- 1Department of Biomedical, Experimental and Clinical Sciences, University of Florence, Careggi University Hospital, Florence, Italy
- 2High Risk Pregnancy Unit, Careggi University Hospital, Florence, Italy
- 3Department of Molecular and Developmental Medicine, University of Siena, Siena, Italy
Objective: Maternal characteristics and OGTT values of pregnancies complicated by gestational diabetes mellitus (GDM) were evaluated according to treatment strategies. The goal was to identify different maternal phenotypes in order to predict the appropriate treatment strategy.
Methods: We conducted a retrospective study among 1,974 pregnant women followed up for GDM in a tertiary referral hospital for high-risk pregnancies (Careggi University Hospital, Florence, Italy) from 2013 to 2018. We compared nutritional therapy (NT) alone (n = 962) versus NT and insulin analogues (n = 1,012) group. Then, we focused on different insulin analogues groups: long acting (D), rapid acting (R), both D and R. We compared maternal characteristics of the three groups, detecting which factors may predict the use of rapid or long-acting insulin analogue alone versus combined therapy.
Results: Among women included in the analysis, 51.3% of them needed insulin therapy for glycemic control: 61.8% D, 28.3% combined D and R, and 9.9% R alone. Age >35 years, pre-pregnancy BMI >30, family history of diabetes, previous GDM, altered fasting plasma glucose (FPG), hypothyroidism, and assisted reproductive technologies (ART) were identified as maternal variables significantly associated with the need of insulin therapy. Altered 1-h and 2-h glucose plasma glucose level at OGTT, age >35 years, and previous GDM were found as independent predicting factors for the use of combined therapy with rapid and long acting analogues for glycemic control. On the contrary, pre-pregnancy BMI <25 and normal fasting plasma glucose values at OGTT were found to be significantly associated to the use of rapid insulin analogue only.
Conclusion: A number of maternal and metabolic variables may be identified at the diagnosis of GDM, in order to identify different GDM phenotypes requiring a personalized treatment for glycemic control.
Introduction
Gestational diabetes mellitus (GDM) is considered a major global health problem because of its increasing prevalence and the well-known association between hyperglycemia in pregnancy and feto-maternal morbidity (1, 2). Fetal outcomes correlate with maternal hyperglycemia severity, determining not only perinatal and neonatal complications in the short term (such as macrosomia, shoulder dystocia, respiratory distress, hypoglycemia, polycythemia, and hyperbilirubinemia), but also long-term sequelae with increased risk of obesity and diabetes later in life (3). Therefore, it is mandatory to perform a timely diagnosis of GDM and to improve therapeutic strategies in order to achieve the optimal glycemic control during pregnancy in the least amount of time.
Insulin therapy is one of the key strategies in managing diabetes in pregnancy, when nutritional therapy (NT) and lifestyle changes alone are not enough for an adequate glycemic control. In the last 10 years short and long-acting insulin analogues have replaced human-derived insulin, with a number of advantages for glycemic control. Short-acting analogues (Lispro and Aspart) have faster onset and shorter duration of action determining a more overlapping peak to the one of the endogenous insulin, reducing the risk of post-meal hypoglycemia and improving glycemic control in women with GDM (4, 5). The long-acting analogues (Glargine and Detemir) and their good pharmacokinetic characteristics allow to control basal glycemia providing a flat and protracted pharmacodynamic profile (4, 6). Their use has increased in the last decade because of the rising attention to fasting glucose profile control rather than post meal glycemic values. Maternal 1-h postprandial glycemic peak is one of the best predictor of fetal macrosomia, but fasting glucose levels also significantly influence perinatal and neonatal outcome (7, 8).
Choosing the best therapeutic strategy in terms of type and timing of insulin therapy is essential to improve glycemic control in pregnancy. A number of maternal features and clinical data at diagnosis of GDM are reported to be associated with failure of NT and subsequent need for pharmacological treatment. For instance, early diagnosis of GDM (<24 weeks of gestation), older maternal age, pre-pregnancy BMI>30, previous GDM, family history of diabetes are reported as associated factors to insulin treatment (9–12). Biochemical parameters indicating poor metabolic glycemic control at the diagnosis of GDM can also predict the need of insulin therapy: HbA1c at diagnosis >5.5% and elevated 1-h and 2-h plasma glucose level at OGTT has been reported to be predictors of pharmacological treatment (13, 14).
The aim of the present study is to analyze maternal characteristics and OGTT values in pregnancies complicated by GDM according to different treatment strategies (diet alone or diet plus various combination of long- and short-term insulin analogues). The goal is to identify different maternal phenotypes in order to plan, since the diagnosis of GDM, the appropriate treatment strategy.
Materials and Methods
We performed a retrospective cohort study among 1,974 women with singleton pregnancy affected by GDM, who were referred to High Risk Pregnancy Unit in Careggi University Hospital (Florence, Italy) from 2013 to 2018. We compared women treated with nutritional therapy (NT) versus women treated with NT and insulin analogues. Women with pre-pregnancy diabetes or glucose intolerance, previous insulin treatment, current consuming oral glucose-lowering drugs, such as metformin, and twin pregnancies were excluded from the study.
According to Figo guidelines (3) all women underwent universal screening for GDM by 75 g oral glucose tolerance test (OGTT) at 24–28 weeks of gestation. Women considered at high risk to develop GDM [previous GDM, BMI >30, fasting plasma glucose (FPG) 100–125 mg/dl at first trimester blood test] were screened at 16–18 weeks and in case of normal glucose values, they repeated OGTT at 24–28 weeks (15). The diagnosis of GDM was made if one or more of the glucose values was equal or over the cutoff proposed by IADPSG Consensus Panel 2010 (16).
After diagnosis of GDM, patients were educated to self-monitoring of blood glucose. Finger sticks were obtained four to seven times a day: preprandial, postprandial (1 h and 2 h after breakfast, lunch, or dinner) and during the night. A nutritional counseling with an expert dietician devoted to GDM patients was provided every 2 weeks. Nutrition plan included a diet with adequate energy content, macronutrient distribution, quality and amount, in order to preserve mother’s weight gain and fetal growth, as well as maintaining near-normoglycemia and avoiding the development of ketone bodies. Also lifestyle interventions were proposed at every consultation.
The glucose reference values recommended by Italian National Guidelines were used: fasting (90 mg/dl), 1 h (130 mg/dl), and 2 h after a meal (120 mg/dl) (15). Insulin treatment was started with long-acting analogue (Detemir) (D) when glycemic values at fasting or during night were > 90 mg/dl in more than half of measurements during 2 weeks assessment. We prescribed Detemir in the morning or in the evening considering if glucose values were more altered during day or nighttime, but we often prescribed two shot-therapy with injections every 12 h. We used rapid analogue (Aspart, Lispro) (R) only when we were unable to normalize postprandial blood glucose levels with Detemir or when we had optimal fasting glycemic control but high postprandial values, despite an adequate NT: in this latter case we used only rapid insulin analogue.
Clinical data were extracted by reviewing obstetric records of all women followed up in our GDM clinic in a standardized electronic database. Collected data included maternal characteristics (age, ethnicity, BMI, family history, obstetric history, mode of conception, pre-pregnancy diseases), OGTT values, and GDM management (diet vs insulin treatment; short- and long-acting insulin analogues) until delivery. The last reported treatment was considered to classify each case according to the type of therapeutic approach for glycemic control. We also collected data on delivery and neonatal outcome, including gestational age at delivery, induction of labor, mode of delivery, small for gestational age (SGA), and large for gestational age (LGA) neonates, birthweight >4,000g, Apgar score at 10 min. In our department, according to national guidelines, if glycemic values are compensated by only NT, induction of labor is scheduled for GDM after 40 weeks + 6 days, whereas in case of insulin treatment women are induced by 40 weeks or earlier if glycemic values are not within the ranges.
The research protocol involved only existing records, based on information routinely collected and stored in a de-identified dataset and hence was considered exempt from ethical review board approval. However, all women referred to our GDM clinic signed an informed consent for clinical data treatment.
We analyzed maternal characteristics between the group of women treated with NT alone vs those treated with NT and insulin, identifying those maternal factors and metabolic variables at OGTT which could predict the need and type of insulin therapy. Then, among women treated with NT and insulin, we identified three groups under treatment with different types of insulin analogues: long-acting analogue, long- and rapid-acting analogues, only rapid-acting analogue. We compared maternal characteristics of the three groups, detecting which factors may predict the use of rapid or long-acting insulin analogue alone versus combined therapy using. Also the perinatal outcomes were compared between the group of women under NT vs those under NT and insulin treatment, and differentiating among the three insulin analogues treatment groups.
The collected data were entered in an electronic database and analyzed using the software SPSS (Statistical Package for Social Science) (IBM SPSS Statistics 23, IBM Corporation). Percentages, means, and standard deviations (SD) have been extrapolated, followed by the creation of charts and tables for a descriptive analysis. Data distribution was evaluated by the D’Agostino & Pearson omnibus normality test. Baseline characteristics were compared by using chi-square test with continuity correction or Fisher’s exact test for binomial variables. Mann-Whitney U, t test for unpaired or one-way ANOVA were used for continuous variables. A multivariable stepwise (conditional) logistic regression was used to identify the variables associated with NT vs insulin therapy and, similarly, those related to the different regimen of insulin therapy. Odds Ratios (OR) and 95% CI were calculated. A receiving operating curve (ROC) analysis was further performed on combined maternal and metabolic variables, significantly associated to the need of insulin treatment. A p value <0.05 was considered statistically significant.
Results
In the study group 962 (48.7%) women were treated with NT alone, whereas 1,012 (51.3%) women received diet and insulin therapy. Maternal characteristics of the two groups are reported in Table 1. Regarding timing of GDM diagnosis, 11.2% (n = 221) of women had a positive OGTT at 16–18 weeks. Comparing the diet and insulin groups, we observed that patients who needed insulin therapy had higher pre-pregnancy BMI (23.9 ± 4.91 vs 25.2 ± 5.49, p < 0.001) and were more likely to be obese (6.3 vs 12%, p < 0.001). They had also more frequently family history of diabetes (23.2 vs 30.5%, p < 0.001) and previous GDM (8.8 vs 14.9%, p < 0.001). Furthermore, patients in therapy with insulin were significantly more affected by chronic hypertension (0.5 vs 3.2%, p < 0.001) and hypothyroidism (6.1 vs 9.9%, p 0.002), and their pregnancy was more likely achieved using assisted reproductive technology (ART) (4.2 vs 7.3%, p 0.003). We didn’t find any difference in age, ethnicity, and rate of previous macrosomia between the two groups.
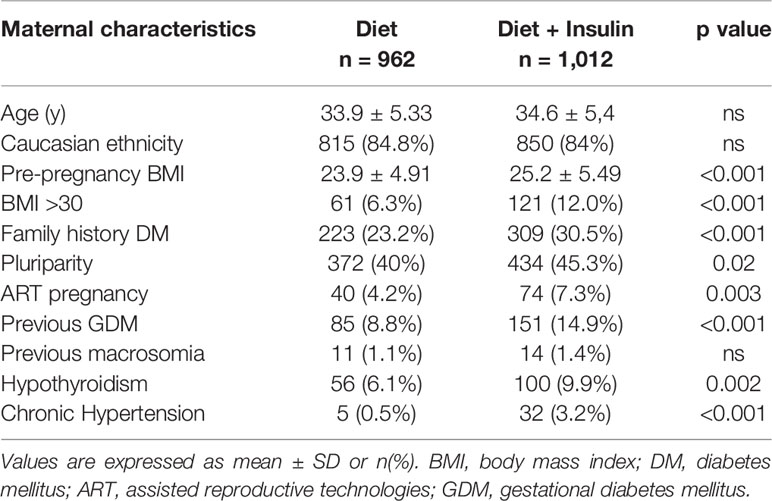
Table 1 Maternal characteristics, including anthropometric data, family and clinical history, of women with GDM treated with only diet and those requiring also insulin.
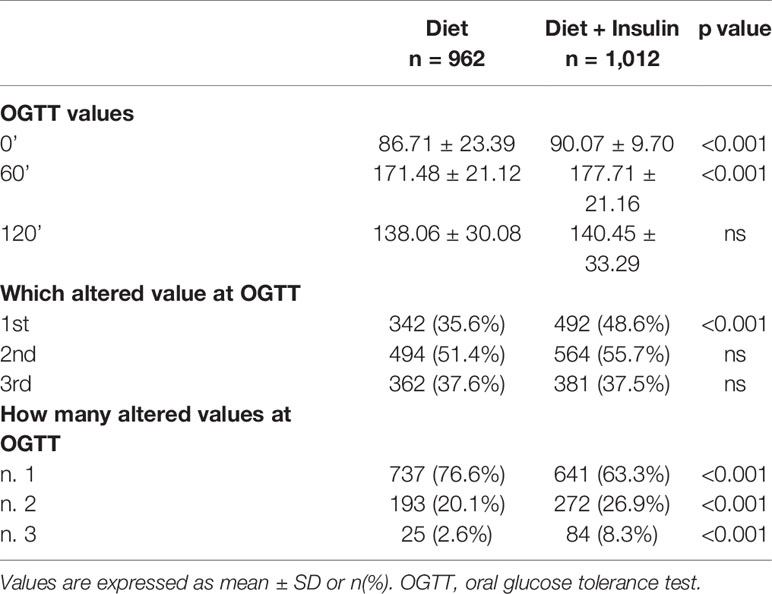
When we compared OGTT values between the two groups, we found that the insulin therapy group had higher fasting plasma glucose (FPG) values (86.7 ± 23.4 mg/dl vs 90.1 ± 9.7 mg/dl, p < 0.001), higher 1-h glucose values (171.5 ± 21.1 mg/dl vs 177.7 ± 21.2 mg/dl, p < 0,001), and higher numbers of altered glucose values at OGTT (n.3 altered values 2.6 vs 8.3%, p < 0.001) (Table 2).
After multivariate logistic regression analysis, pre-pregnancy BMI >30 (OR 1.86, 95% CI 1.42–2.45), family history of diabetes (OR 1.14, 95% CI 1.01–1.29), previous GDM (OR 1.81, 95% CI 1.36–2.43), hypothyroidism (OR 1.52, 95% CI 1.07–2.15), and ART pregnancy (OR 1.81, 95% CI 1.19–2.73) were identified as maternal variables significantly associated with the need of insulin therapy. Similarly, patients who needed insulin therapy were significantly older than women in nutritional therapy (age >35 y OR 1.23, 95% CI 1.02–1.48) and they had more frequently altered FPG at OGTT (OR 1.62, 95% CI 1.34–1.96) (Table 3). By integrating all the maternal variables found to be significantly associated to the need of insulin therapy, a ROC curve analysis was performed and the prediction model based on these seven maternal risk factors (altered FPG at OGTT, pre-gestational BMI>30, age >35 years, familiar history of diabetes, ART pregnancy, previous GDM, hypothyroidism) showed acceptable screening performance. The area under curve (AUC) was 0.647 (95% CI 0.626–0.668) and the model has 55.3% sensitivity and 67.3% specificity (Figure 1).
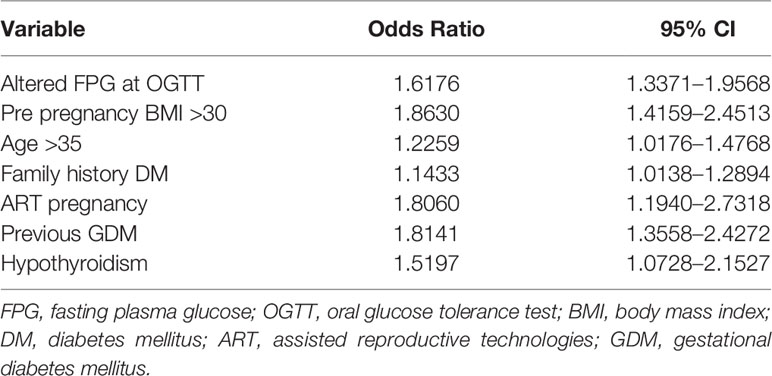
Table 3 Variables significantly associated to insulin treatment for GDM from multivariate stepwise logistic regression.
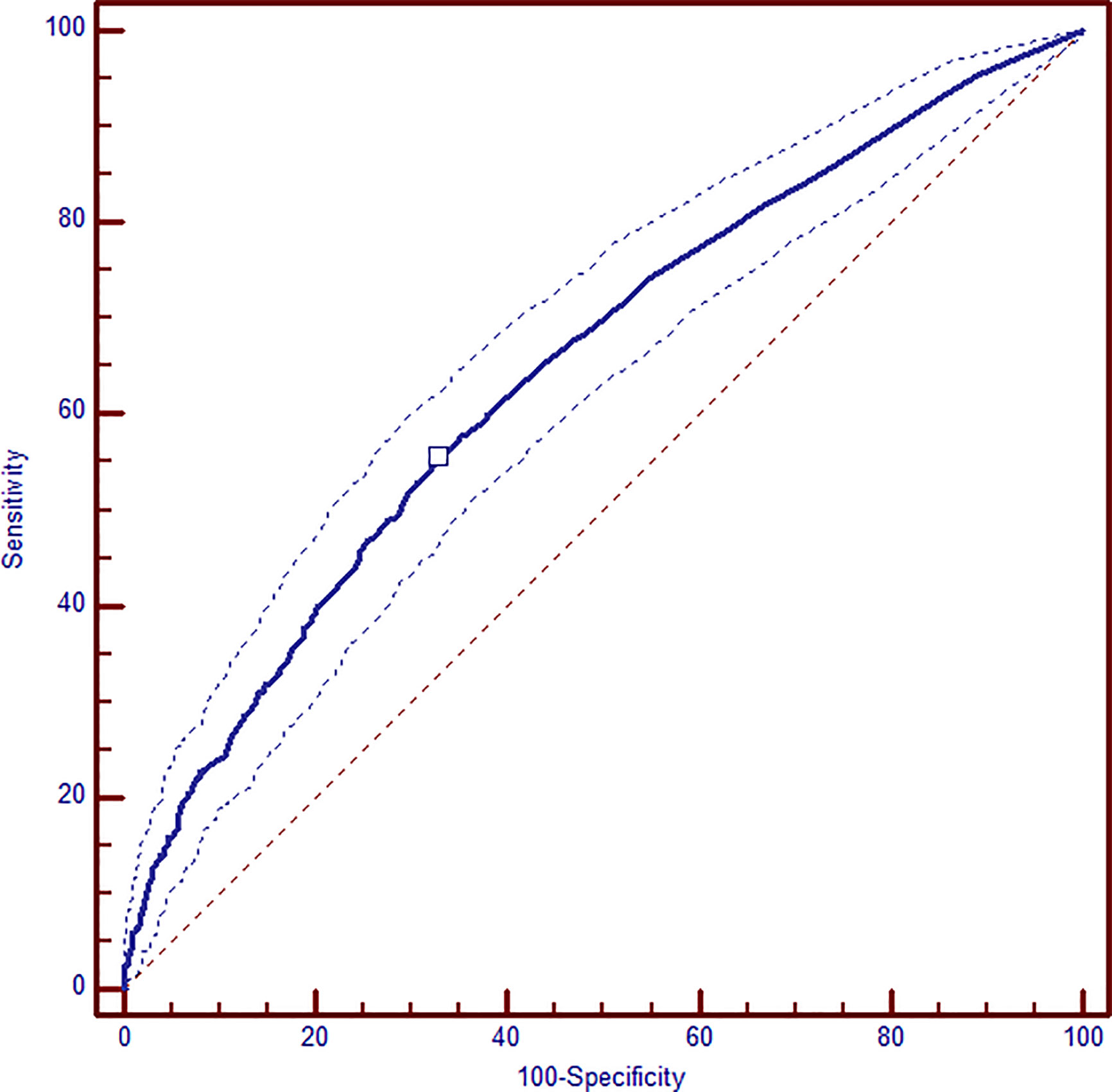
Figure 1 ROC analysis of combined maternal and metabolic significant variables: altered FPG at OGTT, pre-gestational BMI >30, age >35 years, familiar history of diabetes, ART pregnancy, previous GDM, hypothyroidism. AUC 0.647 (95% CI 0.626–0.668); sensitivity 55.3% and specificity 67.3%.
Focusing on the 1,012 patients treated with both diet and insulin, we identified 626 (61.8%) women in therapy with long-acting analogues (Detemir), 286 (28.3%) who received both long and rapid analogues, and 100 (9.9%) patients on rapid analogue only treatment.
Maternal characteristics of the three groups are reported in Table 4. Patient who needed combined therapy and rapid insulin analogue were older than those who received only Detemir (D 34.2 ± 5.4 vs D+R 35.4 ± 5.5 vs 35.3 ± 4.8 years; p = 0.005). Furthermore, patients treated with Detemir or combined therapy had higher pre-pregnancy weight (D 68.0 ± 16.3 vs D+R 71.1 ± 17.3 vs R 61.5 ± 9.9 kg; p < 0.001) and were more likely to be obese (D 17.9% vs D+R 20.9% vs R 4%; p = 0.001). They also had more frequently history of previous GDM (D 44.5 vs D+R 47.8 vs R 10%; p = 0.008). We didn’t find any difference between the three groups in terms of family history of diabetes, pluriparity, chronic hypertension, ART pregnancy, and hypothyroidism.
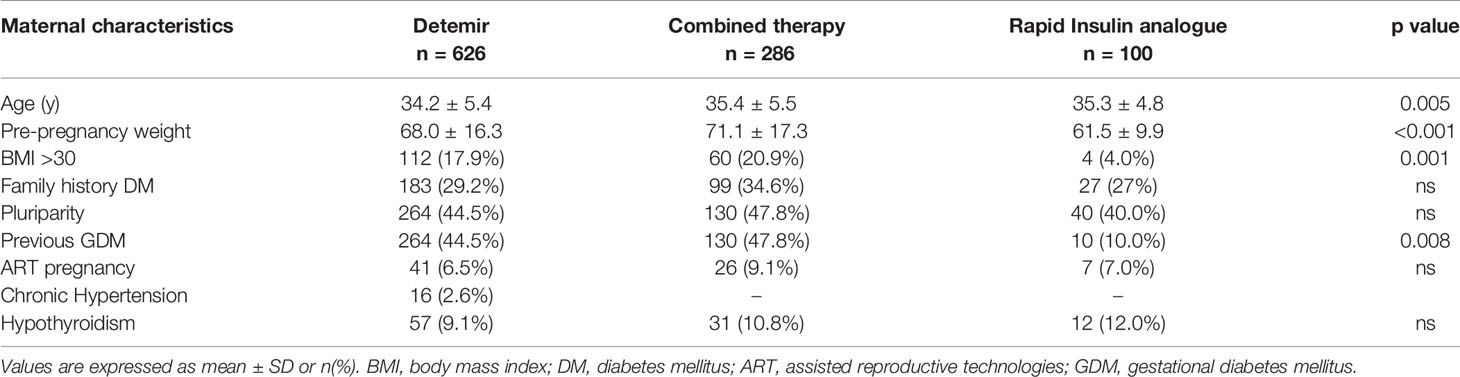
Table 4 Maternal characteristics, including anthropometric data, family and clinical history, of women with GDM treated with long-acting analogues (Detemir) or rapid analogues or combined therapy.
Regarding OGTT values, women who required combined long-acting and rapid analogues therapy had higher basal and post load values than those treated with only rapid analogues (basal value: D+R 92.05 ± 27.38 mg/dl vs R 83.65 ± 8.65 mg/dl; p = 0.0023. 1 h value: D+R 184.67 ± 49.25 mg/dl vs R 180.67 ± 32.97 mg/dl; p < 0.0001. 2 h value: D+R 149.10 ± 48.61 mg/dl vs R 145.92 ± 30.83 mg/dl; p < 0.0001). Basal values at OGTT were higher in Detemir group compared with rapid insulin analogue alone (D 92.08 ± 21.44 mg/dl vs R 83.65 ± 8.65 mg/dl; p = 0.0011), 2 h post load glucose values were instead higher in short- vs long-acting analogues groups (D 133.78 ± 47.27 mg/dl vs R 145.92 ± 30.08 mg/dl; p = 0.021) (Table 5).
Adjusting for confounding factors, pre-pregnancy BMI < 25 (OR 2.77, 95% CI 1.69–4.55), and normal fasting plasma glucose values at OGTT (OR 2.77, 95% CI 1.68–4.26) were found to be two statistically significant variables for the use of rapid insulin analogue alone (Table 6).
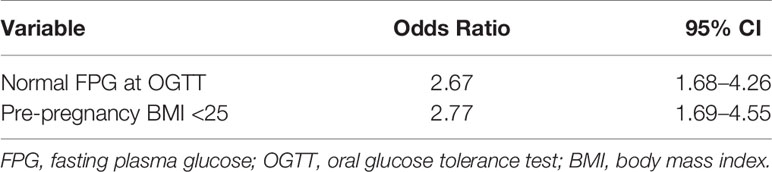
Table 6 Variables significantly associated to rapid analogues insulin treatment for GDM from multivariate stepwise logistic regression.
The multivariate logistic regression analysis identified altered 1-h plasma glucose level at OGTT (OR 1.51 95% CI 1.11–2.04), 2-h glucose level (OR 1.39 95% CI 1.03–1.88), age >35 years (OR 1.61 95% CI 1.19–2.17), and previous GDM (OR 1.62 95% CI 1.10–2.39) as independent predicting factors for the use of combined therapy with rapid and long-acting analogues for glycemic control during pregnancy (Table 7).
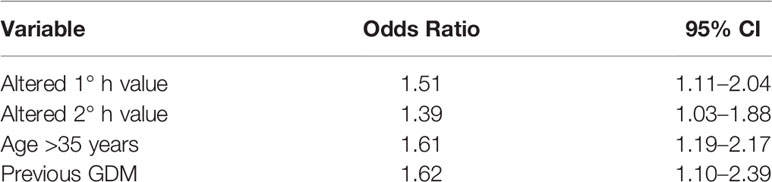
Table 7 Variables significantly associated to the combined long-acting and rapid analogues insulin treatment for GDM from multivariate stepwise logistic regression.
Finally, we compared numbers of injections in the three groups and we observed an average of 1.81 (± 0.39) in Detemir group, 4.07 (± 1.00) in combined therapy group and 2.46 (± 0.80) in rapid analogue group. The number of shots was significantly lower in Detemir group compared with combined therapy group (p < 0.0001, estimate difference −2.27), in Detemir group compared with rapid group (p < 0.0001, ED −0.65) and in rapid group compared with combined therapy group (p < 0.0001 ED −1.62).
The analysis of perinatal outcomes showed no differences in terms of gestational age at delivery, preterm birth, mode of delivery, SGA and LGA rate, birthweight >4,000 g, and Apgar score at 10 min between women on NT versus those on NT and insulin, and among groups according to type of insulin treatment. However, a higher induction of labor rate was found (NT 29.7% vs NT+insulin 41% p < 0.001) in women treated with insulin, especially if under combined long-acting and rapid insulin analogues (D 24.6% vs D+R 33.6% vs R 27%, p = 0.009) (Supplementary Tables 1 and 2).
Discussion
The present study shows relevant differences between GDM women according to the treatment they need, highlighting that, since the diagnosis of GDM, it is possible to identify those who would be more likely to be treated by insulin and which type of regimen would fit better for them. The identification of specific maternal and OGTT features at diagnosis of GDM would allow to develop orientated therapeutic strategies, in order to timely personalize the nutritional and medical approach to GDM.
The significant variables associated to insulin therapy were maternal age, obesity, previous GDM, altered FPG at OGTT, hypothyroidism, ART pregnancy, family history of DM. Our findings were consistent with previous studies (12, 14, 17) and this risk factors profile identifies a phenotype which is already available at the diagnosis of GDM, allowing to adequately counsel the patient on the increased risk of need for insulin treatment to control glucose, if NT would not be sufficient.
In a retrospective cohort study Meshel reported similar risk factors for insulin therapy (GMD in previous pregnancy, pre-pregnancy obesity, and maternal age >30 y). Only fasting glucose values >95 mg/dl was found to predict insulin therapy but no other abnormal values at OGTT (17). Although not associated with significant differences in maternal and neonatal outcomes, a poor metabolic profile demonstrated by fasting hyperglycemia at OGTT predicted a greater need of insulin therapy and a higher risk of impaired glucose tolerance persistence after childbirth (18). Obesity is a well-known risk factor for GDM and insulin treatment, due to increased insulin resistance (19, 20). Also hypothyroidism has been recently demonstrated to be associated with increased risk of GDM, as thyroid hormones affect glucose regulation during pregnancy and their abnormality may contribute to insulin resistance, aside from the specific role played by thyroid autoimmunity (21). Furthermore, as shown by our results, ART seems to be an independent prognostic factor predicting insulin therapy in women affected by GDM (22–24).
Barnes indicated a model to predict the need of insulin therapy based on maternal characteristic and metabolic profile demonstrating that the number of risk factors was associated with worse pregnancy outcomes (12) and the prediction model had an acceptable accuracy (AUC 0.71 (95% CI 0.693–0.731), even though this does not represent the optimal prediction. We obtained similar results with our prediction model, integrating all the maternal and metabolic significant variables associated to the need of insulin. Nonetheless, Ducarme failed to combine maternal characteristics and biological parameters to develop an accurate model (13).
Regarding the importance of metabolic profile at OGTT, alongside maternal variables, another retrospective analysis in which 1 h and 2 h glucose values at OGTT and the number of abnormal values in 75 g OGTT predicted the requirement of insulin therapies, confirming our findings (14). In the present study population, not only an altered FPG was significantly associated with the failure of NT, but also 1-h glycemic value at OGTT and the risk was higher if one, two, or three values were altered. In those cases, a combined long-acting and rapid analogues therapy was necessary to keep under control glycemic values. On the contrary, the phenotype of a GDM women with normal BMI and normal FPG is significantly associated with the need of only rapid insulin analogues, if NT has failed.
Focusing on insulin therapy we achieved optimal glycemic control with Detemir in 60% of GDM women reducing numbers of daily injections, hypothesizing a better maternal compliance and adherence to therapy. A recent meta-analysis showed no differences between Detemir and Neutral Protamine Hagedorn in term of glycemic control and fetal outcomes in GDM women (5). The use of Detemir in pregnancy is considered safe because it doesn’t cross the human placenta and, unlike Galrgine, it has low affinity for the IGF-1 receptor and this could prove beneficial in the treatment of pregnant women, particularly as regards the effect on birthweight and maternal complications (25).
Data on the use of Detemir in women with GDM are increasing, showing that it allows to achieve a good glycemic control, similar to NPH (26, 27). Studies about Detemir and type 1 diabetes in pregnancy published in recent years showed that Detemir is as effective as NPH when used as a basal insulin in pregnant women affected by type 1 diabetes with the potential to offer some clinical benefits in terms of fasting plasma glucose control (28–30). Detemir is as well tolerated as NPH regarding perinatal outcomes and no safety issues were identified. Results from a recent large international prospective cohort study will provide updated information on the occurrence of malformations and perinatal outcome in women under Detemir treatment compared to other long-acting insulins (31).
By using long-acting analogue treatment fasting glucose values may be normalized and this goal seems to be the major determinant of pregnancy and neonatal outcomes. Furthermore, the use of one injection every 12 h allows to provide a stable level of insulin all-day, managing to drop also postprandial glycemic values, with an irrelevant risk of hypoglycemia. In this scenario postprandial glucose values may play a key role in determining fetal overgrowth in women with severe GDM that require combined therapy (8).
According to our results, we found that women who required long-acting analogues or combined therapy presented a specific phenotype: they more often older, affected by GDM in previous pregnancy, and more insulin resistant (elevated fasting glucose values and more altered OGTT) compared to women who required only rapid analogues who were slimmer and had normal fasting glycemic values. Similar findings are reported in a recent multicenter prospective cohort study (32), where GDM subtypes with different degrees of insulin resistance had distinct phenotype and risks of adverse pregnancy outcomes. Women with GDM and normal insulin sensibility could be assimilated to normal glucose tolerance women in terms of adverse outcomes and pre-pregnancy characteristics. Moreover, women affected by GDM with higher insulin resistance represented a more adverse metabolic profile with greater risk of adverse pregnancy outcomes.
Risk factors for insulin therapy identified in our population are concordant in recognizing a poor metabolic profile that can be the substrate of maladaptation to the insulin resistance state physiologically induced by pregnancy. That is especially true for patients who required combined therapy, that was at high risk of poor glycemic control according history and metabolic factors.
Therefore, it is important to promptly identify patients who need insulin therapy in order to start adequate treatment as soon as possible improving glycemic control and avoiding adverse pregnancy outcomes. Women with insulin resistance and metabolic syndrome appears to be the highest risk group, that requires a strict management in order to improve fetal and neonatal outcomes.
The strength of this study is that it included a large number of patients in each group and that it was performed in a single center where the screening of GDM and the glycemic goals for treatment have been constant during the study period. The major limitation is the retrospective nature of the study.
In conclusion, some maternal constitutional and metabolic factors of GDM can promptly predict the need for insulin therapy. This approach can identify a high-risk group in GDM patients and can allow the prescription of a timely and appropriate therapy in order to improve obstetric outcomes and reducing the time of fetal exposure to hyperglycemia. This can also improve fetal programming thereby reducing short- and long-term consequences.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. The research protocol involved only existing records, based on information routinely collected and stored in a de-identified dataset and hence was considered exempt from ethical review board approval.
Author Contributions
FM contributed to the conception and design of the study. FL, SO, CS, MR, and SS contributed to patient recruitment and collection of data. SV and FL collaborated to data analysis and first draft writing. FM and FP substantially contributed to interpretation of data. FM, SV, and FP revised the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank all the senior and junior doctors, midwives, and dieticians involved in the management of GDM patients in our High Risk Pregnancy Unit, in Careggi University Hospital, Florence, Italy.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.630903/full#supplementary-material
References
1. International Diabetes Federation. IDF Diabetes Atlas. 9th edn. Brussels, Belgium: International Diabetes Federation (2019).
2. Mitanchez D, Yzydorczyk C, Siddeek B, Boubred F, Benahmed M, Simeoni U. The offspring of the diabetic mother. Short- and long-term implications. Best Pract Res Clin Obstet Gynaecol (2015) 29:256–9. doi: 10.1016/j.bpobgyn.2014.08.004
3. Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, Di Renzo GC, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on Gestational Diabetes Mellitus: A Pragmatic Guide for Diagnosis, Management, and Care. Int J Gynecol Obstet (2015) 131 S3:S173–211. doi: 10.1016/S0020-7292(15)30033-3
4. Toledano Y, Hadar E, Hod M. Pharmacotherapy for Hyperglycemia in Pregnancy- The New Insulins. Diabetes Res Clin Pract (2018) 145:59–66. doi: 10.1016/j.diabres.2018.04.035
5. Lv S, Wang J, Xu Y. Safety of insulin analogs during pregnancy: a meta-analysis. Arch Gynecol Obs (2015) 292:749–56. doi: 10.1007/s00404-015-3692-3
6. Herrera KM, Rosenn BM, Foroutan J, Bimson BE, Ibraheemi Z, Moshier EL, et al. Randomized controlled trial of insulin detemir versus NPH for the treatment of pregnant women with diabetes. Am J Obstet Gynecol (2015) 213:426.e1–7. doi: 10.1016/j.ajog.2015.06.010
7. Bacon S, Feig DS. Glucose Targets and Insulin Choice in Pregnancy: What Has Changed in the Last Decade? Curr Diabetes Rep (2018) 18:77. doi: 10.1007/s11892-018-1054-9DIABETES
8. Durnwald CP, Mele L, Spong CY, Ramin SM, Varner MW, Rouse DJ, et al. Glycemic Characteristics and Neonatal Outcomes of Women Treated for Mild Gestational Diabetes. Obstet Gynecol (2011) 117:819–27. doi: 10.1097/AOG.0b013e31820fc6cf
9. Pertot T, Molyneaux L, Tan K, Ross GP, Yue DK, Wong J. Can common clinical parameters be used to Identify Patients Who Will Need Diabetes Mellitus? Diabetes Care (2011) 34:2214–6. doi: 10.2337/dc11-0499
10. Wong VW, Jalaludin B. Gestational diabetes mellitus: Who requires insulin therapy? Aust New Zeal J Obstet Gynaecol (2011) 51:432–6. doi: 10.1111/j.1479-828X.2011.01329.x
11. Bakiner O, Bozkirli E, Ozsahin K, Sariturk C, Ertorer E. Risk Factors That can Predict Antenatal Insulin Need in Gestational Diabetes. J Clin Med Res (2013) 5:381–8. doi: 10.4021/jocmr1515w
12. Barnes RA, Wong T, Ross GP, Jalaludin BB, Wong VW, Smart CE, et al. A novel validated model for the prediction of insulin therapy initiation and adverse perinatal outcomes in women with gestational diabetes mellitus. Diabetologia (2016) 59:2331–8. doi: 10.1007/s00125-016-4047-8
13. Ducarme G, Desroys F, Grange J, Vital M, Le A, Ingrid T. Predictive factors of subsequent insulin requirement for glycemic control during pregnancy at diagnosis of gestational diabetes mellitus. Int J Gynecol Obs (2019) 144:265–70. doi: 10.1002/ijgo.12753
14. Watanabe M, Katayama A, Kagawa H, Ogawa D, Wada J. Risk Factors for the Requirement of Antenatal Insulin Treatment in Gestational Diabetes Mellitus. J of Diabetes Res (2016) 2016:9648798. doi: 10.1155/2016/9648798
15. SID AMD. Standard italiani per la cura del diabete mellito 2018. (2018). Available at: https://www.siditalia.it/clinica/standard-di-cura-amd-sid.
16. International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International association of diabetes and pregnancy study groups recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care (2010) 33:676–82. doi: 10.2337/dc09-1848
17. Meshel S, Schejter E, Harel T, Maslovitz S, Elimelech B, Cohen B, et al. Can we predict the need for pharmacological treatment according to demographic and clinical characteristics in gestational diabetes? J Matern Neonatal Med (2015) 29:2062–6. doi: 10.3109/14767058.2015.1077225
18. Parrettini S, Ranucci L, Caroli A, Bini V, Calafiore R, Torlone E. Gestational diabetes: A link between OGTT, maternal-fetal outcomes and maternal glucose tolerance after childbirth. Nutr Metab Cardiovasc Dis (2020) 30:2389–97. doi: 10.1016/j.numecd.2020.08.002
19. Yao D, Chang Q, Wu QJ, Gao SY, Zhao H, Liu YS, et al. Relationship between Maternal Central Obesity and the Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Cohort Studies. J Diabetes Res (2020) 30:2070–6. doi: 10.1155/2020/6303820
20. Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care (2007). doi: 10.2337/dc06-2559a
21. Jia M, Wu Y, Lin B, Shi Y, Zhang Q, Lin Y, et al. Meta-analysis of the association between maternal subclinical hypothyroidism and gestational diabetes mellitus. Int J Gynecol Obstet (2019) 144:239–47. doi: 10.1002/ijgo.12751
22. Maroufizadeh S, Navid B, Alizadeh A, Amini P, Mohammadi M, Morasae EK, et al. Risk of gestational diabetes mellitus following assisted reproductive technology: systematic review and meta-analysis of 59 cohort studies. J Matern Neonatal Med (2019) 1:1–10. doi: 10.1080/14767058.2019.1670790
23. Giannakou K, Evangelou E, Yiallouros P, Christophi CA, Middleton N, Papatheodorou E, et al. Risk factors for gestational diabetes: An umbrella review of meta-analyses of observational studies. PloS One (2019) 14(4):e0215372. doi: 10.1371/journal.pone.0215372
24. Kouhkan A, Baradaran HR, Hosseini R, Arabipoor A, Moini A, Pirjani R, et al. Assisted conception as a potential prognostic factor predicting insulin therapy in pregnancies complicated by gestational diabetes mellitus. Reprod Biol Endocrinol (2019) 17:83. doi: 10.1186/s12958-019-0525-4
25. Suffecool K, Rosenn B, Niederko EE, Kiernan UA, Foroutan J, Antwi K, et al. Insulin Detemir Does Not Cross the Human Placenta. Diabetes Care (2015) 38:e20–1. doi: 10.2337/dc14-2090
26. Patti AM, Giglio RV, Pafili K, Rizzo M, Papanas N. Pharmacotherapy for gestational diabetes. Expert Opin Pharmacother (2018) 19:1407–14. doi: 10.1080/14656566.2018.1509955
27. Herrera KM, Rosenn BM, Foroutan J, Bimson BE, Al Ibraheemi Z, Moshier EL, et al. Randomized controlled trial of insulin detemir versus NPH for the treatment of pregnant women with diabetes. in. Am J Obstet Gynecol (2015) 213:426.e1–7. doi: 10.1016/j.ajog.2015.06.010
28. Callesen NF, Damm J, Mathiesen JM, Ringholm L, Damm P, Mathiesen ER. Treatment with the long-acting insulin analogues detemir or glargine during pregnancy in women with type 1 diabetes: comparison of glycaemic control and pregnancy outcome. J Matern Neonatal Med (2013) 26:588–92. doi: 10.3109/14767058.2012.743523
29. Hod M, Mathiesen ER, Jovanovic L, McCance DR, Ivanisevic M, Duràn-Garcia S, et al. A randomized trial comparing perinatal outcomes using insulin detemir or neutral protamine Hagedorn in type 1 diabetes. J Matern Fetal Neonatal Med (2014) 27(1):7–13. doi: 10.3109/14767058.2013.799650
30. Mello G, Biagioni S, Ottanelli S, Nardini C, Serena C, Marchi L, et al. Continuous subcutaneous insulin infusion (CSII) versus multiple daily injections (MDI) of rapid-acting insulin analogues and detemir in type 1 diabetic (T1D) pregnant women injections (MDI) of rapid-acting insulin analogues and detemir in type 1 diabetic. J Matern Neonatal Med (2015) 28(3):276–80. doi: 10.3109/14767058.2014.914922
31. Mathiesen ER, Andersen H, Kring SII, Damm P. Design and rationale of a large, international, prospective cohort study to evaluate the occurrence of malformations and perinatal/neonatal death using insulin detemir in pregnant women with diabetes in comparison with other long-acting insulins. BMC Pregnancy Childbirth (2017) 17:38. doi: 10.1186/s12884-016-1177-4
Keywords: body mass index (BMI), diet, gestational diabetes mellitus (GDM), glycemic control, hyperglycemia, insulin analogues, obesity, oral glucose tolerance test (OGTT)
Citation: Mecacci F, Lisi F, Vannuccini S, Ottanelli S, Rambaldi MP, Serena C, Simeone S and Petraglia F (2021) Different Gestational Diabetes Phenotypes: Which Insulin Regimen Fits Better? Front. Endocrinol. 12:630903. doi: 10.3389/fendo.2021.630903
Received: 18 November 2020; Accepted: 18 January 2021;
Published: 09 March 2021.
Edited by:
Elena Succurro, University of Magna Graecia, ItalyReviewed by:
Amelia Caretto, San Raffaele Hospital (IRCCS), ItalyYoshifumi Saisho, Keio University, Japan
Copyright © 2021 Mecacci, Lisi, Vannuccini, Ottanelli, Rambaldi, Serena, Simeone and Petraglia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvia Vannuccini, c2lsdmlhLnZhbm51Y2NpbmlAdW5pZmkuaXQ=
 Federico Mecacci1,2
Federico Mecacci1,2 Silvia Vannuccini
Silvia Vannuccini Serena Ottanelli
Serena Ottanelli Felice Petraglia
Felice Petraglia