- Department of Urology, Affiliated Hospital of North Sichuan Medical College, Nanchong, China
Purpose: Male infertility is a global public health issue recognized by the WHO. Recently, antioxidants are increasingly used to treat idiopathic male infertility. However, the lack of available evidence has led to the inability to rank the effects of antioxidants on the sperm quality parameters and pregnancy rate of infertile men. This network meta-analysis studied the effects of different antioxidants on the sperm quality and pregnancy rate of idiopathic male infertility.
Methods: We searched PubMed, Embase, Web of Science, and Cochrane Library databases for randomized controlled trials (RCTs). The weighted mean difference (WMD) and odds ratio (OR) were applied for the comparison of continuous and dichotomous variables, respectively, with 95% CIs. The outcomes were sperm motility, sperm concentration, sperm morphology, and pregnancy rate.
Results: A total of 23 RCTs with 1,917 patients and 10 kids of antioxidants were included. L-Carnitine, L-carnitine+L-acetylcarnitine, coenzyme-Q10, ω-3 fatty acid, and selenium were more efficacious than placebo in sperm quality parameters. L-Carnitine was ranked first in sperm motility and sperm morphology (WMD 6.52% [95% CI: 2.55% to 10.05%], WMD 4.96% [0.20% to 9.73%]). ω-3 fatty acid was ranked first in sperm concentration (WMD 9.89 × 106/ml, [95% CI: 7.01 to 12.77 × 106/ml]). In terms of pregnancy rate, there was no significant effect as compared with placebo.
Conclusions: L-Carnitine was ranked first in sperm motility and sperm morphology. ω-3 fatty acid was ranked first in sperm concentration. Coenzyme-Q10 had better effective treatment on sperm motility and concentration. Furthermore, high-quality RCTs with adequate sample sizes should be conducted to compare the outcomes of different antioxidants.
Introduction
Increasing evidence indicates that the incidence of infertility has gradually increased over the past decades. Infertility affects about 15% of couples globally (1). Male factors are estimated to be present in about 50% of cases, and 20% of cases have common contributing female factors (2, 3). Although the current research on the causes of male infertility has made great progress, idiopathic male infertility is still a challenging condition to diagnose and manage (2). Unlike unexplained male infertility (UMI) with normal sperm parameters, the patients diagnosed with idiopathic male infertility have the presence of altered sperm characteristics without an identifiable cause. We have to rule out the cause of female factor infertility (4, 5).
Overwhelming evidence suggests that oxidative stress (OS) plays a vital role in the etiology of male infertility (6). OS is an imbalance between reactive oxygen species (ROS) and protective antioxidants (7). OS could lead to abnormal sperm parameters and high levels of sperm deoxyribonucleic acid fragmentation (8). Recently, the concept “male oxidative stress infertility” (MOSI) has been proposed and pointed out that about 30%–80% of infertile men have increased ROS in sperm, which affected about 37.2 million infertile men (4). Therefore, using antioxidants to combat excessive OS in the treatment of infertility is a potential option. Several studies have reported the beneficial effects of oral antioxidants on sperm parameters. Supplementing exogenous L-carnitine, L-carnitine+L-acetylcarnitine, coenzyme-Q10, ω-3 fatty acid, selenium, zinc, vitamin E, and vitamin C has beneficial effects on sperm parameters. However, there were limited data available on pregnancy rates for interventions (9, 10–13).
Double-blind randomized controlled trials (RCTs) of direct comparison between drugs can provide clear evidence on health technology profiles. However, the high cost and a large number of patients could limit their development (14, 15). Network meta-analysis (NMA) allows the comparison of more interventions simultaneously, even if they are not directly compared by clinical trials. Compared with pairwise meta-analyses, the NMA could facilitate clinicians to mold interventions according to their choice to achieve desired outcomes in idiopathic male infertility (16).
There have been many RCTs of antioxidants versus placebo, which have demonstrated their effectiveness. However, there have been few head-to-head trials of one kind of antioxidant vs. another. So the relative efficacy of available treatment options for infertility is unclear. There are several parameters involved in the evaluation of sperm quality. Furthermore, it is uncertain, which antioxidants have the best effect on a certain parameter of sperm. Last, many articles also did not report the effect of antioxidants on pregnancy rates. So we conducted an NMA to resolve this uncertainty and found the most effective drug for male infertility.
Methods
Search Strategy
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (17). We conducted a systematic electronic literature search in February 2021 in PubMed, Embase, Web of Science, and Cochrane Library databases. The search was limited to English. We used medical subject heading (MeSH and Emtree) terms combined with Boolean logical operators (for searching strategies, see Table S1).
Inclusion and Exclusion Criteria
Studies were included if they met the following criteria: 1) only RCT studies; 2) idiopathic infertility was diagnosed; 3) the patients have sperm parameter abnormalities; and 4) the primary outcomes have to include sperm motility, sperm concentration, sperm morphology, pregnancy rate, alone or in combination. Studies were excluded if they met the following criteria: 1) case–control, cross-sectional, or retrospective studies; 2) men with varicocele or other fertility-related diseases; 3) men with drug interventions; and 4) combined use of different types of antioxidants.
Data Extraction
Two independent reviewers extracted the following data. Data extracted for the individual study included 1) general information related to the manuscript: first author, year of publication, and country. 2) Characteristics of the population: sample size, groups, age, and diagnosis. 3) Type of treatment, duration, and dosage. 4) Primary outcomes: sperm motility, sperm concentration, sperm morphology, and pregnancy rate. In cases that have multiple visits between these intervals, the longest one would be selected. We tried to make the included articles have similar characteristics to ensure the optimal transitivity of the NMA (18). Any dispute was resolved by consensus or consultation with a third reviewer.
Quality Assessment
The quality of RCTs was evaluated according to the Cochrane Collaboration’s tool (19), including random sequence generation, allocation concealment, blinding of participants, personnel and outcome assessment, incomplete outcome data, selective reporting, and other sources of bias.
Statistical Analysis
All the analyses were performed using Stata statistical package version 14.1 software; all statistical analyses were conducted to calculate the weighted mean difference (WMD) for continuous data and odds ratio (OR) for dichotomous variables, together with the corresponding 95% CIs. For the traditional pairwise meta-analysis, the Mantel–Haenszel random-effects model was used (20). The heterogeneity in each pairwise comparison used I2 statistics and was judged as low (<25%), moderate (25%–75%), or high (>75%). p-Values of <0.05 were regarded as statistically significant (21). The random-effects NMA was conducted as a frequentist approach (22). The model inconsistency must be evaluated because the presence of inconsistency is often regarded as a statistical manifestation of intransitivity (18). Both consistent and inconsistent models were analyzed and compared. Furthermore, the design-by-treatment interaction model and loop consistency were used to assess the inconsistency in the NMA. The loop-specific approach estimated inconsistency factors and their 95% CIs within every closed triangular or quadratic loop (23–25). The relative ranking of treatments was done using the surface under the cumulative ranking (SUCRA). The ranks for each outcome were also presented (26, 27). Additionally, publication bias of NMA and potential small-study effects were tested for using a comparison-adjusted funnel plot (28). Multiple sensitivity analyses were performed to address the robustness of our findings, including 1) exclusion of studies with a significant risk of bias, 2) exclusion of studies published before 2002, and 3) exclusion of sample size of less than 30.
Results
Literature Search Results and Characteristics of Included Randomized Controlled Trials
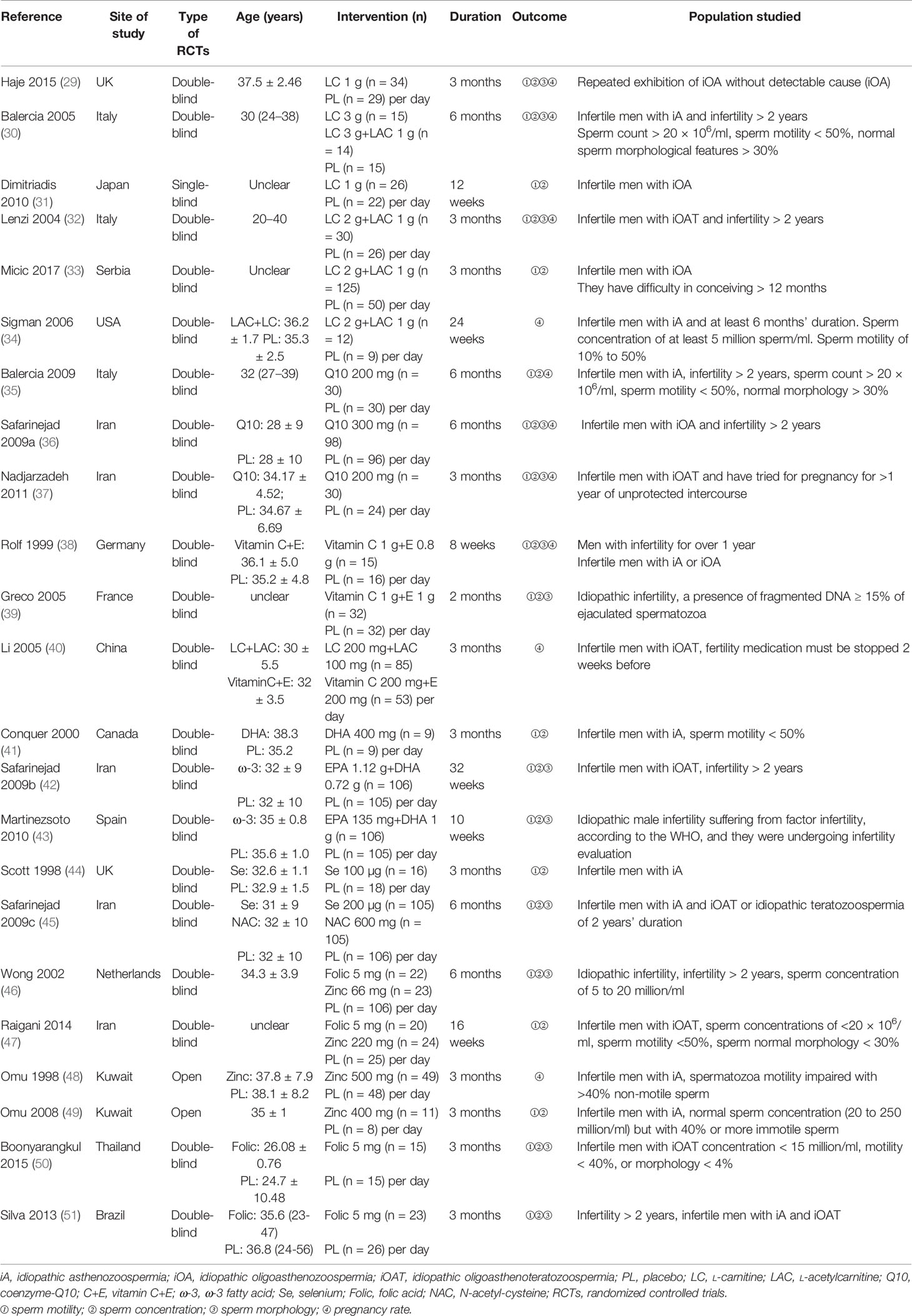
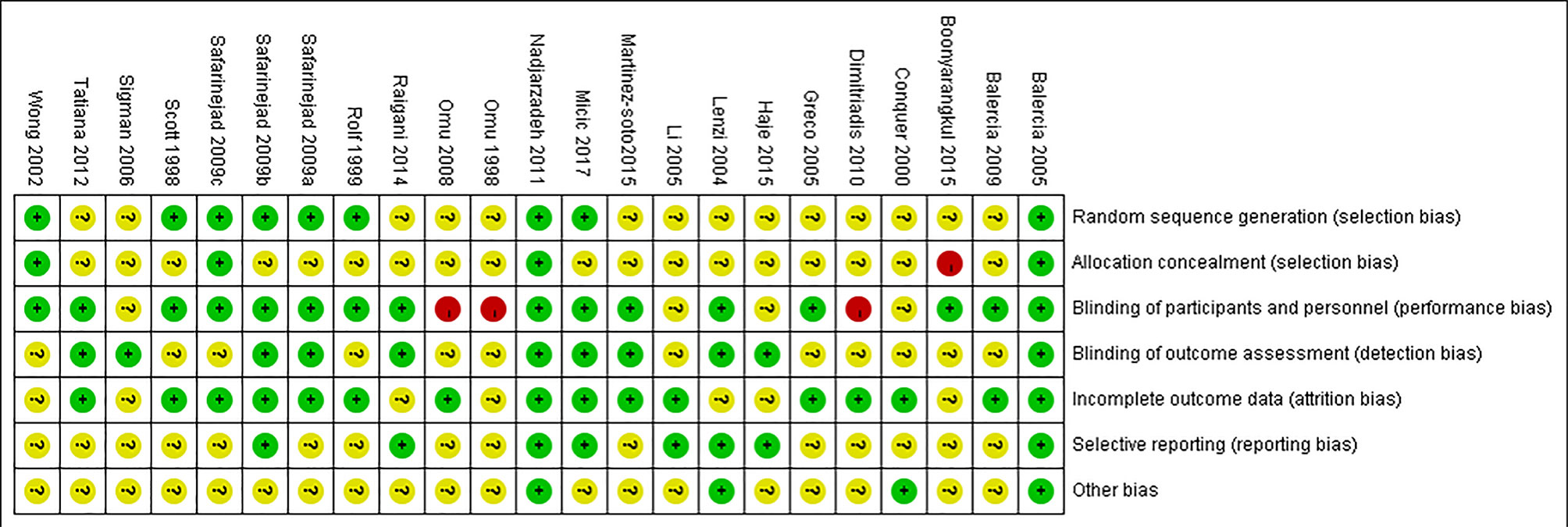
According to the literature search and the inclusion criteria, we included 1,917 patients in 23 studies for meta-analysis (29–51) (Figure 1). The details are in Table 1. Those studies were published from 1998 to 2017 and evaluated 10 antioxidants including L-carnitine, L-carnitine+L-acetylcarnitine, coenzyme-Q10, ω-3 fatty acid, selenium, zinc, vitamin E+vitamin C, folic, and N-acetyl-cysteine. All the patients were diagnosed with idiopathic infertility with abnormal sperm parameters. Most studies included a two-arm study design, but 4 studies included a three-arm design. Of 23 studies, 2 were rated as low risk and 4 studies as high risk (Figure 2).
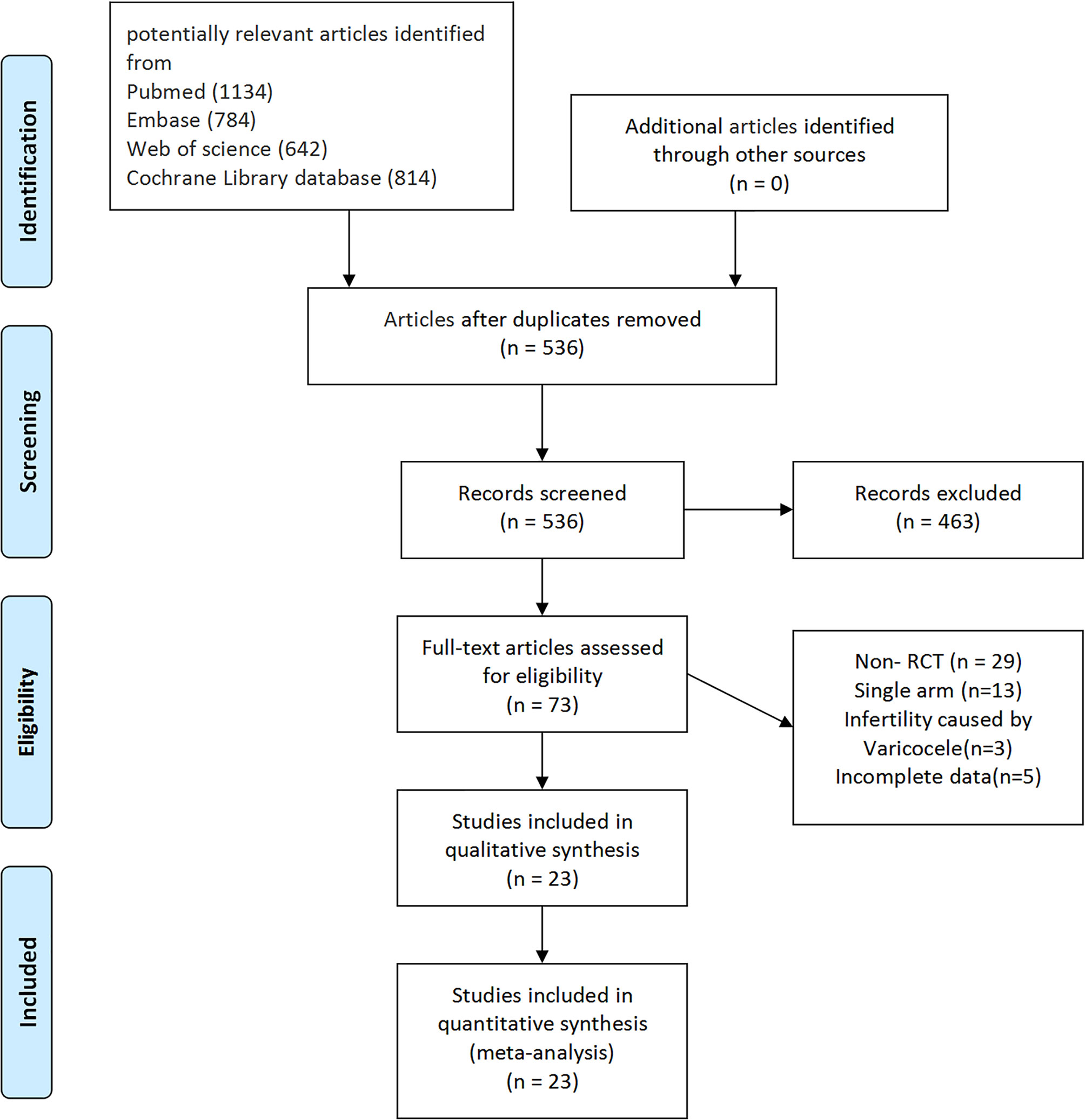
Figure 1 PRISMA flowchart of study selection. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Pairwise Meta-Analysis
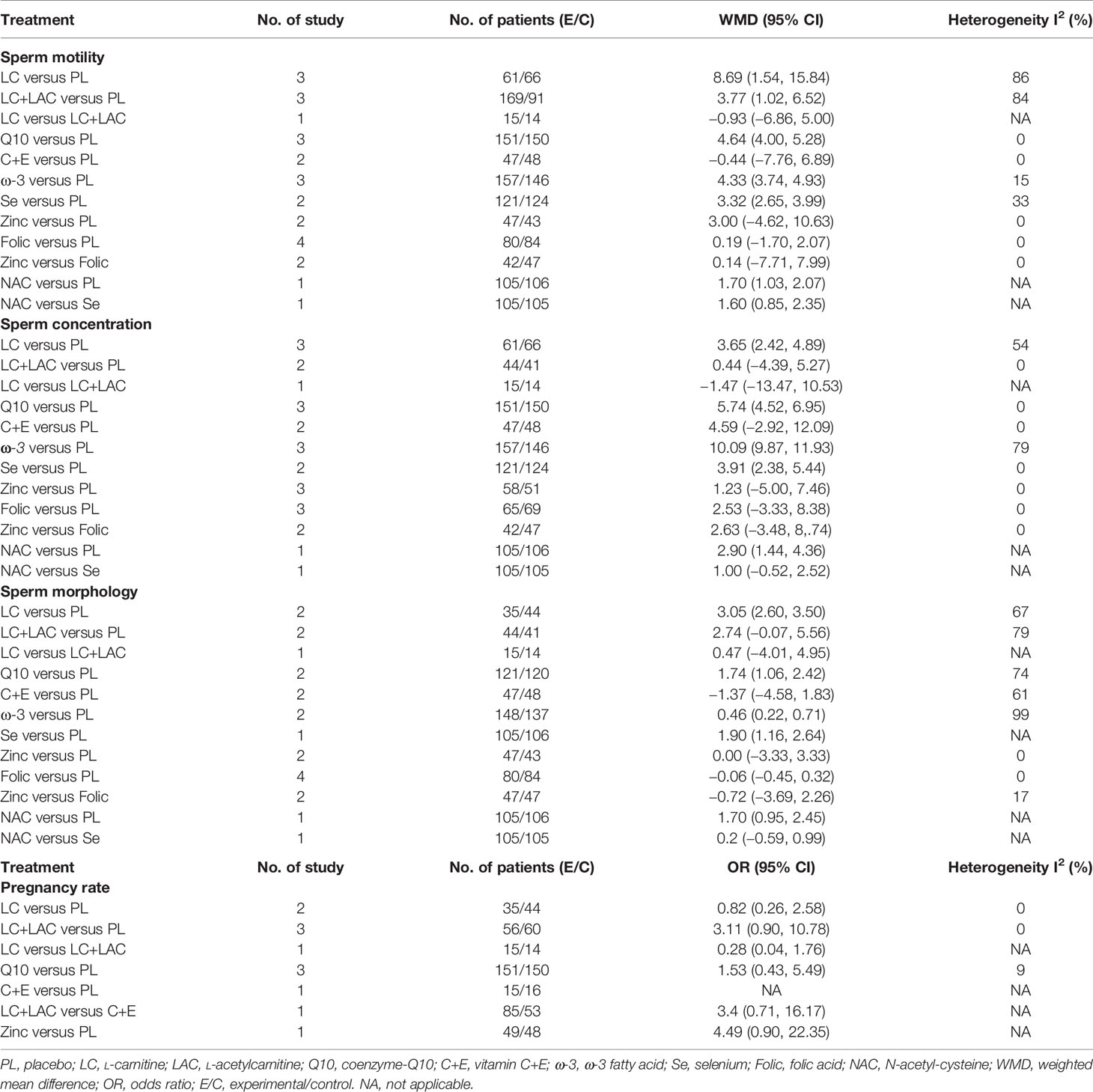
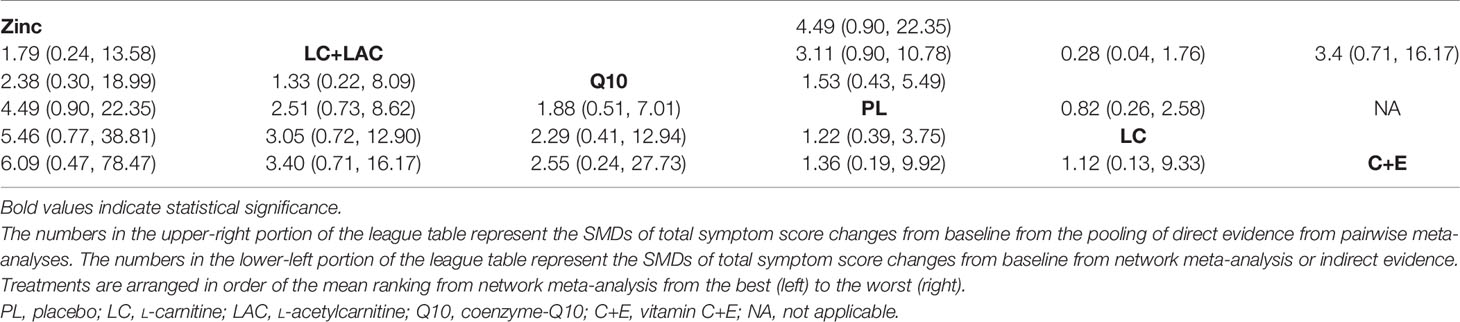
The results of the pairwise meta-analysis are shown in Table 2. We conducted the results of a pairwise meta-analysis that included two or more RCTs. In terms of sperm motility, LC (WMD 8.69% [95% CI: 1.54% to 15.84%]), LC+LAC (WMD 3.77% [95% CI: 1.02% to 6.52%]), Q10 (WMD 4.64% [95% CI: 4.00% to 5.28%]), ω-3 (WMD 4.33% [95% CI: 3.74% to 4.93%]), and Se (WMD 3.32% [95% CI: 2.65% to 3.99%]) had significantly increased sperm motility as compared with placebo. In terms of sperm concentration, the result showed that four antioxidants could improve sperm concentration compared with placebo—LC (WMD 3.65 × 106/ml [95% CI: 2.42 to 4.89 × 106/ml]), Q10 (WMD 5.74 × 106/ml [95% CI: 4.52 to 6.95 × 106/ml]), ω-3 (WMD 10.09 × 106/ml [95% CI: 9.87 to 11.93 × 106/ml]), and Se (WMD 3.91 × 106/ml [95% CI: 2.85 to 5.44 × 106/ml]). In terms of sperm morphology, LC (WMD 3.05% [95% CI: 2.60% to 3.50%]), CoQ10 (WMD 1.74% [95% CI: 1.06% to 2.42%]), and ω-3 (WMD 0.46% [95% CI: 0.22% to 0.71%]) were shown to have treatment effects with statistical significance. In terms of pregnancy rate, 12 studies reported the pregnancy rate, but no significant differences in the pregnancy rate were observed between antioxidants and placebo.
Network Meta-Analysis
Sperm Motility
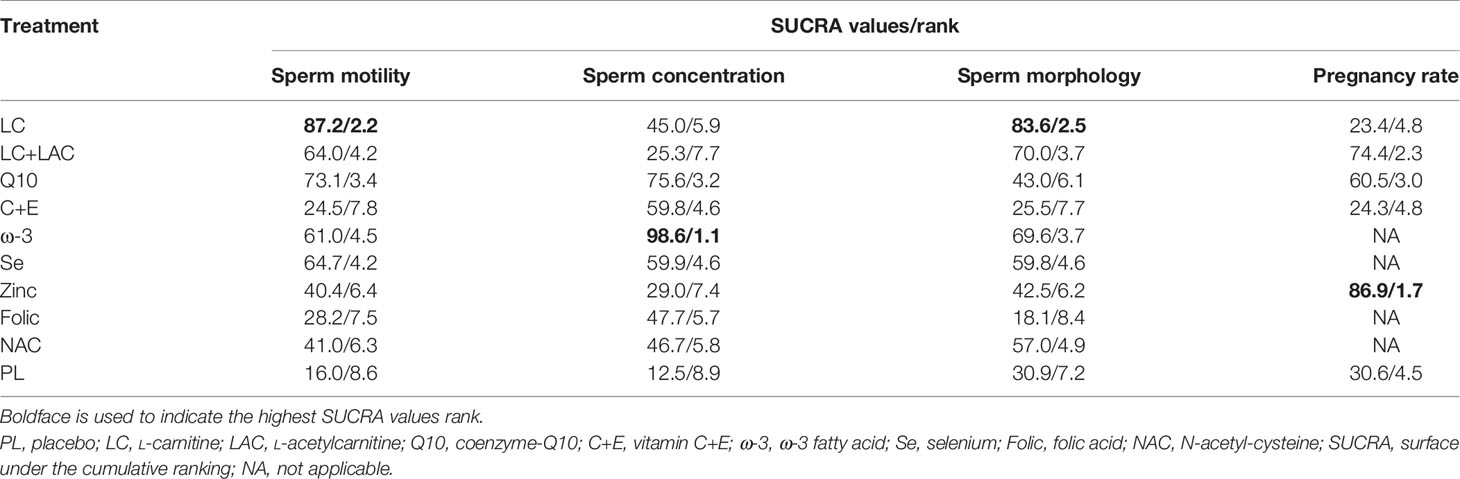
Sperm motility was reported in 18 RCTs, which included 9 different treatments that led to 27 pairwise comparisons. Compared with placebo, LC (WMD 6.52% [95% CI: 2.55% to 10.50%]), CoQ10 (WMD 4.92% [95% CI: 1.49% to 8.35%]), LC+LAC (WMD 4.21% [95% CI: 0.21% to 8,21%]), ω-3 (4.21% [95% CI: 0.21% to 8.21%]) had significantly increased sperm motility. We did not find other statistically significant differences between the remainder of the active treatments and placebo. Based on SUCRA, the LC had the highest probability of being the most effective treatment to increase sperm motility, followed by CoQ10 and LC+LAC. The network plot is shown in Figure 3. The ranking and SUCRA values are shown in Table 3 and Figure 4. Overall results of sperm motility are described in Table 4.
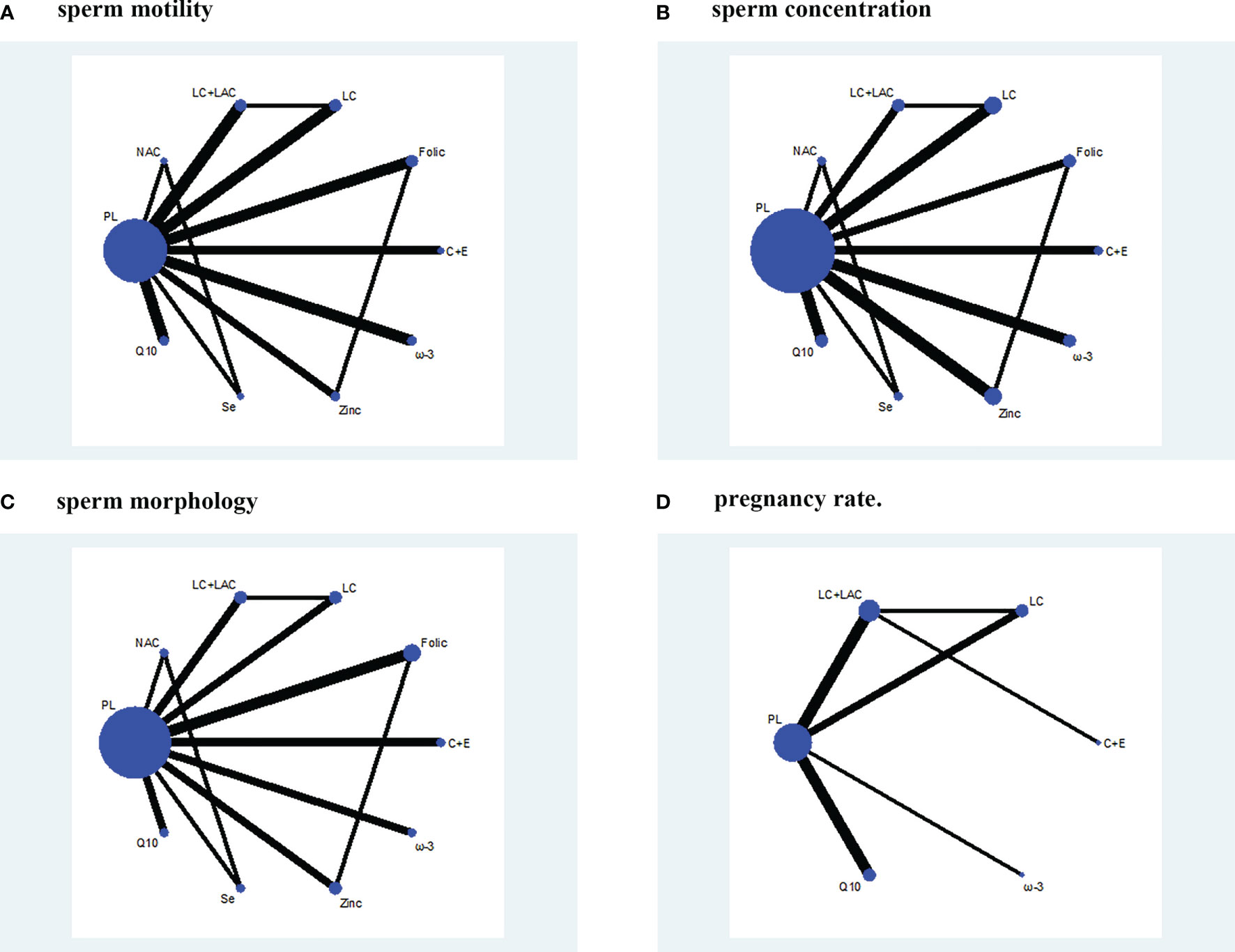
Figure 3 Network plot. (A) Sperm motility. (B) Sperm concentration. (C) Sperm morphology. (D) Pregnancy rate. PL, placebo; LC, L-carnitine; LAC, L-acetylcarnitine; Q10, coenzyme-Q10; C+E, vitamin C+E; ω-3, ω-3 fatty acid; Se, selenium; Folic, folic acid; NAC, N-acetyl-cysteine.
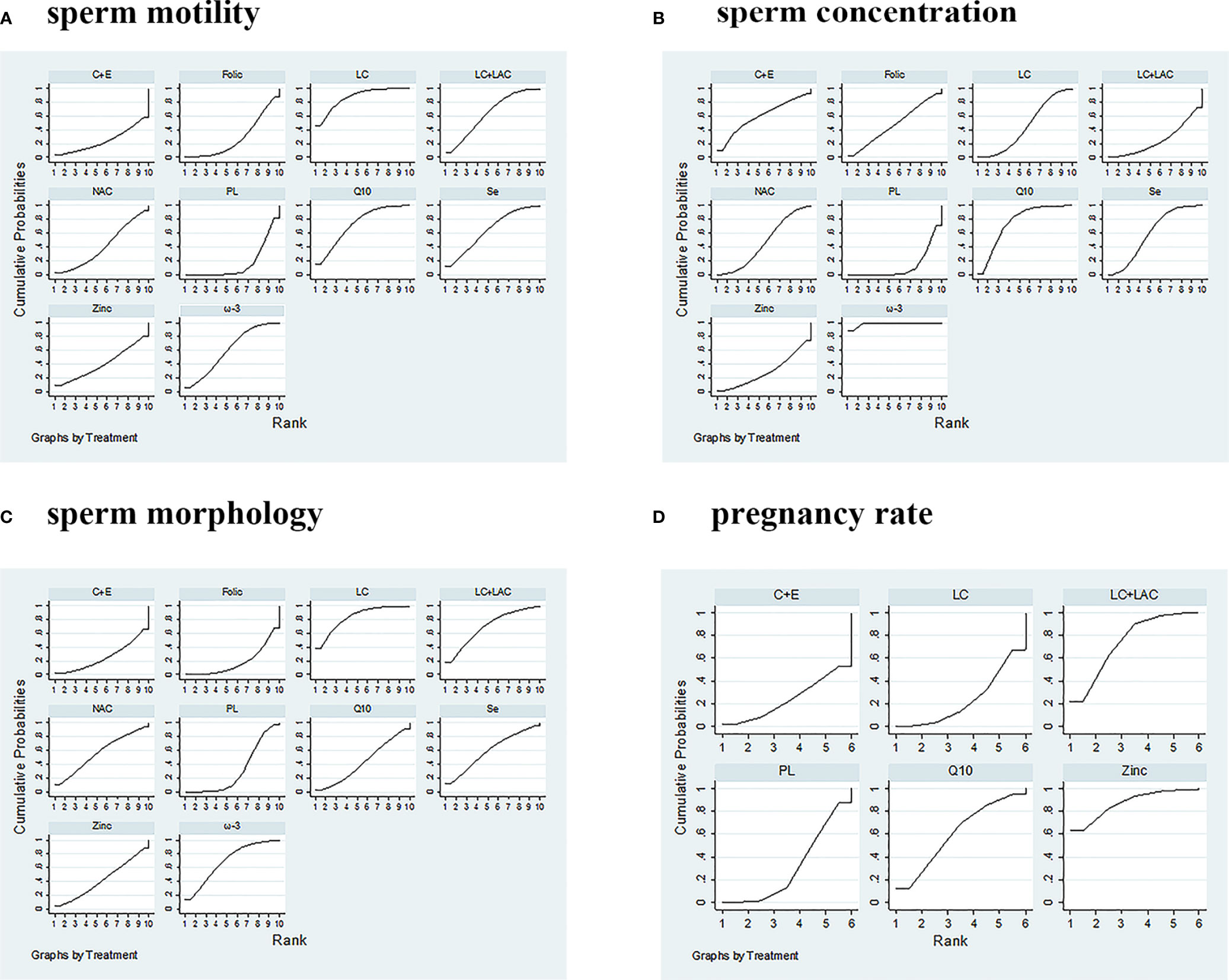
Figure 4 SUCRA ranking curves. (A) Sperm motility. (B) Sperm concentration. (C) Sperm morphology. (D) Pregnancy rate. PL, placebo; LC, L-carnitine; LAC, L-acetylcarnitine; Q10, coenzyme-Q10; C+E, vitamin C+E; ω-3, ω-3 fatty acid; Se, selenium; Folic, folic acid; NAC, N-acetyl-cysteine; SUCRA, surface under the cumulative ranking.
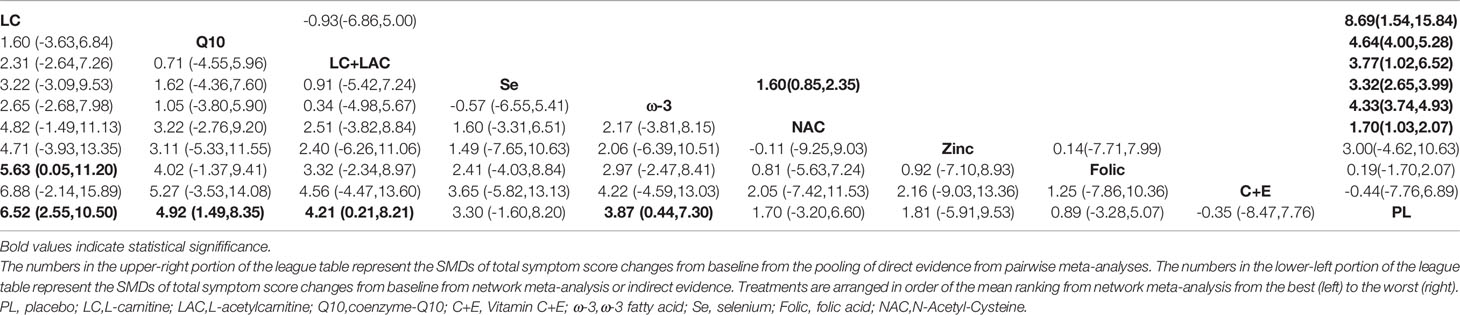
Table 4 League table of weighted mean difference (WMD) for idiopathic male infertility with sperm motility.
Sperm Concentration
A summary of 19 RCTs with 9 different treatments was assessed for the sperm concentration. Compared with placebo, ω-3 (WMD 9.89 × 106/ml [95% CI: 7.01 to 12.77 × 106/ml]), Q10 (WMD 5.44 × 106/ml [95% CI: 2.15 to 8.73 × 106/ml]), Se (WMD 3.96 × 106/ml [95% CI: 0.06 to 7.86 × 106/ml]), and LC (WMD 2.75 × 106/ml [95% CI: 0.05 to 5.44 × 106/ml]) had significantly increased sperm concentration (Table 5). In comparison, no treatment significantly outperformed the other antioxidants. Based on SUCRA, ω-3 had the highest probability to improve concentration, followed by CoQ10, Se, and LC. The network plot is shown in Figure 3. The details as regards ranking and SUCRA values are shown in Table 3 and Figure 4.
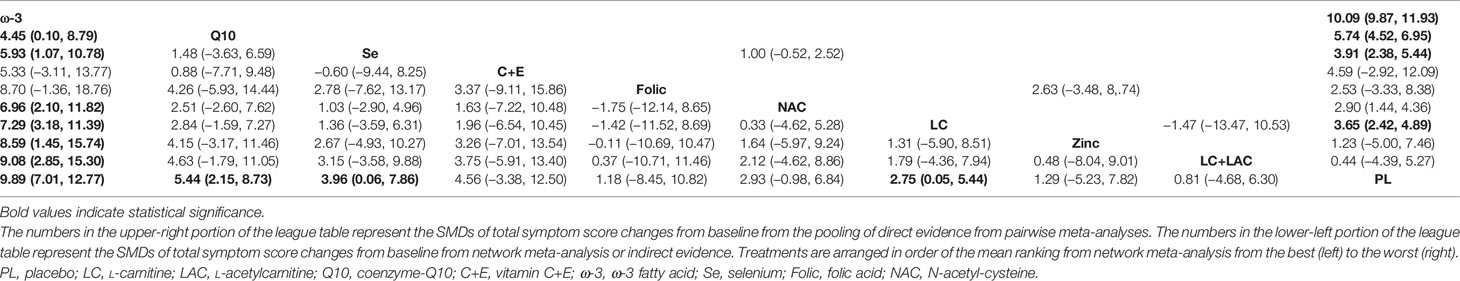
Table 5 League table of weighted mean difference (WMD) for idiopathic male infertility with sperm concentration.
Sperm Morphology
Sperm morphology data were involved in 14 RCTs. In comparison with placebo, only LC (WMD 3.86% [95% CI: 0.91% to 7.53%]) was shown to have treatment effects with statistical significance (Table 6). On the other hand, LC was the only treatment that showed significant superiority to another active treatment, folic (WMD 4.96% [95% CI: 0.20% to 9.73%]). Based on SUCRA, LC was ranked the highest over the other antioxidants. The network plot is shown in Figure 3. The ranking and SUCRA values are shown in Table 3 and Figure 4.
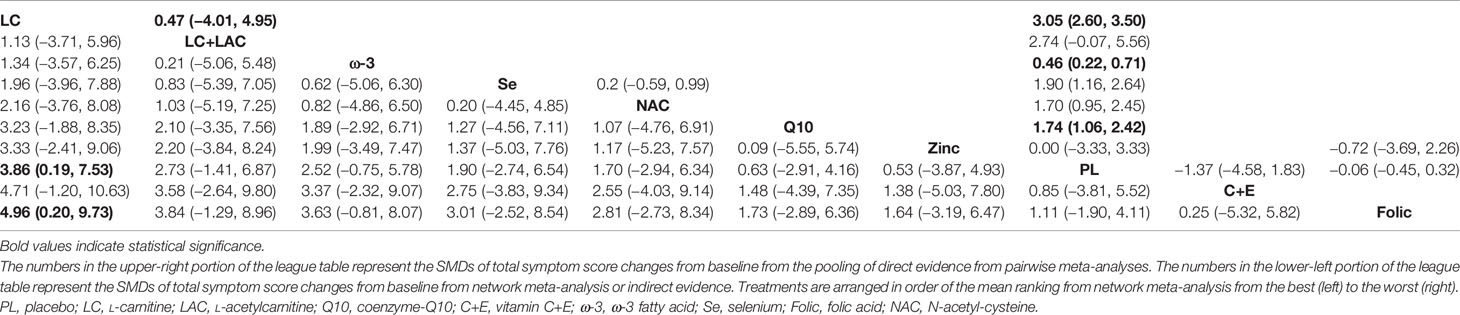
Table 6 League table of weighted mean difference (WMD) for idiopathic male infertility with sperm morphology.
Pregnancy Rate
Although we wanted to find out the effect of antioxidants on pregnancy rate after treatment, only 12 RCTs reported the pregnancy rate, which included 6 different treatments. Despite LC+LAC and CoQ10 having a high ranking in treatment effects as compared with other antioxidants, there is no statistical significance between all antioxidants and placebo (Table 7). Furthermore, none of these treatments had shown any statistical superiority to other antioxidants. The network plot is shown in Figure 3. The ranking based on SUCRA values in terms of pregnancy rate is shown in Table 3 and Figure 4.
The Heterogeneity and Inconsistency
From the results of the meta-analysis, there was some evidence of low-to-moderate statistical heterogeneity on included studies, especially in the outcomes of the sperm motility and sperm concentration (Table 2). There are no significant inconsistency factors found in the loops of treatment efficacy (p > 0.05). However, the inconsistency between direct and indirect evidence was identified from the global design-by-treatment interaction model in outcomes of sperm motility (p = 0.043). In the remaining results, we did not find the global inconsistency (p > 0.05) (Table S2).
Publication Bias and Sensitivity Analysis
There is no evidence of publication bias in comparison-adjusted funnel plots. The funnel plots are shown in Figure 5. In terms of sensitivity, excluding published before 2002 and smaller studies also showed no obvious change in the primary outcomes. So it showed the stability of the results. The results are shown in Table S3.
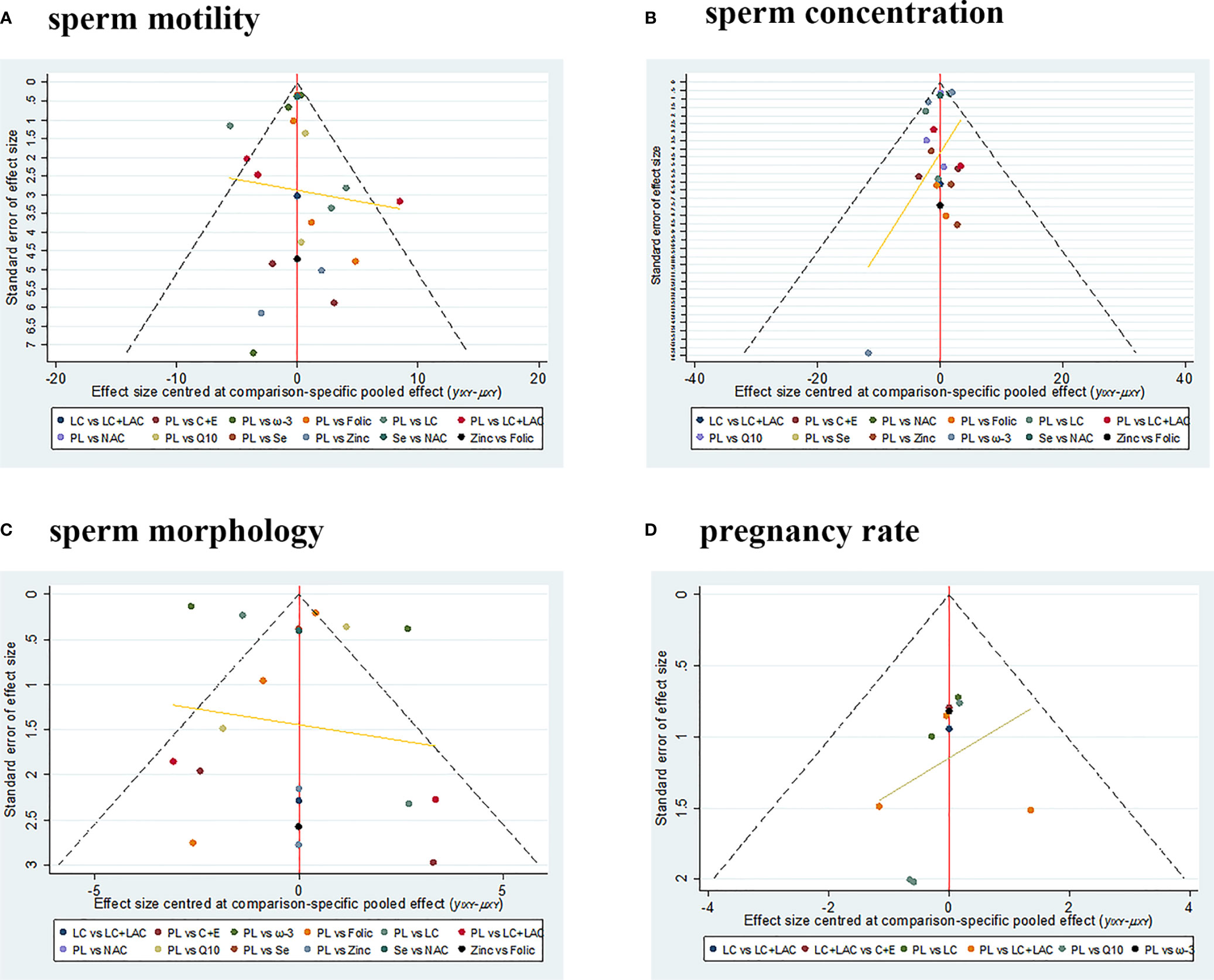
Figure 5 The funnel plots. (A) Sperm motility. (B) Sperm concentration. (C) Sperm morphology. (D) Pregnancy rate. PL, placebo; LC, L-carnitine; LAC, L-acetylcarnitine; Q10, coenzyme-Q10; C+E, vitamin C+E; ω-3, ω-3 fatty acid; Se, selenium; Folic, folic acid; NAC, N-acetyl-cysteine.
Discussion
Up until the present, this study is the first study to examine the comparative efficacy of various antioxidants in patients with idiopathic male infertility. Furthermore, the pregnancy rate of patients was also summarized. We found that various antioxidants have different effects on the aspects of sperm parameters.
Sperm Motility
LC, LC+LCA, CoQ10, and ω-3 showed beneficial effects on sperm motility as compared with placebo. Previous meta-analyses conducted by Buhling (52) and Zhang (53) also demonstrated that carnitine had a favorable effect on sperm motility as compared with placebo. Unlike previous studies’ previous pairwise meta-analyses, our NMA demonstrated that LC was the most effective treatment to increase sperm motility, followed by CoQ10. On the other hand, basic research has been reported that LC and LAC participate in the transportation of long-chain fatty acids to the mitochondrial matrix for β-oxidation, providing energy for β-oxidation of spermatozoa in the epididymis (54). So LC and LAC play a vital role in sperm motility, spermatogenic process, and maturation by enhancing energy metabolism (55). It seems to explain why LC has a better effect on sperm motility. However, some studies had an unclear randomization method and a low number of patients. So the results should be interpreted with caution.
Sperm Concentration
Compared with placebo, ω-3, CoQ10, Se, and LC had significantly increased sperm concentration. Our NAM demonstrated that ω-3 had the highest probability of being the most effective treatment to increase sperm concentration, followed by CoQ10. Recently, basic research demonstrated that ω-3 fatty acids have anti-inflammatory and antioxidant properties, potentially protecting the composition and function of cell membranes. Furthermore, the successful fertilization of spermatozoa is related to the lipid composition of the spermatozoa membrane (56, 57). Consistent with this finding, the previous meta-analysis also showed that ω-3 fatty acids had a positive effect on sperm concentration compared with placebo (58). However, there are few reports on the influence of ω-3 on pregnancy rate, and no major side effects were reported from the included studies. So more RCTs conducted in large samples of participants are needed to verify the therapeutic effects of ω-3 on pregnancy rate and side effects.
Sperm Morphology
The NMA demonstrated that CoQ10 and ω-3 were near the critical statistical significance as compared with placebo. But only LC was shown to have treatment effects with statistical significance. Contrastingly, our pairwise meta-analysis demonstrated that ω-3 and CoQ10 were shown to have treatment effects with statistical significance. The previous meta-analysis conducted by Salas (59) also demonstrated that ω-3 and CoQ10 had a favorable effect on sperm morphology as compared with placebo. The controversy of this result might be related to the lack of study and the differences in data after mixed comparison in the NMA. So we need more RCTs to verify the therapeutic effects on the morphology of ω-3 and CoQ10. However, there is no doubt that LC has the best therapeutic effect on sperm morphology.
Pregnancy Rate
The most objective outcome to show the effect of treatment on male fertility must be the pregnancy rate. However, only 12 RCTs reported the pregnancy rate. There is no statistical significance between treatment and placebo. Most studies have reported effects on sperm parameters but have not described the pregnancy rate in detail. On the other hand, the shorter follow-up time would result in lower positive rates. We must point out that “fertility” also depends on the fertility status of the female partner. It would also clearly influence the outcome of medical intervention in the male partner, although the previous meta-analysis showed that supplementation with antioxidants has a positive effect on pregnancy rate as compared with placebo (60). However, due to the lack of data in each study, the results are not applicable to idiopathic male infertility.
Although CoQ10 was not ranked first in four aspects of sperm parameters in this study, CoQ10 has an obvious therapeutic effect on sperm motility and sperm concentration. A previous meta-analysis study also concludes that CoQ10 has a profound effect on sperm motility and concentration (61). In the electron-transport system, CoQ10 is an antioxidant molecule that plays a vital role, and CoQ10 could inhibit the formation of organic peroxides in sperm to reduce sperm cell OS (62, 63). Based on our result, CoQ10 could be a good choice for idiopathic male infertility.
It is worth emphasizing that previous RCTs and meta-analyses did not analyze the optimal dosage and medication time. Until now, the exact dosages and regimens have not been clearly defined (64). Recently, a study has shown that excessive intake of antioxidants can change the oxidation–reduction equilibrium into reductive stress, and it has the same adverse effect on sperm quality as OS (65). In our study, the follow-up period was from 3 to 8 months, and the dosage of the same type of antioxidants was roughly the same. Compared with placebo, some antioxidants such as zinc, vitamin E+vitamin C, and folic were of no statistical significance. But we do not know whether the reductive stress was caused by a high amount/dose of antioxidants or lack of follow-up time. On the other hand, some studies have reported that hormones and SERMs increased sperm quality (66). We need more experimental studies on these interventions with significant effect sizes so that a better treatment could be planned to improve the outcome.
Limitations and Future Studies
Several limitations of our NMA should be stated. The internal effectiveness of NMA mainly relies on the realization of the required assumptions, like heterogeneity, inconsistency, and transitivity (67). First, although the results of inconsistency factors were not found in the loops of treatment efficacy, the global inconsistency was existing in the outcome of sperm motility. Second, we found that some treatments had high statistical heterogeneity based on I2. It could be caused by methodological heterogeneity (clinical measurement tool and point of evaluation) and clinical heterogeneity (age, duration of infertility, or dosage). So considering the heterogeneity between studies, we used the random-effects model to pool the pairwise estimates. Third, many treatment pairs include two studies or at the most a maximum of three studies, which were not sufficient to correctly assess the transitivity assumption. So we are looking forward to getting more clinical data in the future. Furthermore, the applicability of our findings might be limited, because the NMA was based on indirect comparisons but not direct comparisons. At last, we have included the main outcomes of sperm motility, sperm morphology, sperm concentration, and pregnancy rate. Because of the lack of data in each study, we could not include some outcomes like forwarding sperm motility and sperm volume. Therefore, we have to be cautious when evaluating the outcomes of different treatments.
We also hope that this article will give some enlightenment to the focus in our future research. First of all, some RCTs have gradually begun to use combinations of different types of antioxidants, for example, using the mixture of selenium+zinc+vitamin E or LC+CoQ10+vitamin C+zinc (68, 69). Those combinations were also more efficacious than placebo in sperm quality parameters. However, due to the lack of research literature and difficulty in distinguishing the effects of combinations of various antioxidants, those studies were not included. Therefore, in the future, we need more RCTs to verify the therapeutic effects of combining different types of antioxidants.
Conclusion
Our results suggest that compared with other antioxidants, L-carnitine had the highest probability of being the most effective treatment to increase sperm motility and morphology. ω-3 fatty acids have the most positive effect on sperm concentration. Coenzyme-Q10 is a better effective treatment for sperm motility and concentration. In terms of pregnancy rate, there is no statistical significance between treatment and placebo, and further evidence is awaited to verify the effect of antioxidants on the pregnancy rate.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
K-pL: protocol development, data collection and management, data analysis, and manuscript writing. X-sY: protocol development, data collection and management, data analysis, and manuscript writing. TW: protocol development, data management, data analysis, and manuscript writing.
Funding
This work was supported by the City of Nanchong Strategic Cooperation with Local Universities Foundation of technology (20SXQT0305); the Application and Basic Research Program of the Sichuan Science and Technology Department (2020YJ0185); and The Primary Health Development Research Center of Sichuan Province Program (SWFZ21-C-98).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Ms Miao He for providing continuous encouragement to K-pL to pursue his career in medicine.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.810242/full#supplementary-material
References
1. Hussein TM, Eldabah N, Zayed HA. Assessment of Serum Vitamin D Level and Seminal Vitamin D Receptor Gene Methylation in a Sample of Egyptian Men With Idiopathic Infertility. Andrologia (2021) 53:e14172. doi: 10.1111/and.14172
2. Kokubu D, Ooba R, Abe Y, Ishizaki H, Yoshida S, Asano A, et al. Angelica Keiskei (Ashitaba) Powder and its Functional Compound Xanthoangelol Prevent Heat Stress-Induced Impairment in Sperm Density and Quality in Mouse Testes. J Reprod Dev (2019) 65:139–46. doi: 10.1262/jrd.2018-141
3. Illiano E, Trama F, Zucchi A, Iannitti RG, Fioretti B, Costantini E. Resveratrol-Based Multivitamin Supplement Increases Sperm Concentration and Motility in Idiopathic Male Infertility: A Pilot Clinical Study. J Clin Med (2020) 9:4017. doi: 10.3390/jcm9124017
4. Agarwal A, Parekh N. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J Mens Health (2019) 37:296–312. doi: 10.5534/wjmh.190055
5. Ko EY, Siddiqi K, Brannigan RE, Sabanegh ES Jr. Empirical Medical Therapy for Idiopathic Male Infertility: A Survey of the American Urological Association. J Urol (2012) 187:973–8. doi: 10.1016/j.juro.2011.10.137
6. Russo A, Troncoso N, Sanchez F, Garbarino JA, Vanella A. Propolis Protects Human Spermatozoa From DNA Damage Caused by Benzo[a]Pyrene and Exogenous Reactive Oxygen Species. Life Sci (2006) 78:1401–6. doi: 10.1016/j.lfs.2004.10.085
7. Adeoye O, Olawumi J, Opeyemi A, Christiania O. Review on the Role of Glutathione on Oxidative Stress and Infertility. JBRA Assist Reprod (2018) 22:61–6. doi: 10.5935/1518-0557.20180003
8. Kumar N, Singh AK. Reactive Oxygen Species in Seminal Plasma as a Cause of Male Infertility. J Gynecol Obstet Hum Reprod (2018) 47:565–72. doi: 10.1016/j.jogoh.2018.06.008
9. Majzoub A, Agarwal A, Esteves SC. Antioxidants for Elevated Sperm DNA Fragmentation: A Mini Review. Transl Androl Urol (2017) 6:S649–s53. doi: 10.21037/tau.2017.07.09
10. Arafa M, Agarwal A. Efficacy of Antioxidant Supplementation on Conventional and Advanced Sperm Function Tests in Patients With Idiopathic Male Infertility. Antioxid (Basel) (2020) 9:219. doi: 10.3390/antiox9030219
11. Omar MI, Pal RP, Kelly BD, Bruins HM, Yuan Y, Diemer T, et al. Benefits of Empiric Nutritional and Medical Therapy for Semen Parameters and Pregnancy and Live Birth Rates in Couples With Idiopathic Infertility: A Systematic Review and Meta-Analysis. Eur Urol (2019) 75:615–25. doi: 10.1016/j.eururo.2018.12.022
12. Noh S, Go A, Kim DB, Park M, Jeon HW, Kim B. Role of Antioxidant Natural Products in Management of Infertility: A Review of Their Medicinal Potential. Antioxid (Basel) (2020) 9:957. doi: 10.3390/antiox9100957
13. Ali M, Martinez M, Parekh N. Are Antioxidants a Viable Treatment Option for Male Infertility? Andrologia (2020) 53:e13644. doi: 10.1111/and.13644
14. Song F, Loke YK, Walsh T, Glenny AM, Eastwood AJ, Altman DG. Methodological Problems in the Use of Indirect Comparisons for Evaluating Healthcare Interventions: Survey of Published Systematic Reviews. Bmj (2009) 338:b1147. doi: 10.1136/bmj.b1147
15. Song F, Xiong T, Parekh-Bhurke S, Loke YK, Sutton AJ, Eastwood AJ, et al. Inconsistency Between Direct and Indirect Comparisons of Competing Interventions: Meta-Epidemiological Study. Bmj (2011) 343:d4909. doi: 10.1136/bmj.d4909
16. Tonin FS, Rotta I. Network Meta-Analysis: A Technique to Gather Evidence From Direct and Indirect Comparisons. Pharm Pract (Granada) (2017) 15:943. doi: 10.18549/PharmPract.2017.01.943
17. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann Intern Med (2015) 162:777–84. doi: 10.7326/m14-2385
18. Rouse B, Chaimani A, Li T. Network Meta-Analysis: An Introduction for Clinicians. Intern Emerg Med (2017) 12:103–11. doi: 10.1007/s11739-016-1583-7
19. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. Bmj (2011) 343:d5928. doi: 10.1136/bmj.d5928
20. DerSimonian R, Laird N. Meta-Analysis in Clinical Trials Revisited. Contemp Clin Trials (2015) 45:139–45. doi: 10.1016/j.cct.2015.09.002
21. Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE Guidelines: 7. Rating the Quality of Evidence–Inconsistency. J Clin Epidemiol (2011) 64:1294–302. doi: 10.1016/j.jclinepi.2011.03.017
22. Lu G, Ades AE. Combination of Direct and Indirect Evidence in Mixed Treatment Comparisons. Stat Med (2004) 23:3105–24. doi: 10.1002/sim.1875
23. Mills EJ, Thorlund K, Ioannidis JP. Demystifying Trial Networks and Network Meta-Analysis. Bmj (2013) 346:f2914. doi: 10.1136/bmj.f2914
24. Chaimani A, Salanti G, Leucht S, Geddes JR, Cipriani A. Common Pitfalls and Mistakes in the Set-Up, Analysis and Interpretation of Results in Network Meta-Analysis: What Clinicians Should Look for in a Published Article. Evid Based Ment Health (2017) 20:88–94. doi: 10.1136/eb-2017-102753
25. Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G. Evaluation of Inconsistency in Networks of Interventions. Int J Epidemiol (2013) 42:332–45. doi: 10.1093/ije/dys222
26. Salanti G, Ades AE, Ioannidis JP. Graphical Methods and Numerical Summaries for Presenting Results From Multiple-Treatment Meta-Analysis: An Overview and Tutorial. J Clin Epidemiol (2011) 64:163–71. doi: 10.1016/j.jclinepi.2010.03.016
27. Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical Tools for Network Meta-Analysis in STATA. PloS One (2013) 8:e76654. doi: 10.1371/journal.pone.0076654
28. Egger M, Davey Smith G, Schneider M, Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. Bmj (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
29. Haje M, Naoom K. Combined Tamoxifen and L-Carnitine Therapies for the Treatment of Idiopathic Male Infertility Attending Intracytoplasmic Sperm Injection: A Randomized Controlled Trial. Int J Infertil Fetal Med (2015) 6:20–4. doi: 10.5005/jp-journals-10016-1096
30. Balercia G, Regoli F, Armeni T, Koverech A, Mantero F, Boscaro M. Placebo-Controlled Double-Blind Randomized Trial on the Use of L-Carnitine, L-Acetylcarnitine, or Combined L-Carnitine and L-Acetylcarnitine in Men With Idiopathic Asthenozoospermia. Fertil Steril (2005) 84:662–71. doi: 10.1016/j.fertnstert.2005.03.064
31. Dimitriadis F, Tsambalas S, Tsounapi P, Kawamura H, Vlachopoulou E, Haliasos N, et al. Effects of Phosphodiesterase-5 Inhibitors on Leydig Cell Secretory Function in Oligoasthenospermic Infertile Men: A Randomized Trial. BJU Int (2010) 106:1181–5. doi: 10.1111/j.1464-410X.2010.09243.x
32. Lenzi A, Sgrò P, Salacone P, Paoli D, Gilio B, Lombardo F, et al. A Placebo-Controlled Double-Blind Randomized Trial of the Use of Combined L-Carnitine and L-Acetyl-Carnitine Treatment in Men With Asthenozoospermia. Fertil Steril (2004) 81:1578–84. doi: 10.1016/j.fertnstert.2003.10.034
33. Micic S, Lalic N, Djordjevic D, Bojanic N, Bogavac-Stanojevic N, Busetto GM. Double-Blind, Randomised, Placebo-Controlled Trial on the Effect of L-Carnitine and L-Acetylcarnitine on Sperm Parameters in Men With Idiopathic Oligoasthenozoospermia. Andrologia (2019) 51:e13267. doi: 10.1111/and.13267
34. Sigman M, Glass S, Campagnone J, Pryor JL. Carnitine for the Treatment of Idiopathic Asthenospermia: A Randomized, Double-Blind, Placebo-Controlled Trial. Fertil Steril (2006) 85:1409–14. doi: 10.1016/j.fertnstert.2005.10.055
35. Balercia G, Buldreghini E, Vignini A, Tiano L, Paggi F, Amoroso S, et al. Coenzyme Q10 Treatment in Infertile Men With Idiopathic Asthenozoospermia: A Placebo-Controlled, Double-Blind Randomized Trial. Fertil Steril (2009) 91:1785–92. doi: 10.1016/j.fertnstert.2008.02.119
36. Safarinejad MR. Efficacy of Coenzyme Q10 on Semen Parameters, Sperm Function and Reproductive Hormones in Infertile Men. J Urol (2009) 182:237–48. doi: 10.1016/j.juro.2009.02.121
37. Nadjarzadeh A, Sadeghi MR, Amirjannati N, Vafa MR, Motevalian SA, Gohari MR, et al. Coenzyme Q10 Improves Seminal Oxidative Defense But Does Not Affect on Semen Parameters in Idiopathic Oligoasthenoteratozoospermia: A Randomized Double-Blind, Placebo Controlled Trial. J Endocrinol Invest (2011) 34:e224–8. doi: 10.3275/7572
38. Rolf C, Cooper TG, Yeung CH, Nieschlag E. Antioxidant Treatment of Patients With Asthenozoospermia or Moderate Oligoasthenozoospermia With High-Dose Vitamin C and Vitamin E: A Randomized, Placebo-Controlled, Double-Blind Study. Hum Reprod (1999) 14:1028–33. doi: 10.1093/humrep/14.4.1028
39. Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, Tesarik J. Reduction of the Incidence of Sperm DNA Fragmentation by Oral Antioxidant Treatment. J Androl (2005) 26:349–53. doi: 10.2164/jandrol.04146
40. Li Z GR, Liu Y, Xiang Z, Cao X, Han Y. Curative Elect of L- Carnitine Supplementation in the Treatment of Male Infertility. Acad J Shanghai Second Med Univ (2005) 25:292–4.
41. Conquer JA, Martin JB, Tummon I, Watson L, Tekpetey F. Effect of DHA Supplementation on DHA Status and Sperm Motility in Asthenozoospermic Males. Lipids (2000) 35:149–54. doi: 10.1007/bf02664764
42. Safarinejad MR. Effect of Omega-3 Polyunsaturated Fatty Acid Supplementation on Semen Profile and Enzymatic Anti-Oxidant Capacity of Seminal Plasma in Infertile Men With Idiopathic Oligoasthenoteratospermia: A Double-Blind, Placebo-Controlled, Randomised Study. Andrologia (2011) 43:38–47. doi: 10.1111/j.1439-0272.2009.01013.x
43. Martínez-Soto JC, Domingo JC, Cordobilla B, Nicolás M, Fernández L, Albero P, et al. Dietary Supplementation With Docosahexaenoic Acid (DHA) Improves Seminal Antioxidant Status and Decreases Sperm DNA Fragmentation. Syst Biol Reprod Med (2016) 62:387–95. doi: 10.1080/19396368.2016.1246623
44. Scott R, MacPherson A, Yates RW, Hussain B, Dixon J. The Effect of Oral Selenium Supplementation on Human Sperm Motility. Br J Urol (1998) 82:76–80. doi: 10.1046/j.1464-410x.1998.00683.x
45. Safarinejad MR, Safarinejad S. Efficacy of Selenium and/or N-Acetyl-Cysteine for Improving Semen Parameters in Infertile Men: A Double-Blind, Placebo Controlled, Randomized Study. J Urol (2009) 181:741–51. doi: 10.1016/j.juro.2008.10.015
46. Wong WY, Merkus HM, Thomas CM, Menkveld R, Zielhuis GA, Steegers-Theunissen RP. Effects of Folic Acid and Zinc Sulfate on Male Factor Subfertility: A Double-Blind, Randomized, Placebo-Controlled Trial. Fertil Steril (2002) 77:491–8. doi: 10.1016/s0015-0282(01)03229-0
47. Raigani M, Yaghmaei B, Amirjannti N, Lakpour N, Akhondi MM, Zeraati H, et al. The Micronutrient Supplements, Zinc Sulphate and Folic Acid, did Not Ameliorate Sperm Functional Parameters in Oligoasthenoteratozoospermic Men. Andrologia (2014) 46:956–62. doi: 10.1111/and.12180
48. Omu AE, Dashti H, Al-Othman S. Treatment of Asthenozoospermia With Zinc Sulphate: Andrological, Immunological and Obstetric Outcome. Eur J Obstet Gynecol Reprod Biol (1998) 79:179–84. doi: 10.1016/s0301-2115(97)00262-5
49. Omu AE, Al-Azemi MK, Kehinde EO, Anim JT, Oriowo MA, Mathew TC. Indications of the Mechanisms Involved in Improved Sperm Parameters by Zinc Therapy. Med Princ Pract (2008) 17:108–16. doi: 10.1159/000112963
50. Boonyarangkul A, Vinayanuvattikhun N, Chiamchanya C, Visutakul P. Comparative Study of the Effects of Tamoxifen Citrate and Folate on Semen Quality of the Infertile Male With Semen Abnormality. J Med Assoc Thai (2015) 98:1057–63.
51. Silva T, Maia M, Arruda J, Approbato F, Mendonça C, Approbato M. Folic Acid Does Not Improve Semen Parametrs in Subfertile Men: A Double-Blin, Randomized, Placebo-Controlled Study. J Bras Reprod Assist (2013) 17:152–7. doi: 10.5935/1518-0557.20130052
52. Buhling K, Schumacher A, Eulenburg CZ, Laakmann E. Influence of Oral Vitamin and Mineral Supplementation on Male Infertility: A Meta-Analysis and Systematic Review. Reprod BioMed Online (2019) 39:269–79. doi: 10.1016/j.rbmo.2019.03.099
53. Zhang X, Cui Y. The Efficacy of Combined L-Carnitine and L-Acetyl Carnitine in Men With Idiopathic Oligoasthenoteratozoospermia: A Systematic Review and Meta-Analysis. Andrologia (2020) 52:e13470. doi: 10.1111/and.13470
54. Enomoto A, Wempe MF, Tsuchida H, Shin HJ, Cha SH, Anzai N, et al. Molecular Identification of a Novel Carnitine Transporter Specific to Human Testis. Insights Into the Mechanism of Carnitine Recognition. J Biol Chem (2002) 277:36262–71. doi: 10.1074/jbc.M203883200
55. Agarwal A, Said TM. Carnitines and Male Infertility. Reprod BioMed Online (2004) 8:376–84. doi: 10.1016/s1472-6483(10)60920-0
56. Aksoy Y, Aksoy H, Altinkaynak K, Aydin HR, Ozkan A. Sperm Fatty Acid Composition in Subfertile Men. Prostaglandins Leukot Essent Fatty Acids (2006) 75:75–9. doi: 10.1016/j.plefa.2006.06.002
57. Safarinejad MR, Safarinejad S. The Roles of Omega-3 and Omega-6 Fatty Acids in Idiopathic Male Infertility. Asian J Androl (2012) 14:514–5. doi: 10.1038/aja.2012.46
58. Hosseini B, Nourmohamadi M, Hajipour S, Taghizadeh M, Asemi Z, Keshavarz SA, et al. The Effect of Omega-3 Fatty Acids, EPA, and/or DHA on Male Infertility: A Systematic Review and Meta-Analysis. J Diet Suppl (2019) 16:245–56. doi: 10.1080/19390211.2018.1431753
59. Salas-Huetos A, Rosique-Esteban N, Becerra-Tomás N, Vizmanos B, Bulló M, Salas-Salvadó J. The Effect of Nutrients and Dietary Supplements on Sperm Quality Parameters: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Adv Nutr (2018) 9:833–48. doi: 10.1093/advances/nmy057
60. Smits RM, Mackenzie-Proctor R, Yazdani A, Stankiewicz MT, Jordan V, Showell MG. Antioxidants for Male Subfertility. Cochrane Database Syst Rev (2019) 3:Cd007411. doi: 10.1002/14651858.CD007411.pub4
61. Vishvkarma R, Alahmar AT. Coenzyme Q10 Effect on Semen Parameters: Profound or Meagre? Andrologia (2020) 52:e1357. doi: 10.1111/and.13570
62. Gvozdjáková A, Kucharská J, Ostatníková D, Babinská K, Nakládal D, Crane FL. Ubiquinol Improves Symptoms in Children With Autism. Oxid Med Cell Longev (2014) 2014:798957. doi: 10.1155/2014/798957
63. Hernández-Camacho JD, Bernier M, López-Lluch G, Navas P. Coenzyme Q(10) Supplementation in Aging and Disease. Front Physiol (2018) 9:44. doi: 10.3389/fphys.2018.00044
64. Agarwal A, Nallella KP, Allamaneni SS, Said TM. Role of Antioxidants in Treatment of Male Infertility: An Overview of the Literature. Reprod BioMed Online (2004) 8:616–27. doi: 10.1016/s1472-6483(10)61641-0
65. Henkel R, Sandhu IS, Agarwal A. The Excessive Use of Antioxidant Therapy: A Possible Cause of Male Infertility? Andrologia (2019) 51:e13162. doi: 10.1111/and.13162
66. Nieschlag E, Bouloux PG, Stegmann BJ, Shankar RR, Guan Y, Tzontcheva A, et al. An Open-Label Clinical Trial to Investigate the Efficacy and Safety of Corifollitropin Alfa Combined With hCG in Adult Men With Hypogonadotropic Hypogonadism. Reprod Biol Endocrinol (2017) 15:17. doi: 10.1186/s12958-017-0232-y
67. Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the Quality of Evidence From a Network Meta-Analysis. PloS One (2014) 9:e99682. doi: 10.1371/journal.pone.0099682
68. Sivkov AV, Oshchepkov VN, Evdokimov VV, Keshishev NG, Skabko OV. [Selzink Plus Study in Patients With Chronic non-Infectious Prostatitis and Abnormal Fertility]. Urologiia (2011) 27-8:30–3.
Keywords: antioxidants, idiopathic male infertility, sperm quality parameters, pregnancy rate, network meta-analysis, randomized controlled trials
Citation: Li K-p, Yang X-s and Wu T (2022) The Effect of Antioxidants on Sperm Quality Parameters and Pregnancy Rates for Idiopathic Male Infertility: A Network Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 13:810242. doi: 10.3389/fendo.2022.810242
Received: 06 November 2021; Accepted: 24 January 2022;
Published: 21 February 2022.
Edited by:
Davor Jezek, University of Zagreb, CroatiaReviewed by:
Gian Maria Busetto, University of Foggia, ItalyMojtaba Ziaee, Maragheh University of Medical Sciences, Iran
Copyright © 2022 Li, Yang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Wu, YWxoYXdraW5nQDE2My5jb20=
†These authors have contributed equally to this work
 Kun-peng Li†
Kun-peng Li† Xue-song Yang
Xue-song Yang Tao Wu
Tao Wu