- 1Department of Geriatrics, Tianjin Medical University General Hospital, Tianjin Key Laboratory of Elderly Health, Tianjin Geriatrics Institute, Tianjin, China
- 2Department of Toxicology and Sanitary Chemistry, School of Public Health, Tianjin Medical University, Tianjin, China
- 3Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, Tianjin, China
- 4National Center for Chronic and Noncommunicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China
- 5Tianjin Key Laboratory of Environment, Nutrition and Public Health, Tianjin, China
- 6National Demonstration Center for Experimental Preventive Medicine Education, Tianjin Medical University, Tianjin, China
Objective: The effect of passive smoking exposure on the risk of type 2 diabetes has not been systematically studied. A meta-analysis was conducted to assess the association between passive smoking exposure and the risk of diabetes.
Methods: We searched three major databases up to 31 October 2022 to identify relevant prospective cohort studies on the association between passive smoking and the risk of type 2 diabetes. The pooled relative risk (RR) and 95% confidence interval (CI) for the association between passive smoking exposure and the risk of type 2 diabetes were analyzed using a fixed-effect model.
Results: Ten prospective cohort studies were included in this meta-analysis, with a total of 251,620 participants involved. The pooled RR showed a significantly positive association between nonsmokers exposed to passive smoking and type 2 diabetes as compared to non-smokers who were not exposed to passive smoking [RR = 1.27; 95% CI (1.19, 1.36); p < 0.001]. Sensitivity analysis indicated that the pooled RR was not substantially affected by any of the individual studies.
Conclusion: Exposure to passive smoking increases the risk of type 2 diabetes. This study may have a positive effect on the prevention of type 2 diabetes.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42023372532.
1 Introduction
Diabetes is a chronic metabolic disease, and its rising prevalence is a serious public health concern globally (1). In particular, in developing countries, the prevalence of diabetes and the number of adults with the disease are increasing faster than in high-income countries (2, 3). Type 2 diabetes is the most common type of diabetes, accounting for more than 90% of patients with diabetes. As we all know, it can give rise to secondary complications such as cardiovascular disease, stroke, and diabetes retinopathy (4–6). Therefore, the identification of risk factors of type 2 diabetes is of significant importance to prevent or delay the occurrence of the disease.
Although tobacco control is a major success story in public health after the adoption of the Framework Convention on Tobacco Control, smoking remains a leading risk of premature death and disability worldwide (7). Environmental tobacco smoke contains more than 4,000 chemicals, including at least 40 carcinogens (8). In addition, in a retrospective analysis, 40% of children, 33% of male non-smokers, and 35% of female non-smokers were exposed to passive smoking worldwide (9). It has been shown that passive smoking is associated with a variety of diseases and causes a serious healthcare burden (10).
Evidence of a significant association between type 2 diabetes and active smoking has been documented (11, 12). In a meta-analysis of 25 studies, active smokers had a 44% higher risk of developing type 2 diabetes than never smokers (13). In addition, some studies have also shown that passive smoking is one of the risk factors for type 2 diabetes (14, 15). Prospective cohort studies observed that passive smoking significantly increased the risk of type 2 diabetes in never smokers, and there was a positive dose–response relationship between the passive smoking exposure level and type 2 diabetes in never smokers (16, 17). Thus, reducing exposure to tobacco smoke through effective public health and clinical interventions may avert the onset of type 2 diabetes and other related diseases.
To provide an up-to-date quantitative assessment of the association between passive smoking and type 2 diabetes, we conducted a meta-analysis based on the currently published prospective cohort studies to provide scientific and theoretical evidence for the prevention of type 2 diabetes.
2 Methods
The design, implementation, analysis, and reporting of our meta-analysis were reported in accordance with the PRISMA statement (Table S1). In addition, our study has been registered on PROSPERO (CRD42023372532).
2.1 Search strategy
We conducted a systematic search of PubMed, Web of Science, and Cochrane Library up to 31 October 2022 to identify relevant prospective cohort studies on the association between passive smoking and the risk of type 2 diabetes. We used the following Mesh terms and combinations of words: [“diabetes mellitus, type 2” (Mesh) OR “non-insulin dependent diabetes” OR “diabetes mellitus, type II” OR “type 2 diabetes” OR “T2DM” OR “prediabetic state” OR “glucose metabolism disorders” OR “insulin resistance” OR “hyperglycemia” OR “impaired fasting glucose”] AND [“tobacco smoke pollution” (Mesh) OR “passive smoking” OR “secondhand smoking” OR “environmental tobacco smoke pollution” OR “air tobacco smoke pollution” OR “involuntary smoking”]. The search terms used in different databases are shown in Table S2. The searches were limited to human studies.
2.2 Selection criteria and exclusion criteria
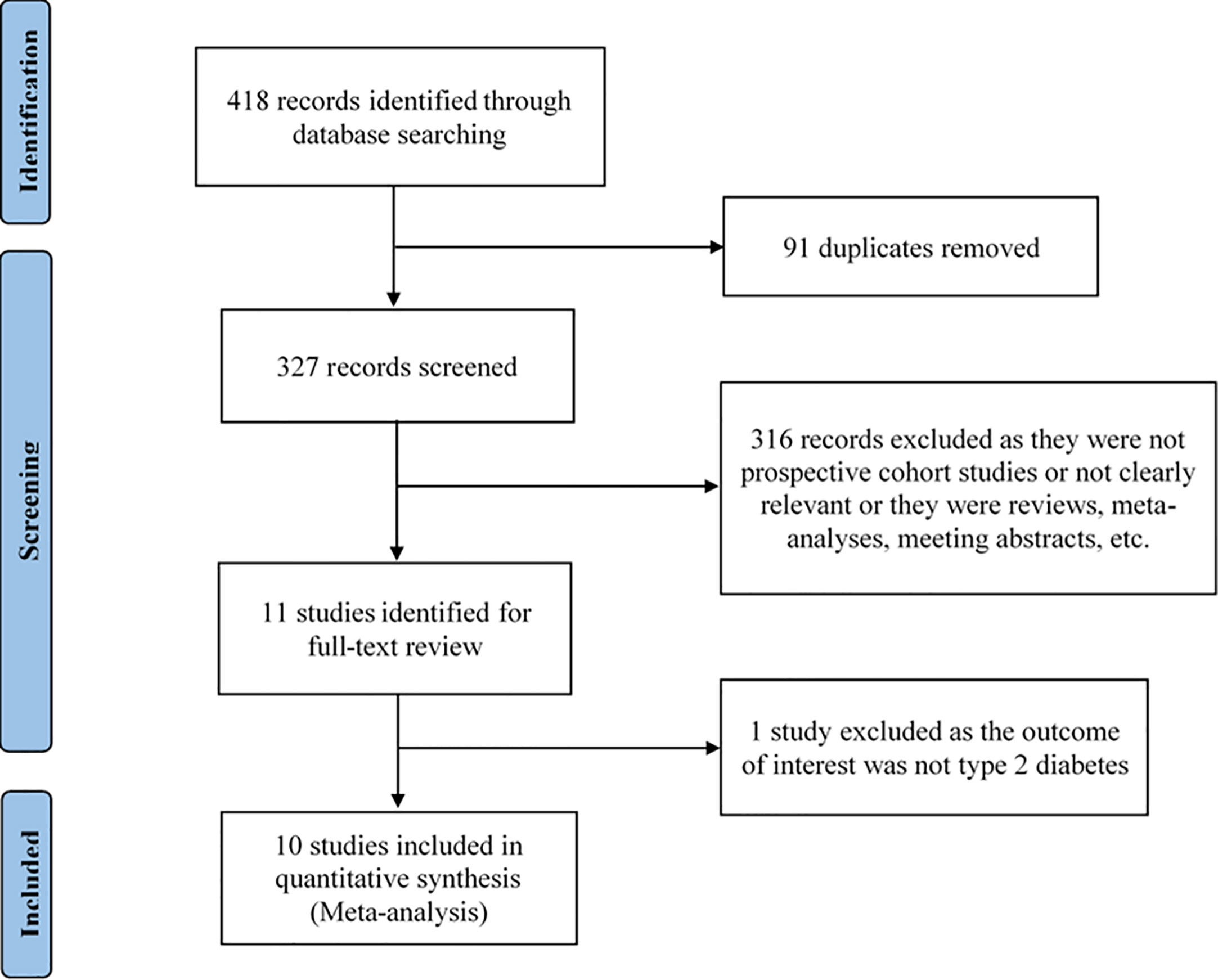
We identified the possible eligible studies by performing an initial screen of identified abstracts or titles and then read the full articles to include eligible studies. The studies were selected if they met all of the following criteria: (1) being a prospective cohort study; (2) the exposure was passive smoking and the outcome was type 2 diabetes; (3) reported estimates of the relative risk (RR) or odds ratio (OR) or hazard ratio (HR) and its 95% confidence interval (CI). The studies were excluded if they met any of the following criteria: (1) being duplicate publications; (2) not being a prospective cohort study; (3) not being relevant; (4) being reviews, meta-analyses, meeting abstracts, and letters; (5) RR or OR or HR and its 95% CI not be reported. Studies with larger sample sizes were chosen among duplicate publications from the same prospective cohort study. Reviews or studies that did not report available information were excluded. The flow chart of the selection of studies is shown in Figure 1.
2.3 Data extraction and quality assessment
Two investigators independently evaluated all relevant articles and identified eligible studies from the databases. Any disagreement about whether a study met the inclusion criteria was resolved through discussion to come to an agreement. The following information of the identified studies was recorded: first author, year of publication, sample size, new cases of type 2 diabetes, follow-up year, RR or OR or HR with corresponding 95% CI, age range, percentage of female, country, define of exposed groups, exposure site, and adjusted variables for each study.
The Newcastle–Ottawa Scale (NOS) was used to assess study quality (18). This scale assigns a maximum of nine points to each study: four scores were assigned for selection of study groups, two scores were assigned for comparability of study groups, and three scores were assigned for assessment of outcomes and adequacy of follow-up. Studies with scores greater than or equal to 7 were defined as a high quality.
2.4 Statistical analysis
When the incidence of an outcome of interest in the study population is low, OR is close to RR (19). In addition, as HR was broadly equivalent to RR, HR was directly considered as RR (14). STATA/MP 17 for Windows was used to analyze the data. In this meta-analysis, RRs and 95% CIs were considered as the effect size for all studies. The values and corresponding standard errors of each study were converted into their natural logarithms to stabilize the variance and normalize its distribution. The fixed-effect model was used to estimate the pooled RR with corresponding 95% CI, and the inverse of the variance was weighted. The I2 statistic was used to estimate the heterogeneity among the studies, and a larger value of I2 indicates a greater heterogeneity, with a p-value < 0.10 deemed to be significant. The RRs and 95% CIs in the included studies were visualized using forest plot. A funnel plot was used to visualize potential publication bias, Egger’s linear regression test was used to measure the asymmetry of the funnel plot, and a p-value < 0.05 was considered significant. Sensitivity analysis was used to examine the impact of a single study. When heterogeneity is high, possible sources of heterogeneity need to be explored by subgroup analyses. All tests were two-tailed, and statistical significance was defined as p < 0.05.
3 Results
3.1 Study selection
The selection process of the study is shown in Figure 1. A total of 418 articles were identified by the search strategy. We first removed 91 articles as they were duplicates, and, then, 327 remaining articles were available for screening. By screening the titles and abstracts, 316 articles excluded as they were not prospective cohort studies or not clearly relevant or they were reviews, meta-analyses, meeting abstracts, etc. After reading the full-text of the remaining 11 articles, one article was excluded as the outcome of interest was not type 2 diabetes. Finally, we selected 10 articles meeting the inclusion criteria for data analysis.
3.2 Study characteristics
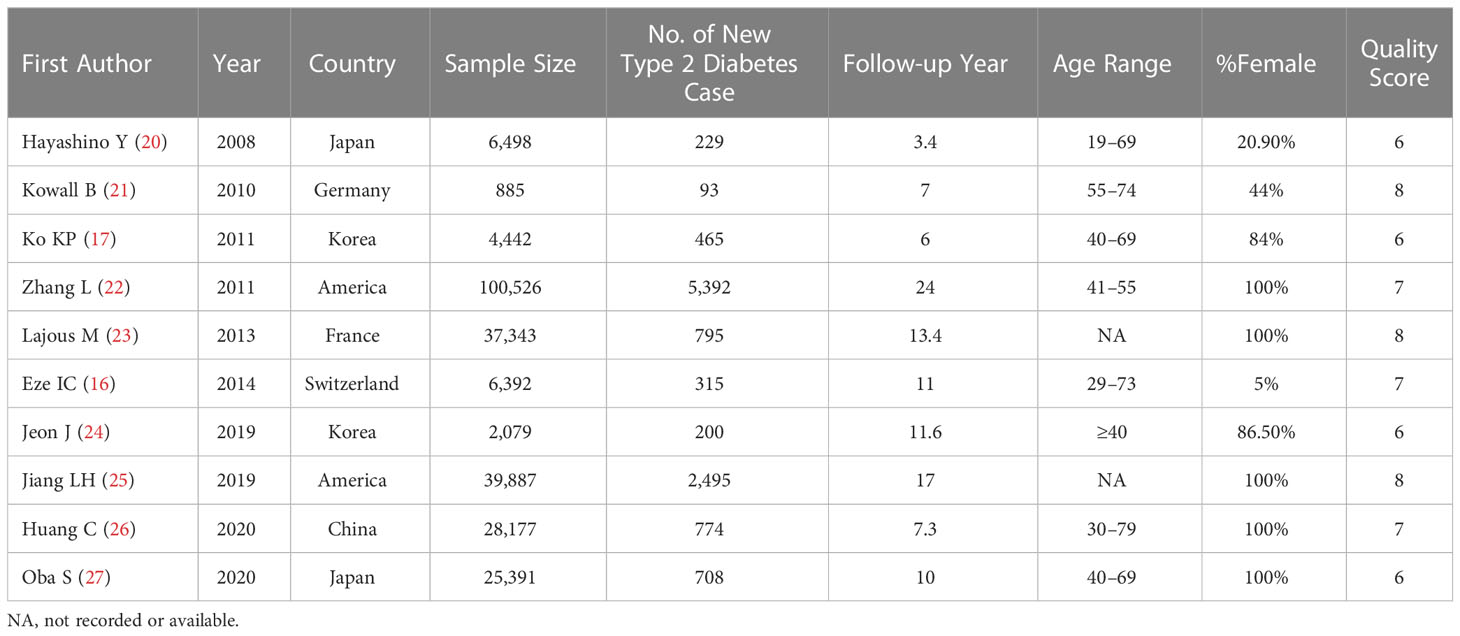
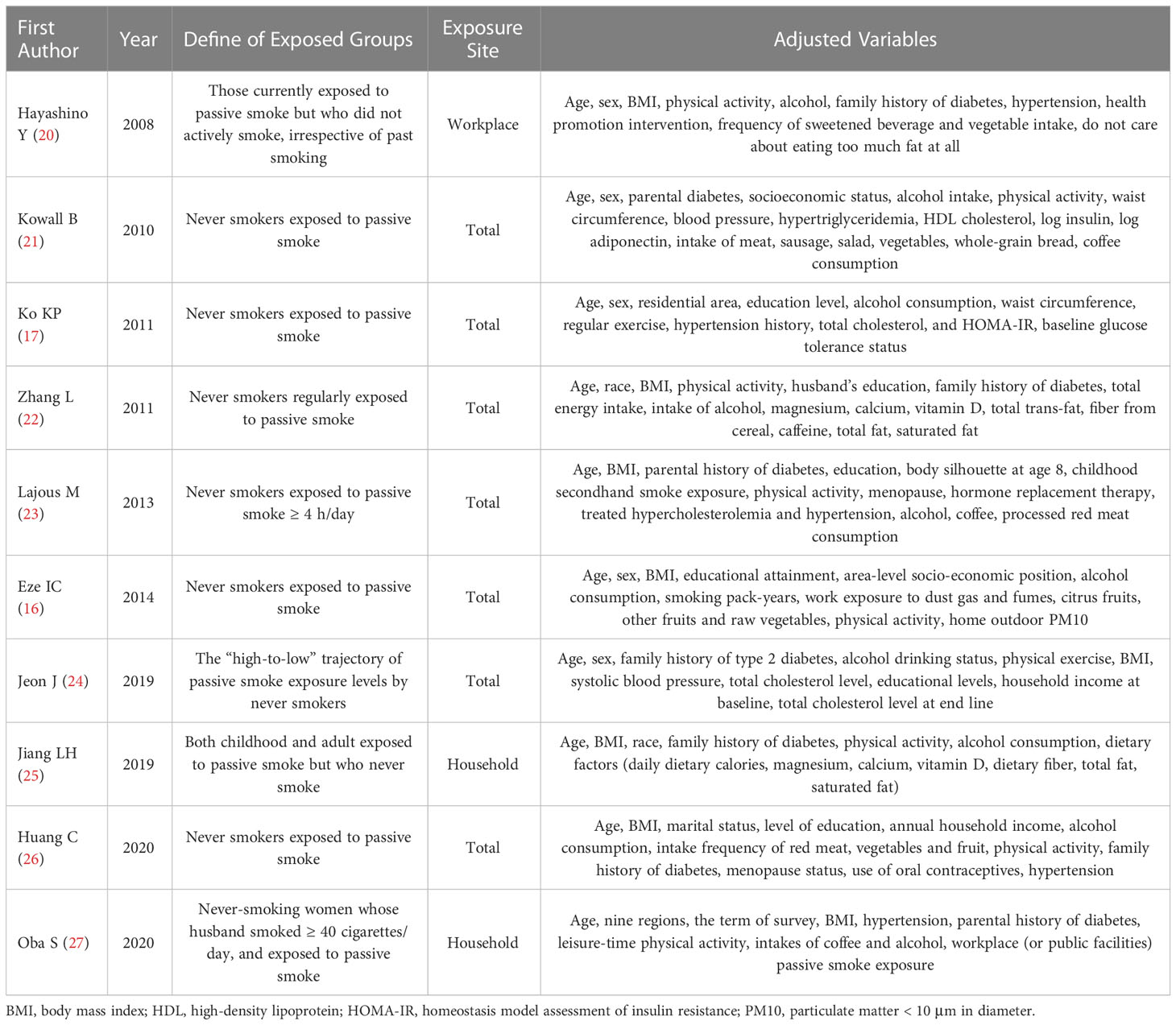
Table 1 summarizes the relevant characteristics of the 10 studies included in the meta-analysis. The population size for each study ranged from 885 to 100,526, involving a total of 251,620 participants from seven countries (two studies from Korea, two studies from Japan, two studies from America, one study from China, one study from Germany, one study from France, and one study from Switzerland). Relevant details of the exposure groups and the adjusted variables for each study are shown in Table 2. The NOS was used to assess the quality of the included articles, and the quality score results are shown in Table 1. In addition, six studies were identified as of relatively high quality (≥7 points). Other characteristics of the included studies are also listed in Table 1.
3.3 Effect of passive smoking exposure on risk of type 2 diabetes
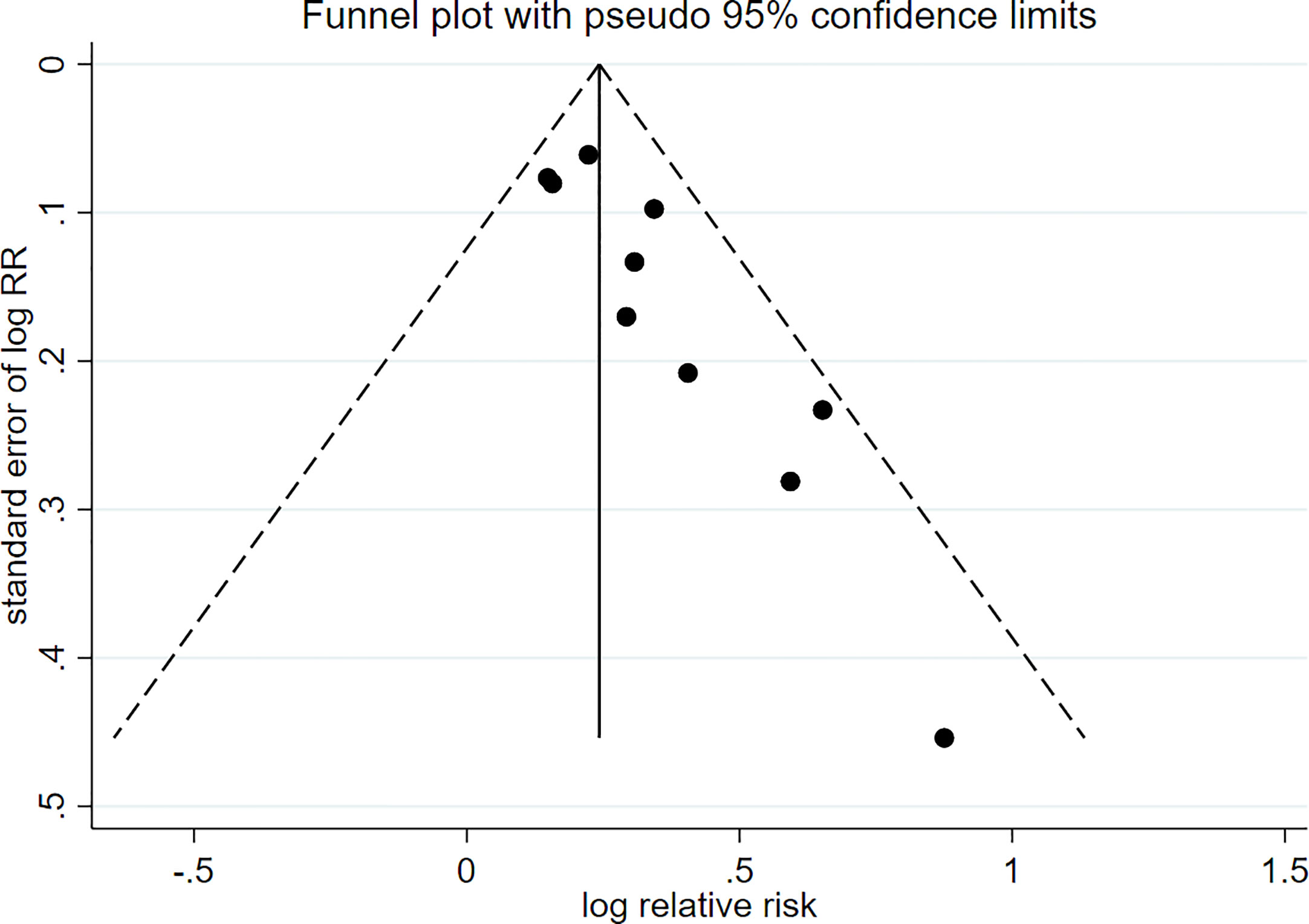
There were 10 prospective cohort studies evaluating the risk of passive smoking with type 2 diabetes. Figure 2 shows a forest plot of the pooled analysis results (pooled RR, 1.27; 95% CI, 1.19–1.36). Compared with non-smokers without passive smoking exposure, non-smokers exposed to passive smoking have an increased risk of type 2 diabetes. There was low heterogeneity between studies (p = 0.252, I2 = 20.8%). The funnel plot in Figure 3 indicates that publication bias was found in the meta-analysis (p < 0.05, Egger’s test).
3.4 Sensitivity analysis
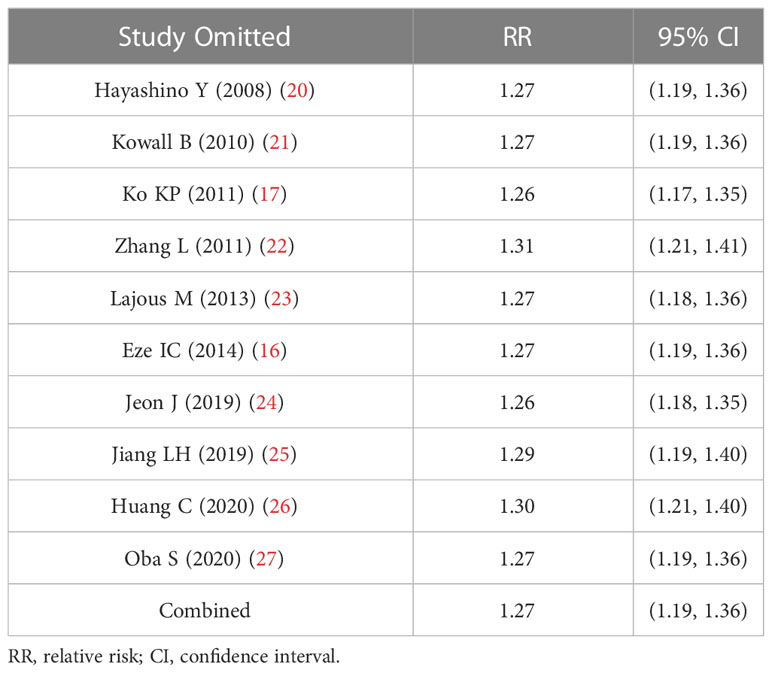
Sensitivity analysis examined the effect on the pooled RR by deleting one study each time. Table 3 shows that passive smoking was significantly associated with a greater risk of type 2 diabetes compared with non-exposed group after deleting individual studies one by one, indicating that the pooled RR was not substantially affected by any of the individual studies.
4 Discussion
Previous epidemiological studies have found that passive smoking is associated with the risk of type 2 diabetes (26, 27). On the basis of the data from 10 prospective cohort studies, this meta-analysis found that exposure to passive smoking increased the risk of type 2 diabetes in non-smokers compared to non-smokers who were not exposed to passive smoking [overall RR = 1.27; 95% CI (1.19, 1.36); p < 0.001].
Type 2 diabetes is influenced by a variety of environmental factors, among which tobacco exposure may be an important risk factor (28). Active smoking has been widely established as a risk factor for type 2 diabetes, although there are relatively few studies on the association between passive smoking and type 2 diabetes (21). The main component of environmental tobacco smoke is sidestream smoke, whose emissions are generally larger than those of mainstream smoke (8). Many toxic components are found in higher concentrations in sidestream smoke than those directly inhaled from cigarettes, such as about five times as much carbon monoxide, about three times as much cigarette tar in sidestream smoke as smoke inhaled directly from cigarettes (29, 30). Compared with active smoking, the negative effects of passive smoking are more widespread, especially for children and adolescents (31). We cannot ignore the fact that passive smoking can occur in many sites, such as household, public places, and even in the workplace. In addition, some studies have found a positive dose–response relationship between the level of environmental tobacco smoke exposure and type 2 diabetes in non-smokers (16, 25). Thus, reducing exposure to passive smoking may have a positive effect on the prevention of type 2 diabetes.
Our findings suggest that passive smoking is associated with type 2 diabetes, which is consistent with other research findings (14, 15). This association may involve several mechanisms: (1) Environmental tobacco smoke contains a large number of toxic substances, among which nicotine is an important ingredient. Nicotine can develop insulin resistance by negatively affecting insulin action (32). In addition, nicotine was found to induce β cell damage through direct interaction with acetylcholine receptors, resulting in reduced insulin production (33, 34). (2) Tobacco smoke exposure decreases insulin secretion by reducing antioxidant production and inducing pancreatic β cells sensitivity to reactive oxygen species, thereby promoting the occurrence of type 2 diabetes (35, 36). (3) Environmental tobacco smoke contains at least 40 carcinogens, and studies have shown that tobacco smoke may cause pancreatic cancer by inducing chronic inflammatory responses (37). This suggests that carcinogens in tobacco smoke have a chronic toxic effect on the pancreas, which, in turn, affects glucose metabolism.
In recent years, environmental pollution caused by passive smoking has received more attention. Passive smoking not only affects the occurrence of type 2 diabetes but also is associated with respiratory diseases, cardiovascular diseases, and cancer (38, 39). In addition, the existence of diabetes will also lead to a series of secondary diseases and affect all organ systems in the body, including diabetic retinopathy, diabetic nephropathy, and diabetic neuropathy (40–42). The hazards of passive smoking are complex, and we should try to avoid tobacco smoke exposure to achieve the purpose of preventing type 2 diabetes.
There are several potential limitations in this study. First, our meta-analysis included only the literature published in English, and, thus, the actual total number of eligible studies may be larger than the number of studies that we included. Second, because the exposure status of passive smoking is self-reported, there may be inaccurate reporting by participants that influence the risk estimates. Finally, on the basis of the fact that the research studies that we included are observational studies, they cannot clarify the causation. Furthermore, there was publication bias in our meta-analysis, whose main source was due to negative results that are not reported.
5 Conclusions
In conclusion, our study suggests that exposure to passive smoking increases the risk of developing type 2 diabetes. The finding has important public health implications for guiding the prevention of type 2 diabetes. By taking appropriate measures to reduce indoor tobacco smoke exposure, the risk of type 2 diabetes may be decreased. An additional meta-analysis that includes well-designed cohort or intervention studies is needed to confirm the current findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Conception and design: QZ and Z-ZF. Search strategy, data extraction, analysis, and writing: G-QQ and LC. Statistical expertise and corrections: JZ, X-MW, YL, KY, and T-FL. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key Research and Development Program of China (2021YFA1301202), National Natural Science Foundation of China (82273676), Liaoning Province Scientific and Technological Project (2021JH2/10300039). Major Research Plan of National Natural Science Foundation of China (Grant No.92163213) and General Program of National Natural Science Foundation of China (Grant No. 81970085).
Acknowledgments
We express deep thanks to the authors of the original studies who provided additional data about their prospective cohort studies, which was critical to this meta-analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1195354/full#supplementary-material
References
1. Zimmet P, Alberti KG, Magliano DJ, Bennett PH. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol (2016) 12(10):616–22. doi: 10.1038/nrendo.2016.105
2. Rawal LB, Tapp RJ, Williams ED, Chan C, Yasin S, Oldenburg B. Prevention of type 2 diabetes and its complications in developing countries: a review. Int J Behav Med (2012) 19(2):121–33. doi: 10.1007/s12529-011-9162-9
3. Collaboration N.C.D.R.F. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet (2016) 387(10027):1513–30. doi: 10.1016/S0140-6736(16)00618-8
4. Balakumar P, Maung UK, Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol Res (2016) 113(Pt A):600–9. doi: 10.1016/j.phrs.2016.09.040
5. Chen R, Ovbiagele B, Feng W. Diabetes and stroke: epidemiology, pathophysiology, pharmaceuticals and outcomes. Am J Med Sci (2016) 351(4):380–6. doi: 10.1016/j.amjms.2016.01.011
6. Lin KY, Hsih WH, Lin YB, Wen CY, and Chang TJ. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J Diabetes Investig (2021) 12(8):1322–5. doi: 10.1111/jdi.13480
7. Collaborators GBDT. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the global burden of disease study 2015. Lancet (2017) 389(10082):1885–906. doi: 10.1016/S0140-6736(17)30819-X
8. Taylor AE, Johnson DC, Kazemi H. Environmental tobacco smoke and cardiovascular disease. a position paper from the council on cardiopulmonary and critical care, American heart association. Circulation (1992) 86(2):699–702. doi: 10.1161/01.cir.86.2.699
9. Oberg M, Jaakkola MS, Woodward A, Peruga A, Pruss-Ustun A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet (2011) 377(9760):139–46. doi: 10.1016/S0140-6736(10)61388-8
10. Hauri DD, Lieb CM, Rajkumar S, Kooijman C, Sommer HL, Roosli M. Direct health costs of environmental tobacco smoke exposure and indirect health benefits due to smoking ban introduction. Eur J Public Health (2011) 21(3):316–22. doi: 10.1093/eurpub/ckq142
11. Manson JE, Ajani UA, Liu SM, Nathan DM, Hennekens CH. A prospective study of cigarette smoking and the incidence of diabetes mellitus among US male physicians. Am J Med (2000) 109(7):538–42. doi: 10.1016/S0002-9343(00)00568-4
12. Houston TK, Person SD, Pletcher MJ, Liu K, Iribarren C, Kiefe CI. Active and passive smoking and development of glucose intolerance among young adults in a prospective cohort: CARDIA study. BMJ (2006) 332(7549):1064–9. doi: 10.1136/bmj.38779.584028.55
13. Willi C, Bodenmann P, Ghali W. A, Faris P. D, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA (2007) 298(22):2654–64. doi: 10.1001/jama.298.22.2654
14. Wang Y, Ji J, Liu YJ, Deng X, He QQ. Passive smoking and risk of type 2 diabetes: a meta-analysis of prospective cohort studies. PloS One (2013) 8(7):e69915. doi: 10.1371/journal.pone.0069915
15. Sun K, Liu D, Wang C, Ren M, Yang C, Yan L. Passive smoke exposure and risk of diabetes: a meta-analysis of prospective studies. Endocrine (2014) 47(2):421–7. doi: 10.1007/s12020-014-0194-1
16. Eze IC, Schaffner E, Zemp E, von Eckardstein A, Turk A, Bettschart R, et al. Environmental tobacco smoke exposure and diabetes in adult never-smokers. Environ Health (2014) 13:74. doi: 10.1186/1476-069X-13-74
17. Ko KP, Min H, Ahn Y, Park SJ, Kim CS, Park JK, et al. A prospective study investigating the association between environmental tobacco smoke exposure and the incidence of type 2 diabetes in never smokers. Ann Epidemiol (2011) 21(1):42–7. doi: 10.1016/j.annepidem.2010.10.006
18. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
19. Zhang J, Yu KF. What's the relative risk? a method of correcting the odds ratio in cohort studies of common outcomes. JAMA (1998) 280(19):1690–1. doi: 10.1001/jama.280.19.1690
20. Hayashino Y, Fukuhara S, Okamura T, Yamato H, Tanaka H, Tanaka T, et al. A prospective study of passive smoking and risk of diabetes in a cohort of workers: the high-risk and population strategy for occupational health promotion (HIPOP-OHP) study. Diabetes Care (2008) 31(4):732–4. doi: 10.2337/dc07-1905
21. Kowall B, Rathmann W, Strassburger K, Heier M, Holle R, Thorand B, et al. Association of passive and active smoking with incident type 2 diabetes mellitus in the elderly population: the KORA S4/F4 cohort study. Eur J Epidemiol (2010) 25(6):393–402. doi: 10.1007/s10654-010-9452-6
22. Zhang L, Curhan GC, Hu FB, Rimm EB, Forman JP. Association between passive and active smoking and incident type 2 diabetes in women. Diabetes Care (2011) 34(4):892–7. doi: 10.2337/dc10-2087
23. Lajous M, Tondeur L, Fagherazzi G, de Lauzon-Guillain B, Boutron-Ruaualt MC, Clavel-Chapelon F, et al. Childhood and adult secondhand smoke and type 2 diabetes in women. Diabetes Care (2013) 36(9):2720–5. doi: 10.2337/dc12-2173
24. Jeon J, Jung KJ, Kimm H, Jee SH. Changes in secondhand smoke exposure levels and risk of type 2 diabetes in middle age: the Korean genome and epidemiology study (KoGES). BMJ Open Diabetes Res Care (2019) 7(1):e000859. doi: 10.1136/bmjdrc-2019-000859
25. Jiang LH, Chang J, Ziogas A, Deapen D, Reynolds P, Bernstein L, et al. Secondhand smoke, obesity, and risk of type II diabetes among California teachers. Ann Epidemiol (2019) 32:35–42. doi: 10.1016/j.annepidem.2019.01.011
26. Huang CY, Chen G, Zhang MZ, Lu Y, Hua YJ, Hu YH, et al. Association between environmental tobacco smoke exposure and risk of type 2 diabetes mellitus in Chinese female never smokers: a population-based cohort study. J Diabetes (2020) 12(4):339–46. doi: 10.1111/1753-0407.13001
27. Oba S, Goto A, Mizoue T, Inoue M, Sawada N, Noda M, et al. Passive smoking and type 2 diabetes among never-smoking women: the Japan public health center-based prospective study. J Diabetes Investig (2020) 11(5):1352–8. doi: 10.1111/jdi.13259
28. Akter S, Goto A, Mizoue T. Smoking and the risk of type 2 diabetes in Japan: a systematic review and meta-analysis. J Epidemiol (2017) 27(12):553–61. doi: 10.1016/j.je.2016.12.017
29. Glantz SA, Parmley WW. Passive smoking and heart disease. epidemiology, physiology, and biochemistry. Circulation (1991) 83(1):1–12. doi: 10.1016/0160-4120(91)90028-O
30. Lofroth G. Environmental tobacco smoke: overview of chemical composition and genotoxic components. Mutat Res (1989) 222(2):73–80. doi: 10.1016/0165-1218(89)90021-9
31. Couriel JM. Passive smoking and the health of children. Thorax (1994) 49(8):731–4. doi: 10.1136/thx.49.8.731
32. Maddatu J, Anderson-Baucum E, Evans-Molina C. Smoking and the risk of type 2 diabetes. Transl Res (2017) 184:101–7. doi: 10.1016/j.trsl.2017.02.004
33. Yoshikawa H, Hellstrom-Lindahl E, Grill V. Evidence for functional nicotinic receptors on pancreatic beta cells. Metabolism (2005) 54(2):247–54. doi: 10.1016/j.metabol.2004.08.020
34. Mukharjee S, Bank S, Maiti S. Chronic tobacco exposure by smoking develops insulin resistance. Endocr Metab Immune Disord Drug Targets (2020) 20(6):869–77. doi: 10.2174/1871530320666200217123901
35. Ashcroft FM, Rorsman P. ATP-sensitive k+ channels: a link between b-cell metabolism and insulin secretion. Biochem Soc Trans (1990) 18(1):109–11. doi: 10.1042/bst0180109
36. Aravamudan B, Kiel A, Freeman M, Delmotte P, Thompson M, Vassallo R, et al. Cigarette smoke-induced mitochondrial fragmentation and dysfunction in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol (2014) 306(9):L840–54. doi: 10.1152/ajplung.00155.2013
37. Malfertheiner P, Schutte K. Smoking–a trigger for chronic inflammation and cancer development in the pancreas. Am J Gastroenterol (2006) 101(1):160–2. doi: 10.1111/j.1572-0241.2006.00402.x
38. Simkovich SM, Goodman D, Roa C, Crocker ME, Gianella GE, Kirenga BJ, et al. The health and social implications of household air pollution and respiratory diseases. NPJ Prim Care Respir Med (2019) 29(1):12. doi: 10.1038/s41533-019-0126-x
39. Khoramdad M, Vahedian-azimi A, Karimi L, Rahimi-Bashar F, Amini H, Sahebkar A. Association between passive smoking and cardiovascular disease: a systematic review and meta-analysis. IUBMB Life (2020) 72(4):677–86. doi: 10.1002/iub.2207
40. ValdezGuerrero AS, Quintana-Perez JC, Arellano-Mendoza MG, Castaneda-Ibarra FJ, Tamay-Cach F, Aleman-Gonzalez-Duhart D. Diabetic retinopathy: important biochemical alterations and the main treatment strategies. Can J Diabetes (2021) 45(6):504–11. doi: 10.1016/j.jcjd.2020.10.009
41. Thipsawat S. Early detection of diabetic nephropathy in patient with type 2 diabetes mellitus: a review of the literature. Diabetes Vasc Dis Res (2021) 18(6):14791641211058856. doi: 10.1177/14791641211058856
Keywords: passive smoking, type 2 diabetes, prospective cohort studies, meta-analysis, relative risk
Citation: Qin G-Q, Chen L, Zheng J, Wu X-M, Li Y, Yang K, Liu T-F, Fang Z-Z and Zhang Q (2023) Effect of passive smoking exposure on risk of type 2 diabetes: a systematic review and meta-analysis of prospective cohort studies. Front. Endocrinol. 14:1195354. doi: 10.3389/fendo.2023.1195354
Received: 28 March 2023; Accepted: 12 June 2023;
Published: 31 July 2023.
Edited by:
S. R. Murthy Madiraju, University of Montreal Hospital Centre (CRCHUM), CanadaReviewed by:
Yifan Bu, Beth Israel Deaconess Medical Center and Harvard Medical School, United StatesDongwei Zhang, Beijing University of Chinese Medicine, China
Copyright © 2023 Qin, Chen, Zheng, Wu, Li, Yang, Liu, Fang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Zhang, emhhbmdxaWFuZ3l1bHZAMTYzLmNvbQ==; Zhong-Ze Fang, ZmFuZ3pob25nemVAdG11LmVkdS5jbg==
†These authors have contributed equally to this work
 Guo-Qiang Qin1,2†
Guo-Qiang Qin1,2† Xiao-Min Wu
Xiao-Min Wu Zhong-Ze Fang
Zhong-Ze Fang