- Section on Endocrinology and Genetics, Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Institutes of Health (NIH), Bethesda, MD, United States
The genetic alterations that cause the development of glucocorticoid and/or mineralocorticoid producing benign adrenocortical tumors and hyperplasias have largely been elucidated over the past two decades through advances in genomics. In benign aldosterone-producing adrenocortical tumors and hyperplasias, alteration of intracellular calcium signaling has been found to be significant in aldosterone hypersecretion, with causative defects including those in KCNJ5, ATP1A1, ATP2B3, CACNA1D, CACNA1H, and CLCN2. In benign cortisol-producing adrenocortical tumors and hyperplasias abnormal cyclic adenosine monophosphate-protein kinase A signaling has been found to play a central role in tumorigenesis, with pathogenic variants in GNAS, PRKAR1A, PRKACA, PRKACB, PDE11A, and PDE8B being implicated. The role of this signaling pathway in the development of Cushing’s syndrome and adrenocortical tumors was initially discovered through the study of the underlying genetic defects causing the rare multiple endocrine neoplasia syndromes McCune-Albright syndrome and Carney complex with subsequent identification of defects in genes affecting the cyclic adenosine monophosphate-protein kinase A pathway in sporadic tumors. Additionally, germline pathogenic variants in ARMC5, a putative tumor suppressor, were found to be a cause of cortisol-producing primary bilateral macronodular adrenal hyperplasia. This review describes the genetic causes of benign cortisol- and aldosterone-producing adrenocortical tumors.
Introduction
The discovery of the genetic drivers of adrenocortical tumorigenesis has been facilitated by advances in genomics over recent years and has provided new insights on the molecular pathogenesis of adrenocortical disease. The identification of disease-causing germline and somatic pathogenic variants in primary aldosteronism (PA) and Cushing’s syndrome (CS) is ushering in a new era of “precision-medicine,” though the challenge in future years is to translate these discoveries into new diagnostic and therapeutic modalities. Furthermore, these discoveries have allowed for a more specific classification of adrenocortical hyperplasias, that goes beyond pathology and is gene-based, allowing for more patient-specific genetic screening and counseling. In PA, these discoveries include the identification of the crucial role of aberrant intracellular calcium signaling in aldosterone hypersecretion, with defects in genes that encode ion channels, including KCNJ5, CLCN2, CACNA1H and CACNA1D, and ATPases, such as ATP1A1 and ATP2B3, being implicated in this pathogenesis. In CS abnormal cyclic adenosine monophosphate (cAMP)-protein kinase A (PKA) signaling has been implicated in the development of most benign cortisol-producing adrenocortical tumors and hyperplasias (1–4). Aberrant cAMP-PKA signaling was first associated with the development CS due to primary bimorphic adrenocortical disease (PBAD) in the rare tumor disorder McCune-Albright syndrome (MAS), which is caused by early embryonic postzygotic somatic activating defects in GNAS, the gene that encodes the α-subunit of the stimulatory G protein (Gsα) (2, 4). This pathway was further implicated in the development of CS due to primary pigmented nodular adrenocortical disease (PPNAD) through the study of another rare familial tumor syndrome, Carney complex (CNC), that is predominantly due to germline inactivating defects in PRKAR1A, which encodes the regulatory subunit type 1α (R1α) of PKA (3). Subsequently aberrant cAMP-PKA signaling was identified as a significant cause of cortisol-producing adrenocortical adenomas (CPAs) through activating somatic defects in PRKACA, the gene that encodes the catalytic subunit Cα of PKA (1). In bilateral macronodular adrenal hyperplasia (PBMAH), another form of adrenocortical hyperplasia, germline defects in the tumor suppressor gene ARMC5 were found to be the most common underlying genetic defect (5).
Primary adrenocortical tumors (ACTs) are primarily comprised of benign adenomas and/or hyperplasias and less frequently carcinoma. These tumors may be sporadic or familial, unilateral or bilateral, and secreting or non-secreting. In a retrospective population-based cohort study that evaluated the standardized incidence rate of adrenal tumors in all patients with tumors who lived in Olmsted County, Minnesota, USA from 1995 to 2017, the overall mean sex-standardized and age-standardized incidence rates of adrenal tumors diagnosed from 1995 to 2017 was 47 (95% CI 45–50) per 100,000 person-years, with the incidence of adrenal tumors increasing ten times from 1995 to 2017 paralleling the increased use of abdominal imaging (6). The prevalence of adrenal tumors in 2017 was 0.53%. Of the 1287 patients identified as having adrenal tumors, 93.7% had adrenocortical adenoma and nodular hyperplasia, 8.6% had malignant masses, 6.6% had other benign masses, and 1.1% had pheochromocytoma, with 4.1% having overt adrenocortical hormone excess. This review focuses on the reported causative genomic alterations in benign cortisol- and/or aldosterone-producing ACTs.
Aldosterone-Producing Adrenocortical Tumors
PA is the most common cause of secondary hypertension, and is responsible for approximately 8% of cases. It is characterized by aldosterone secretion that is relatively autonomous of the major regulators of secretion and inappropriately high for sodium status, and is not suppressed by sodium loading. This results in sodium retention, suppression of plasma renin, hypertension, and increased potassium excretion (7–9). Bilateral adrenocortical hyperplasia (BAH) and aldosterone-producing adenomas (APAs) account for 65% and 35% of cases of PA, respectively, with less common causes including unilateral hyperplasia (2%), pure aldosterone-producing ACC (<1%), familial hyperaldosteronism (FH) (FH type 1 <1%), and ectopic aldosterone-producing adenoma or carcinoma (<0.1%) (9). Intravascular volume depletion and elevation in plasma potassium are the main stimuli for aldosterone synthesis. Intravascular volume depletion causes activation of the renin-angiotensin system with release of angiotensin II (AT-II) which binds to a G-protein coupled receptor (GPCR) on the adrenocortical zona glomerulosa cells, while increased potassium directly raises the production of aldosterone in adrenocortical zona glomerulosa cells, whose resting potential is set by potassium channel activity (10). These physiologic stimuli exert their effects through the generation of a cytoplasmic calcium signal through membrane depolarization with activation of voltage-gated calcium channels and increased intracellular calcium. This leads to increased expression of aldosterone synthase (CYP11B2), increased aldosterone production, and glomerulosa cell proliferation. Aldosterone acts on the mineralocorticoid receptor in the renal distal convoluted tubule, connecting tubule, and cortical collecting duct, amongst other tissues, with resulting increased renal sodium reabsorption and potassium excretion.
Familial Hyperaldosteronism
FH is inherited in an autosomal dominant manner. Four major types of FH, type I through IV, have been described, with FH comprising 1% to 5% of PA cases. FH type I (FH-I) or glucocorticoid remediable hyperaldosteronism (GRA) is the result of aldosterone overproduction due to ACTH-dependent activation of aldosterone synthase and was initially described in 1966 in a single family, with identification of the causative chimeric gene 26 years later (11, 12). GRA is the result of the formation of a chimeric gene from unequal crossing over between 2 highly homologous genes that encode isozymes of 11-beta-hydroxylase on chromosome 8: CYP11B1, which encodes 11β-hydroxylase (catalyzes conversion of 11-deoxycortisol to cortisol), and CYP11B2, which encodes aldosterone synthase (converts deoxycorticosterone to corticosterone and 18-hydroxycorticosterone to aldosterone). The fusion of the promoter region of CYP11B1 with CYP11B2, leads to ectopic expression of CYP11B2 in the zona fasciculata with ACTH-dependent activation of the aldosterone synthase. This condition can be treated with intermediate-acting glucocorticoids administered at bedtime at the smallest effective dose, with or without mineralocorticoid antagonist therapy (13). Glucocorticoids diminish ACTH release and can reverse the hypersecretion of aldosterone. FH type II (FH-II) was first described in 1992 as familial PA due to APA and/or BAH without response to glucocorticoid administration and did not have a known genetic etiology until recently. Initially, in the year 2000, a locus for FH-II was identified on the short arm of chromosome 7 corresponding to the band 7p22 (14). Subsequently it was discovered that gain-of-function defects in the CLCN2 gene, which encodes the chloride channel ClC-2, were the causative pathogenic variants in a subset of patients (15). These CLCN2 defects cause voltage-gated calcium influx due to increased chloride permeability and depolarization (15, 16). A study that included a family with FH-II and 80 additional probands with unsolved early-onset PA initially identified defects in CLCN2 as the cause of FH-II, with eight probands carrying heterozygous variants in CLCN2, including two de novo defects and all relatives with early-onset PA harboring the CLCN2 variant found in the probands (16). FH type III (FH-III) is the result of germline defects in the KCNJ5 gene, and was first described in 2008 in a family presenting with a novel form of glucocorticoid-refractory PA (17). KCNJ5 encodes GIRK4 (G-protein–activated inward rectifier potassium channel 4), an inwardly rectifying potassium channel. Defects in this gene result in altered channel selectivity that causes increased sodium conductance and cell depolarization, ultimately leading to increased intracellular calcium and calcium signaling (17). A case of early-onset PA with BAH caused by mosaicism for a KCNJ5 defect was also recently described (18). Somatic defects in KCNJ5 are the most common genetic defect associated with APAs, with APA-causing somatic pathogenic KCNJ5 variants leading to a more severe phenotype when found in the germline, as opposed to KCNJ5 defects identified only in the germline, which tend to lead to a milder phenotype, though there are some exceptions. FH type IV is the result of germline defects in the CACNA1H gene and was first described in 2015 (19). CACNA1H encodes a T-type calcium channel, with pathogenic variants in this gene causing increased intracellular calcium through impaired channel inactivation and activation at more hyperpolarized potentials (19). Germline CACNA1H defects were initially identified in five unrelated individuals with early-onset PA with family analysis being suggestive of incomplete penetrance and showing de novo occurrence in two kindreds. Germline defects in CACNA1D, which encodes an L-type calcium channel, cause early-onset PA, seizures, and neurologic abnormalities, referred to as PA with seizures and neurological abnormalities or PASNA syndrome (20). Mutant channels are activated at less depolarized membrane potentials and show impaired inactivation, with resulting increased calcium influx. Due to the severity of the associated disease, these variants occur exclusively de novo and are not inherited (20). Germline ARMC5 pathogenic variants have also been associated with PA and germline variants in the phosphodiesterase 2A (PDE2A) and 3B (PDE3B) genes, were recently associated with PA caused by BAH, however these genetic defects have not yet been designated as causes of FH (21, 22). In one study that included 56 subjects with PA, some of whom had BAH, six subjects (10.7%) harbored a germline ARMC5 variant that was predicted to be pathogenic by in silico analysis, with all six of these subjects being African American (22). However, this was not confirmed in a subsequent study of 39 primarily Caucasian patients (37 Caucasian and 2 African American subjects) with PA and BAH, where no germline pathogenic ARMC5 variants were identified (23).
Aldosterone-Producing Adrenocortical Adenomas
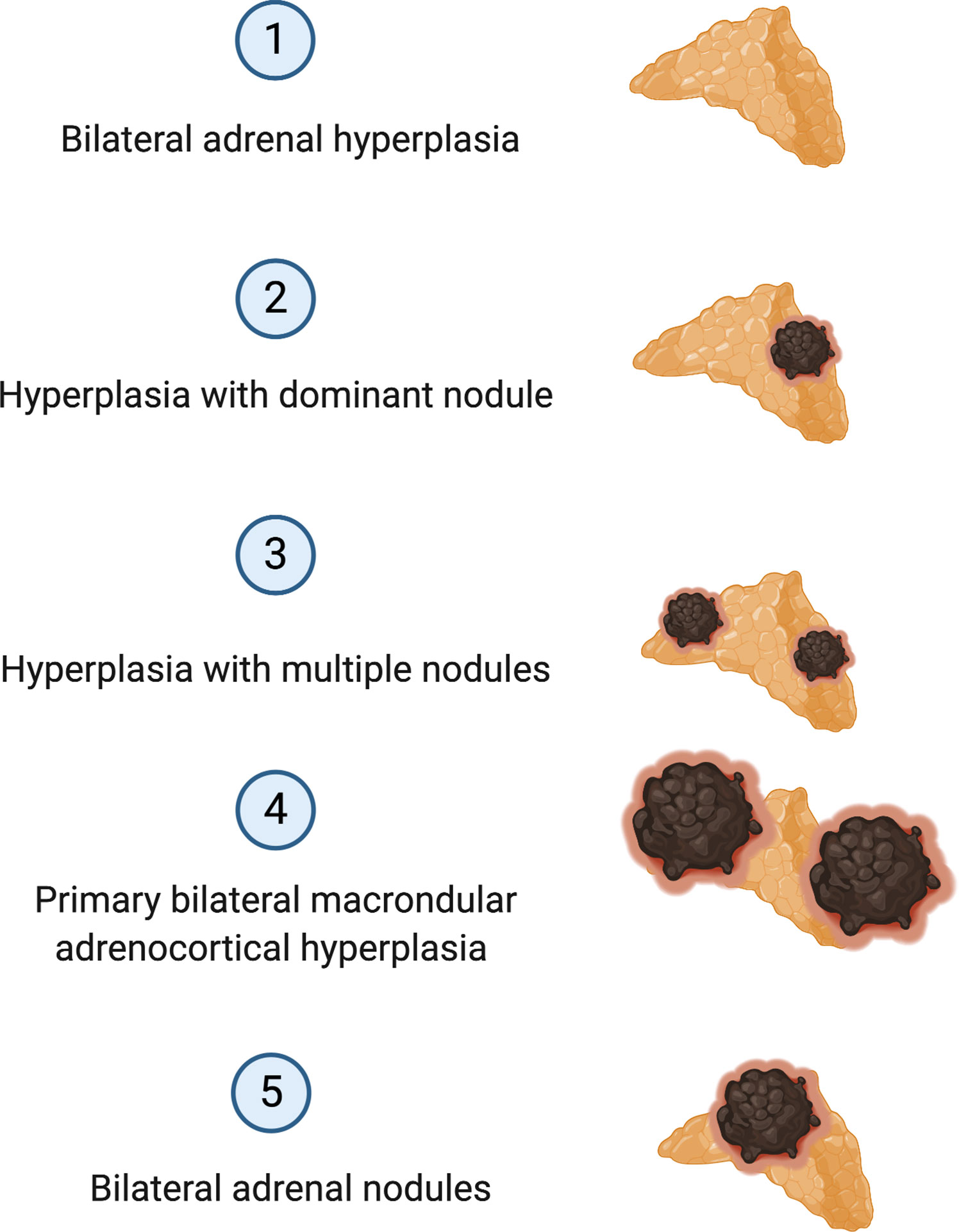
Approximately 90% of APAs are caused by somatic pathogenic variants in genes encoding ion channels or transporters, such as those in KCNJ5, CACNA1D, ATP1A1 and ATP2B3 (20, 24–26) (Figure 1). Somatic variants in KCNJ5 are associated with 40% of APAs, with two hotspots, p.G151R and p.L168R, in and near the selectivity filter of KCNJ5 being responsible for the majority of KCNJ5 defects in APAs. Defects in KCNJ5 lead to more severe PA at a younger age, with larger APAs, and are more common in females than in males (53–63% vs. 22–31%), with a higher frequency in some Asian cohorts (60–70% of APAs) compared to European cohorts (26–31) (Figure 1). Somatic defects in CACNA1D account for 21% to 42% of APAs, making this the second most common defect in these tumors (32). In contrast to patients of European and Asian decent where somatic KCNJ5 defects are the most prevalent APA-causing genetic alterations, in Blacks, somatic CACNA1D defects were found to be the most prevalent genetic defect in APAs (Figure 1) (29–31, 33). Defects in CACNA1D were more frequent in APAs from Black males as opposed to Black females, who unlike males still have a high rate of KCNJ5 pathogenic variants (33). Forty-two percent of APAs in Blacks harbored CACNA1D defects, followed by KCNJ5 in 34%, ATP1A1 in 8%, and ATP2B3 in 4% (33). Three to 17% of APAs result from gain of function somatic pathogenic variants in the ATPase genes ATP1A1 and ATP2B3 (25, 32). Defects in these genes result in abnormal Na+ or H+ permeability and increased aldosterone production. The wingless-type (Wnt)–β-catenin pathway has also been associated with APA formation, with 2% to 5% of APAs harboring activating somatic pathogenic variants in the β-catenin gene CTNNB1 and a significant proportion of APAs demonstrating constitutive activation of the Wnt-β-catenin pathway (20, 34–37). The cytoplasmic protein β-catenin is the main player in this pathway. Its stability is regulated by the Axin complex which is comprised of the scaffolding protein Axin, the tumor suppressor adenomatous polyposis coli (APC) gene product, casein kinase 1 (CK1) and glycogen synthase kinase 3 (GSK3). When Wnt is absent, β-catenin is continually degraded by the action of the Axin complex. This does not allow β-catenin to translocate to the nucleus, with resulting repression of Wnt target genes by the DNA-bound T cell factor/lymphoid enhancer factor (TCF/LEF) family of proteins. Binding of Wnt ligands to a receptor complex including a member of the frizzled family of seven-transmembrane receptors and a member of the LDL receptor family (LRP 5 or 6) activates the Wnt-β-catenin pathway, with subsequent inhibition of the Axin complex, and stabilization and accumulation of β-catenin. Beta-catenin then translocates to the nucleus where it activates TCF/LEF transcription factors thereby activating Wnt target gene expression (38). Two women with APAs that presented in pregnancy were also found to have somatic pathogenic variants in CTNNB1 and had significantly upregulated adrenocortical expression of the LH/hCG receptor and gonadotropin releasing hormone (GnRH) receptor (39). However, a subsequent study that genotyped subjects with PA and evaluated in vivo for GnRH/LH responsive aldosterone secretion, found that aberrant aldosterone regulation occurred frequently in PA, but was not often associated with CTNNB1 pathogenic variants (40). Recently somatic CACNA1H pathogenic variants were identified as a cause of APAs, however the prevalence of these pathogenic variants in APAs is low (41). Somatic pathogenic variants in CLCN2 were also recently described in APAs with a prevalence of 1.74% in one small study (42). The histologic classification of PA is a spectrum, ranging from BAH to PBMAH, as shown in Figure 2 (43).
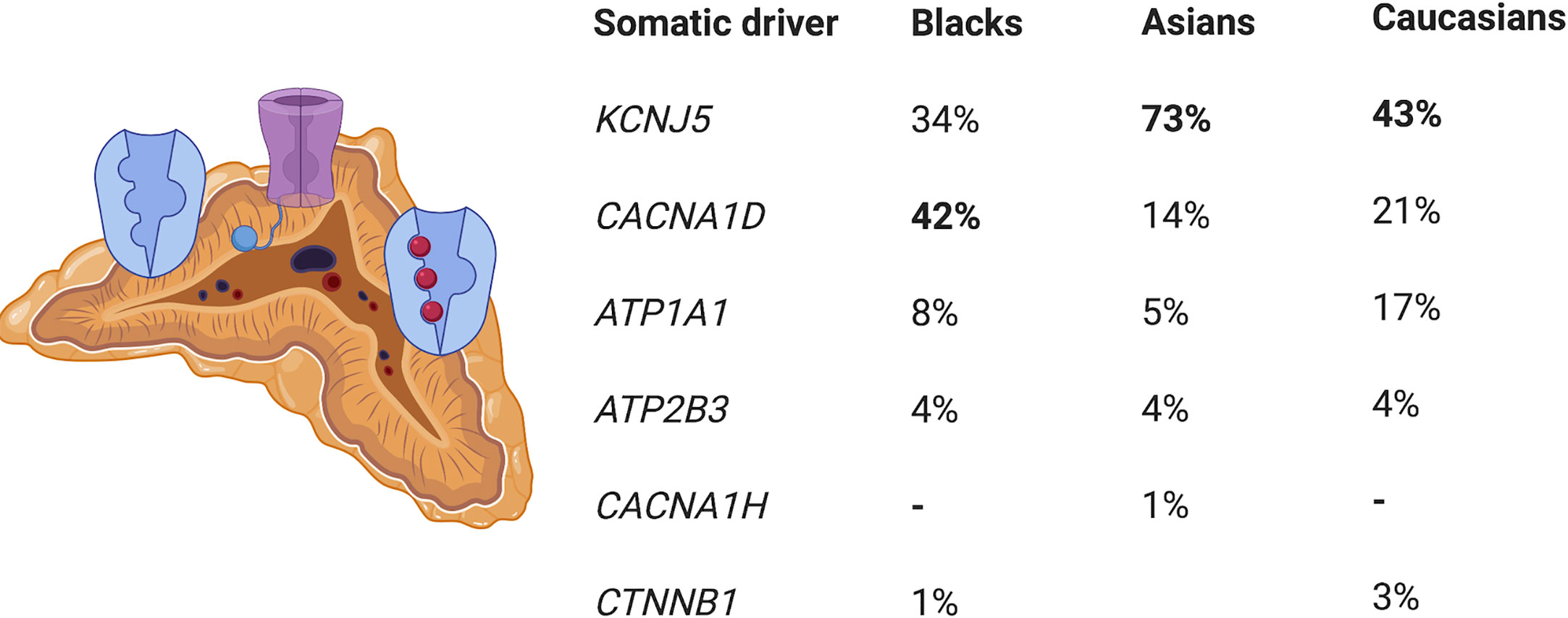
Figure 1 Prevalence of somatic driver pathogenic variants in aldosterone-producing adenomas. KCNJ5, potassium channel, inwardly rectifying, subfamily J, member 5; CACNA1D, calcium channel, voltage dependent, L-type, alpha-1D subunit; ATP1A1 ATPase, NA+/K+ transporting, alpha-1 polypeptide,; ATP2B3, ATPase, Ca(2+)-transporting, plasma membrane, 3; CACNA1H, calcium channel, voltage dependent, T-type, alpha-1H subunit; CTNNBI, catenin, beta-1.
Cortisol-Producing Adrenocortical Tumors
CS has an incidence of two to three cases per one million inhabitants per year, and is comprised of signs and symptoms that develop due to prolonged tissue exposure to excess glucocorticoids, with approximately 20% of cases of endogenous CS being caused by primary adrenocortical hyperfunction (10–22% CPAs, 1–2% adrenocortical hyperplasia which is mostly bilateral, and 5–7% adrenocortical carcinoma [ACC]) (44–46). Specifically, BAH is characterized by multiple adrenocortical nodules and is classified as micronodular or macronodular depending on whether the radiological size of most of the nodules is less than or greater than 1 cm in diameter, respectively. BAH is further subclassified histologically according to the histological presence of pigment (lipofuscin) inside the nodules or in the surrounding adrenal cortex and/or the presence of atrophy or hyperplasia of the internodular cortex. The bilateral nature of the ACTs in BAH suggests an underlying genetic predisposition which has been confirmed in many cases, and a gene-based subclassification was recently proposed (Table 1 and Figure 3) (47, 48).
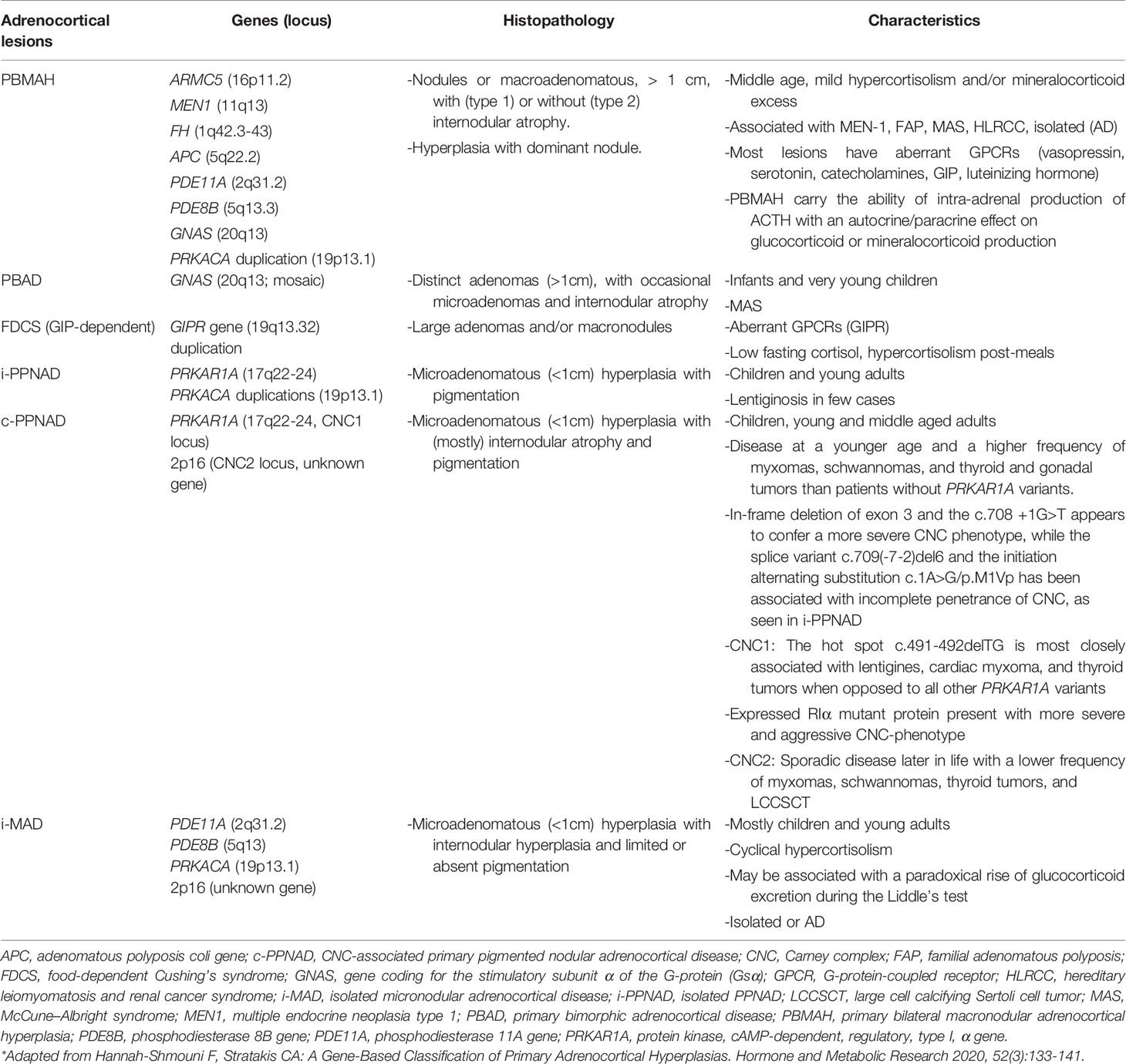
Table 1 Classification and characteristics of primary cortisol-producing adrenocortical hyperplasia.*
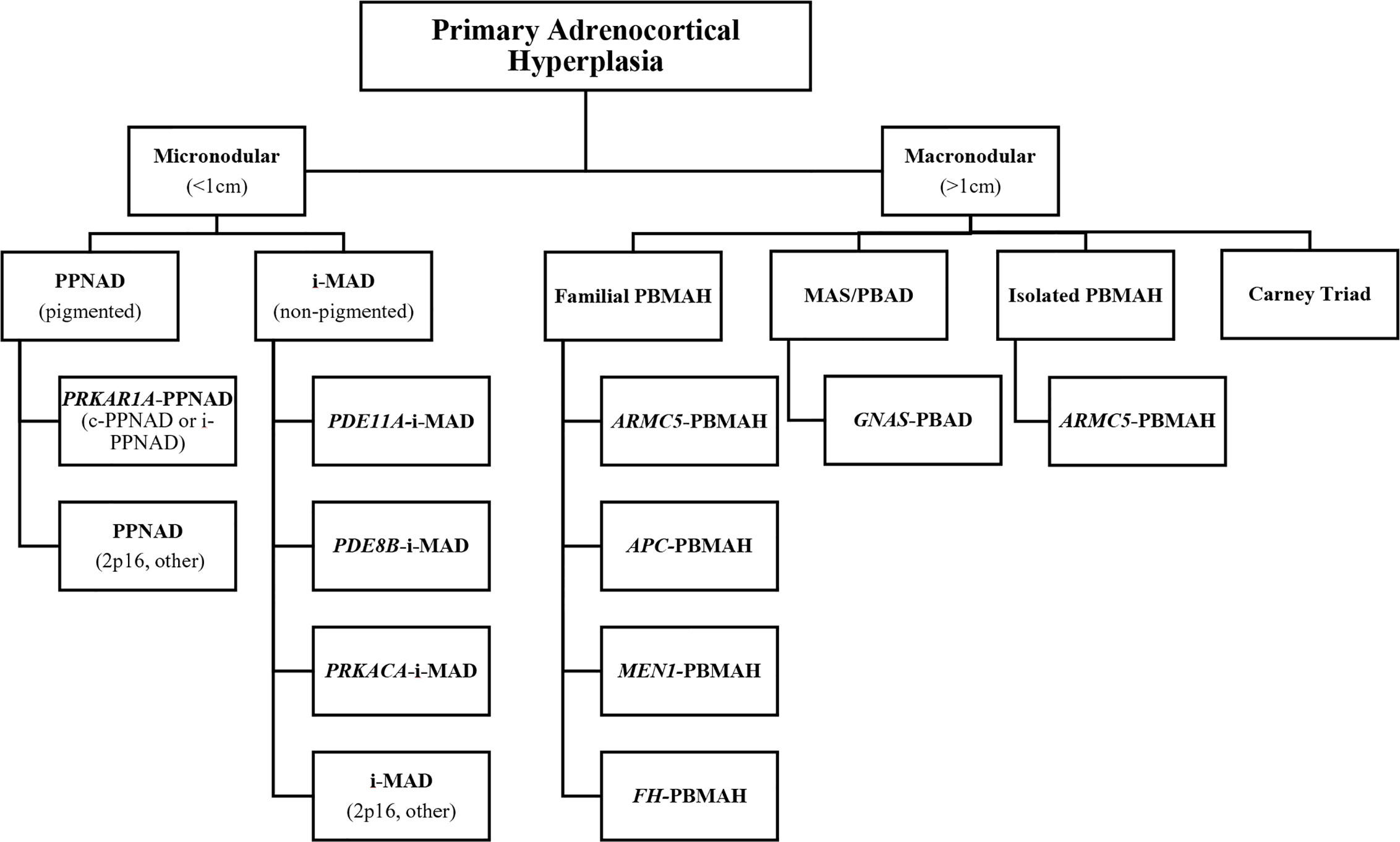
Figure 3 Gene-based diagnostic algorithm for primary cortisol-producing adrenocortical hyperplasias. *APC, adenomatous polyposis coli gene; ARMC5, armadillo repeat-containing protein 5; c-PPNAD, CNC-associated primary pigmented nodular adrenocortical disease; CNC, Carney complex; FH, fumarate hydratase; GNAS, gene coding for the stimulatory subunit α of the G-protein (Gsα); i-MAD, isolated micronodular adrenocortical disease; i-PPNAD, isolated PPNAD; MAS, McCune–Albright syndrome; MEN1, multiple endocrine neoplasia type 1; PBAD, primary bimorphic adrenocortical disease; PBMAH, primary bilateral macronodular adrenocortical hyperplasia; PDE8B, phosphodiesterase 8B gene; PDE11A, phosphodiesterase 11A gene PPNAD, primary pigmented nodular adrenocortical disease; PRKACA, protein kinase, cAMP-dependent, catalytic, alpha; PRKAR1A, protein kinase, cAMP-dependent, regulatory, type I, α gene. *Adapted from Kamilaris CDC, Stratakis CA, Hannah-Shmouni F: Adrenocortical tumorigenesis: Lessons from genetics. Best Practice & Research Clinical Endocrinology & Metabolism 2020,34(3):101428.
The cAMP-PKA pathway is central to the regulation of adrenocortical cell development, proliferation, and function, with aberrant cAMP-PKA signaling playing a significant role in the development of the majority of benign cortisol-producing ACTs. The role of abnormal cAMP-PKA signaling in adrenocortical tumorigenesis was first described in 1991 when early embryonic postzygotic somatic activating defects of the GNAS gene were implicated in the pathogenesis McCune-Albright syndrome (MAS). MAS manifests as café-au-lait skin macules, skeletal fibrous dysplasia, and multiple endocrinopathies including precocious puberty, testicular and thyroid lesions, phosphate wasting, growth hormone excess, and, rarely, neonatal hypercortisolism primarily due to bilateral adrenocortical hyperplasia. This form of BAH develops from adrenocortical cells with fetal features and is termed PBAD (2, 49). GNAS encodes the α-subunit of the stimulatory G protein (Gsα). Mosaic gain-of-function pathogenic variants in GNAS cause constitutive activation of the cAMP-PKA pathway (4).
PKA plays a role in the control of a variety of cellular processes including metabolism, transcription, cell cycle progression, and apoptosis. It is a ubiquitous cAMP-dependent serine-threonine kinase, with four isoforms for both its regulatory subunits (R1α, R1β, R2α, and R2β) and catalytic subunits (Cα, Cβ, Cγ, and PRKX), with each isoform having individual localization and specificity. The cAMP-PKA pathway is activated in the adrenocortical cell by the binding of ACTH to the ACTH receptor, a G-protein coupled receptor (GPCR) encoded by the MC2R gene. This leads to exchange of guanosine triphosphate (GTP) for guanosine diphosphate (GDP) on the α subunit of the associated heterotrimeric G protein and dissociation of the α subunit (encoded by GNAS) from the βγ dimer. The α subunit then binds to adenylyl cyclase (AC) with activation of its enzymatic activity and subsequent production of cAMP from adenosine triphosphate (ATP). cAMP then binds to the regulatory subunits (R) of PKA, which allows for the release of the catalytic subunits (C) of PKA. The catalytic subunits of PKA then mediate serine-threonine phosphorylation of target molecules, including the transcription factor CREB (cAMP response element-binding protein). Phosphodiesterases (PDEs) are the only known enzymes that degrade cyclic nucleotides and regulate the cAMP-PKA pathway through the regulation of cAMP levels (50) (Figure 4).
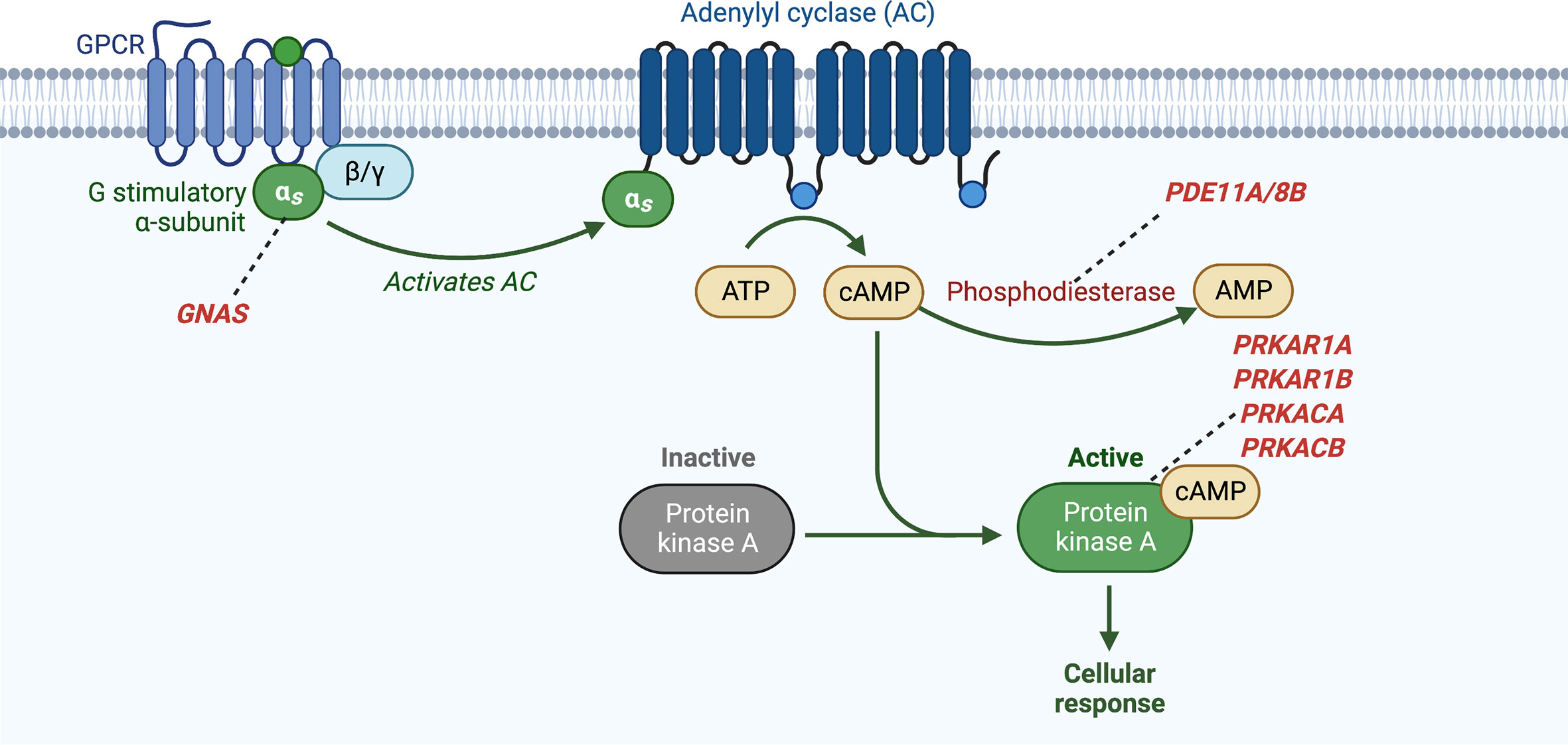
Figure 4 Activation of cyclic AMP pathway through various genetic defects in adrenocortical tumors and hyperplasias. GNAS, gene coding for the stimulatory subunit α of the G-protein (Gsα); PDE8B, phosphodiesterase 8B gene; PDE11A, phosphodiesterase 11A gene; PRKACA, protein kinase, cAMP-dependent, catalytic, alpha; PRKAR1A, protein kinase, cAMP-dependent, regulatory, type I, α gene.
Micronodoular Adrenocortical Hyperplasia
Micronodular BAH accounts for <2% of cases of endogenous CS and may be familial, and inherited in an autosomal dominant manner, or sporadic. It can be divided histologically into at least two subclasses: PPNAD and isolated micronodular adrenocortical disease (i-MAD). PPNAD is characterized by pigmented adrenocortical nodules with atrophic surrounding adrenocortical tissue and is more common than and more frequently familial than i-MAD, whereas i-MAD has limited or absent nodular pigment with hyperplasia in the surrounding zona fasciculata (51). Ninety percent of cases of PPNAD are associated with Carney complex (CNC) (c-PPNAD) though PPNAD may be isolated (i-PPNAD) (52, 53).
CNC is a rare, autosomal dominant, multiple endocrine neoplasia and lentiginosis syndrome comprised of abnormal cutaneous and mucosal pigmentation, myxomas predominantly of the heart, skin, and breast, psammomatous melanotic schwannomas, breast ductal adenomas, osteochondromyxomas, endocrine neoplasms, and other non-endocrine tumors. C-PPNAD is diagnosed in 25% to 60% of patients with CNC and is the most prevalent endocrine neoplasm in this syndrome, identified in almost all patients on autopsy. Pituitary adenomas or hyperplasia, thyroid tumors, and gonadal tumors make up the remaining endocrine tumors associated with CNC (54–56). CNC may be familial in up to 70% of cases and is predominantly caused by inactivating defects PRKAR1A (17q24.2–24.3 locus [CNC1 locus] of the long arm of chromosome 17), which encodes the regulatory subunit type 1α (R1α) of PKA. CNC was the first disease found to be the result of a genetic defect in a gene encoding a component of the PKA enzyme, with inactivating PRKAR1A defects causing loss of regulation of the catalytic subunits of PKA and constitutive activation of the cAMP-PKA pathway. Thirty-seven percent of patients with sporadic CNC and more than 70% of patients with familial CNC carry PRKAR1A pathogenic variants, with almost 100% penetrance (3, 52, 57). Data initially suggested that PRKAR1A functions as a “classic” tumor-suppressor gene, with loss of heterozygosity at the PRKAR1A locus in tumor tissue, however some data show that haploinsufficiency of PRKAR1A may be adequate for increased PKA activity and the early development of certain tumors (58, 59). Most pathogenic PRKAR1A variants lead to PRKAR1A haploinsufficiency due to mRNA nonsense-mediated decay of the mutant sequence, that leads to predicted absence of the mutant protein products in affected cells (52, 60). A second affected locus on chromosome 2p16 (CNC2 locus) was identified by genetic linkage analysis of tumors in most of the remaining patients with CNC that do not carry a germline PRKAR1A defect, though the responsible gene at this locus has not yet been identified (61, 62). In addition, copy number gains of the PRKACB gene locus, on chromosome 1, which encodes the catalytic subunit β (Cβ) of PKA, were identified in a patient with CNC presenting with abnormal skin pigmentation, myxomas, and acromegaly, though defects in this gene have not been associated with c-PPNAD (63). Possibly pathogenic germline PRKACB variants were also recently reported in two of 148 patients with PPNAD and related disorders that did not have other PKA-related defects. The first subject with the PRKACB gene variant (c.858_860GAA [p.K286del]) presented with short stature, multiple skeletal developmental malformations, and severe developmental delay suggesting a role for PRKACB defects in bone pathology, with functional studies demonstrating that this variant affected PRKACB protein stability and led to increased PKA signaling. The other subject carried the c.899C>T (p.T300M or p.T347M in another isoform) PRKACB variant and presented with a PPNAD-like phenotype without any other manifestations of CNC though functional studies demonstrated that this variant did not affect protein stability or response to cAMP and its pathogenicity remains uncertain (64). PRKAR1A defects have also been implicated in i-PPNAD, where there is a genotype-phenotype correlation. A study that included 353 subjects with germline PRKAR1A pathogenic variants or a diagnosis of CNC and/or PPNAD showed that among subjects with i-PPNAD and PRKAR1A defects the germline c.709–7del6 defect was more frequent whereas the remainder of these patients carried the p.Met1Val defect, and subjects less than 8 years of age rarely had PRKAR1A defects (52). Somatic defects in PRKAR1A have also been described in cortisol-producing ACTs, though less frequently, including CPAs and ACCs (65). ACC was also described in two patients with c-PPNAD due to germline PRKAR1A pathogenic variants (65, 66). ACTH-dependent CS due to an ACTH- producing pituitary adenoma has rarely been described in CNC (67, 68). Genetic alterations in PRKAR1B, which encodes the regulatory subunit 1β of PKA have been described in ACTs, with germline PRKAR1B variants identified in i-MAD and somatic PRKAR1B copy number gains (CNGs) found in CPAs. However, the contribution of genetic alterations in PRKAR1B to adrenocortical tumorigenesis may be different than those from defects affecting other subunits of PKA, as PRKAR1B variants and PRKAR1B CNGs led to decreased (rather than increased) overall PKA activity in vitro (69).
Genetic alterations in genes encoding cyclic nucleotide PDEs, that lead to aberrant cAMP-PKA signaling, have also been implicated in the development of micronodular BAH and other cortisol-producing ACTs. A single-nucleotide polymorphism-based genome-wide association study that included patients with i-MAD or i-PPNAD not caused by known genetic defects (defects in GNAS or PRKAR1A) showed that abnormalities in genetic loci harboring PDE genes were most likely to be associated with the disease. Inactivating defects PDE11A, which encodes phosphodiesterase type 11A, were the most frequently linked, followed by defects in PDE8B, which encodes phosphodiesterase type 8B (70). A higher frequency of PDE11A variants has also been found in patients with CNC, with PDE11A defects being associated with increased development of PPNAD and/or testicular large-cell calcifying Sertoli cell tumors (LCCSCT) in those with PRKAR1A defects, possibly acting as a genetic modifying factor in these patients (71). Heterozygous germline defects in PDE11A were also found to be more prevalent in patients with other ACTs, including ACC, CPAs, and PBMAH, when compared to age and/or sex-matched controls and were described in one patient with a non-secreting adrenocortical adenoma (72). Inactivating defects in PDE11A have also been implicated in the development of other tumors including prostate cancer and testicular germ cell tumors (71, 73, 74). A single germline PDE8B missense substitution was first described in a pediatric patient with i-MAD and CS. The patient’s father who harbored the same PDE8B defect, was not diagnosed with CS but did have elevated serum midnight cortisol (75). Subsequently in a case-control study of 216 unrelated patients with adrenocortical tumors (including PPNAD, PBMAH, CPAs, non-secreting adrenocortical tumors, and ACC) and 192 controls, nine different PDE8B sequence changes were found in the patients and controls with two variants that were identified only in the patient group demonstrating significant potential to impair protein function in vitro and in silico (76).
Defects in genes encoding the catalytic subunits of PKA that lead to increased PKA activity have also been found to play a role the pathogenesis of micronodular BAH. Germline copy number gains resulting in amplification of PRKACA, the gene that encodes the catalytic subunit (Cα) of PKA, have been implicated in the development of i-MAD. Three patients with sporadic i-MAD and 2 patients with familial PBMAH were the first patients found to harbor such germline copy number gains of the genomic region on chromosome 19p which includes the entire PRKACA gene (1, 77).
Finally, micronodular BAH has also been associated with abnormalities of the Wnt–β-catenin pathway, which may act as a genetic modifier. One study identified somatic defects in CTNNB1 in 11% of patients with PPNAD (a germline PRKAR1A defect was identified in 1 of the 2 patients with somatic CTNNB1 defects). These CTNNB1 defects occurred in relatively large adrenocortical adenomas that developed in the background of PPNAD and were not found in the surrounding hyperplastic adrenocortical tissue (78). In a second study that included tissue from nine subjects with PPNAD (with eight of the nine harboring PRKAR1A defects), including five with macronodules, activating somatic CTNNB1 defects were identified in two of the five macronodules but not in the micronodules or in the contralateral adrenal gland, whereas all PPNAD tissues had β-catenin accumulation including within the macronodules, micronodules, and internodular tissue (79).
Macronodular Adrenocortical Hyperplasia
PBMAH is a rare cause of adrenal CS, accounting for <2% of cases, and is mostly isolated or sporadic, and inherited in an autosomal dominant fashion when hereditary. Adrenal cortisol secretion in this disease was initially considered to be ACTH-independent and thus this form of BAH was formerly called ACTH-independent macronodular adrenal hyperplasia (AIMAH); however, studies have since demonstrated that ACTH secretion from clusters of adrenocortical cells in PBMAH may in part regulate cortisol secretion by paracrine action (80). PBMAH has also previously been termed bilateral macronodular adrenal hyperplasia (BMAH), primary macronodular adrenal hyperplasia (PMAH), massive macronodular adrenocortical disease (MMAD), autonomous macronodular adrenal hyperplasia (AMAH), ACTH-independent massive bilateral adrenal disease (AIMBAD), and “giant” or “huge” macronodular adrenal disease. Histologically, PBMAH can be subclassified into type I PBMAH which is characterized by internodular atrophy, and the more common type II PBMAH, which is diffusely hyperplastic without residual normal or atrophic internodular tissue (81). Adrenocortical cells in PBMAH express aberrant (ectopic or excessive) hormone receptors in 77% to 87% of cases whereas such aberrant receptor expression has been found less frequently in adrenocortical adenomas and ACC (81). These aberrant receptors are members of the GPCR family and are linked to steroidogenesis. Such receptors include those for glucose-dependent insulinotropic peptide (GIP) (implicated in food-dependent CS), vasopressin, β-adrenergic agonists, luteinizing hormone/choriogonadotropin (LH/hCG), serotonin, angiotensin II, and glucagon (82–92). The underlying genetic changes leading to this ectopic receptor expression have not yet fully been described. Food dependent CS is a rare subtype of macronodular BAH associated with ectopic expression of the GIP receptor (GIPR). The molecular pathogenesis of ectopic GIPR expression in this disease was investigated in a study that included adrenal tissue from 15 ACTs including CPAs and macronodular BAH. This study showed that GIPR expression occurred due somatic duplications in chromosome region 19q13.32, that contains the GIPR locus, with resulting transcriptional activation of a single allele of the GIPR gene in three of the ACTs (2 CPAs and 1 macronodular BAH). In the CPAs, the duplicated 19q13.32 region was rearranged with other chromosome regions however these chromosome rearrangements did not result in gene fusion but instead placed the GIPR gene in a genomic environment near cis-acting regulatory regions favoring transcriptional activation. In the macronodular BAH sample, a duplication of 19q without chromosome rearrangement was identified (93).
The bilateral nature PBMAH as well as the cases of familial PBMAH and the association of PBMAH with familial tumor syndromes suggested that this disease could be caused by underlying germline genetic defect(s). In 2013, germline inactivating defects in ARMC5 were linked to this disease when genotyping of 33 patients with PBMAH showed defects in ARMC5 in 55% (18/33) of these patients (5). In subsequent studies, the prevalence of ARMC5 defects in patients with PBMAH has been estimated to be 21% to 26% (94–96) ARMC5 is a tumor suppressor gene which encodes a cytosolic protein without enzymatic activity that has an armadillo repeat domain, similar to the gene for β-catenin that also contains armadillo repeats (97, 98). In this initial study of 33 patients with PBMAH, functional studies showed that inactivation of ARMC5 led to reduced expression of steroidogenic enzymes and MC2R with abnormal cortisol production. A gradual process of adrenocortical cell dedifferentiation and growth of bilateral masses was evident in these patients, with the hypercortisolemia being more likely a result of the increased adrenocortical mass than cortisol overproduction. Enlargement of the adrenal glands may be due to loss of the ability to induce apoptosis in adrenocortical cells with ARMC5 defects, as shown experimentally in human adrenocortical cell lines when compared to wild-type cell lines (5, 94). Studies in Armc5 knockout mice have shown that this gene may play a significant role in in fetal development, T-cell function, and adrenal gland growth homeostasis. Armc5 haploinsufficiency in these mice manifests as CS later in life, with implication of both the cAMP-PKA and the Wnt-β−catenin pathways (97, 99). ARMC5 variants may act as genetic modifiers in PPNAD due to a PRKAR1A defect, and may affect the presence and severity of hypercortisolemia in patients harboring these variants (100). In addition, in 2015 an association between ARMC5 defects and primary aldosteronism was first described (22). ARMC5 defects have also been linked to the development of meningiomas, as shown in one family with meningioma and adrenal hyperplasia with ARMC5 loss of heterozygosity in the meningioma DNA (101).
Rarely, PBMAH is a component of autosomal dominant multiple tumor syndromes including familial adenomatous polyposis (FAP), multiple endocrine neoplasia type 1 (MEN1), or hereditary leiomyomatosis and renal cell carcinoma (HLRCC) (102–104). The causative genetic defects in these familial tumor syndromes were the only genetic defects associated with PBMAH until inactivating defects of ARMC5 were found to cause this disease (5). Germline inactivating defects in the tumor suppressor gene adenomatous polyposis coli (APC), which encodes the APC protein, cause FAP. The APC protein is a component of the β-catenin Axin degradation complex which negatively regulates the Wnt-β-catenin signaling pathway, with biallelic APC inactivation leading to transcriptional activation of the Wnt-signaling pathway. Classic FAP is comprised of multiple colorectal adenomas (>100) that predispose to colorectal cancer, fundic gland polyps, and duodenal adenomas that predispose to duodenal cancer. FAP may also have extraintestinal manifestations such as follicular or papillary thyroid cancer, childhood hepatoblastoma, central nervous system tumors, desmoid tumors, sebaceous or epidermoid cysts, lipomas, osteomas, fibromas, supernumerary teeth, and juvenile nasopharyngeal angiofibromas. FAP has also been associated with ACTs including PBMAH, adrenocortical adenomas, and ACC. In one cohort, 16% of patients had adrenal masses of which 97% were benign and 80% were adenomas, with 23% of the adrenal masses being bilateral. At diagnosis, the median diameter of these adrenal masses was 1.7 cm (interquartile range (IQR) 1.4–3.0) with median maximal diameter of 2.5 cm (IQR 1.7–4.1) (105).
MEN1 is caused by inactivating defects in MEN1, a tumor suppressor gene located at the 11q13 locus (106, 107). MEN1 encodes the protein menin, whose exact role in tumorigenesis is yet to be identified, though it has been implicated in the regulation of transcription, genome stability, cell division, and cell proliferation (106–109). This syndrome leads to a predisposition to a multitude of endocrine neoplasms predominantly of parathyroid, enteropancreatic, and anterior pituitary origin with other endocrine tumors including foregut carcinoid tumors, ACTs, and rarely pheochromocytoma. Nonendocrine tumors associated with MEN1 include meningiomas and ependymomas, lipomas, angiofibromas, collagenomas, and leiomyomas (110). In a retrospective cohort study of 715 patients with MEN1, 20.4% had adrenal enlargement. Adrenal tumors greater than 1 centimeter were described in 58.1% of these cases with bilateral tumors being present in 12.5% of cases. 15.3% of patients had hormonal hypersecretion, which was found only in patients with ACTs. When compared to controls with adrenal incidentalomas, MEN1-related adrenal tumors exhibited more cases of primary aldosteronism and ACC (102).
HLRCC is a syndrome caused by germline defects in fumarate hydratase (FH), a possible tumor suppressor gene (111). FH encodes fumarate hydratase, an enzyme that is a component of the mitochondrial Krebs or tricarboxylic acid cycle. Defects in FH may lead to HLRCC through increased cellular dependence on glycolysis and pseudohypoxia, though the molecular pathogenesis of HLRCC has not been completely elucidated (112, 113). Patients with HLRCC develop cutaneous and uterine leiomyomas (rarely leiomyosarcomas) and renal cell carcinoma. Approximately 7.8% of patients with HLRCC develop ACTs including PBMAH and adrenocortical adenomas that may be cortisol-producing or non-functional (104, 114). Germline FH pathogenic variants have also been implicated in the development of pheochromocytomas and paragangliomas (115, 116).
The development of PBMAH has also been linked to genetic defects that lead to aberrant cAMP-PKA signaling. Such defects include inactivating germline PDE11A variants that are found in 24% to 28%, of cases of PBMAH, as well as inactivating germline PDE8B defects, activating somatic GNAS defects without MAS, and PRKACA copy number gains (72, 76, 81, 117). Another subtype of macronodular BAH, PBAD, has been described in patients with MAS and CS (2, 118). PRKAR1A defects have not been identified in PBMAH, however somatic losses of the 17q22–24 region in PBMAH lead to PKA subunit and enzymatic activity changes and altered PKA signaling that is similar to that of other adrenal tumors with PRKAR1A defects or 17q losses (119). A single case of PBMAH caused by two defects in the same allele of MC2R which encodes the ACTH or melanocortin 2 receptor has been described. The presence of both of these defects (p.C21R and p.S247G defects) in the same molecule led to constitutive activity of the receptor, with the co-expression of the normal MC2R allele leading to retention of a normal response to ACTH. These defects ultimately resulted in abnormal activation of the cAMP-PKA pathway and clinical hypersensitivity to ACTH, though either defect alone would have produced an inactive receptor (120). Additionally, the role of the cAMP-PKA pathway in the pathogenesis of PBMAH is demonstrated in cases of PBMAH with aberrant receptor expression as the majority of these receptors are GPCRs that stimulate AC, with resulting increased cAMP-PKA signaling.
Additional possible genetic alterations including somatic defects in DOTIL, which encodes a histone H3 lysine methyltransferase, and HDAC9, which encodes a histone deacetylase, have been reported in a small number of patients with PBMAH. Both of these genes play a role in histone modification, chromatin organization and modification of gene transcription. A defect in another gene, the Endothelin receptor type A (EDNRA) gene, which encodes a G-coupled protein, was found in adrenal tissue from two siblings from a family with familial PBMAH (121), but its contribution to adrenocortical tumor development remains questionable.
Cortisol-Producing Adrenocortical Adenomas
As in BAH, the cAMP-PKA pathway also plays an important role in the development of CPAs. However in contrast to BAH where germline defects leading to aberrant cAMP-PKA signaling are most prominent, somatic genetic alterations affecting this pathway predominate in CPAs (122). In 2013, whole exome sequencing of tumor-tissue specimens from patients with unilateral CPAs identified somatic PRKACA in 8 out of 10 adenomas, with additional sequencing of another 129 adenomas demonstrating a p.Leu206Arg variant in 14 of these 129 adrenocortical adenomas. Defects in PRKACA were associated with a more severe phenotype and were found only in patients with overt CS (1). Another 3 studies from China, Japan, and the United States subsequently showed similar findings, with PRKACA defects found in 42% of patients with CPAs with overt CS (123). Activating PRKACA defects lead to constitutive activation of PKA by abolishing the interaction between the regulatory and catalytic subunits of PKA and may also modify substrate specificity with hyperphosphorylation of certain PKA substrates (124). Recently, an activating somatic defect in PRKACB was also described in a patient with a CPA,with increased sensitivity to cAMP demonstrated in in vitro studies (125). PRKACA defects have also been found in cardiac myxomas, and chromosomal PRKACA rearrangements were identified in fibrolamellar hepatocellular carcinoma and in intraductal oncocytic papillary neoplasms of the pancreas and bile duct, along with PRKACB defects (126–128). Inactivating somatic defects in PRKAR1A as well as activating somatic defects in GNAS have also been described in CPAs with a prevalence of 5%, and 4.5% to 11%, respectively (60, 65). Both PRKAR1A and GNAS defects can cause increased cAMP-PKA signaling, however a whole genome expression profile study revealed that not all cAMP activation is the same. In this study, overexpression of the MAPK and p53 signaling pathways was demonstrated in adrenal lesions with both PRKAR1A or GNAS defects, however PRKAR1A-mutant tissues overexpressed genes related to the Wnt-signaling pathway (CCND1, CTNNB1, LEF1, LRP5, WISP1, and WNT3), whereas GNAS-mutant tissues showed increased expression of genes involved in extracellular matrix receptor interaction and focal adhesion pathways (NFKB, NFKBIA, and TNFRSF1A). CPAs without defects in GNAS, PRKAR1A, PDE11A, or PDE8B were also found to have abnormalities in the cAMP-signaling pathway with variant-negative CPAs having significantly decreased PDE activity (129).
Conclusion
Significant advances have been made in understanding the genomic underpinnings of PA and CS in recent years. The use of genomic tools and next generation sequencing have allowed for the discovery that aberrant intracellular calcium signaling plays an integral role in the development of PA and that abnormalities in the cAMP-PKA pathway and/or ARMC5 are central in the development of benign cortisol-producing tumors. The role of abnormal Wnt-β-catenin signaling in ACT development has also been highlighted. These findings have built a foundation for the discovery of more targeted diagnostic, therapeutic, and prognostic tools that may lead to less invasive diagnostic and therapeutic methods, as well as for the development of future gene-based tumor classifications that will allow improved genetic counseling and screening for familial cases, and better prognosis.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the research project Z01-HD008920 (Principal Investigator: CS) of the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Institutes of Health (NIH), Bethesda, MD, USA.
Conflict of Interest
CS holds patents on the PRKAR1A, PDE11A, and GPR101 genes and/or their function and has received research funding from Pfizer Inc. on the genetics and treatment of abnormalities of growth hormone secretion.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Beuschlein F, Fassnacht M, Assie G, Calebiro D, Stratakis CA, Osswald A, et al. Constitutive activation of PKA catalytic subunit in adrenal Cushing’s syndrome. N Engl J Med (2014) 370(11):1019–28. doi: 10.1056/NEJMoa1310359
2. Carney JA, Young WF, Stratakis CA. Primary bimorphic adrenocortical disease: cause of hypercortisolism in McCune-Albright syndrome. Am J Surg Pathol (2011) 35(9):1311–26. doi: 10.1097/PAS.0b013e31821ec4ce
3. Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, et al. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet (2000) 26(1):89–92. doi: 10.1038/79238
4. Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med (1991) 325(24):1688–95. doi: 10.1056/NEJM199112123252403
5. Assie G, Libe R, Espiard S, Rizk-Rabin M, Guimier A, Luscap W, et al. ARMC5 mutations in macronodular adrenal hyperplasia with Cushing’s syndrome. N Engl J Med (2013) 369(22):2105–14. doi: 10.1056/NEJMoa1304603
6. Ebbehoj A, Li D, Kaur RJ, Zhang C, Singh S, Li T, et al. Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: a population-based cohort study. Lancet Diabetes Endocrinol (2020) 8(11):894–902. doi: 10.1016/S2213-8587(20)30314-4
7. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2016) 101(5):1889–916. doi: 10.1210/jc.2015-4061
8. Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol (2006) 48(11):2293–300. doi: 10.1016/j.jacc.2006.07.059
9. Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf) (2007) 66(5):607–18. doi: 10.1111/j.1365-2265.2007.02775.x
10. Spat A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev (2004) 84(2):489–539. doi: 10.1152/physrev.00030.2003
11. Sutherland DJ, Ruse JL, Laidlaw JC. Hypertension, increased aldosterone secretion and low plasma renin activity relieved by dexamethasone. Can Med Assoc J (1966) 95(22):1109–19.
12. Lifton RP, Dluhy RG, Powers M, Rich GM, Cook S, Ulick S, et al. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature (1992) 355(6357):262–5. doi: 10.1038/355262a0
13. Stowasser M, Bachmann AW, Huggard PR, Rossetti TR, Gordon RD. Treatment of familial hyperaldosteronism type I: only partial suppression of adrenocorticotropin required to correct hypertension. J Clin Endocrinol Metab (2000) 85(9):3313–8. doi: 10.1210/jcem.85.9.6834
14. Lafferty AR, Torpy DJ, Stowasser M, Taymans SE, Lin JP, Huggard P, et al. A novel genetic locus for low renin hypertension: familial hyperaldosteronism type II maps to chromosome 7 (7p22). J Med Genet (2000) 37(11):831–5. doi: 10.1136/jmg.37.11.831
15. Fernandes-Rosa FL, Daniil G, Orozco IJ, Goppner C, El Zein R, Jain V, et al. A gain-of-function mutation in the CLCN2 chloride channel gene causes primary aldosteronism. Nat Genet (2018) 50(3):355–61. doi: 10.1038/s41588-018-0053-8
16. Scholl UI, Stolting G, Schewe J, Thiel A, Tan H, Nelson-Williams C, et al. CLCN2 chloride channel mutations in familial hyperaldosteronism type II. Nat Genet (2018) 50(3):349–54. doi: 10.1038/s41588-018-0048-5
17. Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Sci (N Y NY) (2011) 331(6018):768–72. doi: 10.1126/science.1198785
18. Maria AG, Suzuki M, Berthon A, Kamilaris C, Demidowich A, Lack J, et al. Mosaicism for KCNJ5 Causing Early-Onset Primary Aldosteronism due to Bilateral Adrenocortical Hyperplasia. Am J Hypertens (2020) 33(2):124–30. doi: 10.1093/ajh/hpz172
19. Scholl UI, Stolting G, Nelson-Williams C, Vichot AA, Choi M, Loring E, et al. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. Elife (2015) 4:e06315. doi: 10.7554/eLife.06315
20. Scholl UI, Goh G, Stolting G, de Oliveira RC, Choi M, Overton JD, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet (2013) 45(9):1050–4. doi: 10.1038/ng.2695
21. Rassi-Cruz M, Maria AG, Faucz FR, London E, Vilela LAP, Santana LS, et al. Phosphodiesterase 2A and 3B variants are associated with primary aldosteronism. Endocr Relat Cancer (2021) 28(1):1–13. doi: 10.1530/ERC-20-0384
22. Zilbermint M, Xekouki P, Faucz FR, Berthon A, Gkourogianni A, Schernthaner-Reiter MH, et al. Primary Aldosteronism and ARMC5 Variants. J Clin Endocrinol Metab (2015) 100(6):E900–9. doi: 10.1210/jc.2014-4167
23. Mulatero P, Schiavi F, Williams TA, Monticone S, Barbon G, Opocher G, et al. ARMC5 mutation analysis in patients with primary aldosteronism and bilateral adrenal lesions. J Hum Hypertens (2016) 30(6):374–8. doi: 10.1038/jhh.2015.98
24. Azizan EA, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet (2013) 45(9):1055–60. doi: 10.1038/ng.2716
25. Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet (2013) 45(4):440–4, 444e441-442. doi: 10.1038/ng.2550
26. Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension (2014) 64(2):354–61. doi: 10.1161/HYPERTENSIONAHA.114.03419
27. Akerstrom T, Crona J, Verdugo AD, Starker LF, Cupisti K, Willenberg HS, et al. Comprehensive Re-Sequencing of Adrenal Aldosterone Producing Lesions Reveal Three Somatic Mutations near the KCNJ5 Potassium Channel Selectivity Filter. PloS One (2012) 7(7):e41926. doi: 10.1371/journal.pone.0041926
28. Boulkroun S, Beuschlein F, Rossi GP, Golib-Dzib JF, Fischer E, Amar L, et al. Prevalence, clinical, and molecular correlates of KCNJ5 mutations in primary aldosteronism. Hypertension (2012) 59(3):592–8. doi: 10.1161/HYPERTENSIONAHA.111.186478
29. Hong AR, Kim JH, Song YS, Lee KE, Seo SH, Seong MW, et al. Genetics of Aldosterone-Producing Adenoma in Korean Patients. PloS One (2016) 11(1). doi: 10.1371/journal.pone.0147590
30. Lenzini L, Rossitto G, Maiolino G, Letizia C, Funder JW, Rossi GP. A Meta-Analysis of Somatic KCNJ5 K+ Channel Mutations In 1636 Patients With an Aldosterone-Producing Adenoma. J Clin Endocr Metab (2015) 100(8):E1089–95. doi: 10.1210/jc.2015-2149
31. Wu VC, Huang KH, Peng KY, Tsai YC, Wu CH, Wang SM, et al. Prevalence and clinical correlates of somatic mutation in aldosterone producing adenoma-Taiwanese population. Sci Rep-Uk (2015) 5. doi: 10.1038/srep11396
32. Seidel E, Schewe J, Scholl UI. Genetic causes of primary aldosteronism. Exp Mol Med (2019) 51(11):1–12. doi: 10.1038/s12276-019-0337-9
33. Nanba K, Omata K, Gomez-Sanchez CE, Stratakis CA, Demidowich AP, Suzuki M, et al. Genetic Characteristics of Aldosterone-Producing Adenomas in Blacks. Hypertension (2019) 73(4):885–92. doi: 10.1161/HYPERTENSIONAHA.118.12070
34. Akerstrom T, Maharjan R, Sven Willenberg H, Cupisti K, Ip J, Moser A, et al. Activating mutations in CTNNB1 in aldosterone producing adenomas. Sci Rep (2016) 6:19546. doi: 10.1038/srep19546
35. Berthon A, Drelon C, Ragazzon B, Boulkroun S, Tissier F, Amar L, et al. WNT/beta-catenin signalling is activated in aldosterone-producing adenomas and controls aldosterone production. Hum Mol Genet (2014) 23(4):889–905. doi: 10.1093/hmg/ddt484
36. Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, et al. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin Endocrinol (Oxf) (2015) 83(6):779–89. doi: 10.1111/cen.12873
37. Tadjine M, Lampron A, Ouadi L, Bourdeau I. Frequent mutations of beta-catenin gene in sporadic secreting adrenocortical adenomas. Clin Endocrinol (Oxf) (2008) 68(2):264–70. doi: 10.1111/j.1365-2265.2007.03033.x
38. MacDonald BT, Tamai K, He X. Wnt/beta-Catenin Signaling: Components, Mechanisms, and Diseases. Dev Cell (2009) 17(1):9–26. doi: 10.1016/j.devcel.2009.06.016
39. Teo AE, Garg S, Shaikh LH, Zhou J, Karet Frankl FE, Gurnell M, et al. Pregnancy, Primary Aldosteronism, and Adrenal CTNNB1 Mutations. N Engl J Med (2015) 373(15):1429–36. doi: 10.1056/NEJMoa1504869
40. Gagnon N, Caceres-Gorriti KY, Corbeil G, El Ghoyareb N, Ludwig N, Latour M, et al. Genetic Characterization of GnRH/LH-Responsive Primary Aldosteronism. J Clin Endocrinol Metab (2018) 103(8):2926–35. doi: 10.1210/jc.2018-00087
41. Nanba K, Blinder AR, Rege J, Hattangady NG, Else T, Liu CJ, et al. Somatic CACNA1H Mutation As a Cause of Aldosterone-Producing Adenoma. Hypertension (2020) 75(3):645–9. doi: 10.1161/HYPERTENSIONAHA.119.14349
42. Rege J, Nanba K, Blinder AR, Plaska S, Udager AM, Vats P, et al. Identification of Somatic Mutations in CLCN2 in Aldosterone-Producing Adenomas. J Endocr Soc (2020) 4(10):bvaa123. doi: 10.1210/jendso/bvaa123
43. Williams TA, Gomez-Sanchez CE, Rainey WE, Giordano TJ, Lam AK, Marker A, et al. International histopathology consensus for unilateral primary aldosteronism. J Clin Endocrinol Metab (2021) 106(1):42–54. doi: 10.1210/clinem/dgaa484
44. Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet (2015) 386(9996):913–27. doi: 10.1016/S0140-6736(14)61375-1
45. Etxabe J, Vazquez JA. Morbidity and mortality in Cushing’s disease: an epidemiological approach. Clin Endocrinol (Oxf) (1994) 40(4):479–84. doi: 10.1111/j.1365-2265.1994.tb02486.x
46. Lindholm J, Juul S, Jorgensen JO, Astrup J, Bjerre P, Feldt-Rasmussen U, et al. Kristensen L et al: Incidence and late prognosis of cushing’s syndrome: a population-based study. J Clin Endocrinol Metab (2001) 86(1):117–23. doi: 10.1210/jcem.86.1.7093
47. Hannah-Shmouni F, Stratakis CA. A Gene-Based Classification of Primary Adrenocortical Hyperplasias. Horm Metab Res (2020) 52(3):133–41. doi: 10.1055/a-1107-2972
48. Stratakis CA, Boikos SA. Genetics of adrenal tumors associated with Cushing’s syndrome: a new classification for bilateral adrenocortical hyperplasias. Nat Clin Pract Endocrinol Metab (2007) 3(11):748–57. doi: 10.1038/ncpendmet0648
49. Boyce AM, Florenzano P, de Castro LF, Collins MT. Fibrous Dysplasia/McCune-Albright Syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, editors. GeneReview® [Internet]. Seattle (WA): University of Washington (1993).
50. Taylor SS, Ilouz R, Zhang P, Kornev AP. Assembly of allosteric macromolecular switches: lessons from PKA. Nat Rev Mol Cell Biol (2012) 13(10):646–58. doi: 10.1038/nrm3432
51. Tirosh A, Valdes N, Stratakis CA. Genetics of micronodular adrenal hyperplasia and Carney complex. Presse Med (2018) 47(7-8 Pt 2):e127–37. doi: 10.1016/j.lpm.2018.07.005
52. Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, et al. Mutations in regulatory subunit type 1A of cyclic adenosine 5’-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab (2009) 94(6):2085–91. doi: 10.1210/jc.2008-2333
53. Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Med (Baltimore) (1985) 64(4):270–83. doi: 10.1097/00005792-198507000-00007
55. Kamilaris CDC, Faucz FR, Voutetakis A, Stratakis CA. Carneya Complex. Exp Clin Endocrinol Diabetes (2019) 127(2-03):156–64. doi: 10.1055/a-0753-4943
56. Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab (2001) 86(9):4041–6. doi: 10.1210/jcem.86.9.7903
57. Cazabat L, Ragazzon B, Groussin L, Bertherat J. PRKAR1A mutations in primary pigmented nodular adrenocortical disease. Pituitary (2006) 9(3):211–9. doi: 10.1007/s11102-006-0266-1
58. Tsilou ET, Chan CC, Sandrini F, Rubin BI, Shen DF, Carney JA, et al. Eyelid myxoma in Carney complex without PRKAR1A allelic loss. Am J Med Genet A (2004) 130A(4):395–7. doi: 10.1002/ajmg.a.30279
59. Robinson-White A, Hundley TR, Shiferaw M, Bertherat J, Sandrini F, Stratakis CA. Protein kinase-A activity in PRKAR1A-mutant cells, and regulation of mitogen-activated protein kinases ERK1/2. Hum Mol Genet (2003) 12(13):1475–84. doi: 10.1093/hmg/ddg160
60. Bonnet-Serrano F, Bertherat J. Genetics of tumors of the adrenal cortex. Endocr Relat Cancer (2018) 25(3):R131–52. doi: 10.1530/ERC-17-0361
61. Matyakhina L, Pack S, Kirschner LS, Pak E, Mannan P, Jaikumar J, et al. Chromosome 2 (2p16) abnormalities in Carney complex tumours. J Med Genet (2003) 40(4):268–77. doi: 10.1136/jmg.40.4.268
62. Stratakis CA, Carney JA, Lin JP, Papanicolaou DA, Karl M, Kastner DL, et al. Carney complex, a familial multiple neoplasia and lentiginosis syndrome. Analysis of 11 kindreds and linkage to the short arm of chromosome 2. J Clin Invest (1996) 97(3):699–705. doi: 10.1172/JCI118467
63. Forlino A, Vetro A, Garavelli L, Ciccone R, London E, Stratakis CA, et al. PRKACB and Carney complex. N Engl J Med (2014) 370(11):1065–7. doi: 10.1056/NEJMc1309730
64. Espiard S, Drougat L, Settas N, Haydar S, Bathon K, London E, et al. PRKACB variants in skeletal disease or adrenocortical hyperplasia: effects on protein kinase A. Endocr Relat Cancer (2020) 27(11):647–56. doi: 10.1530/ERC-20-0309
65. Bertherat J, Groussin L, Sandrini F, Matyakhina L, Bei T, Stergiopoulos S, et al. Molecular and functional analysis of PRKAR1A and its locus (17q22-24) in sporadic adrenocortical tumors: 17q losses, somatic mutations, and protein kinase A expression and activity. Cancer Res (2003) 63(17):5308–19.
66. Bertherat J. Adrenocortical cancer in Carney complex: a paradigm of endocrine tumor progression or an association of genetic predisposing factors? J Clin Endocrinol Metab (2012) 97(2):387–90. doi: 10.1210/jc.2011-3327
67. Hernandez-Ramirez LC, Tatsi C, Lodish MB, Faucz FR, Pankratz N, Chittiboina P, et al. Corticotropinoma as a Component of Carney Complex. J Endocr Soc (2017) 1(7):918–25. doi: 10.1210/js.2017-00231
68. Kiefer FW, Winhofer Y, Iacovazzo D, Korbonits M, Wolfsberger S, Knosp E, et al. PRKAR1A mutation causing pituitary-dependent Cushing disease in a patient with Carney complex. Eur J Endocrinol (2017) 177(2):K7–K12. doi: 10.1530/EJE-17-0227
69. Drougat L, Settas N, Ronchi CL, Bathon K, Calebiro D, Maria AG, et al. Genomic and sequence variants of protein kinase A regulatory subunit type 1beta (PRKAR1B) in patients with adrenocortical disease and Cushing syndrome. Genet Med (2021) 23(1):174–82. doi: 10.1038/s41436-020-01018-4
70. Horvath A, Boikos S, Giatzakis C, Robinson-White A, Groussin L, Griffin KJ, et al. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet (2006) 38(7):794–800. doi: 10.1038/ng1809
71. Libe R, Horvath A, Vezzosi D, Fratticci A, Coste J, Perlemoine K, et al. Frequent phosphodiesterase 11A gene (PDE11A) defects in patients with Carney complex (CNC) caused by PRKAR1A mutations: PDE11A may contribute to adrenal and testicular tumors in CNC as a modifier of the phenotype. J Clin Endocrinol Metab (2011) 96(1):E208–14. doi: 10.1210/jc.2010-1704
72. Libe R, Fratticci A, Coste J, Tissier F, Horvath A, Ragazzon B, et al. Phosphodiesterase 11A (PDE11A) and genetic predisposition to adrenocortical tumors. Clin Cancer Res (2008) 14(12):4016–24. doi: 10.1158/1078-0432.CCR-08-0106
73. Faucz FR, Horvath A, Rothenbuhler A, Almeida MQ, Libe R, Raffin-Sanson ML, et al. Phosphodiesterase 11A (PDE11A) genetic variants may increase susceptibility to prostatic cancer. J Clin Endocrinol Metab (2011) 96(1):E135–40. doi: 10.1210/jc.2010-1655
74. Pathak A, Stewart DR, Faucz FR, Xekouki P, Bass S, Vogt A, et al. Rare inactivating PDE11A variants associated with testicular germ cell tumors. Endocr Relat Cancer (2015) 22(6):909–17. doi: 10.1530/ERC-15-0034
75. Horvath A, Mericq V, Stratakis CA. Mutation in PDE8B, a cyclic AMP-specific phosphodiesterase in adrenal hyperplasia. N Engl J Med (2008) 358(7):750–2. doi: 10.1056/NEJMc0706182
76. Rothenbuhler A, Horvath A, Libe R, Faucz FR, Fratticci A, Raffin Sanson ML, et al. Identification of novel genetic variants in phosphodiesterase 8B (PDE8B), a cAMP-specific phosphodiesterase highly expressed in the adrenal cortex, in a cohort of patients with adrenal tumours. Clin Endocrinol (Oxf) (2012) 77(2):195–9. doi: 10.1111/j.1365-2265.2012.04366.x
77. Lodish MB, Yuan B, Levy I, Braunstein GD, Lyssikatos C, Salpea P, et al. Germline PRKACA amplification causes variable phenotypes that may depend on the extent of the genomic defect: molecular mechanisms and clinical presentations. Eur J Endocrinol (2015) 172(6):803–11. doi: 10.1530/EJE-14-1154
78. Tadjine M, Lampron A, Ouadi L, Horvath A, Stratakis CA, Bourdeau I. Detection of somatic beta-catenin mutations in primary pigmented nodular adrenocortical disease (PPNAD). Clin Endocrinol (Oxf) (2008) 69(3):367–73. doi: 10.1111/j.1365-2265.2008.03273.x
79. Gaujoux S, Tissier F, Groussin L, Libe R, Ragazzon B, Launay P, et al. Wnt/beta-catenin and 3’,5’-cyclic adenosine 5’-monophosphate/protein kinase A signaling pathways alterations and somatic beta-catenin gene mutations in the progression of adrenocortical tumors. J Clin Endocrinol Metab (2008) 93(10):4135–40. doi: 10.1210/jc.2008-0631
80. Louiset E, Duparc C, Young J, Renouf S, Tetsi Nomigni M, Boutelet I, et al. Intraadrenal corticotropin in bilateral macronodular adrenal hyperplasia. N Engl J Med (2013) 369(22):2115–25. doi: 10.1056/NEJMoa1215245
81. Hsiao HP, Kirschner LS, Bourdeau I, Keil MF, Boikos SA, Verma S, et al. Clinical and genetic heterogeneity, overlap with other tumor syndromes, and atypical glucocorticoid hormone secretion in adrenocorticotropin-independent macronodular adrenal hyperplasia compared with other adrenocortical tumors. J Clin Endocrinol Metab (2009) 94(8):2930–7. doi: 10.1210/jc.2009-0516
82. Gagliardi L, Hotu C, Casey G, Braund WJ, Ling KH, Dodd T, et al. Familial vasopressin-sensitive ACTH-independent macronodular adrenal hyperplasia (VPs-AIMAH): clinical studies of three kindreds. Clin Endocrinol (2009) 70(6):883–91. doi: 10.1111/j.1365-2265.2008.03471.x
83. Hofland J, Hofland LJ, van Koetsveld PM, Steenbergen J, de Herder WW, van Eijck CH, et al. ACTH-independent macronodular adrenocortical hyperplasia reveals prevalent aberrant in vivo and in vitro responses to hormonal stimuli and coupling of arginine-vasopressin type 1a receptor to 11 beta-hydroxylase. Orphanet J Rare Dis (2013) 8. doi: 10.1186/1750-1172-8-142
84. Imohl M, Koditz R, Stachon A, Muller KM, Nicolas V, Pfeilschifter J, et al. Catecholamine-dependent hereditary Cushing’s syndrome - Follow-up after unilateral adrenalectomy. Med Klin (2002) 97(12):747–53. doi: 10.1007/s00063-002-1220-2
85. Lacroix A, Bolte E, Tremblay J, Dupre J, Poitras P, Fournier H, et al. Gastric inhibitory polypeptide-dependent cortisol hypersecretion–a new cause of Cushing’s syndrome. N Engl J Med (1992) 327(14):974–80. doi: 10.1056/NEJM199210013271402
86. Lacroix A, Hamet P, Boutin JM. Leuprolide acetate therapy in luteinizing hormone–dependent Cushing’s syndrome. N Engl J Med (1999) 341(21):1577–81. doi: 10.1056/NEJM199911183412104
87. Lee S, Hwang R, Lee J, Rhee Y, Kim DJ, Chung UI, et al. Ectopic expression of vasopressin V1b and V2 receptors in the adrenal glands of familial ACTH-independent macronodular adrenal hyperplasia. Clin Endocrinol (2005) 63(6):625–30. doi: 10.1111/j.1365-2265.2005.02387.x
88. Libe R, Coste J, Guignat L, Tissier F, Lefebvre H, Barrande G, et al. Aberrant cortisol regulations in bilateral macronodular adrenal hyperplasia: a frequent finding in a prospective study of 32 patients with overt or subclinical Cushing’s syndrome. Eur J Endocrinol (2010) 163(1):129–38. doi: 10.1530/EJE-10-0195
89. Mircescu H, Jilwan J, N’Diaye N, Bourdeau I, Tremblay J, Hamet P, et al. Are ectopic or abnormal membrane hormone receptors frequently present in adrenal Cushing’s syndrome? J Clin Endocrinol Metab (2000) 85(10):3531–6. doi: 10.1210/jc.85.10.3531
90. Miyamura N, Taguchi T, Murata Y, Taketa K, Iwashita S, Matsumoto K, et al. Inherited adrenocorticotropin-independent macronodular adrenal hyperplasia with abnormal cortisol secretion by vasopressin and catecholamines - Detection of the aberrant hormone receptors on adrenal gland. Endocrine (2002) 19(3):319–25. doi: 10.1385/ENDO:19:3:319
91. Reznik Y, Allali-Zerah V, Chayvialle JA, Leroyer R, Leymarie P, Travert G, et al. Food-dependent Cushing’s syndrome mediated by aberrant adrenal sensitivity to gastric inhibitory polypeptide. N Engl J Med (1992) 327(14):981–6. doi: 10.1056/NEJM199210013271403
92. Vezzosi D, Cartier D, Regnier C, Otal P, Bennet A, Parmentier F, et al. Familial adrenocorticotropin-independent macronodular adrenal hyperplasia with aberrant serotonin and vasopressin adrenal receptors. Eur J Endocrinol (2007) 156(1):21–31. doi: 10.1530/eje.1.02324
93. Lecoq AL, Stratakis CA, Viengchareun S, Chaligne R, Tosca L, Demeocq V, et al. Adrenal GIPR expression and chromosome 19q13 microduplications in GIP-dependent Cushing’s syndrome. JCI Insight (2017) 2(18):e92184. doi: 10.1172/jci.insight.92184
94. Espiard S, Drougat L, Libe R, Assie G, Perlemoine K, Guignat L, et al. ARMC5 Mutations in a Large Cohort of Primary Macronodular Adrenal Hyperplasia: Clinical and Functional Consequences. J Clin Endocrinol Metab (2015) 100(6):E926–35. doi: 10.1210/jc.2014-4204
95. Faucz FR, Zilbermint M, Lodish MB, Szarek E, Trivellin G, Sinaii N, et al. Macronodular adrenal hyperplasia due to mutations in an armadillo repeat containing 5 (ARMC5) gene: a clinical and genetic investigation. J Clin Endocrinol Metab (2014) 99(6):E1113–9. doi: 10.1210/jc.2013-4280
96. Gagliardi L, Schreiber AW, Hahn CN, Feng J, Cranston T, Boon H, et al. ARMC5 mutations are common in familial bilateral macronodular adrenal hyperplasia. J Clin Endocrinol Metab (2014) 99(9):E1784–92. doi: 10.1210/jc.2014-1265
97. Hu Y, Lao L, Mao J, Jin W, Luo H, Charpentier T, et al. Armc5 deletion causes developmental defects and compromises T-cell immune responses. Nat Commun (2017) 8:13834. doi: 10.1038/ncomms13834
98. Tissier F, Cavard C, Groussin L, Perlemoine K, Fumey G, Hagnere AM, et al. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res (2005) 65(17):7622–7. doi: 10.1158/0008-5472.CAN-05-0593
99. Berthon A, Faucz FR, Espiard S, Drougat L, Bertherat J, Stratakis CA. Age-dependent effects of Armc5 haploinsufficiency on adrenocortical function. Hum Mol Genet (2017) 26(18):3495–507. doi: 10.1093/hmg/ddx235
100. Maria AG, Tatsi C, Berthon A, Drougat L, Settas N, Hannah-Shmouni F, et al. ARMC5 variants in PRKAR1A-mutated patients modify cortisol levels and Cushing’s syndrome. Endocr Relat Cancer (2020) 27(9):509–17. doi: 10.1530/ERC-20-0273
101. Elbelt U, Trovato A, Kloth M, Gentz E, Finke R, Spranger J, et al. Molecular and clinical evidence for an ARMC5 tumor syndrome: concurrent inactivating germline and somatic mutations are associated with both primary macronodular adrenal hyperplasia and meningioma. J Clin Endocrinol Metab (2015) 100(1):E119–28. doi: 10.1210/jc.2014-2648
102. Gatta-Cherifi B, Chabre O, Murat A, Niccoli P, Cardot-Bauters C, Rohmer V, et al. Adrenal involvement in MEN1. Analysis of 715 cases from the Groupe d’etude des Tumeurs Endocrines database. Eur J Endocrinol (2012) 166(2):269–79. doi: 10.1530/EJE-11-0679
103. Gaujoux S, Pinson S, Gimenez-Roqueplo AP, Amar L, Ragazzon B, Launay P, et al. Inactivation of the APC gene is constant in adrenocortical tumors from patients with familial adenomatous polyposis but not frequent in sporadic adrenocortical cancers. Clin Cancer Res (2010) 16(21):5133–41. doi: 10.1158/1078-0432.CCR-10-1497
104. Shuch B, Ricketts CJ, Vocke CD, Valera VA, Chen CC, Gautam R, et al. Adrenal nodular hyperplasia in hereditary leiomyomatosis and renal cell cancer. J Urol (2013) 189(2):430–5. doi: 10.1016/j.juro.2012.07.139
105. Shiroky JS, Lerner-Ellis JP, Govindarajan A, Urbach DR, Devon KM. Characteristics of Adrenal Masses in Familial Adenomatous Polyposis. Dis Colon Rectum (2018) 61(6):679–85. doi: 10.1097/DCR.0000000000001008
106. Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert-Buck MR, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Sci (N Y NY) 1997 (5311) 276:404–7. doi: 10.1126/science.276.5311.404
107. Lemmens I, VandeVen WJM, Kas K, Zhang CX, Giraud S, Wautot V, et al. Identification of the multiple endocrine neoplasia type 1 (MEN1) gene. Hum Mol Genet (1997) 6(7):1177–83. doi: 10.1093/hmg/6.7.1177
108. Lemos MC, Thakker RV. Multiple endocrine neoplaslia type 1 (MEN 1): Analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat (2008) 29(1):22–32. doi: 10.1002/humu.20605
109. Matkar S, Thiel A, Hua XX. Menin: a scaffold protein that controls gene expression and cell signaling. Trends Biochem Sci (2013) 38(8):394–402. doi: 10.1016/j.tibs.2013.05.005
110. Kamilaris CDC, Stratakis CA. Multiple Endocrine Neoplasia Type 1 (MEN1): An Update and the Significance of Early Genetic and Clinical Diagnosis. Front Endocrinol (Lausanne) (2019) 10:339. doi: 10.3389/fendo.2019.00339
111. Alam NA, Bevan S, Churchman M, Barclay E, Barker K, Jaeger EE, et al. Localization of a gene (MCUL1) for multiple cutaneous leiomyomata and uterine fibroids to chromosome 1q42.3-q43. Am J Hum Genet (2001) 68(5):1264–9. doi: 10.1086/320124
112. Pollard P, Wortham N, Barclay E, Alam A, Elia G, Manek S, et al. Evidence of increased microvessel density and activation of the hypoxia pathway in tumours from the hereditary leiomyomatosis and renal cell cancer syndrome. J Pathol (2005) 205(1):41–9. doi: 10.1002/path.1686
113. Sudarshan S, Pinto PA, Neckers L, Linehan WM. Mechanisms of disease: hereditary leiomyomatosis and renal cell cancer–a distinct form of hereditary kidney cancer. Nat Clin Pract Urol (2007) 4(2):104–10. doi: 10.1038/ncpuro0711
114. Matyakhina L, Freedman RJ, Bourdeau I, Wei MH, Stergiopoulos SG, Chidakel A, et al. Hereditary leiomyomatosis associated with bilateral, massive, macronodular adrenocortical disease and atypical cushing syndrome: a clinical and molecular genetic investigation. J Clin Endocrinol Metab (2005) 90(6):3773–9. doi: 10.1210/jc.2004-2377
115. Castro-Vega LJ, Buffet A, De Cubas AA, Cascon A, Menara M, Khalifa E, et al. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum Mol Genet (2014) 23(9):2440–6. doi: 10.1093/hmg/ddt639
116. Clark GR, Sciacovelli M, Gaude E, Walsh DM, Kirby G, Simpson MA, et al. Germline FH mutations presenting with pheochromocytoma. J Clin Endocrinol Metab (2014) 99(10):E2046–50. doi: 10.1210/jc.2014-1659
117. Vezzosi D, Libe R, Baudry C, Rizk-Rabin M, Horvath A, Levy I, et al. Phosphodiesterase 11A (PDE11A) gene defects in patients with acth-independent macronodular adrenal hyperplasia (AIMAH): functional variants may contribute to genetic susceptibility of bilateral adrenal tumors. J Clin Endocrinol Metab (2012) 97(11):E2063–9. doi: 10.1210/jc.2012-2275
118. Brown RJ, Kelly MH, Collins MT. Cushing syndrome in the McCune-Albright syndrome. J Clin Endocrinol Metab (2010) 95(4):1508–15. doi: 10.1210/jc.2009-2321
119. Bourdeau I, Matyakhina L, Stergiopoulos SG, Sandrini F, Boikos S, Stratakis CA. 17q22-24 chromosomal losses and alterations of protein kinase a subunit expression and activity in adrenocorticotropin-independent macronodular adrenal hyperplasia. J Clin Endocrinol Metab (2006) 91(9):3626–32. doi: 10.1210/jc.2005-2608
120. Swords FM, Noon LA, King PJ, Clark AJ. Constitutive activation of the human ACTH receptor resulting from a synergistic interaction between two naturally occurring missense mutations in the MC2R gene. Mol Cell Endocrinol (2004) 213(2):149–54. doi: 10.1016/j.mce.2003.10.052
121. Zhu J, Cui L, Wang W, Hang XY, Xu AX, Yang SX, et al. Whole exome sequencing identifies mutation of EDNRA involved in ACTH-independent macronodular adrenal hyperplasia. Fam Cancer (2013) 12(4):657–67. doi: 10.1007/s10689-013-9642-y
122. Stratakis CA. Cyclic AMP-dependent protein kinase catalytic subunit A (PRKACA): the expected, the unexpected, and what might be next. J Pathol (2018) 244(3):257–9. doi: 10.1002/path.5014
123. Lodish M, Stratakis CA. A genetic and molecular update on adrenocortical causes of Cushing syndrome. Nat Rev Endocrinol (2016) 12(5):255–62. doi: 10.1038/nrendo.2016.24
124. Bathon K, Weigand I, Vanselow JT, Ronchi CL, Sbiera S, Schlosser A, et al. Alterations in Protein Kinase A Substrate Specificity as a Potential Cause of Cushing Syndrome. Endocrinology (2019) 160(2):447–59. doi: 10.1210/en.2018-00775
125. Espiard S, Knape MJ, Bathon K, Assie G, Rizk-Rabin M, Faillot S, et al. Activating PRKACB somatic mutation in cortisol-producing adenomas. JCI Insight (2018) 3(8):e98296. doi: 10.1172/jci.insight.98296
126. Honeyman JN, Simon EP, Robine N, Chiaroni-Clarke R, Darcy DG, Lim II, et al. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Sci (N Y NY) 2014 (6174) 343:1010–4. doi: 10.1126/science.1249484
127. Singhi AD, Wood LD, Parks E, Torbenson MS, Felsenstein M, Hruban RH, et al. Recurrent Rearrangements in PRKACA and PRKACB in Intraductal Oncocytic Papillary Neoplasms of the Pancreas and Bile Duct. Gastroenterology (2020) 158(3):573–82.e572. doi: 10.1053/j.gastro.2019.10.028
128. Tseng IC, Huang WJ, Jhuang YL, Chang YY, Hsu HP, Jeng YM. Microinsertions in PRKACA cause activation of the protein kinase A pathway in cardiac myxoma. J Pathol (2017) 242(2):134–9. doi: 10.1002/path.4899
Keywords: Cushing’s syndrome, genetics, primary aldosteronism, adrenocortical hyperplasia, adrenocortical adenoma
Citation: Kamilaris CDC, Stratakis CA and Hannah-Shmouni F (2021) Molecular Genetic and Genomic Alterations in Cushing’s Syndrome and Primary Aldosteronism. Front. Endocrinol. 12:632543. doi: 10.3389/fendo.2021.632543
Received: 23 November 2020; Accepted: 01 February 2021;
Published: 12 March 2021.
Edited by:
Antongiulio Faggiano, Sapienza University of Rome, ItalyReviewed by:
Andre Lacroix, Université de Montréal, CanadaAshley Grossman, Queen Mary University of London, United Kingdom
Copyright © 2021 Kamilaris, Stratakis and Hannah-Shmouni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fady Hannah-Shmouni, ZmFkeS5oYW5uYWgtc2htb3VuaUBuaWguZ292
 Crystal D. C. Kamilaris
Crystal D. C. Kamilaris Constantine A. Stratakis
Constantine A. Stratakis Fady Hannah-Shmouni
Fady Hannah-Shmouni