- Department of Endocrinology, Key Laboratory of Endocrinology of Ministry of Health, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
Background: The increase in diabetes worldwide is alarming. Decreased acute insulin response to intravenous glucose tolerance test (IVGTT) during first-phase insulin secretion (FPIS) is a characteristic of diabetes. However, knowledge of the insulin secretion characteristics identified by different time to glucose peak in subjects with different metabolic state is sparse.
Aims: This study aimed to find different patterns of FPIS in subjects with normal glucose tolerance (NGT) and analyzed the relationship between insulin secretion patterns and the risk for development of type 2 diabetes mellitus (T2DM).
Methods: A total of 126 subjects were divided into three groups during a 10-min IVGTT, including NGT with time to glucose peak after 3 min (G1, n = 21), NGT with time to glucose peak at 3 min (G2, n = 95), and prediabetes or diabetes with time to glucose peak at 3 min (G3, n = 10). Glucose, insulin, and C-peptide concentrations at 0, 3, 5, 7, and 10 min during the IVGTT were tested. IVGTT-based indices were calculated to evaluate the insulin secretion and insulin sensitivity.
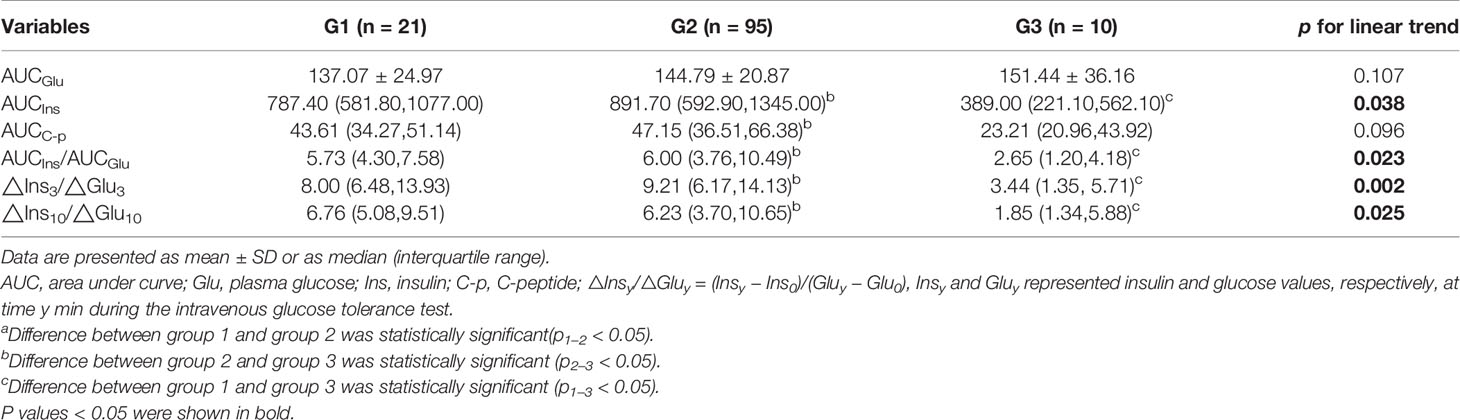
Results: Age, body mass index (BMI), waist-to-hip ratio, triglyceride (TG), and hemoglobin A1c (HbA1c) of subjects were gradually higher, while high-density lipoprotein cholesterol (HDL-C) was gradually lower from G1 to G3 (p for linear trend <0.05), and the differences between G1 and G2 were also statistically significant (p < 0.05). Glucose peak of most participants in G1 converged at 5 min, and the curves shape of insulin and C-peptide in G2 were the sharpest among three groups. There was no significant difference in all IVGTT-based indices between G1 and G2, but AUCIns, AUCIns/AUCGlu, and △Ins3/△Glu3 in G2 were the highest, and the p-value for linear trend of those indices among three groups were statistically significant (p < 0.05).
Conclusions: Two patterns of FPIS were in subjects with NGT, while subjects with later time to glucose peak during FPIS might be less likely to develop T2DM in the future.
Introduction
Diabetes is one of the most common metabolic diseases worldwide, the prevalence of which in 2019 is estimated to be 9.3% (463 million people), rising to 10.2% (578 million) by 2030, and half of the people do not know that they have diabetes. Given the present trends, the number of people with diabetes will increase to a staggering 700 million (10.9%) by 2045, being accompanied by a huge economic burden (1). The pathogenesis of type 2 diabetes (T2DM) mellitus is complex, but it is acknowledged that the combination of defective insulin secretion and insulin resistance plays a critical role in the development of T2DM (2). β-cells respond to increased plasma glucose with a biphasic insulin secretion, appearing a sharp first-phase peak and a slowly rising second phase (3). First-phase insulin secretion (FPIS) plays a significant role in maintaining plasma glucose homeostasis, and decline or loss of FPIS is common in T2DM. It seems that the restoration of FPIS and improvement of plasma glucose in T2DM is interacted, which has thus prompted many studies into its regulation (4, 5).
Intravenous glucose tolerance testing (IVGTT) is a common test of β-cell function. Acute increased plasma glucose can induce an apparent FPIS, and insulin and C-peptide responses are also monitored. Hulman et al. (6) analyzed data from participants without diabetes from the observational cohort from the European Group for the Study of Insulin Resistance and identified four different glucose response patterns according to time to glucose peak and glucose response curve during oral glucose tolerance test (OGTT). However, studies related to FPIS identified by time to glucose peak during IVGTT are sparse. In this study, we examined the insulin secretion in subjects with different metabolic state and glucose response curve during FPIS through IVGTT, aiming to find different patterns of FPIS in subjects with normal glucose tolerance (NGT) and analyzed the relationship between insulin secretion patterns and the risk for development of T2DM.
Materials and Methods
Study Population
A total of 126 participants, including 116 healthy subjects, 5 patients with prediabetes, and 5 patients with T2DM, were consecutively recruited in this study in the Department of Endocrinology of Peking Union Medical College Hospital from November 2020 to April 2021. The exclusion criteria were as follows: (1) acute complications of diabetes, (2) hepatic or renal disease and systemic corticosteroid treatment, and (3) women who were currently pregnant and breastfeeding. All experimental protocols were approved by the Ethics Committee of Peking Union Medical College Hospital and performed in accordance with the Declaration of Helsinki as revised in 2013. Written informed consent was obtained from each participant.
Measurements
Clinical parameters such as height, body weight, waist circumferences (Wc), hip circumferences (Hc), biceps circumferences (Bc), thigh circumferences (Tc), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were collected. Systolic and diastolic blood pressure was measured three times by a standard mercury manometer with the subjects seated. The past and family history were recorded using a questionnaire.
Overnight fasting blood samples were collected before IVGTT. Alanine transaminase (ALT), aspartate aminotransferase (AST), serum creatinine (SCr), uric acid (UA), hemoglobin A1c (HbA1c), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured in all subjects.
After an overnight fasting, IVGTT was performed in all subjects. Glucose solution (25 g; 50 ml of 50% glucose) was injected intravenously within 1 min, and blood samples were obtained from the opposite arm before (0 min) and at 3, 5, 7, and 10 min after glucose infusion for plasma glucose, insulin, and C-peptide determinations.
Seven patients among subjects with prediabetes or diabetes underwent hyperinsulinemic–euglycemic glucose clamp test before IVGTT. There was more than 1-h interval between the two tests. However, plasma glucose at 0 min before IVGTT was lower than their fasting plasma glucose (FPG). To revise the plasma glucose level value without changing the glucose curve shape, FPG was recorded as plasma glucose at 0 min (Glu0), and plasma glucose at each point was recorded as real plasma glucose + (FPG-Glu0).
Related calculation formula were as follows. Body mass index (BMI) was determined by dividing body weight in kilograms by height in meters squared. Waist-to-hip ratio (WHR) was defined as Wc/Hc, and biceps-to-thigh ratio (BTR) was defined as Bc/Tc. The area under curve (AUC) of glucose (AUCGlu), insulin (AUCIns), and C-peptide (AUCC-p) were calculated by GraphPad Prism (Version 8.0). The following IVGTT-based indices of insulin secretion were studied (7): (a) insulinogenic index was calculated as (Insy − Ins0)/(Gluy − Glu0), where Insy and Gluy represent insulin and glucose values, respectively, at time y min during the IVGTT, and (b) the ratio of the total AUCIns to the total AUCGlu was calculated to evaluate insulin sensitivity.
Statistical Analysis
All analyses were conducted using the statistical program SPSS (version 24, SPSS, Chicago, IL). Continuous variables were tested for normality of distribution. Variables with approximately normal distributions are presented as mean ± SD, and those with skewed distributions are presented as median (interquartile range). Categorical variables are presented as percentage (number). One-way ANOVA with least significant difference (LSD) post-hoc test was performed for multiple comparisons. To determine whether there was a significant graded change of variables among the three groups, the p-value for the linear trend was calculated. Relationships between variables were assessed using Spearman’s correlation analysis. Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cutoff points of age and BMI for the prediction of glucose peak time at 3 min. Statistical significance was inferred from two-sided p-values <0.05.
Results
One hundred twenty-six subjects were divided into three groups according to different glucose metabolic state and time to glucose peak, including 21 subjects with NGT and time to glucose peak after 3 min (G1), 95 subjects with NGT and time to glucose peak at 3 min (G2), and 10 subjects with prediabetes or diabetes mellitus and time to glucose peak at 3 min (G3). A comparison of clinical and laboratory characteristics among the three groups is shown in Table 1. Age, BMI, WHR, TG, and HbA1c of subjects in G1 and G2 were higher than those in G3 (all p < 0.05) and were gradually higher from G1 to G3 (p for linear trend < 0.05). HDL-C in G1 was lower than G2 and G3 (p < 0.05), and the decreasing trend was statistically different (p for linear trend <0.05). UA level among the three groups was increasing, although the difference was not significant (p > 0.05).
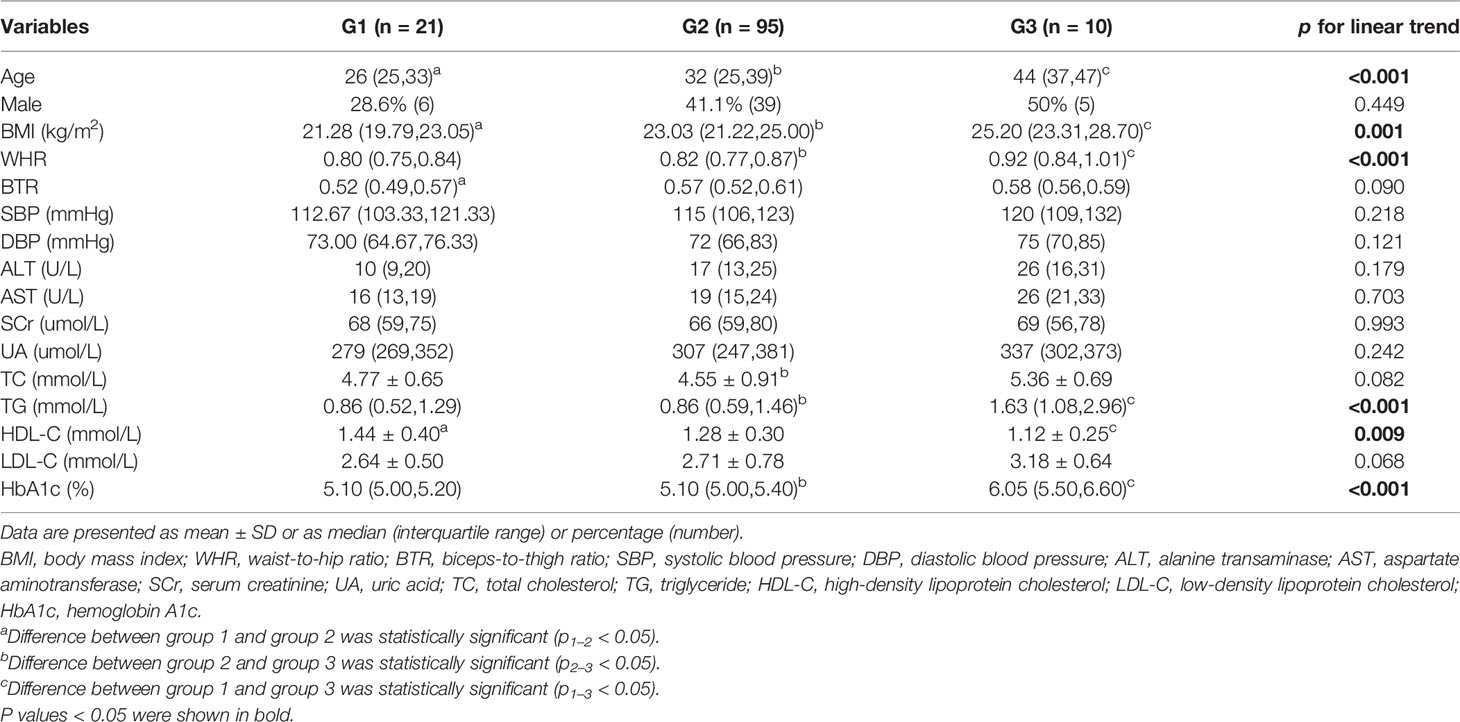
Table 1 Clinical and laboratory characteristics of participants by different glucose metabolic state and time to glucose peak at baseline.
The glucose curves among the three groups are plotted in Figure 1A alongside their corresponding insulin and C-peptide trajectories (Figures 1B, C). Glucose peak at 3 min in G2 was the highest with a sharp decreased trend following, which consisted with the trends of insulin and C-peptide. The glucose peak in G3 also appeared at 3 min, whereas the glucose peak of most participants in G1 was at 5 min. Insulin peak time among the three groups were roughly consistent with the glucose peak time. C-peptide peak time was at 7 min in G1 an G2, while it was at 3 min in G3. On the contrary to G2, insulin and C-peptide curves in G1 and G3 were relatively flat after glucose peak. Insulin and C-peptide were significantly lower in G3 among the three groups. There were no significant differences in glucose, insulin and C-peptide at other points in time between G1 and G2 except at 3-min point (Supplemental Table S1).
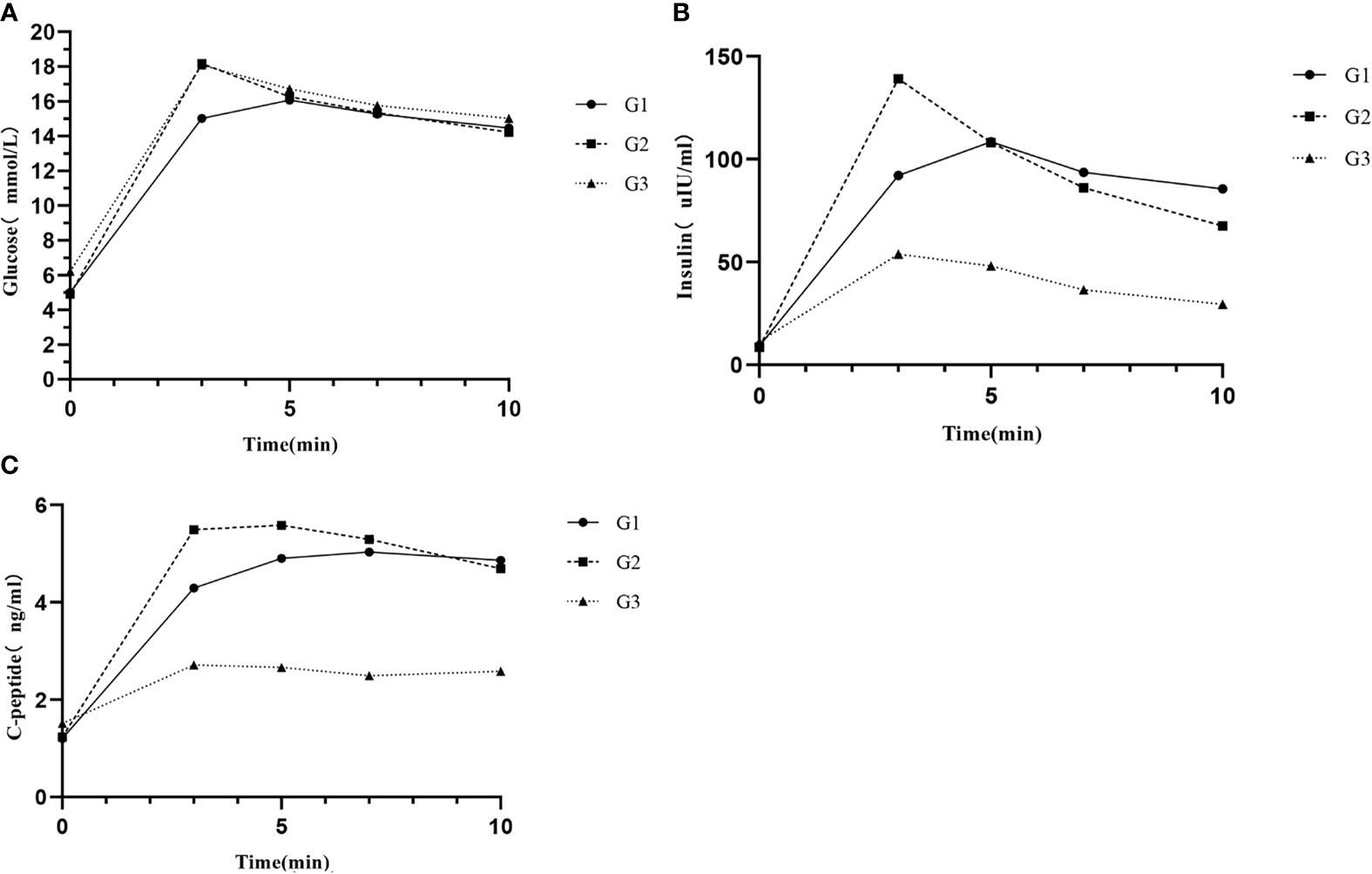
Figure 1 (A) Glucose responses, (B) insulin responses, and (C) C-peptide responses during IVGTT among different groups. G1, subjects with normal glucose tolerance (NGT) and time to glucose peak after 3 min; G2, subjects with NGT and time to glucose peak at 3 min; and G3, subjects with prediabetes or diabetes mellitus and time to glucose peak at 3 min.
Table 2 shows different IVGTT-based indices during 10-min IVGTT. There were no significant differences in all indices between G1 and G2. AUCIns, AUCIns/AUCGlu, △Ins3/△Glu3, and △Ins10/△Glu10 in G1 and G2 were all significantly higher than those in G3 (p < 0.05), and △Ins10/△Glu10 was gradually decreased from G1 to G3 (p for linear trend < 0.05). AUCIns, AUCIns/AUCGlu, and△Ins3/△Glu3 in G2 were the highest, and the p value for the linear trend of those indices among the three groups was statistically significant.
In consideration of the large sample difference of participants between G1 and G2, and the different glucose metabolic states between prediabetes and T2DM, a random selection of 21 subjects from G2 as G2r were compared to G1, and participants in G3 were divided into G3a (prediabetes, n = 5) and G3b (T2DM, n = 5); hence a more detailed grouping is shown in Supplemental Table S2. The trends of variables among the four groups were similar to these in the initial groups and the statistical significance still holds.
A further analysis on relationships between age, BMI, and glucose metabolic characteristics is shown in Supplemental Table S3, and receiver operating characteristic (ROC) curves (Supplemental Figure S1) were used to trying to determine the optimal cutoff points of age and BMI for the prediction of different patterns of insulin secretion. The cutoff points of age, BMI, and age combined with BMI for predicting glucose peak at 3 min were 26.5, 21.35, and 0.872 (AUC, 0.689; 95% CI, 0.579–0.799, p = 0.006; AUC, 0.695; 95% CI, 0.576-0.813, p = 0.005; AUC, 0.742; 95% CI 0.642–0.842, p < 0.001), respectively.
Discussion
In this study, we not only demonstrated a new way of evaluating FPIS by glucose responses during IVGTT but also provided the metabolic state of subjects with different time to glucose peak and insulin curve shapes. According to our results, glucose peak at 3 min and after 3 min might be two patterns of FPIS in adults with NGT, but the former one might be a T2DM susceptibility pattern while subjects with later glucose peak might be less likely to develop metabolic disorders in future. To our knowledge, we were the first study to propose this view in subjects with NGT.
Insulin is released from the pancreatic β cells to respond to increased plasma glucose concentration with a biphasic pattern. Although the mechanisms of biphasic pattern of insulin remain incompletely understood, insulin granules with differing release probabilities is an acceptable interpretation. The released competent insulin granules are adjacent to the plasma membranes, releasing to peripheral blood immediately to respond to elevated plasma glucose, contributing to a sharp increase in plasma insulin, which is known as FPIS (8). The FPIS consists of a brief spike lasting about 10 min followed by a second phase, which is slow but can reach a plateau at 2–3 h (9).
Previous studies have reported that decreased FPIS is an early marker of β-cell dysfunction during development of T2DM, and even mild chronic hyperglycemia is also harmful for β-cell function (10, 11). The classic interpretation of the decreased insulin secretion is the lack of releasable insulin granules in the critical pool (12). A recent study reported that human insulin secretion and exocytosis critically depend on the availability of membrane-docked granules and that T2DM is associated with a strong reduction in granule docking. Besides, the recovery of releasable granules is much slower in cells from subjects with T2DM than in those of subjects with NGT (13). Hong et al. (14) evaluated FPIS in Chinese obese subjects with different categories of impaired glucose regulation and found the FPIS in the NGT group was significantly greater than that in healthy lean control group, but then, it was progressively decreased in impaired glucose tolerance and T2DM group. In the present study, insulin and C-peptide level in G3, especially in G3b (participants with T2DM), were much lower than those in subjects with NGT, implying an impaired β-cell function, which was consistent with previous studies.
However, few studies reported the potential difference among healthy adults identified by different glucose responses patterns. In the present study, although the comparison of glucose, insulin, and C-peptide during FPIS did not have significant difference between G1 and G2 except those at 3-min point, subjects in G1 appeared to have more flat curve no matter the glucose, insulin, or C-peptide than in G2, and the p for linear trend of AUCIns, AUCIns/ACUGlu, and △Ins10/△Glu10 among the three groups were statistically significant, which implied that there might be two patterns of FPIS in subjects with NGT. Relatively higher AUCIns and AUCIns/ACUGlu suggested higher insulin response and lower insulin sensitivity, respectively, which were similar to FPIS in African Americans (AAs) compared with non-Hispanic whites (NHWs) (15, 16). Previous studies found that healthy AAs have a higher insulin response following an intravenous glucose load when compared with NHWs (17). A traditional view is that lower insulin sensitivity may be a driver of compensatory increases in β-cell function and hyperinsulinemia (18). The other opinion is that increased insulin secretion is primary hypersecretion independent of compensatory response to insulin resistance (19). Recently, Armiyaw et al. (20) did further studies among AAs and NHWs by IVGTT and hyperinsulinemic–euglycemic glucose clamp technique to support this opinion. According to our study, it seems that the two patterns of first-phase insulin and C-peptide response exist not only in different races but also in healthy participants in the same race.
The curves of subjects in G2 suggested faster and higher insulin secretion and C-peptide compared with subjects in G1, and relatively lower △Ins10/△Glu10 implied the lack of sustainability of the insulin secretion in G2. Thus, the stabilization points of insulin secretion in G1 and G2 might be different. Keiichi Kodama et al. (21) reported that there were ethnic differences in the optimal states in the relationship between insulin sensitivity and insulin response. Even a small change may cause a rapid increase in the amount of insulin secretion required to maintain NGT in Africans. They assumed that this unstable feedback loop may be implicated in this group’s vulnerability of canalization of blood glucose levels. This genetic background of Africans makes them more and differentially susceptible to diabetes than Caucasians. We hypothesized that this difference also exists in the same race. The insulin response in G1 was in the most stable optimum zone during FPIS, while it was located around an unstable extreme point in G2. The other potential interpretation of the difference between G1 and G2 may be related to the insulin granule dynamics in pancreatic β cells (22). Although β-cell function in G2 was still enough to respond to glucose stimulation, recovery of new releasable granule β cells was much slower, and they did not have enough ability to sustain a higher insulin secretion. However, the exact mechanism is not clear and needs further investigation.
There were other results that should be noticed. Hong et al. (23) have reported that Chinese obese subjects with NGT have increased FPIS compared with lean subjects. In this study, we found time to glucose peak in subjects with prediabetes or diabetes at 3 min, and there were also significant differences in some clinical and laboratory characteristics related to metabolic state between NGT subjects with time to glucose peak at and after 3 min. Older age and BMI and lower LDL-C, which implied higher risk of metabolic disorders, were found in G2. It is interesting that compared with NHWs, healthy AAs also have a higher risk for the development of T2DM (24). Given the characteristics of insulin sensitivity, acute insulin response, and insulin secretion curves in G1 and G2 were all similar to those in NHWs and AAs, we provided a hypothesis that time to glucose peak during FPIS may be related to the risk of T2DM. People with later time to glucose peak in G1 might be less likely to develop T2DM in the future. However, follow-up in these subjects is needed to confirm this view.
This study has a few limitations. First, although all our subjects in G1 and G2 were non-diabetic, glucose tolerance state following an oral glucose tolerance test was not assessed directly. Second, several subjects in G3 underwent hyperinsulinemic–euglycemic glucose clamp test before IVGTT. Although there was an interval between the two tests and adjusted glucose levels were used in analysis, it was difficult to totally eliminate potential impact. Moreover, the time points for evaluating glucose and insulin level were not earlier and closer enough. According to a previous study, glucose peak in normal people is usually within 3–6 min in FPIS. In this study, we cannot exclude that glucose peak time in some participants were out of this range and might appear before 3 min, and the actual peak might be missed. Lastly, the relatively small sample size in each group, especially in prediabetes and T2DM groups, was an additional limitation. Our finding should be investigated in further large studies.
Conclusions
In conclusion, different time to glucose peak implied two patterns of insulin secretion during FPIS in subjects with NGT, which might be related to varying degrees of risk for T2DM. People with later time to glucose peak might be less likely to develop T2DM in the future.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Committees of Peking Union Medical College Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization: TY, SS, and WZ. Investigation: TY, SS, TZ, YaD, SW, JG, SL, YiD, RL, and YF. Methodology: TY and SS. Clinical data collection: SS, TZ, YaD, SW, JG, SL, YiD, RL, and YF. Writing—original draft: TY and SS. Writing—review editing: WZ and TY. Supervision: WZ and TY. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by CAMS Innovation Fund for Medical Science (CIFMS), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences under Grant No. 2016-I2M-4-001 (to TY).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank all the participants in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.738427/full#supplementary-material
References
1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results From the International Diabetes Federation Diabetes Atlas, 9(Th) Edition. Diabetes Res Clin Pract (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
2. Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci (2020) 21(17):6275. doi: 10.3390/ijms21176275
3. Pedersen MG, Tagliavini A, Henquin JC. Calcium Signaling and Secretory Granule Pool Dynamics Underlie Biphasic Insulin Secretion and its Amplification by Glucose: Experiments and Modeling. Am J Physiol Endocrinol Metab (2019) 316(3):E475–86. doi: 10.1152/ajpendo.00380.2018
4. Del Prato S, Tiengo A. The Importance of First-Phase Insulin Secretion: Implications for the Therapy of Type 2 Diabetes Mellitus. Diabetes Metab Res Rev (2001) 17(3):164–74. doi: 10.1002/dmrr.198
5. Fehse F, Trautmann M, Holst JJ, Halseth AE, Nanayakkara N, Nielsen LL, et al. Exenatide Augments First- and Second-Phase Insulin Secretion in Response to Intravenous Glucose in Subjects With Type 2 Diabetes. J Clin Endocrinol Metab (2005) 90(11):5991–7. doi: 10.1210/jc.2005-1093
6. Hulman A, Witte DR, Vistisen D, Balkau B, Dekker JM, Herder C, et al. Pathophysiological Characteristics Underlying Different Glucose Response Curves: A Latent Class Trajectory Analysis From the Prospective EGIR-RISC Study. Diabetes Care (2018) 41(8):1740–8. doi: 10.2337/dc18-0279
7. Kim SH, Silvers A, Viren J, Reaven GM. Relationship Between Insulin Sensitivity and Insulin Secretion Rate: Not Necessarily Hyperbolic. Diabetes Med (2016) 33(7):961–7. doi: 10.1111/dme.13055
8. Omar-Hmeadi M, Idevall-Hagren O. Insulin Granule Biogenesis and Exocytosis. Cell Mol Life Sci (2021) 78(5):1957–70. doi: 10.1007/s00018-020-03688-4
9. Gerich JE. Is Reduced First-Phase Insulin Release the Earliest Detectable Abnormality in Individuals Destined to Develop Type 2 Diabetes? Diabetes (2002) 51 Suppl 1:S117–21. doi: 10.2337/diabetes.51.2007.s117
10. Henquin JC, Dufrane D, Kerr-Conte J, Nenquin M. Dynamics of Glucose-Induced Insulin Secretion in Normal Human Islets. Am J Physiol Endocrinol Metab (2015) 309(7):E640–50. doi: 10.1152/ajpendo.00251.2015
11. Cerasi E, Ependić S, Luft R. Dose-Response Relation Between Plasma-Insulin and Blood-Glucose Levels During Oral Glucose Loads in Prediabetic and Diabetic Subjects. Lancet (1973) 1(7807):794–7. doi: 10.1016/s0140-6736(73)90599-0
12. Rosengren AH, Braun M, Mahdi T, Andersson SA, Travers ME, Shigeto M, et al. Reduced Insulin Exocytosis in Human Pancreatic β-Cells With Gene Variants Linked to Type 2 Diabetes. Diabetes (2012) 61(7):1726–33. doi: 10.2337/db11-1516
13. Gandasi NR, Yin P, Omar-Hmeadi M, Ottosson Laakso E, Vikman P, Barg S. Glucose-Dependent Granule Docking Limits Insulin Secretion and Is Decreased in Human Type 2 Diabetes. Cell Metab (2018) 27(2):470–8.e4. doi: 10.1016/j.cmet.2017.12.017
14. Hong J, Gu WQ, Zhang YF, Yang YS, Shen CF, Xu M, et al. The Interplay of Insulin Resistance and Beta-Cell Dysfunction Involves the Development of Type 2 Diabetes in Chinese Obeses. Endocrine (2007) 31(2):93–9. doi: 10.1007/s12020-007-0002-2
15. Lorenzo C, Wagenknecht LE, D'Agostino RB Jr, Rewers MJ, Karter AJ, Haffner SM. Insulin Resistance, Beta-Cell Dysfunction, and Conversion to Type 2 Diabetes in a Multiethnic Population: The Insulin Resistance Atherosclerosis Study. Diabetes Care (2010) 33(1):67–72. doi: 10.2337/dc09-1115
16. Arslanian S. Insulin Secretion and Sensitivity in Healthy African-American vs American White Children. Clin Pediatr (Phila) (1998) 37(2):81–8. doi: 10.1177/000992289803700204
17. Osei K, Cottrell DA, Harris B. Differences in Basal and Poststimulation Glucose Homeostasis in Nondiabetic First Degree Relatives of Black and White Patients With Type 2 Diabetes Mellitus. J Clin Endocrinol Metab (1992) 75(1):82–6. doi: 10.1210/jcem.75.1.1619033
18. Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin Resistance and Hyperinsulinemia: Is Hyperinsulinemia the Cart or the Horse? Diabetes Care (2008) 31 Suppl 2:S262–8. doi: 10.2337/dc08-s264
19. Tricò D, Natali A, Arslanian S, Mari A, Ferrannini E. Identification, Pathophysiology, and Clinical Implications of Primary Insulin Hypersecretion in Nondiabetic Adults and Adolescents. JCI Insight (2018) 3(24):e124912. doi: 10.1172/jci.insight.124912
20. Armiyaw L, Sarcone C, Fosam A, Muniyappa R. Increased β-Cell Responsivity Independent of Insulin Sensitivity in Healthy African American Adults. J Clin Endocrinol Metab (2020) 105(7):e2429–38. doi: 10.1210/clinem/dgaa234
21. Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic Differences in the Relationship Between Insulin Sensitivity and Insulin Response: A Systematic Review and Meta-Analysis. Diabetes Care (2013) 36(6):1789–96. doi: 10.2337/dc12-1235
22. Rorsman P, Renström E. Insulin Granule Dynamics in Pancreatic Beta Cells. Diabetologia (2003) 46(8):1029–45. doi: 10.1007/s00125-003-1153-1
23. Hong J, Zhang YF, Gu WQ, Zhang YW, Su YX, Chi ZN, et al. Insulin Sensitivity and First-Phase Insulin Secretion in Obese Chinese With Hyperglycemia in 30 and/or 60 Min During Glucose Tolerance Tests. Endocrine (2008) 34(1-3):75–80. doi: 10.1007/s12020-008-9106-6
Keywords: normal glucose tolerance, type 2 diabetes mellitus, intravenous glucose tolerance test, first-phase insulin secretion, insulin sensitivity
Citation: Yuan T, Song S, Zhao T, Duo Y, Wang S, Gao J, Liu S, Dong Y, Li R, Fu Y and Zhao W (2021) Patterns of Insulin Secretion During First-Phase Insulin Secretion in Normal Chinese Adults. Front. Endocrinol. 12:738427. doi: 10.3389/fendo.2021.738427
Received: 08 July 2021; Accepted: 18 October 2021;
Published: 17 November 2021.
Edited by:
Maria Perticone, University of Magna Graecia, ItalyReviewed by:
Aaron Hanukoglu, Tel Aviv University, IsraelDavid H. Wagner, University of Colorado Denver, United States
Copyright © 2021 Yuan, Song, Zhao, Duo, Wang, Gao, Liu, Dong, Li, Fu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weigang Zhao, eGllaGV6aGFvd2VpZ2FuZ0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Tao Yuan
Tao Yuan Shuoning Song
Shuoning Song Tianyi Zhao
Tianyi Zhao Yanbei Duo
Yanbei Duo Shihan Wang
Shihan Wang Shixuan Liu
Shixuan Liu Weigang Zhao
Weigang Zhao