- 1Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
- 2Department of Internal Medicine B, Rabin Medical Center, Beilinson Campus, Petah Tikva, Israel
- 3Department of Internal Medicine E, Rabin Medical Center, Beilinson Campus, Petah Tikva, Israel
- 4Department of Internal Medicine A, Shamir (Assaf Harofeh) Medical Center, Zerifin, Israel
- 5Department of Internal Medicine D, Rabin Medical Center, Hasharon Campus, Petah Tikva, Israel
Objective: To assess the effect of linagliptin vs. standard therapy in improving clinical outcomes in patients hospitalized with diabetes and coronavirus disease 2019 (COVID-19).
Materials and Methods: We did an open-label, prospective, multicenter, randomized clinical trial in 3 Israeli hospitals between October 1, 2020, and April 4, 2021. Eligible patients were adults with type 2 diabetes mellitus and a diagnosis of COVID-19. A total of 64 patients, 32 in each group, were randomized to receive linagliptin 5 mg PO daily throughout the hospitalization or standard of care therapy. The primary outcome was time to clinical improvement within 28 days after randomization, defined as a 2-point reduction on an ordinal scale ranging from 0 (discharged without disease) to 8 (death).
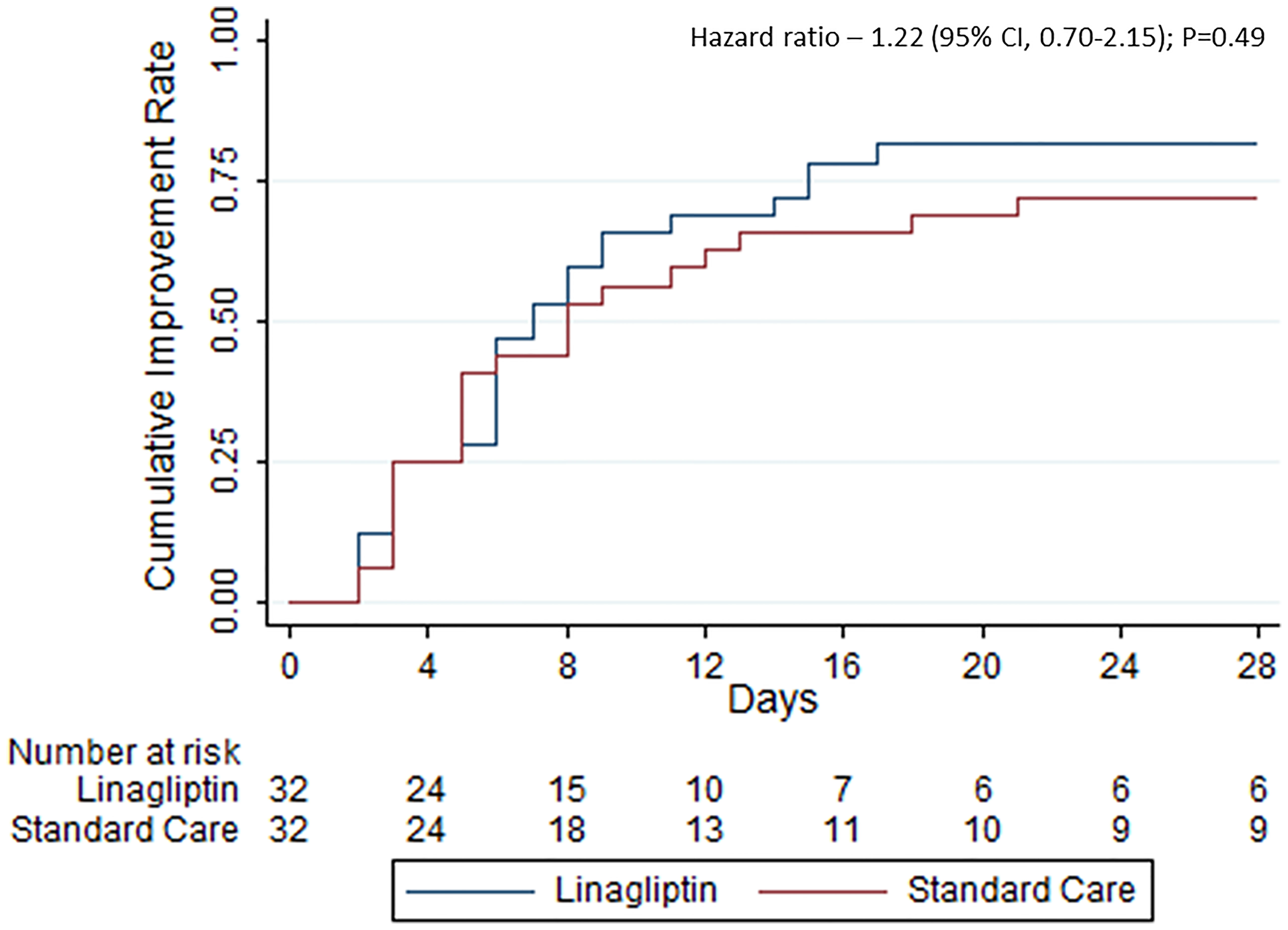
Results: The mean age was 67 ± 14 years, and most patients were male (59.4%). Median time to clinical improvement was 7 days (interquartile range (IQR) 3.5-15) in the linagliptin group compared with 8 days (IQR 3.5–28) in the standard of care group (hazard ratio, 1.22; 95% CI, 0.70–2.15; p = 0.49). In-hospital mortality was 5 (15.6%) and 8 (25.0%) in the linagliptin and standard of care groups, respectively (odds ratio, 0.56; 95% CI, 0.16–1.93). The trial was prematurely terminated due to the control of the COVID-19 outbreak in Israel.
Conclusions: In this randomized clinical trial of hospitalized adult patients with diabetes and COVID-19 who received linagliptin, there was no difference in the time to clinical improvement compared with the standard of care.
Clinical Trial Registration: ClinicalTrials.gov, identifier NCT04371978.
Introduction
The coronavirus disease 2019 (COVID-19) is an emerging pandemic in 2020–2021 caused by a novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1, 2). Diabetes confers a significant additional risk for COVID-19 patients (3–5). Currently, there are only a few specific effective therapeutic agents for the treatment of COVID-19 despite a large number of clinical trials that evaluated a wide range of therapies (6–10).
Dipeptidyl peptidase 4 (DPP-4) is a transmembrane glycoprotein expressed ubiquitously in many tissues and originally known as T-cell surface marker cluster of differentiation 26 (CD26) (11). In addition to its effect on glucose levels, DPP-4 has various effects on the immune system and several diseases, including lung diseases (11, 12). DPP-4 serves as the functional receptor for the Middle East respiratory syndrome coronavirus (MERS-CoV) (13), and potential interactions of SARS-CoV-2 spike glycoprotein and DPP-4 may play a significant role in the viral process of hijacking the human host cells (14).
In addition to their role in treating diabetes, several studies have evaluated the potential of DPP-4 inhibitors as immune-modulating agents and as a treatment for chronic allograft dysfunction following lung transplantation (11, 15). The anti-inflammatory effects of DPP-4 inhibitors and the possible involvement in the process of entering human cells are the basis for the assumption that these agents may be beneficial for the treatment of COVID-19 (16, 17). Two retrospective studies from Italy found that the use of DPP-4 inhibitors as a group or sitagliptin specifically reduced mortality and improved outcomes of patients hospitalized with COVID-19 (18, 19). Nevertheless, a meta-analysis based on retrospective studies concluded that the current data are insufficient and that the combined estimate of the risk ratio for mortality reduction by DPP-4 inhibitors is neutral (20).
Linagliptin is a DPP-4 inhibitor approved by the Food and Drug Administration in 2011 as a treatment for type 2 diabetes (21) and is as effective as other DPP-4 inhibitors (22). In contrast to other DPP-4 inhibitors, no dosage adjustment is necessary for renal or hepatic impairment, and linagliptin does not affect the risk of heart failure (23). In this multicenter randomized, controlled, open-label trial, we investigated the safety and efficacy of linagliptin in hospitalized patients with COVID-19 and diabetes.
Materials and Methods
Design
This was an investigator-initiated, multicenter, open-label randomized clinical trial aimed to assess the safety and efficacy of linagliptin vs. standard therapy in hospitalized patients with diabetes and COVID-19. A total of 3 hospitals in Israel enrolled patients between October 1, 2020, and April 4, 2021, from designated COVID-19 departments. Patients were randomized by the study coordinators using a web-based system with blocks of variable size to a 1:1 allocation ratio. Patients were randomized to receive linagliptin 5 mg daily PO plus standard of care or standard of care alone. The standard of care for patients with diabetes included holding oral drugs and initiating insulin therapy according to a basal bolus protocol (24). The goal of therapy was to keep blood glucose levels in the range of 140–180 mg/dl (25). COVID-19-specific treatments in both groups were according to the updated evidence (26). Treatment with linagliptin was continued from randomization to hospital discharge. The randomization was stratified by the hospital and in each hospital by age (≤70 or >70 years) and oxygen use at randomization (if supplemental oxygen was required or not). Patients were followed up until 28 days following randomization when they were contacted by the study staff by telephone. This trial followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines. Due to the control of the COVID-19 outbreak in Israel (27, 28), no patients were enrolled after April 4, 2021, and the trial was terminated prematurely on May 4, 2021.
Patients
Hospitalized patients 18 years and older, with a diagnosis of COVID-19 confirmed by a positive reverse-transcriptase PCR (RT-PCR) assay for SARS-CoV-2 in a respiratory tract specimen, were all evaluated for eligibility for the study. Other inclusion criteria were a previous diagnosis of type 2 diabetes mellitus or a diagnosis initially made during hospitalization, according to the American Diabetes Association (ADA) guidelines (29), 10 days from COVID-19 symptom onset or within 48 h after positive RT-PCR test. Exclusion criteria included a need for mechanical ventilation or vasopressor medications prior to randomization; expected immediate need of intensive care unit (ICU) admission or immediate surgical intervention; current treatment with any DPP-4 inhibitor; pregnancy; or known hypersensitivity to DPP-4 inhibitors.
Outcomes
The primary clinical endpoint was time to clinical improvement within 28 days after randomization. Clinical improvement was defined as a 2-point reduction in patients’ admission status on a 9-point ordinal scale. This scale has been used in different COVID-19 therapeutic trials (30–32) and was recommended by the R&D Blueprint of the WHO (33). The 9-point scale was as follows: 0, discharged, no clinical or virological evidence of infection; 1, discharged, no limitation of activities; 2, discharged, limitation of activities or ambulatory oxygen use; 3, hospitalized, no oxygen therapy; 4, hospitalized, oxygen by mask or nasal prongs; 5, hospitalized, non-invasive ventilation or high-flow oxygen; 6, hospitalized, intubation and mechanical ventilation; 7, hospitalized, ventilation + additional organ support—vasopressors, renal replacement therapy, extracorporeal membrane oxygenation; and 8, death.
Secondary outcomes were the proportion of patients with 2-point reduction in patients’ admission status on a 9-point ordinal scale; all-cause mortality at 28 days; in-hospital death; length of hospitalization; ICU admissions; mechanical ventilation; supplemental oxygen-free days at 28 days; ventilator-free days at 28 days; and the proportion of patients with 50% decrease in C-reactive protein (CRP) levels. Safety outcomes included treatment-related adverse events, serious adverse events, and premature discontinuations of the study drug.
Statistical Analysis
During the initial design of the study and based on the trial of Cao et al. (30), assuming a decrease in the primary endpoint measure from a median of 16 days to a median of 8 days between the two trial groups with overall power of 80% and significance of 0.05, the sample size required for the trial was 88 patients. After publications of further trials, it was assumed that a larger sample size is required for such power, but the wide vaccine campaign and outbreak control in Israel (27, 28) led to premature termination of the trial.
Primary endpoint analysis was based on intention-to-treat analysis and included all the patients who had undergone randomization. The time to clinical improvement was assessed after all patients had reached day 28, with failure to reach clinical improvement or death before day 28 and before clinical improvement was considered as right-censored at day 28. The time to clinical improvement was portrayed by the Kaplan–Meier plot and compared with a log-rank test. Hazard ratios with 95% CIs were calculated by Cox proportional hazards model. Secondary outcomes such as mortality at 28 days, the proportion of ICU admission, the proportion of mechanical ventilation, and the proportion of CRP reduction were compared using the χ2 test. Only the p-value for the primary efficacy analysis (2-tailed; significance defined as p ≤ 0.05) was reported, and bilateral 95% CI was not adjusted for multiplicity for all the other comparisons. Statistical analyses were performed with Stata, version 13.0 (StataCorp, Texas, USA) and SPSS, version 25 (IBM Corp). This trial is registered with ClinicalTrials.gov, NCT04371978.
Results
Patients
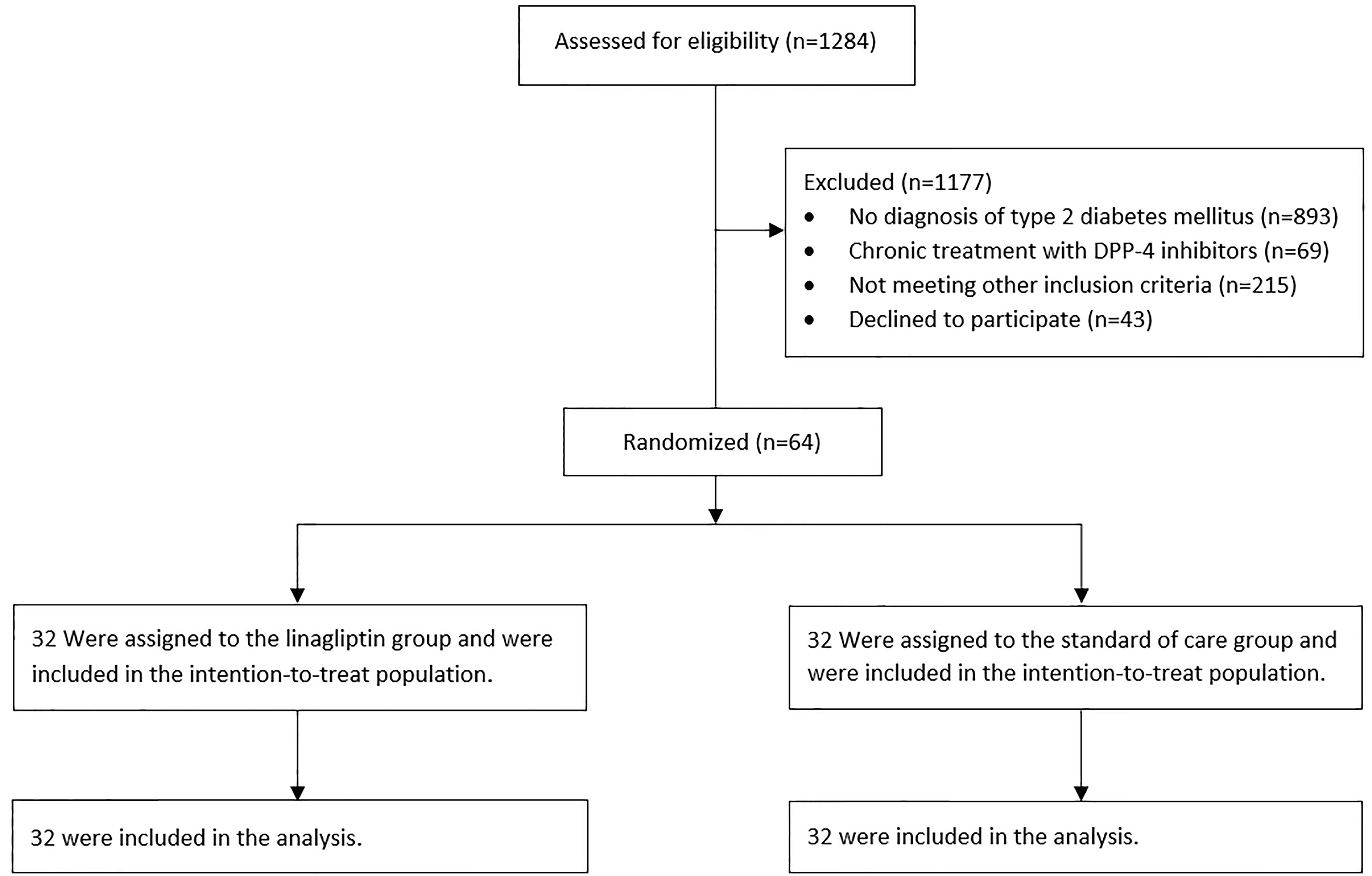
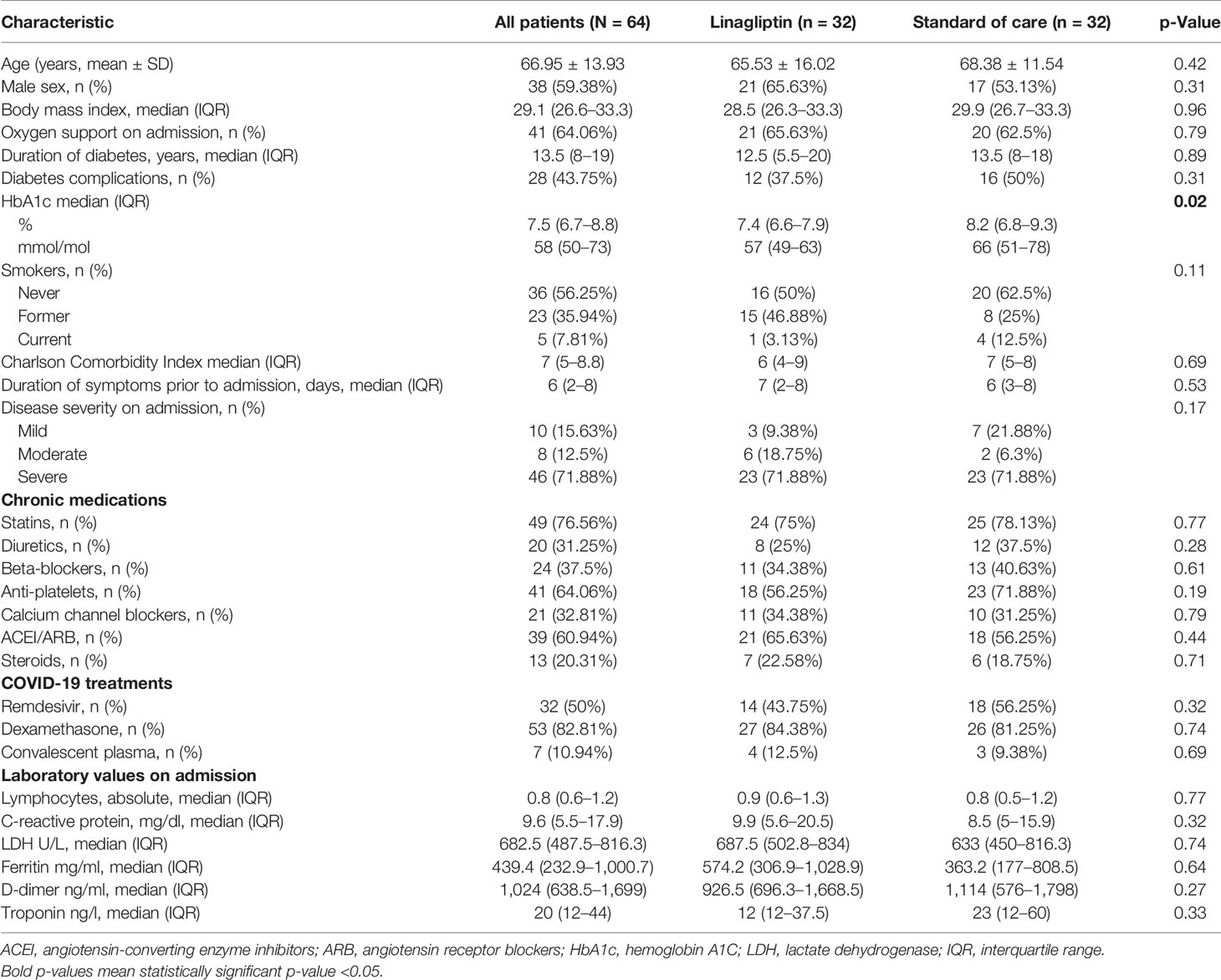
A total of 64 patients with COVID-19 from three hospitals in Israel underwent randomization and were included in this analysis. All patients were included in the intention-to-treat analysis (Figure 1). No patient withdrew from the trial. Demographic and baseline clinical characteristics are summarized in Table 1. Most of the patients were male (59.4%) with a mean age of 66.95 ± 13.93 years. Generally, the baseline clinical and laboratory parameters were balanced between the two groups, except for HbA1c, which was lower in the linagliptin group. COVID-19 disease classification was defined according to the National Institute of Health guidelines (10), and more than 70% of patients were classified as severe on admission. Of the patients, 84.4% and 81.3% received dexamethasone and 43.8% and 56.3% received remdesivir in the linagliptin and standard of care groups, respectively.
Primary Outcome
A total of 26 patients (81.3%) in the linagliptin group and 23 (71.9%) in the standard of care group showed clinical improvement, i.e., 2-point reduction in the WHO scale, within 28 days of randomization (odds ratio, 0.59; 95% CI, 0.18–1.91) (Table 2). The time to improvement was also similar between the groups—median time of 7 days (interquartile range (IQR) 3.5–15) in the linagliptin group compared with 8 days (IQR 3.5–28) in the standard of care group (hazard ratio, 1.22; 95% CI, 0.70–2.15; p = 0.49, Figure 2). Different sensitivity analyses showed similar results.
Adverse events related to the study drug were rare. Only two patients had a related adverse event, hypoglycemia, with one in the linagliptin group and the other in the standard of care group.
Secondary Outcomes
There were no major differences in the rate of ICU admission between the groups, with 7 (21.9%) in the linagliptin group and 4 (12.5%) in the standard of care group (odds ratio, 1.96; 95% CI, 0.51–7.50) (Table 2). All-cause mortality at 28 days was 7 (21.9%) and 9 (28.1%) in the linagliptin and standard of care groups, respectively (odds ratio, 0.72; 95% CI, 0.23–2.23). The length of hospitalization was also similar in both groups with a median of 7 days (IQR 3–12) for the entire cohort (mean difference, 0.44; 95% CI, −2.83 to 3.71). One patient in the linagliptin group was still hospitalized in the ICU after 28 days from randomization.
Discussion
Observational studies have suggested that DPP-4 inhibitors are effective in reducing mortality in patients with COVID-19 (18, 19). However, this randomized trial found that in hospitalized adults with COVID-19 and diabetes, linagliptin treatment added to the standard of care failed to significantly improve the time to resolution of symptoms or day 28 mortality. Furthermore, no difference between the study groups was observed for any of the secondary outcomes, including the proportion of patients admitted to an ICU, mechanical ventilation rates, length of hospitalization, and supplemental oxygen use. To our knowledge, this is the first randomized clinical trial evaluating the efficacy and safety of a DPP-4 inhibitor in patients with COVID-19.
As in many COVID-19 trials (31, 34–36), our study also had a male predominance in both groups, but the mean age was higher. The death and complications rates of our patients were higher than in most other clinical trials since our population included only patients with diabetes mellitus, a known risk factor for severe COVID-19 (3–5). The number of comorbidities was also high as reflected in the Charlson Comorbidity Index with a median score of 7, which is a strong predictor for mortality (37). As in other places in the world (38), hospital resources became scarce during the pandemic, and ambulatory treatment models were widely adopted. That narrowed hospitalizations only to those who needed them the most and explains why more than 70% of the patients were classified with severe disease on admission. Patients with a mild or moderate disease on admission were at high risk of progression to severe disease and/or with several additional acute illnesses.
The timing of linagliptin administration during the course of the disease might also be of importance. There are two suggested mechanisms for the effects of linagliptin on COVID-19. The first is the interaction of the virus with the human host cell (14), and the second is the anti-inflammatory effect (39). The first encourages use during the initial infection, while most patients are asymptomatic/presymptomatic, while the second is aimed for a later stage in the natural history of the disease, the inflammatory stage (40). Since we enrolled only hospitalized patients, treatment during the presymptomatic stage was not practical, but we enrolled patients only until the 10th day of symptoms. The results of previous observational studies support the hypothesis of the anti-inflammatory effect, as patients treated with DPP-4 inhibitors prior to COVID-19 infection had similar outcomes compared with those not being treated, as opposed to the studies in which DPP-4 inhibitors were used during the hospitalization, in which mortality reduction was shown (20). In our study, analysis of patients with symptoms shorter or longer than 5 days prior to admission showed no significant difference in the primary outcome. In addition, since most patients in our study were hospitalized with a disease classified as severe, it is possible that treatment of patients with diabetes and mild COVID-19 in the symptomatic stage may lead to different clinical outcomes. The use of DPP-4 inhibitors as anti-inflammatory agents in patients without diabetes may also lead to different clinical outcomes than those presented in this manuscript. Glucagon-like peptide 1 receptor (GLP-1R) agonists may have a similar anti-inflammatory effect in patients with diabetes and COVID-19, as they are known to reduce the levels of several anti-inflammatory markers (41). This should be assessed in further studies.
The Italian observational studies that showed mortality reduction with DPP-4 inhibitors were conducted during the early phase of the pandemic, February–April 2020 (18, 19). The rapid changes of clinical guidelines as new data emerged weekly led to a substantial difference between the treatment of COVID-19 patients in April 2020 and April 2021 (26, 42). Thus, it is possible that DPP-4 inhibitors have a beneficial effect on COVID-19 compared with supportive care alone, but this effect is eliminated when other therapies such as remdesivir and dexamethasone are added, as was for most of the patients in our study. The relation between the immunomodulatory effect of DPP-4 inhibitors and corticosteroids is unknown, and it is possible that dexamethasone diminished the immunomodulatory effect of linagliptin (43, 44). It should be noted that while sitagliptin specifically was evaluated in a previous study (18), we evaluated linagliptin, but if DPP-4 inhibitors are beneficial, then this is probably a class effect.
The very low rate of adverse events can be explained by the fairly good safety profile of linagliptin, especially with regard to severe adverse events (45). Another explanation is the short treatment period, with most patients treated for less than 10 days, while for diabetes, it is a chronic treatment, and most trials that evaluated the safety of the drug followed up patients for 6–24 months.
Our study has several limitations. The first limitation is the sample size and the premature discontinuation of the trial due to the end of the COVID-19 outbreak in Israel (27, 28). This makes our study underpowered to detect possible differences in the primary outcome and in mortality. The second limitation is the fact that the trial was open-label. We considered conducting a double-blind placebo-controlled trial, but that was not feasible due to the time constraints.
Conclusions
The administration of linagliptin in patients with COVID-19 and diabetes did not improve the time to clinical improvement or 28-day mortality. Further large-scale, blinded, placebo-controlled randomized clinical trials are needed to confirm the results and to explore possible applications of DPP-4 inhibitors in different stages of the disease in patients with and without diabetes.
Data Availability Statement
The data used in the analysis of this study are not publicly available due to the requirements of the IRB Committee but are available from the corresponding author upon request.
Ethics Statement
The trial was approved by the institutional review board of Rabin Medical Center (confirmation number 0303-20-RMC) and Shamir Medical Center (confirmation number 0010-21-ASF). Written informed consent was obtained from all patients. The trial was conducted in accordance with the principles of the Declaration of Helsinki. The authors were responsible for designing the trial and for compiling and analyzing the data. The authors vouch for the completeness and accuracy of the data and for the adherence of the trial to the protocol. The participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization: RA, AG, and IA-D. Methodology: RA and IA-D. Formal analysis: RA, NZ, MK, and DD. Investigation: RA and AG. Resources: AG. Data curation: RA. Writing—original draft preparation: all authors. Writing—review and editing: RA, AG, and RK. Supervision: AG and RK. Funding acquisition: AG. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the internal budget of the Department of Internal Medicine B, Beilinson Campus, Rabin Medical Center.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.794382/full#supplementary-material.
References
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus From Patients With Pneumonia in China, 2019. N Engl J Med (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
2. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical Features of Patients Infected With 2019 Novel Coronavirus in Wuhan, China. Lancet (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
3. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
4. Maddaloni E, Buzzetti R. Covid-19 and Diabetes Mellitus: Unveiling the Interaction of Two Pandemics. Diabetes Metab Res Rev (2020) 36:e3321. doi: 10.1002/dmrr.3321
5. Iacobellis G. COVID-19 and Diabetes: Can DPP4 Inhibition Play a Role? Diabetes Res Clin Pract (2020) 162:108125. doi: 10.1016/j.diabres.2020.108125
6. Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, Liu J. The Effect of Corticosteroid Treatment on Patients With Coronavirus Infection: A Systematic Review and Meta-Analysis. J Infect (2020) 81:e13–20. doi: 10.1016/j.jinf.2020.03.062
7. Siemieniuk RAC, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Pardo-Hernandez H, et al. Drug Treatments for Covid-19: Living Systematic Review and Network Meta-Analysis. BMJ (2020) 370:m2980. doi: 10.1136/bmj.m2980
8. Piscoya A, Ng-Sueng LF, del Riego AP, Cerna-Viacava R, Pasupuleti V, Roman YM, et al. Efficacy and Harms of Remdesivir for the Treatment of COVID-19: A Systematic Review and Meta-Analysis. PloS One (2020) 15:e0243705. doi: 10.1371/journal.pone.0243705
9. Putman M, Chock YPE, Tam H, Kim AHJ, Sattui SE, Berenbaum F, et al. Antirheumatic Disease Therapies for the Treatment of COVID-19: A Systematic Review and Meta-Analysis. Arthritis Rheumatol (2021) 73:36–47. doi: 10.1002/art.41469
10. COVID-19 Treatment Guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/ (Accessed May 13, 2021).
11. Shao S, Xu QQ, Yu X, Pan R, Chen Y. Dipeptidyl Peptidase 4 Inhibitors and Their Potential Immune Modulatory Functions. Pharmacol Ther (2020) 209:107503. doi: 10.1016/j.pharmthera.2020.107503
12. Zou H, Zhu N, Li S. The Emerging Role of Dipeptidyl-Peptidase-4 as a Therapeutic Target in Lung Disease. Expert Opin Ther Targets (2020) 24:147–53. doi: 10.1080/14728222.2020.1721468
13. Stalin Raj V, Mou H, Smits SL, W Dekkers DH, Müller MA, Dijkman R, et al. Dipeptidyl Peptidase 4 Is a Functional Receptor for the Emerging Human Coronavirus-EMC. Nature (2013) 495:251–4. doi: 10.1038/nature12005
14. Vankadari N, Wilce JA. Emerging WuHan (COVID-19) Coronavirus: Glycan Shield and Structure Prediction of Spike Glycoprotein and Its Interaction With Human CD26. Emerg Microbes Infect (2020) 9:601–4. doi: 10.1080/22221751.2020.1739565
15. Yamada Y, Nishikawa S, Tanaka S, Hamaji M, Nakajima D, Ohsumi A, et al. CD26/DPP4 Inhibitor: A Novel Prophylactic Drug for Chronic Allograft Dysfunction After Clinical Lung Transplantation. J Hear Lung Transplant (2020) 39:S66. doi: 10.1016/j.healun.2020.01.1269
16. Scheen AJ. DPP-4 Inhibition and COVID-19: From Initial Concerns to Recent Expectations. Diabetes Metab (2021) 47:101213. doi: 10.1016/j.diabet.2020.11.005
17. Solerte SB, Di Sabatino A, Galli M, Fiorina P. Dipeptidyl Peptidase-4 (DPP4) Inhibition in COVID-19. Acta Diabetol (2020) 57:779–83. doi: 10.1007/s00592-020-01539-z
18. Solerte SB, D’Addio F, Trevisan R, Lovati E, Rossi A, Pastore I, et al. Sitagliptin Treatment at the Time of Hospitalization Was Associated With Reduced Mortality in Patients With Type 2 Diabetes and COVID-19: A Multicenter, Case-Control, Retrospective, Observational Study. Diabetes Care (2020) 42:dc201521. doi: 10.2337/dc20-1521
19. Mirani M, Favacchio G, Carrone F, Betella N, Biamonte E, Morenghi E, et al. Impact of Comorbidities and Glycemia at Admission and Dipeptidyl Peptidase 4 Inhibitors in Patients With Type 2 Diabetes With Covid-19: A Case Series From an Academic Hospital in Lombardy, Italy. Diabetes Care (2020) 43:3042–9. doi: 10.2337/dc20-1340
20. Bonora BM, Avogaro A, Fadini GP. Disentangling Conflicting Evidence on DPP-4 Inhibitors and Outcomes of COVID-19: Narrative Review and Meta-Analysis. J Endocrinol Invest (2021) 1:1–8. doi: 10.1007/s40618-021-01515-6
21. FDA. Drug Approval Package: Tradjenta (Linagliptin) (2011). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/201280Orig1s000TOC.cfm (Accessed April 13, 2020).
22. Keshavarz K, Lotfi F, Sanati E, Salesi M, Hashemi-Meshkini A, Jafari M, et al. Linagliptin Versus Sitagliptin in Patients With Type 2 Diabetes Mellitus: A Network Meta-Analysis of Randomized Clinical Trials. DARU J Pharm Sci (2017) 25:25–23. doi: 10.1186/s40199-017-0189-6
23. McGuire DK, Alexander JH, Johansen OE, Perkovic V, Rosenstock J, Cooper ME, et al. Linagliptin Effects on Heart Failure and Related Outcomes in Individuals With Type 2 Diabetes Mellitus at High Cardiovascular and Renal Risk in CARMELINA. Circulation (2019) 139:351–61. doi: 10.1161/CIRCULATIONAHA.118.038352
24. Association AD. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes-2021. Diabetes Care (2021) 44:S211–20. doi: 10.2337/dc21-s015
25. Association AD. 15. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes—2020. Diabetes Care (2020) 43:S193–202. doi: 10.2337/DC20-S015
26. Scavone C, Mascolo A, Rafaniello C, Sportiello L, Trama U, Zoccoli A, et al. Therapeutic Strategies to Fight COVID-19: Which Is the Status Artis? Br J Pharmacol (2021) 1-21:bph.15452. doi: 10.1111/bph.15452
27. Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med (2021) 384:1412–23. doi: 10.1056/nejmoa2101765
28. Leshem E, Wilder-Smith A. COVID-19 Vaccine Impact in Israel and a Way Out of the Pandemic. Lancet (London England) (2021) 397:1783–5. doi: 10.1016/S0140-6736(21)01018-7
29. Association AD. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care (2021) 44:S15–33. doi: 10.2337/DC21-S002
30. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized With Severe Covid-19. N Engl J Med (2020) 382:1787–99. doi: 10.1056/nejmoa2001282
31. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in Adults With Severe COVID-19: A Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial. Lancet (2020) 395:1569–78. doi: 10.1016/S0140-6736(20)31022-9
32. López-Medina E, López P, Hurtado IC, Dávalos DM, Ramirez O, Martínez E, et al. Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19: A Randomized Clinical Trial. JAMA - J Am Med Assoc (2021) 325:1426–35. doi: 10.1001/jama.2021.3071
33. World Health Organization. WHO R&D Blueprint Novel Coronavirus COVID-19 Phase IIb/III Vaccine Trial Synopsis. Geneva, Switzerland: World Health Organization (2020).
34. Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in Patients Hospitalized With Covid-19 Pneumonia. N Engl J Med (2021) 384:20–30. doi: 10.1056/nejmoa2030340
35. Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, et al. Effect of Tocilizumab vs Standard Care on Clinical Worsening in Patients Hospitalized With COVID-19 Pneumonia: A Randomized Clinical Trial. JAMA Intern Med (2021) 181:24–31. doi: 10.1001/jamainternmed.2020.6615
36. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19 — Final Report. N Engl J Med (2020) 383:1813–26. doi: 10.1056/nejmoa2007764
37. Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data From 6 Countries. Am J Epidemiol (2011) 173:676–82. doi: 10.1093/aje/kwq433
38. Lisker G, Narasimhan M, Greenberg H, Ramdeo R, McGinn T. Ambulatory Management of Moderate to High Risk COVID-19 Patients: The Coronavirus Related Outpatient Work Navigators (CROWN) Protocol. Home Heal Care Manag Pract (2021) 33:49–53. doi: 10.1177/1084822320964196
39. Strollo R, Pozzilli P. DPP4 Inhibition: Preventing SARS-CoV-2 Infection and/or Progression of COVID-19? Diabetes Metab Res Rev (2020) 36:e3330. doi: 10.1002/dmrr.3330
40. Parasher A. COVID-19: Current Understanding of Its Pathophysiology, Clinical Presentation and Treatment. Postgrad Med J (2020) 97:postgradmedj–2020–138577. doi: 10.1136/postgradmedj-2020-138577
41. Shao S, Yang Q, Pan R, Yu X, Chen Y. Interaction of Severe Acute Respiratory Syndrome Coronavirus 2 and Diabetes. Front Endocrinol (Lausanne) (2021) 12:731974/BIBTEX. doi: 10.3389/FENDO.2021.731974/BIBTEX
42. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA (2020) 323(18):1824–36. doi: 10.1001/jama.2020.6019
43. Bassendine MF, Bridge SH, McCaughan GW, Gorrell MD. COVID-19 and Comorbidities: A Role for Dipeptidyl Peptidase 4 (DPP4) in Disease Severity? J Diabetes (2020) 12:649–58. doi: 10.1111/1753-0407.13052
44. Valencia I, Peiró C, Lorenzo Ó, Sánchez-Ferrer CF, Eckel J, Romacho T. DPP4 and ACE2 in Diabetes and COVID-19: Therapeutic Targets for Cardiovascular Complications? Front Pharmacol (2020) 11:1161. doi: 10.3389/FPHAR.2020.01161
Keywords: COVID-19, diabetes, linagliptin, DPP-4 inhibitors, hospital management
Citation: Abuhasira R, Ayalon-Dangur I, Zaslavsky N, Koren R, Keller M, Dicker D and Grossman A (2021) A Randomized Clinical Trial of Linagliptin vs. Standard of Care in Patients Hospitalized With Diabetes and COVID-19. Front. Endocrinol. 12:794382. doi: 10.3389/fendo.2021.794382
Received: 13 October 2021; Accepted: 30 November 2021;
Published: 22 December 2021.
Edited by:
Susanna Hofmann, Helmholtz Association of German Research Centres (HZ), GermanyReviewed by:
Oscar Lorenzo, Health Research Institute Foundation Jimenez Diaz (IIS-FJD), SpainAsimina Mitrakou-Fanariotou, National and Kapodistrian University of Athens, Greece
Copyright © 2021 Abuhasira, Ayalon-Dangur, Zaslavsky, Koren, Keller, Dicker and Grossman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ran Abuhasira, cmFuYWJ1QHBvc3QuYmd1LmFjLmls
 Ran Abuhasira
Ran Abuhasira Irit Ayalon-Dangur1,3
Irit Ayalon-Dangur1,3