- 1Department of Radiology, Guangzhou Red Cross Hospital (Guangzhou Red Cross Hospital of Jinan University), Guangzhou, China
- 2Department of Radiology, Guangzhou First People’s Hospital, Guangzhou, Guangdong, China
- 3Department of Radiology, Wuhan Third Hospital, Tongren Hospital of Wuhan University, Wuhan, Hubei, China
- 4Department of Radiology, Hubei 672 Integrated Traditional Chinese and Western Medicine Orthopedic Hospital, Wuhan, Hebei, China
Objective: To develop and validate an artificial intelligence diagnostic system based on X-ray imaging data for diagnosing vertebral compression fractures (VCFs)
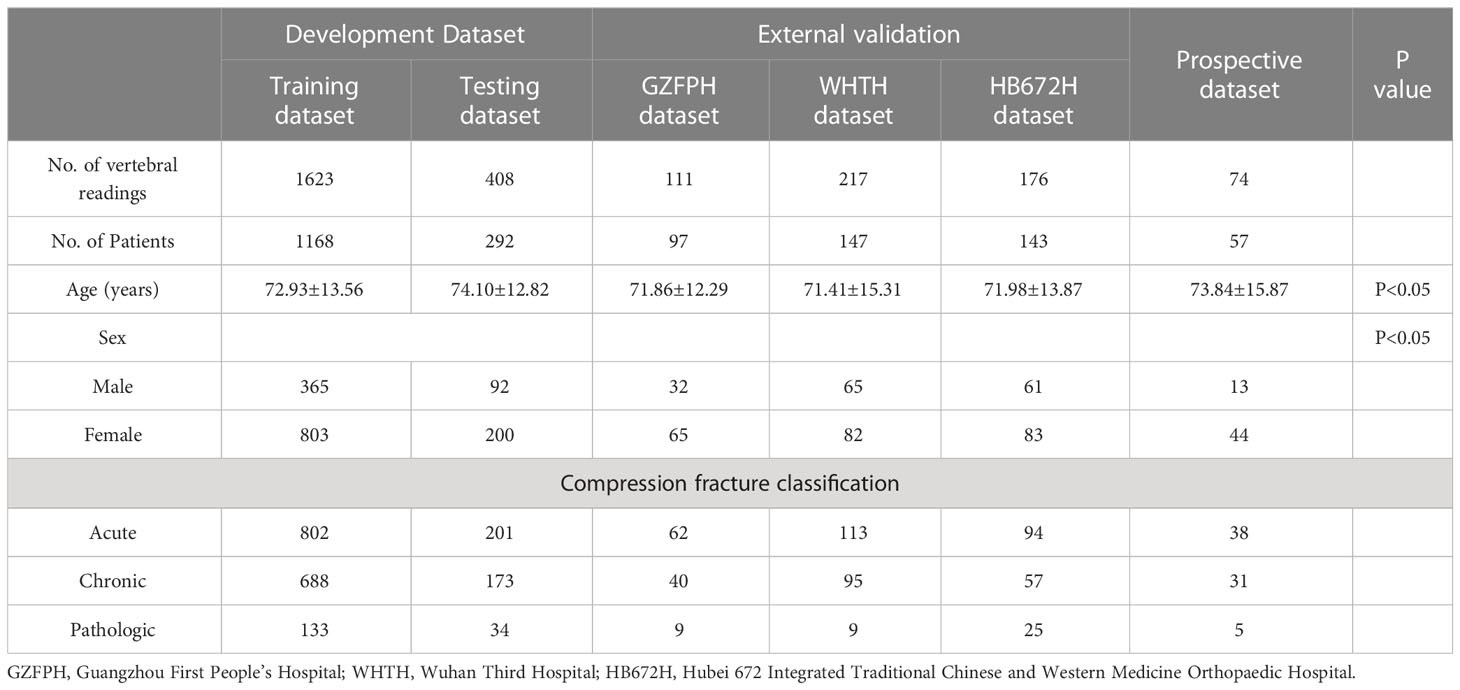
Methods: In total, 1904 patients who underwent X-ray at four independent hospitals were retrospectively (n=1847) and prospectively (n=57) enrolled. The participants were separated into a development cohort, a prospective test cohort and three external test cohorts. The proposed model used a transfer learning method based on the ResNet-18 architecture. The diagnostic performance of the model was evaluated using receiver operating characteristic curve (ROC) analysis and validated using a prospective validation set and three external sets. The performance of the model was compared with three degrees of musculoskeletal expertise: expert, competent, and trainee.
Results: The diagnostic accuracy for identifying compression fractures was 0.850 in the testing set, 0.829 in the prospective set, and ranged from 0.757 to 0.832 in the three external validation sets. In the human and deep learning (DL) collaboration dataset, the area under the ROC curves(AUCs) in acute, chronic, and pathological compression fractures were as follows: 0.780, 0.809, 0.734 for the DL model; 0.573, 0.618, 0.541 for the trainee radiologist; 0.701, 0.782, 0.665 for the competent radiologist; 0.707,0.732, 0.667 for the expert radiologist; 0.722, 0.744, 0.610 for the DL and trainee; 0.767, 0.779, 0.729 for the DL and competent; 0.801, 0.825, 0.751 for the DL and expert radiologist.
Conclusions: Our study offers a high-accuracy multi-class deep learning model which could assist community-based hospitals in improving the diagnostic accuracy of VCFs.
Introduction
Vertebral compression fractures (VCFs) are common diseases that seriously affect human life and pose a very large challenge to the health care system (1). With the rising prevalence of population aging, the occurrence of VCFs due to trauma and osteoporosis is increasing year by year, which increases societal and familial economic burdens. Moreover, pathologic fractures resulting from neoplasms are another leading cause of VCFs worldwide. All types of VCFs foreshadow a high risk of poor outcomes, so early, personalized and effective medical intervention is strongly advised. Therefore, it is desirable to find an accurate and effective method to detect and identify acute, chronic and pathological VCFs.
In recent years, the incidence of back pain due to compression fractures has increased in patients. Many imaging methods are available for early screening and differentiation of compression fractures, such as X-ray (XR) images, Computed tomography (CT) and magnetic resonance imaging (MRI). Among these procedures, CT is the modality of choice for the evaluation of bone structure and fragments. MRI may be the most useful imaging technique based on its excellent soft tissue contrast that shows the change in the signal intensity and morphological characteristics of the collapsed vertebrae. Acute compression fractures show hyperintensity with bone marrow edema, while chronic compression fractures show no bone marrow edema and are isointense on T2WI fat-suppression sequences. The pathologic VCFs shows low signal intensity on T1WI, isointensity or high signal intensity on T2WI, and homogeneous or inhomogeneous enhancement (Figure 1). However, the availability of CT and MRI is limited for overall population diagnosis due to their complexity, high time consuming and high-cost factor (2). In contrast, X-ray images with effective cost and time may be an attractive and widespread method in diagnosis of VCFs, although it could only provide limited detail about 3D anatomy structure or pathology of VCFs.
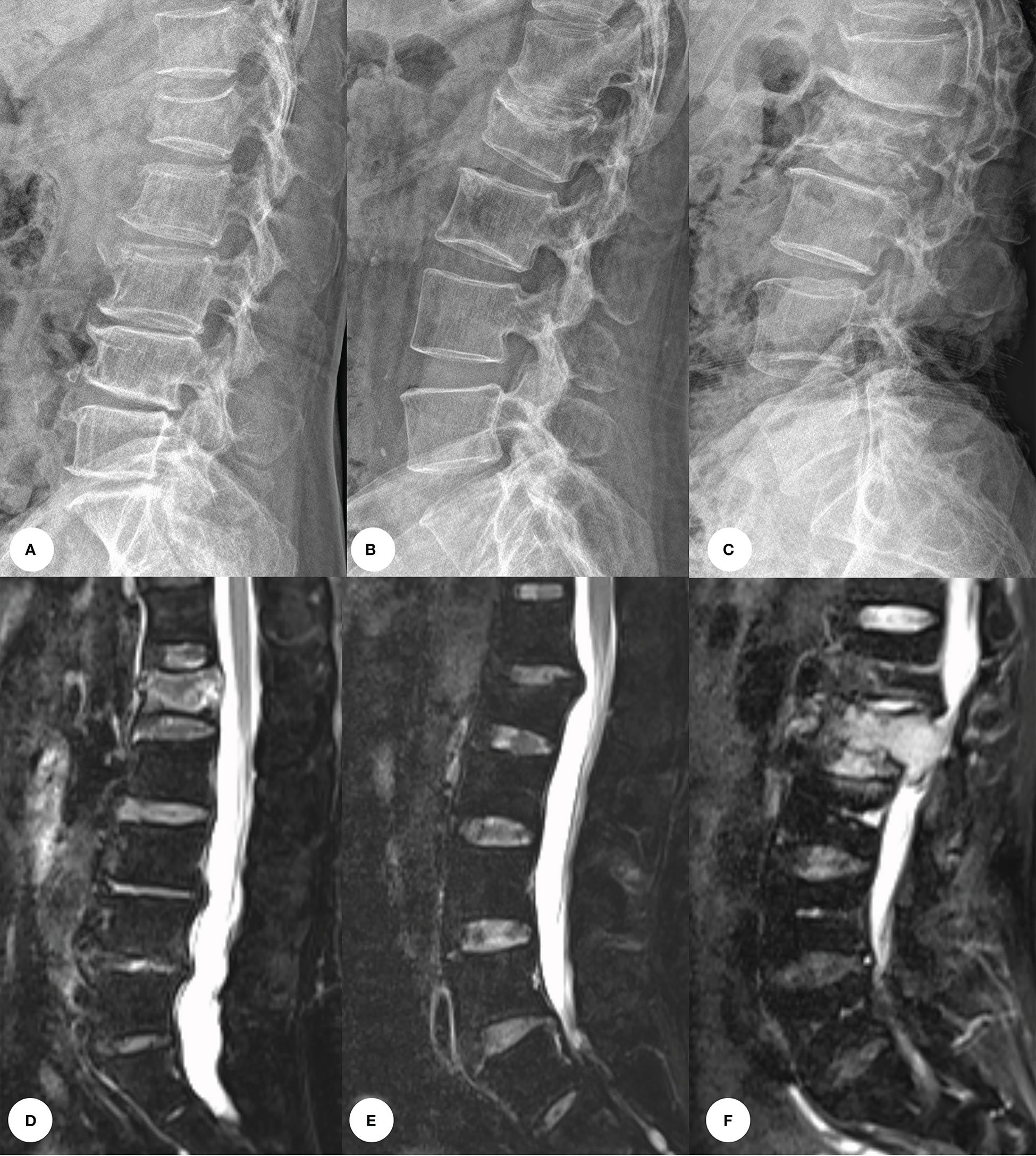
Figure 1 Images of vertebral compression fractures types. (A, D) Acute compression fracture of the L1 vertebral; (B, E) Chronic compression fracture of the L1 vertebral; (C, F) Pathologic compression fracture of the L2 vertebral.
Deep learning (DL), a branch of machine learning, has already shown potential for assisting humans in various medical fields (3–6). A convolutional neural network(CNN) is a deep learning algorithm that is mainly designed to process image data and has grown to be a fundamental aspect of the medical field (7). In recent years, more and more radiomics model algorithms based on plain X-ray films have been developed in the wrist, humerus, hip, femur, shoulder, hand and foot (8–13). However, very few works are carried out using X-ray -based radiomics to predict VCFs. Recently, Chen et al have developed a DL–based model that distinguish fresh VCFs from digital radiography (DR) with sensitivity, specificity and AUC of 80%, 68% and 0.80 respectively. However, the clinical feasibility and benefit of DL–based model remain to be confirmed because no external and multicenter validation was performed in their study (14).
Thus, the aims of this study were to develop an X-ray-based deep learning model using a four-center dataset and determine whether the model could distinguish the type of VCFs and validate these findings in an independent external cohort.
Materials and methods
Datasets
This multicohort diagnostic study was performed with data from four hospitals in China. This study was approved by the institutional review board and ethics committee of the hospital (IRB 2022-108-01). Our retrospective study was approved by the institutional review board of the hospitals with a waiver for written informed consent. Patients in the prospective validation set of compression fractures provided written informed consent prior to participation.
For the development dataset, we evaluated the medical radiology reports of lumbar spine MRI in Site 1 from 1 January 2014 to 31 October 2021 to determine acute, chronic, and pathological compression fractures. The inclusion criteria were as follows: (1) less than 2 weeks between Digital radiography (DR) and MRI examinations; and (2) the height of the vertebral body was reduced by at least 20% or 4 mm from the baseline height on the lateral radiography of the lumbar spine (15). The exclusion criteria were as follows: (1) surgical treatment for compression vertebral bodies such as internal fixation or bone cement filling; (2) lumbar spine presenting serious scoliosis or deformity; and (3) images with a low signal-to-noise ratio or foreign matter present.
To verify the applicability of the classified diagnosis deep learning model in clinical practice, from October 1, 2019, to September 31, 2021, lumbar spine lateral X-ray images were also obtained from three hospitals across China: Site 2; Site 3 and Site 4. In total, 2609 vertebrae from 1904 participants who underwent X-ray were enrolled at four independent hospitals (Figure 2).
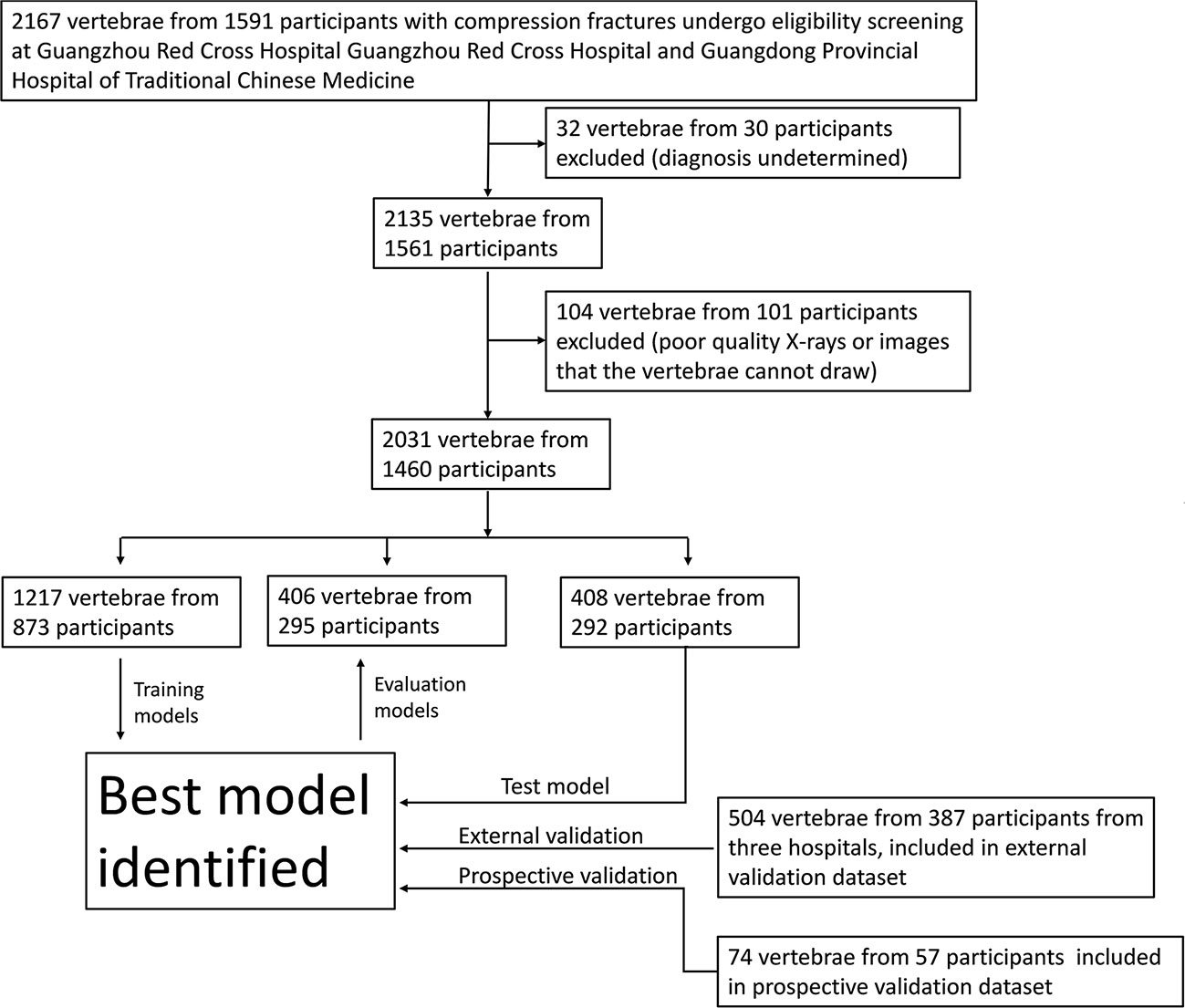
Figure 2 Workflow diagram for the development and evaluation of the deep learning model for compression fracture classification.
From Nov 1, 2021, an independent dataset of consecutive participants undergoing lumbar spine X-ray in Site 1 was prospectively enrolled. These participants were defined as the prospective validation set. In total, 74 vertebrae from 57 participants were enrolled at Site 1 (Figure 2).
Image reading and annotation
Delineated images of acute and chronic compression fractures are based on MRI diagnosis, and pathological compression fractures are diagnosed using MRI, positron emission tomography (PET) or histopathological results. When only MR images are available for a patient, at least two senior diagnosing physicians complete the diagnosis.
Lesion regions of interest (ROIs) were manually represented with bounding boxes on lateral radiographs of the lumbar spine by an experienced radiologist using the LabelImg software (https://pypi.org/project/labelImg/) and annotated (224×224) (Figure 3).
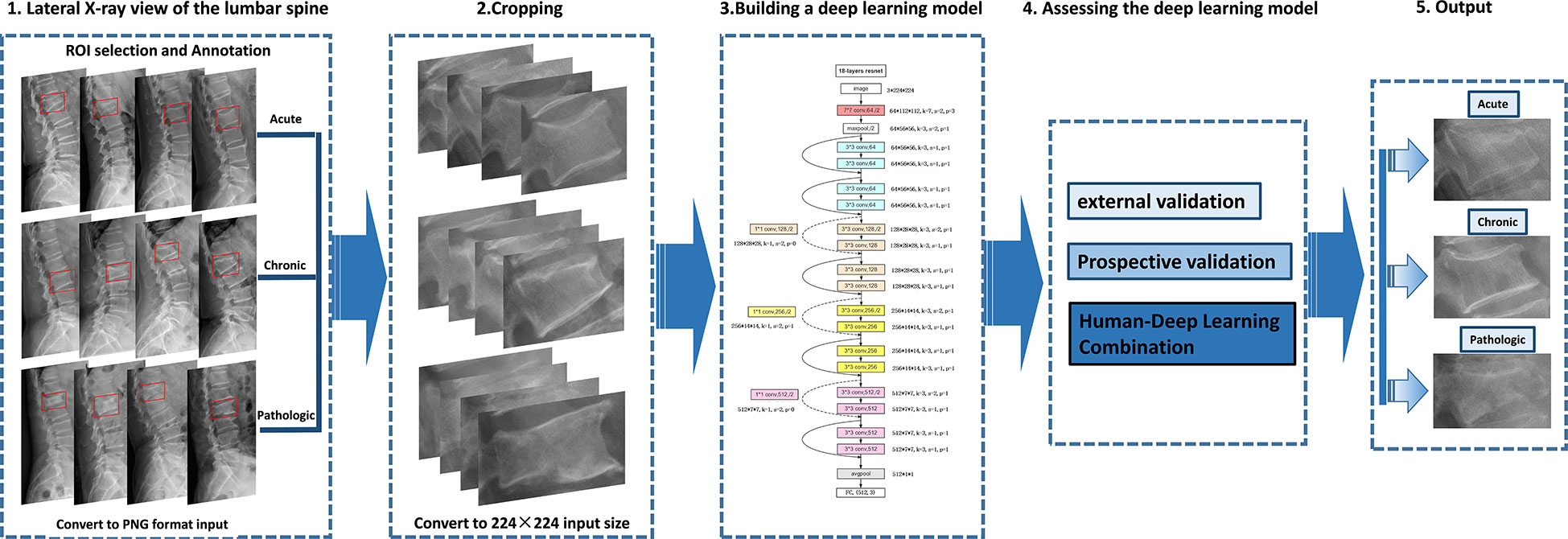
Figure 3 Deep learning architecture overview. First (step 1), compression fractures were reliably delineated and annotated on radiographs using labelImg software. For the step, the radiograph was converted to PNG format. Then (step 2), the cropped lateral X-ray of the lumbar spine was resized to 224×224 pixels and used as the input for a deep learning model. The third step (step 3) is to build a compression fracture classification model based on the Resnet-18 algorithm. Model performance was evaluated using external and prospective data and further validated using a radiologist-deep learning combination (step 4). As a result, we can provide adjunctive evaluation of lumbar compression fractures (acute, chronic, and pathological) (step 5).
Model training
The images from the development dataset were randomly assigned with a ratio of 8:2 for the training datasets (the deep learning model for compression fracture classification) and the testing datasets (for evaluating the performance of the deep learning model). The image of the training set was enhanced, using horizontal flips, vertical flips, and rotations at random angles.
ResNet is a type of CNN where the input from the previous layer is added to the output of the current layer. This skip connection makes it easier for the network to learn and brings better performance. The ResNet architecture has been successful in many tasks, including image classification, objection detection, and semantic segmentation. In addition, because ResNet is composed of layers, these networks can obtain any level of spatial representation at any depth. Each ResNet block has 2 convolutional layers (excluding the 1×1 onvolutional layer), and we connect these two residual blocks as a module. We use 4 such modules, so there are a total of 16 convolutional layers (Figure 4). Together with the first 7×7 onvolutional layer and the final fully connected layer, there are 18 convolutional layers in total, which is ResNet-18 (16).
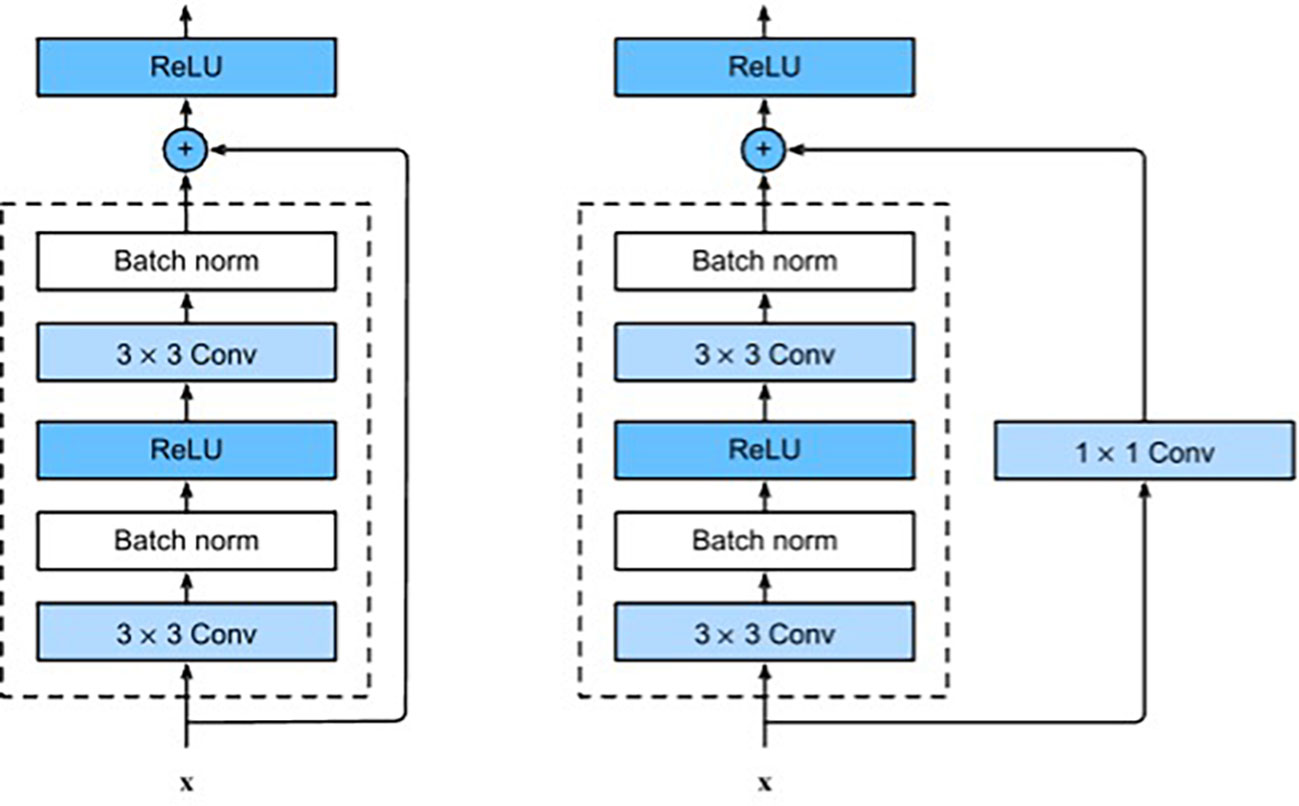
Figure 4 ResNet block with and without 1×1 onvolution, which transforms the input into the desired shape for the addition operation.
In this study, we used the ResNet-18 architecture model, and the input image was resized to 224 × 224 pixels and was normalized with mean= [0.485,0.456,0.406] and std= [0.229,0.224,0.225]. We then fine-tuned the model using a dataset of lateral lumbar spine radiographs of acute, chronic, and pathological compression fractures. Codes are available at https://github.com/Xiongyuchao/VCFNet.
Validation of the model
We first validated the performance of the model in classifying VCFs using the testing dataset. We then evaluated the robustness of this model using an external validation dataset from three participating hospitals. Finally, the model was evaluated using prospective data including 74 vertebrae of 57 patients from Site 1.
Validation of the radiologists’ visual diagnoses combined with DL-model based on collaboration dataset
In addition to the classification research of independent observers, the collaborative research of human and deep learning was also carried out to simulate a real clinical setting. We randomly selected 30% of the images from all external validation sets as the “collaboration dataset”. Three radiologists with different levels of expertise (trainee, competent, and expert) were asked to independently complete the same test images and compare their results with those of the model. Then the three radiologists reevaluated all the same test images independently after knowing the DL-model diagnosis. These radiologists were not involved in the selection and labeling of images, and the images were obfuscated and unidentified prior to evaluation. The expert radiologist was a professor with more than 20 years of experience in musculoskeletal diagnosis. The competent radiologist was a radiologist with 7 years of experience and completed standardized training for practicing physicians. The trainee is a radiologist with 2 years of experience and obtained the qualification certificate of a licensed doctor.
Statistical analysis
All computer codes used for data analysis are stored in GitHub(https://github.com/Xiongyuchao/VCFNet). We used receiver operating characteristic (ROC) curves to demonstrate the ability of deep learning algorithms to classify VCFs. An ROC curve is generated by plotting the ratio of true positive cases (sensitivity) to false positive cases (1-specificity) by varying the predicted probability threshold. A larger area under the ROC curve (AUC) indicates better diagnostic performance.
Results
VCF and clinical datasets
Table 1 provides an overview of the participant characteristics and the VCF classification data. 1003 vertebrae of acute compression fractures, 861 vertebrae of chronic compression fractures, and 167 vertebrae of pathologic vertebrae were included in the development dataset. In addition, 504 vertebrae from 387 participants were used to test the deep learning classification model at three external participating hospitals, and 74 vertebrae from 57 participants were prospectively collected at Site 1 for the prospective validation dataset (Figure 2).
Establishment of deep learning model
After 25 epochs, the training procedure was ended, with no further improvement in accuracy and cross-entropy loss on training, and testing. An accuracy of up to 95.9% was observed in the training set and 77.7% in the testing set (Appendix).
Deep learning model performance on the VCF test set
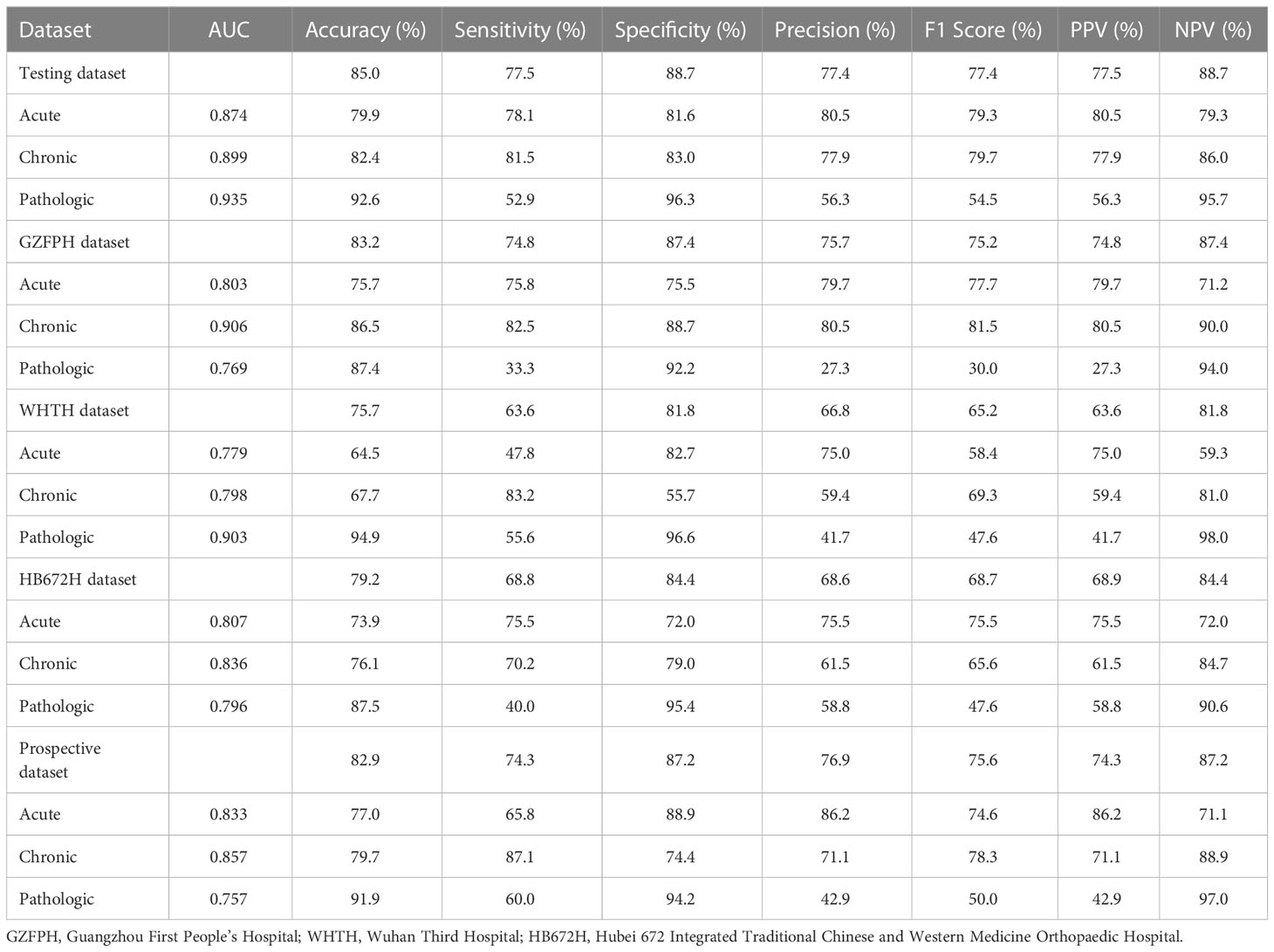
Table 2 shows the performance of the deep learning model in the classification of compression fractures in all five testing sets (Figure 5). Classification accuracies were 0.850 for the testing dataset and 0.829 for the prospective validation dataset. Classification accuracies for the external validation were 0.832 for Site 2, 0.757 for Site 3, and 0.792 for Site 4. Using the proposed model to assess the ability of each compression classification, the AUCs in acute, chronic, and pathological compression fractures were as follows: 0.874 (95% CI: 0.873, 0.875), 0.899 (95% CI: 0.898, 0.900) and 0.935 (95% CI: 0.935, 0.937) in the testing dataset, respectively; 0.803 (95% CI: 0.801, 0.806), 0.906 (95% CI: 0.905, 0.909) and 0.769 (95% CI: 0.771, 0.780) in the GZFPH dataset, respectively; 0.779 (95% CI: 0.777, 0.781), 0.798 (95% CI: 0.796, 0.800) and 0.903 (95% CI: 0.900, 0.907) in the WHTH dataset; 0.807 (95% CI: 0.805, 0.809), 0.836 (95% CI: 0.834, 0.838) and 0.796 (95%CI: 0.793, 0.800) in the HB672H dataset, respectively.
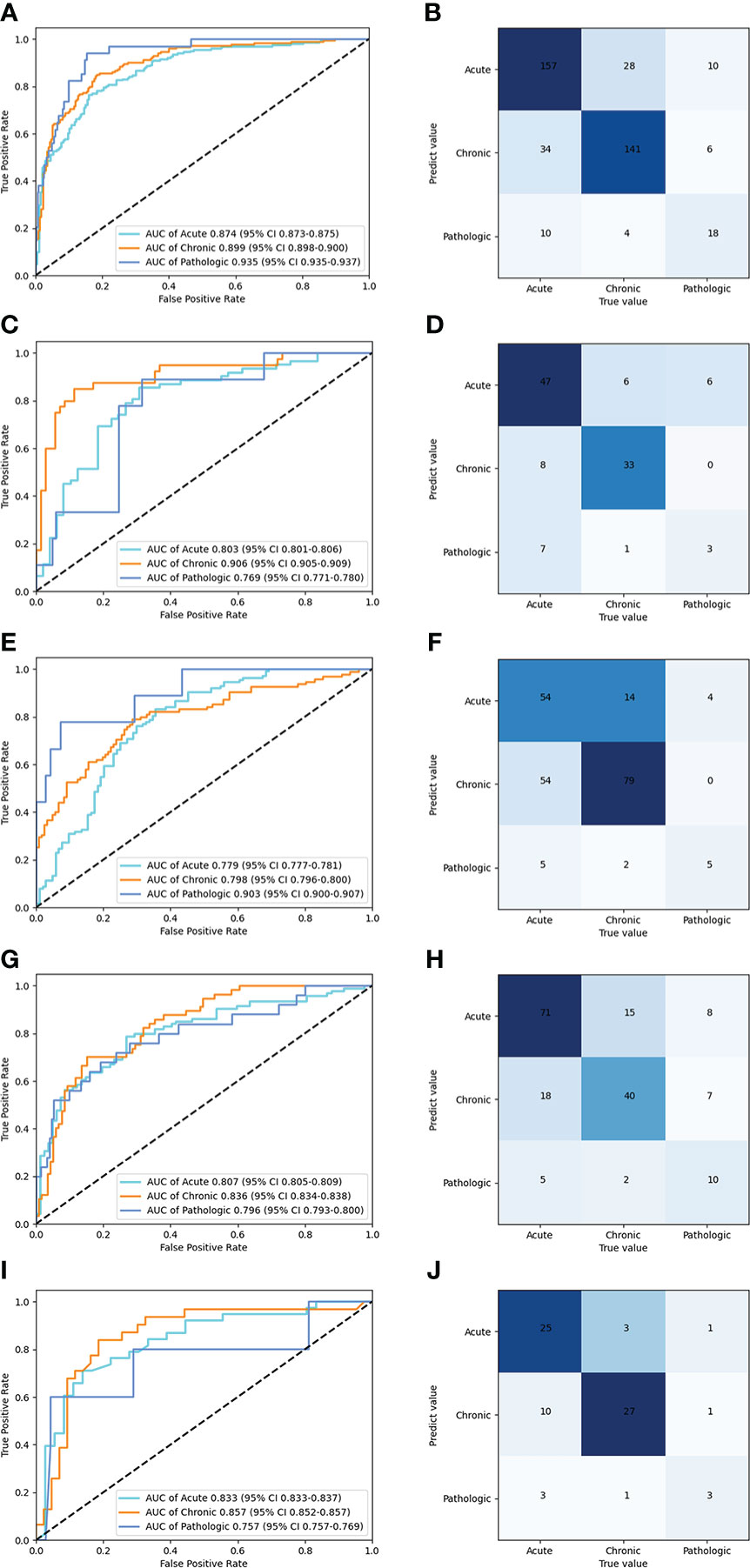
Figure 5 Performance of the deep learning model in the classification of acute, chronic, and pathologic compression fracture in X-ray images, in the internal and external validation datasets. ROC (A) and normalized confusion matrices (B) of the classification mode in the testing dataset. ROC (C) and normalized confusion matrices (D) of the classification mode in the GZFPH dataset. ROC (E) and normalized confusion matrices (F) of the classification mode in WHTH dataset. ROC (G) and normalized confusion matrices (H) of the classification mode in HB672H dataset. ROC (I) and normalized confusion matrices (J) of the prospective dateset.
Human and deep learning collaboration
In the human and deep learning collaboration dataset, 80 vertebrae with acute compression fractures, 57 vertebrae with chronic compression fractures, and 13 vertebrae with pathologic compression fractures were included. The classification results of images from human and deep learning collaboration dataset by the deep learning and radiologists are shown in Table 3. The AUCs in acute, chronic, and pathological compression fractures were as follows: 0.780, 0.809, 0.734 for the deep learning model; 0.573, 0.618, 0.541 for the trainee radiologist; 0.701, 0.782, 0.665 for the competent radiologist; 0.707,0.732, 0.667 for the expert radiologist; 0.722, 0.744, 0.610 for the DL and trainee; 0.767, 0.779, 0.729 for the DL and competent; 0.801, 0.825, 0.751 for the DL and expert radiologist. The overall accuracy of the deep learning model was 0.764, which was significantly higher than that of the trainee radiologist (0.707), similar to the competent radiologist (0.769), and slightly lower than the expert radiologist (0.782). When combined with the deep learning model, the expert, competent, and trainee radiologists’ accuracy all increased significantly (0.853, 0.816, and 0.778, respectively). For sensitivity, combined with the deep learning model, the trainee, competent, and expert radiologists also significantly improved (0.560 vs. 0.667, 0.653 vs. 0.727, and 0.673 vs. 0.776, respectively). For the classification of pathological compression fractures, the sensitivity of expert radiologists was up to 0.385 and the deep learning model was only 0.308. However, when combined with the deep learning model, the expert radiologist, competent radiologist, and trainee radiologist all had increased sensitivity to pathological compression fracture (0.385 vs. 0.538, 0.462 vs. 0.538, and 0.154 vs. 0.308, respectively) (Figure 6). In addition, for pathological compression fractures, 6 out of 13 vertebrae were misjudged by all radiologists and the deep learning model.
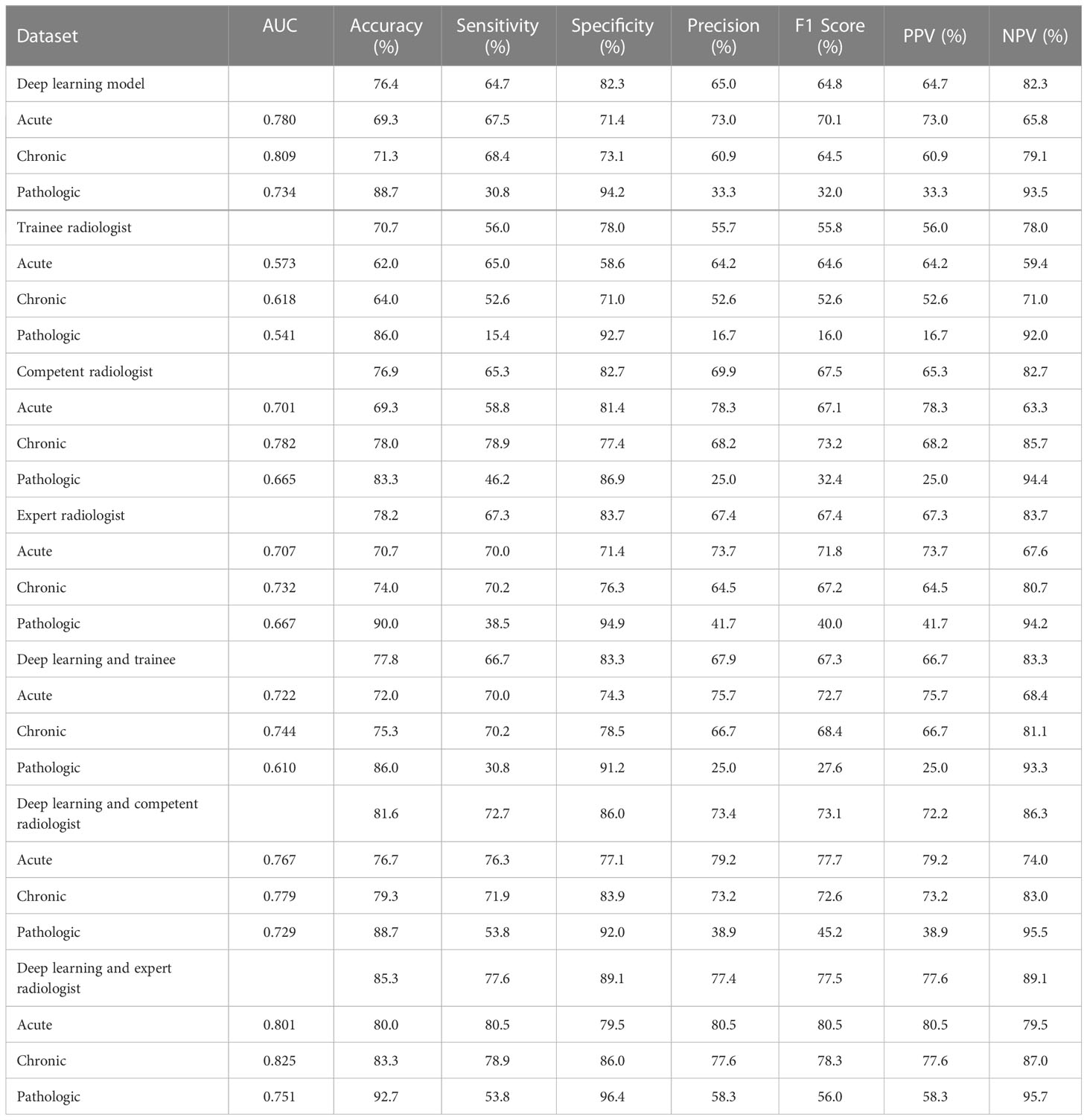
Table 3 Performance of the deep learning versus radiologists in classifying compression fractures in the human and deep learning collaboration dataset.
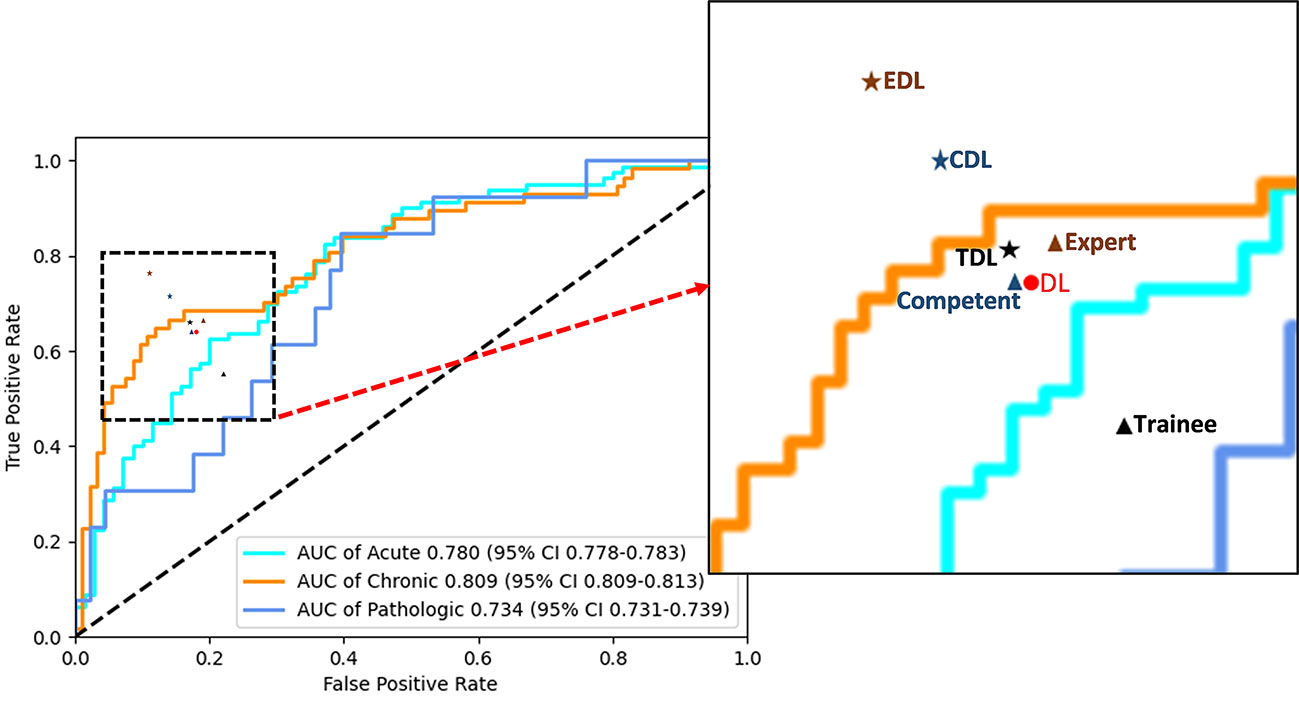
Figure 6 Performance of the deep learning and radiologists in classifying VCFs from X-ray images in the human and deep learning collaboration dataset. ROC of the classification mode in the human and deep learning collaboration dataset. The red dot, black triangle, black star, orange triangle, orange star, blue triangle, and blue star indicate the performance of deep learning (DL), trainee, trainee-deep learning collaboration (TDL), competent, competent-deep learning collaboration (CDL), expert, and expert-deep learning collaboration (EDL), respectively.
Discussion
In this multicenter study, we successfully developed a classification model for acute, chronic and pathological compression fractures by using deep learning neural networks. The model demonstrated high accuracy and specificity in classifying compression fractures in retrospectively stored images as well as in a prospective observational setting. Furthermore, the diagnostic efficiency of deep learning model is higher than that of the trainee radiologists, similar to the competent radiologist, and slightly lower than the expert radiologist. The deep learning model combined with all three expertise levels of radiologists significantly improved the accuracy, specificity, and sensitivity of evaluating compression fractures. To the best of our knowledge, this is the first multicenter study to apply deep learning with CNNs to the classification of acute, chronic, and pathological compression fractures.
Previous studies have used information from radiographs to classify compression fractures. Usually, plain radiographs are initially performed to diagnose acute compression fractures by observing small changes such as endplate rupture and anterior wall protrusion (17) and diagnosis of pathological compression by cortical penetration, trabecular bone destruction, vertebral bone density, and special compression morphology. Although studies of these conventional features have provided guidance for the classification of compression fractures, the information provided by lumbar spine X-rays is limited, and compression fractures can only be simply assessed from morphological and partial imaging signs. The diagnosis of compression fractures, which is subjective and largely depends on the skills and experience of the diagnosing physician, needs to be based on professional knowledge and the accumulation of diagnostic experience to improve the accuracy of diagnosis. CT (18, 19), MRI (19–22), and PET (23) have shown great advantages in the classification of compression fractures, but their clinical applicability has been limited because of patient noncooperation, high costs, and the need for specialized training in tomographic image interpretation. The proposed deep learning CNN has instead been found to provide auxiliary diagnosis to non-professional radiologists to improve performance (competent from 0.756 to 0.816 and trainee from 0.707 to 0.796) on compression fracture classification, both exceeded the expert level (0.764). The deep learning model demonstrated high accuracy and specificity in classifying compression fractures in retrospectively stored images as well as in a prospective observational setting. Furthermore, the diagnostic efficiency of deep learning model is higher than that of the trainee radiologists, similar to the competent radiologist, and slightly lower than the expert radiologist (Figure 6). The deep learning model combined with all three expertise levels of radiologists significantly improved the accuracy, specificity, and sensitivity of evaluating compression fractures. Thus, for developing countries such as China or countries with scarce medical resources, where there is an unequal distribution of urban and rural medical resources, this deep learning CNN can help bridge the classification of compression fractures between national and primary hospitals. In addition, with deep learning model assistance, the classification accuracy of three radiologists with different levels of experience was also improved significantly.
The classification sensitivity of pathological compression fractures was found to be low for all three radiologists and the deep learning model. Even the combination of all three radiologists and the deep learning model was still low. In addition, 6 vertebrae with pathological compression fractures were misdiagnosed by all three radiologists and the deep learning model. We speculate that the main reasons for these false negatives are the low contrast of X-ray images, the fact that there were no obvious signs of bone damage on the vertebral body, intestinal gas interference and bilateral shadows of the vertebral body, which may be an insurmountable limitation caused by the principle of X-ray imaging. However, deep learning model-assisted diagnosis can improve the sensitivity of pathological compression fracture classification, albeit still at a low level. Furthermore, given the high accuracy of classification of acute and chronic compression fractures, deep learning could be considered cost-effective.
There are few studies on compression fractures using deep learning methods, especially based on conventional X-ray images. Only one study used deep learning to evaluate acute and chronic compression fractures on radiographs, which included 1099 patients and used image data from anteroposterior and lateral lumbar spine radiographs as input to a neural network. This study achieved a sensitivity, specificity and AUC of 0.80, 0.68, and 0.80, respectively (14). The clinical applicability of CNN models may be limited as a result of dichotomous disease surveys and retrospective and single-institution studies in homogeneous hospitals. By comparison, the deep learning model of this study demonstrated an overall high accuracy in classifying compression fractures in three retrospective validation sets, suggesting that the model may be generalizable across a variety of scenarios.
Despite these remarkable results, our study has some limitations. First, the subjects who received internal fixation or cementation or who presented with severe scoliosis/deformity were excluded in the development dataset, which may lead to bias. Second, from the nature of CNNs, since a neural network only provides a classification of an image and associated compression fractures, there is no explicit feature definition. Third, the ROIs of the compression fracture were delineated by a manual rectangle. The training of the model relies on the accurate identification of compressed vertebral bodies by radiologists, which requires manual delineation by radiologists. Although there are some differences in the ROIs drawn by different doctors, they will be uniformly processed and then classified after the images are imported into the model. Currently ROI delineation could be finished using automatic, semi-automatic and hand-crafted methods. However, automatic and semi-automatic methods may have a certain deviation which need to be further adjusted manually. Fourth, due to the small numbers of pathological compression fractures in this study with low sensitivity, it is likely the features of pathological fracture might be different from the osteoporotic/traumatic compression fracture and will require a large and specific pathological database to clarify the utilization of deep learning model assistance. Therefore, more studies with larger numbers of patients are required to provide stronger evidence for the accuracy of deep learning models in the prediction of pathological compression fractures. Finally, deep learning model alone was not adequate to detect pathological compression fracture due to the absence of clinical information. Thus, the general applicability of our results in clinical practice could be affected. Clinical information is required in future study to validate the performance of deep learning models.
In conclusion, a multiclass deep learning model for compression fractures on radiographs was developed and validated. The classification performance of the model surpassed that of trainee radiologists and was comparable to that of experienced radiologists. When the skills of the radiologists were combined with a deep learning model, better diagnostic performance was observed, which could improve the accuracy of diagnostic classification, thereby improving the diagnostic workflow for patients with compression fractures. We expect to establish an artificial intelligence-assisted diagnosis platform for compression fracture based on X-ray images to provide patients and clinicians with free access to telemedicine assistance, aiming to eliminate the diagnosis and treatment gap between national hospitals and grassroots hospitals.
Data availability statement
The excel files containing raw data included in the main figures and tables can be found in supplementary material. The lumbar spine X-ray imaging data and clinical information are not publicly available for patient privacy reasons but are available from the corresponding authors upon reasonable request (FX, YCX and XWZ). The models and the code used to test and evaluate the model is available on GitHub (https://github.com/Xiongyuchao/VCFNet). The remaining data are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the institutional review board and ethics committee of the Guangzhou Red Cross Hospital (IRB 2022-108-01). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conception and design: FX, YCX; acquisition of additional data: FX, YCX, GXY, YYL, QPD, WYJ, DLW, SC; analysis and interpretation of data: FX, YCX, GXY, ZPL, XWZ; statistical analysis: FX, YYL, GXY; drafting of the manuscript: FX, YCX; critical revision of the manuscript: FX, XWZ; study supervision: XWZ. All authors listed have contributed substantially to the design, data collection and analysis, and editing of the manuscript.
Funding
This study has received funding by the Guangzhou Science and Technology Project of Health, China [grant numbers: 20231A011021] (YCX). Guangzhou Planned Project of Science and Technology, China [grant numbers: 202102010102] (XWZ). Guangzhou Science and Technology Project of Health, China [grant numbers: 20211A010019] (FX), Guangzhou Planned Project of Science and Technology [grant numbers: 202102010031] (YYL). Guangdong Basic and Applied Basic Research Foundation [grant numbers: 2021A1515110703] (YYL).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1025749/full#supplementary-material
The models and the code used to test and evaluate the model is available on GitHub (https://github.com/Xiongyuchao/VCFNet).
Abbreviations
VCFs, Vertebral compression fractures; XR, X-ray; CT, Computed tomography; MRI, magnetic resonance imaging; DL, Deep learning; CNN, convolutional neural network; DR, digital radiography; GZFPH, Guangzhou First People's Hospital, School of Medicine, South China University of Technology, Guangzhou; WHTH, Wuhan Third Hospital, Tongren Hospital of Wuhan University, Wuhan; HB672H, Hubei 672 Integrated Traditional Chinese and Western Medicine Orthopedic Hospital, Wuhan; PET, positron emission tomography; ROIs, Lesion regions of interest; ROC, receiver operating characteristic; AUC, area under the ROC curve; PACS, picture archiving and communication systems; CI, confidence interval.
References
1. Parreira PCS, Maher CG, Megale RZ, March L, Ferreira ML. An overview of clinical guidelines for the management of vertebral compression fracture: A systematic review. Spine J (2017) 17:1932–8. doi: 10.1016/j.spinee.2017.07.174
2. Musbahi O, Ali AM, Hassany H, Mobasheri R. Vertebral compression fractures. Br J Hosp Med (Lond) (2018) 79:36–40. doi: 10.12968/hmed.2018.79.1.36
3. Esteva A, Robicquet A, Ramsundar B, Kuleshov V, DePristo M, Chou K, et al. A guide to deep learning in healthcare. Nat Med (2019) 25:24–9. doi: 10.1038/s41591-018-0316-z
4. Li X, Zhang S, Zhang Q, Wei X, Pan Y, Zhao J, et al. Diagnosis of thyroid cancer using deep convolutional neural network models applied to sonographic images: a retrospective, multicohort, diagnostic study. Lancet Oncol (2019) 20:193–201. doi: 10.1016/S1470-2045(18)30762-9
5. Luo H, Xu G, Li C, He L, Luo L, Wang Z, et al. Real-time artificial intelligence for detection of upper gastrointestinal cancer by endoscopy: a multicentre, case-control, diagnostic study. Lancet Oncol (2019) 20:1645–54. doi: 10.1016/S1470-2045(19)30637-0
6. Razzak I, Naz S. Unit-vise: Deep shallow unit-vise residual neural networks with transition layer for expert level skin cancer classification. IEEE/ACM Trans Comput Biol Bioinform PP (2022) 19(2):1225–34. doi: 10.1109/TCBB.2020.3039358
8. Chung SW, Han SS, Lee JW, Oh K-S, Kim NR, Yoon JP, et al. Automated detection and classification of the proximal humerus fracture by using deep learning algorithm. Acta Orthop (2018) 89:468–73. doi: 10.1080/17453674.2018.1453714
9. Gan K, Xu D, Lin Y, Shen Y, Zhang T, Hu K, et al. Artificial intelligence detection of distal radius fractures: a comparison between the convolutional neural network and professional assessments. Acta Orthop (2019) 90:394–400. doi: 10.1080/17453674.2019.1600125
10. Olczak J, Fahlberg N, Maki A, Razavian A, Jilert A, Stark A, et al. Artificial intelligence for analyzing orthopedic trauma radiographs. Acta Orthop (2017) 88:581–6. doi: 10.1080/17453674.2017.1344459
11. Kim DH, MacKinnon T. Artificial intelligence in fracture detection: transfer learning from deep convolutional neural networks. Clin Radiol (2018) 73:439–45. doi: 10.1016/j.crad.2017.11.015
12. Ozkaya E, Topal FE, Bulut T, Gursoy M, Ozuysal M, Karakaya Z. Evaluation of an artificial intelligence system for diagnosing scaphoid fracture on direct radiography. Eur J Trauma Emerg Surg (2022) 48(1):585–92. doi: 10.1007/s00068-020-01468-0
13. Mutasa S, Varada S, Goel A, Wong TT, Rasiej MJ. Advanced deep learning techniques applied to automated femoral neck fracture detection and classification. J Digit Imaging (2020) 33:1209–17. doi: 10.1007/s10278-020-00364-8
14. Chen W, Liu X, Li K, Luo Y, Bai S, Wu J, et al. A deep-learning model for identifying fresh vertebral compression fractures on digital radiography. Eur Radiol (2022) 32(3):1496–505. doi: 10.1007/s00330-021-08247-4
15. McCarthy J, Davis A. Diagnosis and management of vertebral compression fractures. Am Fam Physician (2016) 94:44–50.
16. He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition. Proc IEEE Conf Comput Vision Pattern Recognition (2016) 2016:770–8. doi: 10.1109/CVPR.2016.90
17. Yabu A, Hoshino M, Tabuchi H, Takahashi S, Masumoto H, Akada M, et al. Using artificial intelligence to diagnose fresh osteoporotic vertebral fractures on magnetic resonance images. Spine J (2021) 21:1652–8. doi: 10.1016/j.spinee.2021.03.006
18. Laredo JD, Lakhdari K, Bellaiche L, Hamze B, Janklewicz P, Tubiana JM. Acute vertebral collapse: CT findings in benign and malignant nontraumatic cases. Radiology (1995) 194:41–8. doi: 10.1148/radiology.194.1.7997579
19. Schwaiger BJ, Gersing AS, Baum T, Krestan CR, Kirschke JS. Distinguishing benign and malignant vertebral fractures using CT and MRI. Semin Musculoskelet Radiol (2016) 20:345–52. doi: 10.1055/s-0036-1592433
20. Zampa V, Cosottini M, Michelassi C, Ortori S, Bruschini L, Bartolozzi C. Value of opposed-phase gradient-echo technique in distinguishing between benign and malignant vertebral lesions. Eur Radiol (2002) 12:1811–8. doi: 10.1007/s00330-001-1229-6
21. Wang KC, Jeanmenne A, Weber GM, Thawait SK, Carrino JA. An online evidence-based decision support system for distinguishing benign from malignant vertebral compression fractures by magnetic resonance imaging feature analysis. J Digit Imaging (2011) 24:507–15. doi: 10.1007/s10278-010-9316-3
22. Suh CH, Yun SJ, Jin W, Lee SH, Park SY, Ryu CW. ADC As a useful diagnostic tool for differentiating benign and malignant vertebral bone marrow lesions and compression fractures: a systematic review and meta-analysis. Eur Radiol (2018) 28:2890–902. doi: 10.1007/s00330-018-5330-5
23. Kim SJ, Lee JS. Diagnostic performance of f-18 fluorodeoxyglucose positron emission tomography or positron emission Tomography/Computed tomography for differentiation of benign and malignant vertebral compression fractures: A meta-analysis. World Neurosurg (2020) 137:e626–33. doi: 10.1016/j.wneu.2020.02.085
Keywords: vertebral compression fractures, DL: deep learning, DR: digital radiography, x-ray, CNN
Citation: Xu F, Xiong Y, Ye G, Liang Y, Guo W, Deng Q, Wu L, Jia W, Wu D, Chen S, Liang Z and Zeng X (2023) Deep learning-based artificial intelligence model for classification of vertebral compression fractures: A multicenter diagnostic study. Front. Endocrinol. 14:1025749. doi: 10.3389/fendo.2023.1025749
Received: 23 August 2022; Accepted: 03 March 2023;
Published: 22 March 2023.
Edited by:
Cristina Vassalle, Gabriele Monasterio Tuscany Foundation (CNR), ItalyCopyright © 2023 Xu, Xiong, Ye, Liang, Guo, Deng, Wu, Jia, Wu, Chen, Liang and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuwen Zeng, Z3pzaHN6aHl5ZnNrQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Fan Xu
Fan Xu Yuchao Xiong
Yuchao Xiong Guoxi Ye
Guoxi Ye Yingying Liang
Yingying Liang Wei Guo
Wei Guo Qiuping Deng4
Qiuping Deng4 Zhiping Liang
Zhiping Liang Xuwen Zeng
Xuwen Zeng