Abstract
Over the years, the vaste expansion of plastic manufacturing has dramatically increased the environmental impact of microplastics [MPs] and nanoplastics [NPs], making them a threat to marine and terrestrial biota because they contain endocrine disrupting chemicals [EDCs] and other harmful compounds. MPs and NPs have deleteriouse impacts on mammalian endocrine components such as hypothalamus, pituitary, thyroid, adrenal, testes, and ovaries. MPs and NPs absorb and act as a transport medium for harmful chemicals such as bisphenols, phthalates, polybrominated diphenyl ether, polychlorinated biphenyl ether, organotin, perfluorinated compounds, dioxins, polycyclic aromatic hydrocarbons, organic contaminants, and heavy metals, which are commonly used as additives in plastic production. As the EDCs are not covalently bonded to plastics, they can easily leach into milk, water, and other liquids affecting the endocrine system of mammals upon exposure. The toxicity induced by MPs and NPs is size-dependent, as smaller particles have better absorption capacity and larger surface area, releasing more EDC and toxic chemicals. Various EDCs contained or carried by MPs and NPs share structural similarities with specific hormone receptors; hence they interfere with normal hormone receptors, altering the hormonal action of the endocrine glands. This review demonstrates size-dependent MPs’ bioaccumulation, distribution, and translocation with potential hazards to the endocrine gland. We reviewed that MPs and NPs disrupt hypothalamic-pituitary axes, including the hypothalamic-pituitary-thyroid/adrenal/testicular/ovarian axis leading to oxidative stress, reproductive toxicity, neurotoxicity, cytotoxicity, developmental abnormalities, decreased sperm quality, and immunotoxicity. The direct consequences of MPs and NPs on the thyroid, testis, and ovaries are documented. Still, studies need to be carried out to identify the direct effects of MPs and NPs on the hypothalamus, pituitary, and adrenal glands.
1 Introduction
xIn recent decades, plastic pollution has become one of the most widespread and enduring anthropogenic alterations in all environmental compartments of our planet’s surface (1) and therefore, considered as a stratigraphic marker for the Anthropocene (2). A culture of single-use plastic, rapid and inexpensive plastic production and non-circular economic models led to the creation of over 368 million metric tons (Mt) of single-use plastic in 2019 (3). It is expected that if the current production and waste management trends continue, approximately 12,000 Mt of plastic waste will end up in the natural environment by 2050 (4). To date, plastic debris has affected 3876 species only in the aquatic environment (https://litterbase.awi.de/interaction_detail; date assessed July 28, 2022), and by the year 2050, plastic will be found in the digestive tract of 99% of all sea bird species (5). Globally mammals are already at-risk due to several reasons, including climate change (6), however, bioaccumulation of the microplastic (MP) and associated toxic chemical additives are accelerating the risk of extinction (7, 8). Despite its ubiquitous distribution, current knowledge about the health effects of MP and associated chemicals in mammals is limited. Therefore, we aim to highlight how MP affects mammalian endocrine glands, which could contribute to their conservation and management, especially in vulnerable populations.
1.1 Rising global plastic and MPs pollution in the terrestrial and aquatic environment
Plastic waste is a contemporary societal and ecological issue due to its indispensable nature and ubiquitous use in daily life, associated with long-term detrimental effects on organisms (9). Plastic consumption will continue to rise in response to global population growth, and as plastic degrades, microplastics (<5 mm) and nanoplastics (< 1 nm) enter the terrestrial ecosystem in several ways (10). Depending on their manufactured and fragmented origin, MPs can be classified as primary or secondary (11). Primary MPs are primarily designed into small sizes for commercial practices like personal care products, whereas secondary MPs are fragmented from larger plastics by various physical and biological methods (10), such as UV radiation, temperature changes, and wave action (12). Due to sewage sludge applications, each year, 63–430 and 44–300 thousand tons of MPs are added to agro-ecosystems in Europe and North America (13). In marine ecosystems, approximately 5-13 million tons of plastic debris enter the ocean each year (8). Consequently, the world’s upper ocean currently comprises 24.4 trillion pieces, (8.2 × 104 ~ 57.8 × 104 tons) of micro-plastic (14), and its concentration might exceed 250 million metric tons by 2025 (8, 15). In 2015, Oceanographers estimated 15-51 trillion MP particles floating on water surfaces worldwide (16). In Europe, approximately, 63,000–430,000 tonnes of MPs entered the farmlands annually (17), while in most part of the world, data regarding MP loading in agriculture farmlands are unavailable.
1.2 Chemical composition of MPs and their endocrine-disrupting effects
MPs generated from plastic degradation persist for hundreds and thousands of years in the environment (18). Plastic bottles, disposable diapers, and polystyrene foam have a life span of 450, 500, and >5000 years [https://www.goecopure.com/lifespan-of-plastic.aspx]. Plastic additives used during plastic processing contribute up to 70% of plastics (18). More than 10,000 chemicals are identified as plastic additives, and 2400 chemicals have been classified as detrimental to marine and terrestrial biota (19). These additives include plasticizers, antioxidants, UV stabilizers, dyes, and flame retardants. Some of them are of serious concern, such as alkylphenol (20), polybrominated diphenyl ethers [PBDEs] (21), phthalates, organotins, perfluorinated compounds, dioxin (22), bisphenol A [BPA] and heavy metals like chromium, lead, and cadmium (8, 23). Approximately, 1000 chemicals classified as endocrine disruptor chemicals (EDCs) alter the expression of various hormone receptors and interfere with the synthesis, secretion, transport, and action of hormones, leading to endocrine and developmental abnormalities (23, 24). Nearly nine different forms of MPs are reported in human feces from multiple countries, clearly validating the presence of MPs in the human food chain and warning us about their harmful effects on human health (25). MPs and their composite toxic additives can cross various biological membranes, blood-brain barriers and both can interfere with various hormone receptors, thereby disrupting different hypothalamic axes such as the hypothalamic-pituitary-thyroid axis [HPT], the hypothalamic-pituitary-adrenal axis [HPA], and hypothalamic-pituitary-gonadal axis (HPG; Figure 1) (23, 26–29). Various metabolic disorders, gut dysbiosis, and intestinal barrier dysfunction induced by MPs have been explored using rats and mice as model organisms. Similarly, neurobehavioral changes, disrupted thyroid status, and biochemical stress are the direct consequences of MPs exposure in rats (30).
Figure 1
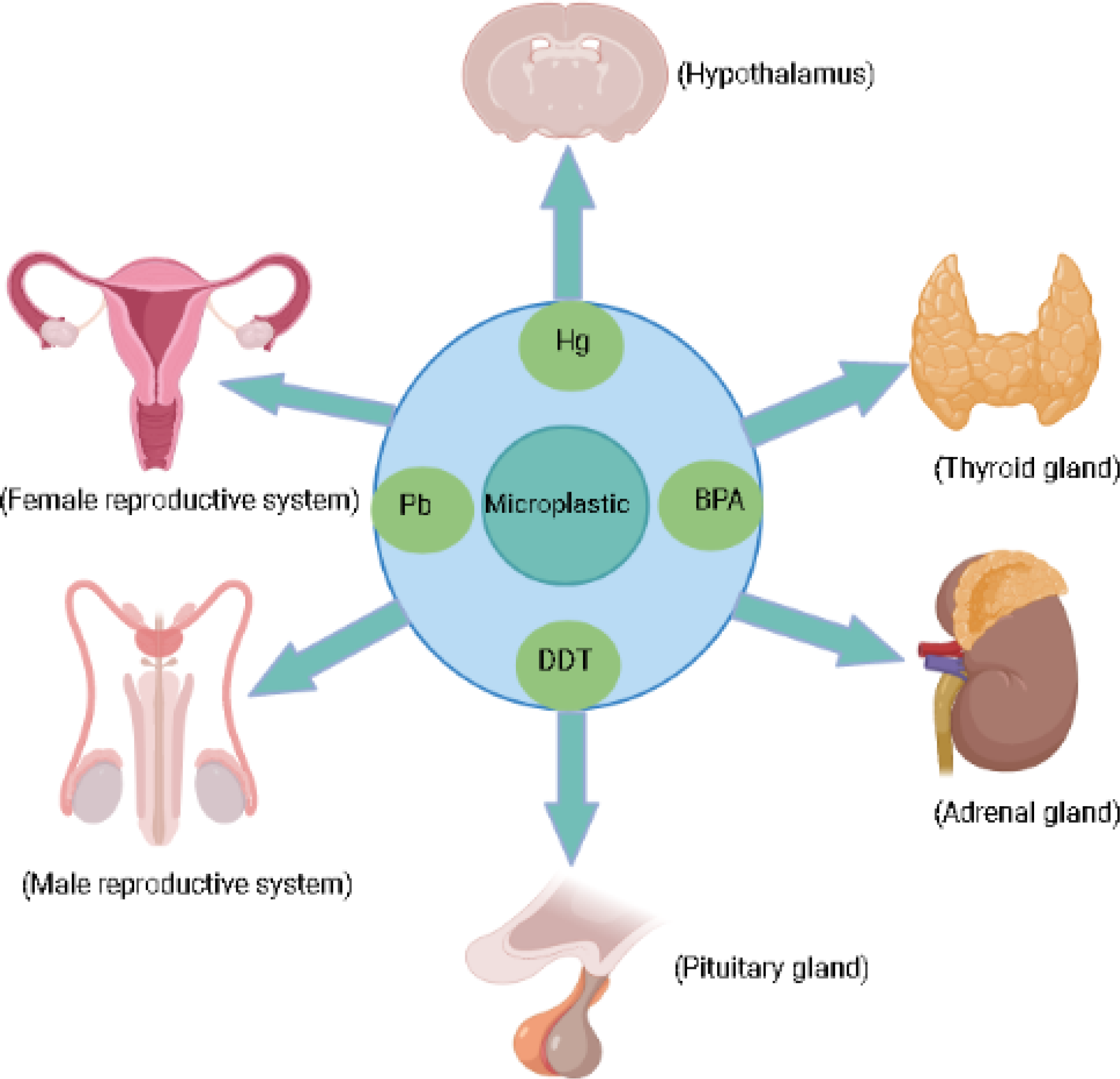
Micoplastic and their associated chemicals exposure can effect endocrine glands.
1.3 MPs bioaccumulation and biomagnification due to their “Trojan Horse” effects in mammals
The large surface area and hydrophobic surface of MPs make them a suitable medium for carrying many pollutants such as EDCs, heavy metals, and other toxic organic chemicals, making them harmful to mammals through bioaccumulation and biomagnification processes (31, 32). These are called “Trojan Horse Effects” of MPs (33), and induce several synergistic, behavioral, histological, and biomolecular alternations (32). Many EDCs and other pollutants are added as additives or absorbed by MPs; after being consumed directly or indirectly through the food web, MPs increase their bioaccumulation in mammals (8, 21, 34). MPs accumulate in various body parts and are involved in biochemical pathways, affecting cell functioning by crossing biological membranes in a size-dependent manner (35). Studies have shown that MPs of size 0.1-10 µm can cross biological membranes, blood-brain barrier, and even placenta, enhancing the possibilities of their bio-accumulation in secondary tissues such as the liver and brain (23). While MPs <150µm can cross the gastrointestinal tract, those <5µm can accumulate in macrophages and be carried to the blood circulation and the spleen (22). Similarly, MPs <10µm trans-locate from the gut to the circulatory system and can accumulate in the liver, kidney, and brain (36).
This review warns about the accumulation of MPs in marine and terrestrial ecosystems. Furthermore, it implies that studies need to be carried out to deeply understand the action mechanism of MPs, nanoplastics [NPs], and associated chemicals in aquatic and terrestrial biota and their long-term detrimental consequences on the mammalian endocrine system.
2 Effects of MPs, NPs, and associated chemicals on the mammalian thyroid gland
The thyroid gland is an essential endocrine gland responsible for normal brain function, growth, and neurological development of all animals (37). The thyroid functions under the HPT axis and affects almost every organ in the body (38), therefore disruption in thyroid homeostasis can be detrimental and will affect the body’s overall health status. Long-term exposure to plastic particles and associated chemicals has been shown to exhaust thyroid endocrine function by weakening its driving forces in regulating growth, development, metabolism, and reproduction (39). MPs additives and pollutants, such as PBDEs, BPA, phthalates, and organotin act as thyroid-disrupting chemicals [TDCs] (22) (Table 1). Similarly, MPs cause thyroid dysfunction and developmental abnormalities once ingested with associated POPs and EDCs (43). Phthalate causes a reduction in thyroid weight during childhood exposure and associated with developmental abnormalities and hyperactivity of the thyroid gland (44, 45). These TDCs associated with plastic enter the body through the gastrointestinal tract and interfere with T4 and T3 biochemical pathways, while their circulation adversely affects thyroid hormone production and metabolism, affecting other organs like the brain in primary developmental stages (46). Several TDCs circulating in the blood form complexes with protein of thyroid hormones and eventually reach the brain and bind with thyroid hormone receptors, disrupting thyroid health (46). TDCs are also responsible for the prevalence of subclinical thyroid conditions known as “subclinical thyroid disease” [SCTD]. In SCTD, the body observes abnormal low or high levels of thyroid-stimulating hormones [TSH] (47). Similarly, BPA can interfere with thyroid hormone action and impairs thyroid functions by inhibiting T3 binding to its receptor and suppressing transcriptional activity mediated by thyroid hormone receptors (48). Phthalates also disturb the normal thyroid system by interfering with gene expression in the HPT axis and metabolic activity (27). It impairs thyroid function through various mechanisms, including inhibition of T3 protein binding, antagonistic interactions, and disruption of throid receptor’s transcriptional activity (49).
Table 1
| Plastics | Species | Thyroid disrupting consequences | References |
|---|---|---|---|
| MPs | Humans | Thyroid dysfunction and metabolic and developmental abnormalities once ingested with associated POPs and EDCs | (40) |
| NPs | Rats | T3 and circulating THs levels were decreased after exposure to PS NPs, while TSH significantly increased. | (41) |
| MPs | Rats | Remarkable lesions Ectopic thymus Ultimobranchial cyst. | (42) |
| MPs | Rats | Increased level of T3, FT3/FT4 ratio, and decreased level of TSH | (41) |
The effects of MPs on the mammalian thyroid gland.
PBDEs and polybrominated biphenyl [PBBs] as flame retardants in plastic (50) can decrease the circulating level of thyroid hormones and are associated with impaired thyroid function (51). It has been reported that five weeks of rats’ exposure to 1, 3, 6, and 10mg/kg/day of polystyrene nanoplastics [PS-NPs] suppress the serum level of T3, FT3, and FT4 synthesis and circulating level of thyroid hormones (41) (Table 2). Few researchers have demonstrated phthalates association with altered FT4 and total T3 in pregnant women (53). It has been shown that BPA and phthalates cause endocrine toxicity at all levels in animals (45).
Table 2
| Endocrine disrupters | Species | Thyroid disrupting consequences | Reference |
|---|---|---|---|
| Phthalates | Humans | Thyroid epithelial cell hypertrophy and hyperplasia Thyroid hyperactivity, gene expression disruption of the hypothalamic-pituitary-thyroid [HPT] axis, thyroid antagonistic interaction, altered FT3 and FT4 |
(52) (44) (27) (49) (53) |
| Bisphenol A [BPA] | Rats | Inhibits T3 receptor binding ability, thyroid antagonist, thyroid oxidative damage | (48, 54) |
| Polybrominated diphenyl ethers [PBDEs] | Rats, Humans |
Serum T4 reduction, the prevalence of hypothyroidism, disturb T4 levels in umbilical-cord blood, altered T3 and T4 levels | (51, 55) (56) |
| Tributyltin [TBT] | Rats | Dysregulated HPT axis, thyroid follicle reduction, decreased FT4 level | (57) |
| Polychlorinated Biphenyls (PCBs) |
Rats | Reduced TT4 and FT4 levels | (58) |
| Hexabromocyclododecane (HBCD) | Rats | Thyroid follicular cell hypertrophy, reduced concentration of serum T3 | (59) |
| Mercury | Humans | Contribute to thyroid cancer, hypothyroidism, and autoimmune thyroiditis | (60) |
| Dichlorodiphenyltrichloroethane [DDT] | Rats | Reduced T4 level and decreased size of follicles | (61) |
The MPs additives effects on the mammalian thyroid gland.
Thyroid hormone levels in pregnant rats and their progeny are altered by brominated flame retardant chemicals, resulting in obesity, heart illness, early puberty, and insulin resistance in their children (62). The PBDEs and PBBs can dissolve in lipids and fats, so their accumulation is easy in wildlife and marine animals once exposed to these chemicals (21). PBDEs reduce the circulating levels of thyroid hormones through changes in T4 binding, and reduction in serum T4 (55). PBDEs disrupt the HPT axis (63) by altering the gene transcriptions of multiple genes like thyroid stimulating hormone subunit [tsh], deiodinase type 2 [deio2], and NK2 homeobox 1 [nkx2.1] (64). These genes, regulating thyroid development and TSH synthesis, are extremely sensitive to PBDEs (64).
3 Effect of MPs, NPs, and associated chemicals on the male reproductive system
Due to the small size of MPs, they can easily enter the organism’s reproductive cells, tissues, and organs altering normal morphology, histology, and physiological functions of the male reproductive system (65). In recent years MPs’ toxicity to the reproductive system got much more attention because they have caused widespread male reproductive abnormalities in mice making them a potential hazard (66) (Table 3). Currently, this environmental issue with respect to mammalian reproductive health is poorly understood (65). However, the harmful effects of MPs on mice’s reproductive health might provide new insight into the deleterious effects of MPs on the mammalian reproductive system.
Table 3
| Plastics | Species | Consequences on male reproductive system | References |
|---|---|---|---|
| MPs | Mice | Recused sperm quality, abnormal testicular spermatogenesis | (67) |
| MPs | Mice | Testicular transcriptomic alterations, altered spermatogenesis | (68) |
| MPs | Mice | Decreased testosterone levels, disruption of Blood Testes Barrier [BTB], testicular inflammation | (66) |
| MPs | Swine | Increased apoptosis and necrosis in testes, decreased viability of testicular cells | (69) |
| MPs | Mice | Decreased testicle weight and sperm quality, altered sperm phenotype | (70) |
| MPs | Rats | Damaged seminiferous tubule, destruction of BTB, spermatogenic cell apoptosis | (71) |
| PS-MPs | Mice | Oxidative stress in testes reduced sperm motility | (72) |
MPs effects on the mammalian male reproductive system.
The limited preliminary studies have suggested MPs bioaccumulation in mammalian testes with subsequent adverse reproductive outcomes (65). MPs ≤10 μm have been observed to accumulate in mice testes reducing testosterone [T] concentration, sperm quality and causes testicular inflammation (66). It also penetrates the testicular tissues such as Leydig cells, germ cells, and Sertoli cells (66). The accumulation of MPs in these cells may profoundly contribute to our understanding of MPs’ effects on mammalian reproductive health.
MPs contaminated with phthalate esters [PAEs] accumulated in the testes and altered testicular weight and sperm physiology by reducing sperm number and vitality (68). MPs cause morphological alternation in sperm like loss of sperm acrosome, cephalic having a small head, acephalia having no head, and tailless sperm (66). Exposure to these plastic particles at developmental stages resulted in shrank germ cells and decreased sperm density in the seminiferous tubules (73). MPs also induces irregular rearrangement of spermatid in testicular seminiferous tubules reducing spermatids’ number (67). A recent study (67) suggested PS-MPs reduce sperm production in mice through testicular injury. Deng et al. (68) observed spermatogenic disruption through altered acid phosphatase [ACP], superoxide dismutase [SOD], and malonaldehyde [MDA] levels in testes. ACP present in Sertoli cells (74) providing structural and nutritional support to spermatogenesis, were significantly increased while SOD and MDA levels were also increased inducing oxidative stress in testes (68). Oxidative stress is the key factor responsible for male infertility due to the increased cell division rate and mitochondrial oxygen consumption in testicular tissues (75). PS-MPs not only cause spermatogenic defects and testicular abnormalities but also penetrate the blood-testis-barrier [BTB] (66) in Sertoli cells and accumulate in the organism testes (30). According to (67) mice exposed to 5 µm of MPs shows a reduction in sperm activity and testicular tissue damage by activating the p38 mitogen-activated protein kinases [MAPK] pathway.
Spermatogenesis is an essential differentiation mechanism that requires T secretion, nutritional support provided by the sertoli cell, and a suitable environment for the production of sperm protected by BTB (66, 76). Following exposure to MPs, the T secretion declined and causes BTB disruption (66) with dysregulated testicular spermatogenesis (67). MPs enter testicular tissues by disrupting BTB, which serves as a physical barrier preventing the penetration of toxic chemicals to the testis, thus providing a healthy environment for spermatogenesis (66, 77) (Table 3). Due to BTB disruption, some proteins expression linked with BTB like Claudin 11, N-Cadherin, Connexin and Occludin were significantly reduced (71). Excess bioaccumulation of MPs induces increased germ cell apoptosis and disrupted spermatogenesis by causing abscission and irregular arrangement of spermatogenic cells (72) and sperm DNA fragmentation; a primary factor responsible for reproductive impairment (30). In mice, MPs exposure resulted in the shedding of spermatogenic cells and the structural disruption of seminiferous tubules (71).
The development and regulation of the reproductive system depend on the HPG axis, which alludes to the connection between the hypothalamus, pituitary, and gonads (78). Reproductive regulation initiates at the hypothalamus level due to gonadotropin-releasing hormone (GnRH) secretion by neurosecretory cells to the hypothalamic-hypophysial system (79). In response to GnRH secretion, pituitary releases FSH and LH that control gonadal functions (79). In males, the HPG axis is responsible for T secretion and regulation of spermatogenesis (80). MPs disrupt the HPG axis (26) as their exposure in male mice reduces the serum concentration of FSH, LH, and T while estradiol level significantly increases (81). Therefore the reproductive abnormalities caused by MPs due to HPG axis disruption include delayed gonadal maturation and the altered ratio of sex hormones that hindered reproductive development (26).
Some studies have shown PS-MPs increase reactive oxygen species [ROS] in male zebrafish liver and gonads. Their exposure to MPs increases apoptosis in testes, affecting gamete production (82) and interact with plasma membrane permeability of gametes, preventing gamete binding and offspring growth (26). In zebrafish testis, silver nanoparticles induce increased cell apoptosis due to overexpression of apoptotic genes like BAX, caspase-3 and caspase-9 (83). MPs also cause the thickness of the basement membrane of the zebrafish testis, due to which the production of spermatozoa is attenuated and undergoes the atrophy of seminiferous tubules (82).
BPA as essential plasticizers causes abnormal spermatogenesis, disruption of BTB, production of poor semen quality, and oxidative stress (84) (Table 4). BPA and BPS consumption alters T secretions and causes cell proliferation by interfering with many receptors (91). PBDEs as persistent flame retardants alter sperm DNA methylation by disrupting the hypothalamic-pituitary-testicular axis, affecting the functional ability of Leydig cells and spermatogenesis (86). MPs contaminated with phthalates can also cause oxidative stress in testes, change the sperm physiology (68), and their esters like dibutyl phthalate [DBP], diethylhexyl phthalate [DEHP], and diisopentyl phthalate [DiPeP] are recognized as anti-androgenic endocrine disrupters (85). Nonylphenol as a persistent pollutant inhibits steroidogenesis and alters enzyme localization like P450 aromatase, 3β-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase in Podarcis Siculus (92). Similarly, DDT causes testicular injury and possible transgenerational effects on epigenomes and transcriptomes of future generations (93). Some anthropogenic EDCs such as cadmium [Cd] and lead [Pb] beyond a certain levels have a close association with infertility in mice and humans (94) (Table 4). Both Cd and Pb adversely alters the HPG axis that harms testicular tissues directly (40) and disrupt spermatogenesis, spermiogenesis, and steroidogenesis (95). Mice administration to varying doses of PSMPs for six weeks induced a significant increase in sperm deformities rate and a decrease in sperm motility (72). PSMPs also cause a reduction in mice’s serum T levels and decrease the functional activity of various enzymes involved in sperm metabolisms such as succinate dehydrogenase and lactase dehydrogenase (72).
Table 4
| Endocrine disrupters | Species | Consequences on male reproductive system | Reference |
|---|---|---|---|
| Phthalates | Rats and Mice | Oxidative stress in testes, altered sperm’s physiology, anti-androgenic effects |
(68, 85) |
| BPA | Mice | Abnormal spermatogenesis, blood-testis-barrier [BTB] disruption, poor semen quality, DNA damage in sperm cells | (84) |
| PBDEs | Male | Dysregulated sperm DNA methylation, altered spermatogenesis | (86) |
| TBT | Syrian hamsters | Adverse steroidogenic enzymes activity, impaired testosterone production, defective spermatozoa | (87) |
| PCBs | Harbour porpoises | Decreased testes weight, Reduced sperm and spermatid numbers, small seminal vesicles |
(88, 89) |
| Chromium, lead and Mercury | Mice, Rabbits | Leydig cell tumors, attenuates serum level of luteinizing hormone [LH], testosterone, follicle-stimulating hormone, testicular stroma | (90) |
MPs additives effects on the male mammalian reproductive system.
4 Effects of MPs, NPs, and their associated chemicals on the female reproductive system
Little is known about the harmful effects of MPs and NPs on the ovaries of mammals but studies conducted on rats and aquatic organisms may provide new insights into deadly consequences caused by plastic particles in ovaries. The harmful effects of MPs and their compound substances are linked to a dysfunctional female reproductive system (26) (Table 5).
Table 5
| Plastic | Species | Consequences on the female reproductive system | References |
|---|---|---|---|
| MPs | Mice | Oxidative stress in ovaries, decrease the number of ovarian antral follicles and malondialdehyde [MDA] levels in ovaries |
(96) |
| MPs | Mice | Decreased pregnancies and increased mortality | (97) |
| MPs | Mice | Spontaneous abortion, decreased diameter of uterine arterioles, decreased uterine blood supply | (98) |
| MPs | Rats | Granulosa cell apoptosis, ovary fibrosis, and pyroptosis | (99) |
| MPs | Rats | Granulosa cells pryptosis through NLRP3/Caspase-1 signaling mechanism, | (100) |
Microplastics effects on the mammalian female reproductive system.
Research has shown MPs accumulation in rat’s ovaries and granulosa cells, reducing the growth of the follicles, decreasing the level of anti-Mullerian hormone [AMH] (99), estradiol, and causing an irregular estrous cycle and abnormal folliculogenesis (101). Granulosa cells are the essential somatic cells of the ovary responsible for normal ovarian development and maturation and play a significant role in folliculogenesis (102). In addition, PS-MPs also induce fibrosis of the ovary through activation of the Wnt/β-Catenin signaling pathway and apoptosis of granulosa cells by oxidative stress, reducing normal ovarian reserve capacity in rats (99, 103). The wnt/β-Catenin signaling pathway is essential for maintaining tissue homeostasis and regulating embryonic maturation, cell proliferation, and apoptosis (104).
MPs as a transport medium for their composite EDCs (105) induce various endocrine disorders like infertility, precocious puberty, hormone-based tumors, several metabolic problems, disruption of granulosa cell steroidogenesis, and polycystic ovary syndrome [PCOS] (106, 107) (Table 6). Endocrine disrupting plastic additives like PBDEs, BPA, phthalates, organotins (20), nonylphenols, octylphenols (113), and biocides like TBT, mercury, arsenic, copper, cadmium, and lead (114) can transfer from pregnant women to the fetal bloodstream through a placental barrier causing neurodevelopmental abnormalities in infants (115). In mice and monkeys, BPA disrupts oocyte development (116) and induces impairment and disruption of steroidogenesis in humans, ovine, swine (107), and murine granulosa cells (117). Several concerns have been raised demonstrating phthalates effect on granulosa cell steroidogenesis in humans (118), mice (108), and rats (119), and increase cell proliferation in swine when exposed to NPs with their composite EDCs (120). Similarly, cadmium disrupts gonadal steroidogenesis and inhibits the binding of FSH to its specific receptor, and alters steroid production of ovarian granulosa cells (121).
Table 6
| Endocrine disrupters | Species | Consequences on the female reproductive system | References |
|---|---|---|---|
| Phthalates | Mice | Reduced LH, defective ovarian steroidogenesis | (108) |
| BPA | Humans | Inhibiting secretion of progesterone and oestradiol, decreases the expression of CYP11A1 | (107) |
| PBDEs | Humans | Increased menstrual cycle and bleeding time | (109) |
| TBT | Rats | Irregular ovarian adipogenesis, Ovarian fibrosis | (110) |
| PCBs | Mice | Follicular atresia, suppressed level of LH, and progesterone | (111, 112) |
| Chromium, lead and Mercury | Mice and Rabbits | Follicular astresia, low follicle growth, corpus luteum | (90) |
The effects of MPs additives on the female mammalian reproductive system.
Some aquatic studies have also shown the bioaccumulation of PS-MPs in female fish embryo yolk sacs and female eggs, affecting the normal physiology of offspring in female fish (122, 123). The ability of polystyrene MPs to interfere with plasma proteins connected to oocytes facilitates MPs’ cross-generational transfer (123). In addition, PSMPs delay ovarian development, decline the reproductive ability of marine medaka (26) and reduce superoxide dismutase [SOD], catalase [CAT], glutathione S-transferase [GST], and glutathione peroxidase [GSH-PX] in Oryzias melastigma ovary (99, 123). Similarly, MPs disrupt the HPG axis by down-regulating the transcription of genes like GnRH, vitellogenin [Vtg], and choriogenin [Chg] in the steroidogenesis pathway (26) while its combined exposure with phenanthrene [Phe] increases the accumulation of Phe in the ovaries of marine medaka, disrupting ovarian development and the HPG axis (124).
5 Effects of MPs, NPs, and their additives on the hypothalamus
Hypothalamus is an essential part of the endocrine system that connects the nervous system to the endocrine system and secretes both inhibiting and releasing hormones that signal the pituitary gland to release various important hormones to the whole endocrine system (125). The detrimental consequences of MPs on the mammalian hypothalamus are poorly documented. However, there is clear evidence of mammalian hypothalamic-pituitary axes disruption caused by MPs and their composite EDCs that alter hormonal balance through feedback mechanisms (126, 127) (Table 7).
Table 7
| Endocrine disrupters | Species | Harmful effects | References |
|---|---|---|---|
| PBDEs | Rats | Dysregulation of HPT and hypothalamic-pituitary-gonadal [HPG] axis | (126) |
| BPA | Mice | Cause significant decrease in hypothalamic neurons, Cause astrocyte activation Impairs the function of proopiomelanocortin [POMC] neurons in the hypothalamic arcuate nucleus [ARC], Astrocyte-dependent hypothalamic inflammation |
(128) |
| Phthalates | Rats | Dysregulation of the HPG axis, induce early puberty in female rats by inducing upregulation of hypothalamic IGF-1 expression, prolong the female estrous cycle, affects mRNA and protein expression of KiSS1, GPR54, and GnRH in the hypothalamus |
(129) |
| PCBs | Rats | Oxidative stress in the hypothalamus, decreased hypothalamic weight, decreased acetylcholinesterase (AChE) activity | (130) |
| TBT | Rats | Disrupts the functional ability of the female HPG axis reducing some hormones like hypothalamic GnRH and decreasing secretion of pituitary LH. Distorting gene expression and provoking thyroid homeostasis to various morphological alternations like significant changes in TSH. |
(131) (57) |
| Mercury | Rats and Mice | Decreased Luteinizing hormone-releasing hormone [LHRH], changes in hypothalamic neuropeptides, decreased Hypothalamic insulin receptor [Insr] mRNA |
(132, 133) |
| Chromium | Rats | Chromium in combination with benzene causes significant alternations in the neuroendocrine and lymphoid systems by disrupting the hypothalamic-pituitary-adrenocortical axis, increased MT-3 mRNA expression in the hypothalamus |
(134) (135) |
MPs additives effects on the mammalian hypothalamus.
MPs and NPs accumulation have been observed in fish brain tissues, crossing the blood-brain barrier and causing neurotoxic effects (136). MPs also decrease hypothalamic kisspeptin level in zebrafish, responsible for their reproduction (137), and interferes with the HPT axis that distorts their gene expression (138).
Plastic additives like BPA and BPS cause decreased hypothalamic neurons and neuroendocrine disruption (139) while polybisphenols disturb the expression of HPT-axis genes (140). Mice exposed to BPA have shown astrocyte activation and various inflammatory actions in the hypothalamus through activation of the toll-like receptor [TLR4], a receptor that plays a vital role in inflammatory responses in the central nervous system (128). The hypothalamic inflammation induced by BPA impairs the function of proopiomelanocortin [POMC] neurons in the hypothalamic arcuate nucleus [ARC] (128). BPA also alters regulatory and inhibitory responses in the HPG axis and its chronic exposure increases expression levels of GnRH1, Kiss1, and FSH in exposed male and female rodents (141). Similarly, BPA induces increased anteroventral periventricular nucleus [AVPV] Kiss1 neurons in male offspring and enhanced Kiss1 cell number in the rostral periventricular area of the third ventricle of female offspring (126).
Phthalates cause hormonal imbalance by interacting with nuclear receptors, hormonal receptors, signaling pathways, and modulate gene expression linked with reproduction thereby, disrupting the HPG axis that affects fertility (142, 143). Due to HPG axis disruption, phthalates alter the levels of GnRH by interacting with genes of G protein-coupled receptors [GPCRs] on pituitary cells and disrupt FSH and LH ratio by interfering with their receptors on Leydig cells, which consequently disrupt the normal activity of steroidogenic enzymes and steroid hormones (142). DEHP exposure induces early puberty in female rats by inducing upregulation of hypothalamic insulin-like Growth Factor-1 [IGF-1] expression which alters normal hormonal levels of growth hormone [GH] and IGF-1 in the hypothalamus (129). DEHP also adversely affects mRNA and protein expression of KiSS1, GPR54, and GnRH in the hypothalamus thereby interfering with the hypothalamic regulatory mechanism affecting normal gonadal development and hypothalamic hormonal balance in pubertal rats (129).
Similarly, nonylphenol disrupts the negative feedback mechanism of the hypothalamic-pituitary-adrenal [HPA] axis by inhibiting estrogen binding with its receptor (28). TBT disrupts the functional ability of the female HPG axis reducing hormones like hypothalamic GnRH and decreasing the secretion of pituitary LH (125). These abnormalities induced by TBT are associated with dysregulated ovarian steroidogenesis, irregular folliculogenesis, oxidative stress, fibrosis, and abnormal alternations in estrogen and testosterone levels (144). Furthermore, TBT is capable of reducing thyroid follicles, distorting gene expression in the HPT axis and provoking thyroid homeostasis to various morphological alternations like changes in TSH and FT4 levels (57).
Mercury exposure causes adverse alternation in the circulating level of some hormones like FSH, LH, inhibin, and androgen, causing reproductive disruption (121, 145) through its pathogenic changes in the HPA axis and HPG axis (146). Studies found a higher concentration of HPA axis hormones in people exposed to heavy metals (147). Some heavy metal present in electronic waste of electronic devices alters the HPA axis and increases the secretion of corticotropin-releasing hormone [CRH], and adrenocorticotropic hormone [ACTH] (147). Lead (Pb) distorts the mechanism of neurotransmission (148) in the brain and causes variations in the regulation of hypothalamic neurotransmitters that affect the functional ability of gonadotropic hormones by influencing the control of the HPG axis (121, 149). Chromium is a neurotoxicant that increases GR activity and metallothionein isoform 3 [MT3] in the hypothalamus (150). Chromium in combination with benzene causes alternations in the neuroendocrine and lymphoid systems by disrupting the hypothalamic-pituitary-adrenocortical axis (134).
6 Effects of MPs, NPs, and their associated chemicals on the pituitary gland
The pituitary gland is a neuroendocrine organ having an essential role in major physiological functions such as growth, sexual development, metabolism, and stress responses (151). Through various regulatory axes, the hypothalamus and pituitary gland regulate neuroendocrine actions including the HPT axis, HPG axis, HPA axis, and HP-somatotrophic axis (126). The hypothalamus-pituitary [HP] axis plays a major integrative role in controlling the mammalian endocrine system. It maintains a balanced homeostatic condition and is responsible for essential hormone secretion that regulates the thyroid, adrenal gland, gonads, somatic growth, and many other functions (126). The hormones secreted by the hypothalamus play a major role in controlling pituitary functions such as metabolism, lactation, growth, and milk secretion (126). Pituitary glands consist of two lobes, the anterior pituitary, and posterior pituitary, linked by the intermediate lobe. The hormones secreted by the anterior pituitary gland into the bloodstream are adrenocorticotrophic hormone, follicle-stimulating hormones, luteinizing hormones, thyroid-stimulating hormone, growth hormone, and prolactin while the posterior pituitary releases oxytocin and antidiuretic hormone (152).
There is a dearth of studies regarding the harmful effects of MPs on the pituitary and we did not find out any research data that have explored the MPs toxicity on the mammalian pituitary gland. However, there is some evidence on the dysregulation of hypothalamic-pituitary axes caused by MPs and their composite EDCs (126, 127) like the HPT axis (138) and HPG axis (127) (Table 8).
Table 8
| Endocrine disrupter | Species | Harmful effects | References |
|---|---|---|---|
| Phthalates | Rats | Altering levels of GnRH, LH, and FSH, increases corticosterone and ACTH levels | (68) (142) (85) |
| Bisphenols | Rats | Effect the pituitary directly by altering its response to TRH released by the hypothalamus | (153) |
| PBDEs | Rats | Significantly alter TH balance at multiple stages of HPT-axis thereby, disrupting normal HPT-axis, and exerting its carcinogenic effects in the pituitary of male rats and the uterus of female rats | (154) (155) |
| TBT | Rats | Decreased secretion of GnRH and LH. | (144) |
| HBCD | Rats | degeneration of the adrenal cortex | (59) |
| Organophosphate | Rats | Decreases fertility by affecting the pituitary gonadotrophins, Cortical hypertrophy of zona fasciculate | (156) (157) |
| Mercury | Human | Inhibits LH and FSH secretion, menstruation disorders, Leydig cells deformation, impaired follicular development | (158) (159) |
| Lead [Pb] | Rats | Suppressed serum FSH | (160) |
| Chromium | Rats | Increased superoxide dismutase activity in the anterior pituitary, oxidative stress in the pituitary gland | (135) |
| Cadmium | Rats | Decreased circulating levels of LH and FSH | (161) |
The effects of [MPs] additives on the mammalian pituitary gland.
HP axis is vulnerable to a variety of MPs composite EDCs (105) such as BPA (162), PCBs, PBDEs, PBBs, dichlorodiphenyltrichloroethane [DDT] (163), and TBT (164) (Table 8). Extensive use of these EDCs in synthetic products, as well as their incorrect disposal, results in a range of environmental contamination, leading to endocrine disruption (126). The consequences of EDCs carried by MPs and NPs (105) on the pituitary gland are the induction of a non-cancerous pituitary tumor known as prolactinoma and stimulation of pituitary hormones like prolactin and TSH (165). The minimal amount of estrogen required to induce tumor is far higher than the normal level so, it is questionable and doubtful whether weak estrogenic disrupters might act as carcinogenic in the pituitary gland (165).
BPA as an essential additive (126) disrupts the regulatory mechanism of the HPT axis through altered TSH levels and affects the pituitary directly by altering its response to Thyrotropin-releasing hormone [TRH] released by the hypothalamus (153).
Mice studies have shown a significant decrease in the expression of pituitary Esr1 with reduced hypothalamic Esr1 expression in rats exposed to 10mg/kg/day diisopentyl phthalate (85). PBDEs as flame retardants alter TH balance at multiple stages of the HPT-axis (154, 155) and exert carcinogenic effects in the thyroid and pituitary of male rats and the uterus of the female rats (154).
Mercury bio-accumulates in the pituitary and thyroid glands and causes endocrine toxicity by altering HP thyroid/gonadal axis (158). Cadmium and arsenic, among the most harmful EDCs, adversely affect the endocrine by altering the secretion of hormones (166).
Mercury inhibits pituitary gland LH and FSH secretion, causing spermatogenesis and sperm count disruption in males and ovarian dysfunction and dysregulated menstruation in females (146). Both Cadmium and arsenic exert xenoestrogenic effects on the interior part of the pituitary gland and reduce LH secretion (167). Arsenic causes neurological abnormalities, and increases mRNA expressions of genes responsible for oxidative responses thus, inducing oxidative stress and apoptosis (168). Similarly, the combined exposure of Pb and cadmium affects the LH and FSH levels in proestrus rats while Pb exposure alone causes a reduction in the fluidity of the pituitary membrane (94).
7 Effects of MPs, NPs, and their associated chemicals on the adrenal gland
The adrenal gland is an essential endocrine gland located on the top of the kidneys and composed of the adrenal cortex and adrenal medulla that release hormones like cortisol, aldosterone, epinephrine, and norepinephrine (169). Like the hypothalamus and pituitary, we did not find any research-based analysis about the direct consequences of MPs on the mammalian adrenal gland, except the findings of Stojanović et al. (170) that have identified an increase in the relative weight of rat’s adrenal gland. However, in zebrafish, PS-NPs affect glucose homeostasis by increasing cortisol secretion and such alternation in cortisol levels causes behavioral changes by interfering with brain cells’ electrical activity that alters important molecules like neurotransmitters, enzymes, and receptors (171).
Toxicological findings have recognized the adrenal gland as the most sensitive organ to EDCs because of the critical role of glucocorticoids secreted by the adrenal cortex in maintaining homeostasis (172) (Table 9). EDCs may disrupt HPA (186), which induces stress responses causing altered behavioral, neuronal, and immune functions while other abnormalities associated with disrupted HPA axis include anxiety, metabolic disorders, and post-traumatic stress disorder [PTSD] (187).
Table 9
| Endocrine disrupter | Species | Harmful effects | References |
|---|---|---|---|
| BPA | Rats | Increases the adrenal gland weight in offspring Stimulates a high level of plasma corticosterone by elevating steroidogenic acute regulatory protein (StAR) concentration Exerts adverse consequences on adrenal cell proliferation through ERβ and SHH signaling mechanism, activating cyclin D1 and cyclin D2 |
(173) (174) |
| Phthalates | Rats | Results in decreased expression of angiotensin II in the adult adrenal gland, reducing aldosterone levels | (172) (175) |
| PBDEs | Rats | 4-bromodiphenyl ether (BDE3) increases serum aldosterone and corticosterone levels. It also up-regulates Cyp11b1 expression and causes AMPK signaling disruption by decreasing its phosphorylation |
(176) |
| TBT | Mice | Increases intracellular storage and causes the accumulation of lipids and cholesterol in adrenal cells, which results in weakened cholesterol utilization and increased cholesterol levels | (177) (178) |
| Organophosphates | Rats | Isopropylated triphenyl phosphate [IPTPP] causes hypertrophy of the adrenal cortical and increased relative weight of the adrenal glands | (156) |
| Phenols | Rats | Damages the endogenous estrogenic cascade in the adrenal gland, cause changes in the regions of the cortex medulla, Causes cytoplasmic decomposition in cells of the cortex and hemorrhage in the tissue interface |
(179) |
| DDT | Rats | Decreased level of catecholamines, norepinephrine, and epinephrine, Impaired aldosterone secretion, reduced the size of zona glomerulosa |
(180) (181) |
| Mercury | Human | Alter the metabolism of catecholamines in the medulla of the adrenal gland leading to an elevated level of plasma nor-adrenaline with aging, pathogenesis of hypertension, and metabolic syndromes. lower corticosterone level | (182) (183) |
| Chromium | Rats | Increased adrenal Δ53β-hydroxysteroid dehydrogenase [HSD] activity, adrenal weight, and serum corticosterone level | (184) |
| Nickel and cobalt | Rats | Increased mass of fascicular zone and secretion of glucocorticoids | (185) |
The effects of [MPs] additives on the mammalian pituitary gland.
BPA as EDC plays an essential role in the development of non-functional adrenal incidentaloma [NFAI] (174) and causes increased adrenal gland weight in offspring of both male and female rats when exposed to food containing BPA of 25mg/kg (173). BPA causes high level of plasma corticosterone by elevating steroidogenic acute regulatory protein [StAR] concentration (174) and altering adrenal cell proliferation through ERβ and Sonic Hedgehog Signaling [SHH], activating cyclin D1 and cyclin D2 (188). BPA also reduces the immunoreactivity of smooth muscle actin [SMA] in smooth muscles of the adrenal capsule and alters the immunoreactivity of adrenal contractile proteins in rats (189). It has been observed that BPA induces a considerable increase in the adrenal index, vascular congestion, cellular destruction, reduced antioxidant enzymes, and decreased expression of vimentin proteins as well as alpha-smooth muscle actin (189). DEHP as an essential plasticizer is associated with decreased expression of angiotensin II in the adult adrenal gland, reducing aldosterone levels (172). Postpartum exposure to 300 mg/kg of DEHP significantly reduced corticosterone levels while 500 mg/kg of DEHP increases corticosterone and ACTH levels and 10 mg/kg of DEHP triggers glucocorticoid receptor [GR] in the HPA axis, resulting in anxiety-like behavior in premature rats (175). PBDEs, such as 4-bromodiphenyl ether [BDE3] increased the level of serum aldosterone and corticosterone (176). BDE3 up-regulates Cyp11b1 expression and causes AMPK signaling disruption by decreasing its phosphorylation in rats exposed to 200mg/kg of BDE3 (176). TBT is an oxidative endocrine disrupter, which increases intracellular storage (177) and causes the accumulation of lipids and cholesterol in adrenal cells, which results in weakened cholesterol utilization and increased cholesterol levels (178). Organophosphates like isopropylated triphenyl phosphate [IPTPP] cause hypertrophy of the adrenal cortical in zona fasciculate and increase the relative weight of the adrenal gland (156).
Nonyl phenols and octylphenol derived from ethoxylates (190) act as an ED, damaging the endogenous estrogenic cascade in the adrenal gland. Nonylphenol causes adrenal disruption by decreasing the noradrenaline cells leading to lethargy and altering stress response in the body (28). While octyl phenol causes changes in the regions of the cortex medulla, cytoplasmic decomposition in cortex cells, and hemorrhage in the tissue interface of pregnant rats (179). DDT, a widespread ED causes cell atrophy and degenerative effects in the adrenal cortex mainly in the zona fasciculate and zona reticularis (191). DDT can bio-accumulate in the thymus, brain, and even in adipose tissue, and induces impairments of both cortex and medulla of the adrenal gland, disrupting hormonal secretion in cortical and chromaffin cells as well as suppressing the thyroxine hydroxylase production in chromaffin cells (191).
The adrenal gland is also vulnerable to heavy metals like mercury, cadmium, cobalt, and copper that affect the zona glomerulosa of the rat adrenal gland (192) and dysregulate HPA axis, altering the hormonal secretion of corticosterone in response to various stressors and interfering with steroid hormones metabolism (183). Among these toxic metals, mercury alters the metabolism of catecholamines in the adrenal medulla, leading to an increased level of plasma nor-adrenaline with aging. Its chronic exposure is associated with the pathogenesis of hypertension and metabolic syndromes (183).
8 Conclusion
The abundance and distribution of MPs derived from plastic degradation across the globe are so extensive that we can claim of living in a plastic world. Endocrine toxicity induced by MPs is an emerging issue, despite the subject being rarely documented, there is growing evidence for ingested MPs bioaccumulation in mammalian tissues and organs with deleterious outcomes including endocrine abnormalities, reproductive toxicity, gut microbiota dysbiosis, and defective immunological responses in rodents, rats and mice. Various EDCs or toxic chemicals present in plastic as an additive or adsorbed by MPs enter the body easily, acting as agonists or antagonists for a wide range of hormonal receptors, and induce endocrine toxicity. The identification of adverse consequences of MPs on the mammalian endocrine system is a great challenge due to their rising levels in both terrestrial and aquatic ecosystems. However, there are still no conclusive research reports that have determined the direct consequences of MPs and NPs on the hypothalamus, pituitary, and adrenal gland. So further research studies are essential to be performed to determine the potential hazards of MPs and NPs to regulate laws that reduce exposure to these small plastic particles.
Statements
Author contributions
Conceptualization, SU (1st author) and GN. methodology, SU (1st author) and GN. writing—original draft preparation, SU (1st author), SaU, and GN. writing—review and editing, SU (1st author), SA, XG, SaU, SU (5th author), GN. funding acquisition, XG, KW. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the Natural Science Foundation of Qinghai Province (2022-ZJ-936Q) and Qinghai Kunlun Ying Cai Action Project No. Qing Ren Zi (2020) 18.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Deonie A Allen S Abbasi S Baker A Bergmann M Brahney J et al . Microplastics and nanoplastics in the marine-atmosphere environment. Nat Rev Earth Environ (2022) 3:1–13. doi: 10.1038/s43017-022-00292-x
2
Reinhold L Wagreich M Williams M Wolfe AP . The geological cycle of plastics and their use as a stratigraphic indicator of the Anthropocene. Anthropocene (2016) 13:4–17. doi: 10.1016/j.ancene.2016.01.002
3
Europe, Plastic. “Plastics–the facts 2020. PlasticEurope (2020) 1:1–64.
4
Roland G Jambeck JR Law KL . Production, use, and fate of all plastics ever made. Sci Adv (2017) 3(7):e1700782. doi: 10.1126/sciadv.1700782
5
Chris W Sebille EV Hardesty BD . Threat of plastic pollution to seabirds is global, pervasive, and increasing. Proc Natl Acad Sci (2015) 112(38):11899–904. doi: 10.1073/pnas.1502108112
6
Davidson AD Shoemaker KT Weinstein B Costa GC Brooks TM Ceballos G et al . Geography of current and future global mammal extinction risk. PloS One (2017) 12(11):e0186934. doi: 10.1371/journal.pone.0186934
7
Santos RG Machovsky-Capuska GE Andrades R . Plastic ingestion as an evolutionary trap: Toward a holistic understanding. Science (2021) 373(6550):56–60. doi: 10.1126/science.abh0945
8
Ghulam N Ahmad S Ullah S Zada S Sarfraz M Guo X et al . The adverse health effects of increasing microplastic pollution on aquatic mammals. J King Saud University-Science (2022) 34(4):102006. doi: 10.1016/j.jksus.2022.102006
9
Galloway TS Cole M Lewis C . Interactions of microplastic debris throughout the marine ecosystem. Nat Ecol Evol (2017) 1(5):1–8. doi: 10.1038/s41559-017-0116
10
Benson NU Agboola OD Fred-Ahmadu OH De-la-Torre GE Oluwalana A Williams A . Micro (Nano) plastics prevalence, food web interactions and toxicity assessment in aquatic organisms: A review. Front Mar Sci (2022) 9:291. doi: 10.3389/fmars.2022.851281
11
Kershaw PJ Rochman CM . Sources, fate and effects of microplastics in the marine environment: part 2 of a global assessment. Reports and Studies-IMO/FAO/Unesco-IOC/WMO/IAEA/UN/UNEP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection (GESAMP) (2015) Eng No. 93
12
Jaikumar G Brun NR Vijver MG Bosker T . Reproductive toxicity of primary and secondary microplastics to three cladocerans during chronic exposure. Environ pollut (2019) 249:638–46. doi: 10.1016/j.envpol.2019.03.085
13
Baho DL Bundschuh M Futter MN . Microplastics in terrestrial ecosystems: Moving beyond the state of the art to minimize the risk of ecological surprise. Global Change Biol (2021) 27(17):3969–86. doi: 10.1111/gcb.15724
14
Atsuhiko I Azuma T Cordova MR Cózar A Galgani F Hagita R et al . A multilevel dataset of microplastic abundance in the world’s upper ocean and the laurentian great lakes. Microplastics Nanoplastics (2021) 1(1):1–14. doi: 10.1186/s43591-021-00013-z
15
Jambeck JR Geyer R Wilcox C Siegler TR Perryman M Andrady A et al . Plastic waste inputs from land into the ocean. Science (2015) 347(6223):768–71. doi: 10.1126/science.1260352
16
Lim X . Microplastics are everywhere—but are they harmful? Nature (2021) 593:22–5
17
Luca N Futter M Langaas S . Environmental Science & Technology (2016) 50(20):10777–9. doi: 10.1021/acs.est.6b04140
18
Laura R Marcos R Hernández A . Potential adverse health effects of ingested micro-and nanoplastics on humans. lessons learned from in vivo and in vitro mammalian models. J Toxicol Environ Health Part B (2020) 23(2):51–68. doi: 10.1080/10937404.2019.1700598
19
Helene W Wang Z Hellweg S . Deep dive into plastic monomers, additives, and processing aids. Environ Sci Technol (2021) 55(13):9339–51. doi: 10.1021/acs.est.1c00976
20
Chen Q Allgeier A Yin D Hollert H . Leaching of endocrine disrupting chemicals from marine microplastics and mesoplastics under common life stress conditions. Environ Int (2019) 130:104938. doi: 10.1016/j.envint.2019.104938
21
Zoeller TR . Environmental chemicals targeting thyroid. Hormones (2010) 9(1):28–40. doi: 10.14310/horm.2002.1250
22
Kurunthachalam K Vimalkumar K . A review of human exposure to microplastics and insights into microplastics as obesogens. Front Endocrinol (2021) 12:978. doi: 10.3389/fendo.2021.724989
23
Campanale C Massarelli C Savino I Locaputo V Uricchio VF . A detailed review study on potential effects of microplastics and additives of concern on human health. Int J Environ Res Public Health (2020) 17(4):1212. doi: 10.3390/ijerph17041212
24
Yilmaz B Terekeci H Sandal S Kelestimur F . Endocrine disrupting chemicals: Exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev endocr Metab Disord (2020) 21(1):127–47. doi: 10.1007/s11154-019-09521-z
25
Schwabl P Köppel S Königshofer P Bucsics T Trauner M Reiberger T et al . Detection of various microplastics in human stool: A prospective case series. Ann Internal Med (2019) 171(7):453–57. doi: 10.7326/M19-0618
26
Wang J Li Y Lu L Zheng M Zhang X Tian H et al . Polystyrene microplastics cause tissue damages, sex-specific reproductive disruption and transgenerational effects in marine medaka (Oryzias melastigma). Environ pollut (2019) 254:113024. doi: 10.1016/j.envpol.2019.113024
27
Derakhshan A Shu H Broeren MAC Lindh CH Peeters RP Kortenkamp A et al . Association of phthalate exposure with thyroid function during pregnancy. Environ Int (2021) 157:106795. doi: 10.1016/j.envint.2021.106795
28
De Falco M Sellitti A Sciarrillo R Capaldo A Valiante S Iachetta G et al . Nonylphenol effects on the hpa axis of the bioindicator vertebrate, Podarcis sicula lizard. Chemosphere (2014) 104:190–96. doi: 10.1016/j.chemosphere.2013.11.014
29
Van Sebille E Wilcox C Lebreton L Maximenko N Hardesty BD Van Franeker JA et al . A global inventory of small floating plastic debris. Environ Res Lett (2015) 10(12):124006. doi: 10.1088/1748-9326/10/12/124006
30
Amereh F Babaei M Eslami A Fazelipour S Rafiee M . The emerging risk of exposure to nano (Micro) plastics on endocrine disturbance and reproductive toxicity: From a hypothetical scenario to a global public health challenge. Environ pollut (2020) 261:114158. doi: 10.1016/j.envpol.2020.114158
31
De Sá LC Oliveira M Ribeiro F Lopes Rocha T Futter MN . Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci total Environ (2018) 645:1029–39. doi: 10.1016/j.scitotenv.2018.07.207
32
Hu L Zhao Y Xu H . Trojan Horse in the intestine: A review on the biotoxicity of microplastics combined environmental contaminants. J Hazard Mater (2022) 439:129652. doi: 10.1016/j.jhazmat.2022.129652
33
Schell T Rico A Cherta L Nozal L Dafouz R Giacchini R et al . Influence of microplastics on the bioconcentration of organic contaminants in fish: Is the “Trojan horse” effect a matter of concern? Environ pollut (2022) 306:119473. doi: 10.1016/j.envpol.2022.119473
34
Alava JJ . Modeling the bioaccumulation and biomagnification potential of microplastics in a cetacean foodweb of the northeastern pacific: A prospective tool to assess the risk exposure to plastic particles. Front Mar Sci (2020) 7:793. doi: 10.3389/fmars.2020.566101
35
De Felice B Sabatini V Antenucci S Gattoni G Santo N Bacchetta R et al . Polystyrene microplastics ingestion induced behavioral effects to the Cladoceran daphnia magna. Chemosphere (2019) 231:423–31. doi: 10.1016/j.chemosphere.2019.05.115
36
Banerjee A Shelver WL . Micro-and nanoplastic-mediated pathophysiological changes in rodents, rabbits, and chickens: A review. J Food Prot (2021) 84(9):1480–95. doi: 10.4315/JFP-21-117
37
Bernal J . Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab (2007) 3(3):249–59. doi: 10.1038/ncpendmet0424
38
Shahid MA Ashraf MA Sharma S . Physiology, Thyroid Hormone. In: StatPearls. StatPearls Publishing, Treasure Island (FL); (2022). Available at: https://www.ncbi.nlm.nih.gov/books/NBK500006/.
39
Kloas W Urbatzka R Opitz R Würtz S Behrends T Hermelink B et al . Endocrine disruption in aquatic vertebrates. Ann New York Acad Sci (2009) 1163(1):187–200. doi: 10.1111/j.1749-6632.2009.04453.x
40
Saradha B Mathur PP . Effect of environmental contaminants on Male reproduction. Environ Toxicol Pharmacol (2006) 21(1):34–41. doi: 10.1016/j.etap.2005.06.004
41
Amereh F Eslami A Fazelipour S Rafiee M Zibaii MI Babaei M . Thyroid endocrine status and biochemical stress responses in adult Male wistar rats chronically exposed to pristine polystyrene nanoplastics. Toxicol Res (2019) 8(6):953–63. doi: 10.1039/c9tx00147f
42
Kim J Maruthupandy M An KS Lee KH Jeon S Kim J-S et al . Acute and subacute repeated oral toxicity study of fragmented microplastics in sprague-dawley rats. Ecotoxicol Environ Saf (2021) 228:112964. doi: 10.1016/j.ecoenv.2021.112964
43
Gallo F Fossi C Weber R Santillo D Sousa J Ingram I et al . Marine litter plastics and microplastics and their toxic chemicals components: The need for urgent preventive measures. Environ Sci Europe (2018) 30(1):1–14. doi: 10.1186/s12302-018-0139-z
44
Boas M Frederiksen H Feldt-Rasmussen U Skakkebæk NE Hegedüs L Hilsted L et al . Childhood exposure to phthalates: Associations with thyroid function, insulin-like growth factor I, and growth. Environ Health Perspect (2010) 118(10):1458–64. doi: 10.1289/ehp.0901331
45
Mathieu-Denoncourt J Wallace SJ de Solla SR Langlois VS . Plasticizer endocrine disruption: Highlighting developmental and reproductive effects in mammals and non-mammalian aquatic species. Gen Comp Endocrinol (2015) 219:74–88. doi: 10.1016/j.ygcen.2014.11.003
46
Andra SS Makris KC . Thyroid disrupting chemicals in plastic additives and thyroid health. J Environ Sci Health Part C (2012) 30(2):107–51. doi: 10.1080/10590501.2012.681487
47
Ayala AR Danese MD Ladenson PW . When to treat mild hypothyroidism. Endocrinol Metab Clinics (2000) 29(2):399–415. doi: 10.1016/S0889-8529(05)70139-0
48
Moriyama K Tagami T Akamizu T Usui T Saijo M Kanamoto N et al . Thyroid hormone action is disrupted by bisphenol a as an antagonist. J Clin Endocrinol Metab (2002) 87(11):5185–90. doi: 10.1210/jc.2002-020209
49
Ishihara A Sawatsubashi S Yamauchi K . Endocrine disrupting chemicals: Interference of thyroid hormone binding to transthyretins and to thyroid hormone receptors. Mol Cell Endocrinol (2003) 199(1-2):105–17. doi: 10.1016/S0303-7207(02)00302-7
50
Shao M Jiang J Li M Wu L Hu M . Recent developments in the analysis of polybrominated diphenyl ethers and polybrominated biphenyls in plastic. Rev Anal Chem (2016) 35(3):133–43. doi: 10.1515/revac-2016-0012
51
Oulhote Y Chevrier J Bouchard MF . Exposure to polybrominated diphenyl ethers (Pbdes) and hypothyroidism in Canadian women. J Clin Endocrinol Metab (2016) 101(2):590–98. doi: 10.1210/jc.2015-2659
52
Liu C Zhao L Wei L Li L . Dehp reduces thyroid hormones Via interacting with hormone synthesis-related proteins, deiodinases, transthyretin, receptors, and hepatic enzymes in rats. Environ Sci pollut Res (2015) 22(16):12711–19. doi: 10.1007/s11356-015-4567-7
53
Huang P-C Tsai C-H Liang W-Y Li S-S Huang H-B Kuo P-L . Early phthalates exposure in pregnant women is associated with alteration of thyroid hormones. PloS One (2016) 11(7):e0159398. doi: 10.1371/journal.pone.0159398
54
Mohammed ET Khalid S H Ahmed AE Tarek Aly M Aleya L Abdel-Daim MM . Ginger extract ameliorates bisphenol a (Bpa)-induced disruption in thyroid hormones synthesis and metabolism: Involvement of nrf-2/Ho-1 pathway. Sci Total Environ (2020) 703:134664. doi: 10.1016/j.scitotenv.2019.134664
55
Stoker TE Laws SC Crofton KM Hedge JM Ferrell JM Cooper RL . Assessment of de-71, a commercial polybrominated diphenyl ether (Pbde) mixture, in the edsp Male and female pubertal protocols. Toxicol Sci (2004) 78(1):144–55. doi: 10.1093/toxsci/kfh029
56
Abdelouahab N Langlois M-F Lavoie L Corbin F Pasquier J-C Takser L . Maternal and cord-blood thyroid hormone levels and exposure to polybrominated diphenyl ethers and polychlorinated biphenyls during early pregnancy. Am J Epidemiol (2013) 178(5):701–13. doi: 10.1093/aje/kwt141
57
Andrade MN Santos-Silva AP Rodrigues-Pereira P Paiva-Melo FD Correa de Lima NJ Pires Teixeira M et al . The environmental contaminant tributyltin leads to abnormalities in different levels of the hypothalamus-Pituitary-Thyroid axis in female rats. Environ pollut (2018) 241:636–45. doi: 10.1016/j.envpol.2018.06.006
58
Hallgren S Sinjari T Håkansson H Darnerud P . Effects of polybrominated diphenyl ethers (Pbdes) and polychlorinated biphenyls (Pcbs) on thyroid hormone and vitamin a levels in rats and mice. Arch Toxicol (2001) 75(4):200–08. doi: 10.1007/s002040000208
59
Saegusa Y Fujimoto H Woo G-H Inoue K Takahashi M Mitsumori TK Hirose M Nishikawa A Shibutani M et al . Developmental toxicity of brominated flame retardants, tetrabromobisphenol a and 1, 2, 5, 6, 9, 10-hexabromocyclododecane, in rat offspring after maternal exposure from mid-gestation through lactation. Reprod Toxicol (2009) 28(4):456–67. doi: 10.1016/j.reprotox.2009.06.011
60
Pamphlett R Doble PA Bishop DP . Mercury in the human thyroid gland: Potential implications for thyroid cancer, autoimmune thyroiditis, and hypothyroidism. PloS One (2021) 16(2):e0246748. doi: 10.1371/journal.pone.0246748
61
Yaglova NV Yaglov VV . Cytophysiological changes in the follicular epithelium of the thyroid gland after long-term exposure to low doses of dichlorodiphenyltrichloroethane (Ddt). Bull Exp Biol Med (2017) 162(5):699–702. doi: 10.1007/s10517-017-3691-4
62
Gore AC Crews D Doan LL La Merrill M Patisaul H Zota A . Introduction to endocrine disrupting chemicals (Edcs). A guide Public interest organ policy-makers (2014), 21–2.
63
Yu L Han Z Liu C . A review on the effects of PBDEs on thyroid and reproduction systems in fish. Gen Comp Endocrinol (2015) 219:64–73. doi: 10.1016/j.ygcen.2014.12.010
64
Yu L Deng J Shi X Liu C Yu K Zhou B . Exposure to DE-71 alters thyroid hormone levels and gene transcription in the hypothalamic–pituitary–thyroid axis of zebrafish larvae. Aquat Toxicol (2010) 97(3):226–33. doi: 10.1016/j.aquatox.2009.10.022
65
D’Angelo S Meccariello R . Microplastics: A threat for Male fertility. Int J Environ Res Public Health (2021) 18(5):2392. doi: 10.3390/ijerph18052392
66
Jin H Ma T Sha X Liu Z Zhou Y Meng X et al . Polystyrene microplastics induced Male reproductive toxicity in mice. J hazard mater (2021) 401:123430. doi: 10.1016/j.jhazmat.2020.123430
67
Hou B Wang F Liu T Wang Z . Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice. J hazard mater (2021) 405:124028. doi: 10.1016/j.jhazmat.2020.124028
68
Deng Y Yan Z Shen R Huang Y Ren H Zhang Y . Enhanced reproductive toxicities induced by phthalates contaminated microplastics in Male mice (Mus musculus). J hazard mater (2021) 406:124644. doi: 10.1016/j.jhazmat.2020.124644
69
Wang X Zhang X Sun K Wang S Gong D . Polystyrene microplastics induce apoptosis and necroptosis in swine testis cells Via Ros/Mapk/Hif1α pathway. Environ Toxicol (2022) 37(10):2483–92. doi: 10.1002/tox.23611
70
Deng Y Chen H Huang Y Wang Q Chen W Chen D . Polystyrene microplastics affect the reproductive performance of Male mice and lipid homeostasis in their offspring. Environ Sci Technol Lett (2022) 9(9):752–57. doi: 10.1021/acs.estlett.2c00262
71
Li S Wang Q Yu H Yang L Sun Y Xu N et al . Polystyrene microplastics induce blood–testis barrier disruption regulated by the mapk-Nrf2 signaling pathway in rats. Environ Sci pollut Res (2021) 28(35):47921–31. doi: 10.1007/s11356-021-13911-9
72
Xie X Ting D Duan J Xie J Yuan J Chen M . Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the P38 mapk signaling pathway. Ecotoxicol Environ Saf (2020) 190:110133. doi: 10.1016/j.ecoenv.2019.110133
73
Huang T Zhang W Lin T Liu S Sun Z Liu F et al . Maternal exposure to polystyrene nanoplastics during gestation and lactation induces hepatic and testicular toxicity in Male mouse offspring. Food Chem Toxicol (2022) 160:112803. doi: 10.1016/j.fct.2021.112803
74
Peruquetti RL Taboga SR Vilela de Azeredo Oliveira MT . Expression of acid phosphatase in the seminiferous epithelium of zebrrates. Genet Mol Res (2010) 9:620–28. doi: 10.4238/vol9-2gmr730
75
Asadi N Bahmani M Kheradmand A Rafieian-Kopaei M . The impact of oxidative stress on testicular function and the role of antioxidants in improving it: A review. J Clin Diagn res: JCDR (2017) 11(5):IE01. doi: 10.7860/JCDR/2017/23927.9886
76
C Yan C Mruk DD . The blood-testis barrier and its implications for Male contraception. Pharmacol Rev (2012) 64(1):16–64. doi: 10.1124/pr.110.002790
77
Mruk DD Cheng CY . The mammalian blood-testis barrier: Its biology and regulation. Endocr Rev36(5) (2015) 36:564–91. doi: 10.1210/er.2014-1101
78
Peper JS Brouwer RM van Leeuwen M Schnack HG Boomsma DI Kahn RS et al . Hpg-axis hormones during puberty: A study on the association with hypothalamic and pituitary volumes. Psychoneuroendocrinology (2010) 35(1):133–40. doi: 10.1016/j.psyneuen.2009.05.025
79
Kurylowicz A . Endocrine disorders accompanying obesity-effect or cause? Role Obes Hum Health Dis (2021) 13. doi: 10.5772/intechopen.98793
80
Dwyer AA Quinton R . Anatomy and physiology of the hypothalamic-Pituitary-Gonadal (Hpg) axis. In Advanced Pract Endocrinol Nurs (2019), 839–52. doi: 10.1007/978-3-319-99817-6_43
81
Wei Z Wang Y Wang S Xie J Han Q Chen M . Comparing the effects of polystyrene microplastics exposure on reproduction and fertility in Male and female mice. Toxicology (2022) 465:153059. doi: 10.1016/j.tox.2021.153059
82
Qiang L Cheng J . Exposure to polystyrene microplastics impairs gonads of zebrafish (Danio rerio). Chemosphere (2021) 263:128161. doi: 10.1016/j.chemosphere.2020.128161
83
Ma Y-B Lu C-J Junaid M Jia P-P Yang L Zhang J-H et al . Potential adverse outcome pathway (Aop) of silver nanoparticles mediated reproductive toxicity in zebrafish. Chemosphere (2018) 207:320–28. doi: 10.1016/j.chemosphere.2018.05.019
84
Chioccarelli T Manfrevola F Migliaccio M Altucci L Porreca V Fasano S et al . Fetal-perinatal exposure to bisphenol-a affects quality of spermatozoa in adulthood mouse. Int J Endocrinol (2020) 2020. doi: 10.1155/2020/2750501
85
da Silva N Tatiana G Curi Z Lima Tolouei SE Tapias Passoni M Sari Hey GB et al . Effects of diisopentyl phthalate exposure during gestation and lactation on hormone-dependent behaviours and hormone receptor expression in rats. J Neuroendocrinol (2019) 31(12):e12816. doi: 10.1111/jne.12816
86
Yu Y-j Lin B-g Chen X-c Qiao J Li L-z Liang Y et al . Polybrominated diphenyl ethers in human serum, semen and indoor dust: Effects on hormones balance and semen quality. Sci Total Environ (2019) 671:1017–25. doi: 10.1016/j.scitotenv.2019.03.319
87
Kanimozhi V Palanivel K Akbarsha MA Kadalmani B . Molecular mechanisms of tributyltin-induced alterations in cholesterol homeostasis and steroidogenesis in hamster testis: In vivo and in vitro studies. J Cell Biochem (2018) 119(5):4021–37. doi: 10.1002/jcb.26564
88
Williams RS Curnick DJ Brownlow A Barber JL Barnett J Davison NJ et al . Polychlorinated biphenyls are associated with reduced testes weights in harbour porpoises (Phocoena phocoena). Environ Int (2021) 150:106303. doi: 10.1016/j.envint.2020.106303
89
Kuriyama SN Chahoud I . In utero exposure to low-dose 2, 3′, 4, 4′, 5-pentachlorobiphenyl (Pcb 118) impairs Male fertility and alters neurobehavior in rat offspring. Toxicology (2004) 202(3):185–97. doi: 10.1016/j.tox.2004.05.006
90
Massányi P Massányi M Madeddu R Stawarz R Lukáč N . Effects of cadmium, lead, and mercury on the structure and function of reproductive organs. Toxics (2020) 8(4):94. doi: 10.3390/toxics8040094
91
Ullah A Pirzada M Jahan S Ullah H Shaheen G Rehman H et al . Bisphenol a and its analogs bisphenol b, bisphenol f, and bisphenol s: Comparative in vitro and in vivo studies on the sperms and testicular tissues of rats. Chemosphere (2018) 209:508–165. doi: 10.1016/j.chemosphere.2018.06.089
92
Di Lorenzo M Mileo A Laforgia V De Falco M Rosati L . Alkyphenol exposure alters steroidogenesis in male lizard Podarcis siculus. Animals (2021) 11(4):1003. doi: 10.3390/ani11041003
93
Burgos-Aceves MA Migliaccio V Di Gregorio I Paolella G Lepretti M Faggio C et al . 1, 1, 1-trichloro-2, 2-bis (p-chlorophenyl)-ethane (DDT) and 1, 1-Dichloro-2, 2-bis (p, p’-chlorophenyl) ethylene (DDE) as endocrine disruptors in human and wildlife: A possible implication of mitochondria. Environ Toxicol Pharmacol (2021) 87:103684. doi: 10.1016/j.etap.2021.103684
94
Plunk EC Richards SM . Endocrine-disrupting air pollutants and their effects on the hypothalamus-Pituitary-Gonadal axis. Int J Mol Sci (2020) 21(23):9191. doi: 10.3390/ijms21239191
95
Pandya C Pillai P Nampoothiri LP Bhatt N Gupta S Gupta S . Effect of lead and cadmium Co-exposure on testicular steroid metabolism and antioxidant system of adult Male rats. Andrologia (2012) 44:813–225. doi: 10.1111/j.1439-0272.2010.01137.x
96
Liu Z Zhuan Q Zhang L Meng L Fu X Hou Y . Polystyrene microplastics induced female reproductive toxicity in mice. J hazard mater (2022) 424:127629. doi: 10.1016/j.jhazmat.2021.127629
97
Park E-J Han J-S Park E-J Seong E Lee G-H Kim D-W et al . Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation. Toxicol Lett (2020) 324:75–85. doi: 10.1016/j.toxlet.2020.01.008
98
Hu J Qin X Zhang J Zhu Y Zeng W Lin Y et al . Polystyrene microplastics disturb maternal-fetal immune balance and cause reproductive toxicity in pregnant mice. Reprod Toxicol (2021) 106:42–50. doi: 10.1016/j.reprotox.2021.10.002
99
An R Xifeng W Yang L Zhang J Wang N Xu F et al . Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology (2021) 449:152665. doi: 10.1016/j.tox.2020.152665
100
Hou J Lei Z Cui L Hou Y Yang L An R et al . Polystyrene microplastics lead to pyroptosis and apoptosis of ovarian granulosa cells Via Nlrp3/Caspase-1 signaling pathway in rats. Ecotoxicol Environ Saf (2021) 212:112012. doi: 10.1016/j.ecoenv.2021.112012
101
Haddadi A Kessabi K Boughammoura S Ben Rhouma M Mlouka R Banni M et al . Exposure to microplastics leads to a defective ovarian function and change in cytoskeleton protein expression in rat. Environ Sci pollut Res (2022) 29:1–13. doi: 10.1007/s11356-021-18218-3
102
Tu J Hoi-Hung Cheung A Leung-Kwok Chan C Chan W-Y . The role of micrornas in ovarian granulosa cells in health and disease. Front Endocrinol (2019) 10:174. doi: 10.3389/fendo.2019.00174
103
Li X Zhang T Lv W Wang H Chen H Xu Q et al . Intratracheal administration of polystyrene microplastics induces pulmonary fibrosis by activating oxidative stress and Wnt/B-catenin signaling pathway in mice. Ecotoxicol Environ Saf (2022) 232:113238. doi: 10.1016/j.ecoenv.2022.113238
104
Silva-García O Valdez-Alarcón JJ Baizabal-Aguirre VM . Wnt/B-catenin signaling as a molecular target by pathogenic bacteria. Front Immunol (2135) 2019). doi: 10.3389/fimmu.2019.02135
105
Laskar N Upendra K . Plastics and microplastics: A threat to environment. Environ Technol Innovation (2019) 14:100352. doi: 10.1016/j.eti.2019.100352
106
Konieczna A Rutkowska A Rachon D . Health risk of exposure to bisphenol a (Bpa). Roczniki Państwowego Zakładu Higieny (2015) 66(1):5–11.
107
Amar S Binet A Téteau O Desmarchais A Papillier P Lacroix M et al . Bisphenol s impaired human granulosa cell steroidogenesis in vitro. Int J Mol Sci (2020) 21(5):1821. doi: 10.3390/ijms21051821
108
Lai F-N Liu J-C Li L Ma J-Y Liu X-L Liu Y-P et al . Di (2-ethylhexyl) phthalate impairs steroidogenesis in ovarian follicular cells of prepuberal mice. Arch Toxicol (2017) 91(3):1279–92. doi: 10.1007/s00204-016-1790-z
109
Shi X Wang X Peng L Chen Y Liu C Yang Q et al . Associations between polybrominated diphenyl ethers (Pbdes) levels in adipose tissues and female menstrual cycle and menstrual bleeding duration in shantou, China. Environ pollut (2022) 301:119025. doi: 10.1016/j.envpol.2022.119025
110
de Araújo JFP Podratz PL Sena GC Merlo E Freitas-Lima LC Mori Ayub JG et al . The obesogen tributyltin induces abnormal ovarian adipogenesis in adult female rats. Toxicol Lett (2018) 295:99–114. doi: 10.1016/j.toxlet.2018.06.1068
111
Pocar P Fiandanese N Secchi C Berrini A Fischer B Schmidt J-S et al . Effects of polychlorinated biphenyls in cd-1 mice: Reproductive toxicity and intergenerational transmission. Toxicol Sci (2012) 126(1):213–26. doi: 10.1093/toxsci/kfr327
112
Steinberg RM Walker DM Juenger TE Woller MJ Gore AC . Effects of perinatal polychlorinated biphenyls on adult female rat reproduction: Development, reproductive physiology, and second generational effects. Biol Reprod (2008) 78(6):1091–101. doi: 10.1095/biolreprod.107.067249
113
Loyo-Rosales JE Rosales-Rivera GC Lynch AM Rice CP Torrents A . Migration of nonylphenol from plastic containers to water and a milk surrogate. J Agric Food Chem (2004) 52:2016–20. doi: 10.1021/jf0345696
114
Marta S Pereiro P Figueras A Novoa B . An integrative toxicogenomic analysis of plastic additives. J hazard mater (2021) 409:124975. doi: 10.1016/j.jhazmat.2020.124975
115
Ong H-T Samsudin H Soto-Valdez H . Migration of endocrine-disrupting chemicals into food from plastic packaging materials: An overview of chemical risk assessment, techniques to monitor migration, and international regulations. Crit Rev Food Sci Nutr (2022) 62(4):957–79. doi: 10.1080/10408398.2020.1830747
116
Hunt PA Koehler KE Susiarjo M Hodges CA Ilagan A Voigt RC et al . Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr Biol 13(7) (2003) 13:546–53. doi: 10.1016/S0960-9822(03)00189-1
117
Yong C Ying Q Valiyaveettil S Luen Tang B . Toxicity of microplastics and nanoplastics in mammalian systems. Int J Environ Res Public Health (2020) 17(5):1509. doi: 10.3390/ijerph17051509
118
Ma Y Zhang J Zeng R Qiao X Cheng R Nie Y et al . Effects of the dibutyl phthalate (Dbp) on the expression and activity of aromatase in human granulosa cell line kgn. Ann Clin Lab Sci (2019) 49(2):175–82.
119
Yu G . Effect of mono-(2-ethylhexyl) phthalate (MEHP) on proliferation of and steroid hormone synthesis in rat ovarian granulosa cells in vitroJ Cell Physiol (2018) 4:3629–37. doi: 10.1002/jcp.26224
120
Basini G Bussolati S Andriani L Grolli S Ramoni R Bertini S et al . Nanoplastics impair in vitro swine granulosa cell functions. Domest Anim Endocrinol (2021) 76:1066115. doi: 10.1016/j.domaniend.2021.106611
121
Chakraborty SB . Non-essential heavy metals as endocrine disruptors: Evaluating impact on reproduction in teleosts. Pap presented at Proc Zool Soc (2021) 74:417–431. doi: 10.1007/s12595-021-00399-x
122
Pitt JA Kozal JS Jayasundara N Massarsky A Trevisan R Geitner N et al . Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio). Aquat Toxicol (2018) 194:185–94. doi: 10.1016/j.aquatox.2017.11.017
123
Pitt JA Trevisan R Massarsky A Kozal JS Levin ED Di Giulio RT . Maternal transfer of nanoplastics to offspring in zebrafish (Danio rerio): A case study with nanopolystyrene. Sci Total Environ (2018) 643:324–34. doi: 10.1016/j.scitotenv.2018.06.186
124
Li Y Yang G Wang J Lu L Li X Zheng Y et al . Microplastics increase the accumulation of phenanthrene in the ovaries of marine medaka (Oryzias melastigma) and its transgenerational toxicity. J hazard mater (2022) 424:127754. doi: 10.1016/j.jhazmat.2021.127754
125
Hiller-Sturmhöfel S Bartke A . The endocrine system: An overview. Alcohol Health Res World (1998) 22(3):153.
126
Graceli, Jones B Dettogni RS Merlo E Niño O da Costa CS Zanol JF et al . The impact of endocrine-disrupting chemical exposure in the mammalian hypothalamic-pituitary axis. Mol Cell Endocrinol (2020) 518:110997. doi: 10.1016/j.mce.2020.110997
127
Haibo J Yan M Pan C Liu Z Sha X Jiang C et al . Chronic exposure to polystyrene microplastics induced Male reproductive toxicity and decreased testosterone levels Via the lh-mediated Lhr/Camp/Pka/Star pathway. Part fibre Toxicol (2022) 19(1):1–17. doi: 10.1186/s12989-022-00453-2
128
Ma Q Deng P Lin M Yang L Li L Guo L et al . Long-term bisphenol a exposure exacerbates diet-induced prediabetes Via Tlr4-dependent hypothalamic inflammation. J hazard mater (2021) 402:123926. doi: 10.1016/j.jhazmat.2020.123926
129
Liu T Wang Y Yang M Shao P Duan L Li M et al . Di-(2-Ethylhexyl) phthalate induces precocious puberty in adolescent female rats. Iranian J Basic Med Sci (2018) 21(8):848. doi: 10.22038/IJBMS.2018.28489.6905
130
Muthuvel R Venkataraman P Krishnamoorthy G Gunadharini DN Kanagaraj P Jone Stanley A et al . Antioxidant effect of ascorbic acid on pcb (Aroclor 1254) induced oxidative stress in hypothalamus of albino rats. Clin Chimica Acta (2006) 365(1-2):297–303. doi: 10.1016/j.cca.2005.09.006
131
Sena GC Freitas-Lima LC Merlo E Podratz PL de Araújo JFP Brandão PAA et al . Environmental obesogen tributyltin chloride leads to abnormal hypothalamic-Pituitary-Gonadal axis function by disruption in Kisspeptin/Leptin signaling in female rats. Toxicol Appl Pharmacol (2017) 319:22–38. doi: 10.1016/j.taap.2017.01.021
132
Oliveira FRT Ferreira JR Corrêa dos Santos CM Miranda Macêdo LE de Oliveira RB Rodrigues JA et al . Estradiol reduces cumulative mercury and associated disturbances in the hypothalamus–pituitary axis of ovariectomized rats. Ecotoxicol Environ Saf (2006) 63(3):488–93. doi: 10.1016/j.ecoenv.2004.12.024
133
Ferrer B Peres TV Dos Santos AA Bornhorst J Morcillo P Ludvig Gonçalves C et al . Methylmercury affects the expression of hypothalamic neuropeptides that control body weight in C57bl/6j mice. Toxicol Sci (2018) 163(2):557–68. doi: 10.1093/toxsci/kfy052
134
Karaulov AV Smolyagin AI Mikhailova IV Stadnikov AA Ermolina EV Filippova YV Kuzmicheva NA Vlata Z Djordjevic AB Tsitsimpikou C et al . Assessment of the combined effects of chromium and benzene on the rat neuroendocrine and immune systems. Environ Res (2022) 207:112096. doi: 10.1016/j.envres.2021.112096
135
Nudler SI Quinteros FA Miler EA Cabilla JP Ronchetti SA Duvilanski BH . Chromium vi administration induces oxidative stress in hypothalamus and anterior pituitary gland from Male rats. Toxicol Lett (2009) 185(3):187–92. doi: 10.1016/j.toxlet.2009.01.003
136
Prüst M Meijer J Westerink RHS . The plastic brain: Neurotoxicity of micro-and nanoplastics. Part fibre Toxicol (2020) 17(1):1–16. doi: 10.1186/s12989-020-00358-y
137
Sarasamma S Audira G Siregar P Malhotra N Lai Y-H Liang S-T et al . Nanoplastics cause neurobehavioral impairments, reproductive and oxidative damages, and biomarker responses in zebrafish: Throwing up alarms of wide spread health risk of exposure. Int J Mol Sci (2020) 21(4):1410. doi: 10.3390/ijms21041410
138
Zhao X Liu Z Ren X Duan X . Parental transfer of nanopolystyrene-enhanced tris (1, 3-Dichloro-2-Propyl) phosphate induces transgenerational thyroid disruption in zebrafish. Aquat Toxicol (2021) 236:105871. doi: 10.1016/j.aquatox.2021.105871
139
Rosenfeld CS . Neuroendocrine disruption in animal models due to exposure to bisphenol a analogues. Front Neuroendocrinol (2017) 47:123–335. doi: 10.1016/j.yfrne.2017.08.001
140
Wang J Li X Li P Li L Zhao L Ru S et al . Porous microplastics enhance polychlorinated biphenyls-induced thyroid disruption in juvenile Japanese flounder (Paralichthys olivaceus). Mar pollut Bull (2022) 174:113289. doi: 10.1016/j.marpolbul.2021.113289
141
Xi W Lee CKF Yeung WSB Giesy JP Wong MH Zhang X et al . Effect of perinatal and postnatal bisphenol a exposure to the regulatory circuits at the hypothalamus–Pituitary–Gonadal axis of cd-1 mice. Reprod Toxicol (2011) 31(4):409–17. doi: 10.1016/j.reprotox.2010.12.002
142
Hlisníková H Petrovičová I Kolena B Šidlovská M Sirotkin A . Effects and mechanisms of phthalates’ action on reproductive processes and reproductive health: A literature review. Int J Environ Res Public Health (2020) 17(18):6811. doi: 10.3390/ijerph17186811
143
Li X-N Li H-X Yang T-N Li X-W Huang Y-Q Zhu S-Y et al . Di-(2-Ethylhexyl) phthalate induced developmental abnormalities of the ovary in quail (Coturnix japonica) Via disruption of the hypothalamic-Pituitary-Ovarian axis. Sci Total Environ (2020) 741:140293. doi: 10.1016/j.scitotenv.2020.140293
144
Barbosa KL Dettogni RS da Costa CS Gastal EL Raetzman LT Flaws JA et al . Tributyltin and the female hypothalamic-Pituitary-Gonadal disruption. Toxicol Sci (2021) 186:179–89. doi: 10.1093/toxsci/kfab141
145
Kumari S Jamwal R Mishra N Singh DK . Recent developments in environmental mercury bioremediation and its toxicity: A review. Environ Nanotechnol Monit Manage (2020) 13:100283. doi: 10.1016/j.enmm.2020.100283
146
Rice KM Walker EM Jr Wu M Gillette C Blough ER . Environmental mercury and its toxic effects. J Prev Med Public Health (2014) 47(2):74. doi: 10.3961/jpmph.2014.47.2.74
147
Li Z Li X Qian Y Guo C Wang Z Wei Y . The sustaining effects of e-Waste-Related metal exposure on hypothalamus-Pituitary-Adrenal axis reactivity and oxidative stress. Sci Total Environ (2020) 739:139964. doi: 10.1016/j.scitotenv.2020.139964
148
Kim J-H Kang J-C . Effects of Sub-chronic exposure to lead (Pb) and ascorbic acid in juvenile rockfish: Antioxidant responses, Mt gene expression, and neurotransmitters. Chemosphere (2017) 171:520–27. doi: 10.1016/j.chemosphere.2016.12.094
149
Qu J Niu H Wang J Wang Q Li Y . Potential mechanism of lead poisoning to the growth and development of ovarian follicle. Toxicology (2021) 457:152810. doi: 10.1016/j.tox.2021.152810
150
Wise JJP Young JL Cai J Cai L . Current understanding of hexavalent chromium [Cr (Vi)] neurotoxicity and new perspectives. Environ Int (2022) 158:106877. doi: 10.1016/j.envint.2021.106877
151
Scanes CG . Pituitary gland. In: Sturkie’s avian physiology. Elsevier (2022). p. 739–93.
152
El Sayed SA Fahmy MW Schwartz J . Physiology, pituitary gland. (2017).
153
Fernandez MO Bourguignon NS Arocena P Rosa M Libertun C Lux-Lantos V . Neonatal exposure to bisphenol a alters the hypothalamic-Pituitary-Thyroid axis in female rats. Toxicol Lett (2018) 285:81–6. doi: 10.1016/j.toxlet.2017.12.029
154
Dunnick JK Pandiri AR Merrick BA Kissling GE Cunny H Mutlu E et al . Carcinogenic activity of pentabrominated diphenyl ether mixture (De-71) in rats and mice. Toxicol Rep (2018) 5:615–245. doi: 10.1016/j.toxrep.2018.05.010
155
Butt CM Miranda ML Stapleton HM . Development of an analytical method to quantify pbdes, oh-bdes, hbcds, 2, 4, 6-tbp, eh-tbb, and beh-tebp in human serum. Anal bioanal Chem (2016) 408(10):2449–59. doi: 10.1007/s00216-016-9340-3
156
Wade MG Kawata A Rigden M Caldwell D Holloway AC . Toxicity of flame retardant isopropylated triphenyl phosphate: Liver, adrenal, and metabolic effects. Int J Toxicol (2019) 38(4):279–90. doi: 10.1177/1091581819851502
157
Sarkar R Mohanakumar KP Chowdhury M . Effects of an organophosphate pesticide, quinalphos, on the hypothalamo-Pituitary-Gonadal axis in adult Male rats. J Reprod Fertil (2000) 118(1):29–38. doi: 10.1530/reprod/118.1.29
158
Bjørklund G Chirumbolo S Dadar M Pivina L Lindh U Butnariu M et al . Mercury exposure and its effects on fertility and pregnancy outcome. Basic Clin Pharmacol Toxicol (2019) 125(4):317–27. doi: 10.1111/bcpt.13264
159
McGregor AJ Mason HJ . Occupational mercury vapour exposure and testicular, pituitary and thyroid endocrine function. Hum Exp Toxicol (1991) 10(3):199–203. doi: 10.1177/096032719101000309
160
Petrusz P Weaver CM Grant LD Mushak P Krigman MR . Lead poisoning and reproduction: Effects on pituitary and serum gonadotropins in neonatal rats. Environ Res (1979) 19(2):383–91. doi: 10.1016/0013-9351(79)90063-X
161
Lafuente A Márquez N Perez-Lorenzo M Pazo D Esquifino AI . Cadmium effects on hypothalamic-Pituitary-Testicular axis in Male rats. Exp Biol Med (2001) 226(6):605–11. doi: 10.1177/153537020122600615
162
Fernández M Bianchi M Lux-Lantos V Libertun C . Neonatal exposure to bisphenol a alters reproductive parameters and gonadotropin releasing hormone signaling in female rats. Environ Health Perspect (2009) 117(5):757–62. doi: 10.1289/ehp.0800267
163
Yaglova NV Tsomartova DA Obernikhin SS Nazimova SV . Alteration of rat adrenal cortex after low-dose exposure to endocrine disrupting chemical dichlorodiphenyltrichloroethane since the first day of postnatal development. Voprosy Pitaniia (2017) 86(4):70–6. doi: 10.24411/0042-8833-2017-00061
164
Merlo E Podratz PL Sena GC De Araújo JFP Lima LCF Alves ISS et al . The environmental pollutant tributyltin chloride disrupts the hypothalamic-Pituitary-Adrenal axis at different levels in female rats. Endocrinology (2016) 157(8):2978–95. doi: 10.1210/en.2015-1896
165
Fujimoto N . Effects of endocrine disruptors on the pituitary gland. J Toxicol Pathol (2001) 14(1):65–5. doi: 10.1293/tox.14.65
166
Sabir S Hamid Akash MS Fiayyaz F Saleem U Mehmood MH Rehman K . Role of cadmium and arsenic as endocrine disruptors in the metabolism of carbohydrates: Inserting the association into perspectives. Biomed Pharmacother (2019) 114:108802. doi: 10.1016/j.biopha.2019.108802
167
Ronchetti SA Novack GV Bianchi MS Crocco MC Duvilanski BH Cabilla JP . In vivo xenoestrogenic actions of cadmium and arsenic in anterior pituitary and uterus. Reproduction (2016) 152(1):1–10. doi: 10.1530/REP-16-0115
168
Ronchetti SA Bianchi MS Duvilanski BH Cabilla JP . In vivo and in vitro arsenic exposition induces oxidative stress in anterior pituitary gland. Int J Toxicol (2016) 35(4):463–75. doi: 10.1177/1091581816645797
169
Rosol TJ Yarrington JT Latendresse J Capen CC . Adrenal gland: Structure, function, and mechanisms of toxicity. Toxicol Pathol (2001) 29(1):41–8. doi: 10.1080/019262301301418847
170
Stojanović Z Todorović A Filipović N Veljković F Guševac Stojanović I . A single dose of microplastic particles induces changed in organ weight of Male wistar rats. Int Conf Fundam Appl Aspects Phys Chemistry-Phys Chem (2021) 2021:119–22
171
Brun NR Van Hage P Hunting ER Haramis A-PG Vink SC Vijver MG et al . Polystyrene nanoplastics disrupt glucose metabolism and cortisol levels with a possible link to behavioural changes in larval zebrafish. Commun Biol (2019) 2(1):1–9. doi: 10.1038/s42003-019-0629-6
172
Campioli E Martinez-Arguelles DB Papadopoulos V . In utero exposure to the endocrine disruptor di-(2-Ethylhexyl) phthalate promotes local adipose and systemic inflammation in adult Male offspring. Nutr Diabetes (2014) 4(5):e115–e15. doi: 10.1038/nutd.2014.13
173
Medwid S Guan H Yang K . Prenatal exposure to bisphenol a disrupts adrenal steroidogenesis in adult mouse offspring. Environ Toxicol Pharmacol (2016) 43:203–08. doi: 10.1016/j.etap.2016.03.014
174
Eker F Gungunes A Durmaz S Kisa U Celik ZR . Nonfunctional adrenal incidentalomas may be related to bisphenol-a. Endocrine (2021) 71(2):459–66. doi: 10.1007/s12020-020-02502-2
175
Hlisníková H Petrovičová I Kolena B Šidlovská M Sirotkin A . Effects and mechanisms of phthalates’ action on neurological processes and neural health: A literature review. Pharmacol Rep (2021) 73(2):386–404. doi: 10.1007/s43440-021-00215-5
176
Chen X Mo J Zhang S Li X Huang T Zhu Q et al . 4-bromodiphenyl ether causes adrenal gland dysfunction in rats during puberty. Chem Res Toxicol (2019) 32(9):1772–79. doi: 10.1021/acs.chemrestox.9b00123
177
Yan ,H Hao G Cheng D Kou R Zhang C Si J . Tributyltin reduces the levels of serum adiponectin and activity of akt and induces metabolic syndrome in Male mice. Environ Toxicol (2018) 33(7):752–58. doi: 10.1002/tox.22562
178
Solaiman A Seddik S . Histological study of the effect of tributyltin on the adrenal cortical cells of adult Male albino rats. Egypt J Histol (2020) 43(1):104–21. doi: 10.21608/EJH.2019.13126.1127
179
Di Lorenzo M Sciarrillo R Rosati L Sellitti A Barra T De Luca A et al . Effects of alkylphenols mixture on the adrenal gland of the lizard Podarcis sicula. Chemosphere (2020) 258:127239. doi: 10.1016/j.chemosphere.2020.127239
180
Yaglova NV Tsomartova DA Yaglov VV . Effect of prenatal and postnatal exposure to low doses of ddt on catecholamine secretion in rats in different period of ontogeny. Bull Exp Biol Med (2017) 163(4):422–24. doi: 10.1007/s10517-017-3819-6
181
Yaglova NV Obernikhin SS Yaglov VV Tsomartova DA Nazimova SV Timokhina EP . Ultrastructural mechanisms of impaired aldosterone synthesis in rats exposed to ddt during prenatal and postnatal development. Bull Exp Biol Med (2020) 170(1):101–05. doi: 10.1007/s10517-020-05013-2
182
Pamphlett R Kum Jew S Doble PA Bishop DP . Mercury in the human adrenal medulla could contribute to increased plasma noradrenaline in aging. Sci Rep (2021) 11(1):1–14. doi: 10.1038/s41598-021-82483-y
183
Soto M Lewis R Curtis JT . Chronic exposure to inorganic mercury alters stress responses in Male prairie voles (Microtus ochrogaster). Horm Behav (2019) 109:53–5. doi: 10.1016/j.yhbeh.2019.02.008
184
Chandra AK Chatterjee A Ghosh R Sarkar M Chaube SK . Chromium induced testicular impairment in relation to adrenocortical activities in adult albino rats. Reprod Toxicol (2007) 24(3-4):388–96. doi: 10.1016/j.reprotox.2007.07.009
185
Mikheeva EV Zhigal’skiĭ OA Mamina VP Baĭtimirova EA . Adaptation of the bank vole (Clethrionomys glareolus schreber) to conditions of biogeochemical province with abnormally high content of nickel, cobalt and chromium. Zhurnal Obshchei Biol (2006) 67(3):212–21. doi: 10.1016/j.ecoenv.2021.112034
186
Lauretta R Sansone A Sansone M Romanelli F Appetecchia M . Endocrine disrupting chemicals: Effects on endocrine glands. Front Endocrinol (2019) 10:178. doi: 10.3389/fendo.2019.00178
187
Kinlein SA Wilson CD Karatsoreos IN . Dysregulated hypothalamic–Pituitary–Adrenal axis function contributes to altered endocrine and neurobehavioral responses to acute stress. Front Psychiatry (2015) 6:31. doi: 10.3389/fpsyt.2015.00031
188
Medwid S Guan H Yang K . Bisphenol a stimulates adrenal cortical cell proliferation via erβ-mediated activation of the sonic hedgehog signalling pathway. J Steroid Biochem Mol Biol (2018) 178:254–62. doi: 10.1016/j.jsbmb.2018.01.004
189
Olukole SG Olaniyi Lanipekun D Ola-Davies EO Bankole OO . Melatonin attenuates bisphenol a-induced toxicity of the adrenal gland of wistar rats. Environ Sci pollut Res (2019) 26(6):5971–82. doi: 10.1007/s11356-018-4024-5
190
Asimakopoulos AG Thomaidis NS Koupparis MA . Recent trends in biomonitoring of bisphenol a, 4-T-Octylphenol, and 4-nonylphenol. Toxicol Lett (2012) 210(2):141–54. doi: 10.1016/j.toxlet.2011.07.032
191
Timokhina EP Yaglov VV Nazimova SV . Dichlorodiphenyltrichloroethane and the adrenal gland: From toxicity to endocrine disruption. Toxics (2021) 9(10):243. doi: 10.3390/toxics9100243
192
Ng TB Liu WK . Toxic effect of heavy metals on cells isolated from the rat adrenal and testis. In Vitro Cell Dev Biol (1990) 26(1):24–8. doi: 10.1007/BF02624150
Summary
Keywords
microplastics, nanoplastics, mammalian endocrine system, endocrine abnormalities, endocrine disrupting chemicals, plastic additives, environmental pollution
Citation
Ullah S, Ahmad S, Guo X, Ullah S, Ullah S, Nabi G and Wanghe K (2023) A review of the endocrine disrupting effects of micro and nano plastic and their associated chemicals in mammals. Front. Endocrinol. 13:1084236. doi: 10.3389/fendo.2022.1084236
Received
30 October 2022
Accepted
05 December 2022
Published
16 January 2023
Volume
13 - 2022
Edited by
Bodil Holst, Swedish University of Agricultural Sciences, Sweden
Reviewed by
Luigi Rosati, University of Naples Federico II, Italy; Sergio Minucci, Università della Campania Luigi Vanvitelli, Italy
Updates
Copyright
© 2023 Ullah, Ahmad, Guo, Ullah, Ullah, Nabi and Wanghe.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ghulam Nabi, ghulamnabiqau@gmail.com; Kunyuan Wanghe, wanghekunyuan@gmail.com
†These authors have contributed equally to this work and share first authorship
This article was submitted to Experimental Endocrinology, a section of the journal Frontiers in Endocrinology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.