- 1Pediatric Unit, Department of Medical and Surgical Sciences of the Mother, Children and Adults, University of Modena and Reggio Emilia, Modena, Italy
- 2Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Modena, Italy
- 3Clinical and Experimental Medicine PhD Program, University of Modena and Reggio Emilia, Modena, Italy
- 4Post graduate School of Pediatrics, Department of Medical and Surgical Sciences of the Mother, Children and Adults, University of Modena and Reggio Emilia, Modena, Italy
- 5School of Medicine, University of Modena and Reggio Emilia, Modena, Italy
- 6Neonatology Unit, Department of Medical and Surgical Sciences of the Mother, Children and Adults, University of Modena and Reggio Emilia, Modena, Italy
- 7Unit of Obstetrics and Gynecology, Department of Medical and Surgical Sciences of the Mother, Children and Adults, University of Modena and Reggio Emilia, Modena, Italy
Background: Phthalates are non-persistent chemicals largely used as plasticizers and considered ubiquitous pollutants with endocrine disrupting activity. The exposure during sensible temporal windows as pregnancy and early childhood, may influence physiological neurodevelopment.
Aims and Scope: The aim of this study is to analyze the relationship between the urinary levels of phthalate metabolites in newborn and infants and the global development measured by the Griffiths Scales of Children Development (GSCD) at six months.
Methods: Longitudinal cohort study in healthy Italian term newborn and their mothers from birth to the first 6 months of life. Urine samples were collected at respectively 0 (T0), 3 (T3), 6 (T6) months, and around the delivery for mothers. Urine samples were analyzed for a total of 7 major phthalate metabolites of 5 of the most commonly used phthalates. At six months of age a global child development assessment using the third edition of the Griffith Scales of Child Development (GSCD III) was performed in 104 participants.
Results: In a total of 387 urine samples, the seven metabolites analyzed appeared widespread and were detected in most of the urine samples collected at any time of sampling (66-100%). At six months most of the Developmental Quotients (DQs) falls in average range, except for the subscale B, which presents a DQ median score of 87 (85-95). Adjusted linear regressions between DQs and urinary phthalate metabolite concentrations in mothers at T0 and in infants at T0, T3 and T6 identified several negative associations both for infants’ and mothers especially for DEHP and MBzP. Moreover, once stratified by children’s sex, negative associations were found in boys while positive in girls.
Conclusions: Phthalates exposure is widespread, especially for not regulated compounds. Urinary phthalate metabolites were found to be associated to GSCD III scores, showing inverse association with higher phthalate levels related to lower development scores. Our data suggested differences related to the child’s sex.
1 Introduction
Phthalates are synthetic chemical compounds obtained through a process of esterification between phthalic anhydride and an alcohol of variable size, from methanol to tridecanol. This family of non-persistent chemicals are generally used as plasticizers to give flexibility, malleability and temperature tolerance to several materials, such as polyvinylchloride (PVC) (1).
Phthalates have a weak interaction with the polymer matrix where they are dispersed and so they are quickly released from their plastic embodiment into the environment. Since they are used in a wide range of everyday consumer products, these compounds are considered ubiquitous pollutants. Consequently, the potential human exposure occurs through multiple sources and is widespread, as well as continuous over time (2–4). One of the main sources of exposure during neonatal life is represented by breast or formula milk. During weaning, exposure due to food contamination is also frequent. Moreover, in the first months and years of life, ingestion from non-food origin occurs due to some typical newborn and toddler behaviors (use of pacifiers and teethers, frequent hand-to-mouth contact, crawling on vinyl and household floors) (5, 6). Phthalates are easily absorbed, distributed by circulation and quickly metabolized by intestinal lipase and esterases into their respective primary and secondary metabolites, and finally eliminated, mainly in the urine (7, 8).
Phthalates are classified as Endocrine-disrupting Chemicals (EDCs) because they may interfere with the regular hormonal activity. In particular, phthalates compete with endogenous hormones-receptor binding and with hormone transport proteins modifying normal gene expression within the cell (9–13). For this reason, over the past decades, European and international regulations have worked on restricting their use in products such as toys, childcare products, food packaging materials and so on (14).
Early life exposure to EDCs, and in particular to phthalates, is suspected to affect child health including neurodevelopment (15–19). The risk is higher when exposure occurs during windows of vulnerability, such as prenatal, perinatal, and early childhood life. In fact, during the third trimester of pregnancy the brain growth spurt (BGS) occurs. Moreover, the development of the central nervous system (CNS) is completed within the first two years after birth. During this time, the CNS is particularly susceptible to external stressors, which if present, can influence neurodevelopment in different domains (i.e. sensory, motor and cognitive functions) (20–24). Several epidemiological studies have described harmful associations between prenatal exposure to phthalates and child behavior or cognition (25, 26).
The aim of this study is to analyze the relationship between the urinary levels of phthalate metabolites and the global development measured by the Griffiths Scales of Children Development (GSCD) at six months.
2 Methods
2.1 Participants and sample collection
Participants were recruited as part of the Modena cohort study, a longitudinal cohort study started in 2019 with the aim of investigating the relationship between peri and postnatal exposure to phthalates and the possible relationship with hormonal, anthropometric and neurocognitive development, in healthy Italian children during the first 3 years of life. Mothers were recruited during their hospital stay in the Obstetric ward between March 2019 and October 2020, as soon as possible after delivery. Newborns with mothers of legal age (>18 years old) at delivery, who understood the Italian language and had a singleton pregnancy were eligible for participation in the main cohort study. Other inclusion criteria were: at term delivery (37-41 weeks), appropriate-for-gestational-age (AGA) infant, Apgar score >7 five minutes after birth.
The study was approved by Area Vasta Emilia Nord Ethics Committee (2018/num715). Informed consent to participation and publication of the data was obtained from caregivers of all individual participants included in the study.
The study is a sub-sample of the Modena cohort study. The eligibility criteria were: 1. having at least one urine sample for exposure assessment from birth to 6 months or from the mother within a few days from delivery and 2. having completed the global development assessment with GSCD at six months. Sample size calculation was performed for the main study, and it was estimated that about 200 newborns were needed. Therefore, we enrolled 197 mothers and child pairs. Among them, 104 infants completed the global development assessment at 6 months, and this determined the sample size for the present sub-study.
2.2 Urine sampling for phthalate exposure assessment
Children underwent consecutive examinations, from birth to sixth month of age, at respectively 0 (T0), 3 (T3), 6 (T6) months. At each evaluation spot urine samples were collected.
Urine samples were chosen as preferred biological matrix to assess phthalate metabolites concentration in mothers and children (27). Children spot urine samples were collected, using phthalate-free pediatric urine bags attached to the skin of external genitalia. Mothers were asked to provide a spot urine sample in phthalate-free plastic containers within a few days from delivery. Urine samples obtained by newborns were collected by trained hospital personnel during their hospital stay, as well as during follow-up visits, until the COVID pandemic outbreak. From that moment on, parents were instructed on correct at-home sampling techniques and were provided with tested phthalate-free urine sampling kits. Once the sample was obtained, it was collected by researchers and immediately labeled and stored in glass vials at -20°C until extraction and analysis procedures.
2.3 Phthalate analysis
Urine samples were analyzed for a total of 7 major phthalate metabolites of 5 of the most commonly used phthalates (Table 1). Urine sample preparation was performed in the laboratories of the Department of Biomedical, Metabolic and Neural Sciences, while phthalate qualitative and quantitative determination was conducted at the CIGS (Centro Interdipartimentale Grandi Strumenti) of the University of Modena and Reggio Emilia. Abbreviation on phthalates, metabolites and their characteristics may be found in Table 1. Analytical procedures for sample preparation were optimized following published methods by the USA Centers for Disease Control and Prevention (CDC)_Environmental Health Department. Urine samples were defrosted at room temperature sampling 1 ml of urine. β-glucuronidase enzyme was used to hydrolyze the glucuronidated phthalate metabolites (adding 200 μL of 6% β-glucuronidase enzyme in 6.5 pH acetate buffer and then using 10 ppb of internal standard). They were incubated for 120 minutes at 38°C to allow the cleavage of monoester glucuronides by the enzyme. The reaction was blocked by adding glacial acetic acid and 5% acetonitrile in ultrapure water to the sample solution. The samples were then purified by solid phase extraction (SPE), in order to prevent impurities that may affect matrix compounds and alter the sample selectivity and specificity. The cartridges were conditioned with 2 mL of methanol and 2 mL ultra-pure water. Urine sample was then loaded into the cartridge phase which aims to block interferents and phthalates. The cartridge was then washed with 2 mL of 0.1% acetic acid in ultra-pure water. The samples were eluted with 1 mL of 0.1% acetic acid in acetonitrile. All phthalate metabolites were quantified using reversed-phase-high-performance liquid chromatography–mass spectrometry (RP-HPLC–MS).
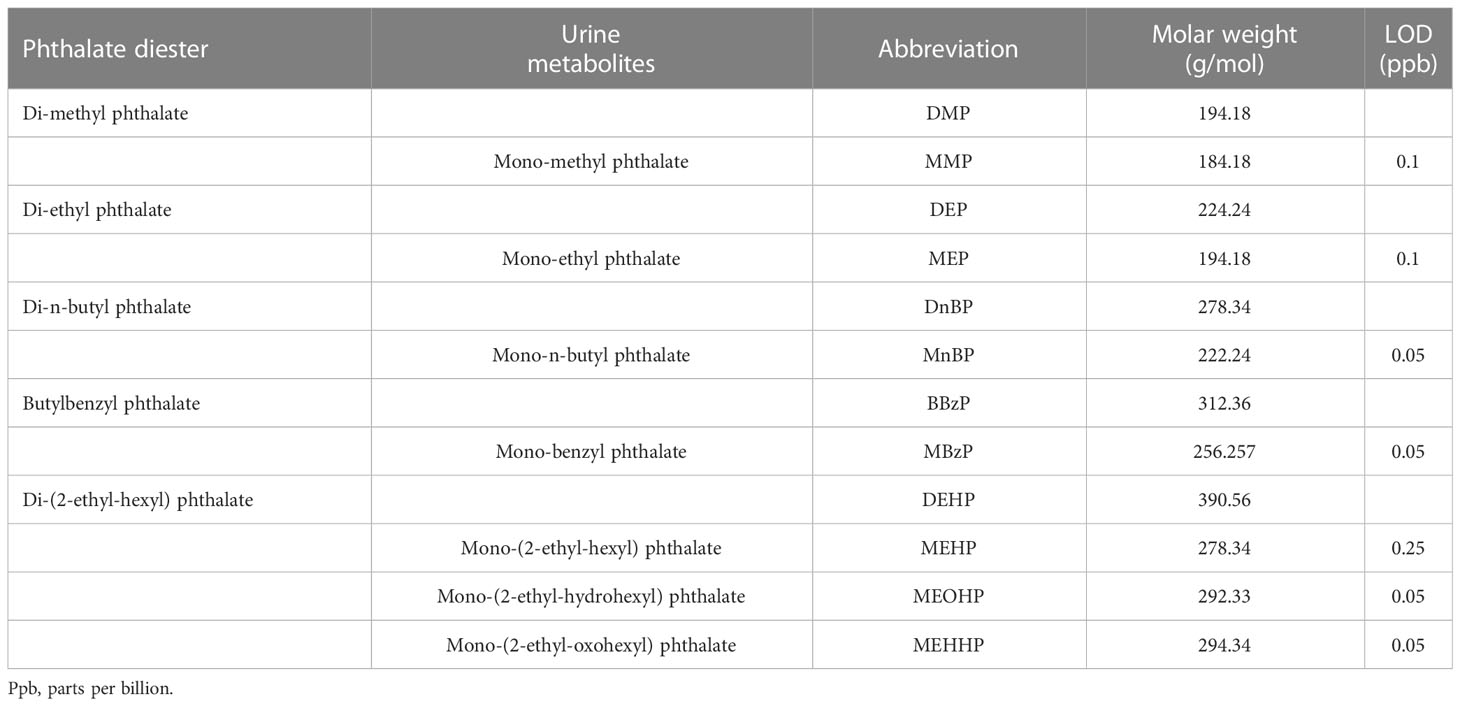
Table 1 Names, abbreviations, molar weight and limit of detection (LOD) of investigated phthalate parents and their metabolites.
2.4 Assessment of neurocognitive development
From 6 months onwards, the study design included a global child development assessment of the enrolled children using the third edition of the Griffith Scales of Child Development (GSCD III) (28, 29). This widely used specialistic tool explores five key evolutionary areas: foundations of learning (scale A), language and communication (scale B), eye and hand coordination (scale C), personal-social-emotional (scale D), and gross motor (scale E). Subscale scores and a total score, the so called General Development Score (GDS), were calculated. Subscale and GDS raw scores were standardized for sex and age according to GSCD normative scoring tables and guidelines, and standardized “Developmental Quotient (DQ)” scores were produced. In the present study, we used the Italian validated version GSCD questionnaire addressed to 0–12 months children and the normative scoring tables produced for the Italian population (30, 31).
According to the Griffiths III administration manual, the observed standardized DQ can be divided into seven categories for score interpretation: Extremely High (DQ ≥ 130), High (DQ = 120-129), Above Average (DQ = 110-119), Average (DQ = 90-109), Below Average (DQ 80-89), Borderline (DQ = 70-79), Extremely Low (DQ ≤ 69). In the present study, a trained team of pediatricians and a psychologist administered the GSCD at the six-month follow-up visit. After February 2020 due to pandemic restrictions, some of the 6-month follow-up visits were performed online. Staff and families were trained to accurately reproduce the procedures and assessments of in-person GSCD visits.
2.5 Covariates
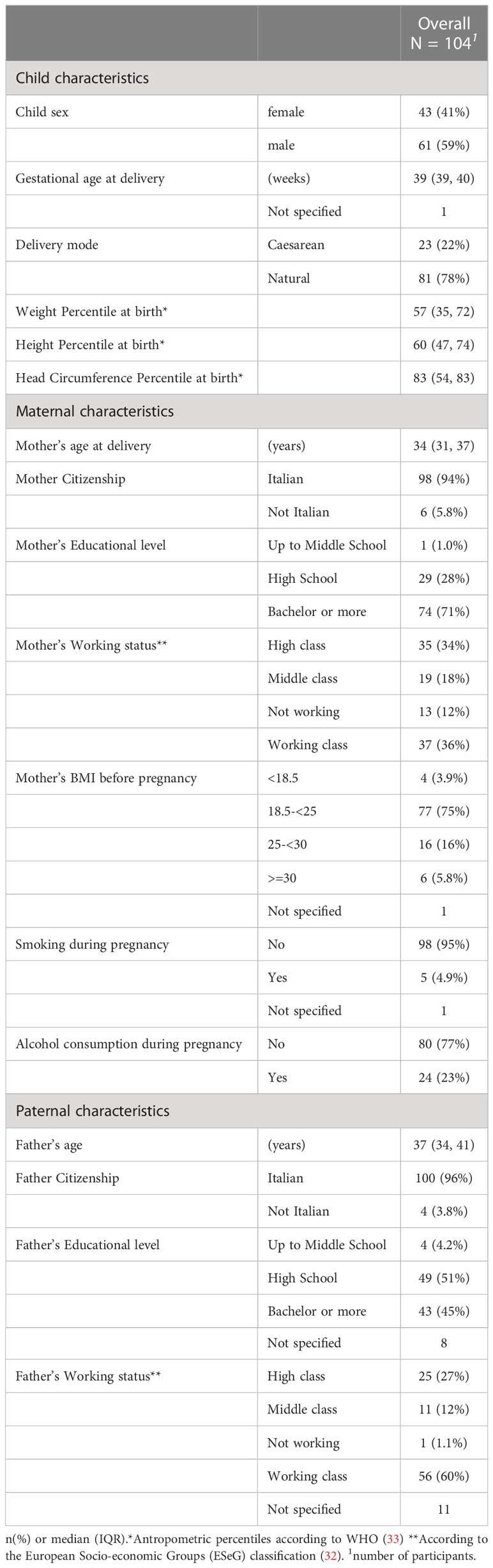
Given that child development is a complex process due to the interaction with multiple factors derived from the environment, society and the family background, we collected as covariates the items that majorly interact with the GSCD paradigm of development, and which are related to social-economical-cultural background of the parents (31). Data on socio-demographic characteristics of caregivers was collected during the enrollment visit. To specifically examine the relationship between the parents’ cultural background and the baby’s cognitive performance according to the GSCD scales, we defined three educational levels (1 = up to middle school; 2 = high school; 3 = bachelor or more). Working groups were defined according to European Socio-economic Groups (ESeG) (32) classification composed of 3 classes of 9 categories: high class (managers, professionals), middle class(technicians and associated professionals employees, small entrepreneur), working class (clerks and skilled service employees, skilled industrial employees, lower status employees) and not working. Maternal age at delivery, pre-gravidic weight, weight at delivery and height were collected for each mother and pre-gravidic BMI was calculated. Lifestyle habits of mothers during pregnancy were collected. Finally data on whether the evaluation was conducted before or after the pandemic outbreak was collected. Variable details are described in Table 2.
2.6 Study size
The Present study involves 104 participants.
2.7 Statistical analysis
Categorical variables characteristics were summarized by absolute and relative frequencies. Median and interquartile range (IQR) were used to summarize continuous variables. Phthalate concentrations below the respective level of detection (LOD) are expressed as the LOD value divided by the square root of 2 (<LOD= LOD/√2). LOD values for each metabolite are reported in Table 1. Metabolites of DEHP were expressed combined as the sums ∑DEHPm, by using equation (1):
where ∑DEHPm is the summed urinary concentration of metabolites of DEHP (MEHP, MEHHP, MEOHP) expressed as the urinary concentration of parent compound; UCm1, UCm2 etc. are the urinary concentration of the individual metabolites and MWm1, MWm2 etc. indicate molecular masses of each metabolite, respectively, while MWp is the molecular mass of the parent compound. Molecular masses are reported in Table 1.
When considering phthalate metabolite concentration, aside from descriptive analyses, statistical analyses were conducted after natural logarithmic transformation to minimize the influence of highly skewed data. Pearson’s chi-square test or Fisher’s exact test was used to compare categorical variables. T-student test or Wilcoxon rank sum test (also known as Mann Whitney U test) were used to analyze differences in two groups of numeric variables depending on their distribution. We examined potential confounding variables by literature search and included those factors likely to be associated both with phthalate exposure and adverse neurodevelopment (details in Table 2). Given the relatively small sample size, we ran regression models with all candidate covariates and no exposure with each GSCD score. Covariates with p-values less than 0.20 in at least one of the models were retained in all final models, The retained covariates were: child sex, gestational age at delivery, delivery mode, mother’s educational level, father’s educational leveland if GSCD was performed before or after the pandemic outbreak.
Several studies reported effect modification for numerous neurodevelopmental outcomes depending on the sex of the child (34–40), We performed regression models stratifying population by sex.
Finally, some of the visits (n:35) were conducted online due to pandemic restrictions. Staff attempted to replicate the procedures and assessments of in-person GSCD visits as accurately as possible; however, given the possibility of bias in test evaluation during online visits, as well as the higher variability of the scores producing some outlier scores, we performed a subgroup analysis on infants visited exclusively in presence. This affected our sample size, but it allowed us to exclude outliers from the analysis. False discovery rate was controlled by using the Benjamini-Hochberg procedure.
Missing data were dealt with pairwise-case data analysis. Statistical software IBM SPSS Statistics®, version 27 and the R software version 4.2.1 (The R Foundation for Statistical Computing) for plotting and advanced statistical analysis, with packages including tidyverse, ggplot2, ggh4x, gtsummary. Code for linear model figure plotting was adapted from Rolland et al. (41).
3 Results
3.1 Description of the population
Table 2 describes the overall population of our study involving 104 children, with regards to demographic-socio-economic characteristics of infants and caregivers.
The comparison between the 104 children who underwent the GSCD III evaluation at T6 and were included in the present study (the ‘included’ group), and those who were not is described in Supplementary Table S1. Significant differences between the two groups were detected for mother’s scholarization (higher educational level in the included group), pre-gravidic BMI (higher normo-BMI in the included group) and smoking habit during pregnancy (lower smoking in the included group). No differences were found for the paternal socio-economic characteristics.
3.2 Urinary phthalate metabolite concentrations
For the present study we collected 387 urine samples from 103 mothers, 95 newborns and respectively 92 and 97 infants of three and six months.
Metabolite concentrations detected in urine samples of mothers and children overtime are presented in Table 3. In a total of 387 urine samples, the seven metabolites analyzed appeared widespread and were detected in most of the urine samples collected at any time of sampling (66-100%).
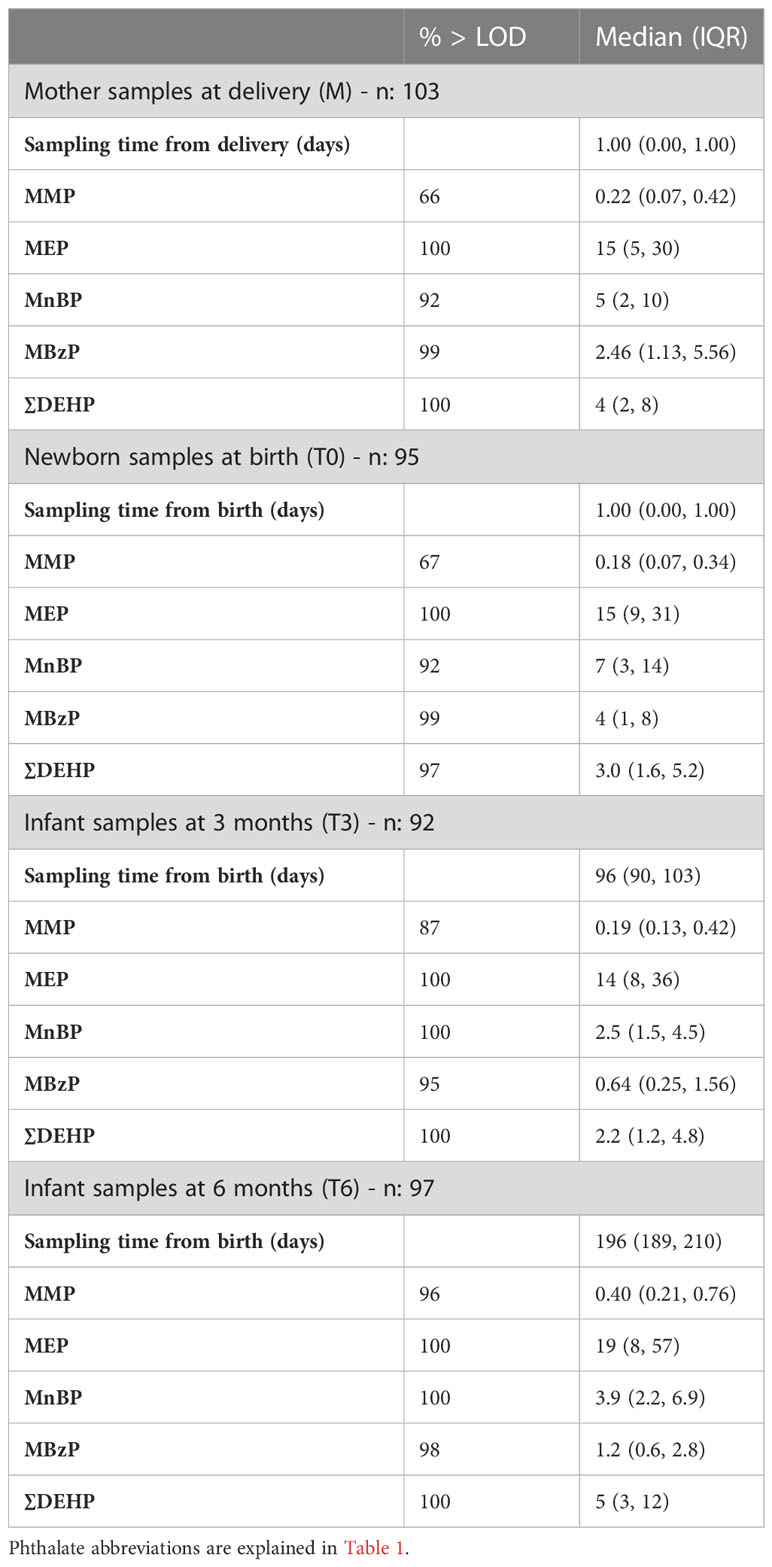
Table 3 Phthalate metabolite concentration (ng/mL) in 387 urine samples from mothers close to delivery, and in their children at birth, three and six months.
Comparison between ‘included’ and ‘not included’ groups are shown in Supplementary Table S2. Overall, the characteristics and urinary concentrations of the ‘included’ group, did not differ significantly from the ‘not included’.
3.3 Assessment of child development
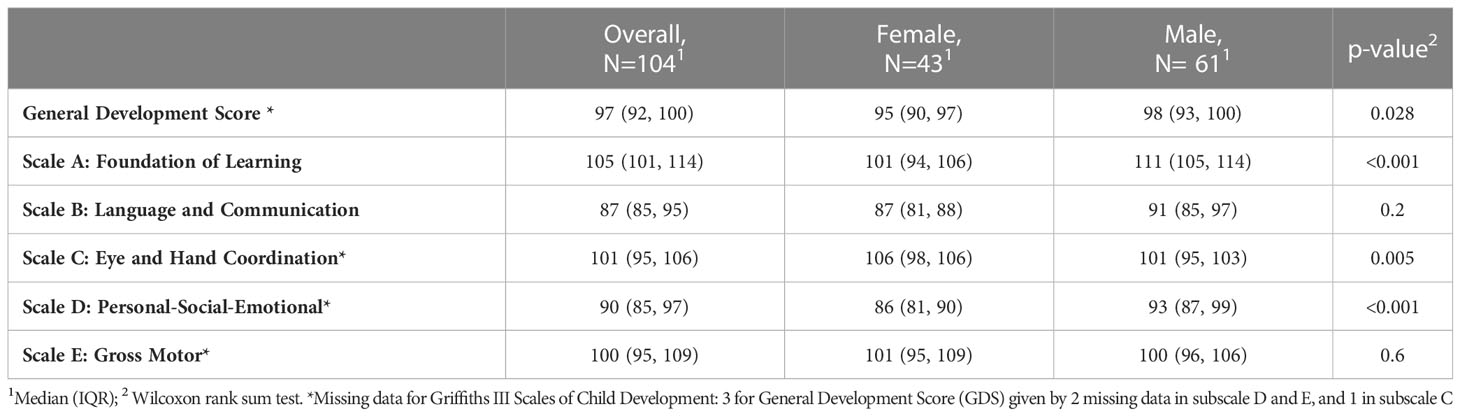
Developmental quotients at 6 months, expressed as median and IQRs, are shown in Table 4. Due to the pandemic restrictions 35 evaluations (33.7%) were performed online, while the ‘in-person’ evaluations were 69 (66.3%). Less than 3% of the study participants had missing outcome data. According to Table 2 most of the DQs fall in average range, except for the subscale B, which present a DQ median score of 87 (85–95). In particular, 46 infants (44%) scored in average or above, while 46 (44%) below average, 8 (7.7%) borderline and 4 (3.8%) extremely low. Table 4 shows the standardized scores obtained at 6 months for the overall population and categorized by children’s sex. The General development and the scales A and D scores, are significantly higher in males; while the scale C had significantly higher score for females. For scale D there is a median score of 93 in boys, which is in average, while in girls there is a median score of 86, which falls below average.
3.4 Adjusted associations between urinary phthalate metabolite concentrations and Griffith’s developmental quotients scores
Figure 1 shows the adjusted linear regressions between Griffith’s developmental quotients scores and urinary phthalate metabolite concentrations in mothers at T0 and in infants at T0, T3 and T6. Several trends both for infants’ and mothers concentrations were found, for each exposure period. Numerical betas and confidence intervals are shown in Supplementary Table 4.
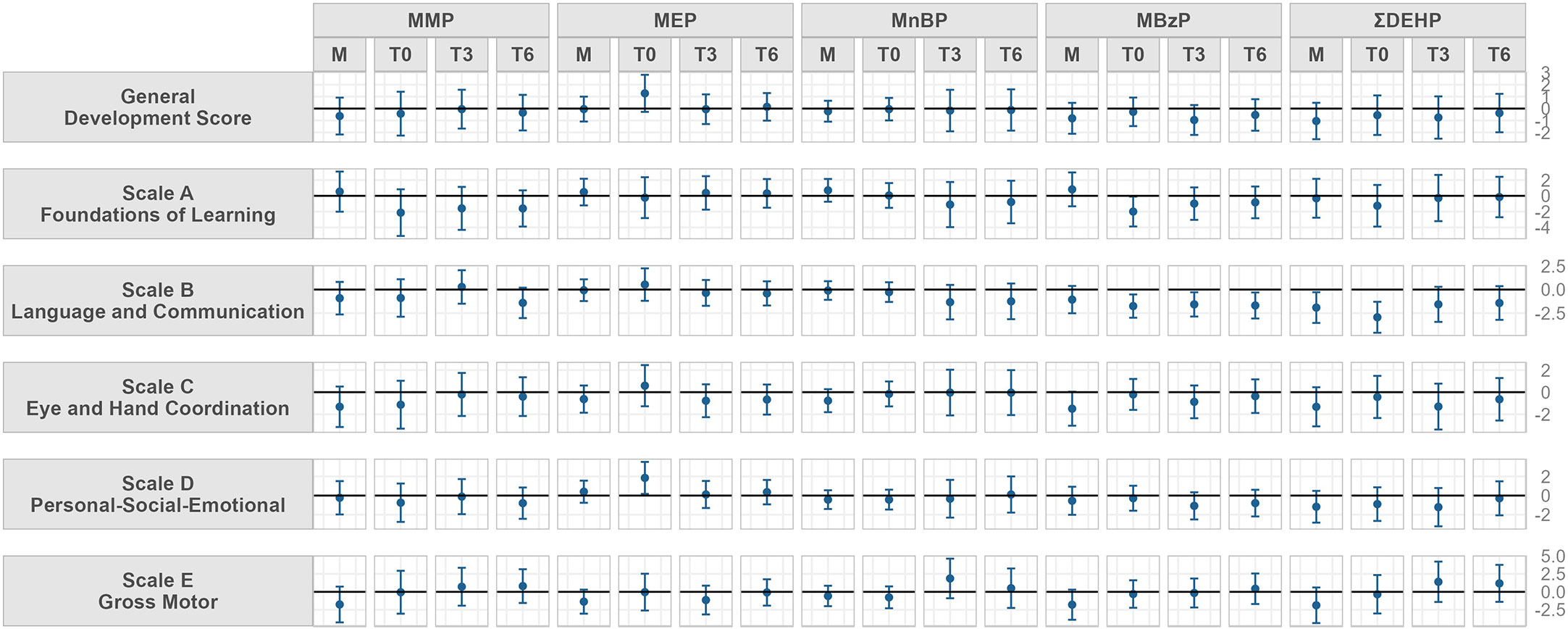
Figure 1 Model adjusted for child sex, gestational age at delivery, delivery mode, mother’s educational level, father’s educational level and if GSCD was performed before or after the pandemic outbreak. Beta and 95% confidence interval of adjusted linear regression models between urinary concentrations of phthalate metabolite and parent compound and Griffith Scales of Child Development (GSCD) measured at 6 months in the Modena cohort study, stratified by sampling timing (from mothers close to delivery, M, and in their children at birth, T0, three, T3, and six months, T6). Values may be found in Supplementary Material; Table S4. Phthalate abbreviations are explained in Table 1.
Maternal urinary MnBP concentrations showed a negative trend with the scale C scores at six months while MBzP concentrations showed a negative trend with scale B, scale C and scale E scores. Maternal DEHP concentrations were negatively associated to scale B scores at six months.
At T0, neonatal concentrations of MBzP and DEHP metabolites showed an inverse trend to the scale B at T6. MBzP also showed negative trends with scale A scores. Neonatal MEP concentrations at T0 were positively associated to scale D scores.
MnBP urinary concentrations at T3 showed positive trends with scale E at three and six months; MBzP for all sampling timings was negatively associated to subscale B scores.
Figure 2 shows the adjusted linear regressions between Griffith’s developmental quotients scores and urinary phthalate metabolite concentrations in mothers at T0 and in infants at T0, T3 and T6 stratified by children’s sex. Numerical betas and confidence intervals are shown in Supplementary Table 5 for female and in Supplementary Table 6 for male.
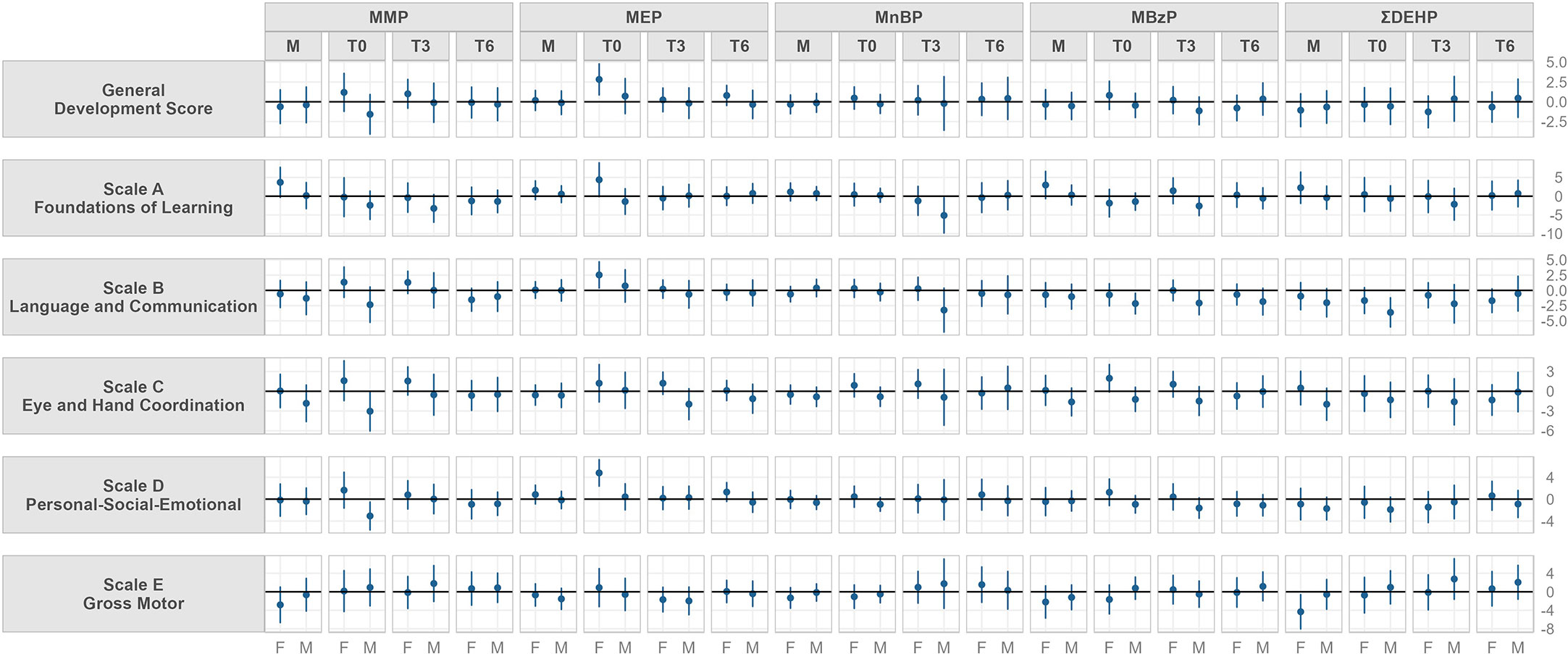
Figure 2 Model adjusted for gestational age at delivery, delivery mode, mother’s educational level, father’s educational leveland if GSCD was performed before or after the pandemic outbreak. Beta and 95% confidence interval of adjusted linear regression models stratified by sex between urinary concentrations of phthalate metabolite and parent compound and Griffith Scales of Child Development (GSCD) measured at 6 months in the Modena cohort study, stratified by sampling timing (from mothers close to delivery, M, and in their children at birth, T0, three, T3, and six months, T6). Values may be found in Supplementary Material; Table S5, 6. Phthalate abbreviations are explained in Table 1.
Maternal urinary concentrations had a positive relationship with female scale A scores for MMP and MBzP, while showed a negative association to scale E for DEHP. In boys, negative trends were found for scale C and maternal MMP, MBzP and DEHP concentrations, and for scale B and D and DEHP concentrations.
Neonatal urinary concentrations (T0) showed a positive trend in females with: scale C for MMP, General development score, scale A, B, C and D for MEP, scale C for MBzP. Moreover, in girls a negative trend was found between scale E and MBzP.
In males, neonatal urinary concentrations (T0) were found to be negatively associated to: scale B, C and D for MMP; scale B for MBzP and for DEHP.
At three months of age (T3) a positive trend between female urinary MMP concentrations and scale C was found.
In males, negative associations at T3 was found between MnBP and MBzP and scale A and B.
Urinary phthalate concentrations at T6 did not show relevant trends with DQs at six months in girls and boys.
Finally, given the possible bias in test evaluation during online visits, we performed a subgroup analysis on infants visited exclusively in presence. Even if due to sample size reduction relationships appear less strong, overall associations and patterns were similar to those found in the main models.
4 Discussion
4.1 Main findings
The present study evaluates peri- and postnatal exposure to phthalates in an Italian pediatric cohort of full term babies and their mothers and its possible associations with the child’s neurodevelopment. The population showed homogeneous characteristics: all the babies were born from a single center, at term, with no perinatal complications. There is a relatively equal division between males and females and the anthropometric characteristics were in line with healthy newborns for both sexes. As shown in Supplementary Material, few differences between ‘included’ and ‘not included’ groups were found, in particular for mothers (educational status, pre-gravidic BMI and smoking habits). Despite these differences, phthalate metabolites concentrations resulted almost equally distributed within the two groups (as shown in Supplementary Table S2). Our results showed that infants and mothers were exposed to several phthalates during the whole study period.
4.1.1 Neurocognitive development
The neurocognitive assessment performed for 104 children at six months through the Griffith Scales of Child Development (GSCD III) showed scores within the normal range for General development score and scales A, C, D, and E. This was expected because the children involved in our study had no significant neurodevelopment risk factors, due to the study inclusion criteria.
For scale B, 46% of our population scored below average. This result could therefore be justified due to the social distancing measures introduced by the pandemic outbreak and the consequent prolonged reduced socialization (42–44). In addition, the use of the facial mask may have influenced and slowed learning, since it hinders labial vision. The evidence that COVID-19 somehow affected children’s neurocognitive development was shown in a recent cross-sectional study. In fact, significantly lower scores in both general development and subscales scores were observed in children assessed during the pandemic period (31).
The surrounding environment, external cues, and lifestyle habits are very important in determining and eliciting neurocognitive development in children. In a study conducted on Filipino children, the Griffith’s Mental Developmental Scales were evaluated by comparing the performance of 742 Filipino children longitudinally at 6, 12 and 24 months old to those of their British counterparts.
Although the performance of the Filipino children in the Griffiths test were within average for age, their performances on developmental sub quotients at later ages of 12 and 24 months were significantly lower than British children and may have been influenced by differences in ethnicity, cultural traditions and limited environmental resources (44).
Our results have also shown differences in the GSCD III scores according to sex, although scores are adjusted for age and sex.
In particular, males reported higher scores in scale A (Foundations of Learning) in scale D (Personal-Social-Emotional) and in General developmental score; while girls, reported higher scores in scale C (hand-eye coordination). From an overall view, in our population boys have higher scores than girls in different areas of development. It is known that there are anatomical and functional differences between the central nervous system maturation of males and females. Hormonally dependent, sex-specific changes in the ultrastructure of the developing central nervous system are known. Higher prenatal androgens in males than in females modulates the masculinization of external genitalia, the developing neural system, and the behavioral development. In particular, the androgen levels during critical periods in early development are thought to contribute to the between- and within-sex variability in human social and cognitive behavior (4, 45). In literature, data comparing the differences between neurodevelopmental assessment of male and female are present, especially using a different tool compared to our study, the Bayley III scales (40). From a general overview, females present higher scores for language and fine motor performance, while males for gross motor functions and oculo-manual coordination, although these differences are quite clear from 12 months onwards (46).
In a 2019 Danish study (45), the authors investigated whether gender differences were present in Bayley-III scores and behavior in a sample of typically developing children at ages 4, 7, 10, 13, 24 and 36 months. The results demonstrated the existence of gender differences in test scores, but the pattern of differences varied across scales and subtests. In the cognitive scale, gender differences were found at 24 and 36 months with higher scores in girls rather than boys; in the language scale at 10, 13, 24 and 36 months, which found girls’ performances to be superior to those of the boys as well as in the motor scale at 36 months. In a Chinese study, conducted in 2019 to detect possible sex differences in term children using the Bayley-III cognitive scales, 1486 children aged 16 days to 42 months were analyzed. In this study, it was found that girls in the 0-5, 6-11, 12-17 and 18-23-months ranges reported higher scores than boys, although the only significance was reported in the 18-23 months range (38). It is therefore necessary to investigate further, particularly with regard to the use of the GSCD III in the assessment of healthy, term-born children.
4.1.2 Possible associations between phthalates exposure and neurocognitive development
This study detected several possible associations between urinary phthalate concentrations in mothers and children, at three different time points, and GSCD III scores conducted at 6 months.
A large number of tests were carried out, hence, we cannot exclude that some statistically significant results were random. Furthermore, the small sample size may account for the change in significance observed between the unadjusted and adjusted model. For this reason, attention should be focused mainly on the more consistent associations.
As shown in Figure 1, MBzP and DEHP metabolites seem to be the most negatively associated to GSCD III, in particular to scale B and C. MBzP itself also showed a negative association with scale A and E. As previously mentioned, these are two of the most regulated phthalates due to their anti-androgenic and endocrine disrupting effects. Although found at low concentrations, both MBzP and DEHP metabolites have shown significant negative associations with different scales of development, confirming previous literature data (47).
MMP, MnBP, DEHP showed positive relation with scale E. The positive association to the gross motor function may be explained with the tendency at six months, for good scale-E performants, to bring objects to the mouth, to suck toys and to start exploring the playground. Maternal levels of MnBP were also found to be negatively associated to scale C, enforcing the negative effect of MBzP and DEHP on neurodevelopment (48).
MEP at T0 was found to be positively associated to scale D performed at six months. MEP is a metabolite of one of the not restricted phthalate (DEP) currently used mainly in self-care products. Our personal interpretation of this direct association with scale D, is linked to the high level of personal cure and hygiene that families of babies with high scores at scale D may present. The more the baby is keen to socialization, the more probably the parent are keen to cure themselves and the baby and vice-versa. However no results in literature are available to confirm this possibility (49). Child development is an ongoing process within the first years of life, influenced by several different factors as: the relationship with parents and care-givers, appropriate sensorial stimulations or unappropriated deprivation, energy intake, quality of sleep etc. Being able to detect an association within phthalates exposure and GSCD III scores at six months of age in otherwise healthy children with no apparent neuro-developmental risk factors, brings us to be very careful in results interpretation. However, as clinicians, we need to consider these findings as an alert for the future child development. We need to ask ourselves if GSCD III lower scores are due to a delayed acquisition of the development milestones or to a proper modulation of the neurodevelopment process. Moreover, seen that phthalates are ubiquitous, our children will experienced a prolonged exposure to chemical compounds during a very vulnerable temporal window, leading to a possible summatory effect. Longitudinal follow up of children becomes a priority for brain health.
Once we stratified the population according to sex, associations between GSCD III scores and phthalates concentrations changed. In particular, from an overall view of Figure 2 we detected positive associations in female, while negative associations in male.
MMP, MnBP, MBzP and DEHP metabolites showed several negative associations with all the scale of GSCD III in male, during all the different timing of the study.
These negative associations were previously detected in literature, indicating that there is a negative influence of these compounds on neurodevelopment, especially in males, probably linked to the anti-androgenic effects of many of them.
A recent study suggested that prenatal exposure to phthalates could be inversely associated with the MDI and PDI of infants, particularly in males, at 6 months (34). Other studies (35–40) showed a negative correlation between phthalate levels and neurodevelopment: in all cases a significant inverse associations between prenatal exposure to DBP and cognitive and psychomotor development was found.
On the contrary, positive associations between GSCD III scores in females and urinary phthalates concentrations were found. This was seen especially for MEP, who showed associations with general development score, scale A, B and D for neonatal urinary concentrations.
In females, negative association were found with scale E and MBzP and DEHP concentrations.
Similar associations were previously found by Hyland C et al, who found slightly higher IQ scores in girls with higher phthalates concentrations (50) compared to boys, although age of assessment and urinary collection was completely different from our study. In fact, we need to remember how, in our population, general quotient, scale A and scale D scores were higher in male.
The positive associations in girls may depend on the different body composition of female neonates and children, who present an higher percentage of body fat mass, than male peers.
Differences in body composition between female and male neonates are confirmed, with girls having a higher fat mass divided by total body mass (%) (11.1% vs. 9.6%) and lower free fat mass (2827 g vs. 2979 g), which seems to be maintained throughout life (51, 52).
Full term males are heavier at birth and have a higher lean body mass, whereas females have more subcutaneous fat (53). These sex-related differences have been primarily attributed to the action of fetal sex steroid hormones, which presumably enhances lean body mass growth in utero and later during the first months of life, determining a metabolic imprinting of the body fat mass in males (54).
Boys may be more vulnerable to the neurotoxicity in several areas of development, due to anti-androgenic effects of phthalates, although their global scores were within the normal range except for scale B (55–57).
Females may be keener to the accumulation of phthalates in body fat mass. This may lead to a positive association within phthalates concentrations and different developmental domains, although lower scores than males were often found, except for scale C.
4.2 Strengths and limitation
The small number of subjects enrolled in the study is strictly dependant by the complexity of urine sampling in newborn during their very first days of life, which is especially true when conducting stratification by sex. Given this problem, even if the sample appears small, this study is one of the few European studies to assess exposure at this timepoint and, at the moment, one of the largest (34–39). COVID-19 disruption of study protocol affected timing and logistic of sampling collection. After the outbreak, caregivers were asked to collect urine samples. To avoid contamination, we carefully trained them on proper sample techniques and provided them with phthalate-free sampling kits. Finally, researchers collected and stored urine samples as quickly as feasible after sampling. While attempts were made to minimize contamination in our investigation, the chance of contamination exists. Secondly, the use of single spot urine, especially with short half-life compounds, limits the generalizability of our findings to everyday life or chronic exposure. The use of multiple single spot urine over the first six months of life enabled us to assess time trends in early life exposure to phthalates. Furthermore, some authors argued that a single sample may also reasonably predict average metabolite concentrations over a period of several months (58–60). Even though the first year of life is a critical period for brain development, very few studies assessed phthalate concentrations during the first year of life (41, 61), hence the collection of three sample points in the first six months of life is a great strength of our study.
It is noteworthy that the associations of urinary phthalate metabolites collected at 6 months and the GSCD scores collected at the same timing are cross-sectional and need to be interpreted with caution.
Due to the presence of many exposures as well as outcomes, multiple comparisons were performed and we cannot exclude that the some of the association we found were due to chance. Associations found with MMP must be interpreted cautiously, given that the percent of detected samples but lower than LOD was lower than 90%.
Finally, the collection of information regarding the family’s socio-economical-cultural background, perinatal and child factors, allowed us to adjust for potential confounder. However, given the complexity of environmental exposure, there may be confounding from residual covariates.
5 Conclusion
Our results highlighted how phthalates exposure in the restricted geographic area analyzed, is widespread. At six months of age, our population of healthy term newborn scored within the normal range in all the different domains of development, except for the language development. This could be related to the social distancing and the use of masks due to pandemic restrictions.
Urinary phthalate metabolites were found to be associated to GSCD III scores, showing negative associations within ‘language’ and ‘hand-eye coordination’ and MBzP and DEHP concentrations. The impact of phthalates on neurodevelopment showed a clear sex-related difference with several negative associations in boys within GSCD III scores and anti-androgenic phthalates, while several positive associations in girls, especially with non-regulated compounds.
As phthalates are almost ubiquitous compounds and it is therefore quite impossible to avoid them in daily life, some precautions can be used to minimize exposure. Intervention strategies to reduce the exposure to phthalates in children may include: to prefer fresh foods, to avoid the use of environmental deodorants or cosmetics and personal care products if not phthalates-free; to pay attention to the labels of products purchased, making sure they are phthalate-free; to dispose of older toys or food contact materials that were made prior to the introduction to phthalate regulations, preferring the use of materials that come from countries that currently have in place regulations that limit the use of such compounds (I.e.: European Union)t (62).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Comitato Etico Area Vasta Emilia Nord. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
LL, LP, ER conceptualized and designed the study, coordinated and supervised data collection, drafted the initial manuscript, and reviewed and revised the manuscript. EP, VT, FC, EF, GT and NB collected data. AF analyzed urinary samples. LL, LP and ER critically analyzed and interpreted data. FF, BP and LI critically reviewed the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. LL and LP have contributed equally to this work and share first authorship. All authors contributed to the article and approved the submitted version.
Funding
This study was partially funded by the research grant ‘University funding grant for interdisciplinary proposals year 2018’ emitted by the University of Modena and Reggio Emilia, Italy (Fondo di Ateneo per la Ricerca anno 2018. Progetto di ricerca interdisciplinare).
Acknowledgments
We would like to thank all the families and patients who decided to take part to the study. Moreover we would like to thank the doctors of the Post graduate School of Pediatrics who helped in the recruitment process of the study, in particular: Dr. Righi B, Miceli A, Marrozzini L, Luglio A, Cattini U, Poluzzi F, Plessi C and to the residents in Hygiene and Preventive Medicine who helped with data and sample collecting. We thank Dr. Diego Pinetti of the CIGS for his precious help on processing the urinary samples for phthalate metabolites dosage.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1172743/full#supplementary-material
References
1. Zhang YJ, Guo JL, Xue JC, Bai CL, Guo Y. Phthalate metabolites: characterization, toxicities, global distribution, and exposure assessment. Environ Pollut (2021) 291:118106. doi: 10.1016/j.envpol.2021.118106
2. Benjamin S, Masai E, Kamimura N, Takahashi K, Anderson RC, Faisal PA. Phthalates impact human health: epidemiological evidences and plausible mechanism of action. J Hazard Mater (2017) 340:360–83. doi: 10.1016/j.jhazmat.2017.06.036
3. Lucaccioni L, Trevisani V, Passini E, Righi B, Plessi C, Predieri B, et al. Perinatal exposure to phthalates: from endocrine to neurodevelopment effects. Int J Mol Sci (2021) 22(8):4063. doi: 10.3390/ijms22084063
4. Wang Y, Zhu H, Kannan K. A review of biomonitoring of phthalate exposures. Toxics (2019) 7(2):21. doi: 10.3390/toxics7020021
5. Qian Y, Shao H, Ying X, Huang W, Hua Y. The endocrine disruption of prenatal phthalate exposure in mother and offspring. Front Public Health (2020) 8:366. doi: 10.3389/fpubh.2020.00366
6. Frederiksen H, Skakkebaek NE, Andersson AM. Metabolism of phthalates in humans. Mol Nutr Food Res (2007) 51(7):899–911. doi: 10.1002/mnfr.200600243
7. Wittassek M, Koch HM, Angerer J, Brüning T. Assessing exposure to phthalates - the human biomonitoring approach. Mol Nutr Food Res (2011) 55(1):7–31. doi: 10.1002/mnfr.201000121
8. Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health (2007) 210(5):623–34. doi: 10.1016/j.ijheh.2007.07.011
9. Kahn LG, Philippat C, Nakayama SF, Slama R, Trasande L. Endocrine-disrupting chemicals: implications for human health. Lancet Diabetes Endocrinol (2020) 8(8):703–18. doi: 10.1016/S2213-8587(20)30129-7
10. European Commission (EC). 4th report on the implementation of the “Community strategy for endocrine disruptors” a range of substances suspected of interfering with the hormone systems of humans and wildlife (COM (1999) 706). Brussels, Belgium: European Commission (2011). Commission staff working paper.
11. Martínez-Ibarra A, Martínez-Razo LD, MacDonald-Ramos K, Morales-Pacheco M, Vázquez-Martínez ER, López-López M, et al. Multisystemic alterations in humans induced by bisphenol a and phthalates: experimental, epidemiological and clinical studies reveal the need to change health policies. Environ Pollut (2021) 271:116380. doi: 10.1016/j.envpol.2020.116380
12. Sathyanarayana S. Phthalates and children's health. Curr Probl Pediatr Adolesc Health Care (2008) 38(2):34–49. doi: 10.1016/j.cppeds.2007.11.0
13. Poopal RK, Ramesh M, Maruthappan V, Babu Rajendran R. Potential effects of low molecular weight phthalate esters (C16H22O4 and C12H14O4) on the freshwater fish. Cyprinus carpio. Toxicol Res (Camb). (2017) 6(4):505–20. doi: 10.1039/c7tx00084
14. ECHA (European Chemicals Agency). Hot topics: phthalates (2022). Available at: https://echa.europa.eu/en/web/guest/hot-topics/phthalates.
15. Nidens N, Vogel M, Körner A, Kiess W. Prenatal exposure to phthalate esters and its impact on child development. Best Pract Res Clin Endocrinol Metab (2021) 35(5):101478. doi: 10.1016/j.beem.2020.101478
16. Iughetti L, Lucaccioni L, Street ME, Bernasconi S. Clinical expression of endocrine disruptors in children. Curr Opin Pediatr (2020) 32(4):554–9. doi: 10.1097/MOP.0000000000000926
17. Le Magueresse-Battistoni B, Labaronne E, Vidal H, Naville D. Endocrine disrupting chemicals in mixture and obesity, diabetes and related metabolic disorders. World J Biol Chem (2017) 8(2):108–19. doi: 10.4331/wjbc.v8.i2.108
18. Broe A, Pottegård A, Hallas J, Ahern TP, Lamont RF, Damkier P. Phthalate exposure from drugs during pregnancy and possible risk of preterm birth and small for gestational age. Eur J Obstet Gynecol Reprod Biol (2019) 240:293–9. doi: 10.1016/j.ejogrb.2019.07.023
19. Chin HB, Jukic AM, Wilcox AJ, Weinberg CR, Ferguson KK, Calafat AM, et al. Association of urinary concentrations of early pregnancy phthalate metabolites and bisphenol a with length of gestation. Environ Health (2019) 18(1):80. doi: 10.1186/s12940-019-0522-2
20. de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when? Early Hum Dev (2006) 82(4):257–66. doi: 10.1016/j.earlhumdev.2005.10.013
21. Ferguson KK, McElrath TF, Chen YH, Mukherjee B, Meeker JD. Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: a repeated measures analysis [published correction appears in environ health perspect. Environ Health Perspect (2015) 123(3):210–6. doi: 10.1289/ehp.1307996
22. Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol (2004) 16(10):809–18. doi: 10.1111/j.1365-2826.2004.01243.x
23. Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol (2017) 13(3):161–73. doi: 10.1038/nrendo.2016.186
24. Holahan MR, Smith CA. Phthalates and neurotoxic effects on hippocampal network plasticity. Neurotoxicology (2015) 48:21–34. doi: 10.1016/j.neuro.2015.02.008
25. Shea KM. American Academy of pediatrics committee on environmental health. pediatric exposure and potential toxicity of phthalate plasticizers. Pediatrics (2003) 111(6 Pt 1):1467–74. doi: 10.1542/peds.111.6.1467
26. Langer S, Bekö G, Weschler CJ, Brive LM, Toftum J, Callesen M, et al. Phthalate metabolites in urine samples from Danish children and correlations with phthalates in dust samples from their homes and daycare centers. Int J Hygiene Environ Health (2014) 217(1):78–87. doi: 10.1016/j.ijheh.2013.03.014
27. Lanfranchi S, Rea M, Vianello R, Ferri R. GRIFFITHS III - griffiths scales of child development: part I manual: overview, development and psychometric properties. In: Italian Edition, 2nd ed. Florence: Hogrefe (2017).
29. Lanfranchi S, Rea M, Vianello R, Ferri R. GRIFFITHS III - griffiths scales of child development: part II manual: administration and scoring. In: Italian Edition, 2nd ed. Florence: Hogrefe (2017).
30. Lanfranchi S, Rea M, Vianello R, Ferri R. GRIFFITHS III - griffiths scales of child development: part III manual: Italian standards. In: Girls and boys. Italian edition, 2nd ed. Florence: Hogrefe (2019).
31. Ferrari E, Palandri L, Lucaccioni L, Talucci G, Passini E, Trevisani V, et al. The kids are alright (?). infants’ development and COVID-19 pandemic: a cross-sectional study. Int J Public Health (2022) 67:1604804. doi: 10.3389/ijph.2022.1604804
32. Bohr J. EU-AES Tools: implementation of the European socioeconomic groups classification (ESeG) using adult education survey microdata. GESIS Papers (2018) 24. doi: 10.21241/ssoar.57622
33. Available at: https://www.who.int/tools/child-growth-standards/standards.
34. Park S, Zimmerman E, Huerta-Montañez G, Rosario-Pabón Z, Vélez-Vega CM, Cordero JF, et al. Gestational exposure to phthalates and phthalate replacements in relation to neurodevelopmental delays in early childhood. Toxics (2023) 11(1):65. doi: 10.3390/toxics11010065
35. Kim Y, Ha EH, Kim EJ, Park H, Ha M, Kim JH, et al. Prenatal exposure to phthalates and infant development at 6 months: prospective mothers and children's environmental health (MOCEH) study. Environ Health Perspect (2011) 119(10):1495–500. doi: 10.1289/ehp.1003178
36. Polanska K, Ligocka D, Sobala W, Hanke W. Phthalate exposure and child development: the polish mother and child cohort study. Early Hum Dev (2014) 90(9):477–85. doi: 10.1016/j.earlhumdev.2014.06.006
37. Factor-Litvak P, Insel B, Calafat AM, Liu X, Perera F, Rauh VA, et al. Persistent associations between maternal prenatal exposure to phthalates on child IQ at age 7 years. PloS One (2014) 9(12):e114003. doi: 10.1371/journal.pone.0114003
38. Zhang Q, Chen XZ, Huang X, Wang M, Wu J. The association between prenatal exposure to phthalates and cognition and neurobehavior of children-evidence from birth cohorts. Neurotoxicology (2019) 73:199–212. doi: 10.1016/j.neuro.2019.04.007
39. Ponsonby AL, Symeonides C, Saffery R, Mueller JF, O'Hely M, Sly PD, et al. Prenatal phthalate exposure, oxidative stress-related genetic vulnerability and early life neurodevelopment: a birth cohort study. Neurotoxicology (2020) 80:20–8. doi: 10.1016/j.neuro.2020.05.006
40. Sathyanarayana S, Butts S, Wang C, Barrett E, Nguyen R, Schwartz SM, et al. Early prenatal phthalate exposure, sex steroid hormones, and birth outcomes. J Clin Endocrinol Metab (2017) 102(6):1870–8. doi: 10.1210/jc.2016-3837
41. Rolland M, Lyon-Caen S, Thomsen C, Sakhi AK, Sabaredzovic A, Bayat S, et al. Effects of early exposure to phthalates on cognitive development and visual behavior at 24 months. Environ Res (2023) 219:115068. doi: 10.1016/j.envres.2022.115068
42. Weyers S, Rigó M. Child health and development in the course of the COVID-19 pandemic: are there social inequalities? Eur J Pediatr (2023) 182(3):1173–81. doi: 10.1007/s00431-022-04799-9
43. Crimon C, Barbir M, Hagihara H, de Araujo E, Nozawa S, Shinya Y, et al. Mask wearing in Japanese and French nursery schools: the perceived impact of masks on communication. Front Psychol (2022) 13:874264. doi: 10.3389/fpsyg.2022.874264
44. Reyes A, Pacifico R, Benitez B, Villanueva-Uy E, Lam H, Ostrea EM Jr. Use of the griffiths mental development scales in an agro-industrial province in the Philippines. Child Care Health Dev (2010) 36(3):354–60. doi: 10.1111/j.1365-2214.2010.01080.x
45. Krogh MT, Væver MS. Does gender affect bayley-III scores and test-taking behavior? Infant Behav Dev (2019) 57:101352. doi: 10.1016/j.infbeh.2019.101352
46. Kurth F, Gaser C, Luders E. Development of sex differences in the human brain. Cognit Neurosci (2021) 12(3-4):155–62. doi: 10.1080/17588928.2020.1800617
47. Vilmand M, Beck IH, Bilenberg N, Andersson AM, Juul A, Schoeters G, et al. Prenatal and current phthalate exposure and cognitive development in 7-year-old children from the odense child cohort. Neurotoxicol Teratol (2023) 96:107161. doi: 10.1016/j.ntt.2023.107161
48. Engel SM, Patisaul HB, Brody C, Hauser R, Zota AR, Bennet DH, et al. Neurotoxicity of ortho-phthalates: recommendations for critical policy reforms to protect brain development in children. Am J Public Health (2021) 111(4):687–95. doi: 10.2105/AJPH.2020.306014
49. Birnbaum LS, Bornehag CG. Phthalates should be regulated as a class to protect the brains of our children. Am J Public Health (2021) 111(4):551–2. doi: 10.2105/AJPH.2021.306193
50. Hyland C, Mora AM, Kogut K, Calafat AM, Harley K, Deardorff J, et al. Prenatal exposure to phthalates and neurodevelopment in the CHAMACOS cohort. Environ Health Perspect (2019) 127(10):107010. doi: 10.1289/EHP5165
51. Wiechers C, Kirchhof S, Maas C, Poets CF, Franz AR. Neonatal body composition by air displacement plethysmography in healthy term singletons: a systematic review. BMC Pediatr (2019) 19(1):489. doi: 10.1186/s12887-019-1867-y
52. Abernathy RP, Black DR. Healthy body weights: an alternative perspective. Am J Clin Nutr (1996) 63(3 Suppl):448S–51S. doi: 10.1093/ajcn/63.3.448
53. Taylor RW, Gold E, Manning P, Goulding A. Gender differences in body fat content are present well before puberty. Int J Obes Relat Metab Disord (1997) 21(11):1082–4. doi: 10.1038/sj.ijo.0800522
54. Simon L, Borrego P, Darmaun D, Legrand A, Roze JC, Chauty-Frondas A. Effect of sex and gestational age on neonatal body composition. Br J Nutr (2013) 109(6):1105–8. doi: 10.1017/S0007114512002991
55. Forest MG, de Peretti E, Bertrand J. Testicular and adrenal androgens and their binding to plasma proteins in the perinatal period: developmental patterns of plasma testosterone, 4-androstenedione, dehydroepiandrosterone and its sulfate in premature and small for date infants as compared with that of full-term infants. J Steroid Biochem (1980) 12:25–36. doi: 10.1016/0022-4731(80)90247-2
56. Ferguson KK, Bommarito PA, Arogbokun O, Rosen EM, Keil AP, Zhao S, et al. Prenatal phthalate exposure and child weight and adiposity from in utero to 6 years of age. Environ Health Perspect (2022) 130(4):47006. doi: 10.1289/EHP10077
57. Scheurer JM, Zhang L, Plummer EA, Hultgren SA, Demerath EW, Ramel SE. Body composition changes from infancy to 4 years and associations with early childhood cognition in preterm and full-term children. Neonatology (2018) 114(2):169–76. doi: 10.1159/000487915
58. Bekö G, Weschler CJ, Langer S, Callesen M, Toftum J, Clausen G. Children’s phthalate intakes and resultant cumulative exposures estimated from urine compared with estimates from dust ingestion, inhalation and dermal absorption in their homes and daycare centers. PloS One (2013) 8(4):e62442. doi: 10.1371/journal.pone.0062442
59. Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the united states. Environ Res (2008) 106(2):257–69. doi: 10.1016/j.envres.2007.09.010
60. Fromme H, Bolte G, Koch HM, Angerer J, Boehmer S, Drexler H, et al. Occurrence and daily variation of phthalate metabolites in the urine of an adult population. Int J Hygiene Environ Health (2007) 210(1):21–33. doi: 10.1016/j.ijheh.2006.09.005
61. Li N, Papandonatos GD, Calafat AM, Yolton K, Lanphear BP, Chen A, et al. Identifying periods of susceptibility to the impact of phthalates on children’s cognitive abilities. Environ Res (2019) 172:604–14. doi: 10.1016/j.envres.2019.03.009
Keywords: neurodevelopment, phthalates, perinatal exposure, endocrine disruptors, infants, newborns, Griffiths development scales
Citation: Lucaccioni L, Palandri L, Passini E, Trevisani V, Calandra Buonaura F, Bertoncelli N, Talucci G, Ferrari A, Ferrari E, Predieri B, Facchinetti F, Iughetti L and Righi E (2023) Perinatal and postnatal exposure to phthalates and early neurodevelopment at 6 months in healthy infants born at term. Front. Endocrinol. 14:1172743. doi: 10.3389/fendo.2023.1172743
Received: 23 February 2023; Accepted: 08 May 2023;
Published: 24 May 2023.
Edited by:
Ashu Johri, Independent Researcher, New York, NY, United StatesReviewed by:
Robert Gunier, University of California, Berkeley, United StatesWojciech Hanke, Nofer Institute of Occupational Medicine, Poland
Copyright © 2023 Lucaccioni, Palandri, Passini, Trevisani, Calandra Buonaura, Bertoncelli, Talucci, Ferrari, Ferrari, Predieri, Facchinetti, Iughetti and Righi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lorenzo Iughetti, bG9yZW56by5pdWdoZXR0aUB1bmltb3JlLml0
†These authors have contributed equally to this work and share first authorship
 Laura Lucaccioni
Laura Lucaccioni Lucia Palandri
Lucia Palandri Erica Passini
Erica Passini Viola Trevisani
Viola Trevisani Filippo Calandra Buonaura
Filippo Calandra Buonaura Natascia Bertoncelli
Natascia Bertoncelli Giovanna Talucci6
Giovanna Talucci6 Angela Ferrari
Angela Ferrari Barbara Predieri
Barbara Predieri Lorenzo Iughetti
Lorenzo Iughetti