- 1Department of Internal Medicine, National Taiwan University College of Medicine, Taipei, Taiwan
- 2Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
- 3Division of Environmental Health and Occupational Medicine of the National Health Research Institutes, Zhunan, Taiwan
- 4Chong’s Physical Medicine and Rehabilitation Center, Taipei, Taiwan
- 5Department of Neurology, Taipei Medical University Hospital, Taipei, Taiwan
Background: Diabetic sensory neuropathy has rarely been studied in the Asian populations. This study investigated the prevalence and risk factors of sensory symptoms (SS) in the Taiwanese diabetes patients.
Methods: A total of 1,400 diabetes patients received a health examination together with a structured questionnaire interview for three categories of abnormal sensation of numbness or tingling pain, electric shock, and skin thickness sensation on seven anatomical sites on upper limbs and six sites on lower limbs. Prevalence of SS was defined using nine different criteria, with the least stringent criterion of “any positive symptom on at least 1 site” and the most stringent criterion of “any positive symptom on at least bilateral and symmetrical 2 sites involving the lower limb.” Logistic regression was used to estimate the odds ratios and their 95% confidence interval for SS by the different definitions. Fasting plasma glucose and hemoglobin A1c were entered in separate models to avoid hypercollinearity.
Results: The prevalence of SS was 14.4 and 54.0% when using the most stringent and least stringent criterion, respectively. Women consistently had a significantly higher prevalence than men did. Among the three categories of symptoms, numbness or tingling pain was the most common, and fingers and toes were the most commonly involved anatomical sites. For any symptoms, 37.1% of the patients had any symptoms on the upper limbs and 41.7% had any symptoms on the lower limbs. Female sex, diabetes duration, hemoglobin A1c, and hypertension were associated with SS in all models.
Conclusions: Taiwanese diabetes patients may have a high prevalence of SS if a structured questionnaire is used for screening. Female sex, diabetes duration, hemoglobin A1c, and hypertension are associated with SS.
Introduction
Diabetes mellitus is a chronic non-communicable disease characterized by various vascular complications involving the arterial system (macrovascular complications) and the capillaries (microvascular complications). “Diabetes triopathy” has been used to refer to the microvascular complications including retinopathy, nephropathy, and neuropathy (1). Diabetic polyneuropathy (DPN) may involve the sensory, motor, and/or autonomic nervous system. Sensory neuropathy can be divided clinically into painful and non-painful subtypes (2, 3). Painful diabetic peripheral neuropathy (pDPN) may impair the patients’ mood, daily function, sleep, and quality of life; and its humanistic and economic burdens are immense (4, 5).
The prevalence of pDPN varies from 6 to 34% in different reports by using different diagnostic criteria in Europe (4). A recent phone/internet survey conducted in the USA showed an ethnic difference in the prevalence of pDPN: 49% in Hispanics, 65% in African-Americans, and 87% in Caucasians (P < 0.05) (6).
Epidemiological studies on DPN are rare in Asian populations. In Taipei City of Taiwan, among 217 diabetes patients derived from an epidemiological survey in 1978, 44 patients (20.3%) were diagnosed as having DPN which was defined as a motor nerve conduction velocity below the normal mean minus 2 standard deviations (1). While comparing patients with and without DPN in that study, only diabetes duration, fasting glucose level, insulin use, and blood urea nitrogen (but not creatinine level) differed significantly between the two groups (1). A nationwide hospital-based study conducted in Korea suggested a prevalence of 14.4% for pDPN in patients with type 2 diabetes mellitus (7). This Korean study identified age, female sex, glycemic control, hypertension, and previous cardiovascular events as risk factors of pDPN (7). In Myanmar, a study conducted in 975 diabetes patients who attended the outpatient clinics of four hospitals reported a prevalence rate of 33.7% for DPN and 59.5% for pDPN (8). The investigators found an association between DPN and older age, longer diabetes duration, and smoking (8). In a cross-sectional study that aimed at investigating the prevalence of diabetic retinopathy in 1,008 diabetes patients enrolled from a hospital in Shijiazhuang, Hebei, China, the investigators reported a prevalence of 11% for DPN among the patients and found a close association between DPN and diabetic retinopathy (9). Another recently published cross-sectional study that enrolled patients from 17 primary care clinics across Japan showed a prevalence of 27.7% for DPN among 9,914 patients surveyed (10). Among the patients with DPN, 61.5% had DPN-related sensory symptoms/signs, which were significantly associated with female sex, smoking, and alcohol drinking (10).
Under-diagnosis is very common for pDPN in clinical practice (11). A USA study showed that while 83% of diabetes patients might report symptoms of pDNP at study, only 41% of them had ever been diagnosed as having DPN by healthcare practitioners (11). Another Japanese study showed that although 22.1% of the 298 studied patients might have pDPN, only 36.4% of them were recognized by the physicians (12). A recent multinational survey conducted in five countries in South-East Asia indicated that there might be significant gaps between physicians and patients in the perception of pDPN (13). The investigators advocated physician-patient dialogue to maximize patient outcomes (13).
Because reports on diabetic sensory neuropathy are rare and its true prevalence requires careful and systematic evaluation, the present study aimed at investigating the prevalence and risk factors of sensory symptoms (SS) in a representative cohort of diabetes patients derived from an epidemiological study in Taiwan.
Materials and Methods
Study Subjects
The study was approved by an ethics committee of the Department of Health of Taiwan (DOH89-TD-1035) and the subjects participated in the study voluntarily. The participants were informed of the purpose of the study and the funding of the study by the Department of Health of Taiwan. They were able to opt out of the study according to their free will. At the time of questionnaire interview and blood sampling, signed inform consent was not required according to local regulations. More than 96% of the Taiwanese population is covered by a universal and compulsory National Health Insurance at the time of the study. A total of 256,036 diabetes patients using this health insurance were assembled from 1995 to 1998 to investigate a series of epidemiologic issues (14). Baseline data on the onset symptoms and confirmation of diabetes diagnosis were collected by a questionnaire from 93,484 patients (15). At random, 4,164 patients living in the Northern Region of Taiwan from the main cluster of 93,484 patients were selected and invited to participate in a health examination. From March 1998 to September 2002, a total of 1,441 patients participated in the health examination. No significant differences in age or sex were noted among the main national sample and those who participated in the health examination (16, 17).
Questionnaire Interview for Sensory Symptoms
For those who participated in the health examination, a structured questionnaire (Supplementary File) was interviewed by a well-trained interviewer for the symptoms of three categories of sensory abnormalities: 1) numbness or tingling pain; 2) electric shock; and 3) skin thickness. Seven sites on each upper limb (i.e., fingertip, other parts of the finger, palm, dorsum of hand, wrist, lower arm, and upper arm) and six sites on each lower limb (toe tip, other parts of the toe, plantar surface of foot, dorsum of foot, lower leg, and thigh) were recorded for the respective symptoms.
Measurements of Covariates
Age, sex, diabetes duration, fasting plasma glucose/hemoglobin A1c (A1C), smoking, obesity, hypertension, dyslipidemia, proteinuria, and use of insulin were treated as covariates.
Diabetes duration was defined as the time period in years between the time of receiving health examination and the time when diabetes was diagnosed. Patients who smoked one or more cigarettes per day were defined as smokers.
Anthropometric factors including body height, body weight, and waist circumference were measured as described in detail previously (18, 19). Body mass index was calculated as body weight in kg divided by the square of body height in meters. Obesity was defined as a body mass index ≥25 kg/m2 (20), and/or a waist circumference ≥90 cm for men or ≥80 cm for women.
A mercury sphygmomanometer was used to measure blood pressure on the right arm after 20 min rest in a sitting position. Definition of hypertension was based on one of the following three criteria: 1) being under treatment with antihypertensive drugs; 2) having systolic blood pressure ≥140 mmHg; or 3) having diastolic blood pressure ≥90 mmHg.
Subjects were instructed to avoid any vigorous physical activities one day before attending the health examination, to prevent any undue influence on the urinary excretion of albumin. In the early morning of the date of health examination, urine and blood samples were collected after fasting for a minimum duration of 12 h. First voided mid-stream urine was collected and then venous blood sample was taken. Urinary albumin concentration was measured by a particle-enhanced turbidimetric immunoassay (Biolatex®, Logroño, Spain) (21, 22) and urinary creatinine concentration was measured after dilution (×10) on an automated chemistry analyzer (Cobas Mira S, Roche Diagnostica, Basel, Switzerland) with reagents obtained from Randox Laboratories Ltd. (Antrim, UK). Proteinuria was defined by an albumin-to-creatinine ratio ≥300 μg/mg. Fasting plasma glucose and serum lipid profiles were measured by an automatic biochemistry analyzer (Cobas Mira S, Roche Diagnostica, Basel, Switzerland) with reagents obtained from Randox Laboratories Ltd. (Antrim, UK). A1C was measured by means of boronate affinity chromatography with reagents obtained from the Primus Corporation (Primus CLC385, Kansas City, MO, USA). Dyslipidemia was defined as a triglyceride level ≥1.7 mmol/L and/or low-density lipoprotein cholesterol ≥2.59 mmol/L and/or high-density lipoprotein cholesterol <0.9 mmol/L for men or <1.0 mmol/L for women, and/or those undergoing treatment for lipid disorder.
Statistical Analyses
Analyses were conducted using SAS statistical software, version 9.4 (SAS Institute, Cary, NC, USA). P-value <0.05 was considered statistically significant.
The distributions of the three categories of sensory abnormalities (i.e., numbness or tingling pain, electric shock, and skin thickness) and any symptom by sites on upper and lower limbs were first tabulated. SS was defined by nine different criteria with positive symptom: 1) any positive symptom on at least one site; 2) any positive symptom on at least one site involving the lower limb; 3) any positive symptom on at least two sites with at least one involving the lower limb; 4) any positive symptom on at least three sites with at least one involving the lower limb; 5) any positive symptom on at least four sites with at least one involving the lower limb; 6) any positive symptom on at least two sites with at least two involving the lower limb; 7) any positive symptom on at least three sites with at least two involving the lower limb; 8) any positive symptom on at least four sites with at least two involving the lower limb; and 9) any positive symptom on at least bilateral and symmetrical two sites involving the lower limb.
The prevalence of SS according to the different criteria was then calculated for all patients and for men and women, respectively. Chi square test was used to compare the difference of SS prevalence between men and women.
To identify potential risk factors of SS, logistic regression models were created to estimate the odds ratios and their 95% confidence intervals. SS defined by different criteria was treated as a dependent variable, and all covariates including age, sex, diabetes duration, fasting plasma glucose/A1C, smoking, obesity, hypertension, dyslipidemia, proteinuria, and use of insulin were treated as independent variables. Because fasting plasma glucose and A1C were highly correlated, separate models were created for these two covariates.
Results
A total of 1,400 patients received questionnaire interview for the sensory symptoms. Among them, 1,395 patients received health examination and had complete data of the measured covariates.
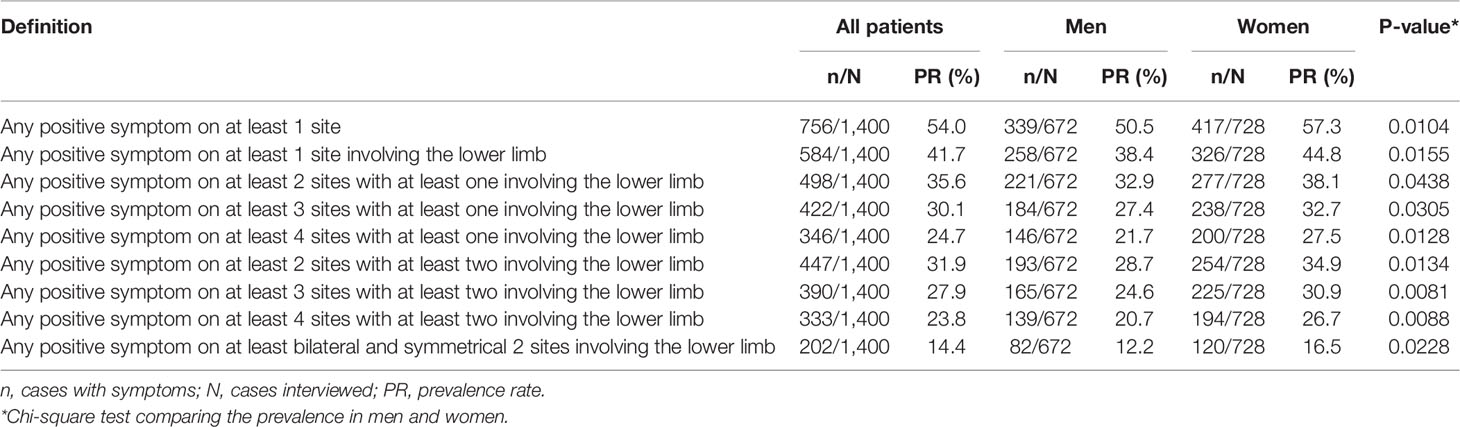
Table 1 shows the distribution of the three categories of sensory abnormalities by sites on the upper limbs and lower limbs, respectively. The most common complaint was numbness or tingling pain, which involved mainly the fingers or toes. For any symptoms, 37.1% of the patients had any symptoms on the upper limbs and 41.7% had any symptoms on the lower limbs.
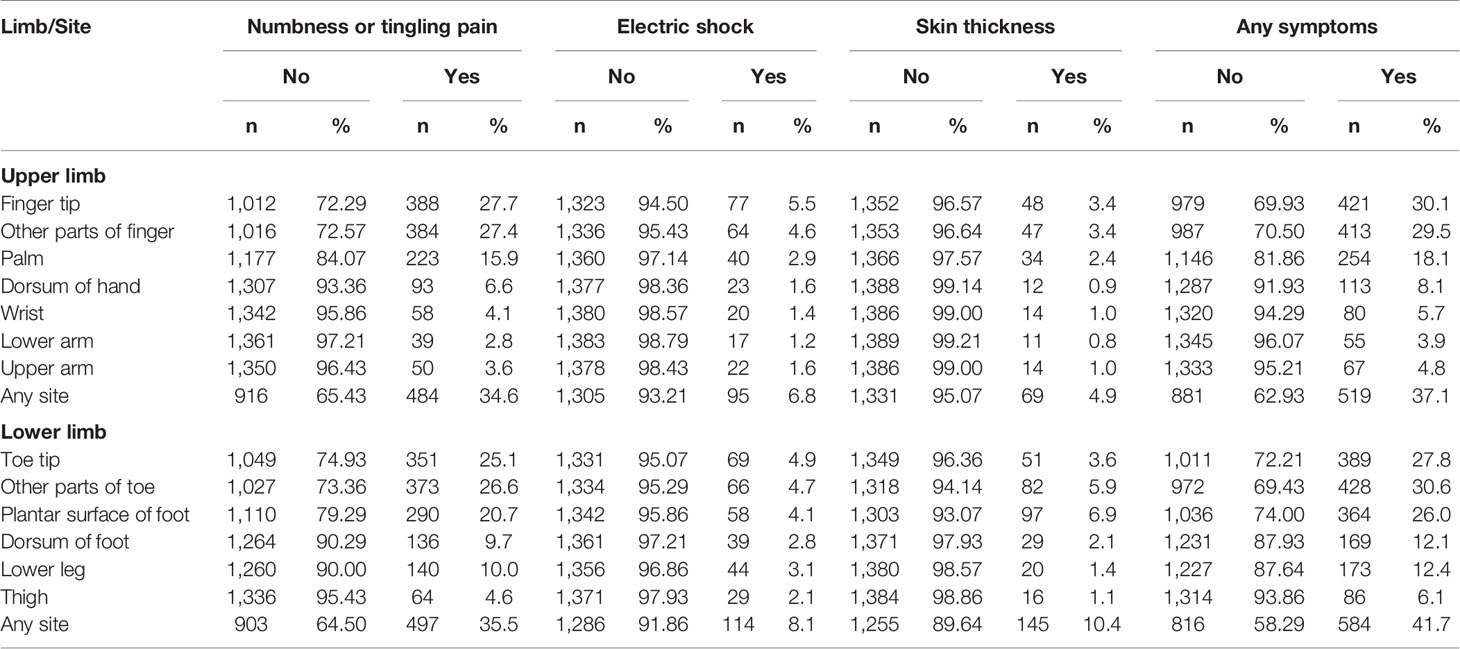
Table 1 Distribution of positive sensory symptoms by sites on upper and lower limbs in 1,400 diabetes patients.
Table 2 shows the prevalence of SS by using the nine different definitions. If any positive symptom on any one site was defined as SS, then 54.0% of the patients would have SS. If the most stringent definition was applied (i.e., any positive symptom on at least bilateral and symmetrical two sites involving the lower limb), then 14.4% of the patients would have SS. When comparing the prevalence between men and women, it is evident that a significantly higher prevalence was observed in women disregarding the definitions used.
Tables 3 and 4 show the odds ratios and their 95% confidence intervals in models using the nine different definitions of SS. Table 3 shows the models created with fasting plasma glucose and Table 4 shows the models created with A1C. Female sex, diabetes duration, A1C, and hypertension were the four variables that were significantly associated with SS in all models. Fasting plasma glucose was not as good as A1C in the association with SS. Smoking, obesity, and dyslipidemia were not associated with SS in all models. Except for model II in Table 4, age was not associated with SS. Other covariates including proteinuria and use of insulin were not consistently associated with SS.
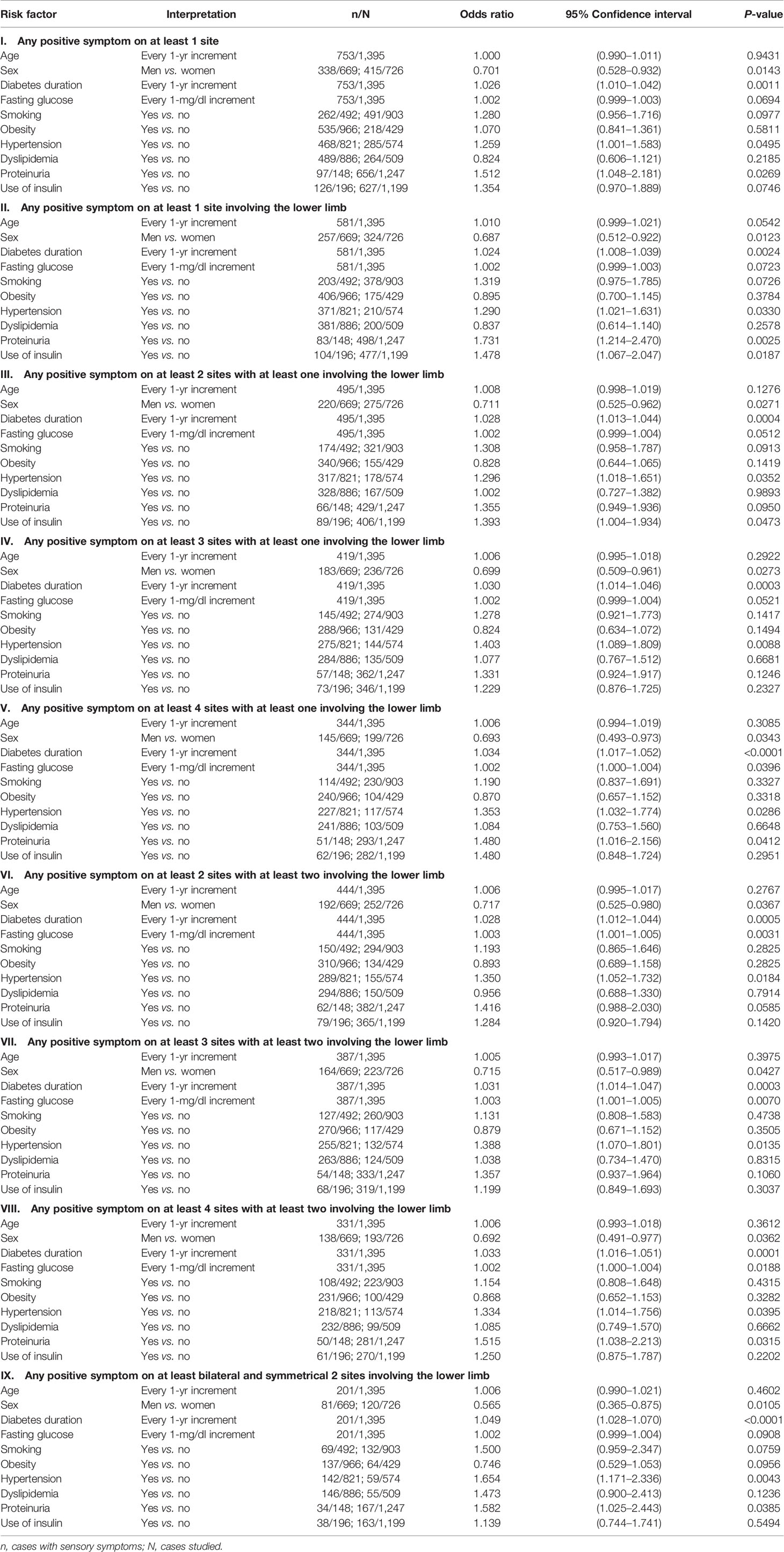
Table 3 Logistic regression evaluating risk factors of sensory symptoms according to different definitions (models with fasting plasma glucose).
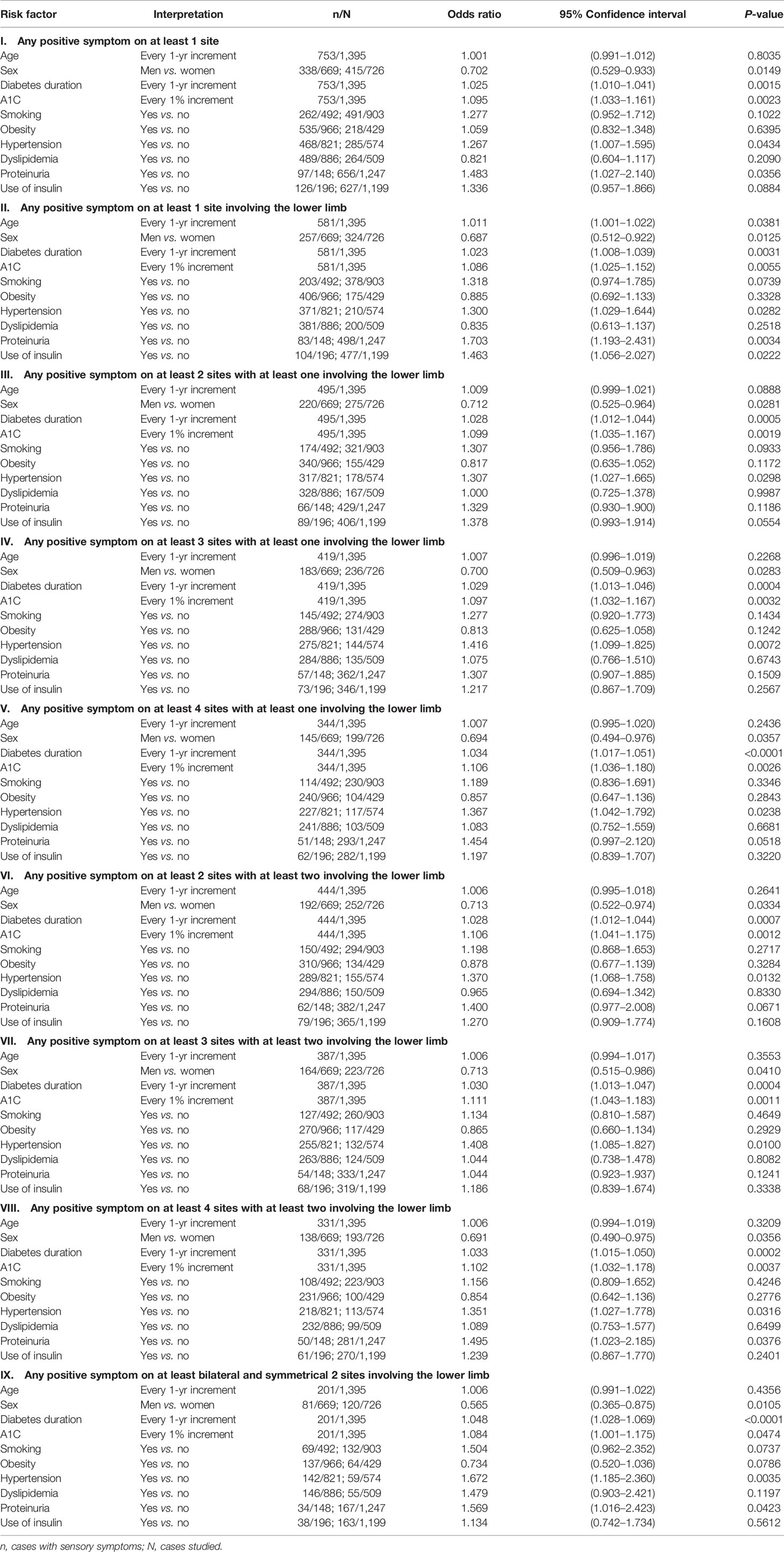
Table 4 Logistic regression evaluating risk factors of sensory symptoms according to different definitions (models with A1C).
Discussion
This is the first population-based observational study evaluating the sensory abnormalities in Taiwanese diabetes patients. The prevalence of 14.4% by using the most stringent criterion in the present study was lower than the reported 20.3% in our early study conducted in 1978 in Taipei City by using a definition of a slowed motor nerve conduction velocity (1). However, this prevalence rate was the same as that reported in the nationwide hospital-based survey conducted in Korea (7). The identified independent risk factors of female sex and hypertension in the present study (Tables 3 and 4) were also observed in the Korean study (7). The present study suggested that diabetes duration was significantly associated with SS after multivariate adjustment (Tables 3 and 4). On the contrary, the Korean study showed that age but not diabetes duration was significantly associated with pDPN (7). Glycemic control, especially when indicated by A1C, was significantly associated with SS in the present study (Tables 3 and 4), but the Korean study suggested that fasting plasma glucose was better associated with pDPN than A1C (7). The higher risk associated with the use of insulin in our previous study (1) and in the present cross-sectional study in some models (Tables 3 and 4) might just indicate a poor glycemic control in patients who required insulin for treatment rather than a true cause-effect relationship.
Sex differences in sensory perception have long been recognized either in animals or humans (23–25). Females are more sensitive to pain than males (23–25). The real mechanisms for such a sex discrepancy in sensory perception remain to be explored, but biological, sociocultural, and psychological factors may play some roles (24, 25). Recent studies suggested a role of estrogen in the regulation of pain processing pathway with the involvement of the adaptive immune system (22, 26, 27). It is not known whether the higher prevalence of SS in women than in men was due to sex difference in the thresholds of sensory perception. A female preponderance of pDPN was also observed in the Korean study (7) and the Japanese study (10). Animal in vitro and in vivo studies suggested that estradiol may upregulate the nocisensor transient receptor potential (TRP) vanilloid 1 receptor in sensory neurons, resulting in lowered thresholds of sensation (28). Animal studies also supported that female sex may experience greater pain related to inflammation, because of the upregulation of TRP vanilloid 1, TRP ankyrin 1, and TRP melastatin 8 by prolactin; and such effects are more prominent in female than in male rats (29, 30). Studies conducted in humans also suggested sex differences in the response to mechanical pressure pain (31) and cold pressor pain (32), which might be related to the differences in sex hormone levels between men and women and during different phases of menstruation cycle in women.
DPN may develop at an early stage of hyperglycemia including the prediabetes status (33). Therefore, the nerve damages caused by high glucose levels can develop insidiously during the long period of prediabetes status. The consistency of diabetes duration (but not age) and A1C in the association with SS (Tables 3 and 4) suggested that the SS might be diabetes-specific and related to glycemic control.
A recent study showed an association between pDPN and nondipping in blood pressure during midnight (34). Because the circadian change in blood pressure is controlled by autonomic nerve and diabetes patients with hypertension is highly associated with non-dipping (35), the link between hypertension and SS, but not other major atherosclerotic risk factors such as smoking, obesity, and dyslipidemia (Tables 3 and 4), suggested a potential involvement of autonomic neuropathy.
This study has several strengths. First, it was conducted in a population-based representative cohort of diabetes patients and might be more readily used for generalization of the findings. Second, the questionnaire covered three categories of symptoms on different anatomical sites for a better description of the clinical distributions of different symptoms. Third, by using the different definitions of SS, it was possible for us to evaluate the prevalence according to different definitions (Table 2) and to test the consistency of findings by using different definitions (Tables 3 and 4).
There are some limitations. First, this study was conducted in 1990s, and therefore, it remains unknown whether the prevalence might have changed significantly in recent years. However, a long-term follow-up of the patients would allow us to conduct studies in the near future to evaluate the impact of SS on the mortality of the patients by matching the national death certificates database in Taiwan. Second, because the study was conducted at a time when the currently used tools such as LANSS (Leeds Assessment of Neuropathic Symptoms and Signs) (36) and DN4 (Douleur Neuropathique 4) (37) were not yet available, we used a questionnaire developed by ourselves. Third, we recognized that the validity and reliability of the questionnaire used in the study had not been tested. Therefore, we still need to take some effort to test the validity and reliability of this questionnaire and to examine its usefulness as a clinical tool for predicting the development of diabetes complications and mortality. The inclusion of neurological examinations and objective laboratory tests such as Achilles tendon reflex, vibration threshold and nerve conduction velocity, etc. in our future studies would be helpful for evaluating the usefulness of the questionnaire. Fourth, the cross-sectional nature of the study did not allow a direct interpretation of cause-effect relationship between the evaluated covariates and SS. Fifth, this study was not able to evaluate the prevalence of painless neuropathy, which is related to the development of diabetic foot (38). Sixth, symptom severity was not evaluated in the present study. Seventh, the abnormal sensation obtained from the interview might not be really due to DPN. Other diseases such as carpal tunnel syndrome or spinal stenosis could not be excluded because of similar sensory presentations.
In summary, the presentation of SS is very common in the Taiwanese diabetes patients. The prevalence may range from 14.4 to 54.0% by using the most stringent criterion to using the least stringent criterion. Women are more prone to report sensory abnormalities than men. SS may be diabetes-specific and related to female sex, diabetes duration, A1C, and hypertension. However, age, and other atherosclerotic risk factors such as smoking, obesity, and dyslipidemia are not associated with SS. Prospective cohort studies are required to explore the cause-effect relationship of some covariates.
Data Availability Statement
The datasets presented in this article are not readily available because according to the Personal Information Protection Act enacted in Taiwan, individualized data cannot be released for the protection of privacy. Requests to access the datasets should be directed to Y2NrdHNoQG1zNi5oaW5ldC5uZXQ=.
Ethics Statement
The studies involving human participants were reviewed and approved by the Department of Health, Taiwan. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
C-HT researched the data and wrote manuscript. C-KC designed the questionnaire and trained interviewers. J-JS designed the questionnaire and controlled the quality of the questionnaire. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by the Department of Health (DOH89-TD-1035 and DOH97-TD-D-113-97009) of Taiwan. Analyses of the data were supported partly by the Ministry of Science and Technology (MOST 107-2221-E-002-129-MY3) of Taiwan, the Yee Fong Charity Foundation, and the Pfizer Biopharmaceuticals Group, Taiwan. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Ms. Chai-Yen Yang and Ms. Ting-Ting Chan for their respective excellent help in conducting the questionnaire interview and the statistical analyses.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2020.580426/full#supplementary-material
References
1. Tai TY, Tseng CH, Sung SM, Huang RF, Chen CZ, Tsai SH. Retinopathy, neuropathy and nephropathy in non-insulin-dependent diabetic patients. J Formosan Med Assoc (1991) 90:936–40.
2. Spallone V, Greco C. Painful and painless diabetic neuropathy: one disease or two? Curr Diabetes Rep (2013) 13:533–49. doi: 10.1007/s11892-013-0387-7
3. Themistocleous AC, Ramirez JD, Shillo PR, Lees JG, Selvarajah D, Orengo C, et al. The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain (2016) 157:1132–45. doi: 10.1097/j.pain.0000000000000491
4. Alleman CJ, Westerhout KY, Hensen M, Chambers C, Stoker M, Long S, et al. Humanistic and economic burden of painful diabetic peripheral neuropathy in Europe: A review of the literature. Diabetes Res Clin Pract (2015) 109:215–25. doi: 10.1016/j.diabres.2015.04.031
5. Sadosky A, Mardekian J, Parsons B, Hopps M, Bienen EJ, Markman J. Healthcare utilization and costs in diabetes relative to the clinical spectrum of painful diabetic peripheral neuropathy. J Diabetes Complications (2015) 29:212–7. doi: 10.1016/j.jdiacomp.2014.10.013
6. Eichholz M, Alexander AH, Cappelleri JC, Hlavacek P, Parsons B, Sadosky A, et al. Perspectives on the impact of painful diabetic peripheral neuropathy in a multicultural population. Clin Diabetes Endocrinol (2017) 3:12. doi: 10.1186/s40842-017-0051-2
7. Kim SS, Won JC, Kwon HS, Kim CH, Lee JH, Park TS, et al. Prevalence and clinical implications of painful diabetic peripheral neuropathy in type 2 diabetes: results from a nationwide hospital-based study of diabetic neuropathy in Korea. Diabetes Res Clin Pract (2014) 103:522–9. doi: 10.1016/j.diabres.2013.12.003
8. Win MMTM, Fukai K, Nyunt HH, Hyodo Y, Linn KZ. Prevalence of peripheral neuropathy and its impact on activities of daily living in people with type 2 diabetes mellitus. Nurs Health Sci (2019) 21:445–53. doi: 10.1111/nhs.12618
9. Yin L, Zhang D, Ren Q, Su X, Sun Z. Prevalence and risk factors of diabetic retinopathy in diabetic patients: A community based cross-sectional study. Med (Baltimore) (2020) 99:e19236. doi: 10.1097/MD.0000000000019236
10. Yokoyama H, Tsuji T, Hayashi S, Kabata D, Shintani A. Factors associated with diabetic polyneuropathy-related sensory symptoms and signs in patients with polyneuropathy: A cross-sectional Japanese study (JDDM 52) using a non-linear model. J Diabetes Investig (2020) 11:450–7. doi: 10.1111/jdi.13117
11. Sadosky A, Hopper J, Parsons B. Painful diabetic peripheral neuropathy: results of a survey characterizing the perspectives and misperceptions of patients and healthcare practitioners. Patient (2014) 7:107–14. doi: 10.1007/s40271-013-0038-8
12. Tsuji M, Yasuda T, Kaneto H, Matsuoka TA, Hirose T, Kawamori R, et al. Painful diabetic neuropathy in Japanese diabetic patients is common but underrecognized. Pain Res Treat (2013) 2013:318352. doi: 10.1155/2013/318352
13. Malik RA, Aldinc E, Chan SP, Deerochanawong C, Hwu CM, Rosales RL, et al. Perceptions of painful diabetic peripheral neuropathy in South-East Asia: results from patient and physician surveys. Adv Ther (2017) 34:1426–37. doi: 10.1007/s12325-017-0536-5
14. Tseng CH. Mortality and causes of death in a national sample of diabetic patients in Taiwan. Diabetes Care (2004) 27:1605–9. doi: 10.2337/diacare.27.7.1605
15. Tseng CH. Prevalence of lower-extremity amputation among patients with diabetes mellitus: Is height a factor? CMAJ (2006) 174:319–23. doi: 10.1503/cmaj.050680
16. Tseng CH. Waist-to-height ratio and coronary artery disease in Taiwanese type 2 diabetic patients. Obesity (2008) 16:2754–9. doi: 10.1038/oby.2008.430
17. Tseng CH, Chong CK, Tseng CP, Shau WY, Tai TY. Hypertension is the most important component of metabolic syndrome in the association with ischemic heart disease in Taiwanese type 2 diabetic patients. Circ J (2008) 72:1419–24. doi: 10.1253/circj.CJ-08-0009
18. Tseng CH. Body composition as a risk factor for coronary artery disease in Chinese type 2 diabetic patients in Taiwan. Circ J (2003) 67:479–84. doi: 10.1253/circj.67.479
19. Tseng CH. Sexual difference in the distribution of atherosclerotic risk factors and their association with peripheral arterial disease in Taiwanese type 2 diabetic patients. Circ J (2007) 71:1131–6. doi: 10.1253/circj.71.1131
20. World Health Organization. International Obesity Taskforce & International Association for the Study of Obesity. In: The Asia–Pacific perspective: redefining obesity and its treatment. Hong Kong: WHO, IOTF & IASO (2000).
21. Tseng CH. Lipid abnormalities associated with urinary albumin excretion rate in Taiwanese type 2 diabetic patients. Kidney Int (2005) 67:1547–53. doi: 10.1111/j.1523-1755.2005.00235.x
22. Tseng CH. Correlation of uric acid and urinary albumin excretion rate in patients with type 2 diabetes mellitus in Taiwan. Kidney Int (2005) 68:796–801. doi: 10.1111/j.1523-1755.2005.00459.x
23. Amandusson Å, Blomqvist A. Estrogenic influences in pain processing. Front Neuroendocrinol (2013) 34:329–49. doi: 10.1016/j.yfrne.2013.06.001
24. Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med (2005) 2:137–45. doi: 10.1016/S1550-8579(05)80042-7
25. Sorge RE, Totsch SK. Sex differences in pain. J Neurosci Res (2017) 95:1271–81. doi: 10.1002/jnr.23841
26. Rosen S, Ham B, Mogil JS. Sex differences in neuroimmunity and pain. J Neurosci Res (2017) 95:500–8. doi: 10.1002/jnr.23831
27. Mapplebeck JC, Beggs S, Salter MW. Molecules in pain and sex: a developing story. Mol Brain (2017) 10:9. doi: 10.1186/s13041-017-0289-8
28. Payrits M, Sághy É, Cseko K, Pohóczky K, Bölcskei K, Ernszt D, et al. Estradiol sensitizes the Transient Receptor Potential Vanilloid 1 Receptor in pain responses. Endocrinology (2017) 158:3249–58. doi: 10.1210/en.2017-00101
29. Patil MJ, Ruparel SB, Henry MA, Akopian AN. Prolactin regulates TRPV1, TRPA1, and TRPM8 in sensory neurons in a sex-dependent manner: Contribution of prolactin receptor to inflammatory pain. Am J Physiol Endocrinol Metab (2013) 305:E1154–64. doi: 10.1152/ajpendo.00187.2013
30. Patil MJ, Green DP, Henry MA, Akopian AN. Sex-dependent roles of prolactin and prolactin receptor in postoperative pain and hyperalgesia in mice. Neuroscience (2013) 253:132–41. doi: 10.1016/j.neuroscience.2013.08.035
31. Kowalczyk WJ, Sullivan MA, Evans SM, Bisaga AM, Vosburg SK, Comer SD. Sex differences and hormonal influences on response to mechanical pressure pain in humans. J Pain (2010) 11:330–42. doi: 10.1016/j.jpain.2009.08.004
32. Kowalczyk WJ, Evans SM, Bisaga AM, Sullivan MA, Comer SD. Sex differences and hormonal influences on response to cold pressor pain in humans. J Pain (2006) 7:151–60. doi: 10.1016/j.jpain.2005.10.004
33. Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol (2012) 11:521–34. doi: 10.1016/S1474-4422(12)70065-0
34. D’Amato C, Morganti R, Di Gennaro F, Greco C, Marfia GA, Spallone V. A novel association between nondipping and painful diabetic polyneuropathy. Diabetes Care (2014) 37:2640–2. doi: 10.2337/dc14-0528
35. Cardoso CR, Leite NC, Freitas L, Dias SB, Muxfeld ES, Salles GF. Pattern of 24-hour ambulatory blood pressure monitoring in type 2 diabetic patients with cardiovascular dysautonomy. Hypertens Res (2008) 31:865–72. doi: 10.1291/hypres.31.865
36. Bennett M. The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain (2001) 92:147–57. doi: 10.1016/S0304-3959(00)00482-6
37. Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain (2005) 114:29–36. doi: 10.1016/j.pain.2004.12.010
Keywords: diabetes mellitus, sensory symptoms, epidemiology, risk factors, Taiwan
Citation: Tseng C-H, Chong C-K and Sheu J-J (2021) Prevalence and Risk Factors of Sensory Symptoms in Diabetes Patients in Taiwan. Front. Endocrinol. 11:580426. doi: 10.3389/fendo.2020.580426
Received: 06 July 2020; Accepted: 26 November 2020;
Published: 08 January 2021.
Edited by:
Hiroki Mizukami, Hirosaki University, JapanCopyright © 2021 Tseng, Chong and Sheu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chin-Hsiao Tseng, Y2NrdHNoQG1zNi5oaW5ldC5uZXQ=
 Chin-Hsiao Tseng
Chin-Hsiao Tseng Choon-Khim Chong4
Choon-Khim Chong4