- 1Center for Reproductive Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Henan Key Laboratory of Reproduction and Genetics, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Oocyte retrieval is a routine procedure during the application of assisted reproduction technology. However, technical difficulties experienced during oocyte retrieval and the subsequent unsatisfactory number of oocytes obtained are rarely reported. The current study included 10,624 oocyte retrieval cycles from April 2015 to June 2018, and patients were followed up until February 2019. Patients were divided into two groups depending on whether the oocyte number obtained reached the >14-mm follicle number on the day of hCG administration. In the oocyte retrieval not satisfactory (ORNS) group, there were 1,294 cycles, and in the oocyte retrieval satisfactory (ORS) group, there were 9,330 cycles. ORNS patients were older, had a longer duration of infertility, had higher follicle-stimulating hormone, and were more likely to have endometriosis. The ORS group had a higher rate of the use of a follicular phase long-acting gonadotropin-releasing hormone (GnRH) agonist long ovarian stimulation protocol and a lower rate of the use of a luteal phase short-acting GnRH agonist long protocol. The ORNS group had fewer total number of days of FSH stimulation. On human chorionic gonadotropin day, the ORNS group had higher luteinizing hormone (LH), lower estradiol, and lower progesterone levels. After oocyte retrieval, the oocyte quality and fresh cycle transplantation rate were higher in the ORNS group. An unsatisfactory oocyte retrieval number did not influence the clinical pregnancy rate, miscarriage rate, or live birth rate during the fresh cycles. The cumulative pregnancy rate and the live birth rate were lower in the ORNS group. In conclusion, with a similar number of matured follicles, ORNS was more likely to occur in ovarian dysfunction patients. The follicular phase long-acting GnRH agonist long protocol had lower oocyte retrieval difficulty during IVF/ICSI. ORNS does not affect embryo quality or the fresh cycle pregnancy rate, but it significantly reduces the cumulative pregnancy rate and the live birth rate.
Introduction
Transvaginal oocyte retrieval is a regular procedure in each in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) cycle. The aim is to obtain matured oocyte–cumulus complexes after controlled ovarian stimulation. Sometimes, however, despite the operator trying to obtain oocytes from each >14-mm follicle, an unsatisfactory number of oocytes is retrieved.
Neither the reasons for oocyte retrieval difficulty nor the effects that arise from it have been thoroughly investigated. Follicular flushing has been used in attempts to negate unsatisfactory oocyte retrieval. In patients in whom follicular aspiration was difficult, oocytes could reportedly be obtained by repeated follicular flushing. In a survey of Australian assisted reproduction technology (ART) units, >50% of operators used follicular flushing in addition to direct aspiration during oocyte retrieval (1). Follicular flushing increased the number of oocytes retrieved (2), but in randomized controlled IVF/ICSI trials, direct aspiration and follicular flushing were associated with similar outcomes, including the numbers of oocytes retrieved, fertilization rates, embryo quality, and pregnancy rates in both normal and poor ovarian responders (3–10). Thus, follicular flushing has become a safe and efficient way to increase the number of oocytes obtained.
Oocyte retrieval difficulty usually manifested as the number of oocytes acquired after four to six follicular flushes remains unsatisfactory. Not all of these patients are poor ovarian responders. Some patients even have more than 10 follicles according to our observation. The reasons for this phenomenon are unclear, and few studies have focused on this population. Only endometriosis has been definitively identified as a cause of oocyte retrieval difficulty (11), and this causative association may be related to insufficient cumulus expansion, where cumulus expansion-relative genes were decreased in endometriosis patients (12). Our investigation showed that patients with unsatisfactory oocyte retrieval numbers (i.e., the final oocyte number obtained was less than the >14-mm follicle number) were accounted for 12.18% of all IVF/ICSI cycles. The reasons for unsatisfactory oocyte retrieval numbers and its influence on ART outcome remain to be determined.
Materials and Methods
Study Design and Population
Women undergoing IVF or ICSI at the First Affiliated Hospital of Zhengzhou University were enrolled in the study. The study was conducted from February 2015 to June 2018 and was approved by the Independent Ethics Committee of Zhengzhou University. Patients with a body mass index >15 and <36, between the ages of 22 and 45 years, with an indication for IVF or ICSI, and presenting with at least one follicle >14 mm in both ovaries combined were considered eligible for inclusion. The exclusion criteria were mini cycles or natural stimulation cycles, no oocyte unoccupied cycles, or preimplantation genetic diagnosis cycles. A total of 10,624 oocyte retrieval cycles were included in the study.
Final oocyte maturation was conventionally induced by 6,500 IU recombinant human chorionic gonadotropin (hCG) and 2,000 IU urinary hCG. In patients at high risk of ovarian hyperstimulation syndrome, 6,500 IU recombinant hCG or 3,000–5,000 IU urinary hCG was used. Oocyte pickup was scheduled 34–37 h thereafter. The numbers and sizes of follicles were assessed and recorded just before beginning the retrieval process. The main aim was to procure follicles with diameters >14 mm. Oocyte retrieval was performed together with oocyte pickup under a microscope by two embryologists. Follicles >14 mm were aspirated or flushed.
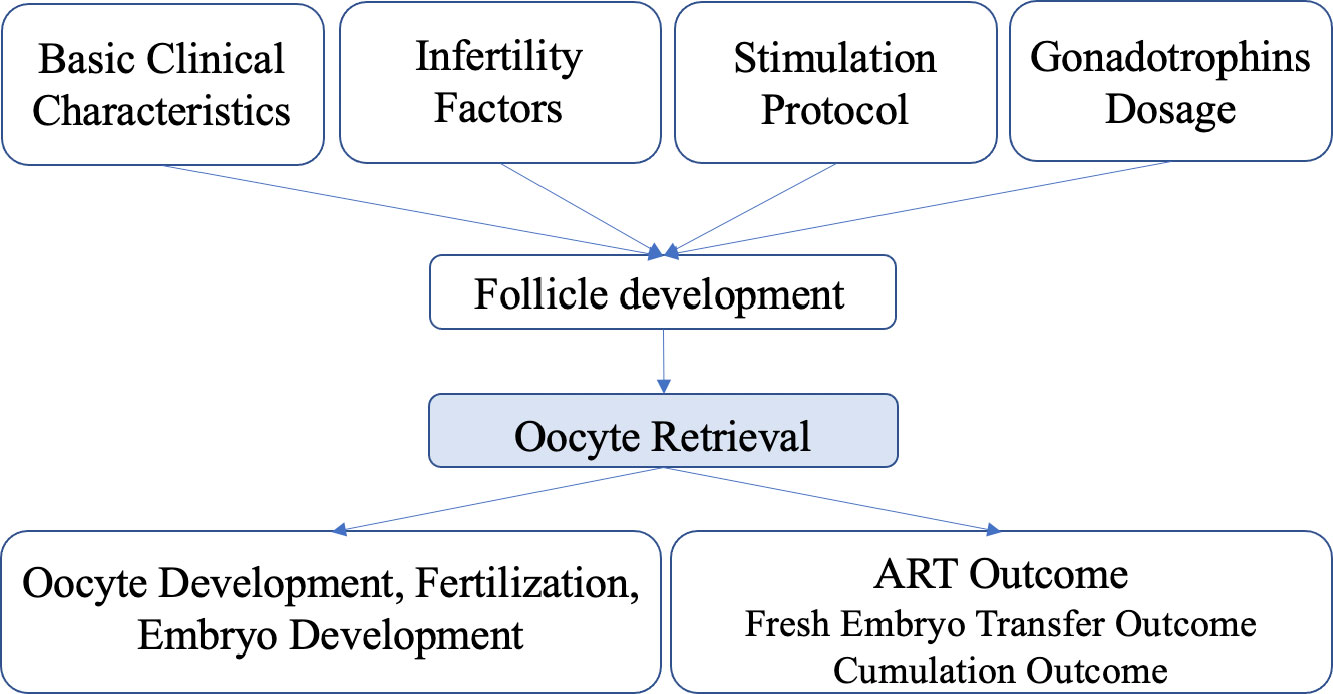
Aspiration was performed first so that the follicle completely collapsed. Then flushing was performed to expand the follicle back to its original diameter in each round with a steady pressure of 125–140 mmHg. The flushing system was prefilled and prewarmed, and a mechanical pump with a foot pedal was used. Aspirated and flushed follicular fluid was collected into stacked tubes on a warming rack. The decision as to whether to perform flushing or not was made by the operator based on the following criteria: if the oocyte was obtained from the original follicular fluid, the follicular cavity would not be flushed; if the oocyte could not be acquired from the original follicular fluid, the follicular cavity would be flushed two to four times. The maximum number of flushes was six. The patients were divided into two groups based on whether the number of >14-mm follicles was consistent with the number of oocytes obtained: an oocyte retrieval satisfactory (ORS) group and an oocyte retrieval not satisfactory (ORNS) group. The causes of unsatisfactory oocyte retrieval and subsequent outcomes associated with it were analyzed (Figure 1).
Data Collection
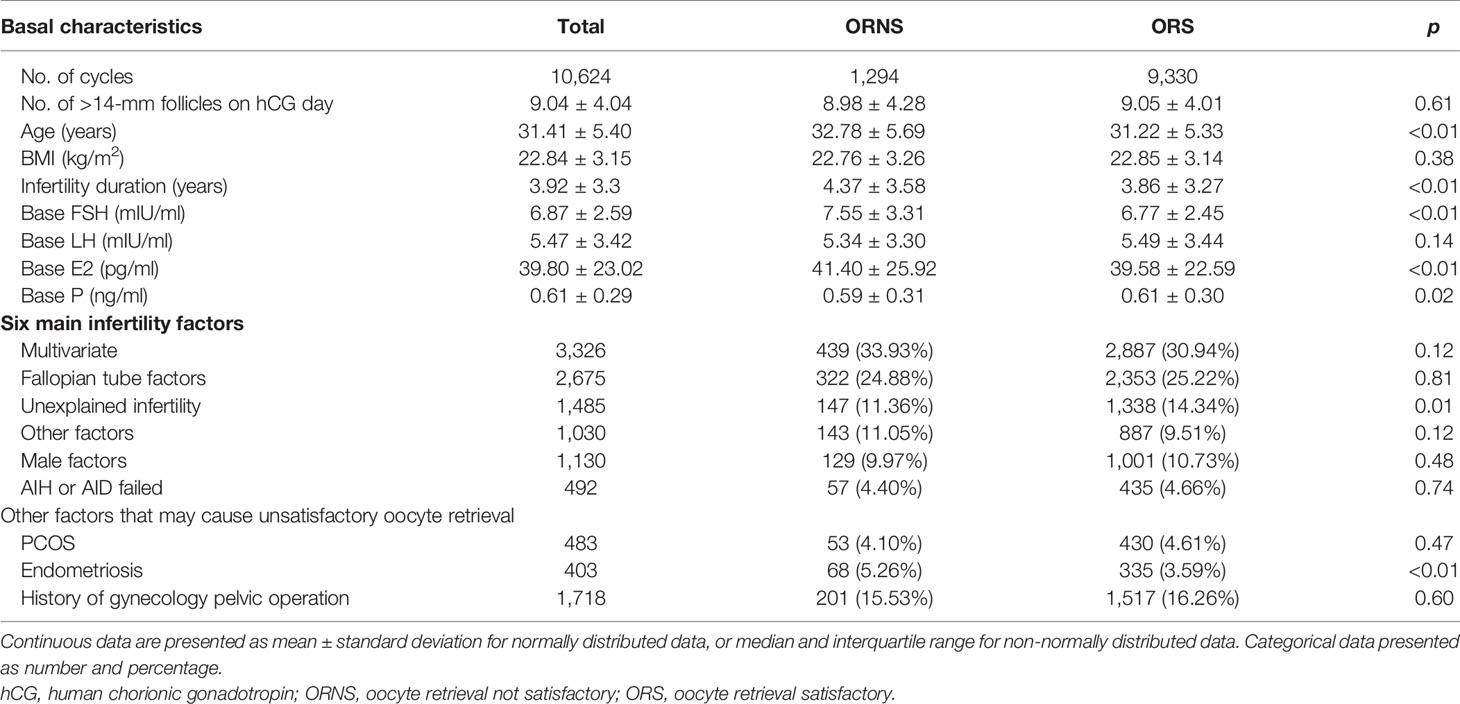
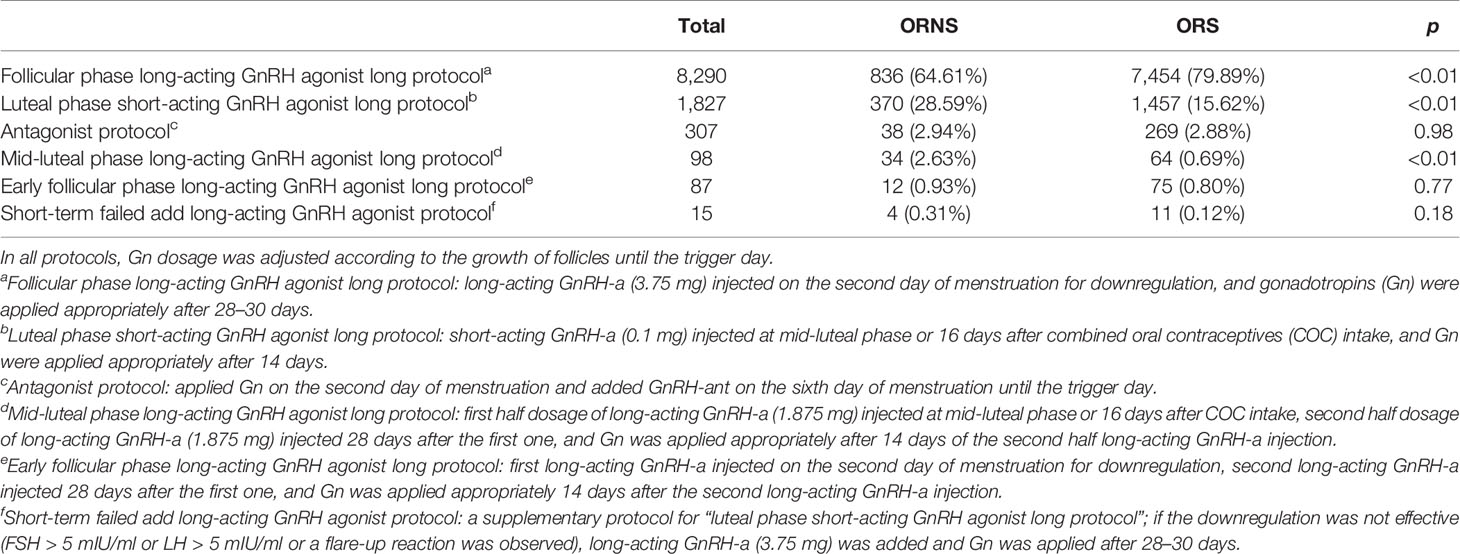
Basic clinical characteristics analyzed included age, body mass index, infertility duration, baseline follicle-stimulating hormone (FSH), baseline luteinizing hormone, baseline estradiol, and baseline progesterone. Infertility factors and the stimulation protocol used were also analyzed. Infertility factors were divided into six categories: multivariate fallopian tube factors; unexplained infertility (routine IVF examinations did not find any potential causes of infertility); other (uncertain single factors such as scarred uterus, benign ovarian cysts, simple oligomenorrhea, unicornuate uterus, mediastinal uterus, and uterine fibroids); male factors; artificial insemination by husband (AIH) or artificial insemination with donor semen (AID) failed; and polycystic ovary syndrome (PCOS). Because endometriosis and pelvic surgery can cause unsatisfactory oocyte retrieval, they were analyzed separately (13). The stimulation protocols included the follicular phase long-acting GnRH agonist long protocol, luteal phase short-acting GnRH agonist long protocol, antagonist protocol, mid-luteal phase long-acting GnRH agonist long protocol, early follicular phase long-acting GnRH agonist long protocol, and the short-term failed add long-acting GnRH agonist protocol.
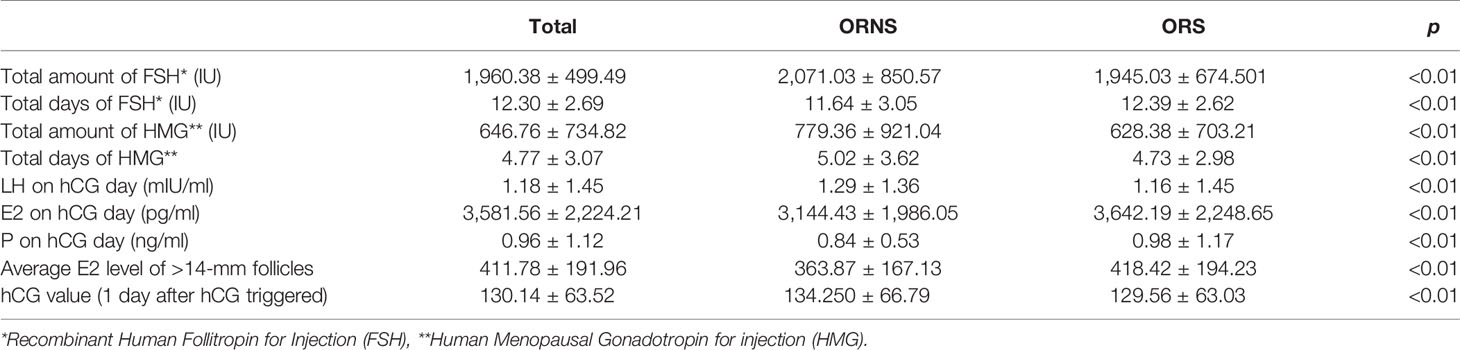
On hCG day, the following were analyzed: total amount of FSH; total days of FSH; total amount of human menopausal gonadotropin (HMG); total days of HMG; levels of FSH, estradiol, and progesterone; number of >14-mm follicles under ultrasound; and average estradiol level of >14-mm follicles. hCG values after hCG was triggered were also analyzed. The number of oocytes retrieved was recorded, and then they were fertilized using the scheduled plan. Rescue ICSI was performed if the conventional fertilization method failed. The fertilization method, metaphase II rate, fertilization rate, cleavage rate, and high-quality embryo rate were recorded. Information pertaining to embryo transfer, the freezing of embryos, or a lack of transplantable embryos was also recorded.
Biochemical pregnancy tests were considered positive if the plasma concentration of β-hCG was ≥50 IU/L 14 to 18 days after embryo transplantation. Clinical pregnancy tests were considered positive if a fetal heartbeat was detected via ultrasound on day 35 after transplantation. At this time, if the gestational sac appeared in the uterus, it was considered an intrauterine pregnancy. The clinical outcomes recorded included clinical pregnancy rate for fresh embryo transfer, cumulative pregnancy rate (followed up to at least one clinical pregnancy or until all embryos were used; the percentage of cycles that resulted in at least one clinical pregnancy), and cumulative live birth rate (followed up to at least one live birth or until all embryos were used; the percentage of cycles that resulted in at least one live birth). Unpregnant cycles for which embryos were not used up until February 2019 were excluded when calculating the cumulative pregnancy and cumulative live birth rates. Pregnancy loss before 28 weeks of gestation was recorded as miscarriage. Cumulative pregnancy/live birth rate was the number of pregnancies/live births that had been achieved from the first to the current cycle divided by the initial number of patients, assuming no patient dropout (14).
Statistical Analysis
Numerical variables are presented as means ± standard deviations, and categorical variables are presented as frequencies. Differences between two groups were compared via t-tests, and differences between three or more groups were compared via one-way analysis of variance. Differences between categorical variables were compared via chi-squared tests. All analyses were performed using SPSS 22.0 software (IBM Corp., Armonk, NY, USA). Differences were considered significant at p <0.05.
Results
Grouping Method and Baseline Characteristics
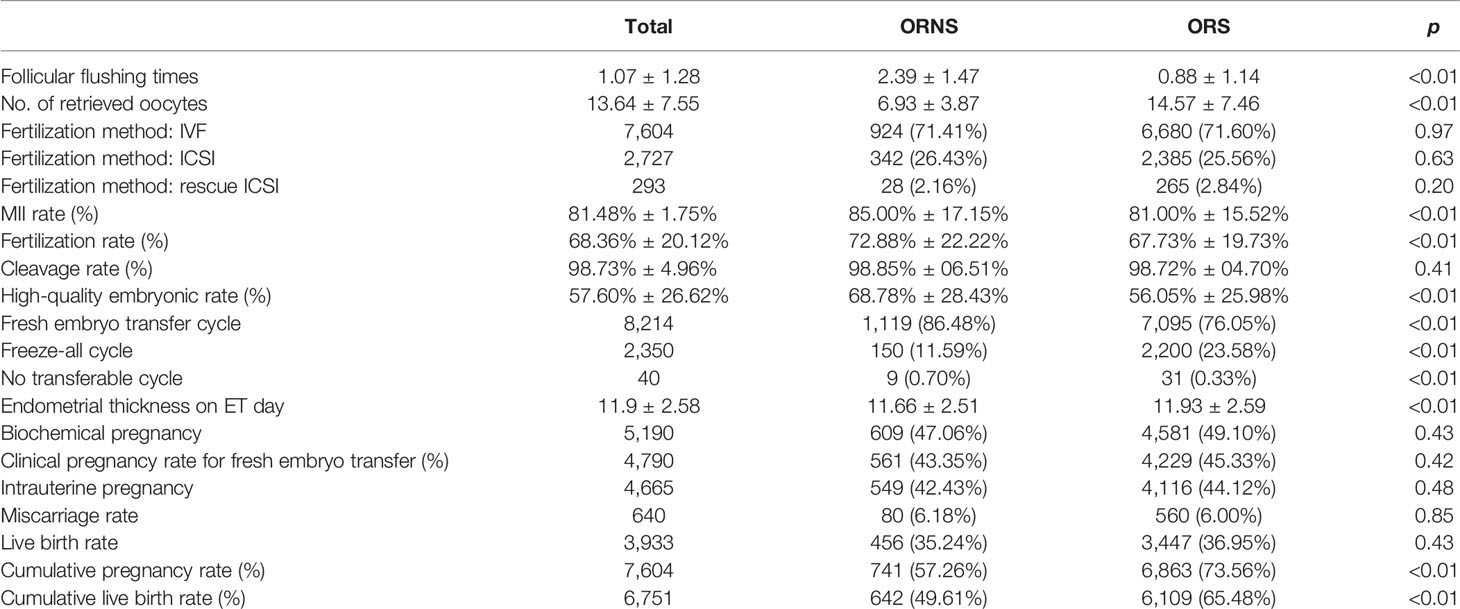
A total of 10,624 cycles were included in the study. Their ages ranged from 22 to 45 years. The oocyte numbers obtained ranged from 1 to 65. The oocyte numbers obtained and the frequencies of differences in oocyte numbers obtained in two groups are shown in Figure 2. During clinical procedures, if the number of oocytes obtained did not match, the operator usually subjectively increased follicular flushing times. The relationships between follicular flushing and different oocyte numbers obtained are shown in Figure 3A (r = 0.53, p < 0.01), and the follicular flushing times between the two groups are shown in Figure 3B.
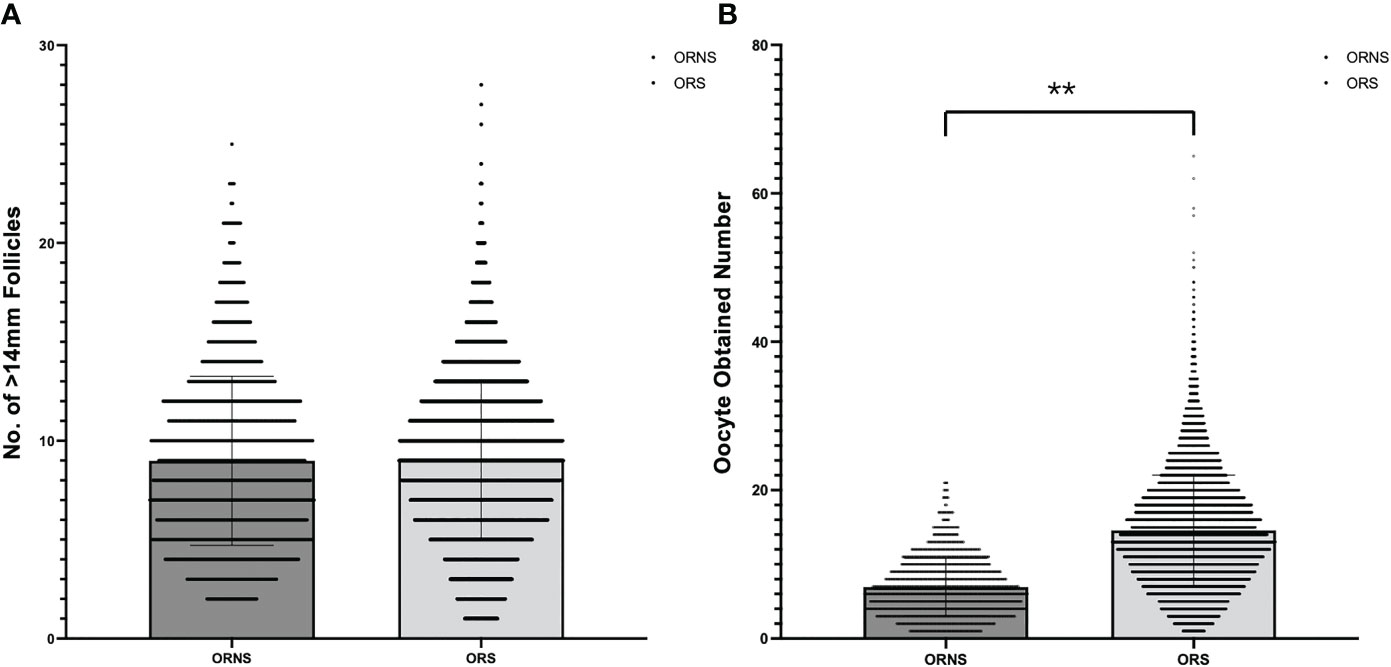
Figure 2 Frequency of >14-mm follicle number (A) and oocyte retrieval number (B) differences between the two groups (**P < 0.01).
Figure 3 Relationship between follicular flushing and difference in oocytes obtained. (A) Relationship between follicular flushing times and difference in oocytes obtained. (B) Follicular flushing difference between the ORNS and ORS groups (**P < 0.01).
In the ORS group, 9,330 oocytes were retrieved, and in the ORNS group, 1,294 oocytes were retrieved. The numbers of follicles >14 mm on hCG day were similar in the two groups. The significant indicators of reduced ovarian function that were higher in the ORNS group were age (32.78 ± 5.69 vs. 31.22 ± 5.33, p < 0.01), infertility duration (4.37 ± 3.58 vs. 3.86 ± 3.27, p < 0.01), baseline FSH (7.55 ± 3.31 vs. 6.77 ± 2.45, p < 0.01), and baseline estradiol (41.40 ± 25.92 vs. 39.58 ± 22.59, p < 0.01). Baseline progesterone was significantly lower in the ORNS group (0.59 ± 0.31 vs. 0.61 ± 0.30, p = 0.02) (Table 1, top). Of the six above-described infertility factors analyzed, only unexplained infertility was significantly lower in the ORNS group (11.36% vs. 14.34%, p < 0.01) (Table 1, middle). In comparison of endometriosis and pelvic surgery history, there was a significantly larger number of endometriosis patients in the ORNS group (5.26% vs. 3.59%, p < 0.01) (Table 1, bottom).
Stimulation Protocol Comparisons
In comparison of the six above-described stimulation protocols used in the two groups, the luteal phase short-acting GnRH agonist long protocol was used significantly more often in the ORNS group (28.59% vs. 15.62%, p < 0.01). Conversely, the follicular phase long-acting GnRH agonist long protocol was used significantly less often in the ORNS group (64.61% vs. 79.89%, p < 0.01). There were no significant differences in the rates of use of other stimulation protocols between the two groups (Table 2). Subgroup analysis of poor and normal response analysis is listed in Supplementary Table 1, and subgroup analyses of the three main stimulation protocols are listed in Supplementary Table 2.
Gonadotropin Dosage and Hormone Level Comparisons
The total amount of FSH was significantly higher in the ORNS group than in the ORS group (2,071.03 ± 850.57 vs. 1,945.03 ± 674.50 IU, p < 0.01). The total number of days of FSH was significantly lower in ORNS group than in the ORS group (11.64 ± 3.05 vs. 12.39 ± 2.62, p < 0.01). The total amount of HMG was greater in the ORNS group (779.36 ± 921.04 vs. 628.38 ± 703.21 IU, p < 0.01), as was the total number of HMG days (5.02 ± 3.62 vs. 4.73 ± 2.98, p < 0.01). One day after hCG was triggered, luteinizing hormone was significantly higher in the ORNS group (1.29 ± 1.36 vs. 1.16 ± 1.45 U/L, p < 0.01). Estradiol was significantly lower (3,144.43 ± 1,986.05 vs. 3,642.19 ± 2,248.65, p < 0.01), as were the mean estradiol levels of >14-mm follicles (363.87 ± 167.13 vs. 418.42 ± 194.23, p < 0.01) and progesterone (0.84 ± 0.53 vs. 0.98 ± 1.17, p < 0.01) and the hCG value (134.250 ± 66.79 vs. 129.56 ± 63.03, p < 0.01) (Table 3).
Comparisons After Oocyte Retrieval to ART Outcome
The numbers of >14-mm follicles were similar in the ORNS and ORS groups after oocyte retrieval, but the follicular flushing times and numbers of oocytes obtained differed significantly [mean follicular flushing times 2.39 ± 1.47 (ORNS) vs. 0.88 ± 1.14 (ORS), p < 0.01; mean numbers of oocytes retrieved 6.93 ± 3.87 vs. 14.57 ± 7.46, p < 0.01). The rates of IVF (71.41% vs. 71.60%), ICSI (26.43% vs. 25.56%), and rescue ICSI (2.16% vs. 2.84%) were similar in the two groups. The cleavage rate was similar in the two groups (98.85% ± 6.51% vs. 98.72% ± 4.70%), but the metaphase II rate, fertilization rate, and high-quality embryo rate were all significantly higher in the ORNS group than in the ORS group (metaphase II rate 85.00% ± 17.15% vs. 81.00% ± 15.52%, p < 0.01; fertilization rate 72.88% ± 22.22% vs. 67.73% ± 19.73%, p < 0.01; high-quality embryo rate 68.78% ± 28.43% vs. 56.05% ± 25.98%, p < 0.01). In the ORNS group, there were more fresh embryo transfer cycles (86.48% vs. 76.05%, p < 0.01), and the rate of no transferable embryos was higher (0.70% vs. 0.33%, p < 0.01), whereas in the ORS group, there were more freeze-all cycles (11.59% vs. 23.58%, p < 0.01). Endometrial thickness on embryo transfer (ET) day was lower in the ORNS group than in the ORS group (11.66 ± 2.51 vs. 11.93 ± 2.59, p < 0.01). Fresh cycles, biochemical pregnancy rate, clinical pregnancy rate, intrauterine pregnancy rate, miscarriage rate, and live birth rate were all similar in the two groups. The cumulative pregnancy rate and cumulative live birth rate were significantly lower in the ORNS group (Table 4).
Discussion
Oocyte retrieval is a routine procedure during ART, but technical difficulties associated with oocyte retrieval and subsequent unsatisfactory numbers of oocytes obtained have rarely been reported. Follicles >14 mm as determined via ultrasound are usually considered to contain a mature oocyte. In the current study, however, in 12.18% of cycles (1,294/10,624), the final number of oocytes obtained was less than the number of >14-mm follicles on hCG day. Thus, all cycles were divided into two groups (ORNS vs. ORS) based on this standard. Data showed ORNS patients tended to be older, have a longer duration of infertility, and have higher FSH, indicating worse ovarian reserve and a higher incidence of infertility caused by endometriosis. There were also significant differences in stimulation protocols between the two groups. The use of a follicular phase long-acting GnRH agonist long protocol was more common in the ORS group. It is reasonable that the ORNS group used more gonadotropins due to a poor ovarian reserve, but the ORNS group had fewer total FSH days than the ORS group. On hCG day, the ORNS group had higher luteinizing hormone and lower estradiol and progesterone. After oocyte retrieval, even though the obtained oocyte numbers were significantly lower in the ORNS group, the proportion of high-quality embryos was even higher than ORS cycles. An unsatisfactory oocyte retrieval number did not influence the biochemical pregnancy rate, clinical pregnancy rate, intrauterine pregnancy rate, miscarriage rate, or live birth rate during fresh cycles, but it was associated with the cumulative pregnancy rate and the cumulative live birth rate.
Ovarian dysfunction is one of the biggest contributors to an unsatisfactory number of oocytes being retrieved and a poor ART outcome. In the present study, of the 1,040 poor response cycles, 38.17% (397/1,040) were ORNS cycles, while in the normal response cycles, this was only 9.36% (897/9,584). This also indicated that ovarian dysfunction patients were more likely to experience ORNS. Besides, poor responders cannot be regarded as belonging to the same populations (15). A systematic review of 19 poor ovarian responder studies indicated that even though the final consequence was lower pregnancy, and further pregnancy prospects were reduced when fewer oocytes were retrieved, if >2 oocytes were retrieved, the poor responders exhibited a more favorable outcome (15). That study further illustrated that an insufficient number of oocytes obtained is associated with pregnancy rate, especially in poor ovarian responders. There are usually a greater focus on follicle development during ovarian stimulation and a little focus on the how many follicles contain mature oocytes and can be considered as “an actual follicle.” After ovarian stimulation, the ORNS group had lower average estradiol levels and higher hCG levels, indicating that during follicle development the function of granulosa cells was not as good as that in the ORS group. It may also be that those small follicles contributed to the estradiol level, especially in the ORS group. The specific reason remains to be further investigated. The surgical records of these patients also provided some potentially relevant information. They usually described poor follicle surface tension, not enough granulosa cells observed, variable flushing times without oocytes harvested, premature rupture of some follicles, and sticky follicular fluid. Thus, the results of the current study provide evidence for future research on follicle development and infertility treatment.
Ovarian stimulation is an extremely important component of IVF. It is closely related to the quality of oocytes and embryos and the occurrence of IVF complications. Two main hyperstimulation protocols are currently used: the follicular phase long-acting GnRH agonist long protocol and the luteal phase short-acting GnRH agonist long protocol. The latter is considered a classical ovarian stimulation protocol due to its stable successful rate. Notably, however, in-depth research of downregulation indicates that long-acting GnRH agonist protocol can improve the receptivity of the endometrium and increase the embryo implantation rate, significantly improving the clinical pregnancy rate associated with IVF cycles (16). Therefore, the long-acting GnRH protocol and long-term follicular phase have been widely used. In the present study, it was associated with an additional advantage: a greater number of mature follicles obtained.
Endometriosis is a relatively clear cause of oocyte retrieval difficulty (11), which may be related to insufficient cumulus expansion (12). Endometriosis may also interfere with the ovarian response during hyper-stimulation by follicular fluid contaminated with endometrioma content (17). In a recent meta-analysis, lower numbers of mature oocytes were retrieved in patients with endometriosis (18). Our conclusions were consistent with these studies, further illustrating the negative effects of endometriosis on ART.
However, no article clearly pointed out which follicle size had matured oocytes, or which follicle size had unmatured oocytes, indicating no aspiration value. Several studies provided the relationship between follicle size and oocyte development potential (19–22). From these studies, we found that the size of the follicle and the maturation of oocytes were relative. Larger follicles had a higher chance of having mature oocytes. In our center, all >12-mm follicles would be recorded and aspirated. However, >14-mm follicle numbers would be used to evaluate whether the retrieval process was satisfactory (the actual obtained oocyte number should be same or >14-mm follicle number). If the obtained oocyte number reached or exceeded the >14-mm follicle number, the operator could finish aspiration. Otherwise, the operator would increase the flushing times or puncture smaller follicles. Follicular flushing is widely used in ART centers. Over one-third of the centers (38%, 9/24) performed selective follicular flushing in poor responders or those with other specific indications (e.g., when follicular aspiration did not yield oocytes at the beginning of the operation). Two centers (8%) did not perform follicular flushing (2). Randomized controlled trial results have demonstrated the safety of follicular flushing in normal and poor responders (3–9), and in observational studies, follicular flushing increased oocyte yield, which was associated with a higher cumulative live birth rate (23). Meta-analyses have concluded that flushing did not change the oocyte yield (24–26). At our clinic, follicular flushing was used in 53.2% of the cycles. It may be useful for ART centers to investigate the value of this practice. All these data indicate that follicular flushing is safe and that it does not affect the rate of retrieval of a satisfactory number of oocytes.
In conclusion, ovarian dysfunction patients were more likely to experience ORNS. The follicular phase long-acting GnRH agonist long protocol was more advanced to reduce the occurrence of oocyte retrieval difficulty. Although ORNS does not affect embryo quality or the fresh cycle pregnancy rate, it significantly reduces the cumulative pregnancy rate due to fewer transplantable embryos.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Independent Ethics Committee of Zhengzhou University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YW and MZ participated in designing the study. SY and QL recruited the subjects and collected the data. HS analyzed the data. YW and MZ wrote the manuscript. All authors have reviewed the data and analysis and contributed to the revision of the manuscript. The final manuscript and the order of authorship have been approved by all authors.
Funding
This work was supported by National Natural Science Foundation of China (No.82101742) for MZ , Henan Province Medical Science and Technology Co-construction Project for HS and MZ, the Youth Innovation Fund of the First Affiliated Hospital of Zhengzhou University for YW. This work was also supported by the National Natural Science Foundation of China for the National Key R&D Program of China (2019YFA 0110900) and the International (Regional) Cooperation and Exchange Projects (81820108016) to Y-pS.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge the attending physicians, fellows, embryologists, nurses, and research staff at the Reproductive Medical Center of the First Affiliated Hospital of Zhengzhou University, China. We acknowledge the assistance with the access of analytic instruments from Translational Medical Center at The First Affiliated Hospital of Zhengzhou University. We also acknowledge Anna Michelle Scott, Jeremy Corbett, and Guillermo Cantarutti Dumont for their help with language polishing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.564344/full#supplementary-material
Supplementary Table 1 | Subgroup analysis of poor and normal respond cycles in ORNS and ORS. *Chi squre compare the ratio of ORNS and ORS in poor responder and normal responder groups.
Supplementary Table 2 | Subgroup analysis of different stimulation protocols ORNS and ORS. *Chi squre compare the ratio of ORNS and ORS in poor responder and normal responder groups.
References
1. Knight DC, Tyler JP, Driscoll GL. Follicular Flushing at Oocyte Retrieval: A Reappraisal. Aust N Z J Obstet Gynaecol (2001) 41:210–13. doi: 10.1111/j.1479-828X.2001.tb01212.x
2. Neyens S, De Neubourg D, Peeraer K, De Jaegher N, Spiessens C, Debrock S, et al. Is There a Correlation Between the Number of Follicular Flushings, Oocyte/Embryo Quality and Pregnancy Rate in Assisted Reproductive Technology Cycles? Results From a Prospective Study. Gynecol Obstet Invest (2016) 81:34–40. doi: 10.1159/000434750
3. Tan SL, Waterstone J, Wren M, Parsons J. A Prospective Randomized Study Comparing Aspiration Only With Aspiration and Flushing for Transvaginal Ultrasound-Directed Oocyte Recovery. Fertil Steril (1992) 58:356–60. doi: 10.1016/S0015-0282(16)55230-3
4. Haines CJ, Emes AL, O’Shea RT, Weiss TJ. Choice of Needle for Ovum Pickup. J In Vitro Fert Embryo Transf (1989) 6:111–2. doi: 10.1007/BF01130737
5. Scott RT, Hofmann GE, Muasher SJ, Acosta AA, Kreiner DK, Rosenwaks Z. A Prospective Randomized Comparison of Single- and Double-Lumen Needles for Transvaginal Follicular Aspiration. J In Vitro Fert Embryo Transf (1989) 6:98–100. doi: 10.1007/BF01130734
6. Kingsland CR, Taylor CT, Aziz N, Bickerton N. Is Follicular Flushing Necessary for Oocyte Retrieval? A Randomized Trial Hum Reprod (1991) 6:382–3. doi: 10.1093/oxfordjournals.humrep.a137344
7. Haydardedeoglu B, Cok T, Kilicdag EB, Parlakgumus AH, Simsek E, Bagis T. In Vitro Fertilization–Intracytoplasmic Sperm Injection Outcomes in Single- Versus Double-Lumen Oocyte Retrieval Needles in Normally Responding Patients: A Randomized Trial. Fertil Steril (2011) 95:812–4. doi: 10.1016/j.fertnstert.2010.09.013
8. Levens ED, Whitcomb BW, Payson MD, Larsen FW. Ovarian Follicular Flushing Among Low-Responding Patients Undergoing Assisted Reproductive Technology. Fertil Steril (2009) 91:1381–4. doi: 10.1016/j.fertnstert.2008.04.034
9. Mok-Lin E, Brauer AA, Schattman G, Zaninovic N, Rosenwaks Z, Spandorfer S. Follicular Flushing and In Vitro Fertilization Outcomes in the Poorest Responders: A Randomized Controlled Trial. Hum Reprod (2013) 28:2990–5. doi: 10.1093/humrep/det350
10. Georgiou EX, Melo P, Brown J, Granne IE. Follicular Flushing During Oocyte Retrieval in Assisted Reproductive Techniques. Cochrane Database Syst Rev (2018) 4:CD004634. doi: 10.1002/14651858.CD004634.pub3
11. Benaglia L, Busnelli A, Biancardi R, Vegetti W, Reschini M, Vercellini P, et al. Oocyte Retrieval Difficulties in Women With Ovarian Endometriomas. Reprod BioMed Online (2018) 37:77–84. doi: 10.1016/j.rbmo.2018.03.020
12. Yin Y, Mao Y, Liu A, Shu L, Yuan C, Cui Y, et al. Insufficient Cumulus Expansion and Poor Oocyte Retrieval in Endometriosis-Related Infertile Women. Reprod Sci (2021) 28:1412–20. doi: 10.1007/s43032-020-00410-4
13. Greenberger C, Matot I, Artsi H, Samara N, Azem F. High Level of Satisfaction Among Women Who Underwent Oocyte Retrieval Without Anesthesia. Fertil Steril (2020) 114:354–60. doi: 10.1016/j.fertnstert.2020.03.033
14. Raz N, Shalev A, Horowitz E, Weissman A, Mizrachi Y, Ganer Herman H, et al. Cumulative Pregnancy and Live Birth Rates Through Assisted Reproduction in Women 44-45 Years of Age: Is There Any Hope? J Assist Reprod Genet (2018) 35:441–7. doi: 10.1007/s10815-017-1094-0
15. Blumenfeld Z. What Is the Best Regimen for Ovarian Stimulation of Poor Responders in ART/IVF? Front Endocrinol (Lausanne) (2020) 11:192. doi: 10.3389/fendo.2020.00192
16. Ren J, Sha A, Han D, Li P, Geng J, Ma C. Does Prolonged Pituitary Down-Regulation With Gonadotropin-Releasing Hormone Agonist Improve the Live-Birth Rate in In Vitro Fertilization Treatment? Fertil Steril (2014) 102:75–81. doi: 10.1016/j.fertnstert.2014.03.030
17. Somigliana E, Benaglia L, Paffoni A, Busnelli A, Vigano P, Vercellini P. Risks of Conservative Management in Women With Ovarian Endometriomas Undergoing IVF. Hum Reprod Update (2015) 21:486–99. doi: 10.1093/humupd/dmv012
18. Sanchez AM, Vanni VS, Bartiromo L, Papaleo E, Zilberberg E, Candiani M, et al. Is the Oocyte Quality Affected by Endometriosis? A Review of the Literature. J Ovarian Res (2017) 10:43. doi: 10.1186/s13048-017-0341-4
19. Akbariasbagh F, Lorzadeh N, Azmoodeh A, Ghaseminejad A, Mohamadpoor J, Kazemirad S. Association Among Diameter and Volume of Follicles, Oocyte Maturity, and Competence in Intracytoplasmic Sperm Injection Cycles. Minerva Ginecol (2015) 67:397–403.
20. Mehri S, Levi Setti PE, Greco K, Sakkas D, Martinez G, Patrizio P. Correlation Between Follicular Diameters and Flushing Versus No Flushing on Oocyte Maturity, Fertilization Rate and Embryo Quality. J Assist Reprod Genet (2014) 31:73–7. doi: 10.1007/s10815-013-0124-9
21. Scott RT, Hofmann GE, Muasher SJ, Acosta AA, Kreiner DK, Rosenwaks Z. Correlation of Follicular Diameter With Oocyte Recovery and Maturity at the Time of Transvaginal Follicular Aspiration. J In Vitro Fert Embryo Transf (1989) 6:73–5. doi: 10.1007/BF01130729
22. Salha O, Nugent D, Dada T, Kaufmann S, Levett S, Jenner L, et al. The Relationship Between Follicular Fluid Aspirate Volume and Oocyte Maturity in in-Vitro Fertilization Cycles. Hum Reprod (1998) 13:1901–6. doi: 10.1093/humrep/13.7.1901
23. Zhou J, Wang B, Hu Y, Sun H. Association Between the Number of Oocytes Retrieved and Cumulative Live Birth Rate in Women Aged 35-40 Years Undergoing Long Gnrh Agonist IVF/ICSI Cycles. Arch Gynecol Obstet (2017) 296:1005–12. doi: 10.1007/s00404-017-4503-9
24. Levy G, Hill MJ, Ramirez CI, Correa L, Ryan ME, DeCherney AH, et al. The Use of Follicle Flushing During Oocyte Retrieval in Assisted Reproductive Technologies: A Systematic Review and Meta-Analysis. Hum Reprod (2012) 27:2373–9. doi: 10.1093/humrep/des174
25. Roque M, Sampaio M, Geber S. Follicular Flushing During Oocyte Retrieval: A Systematic Review and Meta-Analysis. J Assist Reprod Genet (2012) 29:1249–54. doi: 10.1007/s10815-012-9869-9
Keywords: assisted reproduction, clinical pregnancy rate, cumulative pregnancy rate (CPR), cumulative live birth rate, oocyte retrieval difficulty
Citation: Wang Y, Zhang M, Shi H, Yi S, Li Q, Su Y, Guo Y, Hu L, Sun J and Sun Y-p (2022) Causes and Effects of Oocyte Retrieval Difficulties: A Retrospective Study of 10,624 Cycles. Front. Endocrinol. 12:564344. doi: 10.3389/fendo.2021.564344
Received: 21 May 2020; Accepted: 22 November 2021;
Published: 03 January 2022.
Edited by:
Katja Teerds, Wageningen University, NetherlandsReviewed by:
Qiuju Chen, Shanghai Jiao Tong University, ChinaDiao Feiyang, Nanjing Medical University, China
Copyright © 2022 Wang, Zhang, Shi, Yi, Li, Su, Guo, Hu, Sun and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying-pu Sun, c3lwMjAwOEB2aXAuc2luYS5jb20=
†These authors have contributed equally to this work
 Yang Wang1,2†
Yang Wang1,2† Meixiang Zhang
Meixiang Zhang Hao Shi
Hao Shi Shiqi Yi
Shiqi Yi Linli Hu
Linli Hu Ying-pu Sun
Ying-pu Sun