- 1Department of Cardiology, First Affiliated Hospital of Dalian Medical University, Dalian, China
- 2Faculty of Medicine, Newcastle University, Newcastle, United Kingdom
Background: Hyperglycemia is associated with an increased risk of developing atrial fibrillation (AF) and atrial flutter (AFL). Sodium-glucose transporter 2 inhibitors (SGLT2i) have been reported to prevent AF/AFL in some studies, but not others. Therefore, a meta-analysis was performed to investigate whether SGLT2i use is associated with lower risks of AF/AFL.
Methods: PubMed, Scopus, Web of Science, Cochrane library databases were searched for randomized placebo-controlled trials comparing SGLT2i and placebo.
Results: A total of 33 trials involving 66,685 patients were included. The serious adverse events (SAEs) of AF/AFL occurrence were significantly lower in the SGLT2i group than the placebo group (0.96% vs. 1.19%; RR 0.83; 95% CI 0.71–0.96; P = 0.01; I2 25.5%). Similarly, the SAEs of AF occurrence was significantly lower in the SGLT2i group (0.82% vs. 1.06%; RR 0.81; 95% CI 0.69–0.95; P = 0.01; I2 10.2%). The subgroup analysis showed that the reduction in AF/AFL was significant only for dapagliflozin (1.02% vs. 1.49%; RR 0.73; 95% CI 0.59–0.89; P = 0.002; I2 0%), but not for canagliflozin (1.00% vs 1.08%; RR 0.83; 95% CI 0.62–1.12; P = 0.23; I2 0%), empagliflozin (0.88% vs 0.70%; RR 1.20; 95% CI 0.76–1.90; P = 0.43; I2 0%), ertugliflozin (1.01% vs 0.96%; RR 1.08; 95% CI 0.66–1.75; P = 0.76; I2 0%), and sotagliflozin (0.16% vs 0.10%; RR 1.09; 95% CI 0.13–8.86; P = 0.93; I2 0%).
Conclusions: SGLT2i use is associated with a 19.33% lower SAEs of AF/AFL compared with the placebo. Dapagliflozin users had the lowest SAEs of AF/AFL incidence. Further studies are needed to determine whether canagliflozin, empagliflozin, ertugliflozin, and sotagliflozin similarly exert protective effects against AF/AFL development.
Introduction
Patients with hyperglycemia such as type 2 diabetes mellitus (T2DM) are at increased risks of developing arrhythmias such as atrial fibrillation (AF) and atrial flutter (AFL) (1–3). Hyperglycemia and fluctuations in blood glucose levels can contribute to cardiac electrophysiological and structural remodeling, particularly in the atria (4, 5). Cardiovascular comorbidities such as heart failure (HF) also play a significant role in increasing AF/AFL incidence (6, 7). Even with optimal medical treatment, patients with T2DM may nevertheless go on to develop AF/AFL (8). Given that AF/AFL is associated with adverse outcomes such as HF and stroke (9), there is a need to identify treatment options that can prevent their development.
The underlying pathophysiology linking T2DM to AF predominantly favors the theory involving the generation of reactive oxygen species (ROS) secondary to hyperglycemia (10), which can lead to atrial cardiomyopathic changes (11, 12). While many interventions ranging from weight loss, angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) to catheter ablation are used to prevent or treat AF, the diabetic medications can also protect against AF development (9, 13).
The sodium-glucose transporter inhibitor (SGLT2i) is a new class of anti-diabetic agents and works by inhibiting the reabsorption of sodium and glucose by the kidneys (14). Their use has been associated with a lower incidence of adverse events including all-cause mortality, cardiovascular mortality, HF, and AF (15–18). In clinical practice, SGLT2i is currently recommended for T2DM as a second- or third-line agent following inadequate glycemic control using metformin and/or sulphonylureas (19–21).
Animal studies have demonstrated that SGLT2i could reduce the oxidative stress in cardiomyocytes, which in turn reverses myocardial structural/electronic remodeling (22, 23). The post-hoc analysis of the DECLARE-TIMI 58 trial confirmed that dapagliflozin has a lower incidence of AF over placebo, indicated the potential benefit of SGLT2i in preventing AF/AFL (24), as confirmed by subsequent meta-analyses (25, 26). Recent studies have reported beneficial effects of SGLT2i in preventing atrial remodeling even in non-diabetic conditions. Therefore, we conducted this systematic review and meta-analysis of placebo-controlled trials to investigate the clinical effectiveness of SGLT2i in AF/AFL prevention among patients with or without T2DM.
Methods
Search Strategy and Data Sources
An electronic search of PubMed, Scopus, Web of Science and Cochrane library databases was conducted until 3rd December, 2020 using searching terms and related items including keywords “sodium-glucose transporter 2 inhibitors,” “sodium-glucose cotransporter 2 inhibitors,” “SGLT2i,” “dapagliflozin,” “BMS 512148,” “empagliflozin,” “BI 10773,” “canagliflozin,” “JNJ 28431754,” “tofogliflozin,” “CSG452,” “luseogliflozin,” “TS071,” “ipragliflozin,” “ASP1941,” “sotagliflozin,” “LX4211,” “ertugliflozin,” and “PF04971729.” The search algorithm is shown in Table S1 in the Supplementary Appendix.
Inclusion and Exclusion Criteria
The inclusion criteria were: (1) randomized placebo-controlled trials registered in ClinicalTrials.gov comparing SGLT2i with matching placebo including recorded AF/AFL outcomes; and (2) involving adult patients (>18 years of age) and iii) published in English language. The exclusion criteria were: (1) non-randomized placebo-controlled trials; (2) lack of information on the occurrences of AF/AFL; and (3) animal studies. This meta-analysis was performed under the recommendation of the preferred reporting items for systemic review and meta-analyses (PRISMA) guidelines, and the retrieved data were reviewed and approved by the principal investigator.
Study Selection and Outcome Identification
All the studies were independently identified, reviewed, and screened by two authors (YL and YWa) based on their titles and abstracts to identify eligible studies. The authors performed a full-text review of the selected articles, and data were summarized in a prespecified spreadsheet in Microsoft Excel. All potentially relevant reports were retrieved as complete manuscripts and assessed for compliance with the inclusion criteria. Decisions of inclusion and exclusion were resolved by consensus between the reviewers. Agreement between reviewers for study selection was examined using the Kappa statistic. A third reviewer (DL) addressed disagreements concerning study inclusion. Citations matching inclusion criteria were included in the final analysis.
Data Extraction and Quality Assessment
The characteristics of the studies (first author, year of publication, study design, and inclusion criteria) were extracted into an Excel file after identifying all relevant articles. Demographic and baseline patient characteristics were collected from all included trials. The Excel file contained the total number of participants in each trial, the number of participants who were in SGLT2i during the period of the trial, and the corresponding total number of AF/AFL occurrences.
AF/AFL outcomes were extracted from the eligible studies. We further retrieved relevant clinical data through clinicaltrials.gov, www.who.int/ictrp, or by browsing supplementary materials. The individual study outcomes were reported according to whether SGLT2i lowers serious adverse events (SAEs), particularly the total number of AF/AFL events during follow-up. The risk of bias of included trials was assessed through the Cochrane Collaboration’s tool for assessing the risk of bias by two reviewers (YL and YWa) independently. Each domain was assigned low, unclear, or high risk of bias. Since the data used in the meta-analysis derive from previously published studies, the approval of the Institutional Review Board was not necessary, and the analytical methods will not be made available to other researchers for the reproduction of the findings or replication of the procedure.
Outcomes
The outcomes of our meta-analysis were SAEs of AF or AFL incidence for SGLT2i as a treatment group versus matching placebo. AF and AFL were defined as reported SAEs among included trials. The source of data on SAE were their Supplementary Materials of the publications for three trials (NCT01730534 [DECLARE-TIMI58 trial], NCT03036150 [DAPA-CKD trial], and NCT01986881). For the remaining 30, we obtained the information from clinicaltrials.gov.
Statistical Analysis
Data were summarized using descriptive statistics including proportions for categorical variables. The overall risk ratio (RR) of efficacy outcomes was estimated using a Mantel-Haenszel random-effects model. The pooled SAEs of AF/AFL incidence events for the SGLT2i group and placebo group were also computed. The I2 statistics were assessed to quantify the heterogeneity in RR across the studies. I2 statistics <25%, 25–75%, and >75% were used to represent low, moderate, and a high degree of heterogeneity, respectively, at Cochrane P-value ≤0.05. Funnel plots were used to assess publication bias. All statistical analyses were conducted with the Review Manager (RevMan, version 5.3, The Cochrane Collaboration, Copenhagen, Denmark).
Results
Literature Search Results
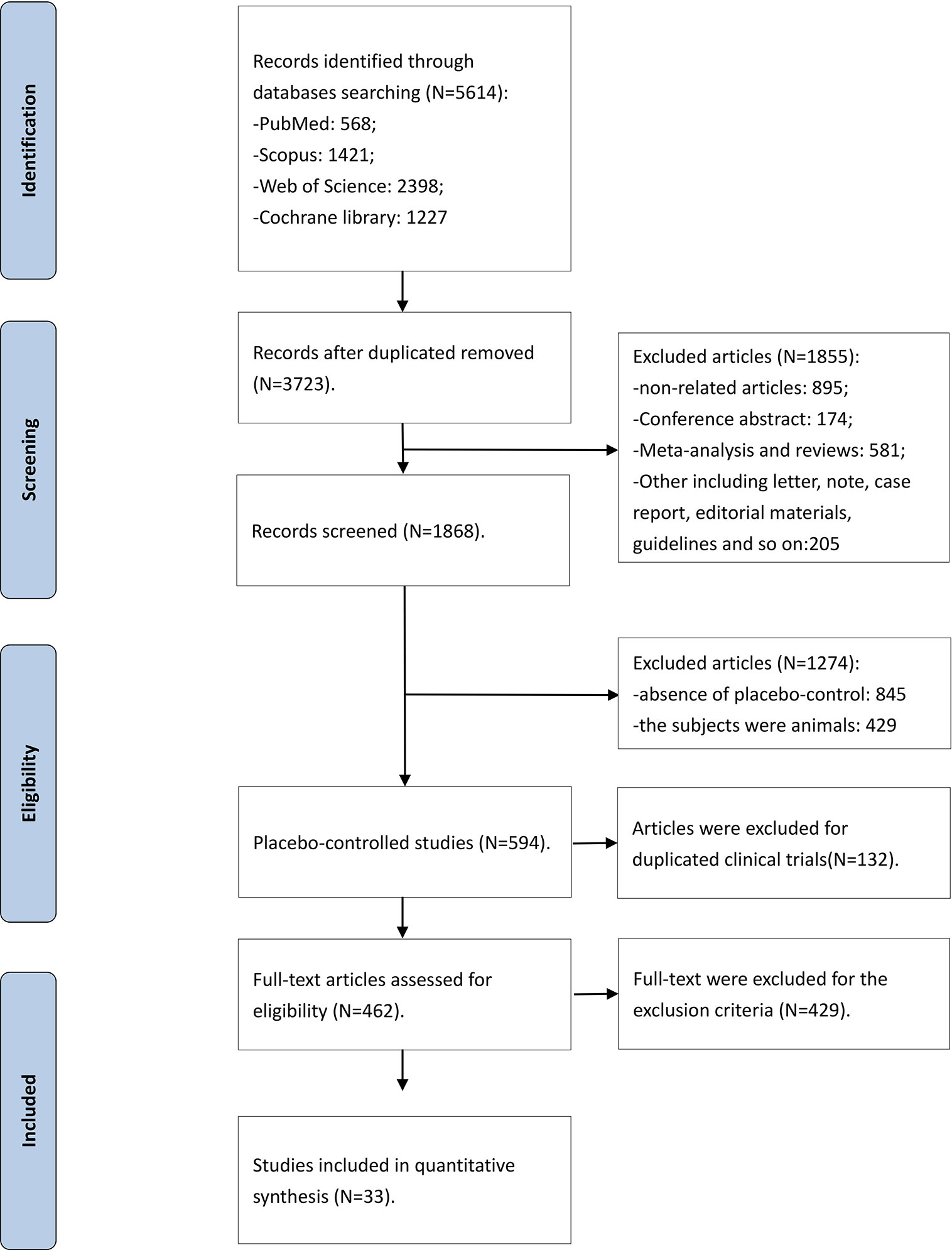
The flow diagram of the detailed searching steps for this meta-analysis is described in Figure 1. Our search strategy yielded a total of 5,614 studies, of which 1,891 were duplicate entries. We screened 3,723 studies based on the inclusion and exclusion criteria. After a thorough assessment, we excluded 1,855 articles: 895 articles were irrelevant, 581 were meta-analyses and reviews, 174 were conference abstract, and 205 were case reports, short letters, comments, and guidelines. An additional 845 trials were excluded due to a lack of randomized placebo-controlled design and 429 trials were excluded because the subjects were animals. Furthermore, 594 articles were reviewed in more detail, and a full-text screening led to exclusion of 132 duplicated trials. The search strategy yielded 33 randomized placebo-controlled trials that met the inclusion criteria. The Kappa statistic of agreement between the two authors was 87.6%.
Study Characteristics and Quality Assessment
A total of 37,068 patients received SGLT2i [dapagliflozin (27–35), canagliflozin (36–43), empagliflozin (44–53), sotagliflozin (54, 55) and ertugliflozin (56–58)] and 29,617 patients received placebo. The baseline characteristics of the studies included in this systematic review are shown in Table 1. The screening methods of AF/AFL as SAEs are shown in Table S2 in the Supplementary Appendix.
All the included trials had a low risk of bias of random sequence generation (selection bias), allocation concealment, incomplete outcome data, selective reporting bias, except that five trials had unclear other bias. The risk of bias based on the quality of the included trials and the summary of the authors’ judgments of the risk of biases are indicated in Figure 2.
Overall Efficacy Outcome
The SAEs of AF/AFL occurred in 355 of 37,068 patients who were on SGLT2i and 353 of 29,617 patients among those in the placebo group. The SAEs of AF/AFL was significantly lower in SGLT2i group than that of the placebo group (0.96% vs. 1.19%; RR 0.83; 95% CI 0.71–0.96; P = 0.01; I2 25.5%). The SAEs of AF occurred in 291 of 35,464 patients who were on SGLT2i and 298 of 28,229 patients in the placebo group (0.82% vs. 1.06%). The SAEs of AF were significantly lower in the SGLT2i group than that of the placebo group (RR 0.81; 95% CI: 0.69–0.95; P = 0.01; I2 10.2%). Given that the DECLARE-TIMI 58 trial contributed the majority of the patients, sensitivity analysis was performed by excluding this trial. The AF/AFL occurrence were still significantly lower in dapagliflozin group than the placebo group (0.67% vs. 1.13%; RR 0.67; 95% CI 0.46–0.97; P = 0.03; I2 0%), which indicated that the exclusion of DECLARE-TIMI 58 trial did not affect the conclusion at least in the subgroup analysis for dapagliflozin. For all SGLT2i, after we excluded data from the DECLARE-TIMI 58 trial, the risks of AF/AFL incidence between SGLT2i and placebo did not demonstrate statistical significance (0.85% vs. 0.97%; RR 0.87; 95% CI 0.72–1.05; P = 0.16). However, the risks of AF incidence between SGLT2i and placebo was borderline significant (0.73% vs. 0.90%; RR 0.83; 95% CI 0.67–1.01; P = 0.07).
Subgroup Outcome
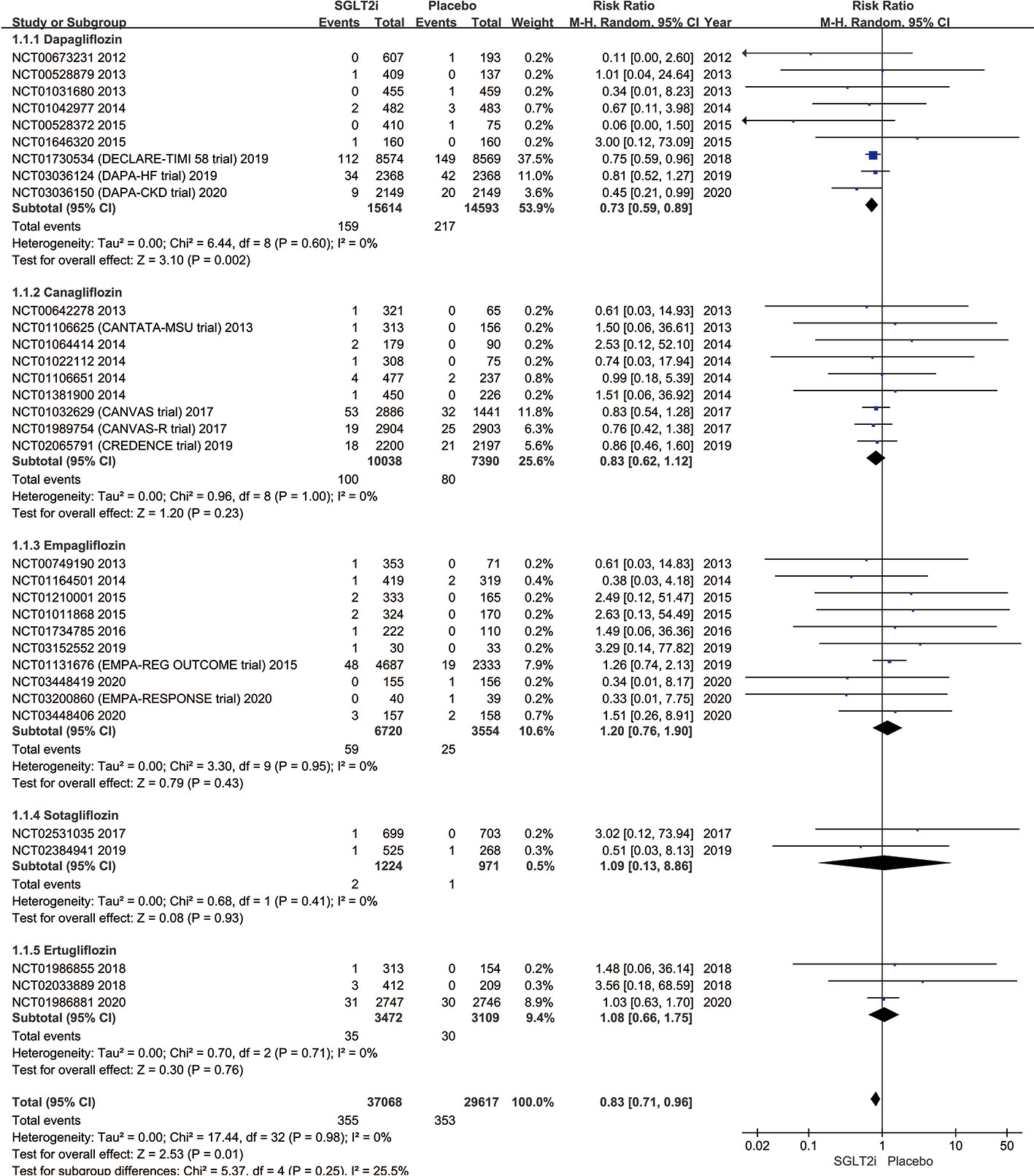
A subgroup analysis of studies comparing SGLT2i (dapagliflozin, canagliflozin, empagliflozin, sotagliflozin, and ertugliflozin) with the placebo group is shown in Figure 3. Altogether, 159 of 15,614 patients in the dapagliflozin group had SAEs of AF/AFL in comparison to a higher incidence of 217 out of 14,593 for the placebo group (1.02% vs. 1.49%; RR 0.73; 95% CI 0.59–0.89; P = 0.002; I2 0%). Though the SAEs incidence of AF/AFL was lower in the canagliflozin group compared with those on placebo, the canagliflozin and placebo groups did not differ in the incidence of AF/AFL (1.00% vs. 1.08%; RR 0.83; 95% CI 0.62–1.12; P = 0.23; I2 0%). Also, there was no difference in the SAEs of AF/AFL in the empagliflozin group (0.88% vs. 0.70%; RR 1.20; 95% CI 0.76–1.90; P = 0.43; I2 0%), sotagliflozin group (0.16% vs. 0.10%; RR 1.09; 95% CI 0.13–8.86; P = 0.93; I2 0%) and ertugliflozin (1.01% vs. 0.96%; RR 1.08; 95% CI 0.66–1.75; P = 0.76; I2 0%) versus matching placebo. Figure 3 illustrates the comparison of AF/AFL events. The symmetrical funnel plot suggests no significant publication bias (Figure S1 in the Supplementary Appendix).
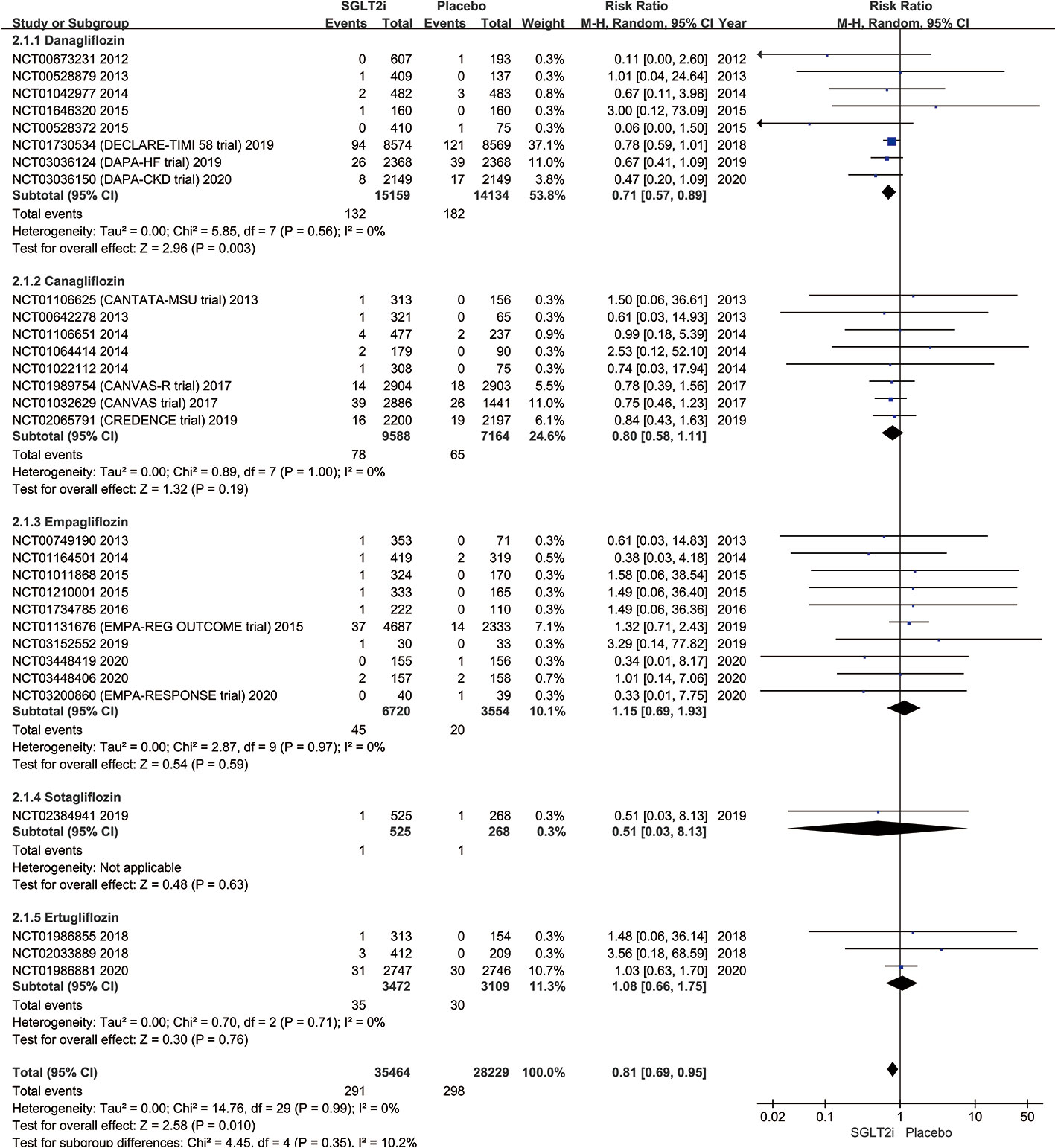
The subgroup analysis of all 33 trials reported the outcomes of 291 SAEs of AF among all SGLT2i classes are given in Figure 4. The SAEs of AF occurred in 132 of 15,159 patients in dapagliflozin group and 182 of 14,134 placebo (0.87% vs. 1.29%; RR 0.71; 95% CI 0.57–0.89; P = 0.003; I2 0%). The pooled SAEs incidence of AF was also lower in the canagliflozin group compared with placebo with no significant difference in the SAEs incidence of AF in the two groups (0.81% vs. 0.91%; RR 0.80; 95% CI 0.58–1.11; P = 0.19; I2 0%). Similarly, there was no significant difference between empagliflozin group (0.67% vs. 0.56%; RR 1.15; 95% CI 0.69–1.93; P = 0.59; I2 0%), sotagliflozin (0.19% vs. 0.37%; RR 0.51; 95% CI 0.03–8.13; P = 0.63; I2 not applicable) or ertugliflozin (1.01% vs. 0.96%; RR 1.08; 95% CI 0.66–1.75; P = 0.76; I2 0%) compared to placebo. A symmetrical funnel plot suggests that there was no significant publication bias (Figure S2 in the Supplementary Appendix).
Discussion
This systematic review and meta-analysis analyzed AF/AFL of approximately 66,685 patients who were on either SGLT2i or placebo. Our results show that SGLT2i was associated with a lower incidence of AF/AFL. The pooled incidence of AF/AFL was 19.33% lower in SGLT2i compared with those on placebo (0.96% vs. 1.19%), with a significant difference in the risk of AF/AFL between the two groups. In the subgroup analysis of the individual SGLT2i drugs, dapagliflozin was associated with an approximate 31.54% lower SAEs of AF/AFL incidence favoring the SGLT2i group over the placebo group. However, there were no significant differences in the incidence of AF/AFL in the other members of the SGLT2i class, namely canagliflozin, empagliflozin, sotagliflozin, and ertugliflozin when compared to placebo.
The results of this meta-analysis are relevant for the growing body of patients who are on SGLT2i especially for T2DM with diabetes-related comorbidities or cardiovascular death risk factors. This systematic review and meta-analysis shows that SGLT2i may reduce AF/AFL occurrence. More specifically, dapagliflozin decreased the incidence of reported SAEs of AF/AFL. According to the CVD-REAL 2 Study (59) and a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) (60), SGLT2i contributes a beneficial effect in reducing cardiovascular comorbidities. Recently, a post-hoc analysis based on the DECLARE-TIMI58 trial found that dapagliflozin lowered the incidence of reported episodes of AF/AFL in high-risk T2DM patients (20). Also, the findings of our meta-analysis buttress the findings of the DAPA-HF and DAPA-CKD trials (22). The ongoing DELIVER trial, a study designed to detect the therapeutic effects of dapagliflozin in HF with preserved ejection fraction (61), is expected to further explain the relationship between AF/AFL and HF in patients without diabetes mellitus.
In this meta-analysis, canagliflozin, empagliflozin, sotagliflozin, and ertugliflozin do not appear to reduce the incidence of reported SAEs of AF/AFL. Canagliflozin use was initially found to reduce AF/AFL incidence in individual studies but was no longer statistically significant in the meta-analysis. This is likely due to the relatively small sample size and low AF/AFL incidence rate. The influence of canagliflozin on AF/AFL occurrence warrants further investigation. Interestingly, in the EMPA-REG OUTCOME trial (49), despite seeing an improvement in HF, patients in the empagliflozin arm were found to have an increased SAEs of AF/AFL incidence.
The post-hoc analysis of the DECLARE-TIMI 58 trial found dapagliflozin reduced the effect of AF/AFL incidence was irrespective of baseline AF/AFL. Moreover, the presence of atherosclerotic cardiovascular disease versus multiple risk factors or a history of HF did not alter the reduction in AF/AFL events. Further, there were no interaction effects with respect to gender, prior ischemic stroke, HbA1c levels, body mass index, blood pressure or estimated glomerular filtration rate. In a recent meta-analysis, Li et al. indicated that the AF/AFL reduction benefit of SGLT2i have no relevance with age, body weight, and systolic blood pressure at baseline (26). In another meta-analysis, Okunrintemi et al. considered the AF/AFL reduction may be associated with decreased uric acid and increased magnesium induced by SGLT2i (25). Moreover, the protective effects of SGLT2i against AF/AFL may be direct actions on cardiac remodeling by reducing oxidative stress (62), which can prevent mitochondrial dysfunction and improve mitochondrial energetics (63). Thus, the current evidence points toward both a systematic and cardio-specific mechanism in preventing arrhythmias (64), while the relative contributions from either pathway remain unclear. The clinical significance our finding is that SGLT2i may reduce mortality, incident HF and HF-related hospitalizations at least partly by reducing AF/AFL occurrences. However, mediation analysis is needed to confirm this. Whether SGLT2i use is associated with a reduced incidence of ischemic stroke remains to be elucidated in future studies.
Previous evidence has confirmed a lower AF incidence with ACEIs and ARBs (65–67). Within the context of T2DM, hypoglycemic medications were rarely reported to reduce the incidence of AF. Published placebo-controlled clinical trials are known to be less susceptible to selection and recall bias compared with observational studies. As such, the latter studies were excluded from the meta-analysis. Recently, two meta-analyses on the relationship between SGLT2i use and AF outcomes have been performed (25, 26). Our current meta-analysis extends these two studies by including the largest number of trials (n = 33) involving 66,685 patients.
Limitations
In this meta-analysis, several limitations can be stated. Firstly, the AF/AFL incidence in the included studies was relatively low, and the AF/AFL incidence was calculated by reported SAEs among trials. This may have underestimated the reported pooled incidence rate of AF/AFL. Secondly, it should be acknowledged that there were differences in baseline characteristics of the patients, such as follow-up duration, sample size, age, and gender. Only the DECLARE-TIMI 58 trial was the most inclusive cardiovascular outcomes trial, with a broad representation of patients encountered in routine clinical practice than those of CANVAS and EMPA-REG OUTCOME (68). Thirdly, our meta-analysis demonstrated intermediate heterogeneity among SGLT2i, although subgroup analysis demonstrated no significant heterogeneity. Fourthly, the dose of some SGLT2i (canagliflozin and empagliflozin) was not consistent between the trials, thus the potential dose-reaction effect may influence the AF/AFL incidence. Finally, few randomized placebo-controlled trials have reported the findings for individual SGLT2i drugs apart from dapagliflozin, thus the potential benefits of these drugs on AF/AFL remain to be elucidated.
Conclusions
The use of SGLT2i is associated with a 19.33% lower risk of AF/AFL compared with the placebo. Dapagliflozin users had the lowest risk of AF/AFL episodes.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
This study was conceived and designed by XY. YL and YWa were responsible for data collection and data analysis. DL wrote the main manuscript text. TH and XY supervised data collection and data analysis. All authors contributed to the article and approved the submitted version.
Funding
Supported by Chang Jiang Scholars Program (T2017124) from Ministry of Education, the People’s Republic of China, the Program of Liaoning Distinguished Professor for YX, and National Natural Science Foundation of China (81970286) for YX.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks to all authors who helped write this article.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.619586/full#supplementary-material
Abbreviations
ACEIs, angiotensin-converting enzyme inhibitors; ADA, American Diabetes Association; AF, atrial fibrillation; AFL, atrial flutter; ARBs, angiotensin receptor blockers; CANVAS, Canagliflozin Cardiovascular Assessment Study; CVD-REAL, comparative effectiveness of cardiovascular outcomes in new users of SGLT-2 inhibitors; DAPA-HF, dapagliflozin in patients with heart failure and reduced ejection fraction; DAPA-CKD, dapagliflozin in patients with chronic kidney disease; DECLARE-TIMI 58, dapagliflozin effect on cardiovascular events–thrombolysis in myocardial infarction 58; DELIVER, dapagliflozin evaluation to improve the LIVEs of patients with preserved ejection fraction heart failure; EASD, European Association for the Study of Diabetes; EMPA-REG OUTCOME, empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes; HF, heart failure; RR, risk ratio; SAEs, serious adverse events; SGLT2i, sodium-glucose transporter 2 inhibitor; T2DM, type 2 diabetes mellitus.
References
1. Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol (2011) 108(1):56–62. doi: 10.1016/j.amjcard.2011.03.004
2. Pallisgaard JL, Schjerning AM, Lindhardt TB, Procida K, Hansen ML, Torp-Pedersen C, et al. Risk of atrial fibrillation in diabetes mellitus: A nationwide cohort study. Eur J Prev Cardiol (2016) 23(6):621–7. doi: 10.1177/2047487315599892
3. Xiong Z, Liu T, Tse G, Gong M, Gladding PA, Smaill BH, et al. A Machine Learning Aided Systematic Review and Meta-Analysis of the Relative Risk of Atrial Fibrillation in Patients With Diabetes Mellitus. Front Physiol (2018) 9:835. doi: 10.3389/fphys.2018.00835
4. Saito S, Teshima Y, Fukui A, Kondo H, Nishio S, Nakagawa M, et al. Glucose fluctuations increase the incidence of atrial fibrillation in diabetic rats. Cardiovasc Res (2014) 104(1):5–14. doi: 10.1093/cvr/cvu176
5. Tadic M, Cuspidi C. Type 2 diabetes mellitus and atrial fibrillation: From mechanisms to clinical practice. Arch Cardiovasc Dis (2015) 108(4):269–76. doi: 10.1016/j.acvd.2015.01.009
6. Lopez JM, Bailey RA, Rupnow MF. Demographic Disparities Among Medicare Beneficiaries with Type 2 Diabetes Mellitus in 2011: Diabetes Prevalence, Comorbidities, and Hypoglycemia Events. Popul Health Manag (2015) 18(4):283–9. doi: 10.1089/pop.2014.0115
7. Zafrir B, Lund LH, Laroche C, Ruschitzka F, Crespo-Leiro MG, Coats AJS, et al. Prognostic implications of atrial fibrillation in heart failure with reduced, mid-range, and preserved ejection fraction: a report from 14 964 patients in the European Society of Cardiology Heart Failure Long-Term Registry. Eur Heart J (2018) 39(48):4277–84. doi: 10.1093/eurheartj/ehy626
8. Fatemi O, Yuriditsky E, Tsioufis C, Tsachris D, Morgan T, Basile J, et al. Impact of intensive glycemic control on the incidence of atrial fibrillation and associated cardiovascular outcomes in patients with type 2 diabetes mellitus (from the Action to Control Cardiovascular Risk in Diabetes Study). Am J Cardiol (2014) 114(8):1217–22. doi: 10.1016/j.amjcard.2014.07.045
9. De Sensi F, De Potter T, Cresti A, Severi S, Breithardt G. Atrial fibrillation in patients with diabetes: molecular mechanisms and therapeutic perspectives. Cardiovasc Diagn Ther (2015) 5(5):364–73. doi: 10.3978/j.issn.2223-3652.2015.06.03
10. Korantzopoulos P, Letsas K, Fragakis N, Tse G, Liu T. Oxidative stress and atrial fibrillation: an update. Free Radical Res (2018) 52(11-12):1199–209. doi: 10.1080/10715762.2018.1500696
11. Tse G, Yan BP, Chan YW, Tian XY, Huang Y. Reactive Oxygen Species, Endoplasmic Reticulum Stress and Mitochondrial Dysfunction: The Link with Cardiac Arrhythmogenesis. Front Physiol (2016) 7:313. doi: 10.3389/fphys.2016.00313
12. Korantzopoulos P, Letsas KP, Tse G, Fragakis N, Goudis CA, Liu T. Inflammation and atrial fibrillation: A comprehensive review. J Arrhythm (2018) 34(4):394–401. doi: 10.1002/joa3.12077
13. Zhang X, Zhang Z, Yang Y, Suo Y, Liu R, Qiu J, et al. Alogliptin prevents diastolic dysfunction and preserves left ventricular mitochondrial function in diabetic rabbits. Cardiovasc Diabetol (2018) 17(1):160. doi: 10.1186/s12933-018-0803-z
14. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation (2016) 134(10):752–72. doi: 10.1161/CIRCULATIONAHA.116.021887
15. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet (London England) (2019) 393(10166):31–9. doi: 10.1016/S0140-6736(18)32590-X
16. Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW, et al. Lower Risk of Heart Failure and Death in Patients Initiated on Sodium-Glucose Cotransporter-2 Inhibitors Versus Other Glucose-Lowering Drugs: The CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation (2017) 136(3):249–59. doi: 10.1161/CIRCULATIONAHA.117.029190
17. Toulis KA, Willis BH, Marshall T, Kumarendran B, Gokhale K, Ghosh S, et al. All-Cause Mortality in Patients With Diabetes Under Treatment With Dapagliflozin: A Population-Based, Open-Cohort Study in The Health Improvement Network Database. J Clin Endocrinol Metab (2017) 102(5):1719–25. doi: 10.1210/jc.2016-3446
18. Ingelfinger JR, Rosen CJ. Cardiovascular Risk and Sodium-Glucose Cotransporter 2 Inhibition in Type 2 Diabetes. N Engl J Med (2015) 373(22):2178–9. doi: 10.1056/NEJMe1512602
19. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J (2020) 41(2):255–323. doi: 10.1093/eurheartj/ehz486
20. O’Meara E, McDonald M, Chan M, Ducharme A, Ezekowitz JA, Giannetti N, et al. CCS/CHFS Heart Failure Guidelines: Clinical Trial Update on Functional Mitral Regurgitation, SGLT2 Inhibitors, ARNI in HFpEF, and Tafamidis in Amyloidosis. Can J Cardiol (2020) 36(2):159–69. doi: 10.1016/j.cjca.2019.11.036
21. Vardeny O, Vaduganathan M. Practical Guide to Prescribing Sodium-Glucose Cotransporter 2 Inhibitors for Cardiologists. JACC Heart Fail (2019) 7(2):169–72. doi: 10.1016/j.jchf.2018.11.013
22. Olgar Y, Turan B. A sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin comparison with insulin shows important effects on Zn(2+)-transporters in cardiomyocytes from insulin-resistant metabolic syndrome rats through inhibition of oxidative stress (1). Can J Physiol Pharmacol (2019) 97(6):528–35. doi: 10.1139/cjpp-2018-0466
23. Yang Y, Zhao J, Qiu J, Li J, Liang X, Zhang Z, et al. Xanthine Oxidase Inhibitor Allopurinol Prevents Oxidative Stress-Mediated Atrial Remodeling in Alloxan-Induced Diabetes Mellitus Rabbits. J Am Heart Assoc (2018) 7(10):e008807. doi: 10.1161/JAHA.118.008807
24. Zelniker TA, Bonaca MP, Furtado R, Mosenzon O, Kuder JF, Murphy SA, et al. Effect of Dapagliflozin on Atrial Fibrillation in Patients with Type 2 Diabetes Mellitus: Insights from the DECLARE-TIMI 58 Trial. Circulation (2020) 141(15):1227–34. doi: 10.1161/CIRCULATIONAHA.119.044183
25. Okunrintemi V, Mishriky BM, Powell JR, Cummings DM. Sodium-glucose co-transporter-2 inhibitors and atrial fibrillation in the cardiovascular and renal outcome trials. Diabetes Obes Metab (2020) 23(1):276–80. doi: 10.1111/dom.14211
26. Li W-j, Chen X-q, Xu L-l, Li Y-q, Luo B-h. SGLT2 inhibitors and atrial fibrillation in type 2 diabetes: a systematic review with meta-analysis of 16 randomized controlled trials. Cardiovasc Diabetol (2020) 19(1):130. doi: 10.1186/s12933-020-01105-5
27. Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med (2020) 383(15):1436–46. doi: 10.1056/NEJMoa2024816
28. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med (2019) 380(4):347–57. doi: 10.1056/NEJMoa1812389
29. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med (2019) 381(21):1995–2008. doi: 10.1056/NEJMoa1911303
30. Mathieu C, Herrera Marmolejo M, González González JG, Hansen L, Chen H, Johnsson E, et al. Efficacy and safety of triple therapy with dapagliflozin add-on to saxagliptin plus metformin over 52 weeks in patients with type 2 diabetes. Diabetes Obes Metab (2016) 18(11):1134–7. doi: 10.1111/dom.12737
31. Bailey CJ, Morales Villegas EC, Woo V, Tang W, Ptaszynska A, List JF. Efficacy and safety of dapagliflozin monotherapy in people with Type 2 diabetes: a randomized double-blind placebo-controlled 102-week trial. Diabetes Med (2015) 32(4):531–41. doi: 10.1111/dme.12624
32. Nct. Efficacy and Safety in Patients With Type 2 Diabetes Mellitus and Cardiovascular Disease. (2010). Availabe at: https://clinicaltrialsgov/show/NCT01042977.
33. Cefalu WT, Leiter LA, de Bruin TW, Gause-Nilsson I, Sugg J, Parikh SJ. Dapagliflozin’s Effects on Glycemia and Cardiovascular Risk Factors in High-Risk Patients With Type 2 Diabetes: A 24-Week, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study With a 28-Week Extension. Diabetes Care (2015) 38(7):1218–27. doi: 10.2337/dc14-0315
34. Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Med (2013) 11:43. doi: 10.1186/1741-7015-11-43
35. Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab (2014) 16(2):124–36. doi: 10.1111/dom.12187
36. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med (2019) 380(24):2295–306. doi: 10.1056/NEJMoa1811744
37. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med (2017) 377(7):644–57. doi: 10.1056/NEJMoa1611925
38. Nct. An Efficacy, Safety, and Tolerability Study of Canagliflozin in Patients With Type 2 Diabetes Mellitus Who Have Moderate Renal Impairment. (2010). Available at: https://clinicaltrialsgov/show/NCT01064414.
39. Bode B, Stenlöf K, Sullivan D, Fung A, Usiskin K. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: a randomized trial. Hosp Pract (1995) (2013) 41(2):72–84. doi: 10.3810/hp.2013.04.1020
40. Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab (2013) 15(12):1136–45. doi: 10.1111/dom.12149
41. Nct. A Efficacy, Safety, and Tolerability Study of Canagliflozin in Patients With Type 2 Diabetes Mellitus With Inadequate Glycemic Control on Metformin Alone or in Combination With a Sulphonylurea. (2011). Available at: https://clinicaltrialsgov/show/NCT01381900.
42. Nct. The CANTATA-MSU Trial (CANagliflozin Treatment And Trial Analysis - Metformin and SUlphonylurea). (2010). Available at: https://clinicaltrialsgov/show/NCT01106625.
43. Rosenstock J, Aggarwal N, Polidori D, Zhao Y, Arbit D, Usiskin K, et al. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care (2012) 35(6):1232–8. doi: 10.2337/dc11-1926
44. Nct. Effects of Empagliflozin on Clinical Outcomes in Patients With Acute Decompensated Heart Failure. (2017). Available at: https://clinicaltrialsgov/show/NCT03200860.
45. Nct. This Study Tests Empagliflozin in Patients With Chronic Heart Failure With Preserved Ejection Fraction (HFpEF). The Study Looks at How Far Patients Can Walk in 6 Minutes and at Their Heart Failure Symptoms. (2018). Available at: https://clinicaltrialsgov/show/NCT03448406.
46. Nct. This Study Tests Empagliflozin in Patients With Chronic Heart Failure With Reduced Ejection Fraction (HFrEF). The Study Looks at How Far Patients Can Walk in 6 Minutes and at Their Heart Failure Symptoms. (2018). Available at: https://clinicaltrialsgov/show/NCT03448419.
47. Nct. A Dose Finding Study to Assess the Effect of LIK066 Compared to Placebo or Empagliflozin in Patients With Type 2 Diabetes Mellitus and Heart Failure. (2017). Available at: https://clinicaltrialsgov/show/NCT03152552.
48. Softeland E, Meier JJ, Vangen B, Toorawa R, Maldonado-Lutomirsky M, Broedl UC. Empagliflozin as Add-on Therapy in Patients With Type 2 Diabetes Inadequately Controlled With Linagliptin and Metformin: A 24-Week Randomized, Double-Blind, Parallel-Group Trial. Diabetes Care (2017) 40(2):201–9. doi: 10.2337/dc16-1347
49. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med (2015) 373(22):2117–28. doi: 10.1056/NEJMoa1504720
50. Kovacs CS, Seshiah V, Merker L, Christiansen AV, Roux F, Salsali A, et al. Empagliflozin as Add-on Therapy to Pioglitazone With or Without Metformin in Patients With Type 2 Diabetes Mellitus. Clin Ther (2015) 37(8):1773–88. doi: 10.1016/j.clinthera.2015.05.511
51. Nct. Efficacy and Safety of BI 10773 in Combination With Insulin in Patients With Type 2 Diabetes. (2009). Available at: https://clinicaltrialsgov/show/NCT01011868.
52. Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol (2014) 2(5):369–84. doi: 10.1016/S2213-8587(13)70208-0
53. Riggs MM, Staab A, Seman L, MacGregor TR, Bergsma TT, Gastonguay MR, et al. Population pharmacokinetics of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in patients with type 2 diabetes. J Clin Pharmacol (2013) 53(10):1028–38. doi: 10.1002/jcph.147
54. Garg SK, Henry RR, Banks P, Buse JB, Davies MJ, Fulcher GR, et al. Effects of Sotagliflozin Added to Insulin in Patients with Type 1 Diabetes. N Engl J Med (2017) 377(24):2337–48. doi: 10.1056/NEJMoa1708337
55. Buse JB, Garg SK, Rosenstock J, Bailey TS, Banks P, Bode BW, et al. Sotagliflozin in Combination With Optimized Insulin Therapy in Adults With Type 1 Diabetes: The North American inTandem1 Study. Diabetes Care (2018) 41(9):1970–80. doi: 10.2337/dc18-0343
56. Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N Engl J Med (2020) 383(15):1425–35. doi: 10.1056/NEJMoa2004967
57. Gallo S, Charbonnel B, Goldman A, Shi H, Huyck S, Darekar A, et al. Long-term efficacy and safety of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: 104-week VERTIS MET trial. Diabetes Obes Metab (2019) 21(4):1027–36. doi: 10.1111/dom.13631
58. Grunberger G, Camp S, Johnson J, Huyck S, Terra S, Mancuso JP, et al. Ertugliflozin in Patients with Stage 3 Chronic Kidney Disease and Type 2 Diabetes Mellitus: The VERTIS RENAL Randomized Study. Diabetes Ther (2018) 9(1):49–66. doi: 10.1007/s13300-017-0337-5
59. Kosiborod M, Lam CSP, Kohsaka S, Kim DJ, Karasik A, Shaw J, et al. Cardiovascular Events Associated With SGLT-2 Inhibitors Versus Other Glucose-Lowering Drugs: The CVD-REAL 2 Study. J Am Coll Cardiol (2018) 71(23):2628–39. doi: 10.1016/j.jacc.2018.03.009
60. Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia (2018) 61(12):2461–98. doi: 10.1007/s00125-018-4729-5
61. Nct. Dapagliflozin Evaluation to Improve the LIVEs of Patients With PReserved Ejection Fraction Heart Failure. (2018). Available at: https://clinicaltrialsgov/show/NCT03619213.
62. Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z, et al. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol (2019) 18(1):165. doi: 10.1186/s12933-019-0964-4
63. Yurista SR, Silljé HHW, Rienstra M, de Boer RA, Westenbrink BD. Sodium-glucose co-transporter 2 inhibition as a mitochondrial therapy for atrial fibrillation in patients with diabetes? Cardiovasc Diabetol (2020) 19(1):5. doi: 10.1186/s12933-019-0984-0
64. Tse G, Lai ET, Tse V, Yeo JM. Molecular and Electrophysiological Mechanisms Underlying Cardiac Arrhythmogenesis in Diabetes Mellitus. J Diabetes Res (2016) 2016:2848759. doi: 10.1155/2016/2848759
65. Chaugai S, Meng WY, Ali Sepehry A. Effects of RAAS Blockers on Atrial Fibrillation Prophylaxis: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Cardiovasc Pharmacol Ther (2016) 21(4):388–404. doi: 10.1177/1074248415619490
66. Mascolo A, Urbanek K, De Angelis A, Sessa M, Scavone C, Berrino L, et al. Angiotensin II and angiotensin 1-7: which is their role in atrial fibrillation? Heart Fail Rev (2020) 25(2):367–80. doi: 10.1007/s10741-019-09837-7
67. Khatib R, Joseph P, Briel M, Yusuf S, Healey J. Blockade of the renin-angiotensin-aldosterone system (RAAS) for primary prevention of non-valvular atrial fibrillation: a systematic review and meta analysis of randomized controlled trials. Int J Cardiol (2013) 165(1):17–24. doi: 10.1016/j.ijcard.2012.02.009
68. Pintat S, Fenici P, Hammar N, Ji L, Khunti K, Medina J, et al. Eligibility of patients with type 2 diabetes for sodium-glucose cotransporter 2 inhibitor cardiovascular outcomes trials: a global perspective from the DISCOVER study. BMJ Open Diabetes Res Care (2019) 7(1):e000627. doi: 10.1136/bmjdrc-2018-000627
Keywords: sodium-glucose transporter 2 inhibitors, dapagliflozin, atrial fibrillation, atrial flutter, prevention
Citation: Li D, Liu Y, Hidru TH, Yang X, Wang Y, Chen C, Li KHC, Tang Y, Wei Y, Tse G and Xia Y (2021) Protective Effects of Sodium-Glucose Transporter 2 Inhibitors on Atrial Fibrillation and Atrial Flutter: A Systematic Review and Meta- Analysis of Randomized Placebo-Controlled Trials. Front. Endocrinol. 12:619586. doi: 10.3389/fendo.2021.619586
Received: 28 October 2020; Accepted: 15 February 2021;
Published: 19 March 2021.
Edited by:
Federico Biscetti, Catholic University of the Sacred Heart, ItalyReviewed by:
Martin Javorský, University of Pavol Jozef Šafárik, SlovakiaMaria Margherita Rando, Catholic University of the Sacred Heart, Italy
Andrea Giaccari, Catholic University of the Sacred Heart, Italy
Copyright © 2021 Li, Liu, Hidru, Yang, Wang, Chen, Li, Tang, Wei, Tse and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunlong Xia, eXVubG9uZ194aWFAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Daobo Li
Daobo Li Yingying Liu
Yingying Liu Tesfaldet Habtemariam Hidru
Tesfaldet Habtemariam Hidru Xiaolei Yang1
Xiaolei Yang1 Yunsong Wang
Yunsong Wang Cheng Chen
Cheng Chen Ka Hou Christien Li
Ka Hou Christien Li Yuqi Tang
Yuqi Tang Yushan Wei
Yushan Wei Gary Tse
Gary Tse Yunlong Xia
Yunlong Xia