- 1Department of Obstetrics and Gynecology, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, China
- 3Reproductive Endocrinology and Regulation Laboratory, West China Second University Hospital, Sichuan University, Chengdu, China
- 4The Joint Laboratory for Reproductive Medicine of Sichuan University, The Chinese University of Hong Kong, Chengdu, China
- 5National Clinical Research Center for Geriatrics, Department of Gerontology and Geriatrics, West China Hospital, Sichuan University, Chengdu, China
Objective: To evaluate the effect of vitamin D supplementation on pregnancy and ovulation in patients with polycystic ovary syndrome.
Method: We searched Pubmed, Medline (via Ovid, 1974 to 2020), EMBASE (via Ovid, 1974 to 2020), Cochrane Central Register of Controlled Trials (via Ovid), Web of Science, CNKI, WangFang and the Vip database from inception until April 2021. Two researchers independently screened articles, collected data and evaluated the quality, with Review manager 5.3 for meta-analysis.
Results: Totally 20 randomized controlled studies with 1961 subjects were included. Meta analysis showed that pregnancy rate [RR=1.44 (1.28, 1.62), p<0.00,001], ovulation rate [RR=1.42 (1.14, 1.78), p=0.002] and matured oocytes rate [RR=1.08 (1.03, 1.13), p=0.002] of vitamin D supplementation group were significantly higher than those of control group. Meanwhile, early miscarriage rate [RR=0.44 (0.30, 0.66), p<0.00,001], androgen level [MD=-2.31 (-3.51, -1.11), p=0.0002], luteinizing hormone [MD=-1.47 (-2.57, -0.36), p=0.009], follicle stimulating hormone [MD=-0.15 (-0.24, -0.05), p=0.002], and premature delivery rate [RR=0.38, 95% CI (0.21, 0.70), p=0.002] were declined significantly than the controls. However, only one article suggested that the progesterone [MD=6.52 (4.52, 8.52), p<0.05] in the vitamin D intervention group was increased. There was no notable difference in the biochemical pregnancy rate [RR=0.95 (0.55, 1.63), p=0.84], gestational hypertension rate [RR=0.40, 95% CI (0.15, 1.11), p=0.08], gestational diabetes mellitus rate [RR=0.27, 95% CI (0.05, 1.39), p=0.11], fertilization rate [RR=1.05 (1.00, 1.10), p=0.04], cleavage rate [RR=1.03 (0.99, 1.06), p=0.17], high-quality embryo rate [RR=1.08 (0.98, 1.20), p=0.10], endometrial thickness [MD=0.10], 77 (-0.23, 1.77), p=0.13], estrogen level [MD=-0.34 (-1.55, 0.87), p=0.59], LH/FSH [MD=-0.14, 95% CI (-0.48, 0.20), p=1.00] and anti-Mullerian hormone [MD=-0.22 (-0.65, 0.21), p=0.32].
Conclusion: Vitamin D supplementation contribute to the higher pregnancy and ovulation rates, and lower androgen, LH, FSH and early miscarriage rates in women with PCOS, regardless of the use of ovulation induction drugs or assisted reproductive technologies. However, no significant improvement was observed in fertilization rate or cleavage rate. Due to the limitation in quality of involved studies, more high-quality RCTs are needed for further validation.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO, identifier CRD42021250284.
1 Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of childbearing period, and 75% of these patients suffer from infertility (1). Existing treatments of infertility in PCOS mainly include monitoring ovulation, ovulation induction, ovarian perforation and assisted reproduction technologies (2). In addition, the supplementation of some nutrients has received some attention as an adjunction, such as vitamins, unsaturated fatty acids, minerals, etc. (3). Notably, about 67 – 85% of PCOS patients have concomitant vitamin D deficiency (4). What’s more, vitamin D deficiency may be involved in the pathogenesis of insulin resistance and metabolic syndrome in PCOS (5). These days, vitamin D supplementation draw some attentions in the preparation for pregnancy in PCOS patients, and the possible mechanism relies on its protection against oxidative stress and keep calcium ion levels to maintain the cell’s resting potential, which in turn benefits fertilization, cleavage, implantation, and placental formation (6).
However, current conclusions regarding the effect of vitamin D supplementation on pregnancy rates are inconsistent. Some studies have shown that vitamin D supplementation was able to increase pregnancy rates in PCOS patients, while others have proved that vitamin D supplementation had no significant improvement (7). Based on this argument, the present study systematically collated the existing evidence of vitamin D adjuvant treatment for infertility in PCOS patients, with the view of seeking evidence for whether vitamin D improves the pregnancy situation of PCOS patients and providing a basis for clinical medication.
2 Methods
2.1 Eligibility criteria, study selection and data extraction
This article followed the agreement registered in the International Prospective Systems Review Register (No. CRD42021250284).
2.1.1 Criteria included
2.1.1.1 Study type
Randomized controlled study.
2.1.1.2 Subjects
Patients with PCOS who had fertility requirements. The diagnostic criteria of PCOS follows the Rotterdam criteria in 2003, that at least two of the following three criteria were met:
2.1.1.2.1 Ovulatory dysfunction (oligoovulation/anovulation);
2.1.1.3 Interventions
Placebo, metformin, short acting contraceptives, ovulation induction drugs and assisted reproductive technology were defined as control group. Vitamin D supplemented based on control group (dosage form, time and dosage are not limited) were considered as the intervention group.
2.1.1.4 Outcome indicators
Main outcomes were clinical pregnancy rate and ovulation rate, and secondary outcomes were related outcomes like cumulative pregnancy rate, biochemical pregnancy rate, pregnancy complications, term delivery rate, premature delivery rate, matured oocytes rate, sex hormone level and adverse events. It was suggested that the ovulation manifestations include progesterone level ≥ 19 nmol/l,or after the dominant follicle (≥ 1.2mm) under ultrasound, the dominant follicle disappeared, collapsed or shrank by ≥ 50%, and a small amount of fluid accumulated in the rectal fossa. If the basal body temperature rose for 3 days and no ovulation was observed by ultrasonography, luteinized unruptured follicle syndrome (LUFS) would be diagnosed. The follow-up duration was 6 months.
2.1.2 Excluding criteria
Conference summary, animal experiment, cohort study, retrospective study and non- randomized controlled intervention study were excluded.
2.1.3 Data extraction
Two researchers (Yang Meina, Peng Jin) independently evaluated the articles and collected information. Inconsistencies were judged through discussion with the third author (Zhang Jing). The two researchers independently collected the following data: basic characteristics (author, year, country); Characteristics of subjects (age, number of people in each group) Outcome indicators (pregnancy related indicators: pregnancy rate, biochemical pregnancy rate, early miscarriage rate, incidence of pregnancy complications, assisted reproduction related indicators: fertilization rate, cleavage rate, high-quality embryo rate, fetal premature delivery rate, ovulation related indicators: ovulation rate, matured oocytes rate, endometrial thickness, sex hormone level and adverse events). We sent emails to study authors for more information.
2.2 Search strategy
We searched Pubmed, MEDLINE(via Ovid, 1974 to 2020), EMBASE (via Ovid, 1974 to 2020), Cochrane Central Register of Controlled Trials (CENTRAL) (via Ovid), Web of Science, CNKI, WangFang and the Vip database from inception until April 2021. We applied “vitamin d” “vitamin d1” “vitamin d2” “vitamin d3” “1, 25(OH)2 vitamin D” “cholecalciferol” “Polycystic Ovary Syndrome” “Randomized Controlled Trial” as search terms. The search strategy is available in Supplementary Information.
2.3 Data synthesis and analysis
Data analyses were conducted with the software Review Manager 5.3. For dichotomous data (ovulation and pregnancy rate for example), we used relative risk (RR). When it comes to continuous data (luteinizing hormone (LH) and follicle-stimulating hormone (FSH) and so on), mean difference (MD) was applied. When different scales were taken for the same, we performed standardized mean difference (SMD) instead. MD, if not mentioned, was calculated from the standard error, interquartile range or 95% confidence interval. If there were at least 5 studies, we synthesized the data. Cochrane risk of bias tool was implemented to access the quality of the evidence. We applied I² test to examine the heterogeneity. The fixed-effects model was applied when I² <50%, otherwise the random-effects model was conducted. Sensitivity analysis was utilized to distinguish the source of heterogeneity in Stata 12.0. We extracted data from images with Engauge Digitizer 4.1 if necessary. Begg’s and Egger’s test were performed to access the public bias.
3 Results
3.1 Studies selection and the flow chart
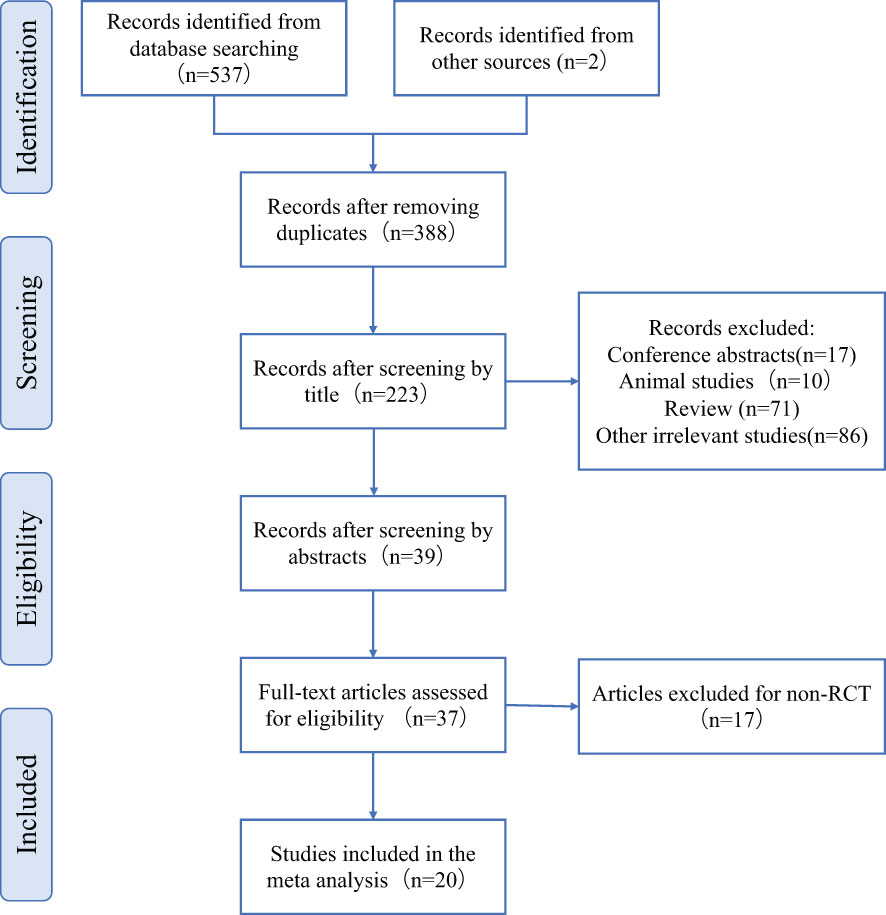
A total of 557 articles were retrieved, including 555 from the database and 2 from the references. After screening by EndnoteX8, 388 articles were left after removing duplicate articles (n=169).39 articles were left after removing conference abstract (n=17), animal experiment (n=10), and irrelevant articles (n=86) after browsing the title. We read the abstracts and carefully screened out non RCT studies (n=17) and no full text studies (n=2). A total of 20 articles were included:8 in English and 12 in Chinese (Figure 1).
3.2 Characteristics of included studies
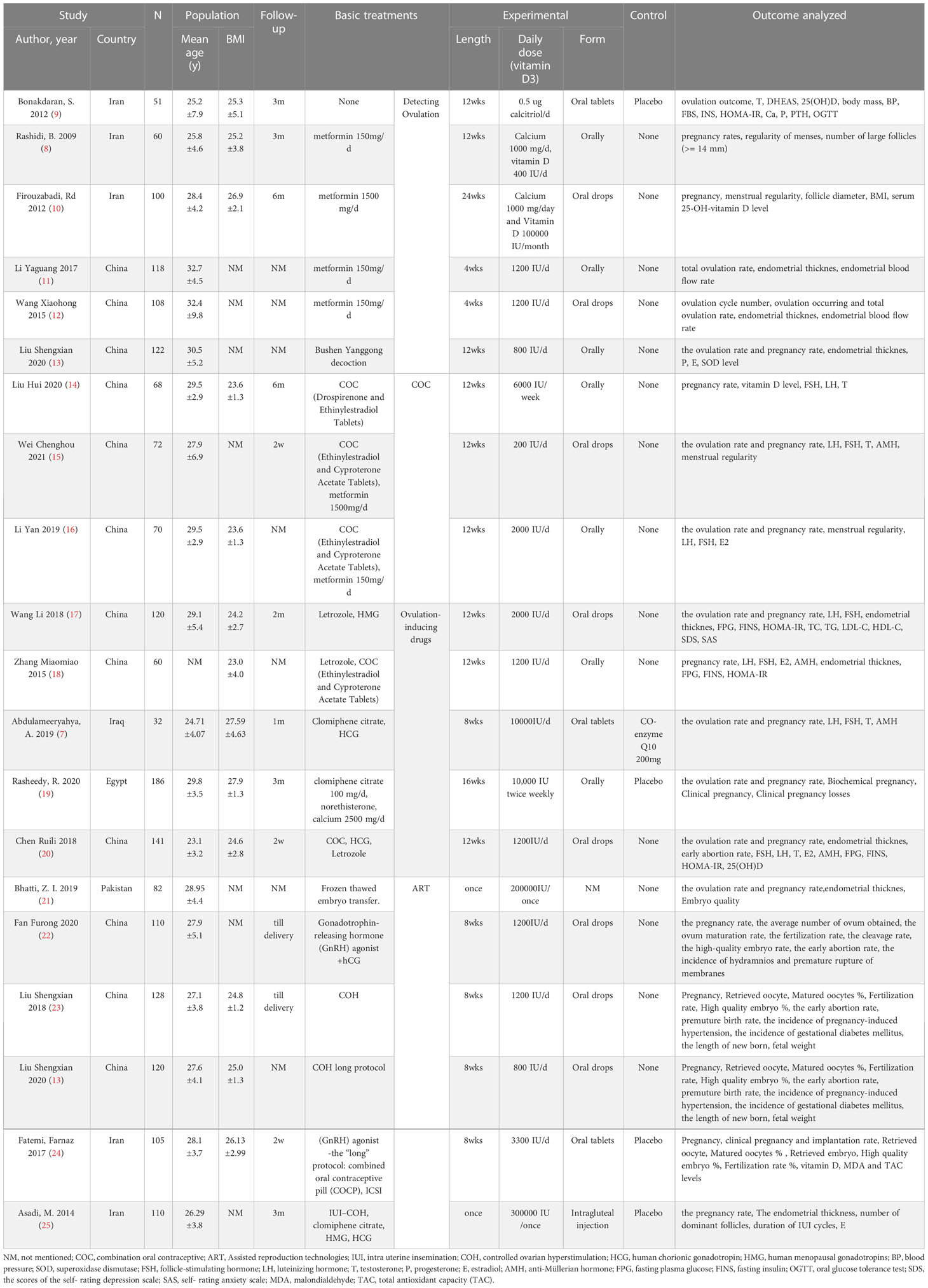
Table 1 presents the basic characteristics of the 20 RCTs including 1961 subjects. The average age of control group was 28.83 ± 4.35 years old, and it was 28.61 ± 4.56 years of age in the vitamin D intervention group. There was no statistical difference in the age of the subjects in studies. The baseline level of vitamin D in the control group was 15.40 ± 6.50 on average, and that in the intervention group was 14.22 ± 4.86. Both groups were in a state of vitamin D deficiency, which were comparable. Only two studies mentioned whether the subjects were secondary infertility, which was balanced and comparable between groups. The other articles did not mention the number of previous pregnancies. All included studies did not mention whether subjects experienced any pregnancy complication in the past. The follow-up time was from 2 weeks to 3 months. Two of the studies reported the delivery situation and subjects with fetal heart rate under ultrasound 5 weeks after embryo implantation and followed up until delivery. The intervention of vitamin D were intramuscular injection or long-term oral administration. The average daily dose varies from 200 IU to 10000 IU, and the intervention duration varies from a single dose, 8 weeks, 12 weeks and 24 weeks. In terms of outcome indicators, 18 studies reported pregnancy rate, 9 included ovulation rate, and 7 investigated matured oocytes rate (Table 1).
The included studies were summarized in Figures 2, 3 according to Cochrane risk of bias tool. Random methods used in 8 studies were random number table, 2 studies used blocked randomization, 1 study was conducted by blocked randomization, and the remaining 9 studies only mentioned randomization in words. In terms of allocation concealment, 2 studies used opaque envelopes, 2 articles mentioned it in words, and the remaining 16 did not mention it. 4 articles were blinding of participants, and the rest 16 articles did not mention, only one article used the blinding of personnel, and the other 19 articles failed to mention. None of the 20 articles mentioned the bias of selective reporting of research results and other sources bias.
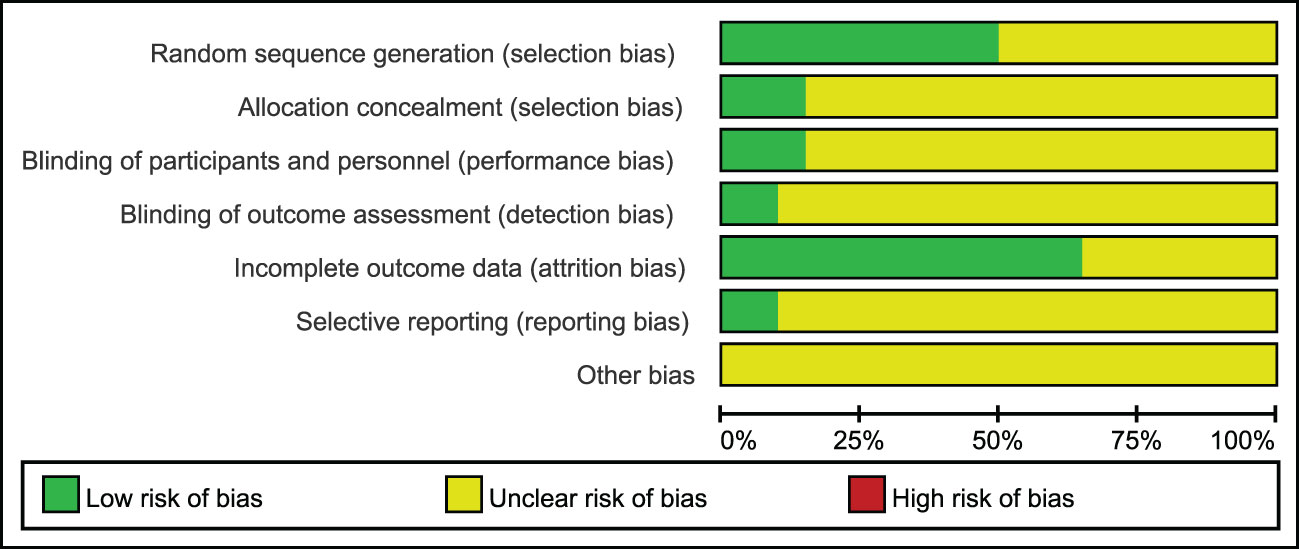
Figure 2 Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
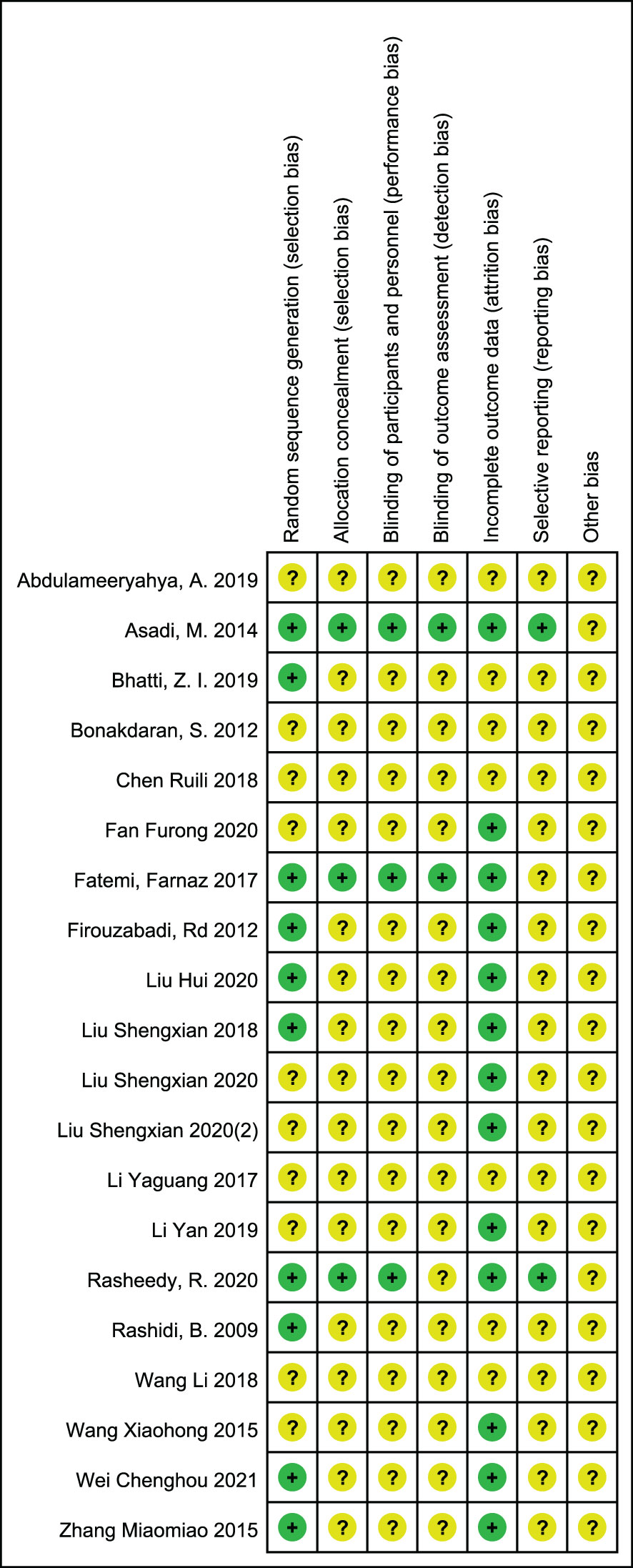
Figure 3 Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.3 Meta analysis
3.3.1 Pregnancy related outcomes
3.3.1.1 Pregnancy rate
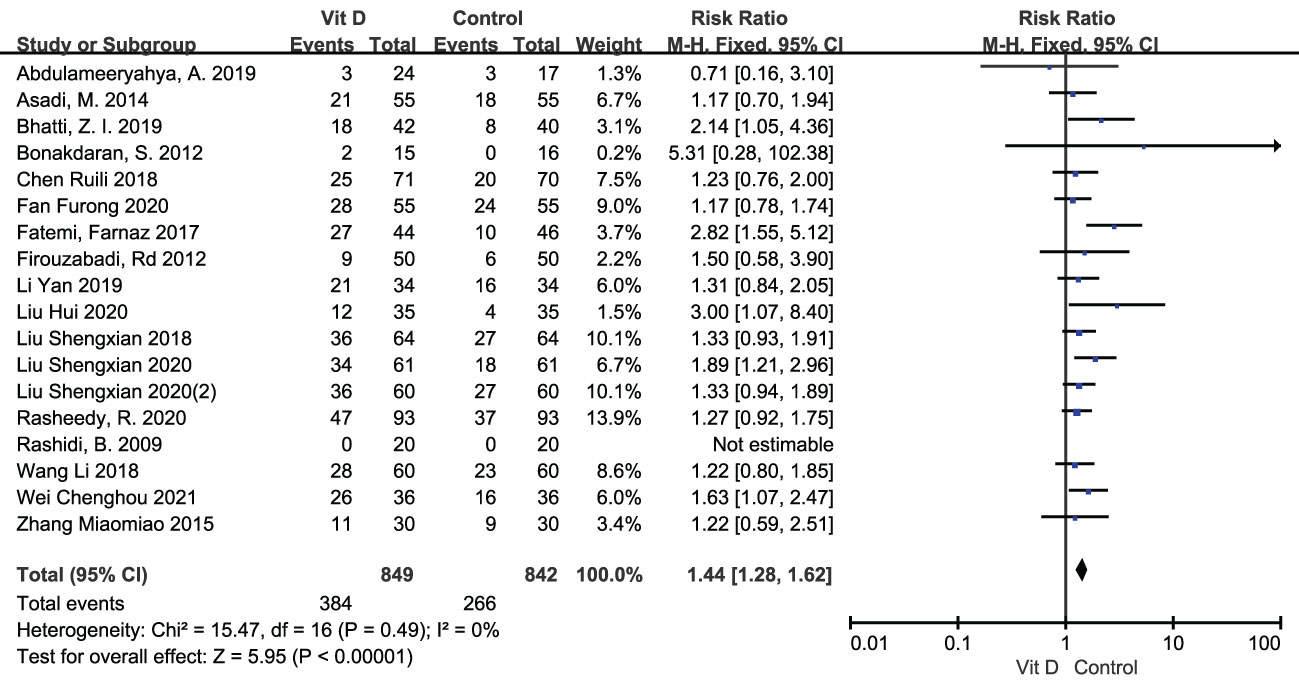
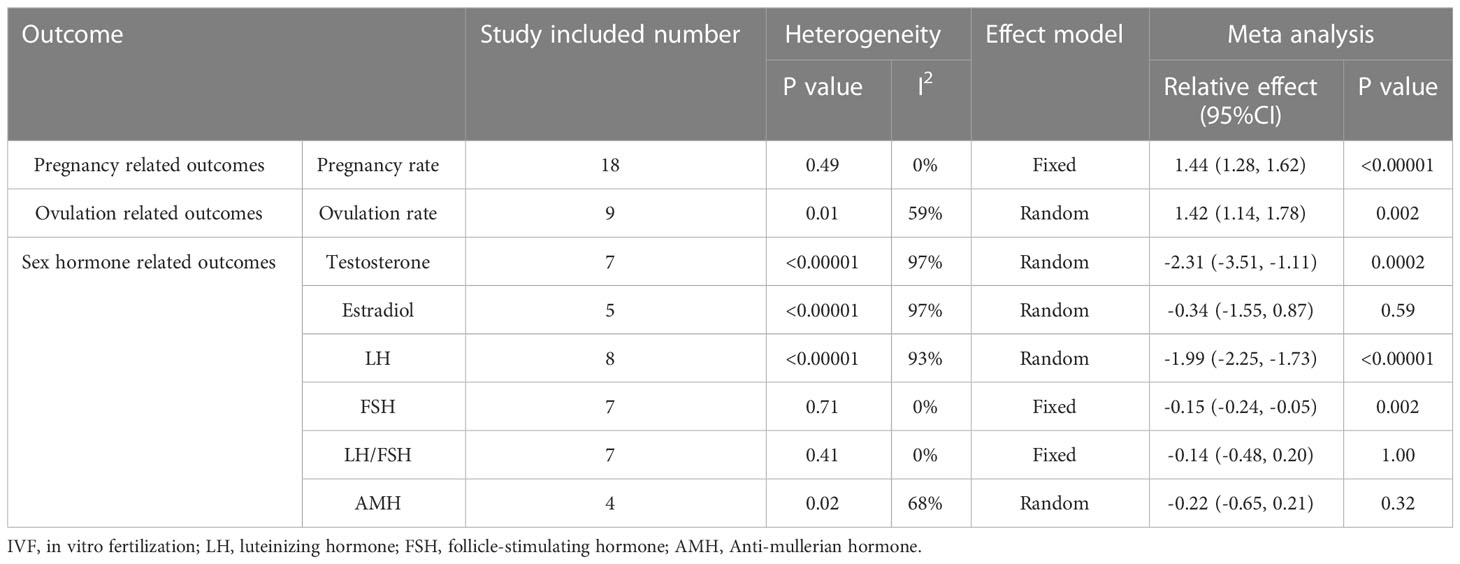
The clinical pregnancy rate was reported in 18 RCTs, including 849 participants in the intervention group and 842 cases in the control group. Meta analysis result of fixed effect model showed that after vitamin D supplementation, the pregnancy rate of PCOS patients significantly increased [RR=1.44, 95% CI (1.28, 1.62), p<0.00001] comparing with the control group (Figure 4; Table 2).
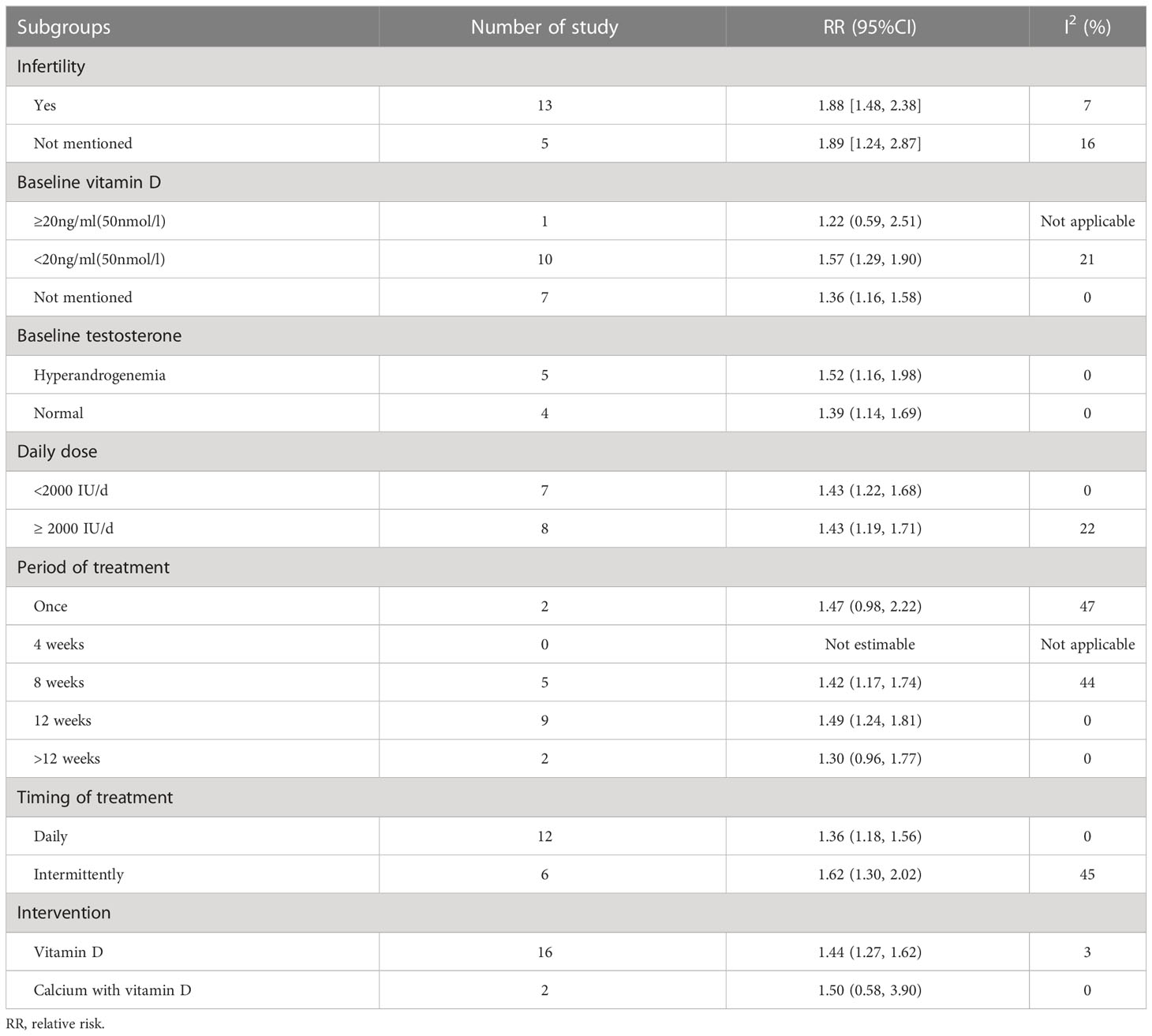
The following conclusions were drawn from the subgroup analyses of patient characteristics (baseline vitamin D level, testosterone level, and history of infertility) and vitamin D supplementation characteristics (average daily dose, calcium was supplemented at the same time, duration and frequency of medication) (see Table 3):
We divided patients into 2 subgroups based on whether they had infertility or not. As a result, a total of 13 studies involved patients with infertility, and the vitamin D intervention group had an increased pregnancy rate when compared with the control group [RR = 1.88, 95% CI (1.48, 2.38), P < 0.00001, I²= 7%]. While those studies without mention infertility in PCOS patients showed the same trend [RR = 1.89, 95% CI (1.24, 2.87), P = 0.003, I² = 16%].
According to Method for vitamin D deficiency screening published by National Health Commission of the People’s Republic of China in June 2020, serum vitamin D content ≥ 20 ng/ml (50 nmol/L) was considered as the normal level whileunders12 ng/ml (30 nmol/L) was defined as vitamin D deficiency status, and between as vitamin D insufficiency status. Only 1 study reported PCOS patients with normal vitamin D at baseline, and there was no significant difference of the pregnancy rate between the vitamin D intervention group and the control group [RR = 1.22, 95% CI (0.59, 2.51), P = 0.59]. Patients in ten studies were in vitamin D insufficiency condition at baseline, and pregnancy rates improved significantly in PCOS patients compared with controls after vitamin D intervention [RR = 1.57, 95% CI (1.29, 1.90), P < 0.00001, I² = 21%]. Whereas 7 studies did not report baseline of vitamin D levels, the pregnancy rate improved in the intervention group compared with the control group [RR = 1.36, 95% CI (1.16, 1.58), P = 0.0001, I² = 0%].
We applied subgroup analysis according to subjects with or without hyperandrogenism at baseline, the result revealed pregnancy rates consistency in patients with [RR = 1.52, 95% CI (1.16, 1.98), P = 0.002, I² = 0%] or without [RR = 1.39, 95% CI (1.14, 1.69), P = 0.0009, I² = 0%] hyperandrogenism.
Subgroup analysis performed by whether daily dose above 2000IUor not. Results showed no difference about pregnancy rates between the lower daily dose (<2000IU/d) [RR=1.43, 95%CI (1.22, 1.68),p<0.00001, I²=0%] and the higher daily dose(>2000IU/d) [RR=1.43, 95%CI (1.19, 1.71),p=0.0001, I²=22%].
Subgroup analysis according to the period of treatment unveiled that 8 weeks [RR = 1.42, 95% CI (1.17, 1.74), P = 0.0004, I² = 44%] or 12 weeks [RR = 1.49, 95% CI (1.24, 1.81), P < 0.00001, I² = 0%] of vitamin D supplementation both improved pregnancy rates. However, either single dose [RR = 1.47, 95% CI (0.98, 2.22), p = 0.06, I² = 47%] or more than 12 weeks [RR = 1.30, 95% CI (0.96, 1.77), p=0.09, I²=0%] did not ameliorate the pregnancy rate. On the other aspect, both continuous daily administration [RR = 1.36, 95% CI (1.18, 1.56), P < 0.0001, I² = 0%] and intermittent administration [RR = 1.62, 95% CI (1.30, 2.02), P < 0.0001, I² = 45%] of vitamin D elevated pregnancy rates in PCOS.
Vitamin D supplementation alone [RR = 1.44, 95% CI (1.27, 1.62), P < 0.00001, I² = 3%] did rise the pregnancy rate. However, it was not significantly changed by concomitant calcium supplementation [RR = 1.50, 95% CI (0.58, 3.90), P = 0.41, I² = 0%], probably due to the small number of relative studies.
3.3.1.2 Cumulative pregnancy rates
Cumulative pregnancy rate equals the number of pregnancies (per patient, clinical pregnancy, or after transfer of all embryos from one oocyte retrieval cycle)/number of all oocyte retrieval cycles × 100% (26). Only one study reported the cumulative pregnancy rate (19). After the first cycle of induced ovulation, both groups had the same cumulative pregnancy rate (0%). Cumulative pregnancy rates were 13% and 12% in the vitamin D intervention and control groups after the second cycle, respectively. After the third cycle, it was 29% in the intervention group and 24% in the control group. We drew data and discovered no statistical difference between both groups for the three cycles, specifically for the first cycle [RR = 0.99, 95% CI (0.89, 1.09)], the second cycle [RR = 0.96, 95% CI (0.83, 1.09)], and the third cycle [RR = 0.87, 95% CI (0.71, 1.07)] (Table 2).
3.3.2 Ovulation related outcomes
3.3.2.1 Ovulation rate
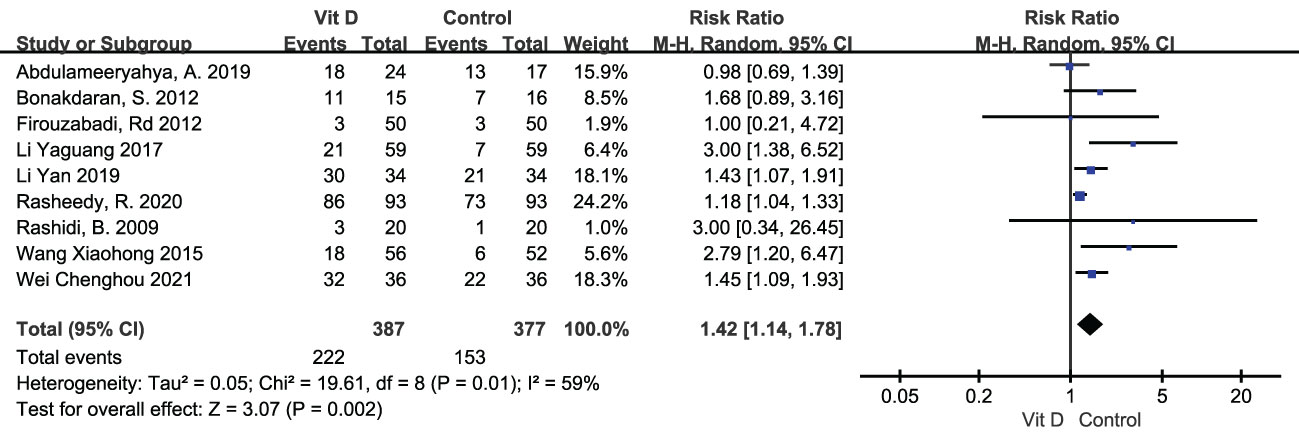
Ovulation rate was displayed in 9 studies, including 387 subjects in the vitamin D group and 377 in the control group. Random effects model showed that ovulation rate was apparently improved with vitamin D supplementation [RR=1.42, 95%CI (1.14, 1.78), p=0.002, I²=59%] (Table 2; Figure 5).
3.3.2.2 Sex hormone related outcomes
3.3.2.2.1 Luteinizing hormone
A total of 8 studies revealed luteinizing hormone levels, including 345 subjects in the intervention group and 340 in the control group. The random effects model showed that LH levels were significantly lower in individuals of the vitamin D supplemented group[MD= -1.47, 95%CI (-2.57, -0.36), p=0.009, I²=93%]. We carried out the sensitivity analysis for obvious heterogeneity. After removing the result in (13), I² decreased to 0%, while LH was still lower in the intervention group [MD= -1.31, 95%CI (-1.60, -1.01), p<0.00001, I²=0%] (Table 2).
3.3.2.2.2 Follicle-stimulating hormone
Follicle stimulating hormone levels were mentioned in 7 studies, involving 284 in the intervention group and 280 in the control group. A fixed effects model resulted that FSH levels were noticeable lower in the vitamin D supplemented group [MD=-0.15, 95%CI (-0.24, -0.05), p=0.002, I²=0%] (Table 2).
3.3.2.2.3 LH/FSH
Seven studies showed both LH and FSH levels, consisted of 284 patients in the vitamin D supplementation group and 279 patients in the control group. LH/FSH was calculated and the fixed effects model found that LH/FSH was not significantly different between two groups [MD=-0.14, 95%CI (-0.48, 0.20), p=1.00, I²=0%] (Table 2).
3.3.2.2.4 Anti-mullerian hormone
There were 4 studies reported anti-mullerian hormone, including 155 participants in the intervention group and 150 in the control group. The random effects model showed that AMH levels were not significantly changed in the vitamin D supplemented group compared with the control group [MD=-0.22, 95%CI (-0.65, 0.21), p=0.32, I²=68%] (Table 2).
3.3.2.2.5 Testosterone
Testosterone levels were reported in 7 studies. A random effect model displayed a significant decrease in the vitamin D supplementation group compared with the control group [MD=-2.31, 95%CI(-3.51, -1.11), p=0.0002, I²=97%]. Heterogeneity remained large after subgroup analyses according to the test period: with basal testosterone levels under a random effects model [MD=-1.48, 95%CI (-2.94, -0.01), p=0.05, I²=95%] and the test period not mentioned [MD=-3.40, 95%CI (-5.99, -0.80), p=0.01, I²=98%], respectively (Table 2).
3.3.2.2.6 Estradiol
Estradiol levels were assessed in 5 literatures, involving 251 patients supplemented with vitamin D and 250 patients in the control group. Random effects model resulted that estradiol levels were not significantly different in two groups [MD=-0.34,95%CI (-1.55, 0.87), p=0.59, I²=97%]. Subgroups analysis according to the examining period demonstrated the same trend under random effects model meta-analysis. There was no noticeable difference between the two groups about basal estradiol levels [MD=0.32, 95%CI (-0.07, 0.71), p=0.11, I²=66%] and estradiol levels that the test time not mentioned [MD=-1.34, 95%CI (-5.49, 2.81), p=0.53, I²=99%] (Table 2).
In summary, we predominantly found vitamin D supplementation could ameliorate outcomes like the premature delivery rate and the matured oocytes rate. While other indicators did not alter significantly (the early miscarriage rate, biochemical pregnancy rate, the fertilization rate, the incidence of gestational hypertension and gestational diabetes mellitus (GDM), the cleavage rate, the high quality embryo rate and endometrial thickness) (Supplementary Information).
3.3.3 Adverse events
Only 4 studies reported the adverse events. One trail showed no obvious adverse reactions in both intervention and control groups. Two of them demonstrated ovarian hyperstimulation syndrome (OHSS) occurred in both groups during ovulation induction, but there was no significant difference between the vitamin D intervention group and the control group. One research revealed that the control group had allergic reactions to short-acting oral contraceptives.
In general, no trail had severe adverse event for vitamin D per se.
3.4 Bias
We exerted funnel plots, Begg’s tests and Egger’s tests. No public bias was found in our research (Figures 6, 7).
4 Discussion
PCOS patients are generally experiencing vitamin D deficiency, and it is not known whether vitamin supplementation is beneficial for pregnancy in PCOS patients till now. This is the first systematic review and meta-analysis of vitamin D supplementation on pregnancy in PCOS patients. A total of 20 RCTs were included. There were 1961 subjects in total, no statistical difference was discovered between the intervention group and the control group in terms of age and vitamin D level at baseline. This systematic review, based on the existing RCTs, provides evidence for PCOS patients to take vitamin D supplements or not before pregnancy.
The results of meta-analysis found that the pregnancy rate, ovulation rate and matured oocytes rate of vitamin D supplementation group were significantly higher than those of the control group. The premature delivery rate, testosterone, LH and FSH decreased evidently. Only one trial suggested that the level of progesterone in vitamin D supplementation group increased. There was no remarkable difference with vitamin D supplemented in chemical purity rate, fertilization rate, cleavage rate, high-quality embryo rate, endometrial thickness, serum estradiol and AMH.
4.1 Pregnancy related outcomes
In this study, the pregnancy rate of PCOS patients was apparently increased with vitamin D supplementation, but vitamin D had no significant impact on other indicators related to assisted reproductive technology, such as fertilization rate, cleavage rate and high-quality embryo rate. To best of our knowledge, there is no systematic review on the gestation of PCOS patients with vitamin D supplementation. Existing results are dominantly on the correlation between vitamin D levels and pregnancy rate in IVF, and the conclusions are not consistent. Specifically, vitamin D levels in serum and follicular fluid may improve, reduce or have no effect on pregnancy rate (5), and the reason for the inconsistency may be the difference of vitamin D levels in patients themselves. The mechanism of how vitamin D improve the pregnancy rate of PCOS patients is not clearly demonstrated. In combination with other results of this study, we speculated that supplementation of vitamin D may improve the pregnancy rate of PCOS patients by mitigating inflammation in granulosa cells, ameliorating ovulation and increasing endometrial thickness.
This study also indicated that the incidence of gestational hypertension and gestational diabetes mellitus (GDB) in the vitamin D supplementation group was not significantly different from that in the control group, but the premature delivery rate was reduced. However, only three articles included in this study mentioned pregnancy complications. Thus the results should be treated with caution. The existing meta-analysis found that vitamin D supplementation during pregnancy can reduce the risk of preeclampsia, but no difference was found about the incidence of gestational diabetes mellitus (27). The different findings between this study and the previous study may because of populations (PCOS or healthy gravidas), timing of vitamin D supplementation (before or during maternity) and doses they used. Some researchers have pointed out that vitamin D plays a role in reducing blood pressure and glucose level. A meta-analysis found that the diastolic blood pressure of adolescents decreased after vitamin D supplement (28). The potential mechanism is that vitamin D reduces the production of endothelium-derived contractile factor and ultimately decreases blood pressure by up-regulating endothelial nitric oxide synthase (eNOS) activity, reducing the levels of reactive oxygen species (ROS) and cyclooxygenase-1 (COX-1) (29). In addition, vitamin D deficiency is positively related to the risk of GBD (30). The underlying mechanism of vitamin D’s improvement on glucose metabolism is (1) to stimulate the expression of insulin receptor, thus increasing the insulin sensitivity of liver, muscle and adipose tissue (2) to stimulate the nuclear vitamin D receptor, thus raising the production of GLUT-4 (3) to promote calcium-dependent insulin secretion (4) to inhibit the production of cytokines from macrophages, and protect islet cells from damage.
Moreover, we found that vitamin D supplementation may reduce the incidence of premature delivery in PCOS offspring in present study. However, it is noteworthy that only two studies have reported on this outcome and were completed by the same researcher, which may cause bias. At present, there is no other research reported the effect of vitamin D supplementation on the occurrence of premature delivery of the fetus in PCOS women, but a meta-analysis found that the risk of premature delivery of pregnant women with vitamin D deficiency is increased (31). Although more studies are still needed to verify, there are some possible grounds for the reduction of premature delivery rate of fetus by vitamin D supplementation for gravidas. Research demonstrates that vitamin D deficiency of pregnant women is positively correlated with the increased risk of premature delivery (32, 33), and vitamin D may reduce the incidence of premature delivery via NF- κ B, which plays an anti-inflammatory role and reduces infections (34).
4.2 Ovulation rate
We found that vitamin D supplementation improved ovulation rate in PCOS patients. Berry et al. claims that 70% infertile PCOS women have a lower level of vitamin D than the healthy (35). Besides, studies unveil that vitamin D deficiency is positively associated with the number of mature oocytes (36) and the ovulation rates (35, 37, 38). Nevertheless, different concepts still exist. Evidence states serum vitamin D in PCOS women are similar to controls (39). The discrepancy may originate from diverse races and the specific type of PCOS. Except for vitamin D receptor and sex hormone related mechanism that will be mentioned later, the mechanism of VD affecting ovulation is that VD may down regulate AMH receptor II, thus promote the activation and maturation of oocytes (40).
4.3 Sex hormone related outcomes
In addition to the above gestation related indicators, the effect of vitamin D on the level of sex hormones in patients with PCOS also deserves attention. In this study, compared with the control group, testosterone, LH and FSH in vitamin D supplementation group decreased significantly, while progesterone level increased, but estradiol, AMH and endometrial thickness did not change.
We found that the serum testosterone level decreased in vitamin D intervention group. Studies manifests that in women with PCOS, the level of 25-hydroxyvitamin D (25 (OH) D) is negatively correlated with testosterone (29). A meta-analysis involving 183 PCOS patients suggests serum testosterone decreases after vitamin D supplementation (41), which complies with this study. Similarly, Nahla et al. demonstrates 50,000 IU per week of VD can decrease testosterone in overweight PCOS women (42). The mechanisms related to vitamin D and testosterone are not obvious. Possible mechanisms might be the improvement of insulin resistance, thus interrupting the vicious cycle of insulin resistance-hyperandrogenism. The increase of aromatase activity promote the conversion of androgen to estrogen, which is described later. In addition, the etiology of PCOS is linked to the effect of vitamin D receptor (VDR) gene polymorphism on LH, SHBG concentration, testosterone level, insulin resistance and serum insulin level (41). In terms of the molecular mechanism, vitamin D deficiency increases the activity of the PI3K/Akt pathway in ovarian tissue, thus interfering with the selection of dominant follicles. What’s more, hyperandrogenism lead to increasing AR activity. Decreased ARA70 (androgen receptor-associated protein 70, which is necessary for androgen receptor (AR) and VDR transcription activity) subsequently inactivates free testosterone by promoting the transcription of VDR/RXR (retinoid X receptor) complex, which induces a vicious cycle of hyperandrogenism. Vitamin D supplementation breaks this cycle by up-regulating VDR (43).
There was no remarkable difference in estrogen level and endometrium in two groups. According to previous studies, calcitriol (1,25 (OH) 2 vitamin D) directly led to the production of estrogen and progesterone in cultured human ovary and placenta cells, which may enhance the luteinization of granulosa cells and ameliorate endometrial environment (33). The reason for the discrepancy could be there are few studies on the relationship about vitamin D and sex hormones, and more studies are still required. In addition, the difference in the basic treatment and monitoring days of each trail trial were included in this study, after further analyzing, the heterogeneity eliminated and the endometrial thickness of vitamin D supplementation group was increased compared with the control group. Only one study suggested that progesterone increased after vitamin D use, which was consistent with previous reports. Besides, the increase of progesterone may reduce the abortion rate.
There was no difference in the level of AMH between the vitamin D intervention group and the control group in present study. Some studies show that serum vitamin D is positively correlated with AMH, while in VD-deficient women with PCOS, VD supplementation improves the abnormally elevated AMH (44, 45). There is discrepancy in our study and previous studies. The reason may be that patients’ conditions possess their own characteristics, and the population analyzed in previous studies has no fertility requirements. Besides, age may be one reason for inconsistency.
In present study, the LH and FSH in the vitamin D intervention group were notably lower than those in the control group in PCOS patients. For extraordinary heterogeneity of LH, we executed sensitivity analysis. After removing Liu shengxian’s study, the heterogeneity decreased to 0%. The heterogeneousness may come from the elementary treatment whether use short-acting contraceptives and ovulation induction drugs or other medicine that affect sex hormones. It indicates that vitamin D supplementation may reduce LH level based on using short-acting contraceptives or ovulation induction drugs. Departed studies indicate that serum vitamin D level is negatively correlated with serum LH and FSH, but no statistical difference (46). While other findings demonstrate that LH and FSH levels are not related to vitamin D level (47), that may be due to most of the previous studies are women of healthy childbearing age, while our study focus on PCOS patients. The possible mechanism involved in vitamin D ameliorating LH of PCOS is that vitamin D attenuates the adverse effects of AGEs on human granulosa cell (GCs) steroids by down-regulating the expression of pro-inflammatory cell membrane receptor for AGEs (RAGE) (48). In addition, in human cumulus granulosa cells, vitamin D increases aromatase activity (49), while the expression of AMH receptor and FSH receptor decrease, thus promoting androgen converts to estrogen. Besides, vitamin D offsets the inhibition of AMH on GC differentiation and allows follicles to maturate and ovulate (49).
Though our study is the first systematic evaluation and meta-analysis of vitamin D on pregnancy in PCOS patients, some limitations should be treated carefully. Firstly, the quality of included studies could be higher. Second, the data of some indicators such as the cumulative pregnancy rate was not published, which induces the analysis limited.
5 Conclusion
In conclusion, the current evidence demonstrated that vitamin D supplementation may improve the ovulation and pregnancy rate of PCOS patients on the basis of routine treatment, providing a certain basis for clinical use of vitamin D in PCOS patients in the future. However, due to the quality and heterogeneity of the included studies, more valuable studies are still needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
MY and JP contributed to the selection of articles, evaluation of evidence quality, and extraction of data. MY and XS performed the data analyses and wrote the manuscript. JZ contributed to the conception of the study. SZ modified this article. DL, LX, and JZ helped perform the analyses with constructive discussions. All authors contributed to the article and approved the submitted version.
Funding
This article was funded by National Natural Science Foundation (No:81971354).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1148556/full#supplementary-material
References
1. Costello MF, Misso ML, Balen A, Boyle J, Devoto L, Garad RM, et al. A brief update on the evidence supporting the treatment of infertility in polycystic ovary syndrome. Aust New Z J Obstet Gynaecol (2019) 59(6):867–73. doi: 10.1111/ajo.13051
3. Faghfoori Z, Fazelian S, Shadnoush M, Goodarzi R. Nutritional management in women with polycystic ovary syndrome: a review study. Diabetes Metab Syndrome (2017) 11 Suppl 1:S429–s32. doi: 10.1016/j.dsx.2017.03.030
4. Menichini D, Facchinetti F. Effects of vitamin d supplementation in women with polycystic ovary syndrome: a review. Gynecol Endocrinol (2020) 36(1):1–5. doi: 10.1080/09513590.2019.1625881
5. Lerchbaum E, Obermayer-Pietsch B. Vitamin d and fertility: a systematic review. Eur J Endocrinol (2012) 166(5):765–78. doi: 10.1530/EJE-11-0984
6. Berridge MJ. Vitamin d deficiency: infertility and neurodevelopmental diseases (attention deficit hyperactivity disorder, autism, and schizophrenia). Am J Physiol Cell Physiol (2018) 314(2):C135–c51. doi: 10.1152/ajpcell.00188.2017
7. Abdulameeryahya A, Abdulridha MK, Al-Rubuyae BJ, Al-Atar HA. The effect of vitamin d and Co-enzyme Q10 replacement therapy on hormonal profile and ovulation statusin women with clomiphene citrate resistant polycystic ovary syndrome. J Pharm Sci Res (2019) 11(1):208–15.
8. Rashidi B, Haghollahi F, Shariat M, Zayerii F. The effects of calcium-vitamin d and metformin on polycystic ovary syndrome: a pilot study. Taiwanese J Obstet Gynecol (2009) 48(2):142–7. doi: 10.1016/S1028-4559(09)60275-8
9. Bonakdaran S, Mazloom Khorasani Z, Davachi B, Mazloom Khorasani J. The effects of calcitriol on improvement of insulin resistance, ovulation and comparison with metformin therapy in PCOS patients: a randomized placebo- controlled clinical trial. Iranian J Reprod Med (2012) 10(5):465–72.
10. Firouzabadi R, Aflatoonian A, Modarresi S, Sekhavat L, Mohammadtaheri S. Therapeutic effects of calcium & vitamin d supplementation in women with PCOS. Complementary Therapies Clin Pract (2012) 18(2):85–8.
11. Li Y. Ovulatory therapy and uterine receptivity in women with PCOS. Guide China Med (2017) 15(31):52.
12. Wang X, Wang Z, Miu L. Effect of vitamin D in combination with metformin in treatment of patients with PCOS ovulation and the receptivity of endometrium. Military Medical Journal of Southeast China (2015) 17(02):142–5.
13. Sheng-Xian L, Fang W. Vitamin D supplementation on ovum quality and pregnancy outcomes in polycystic ovary syndrome. Modern Diagnosis and Treatment (2020) 31(10):1583–4.
14. Hui L. Clinical study of vitamin d on ovulation of patients with polycystic ovary syndrome. Guide China Med (2020) 18(33):68–9.
15. Wei C, Han L, Zhang J. Analysis of the efficacy of vitamin D combined with metformin and ethinylestradiol cyproterone for the treatment of infertility in polycystic ovary syndrome. Chinese Journal of Reproductive Health (2021) 32(02):157–9.
16. Yan L. Analysis of clinical value of metformin combined with vitamin d and ethinylestradiol cycloproterone in treatment of polycystic ovary syndrome infertility. J Pract Gynecological Endo (2019) 6(15):4–6.
17. Wang Li XS. Effects of vitamin d adjuvant therapy on lipid metabolism and related hormone levels and depression in patients with polycystic ovary syndrome. China J Health Psychol (2018) 26(04):539–44.
18. Zhang M, Zhu L, La D. Clinical efficacy of vitamin D combined with dinae-35 in polycystic ovary syndrome. Maternal and Child Health Care of China (2015) 30(32):5704–7.
19. Rasheedy R, Sammour H, Elkholy A, Salim Y. The efficacy of vitamin d combined with clomiphene citrate in ovulation induction in overweight women with polycystic ovary syndrome: a double blind, randomized clinical trial. Endocrine (2020) 69(2):393–401. doi: 10.1007/s12020-020-02315-3
20. Chen R. The effects of vitamin d on AMH,insulin metabolism andovulation induction in PCOS patients. Zhengzhou University (2018).
21. Bhatti ZI, Choudhery KA, Yasin I, Assan MH, Bashir F. Role of vitamin d supplements in improving fertility among sub fertile couples. Pakistan J Med Health Sci (2019) 13(1):28–32.
22. Furong F. Effects of vitamin d on ovum quality and pregnancy outcome in patients with polycystic ovary syndrome. Guide China Med (2020) 18(31):58–9.
23. Sheng-Xian L, Fang W, Ge-Lin L. Influence of vitamin d on ovum quality and pregnancy outcome of patients with polycystic ovary syndrome. Pract Pharm Clin Remedies (2018) 21(03):261–5.
24. Fatemi F, Mohammadzadeh A, Sadeghi MR, Akhondi MM, Mohammadmoradi S, Kamali K, et al. Role of vitamin e and D(3) supplementation in intra-cytoplasmic sperm injection outcomes of women with polycystic ovarian syndrome: a double blinded randomized placebo-controlled trial. Clin Nutr ESPEN (2017) 18:23–30.
25. Asadi M, Matin N, Frootan M, Mohamadpour J, Qorbani M, Tanha FD. Vitamin d improves endometrial thickness in PCOS women who need intrauterine insemination: a randomized double-blind placebo-controlled trial. Arch Gynecology Obstetrics (2014) 289(4):865–70.
26. Cong-Shun T Y C Y-Y M. Comparison of cumulative pregnancy rate between follicular phase long-acting long protocol and luteal phase short-acting long protocol in IVF. J Reprod Med (2020) 29(11):1410–4.
27. Palacios C, De-Regil LM, Lombardo LK, Peña-Rosas JP. Vitamin d supplementation during pregnancy: updated meta-analysis on maternal outcomes. J?Steroid Biochem Mol Biol (2016) 164:148–55. doi: 10.1016/j.jsbmb.2016.02.008
28. Abboud M. Vitamin d supplementation and blood pressure in children and adolescents: a systematic review and meta-analysis. Nutrients (2020) 12(4):1163. doi: 10.3390/nu12041163
29. Latic N, Erben RG. Vitamin d and cardiovascular disease, with emphasis on hypertension, atherosclerosis, and heart failure. Int J Mol Sci (2020) 21(18):6483. doi: 10.3390/ijms21186483
30. Mahendra A, Fall CHD. Maternal vitamin d deficiency and GDM risk: evidence for the case of investing more attention in antenatal clinics. Proc Nutr Soc (2021), 1–7. doi: 10.1017/S0029665121003840
31. Qin LL, Lu FG, Yang SH, Xu HL, Luo BA. Does maternal vitamin d deficiency increase the risk of preterm birth: a meta-analysis of observational studies. Nutrients (2016) 8(5):301. doi: 10.3390/nu8050301
32. Von Websky K, Hasan AA, Reichetzeder C, Tsuprykov O, Hocher B. Impact of vitamin d on pregnancy-related disorders and on offspring outcome. J Steroid Biochem Mol Biol (2018) 180:51–64. doi: 10.1016/j.jsbmb.2017.11.008
33. Wang T, Fu H, Chen L, Xu Y. Pregnancy complications among women with polycystic ovary syndrome in China: a meta-analysis. Zhong Nan Da Xue Xue Bao Yi Xue Ban = J Cent South Univ Med Sci (2017) 42(11):1300–10.
34. Agarwal S, Kovilam O, Agrawal DK. Vitamin d and its impact on maternal-fetal outcomes in pregnancy: a critical review. Crit Rev Food Sci Nutr (2018) 58(5):755–69. doi: 10.1080/10408398.2016.1220915
35. Berry S, Seidler K, Neil J. Vitamin d deficiency and female infertility: a mechanism review examining the role of vitamin d in ovulatory dysfunction as a symptom of polycystic ovary syndrome. J Reprod Immunol (2022) 151:103633. doi:?10.1016/j.jri.2022.103633
36. Eller ABP, Ejzenberg D, Monteleone PAA, Soares JM Jr, Baracat EC. Vitamin d and in vitro fertilization: a systematic review. J Assisted Reprod Genet (2023). doi: 10.1007/s10815-023-02767-2
37. Moslehi N, Zeraattalab-Motlagh S, Rahimi Sakak F, Shab-Bidar S, Tehrani FR, Mirmiran P. Effects of nutrition on metabolic and endocrine outcomes in women with polycystic ovary syndrome: an umbrella review of meta-analyses of randomized controlled trials. Nutr Rev (2022), 555–777. doi: 10.1093/nutrit/nuac075
38. Gorelova IV, Popova PV, Rulev MV. Vitamin d and reproductive health. Problemy Endokrinologii (2020) 66(5):96–101. doi: 10.14341/probl12468
39. Ozyurt R, Karakus C. Follicular fluid 25-hydroxyvitamin d levels determine fertility outcome in patients with polycystic ovary syndrome. Taiwanese J Obstet Gynecol (2022) 61(4):620–5. doi: 10.1016/j.tjog.2022.03.041
40. Grzeczka A, Graczyk S, Skowronska A, Skowronski MT, Kordowitzki P. Relevance of vitamin d and its deficiency for the ovarian follicle and the oocyte: an update. Nutrients (2022) 14(18):3712. doi: 10.3390/nu14183712
41. Azadi-Yazdi M, Nadjarzadeh A, Khosravi-Boroujeni H, Salehi-Abargouei A. The effect of vitamin d supplementation on the androgenic profile in patients with polycystic ovary syndrome: a systematic review and meta-analysis of clinical trials. Hormone Metab Res = Hormon- und Stoffwechselforschung = Hormones Metabolisme (2017) 49(3):174–9. doi: 10.1055/s-0043-103573
42. Al-Bayyari N, Al-Domi H, Zayed F, Hailat R, Eaton A. Androgens and hirsutism score of overweight women with polycystic ovary syndrome improved after vitamin d treatment: a randomized placebo controlled clinical trial. Clin Nutr (Edinburgh Scotland) (2021) 40(3):870–8. doi: 10.1016/j.clnu.2020.09.024
43. Lajtai K, Nagy CT, Tarszabó R, Benkő R, Hadjadj L, Sziva RE, et al. Effects of vitamin d deficiency on proliferation and autophagy of ovarian and liver tissues in a rat model of polycystic ovary syndrome. Biomolecules (2019) 9(9):471. doi: 10.3390/biom9090471
44. Dennis NA, Houghton LA, Jones GT, Van Rij AM, Morgan K, Mclennan IS. The level of serum anti-müllerian hormone correlates with vitamin d status in men and women but not in boys [J]. J Clin Endocrinol Metab (2012) 97(7):2450–5. doi: 10.1210/jc.2012-1213
45. Moridi I, Chen A, Tal O, Tal R. The association between vitamin d and anti-müllerian hormone: a systematic review and meta-analysis. Nutrients (2020) 12(6):1567. doi: 10.3390/nu12061567
46. Hahn S, Haselhorst U, Tan S, Quadbeck B, Schmidt M, Roesler S, et al. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome [J]. Experimental and clinical endocrinology & diabetes: official journal, German Society of Endocrinology [and] German Diabetes Association (2006) 114(10):577–83. doi: 10.1055/s-2006-948308
47. Yildizhan R, Kurdoglu M, Adali E, Kolusari A, Yildizhan B, Sahin HG, et al. Serum 25-hydroxyvitamin D concentrations in obese and non-obese women with polycystic ovary syndrome [J]. Arch Gynecol Obstet (2009) 280(4):559–63. doi: 10.1007/s00404-009-0958-7
48. Parikh G, Varadinova M, Suwandhi P, Araki T, Rosenwaks Z, Poretsky L, et al. Vitamin d regulates steroidogenesis and insulin-like growth factor binding protein-1 (IGFBP-1) production in human ovarian cells. Hormone Metab Res = Hormon- und Stoffwechselforschung = Hormones Metabolisme (2010) 42(10):754–7. doi: 10.1055/s-0030-1262837
Keywords: vitamin D, polycystic ovary syndrome, pregnancy, ovulation, meta-analysis
Citation: Yang M, Shen X, Lu D, Peng J, Zhou S, Xu L and Zhang J (2023) Effects of vitamin D supplementation on ovulation and pregnancy in women with polycystic ovary syndrome: a systematic review and meta-analysis. Front. Endocrinol. 14:1148556. doi: 10.3389/fendo.2023.1148556
Received: 20 January 2023; Accepted: 05 April 2023;
Published: 01 August 2023.
Edited by:
Francesco Pallotti, Kore University of Enna, ItalyReviewed by:
Gianmaria Salvio, University Hospital Ospedali Riuniti Di Ancona, ItalyNahla Al-Bayyari, Al-Balqa Applied University, Jordan
Copyright © 2023 Yang, Shen, Lu, Peng, Zhou, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liangzhi Xu, bGlhbmd6eHVAMTI2LmNvbQ==; Jing Zhang, amluZ3poYW5nMTEwOTE0QDEyNi5jb20=
 Meina Yang
Meina Yang Xiaoyang Shen
Xiaoyang Shen Danhua Lu1,2,3,4
Danhua Lu1,2,3,4 Jin Peng
Jin Peng Jing Zhang
Jing Zhang