- 1Department of Translational Medical Science, Section of Pediatrics, Regional Center of Pediatric Diabetes, Federico II University of Naples, Naples, Italy
- 2Pediatric Unit, S. Chiara Hospital, Trento, Italy
- 3Regional Center for Pediatric Diabetes, University of Verona, Verona, Italy
- 4Department of Human Pathology in Adult and Developmental Age, University of Messina, Messina, Italy
- 5SOD Pediatric Diabetology, Department of Women’s and Children’s, “G. Salesi” Children’s Hospital, AOU Ospedali Riuniti, Ancona, Italy
- 6Department of Woman, Child and Urological Diseases, S. Orsola-Malpighi University Hospital, Bologna, Italy
- 7University of Pavia, Pavia and Department of Pediatrics, "Vittore Buzzi" Children’s Hospital, Milano, Italy
- 8IRCCS Istituto Giannina Gaslini, Genova, Italy
- 9Diabetes Unit, Ospedale Brotzu, Cagliari, Italy
- 10Centro di Riferimento Regionale di Diabetologia Pediatrica A.O.U. Policlinico G. Rodolico, Catania, Italy
- 11Diabetes Unit, Bambino Gesù Children’s Hospital, Rome, Italy
- 12D.A.I. Pediatria, Ospedale Pediatrico Giovanni XXIII, Bari, Italy
- 13Department of Medical and Surgical Sciences of the Mother, Children and Adults - Pediatric Unit, University of Modena and Reggio Emilia, Modena, Italy
- 14Division of Pediatrics, Department of Health Science, University of Piemonte Orientale, Novara, Italy
- 15Diabetes & Nutrition Unit, Pediatrics, ASST Cremona, Cremona, Italy
- 16Pediatric Diabetology Unit, Meyer Children Hospital, Florence, Italy
Cystic fibrosis related diabetes (CFRD) is a comorbidity of cystic fibrosis (CF) that negatively impacts on its clinical course. Prediabetes is an important predictor of either CFRD development and unfavorable prognosis of CF in both pediatric and adult patients. International guidelines recommend insulin only in case of CFRD diagnosis. Whether early detection and treatment of prediabetes may contribute to improve the clinical course of CF is still debated. A subgroup of pediatric diabetologists of the Italian Society for Pediatric Endocrinology and Diabetology (ISPED) performed a systematic review of the literature based on predefined outcomes: impact of pre-diabetes on clinical outcomes and on the risk of developing CFRD; diagnosis of diabetes and pre-diabetes under 10 years of age; effectiveness of therapy on glycemic control, impact of therapy on pulmonary function and nutritional status. Thirty-one papers were selected for the analysis data presented in these papers were reported in tables sorted by outcomes, including comprehensive evidence grading according to the GRADE approach. Following the grading of the quality of the evidence, the entire ISPED diabetes study group achieved consensus for the Italian recommendations based on both evidence and clinical experience. We concluded that in patients with CF, prediabetes should be carefully considered as it can evolve into CFRD. In patients with CF and prediabetic conditions, after complete evaluation of the OGTT trend, glucometrics, glycemic values measured during pulmonary exacerbations and/or steroid therapy, early initiation of insulin therapy could have beneficial effects on clinical outcomes of patients with CF and prediabetes.
Introduction
Improving survival in cystic fibrosis (CF) has caused an increase of the prevalence of comorbidities, with cystic fibrosis-related diabetes (CFRD) being the most common and affecting at least half of the adult CF population (1). CFRD has a negative impact on the clinical course of the disease increasing its mortality (2). In recent years, several lines of evidence have demonstrated that pulmonary function, microbiological colonization and nutritional status start to worsen several years prior to the diagnosis of CFRD (3). Early detection of pre-diabetes, defined in CF patients by the presence of abnormal glucose tolerance (AGT), impaired glucose tolerance (IGT), impaired fasting glucose (IFG) or indeterminate glycaemia (INDET) is therefore essential, although to date few studies have focused on pre-diabetes and its negative significant impact on the course of CF
The main cause of CFRD is insulin insufficiency with insulin secretion being increasingly impaired in correlation with exacerbation of pre-diabetes (4). According to this pathophysiological evidence, current guidelines of the International Society for Pediatric and Adolescent Diabetes (5) recommend insulin therapy initiation for the treatment of CFRD. However, no specific insulin therapies appear to have significantly distinct advantages both for an effective treatment of hyperglycemia and for their possible positive impact on the clinical course of CF (6). In addition, whether CF patients diagnosed with pre-diabetes could benefit from ‘early’ initiation of insulin therapy it is still debated (7).
The screening and diagnosis of CFRD and pre-diabetic conditions, as long as the effectiveness of the therapy for the treatment of CFRD and pre-diabetes represent two topics of major interest in the field of diabetes and CF. For this reason, a subgroup of pediatric diabetologists of Italian Society for Pediatric Endocrinology and Diabetology (ISPED) performed a systematic review of the literature and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) profiles focusing on the above mentioned debated topics.
Methods
A systematic review based on pre-defined outcomes for each question has been performed. The entire diabetes study group of ISPED compiled evidence profiles and achieved consensus for the final recommendations.
In regards to screening and diagnosis of diabetes and pre-diabetes in CF patients, the outcomes were: 1) impact of pre-diabetes on clinical outcomes (pulmonary function, number of pulmonary exacerbations and nutritional status) and 2) on the risk of developing CFRD; 3) diagnosis of diabetes and pre-diabetes in children under 10 years of age.
In regards to effectiveness of therapy in CFRD and pre-diabetes the outcomes were: 1) effectiveness of therapy on biochemical measures of glycemic control, as glycosylated hemoglobin (HbA1c), fasting and 2 h post-meal serum blood glucose values, and data derived from CGM download; 2) effectiveness of therapy in improving pulmonary function, 3) effectiveness of therapy in improving nutritional status.
Pulmonary function was analyzed in terms of forced expiratory volume in the 1st second (FEV1) and forced vital capacity (FVC). Pulmonary exacerbations were analyzed considering the following criteria: increased sputum volume, more frequent coughing, increased dyspnea, weight loss, change in the chest physical examination, absence from school or work because of illness, requiring hospitalization and antibiotic therapy. Pathogens colonization was considered on the results of microbiological investigations on deep pharyngeal aspirates. Pathogens colonization on deep pharyngeal aspirates was considered on the results of microbiological investigations. Nutritional status was evaluated as body mass index (BMI) and standard deviation scores (SDS) of weight and height.
The method used to perform this systematic review was based on the PICOS model (Population, Intervention, Comparison, Results, Study design). Inclusion criteria of studies are listed in Table 1. Exclusion criteria were studies not meeting the established outcomes and studies with animals. No restrictions were applied regarding the published paper’s language and patients’ age. The articles selected for this literature review include all those published from 1/01/2006 to 30/10/2020. The keywords used, also called “mesh” (MEdical Subject Headings) on PubMed, are the following: “diabetes diagnostic test AND cystic fibrosis,” “cystic fibrosis AND diabetes management,” “cystic fibrosis AND AGT,” “cystic fibrosis AND IFG,” “cystic fibrosis AND IGT,” “cystic fibrosis AND INDET,” “cystic fibrosis AND diabetes.” Systematic searches, using relevant keywords and search strings, were conducted on electronic databases (PubMed, Scopus, Google Scholar, CINAHL, Nursing reference center, Up to date and PsycINFO, Embase, CENTRAL) and clinical trial registers (http://clinicaltrials.gov; www.controlled-trials.com).
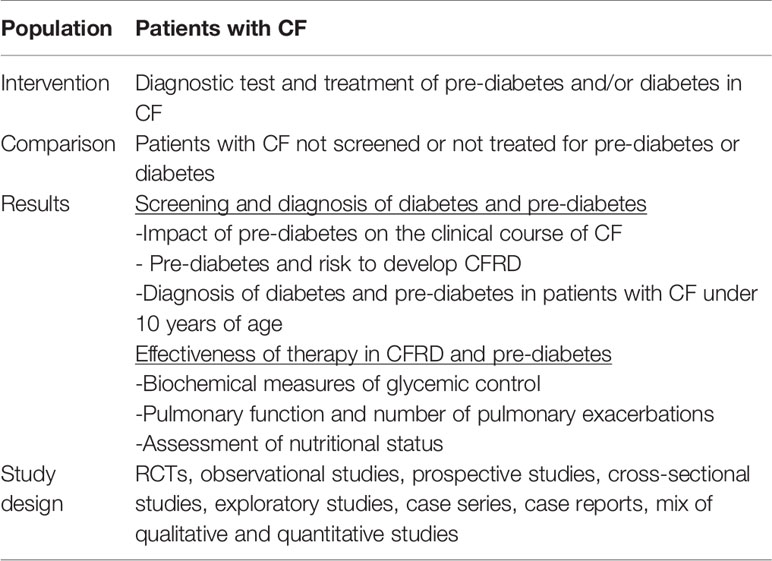
Table 1 PICOS model (population, intervention, comparison, results, study design) adopted in the systematic review.
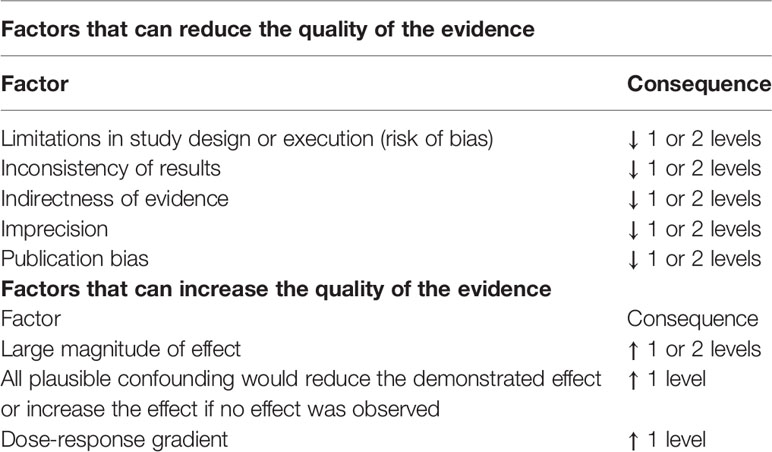
In order to derive recommendations, a GRADE approach to rank the quality of a body of evidence was applied as reported in Table 2 (8, 9). The final assessment of the quality of evidence was discussed and established by the entire subgroup.
For each risk of bias (i.e. imprecision, inconsistency, indirectness, and publication bias), the authors had the option of decreasing their level of certainty one or two levels (e.g., from high to moderate). Since GRADE cannot be implemented mechanically, there is by necessity a considerable amount of subjectivity in each decision.
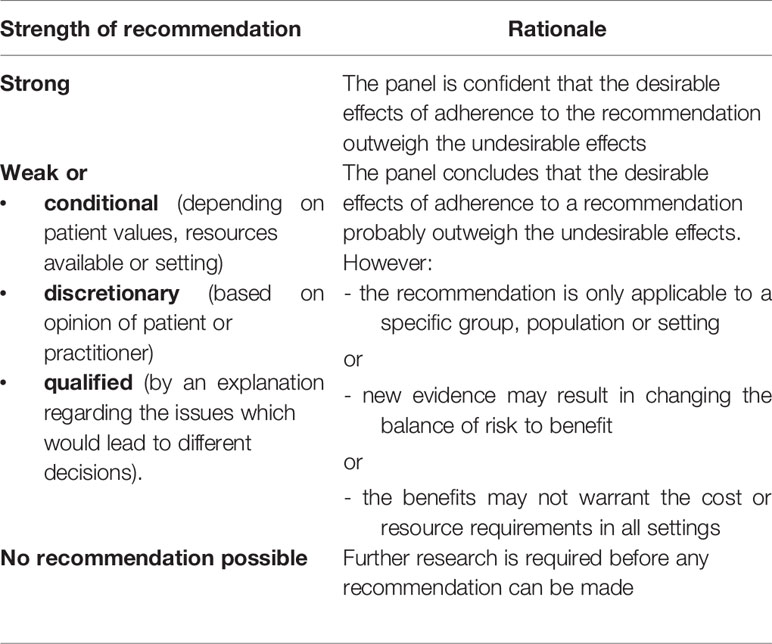
Following the assessment of the quality of the evidence, an assessment of the strength of the recommendation was made. A consensus for the final recommendations was achieved from the entire diabetes study group of the ISPED, and summarized in Table 3. According to the GRADE method, the strength of each recommendation is classified in four mutually exclusive categories: “strong” and “weak or conditional” in favor (positive) or against (negative) the use of a specific intervention, as reported in Table 4 (10).
We used these standard expressions and if sufficient evidence was not available recommendations were based on the panel opinion according to the current daily clinical practice.
Results
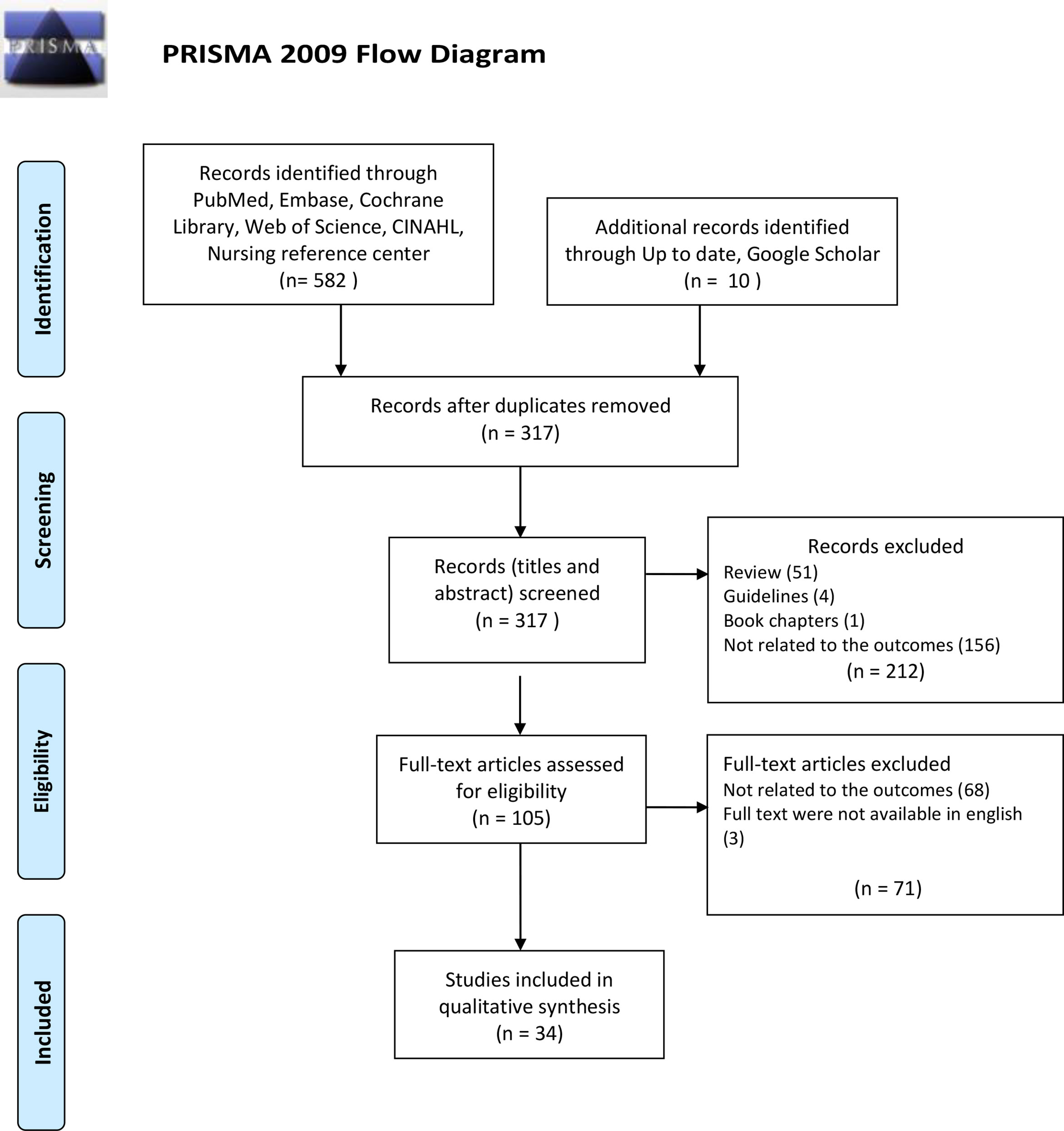
After a careful evaluation of all databases available, 592 papers were found, 317 of whom were removed because they were duplicates (Figure 1). Among the 105 papers left, after reading the full text of each of them, only 34 papers were selected for the analysis because all the inclusion criteria were met.
Data from the 34 studies selected, including comprehensive evidence grading, are presented in Tables 5–10, sorted by outcomes.
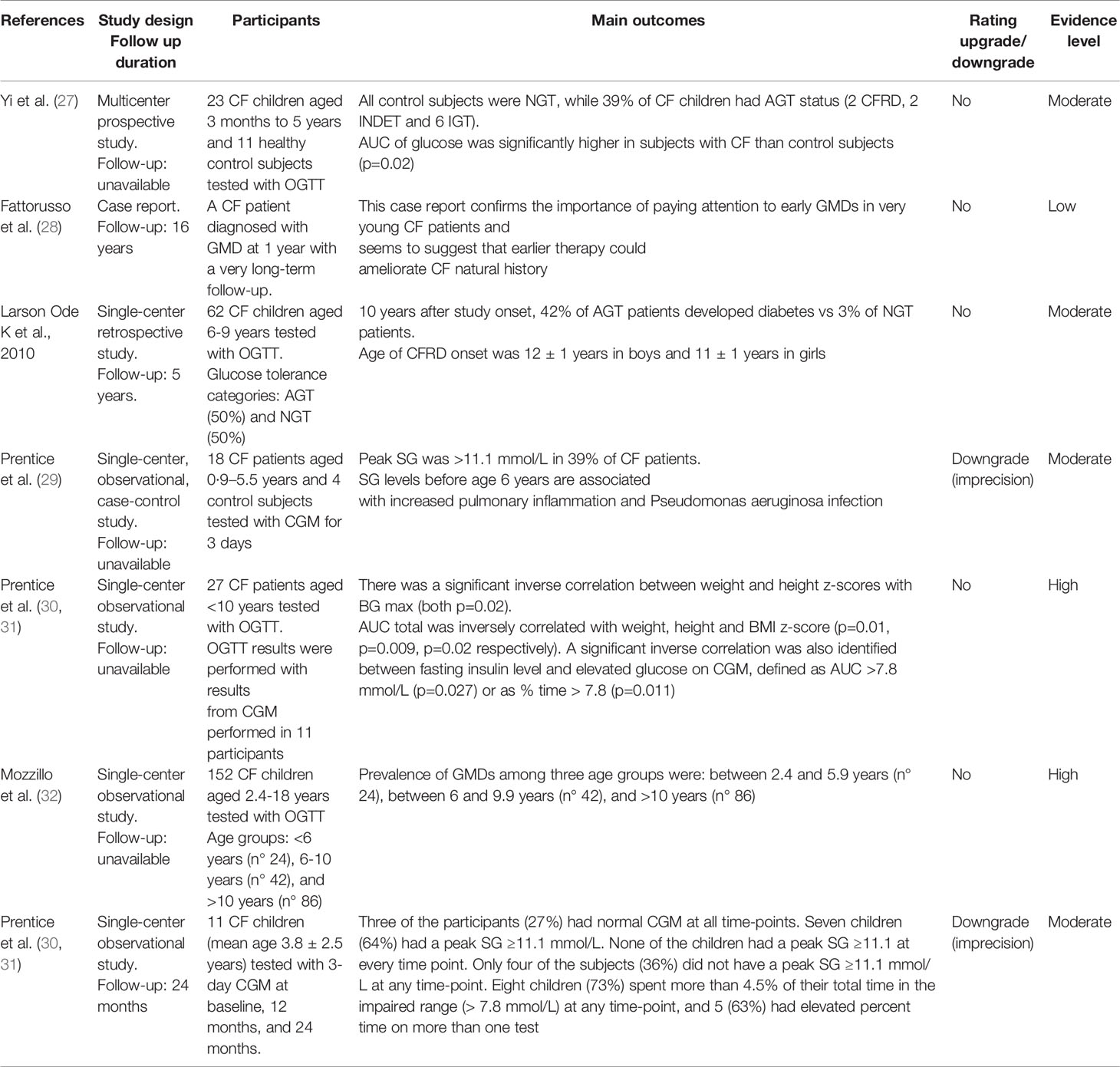
Table 7 Diagnosis of diabetes and prediabetes in children with Cystic Fibrosis under 10 years of age.
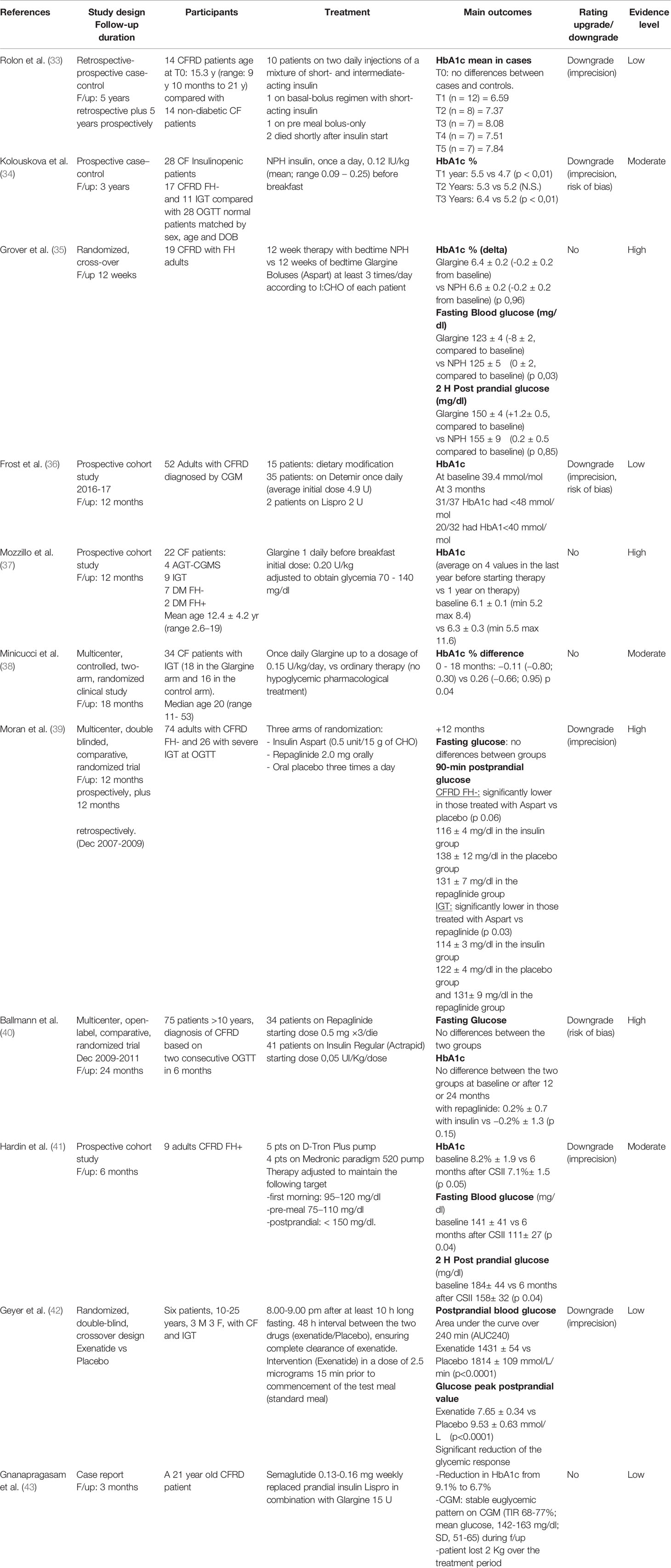
Table 8 Effectiveness of therapy on glycemic control (HbA1c, fasting and 2 h post-meal serum blood sugar values).
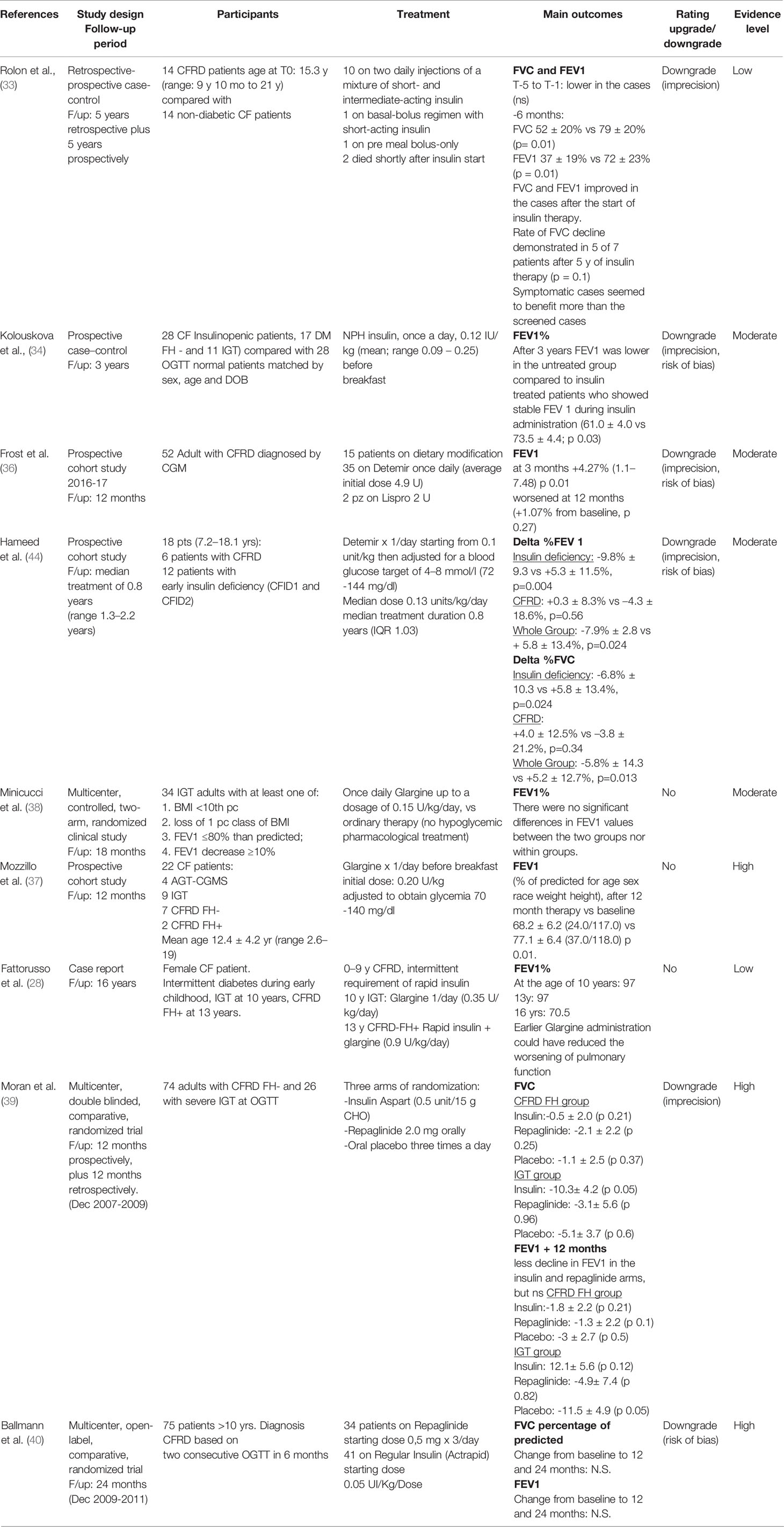
Table 9 Effectiveness of therapy on pulmonary function (eg forced expiratory volume (FEV1) and forced vital capacity (FVC).
According to the ISPAD guidelines (5), the following two diagnostic categories of CFRD have been established for patients screened with oral glucose tolerance test (OGTT) during periods of stable CF clinical conditions, based on fasting and 2-h glucose values: CFRD without fasting hyperglycemia (CFRD-FH-) and CFRD with fasting hyperglycemia (CFRD-FH+) (Table 11). In addition, in symptomatic patients, the CFRD can be diagnosed if random blood glucose level is ≥200 mg/dL (≥11.1 mmol/L) on 2 or more occasions, and if HbA1c is ≥ 48 mmol/mol (6.5%) (48 mmol/mol), even though diagnosis of diabetes can also be made in CF patients that show HbA1c value below this range (45). During flare-up phases of the disease, when intravenous antibiotic therapy and/or systemic corticosteroid therapy is required, the diagnosis of CFRD can be made if a fasting glycemia ≥126 mg/dL (≥7 mmol/L) or a post-prandial blood glucose ≥200 mg/dL (≥11.1 mmol/L) is present for 48 h.
Due to the insidious onset of CFRD, once a year OGTT in all patients aged 10 and above is crucial for the diagnosis of CFRD and for the identification of high-risk subjects (5).
OGTT identifies patients with CFRD and with pre-diabetes using the following diagnostic categories: normal glucose tolerance (NGT); INDET; IGT (Table 11). Two more categories named AGT (4, 37) and impaired fasting glucose (IFG) can also to be considered (46) (Table 11).
Impact of CFRD and Pre-Diabetes on CF Outcomes
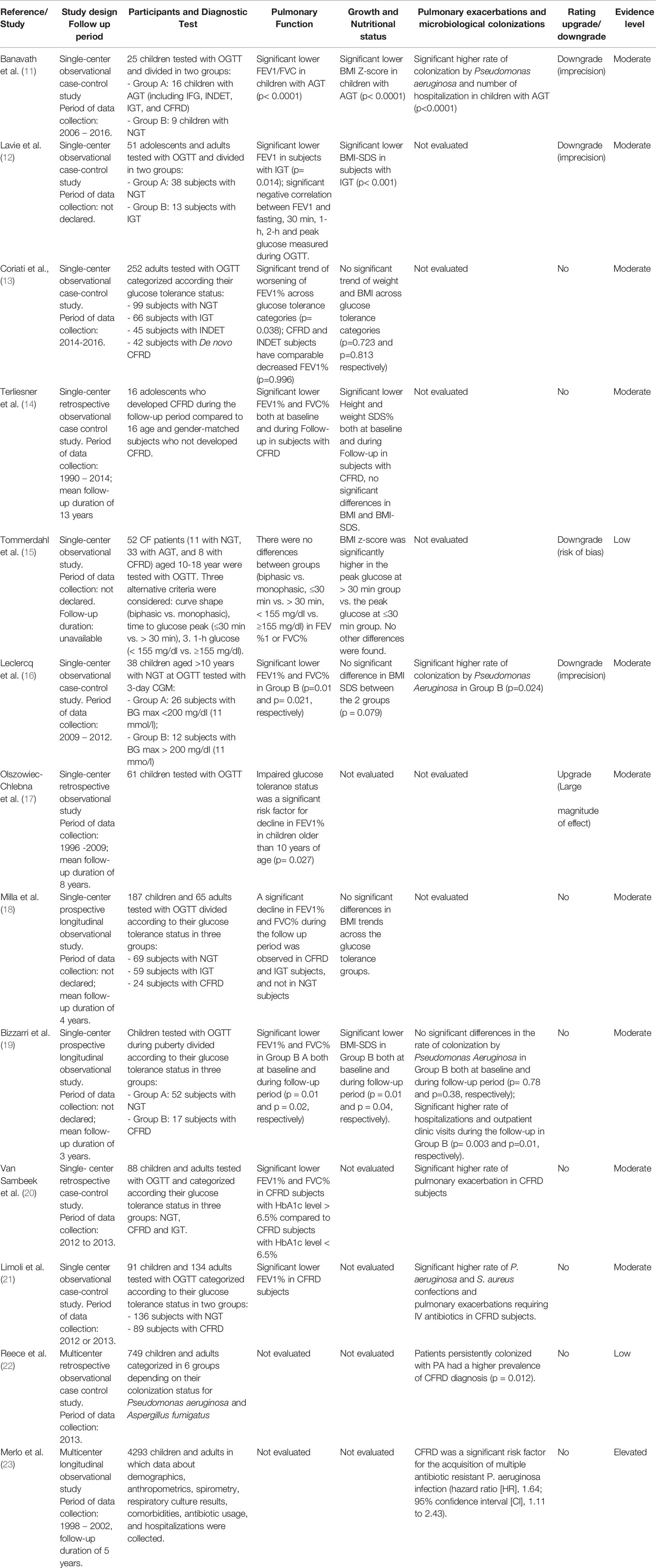
Regarding the outcome “Impact of pre-diabetes on the clinical outcomes of CF”, 13 paper were included in this systematic review, 2 are multi-center and 11 are single-center studies; 8 were prospective observational studies while 5 were retrospective-observational studies. The number of enrolled patients ranged from 16 to 4,293 including both children and adults (Table 5).
Impact on Pulmonary Function
CF patients diagnosed with IGT or INDET had lower FEV1 and FVC levels compared to CF patients without glucose abnormalities (11–14) (evidence Moderate); glucose peaks >200 mg/dl (11.1 mmol/l) during a continuous glucose monitoring were associated with worse spirometry pulmonary function parameters (16) (evidence Low). Pulmonary function is not associated with alternative OGTT criteria (i.e. monophasic curve, glucose peak > 30 min, and/or 1 h ≥155 mg/dl) (15) (evidence Low).
Prediabetes was one of the most relevant predictors of deterioration of lung function defined as a significant decrease in FEV1 predicted value, during a 5 year-follow-up (17, 18) (evidence Moderate).
Impact on Growth and Nutritional Status
Auxological parameters, height-SDS and BMI-SDS, may be negatively influenced by prediabetes status in CF patients (11, 12, 14) (evidence Moderate).
Pediatric CF patients experienced a deterioration of their nutritional status and a negative impact on their final height due to prediabetes (14, 19) (evidence Moderate).
Impact on Pulmonary Exacerbations and Microbiological/Pathogens Colonization
A higher rate of pulmonary exacerbations, hospital admissions and outpatient clinic visits was observed in CF patients with CFRD and early glucose tolerance abnormalities compared to patients with NGT (11, 12, 19–21) (evidence Moderate).
CFRD and prediabetes diagnosis was recognized as independent risk factors for colonization by common CF pathogens, in particular for the acquisition of Pseudomonas Aeruginosa, its multiple antibiotic-resistant infection and its co-infection with other pathogens (11, 12, 19, 21–23) (evidence Moderate).
Regarding the outcome “Impact of prediabetes on the risk to develop CFRD”, 4 papers were included, 1 is a multi-center prospective study, 1 is a single-center prospective study and 2 are single-center retrospective studies. The sample size of the study populations ranged from 17 to 1,093 patients, especially children and adolescents (Table 6).
The results of these studies strongly support the evidence for an early detection of prediabetic conditions in CF patients, because IGT, IFG or INDET presented a five-years CFRD risk at least 10 times higher compared to CF patients with NGT (3, 24, 25). (evidence High). In addition, early glucose tolerance alterations defined by INDET during continuous glucose monitoring were also related to a higher risk of developing CFRD (25, 26) (evidence Moderate).
Regarding the outcome “Diagnosis of diabetes and prediabetes in patients with CF under 10 years of age” we included in this systematic review 7 papers. Among these, one is a multi-center prospective study, 4 are single-center prospective studies, one is a single-center retrospective study, and one is a case report. The number of enrolled patients ranged from 11 to 152 and age ranged from a few months-old to less than 10 years of age (Table 7). The analysis of these studies showed that in infants and young children with CF glucose derangements detected by OGTT are often diagnosed (3, 27–31) (evidence Moderate) and annual diabetes screening program in patients <10 years of age increased the early detection of CFRD (27, 28, 32) (evidence Low), leading to a more prompt and appropriate therapeutic approach (32) (evidence Very Low). Continuous glucose monitoring may be an alternative method for detecting glucose derangements in very young children with CF (29, 30) (evidence Moderate), particularly those with Pseudomonas Aeruginosa colonization (29) (evidence Moderate). Current evidence has also demonstrated that early diagnosis of prediabetes may be related to early clinical deterioration, particularly lung function and auxological parameters (29, 30) (evidence Moderate).
Effectiveness of Therapy in CFRD
Regarding the outcome “Effectiveness on glycemic control”, we included in this systematic review 10 studies, 5 are randomized controlled trials (RCT), 3 are prospective cohort studies, one is a prospective case-control study, and one is a retrospective case-control study. The number of enrolled patients ranged from 9 to 100, from infancy to adulthood. Glycemic control has been analyzed in terms of glycated hemoglobin (HbA1c), fasting and 2 h post-meal blood glucose (Table 8). Different insulin and other drugs used are reported in Table 8.
Among the 3 studies on NPH insulin, in a retrospective - prospective case-control study, 10 pediatric patients with CFRD were treated with a mixture of short and intermediate acting insulin over a 10-year period, with no evidence of HbA1c improvement when compared to 14 non-diabetic matched controls (33) (evidence Low). In 28 adult patients, who were insulinopenic, diagnosed with IGT or CFRD- FH-, NPH insulin treatment (0.12–0.25 IU/kg/day) has been able to improve HbA1c during the first 2 years, but the effect vanished during the third year of treatment. NPH insulin did not increase the risk of hypoglycemia (34) (evidence Moderate). In an RCT, 19 adults with CFRD-FH+ were randomly assigned to treatment with NPH or with glargine while continuing pre-meal insulin aspart (at least 3 administration per day). After 12-week treatment, NPH and glargine had similar efficacy on metabolic control in terms of HbA1c and postprandial glycaemia, whereas in the group treated with glargine, FPG was significantly reduced. No severe hypoglycemia event occurred and the frequency of minor hypoglycemic episodes was not significantly different in the two groups (35) (evidence High).
Only one prospective cohort study used insulin detemir, showing that this insulin administered once a day has been able to maintain HbA1c value below 6.5% (48 mmol/mol) in adult patients with CFRD after 3 months of treatment. Moreover, 62.5% of patients had HbA1c values below 5.8% (40 mmol/mol), with an 8% decrease in time spent above 140 mg/dl (7.8 mmol/l) (36) (evidence Low).
Three studies evaluated the efficacy of basal insulin therapy using glargine, without any apparent effect of this insulin on HbA1c (Table 8). A significant decrease in HbA1c has been observed only after 18-month therapy in one study (38) (evidence Moderate).
Pre-meal insulin (aspart or regular Actrapid) was compared to repaglinide in two studies, with conflicting results (Table 8). A study in 81 adults with CF and FPG or IGT showed repaglinide more effective after 6-month follow-up but not after 12-month follow-up (39) (evidence High). In pediatric patients Actrapid insulin and repaglinide showed similar efficacy, with no severe hypoglycemia episodes in the 2 groups (40) (evidence High).
One study in 9 patients with CFRD-FH+ used insulin pump therapy for 6 months achieving a significant reduction of HbA1c, fasting and postprandial blood glucose, without severe hypoglycemia events (41) (evidence Moderate).
Two studies have been published on the use of GLP-1 receptor agonists (GLP-1 RA) in the treatment of CFRD (42, 43). In a double blind RCT study conducted on 6 adolescents and young adults with IGT, exenatide was administered for 48 h showing a significant reduction in postprandial blood glucose (42) (evidence Low); as well as Semaglutide administered weekly at a low dose, was able to replace prandial Lispro and control glycemia in combination with Glargine (43).
In regard to the outcome “Effectiveness on pulmonary function”, 9 studies were included in this systematic review, 3 are RCT, 3 are prospective cohort studies, and 3 are case-control studies, either prospective or retrospective. The number of enrolled patients ranged from 9 to 100, aged from less than 1 year to adulthood. The following therapies were tested in these studies, intermediate/NPH insulin (2 studies), insulin detemir (2 studies), insulin glargine (3 studies), rapid analogue insulin (2 studies), rapid insulin (1 study), repaglinide (2 studies), with conflicting results as shown in Table 9.
In a few studies, especially in pediatrics, it seems that insulin therapy has beneficial effects either on FVC or FEV1 (33) (evidence Low), while in adult patients the efficacy of insulin therapy seems to disappear after some time (34) (evidence Moderate). Similar data have been observed in other studies using insulin analogs either in adults (37) (evidence High) (36, 38) (evidence Moderate), or in pediatrics (44) (evidence Moderate) (28) (evidence Low).
Insulin aspart treatment compared to repaglinide or placebo, gave significant changes in the annual rate of decline in FVC, but not in FEV1 and in the number of acute exacerbations in subjects with Fc and IGT (39) (evidence High), while others observed that insulin Actrapid did not have any impact (40) (evidence High), or just limited one (28) (evidence Low) on pulmonary function decline.
Regarding the outcome “Effectiveness on nutritional status”, 11 papers were included in this systematic review, four are RCT, four are prospective cohort studies, one is a prospective case-control, and one is a case-control study. The number of enrolled patients ranged from 9 to 100, aged from a few months to adult age (Table 10).
Details about the relationship between the therapies used and the nutritional status of the patients with CF are given in Table 10. Summarizing, there is Low evidence that NPH insulin allows a significant improvement in BMI in children (33), but not in adults (34) (evidence Moderate), even if data are conflicting (35) (evidence Low). Better results have been found using insulin analogues, such as detemir either in pediatric patients (44) (evidence Moderate), or in adults (36) (evidence Moderate).
No significant differences in BMI-SDS were observed either in adolescents treated with Repaglinide compared to those treated with insulin Actrapid (40) (evidence High), or in adults (39) (evidence Moderate). Insulin pump therapy seemed to be effective in increasing weight and lean mass (41) (evidence Moderate).
Discussion
This systematic review of the literature and the GRADE approach (8, 9) were able to demonstrate how much CFRD impacts on the clinical course of CF in terms of pulmonary function, pulmonary exacerbations, pulmonary microbiological colonization and auxological parameters.
Moreover, prediabetes emerged as an important predictor factor of either CFRD or worse prognosis of CF outcomes in both pediatric and adult patients. Evidence of prediabetes in children under 10 years of age is not unusual and is once again associated with a significant risk of progression to CFRD and worse clinical course of CF. A significant decline in pulmonary function was in fact observed in pediatric patients with prediabetes (14), as well as, in adult patients with prediabetes compared to patients with NGT (11–14).
Among prediabetes, IGT represents one of the most relevant predictors of the decline in pulmonary function (17) (18) and both CFRD and IGT have been recognized as independent risk factors for pulmonary exacerbations and colonization by common CF pathogens, in particular by Pseudomonas Aeruginosa, and its form of infection characterized by multi-resistance to antibiotic therapy and co-infection with other pathogens (11, 16, 19, 21–23, 25).
Glycemic peaks higher than 200 mg/dl (11.1 mmol/l) were recorded with continuous glucose monitors in patients screened with OGTT, with normal fasting and 2-h blood glucose, and these glycemic peak were related to a worsening of spirometry parameters (16). A very recent study, not found by the search for the systematic review that was updated till October 2020, analyzed the correlation between intermittent scan CGM (isCGM) and OGTT data in a cohort of 32 children with CF. The isCGM percent of measurements >140 mg/dL (7.8 mmol/L) and the number of peaks per day >200 mg/dL (11 mmol/L) have correlations with intermediate OGTT glucose time points, but not the 2-h glucose value. Moreover patients with abnormal glucose tolerance (AGT) had lower lung function than those with normal glucose tolerance demonstrated by both FEV1% predicted and lung clearance index (47).
Among patients with CF, auxological parameters are significantly impaired in patients with CFRD than patients with NGT, also before diabetes diagnosis (14) and can be negatively influenced by prediabetes (11, 12, 44).
Evaluating the impact of different therapeutic strategies on glycemic control, pulmonary function and auxological parameters in patients with CFRD, glargine seems to be the most studied insulin. Data showed with moderate to high level of evidence that glargine therapy led to a significant improvement in HbA1c, the gold standard metric for long-term glycemic control, only in adult IGT patients (38), but not in children (37). In addition, in CFRD-FH+ adult patients glargine had the same efficacy than NPH insulin (35). Data about detemir are more confusing (36, 44).
A significant improvement in HbA1c value was demonstrated in CFRD patients treated with insulin pump therapy (41).
The analysis of the efficacy of pre-meal insulin or oral antidiabetic agents in controlling postprandial hyperglycemia in CFRD showed that aspart insulin is more effective than repaglinide (39), and regular insulin is as effective as repaglinide in patients with HbA1c <7% (40).
Glargine insulin in CFRD patients did not increase the risk of hypoglycemia when compared to NPH (35), and repaglinide does not increase the number of hypoglycemic events compared to regular insulin (40).
In both, adult and pediatric patients with CFRD or prediabetes, insulin therapy with NPH (32), as well as with Detemir (44) and glargine (37, 38) preserved pulmonary function and reduced the number of respiratory exacerbations, whereas therapy with regular human insulin or rapid analogue insulin or repaglinide had no impact on pulmonary function (28, 39, 40).
Regarding auxological and nutritional status parameters, in adults with diabetes or prediabetes, NPH insulin preserved the decay of BMI-SDS (34). Insulin detemir in pediatric patients with CFRD improved BMI-SDS (44), while this effect was not sustained in adult patients 37. In pediatric subjects diagnosed with AGT, IGT or CFRD, glargine insulin improved the BMI-SDS only in patients with poor nutritional status (BMI SDS <−1) (37, 38). In adults, insulin therapy with rapid analogue increased BMI in those diagnosed with IGT, but not in those with CFRD FH+ (39), while insulin pump therapy produces an increase in weight and lean mass measured with DEXA after 6 months of therapy (41). Repaglinide and regular insulin in CFRD patients have no impact on BMI-SDS (40).
Limitations
To obtain the best evidence to answer our research questions, we have made a great effort to collect studies by establishing strict inclusion criteria. Even if low-quality studies have been excluded, a few limitations still exist, that should be acknowledged for the evaluation and interpretation of the results, and consequently, the recommendation summarized in this review: 1) few RCT studies have been found, particularly for the outcome related to “effectiveness of treatment”; 2) only a few studies had sample sizes larger than 100 patients, and 3) continuous glucose monitoring was used in a limited number of studies.
Conclusions and Recommendations for The Clinical Practice
CF is a complex multi-organ disease requiring a comprehensive multidisciplinary treatment program. CFRD deserves special knowledge and expertise to be adequately diagnosed and treated. For these reasons, after a systematic review of the literature, the Diabetes Study Group of the ISPED has agreed to provide practical recommendations based on both evidence from current literature and clinical experience.
1. In patients with CF, OGTT is the gold-standard method for the screening of glucose abnormalities during periods of clinical stability (absence of respiratory exacerbation and/or use of antibiotic and/or steroid therapy, and in case of organ transplantation). Patients should be tested once a year -Strong in favor-.
2. Random BG and fasting BG measurement are recommended during periods of pulmonary exacerbations and/or use of glucocorticoid therapy, enteral nutrition and in case of diabetes symptoms -Strong in favor-.
3. Current evidence did not support the use of continuous glucose monitoring as a diagnostic and/or screening tool; however, it is considered very useful for monitoring subjects with IFG, IGT or INDET and during high risk conditions (i.e.: pulmonary exacerbations and/or use of steroids) - Strong in favor-.
4. Subjects with prediabetes are at risk of developing CFRD, thus close monitoring of glucose metabolism with OGTT and 2-week CGM at 6-month intervals is recommended -Conditional in favor-.
5. In children <10 years of age, starting from at least 6 years of age or even earlier if possible, it is advisable to screen for alterations of glucose metabolism at least once a year, whenever possible, and if IGT or INDET conditions are diagnosed a close monitoring of BG levels is recommended (OGTT at 6-month intervals; continuous glucose monitoring as a possible adjunctive tool for glucose monitoring during respiratory exacerbations and/or use of steroids), -Conditional in Favor-.
6. Treatment with a basal analogue (glargine) should be started in patients affected by CFRD FH+ at a dosage of 0.2 IU/kg/day -Conditional in Favor-.
7. Postprandial hyperglycemia should be treated with rapid analogue at initial dosage of 0.05 to 0.1 UI/kg before meal or an insulin/carbohydrate ratio starting with 1UI: 15 g and 1UI: 30 g ratio and subsequently modified on the basis of different intakes of the day and of the degree of insulin resistance at each moment. -Conditional in favor-.
8. The risk of hypoglycemia due to insulin therapy is low, -Strong in favor-. Treatment of CFRD with insulin could result in an improvement in fasting and postprandial blood sugar levels more than in the HbA1c value, -Strong in favor-.
9. Insulin pump therapy, although rarely used in these patients, is a valid and effective alternative to multiple daily injections -Strong in favor-.
10. In patients diagnosed with IGT, if clinically compromised, treatment with basal insulin analogue is recommended starting with 0.1 to 0.2 UI/kg/day with subsequent adjustments based on the glycemic trend, -Conditional in Favor-.
11. In patients diagnosed with other prediabetic conditions, in particular INDET and AGT, early initiation of insulin therapy could be beneficial and this possibility should be taken into account after a complete evaluation of the patient on the basis of the annual trend of the OGTT results, glucometrics, glycemic values measured during pulmonary exacerbations and/or steroid therapy, -Conditional in Favor-.
12. In all CF patients with diabetes and prediabetes treated with insulin analysis of glucose metrics with CGM may be useful for monitoring insulin treatment, -Conditional in Favor-.
Collaborators
Collaborators of the Diabetes Study Group of the Italian Society for Pediatric Endocrinology and Diabetology (ISPED). A complete list of the members of the Diabetes Study Group of the ISPED can be found in the supplementary material online.
Author Contributions
EM and RF engaged in literature retrieval of the articles, have analyzed the results, and wrote the manuscript. CP, SP, AC, and DP performed records screening and assessed eligibility, compilation of evidence and of evidence tables. AES reviewed the records screening and contributed to wrote the manuscript. GM, VCal, VCau, VCh, GD, AF, APF, FL, DP, MCM, EP, BP, IR, ST, and SZ discussed and commented on literature analysis. RS and CM critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.673539/full#supplementary-material
References
1. Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic Fibrosis-Related Diabetes: Current Trends in Prevalence, Incidence, and Mortality. Diabetes Care (2009) 32(9):1626–31. doi: 10.2337/dc09-0586
2. Chamnan P, Shine BSF, Haworth CS, Bilton D, Adler AI. Diabetes as a Determinant of Mortality in Cystic Fibrosis. Diabetes Care (2010) 33(2):311–6. doi: 10.2337/dc09-1215
3. Ode KL, Frohnert B, Laguna T, Phillips J, Holme B, Regelmann W, et al. Oral glucose tolerance testing in children with cystic fibrosis. Pediatr Diabetes (2010) 11(7):487–92. doi: 10.1111/j.1399-5448.2009.00632.x
4. Piona C, Volpi S, Zusi C, Mozzillo E, Tosco A, Franzese A, et al. Glucose Tolerance Stages in Cystic Fibrosis Are Identified by a Unique Pattern of Defects of Beta-Cell Function. J Clin Endocrinol Metab (2020), dgaa932. doi: 10.1210/clinem/dgaa932
5. Moran A, Pillay K, Becker D, Granados A, Hameed S, Acerini CL. ISPAD Clinical Practice Consensus Guidelines 2018: Management of cystic fibrosis-related diabetes in children and adolescents. Pediatr Diabetes (2018) 19:64–74. doi: 10.1111/pedi.12732
6. Onady GM, Stolfi A. Drug treatments for managing cystic fibrosis-related diabetes. Cochrane Cystic Fibrosis and Genetic Disorders Group, ed. Cochrane Database Syst Rev (2020) (10):1–48. doi: 10.1002/14651858.CD004730.pub5
7. Nguyen CQT, Denis M-H, Chagnon M, Rabasa-Lhoret R, Mailhot G. Abnormal glucose tolerance in a pediatric cystic fibrosis cohort: Trends in clinical outcomes and associated factors in the preceding years. Nutr Metab Cardiovasc Dis (2021) 31(1):277–85. doi: 10.1016/j.numecd.2020.07.044
9. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol (2011) 64(4):383–94. doi: 10.1016/j.jclinepi.2010.04.026
10. Andrews J, Guyatt G, Oxman AD, Aldersonf P, Dahmg P, Falck-Ytterh Y, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol (2013) 66(7):719–25. doi: 10.1016/j.jclinepi.2012.03.013
11. Banavath LN, Kumar R, Dayal D, Yadav J, Sachdeva N, Mathew JL, et al. Glucose intolerance in children with cystic fibrosis: a developing country’s perspective. J Pediatr Endocrinol Metab (2018) 31(10):1139–46. doi: 10.1515/jpem-2018-0222
12. Lavie M, Fisher D, Vilozni D, Forschmidt R, Sarouk I, Kanety H, et al. Glucose intolerance in cystic fibrosis as a determinant of pulmonary function and clinical status. Diabetes Res Clin Pract (2015) 110(3):276–84. doi: 10.1016/j.diabres.2015.10.007
13. Coriati A, Ziai S, Azar M, Berthiaume Y, Rabasa-Lhoret R. Characterization of patients with cystic fibrosis presenting an indeterminate glucose tolerance (INDET). J Cyst Fibros (2016) 15(1):127–32. doi: 10.1016/j.jcf.2015.03.001
14. Terliesner N, Vogel M, Steighardt A, Gausche R, Henn C, Hentschel J, et al. Cystic-fibrosis related-diabetes (CFRD) is preceded by and associated with growth failure and deteriorating lung function. J Pediatr Endocrinol Metab (2017) 30(8). doi: 10.1515/jpem-2017-0005
15. Tommerdahl KL, Brinton JT, Vigers T, Cree-Green M, Zeitler PS, Nadeau KJ, et al. Delayed glucose peak and elevated 1-hour glucose on the oral glucose tolerance test identify youth with cystic fibrosis with lower oral disposition index. J Cyst Fibros (2020) 2111. doi: 10.1016/j.jcf.2020.08.020
16. Leclercq A, Gauthier B, Rosner V, Weiss L, Moreau F, Constantinescu AA, et al. Early assessment of glucose abnormalities during continuous glucose monitoring associated with lung function impairment in cystic fibrosis patients. J Cyst Fibros (2014) 13(4):478–84. doi: 10.1016/j.jcf.2013.11.005
17. Olszowiec-Chlebna M, Koniarek-Maniecka A, Stelmach W, Smejda K, Jerzyńska J, Majak Paweł, et al. Predictors of deterioration of lung function in Polish children with cystic fibrosis. aoms (2016) 2:402–7. doi: 10.5114/aoms.2016.59268
18. Milla CE, Warwick WJ, Moran A. Trends in Pulmonary Function in Patients with Cystic Fibrosis Correlate with the Degree of Glucose Intolerance at Baseline. Am J Respir Crit Care Med (2000) 162(3):891–5. doi: 10.1164/ajrccm.162.3.9904075
19. Bizzarri C, Montemitro E, Pedicelli S, Ciccone S, Majo F, Cappa M, et al. Glucose tolerance affects pubertal growth and final height of children with cystic fibrosis: Cystic Fibrosis-Related Diabetes and Pubertal Growth. Pediatr Pulmonol (2015) 50(2):144–9. doi: 10.1002/ppul.23042
20. Van Sambeek L, Cowley ES, Newman DK, Kato R. Sputum Glucose and Glycemic Control in Cystic Fibrosis-Related Diabetes: A Cross-Sectional Study. PloS One (2015) 10(3):e0119938. doi: 10.1371/journal.pone.0119938
21. Limoli DH, Yang J, Khansaheb MK, Helfman B, Peng L, Stecenko AA, et al. Staphylococcus aureus and Pseudomonas aeruginosa co-infection is associated with cystic fibrosis-related diabetes and poor clinical outcomes. Eur J Clin Microbiol Infect Dis (2016) 35(6):947–53. doi: 10.1007/s10096-016-2621-0
22. Reece E, Segurado R, Jackson A, McClean S, Renwick J, Greally P. Co-colonisation with Aspergillus fumigatus and Pseudomonas aeruginosa is associated with poorer health in cystic fibrosis patients: an Irish registry analysis. BMC Pulm Med (2017) 17(1):70. doi: 10.1186/s12890-017-0416-4
23. Merlo CA, Boyle MP, Diener-West M, Marshall BC, Goss CH, Lechtzin N. Incidence and Risk Factors for Multiple Antibiotic-Resistant Pseudomonas aeruginosa in Cystic Fibrosis. Chest (2007) 132(2):562–8. doi: 10.1378/chest.06-2888
24. Schmid K, Fink K, Holl RW, Hebestreit H, Ballmann M. Predictors for future cystic fibrosis-related diabetes by oral glucose tolerance test. J Cyst Fibros (2014) 13(1):80–5. doi: 10.1016/j.jcf.2013.06.001
25. Sheikh S, Putt ME, Forde KA, Rubenstein RC, Kelly A. Elevation of one hour plasma glucose during oral glucose tolerance testing: 1-Hour Plasma Glucose and Cystic Fibrosis. Pediatr Pulmonol (2015) 50(10):963–9. doi: 10.1002/ppul.23237
26. Schiaffini R, Brufani C, Russo B, Fintini D, Migliaccio A, Pecorelli L, et al. Abnormal glucose tolerance in children with cystic fibrosis: the predictive role of continuous glucose monitoring system. Eur J Endocrinol (2010) 162(4):705–10. doi: 10.1530/EJE-09-1020
27. Yi Y, Norris AW, Wang K, Sun X, Uc A, Moran A, et al. Abnormal Glucose Tolerance in Infants and Young Children with Cystic Fibrosis. Am J Respir Crit Care Med (2016) 194(8):974–80. doi: 10.1164/rccm.201512-2518OC
28. Fattorusso V, Casale A, Raia V, Mozzillo E, Franzese A. Long-Term Follow-Up in a Girl with Cystic Fibrosis and Diabetes Since the First Year of Life. Diabetes Ther (2017) 8(5):1187–90. doi: 10.1007/s13300-017-0289-9
29. Prentice BJ, Ooi CY, Strachan RE, Hameed S, Ebrahimkhani S, Waters S, et al. Early glucose abnormalities are associated with pulmonary inflammation in young children with cystic fibrosis. J Cyst Fibros (2019) 18(6):869–73. doi: 10.1016/j.jcf.2019.03.010
30. Prentice BJ, Chelliah A, Ooi CY, Hameed S, Verge CF, Plush L, et al. Peak OGTT glucose is associated with lower lung function in young children with cystic fibrosis. J Cyst Fibros (2020) 19(2):305–9. doi: 10.1016/j.jcf.2019.05.005
31. Prentice BJ, Ooi CY, Verge CF, Hameed S, Widger J. Glucose abnormalities detected by continuous glucose monitoring are common in young children with Cystic Fibrosis. J Cyst Fibros (2020) 19(5):700–3. doi: 10.1016/j.jcf.2020.02.009
32. Mozzillo E, Raia V, Fattorusso V, Falco M, Sepe A, Gregorio F, et al. Glucose Derangements in Very Young Children With Cystic Fibrosis and Pancreatic Insufficiency. Diabetes Care (2012) 35(11):e78–8. doi: 10.2337/dc12-0459
33. Rolon MA, Benali K, Munck A, Navarro J, Clement A, Tubiana-Rufi N, et al. Cystic fibrosis-related diabetes mellitus: clinical impact of prediabetes and effects of insulin therapy. Acta Paediatr (2001) 90(8):860–7. doi: 10.1111/j.1651-2227.2001.tb02446.x
34. Koloušková S, Zemková D, Bartošová J, Skalická V, Šumník Z, Vávrová V, et al. Low-dose insulin therapy in patients with cystic fibrosis and early-stage insulinopenia prevents deterioration of lung function: a 3-year prospective study. J Pediatr Endocrinol Metab (2011) 24(7-8). doi: 10.1515/jpem.2011.050
35. Grover P, Thomas W, Moran A. Glargine versus NPH insulin in cystic fibrosis?A3B2 show [#,32] ?> related diabetes. J Cyst Fibros (2008) 7(2):134–6. doi: 10.1016/j.jcf.2007.07.004
36. Frost F, Dyce P, Nazareth D, Malone V, Walshaw MJ. Continuous glucose monitoring guided insulin therapy is associated with improved clinical outcomes in cystic fibrosis-related diabetes. J Cyst Fibros (2018) 17(6):798–803. doi: 10.1016/j.jcf.2018.05.005
37. Mozzillo E, Franzese A, Valerio G, Sepe A, De Simone I, Mazzarella G, et al. One-year glargine treatment can improve the course of lung disease in children and adolescents with cystic fibrosis and early glucose derangements. Pediatr Diabetes (2009) 10(3):162–7. doi: 10.1111/j.1399-5448.2008.00451.x
38. Minicucci L, Haupt M, Casciaro R, De Alessandri A, Bagnasco F, Lucidi V, et al. Slow-release insulin in cystic fibrosis patients with glucose intolerance: a randomized clinical trial. Pediatr Diabetes (2012) 13(2):197–202. doi: 10.1111/j.1399-5448.2011.00810.x
39. Moran A, Pekow P, Grover P, Zorn M, Slovis B, Pilewski J, et al. Insulin Therapy to Improve BMI in Cystic Fibrosis-Related Diabetes Without Fasting Hyperglycemia: Results of the Cystic Fibrosis Related Diabetes Therapy Trial. Diabetes Care (2009) 32(10):1783–8. doi: 10.2337/dc09-0585
40. Ballmann M, Hubert D, Assael BM, Staab D, Hebestreit A, Naehrlich L, et al. Repaglinide versus insulin for newly diagnosed diabetes in patients with cystic fibrosis: a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol (2018) 6(2):114–21. doi: 10.1016/S2213-8587(17)30400-X
41. Hardin DS, Rice J, Rice M, Rosenblatt R. Use of the insulin pump in treat cystic fibrosis related diabetes. J Cyst Fibros (2009) 8(3):174–8. doi: 10.1016/j.jcf.2008.12.001
42. Geyer MC, Sullivan T, Tai A, Morton JM, Edwards S, Martin AJ, et al. Exenatide corrects postprandial hyperglycaemia in young people with cystic fibrosis and impaired glucose tolerance: A randomized crossover trial. Diabetes Obes Metab (2019) 21(3):700–4. doi: 10.1111/dom.13544
43. Gnanapragasam H, Mustafa N, Bierbrauer M, Andrea Providence T, Dandona P. Semaglutide in Cystic Fibrosis-Related Diabetes. J Clin Endocrinol Metab (2020) 105(7):2341–4. doi: 10.1210/clinem/dgaa167
44. Hameed S, Morton JR, Field PI, Belessis Y, Yoong T, Katz T, et al. Once daily insulin detemir in cystic fibrosis with insulin deficiency. Arch Dis Child (2012) 97(5):464–7. doi: 10.1136/adc.2010.204636
45. Choudhury M, Taylor P, Morgan PH, Duckers J, Lau D, George L, et al. Association between HbA 1c and the development of cystic fibrosis-related diabetes. Diabetes Med (2019) 36(10):1251–5. doi: 10.1111/dme.13912
46. Mueller-Brandes C. New criteria for impaired fasting glucose and screening for diabetes in cystic fibrosis. Eur Respir J (2005) 25(4):715–7. doi: 10.1183/09031936.05.00068104
Keywords: cystic fibrosis-related diabetes, prediabetes, oral glucose tolerance test (OGTT), continuous glucose monitoring, abnormal glucose tolerance, systematic review, recommendations, glargine insulin
Citation: Mozzillo E, Franceschi R, Piona C, Passanisi S, Casertano A, Pjetraj D, Maltoni G, Calcaterra V, Cauvin V, Cherubini V, D’Annunzio G, Franzese A, Frongia AP, Lombardo F, Lo Presti D, Matteoli MC, Piccinno E, Predieri B, Rabbone I, Scaramuzza AE, Toni S, Zucchini S, Maffeis C and Schiaffini R (2021) Diabetes and Prediabetes in Children With Cystic Fibrosis: A Systematic Review of the Literature and Recommendations of the Italian Society for Pediatric Endocrinology and Diabetes (ISPED). Front. Endocrinol. 12:673539. doi: 10.3389/fendo.2021.673539
Received: 27 February 2021; Accepted: 29 March 2021;
Published: 29 April 2021.
Edited by:
Brenda Kohn, Pediatric Endocrinology and Diabetes NYU- Langone Medical Center NYU Grossman School of Medicine, United StatesReviewed by:
Raquel Barrio, Pediatric Diabetology D-Medical Clinic, SpainMary Pat Gallagher, New York University, United States
Copyright © 2021 Mozzillo, Franceschi, Piona, Passanisi, Casertano, Pjetraj, Maltoni, Calcaterra, Cauvin, Cherubini, D’Annunzio, Franzese, Frongia, Lombardo, Lo Presti, Matteoli, Piccinno, Predieri, Rabbone, Scaramuzza, Toni, Zucchini, Maffeis and Schiaffini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enza Mozzillo, bW96emlsbG9lbnphQGdtYWlsLmNvbQ==; Roberto Franceschi, cm9iZXJ0by5mcmFuY2VzY2hpQGFwc3MudG4uaXQ=; Riccardo Schiaffini, cmljY2FyZG8uc2NoaWFmZmluaUBvcGJnLm5ldA==
†ORCID: Dorina Pjetraj, orcid.org/0000-0002-0238-7865
‡These authors have contributed equally to this work
 Enza Mozzillo
Enza Mozzillo Roberto Franceschi
Roberto Franceschi Claudia Piona
Claudia Piona Stefano Passanisi
Stefano Passanisi Alberto Casertano
Alberto Casertano Dorina Pjetraj5†
Dorina Pjetraj5† Giulio Maltoni
Giulio Maltoni Valeria Calcaterra
Valeria Calcaterra Valentino Cherubini
Valentino Cherubini Giuseppe D’Annunzio
Giuseppe D’Annunzio Adriana Franzese
Adriana Franzese Fortunato Lombardo
Fortunato Lombardo Elvira Piccinno
Elvira Piccinno Barbara Predieri
Barbara Predieri Ivana Rabbone
Ivana Rabbone Andrea Enzo Scaramuzza
Andrea Enzo Scaramuzza Sonia Toni
Sonia Toni Stefano Zucchini
Stefano Zucchini Claudio Maffeis
Claudio Maffeis Riccardo Schiaffini
Riccardo Schiaffini