- 1Department of Neurosurgery, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China
- 2Department of Neurosurgery, The Fifth Affiliated Hospital of Nanchang University (Fu Zhou First People’s Hospital of Jiangxi Province), Fuzhou, Jiangxi, China
- 3College of Medical, Nanchang University, Nanchang, Jiangxi, China
Purpose: Three dopamine agonists [bromocriptine, cabergoline, and quinagolide (CV)] have been used for hyperprolactinemia treatment for decades. Several studies have reviewed the efficacy and safety of bromocriptine and cabergoline. However, no systematic review or meta-analysis has discussed the efficacy and safety of CV in hyperprolactinemia and prolactinoma treatment.
Methods: Five medical databases (PubMed, Web of Science, Embase, Scopus, and Cochrane Library) were searched up to 9 May 2022 to identify studies related to CV and hyperprolactinemia. A meta-analysis was implemented by using a forest plot, funnel plot, sensitivity analysis, meta-regression, and Egger’s test via software R 4.0 and STATA 12.
Results: A total of 1,211 studies were retrieved from the five medical databases, and 33 studies consisting of 827 patients were finally included in the analysis. The pooled proportions of patients with prolactin concentration normalization and tumor reduction (>50%) under CV treatment were 69% and 20%, respectively, with 95% confidence intervals of 61%–76% and 15%–28%, respectively. The pooled proportion of adverse effects was 13%, with a 95% confidence interval of 11%–16%.
Conclusion: Our study showed that CV is not less effective than cabergoline and bromocriptine in treating hyperprolactinemia, and the side effects were not significant. Hence, this drug could be considered an alternative first-line or rescue treatment in treating hyperprolactinemia in the future.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO, identifier CRD42022347750.
1 Introduction
The synthesis and secretion of prolactin (PRL) are suppressed through the hypothalamic dopamine system (1). Disorders of the hypothalamus usually induce high serum PRL concentrations that subsequently evolve into hyperprolactinemia. The most common cause of hyperprolactinemia is prolactinoma, which is one of the most common pituitary adenomas (2). Patients with hyperprolactinemia can present with bone loss, decreased spinal bone density (3), amenorrhea, galactorrhea, and decreased libido (4). Prolactinoma not only includes the above complications but also may lead to headaches and visual field defects caused by tumor compression or even death due to tumor bleeding. The first-line treatment of hyperprolactinemia and prolactinomas involves the use of dopamine agonists (DAs), the most common of which are cabergoline (CAB) and bromocriptine (BRC), whereas quinagolide (CV) is mainly used in Europe. However, several systematic reviews and meta-analyses have indicated that the remission rate under CAB and BRC treatment is not satisfactory, which is mainly due to recurrence (30%–80%), resistance, and intolerance (5–8).
Unlike CAB and BRC, which are ergot-like DAs, CV is a non-ergot-like DA (9). It may have the potential to overcome the resistance and intolerance to CAB and BRC. In addition, in the study by Colao et al. (10), CV was shown to be more effective than BRC in the implantation inhibition test and lactation inhibition test. Moreover, no systematic review or meta-analysis has discussed the efficacy and safety of CV in hyperprolactinemia and prolactinoma treatment, whereas many clinical trials have explored this concept. Hence, we performed this systematic review and meta-analysis to assess the efficacy and safety of CV in treating hyperprolactinemia and prolactinomas.
2 Methods
2.1 Study registration
This study is registered in PROSPERO (International Prospective Register of Systematic Reviews) (CRD42022347750). The registration information is shown in the following link: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=347750.
2.2 Information sources
Literature retrieval was performed by using five databases, including PubMed, Web of Science, Embase, Scopus, and Cochrane Library. There was no limitation on the published language. Retrieval was restricted by studies published before 9 May 2022.
2.3 Search
The search term was “quinagolide and (prolactinoma or hyperprolactinemia).”
2.4 Study selection
Retrieval studies were loaded into the Reference management software NoteExpress 3.2.0.7276 (AegeanSoft Corporation). Duplicate studies among different databases were removed. In addition, preliminary screening was performed based on the title, abstract, and keywords. The remaining studies were subsequently screened based on inclusion and exclusion criteria under full-text reading.
Both groups were screened simultaneously. YYZ and JCT were in Group A. QLH and WZ were in Group B. The two groups were screened separately by first reviewing the titles, abstracts, and keywords. Disagreements were resolved by discussion. Afterward, full texts were screened. Any objections were resolved via discussions with more experienced researchers (MHL and YZZ).
The inclusion criteria were as follows:
i. Participants were hyperprolactinemia or prolactinoma patients;
ii. CV was the only intervention or one of the interventions;
iii. The proportion of patients with normalized PRL concentrations or tumor reductions (>50%) is reported or can be calculated;
iv. If there were duplicated cohorts, the largest cohort was included in the analysis.
The exclusion criteria were as follows:
i. The normal reference values of PRL were not reported;
ii. The dosage of DAs was not reported;
iii. The smaller cohorts were removed if duplicated cohorts were presented;
iv. Studies had high heterogeneity;
v. Acromegaly, plurihormonal pituitary adenomas, hyperprolactinemia due to drug use, and renal failure.
2.5 Data collection process
All of the authors related to the data collection process were divided into two groups. YYZ and JCT were in Group A. QLH and WZ were in Group B. Group A and Group B separately extracted the related data into an Excel table. Disagreements were resolved by discussions within the two groups. Any disagreements without consensus were discussed with experimental researchers (MHL and YZZ).
2.6 Data items
We extracted the following data: year of publication, research area, study type, cause of hyperprolactinemia, drug used, numbers and ages of the included patients, sex information, methods of tumor detection, methods of serum PRL concentration measurement, initial serum PRL concentrations of the included patients, information on tumor reduction, and information on PRL concentration normalization.
2.7 Summary measures
The proportion of normalized PRL concentrations and tumor reductions (>50%) under DA treatment were the outcome indicators in the individual studies. The pooled proportions, risk ratios (RRs), and 95% confidence intervals (CIs) were the effect sizes in this systematic review and meta-analysis.
2.8 Synthesis of results
Two main units of serum PRL concentration (ng/ml and mIU/L) were used in the included studies. In our study, ng/ml (the conversion factor is 30) was used as the serum PRL concentration (8). Prolactinoma is classified according to size, and the diameter is shown in mm. Microprolactinoma is less than 1 mm, and macroprolactinoma is 1 mm or more (11). The proportion of normalized PRL concentrations and tumor reductions (>50%) under DA treatment were calculated and pooled. These values are presented by using pooled proportions and RRs with 95% CIs. In terms of heterogeneity among the included studies, we performed the I2 test and χ2 statistic test. Significant heterogeneity was indicated if P < 0.1 (χ2 statistic) or I2 > 50% (I2 test), and a random-effects model was used for the meta-analysis; otherwise, a fixed-effects model was performed. Heterogeneity was analyzed via stratified analysis, sensitivity analysis, and meta-regression. In the sensitivity analysis, each study was omitted one by one with a changed effect size. In the stratified analysis and meta-regression, the included studies were divided based on treatment line and the size of the prolactinomas before DA treatment. The source of heterogeneity was indicated according to a statistically significant P value (P < 0.05). Publication bias was evaluated via funnel plots and the Egger’s test, which are qualitative and quantitative methods, respectively. In the Egger’s test, P < 0.05 indicates a statistically significant publication bias. In our study, statistical analyses were performed using R (Version 4.0.3) and STATA 12.
3 Results
3.1 Study selection
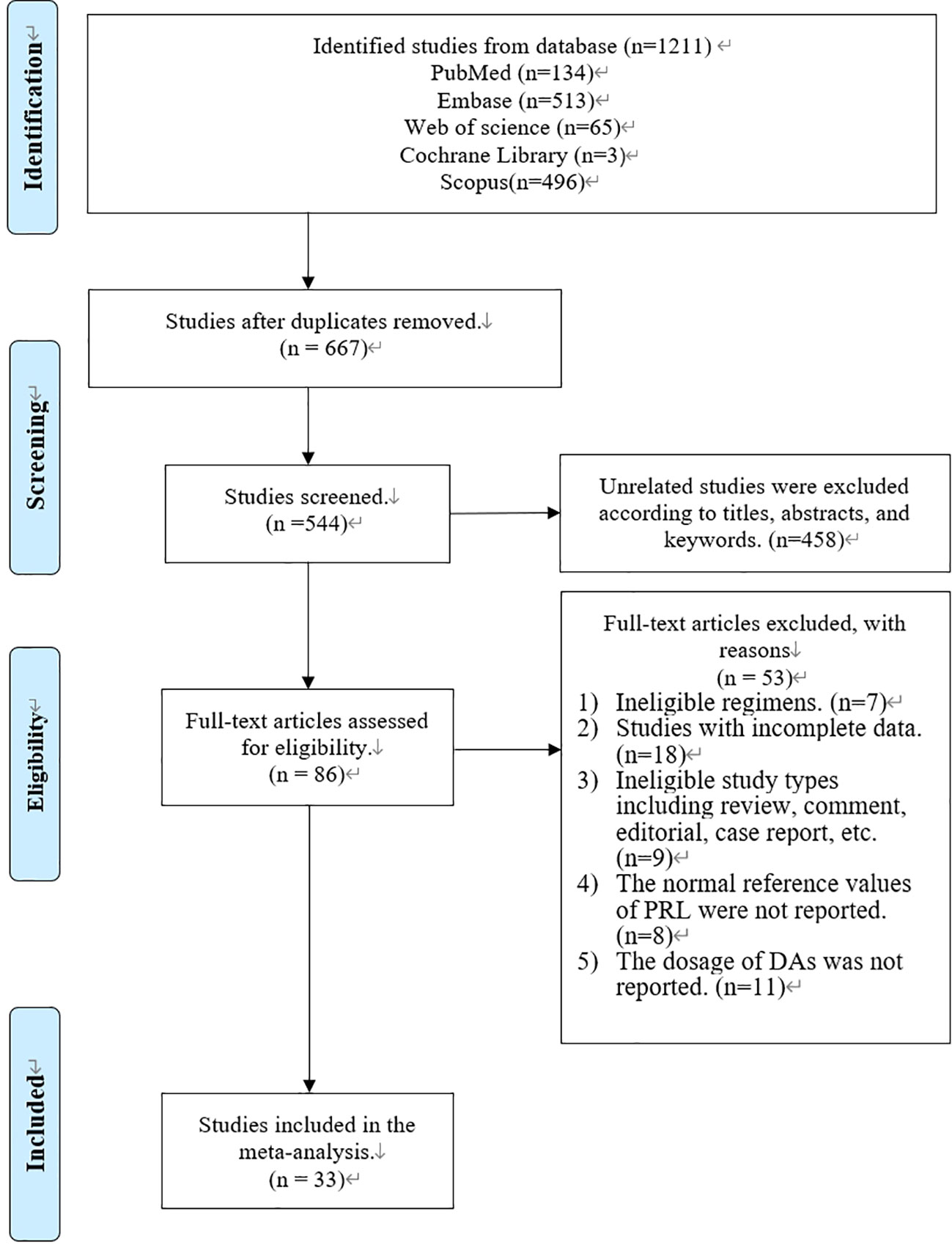
In total, 1,211 articles were retrieved from PubMed (n = 134), Embase (n = 513), Web of Science (n = 65), Cochrane Library (n = 3), and Scopus (n = 496) (Figure 1). A total of 667 articles were removed via a duplication check. The remaining 544 articles were screened according to the titles, abstracts, and keywords, and 458 of the articles were removed. Afterward, 86 articles were screened via a full-text check, and 53 of the articles were removed due to ineligible regimens (n = 7), incomplete data (n = 18), ineligible study types (n = 9), no reference values (n = 8), and no dosage information (n = 11). Finally, 33 studies were included in our systematic review and meta-analysis (Figure 1).
3.2 Study characteristics
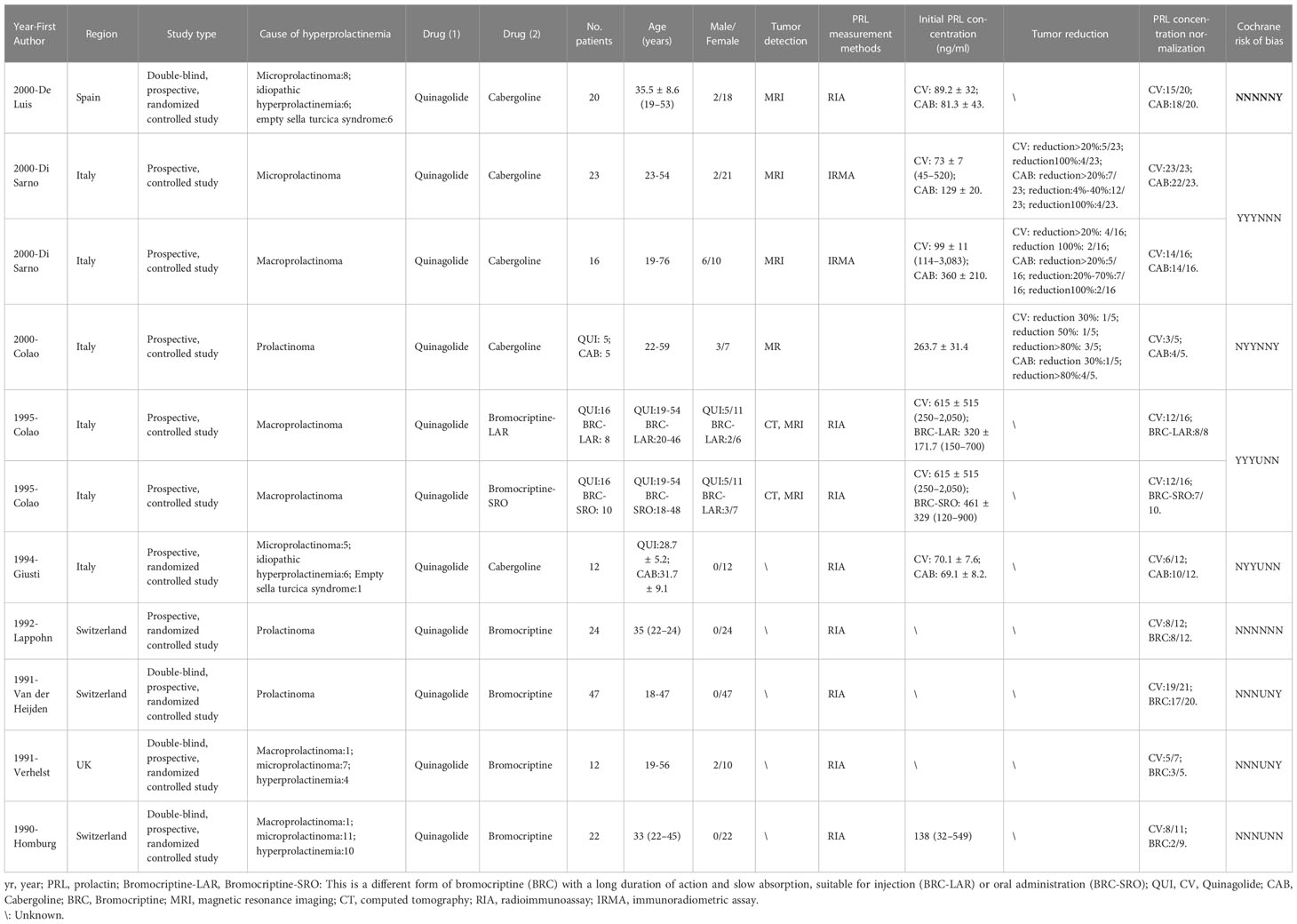
In total, 33 studies with 827 patients and 178 arms were included in our systematic review and meta-analysis (9, 12–43). Among 178 arms, 59 arms referred to the efficacy of CV and 119 arms referred to safety.
In nine studies with double arms, one study was published in Spain (35), four were published in Italy (36–39), three were published in Switzerland (40, 41, 43), and one was published in Britain (42). Four studies compared CV and CAB (35–37, 39), and another five studies compared CV and BRC (38, 40–43). In tumor detection, three studies used magnetic resonance imaging (MRI) (35–37) and one study used both MRI and computed tomography (CT) (38). For serum PRL concentration measurements, seven studies used radioimmunoassay (RIA) (35, 38–43) and one study used immunoradiometric assay (IRMA) (36).
In 24 studies with a single arm, seven studies were retrospective (12–18) and 17 studies were prospective (9, 19–34). In the retrospective studies, for tumor detection, two studies used MRI (12, 16), one study used CT (15), and three studies used both MRI and CT (14, 17, 18). For serum PRL concentration measurements, six studies used RIA (12, 14–18) and one study used both IRMA and immunochemilu-minometric assay (ICMA) (13). In prospective studies, for tumor detection, one study used MRI (28) and four studies used both MRI and CT (19, 20, 22, 31). For serum PRL measurements, most studies used RIA, except for the studies by Vilar et al. (21) and Crottaz et al. (23).
The drug dosage and duration information are listed in Tables S1, S2. Detailed information is shown in Tables 1, 2. For nine studies with double arms, one study included a first week dose of 25–50 µg/day and second week maintenance of 75 µg/day; one study included doses ranging from 25 to 75 µg/day and 150 µg/day after 12 weeks; one study included doses ranging from 25 to 75 µg/day; two studies included maintenance at 75 µg/day; three studies included doses ranging from 75 to 600 µg/day; and one study included a dose of 25 µg/day in the first week, 100 µg/day in the second week, and a maximum dose not to exceed 200 µg/day. In summary, the minimum doses ranged from 25 to 75 µg/day, with final doses not exceeding 600 µg/day. For 24 studies with a single arm, these single-arm trials were unstable, with drug doses below 600 µg/day (except for one study), and 75–300 µg/day was a more common dose.
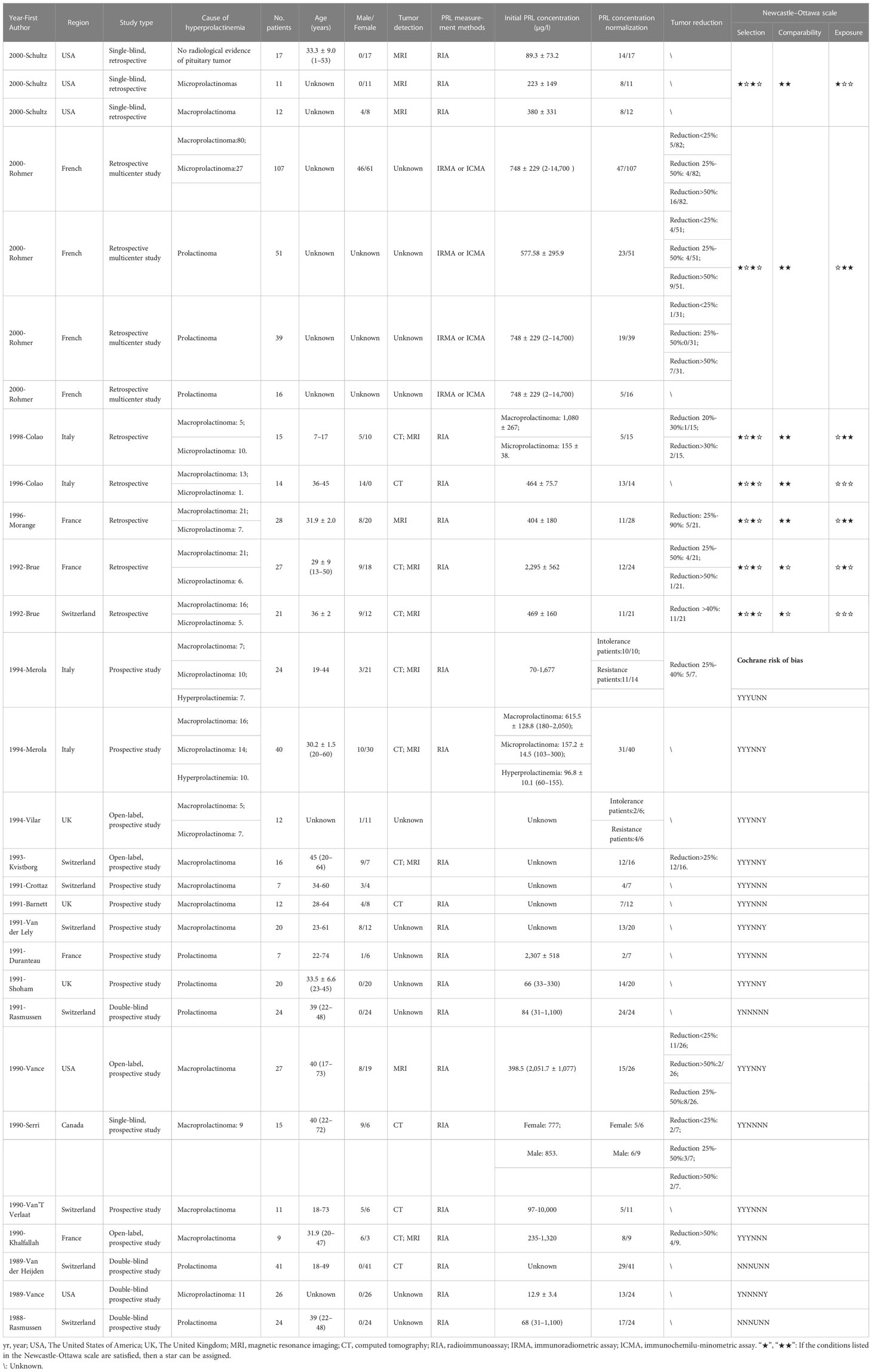
Table 2 Characteristics, Cochrane risk of bias, and Newcastle–Ottawa scale of included studies with single arm.
3.3 The efficacy and safety of quinagolide
3.3.1 Efficacy of quinagolide
3.3.1.1 The efficacy of serum PRL: Prolactin. concentration normalization
There were 24 studies with 41 single and 10 double arms that referred to the efficacy of serum PRL concentration normalization. The pooled proportion of normalized PRL concentration was 69% (95% CI: 61%–76%) (Figure 2A). For different initial serum PRL concentrations, we divided the initial serum PRL concentration into three levels based on the guidelines, which indicated that two thresholds (250 and 500 ng/ml) reflected the situations of hyperprolactinemia (4). The pooled proportions of normalized PRL concentrations for patients with low (<250 ng/ml), moderate (250–500 ng/ml), and high (>500 ng/ml) PRL levels were 0.81 (95% CI: 0.71–0.90), 0.59 (95% CI: 0.44–0.75), and 0.55 (95% CI: 0.43–0.67), respectively (Figure S1). In terms of patients with different sizes of prolactinomas, the pooled proportion of normalized PRL concentrations for patients with microprolactinoma was 90% (95% CI: 68%–100%) and that for patients with macroprolactinoma was 76% (95% CI: 69%–84%) (Figure S2). In terms of different treatment lines, the pooled proportion of normalized PRL concentrations for first-line CV treatment was 89% (95% CI: 77%–100%) and that for second-line treatment CV (patients with CAB/BRC resistance or intolerance) was 56% (95% CI: 46%–67%) (Figure S3). When comparing CAB and BRC, the pooled RRs of the proportion of PRL normalization were 0.97 (95% CI: 0.74–1.27) and 1.06 (95% CI: 0.79–1.42), respectively (Figure 2B).
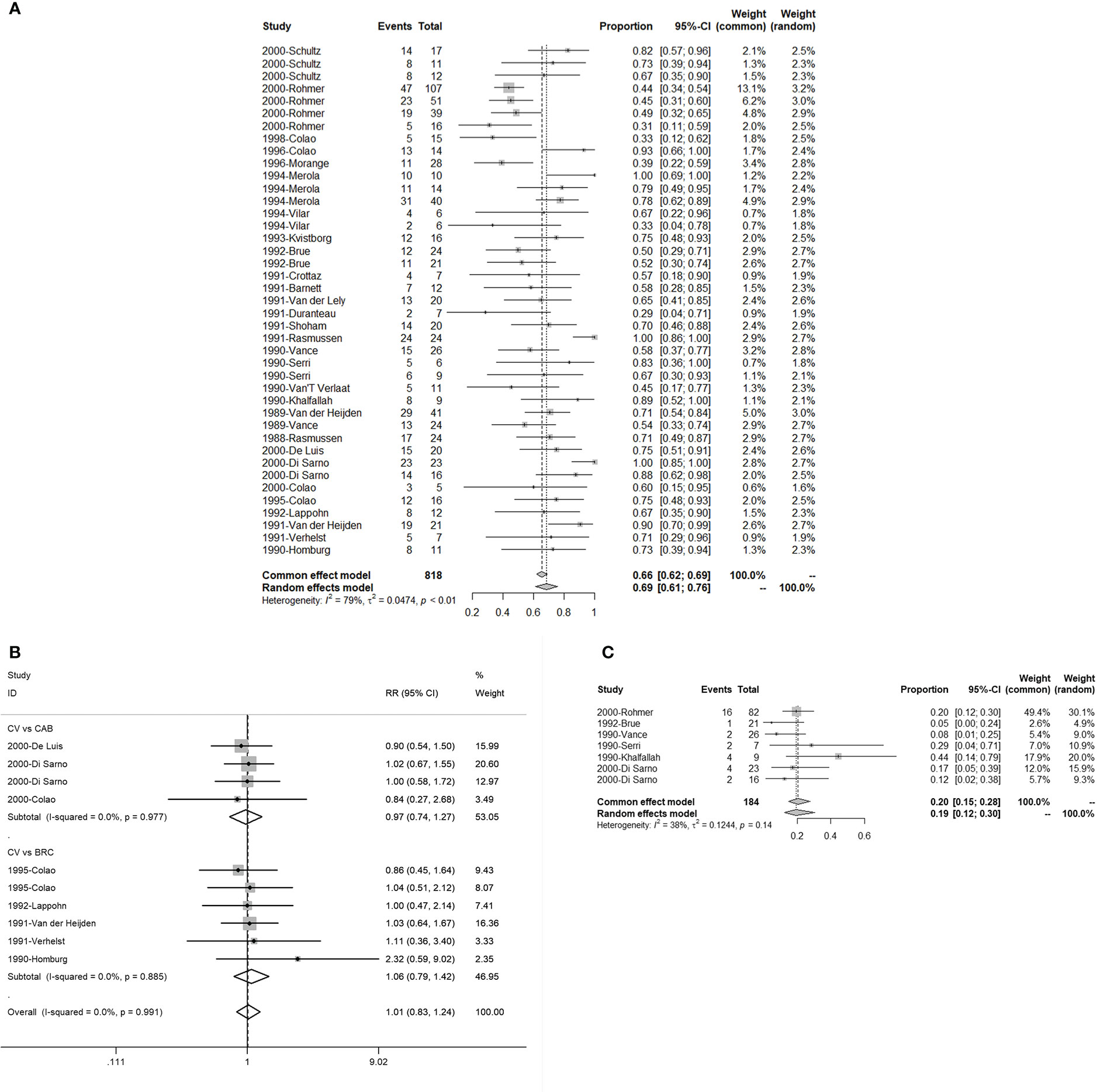
Figure 2 The efficacy of quinagolide (CV) in hyperprolactinemia and prolactinoma treatment. (A) The bulk efficacy of CV treatment in hyperprolactinemia; (B) The pooled risk ratios (RRs) of efficacy between CV vs. cabergoline (CAB) and CV vs. bromocriptine (BRC); (C) The efficacy of CV treatment in prolactinomas.
3.3.1.2 The efficacy of tumor reduction (>50%)
There were seven studies with eight double arms that referred to the efficacy of tumor reduction (>50%). However, the heterogeneity of the seven included studies was statistically significant (Figure S4A). The sensitivity analysis indicated that the study by Colao published in 2000 had a large impact on robustness (Figure S4B) (37). After removing this study, the heterogeneity of the remaining six studies was not significant (Figure 2C). The sensitivity analysis showed that the included studies were robust (Figure 3B). Moreover, a funnel plot did not find a potential publication bias (Figure 4B). The pooled proportion of tumor reduction (>50%) was 20% (95% CI: 15%–28%) (Figure 2C).
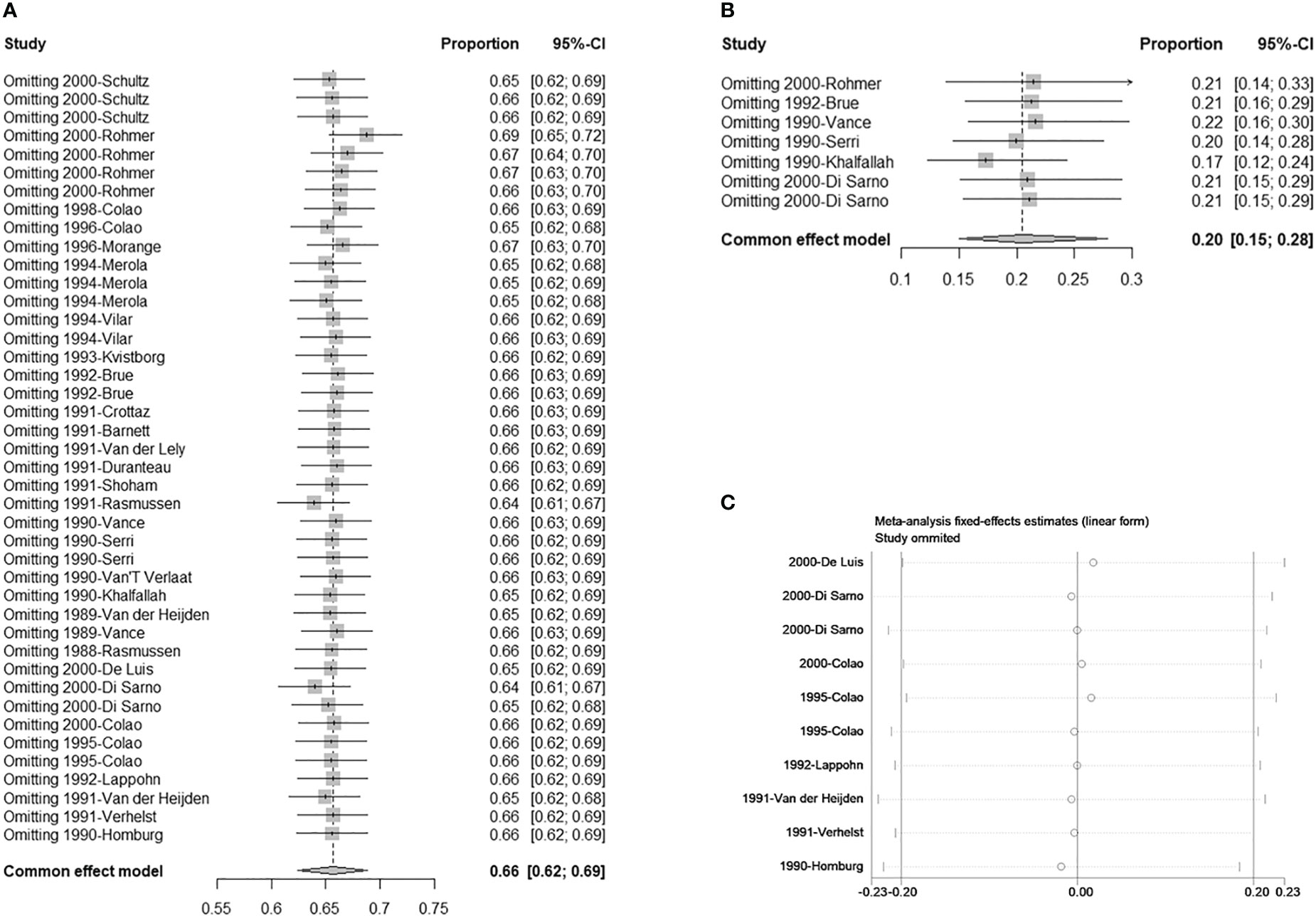
Figure 3 Sensitivity analysis. (A) Single-armed studies related to hyperprolactinemia treatment; (B) Single-armed studies related to prolactinoma treatment; (C) Double-armed studies.
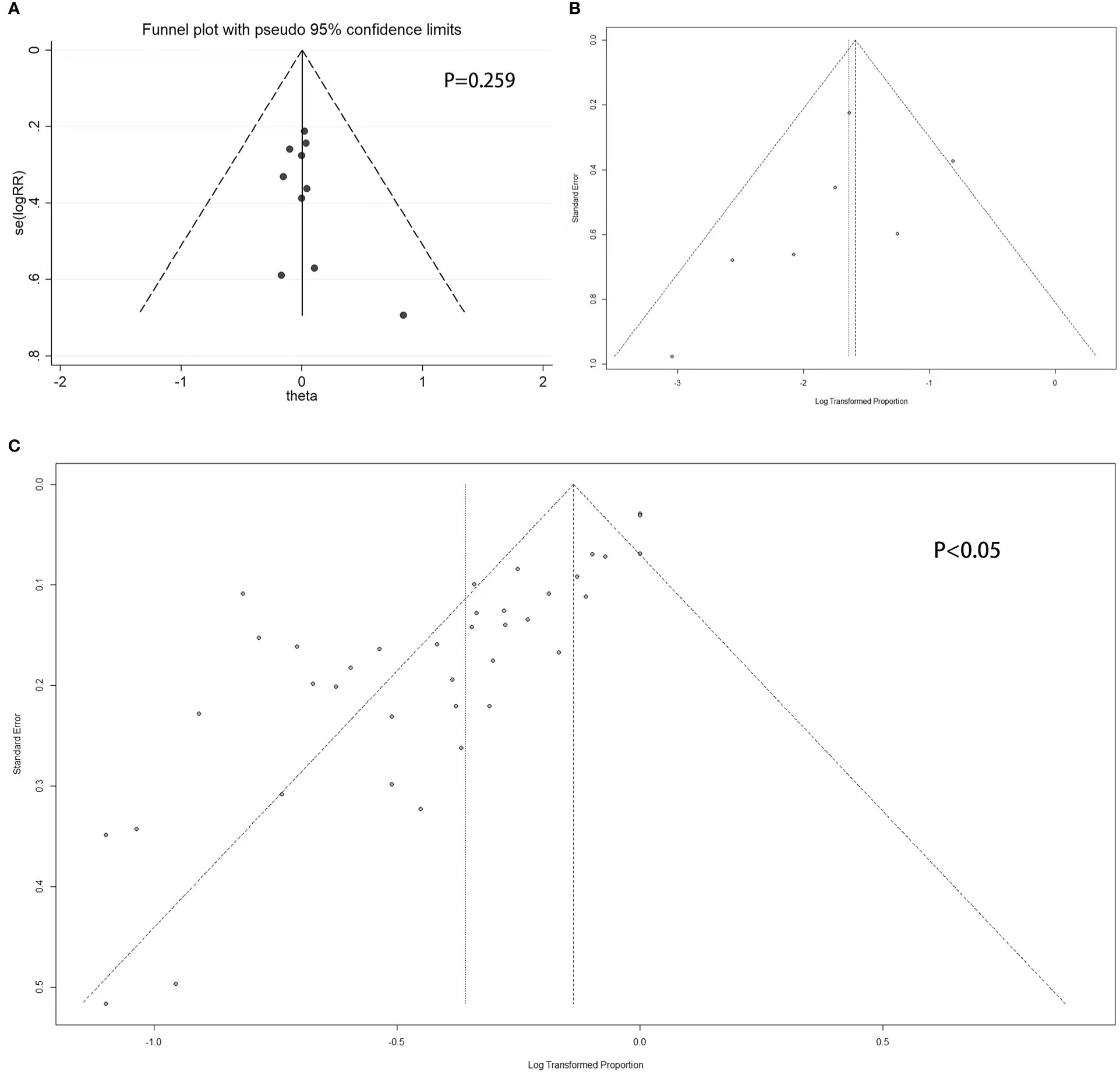
Figure 4 Funnel plot. (A) Double-armed studies; (B) Single-armed studies related to prolactinoma treatment; (C) Single-armed studies related to hyperprolactinemia treatment.
3.3.2 Safety of quinagolide
There were 24 studies with 119 single arms that referred to the safety of CV. The pooled proportion of side effects under CV was 13% (95% CI: 11%–16%) (Figure 5). In terms of the different types of side effects, the pooled proportions of constipation, depression, dizziness, drowsiness, drug discontinuance, fatigue, headache, muscle pain, nasal congestion, nasal stuffiness, nausea, palpitation, tiredness, vomiting, weight loss, other digestive disorders, other mental disorders, postural related disorders, and sleep disorders were 14% (95% CI: 5%–32%), 7% (95% CI: 3%–16%), 20% (95% CI: 14%–27%), 4% (95% CI: 2%–10%), 9% (95% CI: 5%–14%), 29% (95% CI: 20%–40%), 20% (95% CI: 13%–30%), 2% (95% CI: 0%–6%), 11% (95% CI: 4%–28%), 13% (95% CI: 6%–25%), 23% (95% CI: 16%–31%), 23% (95% CI: 10%–46%), 16% (95% CI: 8%–31%), 9% (95% CI: 5%–15%), 6% (95% CI: 1%–25%), 13% (95% CI: 8%–22%), 8% (95% CI: 4%–15%), 12% (95% CI: 4%–31%), and 16% (95% CI: 5%–39%), respectively (Figure 5). Detailed results are shown in Figure 5.
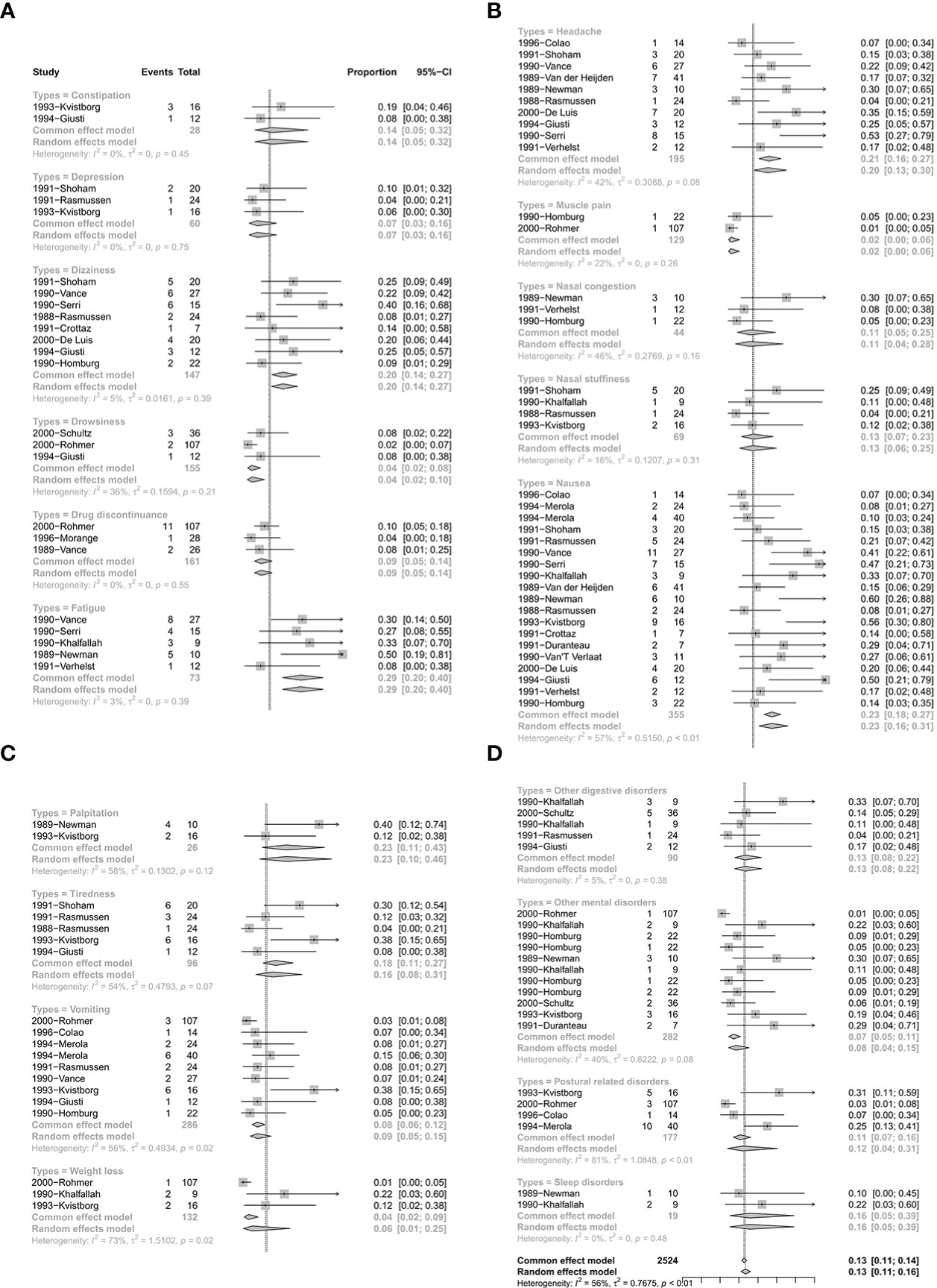
Figure 5 The safety of quinagolide treatment. (A) The pooled proportion of constipation, depression, dizziness, drowsiness, drug discontinuance, fatigue; (B) The pooled proportion of headache, muscle pain, nasal congestion, nasal stuffiness, nausea; (C) The pooled proportion of palpitation, tiredness, vomiting, weight loss; (D) The pooled proportion of other digestive disorders, other mental disorders, postural disorders, sleep disorders.
3.4 Sensitivity analysis, stratified analysis, and meta-regression
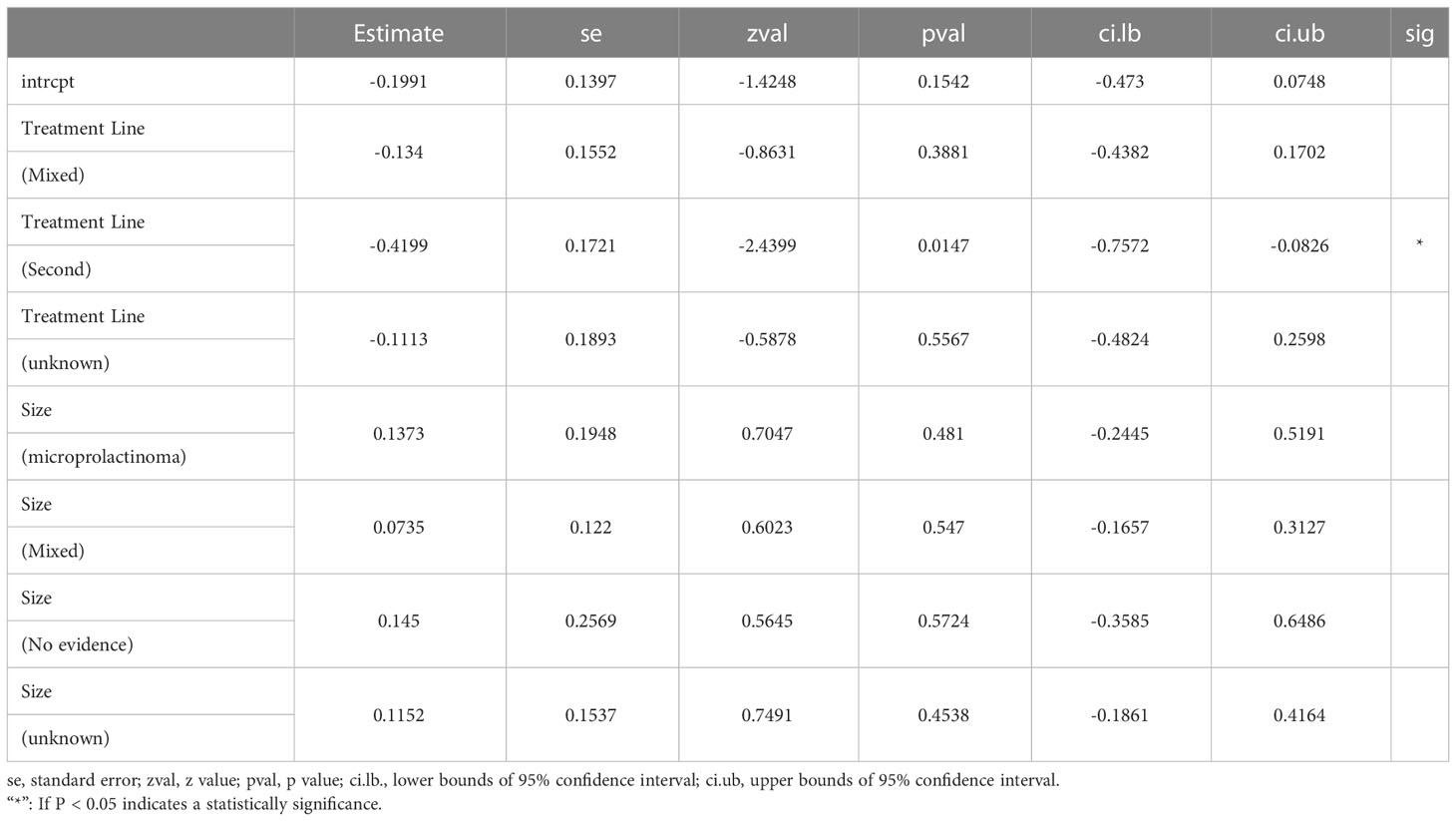
The sensitivity analyses of single arms referring to normalized PRL concentrations and corrected tumor reduction, as well as double arms referring to CV vs. CAB and BRC, were robust (Figures 3A–C). In the stratified analysis and meta-regression, studies were divided according to treatment lines and sizes of prolactinomas. As shown in Table 3, only studies referring to second-line treatments had statistical significance (P = 0.0147 < 0.05), which indicated that they may be the source of the heterogeneity (Table 3).
3.5 Publication bias
For double-armed studies concerning the efficacy of PRL concentration normalization, the funnel plot did not show a potential publication bias, with a non-statistically significant Egger’s test result (P = 0.259 > 0.05) (Figure 4A). For studies related to the efficacy of tumor reduction (>50%), the funnel plot did not show a potential publication bias (Figure 4B), whereas the Egger’s test was not performed due to the small number of studies. For single-armed studies concerning the efficacy of PRL concentration normalization, a funnel plot showed the potential of publication bias, with statistically significant Egger’s test results (P < 0.05) (Figure 4C).
4 Discussion
In our results, the pooled proportion of normalized PRL concentrations was 69% under CV treatment, which is a relatively satisfactory value. The efficacy of CV is affected by the size of prolactinomas that were initially detected, the treatment line, and the initial serum PRL concentration. When the initial serum PRL concentration was more than 500 ng/ml or larger tumors were detected at the time of diagnosis, the efficacy was significantly reduced (Figures S1, S2). Likewise, the use of CV as a first-line treatment resulted in better efficacy (Figure S3). Compared with CAB and BRC, the pooled RRs of the proportion of PRL normalization were 0.97 and 1.06 when compared with CAB and BRC, respectively (Figure 2B). These results indicate that CV is not less effective than CAB and BRC in treating hyperprolactinemia.
For tumor shrinkage, the pooled proportion of more than 50% tumor shrinkage after CV treatment was 20% (Figure 2C), which is less effective than CAB and BRC, wherein these treatments had overall therapeutic outcomes that were reported to be more than 20% and 30%, respectively (6).
When concerning the adverse effects of CV treatment, dizziness (20%), fatigue (29%), headache (20%), nausea (23%), and palpitations (23%) were more likely to occur (pooled proportion ≥20%). Although multiple reports in the literature have confirmed that CAB seems to be safe at the doses that were employed in hyperprolactinemic patients, which is still unsettled. The incidence of asymptomatic tricuspid regurgitation is higher when detected via systematic echocardiography (44–46). It has been reported in the literature that echocardiography is recommended in patients with hyperprolactinemia who are under CAB therapy (46). This undoubtedly increases the difficulty of patient compliance and the costs of treatment. To date, there are no reports of cardiac valve disease associated with CV in the treatment of hyperprolactinemia.
For the robustness of our study, the heterogeneity was statistically significant (Figures 2A, S4A). We then performed a sensitivity analysis, Egger’s test, and funnel plots to evaluate and attempt to determine the source of the heterogeneity. In the sensitivity analysis of studies related to tumor shrinkage, we found that the study by Colao published in 2000 was relatively more sensitive because the pooled proportion was changed from 32% (95% CI: 25%–41%) to 20% (95% CI: 15%–28%) (Figures S4B, 3B). After removing this study, the heterogeneity was significantly reduced, with the value changing from 80% I2 (P < 0.01) to 38% I2 (P = 0.14) (Figures 2C, S4A). However, the sensitivity analysis of the studies related to PRL normalization did not identify the source of the heterogeneity. We further performed a meta-regression analysis to try to verify whether the basic information (including treatment line and tumor size) reduced the robustness. In the results of the meta-regression, studies related to second-line CV treatment may be the source of the heterogeneity (Table 3). Subsequently, the publication bias was evaluated via a funnel plot and Egger’s test. The results showed a publication bias in the studies with a single arm.
Although our systematic review and meta-analysis showed that the overall success rate of PRL normalization after CV was 69%, it is worth mentioning that the current definition of remission is that the serum PRL concentration is normalized and persistent for more than 2 years. So, the data on CV may lack accuracy. To better evaluate the efficacy and safety of CV, more CV-related studies with more than 2 years of follow-up are needed.
Currently, there are many feasible options for hyperprolactinemia and prolactinoma treatments, including surgery, radiotherapy, and DAs. Surgery and radiotherapy are relatively difficult for patients to accept due to their disadvantages, such as considerable trauma and obvious side effects. Hence, DAs represent a first-line treatment. The sole reliance on drugs to treat tumors is also the future trend of tumor prevention and treatment. CV is not less effective than CAB and BRC in treating hyperprolactinemia, and the side effects were not significant. We may consider CV as an alternative first-line or rescue treatment.
There were some limitations in our study. First, due to the fact that the common DAs that are used for hyperprolactinemia and prolactinoma treatments in recent years are CAB and BRC, only a few studies have reported on the efficacy of CV in hyperprolactinemia and prolactinoma treatments; hence, CV-related studies have mostly been published before 2000 without enough follow-up time. Therefore, the overall quality of the included studies was low. There are not enough data to evaluate the long-term remission rate and risk of recurrence after CV treatment. Second, most of the included patients in these studies were from Europe, and it is unclear as to whether CV is suitable for people from continents other than Europe. Thus, we suggest that more CV–hyperprolactinemia-related studies with more than 2 years of follow-up and included patients from different areas around the world be performed.
5 Conclusion
Our study showed that CV is not less effective than CAB and BRC in treating hyperprolactinemia, and the side effects were not significant. More CV-related studies are also needed in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YYZ wrote this article. YZZ and QLH processed the data. YYZ and QLH contributed equally. MHL reviewed all the work, and other authors participated in the whole work. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Natural Science Foundation of China (NSFC) (No. 81860225), the key research and development plan of Jiangxi Province (No. 20203BBG73060), natural science foundation of Jiangxi Province (No. 20212BAB206029), science and technology project of Jiangxi Provincial Health Care Commission (No.20195116), and young talents research and cultivation foundation of the First Affiliated Hospital of Nanchang University (No. YFYPY202038).
Acknowledgments
The authors thank MHL for editorial assistance in preparing this paper and YZZ for the statistical analysis.
Conflict of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1027905/full#supplementary-material
Supplementary Figure 1 | The efficacy of quinagolide treatment in hyperprolactinemia. Subgroup analysis is based on different level of initial serum prolactin concentration.
Supplementary Figure 2 | The efficacy of quinagolide treatment in hyperprolactinemia. Subgroup analysis is based on size of prolactinomas at diagnosis.
Supplementary Figure 3 | The efficacy of quinagolide treatment in hyperprolactinemia. Subgroup analysis is based on treatment time of quinagolide.
Supplementary Figure 4 | The forest plot and sensitivity analysis of studies related to tumor shrinkage before correction. (A) Forest plot; (B) Sensitivity analysis.
References
1. Melmed S. Mechanisms for pituitary tumorigenesis: The plastic pituitary. J Clin Invest (2003) 112:1603–18. doi: 10.1172/JCI20401
2. Chanson P, Maiter D. The epidemiology, diagnosis and treatment of prolactinomas: The old and the new. Best Pract Res Clin Endocrinol Metab (2019) 33:101290. doi: 10.1016/j.beem.2019.101290
3. Schlechte J, el-Khoury G, Kathol M, Walkner L. Forearm and vertebral bone mineral in treated and untreated hyperprolactinemic amenorrhea. J Clin Endocrinol Metab (1987) 64:1021–6. doi: 10.1210/jcem-64-5-1021
4. Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, et al. Diagnosis and treatment of hyperprolactinemia: An endocrine society clinical practice guideline. J Clin Endocrinol Metab (2011) 96:273–88. doi: 10.1210/jc.2010-1692
5. Dekkers OM, Lagro J, Burman P, Jorgensen JO, Romijn JA, Pereira AM. Recurrence of hyperprolactinemia after withdrawal of dopamine agonists: Systematic review and meta-analysis. J Clin Endocrinol Metab (2010) 95:43–51. doi: 10.1210/jc.2009-1238
6. Xia MY, Lou XH, Lin SJ, Wu ZB. Optimal timing of dopamine agonist withdrawal in patients with hyperprolactinemia: A systematic review and meta-analysis. Endocrine (2018) 59:50–61. doi: 10.1007/s12020-017-1444-9
7. Dogansen SC, Selcukbiricik OS, Tanrikulu S, Yarman S. Withdrawal of dopamine agonist therapy in prolactinomas: In which patients and when? Pituitary (2016) 19:303–10. doi: 10.1007/s11102-016-0708-3
8. Hu J, Zheng X, Zhang W, Yang H. Current drug withdrawal strategy in prolactinoma patients treated with cabergoline: A systematic review and meta-analysis. Pituitary (2015) 18:745–51. doi: 10.1007/s11102-014-0617-2
9. van der Lely AJ, Brownell J, Lamberts SW. The efficacy and tolerability of CV 205-502 (a nonergot dopaminergic drug) in macroprolactinoma patients and in prolactinoma patients intolerant to bromocriptine. J Clin Endocrinol Metab (1991) 72:1136–41. doi: 10.1210/jcem-72-5-1136
10. Colao A, Pivonello R, Di Somma C, Savastano S, Grasso LF, Lombardi G. Medical therapy of pituitary adenomas: Effects on tumor shrinkage. Rev Endocr Metab Disord (2009) 10:111–23. doi: 10.1007/s11154-008-9107-z
11. Klibanski A. Clinical practice. prolactinomas. N Engl J Med (2010) 362:1219–26. doi: 10.1056/NEJMcp0912025
12. Schultz PN, Ginsberg L, McCutcheon IE, Samaan N, Leavens M, Gagel RF. Quinagolide in the management of prolactinoma. Pituitary (2000) 3:239–49. doi: 10.1023/A:1012884214668
13. Rohmer V, Freneau E, Morange I, Simonetta C. Efficacy of quinagolide in resistance to dopamine agonists: Results of a multicenter study. club de l'Hypophyse. Ann Endocrinol (Paris) (2000) 61:411–7. doi: 10.1046/j.1365-2265.2000.01016.X
14. Colao A, Loche S, Cappa M, Di Sarno A, Landi ML, Sarnacchiaro F, et al. Prolactinomas in children and adolescents. clinical presentation and long-term follow-up. J Clin Endocrinol Metab (1998) 83:2777–80. doi: 10.1210/jcem.83.8.5001
15. Colao A, De Rosa M, Sarnacchiaro F, Di Sarno A, Landi ML, Iervolino E, et al. Chronic treatment with CV 205-502 restores the gonadal function in hyperprolactinemic males. Eur J Endocrinol (1996) 135:548–52. doi: 10.1530/eje.0.1350548
16. Morange I, Barlier A, Pellegrini I, Brue T, Enjalbert A, Jaquet P. Prolactinomas resistant to bromocriptine: Long-term efficacy of quinagolide and outcome of pregnancy. Eur J Endocrinol (1996) 135:413–20. doi: 10.1530/eje.0.1350413
17. Brue T, Pellegrini I, Priou A, Morange I, Jaquet P. Prolactinomas and resistance to dopamine agonists. Horm Res (1992) 38:84–9. doi: 10.1159/000182496
18. Brue T, Pellegrini I, Gunz G, Morange I, Dewailly D, Brownell J, et al. Effects of the dopamine agonist CV 205-502 in human prolactinomas resistant to bromocriptine. J Clin Endocrinol Metab (1992) 74:577–84. doi: 10.1210/jcem.74.3.1346788
19. Merola B, Sarnacchiaro F, Colao A, Di Somma C, Di Sarno A, Ferone D, et al. Positive response to compound CV 205-502 in hyperprolactinemic patients resistant to or intolerant of bromocriptine. Gynecol Endocrinol (1994) 8:175–81. doi: 10.3109/09513599409072452
20. Merola B, Sarnacchiaro F, Colao A, Di Somma C, Di Sarno A, Landi ML, et al. CV 205-502 in the treatment of tumoral and non-tumoral hyperprolactinemic states. BioMed Pharmacother (1994) 48:167–74. doi: 10.1016/0753-3322(94)90105-8
21. Vilar L, Burke CW. Quinagolide efficacy and tolerability in hyperprolactinaemic patients who are resistant to or intolerant of bromocriptine. Clin Endocrinol (Oxf) (1994) 41:821–6. doi: 10.1111/j.1365-2265.1994.tb02799.x
22. Kvistborg A, Halse J, Bakke S, Bjoro T, Hansen E, Djoseland O, et al. Long-term treatment of macroprolactinomas with CV 205-502. Acta Endocrinol (Copenh) (1993) 128:301–7. doi: 10.1530/acta.0.1280301
23. Crottaz B, Uske A, Reymond MJ, Rey F, Siegel RA, Brownell J, et al. CV 205-502 treatment of macroprolactinomas. J Endocrinol Invest (1991) 14:757–62. doi: 10.1007/BF03347910
24. Barnett PS, Palazidou E, Miell JP, Coskeran PB, Butler J, Dawson JM, et al. Endocrine function, psychiatric and clinical consequences in patients with macroprolactinomas after long-term treatment with the new non-ergot dopamine agonist CV205-502. Q J Med (1991) 81:891–906. doi: 10.1055/s-2008-1063739
25. Duranteau L, Chanson P, Lavoinne A, Horlait S, Lubetzki J, Kuhn JM. Effect of the new dopaminergic agonist CV 205-502 on plasma prolactin levels and tumour size in bromocriptine-resistant prolactinomas. Clin Endocrinol (Oxf) (1991) 34:25–9. doi: 10.1111/j.1365-2265.1991.tb01731.x
26. Shoham Z, Homburg R, Jacobs HS. CV 205-502–effectiveness, tolerability, and safety over 24-month study. Fertil Steril (1991) 55:501–6. doi: 10.1016/S0015-0282(16)54175-2
27. Rasmussen C, Brownell J, Bergh T. Clinical response and prolactin concentration in hyperprolactinemic women during and after treatment for 24 months with the new dopamine agonist, CV 205-502. Acta Endocrinol (Copenh) (1991) 125:170–6. doi: 10.1530/acta.0.1250170
28. Vance ML, Lipper M, Klibanski A, Biller BM, Samaan NA, Molitch ME. Treatment of prolactin-secreting pituitary macroadenomas with the long-acting non-ergot dopamine agonist CV 205-502. Ann Intern Med (1990) 112:668–73. doi: 10.7326/0003-4819-112-9-668
29. Serri O, Beauregard H, Lesage J, Pedneault L, Comtois R, Jilwan N, et al. Long term treatment with CV 205-502 in patients with prolactin-secreting pituitary macroadenomas. J Clin Endocrinol Metab (1990) 71:682–7. doi: 10.1210/jcem-71-3-682
30. van't Verlaat JW, Croughs RJ, Brownell J. Treatment of macroprolactinomas with a new non-ergot, long-acting dopaminergic drug, CV 205-502. Clin Endocrinol (Oxf) (1990) 33:619–24. doi: 10.1111/j.1365-2265.1990.tb03900.x
31. Khalfallah Y, Claustrat B, Grochowicki M, Flocard F, Horlait S, Serusclat P, et al. Effects of a new prolactin inhibitor, CV 205-502, in the treatment of human macroprolactinomas. J Clin Endocrinol Metab (1990) 71:354–9. doi: 10.1210/jcem-71-2-354
32. van der Heijden PF, Lappohn RE, Corbey RS, de Goeij WB, Brownell J, Rolland R. The effectiveness, safety, and tolerability of CV 205-502 in hyperprolactinemic women: A 12-month study. Fertil Steril (1989) 52:574–9. doi: 10.1016/S0015-0282(16)60966-4
33. Vance ML, Cragun JR, Reimnitz C, Chang RJ, Rashef E, Blackwell RE, et al. CV 205-502 treatment of hyperprolactinemia. J Clin Endocrinol Metab (1989) 68:336–9. doi: 10.1210/jcem-68-2-336
34. Rasmussen C, Bergh T, Wide L, Brownell J. Long-term treatment with a new non-ergot long-acting dopamine agonist, CV 205-502, in women with hyperprolactinaemia. Clin Endocrinol (Oxf) (1988) 29:271–9. doi: 10.1111/j.1365-2265.1988.tb01225.x
35. De Luis DA, Becerra A, Lahera M, Botella JI, Varela C. A randomized cross-over study comparing cabergoline and quinagolide in the treatment of hyperprolactinemic patients. J Endocrinol Invest (2000) 23:428–34. doi: 10.1007/BF03343751
36. Di Sarno A, Landi ML, Marzullo P, Di Somma C, Pivonello R, Cerbone G, et al. The effect of quinagolide and cabergoline, two selective dopamine receptor type 2 agonists, in the treatment of prolactinomas. Clin Endocrinol (Oxf) (2000) 53:53–60. doi: 10.1046/j.1365-2265.2000.01016.x
37. Colao A, Ferone D, Lastoria S, Cerbone G, Di Sarno A, Di Somma C, et al. Hormone levels and tumour size response to quinagolide and cabergoline in patients with prolactin-secreting and clinically non-functioning pituitary adenomas: Predictive value of pituitary scintigraphy with 123I-methoxybenzamide. Clin Endocrinol (Oxf) (2000) 52:437–45. doi: 10.1046/j.1365-2265.2000.00951.x
38. Colao A, Merola B, Sarnacchiaro F, Di Sarno A, Landi ML, Marzullo P, et al. Comparison among different dopamine-agonists of new formulation in the clinical management of macroprolactinomas. Horm Res (1995) 44:222–8. doi: 10.1159/000184630
39. Giusti M, Porcella E, Carraro A, Cuttica M, Valenti S, Giordano G. A cross-over study with the two novel dopaminergic drugs cabergoline and quinagolide in hyperprolactinemic patients. J Endocrinol Invest (1994) 17:51–7. doi: 10.1007/BF03344963
40. Lappohn RE, van de Wiel HB, Brownell J. The effect of two dopaminergic drugs on menstrual function and psychological state in hyperprolactinemia. Fertil Steril (1992) 58:321–7. doi: 10.1016/S0015-0282(16)55201-7
41. van der Heijden PF, de Wit W, Brownell J, Schoemaker J, Rolland R. CV 205-502, a new dopamine agonist, versus bromocriptine in the treatment of hyperprolactinaemia. Eur J Obstet Gynecol Reprod Biol (1991) 40:111–8. doi: 10.1016/0028-2243(91)90101-p
42. Verhelst JA, Froud AL, Touzel R, Wass JA, Besser GM, Grossman AB. Acute and long-term effects of once-daily oral bromocriptine and a new long-acting non-ergot dopamine agonist, quinagolide, in the treatment of hyperprolactinemia: A double-blind study. Acta Endocrinol (Copenh) (1991) 125:385–91. doi: 10.1530/acta.0.1250385
43. Homburg R, West C, Brownell J, Jacobs HS. A double-blind study comparing a new non-ergot, long-acting dopamine agonist, CV 205-502, with bromocriptine in women with hyperprolactinaemia. Clin Endocrinol (Oxf) (1990) 32:565–71. doi: 10.1111/j.1365-2265.1990.tb00899.x
44. Stiles CE, Tetteh-Wayoe ET, Bestwick J, Steeds RP, Drake WM. A meta-analysis of the prevalence of cardiac valvulopathy in hyperprolactinemic patients treated with cabergoline. J Clin Endocrinol Metab (2018) 104(2):523–38. doi: 10.1210/jc.2018-01071
45. Vargas ML, Cervantes CE, Hernando CA, Da Costa CV. [Cabergoline in hyperprolactinemia and valvular heart disease]. Endocrinol Nutr (2009) 56:412–7. doi: 10.1016/S1575-0922(09)72711-1
Keywords: hyperprolactinemia, prolactinomas, dopamine agonist, quinagolide, meta-analysis, cabergoline, bromocriptine, efficacy
Citation: Zeng Y, Huang Q, Zou Y, Tan J, Zhou W and Li M (2023) The efficacy and safety of quinagolide in hyperprolactinemia treatment: A systematic review and meta-analysis. Front. Endocrinol. 14:1027905. doi: 10.3389/fendo.2023.1027905
Received: 25 August 2022; Accepted: 02 January 2023;
Published: 24 January 2023.
Edited by:
Gianluca Tamagno, Hermitage Medical Clinic, IrelandReviewed by:
Eliza B. Geer, Memorial Sloan Kettering Cancer Center, United StatesLeila Warszawski, Instituto Estadual de Diabetes e Endocrinologia Luiz Capriglione, Brazil
Copyright © 2023 Zeng, Huang, Zou, Tan, Zhou and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meihua Li, bmR5ZnkwMTgxNUBuY3UuZWR1LmNu; bGltZWlodWEyMDAwQHNpbmEuY29t
†These authors have contributed equally to this work
 Yanyang Zeng
Yanyang Zeng Qingliang Huang2†
Qingliang Huang2† Yunzhi Zou
Yunzhi Zou Wu Zhou
Wu Zhou Meihua Li
Meihua Li