- 1College of Pharmacy, Dali University, Dali, China
- 2Department of Pharmacy, Kunming Fourth People’s Hospital, Kunming, China
Objective: Clinical trials have recently shown a connection between nonalcoholic fatty liver disease (NAFLD) and empagliflozin. This paper aimed at comprehensively assessing the effectiveness and security of empagliflozin in NAFLD patients.
Methods: PubMed, Embase, Web of Science, Cochrane Library, CNKI, CBM, Wan-Fang digital database, VIP, and WHO ICTRP were searched for randomized controlled trials (RCTs) on the role of empagliflozin in NAFLD from inception to November 2, 2021. For continuous dating, we used values of mean differences (MD) to present.
Results: A total of four articles involving 244 NAFLD patients were included. Compared with the control group, empagliflozin could significantly reduce the body mass index (BMI) (MD: −0.98 [95% CI: −1.87, −0.10], p = 0.03), liver stiffness measurement (LSM) (MD: 0.49 [95% CI: −0.93, −0.06], p = 0.03), aspartate aminotransferase (AST) (MD: −3.10 [95% CI: −6.18, −0.02], p = 0.05), homeostasis model assessment of insulin resistance (HOMA-IR) (MD: −0.45 [95% CI: −0.90, 0.00], p = 0.05) of the treatment group.
Conclusions: Empagliflozin can improve body composition, insulin resistance, and liver fibrosis and decrease the hepatic enzymes in patients with NAFLD. Empagliflozin emerges as a new option for treating patients with NAFLD. However, further research shall determine the efficacy and safety of empagliflozin in NAFLD.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is an often neglected etiology of chronic liver disease that is prevalent globally, resulting from the liver fatty acid accumulation and fibrosis without overmuch alcohol consumption (1). Nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH) are the two major subtypes of NAFLD. The patients diagnosed with NASH can subsequently progress to liver fibrosis, which increases the risks of cirrhosis and liver cancer (2, 3). NAFLD is also a dangerous factor that obviously grows the risk of cardiovascular and chronic kidney disease (4, 5). The prevalence of NAFLD is approximately 25% worldwide; furthermore, it is estimated that NAFLD shall be the most common index for liver transplantation by 2030 (6, 7).
The pathogenesis of NAFLD is complex, and several factors have been involved, including Western diet, lifestyle, and genetic susceptibility. NAFLD is most closely related to the metabolic environment, such as insulin resistance, abnormal lipid profile, and metabolic dysfunction, which is also why it has an appropriate new nomenclature as MAFLD (8, 9). Additionally, increased risk of more severe NAFLD is strongly associated with type 2 diabetes (T2DM) and diabetes-related complications (10). Currently, no available drug has been approved for the management of NAFLD (11). Although the insulin sensitizer pioglitazone has been proven to enhance metabolism and liver histology among NAFLD patients, it has limitations in clinical use due to significant adverse events including weight gain, lower extremity edema, and heart failure (12, 13).
Empagliflozin, a sodium-glucose cotransporter 2 (SGLT-2) inhibitor, is a novel oral hypoglycemic agent that inhibits glucose reabsorption, improving insulin resistance, leading to downregulation of SREBP-1c and blockage of new hepatic lipogenesis, thereby improving lipid metabolisms (14, 15). Currently, it has been shown that controlled clinical trials (RCTs) have evaluated the role of empagliflozin in NAFLD patients. However, the role of empagliflozin in NAFLD patients is controversial, and there is no comprehensive analysis on RCTs of empagliflozin. Hence, this research aiming to investigate the effectiveness and security of empagliflozin in NAFLD was made.
Methods
Data Sources and Search Strategy
This study was designed based on Preferred Reporting Items for conducting Systematic Reviews and Meta-Analyses (PRISMA) (16). The agreement of this research was registered with PROSPERO.
We conducted an electronic search in the databases below: Pubmed, Embase, Web of Science, Cochrane Library, China National Knowledge Internet (CNKI), Chinese Biomedical Literature Database (CBM), Wan-Fang digital database, China Science and Technology Journal Database (VIP), and WHO International Clinical Trial Registration Platform (ICTRP). The subject terms were as below: “NAFLD,” “empagliflozin”, and “randomized”. There are no language restrictions, and the last search was made on November 2, 2021. The detailed description of search measure is provided in Supplementary Material 1.
Selection and Eligibility Criteria
The search results were screened by two reviewers for title, abstract, and full text, and the differences were resolved by a third reviewer. Inclusion criteria include RCTs on the effectiveness of empagliflozin in the treatment of NAFLD patients. Only original articles were included. Exclusion criteria were as follows: studies with nonhuman subjects, non-RCTs, systematic reviews, or meta-analyses.
Data Extraction and Outcomes
Reviewers independently extracted relevant data from each study into a predesigned Excel spreadsheets, which included country of origin, year of publication, first author, trial design, inclusion criteria, study duration, study population, intervention and duration, participant gender and age, baseline patient information, and treatment outcomes. The outcomes included are biological indicators of liver function such as: aspartate transaminase (AST), alanine transaminase (ALT), low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides (TG). Body composition includes the following: body weight and body mass index (BMI). Glycemic indices are as follows: fasting blood sugar (FBS), insulin, glycosylated hemoglobin A1c (HbA1C), and homeostatic model assessment of insulin resistance (HOMA-IR). Hepatic steatosis and fibrosis indicators are controlled attenuation parameter (CAP), liver stiffness measurement (LSM), NAFLD fibrosis score (NFS), and fibrosis-4 Index (FIB-4 index). For continuous dating, we extracted the means and standard deviation (SD). In the absence of the mean and SD, the data were transformed based on the existing formulae. Differences were resolved independently by a third reviewer.
Statistical Analysis and Quality Assessment
For results, this meta-analysis selected mean difference (MD) to assess continuous variables and presented with a 95% CI. The degree of heterogeneity was quantified with the I2 statistic. I2 values of 25%, 50%, and 75% represented low, medium, and high heterogeneity, respectively. Random effects models were used to pool measures in all studies. p-values equal or less than 0.05 set as the cutoff for statistical significance. The quality of the RCT was evaluated using the Cochrane Risk of Bias Assessment Tool, including the following six criteria: random sequence generation, allocation concealment, blinding of patients, trialists, blinding of result assessors, incomplete result information, selective reporting, and other biases. Each item was assessed for risk of bias as “low risk”, “high risk”, or “unclear risk” according to the recommendations of the Cochrane Handbook. STATA 16 and RevMan 5.4 were used for statistical analysis and quality assessment.
Results
Search Results
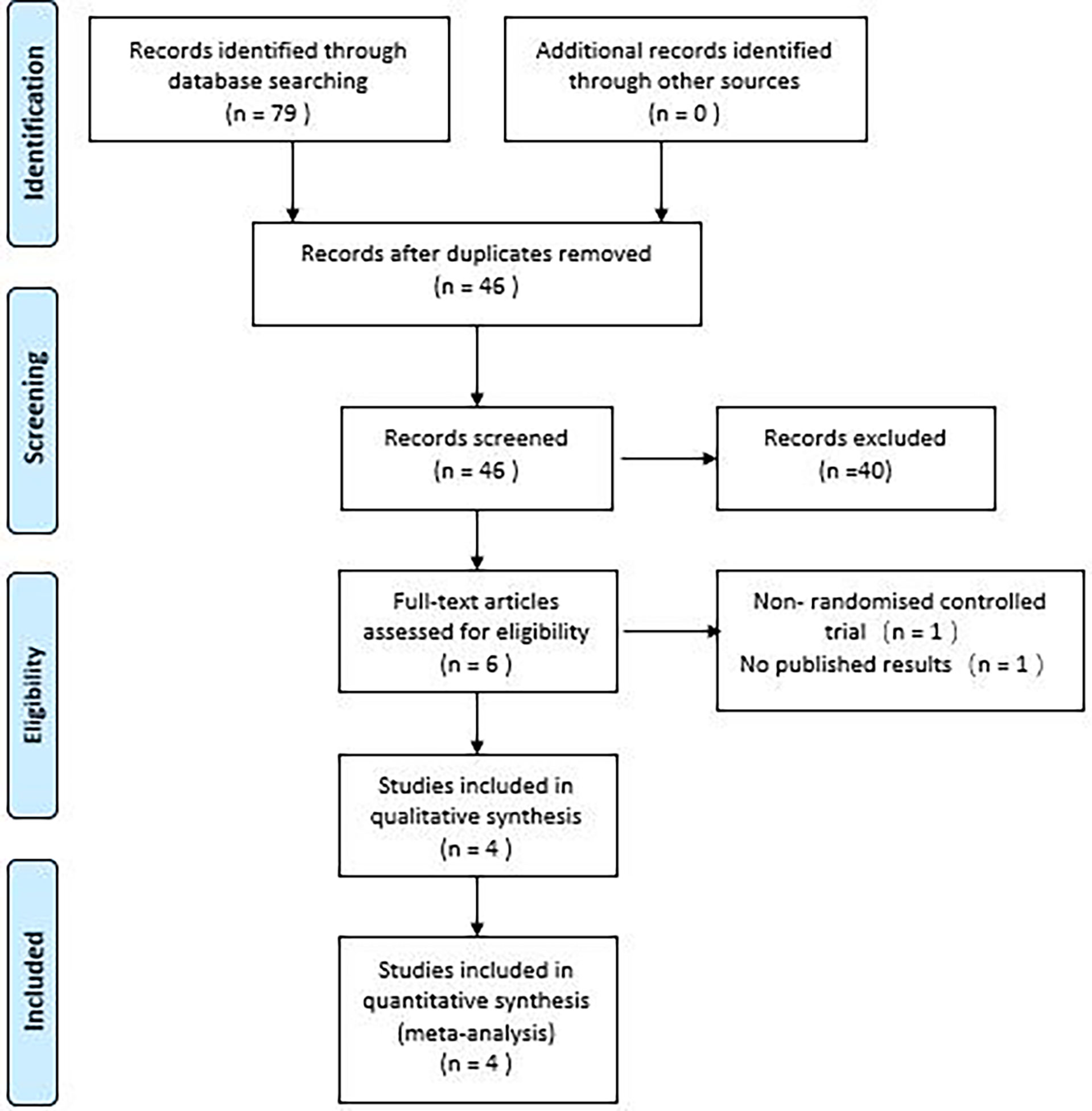
The study initially identified 79 articles, which included 7 studies from PubMed, 18 from the Cochrane Library, 28 from Embase, 20 from the Web of Science, and 2 each from SINOMED, CNKI and Wanfang digital database, respectively. After excluding 33 duplicates, reviewing 46 titles and abstracts, 40 outcomes were excluded, and the remaining 6 outcomes were reviewed in full, resulting in 4 randomized controlled studies of empagliflozin for NAFLD included in the meta-analysis (17–20). The PRISMA flow chart (Figure 1) illustrates the detailed selection process.
Study Characteristics and Quality Assessment
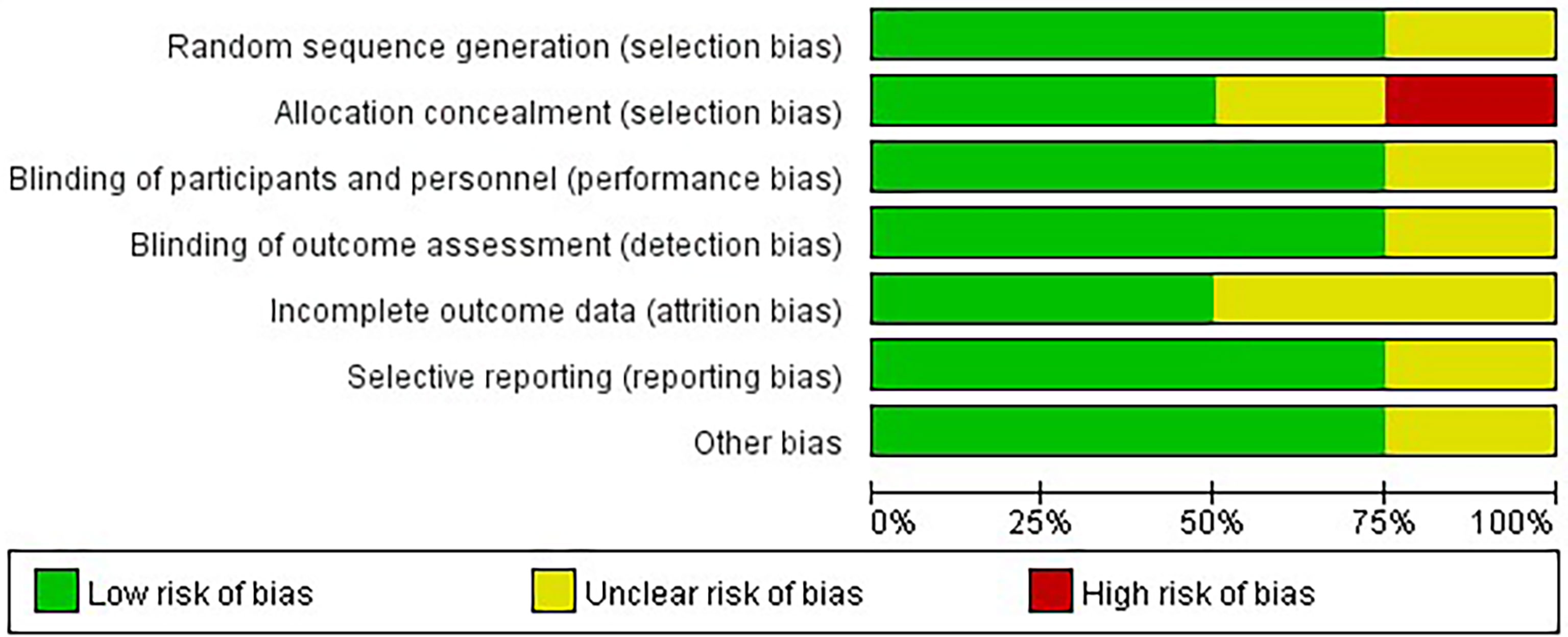
Four studies were conducted in 2018–2021. All studies were RCTs with a total of 244 more participants (in the treatment group, 120 participants used empagliflozin, while in the control group, 107 participants used a placebo in the control group and 20 participants used glimepiride), all patients were diagnosed with NAFLD (three of the study patients had comorbid diabetes and one was nondiabetic), two of the included studies were conducted in Iran, whereas the remaining two were conducted in India, China, and the duration of interventions varied from 20 to 24 weeks (The study’s attributes are detailed in Supplementary Excel). Four RCTs were parallel-group studies and articles of generally moderate and high quality based on the Cochrane Risk of Bias tool. The quality evaluation results of the four RCTs are delineated in Figure 2.
The Effect of Empagliflozin on Liver Biological Indicators
Meta-analysis concluded that empagliflozin was able to decrease AST levels among patients suffering from NAFLD compared with controls; the statistical difference was significant (MD: −3.10, 95% CI: −6.18 to −0.02, p = 0.05, I2 = 0%; Figure 3A). Empagliflozin was likely to reduce the levels of TG (MD: −8.87, 95% CI: −18.21 to 0.46, p = 0.06, I2 = 0%; Figure 3B) and ALT (MD: −3.30, 95% CI: −8.85 to 2.26, p = 0.24, I2 = 0%; Figure 3C), but the diversity was not of statistical significance. Compared with controls, there were no great diversity in HDL (MD: 0.69, 95% CI: −3.00 to 4.38, p = 0.71, I2 = 0%; Figure 3D) and LDL levels (MD: −3.24, 95% CI: −11.77 to 5.29, p = 0.46, I2 = 0%; Figure 3E).
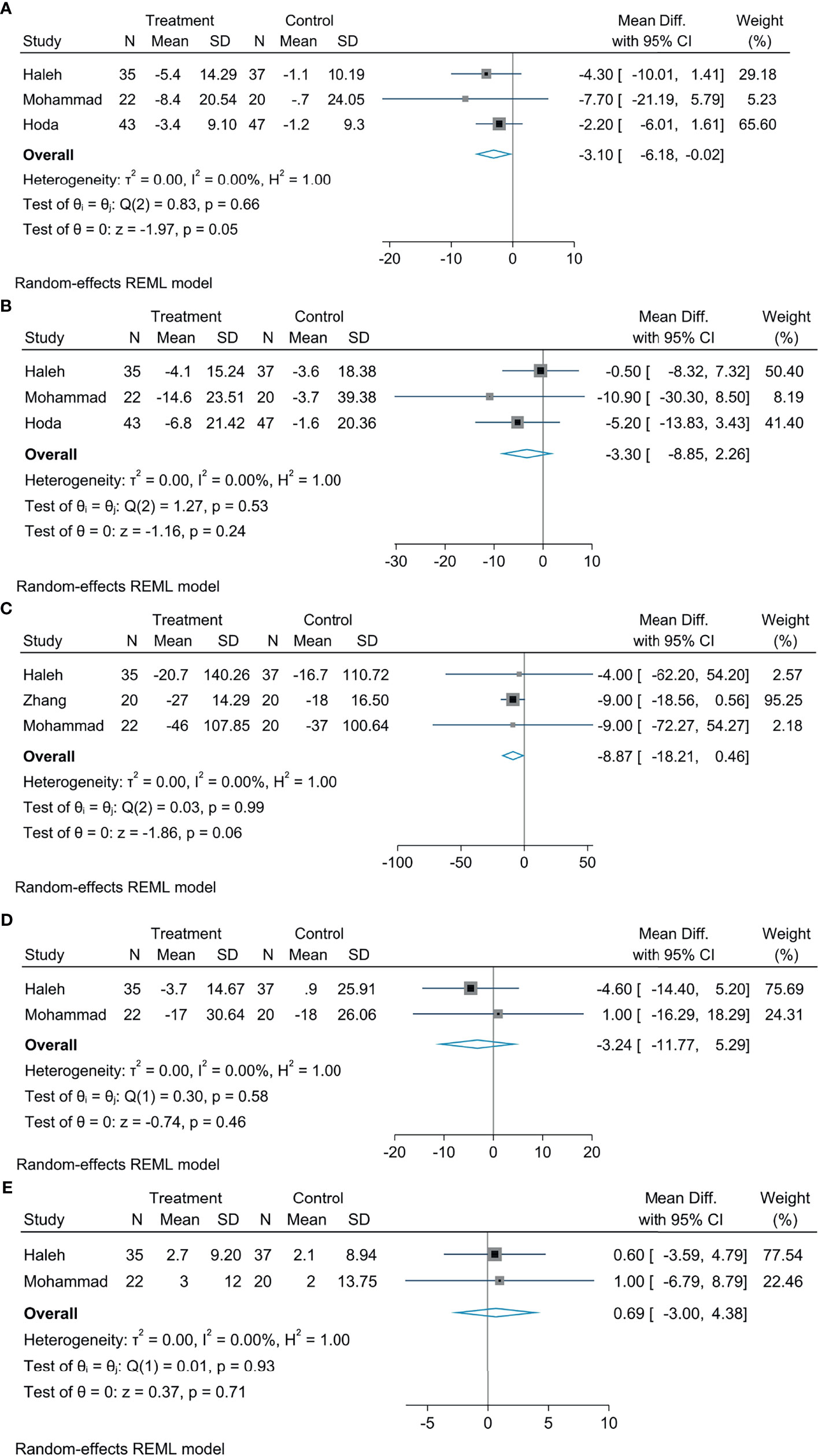
Figure 3 The effect of empagliflozin on AST (A); the effect of empagliflozin on ALT (B) the effect of empagliflozin on TG (C); the effect of empagliflozin on LDL (D); the effect of empagliflozin on HDL (E).
The Effect of Empagliflozin on Glycemic Indices
In comparison with the control group, empagliflozin treatment can attenuate HOMA-IR levels, the statistical difference was significant (MD: −0.45, 95% CI: −0.90 to 0.00, p = 0.05, I2 = 0%; Figure 4A), but there were no significant diversity in HbA1c (MD: −0.10, 95% CI: −0.60 to −0.40, p = 0.70, I2 = 42.79%; Figure 4B), FBS (MD: −1.21, 95% CI: −4.98 to 2.55, p = 0.53, I2 = 0%; Figure 4C), insulin (MD: 3.63, 95% CI: −7.64 to 14.90, p = 0.53, I2 = 90%; Figure 4D), and HOMA2-IR (MD: −0.12, 95% CI: −0.45 to 0.20, p = 0.46, I2 = 0%; Figure 4E).
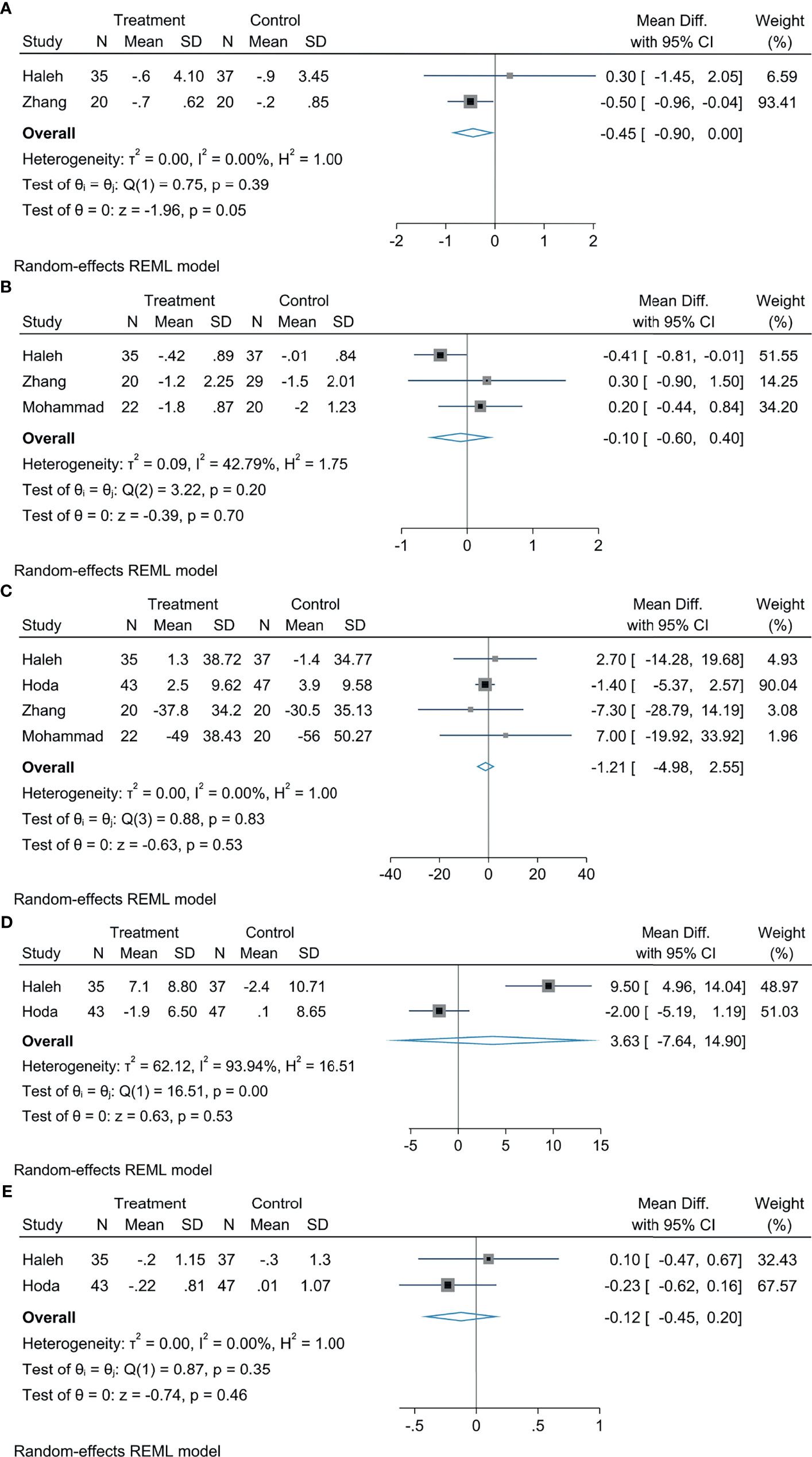
Figure 4 The effect of empagliflozin on HOMA-IR (A); the effect of empagliflozin on HbA1c (B); the effect of empagliflozin on FBS (C); the effect of empagliflozin on insulin (D); the effect of empagliflozin on HOMA2-IR (E).
The Effect of Empagliflozin on Body Composition
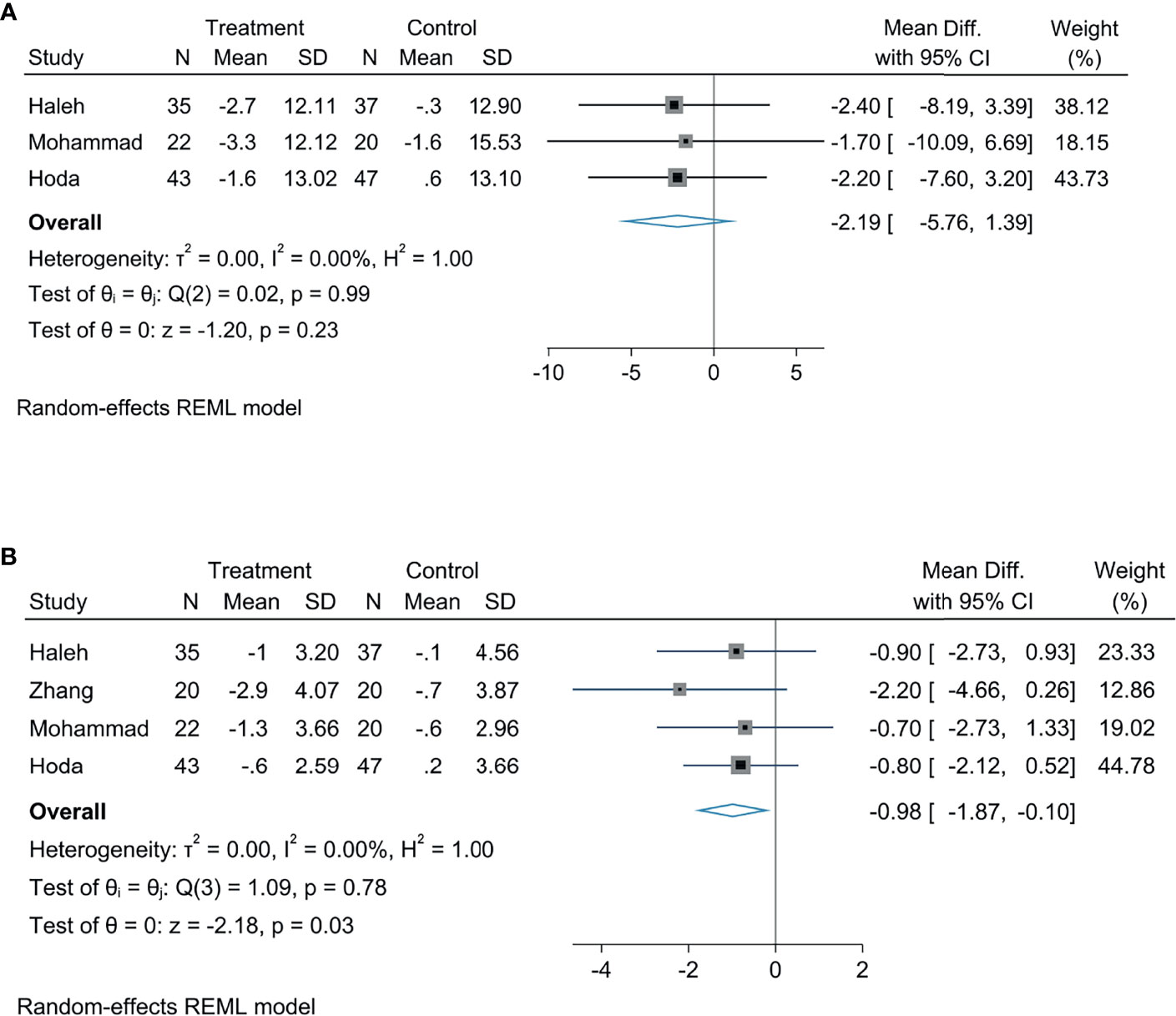
Our analysis demonstrated that empagliflozin obviously reduces the BMI in patients with NAFLD by comparing with controls, which had statistical differences (MD: −0.98, 95% CI: −1.87 to −0.10, p = 0.03, I2 = 0%; Figure 5A), empagliflozin was likely to reduce weight in patients with NAFLD by comparing with controls, but the statistical diversity was not great (MD: −2.19, 95% CI: −5.76 to 1.39, p = 0.23, I2 = 0%; Figure 5B).
The Effect of Empagliflozin on Hepatic Steatosis and Fibrosis
Our results indicated that treatment with empagliflozin provoked a decreased LSM in patients with NAFLD by comparing with controls, with a statistically significant diversity (MD: −0.49, 95% CI: −0.93 to −0.06, p = 0.03, I2 = 0%; Figure 6A), and this analysis indicated that empagliflozin could reduce the CAP in patients with NAFLD, but there was no statistical significance (MD: −8.49, 95% CI: −18.21 to 1.23, p = 0.09, I2 = 0%; Figure 6B). According to the meta-analysis, empagliflozin did not greatly reduce the FIB-4 index (MD: −0.03, 95% CI: −0.15 to 0.09, p = 0.64, I2 = 0%; Figure 6C), APRI (MD: −0.03, 95% CI: −0.07 to 0.02, p = 0.28, I2 = 0%; Figure 6D), and NFS (MD: 0.01, 95% CI: −0.34 to 0.35, p = 0.98, I2 = 0%; Figure 6E) compared with controls.
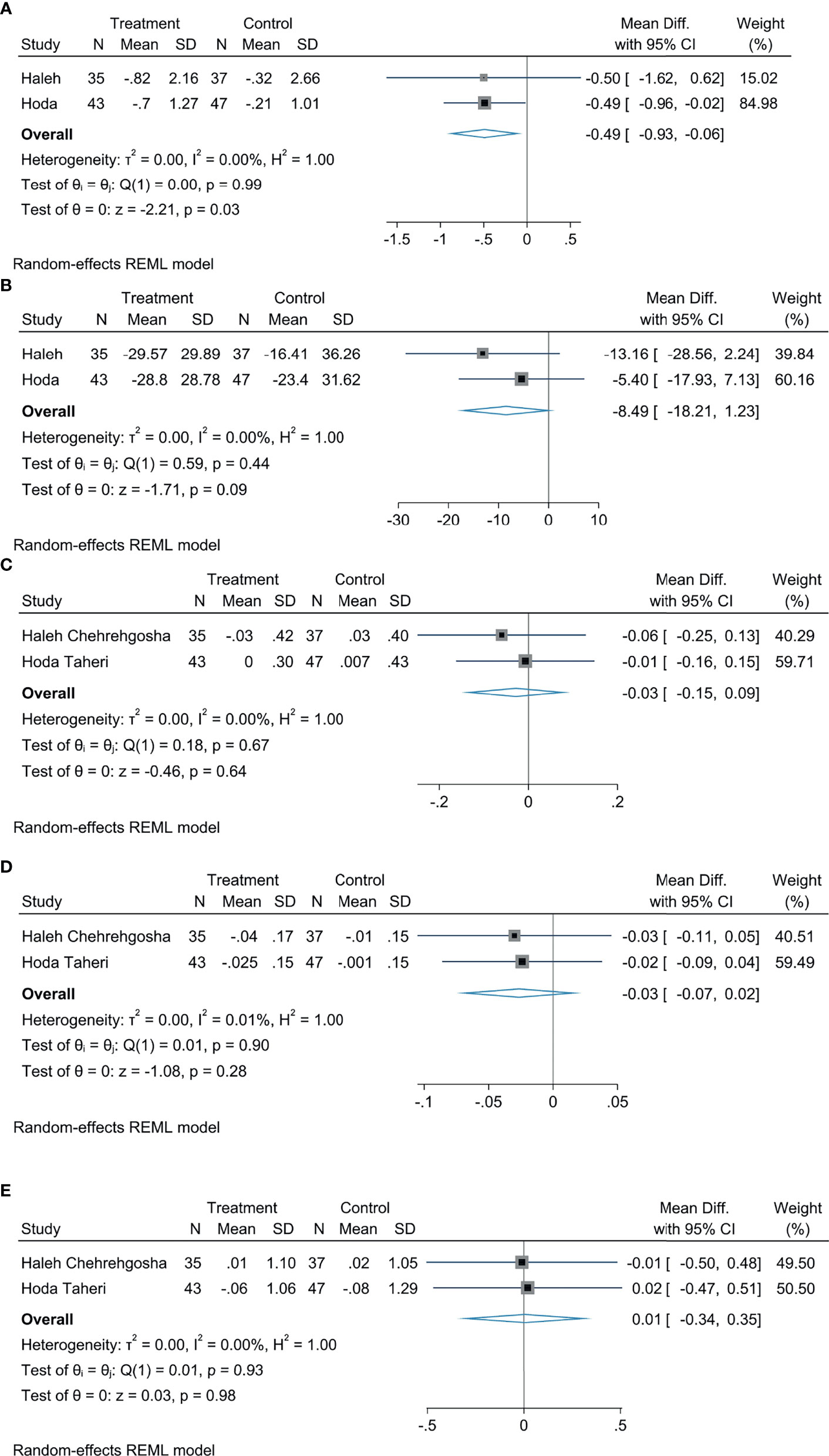
Figure 6 The effect of empagliflozin on LSM (A); the effect of empagliflozin on CAP (B); the effect of empagliflozin on FIB-4 (C); the effect of empagliflozin on APRI (D); the effect of empagliflozin on fasting plasma glucose NFS (E).
Adverse Events
Three RCTs (17, 18, 20) reported adverse events. The major adverse events consisted of nonspecific fatigue, arthralgia, mild hypoglycemia, mild fungal vaginal infections, mild allergic reactions, and urticaria. Overall, all the side effects were relieved after discontinuation of empagliflozin or appropriate local treatment. Mohammad et al. (17) covered that one patient discontinued the treatment after developing balanoposthitis. Haleh et al. (20) revealed that one patient discontinued the treatment owing to severe weakness and fatigue.
Publication Bias
We only included 4 RCTs in this study; publication bias assessments were not conducted due to the insufficient number of articles.
Discussion
This systematic review and meta-analysis of four RCTs was designed to assess the effectiveness and safety of empagliflozin in treating NAFLD patients. Most of these patients have T2DM. This analysis comprehensively assessed the impact of NAFLD patients in terms of glycemic control, body composition, biological indicators of liver function, and hepatic steatosis and fibrosis. The results of our research showed that empagliflozin had significant beneficial effects in decreasing body weight and improving liver function, liver fibrosis, and insulin resistance.
NAFLD has been the most prevalent etiology of chronic liver disease, which is a hot issue in recent research. NAFLD always have associations with changes in metabolic and nonmetabolic processes, such as dyslipidemia, oxidative stress, and insulin resistance. Therefore, attention should be paid to correcting all of these disturbances in the treatment of NAFLD. Current guidelines recommend changing life patterns as first-line therapy strategy for NAFLD. Nevertheless, no pharmacological therapy has been approved for NAFLD (21). Currently, studies have proven that liver histology in T2DM patients and non-T2DM patients with NASH confirmed by biopsy was improved by pioglitazone (3, 22). However, the adverse reactions of pioglitazone limit its use, including sodium and water leakage, weight gain, heart failure, and bone damage (3, 23). Moreover, glucagon-like peptide 1 receptor agonists have indicated beneficial effects in patients with T2DM and NAFLD (24). Several meta-analyses have demonstrated that SGLT2 inhibitors can lower liver enzymes, liver fat and improve body composition (25–27). The role of empagliflozin in NAFLD is not yet fully understood and deserves further investigation.
Empagliflozin is an SGLT2 inhibitor that attenuates blood glucose concentrations by restraining glucose reabsorption and accelerating glucose excretion. Additionally, empagliflozin also has exceptional profits, like cardiovascular protective actions (28), renal protection (29), lowering uric acid levels, and healing NAFLD-associated liver injury (30). In this meta-analysis, our results show reductions in liver function markers AST and ALT after empagliflozin treatment, although the change of ALT did not reach a statistical threshold. Changes in these parameters indicate an amelioration in NAFL or NASH (31). This may be due to the fact that empagliflozin can significantly improve NAFLD-related damage by enhancing autophagy by the liver macrophages and further restraining the inflammatory response (32).
The major underlying pathophysiologic mechanisms of NAFLD are insulin resistance and hepatic steatosis (33, 34). Evidence has demonstrated that patients treated with empagliflozin can improve hypothalamic insulin sensitivity and potentially benefit obesity and systemic metabolism (35). Studies have reported that empagliflozin can reverse fat and insulin resistance through lipid browning and macrophage activation (30). Improvements in obesity and insulin sensitivity may be associated with increased adiponectin levels, a bioactive protein known as adipokines (36). Empagliflozin promoted the replacement activation of macrophages in white adipose tissue and increased the plasma adiponectin level, resulting in obesity-induced inflammation and insulin resistance (30). Our results indicated that empagliflozin could enhance insulin sensitivity, as measured by HOMA-IR and HOMA2-IR, though the differences did not reach statistical significance. Empagliflozin has a beneficial effect on anthropometric parameters; BMI improved significantly in patients after empagliflozin treatment, but the change in weight was not of statistical significance.
Noninvasive biomarkers of liver fibrosis including the FIB-4 index, NFS, and APRI, were used to assess the severity degree of liver fibrosis (37), and the LSM was also adopted to evaluate liver fibrosis (38). End-stage hepatic fibrosis is a key prognostic biomarker for liver-associated outcomes and the total mortality (21). No effective treatment for hepatic fibrosis was found in the guidelines (39). This analysis demonstrated an obvious improvement in LSM scores after empagliflozin treatment; other markers of liver fibrosis including FIB-4 index, APRI, and NFS also depicted an amelioration with empagliflozin, to a large extent, although not statistically significant. This lack of statistically significant difference may be associated with the short duration of follow-up. The development of fibrosis may last for many years, with significant fibrosis reversal observed after long-term treatment (40). Studies with longer follow-up periods are required to verify the role of empagliflozin in the management of liver fibrosis in NAFLD patients. The specific mechanism of action of empagliflozin in liver fibrosis is not clear. However, it is hypothesized that empagliflozin achieves its antifibrosis effect by inhibiting proinflammatory cytokines such as IL-6, TNF-α, and MCP-1 (41)
NAFLD is currently a hot issue in research, and there are no other drugs in the guidelines that could be approved for treating NAFLD. At present, there is no meta-analysis of empagliflozin in the treatment of NAFLD. As we all know, this is the first meta-analysis to evaluate the role of empagliflozin in NAFLD. The advantage of this analysis is to comprehensively assess the roles of empagliflozin in glycemic control, body composition, levels of biological indicators of liver injury, and liver steatosis and fibrosis among patients with T2DM or non-T2DM combined with NAFLD. However, our study has several limitations: (a) Due to the limited large clinical trials, only a few RCTs were eligible, and only four RCTs were included in this paper. Most of the RCTs had small sample sizes, so the results are not significant. (b) The follow-up period of the included studies was short, and the duration was less than 6 months. There is no evidence of additional histological benefits from empagliflozin treatment for more than 6 months. The long-term prognosis and safety of empagliflozin remains unclear, so further research is required. (c) Liver biopsy as a gold standard for evaluating NAFLD (3). The included articles were not based on liver biopsy but relied on ultrasound and proton density fat fraction to diagnose NAFLD.
Conclusions
In conclusion, our results suggest that empagliflozin significantly reduces the BMI, HOMA-IR, CAP score, and LSM score of both diabetic and nondiabetic NAFLD patients. However, the beneficial effects of empagliflozin did not achieve statistical significance in terms of body weight, FBS, HbA1C, LDL, HDL, TG, ALT, AST, insulin, HOMA2-IR, NFS, FIB-4 index, and APRI. Thus, more RCTs with longer duration and larger sample sizes are required to decide the roles of empagliflozin in patients with NAFLD and to establish adequate guidelines for clinical practice.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
YZ conducted a literature search, information extraction and information analysis and wrote the manuscript. XL extracted the data and reviewed the manuscript. XW and HZ were involved in review. All authors made contributions to the article and approved the submitted version.
Funding
The Yunnan Provincial Science and Technology Department Project Fund of China (2017FH001-095) funded this study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.836455/full#supplementary-material
References
1. Abenavoli L, Greco M, Milic N, Accattato F, Foti D, Gulletta E, et al. Effect of Mediterranean Diet and Antioxidant Formulation in Non-Alcoholic Fatty Liver Disease: A Randomized Study. Nutrients (2017) 9(8):870. doi: 10.3390/nu9080870
2. Stefan N, Häring HU, Cusi K. Non-Alcoholic Fatty Liver Disease: Causes, Diagnosis, Cardiometabolic Consequences, and Treatment Strategies. Lancet Diabetes Endocrinol (2019) 7(4):313–24. doi: 10.1016/s2213-8587(18)30154-2
3. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology (2018) 67(1):328–57. doi: 10.1002/hep.29367
4. Targher G, Day CP, Bonora E. Risk of Cardiovascular Disease in Patients With Nonalcoholic Fatty Liver Disease. N Engl J Med (2010) 363(14):1341–50. doi: 10.1056/NEJMra0912063
5. Mantovani A, Zaza G, Byrne CD, Lonardo A, Zoppini G, Bonora E, et al. Nonalcoholic Fatty Liver Disease Increases Risk of Incident Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Metabolism (2018) 79:64–76. doi: 10.1016/j.metabol.2017.11.003
6. Araújo AR, Rosso N, Bedogni G, Tiribelli C, Bellentani S. Global Epidemiology of Non-Alcoholic Fatty Liver Disease/non-Alcoholic Steatohepatitis: What We Need in the Future. Liver Int (2018) 38(Suppl 1):47–51. doi: 10.1111/liv.13643
7. Byrne CD, Targher G. NAFLD: A Multisystem Disease. J Hepatol (2015) 62(1 Suppl):S47–64. doi: 10.1016/j.jhep.2014.12.012
8. Schuppan D, Schattenberg JM. Non-Alcoholic Steatohepatitis: Pathogenesis and Novel Therapeutic Approaches. J Gastroenterol Hepatol (2013) 28(Suppl 1):68–76. doi: 10.1111/jgh.12212
9. Eslam M, Sanyal AJ, George J. International Consensus P: MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology (2020) 158(7):1999–2014.e1991. doi: 10.1053/j.gastro.2019.11.312
10. Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-Alcoholic Fatty Liver Disease and Diabetes. Metabolism (2016) 65(8):1096–108. doi: 10.1016/j.metabol.2016.01.001
11. Polyzos SA, Kountouras J, Mantzoros CS. Obesity and Nonalcoholic Fatty Liver Disease: From Pathophysiology to Therapeutics. Metabolism (2019) 92:82–97. doi: 10.1016/j.metabol.2018.11.014
12. Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A Placebo-Controlled Trial of Pioglitazone in Subjects With Nonalcoholic Steatohepatitis. N Engl J Med (2006) 355(22):2297–307. doi: 10.1056/NEJMoa060326
13. Lian J, Fu J. Pioglitazone for NAFLD Patients With Prediabetes or Type 2 Diabetes Mellitus: A Meta-Analysis. Front Endocrinol (Lausanne) (2021) 12:615409. doi: 10.3389/fendo.2021.615409
14. Tahrani AA, Barnett AH, Bailey CJ. SGLT Inhibitors in Management of Diabetes. Lancet Diabetes Endocrinol (2013) 1(2):140–51. doi: 10.1016/S2213-8587(13)70050-0
15. Ferré P, Foufelle F. Hepatic Steatosis: A Role for De Novo Lipogenesis and the Transcription Factor SREBP-1c. Diabetes Obes Metab (2010) 12(Suppl 2):83–92. doi: 10.1111/j.1463-1326.2010.01275.x
16. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Heal Thcare Interventions: Explanation and Elaboration. BMJ (2009) 339:b2700. doi: 10.1136/bmj.b2700
17. Kuchay M, Krishan S, Mishra S, Farooqui K, Singh M, Wasir J, et al. Effect of Empagliflozin on Liver Fat in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial). Diabetes Care (2018) 41(8):1801–08. doi: 10.2337/dc18-0165
18. Taheri H, Malek M, Ismail-Beigi F, Zamani F, Sohrabi M, Reza babaei M, et al. Effect of Empagliflozin on Liver Steatosis and Fibrosis in Patients With Non-Alcoholic Fatty Liver Disease Without Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial Advances in Therapy. Adv Ther (2020) 37(11):4697–708. doi: 10.1007/s12325-020-01498-5
19. Zhang XL, Wang XT, Wang DJ, Xu WJ. Clinical Study of Engramine in the Treatment of Type 2 Diabetes Mellitus Combined With Non-Alcoholic Fatty Liver. J Pract Diabetol (2020) 16(05):110–11.
20. Chehrehgosha H, Sohrabi M, Ismail-Beigi F, Malek M, Reza BM, Zamani F, et al. Empagliflozin Improves Liver Steatosis and Fibrosis in Patients With Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Diabetes Ther (2021) 12(3):843–61. doi: 10.1007/s13300-021-01011-3
21. Powell EE, Wong VW, Rinella M. Non-Alcoholic Fatty Liver Disease. Lancet (2021) 397(10290):2212–24. doi: 10.1016/S0140-6736(20)32511-3
22. Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, et al. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med (2016) 165(5):305–15. doi: 10.7326/M15-1774
23. Portillo-Sanchez P, Bril F, Lomonaco R, Barb D, Orsak B, Bruder JM, et al. Effect of Pioglitazone on Bone Mineral Density in Patients With Nonalcoholic Steatohepatitis: A 36-Month Clinical Trial. J Diabetes (2019) 11(3):223–31. doi: 10.1111/1753-0407.12833
24. Toplak H, Stauber R, Sourij H. EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease: Guidelines, Clinical Reality and Health Economic Aspects. Diabetologia (2016) 59(6):1148–9. doi: 10.1007/s00125-016-3941-4
25. Xing B, Zhao Y, Dong B, Zhou Y, Lv W, Zhao W. Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Non-Alcoholic Fatty Liver Disease in Patients With Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. J Diabetes Investig (2020) 11(5):1238–47. doi: 10.1111/jdi.13237
26. Coelho FDS, Borges-Canha M, von Hafe M, Neves JS, Vale C, Leite AR, et al. Effects of Sodium-Glucose Co-Transporter 2 Inhibitors on Liver Parameters and Steatosis: A Meta-Analysis of Randomized Clinical Trials. Diabetes Metab Res Rev (2021) 37(6):e3413. doi: 10.1002/dmrr.3413
27. Wei Q, Xu X, Guo L, Li J, Li L. Effect of SGLT2 Inhibitors on Type 2 Diabetes Mellitus With Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. Front Endocrinol (Lausanne) (2021) 12:635556. doi: 10.3389/fendo.2021.635556
28. Jarcho JA. More Evidence for SGLT2 Inhibitors in Heart Failure. N Engl J Med (2020) 383(15):1481–82. doi: 10.1056/NEJMe2027915
29. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and Renal Outcomes With Empagliflozin in Heart Failure. N Engl J Med (2020) 383(15):1413–24. doi: 10.1056/NEJMoa2022190
30. Xu L, Nagata N, Chen G, Nagashimada M, Zhuge F, Ni Y, et al. Empagliflozin Reverses Obesity and Insulin Resistance Through Fat Browning and Alternative Macrophage Activation in Mice Fed a High-Fat Diet. BMJ Open Diabetes Res Care (2019) 7(1):e000783. doi: 10.1136/bmjdrc-2019-000783
31. Takeshita Y, Kanamori T, Tanaka T, Kaikoi Y, Kita Y, Takata N, et al. Study Protocol for Pleiotropic Effects and Safety of Sodium-Glucose Cotransporter 2 Inhibitor Versus Sulfonylurea in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease. Diabetes Ther (2020) 11(2):549–60. doi: 10.1007/s13300-020-00762-9
32. Meng Z, Liu X, Li T, Fang T, Cheng Y, Han L, et al. The SGLT2 Inhibitor Empagliflozin Negatively Regulates IL-17/IL-23 Axis-Mediated Inflammatory Responses in T2DM With NAFLD via the AMPK/mTOR/autophagy Pathway. Int Immunopharmacol (2021) 94:107492. doi: 10.1016/j.intimp.2021.107492
33. Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, et al. Association of Nonalcoholic Fatty Liver Disease With Insulin Resistance. Am J Med (1999) 107(5):450–5. doi: 10.1016/s0002-9343(99)00271-5
34. Lee SM, Pusec CM, Norris GH, De Jesus A, Diaz-Ruiz A, Muratalla J, et al. Hepatocyte-Specific Loss of Pparγ Protects Mice From NASH and Increases the Therapeutic Effects of Rosiglitazone in the Liver. Cell Mol Gastroenterol Hepatol (2021) 11(5):1291–311. doi: 10.1016/j.jcmgh.2021.01.003
35. Kullmann S, Hummel J, Wagner R, Dannecker C, Vosseler A, Fritsche L, et al. Empagliflozin Improves Insulin Sensitivity of the Hypothalamus in Humans With Prediabetes: A Randomized, Double-Blind, Placebo-Controlled, Phase 2 Trial. Diabetes Care (2021) 2021):dc211136. doi: 10.2337/dc21-1136
36. Boutari C, Mantzoros CS. Adiponectin and Leptin in the Diagnosis and Therapy of NAFLD. Metabolism (2020) 103:154028. doi: 10.1016/j.metabol.2019.154028
37. Naguib M, Abou Elfotouh M, Wifi MN. Elevated Serum Cyclophilin D Level is Associated With Nonalcoholic Fatty Liver Disease and Higher Fib Rosis Scores in Patients With Diabetes Mellitus. Int J Gen Med (2021) 14:4665–75. doi: 10.2147/IJGM.S322986
38. Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology (2019) 156(6):1717–30. doi: 10.1053/j.gastro.2019.01.042
39. Glen J, Floros L, Day C, Pryke R. Guideline Development Group. Non-Alcoholic Fatty Liver Disease (NAFLD): Summary of NICE Guidance. BMJ (2016) 354:i4428. doi: 10.1136/bmj.i4428
40. Ellis EL, Mann DA. Clinical Evidence for the Regression of Liver Fibrosis. J Hepatol (2012) 56(5):1171–80. doi: 10.1016/j.jhep.2011.09.024
Keywords: NAFLD, empagliflozin, medication, meta-analysis, systematic review
Citation: Zhang Y, Liu X, Zhang H and Wang X (2022) Efficacy and Safety of Empagliflozin on Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Front. Endocrinol. 13:836455. doi: 10.3389/fendo.2022.836455
Received: 15 December 2021; Accepted: 31 January 2022;
Published: 24 February 2022.
Edited by:
Luca Busetto, Università degli Studi di Padova, ItalyReviewed by:
Anna Di Sessa, University of Campania Luigi Vanvitelli, ItalyAtsushi Nakajima, Yokohama City University, Japan
Copyright © 2022 Zhang, Liu, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaobo Liu, eW5kbHhiQDEyNi5jb20=
 Yuyuan Zhang
Yuyuan Zhang Xiaobo Liu1*
Xiaobo Liu1*