients, administration of 3 mg of melatonin for 4 weeks improved the quality of sleep but did not affect motor symptoms (179). Moreover, by evaluating the effects of slow release melatonin preparation via intracerebroventricular implants in rats injected with 6-OHDA or MPTP, Willis and Armstrong (180) reported that melatonin indeed deteriorated motor performance. Remarkably, other studies reported an enhancement of motor deficit in animal models of PD after the administration of the antagonist of melatonin receptors ML-23 (181). The beneficial effect of melatonin antagonism on motor symptoms of PD could be explained by inhibition of DA release by the methoxyindole (182).
Regardless of these discrepancies, melatonin preventing activity on PD-related neurodegeneration is generally accepted (183–185). For example, melatonin inhibits α-synuclein assembly and attenuated kainic acid-induced neurotoxicity (186) and arsenite-induced apoptosis (187). Melatonin also impaired the augmented expression of α-synuclein in DA containing neurons following amphetamine administration (188, 189). Melatonin blocked α-synuclein fibril formation and destabilized preformed fibrils by inhibiting protofibril formation and secondary structure transitions and by reducing α-synuclein cytotoxicity (169, 190).
An insufficient clearance by the autophagic–lysosomal network (146) can explain the accumulation and spread of oligomeric forms of neurotoxic α-synuclein. In addition, other clearance pathways are compromised, like the ubiquitin–proteasome system, the autophagy mediated by chaperone, extracellular clearance by proteases, or entrance into the general circulation via the glymphatic system (146).
As above mentioned, the elimination of waste products by the glymphatic system considerably contributes to recovery processes in the brain. The role of AQ4 water channels in the glymphatic system seems to be crucial and, remarkably, AQ4 expression is severely disrupted in PD brains (191). It may explain why the CSF α-synuclein levels inversely correlate with symptoms in PD patients (192). The association of loss of sleep with impairment of the glymphatic clearance is important in the case of PD because rapid eye movement sleep behavior disorder (RBD) is a prodrome of PD. Melatonin administration to animals augments glymphatic clearance (101) as well as preserved sleep in patients. Curiously, melatonin was not listed in the myriad of drugs affecting anomalous protein clearance in the brain (146).
Symptomatically, an effective treatment for PD is the supplementation of DA in its precursor form L-dihydroxyphenylalanine (L-DOPA) that crosses the blood brain barrier. However, long-term administration of -L-DOPA leads to motor side effects like dyskinesias (193, 194). Moreover, administration of L-DOPA in high doses leads to production of neurotoxic molecules like 6-OHDA. Therefore, efforts to reduce the intake or to compensate for the side effects of L-DOPA are in the vogue. In MPTP-treated mice, melatonin, but not L-DOPA, restored striatal spine density, supporting the application of melatonin as an adjuvant to L-DOPA therapy in PD (173).
Clinical Use of Melatonin in PD
Approximately 3/4 of the dopaminergic cells in the SNpc need to be lost to uncover motor symptomatology in PD. However, non-motor symptoms like hyposmia, depression, or RBD (characterized by the occurrence of vivid, intense, and violent movements during REM sleep) precede the onset of PD for years and are index of worse prognosis (144). Indeed, up to 65% of patients showing RBD developed PD 10–13 years later (195).
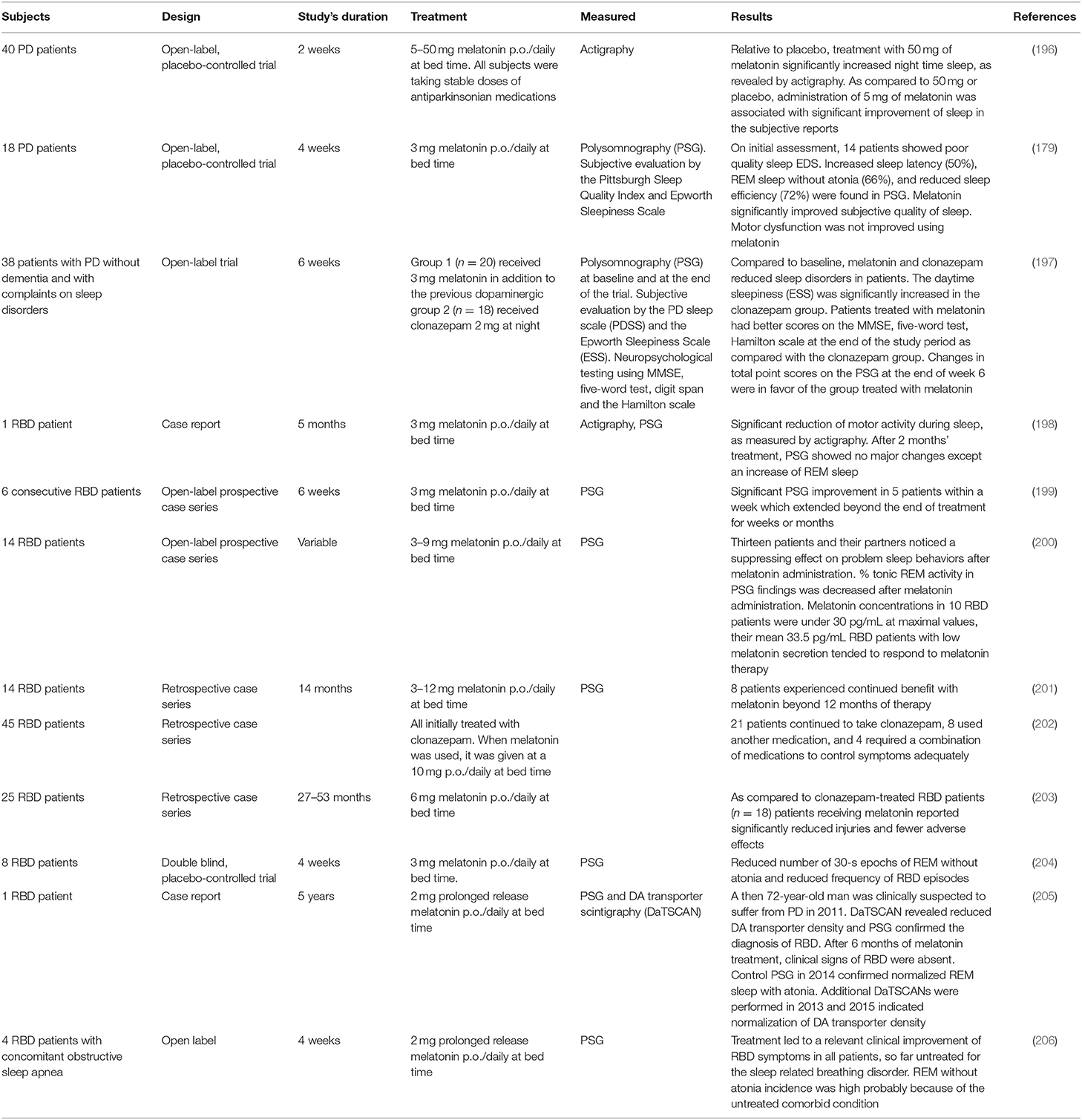
Table 5 summarizes the clinical studies reporting melatonin use in PD. Daily administration of 3–12 mg of melatonin at bedtime is effective in the treatment of RBD (198–206). Polysomnography (PSG) in RBD patients treated with melatonin showed significant decreases in number of R epochs without atonia and in movement time during REM sleep, contrasting with the persistence of muscle tone in R sleep seen with patients treated with clonazepam. Based on these data, a clinical consensus recommended melatonin use in RBD at Level B (207).
Another consensus has claimed for trials with neuroprotective agents in RBD based on the high conversion rate from idiopathic RBD to PD (195). Indeed, the conversion rate to synucleinopathy in clonazepam-treated RBD patients is high (208, 209). Although no comparable data are available yet for melatonin-treated RBD patients, a recent observation by Kunz and Bes deserves to be considered (205). The investigators reported the increase in DA transporter density (as assessed by DA transporter scintigraphy) over successive years in a 72-year-old RBD male patient treated with 2 mg of slow release melatonin daily. After 6 months of gradual improvement, clinical and PSG signs of RBD disappeared. Whereas, the scan prior to melatonin treatment had clear signs of PD, the scan recorded 2 years later was considered borderline, with absence of any sign of PD 4 years after the first scan. The results were interpreted as a possible neuroprotective role for melatonin in synucleinopathy (205).
A phase advance in nocturnal melatonin secretion was reported in L-DOPA-treated parkinsonian patients (210, 211). L-DOPA-treated patients exhibited an increase in daytime melatonin secretion perhaps as an adaptive response to neurodegeneration (211). In a study aiming to examine circadian dysfunction as a cause for excessive sleepiness in PD, blunted circadian rhythms of melatonin were reported (212). The amplitude of the melatonin rhythm decreased in PD patients, mainly in those depicting excessive daytime sleepiness. Thus, a chronobiological approach to improve circadian function, such as timed exposure to melatonin and bright light, could serve as an adjuvant therapy for the non-motor manifestations of PD.
An association between motor fluctuations in PD and diurnal variation in circulating melatonin levels was postulated via possible interactions of melatonin with monoamines (DA, serotonin) in the striatal complex (213). Nearly half of the patients with PD showed L-DOPA-related motor complications after 5 years of treatment. In view of the results obtained in experimental parkinsonism discussed above, the use of melatonin as an adjuvant to decrease the therapeutic dosage of L-DOPA in PD deserves to be considered (214).
Wearing-off episodes in PD could be related to loss of the inhibitory motor effect of melatonin, since stimulation of globus pallidus improved motor symptoms and complications in patients with PD as well as inhibited the increase in daytime plasma melatonin levels found (215). Relevant to the subject of the present review, genetic susceptibility and life-style factors (e.g., smoking) have been entertained to explain the epidemiological that longer years of working night shifts are associated with reduced risk of PD and decreased melatonin levels (216).
Patients with PD showed decreased melatonin MT1 and MT2 receptor density in amygdala and substantia nigra (217). Supporting that a disrupted melatonergic system could be involved in the altered sleep/wake cycle seen in PD, an actigraphic study undertaken in 40 PD patients indicated that melatonin (50 mg/day at bedtime) increased nighttime sleep. Those patients taking 5 mg of melatonin only reported a significant improvement of subjectively evaluated sleep (196).
In another study, 18 PD patients were randomized after performing a basal PSG to receive melatonin (3 mg) or placebo 1 h before bedtime for 4 weeks (179). Although melatonin significantly improved the subjective quality of sleep, the motor dysfunction was not improved (179).
An important experimental study carried out in the MPTP monkey model of PD evaluated the effects of melatonin and L-DOPA on sleep disorders as monitored by PSG (218). The combined treatment of melatonin and L-DOPA significantly curtailed sleep fragmentation at night and sleep episodes during the day seen in MPTP-treated monkeys, thus indicating that melatonin treatment may have the therapeutic potential to treat sleep disorder in PD patients.
Exposure to 1–1.5 h of light (1,000–1,500 lux) prior to bedtime reduced bradykinesia and rigidity in PD patients, as well as agitation and psychiatric side effects (219). The authors concluded that suppressing melatonin secretion with bright light may have a therapeutic value for treating the symptoms of PD (220). However, suppression of melatonin secretion may not be the likely mechanism by which artificial light exerts its therapeutic effect, as shown in depressive patients subjected to phototherapy (221). In any event, the circadian system is considered a novel diagnostic and therapeutic target in PD (212, 222).
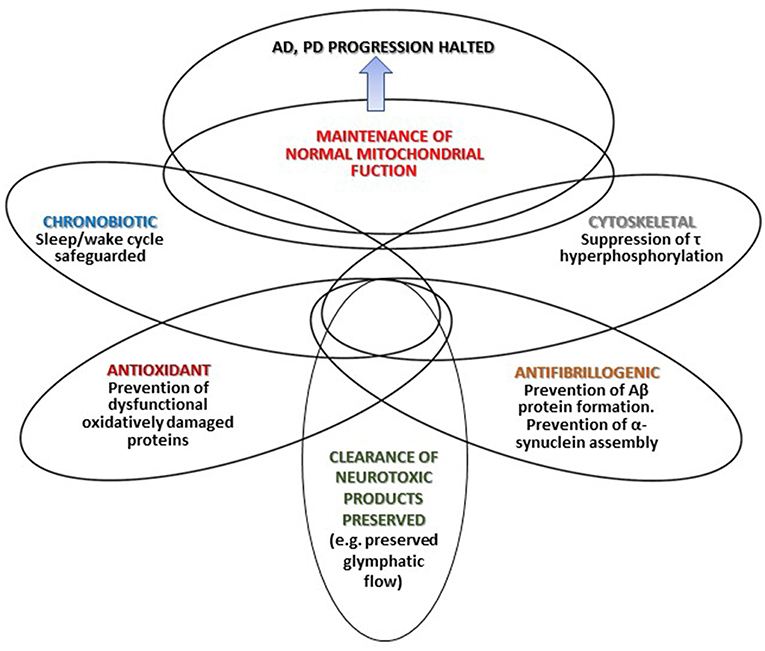
Figure 1 summarizes that different mechanisms by which melatonin may halt AD and PD progression. Depicted intersections in the Figure represent the multiple effects of melatonin and the different degree of overlap (interrelations and mutual influences) discussed in the text.
Conclusions
Because of both hypnotic and chronobiotic properties, the use of melatonin has been recommended for treatment of insomnia (223, 224). Several meta-analyses support such therapeutic role (225–227). A consensus of the British Association for Psychopharmacology on evidence-based treatment of insomnia concluded that melatonin is the first choice treatment when a hypnotic is indicated in patients over 55 years (228).
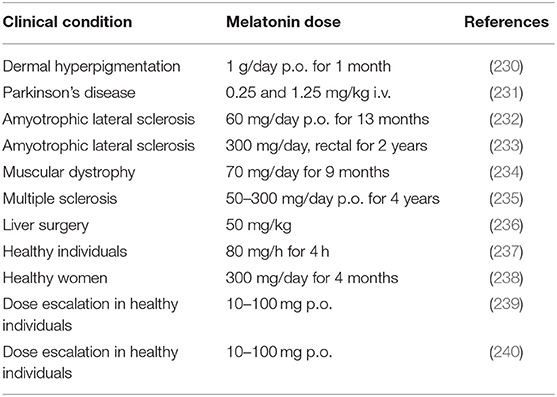
As discussed in this article, studies using 2–5 mg melatonin/day are unsuitable to give appropriate comparison with data on neurodegeneration protection derived from animal studies. Melatonin is remarkably atoxic and its safety is very high. Lethal dose 50 (LD50) for intraperitoneal melatonin injection was 1,131 mg/kg for mice and 1,168 mg/kg for rats. However, LD50 could not be constructed after oral administration of up to 3,200 mg of melatonin/kg in rats, or after subcutaneous injection of up to 1,600 mg/kg in rats and mice (229). In humans, melatonin has a high safety profile and it is usually remarkably well-tolerated (Table 6). Presently, the only option for the incumbent physician interested in the use of melatonin as a cytoprotective is the off-label indication of the drug.
Off-label drugs are defined as drug uses that not included in the indications or dosage regimens listed by the administrative body that registers, controls, and monitors medicines authorized, e.g., the Food and Drug Administration in USA (241). Off label drug use is common in intensive care unit, pediatrics, psychiatry and oncology (242–245). In general, no law prohibits off-label drug use and prescribing off-label is legally accepted in most legislations (246).
In Argentina, the National Administration for Medicaments, Food and Medical Technology (ANMAT) approved melatonin (3 mg capsules or tablets) as an over-the-counter medication in 1995. In 2017 ANMAT authorized a prolonged release preparation of 2 mg melatonin (CircadinR) as a prescription drug. Although ANMAT cannot authorize the use of a medication for an indication not listed in the package leaflet, it does not mean that the indication of a medication for other clinical situations is prohibited. In accordance to ANMAT, The off-label prescriptions are “the sole responsibility of the attending physician, who performs them in the full exercise of their professional activity, based on their experience and the available scientific knowledge, motivated by the need to provide an answer to health problems for which there are no standards of treatment or that, in case of existing, they are very difficult to access.”
In many countries, melatonin is used as a food supplement or dietetic products. Indeed, the European Food Safety Authority (EFSA) has endorsed the health claim that melatonin reduces sleep onset latency (247, 248). Thus, melatonin, melatonin-rich food and bioextracts could now be developed.
Overexpression of melatonin in plants facilitates the germination of seeds and protects plants from abiotic and biotic stress (249–251) and potentially genetically manipulated plants may have use in human nutrition. In parallel, toxicity of long-term melatonin use must be evaluated.
In conclusion, from animal studies several potentially useful effects of melatonin, like those in neurodegenerative disorders, need high doses of melatonin to become apparent. Regardless of the amount of experimental data gathered as far as how melatonin acts in animal and cell models, in most cases it is not known whether it works as a chronobiotic drug, as an endogenous antioxidant or as an immunomodulatory compound. This is an important caveat deserving consideration (252).
Although melatonin is remarkably atoxic and its safety is very high in adults, caution must be exerted with melatonin use in children taking in consideration that melatonin is known to inhibit LH secretion from neonatal pituitary gonadotrophs in the rat (253). Even if similar effect has not yet been documented in human, it cannot be excluded that treatment with high doses of exogenous melatonin could influence the development of the reproductive system.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Jeong S. Molecular and cellular basis of neurodegeneration in Alzheimer's disease. Mol Cells. (2017) 40:613–20. doi: 10.14348/molcells.2017.0096
2. Tan SH, Karri V, Tay NWR, Chang KH, Ah HY, Ng PQ, et al. Emerging pathways to neurodegeneration: Dissecting the critical molecular mechanisms in Alzheimer's disease, Parkinson's disease. Biomed Pharmacother. (2019) 111:765–77 doi: 10.1016/j.biopha.2018.12.101
3. Davies JMS, Cillard J, Friguet B, Cadenas E, Cadet J, Cayce R, et al. The Oxygen Paradox, the French Paradox, and age-related diseases. Geroscience. (2017) 39:499–550. doi: 10.1007/s11357-017-0002-y
4. Tan DX, Zheng X, Kong J, Manchester LC, Hardeland R, Kim SJ, et al. Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: relation to their biological functions. Int J Mol Sci. (2014) 15:15858–90. doi: 10.3390/ijms150915858
5. Claustrat B, Leston J. Melatonin: physiological effects in humans. Neurochirurgie. (2015) 61:77–84. doi: 10.1016/j.neuchi.2015.03.002
6. Dawson D, Armstrong SM. Chronobiotics–drugs that shift rhythms. Pharmacol Ther. (1996) 69:15–36. doi: 10.1016/0163-7258(95)02020-9
7. Cardinali DP, Pandi-Perumal SR, Srinivasan V, Spence DW, Trakht I. Therapeutic potential of melatonin agonists. Exp Rev Endocrinol Metab. (2008) 3:269–79. doi: 10.1586/17446651.3.2.269
8. Cardinali DP. Melatonin. A mammalian pineal hormone. Endocr Rev. (1981) 2:327–46 doi: 10.1210/edrv-2-3-327
9. Lavie P. Melatonin: role in gating nocturnal rise in sleep propensity. J Biol Rhythms. (1997) 12:657–65 doi: 10.1177/074873049701200622
10. Lewy AJ, Emens J, Jackman A, Yuhas K. Circadian uses of melatonin in humans. Chronobiol Int. (2006) 23:403–12. doi: 10.1080/07420520500545862
11. Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Maestroni GJM, Zisapel N, et al. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Progr Neurobiol. (2008) 185:335–53. doi: 10.1016/j.pneurobio.2008.04.001
12. Acuña-Castroviejo D, Escames G, Venegas C, Diaz-Casado ME, Lima-Cabello E, Lopez LC, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. (2014) 71:2997–3025. doi: 10.1007/s00018-014-1579-2
13. Reiter RJ, Rosales-Corral S, Tan DX, Jou MJ, Galano A, Xu B. Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas. Cell Mol Life Sci. (2017) 74: 3863–81. doi: 10.1007/s00018-017-2609-7
14. Tan DX, Reiter R. J. Mitochondria: the birth place, the battle ground and the site of melatonin metabolism. Melatonin Res. (2019) 2: 44–66. doi: 10.32794/nr11250011
15. Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin–a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. (2011) 93:350–84. doi: 10.1016/j.pneurobio.2010.12.004
16. Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International union of basic and clinical pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. (2010) 62:343–80. doi: 10.1124/pr.110.002832
17. Ng KY, Leong MK, Liang H, Paxinos G. Melatonin receptors: distribution in mammalian brain and their respective putative functions. Brain Struct Funct. (2017) 222:2921–39. doi: 10.1007/s00429-017-1439-6
18. Cecon E, Oishi A, Jockers R. Melatonin receptors: molecular pharmacology and signalling in the context of system bias. Br J Pharmacol. (2017) 175:3263–80. doi: 10.1111/bph.13950
19. Oishi A, Cecon E, Jockers R. Melatonin receptor signaling: impact of receptor oligomerization on receptor function. Int Rev Cell Mol Biol. (2018) 338:59–77. doi: 10.1016/bs.ircmb.2018.02.002
20. Suofu Y, Li W, Jean-Alphonse FG, Jia J, Khattar NK, Li J, et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc Natl Acad Sci USA. (2017) 114:E7997–8006. doi: 10.1073/pnas.1705768114
21. Jimenez-Rubio G, Ortiz-Lopez L, Benitez-King G. Melatonin modulates cytoskeletal organization in the rat brain hippocampus. Neurosci Lett. (2012) 511:47–51. doi: 10.1016/j.neulet.2012.01.040
22. Hardeland R. Recent findings in melatonin research and their relevance to the CNS. Cent Nerv Syst Agents Med Chem. (2018) 18:102–14. doi: 10.2174/1871524918666180531083944
23. Venegas C, Garcia JA, Doerrier C, Volt H, Escames G, Lopez LC, et al. Analysis of the daily changes of melatonin receptors in the rat liver. J Pineal Res. (2013) 54:313–21. doi: 10.1111/jpi.12019
24. Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Jou MJ, Acuña-Castroviejo D. Melatonin mitigates mitochondrial meltdown: interactions with SIRT3. Int J Mol Sci. (2018) 19:E2439. doi: 10.3390/ijms19082439
25. Vanecek J, Klein DC. Melatonin inhibits gonadotropin-releasing hormone-induced elevation of intracellular Ca2+ in neonatal rat pituitary cells. Endocrinology. (1992) 130:701–7. doi: 10.1210/en.130.2.701
26. Zemkova H, Vanecek J. Inhibitory effect of melatonin on gonadotropin-releasing hormone-induced Ca2+ oscillations in pituitary cells of newborn rats. Neuroendocrinology. (1997) 65:276–83. doi: 10.1159/000127185
27. Shukla M, Chinchalongporn V, Govitrapong P, Reiter RJ. The role of melatonin in targeting cell signaling pathways in neurodegeneration. NY Acad Sci. (2019) doi: 10.1111/nyas.14005
28. Manchester LC, Coto-Montes A, Boga JA, Andersen LP, Zhou Z, Galano A, et al. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J Pineal Res. (2015) 59:403–19. doi: 10.1111/jpi.12267
29. Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. (2011) 51:1–16. doi: 10.1111/j.1600-079X.2011.00916.x
30. Carrillo-Vico A, Lardone PJ, Alvarez-Sanchez N, Rodriguez-Rodriguez A, Guerrero JM. Melatonin: buffering the immune system. Int J Mol Sci. (2013) 14:8638–83. doi: 10.3390/ijms14048638
31. Hardeland R. Melatonin and inflammation-Story of a double-edged blade. J Pineal Res. (2018) 65:e12525. doi: 10.1111/jpi.12525
32. Cardinali DP, Ritta MN, Fuentes AM, Gimeno MF, Gimeno AL. Prostaglandin E release by rat medial basal hypothalamus in vitro. Inhibition by melatonin at submicromolar concentrations. Eur J Pharmacol. (1980) 67:151–3. doi: 10.1016/0014-2999(80)90025-4
33. Deng WG, Tang ST, Tseng HP, Wu KK. Melatonin suppresses macrophage cyclooxygenase-2 and inducible nitric oxide synthase expression by inhibiting p52 acetylation and binding. Blood. (2006) 108:518–24. doi: 10.1182/blood-2005-09-3691
34. Costantino G, Cuzzocrea S, Mazzon E, Caputi AP. Protective effects of melatonin in zymosan-activated plasma-induced paw inflammation. Eur J Pharmacol. (1998) 363:57–63. doi: 10.1016/S0014-2999(98)00673-6
35. Golombek DA, Pevet P, Cardinali DP. Melatonin effects on behavior: possible mediation by the central GABAergic system. Neurosci Biobehav Rev. (1996) 20:403–12. doi: 10.1016/0149-7634(95)00052-6
36. Caumo W, Levandovski R, Hidalgo MP. Preoperative anxiolytic effect of melatonin and clonidine on postoperative pain and morphine consumption in patients undergoing abdominal hysterectomy: a double-blind, randomized, placebo-controlled study. J Pain. (2009) 10:100–8. doi: 10.1016/j.jpain.2008.08.007
37. Louzada PR, Paula Lima AC, Mendonca-Silva DL, Noel F, De Mello FG, Ferreira ST. Taurine prevents the neurotoxicity of beta-amyloid and glutamate receptor agonists: activation of GABA receptors and possible implications for Alzheimer's disease and other neurological disorders. FASEB J. (2004) 18:511–8. doi: 10.1096/fj.03-0739com
38. Cheng XP, Sun H, Ye ZY, Zhou JN. Melatonin modulates the GABAergic response in cultured rat hippocampal neurons. J Pharmacol Sci. (2012) 119:177–85. doi: 10.1254/jphs.11183FP
39. Giusti P, Lipartiti M, Franceschini D, Schiavo N, Floreani M, Manev H. Neuroprotection by melatonin from kainate-induced excitotoxicity in rats. FASEB J. (1996) 10:891–6. doi: 10.1096/fasebj.10.8.8666166
40. Cho S, Joh TH, Baik HH, Dibinis C, Volpe BT. Melatonin administration protects CA1 hippocampal neurons after transient forebrain ischemia in rats. Brain Res. (1997) 755:335–8. doi: 10.1016/S0006-8993(97)00188-1
41. Furio AM, Fontao R, Falco N, Ruiz JI, Caccuri RL, Cardinali DP. Neuroprotective effect of melatonin on glucocorticoid toxicity in the rat hippocampus. Open Physiol J. (2008) 1:23–7. doi: 10.2174/1874360900901010023
42. Escames G, Leon J, Lopez LC, Acuña-Castroviejo D. Mechanisms of N-methyl-D-aspartate receptor inhibition by melatonin in the rat striatum. J Neuroendocrinol. (2004) 16:929–35. doi: 10.1111/j.1365-2826.2004.01250.x
43. Poliandri AH, Esquifino AI, Cano P, Jimenez V, Lafuente A, Cardinali DP, et al. In vivo protective effect of melatonin on cadmium-induced changes in redox balance and gene expression in rat hypothalamus and anterior pituitary. J Pineal Res. (2006) 41:238–46. doi: 10.1111/j.1600-079X.2006.00360.x
44. Jimenez-Ortega V, Cano P, Scacchi PA, Cardinali DP, Esquifino AI. Cadmium-induced disruption in 24-h expression of clock and redox enzyme genes in rat medial basal hypothalamus: prevention by melatonin. Front Neurol. (2011) 2:13. doi: 10.3389/fneur.2011.00013
45. Shaikh AY, Xu J, Wu Y, He L, Hsu CY. Melatonin protects bovine cerebral endothelial cells from hyperoxia-induced DNA damage and death. Neurosci Lett. (1997) 229:193–7. doi: 10.1016/S0304-3940(97)00307-8
46. Pablos MI, Reiter RJ, Chuang JI, Ortiz GG, Guerrero JM, Sewerynek E, et al. Acutely administered melatonin reduces oxidative damage in lung and brain induced by hyperbaric oxygen. J Appl Physiol. (1997) 83:354–8. doi: 10.1152/jappl.1997.83.2.354
47. Princ FG, Juknat AA, Maxit AG, Cardalda C, Batlle A. Melatonin's antioxidant protection against delta-aminolevulinic acid-induced oxidative damage in rat cerebellum. J Pineal Res. (1997) 23:40–6. doi: 10.1111/j.1600-079X.1997.tb00333.x
48. Erol FS, Topsakal C, Ozveren MF, Kaplan M, Ilhan N, Ozercan IH, et al. Protective effects of melatonin and vitamin E in brain damage due to gamma radiation: an experimental study. Neurosurg Rev. (2004) 27:65–9. doi: 10.1007/s10143-003-0291-8
49. Lee EJ, Wu TS, Lee MY, Chen TY, Tsai YY, Chuang JI, et al. Delayed treatment with melatonin enhances electrophysiological recovery following transient focal cerebral ischemia in rats. J Pineal Res. (2004) 36:33–42. doi: 10.1046/j.1600-079X.2003.00093.x
50. Beni SM, Kohen R, Reiter RJ, Tan DX, Shohami E. Melatonin-induced neuroprotection after closed head injury is associated with increased brain antioxidants and attenuated late-phase activation of NF-kappaB and AP-1. FASEB J. (2004) 18:149–51. doi: 10.1096/fj.03-0323fje
51. Kabadi SV, Maher TJ. Posttreatment with uridine and melatonin following traumatic brain injury reduces edema in various brain regions in rats. Ann N Y Acad Sci. (2010) 1199:105–13. doi: 10.1111/j.1749-6632.2009.05352.x
52. Reiter RJ, Manchester LC, Tan DX. Neurotoxins: free radical mechanisms and melatonin protection. Curr Neuropharmacol. (2010) 8:194–210. doi: 10.2174/157015910792246236
53. Lahiri DK. Melatonin affects the metabolism of the beta-amyloid precursor protein in different cell types. J Pineal Res. (1999) 26:137–46. doi: 10.1111/j.1600-079X.1999.tb00575.x
54. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. (2008) 22:659–61. doi: 10.1096/fj.07-9574LSF
55. Matsubara E, Bryant-Thomas T, Pacheco QJ, Henry TL, Poeggeler B, Herbert D, et al. Melatonin increases survival and inhibits oxidative and amyloid pathology in a transgenic model of Alzheimer's disease. J Neurochem. (2003) 85:1101–8. doi: 10.1046/j.1471-4159.2003.01654.x
56. Feng Z, Chang Y, Cheng Y, Zhang BL, Qu ZW, Qin C, et al. Melatonin alleviates behavioral deficits associated with apoptosis and cholinergic system dysfunction in the APP 695 transgenic mouse model of Alzheimer's disease. J Pineal Res. (2004) 37:129–36. doi: 10.1111/j.1600-079X.2004.00144.x
57. Quinn J, Kulhanek D, Nowlin J, Jones R, Pratico D, Rokach J, et al. Chronic melatonin therapy fails to alter amyloid burden or oxidative damage in old Tg2576 mice: implications for clinical trials. Brain Res. (2005) 1037:209–13. doi: 10.1016/j.brainres.2005.01.023
58. Feng Z, Qin C, Chang Y, Zhang JT. Early melatonin supplementation alleviates oxidative stress in a transgenic mouse model of Alzheimer's disease. Free Radic Biol Med. (2006) 40:101–9. doi: 10.1016/j.freeradbiomed.2005.08.014
59. Garcia T, Ribes D, Colomina MT, Cabre M, Domingo JL, Gomez M. Evaluation of the protective role of melatonin on the behavioral effects of aluminum in a mouse model of Alzheimer's disease. Toxicology. (2009) 265:49–55. doi: 10.1016/j.tox.2009.09.009
60. Olcese JM, Cao C, Mori T, Mamcarz MB, Maxwell A, Runfeldt MJ, et al. Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of Alzheimer disease. J Pineal Res. (2009) 47:82–96. doi: 10.1111/j.1600-079X.2009.00692.x
61. Garcia T, Esparza JL, Nogues MR, Romeu M, Domingo JL, Gomez M. Oxidative stress status and RNA expression in hippocampus of an animal model of Alzheimer's disease after chronic exposure to aluminum. Hippocampus. (2010) 20:218–25. doi: 10.1002/hipo.20612
62. Spuch C, Antequera D, Isabel Fernandez-Bachiller M, Isabel Rodriguez-Franco M, Carro E. A new tacrine-melatonin hybrid reduces amyloid burden and behavioral deficits in a mouse model of Alzheimer's disease. Neurotox Res. (2010) 17:421–31. doi: 10.1007/s12640-009-9121-2
63. Bedrosian TA, Herring KL, Weil ZM, Nelson RJ. Altered temporal patterns of anxiety in aged and amyloid precursor protein (APP) transgenic mice. Proc Natl Acad Sci USA. (2011) 108:11686–91. doi: 10.1073/pnas.1103098108
64. Dragicevic N, Copes N, O'Neal-Moffitt G, Jin J, Buzzeo R, Mamcarz M, et al. Melatonin treatment restores mitochondrial function in Alzheimer's mice: a mitochondrial protective role of melatonin membrane receptor signaling. J Pineal Res. (2011) 51:75–86. doi: 10.1111/j.1600-079X.2011.00864.x
65. Baño OB, Popovic N, Gambini J, Popovic M, Vina J, Bonet-Costa V, et al. Circadian system functionality, hippocampal oxidative stress, and spatial memory in the APPswe/PS1dE9 transgenic model of Alzheimer disease: effects of melatonin or ramelteon. Chronobiol Int. (2012) 29:822–34. doi: 10.3109/07420528.2012.699119
66. Dragicevic N, Delic V, Cao C, Copes N, Lin X, Mamcarz M, et al. Caffeine increases mitochondrial function and blocks melatonin signaling to mitochondria in Alzheimer's mice and cells. Neuropharmacology. (2012) 63:1368–79. doi: 10.1016/j.neuropharm.2012.08.018
67. Garcia-Mesa Y, Gimenez-Llort L, Lopez LC, Venegas C, Cristofol R, Escames G, et al. Melatonin plus physical exercise are highly neuroprotective in the 3xTg-AD mouse. Neurobiol Aging. (2012) 33:1124–9. doi: 10.1016/j.neurobiolaging.2011.11.016
68. McKenna JT, Christie MA, Jeffrey BA, McCoy JG, Lee E, Connolly NP, et al. Chronic ramelteon treatment in a mouse model of Alzheimer's disease. Arch Ital Biol. (2012) 150:5–14. doi: 10.4449/aib.v149i5.1375
69. Di Paolo C, Reverte I, Colomina MT, Domingo JL, Gomez M. Chronic exposure to aluminum and melatonin through the diet: neurobehavioral effects in a transgenic mouse model of Alzheimer disease. Food Chem Toxicol. (2014) 69:320–9. doi: 10.1016/j.fct.2014.04.022
70. Gerenu G, Liu K, Chojnacki JE, Saathoff JM, Martinez-Martin P, Perry G, et al. Curcumin/melatonin hybrid 5-(4-hydroxy-phenyl)-3-oxo-pentanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide ameliorates AD-like pathology in the APP/PS1 mouse model. ACS Chem Neurosci. (2015) 6:1393–9. doi: 10.1021/acschemneuro.5b00082
71. Nie L, Wei G, Peng S, Qu Z, Yang Y, Yang Q, et al. Melatonin ameliorates anxiety and depression-like behaviors and modulates proteomic changes in triple transgenic mice of Alzheimer's disease. Biofactors. (2017) 43:593–611. doi: 10.1002/biof.1369
72. Kozhevnikova OS, Korbolina EE, Stefanova NA, Muraleva NA, Orlov YL, Kolosova NG. Association of AMD-like retinopathy development with an Alzheimer's disease metabolic pathway in OXYS rats. Biogerontology. (2013) 14:753–62. doi: 10.1007/s10522-013-9439-2
73. Rudnitskaya EA, Maksimova KY, Muraleva NA, Logvinov SV, Yanshole LV, Kolosova NG, Stefanova NA. Beneficial effects of melatonin in a rat model of sporadic Alzheimer's disease. Biogerontology. (2015) 16:303–16. doi: 10.1007/s10522-014-9547-7
74. Poeggeler B, Miravalle L, Zagorski MG, Wisniewski T, Chyan YJ, Zhang Y, et al. Melatonin reverses the profibrillogenic activity of apolipoprotein E4 on the Alzheimer amyloid Abeta peptide. Biochemistry. (2001) 40:14995–5001. doi: 10.1021/bi0114269
75. Pappolla M, Bozner P, Soto C, Shao H, Robakis NK, Zagorski M, et al. Inhibition of Alzheimer beta-fibrillogenesis by melatonin. J Biol Chem. (1998) 273:7185–8. doi: 10.1074/jbc.273.13.7185
76. Shukla M, Htoo HH, Wintachai P, Hernandez JF, Dubois C, Postina R, et al. Melatonin stimulates the nonamyloidogenic processing of βAPP through the positive transcriptional regulation of ADAM10 and ADAM17. J Pineal Res. (2015) 58:151–65. doi: 10.1111/jpi.12200
77. Panmanee J, Nopparat C, Chavanich N, Shukla M, Mukda S, Song W, et al. Melatonin regulates the transcription of βAPP-cleaving secretases mediated through melatonin receptors in human neuroblastoma SH-SY5Y cells. J Pineal Res. (2015) 59:308–20. doi: 10.1111/jpi.12260
78. Chinchalongporn V, Shukla M, Govitrapong P. Melatonin ameliorates β42 -induced alteration of βAPP-processing secretases via the melatonin receptor through the Pin1/GSK3beta/NF-κB pathway in SH-SY5Y cells. J Pineal Res. (2018) 64:e12470. doi: 10.1111/jpi.12470
79. Zatta P, Tognon G, Carampin P. Melatonin prevents free radical formation due to the interaction between beta-amyloid peptides and metal ions [Al(III), Zn(II), Cu(II), Mn(II), Fe(II)]. J Pineal Res. (2003) 35:98–103. doi: 10.1034/j.1600-079X.2003.00058.x
80. Feng Z, Zhang JT. Protective effect of melatonin on beta-amyloid-induced apoptosis in rat astroglioma C6 cells and its mechanism. Free Radic Biol Med. (2004) 37:1790–801. doi: 10.1016/j.freeradbiomed.2004.08.023
81. Furio AM, Cutrera RA, Castillo T, V, Perez LS, Riccio P, Caccuri RL, et al. Effect of melatonin on changes in locomotor activity rhythm of Syrian hamsters injected with beta amyloid peptide 25-35 in the suprachiasmatic nuclei. Cell Mol Neurobiol. (2002) 22:699–709. doi: 10.1023/A:1021805023906
82. Shen YX, Xu SY, Wei W, Wang XL, Wang H, Sun X. Melatonin blocks rat hippocampal neuronal apoptosis induced by amyloid beta-peptide 25-35. J Pineal Res. (2002) 32:163–7. doi: 10.1034/j.1600-079x.2002.1o839.x
83. Rosales-Corral S, Tan DX, Reiter RJ, Valdivia-Velazquez M, Martinez-Barboza G, Acosta-Martinez JP, et al. Orally administered melatonin reduces oxidative stress and proinflammatory cytokines induced by amyloid-beta peptide in rat brain: a comparative, In vivo study versus vitamin C and E. J Pineal Res. (2003) 35:80–4. doi: 10.1034/j.1600-079X.2003.00057.x
84. Deng YQ, Xu GG, Duan P, Zhang Q, Wang JZ. Effects of melatonin on wortmannin-induced tau hyperphosphorylation. Acta Pharmacol Sin. (2005) 26:519–26. doi: 10.1111/j.1745-7254.2005.00102.x
85. Li SP, Deng YQ, Wang XC, Wang YP, Wang JZ. Melatonin protects SH-SY5Y neuroblastoma cells from calyculin A-induced neurofilament impairment and neurotoxicity. J Pineal Res. (2004) 36:186–91. doi: 10.1111/j.1600-079X.2004.00116.x
86. Xiong YF, Chen Q, Chen J, Zhou J, Wang HX. Melatonin reduces the impairment of axonal transport and axonopathy induced by calyculin A. J Pineal Res. (2011) 50:319–27. doi: 10.1111/j.1600-079X.2010.00846.x
87. Benitez-King G, Tunez I, Bellon A, Ortiz GG, Anton-Tay F. Melatonin prevents cytoskeletal alterations and oxidative stress induced by okadaic acid in N1E-115 cells. Exp Neurol. (2003) 182:151–9. doi: 10.1016/S0014-4886(03)00085-2
88. Tunez I, Munoz MC, Feijoo M, Munoz-Castaneda JR, Bujalance I, Valdelvira ME, et al. Protective melatonin effect on oxidative stress induced by okadaic acid into rat brain. J Pineal Res. (2003) 34:265–8. doi: 10.1034/j.1600-079X.2003.00039.x
89. Wang YP, Li XT, Liu SJ, Zhou XW, Wang XC, Wang JZ. Melatonin ameliorated okadaic-acid induced Alzheimer-like lesions. Acta Pharmacol Sin. (2004) 25:276–80.
90. Liu SJ, Wang JZ. Alzheimer-like tau phosphorylation induced by wortmannin In vivo and its attenuation by melatonin. Acta Pharmacol Sin. (2002) 23:183–7.
91. Wang XC, Zhang J, Yu X, Han L, Zhou ZT, Zhang Y, et al. Prevention of isoproterenol-induced tau hyperphosphorylation by melatonin in the rat. Sheng Li Xue Bao. (2005) 57:7–12.
92. Schuster C, Williams LM, Morris A, Morgan PJ, Barrett P. The human MT1 melatonin receptor stimulates cAMP production in the human neuroblastoma cell line SH-SY5Y cells via a calcium-calmodulin signal transduction pathway. J Neuroendocrinol. (2005) 17:170–8. doi: 10.1111/j.1365-2826.2005.01288.x
93. Witt-Enderby PA, MacKenzie RS, McKeon RM, Carroll EA, Bordt SL, Melan MA. Melatonin induction of filamentous structures in non-neuronal cells that is dependent on expression of the human mt1 melatonin receptor. Cell Motil Cytoskeleton. (2000) 46:28–42. doi: 10.1002/(SICI)1097-0169(200005)46:1<28::AID-CM4>3.0.CO;2-5
94. Benitez-King G, Rios A, Martinez A, Anton-Tay F. in vitro inhibition of Ca2+/calmodulin-dependent kinase II activity by melatonin. Biochim Biophys Acta. (1996) 1290:191–6. doi: 10.1016/0304-4165(96)00025-6
95. Chan AS, Lai FP, Lo RK, Voyno-Yasenetskaya TA, Stanbridge EJ, Wong YH. Melatonin mt1 and MT2 receptors stimulate c-Jun N-terminal kinase via pertussis toxin-sensitive and -insensitive G proteins. Cell Signal. (2002) 14:249–57. doi: 10.1016/S0898-6568(01)00240-6
96. Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: A beginner's guide. Neurochem Res. (2015) 40:2583–99. doi: 10.1007/s11064-015-1581-6
97. Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. (2012) 4:147ra111. doi: 10.1126/scitranslmed.3003748
98. Boespflug EL, Iliff JJ. The emerging relationship between interstitial fluid-cerebrospinal fluid exchange, amyloid-beta, and sleep. Biol Psychiatry. (2018) 83:328–36. doi: 10.1016/j.biopsych.2017.11.031
99. Achariyar TM, Li B, Peng W, Verghese PB, Shi Y, McConnell E, et al. Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol Neurodegener. (2016) 11:74. doi: 10.1186/s13024-016-0138-8
100. Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. (2014) 34:16180–93. doi: 10.1523/JNEUROSCI.3020-14.2014
101. Pappolla MA, Matsubara E, Vidal R, Pacheco-Quinto J, Poeggeler B, Zagorski M, et al. Melatonin treatment enhances aβ lymphatic clearance in a transgenic mouse model of amyloidosis. Curr Alzheimer Res. (2018) 15:637–42. doi: 10.2174/1567205015666180411092551
102. Monti JM, Alvarino F, Cardinali D, Savio I, Pintos A. Polysomnographic study of the effect of melatonin on sleep in elderly patients with chronic primary insomnia. Arch Gerontol Geriatr. (1999) 28:85–98. doi: 10.1016/S0167-4943(98)00129-0
103. Plog BA, Nedergaard M. The glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol. (2018) 13:379–94. doi: 10.1146/annurev-pathol-051217-111018
104. Liu RY, Zhou JN, van HJ, Hofman MA, Swaab DF. Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer's disease, and apolipoprotein E-epsilon4/4 genotype. J Clin Endocrinol Metab. (1999) 84:323–7. doi: 10.1210/jc.84.1.323
105. Sirin FB, Kumbul DD, Vural H, Eren I, Inanli I, Sutcu R, et al. Plasma 8-isoPGF2alpha and serum melatonin levels in patients with minimal cognitive impairment and Alzheimer disease. Turk J Med Sci. (2015) 45:1073–7. doi: 10.3906/sag-1406-134
106. Ooms S, Ju YE. Treatment of sleep disorders in dementia. Curr Treat Options Neurol. (2016) 18:40. doi: 10.1007/s11940-016-0424-3
107. Cardinali DP, Brusco LI, Liberczuk C, Furio AM. The use of melatonin in Alzheimer's disease. Neuro Endocrinol Lett. (2002) 23(Suppl. 1):20–3.
108. Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. (2008) 299:2642–55. doi: 10.1001/jama.299.22.2642
109. Fainstein I, Bonetto A, Brusco LI, Cardinali DP. Effects of melatonin in elderly patients with sleep disturbance. A pilot study. Curr Ther Res. (1997) 58:990–1000. doi: 10.1016/S0011-393X(97)80066-5
110. Brusco LI, Marquez M, Cardinali DP. Melatonin treatment stabilizes chronobiologic and cognitive symptoms in Alzheimer's disease. Neuroendocrinol Lett. (1998) 19:111–5.
111. Brusco LI, Marquez M, Cardinali DP. Monozygotic twins with Alzheimer's disease treated with melatonin: case report. J Pineal Res. (1998) 25:260–3. doi: 10.1111/j.1600-079X.1998.tb00396.x
112. Cohen-Mansfield J, Garfinkel D, Lipson S. Melatonin for treatment of sundowning in elderly persons with dementia - a preliminary study. Arch Gerontol Geriatr. (2000) 31:65–76. doi: 10.1016/S0167-4943(00)00068-6
113. Mishima K, Okawa M, Hozumi S, Hishikawa Y. Supplementary administration of artificial bright light and melatonin as potent treatment for disorganized circadian rest-activity and dysfunctional autonomic and neuroendocrine systems in institutionalized demented elderly persons. Chronobiol Int. (2000) 17:419–32. doi: 10.1081/CBI-100101055
114. Serfaty M, Kennell-Webb S, Warner J, Blizard R, Raven P. Double blind randomised placebo controlled trial of low dose melatonin for sleep disorders in dementia. Int J Geriatr Psychiatry. (2002) 17:1120–7. doi: 10.1002/gps.760
115. Singer C, Tractenberg RE, Kaye J, Schafer K, Gamst A, Grundman M, et al. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer's disease. Sleep. (2003) 26:893–901. doi: 10.1093/sleep/26.7.893
116. Asayama K, Yamadera H, Ito T, Suzuki H, Kudo Y, Endo S. Double blind study of melatonin effects on the sleep-wake rhythm, cognitive and non-cognitive functions in Alzheimer type dementia. J Nippon Med Sch. (2003) 70:334–41. doi: 10.1272/jnms.70.334
117. Mahlberg R, Kunz D, Sutej I, Kuhl KP, Hellweg R. Melatonin treatment of day-night rhythm disturbances and sundowning in Alzheimer disease: an open-label pilot study using actigraphy. J Clin Psychopharmacol. (2004) 24:456–9. doi: 10.1097/01.jcp.0000132443.12607.fd
118. Mahlberg R, Walther S. Actigraphy in agitated patients with dementia. Monitoring treatment outcomes. Z Gerontol Geriatr. (2007) 40:178–84. doi: 10.1007/s00391-007-0420-z
119. Anderson KN, Jamieson S, Graham AJ, Shneerson JM. REM sleep behaviour disorder treated with melatonin in a patient with Alzheimer's disease. Clin Neurol Neurosurg. (2008) 110:492–5. doi: 10.1016/j.clineuro.2008.01.004
120. Dowling GA, Burr RL, Van Someren EJ, Hubbard EM, Luxenberg JS, Mastick J, et al. Melatonin and bright-light treatment for rest-activity disruption in institutionalized patients with Alzheimer's disease. J Am Geriatr Soc. (2008) 56:239–46. doi: 10.1111/j.1532-5415.2007.01543.x
121. Gehrman PR, Connor DJ, Martin JL, Shochat T, Corey-Bloom J, Ancoli-Israel S. Melatonin fails to improve sleep or agitation in double-blind randomized placebo-controlled trial of institutionalized patients with Alzheimer disease. Am J Geriatr Psychiatry. (2009) 17:166–9. doi: 10.1097/JGP.0b013e318187de18
122. Cardinali DP, Furio AM, Brusco LI. Clinical aspects of melatonin intervention in Alzheimer's disease progression. Curr Neuropharmacol. (2010) 8:218–27. doi: 10.2174/157015910792246209
123. Xu J, Wang LL, Dammer EB, Li CB, Xu G, Chen SD, et al. Melatonin for sleep disorders and cognition in dementia: a meta-analysis of randomized controlled trials. Am J Alzheimers Dis Other Demen. (2015) 30:439–47. doi: 10.1177/1533317514568005
124. Zhang W, Chen XY, Su SW, Jia QZ, Ding T, Zhu ZN, et al. Exogenous melatonin for sleep disorders in neurodegenerative diseases: a meta-analysis of randomized clinical trials. Neurol Sci. (2016) 37:57–65. doi: 10.1007/s10072-015-2357-0
125. Furuya M, Miyaoka T, Yasuda H, Yamashita S, Tanaka I, Otsuka S, et al. Marked improvement in delirium with ramelteon: five case reports. Psychogeriatrics. (2012) 12:259–62. doi: 10.1111/j.1479-8301.2012.00422.x
126. Allan CL, Behrman S, Ebmeier KP, Valkanova V. Diagnosing early cognitive decline-when, how and for whom? Maturitas. (2017) 96:103–8. doi: 10.1016/j.maturitas.2016.11.018
127. Davies L, Wolska B, Hilbich C, Multhaup G, Martins R, Simms G, et al. A4 amyloid protein deposition and the diagnosis of Alzheimer's disease: prevalence in aged brains determined by immunocytochemistry compared with conventional neuropathologic techniques. Neurology. (1988) 38:1688–93. doi: 10.1212/WNL.38.11.1688
128. Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann Neurol. (1999) 45:358–68. doi: 10.1002/1531-8249(199903)45:3<358::AID-ANA12>3.0.CO;2-X
129. Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. (1995) 16:271–8. doi: 10.1016/0197-4580(95)00021-6
130. Braak H, Braak E. Evolution of neuronal changes in the course of Alzheimer's disease. J Neural Transm Suppl. (1998) 53:127–40. doi: 10.1007/978-3-7091-6467-9_11
131. Cespon J, Miniussi C, Pellicciari MC. Interventional programmes to improve cognition during healthy and pathological ageing: cortical modulations and evidence for brain plasticity. Ageing Res Rev. (2018) 43:81–98. doi: 10.1016/j.arr.2018.03.001
132. Jean-Louis G, von GH, Zizi F. Melatonin effects on sleep, mood, and cognition in elderly with mild cognitive impairment. J Pineal Res. (1998) 25:177–83. doi: 10.1111/j.1600-079X.1998.tb00557.x
133. Peck JS, LeGoff DB, Ahmed I, Goebert D. Cognitive effects of exogenous melatonin administration in elderly persons: a pilot study. Am J Geriatr Psychiatry. (2004) 12:432–6. doi: 10.1176/appi.ajgp.12.4.432
134. Wade AG, Ford I, Crawford G, McMahon AD, Nir T, Laudon M, et al. Efficacy of prolonged release melatonin in insomnia patients aged 55-80 years: quality of sleep and next-day alertness outcomes. Curr Med Res Opin. (2007) 23:2597–605. doi: 10.1185/030079907X233098
135. Furio AM, Brusco LI, Cardinali DP. Possible therapeutic value of melatonin in mild cognitive impairment: a retrospective study. J Pineal Res. (2007) 43:404–9. doi: 10.1111/j.1600-079X.2007.00491.x
136. Garzon C, Guerrero JM, Aramburu O, Guzman T. Effect of melatonin administration on sleep, behavioral disorders and hypnotic drug discontinuation in the elderly: a randomized, double-blind, placebo-controlled study. Aging Clin Exp Res. (2009) 21:38–42. doi: 10.1007/BF03324897
137. Cazzola R, Rondanelli M, Faliva M, Cestaro B. Effects of DHA-phospholipids, melatonin and tryptophan supplementation on erythrocyte membrane physico-chemical properties in elderly patients suffering from mild cognitive impairment. Exp Gerontol. (2012) 47:974–8. doi: 10.1016/j.exger.2012.09.004
138. Rondanelli M, Opizzi A, Faliva M, Mozzoni M, Antoniello N, Cazzola R, et al. Effects of a diet integration with an oily emulsion of DHA-phospholipids containing melatonin and tryptophan in elderly patients suffering from mild cognitive impairment. Nutr Neurosci. (2012) 15:46–54. doi: 10.1179/1476830511Y.0000000032
139. Cardinali DP, Vigo DE, Olivar N, Vidal MF, Furio AM, Brusco LI. Therapeutic application of melatonin in mild cognitive impairment. Am J Neurodegener Dis. (2012) 1:280–91.
140. Wade AG, Farmer M, Harari G, Fund N, Laudon M, Nir T, et al. Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer's disease: a 6-month, randomized, placebo-controlled, multicenter trial. Clin Interv Aging. (2014) 9:947–61. doi: 10.2147/CIA.S65625
141. Targum SD, Wedel PC, Fava M. Changes in cognitive symptoms after a buspirone-melatonin combination treatment for major depressive disorder. J Psychiatr Res. (2015) 68:392–6. doi: 10.1016/j.jpsychires.2015.04.024
142. Fan Y, Yuan L, Ji M, Yang J, Gao D. The effect of melatonin on early postoperative cognitive decline in elderly patients undergoing hip arthroplasty: a randomized controlled trial. J Clin Anesth. (2017) 39:77–81. doi: 10.1016/j.jclinane.2017.03.023
143. Rothman SM, Mattson MP. Sleep disturbances in Alzheimer's and Parkinson's diseases. Neuromolecular Med. (2012) 14:194–204. doi: 10.1007/s12017-012-8181-2
144. Nutt JG, Bohnen NI. Non-Dopaminergic Therapies. J Parkinsons Dis. (2018) 8:S73–8. doi: 10.3233/JPD-181472
145. Michel PP, Hirsch EC, Hunot S. Understanding dopaminergic cell death pathways in Parkinson disease. Neuron. (2016) 90:675–91. doi: 10.1016/j.neuron.2016.03.038
146. Boland B, Yu WH, Corti O, Mollereau B, Henriques A, Bezard E, et al. Promoting the clearance of neurotoxic proteins in neurodegenerative disorders of ageing. Nat Rev Drug Discov. (2018) 17:660–88. doi: 10.1038/nrd.2018.109
147. Marmion DJ, Kordower JH. alpha-Synuclein nonhuman primate models of Parkinson's disease. J Neural Transm. (2018) 125:385–400. doi: 10.1007/s00702-017-1720-0
148. Visanji NP, Brotchie JM, Kalia LV, Koprich JB, Tandon A, Watts JC, et al. α-synuclein-based animal models of Parkinson's disease: challenges and opportunities in a new era. Trends Neurosci. (2016) 39:750–62. doi: 10.1016/j.tins.2016.09.003
149. Mack JM, Schamne MG, Sampaio TB, Pertile RA, Fernandes PA, Markus RP, et al. Melatoninergic system in Parkinson's disease: from neuroprotection to the management of motor and nonmotor symptoms. Oxid Med Cell Longev. (2016) 2016:3472032. doi: 10.1155/2016/3472032
150. Herraiz T, Guillen H. Inhibition of the bioactivation of the neurotoxin MPTP by antioxidants, redox agents and monoamine oxidase inhibitors. Food Chem Toxicol. (2011) 49:1773–81. doi: 10.1016/j.fct.2011.04.026
151. Masilamoni GJ, Smith Y. Chronic MPTP administration regimen in monkeys: a model of dopaminergic and non-dopaminergic cell loss in Parkinson's disease. J Neural Transm. (2018) 125:337–63. doi: 10.1007/s00702-017-1774-z
152. Burton S, Daya S, Potgieter B. Melatonin modulates apomorphine-induced rotational behaviour. Experientia. (1991) 47:466–9. doi: 10.1007/BF01959946
153. Acuña-Castroviejo D, Coto-Montes A, Gaia MM, Ortiz GG, Reiter RJ. Melatonin is protective against MPTP-induced striatal and hippocampal lesions. Life Sci. (1997) 60:L23–9. doi: 10.1016/S0024-3205(96)00606-6
154. Jin BK, Shin DY, Jeong MY, Gwag MR, Baik HW, Yoon KS, et al. Melatonin protects nigral dopaminergic neurons from 1-methyl-4-phenylpyridinium (MPP+) neurotoxicity in rats. Neurosci Lett. (1998) 245:61–4. doi: 10.1016/S0304-3940(98)00170-0
155. Joo WS, Jin BK, Park CW, Maeng SH, Kim YS. Melatonin increases striatal dopaminergic function in 6-OHDA-lesioned rats. Neuroreport. (1998) 9:4123–6. doi: 10.1097/00001756-199812210-00022
156. Kim YS, Joo WS, Jin BK, Cho YH, Baik HH, Park CW. Melatonin protects 6-OHDA-induced neuronal death of nigrostriatal dopaminergic system. Neuroreport. (1998) 9:2387–90. doi: 10.1097/00001756-199807130-00043
157. Dabbeni-Sala F, Di SS, Franceschini D, Skaper SD, Giusti P. Melatonin protects against 6-OHDA-induced neurotoxicity in rats: a role for mitochondrial complex I activity. FASEB J. (2001) 15:164–70. doi: 10.1096/fj.00-0129com
158. Aguiar LM, Vasconcelos SM, Sousa FC, Viana GS. Melatonin reverses neurochemical alterations induced by 6-OHDA in rat striatum. Life Sci. (2002) 70:1041–51. doi: 10.1016/S0024-3205(01)01480-1
159. Chen ST, Chuang JI, Hong MH, Li EI. Melatonin attenuates MPP+-induced neurodegeneration and glutathione impairment in the nigrostriatal dopaminergic pathway. J Pineal Res. (2002) 32:262–9. doi: 10.1034/j.1600-079X.2002.01871.x
160. Khaldy H, Escames G, Leon J, Bikjdaouene L, Acuña-Castroviejo D. Synergistic effects of melatonin and deprenyl against MPTP-induced mitochondrial damage and DA depletion. Neurobiol Aging. (2003) 24:491–500. doi: 10.1016/S0197-4580(02)00133-1
161. Sharma R, McMillan CR, Tenn CC, Niles LP. Physiological neuroprotection by melatonin in a 6-hydroxydopamine model of Parkinson's disease. Brain Res. (2006) 1068:230–6. doi: 10.1016/j.brainres.2005.10.084
162. Singh S, Ahmed R, Sagar RK, Krishana B. Neuroprotection of the nigrostriatal dopaminergic neurons by melatonin in hemiparkinsonium rat. Indian J Med Res. (2006) 124:419–26.
163. Saravanan KS, Sindhu KM, Mohanakumar KP. Melatonin protects against rotenone-induced oxidative stress in a hemiparkinsonian rat model. J Pineal Res. (2007) 42:247–53. doi: 10.1111/j.1600-079X.2006.00412.x
164. Huang JY, Hong YT, Chuang JI. Fibroblast growth factor 9 prevents MPP+-induced death of dopaminergic neurons and is involved in melatonin neuroprotection In vivo and in vitro. J Neurochem. (2009) 109:1400–12. doi: 10.1111/j.1471-4159.2009.06061.x
165. Tapias V, Escames G, Lopez LC, Lopez A, Camacho E, Carrion MD, et al. Melatonin and its brain metabolite N1-acetyl-5-methoxykynuramine prevent mitochondrial nitric oxide synthase induction in parkinsonian mice. J Neurosci Res. (2009) 87:3002–10. doi: 10.1002/jnr.22123
166. Patki G, Lau YS. Melatonin protects against neurobehavioral and mitochondrial deficits in a chronic mouse model of Parkinson's disease. Pharmacol Biochem Behav. (2011) 99:704–11. doi: 10.1016/j.pbb.2011.06.026
167. Singhal NK, Srivastava G, Patel DK, Jain SK, Singh MP. Melatonin or silymarin reduces maneb- and paraquat-induced Parkinson's disease phenotype in the mouse. J Pineal Res. (2011) 50:97–109. doi: 10.1111/j.1600-079X.2010.00819.x
168. Gutierrez-Valdez AL, Anaya-Martinez V, Ordonez-Librado JL, Garcia-Ruiz R, Torres-Esquivel C, Moreno-Rivera M, et al. Effect of chronic L-dopa or melatonin treatments after dopamine deafferentation in rats: dyskinesia, motor performance, and cytological analysis. ISRN Neurol. (2012) 2012:360379. doi: 10.5402/2012/360379
169. Brito-Armas JM, Baekelandt V, Castro-Hernandez JR, Gonzalez-Hernandez T, Rodriguez M, Castro R. Melatonin prevents dopaminergic cell loss induced by lentiviral vectors expressing A30P mutant alpha-synuclein. Histol Histopathol. (2013) 28:999–1006. doi: 10.14670/HH-28.999
170. Zaitone SA, Hammad LN, Farag NE. Antioxidant potential of melatonin enhances the response to L-dopa in 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine-parkinsonian mice. Pharmacol Rep. (2013) 65:1213–26. doi: 10.1016/S1734-1140(13)71479-8
171. Bassani TB, Gradowski RW, Zaminelli T, Barbiero JK, Santiago RM, Boschen SL, et al. Neuroprotective and antidepressant-like effects of melatonin in a rotenone-induced Parkinson's disease model in rats. Brain Res. (2014) 1593:95–105. doi: 10.1016/j.brainres.2014.09.068
172. Yildirim FB, Ozsoy O, Tanriover G, Kaya Y, Ogut E, Gemici B, et al. Mechanism of the beneficial effect of melatonin in experimental Parkinson's disease. Neurochem Int. (2014) 79:1–11. doi: 10.1016/j.neuint.2014.09.005
173. Naskar A, Prabhakar V, Singh R, Dutta D, Mohanakumar KP. Melatonin enhances L-DOPA therapeutic effects, helps to reduce its dose, and protects dopaminergic neurons in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in mice. J Pineal Res. (2015) 58:262–74. doi: 10.1111/jpi.12212
174. Ozsoy O, Yildirim FB, Ogut E, Kaya Y, Tanriover G, Parlak H, et al. Melatonin is protective against 6-hydroxydopamine-induced oxidative stress in a hemiparkinsonian rat model. Free Radic Res. (2015) 49:1004–14. doi: 10.3109/10715762.2015.1027198
175. Carriere CH, Kang NH, Niles LP. Chronic low-dose melatonin treatment maintains nigrostriatal integrity in an intrastriatal rotenone model of Parkinson's disease. Brain Res. (2016) 1633:115–25. doi: 10.1016/j.brainres.2015.12.036
176. Li Y, Wang SM, Guo L, Zhu J, Wang Y, Li L, et al. Effects of melatonin levels on neurotoxicity of the medial prefrontal cortex in a rat model of Parkinson's disease. Chin Med J. (2017) 130:2726–31. doi: 10.4103/0366-6999.218025
177. Lopez A, Ortiz F, Doerrier C, Venegas C, Fernandez-Ortiz M, Aranda P, et al. Mitochondrial impairment and melatonin protection in parkinsonian mice do not depend of inducible or neuronal nitric oxide synthases. PLoS ONE. (2017) 12:e0183090. doi: 10.1371/journal.pone.0183090
178. Paul R, Phukan BC, Justin TA, Manivasagam T, Bhattacharya P, Borah A. Melatonin protects against behavioral deficits, dopamine loss and oxidative stress in homocysteine model of Parkinson's disease. Life Sci. (2018) 192:238–45. doi: 10.1016/j.lfs.2017.11.016
179. Medeiros CA, Carvalhedo de Bruin PF, Lopes LA, Magalhaes MC, de Lourdes SM, de Bruin VM. Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson's disease. A randomized, double blind, placebo-controlled study. J Neurol. (2007) 254:459–64. doi: 10.1007/s00415-006-0390-x
180. Willis GL, Armstrong SM. A therapeutic role for melatonin antagonism in experimental models of Parkinson's disease. Physiol Behav. (1999) 66:785–95. doi: 10.1016/S0031-9384(99)00023-2
181. Willis GL, Robertson AD. Recovery from experimental Parkinson's disease in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride treated marmoset with the melatonin analogue ML-23. Pharmacol Biochem Behav. (2005) 80:9–26. doi: 10.1016/j.pbb.2004.10.022
182. Zisapel N, Egozi Y, Laudon M. Inhibition of dopamine release by melatonin: regional distribution in the rat brain. Brain Res. (1982) 246:161–3. doi: 10.1016/0006-8993(82)90157-3
183. Leeboonngam T, Pramong R, Sae-Ung K, Govitrapong P, Phansuwan-Pujito P. Neuroprotective effects of melatonin on amphetamine-induced dopaminergic fiber degeneration in the hippocampus of postnatal rats. J Pineal Res. (2018) 64:e12456. doi: 10.1111/jpi.12456
184. Zampol MA, Barros MH. Melatonin improves survival and respiratory activity of yeast cells challenged by alpha-synuclein and menadione. Yeast. (2018) 35:281–90. doi: 10.1002/yea.3296
185. Phillipson OT. Alpha-synuclein, epigenetics, mitochondria, metabolism, calcium traffic, & circadian dysfunction in Parkinson's disease. An integrated strategy for management. Ageing Res Rev. (2017) 40:149–67. doi: 10.1016/j.arr.2017.09.006
186. Chang AM, Santhi N, St. Hilaire M, Gronfier C, Bradstreet DS, Duffy JF, et al. Human responses to bright light of different durations. J Physiol. (2012) 590:3103–12. doi: 10.1113/jphysiol.2011.226555
187. Lin AM, Fang SF, Chao PL, Yang CH. Melatonin attenuates arsenite-induced apoptosis in rat brain: involvement of mitochondrial and endoplasmic reticulum pathways and aggregation of alpha-synuclein. J Pineal Res. (2007) 43:163–71. doi: 10.1111/j.1600-079X.2007.00456.x
188. Sae-Ung K, Ueda K, Govitrapong P, Phansuwan-Pujito P. Melatonin reduces the expression of alpha-synuclein in the dopamine containing neuronal regions of amphetamine-treated postnatal rats. J Pineal Res. (2012) 52:128–37. doi: 10.1111/j.1600-079X.2011.00927.x
189. Klongpanichapak S, Phansuwan-Pujito P, Ebadi M, Govitrapong P. Melatonin inhibits amphetamine-induced increase in alpha-synuclein and decrease in phosphorylated tyrosine hydroxylase in SK-N-SH cells. Neurosci Lett. (2008) 436:309–13. doi: 10.1016/j.neulet.2008.03.053
190. Chang CF, Huang HJ, Lee HC, Hung KC, Wu RT, Lin AM. Melatonin attenuates kainic acid-induced neurotoxicity in mouse hippocampus via inhibition of autophagy and alpha-synuclein aggregation. J Pineal Res. (2012) 52:312–21. doi: 10.1111/j.1600-079X.2011.00945.x
191. Hoshi A, Tsunoda A, Tada M, Nishizawa M, Ugawa Y, Kakita A. Expression of aquaporin 1 and aquaporin 4 in the temporal neocortex of patients with Parkinson's disease. Brain Pathol. (2017) 27:160–8. doi: 10.1111/bpa.12369
192. Schirinzi T, Sancesario GM, Di LG, Biticchi B, Colona VL, Mercuri NB, et al. CSF alpha-synuclein inversely correlates with non-motor symptoms in a cohort of PD patients. Parkinsonism Relat Disord. (2018) 61:203–6. doi: 10.1016/j.parkreldis.2018.10.018
193. Carta MG, Hardoy MC, Dell'Osso L, Carpiniello B. [Tardive dyskinesia: review of the literature]. Clin Ter. (2004) 155:127–33.
194. Werneke U, Turner T, Priebe S. Complementary medicines in psychiatry: review of effectiveness and safety. Br J Psychiatry. (2006) 188:109–21. doi: 10.1192/bjp.188.2.109
195. Schenck CH, Montplaisir JY, Frauscher B, Hogl B, Gagnon JF, Postuma R, et al. Rapid eye movement sleep behavior disorder: devising controlled active treatment studies for symptomatic and neuroprotective therapy–a consensus statement from the International Rapid Eye Movement Sleep Behavior Disorder Study Group. Sleep Med. (2013) 14:795–806. doi: 10.1016/j.sleep.2013.02.016
196. Dowling GA, Mastick J, Colling E, Carter JH, Singer CM, Aminoff MJ. Melatonin for sleep disturbances in Parkinson's disease. Sleep Med. (2005) 6:459–66. doi: 10.1016/j.sleep.2005.04.004
197. Litvinenko IV, Krasakov IV, Tikhomirova OV. [Sleep disorders in Parkinson's disease without dementia: a comparative randomized controlled study of melatonin and clonazepam]. Zh Nevrol Psikhiatr Im S S Korsakova. (2012) 112:26–30.
198. Kunz D, Bes F. Melatonin effects in a patient with severe REM sleep behavior disorder: case report and theoretical considerations. Neuropsychobiology. (1997) 36:211–4. doi: 10.1159/000119383
199. Kunz D, Bes F. Melatonin as a therapy in REM sleep behavior disorder patients: an open-labeled pilot 9tudy on the possible influence of melatonin on REM-sleep regulation. Mov Disord. (1999) 14:507–11. doi: 10.1002/1531-8257(199905)14:3<507::AID-MDS1021>3.0.CO;2-8
200. Takeuchi N, Uchimura N, Hashizume Y, Mukai M, Etoh Y, Yamamoto K, et al. Melatonin therapy for REM sleep behavior disorder. Psychiatry Clin Neurosci. (2001) 55:267–9. doi: 10.1046/j.1440-1819.2001.00854.x
201. Boeve BF, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med. (2003) 4:281–4. doi: 10.1016/S1389-9457(03)00072-8
202. McCarter SJ, Boswell CL, St. Louis EK, Dueffert LG, Slocumb N, Boeve BF, et al. Treatment outcomes in REM sleep behavior disorder. Sleep Med. (2013) 14:237–42. doi: 10.1016/j.sleep.2012.09.018
203. Anderson KN, Shneerson JM. Drug treatment of REM sleep behavior disorder: the use of drug therapies other than clonazepam. J Clin Sleep Med. (2009) 5:235–9.
204. Kunz D, Mahlberg R. A two-part, double-blind, placebo-controlled trial of exogenous melatonin in REM sleep behaviour disorder. J Sleep Res. (2010) 19:591–6. doi: 10.1111/j.1365-2869.2010.00848.x
205. Kunz D, Bes F. Twenty years after: another case report of melatonin effects on REM sleep behavior disorder, using serial dopamine transporter imaging. Neuropsychobiology. (2017) 76:100–4. doi: 10.1159/000488893
206. Schaefer C, Kunz D, Bes F. Melatonin effects in REM sleep behavior disorder associated with obstructive sleep apnea syndrome: a case series. Curr Alzheimer Res. (2017) 14:1084–9. doi: 10.2174/1567205014666170523094938
207. Aurora RN, Zak RS, Maganti RK, Auerbach SH, Casey KR, Chowdhuri S, et al. Best practice guide for the treatment of REM sleep behavior disorder (RBD). J Clin Sleep Med. (2010) 6:85–95.
208. Mahowald MW, Schenck CH. REM sleep behaviour disorder: a window on the sleeping brain. Brain. (2015) 138:1131–3. doi: 10.1093/brain/awv058
209. Iranzo A, Molinuevo JL, Santamaria J, Serradell M, Marti MJ, Valldeoriola F, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. (2006) 5:572–7. doi: 10.1016/S1474-4422(06)70476-8
210. Fertl E, Auff E, Doppelbauer A, Waldhauser F. Circadian secretion pattern of melatonin in de novo parkinsonian patients: evidence for phase-shifting properties of l-dopa. J Neural Transm Park Dis Dement Sect. (1993) 5:227–34. doi: 10.1007/BF02257677
211. Bordet R, Devos D, Brique S, Touitou Y, Guieu JD, Libersa C, et al. Study of circadian melatonin secretion pattern at different stages of Parkinson's disease. Clin Neuropharmacol. (2003) 26:65–72. doi: 10.1097/00002826-200303000-00005
212. Videnovic A, Noble C, Reid KJ, Peng J, Turek FW, Marconi A, et al. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol. (2014) 71:463–9. doi: 10.1001/jamaneurol.2013.6239
213. Escames G, Acuña CD, Vives F. Melatonin-dopamine interaction in the striatal projection area of sensorimotor cortex in the rat. Neuroreport. (1996) 7:597–600. doi: 10.1097/00001756-199601310-00053
214. Naskar A, Manivasagam T, Chakraborty J, Singh R, Thomas B, Dhanasekaran M, et al. Melatonin synergizes with low doses of L-DOPA to improve dendritic spine density in the mouse striatum in experimental Parkinsonism. J Pineal Res. (2013) 55:304–12. doi: 10.1111/jpi.12076
215. Catala MD, Canete-Nicolas C, Iradi A, Tarazona PJ, Tormos JM, Pascual-Leone A. Melatonin levels in Parkinson's disease: drug therapy versus electrical stimulation of the internal globus pallidus. Exp Gerontol. (1997) 32:553–8. doi: 10.1016/S0531-5565(96)00173-8
216. Schernhammer E, Schulmeister K. Light at night and cancer risk. Photochem Photobiol. (2004) 79:316–8. doi: 10.1562/SA-03-28.1
217. Adi N, Mash DC, Ali Y, Singer C, Shehadeh L, Papapetropoulos S. Melatonin MT1 and MT2 receptor expression in Parkinson's disease. Med Sci Monit. (2010) 16:BR61-BR67
218. Belaid H, Adrien J, Karachi C, Hirsch EC, Francois C. Effect of melatonin on sleep disorders in a monkey model of Parkinson's disease. Sleep Med. (2015) 16:1245–51. doi: 10.1016/j.sleep.2015.06.018
219. Willis GL, Turner EJ. Primary and secondary features of Parkinson's disease improve with strategic exposure to bright light: a case series study. Chronobiol Int. (2007) 24:521–37. doi: 10.1080/07420520701420717
220. Willis GL. Parkinson's disease as a neuroendocrine disorder of circadian function: dopamine-melatonin imbalance and the visual system in the genesis and progression of the degenerative process. Rev Neurosci. (2008) 19:245–316. doi: 10.1515/REVNEURO.2008.19.4-5.245
221. Lewy AJ, Sack RA, Singer CL. Assessment and treatment of chronobiologic disorders using plasma melatonin levels and bright light exposure: the clock-gate model and the phase response curve. Psychopharmacol Bull. (1984) 20:561–5. doi: 10.1016/0022-4731(84)90599-5
222. Willis GL, Freelance CB. Emerging preclinical interest concerning the role of circadian function in Parkinson's disease. Brain Res. (2018) 1678:203–13. doi: 10.1016/j.brainres.2017.09.027
223. Leger D, Laudon M, Zisapel N. Nocturnal 6-sulfatoxymelatonin excretion in insomnia and its relation to the response to melatonin replacement therapy. Am J Med. (2004) 116:91–5. doi: 10.1016/j.amjmed.2003.07.017
224. Zhdanova IV, Wurtman RJ, Regan MM, Taylor JA, Shi JP, Leclair OU. Melatonin treatment for age-related insomnia. J Clin Endocrinol Metab. (2001) 86:4727–30. doi: 10.1210/jcem.86.10.7901
225. Ferracioli-Oda E, Qawasmi A, Bloch MH. Meta-analysis: melatonin for the treatment of primary sleep disorders. PLoS ONE. (2013) 8:e63773. doi: 10.1371/journal.pone.0063773
226. Auld F, Maschauer EL, Morrison I, Skene DJ, Riha RL. Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med Rev. (2017) 34:10–22. doi: 10.1016/j.smrv.2016.06.005
227. Li T, Jiang S, Han M, Yang Z, Lv J, Deng C, et al. Exogenous melatonin as a treatment for secondary sleep disorders: a systematic review and meta-analysis. Front Neuroendocrinol. (2018) 52:22–8. doi: 10.1016/j.yfrne.2018.06.004
228. Wilson SJ, Nutt DJ, Alford C, Argyropoulos SV, Baldwin DS, Bateson AN, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol. (2010) 24:1577–601. doi: 10.1177/0269881110379307
229. Sugden D. Psychopharmacological effects of melatonin in mouse and rat. J Pharmacol Exp Ther. (1983) 227:587–91.
230. Nordlund JJ, Lerner AB. The effects of oral melatonin on skin color and on the release of pituitary hormones. J Clin Endocrinol Metab. (1977) 45:768–74. doi: 10.1210/jcem-45-4-768
231. Anton-Tay F, Diaz JL, Fernandez-Guardiola A. On the effect of melatonin upon human brain. Its possible therapeutic implications. Life Sci I. (1971) 10:841–50. doi: 10.1016/0024-3205(71)90155-X
232. Jacob S, Poeggeler B, Weishaupt JH, Siren AL, Hardeland R, Bahr M, et al. Melatonin as a candidate compound for neuroprotection in amyotrophic lateral sclerosis (ALS): high tolerability of daily oral melatonin administration in ALS patients. J Pineal Res. (2002) 33:186–7. doi: 10.1034/j.1600-079X.2002.02943.x
233. Weishaupt JH, Bartels C, Polking E, Dietrich J, Rohde G, Poeggeler B, et al. Reduced oxidative damage in ALS by high-dose enteral melatonin treatment. J Pineal Res. (2006) 41:313–23. doi: 10.1111/j.1600-079X.2006.00377.x
234. Chahbouni M, Escames G, Lopez LC, Sevilla B, Doerrier C, Munoz-Hoyos A, et al. Melatonin treatment counteracts the hyperoxidative status in erythrocytes of patients suffering from Duchenne muscular dystrophy. Clin Biochem. (2011) 44:853–8. doi: 10.1016/j.clinbiochem.2011.04.001
235. Lopez-Gonzalez A, Alvarez-Sanchez N, Lardone PJ, Cruz-Chamorro I, Martinez-Lopez A, Guerrero JM, et al. Melatonin treatment improves primary progressive multiple sclerosis: a case report. J Pineal Res. (2015) 58:173–7. doi: 10.1111/jpi.12203
236. Nickkholgh A, Schneider H, Sobirey M, Venetz WP, Hinz U, Pelzl lH, et al. The use of high-dose melatonin in liver resection is safe: first clinical experience. J Pineal Res. (2011) 50:381–8. doi: 10.1111/j.1600-079X.2011.00854.x
237. Waldhauser F, Saletu B, Trinchard-Lugan I. Sleep laboratory investigations on hypnotic properties of melatonin. Psychopharmacology. (1990) 100:222–6. doi: 10.1007/BF02244410
238. Voordouw BC, Euser R, Verdonk RE, Alberda BT, de Jong FH, Drogendijk AC, et al. Melatonin and melatonin-progestin combinations alter pituitary-ovarian function in women and can inhibit ovulation. J Clin Endocrinol Metab. (1992) 74:108–17. doi: 10.1210/jcem.74.1.1727807
239. Galley HF, Lowes DA, Allen L, Cameron G, Aucott LS, Webster NR. Melatonin as a potential therapy for sepsis: a phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis. J Pineal Res. (2014) 56:427–38. doi: 10.1111/jpi.12134
240. Andersen LP, Werner MU, Rosenkilde MM, Fenger AQ, Petersen MC, Rosenberg J, et al. Pharmacokinetics of high-dose intravenous melatonin in humans. J Clin Pharmacol. (2015) 56:324–9. doi: 10.1002/jcph.592
241. ASHP statement on the use of medications for unlabeled uses. Am J Hosp Pharm. (1992) 49:2006–8. doi: 10.1093/ajhp/49.8.2006
242. Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. (2011) 20:177–84. doi: 10.1002/pds.2082
243. Bazzano AT, Mangione-Smith R, Schonlau M, Suttorp MJ, Brook RH. Off-label prescribing to children in the United States outpatient setting. Acad Pediatr. (2009) 9:81–8. doi: 10.1016/j.acap.2008.11.010
244. Smithburger PL, Buckley MS, Culver MA, Sokol S, Lat I, Handler SM, et al. A multicenter evaluation of off-label medication use and associated adverse drug reactions in adult medical ICUs. Crit Care Med. (2015) 43:1612–21. doi: 10.1097/CCM.0000000000001022
245. Saiyed MM, Ong PS, Chew L. Off-label drug use in oncology: a systematic review of literature. J Clin Pharm Ther. (2017) 42:251–8. doi: 10.1111/jcpt.12507
246. Aagaard L, Kristensen K. Off-label and unlicensed prescribing in Europe: implications for patients' informed consent and liability. Int J Clin Pharm. (2018) 40:509–12. doi: 10.1007/s11096-018-0646-4
247. EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to melatonin and alleviation of subjective feelings of jet lag (ID 1953), and reduction of sleep onset latency, and improvement of sleep quality (ID 1953) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. (2010) 8:1467. doi: 10.2903/j.efsa.2010.1467
248. EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to melatonin and reduction of sleep onset latency (ID 1698; 1780, 4080) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. (2011) 9:2241. doi: 10.2903/j.efsa.2011.2241
249. Tan DX, Zanghi BM, Manchester LC, Reiter RJ. Melatonin identified in meats and other food stuffs: potentially nutritional impact. J Pineal Res. (2014) 57:213–8. doi: 10.1111/jpi.12152
250. Erland LA, Murch SJ, Reiter RJ, Saxena PK. A new balancing act: The many roles of melatonin and serotonin in plant growth and development. Plant Signal Behav. (2015) 10:e1096469. doi: 10.1080/15592324.2015.1096469
251. Arnao MB, Hernandez-Ruiz J. Functions of melatonin in plants: a review. J Pineal Res. (2015) 59:133–50. doi: 10.1111/jpi.12253
252. Cardinali DP. Melatonin as a chronobiotic/cytoprotector: its role in healthy aging. Biol Rhythm Res. (2019) 50:28–45. doi: 10.1080/09291016.2018.1491200
Keywords: aging, Alzheimer's disease, glymphatic system, melatonin, mild cognitive impairment, neurodegeneration, oxidative stress, Parkinson's disease
Citation: Cardinali DP (2019) Melatonin: Clinical Perspectives in Neurodegeneration. Front. Endocrinol. 10:480. doi: 10.3389/fendo.2019.00480
Received: 11 January 2019; Accepted: 03 July 2019;
Published: 16 July 2019.
Edited by:
James M. Olcese, Florida State University, United StatesReviewed by:
Hana Zemkova, Institute of Physiology (ASCR), CzechiaDun-Xian Tan, The University of Texas Health Science Center at San Antonio, United States
Copyright © 2019 Cardinali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel P. Cardinali, ZGFuaWVsX2NhcmRpbmFsaUB1Y2EuZWR1LmFy
 Daniel P. Cardinali
Daniel P. Cardinali