- Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: Thyroid function is closely involved in cardiovascular diseases. The free triiodothyronine (fT3) to free thyroxine (fT4) ratio has been reported as a risk factor for coronary artery disease, but its prognostic value in euthyroid patients with myocardial infarction with nonobstructive coronary arteries (MINOCA) remains unclear.
Methods: A total of 1162 euthyroid patients with MINOCA were enrolled and divided according to decreased tertiles of fT3/fT4 ratio. The study endpoint was major adverse cardiovascular events (MACE), including all-cause death, nonfatal MI, nonfatal stroke, revascularization, and hospitalization for unstable angina or heart failure. Kaplan-Meier, Cox regression, and receiver-operating characteristic analyses were performed.
Results: Patients with lower fT3/fT4 tertile levels had a significantly higher incidence of MACE (10.0%, 13.9%, 18.2%; p=0.005) over the median follow-up of 41.7 months. The risk of MACE increased with the decreasing fT3/fT4 tertiles even after multivariate adjustment (tertile1 as reference, tertile2: HR 1.58, 95% CI: 1.05-2.39, p=0.030; tertile3: HR 2.06, 95% CI: 1.17-3.11, p=0.006). Lower level of fT3/fT4 ratio remained a robust predictor of MACE in overall (HR 1.64, 95% CI: 1.18-2.29, p=0.003) and in subgroups. When adding fT3/fT4 ratio [area under the curve (AUC) 0.61] into the thrombolysis in myocardial infarction (TIMI) risk score (AUC 0.69), the combined model (AUC 0.74) yielded a significant improvement in discrimination for MACE (ΔAUC 0.05, p=0.023).
Conclusions: Low level of fT3/fT4 ratio was strongly associated with a poor prognosis in euthyroid patients with MINOCA. Routine assessment of fT3/fT4 ratio may facilitate risk stratification in this specific population.
Introduction
Acute myocardial infarction (AMI) remains the leading cause of high morbidity and mortality of cardiovascular (CV) diseases worldwide (1). Recently, a distinct population with myocardial infarction with nonobstructive coronary arteries (MINOCA) has been increasingly recognized due to the widespread use of coronary angiography. MINOCA occurs in 5% to 10% of all AMIs and they are younger and more often women compared to those with AMI and obstructive coronary artery disease (CAD) (2–5). It has been found that the prognosis of MINOCA is not trivial and these patients are still at considerable risks for long-term adverse CV events despite optimal secondary prevention treatments (6–10). Thus, it is of necessity to find potential residual risk factors and improve prognosis for MINOCA population.
Thyroid hormones (TH) have been linked with a variety of CV processes. Subclinical or overt thyroid diseases such as hyperthyroidism and hypothyroidism are significantly associated with the development of atherosclerosis and subsequent worse CV outcomes, and the underlying mechanisms may include inflammation, endothelial injury, changes in blood pressure, dyslipidemia, atherogenesis, and cardiac dysfunction (11, 12). Even in euthyroid individuals, minor alterations in TH concentration may lead to increased CV morbidity and mortality (13, 14). Recently, the ratio of free triiodothyronine (fT3) to free thyroxine (fT4) has been suggested as an indirect index reflecting the conversion of T4 to T3 and the peripheral deiodinase activity (15, 16). As reported, the reduction of fT3/fT4 ratio is commonly seen in CV diseases, especially during acute illness (17–19). Meanwhile, lower fT3/fT4 ratio is closely related to unfavorable prognosis in different cohorts with CAD (20–22). However, the predictive value of fT3/fT4 ratio in euthyroid patients with MINOCA remains unclear. Here, we investigated the association between fT3/fT4 ratio and long-term outcomes after MINOCA and explored whether this ratio might provide significant prognostic information in this population.
Methods
Study Population
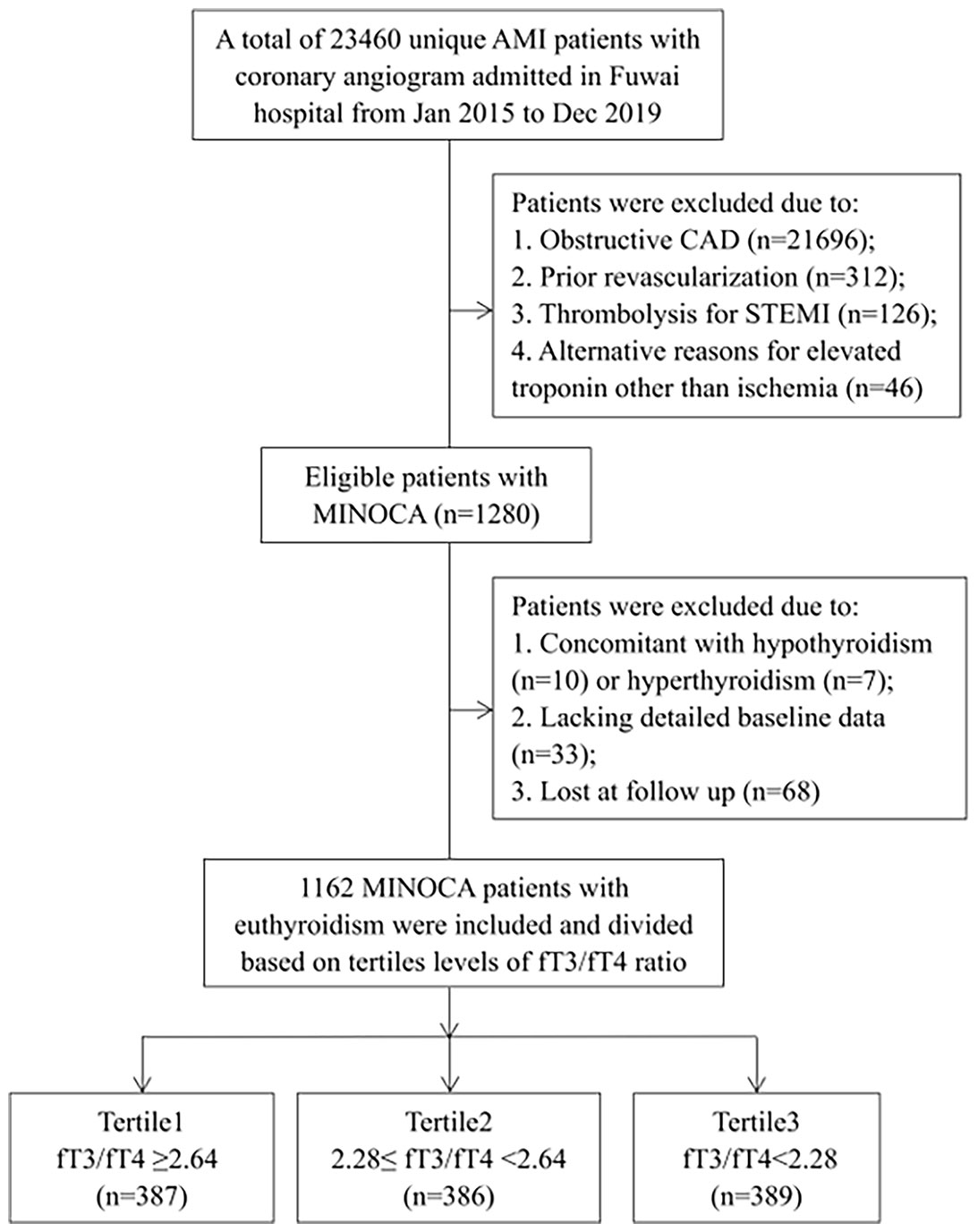
This was a single-center, prospective and observational cohort study of patients with MINOCA. From January 2015 to December 2019, a total of 23460 unique AMI patients with coronary angiogram were consecutively hospitalized in Fuwai hospital, including non ST-segment elevation myocardial infarction (NSTEMI) and ST-segment elevation myocardial infarction (STEMI). Patients were diagnosed with MINOCA if they met the 4th universal definition of AMI (23) and the coronary angiography did not show a stenosis of ≥50% in epicardial coronary arteries (2). Patients were excluded due to: (1) presence of obstructive CAD (n=21696); (2) prior revascularization (n=312); (3) thrombolytic therapy for STEMI since the coronary lesion may be affected by thrombolysis (n=126); (4) alternate explanations for elevated troponin rather than coronary-related causes (e.g., acute heart failure, myocarditis, pulmonary embolism, takotsubo syndrome, n=46); (5) concomitant with hyperthyroidism (n=7) or hypothyroidism (n=10); (6) lack of detailed baseline data (n=33); (7) lost at follow up (n=68). As a result, 1162 eligible MINOCA patients with euthyroid were enrolled in final analysis (Figure 1). Patients were prescribed the evidence-based secondary therapies, including dual anti-platelet therapy (DAPT), statins, β-blocker, and angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor antagonist (ARB) (24, 25). This study was approved by the Ethics Committee of Fuwai hospital and complied with the Declaration of Helsinki. All enrolled subjects provided the written informed consent.
Data Collection
Patients’ demographics, medical history, laboratory test, echocardiographic data and medication were collected and verified from in-person interviews and medical records. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. The thyroid function profiles including fT3, fT4, and TSH were measured using a direct chemiluminescence method (ADVIA Centaur, Siemens, USA). The reference intervals were as follows: fT3, 2.36-4.21 pg/mL; fT4, 0.89-1.76 ng/dL; TSH, 0.55-4.78 μIU/mL. The concentrations of fasting blood glucose (FBG), low density lipoprotein cholesterol (LDL-C), creatinine, and high-sensitive C-reactive protein (hs-CRP) were tested using an automatic biochemistry analyzer. The N-terminal po-B-type natriuretic peptide (NT-proBNP) and cardiac troponin I (TnI) values at admission were recorded. The left ventricular ejection fraction (LVEF) was measured using echocardiography with the biplane Simpson method. The Thrombolysis in Myocardial Infarction (TIMI) risk score was calculated since admission as previously described (26, 27).
Definitions and Outcomes
In this study, euthyroidism was defined as having no history of hyperthyroidism or hypothyroidism and with normal levels of fT3, fT4, and TSH (11). Diabetes was defined with FBG ≥7.0 mmol/L, 2-h plasma glucose ≥11.1 mmol/L, or having a diabetic history (28). Hypertension was defined as repeated blood pressure ≥140/90 mmHg, use of anti-hypertensive drugs, or having a history of hypertension. Dyslipidemia was diagnosed by medical history or having LDL-C≥3.4 mmol/L, high density lipoprotein cholesterol <1.0 mmol/L, or triglyceride≥ 1.7mmol/L (29).
The primary study endpoint was a composite of major adverse cardiovascular events (MACE), including all-cause death, nonfatal MI, revascularization, nonfatal stroke, and hospitalization for unstable angina (UA) or heart failure (HF). The MACE was assessed as time to first event. The secondary endpoints included each component of MACE and the composite “hard” endpoint of death, nonfatal MI, revascularization, and nonfatal stroke. Reinfarction was diagnosed according to the 4th universal definition of MI (23). Revascularization was performed at the operator’s discretion due to recurrent ischemia and progression of coronary lesion. Stroke was defined by the presence of neurological dysfunction and vascular brain injury caused by cerebral ischemia or hemorrhage (30). Hospitalization for UA or HF reflected the clinical status and quality of life after AMI. Patients were regularly followed up at clinics or via telephone by a team of independent researchers. The endpoints were confirmed by at least two professional cardiologists.
Statistical Analysis
Data were expressed as mean ± standard deviation (SD) or median with interquartile range for continuous variables and numbers with percentages for categorical variables. Differences were evaluated using the analysis of variance or Kruskal-Wallis H test for continuous variables and Pearson’s χ2 or Fisher’s exact test for categorical variables. Cumulative incidence of MACE among groups were showed by Kaplan-Meier analysis and compared using log-rank test. The univariable and multivariable Cox proportional regression analyses were used to identify association between levels of fT3/fT4 ratio and outcomes. The risk of MACE was adjusted by age and sex and further adjusted by multiple clinically relevant variables, including age, sex, MI classification (NSTEMI or STEMI), hypertension, diabetes and dyslipidemia. The hazard ratio (HR) with 95% confidence interval (CI) were calculated. Discrimination was defined with areas under the curve (AUC) using a receiver-operating characteristic curve (ROC) analysis. The AUC values were categorized as negligible (≤0.55), small (0.56-0.63), moderate (0.64-0.70) or strong (≥0.71) (31), and compared by Delong’s test (32) with MedCalc version 11.4 (MedCalc Inc., Ostend, Belgium). A combined risk model incorporating fT3/fT4 ratio into the original TIMI risk score was generated using Cox regression. A two-tailed P<0.05 was considered statistically significant. Unless stated otherwise, most of the analyses were performed with SPSS version 22.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline Characteristics
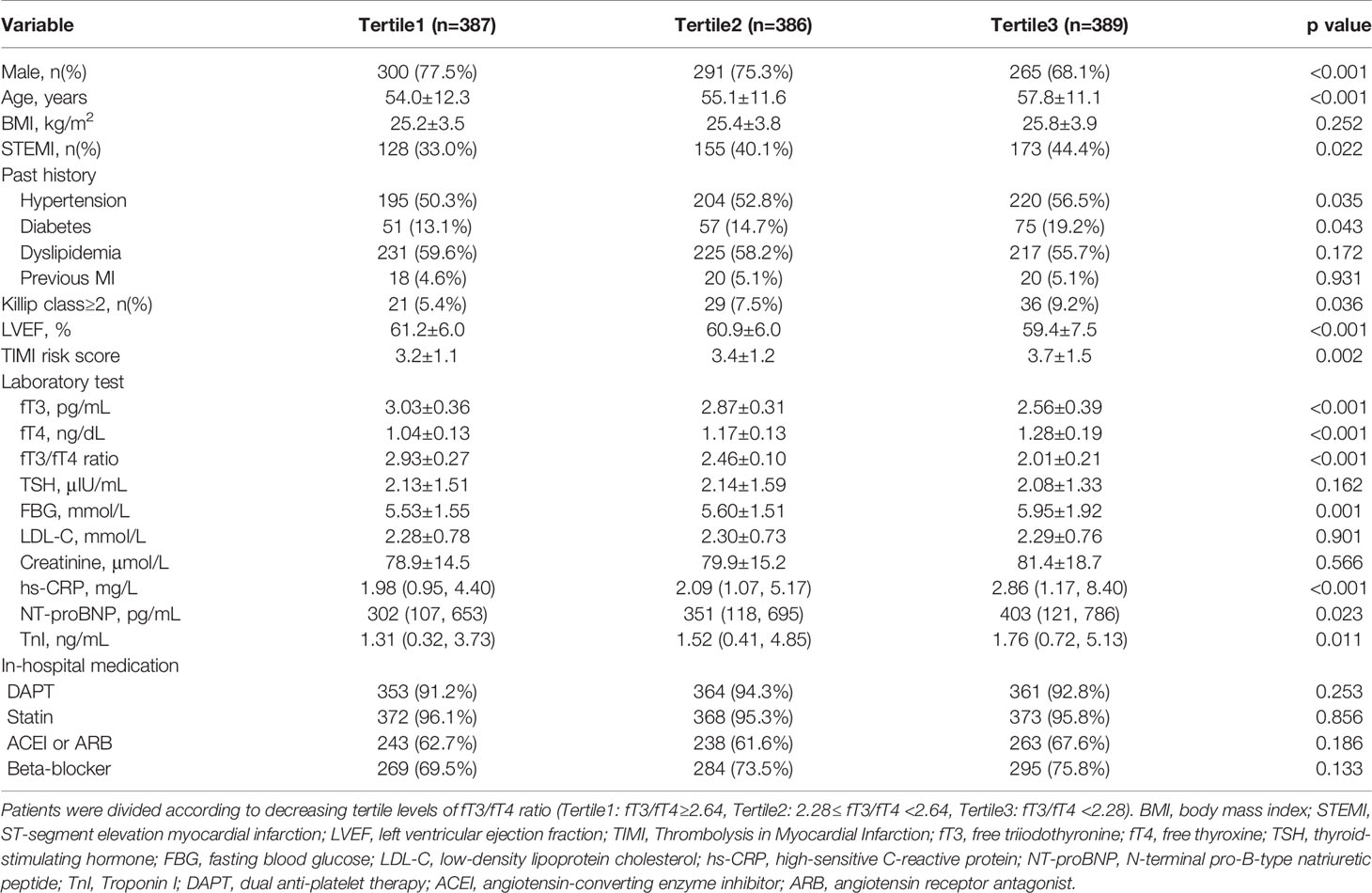
Patients were divided according to decreasing tertile levels of fT3/fT4 ratio (tertile1: fT3/fT4 ≥2.64, n=387; tertile2: 2.28≤ fT3/fT4 <2.64, n=386; tertile3: fT3/fT4 <2.28, n=389) (Figure 1). As shown in Table 1, patients with lower fT3/fT4 tertiles were older and more often female. They had more presence of STEMI and higher prevalence of hypertension and diabetes. They also had higher Killip class, lower LVEF, higher TIMI score, and higher values of FBG, hs-CRP, NT-proBNP and peak TnI. There were no significant differences in BMI, dyslipidemia, prior MI, LDL-C, creatinine levels, and in-hospital medication among the 3 groups. In this regard, patients with lower levels of fT3/fT4 ratio had more baseline risk profiles.
Association Between fT3/fT4 Ratio and Outcomes
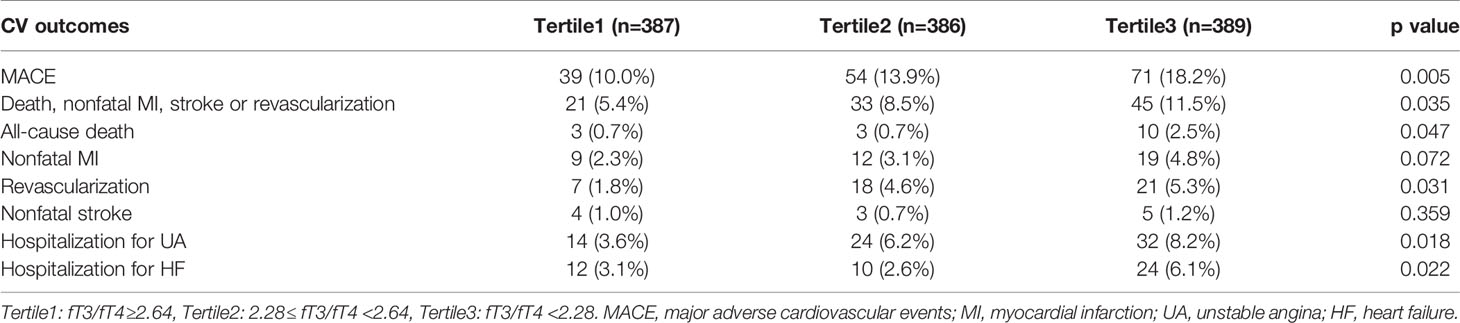
During the median follow-up of 41.7 months, 164 euthyroid patients with MINOCA developed MACE (16 died, 40 had recurrent MI, 46 had revascularization, 12 suffered stroke, 70 was hospitalized for UA and 46 hospitalized for HF) (Table 2). Patients with lower fT3/fT4 had a significantly higher incidence of MACE (10.0%, 13.9%, 18.2%; p=0.005). The rate of the composite endpoint of death, reinfarction, revascularization, or stroke also increased with decreasing fT3/fT4 tertiles (5.4%, 8.5%, 11.5%; p=0.035). In addition, the Kaplan-Meier analysis showed that the cumulative incidence of MACE was significantly higher in patients with lower fT3/fT4 (log rank p=0.011) (Figure 2).
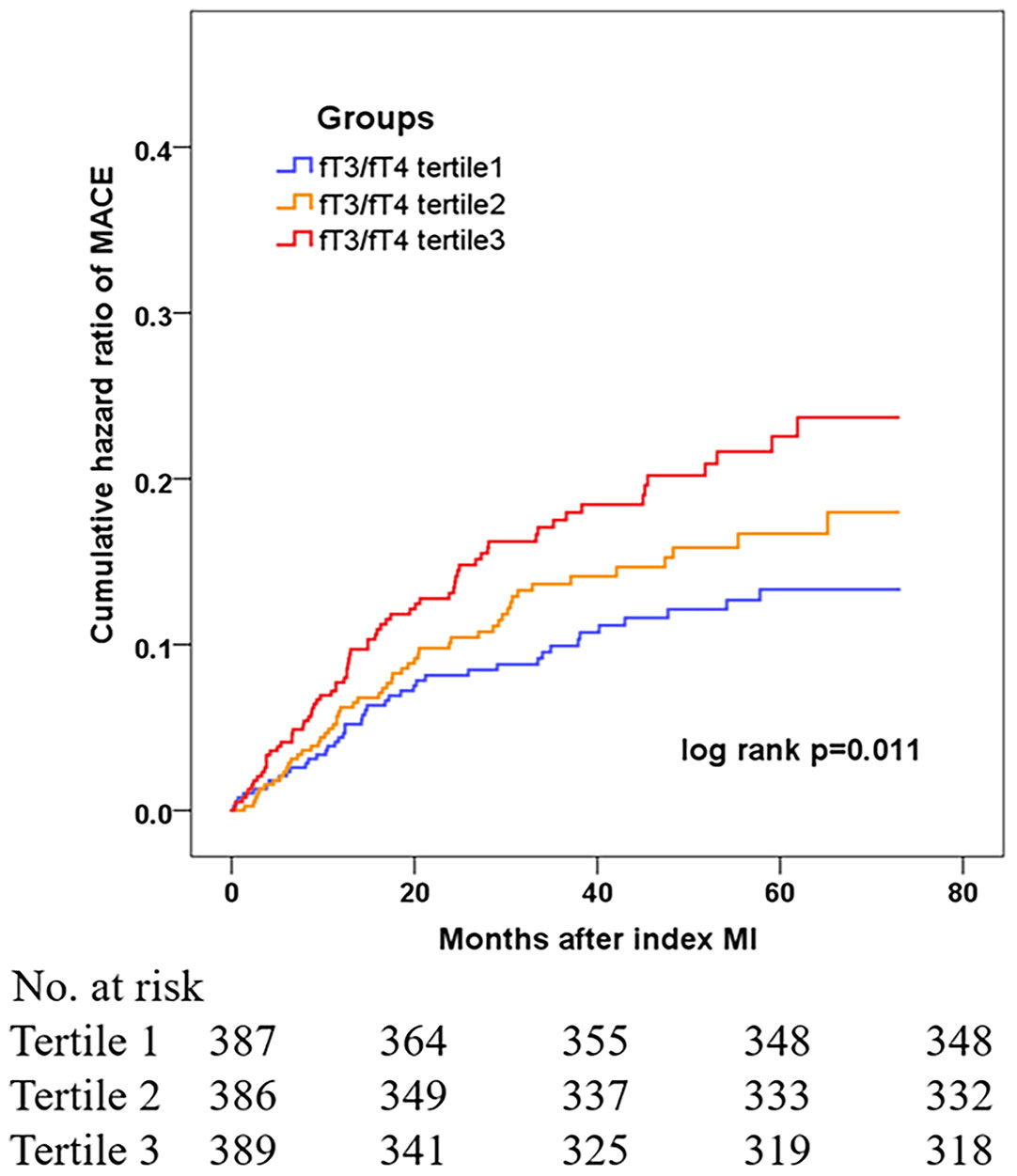
Figure 2 Cumulative hazard ratio of MACE in MINOCA patients based on tertile levels of fT3/fT4 ratio.
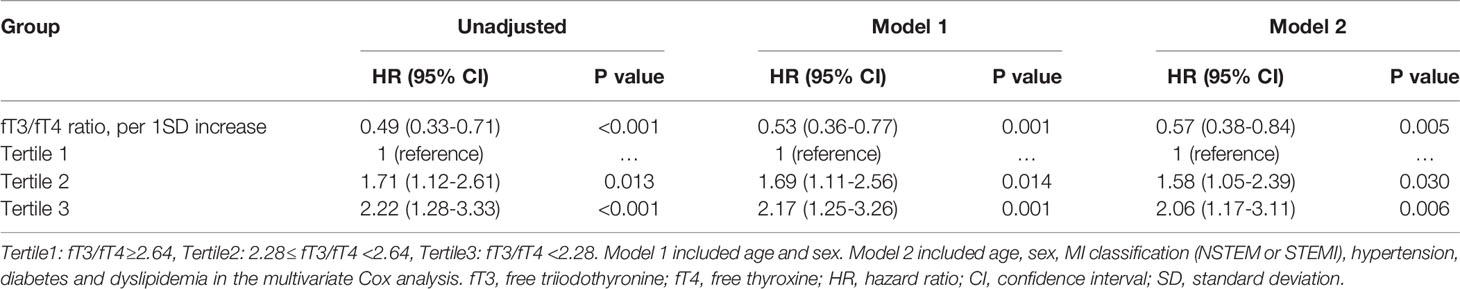
At multivariate Cox analysis, patients with lower fT3/fT4 tertiles had an increased risk of MACE after adjustment for age and sex (tertile 1 as reference; tertile 2: HR 1.69, 95% CI: 1.11-2.56, p=0.014; tertile 3: HR 2.17 95% CI: 1.25-3.26, p=0.001) or after multivariate adjustment (tertile 1 as reference; tertile 2: HR 1.58, 95% CI: 1.05-2.39, p=0.030; tertile 3: HR 2.06, 95% CI: 1.17-3.11, p=0.006) (Table 3). Meanwhile, the fT3/fT4 level was significantly correlated with the adjusted risk of MACE (for per 1SD increase in fT3/fT4, HR 0.57, 95% CI: 0.38-0.84, p=0.005) (Table 3). At ROC analysis, the cutoff of fT3/fT4 that maximized sensitivity and specificity for MACE prediction in all patients was identified as 2.50. Totally, 535 patients (46.0%) had a ratio above the cutoff value. The incidence of MACE was 11.3% and 17.3% (p<0.001) in patients with fT3/fT4 above and below the cutoff, respectively (HR 1.64, 95% CI: 1.18-2.29, p=0.003) (Figure 3). At subgroup analysis, lower fT3/fT4 (<2.50) remained a robust risk factor in subsets of patients stratified by sex, age, BMI, MI classification, history of hypertension, diabetes, and dyslipidemia (all p<0.05) (Figure 3), suggesting that the prognostic effect of fT3/fT4 was not affected by clinically relevant demographic or traditional risk factors.
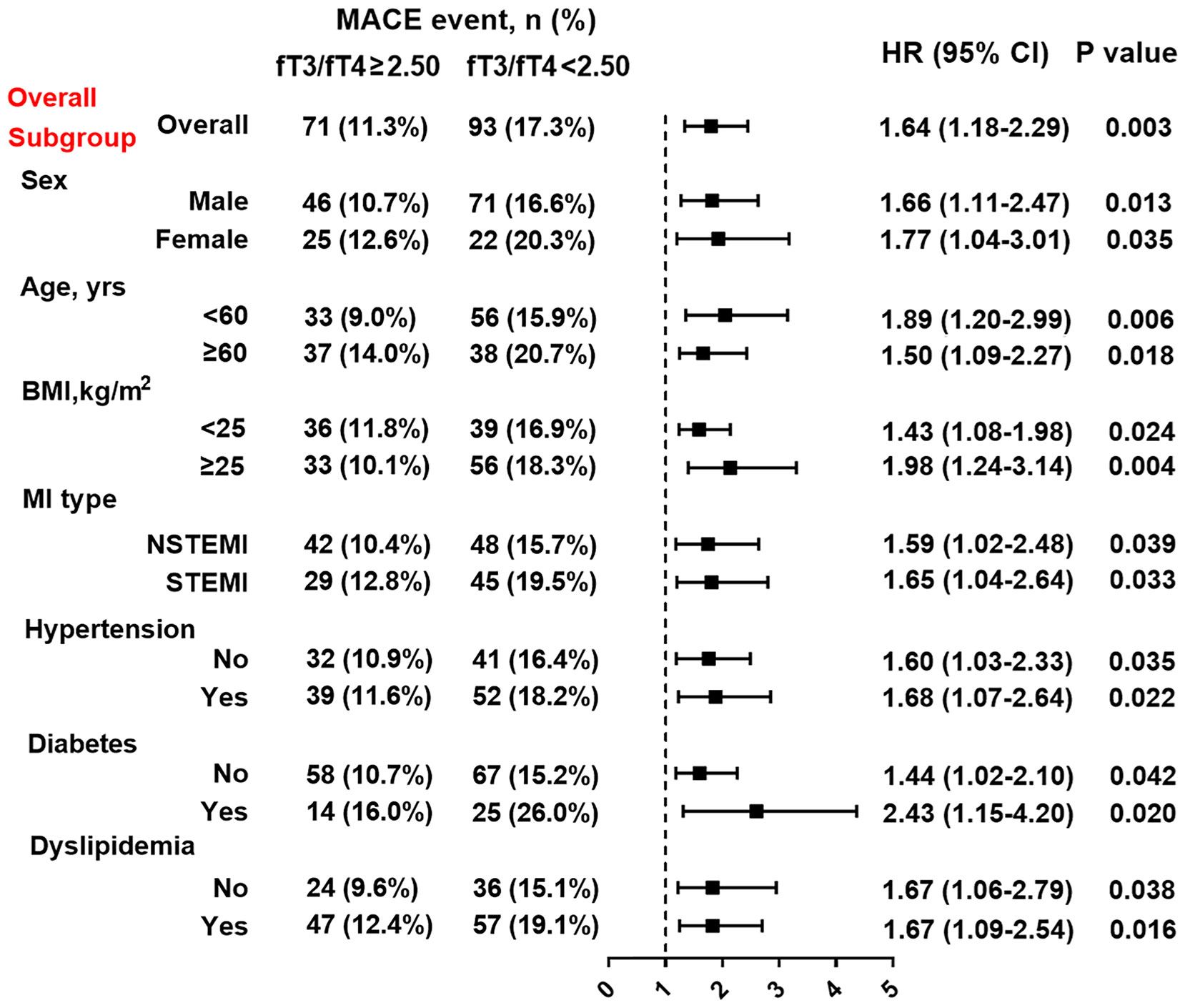
Figure 3 Association between fT3/fT4 ratio and the risk of MACE in overall and subgroups. Subgroup analysis showing the incidence and risk of MACE in patients with fT3/fT4 ratio above and below the cut-off value of 2.50, which was identified with maximum Youden index in all MINOCA patients for MACE prediction. Hazard ratio (HR) was calculated by univariate Cox regression analysis. Vertical dotted line indicated the HR value of 1. BMI, body mass index; STEMI, ST-segment elevation myocardial infarction; NSTEMI; non-ST-segment elevation myocardial infarction, CI, confidence interval.
Predictive Value of fT3/fT4 Ratio for MACE
The ROC analysis confirmed the value of fT3/fT4 ratio for MACE prediction (AUC 0.61, 95% CI: 0.55-0.66, p<0.001) (Figure 4). Meanwhile, the TIMI risk score showed a moderate discrimination for MACE (AUC 0.69, 95% CI: 0.64-0.73, p<0.001). When adding fT3/fT4 ratio to the original TIMI risk score using Cox regression, the combined model yielded a significant improvement in risk prediction (AUC increased from 0.69 to 0.74, ΔAUC 0.05, p=0.023 by DeLong’s test).
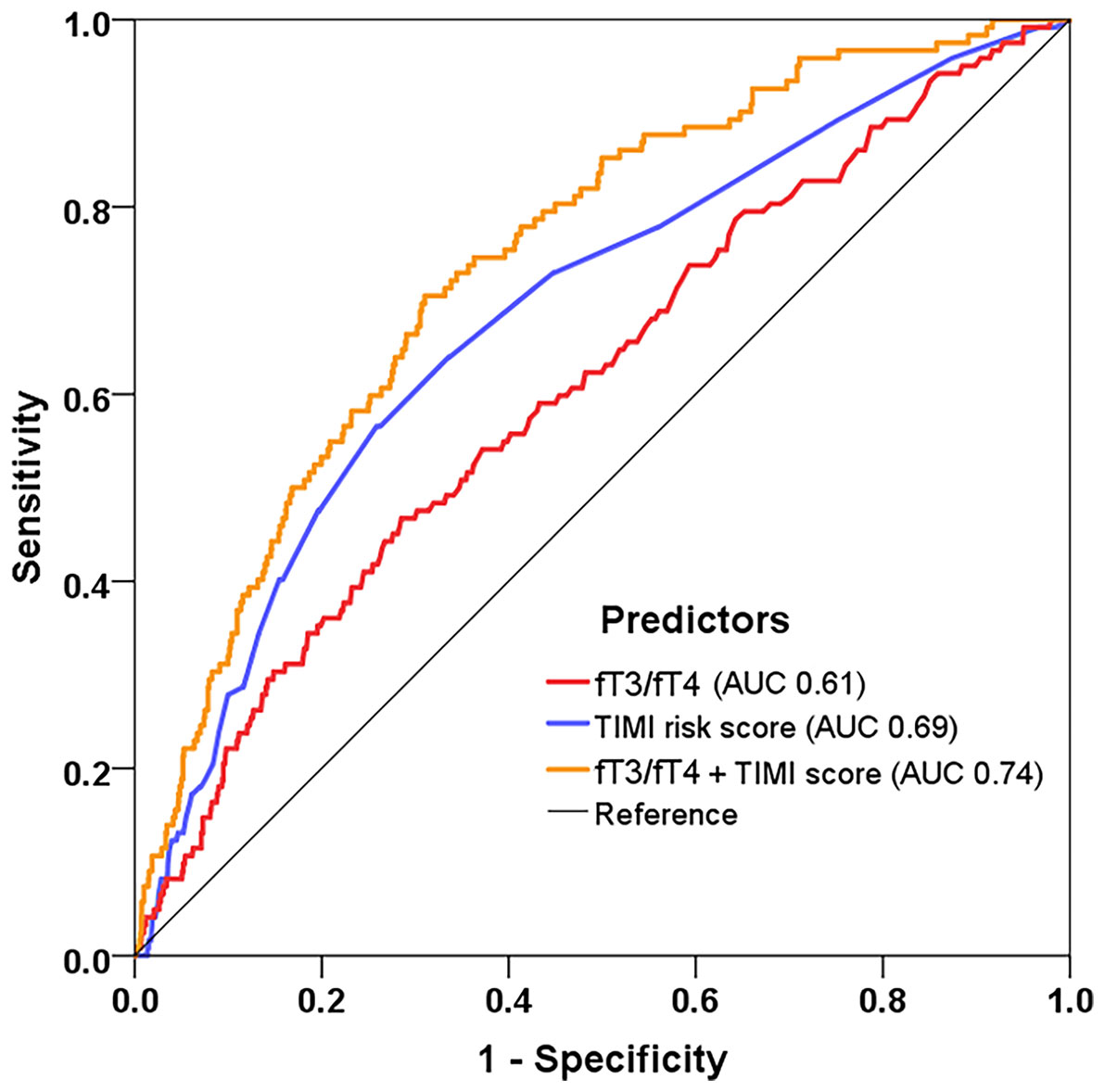
Figure 4 Model improvement in predicting MACE. Receiver operating characteristic curves showing predictive value of the fT3/fT4 ratio, TIMI risk score, and the combined model incorporating fT3/fT4 and TIMI score using Cox regression. fT3, free triiodothyronine; fT4, free thyroxine; TIMI, Thrombolysis in Myocardial Infarction.
Discussion
The present study, for the first time, verified the clinical significance of the fT3/fT4 ratio in euthyroid patients with MINOCA, and found that decreased fT3/fT4 ratio was independently associated with an increased risk of long-term MACE. Adding fT3/fT4 ratio to traditional risk score further improved the outcome prediction. Our data support the utility of fT3/fT4 ratio as a prognostic marker for risk stratification in contemporary real-world management of MINOCA.
MINOCA represents a distinct clinical entity with multiple underlying mechanisms, including plaque rupture or erosion, thromboembolism, spasm, spontaneous dissection, microvascular dysfunction and supply/demand mismatch. Some non-ischemic diseases such as myocarditis may also mimic the presentation of MINOCA (5). More recently, however, it has been used to primarily describe patients with coronary-related ischemia. We adopted this criteria and established a long-term cohort with relatively large sample size. A systematic review estimated the prevalence of MINOCA to be 6% in all AMIs (4), which is close to the prevalence of 5.1% in our study. As reported, nearly one-third of MINOCA would present with STEMI. Patients with MINOCA were more likely to be younger, female, and had fewer comorbidities compared to patients with AMI and obstructive CAD (4). We described the clinical profiles of MINOCA across the fT3/fT4 ratio tertile levels. Meanwhile, we found that the course of MINOCA was not benign. Over the median follow-up of 3.5 years, about 1.4% of MINOCA patients died and 14.1% of them developed MACE. Similarly, previous studies showed that these patients were still at considerable risks for long-term mortality and CV events after MINOCA (4–10). Therefore, the prognosis of MINOCA should be more emphasized and it is necessary to find potential residual risk factors and further improve healthcare for this population.
Thyroid function has a complex relationship with CV physiology (11, 12). Overt thyroid disease or even subclinical thyroid dysfunction can result in alterations in cardiac output, heart rate, vascular resistance, and blood pressure (11). There are also significant changes in atherosclerotic risk factors, including dyslipidemia, hypertension, hypercoagulation, arterial stiffness, and LV dysfunction (12), which may markedly increase CV morbidity and mortality. In addition, euthyroid patients with CV disease may also have alterations in TH concentrations that are associated with worse outcomes (13, 14). TH mainly consist of iodinated T3 and T4 and the free forms of fT3 and fT4. Previous studies have verified the prognostic values of free TH in euthyroid patients with CAD. Lower level of fT3 is commonly seen in AMI, which is not only associated with the severity of myocardial injury and LV dysfunction, but also predicts poor prognosis after AMI (33–35). Meanwhile, high fT4 level is also a potential CV risk factor. Several population-based studies have confirmed that higher fT4 level is independently correlated with atherosclerosis (36) and increased risks of MACE even in euthyroid subjects (37–39). Recent evidence suggest that the combined evaluation of fT3 and fT4 (fT3/fT4 ratio) may serve as a reasonable index of metabolic variation of TH compared with fT3 or fT4 alone and may thus provide a more accurate outcome prediction in different clinical settings. A British cohort study found that lower fT3/fT4 ratio was associated with frailty and long-term mortality in hospitalized older patients (17). The fT3/fT4 ratio was still a robust risk factor for cardiac dysfunction and 1-year mortality in dilated cardiomyopathy (18). In terms of CAD, the fT3/fT4 ratio was reported to be inversely associated with an increased risk of death in euthyroid patients with ACS (19–21). This is not only the case in acute setting, but also in the longer term after recovery from ACS. Another study also indicated that low fT3/fT4 ratio was related to long-term MACE in euthyroid patients with three-vessel disease (22).
In line with previous results, we found that the incidence and adjusted risk of MACE significantly increased with the decreasing tertiles of fT3/fT4 ratio. The fT3/fT4 ratio was inversely correlated with risk of MACE. Meanwhile, lower fT3/fT4 ratio defined by the cut-off of 2.50 remained an independent predictor of MACE in overall and in subgroups. Further, fT3/fT4 ratio provided an incremental predictive value of MACE when added to the TIMI risk score. These results extended the utility of fT3/fT4 ratio to euthyroid patients with MINOCA, suggesting that it might be reasonable to use the ratio as a prognostic marker in daily clinical practice for this specific population.
Actually, both T3 and T4 constitute the active forms of TH, but the majority of T3 is converted from T4 in the process of peripheral deiodination. Moreover, T3 has a higher affinity than T4 for TH receptors in myocardium and vascular tissue, and exerts various bioactive effects on CV system via nongenomic and genomic approaches (40). Thus, the conversion of T4 to T3 is critical for the production of circulating T3 and the TH actions on CV function. Decrease in fT3/fT4 ratio reflects the disturbance of T4 converting to T3, and is commonly seen in acute or chronic processes of myocardial injury as cardiac disease itself may lead to alterations in TH concentrations (17–19). Lower fT3/fT4 ratio, in turn, have deleterious effects on CV systems including reduced cardiac contractility and increased vascular resistance (12–14). Other mechanisms such as cardiac dysfunction, inflammation and oxidative stress have also been proposed (41, 42). Several studies, along with ours, have found that CAD patients with lower fT3/fT4 ratio tended to have more severe myocardial injury (e.g., higher TnI), more impaired LV mechanics (e.g., lower LVEF, higher NT-proBNP) and higher plasma inflammatory markers (e.g., higher hs-CRP) (20–22). All these changes may finally contribute to the increased risks of CV events. However, the pathophysiological and therapeutic relevance of thyroid dysregulation in euthyroid patients after AMI are far from elucidated. Future studies are warranted to confirm our findings and to better understand the biological mechanisms underlying this prognostic association.
Limitation
Some limitations should be mentioned. First, the percentage of women was relatively low in our cohort, possibly due to the large proportion of men in all AMIs treated in our center and a lower rate for women to receive coronary angiography. Given the potential selection bias in single-center studies, future nationwide registry cohorts of MINOCA are warranted to validated our findings. Second, we did not capture and record the exact mechanism for every MINOCA patient. The association between etiology of MINOCA and outcomes should be further investigated. Third, despite multivariate adjustment and subgroup analyses were performed, there might be other unmeasured confounders that may affect the prognosis. Fourth, the fT3/fT4 ratio was only measured at baseline, and the follow-up levels of fT3/fT4 ratio may also be clinically significant.
Conclusion
Decreased fT3/fT4 ratio was an independent predictor of poor outcomes in euthyroid patients with MINOCA. Routine assessment of fT3/fT4 ratio might provide significant prognostic value and facilitate risk stratification and decision making in this population.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Fuwai hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SG conceived and designed the study. SG, WM, SH and XL performed data analysis and interpretation. SG drafted the manuscript. MY reviewed and gave final approval of the version to be published. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (81670415).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.708216/full#supplementary-material
References
1. Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol (2017) 70(1):1–25. doi: 10.1016/j.jacc.2017.04.052
2. Beltrame JF. Assessing Patients With Myocardial Infarction and Nonobstructed Coronary Arteries (MINOCA). J Intern Med (2013) 273(2):182–85. doi: 10.1111/j.1365-2796.2012.02591.x
3. Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, et al. ESC Working Group Position Paper on Myocardial Infarction With Non-Obstructive Coronary Arteries. Eur Heart J (2017) 38(3):143–53. doi: 10.1093/eurheartj/ehw149
4. Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic Review of Patients Presenting With Suspected Myocardial Infarction and Nonobstructive Coronary Arteries. Circulation (2015) 131(10):861–70. doi: 10.1161/CIRCULATIONAHA.114.011201
5. Niccoli G, Scalone G, Crea F. Acute Myocardial Infarction With No Obstructive Coronary Atherosclerosis: Mechanisms and Management. Eur Heart J (2015) 36(8):475–81. doi: 10.1093/eurheartj/ehu469
6. Safdar B, Spatz ES, Dreyer RP, Beltrame JF, Lichtman JH, Spertus JA, et al. Presentation, Clinical Profile, and Prognosis of Young Patients With Myocardial Infarction With Nonobstructive Coronary Arteries (MINOCA): Results From the VIRGO Study. J Am Heart Assoc (2018) 7(13):e009174. doi: 10.1161/JAHA.118.009174
7. Bainey KR, Welsh RC, Alemayehu W, Westerhout CM, Traboulsi D, Anderson T, et al. Population-Level Incidence and Outcomes of Myocardial Infarction With Non-Obstructive Coronary Arteries (MINOCA): Insights From the Alberta Contemporary Acute Coronary Syndrome Patients Invasive Treatment Strategies (COAPT) Study. Int J Cardiol (2018) 264:12–7. doi: 10.1016/j.ijcard.2018.04.004
8. Andersson HB, Pedersen F, Engstrøm T, Helqvist S, Jensen MK, Jørgensen E, et al. Long-Term Survival and Causes of Death in Patients With ST-Elevation Acute Coronary Syndrome Without Obstructive Coronary Artery Disease. Eur Heart J (2018) 39(2):102–10. doi: 10.1093/eurheartj/ehx491
9. Planer D, Mehran R, Ohman EM, White HD, Newman JD, Xu K, et al. Prognosis of Patients With Non-ST-Segment-Elevation Myocardial Infarction and Nonobstructive Coronary Artery Disease: Propensity-Matched Analysis From the Acute Catheterization and Urgent Intervention Triage Strategy Trial. Circ Cardiovasc Interv (2014) 7(3):285–93. doi: 10.1161/CIRCINTERVENTIONS.113.000606
10. Abdu FA, Liu L, Mohammed AQ, Luo Y, Xu S, Auckle R, et al. Myocardial Infarction With Non-Obstructive Coronary Arteries (MINOCA) in Chinese Patients: Clinical Features, Treatment and 1 Year Follow-Up. Int J Cardiol (2019) 287:27–31. doi: 10.1016/j.ijcard.2019.02.036
11. Razvi S, Jabbar A, Pingitore A, Danzi S, Biondi B, Klein I, et al. Thyroid Hormones and Cardiovascular Function and Diseases. J Am Coll Cardiol (2018) 71:1781–96. doi: 10.1016/j.jacc.2018.02.045
12. Jabbar A, Pingitore A, Pearce SH, Zaman A, Iervasi G, Razvi S, et al. Thyroid Hormones and Cardiovascular Disease. Nat Rev Cardiol (2017) 14:39–55. doi: 10.1038/nrcardio.2016.174
13. Fliers E, Bianco AC, Langouche L, Boelen A. Thyroid Function in Critically Ill Patients. Lancet Diabetes Endocrinol (2015) 3:816–25. doi: 10.1016/S2213-8587(15)00225-9
14. Pingitore A, Chen Y, Gerdes AM, Iervasi G. Acute Myocardial Infarction and Thyroid Function: New Pathophysiological and Therapeutic Perspectives. Ann Med (2012) 44(8):745–57. doi: 10.3109/07853890.2011.573501
15. Maia AL, Goemann IM, Meyer EL, Wajner SM. Deiodinases: The Balance of Thyroid Hormone: Type 1 Iodothyronine Deiodinase in Human Physiology and Disease. J Endocrinol (2011) 209(3):283–97. doi: 10.1530/JOE-10-0481
16. Bassols J, Prats-Puig A, Soriano-Rodríguez P, García-González MM, Reid J, Martínez-Pascual M, et al. Lower Free Thyroxin Associates With a Less Favorable Metabolic Phenotype in Healthy Pregnant Women. J Clin Endocrinol Metab (2011) 96(12):3717–23. doi: 10.1210/jc.2011-1784
17. Pasqualetti G, Calsolaro V, Bernardini S, Linsalata G, Bigazzi R, Caraccio N, et al. Degree of Peripheral Thyroxin Deiodination, Frailty, and Long-Term Survival in Hospitalized Older Patients. J Clin Endocrinol Metab (2018) 103(5):1867–76. doi: 10.1210/jc.2017-02149
18. Kozdag G, Ural D, Vural A, Agacdiken A, Kahraman G, Sahin T, et al. Relation Between Free Triiodothyronine/Free Thyroxine Ratio, Echocardiographic Parameters and Mortality in Dilated Cardiomyopathy. Eur J Heart Fail (2005) 7(1):113–8. doi: 10.1016/j.ejheart.2004.04.016
19. Lamprou V, Varvarousis D, Polytarchou K, Varvarousi G, Xanthos T. The Role of Thyroid Hormones in Acute Coronary Syndromes: Prognostic Value of Alterations in Thyroid Hormones. Clin Cardiol (2017) 40(8):528–33. doi: 10.1002/clc.22689
20. Yu T, Tian C, Song J, He D, Wu J, Wen Z, et al. Value of the Ft3/Ft4 Ratio and its Combination With the GRACE Risk Score in Predicting the Prognosis in Euthyroid Patients With Acute Myocardial Infarction Undergoing Percutaneous Coronary Intervention: A Prospective Cohort Study. BMC Cardiovasc Disord (2018) 18(1):181. doi: 10.1186/s12872-018-0916-z
21. Brozaitiene J, Mickuviene N, Podlipskyte A, Burkauskas J, Bunevicius R. Relationship and Prognostic Importance of Thyroid Hormone and N-Terminal Pro-B-Type Natriuretic Peptide for Patients After Acute Coronary Syndromes: A Longitudinal Observational Study. BMC Cardiovasc Disord (2016) 16:45. doi: 10.1186/s12872-016-0226-2
22. Yuan D, Zhang C, Jia S, Liu Y, Jiang L, Xu L, et al. Predictive Value of Free Triiodothyronine (FT3) to Free Thyroxine (FT4) Ratio in Long-Term Outcomes of Euthyroid Patients With Three-Vessel Coronary Artery Disease. Nutr Metab Cardiovasc Dis (2021) 31(2):579–86. doi: 10.1016/j.numecd.2020.10.011
23. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol (2018) 72:2231–64. doi: 10.1016/j.jacc.2018.08.1038
24. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting Without Persistent ST-Segment Elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting Without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J (2016) 37:267–315. doi: 10.1093/eurheartj/ehv320
25. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the Management of Acute Myocardial Infarction in Patients Presenting With ST-Segment Elevation: The Task Force for the Management of Acute Myocardial Infarction in Patients Presenting With ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
26. Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI Risk Score for Unstable Angina/non-ST Elevation MI: A Method for Prognostication and Therapeutic Decision Making. JAMA (2000) 284:835–42. doi: 10.1001/jama.284.7.835
27. Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, de Lemos JA, et al. TIMI Risk Score for ST-Elevation Myocardial Infarction: A Convenient, Bedside, Clinical Score for Risk Assessment at Presentation: An Intravenous nPA for Treatment of Infarcting Myocardium Early II Trial Substudy. Circulation (2000) 102:2031–7. doi: 10.1161/01.cir.102.17.2031
28. American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care (2018) 41:S13–27. doi: 10.2337/dc18-S002
29. Rabar S, Harker M, O’Flynn N, Wierzbicki AS, Guideline Development Group. Lipid Modification and Cardiovascular Risk Assessment for the Primary and Secondary Prevention of Cardiovascular Disease: Summary of Updated NICE Guidance. BMJ (2014) 349:g4356. doi: 10.1136/bmj.g4356
30. Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, et al. 2017 Cardiovascular and Stroke Endpoint Definitions for Clinical Trials. J Am Coll Cardiol (2018) 71:1021–34. doi: 10.1016/j.jacc.2017.12.048
31. Ma H, Bandos AI, Rockette HE, Gur D. On Use of Partial Area Under the ROC Curve for Evaluation of Diagnostic Performance. Stat Med (2013) 32:3449–58. doi: 10.1002/sim.5777
32. Delong ER, Delong DM, ClarkePearson DL. Comparing the Areas Under Two or More Correlated Receiver Operating Characteristic Curves: A Non-Parametric Approach. Biometrics (1988) 44:837–45. doi: 10.2307/2531595
33. Wang WY, Tang YD, Yang M, Cui C, Mu M, Qian J, et al. Free Triiodothyronine Level Indicates the Degree of Myocardial Injury in Patients With Acute ST-Elevation Myocardial Infarction. Chin Med J (Engl) (2013) 126:3926–9. doi: 10.3760/cma.j.issn.0366-6999.20130087
34. Jankauskienė E, Orda P, Barauskienė G, Mickuvienė N, Brožaitienė J, Vaškelytė JJ, et al. Relationship Between Left Ventricular Mechanics and Low Free Triiodothyronine Levels After Myocardial Infarction: A Prospective Study. Intern Emerg Med (2016) 11:391–8. doi: 10.1007/s11739-015-1370-x
35. Wang B, Liu S, Li L, Yao Q, Song R, Shao X, et al. Non-Thyroidal Illness Syndrome in Patients With Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Int J Cardiol (2017) 226:1–10. doi: 10.1016/j.ijcard.2016.10.039
36. Bano A, Chaker L, Mattace-Raso FUS, van der Lugt A, Ikram MA, Franco OH, et al. Thyroid Function and the Risk of Atherosclerotic Cardiovascular Morbidity and Mortality: The Rotterdam Study. Circ Res (2017) 121(12):1392–400. doi: 10.1161/CIRCRESAHA.117.311603
37. Chaker L, van den Berg ME, Niemeijer MN, Franco OH, Dehghan A, Hofman A, et al. Thyroid Function and Sudden Cardiac Death: A Prospective Population-Based Cohort Study. Circulation (2016) 134(10):713–22. doi: 10.1161/CIRCULATIONAHA.115.020789
38. Yeap BB, Alfonso H, Hankey GJ, Flicker L, Golledge J, Norman PE, et al. Higher Free Thyroxine Levels are Associated With All-Cause Mortality in Euthyroid Older Men: The Health In Men Study. Eur J Endocrinol (2013) 169(4):401–8. doi: 10.1530/EJE-13-0306
39. Golledge J, Hankey GJ, Almeida OP, Flicker L, Norman PE, Yeap BB. Plasma Free Thyroxine in the Upper Quartile is Associated With an Increased Incidence of Major Cardiovascular Events in Older Men That Do Not Have Thyroid Dysfunction According to Conventional Criteria. Int J Cardiol (2018) 254:316–21. doi: 10.1016/j.ijcard.2017.10.100
40. Sandler B, Webb P, Apriletti JW, Huber BR, Togashi M, Cunha Lima ST, et al. Thyroxine-Thyroid Hormone Receptor Interactions. J Biol Chem (2004) 279(53):55801–8. doi: 10.1074/jbc.M410124200
41. Mancini A, Di Segni C, Raimondo S, Olivieri G, Silvestrini A, Meucci E, et al. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediators Inflammation (2016) 2016:6757154. doi: 10.1155/2016/6757154
Keywords: thyroid function, FT3/FT4 ratio, myocardial infarction with nonobstructive coronary arteries (MINOCA), cardiovascular outcomes (CV outcomes), euthyroid
Citation: Gao S, Ma W, Huang S, Lin X and Yu M (2021) Predictive Value of Free Triiodothyronine to Free Thyroxine Ratio in Euthyroid Patients With Myocardial Infarction With Nonobstructive Coronary Arteries. Front. Endocrinol. 12:708216. doi: 10.3389/fendo.2021.708216
Received: 11 May 2021; Accepted: 14 July 2021;
Published: 28 July 2021.
Edited by:
Patrick Mueller, University Hospital Münster, GermanyReviewed by:
Stephen Fitzgerald, Royal Adelaide Hospital, AustraliaDavid Duncker, Hannover Medical School, Germany
Copyright © 2021 Gao, Ma, Huang, Lin and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mengyue Yu, eXVteTczQDE2My5jb20=
 Side Gao
Side Gao Mengyue Yu
Mengyue Yu