- 1Department of Endocrine and Breast Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Hepatobiliary, Breast and Thyroid Surgery, The People’s Hospital of Nanchuan, Chongqing, China
Background: Papillary thyroid cancer (PTC) in clinically lymph node-negative (cN0) patients is prone toward lymph node metastasis. As a risk factor for tumor persistence and local recurrence, lateral lymph node metastasis (LLNM) is related to the number of central lymph node metastases (CLNMs).
Methods: We performed LLNM risk stratification based on the number of CLNMs for cN0 PTC patients who underwent thyroidectomy and lymph node dissection between January 2013 and December 2018. A retrospective analysis was applied to the 274 collected patients with 1-2 CLNMs. We examined the clinicopathological characteristics of the patients and constructed a LASSO model.
Results: In the 1–2 CLNM group, tumors >10 mm located in the upper region and nodular goiters were independent risk factors for LLNM. Specifically, tumors >20 mm and located in the upper region contributed to metastasis risk at level II. Hashimoto’s thyroiditis reduced this risk (p = 0.045, OR = 0.280). Age ≤ 30 years and calcification (microcalcification within thyroid nodules) correlated with LLNM. The LASSO model divided the population into low- (25.74%) and high-risk (57.25%) groups for LLNM, with an AUC of 0.715.
Conclusions: For patients with 1–2 CLNMs, young age, calcification, nodular goiter, tumor >10 mm, and tumor in the upper region should alert clinicians to considering a higher occult LLNM burden. Close follow-up and therapy adjustment may be warranted for high-risk patients.
Introduction
Thyroid cancer (TC) is a common endocrine malignancy with an incidence that has constantly increased for decades (1). In 2018, the growth rate rose to 3.1%, with 567,233 new cases (2) and the population experiencing morbidity displaying a younger trend (3). As a major pathological type of TC, papillary thyroid carcinoma (PTC) has a favorable prognosis (4). However, lymph node metastasis (LNM) occurs even in the early stage, with reported incidence rates ranging from 40% to 90% (5). Moreover, LNM correlates with tumor persistence and recurrence and even a poor prognosis (6). It is also reported to occur in more than 80% of recurrence cases (7).
At present, the indications and dissection scopes of lymph node dissection (LND) are still controversial, especially for clinically lymph node-negative (cN0) PTC. In fact, the accuracy of preoperative assessment for cN0 PTC patients is 67.6% and the sensitivity is low (8). Studies show that the incidence of occult central lymph node metastasis (CLNM) ranges from 30% to 80%, with that of occult lateral lymph node metastasis (LLNM) ranging from 18.6% to 64% (8–10). Of note, Asians have a much higher incidence than other populations. According to National Comprehensive Cancer Network (NCCN) (11) and American Thyroid Association (ATA) guidelines (12), experts disagree on prophylactic central lymph node dissection (pCLND), although the Japanese Association of Endocrine Surgeons and the Japanese Society of Thyroid Surgeons recommend routine pCLND (13). By consensus, prophylactic lateral lymph node dissection (pLLND) is not recommended.
China accounts for 37.72% of the world’s new cases (14). Over 80% of Chinese patients have LNM and the 5-year relative survival rate is 84.3%, which is much lower than that in the United States according to the Database Technology Conference China (DTCC) report. Based on research, Chinese expert consensus advises pCLND and selective LLND for high-risk patients with CLNM (15). Many reports have revealed that the number of CLNMs is an independent risk factor for LLNM. The cutoff value differs across studies, ranging from 2 to 3. In general, cN0 PTC patients have a low risk of skip metastasis (central lymph node negative and lateral lymph node positive) (16) and a high risk of LLNM (60%–75%) with ≥3 pathologic CCLMs (17, 18). However, there are few reports on the risk factors for LLNM in CN0 PTC patients with 1–2 pathologic CCLMs.
In this retrospective study, cN0 PTC patients with 1–2 pathologic CCLMs had a median LLNM incidence compared to the skip metastasis and ≥3 CCLM groups. We also investigated the risk factors for LLNM in cN0 PTC patients with 1–2 CCLMs and constructed a prediction model using the LASSO method.
Materials and Methods
Patients
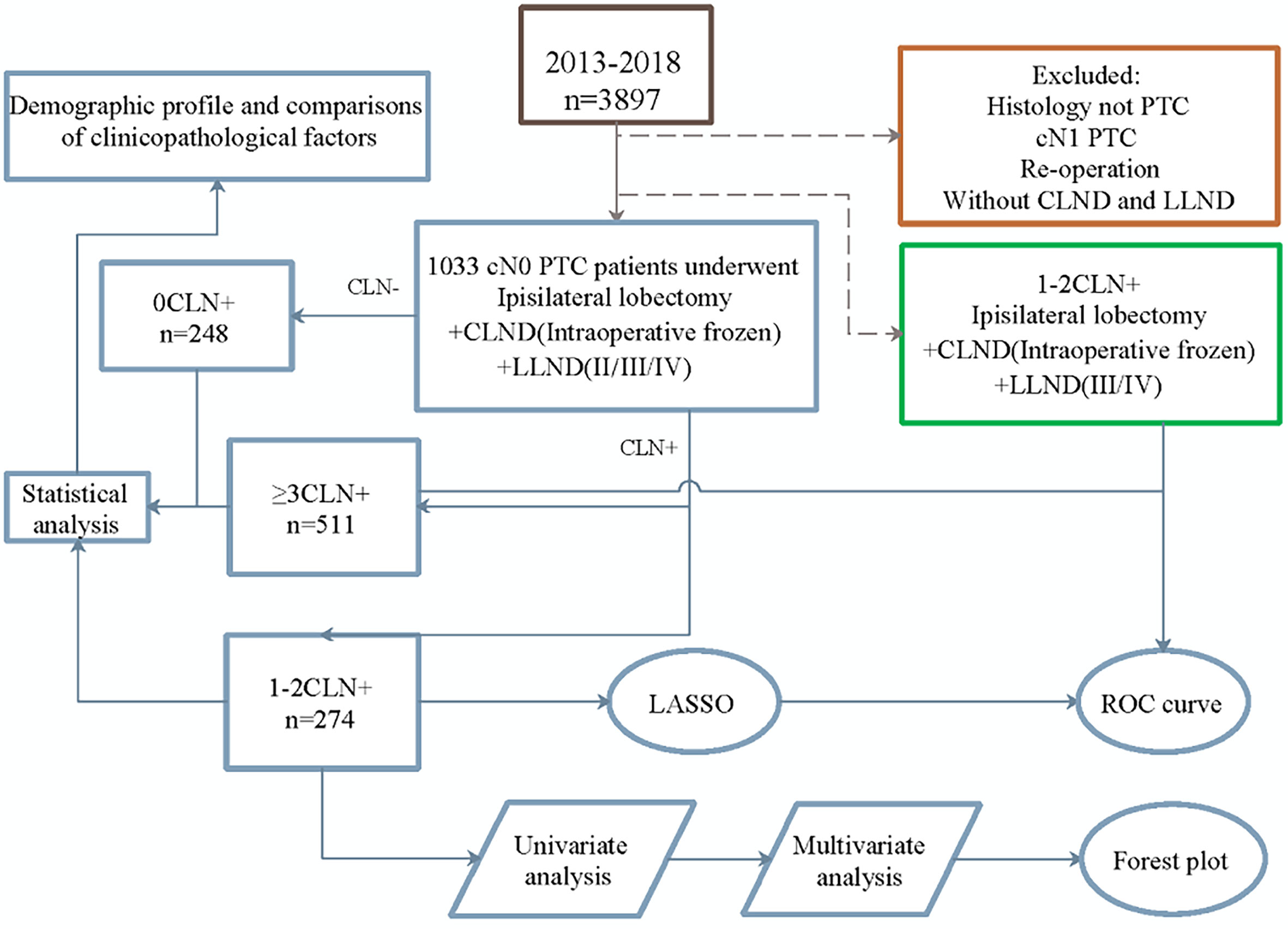
This retrospective study enrolled 1,033 patients at the Department of Endocrine and Breast Surgery from January 2013 to 2018 and was approved by Medical Ethics Committee, the First Affiliated Hospital of Chongqing Medical University. Patients were diagnosed by fine-needle aspiration biopsy (FNAB) and intraoperative frozen and postoperative pathology examined pathologically by three pathologists. Before surgery, physical examination, laryngoscopy, and neck ultrasound were conducted by two experienced ultrasound doctors. The inclusion criteria were as follows: cN0 PTC, complete clinicopathologic data, and primary surgery with ipsilateral lobectomy, CLND, and LLND, including levels II, III, and IV. Intraoperative frozen pathology of CLNs was performed to determine metastasis. For the CN0 assessment criteria, the clinical examination did not address enlarged LNs or swollen LNs that had a soft texture. Ultrasound examination showed no enlarged or swollen LNs that were oval and flat, with clear boundaries between the cortex and medulla, regularly shaped, or had a clear central fat hilum and no obvious malignant signs. We excluded not-PTC, cN1 PTC, reoperation, and patients without LND.
Study Design
The research process is shown in Figure 1. According to the number of CLNMs, patients were divided into three groups: negative, one or two positive, or over three positive. Univariate analysis was performed to explore the differences in demographic and clinicopathological characteristics among the groups.
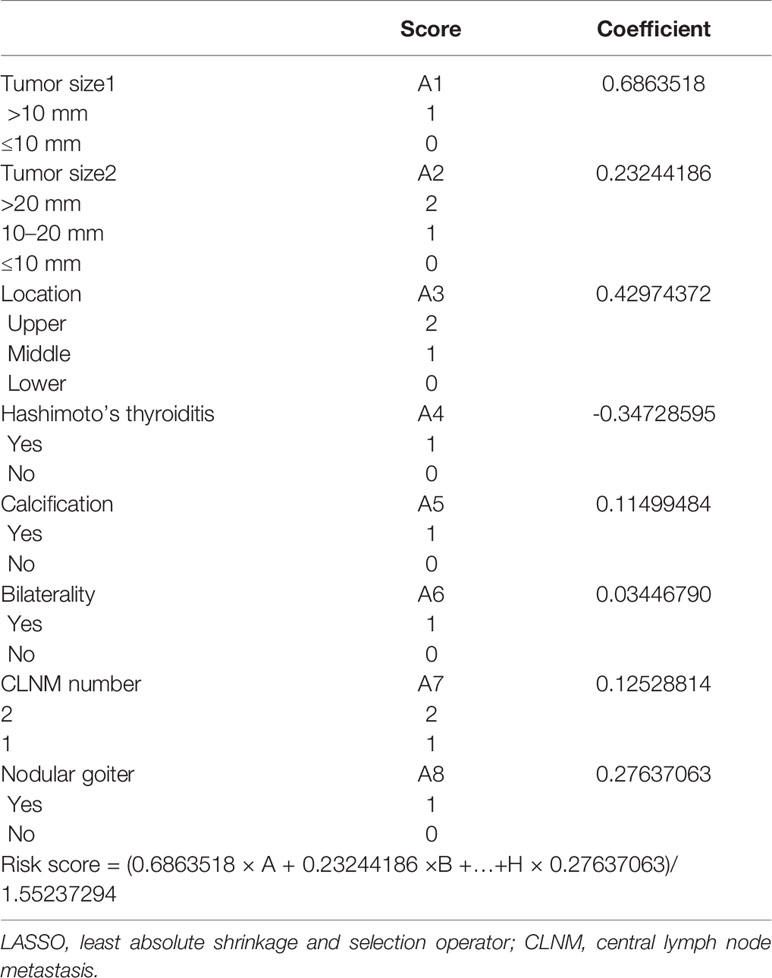
Univariate and multivariate analyses were carried out with patients in the 1–2 CLNM group to determine risk factors associated with LLNM. The LLNM risk prediction model was constructed using the least absolute shrinkage and selection operator (LASSO) method. The method shows good stability and effectively avoids overfitting of the model (19). The risk score was estimated by the sum of the value of each factor multiplied by its corresponding coefficient (α). The standard formula is A1 × α1 + A2× α2 +…An × αn. Every single patient was calculated with a risk score and divided into high- or low-risk groups according to the median score. Meanwhile, to evaluate the quality of the model, time-dependent receiver operating characteristic (ROC) analysis was performed to determine the area under the curve (AUC).
Sex (male, female), age of diagnosis, age (≤30 years, >30 years), tumor size (≤10 mm, >10 mm and ≤20 mm, >20 mm), tumor location (upper/middle/lower pole), nodule number, bilaterality, calcification (microcalcification within thyroid nodules), extrathyroidal extension (ETE), Hashimoto’s thyroiditis (HT), thyroid adenoma, follicular variant, multifocality, CLNM number (2/1), the location of CLNM (prelaryngeal, pretracheal, paratracheal LNM and lymph node posterior to right recurrent laryngeal nerve/LN-prRLN metastasis), number of metastatic and harvested CLNs, number of metastatic and harvested LLNs, and risk (high/low) were included in the analysis.
Statistical Analysis
Continuous variables were analyzed by t test, and categorical variables were analyzed by the chi-squared or Fisher exact test with SPSS version 25.0 (SPSS Inc., Chicago, IL, United States). A p-value <0.05 was considered statistically significant. The LASSO method and ROC curve were applied with the glmnet package and timeROC R package (version 0.3), respectively.
Results
Clinicopathologic Characteristics of CLM
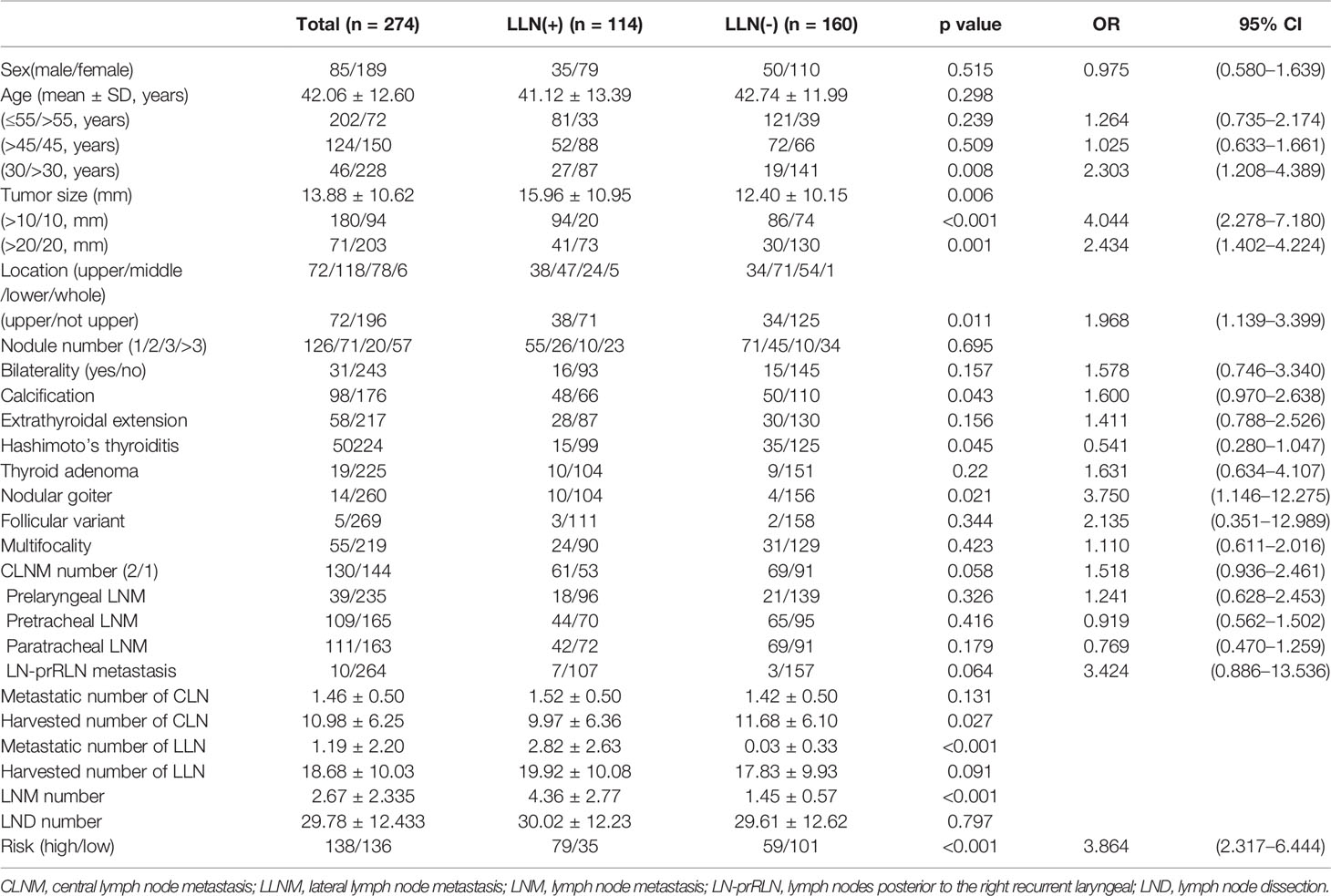
CLM was correlated with sex, age of diagnosis, tumor size, ETE, bilaterality, LLNM, and number of harvested CLNs and LLNs (p < 0.001) (Table 1). Specifically, more patients in the CLMs greater than 3 group had a large tumor size, HT, and bilaterality than those in the other two groups (p < 0.001). Patents with 1–2 CLMs had greater calcification than the other groups (35.77% vs. 10.89%, 35.77% vs. 16.83%), with a nonlinear trend. Differences in LLNM incidences were obvious among the 0, 1–2, and ≥3 CLNM groups (16.53% vs. 41.61% vs. 64.58%, p < 0.001). Based on ATA guidelines, a linear increase in medium–high recurrence risk was observed (29.27% vs. 37.59% vs. 71.82%, respectively; p < 0.001). Two patients experienced recurrence due to LNM in the ≥3 CLNM groups.
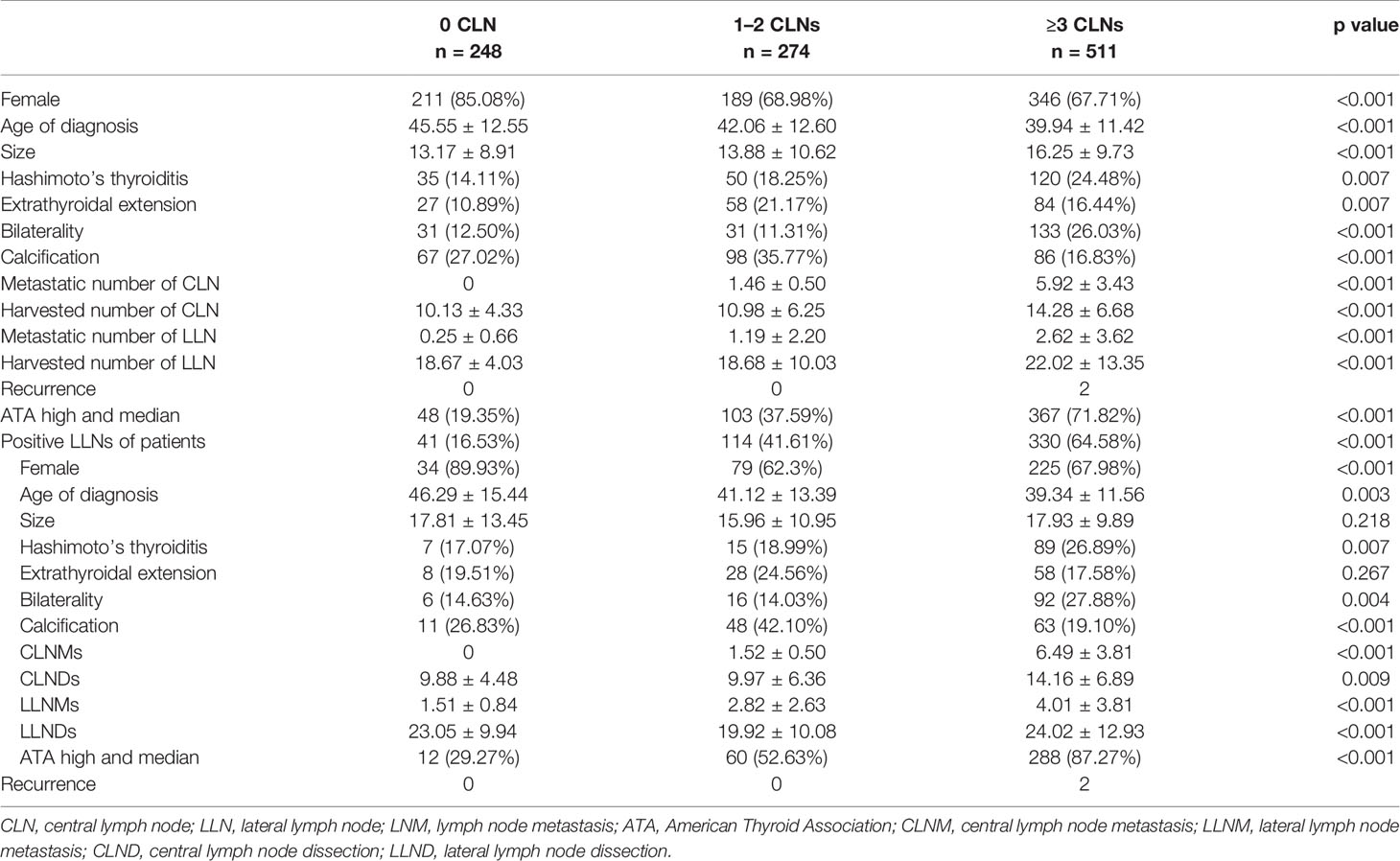
Table 1 Demographic profile and comparisons of clinicopathological factors of the cohort based on the number of CLN-positive cN0 PTC patients.
Clinicopathologic Characteristics of LLNM for Patients With 1–2 CLNMs
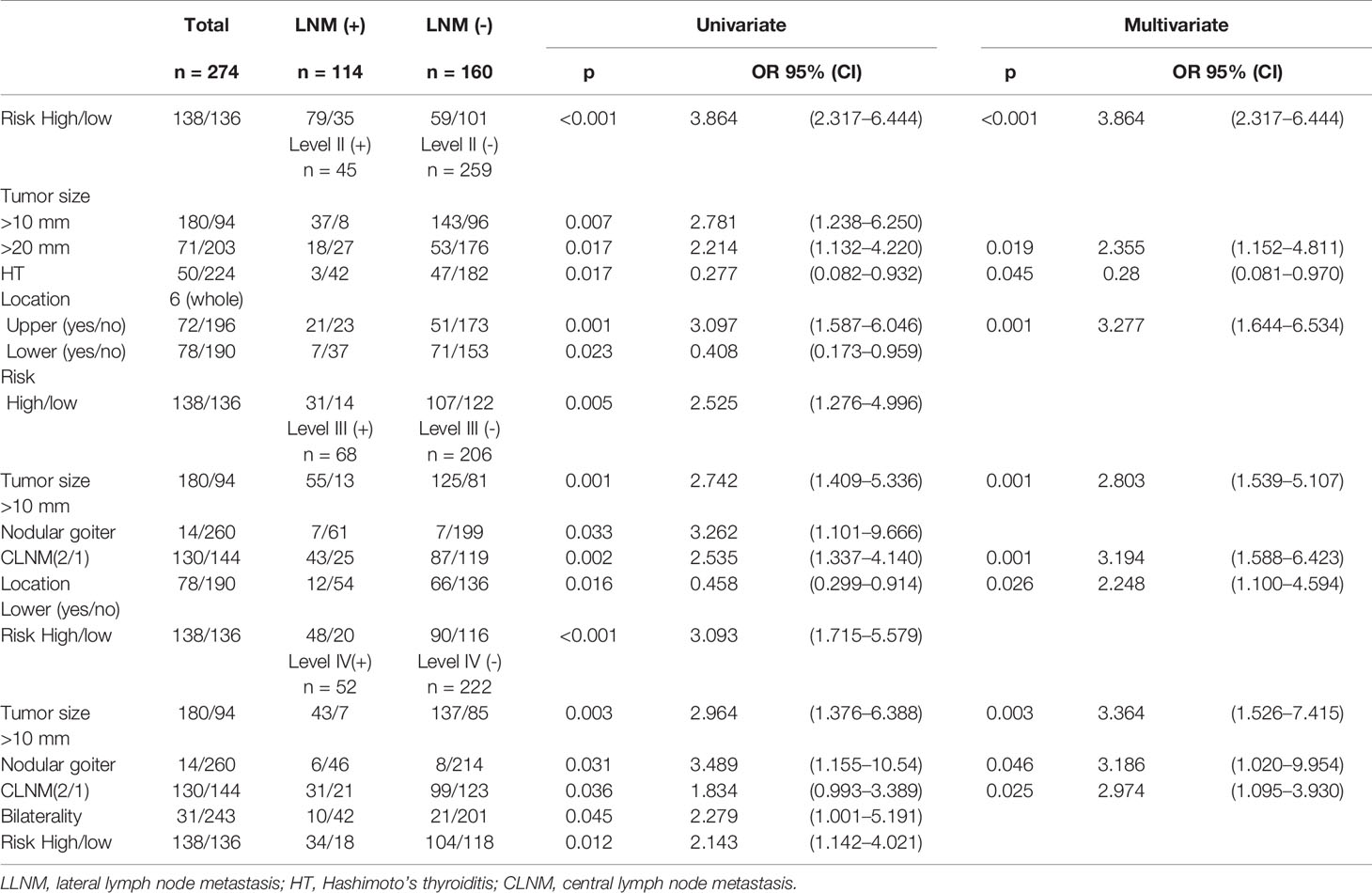
In this cohort, there was no difference between the LLN (+) and LLN (-) groups in terms of sex, age of diagnosis, nodule number, bilaterality, ETE, thyroid adenoma, follicular variant, multifocality, or location and number of CLNMs (Table 2 and Figure S1). However, patients ≤30 years old had a higher risk of LLNM (p = 0.008, OR = 2.303). The number of CLNMs was related to level III (p = 0.002, OR = 2.535) and level IV (p = 0.036, OR = 1.834) LLNMs rather than level II (Table 3 and Figure 2). No recurrence was observed in 1–2 CLNM patients who underwent LLND, although one case of recurrence was observed in the LLND (-) group (Table S2).
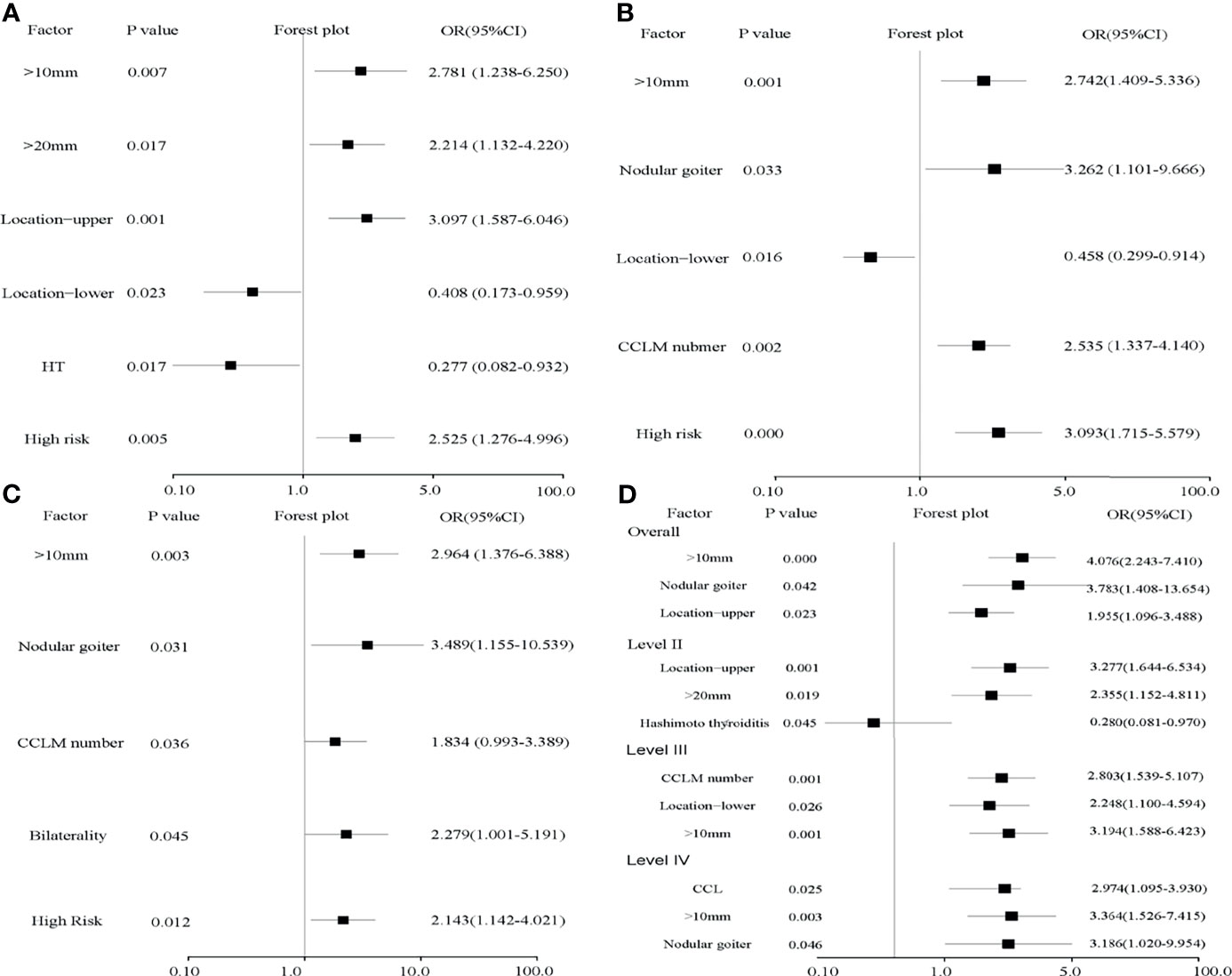
Figure 2 Forest plots of risk factors for levels II–IV (A–C) and independent risk factors for LLNM (D). (A) Tumor sizes over 20 mm and located in the upper region contribute to level II LLNM and HT reduces its risk. (B, C) Tumor size over 10 mm, nodular goiter, and the number of CLNMs were risk factors for level III (p = 0.001, OR = 2.742; p = 0.033, OR = 3.262; p = 0.002, OR = 2.535) and level IV (p = 0.003, OR = 2.964; p = 0.031, OR = 3.489; p = 0.036, OR = 1.834). High risk evaluated by the LASSO model is an independent risk factor for all levels. (D) Tumor size over 10 mm, nodular goiter, and upper location were independent risk factors for LLNM. In detail, tumor size over 20 mm and located in the upper region are independent risk factors and HT is an independent protective factor for level II. Nodular goiter is an independent risk factor for level IV.
Tumors were larger in the LLNM group (15.96 ± 10.95 vs. 12.40 ± 10.15, p = 0.006). Calcification (p = 0.043, OR = 1.600) and nodular goiter (p = 0.021, OR = 3.750) were positively associated with LLNM. Tumors located in the upper region (p = 0.011, OR = 1.968) were also positively related to LLNM, especially for level II (p = 0.001, OR = 3.097). Conversely, HT was negatively related to LLNM (p = 0.045, OR = 0.541), especially for level II (p = 0.017, OR = 0.277) (Figures 2A, D). In multivariate analysis, tumor size over 10 mm, tumor located in the upper region, and nodular goiter were independent risk factors for LLNM. In detail, tumor over 20 mm was an independent risk factor for level II (p = 0.019, OR = 2.355). Non-microcarcinoma and number of CCLMs were independent risk factors for levels III and IV (Table 3 and Figures 2B, C). Meanwhile, an increased number of CLND was associated with LLNM (p = 0.027).
LASSO Model Construction for Risk Stratification of LLNM
All characteristics were analyzed by LASSO (Figure 3). Eight LLNM-related factors were identified including tumor size >10 mm, tumor sizes >20 and 10–20 mm, location (upper, middle, and lower), HT, calcification, bilaterality, CLNM number, and nodular goiter (Table 4). The coefficients of all risk factors were used to calculate the risk score for each patient as follows: (0.6863518 × A + 0.23244186 ×B +…+H × 0.27637063)/1.55237294. Next, patients whose risk score was lower than 0.8729 were assigned to the low-risk group, and the remaining patients were assigned to the high-risk group. The incidence of LLNM in the high-risk group was 57.25% (79/138), and the risk score was an independent risk factor for LLNM (p < 0.001, OR = 3.864). The ROC curve is illustrated in Figure 3, and the AUC was 0.715 (0.654–0.776, 95% CI), with a specificity of 0.604 and sensitivity of 0.729 (Figure 3C). We excluded CLNM number and nodular goiter for a better preoperative prediction of cN0 PTC patients, and the AUC was 0.701 (0.639–0.764, 95% CI). If intraoperative frozen CLNs were available, the AUC of intraoperative prediction was 0.708 (0.647–0.770, 95% CI). In addition, 479 cN0 PTC patients had only level III and IV LLN and were also enrolled to verify the model (Figure 3D and Table S1), with an AUC of 0.732 (0.674–0.789, 95% CI). The AUCs of the preoperative prediction and intraoperative prediction models were 0.726 (0.668–0.784, 95% CI) and 0.733 (0.676–0.791, 95% CI), respectively.
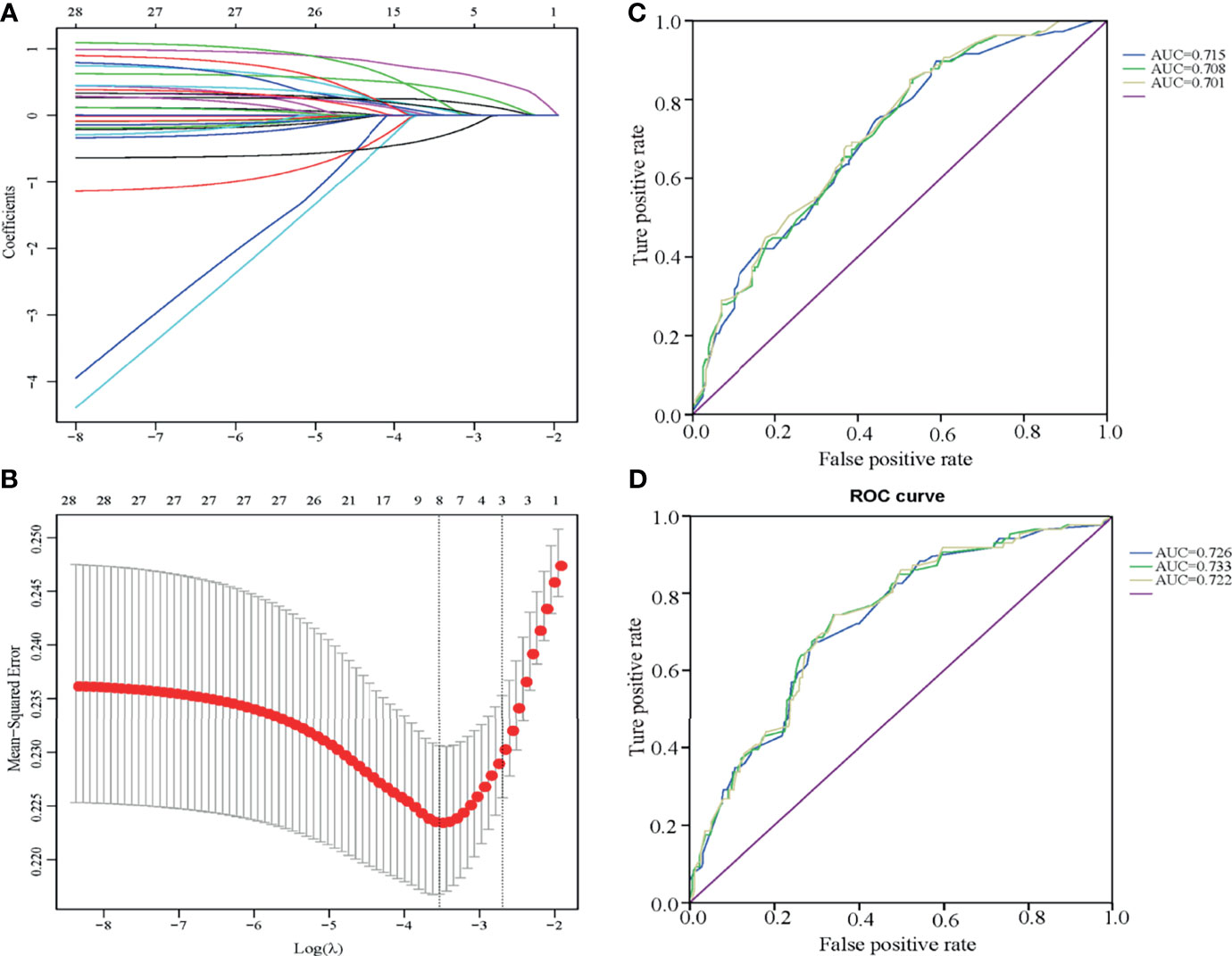
Figure 3 Multivariate risk model constructed by LASSO regression (A, B) and ROC curves of the LLNM risk scores for cN0 PTC patients with one to two CLNMs (C, D). (A, B) The LASSO model is best constructed with eight factors when Log(λ) equals to -3.5. The eight factors were tumor size 1 (over 10 mm), tumor size 2 (over 20 mm), tumor location, HT, calcification, bilaterality, CLNM number, and nodular goiter. (C) The ROC curve of patients who underwent LLND (levels II, III, and IV) presented AUCs of 0.715 (preoperative assessment group with six factors, blue line), 0.708 (intraoperative frozen group with CLNM number enrolled, green line), and 0.701 (postoperative assessment group, yellow line). (D) The ROC curve of patients who underwent LLND (levels III and IV) presented AUCs of 0.726 (preoperative assessment group with six factors, blue line), 0.733 (intraoperative frozen group, green line), and 0.722 (postoperative assessment group, yellow line).
We also verified the model in the ≥3 CLN groups with tumor size >10 mm, tumor sizes >20 and 10–20 mm, location (upper, middle, and lower), HT, calcification, bilaterality, and nodular goiter, and the AUC was 0.716 (0.667–0.765, 95% CI) (Figure S2). We ruled out nodular goiter for preoperative prediction because pathological confirmation is needed after surgery. Moreover, the AUC was 0.721 (0.672–0.770, 95% CI). These data confirm that these LASSO models are suitable for patients with CLNM.
Discussion
PTC has a high morbidity and a low mortality. PTC patients often experience LNM, which is associated with local recurrence (20). Generally, LNM has no significant effect on patient outcome. However, a recent study indicated that it does have a negative impact on survival in high-risk patients (5, 21). LNM is more likely to present a sequential pattern from central to lateral compartments, except in a minority of patients who have skip metastases (22). Currently, CLNM has argues on outcomes. In our study, the increasing number of CLNMs tended to promote regional recurrence and was related to the median (21–36%) and high stratification (68%) of recurrence risk (ATA guidelines for the diagnosis and treatment of thyroid cancer) (12). However, a survival benefit was not observed within the limited time of follow-up. There were also no benefits for cN0 PTC patients who were identified without pathological LNM. Instead, pCLND may increase complications. LLNM has been included as a risk factor for structural recurrence in ATA (12)and has even been shown to influence outcome in some reports (23). As an essential risk factor for LLNM, the number of CCLMs is increasingly being included in risk stratification, with typical cutoff values of 2–3 and 5 (18, 24, 25). We identified number of CLNMs as an independent risk factor for LLNM when patients were stratified into 0, 1–2, and ≥3 CLNM groups. Among them, the skip metastasis risk is 16.53%. In the ≥3 CLNM group, the incidence of LLNM was 64.58% and 71.82% of these patients had a medium–high ATA risk, which should alert clinicians to the possibility of an over 20% local recurrence risk. In the 1–2 CLNM group, the incidences of LLNM and the medium–high recurrence risk were 41.61% and 37.59%, respectively, which were neither high nor low compared to the other groups. Further risk stratification in this group is warranted.
Patients with stage cN0 PTC have a high incidence of occult LNM (26). Experts disagree on the use of prophylactic LND, especially between Western and East Asia. The latter suggests routine pCLND, and the former shows individual variance in pCLND (27). On the one hand, it has been reported that pCLND does not improve outcomes but rather can cause more complications, such as parathyroid and laryngeal nerve injury (28). On the other hand, pCLND has been confirmed to improve the disease-free survival of patients with intermediate and high-risk ATA risk stratification (29).Moreover, pCLND can help with tumor staging, predict LLNM, guide adjuvant radioiodine, reduce postoperative serum thyroglobulin, and decrease the complications of reoperation (30, 31). Indeed, the fifth National Audit Report reports that 37% of patients undergo CLND (32). We performed routine pCLND, and intraoperative frozen pathology results were available. The increasing number of CLNMs was correlated with male sex, younger age, greater tumor size, extrathyroidal extension, bilaterality, calcification, LLNM, and a median-high ATA risk, consistent with previous research (29, 33–36). According to our data, pCLNM can help in effectively screening occult CLNM with an incidence of 75.99% and assess the risk of occult LLNM.
In general, experts do not recommend pLLND for cN0 PTC patients. Some of them perform LLND for high-risk LLNM patients based on clinical features and experience. The number of LNMs helps improve tumor staging and postoperative treatment. There have been numerous risk models to assess LLNM with different methods (25, 37). However, consistency in terms of the LND extent was lacking among the enrolled patients and the results were not precise enough to confirm negative LLNs by imaging and palpation. The traditional extent of LLND is levels II–V, but the debate over level IIb and V dissection remains ongoing due to spinal accessory nerve injuries. Some studies argue that there is no difference in recurrence rates between selective (including II, III, and IV) and traditional neck dissection (38). The incidence of metastasis with level V dissection is low, but the rate of shoulder dysfunction is high (39). Meanwhile, level II is associated with skip metastasis (16), and the incidence of shoulder syndrome is low. Therefore, we adopted levels II–IV as the extent of LND and the incidence of occult LLNM was 46.95%.
In the 1–2 CLNM group, we found that age ≤30 years, tumor size over 10 mm, tumors in the upper region, calcifications, and nodule goiters contributed to LLNM. Hashimoto’s thyroiditis reduced the risk of LLNM. Liu et al. indicated that patients (age ≤30 years old) are susceptible to local recurrence and that patients (<45 years old) with lateral neck LNM are understaged by the 7th edition of the AJCC staging system (40). Our data also showed that 50% (11/22) of patients (age ≤30 years old) with LLNM belong to the medium–high ATA risk group. For this population, genetic testing is a good method for individualized evaluation, such as the RET gene (41). Tumors over 10 mm and those located in the upper region are acknowledged as independent risk factors for LLNM (42). We also confirmed these findings and further reveal that tumor size greater than 20 mm and a tumor located in the upper region are independent risk factors for level II disease. Tumors located in the lower region were an independent risk factor for level III tumors. It has been reported that nodular goiter is an independent risk factor for LLNM (25), which supports our findings. Nonetheless, more data is needed to prove this hypothesis, and the mechanism remains unclear. HT is a controversial topic as some argue that HT has a negative effect on LNM (43), whereas others assert that it has no connection with LNM (44). Some have even argued that its adverse effects on CLNM and antibody status were risk factors of CLNM with a cutoff of 3 (45). In our research, HT was related to level II LLNM (p = 0.045, OR = 0.280) rather than level III or IV. Moreover, HT showed no relationship with LLNM in patients with 1–2 CLNMs who underwent level III and IV LLND (p = 0.399). We found that the morbidity of CLNM in patients with HT was higher than that in patients without HT (170/205 vs. 615/828), although HT showed no connection with the number of positive CLNs in subsequent analyses. We also found that HT was negatively related to ETE (p = 0.040, OR = 0.444). One recurrent male who did not undergo LLN dissection was found to have level III–IV LN metastasis and BRAF, TERT, and PIK3CA mutations. No cases of relapses were observed in the LLND (+) group (Table S2). Survival benefits were not observed for LLND, and recurrence was not common in 1–2 CLNM cN0 PTC patients without LLND. The results show that routine dissection of the lateral lymph nodes is unnecessary, but effort to screen people at high-risk people for occult LLNM should be considered.
We constructed the LASSO model and divided people into low- (25.74%) and high-risk (57.25%) LLNM groups. In the high-risk group (risk score over 0.8729), 78.38% of patients had a median and high ATA risk. LASSO risk stratification is an independent risk factor for LLNM. It aids in the preoperative assessment of LLNM risk for cN1a PTC patients using six factors. Intraoperative frozen pathology results also help intraoperative decisions on pLLND combined with clinical experience to guides postoperative therapy, such as radioactive iodine treatment and TSH inhibition. For high-risk patients, we selectively suggest a polygenetic test to further screen for higher aggressiveness of PTC and identify potential therapeutic targets for subsequent recurrence (46). The results show that RET, TERT, PIK3CA, and fusion mutations are common (Table S3) and longer follow-up periods are needed to determine whether LLND reduces regional recurrence in the high-risk population.
There are several limitations to this study that should be mentioned. Although the rate of occult level II was 16.42%, selection bias regarding the extent of LLND between the III–IV and II–IV groups might exist among surgeons, leading to the relatively high rates of occult LLNM in the latter group. We herein present postoperative TG monitoring, tumor persistence, local recurrence, distant metastasis, and survival data to determine whether LLND is meaningful or harmful for high-risk patients, but long-term follow-up data are also needed.
In summary, for patients with 1–2 CLNMs, young age, calcification, nodular goiter, tumor >10, and tumor in the upper region are risk factors for LLNM. High-risk patients in the LASSO model had a 57.25% chance of occult LLNM and a 78.38% chance of having moderate–high ATA risk. These findings may serve as a reference for selective pLLND, comprehensive therapy, and follow-up.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
This study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University, and each participating patient provided written informed consent.
Author Contributions
YW and XLS conceptualized and designed the study. YW wrote the manuscript and approved the final manuscript. JT provided the study material. CD, HW, XJS, and PY collected the data and specimen. YW and JT analyzed and interpreted the data. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank JT from the First Affiliated Hospital of Chongqing Medical University, Chongqing, for the technical support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.716728/full#supplementary-material
Supplementary Figure 1 | The forest plot of risk factors for LLNM. Sex, age, bilaterality, ETE, thyroid adenoma, follicular variant, multifocality, CLNM number and location were not related to LLNM for cN0 PTC patients with 1-2CLNMs. Tumor size over 10 mm (p=0.000, OR=4.004), upper tumor location (p=0.011, OR=1.968), calcification (p=0.043, OR=1.600), nodular goiter (p=0.021, OR=3.750) and high risk (p=0.000, OR=3.864) were risk factors of LLNM. HT (p=0.045, OR=0.541) was a protective factor against LLNM.
Supplementary Figure 2 | ROC curves of LLNM risk scores for cN0 PTC with ≥3 CLNMs. The ROC curve of patients who underwent LLND (levels II, III and IV) presented AUCs of 0.721 (preoperative assessment group with six factors, blue line) and 0.716 (postoperative assessment group with seven factors, CLNM number excluded, green line).
Abbreviations
TC, thyroid cancer; cN0, clinically lymph node-negative; PTC, papillary thyroid carcinoma; LNM, lymph node metastasis; LND, lymph node dissection; CLNM, central lymph node metastasis; LLNM, lateral lymph node metastasis; NCCN, National Comprehensive Cancer Network; ATA, American Thyroid Association; pCLND, prophylactic central lymph node dissection; pLLND, prophylactic lateral lymph node dissection; DTCC, Database Technology Conference China; FNAB, fine-needle aspiration biopsy; LASSO, least absolute shrinkage and selection operator; ROC, time-dependent receiver operating characteristic; AUC, the area under the ROC curve; ETE, extrathyroidal extension; HT, Hashimoto’s thyroiditis.
References
1. Siegel RL, Miller KF, Jemal A. Cancer Statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Siegel RL, Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, et al. Cancer Statistics for Adolescents and Young Adults, 2020. CA Cancer J Clin (2020) 70(6):443–59. doi: 10.3322/caac.21637
4. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA (2017) 317(13):1338–48. doi: 10.1001/jama.2017.2719
5. Lundgren CI, Hall P, Dickman PW, Dickman Pw, Zedenius J. Clinically Significant Prognostic Factors for Differentiated Thyroid Carcinoma: A Population-Based, Nested Case-Control Study. Cancer (2006) 106(3):524–31. doi: 10.1002/cncr.21653
6. Leboulleux S, Rubino C, Baudin E, Caillou B, Hartl DM, Bidart JM, et al. Prognostic Factors for Persistent or Recurrent Disease of Papillary Thyroid Carcinoma With Neck Lymph Node Metastases and/or Tumor Extension Beyond the Thyroid Capsule at Initial Diagnosis. J Clin Endocrinol Metab (2005) 90(10):5723–9. doi: 10.1210/jc.2005-0285
7. Hay ID, Hutchinson M, Gonzalez-Losada T, McIver B, Reinalda ME, Grant GS, et al. Papillary Thyroid Microcarcinoma: A Study of 900 Cases Observed in a 60-Year Period. Surgery (2008) 144(6):980–7. doi: 10.1016/j.surg.2008.08.035
8. Mulla MG, Knoefel WT, Gilbert J, McGregor A, Schulte KM. Lateral Cervical Lymph Node Metastases in Papillary Thyroid Cancer: A Systematic Review of Imaging-Guided and Prophylactic Removal of the Lateral Compartment. Clin Endocrinol (Oxf) (2012) 77(1):126–31. doi: 10.1111/j.1365-2265.2012.04336.x
9. Shaha AR. Prophylactic Central Compartment Dissection in Thyroid Cancer: A New Avenue of Debate. Surgery (2009) 146(6):1224–7. doi: 10.1016/j.surg.2009.10.020
10. Patron V, Bedfert C, Clech GL, Aubry K, Jegoux F. Pattern of Lateral Neck Metastases in N0 Papillary Thyroid Carcinoma. BMC Cancer (2011) 11:8. doi: 10.1186/1471-2407-11-8
11. Haddad RI, Nasr C, Bischoff L, Busaidy NL, Byrd D, Callender G, et al. NCCN Guidelines Insights: Thyroid Carcinoma, Version 2.2018. J Natl Compr Canc Netw (2018) 16(12):1429–40. doi: 10.6004/jnccn.2018.0089
12. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
13. Takami H, Ito Y, Okamoto T, Yoshida A. Therapeutic Strategy for Differentiated Thyroid Carcinoma in Japan Based on a Newly Established Guideline Managed by Japanese Society of Thyroid Surgeons and Japanese Association of Endocrine Surgeons. World J Surg (2011) 35(1):111–21. doi: 10.1007/s00268-010-0832-6
14. WHO/IARC. World Cancer Report 2020. Lyon: IARC Press (2014) p. 253–7. Available at: https://www.iarc.fr/faq/latest-global-cancer-data-2020-qa/.
15. Chinese Society of Endocrinology and Head and Neck Oncology Committee of Chinese Anti-Cancer Association. Guidelines for Diagnosis and Treatment of Thyroid Nodule and Differentiated Thyroid Carcinoma (In Chinese). Guidelines for Diagnosis and Treatment of Differentiated Thyroid Carcinoma (In Chinese). C J Nucl Med Mol Imaging (2013) 33(2):96–115. doi: 10.3760/cma.j.issn.2095-2848.2013.02.003
16. Hu D, Lin H, Zeng X, Wang T, Deng J, Su X. Risk Factors for and Prediction Model of Skip Metastasis to Lateral Lymph Nodes in Papillary Thyroid Carcinoma. World J Surg (2020) 44(5):1498–505. doi: 10.1007/s00268-019-05332-0
17. Liu C, Xiao C, Chen J, Li X, Feng Z, Gao Q, et al. Risk Factor Analysis for Predicting Cervical Lymph Node Metastasis in Papillary Thyroid Carcinoma: A Study of 966 Patients. BMC Cancer (2019) 19(1):622. doi: 10.1186/s12885-019-5835-6
18. Rajeev P, Ahmed S, Ezzat TM, Ezzat TM, Sadler GP, Mihai R. The Number of Positive Lymph Nodes in the Central Compartment has Prognostic Impact in Papillary Thyroid Cancer. Langenbecks Arch Surg (2013) 398(3):377–82. doi: 10.1007/s00423-012-1041-6
19. Domburg RV, Hoeks S, Kardys I, Lenzen M, Lenzen M, Boersma E. Tools and Techniques–Statistics: How Many Variables Are Allowed in the Logistic and Cox Regression Models? EuroIntervention (2014) 9(12):1472–3. doi: 10.4244/EIJV9I12A245
20. Beasley NJ, Lee J, Eski S, Walfish P, Witterick I, Freeman JL. Impact of Nodal Metastases on Prognosis in Patients With Well-Differentiated Thyroid Cancer. Arch Otolaryngol Head Neck Surg (2002) 128(7):825–8. doi: 10.1001/archotol.128.7.825
21. McConahey WM, Hay ID, Woolner LB, van Heerden JA, Taylor WF. Papillary Thyroid Cancer Treated at the Mayo Clinic, 1946 Through 1970: Initial Manifestations, Pathologic Findings, Therapy, and Outcome. Mayo Clin Proc (1986) 61(12):978–96. doi: 10.1016/s0025-6196(12)62641-x
22. Likhterov I, Reis LL, Urken ML. Central Compartment Management in Patients With Papillary Thyroid Cancer Presenting With Metastatic Disease to the Lateral Neck: Anatomic Pathways of Lymphatic Spread. Head Neck (2017) 39(5):853–9. doi: 10.1002/hed.24568
23. Nixon IJ, Wang LY, Palmer FL, Tuttle RM, Shaha AR, Shah JP, et al. The Impact of Nodal Status on Outcome in Older Patients With Papillary Thyroid Cancer. Surgery (2014) 156(1):137–46. doi: 10.1016/j.surg.2014.03.027
24. Lee YS, Lim YS, Lee JC, Wang SG, Kim IJ, Lee BJ. Clinical Implication of the Number of Central Lymph Node Metastasis in Papillary Thyroid Carcinoma: Preliminary Report. World J Surg (2010) 34(11):2558–63. doi: 10.1007/s00268-010-0749-0
25. Heng Y, Yang Z, Zhou L, Lin J, Cai W, Tao L. Risk Stratification for Lateral Involvement in Papillary Thyroid Carcinoma Patients With Central Lymph Node Metastasis. Endocrine (2020) 68(2):320–8. doi: 10.1007/s12020-020-02194-8
26. Xue S, Wang P, Liu J, Li R, Zhang L, Chen G. Prophylactic Central Lymph Node Dissection in Cn0 Patients With Papillary Thyroid Carcinoma: A Retrospective Study in China. Asian J Surg (2016) 39(3):131–6. doi: 10.1016/j.asjsur.2015.03.015
27. Mazzaferri EL, Doherty GM, Steward DL. The Pros and Cons of Prophylactic Central Compartment Lymph Node Dissection for Papillary Thyroid Carcinoma. Thyroid (2009) 19(7):683–9. doi: 10.1089/thy.2009.1578
28. Napoli DL, Matrone A, Favilla K, Piaggi P, Galleri D, Ambrosini CE, et al. Role of Prophylactic Central Compartment Lymph Node Dissection on the Outcome Of Patients With Papillary Thyroid Carcinoma and Synchronous Ipsilateral Cervical Lymph Node Metastases. Endocr Pract (2020) 26(8):807–17. doi: 10.4158/EP-2019-0532
29. Medas F, Canu GL, Cappellacci F, Anedda G, Conzo G, Erdas E, et al. Prophylactic Central Lymph Node Dissection Improves Disease-Free Survival in Patients With Intermediate and High Risk Differentiated Thyroid Carcinoma: A Retrospective Analysis on 399 Patients. Cancers (Basel) (2020) 12(6):1658. doi: 10.3390/cancers12061658
30. Hartl DM, Leboulleux S, Al Ghuzlan A, Baudin E, Chami L, Schlumberger M, et al. Optimization of Staging of the Neck With Prophylactic Central and Lateral Neck Dissection for Papillary Thyroid Carcinoma. Ann Surg (2012) 255(4):777–83. doi: 10.1097/SLA.0b013e31824b7b68
31. Popadich A, Levin O, Lee JC, Smooke-Praw S, Ro K, Fazel M, et al. A Multicenter Cohort Study of Total Thyroidectomy and Routine Central Lymph Node Dissection for Cn0 Papillary Thyroid Cancer. Surgery (2011) 150(6):1048–57. doi: 10.1016/j.surg.2011.09.003
32. Chadwick D. The British Association of Endocrine and Thyroid Surgeons Fifth National Audit Report. Dendrite Clinical Systems Ltd. (2017) p. 65. Available at: http://www.baets.org.uk/wp-content/uploads/BAETS-Audit-National-Report-2017.pdf.
33. Oh HS, Park S, Kim M, Kwon H, Song E, Sung TY, et al. Young Age and Male Sex Are Predictors of Large-Volume Central Neck Lymph Node Metastasis in Clinical N0 Papillary Thyroid Microcarcinomas. Thyroid (2017) 27(10):1285–90. doi: 10.1089/thy.2017.0250
34. Zhang L, Wei WY, Ji QH, Zhu YX, Wang ZY, Wang Y, et al. Risk Factors for Neck Nodal Metastasis in Papillary Thyroid Microcarcinoma: A Study of 1066 Patients. J Clin Endocrinol Metab (2012) 97(4):1250–7. doi: 10.1210/jc.2011-1546
35. Jiwang L, Yahong L, Kai L, Bo H, Yuejiao Z, Haotian W, et al. Clinicopathologic Factors and Preoperative Ultrasonographic Characteristics for Predicting Central Lymph Node Metastasis in Papillary Thyroid Microcarcinoma: A Single Center Retrospective Study. Braz J Otorhinolaryngol (2020) S1808-8694(20). doi: 10.1016/j.bjorl.2020.05.004
36. Pacini F, Elisei R, Capezzone M, Miccoli P, Molinaro E, Basolo F, et al. Contralateral Papillary Thyroid Cancer Is Frequent at Completion Thyroidectomy With No Difference in Low- and High-Risk Patients. Thyroid (2001) 11(9):877–81. doi: 10.1089/105072501316973145
37. Lin DZ, Qu N, Shi RL, Lu ZW, Ji QH, Wu WL. Risk Prediction and Clinical Model Building for Lymph Node Metastasis in Papillary Thyroid Microcarcinoma. Onco Targets Ther (2016) 9:5307–16. doi: 10.2147/OTT.S107913
38. Won HR, Chang JW, Kang YE, Kang JY, Koo BS. Optimal Extent of Lateral Neck Dissection for Well-Differentiated Thyroid Carcinoma With Metastatic Lateral Neck Lymph Nodes: A Systematic Review and Meta-Analysis. Oral Oncol (2018) 87:117–25. doi: 10.1016/j.oraloncology.2018.10.035
39. Roh JL, Kim JM, Park CI. Lateral Cervical Lymph Node Metastases From Papillary Thyroid Carcinoma: Pattern of Nodal Metastases and Optimal Strategy for Neck Dissection. Ann Surg Oncol (2008) 15(4):1177–82. doi: 10.1245/s10434-008-9813-5
40. Lu Y, Jiang L, Chen C, Chen H, Yao QH. Clinicopathologic Characteristics and Outcomes of Papillary Thyroid Carcinoma in Younger Patients. Med (Baltimore) (2020) 99(15):e19795. doi: 10.1097/MD.0000000000019795
41. Nikiforov YE, Nikiforova MN. Molecular Genetics and Diagnosis of Thyroid Cancer. Nat Rev Endocrinol (2011) 7(10):569–80. doi: 10.1038/s41574-020-00465-y
42. So YK, Kim MJ, Kim S, Son YI. Lateral Lymph Node Metastasis in Papillary Thyroid Carcinoma: A Systematic Review and Meta-Analysis for Prevalence, Risk Factors, and Location. Int J Surg (2018) 50:94–103. doi: 10.1016/j.ijsu.2017.12.029
43. Molnár S, Győry F, Nagy E, Méhes G, Molnár C. Clinico-Pathological Features of Papillary Thyroid Cancer Coexistent With Hashimoto's Thyroiditis. Orv Hetil (2017) 158(5):178–82. doi: 10.1556/650.2017.30647
44. Girardi FM, Barra MB, Zettler CG. Papillary Thyroid Carcinoma: Does the Association With Hashimoto's Thyroiditis Affect the Clinicopathological Characteristics of the Disease? Braz J Otorhinolaryngol (2015) 81(3):283–7. doi: 10.1016/j.bjorl.2014.04.006
45. Wen X, Wang B, Jin Q, Zhang W, Qiu M. Thyroid Antibody Status is Associated With Central Lymph Node Metastases in Papillary Thyroid Carcinoma Patients With Hashimoto's Thyroiditis. Ann Surg Oncol (2019) 26(6):1751–8. doi: 10.1245/s10434-019-07256-4
Keywords: cN0, PTC, LLNM, CLNM, LASSO
Citation: Wang Y, Deng C, Shu X, Yu P, Wang H, Su X and Tan J (2021) Risk Factors and a Prediction Model of Lateral Lymph Node Metastasis in CN0 Papillary Thyroid Carcinoma Patients With 1–2 Central Lymph Node Metastases. Front. Endocrinol. 12:716728. doi: 10.3389/fendo.2021.716728
Received: 29 May 2021; Accepted: 23 September 2021;
Published: 15 October 2021.
Edited by:
Jia Liu, First Affiliated Hospital of Jilin University, ChinaReviewed by:
Lorenzo Scappaticcio, University Hospital “Luigi Vanvitelli”, ItalyFrancesco Latrofa, University of Pisa, Italy
Copyright © 2021 Wang, Deng, Shu, Yu, Wang, Su and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinliang Su, c3V4aW5saWFuZ0AyMWNuLmNvbQ==; Jinxiang Tan, dGp4MTIwMkAxNjMuY29t
 Yuanyuan Wang
Yuanyuan Wang Chang Deng
Chang Deng Xiujie Shu1
Xiujie Shu1 Ping Yu
Ping Yu Huaqiang Wang
Huaqiang Wang Jinxiang Tan
Jinxiang Tan